Abstract
Sheeppox virus and goatpox virus cause systemic disease in sheep and goats that is often associated with high morbidity and high mortality. To increase understanding of the pathogenesis of these diseases, we undertook quantitative time-course studies in sheep and goats following intradermal inoculation of Nigerian sheeppox virus or Indian goatpox virus in their respective homologous hosts. Viremia, determined by virus isolation and real-time PCR, cleared within 2 to 3 weeks post inoculation. Peak shedding of viral DNA and infectious virus in nasal, conjunctival and oral secretions occurred between 10 and 14 days post inoculation, and persisted at low levels for up to an additional 3 to 6 weeks. Although gross lesions developed in multiple organ systems, highest viral titers were detected in skin and in discrete sites within oronasal tissues and gastrointestinal tract. The temporal distribution of infectious virus and viral DNA in tissues suggests an underlying pathogenesis that is similar to smallpox and monkeypox where greatest viral replication occurs in the skin. Our data demonstrate that capripoxvirus infections in sheep and goats provide additional and convenient models which are suitable not only for evaluation of poxvirus-specific vaccine concepts and therapeutics, but also study of poxvirus–host interactions.
Keywords: Poxvirus, Capripoxvirus, Sheeppox, Goatpox, Pathogenesis, Tropism, Shedding, Real-time PCR
Introduction
Sheeppox virus (SPPV) and goatpox virus (GTPV), together with lumpy skin disease virus (LSDV) of cattle, comprise the genus Capripoxvirus in the subfamily Chordopoxvirinae, family Poxviridae (Buller et al., 2005). These viruses are the etiological agents of sheeppox, goatpox and lumpy skin disease, respectively, which collectively constitute the most serious poxvirus diseases of production animals (Damon, 2007, Murphy et al., 1999). SPPV and GTPV cause systemic disease in sheep and goats that is characterized by fever, generalized skin nodules, lesions in the respiratory and gastrointestinal tracts and lymph node enlargement (Kitching, 2004, Kitching and Taylor, 1985a). Transmission is considered to occur primarily via respiratory aerosols (Kitching and Taylor, 1985b). However, the potential for mechanical transmission by insect vectors has also been established experimentally (Kitching and Mellor, 1986, Mellor et al., 1987).
SPPV and GTPV are endemic in Africa (north of the equator), the Middle East, central Asia and the Indian subcontinent (Kitching, 2004, Kitching and Carn, 2004), where they have major impacts on small ruminant production due to the often high morbidity and mortality associated with disease in susceptible sheep and goats (Belwal et al., 1982, Bhanuprakash et al., 2005, Bhanuprakash et al., 2006, Garner et al., 2000, Kitching et al., 1986, Kitching et al., 1987b). In contrast, sheeppox and goatpox are exotic to most developed countries, for which the inadvertent or deliberate introduction of disease would have substantial economic ramifications due to the disruption to trade in livestock and livestock products, and the costs associated with disease control and eradication (Garner and Lack, 1995). Accordingly, sheeppox and goatpox are categorized as notifiable diseases by the World Organization for Animal Health (OIE). They are also considered to be of significance as potential economic bioterrorism agents and are listed as United States Department of Agriculture Select Agents on the National Select Agent Registry. Although effective live-attenuated vaccine strains are available for control of disease in sheep and goats (Bhanuprakash et al., 2004, Hosamani et al., 2004b, Kitching, 2003, Kitching et al., 1987a, Roth and Spickler, 2003), the use of live virus vaccines in non-endemic countries may not be desirable.
Sheeppox and goatpox are indistinguishable clinically and strains of SPPV, GTPV and LSDV cannot be differentiated serologically (Kitching, 1986, Kitching and Taylor, 1985a). Nevertheless, distinct host preferences exist with most strains of SPPV and GTPV causing more severe disease in the homologous host (Bhanuprakash et al., 2006, Kitching et al., 1987b, Kitching and Taylor, 1985a, Sharma et al., 1966, Tantawi et al., 1980). Notable exceptions are particular capripoxvirus strains from Africa (Davies, 1976) and the Middle East (Kitching et al., 1986), each of which appears to be uniformly pathogenic in sheep and goats. These observations led previously to the belief that specific isolates of SPPV and GTPV are strains of a single virus species, with apparent differences in host range a result of virus-host adaptation within different geographic regions (Davies and Otema, 1981, Kitching and Taylor, 1985a). Restriction mapping of SPPV, GTPV and LSDV genomes (Black et al., 1986, Gershon and Black, 1988, Kitching et al., 1989) and, more recently, phylogenetic studies based on partial (Hosamani et al., 2004a) and complete (Tulman et al., 2002) genome sequences suggest, however, that all three viruses are distinct. In particular, despite the close genetic relatedness of capripoxvirus isolates, which average no less than 96% nucleotide identity between strains of SPPV, GTPV and LSDV, the majority of the observed differences between species occur in genes that likely determine virulence and host range (Tulman et al., 2002). Extending these studies to encompass a wider range of geographic isolates, including those that cause disease in both sheep and goats, should facilitate understanding of the genetic basis for specific virulence and host range characteristics.
Despite the considerable threat that sheeppox and goatpox pose to small ruminant production, as well as to global trade in sheep, goats and their products, knowledge of SPPV and GTPV, and the underlying pathogenesis of the diseases that they cause, is comparatively rudimentary. Previous studies investigating the pathology of sheeppox in experimentally infected sheep have focused primarily on lesion development in the skin (Krishnan, 1968, Murray et al., 1973, Plowright et al., 1959). Nevertheless, characteristic gross pathology is usually apparent in multiple other organs as part of the systemic manifestation of disease (Kitching and Taylor, 1985a). In addition, although experimental infections in sheep and goats have enabled the qualitative detection of virus in blood as well as in conjunctival and nasal secretions (Kitching and Taylor, 1985a, Kitching and Taylor, 1985b), no studies regarding the systemic spread of virus in either sheep or goats have been previously described. Furthermore, the development of a prominent exanthem (Damon, 2007, Kitching, 2004, Kitching and Taylor, 1985a) following infection by respiratory aerosol or contact implies an underlying pathogenesis which parallels that of smallpox (Fenner, 1988). Although not closely related to orthopoxviruses, capripoxvirus infections in their natural hosts might therefore provide suitable models with which to study novel strategies for smallpox virus vaccine and therapeutic development, particularly since none of the surrogate animal models currently in use faithfully reproduces all facets of smallpox disease in humans (Jordan and Hruby, 2006, McFadden, 2004).
Here, we describe a quantitative time-course study which utilized real-time PCR and traditional virological assays to improve understanding of the pathogenesis of capripoxvirus infections in sheep and goats. Our results have provided valuable insights regarding the nature of acute disease caused by capripoxviruses in their natural hosts, including the unexpected observation that some animals shed infectious virus for at least 2 months following the onset of clinical disease.
Results
Sensitivity and specificity of the capripoxvirus real-time PCR assay
To enable absolute quantitation of viral DNA concentrations in blood, swab and tissue samples, we developed a quantitative real-time PCR (qPCR) TaqMan assay to amplify and detect an 89 bp region within ORF074 (Tulman et al., 2001, Tulman et al., 2002) of SPPV, GTPV and LSDV genomes. Primers and probe were designed following identification of a conserved region within the 3′ end of this gene by pair wise alignment of all capripoxvirus ORF074 sequences available in GenBank (data not shown). A standard curve was developed by testing 10-fold serial dilutions of a plasmid template, containing ORF074, ranging from 106 to 100 copies per triplicate reaction and the assay was consistently able to detect as few as 10 template copies (data not shown). This defined the lower limit of the assay linear dynamic range, which was at least five orders of magnitude. Although notionally able to detect a single copy of ORF074, threshold cycle (C T) values for such reactions varied between 40 and 45. Accordingly, all test samples with C T values greater than 37 and less than 45 were designated positive, and assigned a template copy number < 10.
The specificity of the capripoxvirus primers and probe was confirmed initially by performing similarity searches using the BLAST network service (Altschul et al., 1997). Subsequent testing of DNA extracted from multiple capripoxvirus and parapoxvirus (orf) isolates grown in lamb testis cell monolayers, as well as clinical materials collected from a control sheep and goat, respectively, demonstrated the capripoxvirus-specific nature of the assay (data not shown).
Clinical signs and gross pathology in sheep and goats
To facilitate the study of capripoxvirus pathogenesis in small ruminants, capripoxviruses with predetermined host specificity for either sheep or goats (Kitching and Taylor, 1985a) were selected. After a period of acclimatization, nine sheep and nine goats were inoculated intradermally on a shaved area of the right chest with either Nigerian SPPV or Indian GTPV, respectively. Although one sheep and one goat were to be necropsied every second day from day post inoculation (DPI) 4 through to 14, the severity of the disease in goats resulted, for welfare reasons, in the unscheduled euthanasia and sampling of two additional goats between DPI 11 and 15. Consequently, only one goat and three sheep were available for monitoring and collection of clinical materials between DPI 15 and the end of the study period at DPI 61 (goat) or DPI 63 to 64 (sheep).
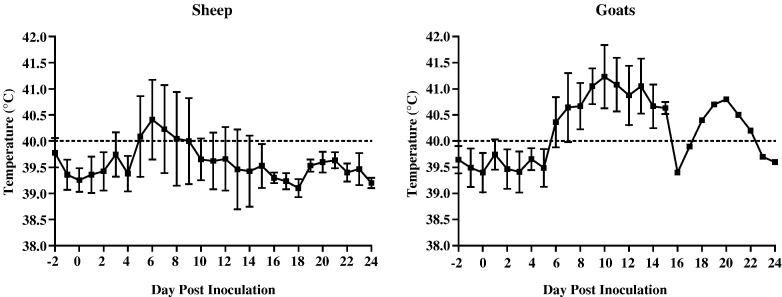
Within 2 to 4 days of virus challenge a well-demarcated, thickened, erythematous primary lesion of up to 5 cm in diameter developed at each inoculation site (Fig. 1A). Rectal temperatures greater than 40 °C, which were indicative of fever, were first observed by DPI 5 in sheep and by DPI 6 in goats. In sheep the duration of fever was, on average, 4 to 5 days with a proportion of sheep showing intermittent low-grade fever for an additional 4 to 5 days. By contrast, fever in goats was generally higher than in sheep and persisted for up to 10 days; in addition, a second temperature spike of lower magnitude and shorter duration was observed in the one goat that survived beyond DPI 15 (Fig. 2 ). In both sheep and goats, the onset of fever coincided with the development of secondary skin lesions, specifically erythematous macules several millimeters in diameter, which faded rapidly and were no longer visible once those animals on DPI 6 were exsanguinated prior to necropsy. Macules progressed to papules within 1 to 2 days and, within the following week, to scabs without either an obvious vesicular or pustular stage. In the most severely affected animals, papules (Fig. 1B) were distributed over more than 50% of the skin surface and were most noticeable in less well-haired or wooled regions including the axillae, groin, perineum, ventral surface of the tail, muzzle and ears. By DPI 6 to 8, coincident with the development of typical pox lesions on mucosal surfaces such as soft palate (Fig. 1C), nasal mucosa, conjunctiva, tongue and buccal surface of the lips, other clinical signs such as rhinitis, conjunctivitis and excessive salivation developed. In addition, superficial lymph node enlargement was also observed at this time and was particularly prominent in prescapular nodes, in several of which petechiae were noted. In the more severely affected goats, which were euthanized and necropsied on DPI 11, 13 or 15, nasal secretions became mucopurulent, and moderate to severe depression was also evident.
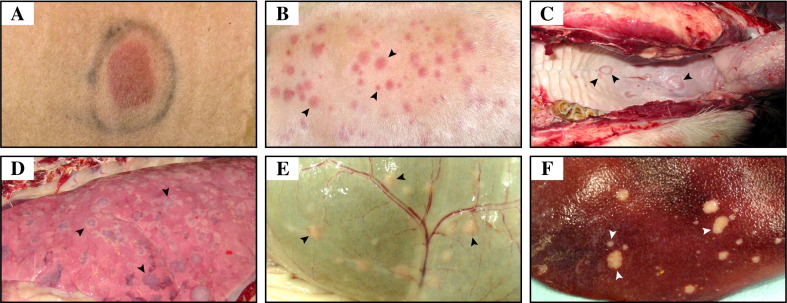
Fig. 1.
Gross lesions following experimental capripoxvirus infection in sheep and goats. (A) Primary lesion on the lateral thorax of a sheep at DPI 4. The lesion was well-demarcated, thickened, red and 4 to 5 cm in diameter. The black mark encircling the lesion was applied to the skin at DPI 0 to identify the site of inoculation. (B) Multifocal papular exanthem on the lateral chest of a goat at DPI 8. Papules (arrowheads) were well-circumscribed, of variable shape, widely distributed and ranged from several millimeters to 1 to 2 cm in diameter. (C) Multifocal ulcerative enanthem in the soft palate of a goat at DPI 15. Ulcerated lesions (arrowheads) were well-circumscribed and ranged from several millimeters to 1 to 2 cm in diameter. Similar ulcerated lesions were present on the tongue, nasal mucosa or buccal mucosa in sheep or goats from DPI 10. (D) Multifocal nodular pneumonia in a goat at DPI 11. Nodules (arrowheads) were pale or red in color, distributed throughout the lung parenchyma and ranged from several millimeters to 4 to 5 cm in diameter. (E) Multifocal nodular enanthem in the rumen mucosa of a goat at DPI 13. Nodules (arrowheads) were pale in color, ranged from several millimeters to 1 to 2 cm in diameter and were readily apparent from the serosal surface of the organ. (F) Multifocal nodular lesions in the liver of a goat at DPI 13. Nodules (arrowheads) were well-circumscribed, pale in color and ranged from pinpoint to 5 mm in diameter.
Fig. 2.
Daily rectal temperatures of sheep and goats infected with Nigerian SPPV or Indian GTPV, respectively. Mean temperatures for all surviving animals during the first three weeks post inoculation are shown, with error bars indicating standard deviations. The upper limit of the normal temperature range (40 °C) is indicated by dashed lines. Note that from DPI 15, data were generated from three sheep and one goat, respectively.
Gross lesions in lungs were first apparent by DPI 6 as red spots on the pleural surface that varied from pinpoint to several millimeters in diameter. By DPI 8 to 10, readily palpable, pale or red nodules, up to several centimeters in diameter, were often present throughout all lung lobes, particularly in those animals that were most affected clinically (Fig. 1D). A marked abdominal lymphadenopathy was also readily apparent from DPI 6 to 8. In addition, discrete nodules were present in rumen (Fig. 1E), abomasum or reticulum from DPI 10 in sheep or goats, while small, well-circumscribed, pale, subcapsular lesions were present in the liver (Fig. 1F) and kidney of goats from DPI 11. The temporal distribution of secondary lesions seen in the six sheep and eight goats that were necropsied between DPI 4 and 15 is summarized in Table 1 .
Table 1.
Distribution of secondary lesions at necropsy in sheep and goats infected with capripoxvirusesa
| DPI | Tissueb |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Skin | Nasal | Pha | Ton | Eso | Tra | Lung | Rum | Abo | Ret | Cec | Liver | Kid | |
| 4 | −,− | −,− | −,− | −,− | −,− | −,− | −,− | −,− | −,− | −,− | −,− | −,− | −,− |
| 6 | +,+ | −,− | −,− | −,− | −,− | −,− | +,+ | −,− | −,− | −,− | −,− | −,− | −,− |
| 8 | +,+ | −,− | −,− | −,− | −,− | −,+ | +,+ | −,− | −,− | −,− | −,− | −,− | −,− |
| 10 | +,+ | −,− | +,− | +,− | −,− | −,− | +,+ | −,− | −,+ | −,− | −,− | −,− | −,− |
| 11 | nc,+ | n,− | n,− | n,+ | n,− | n,+ | n,+ | n,+ | n,+ | n,− | n,+ | n,+ | n,+ |
| 13 | +,+ | −,+ | −,− | −,+ | −,− | −,− | +,+ | −,+ | +,+ | −,− | −,− | −,+ | −,+ |
| 15d | +,++ | −,−− | +,+− | +,+− | +,−− | −,−− | +,++ | +,++ | +,++ | +,−+ | −,−− | −,++ | −,+− |
On any given day post inoculation, the presence (+) or absence (−) of secondary lesions in tissues from one sheep and one goat, respectively, are indicated.
Pha, pharynx; Ton, tongue; Eso, Esophagus; Tra, trachea; Rum, rumen; Abo, abomasum; Ret, reticulum; Cec, cecum; Kid, kidney cortex.
No sample collected.
Data are from one sheep and two goats, respectively.
Detection of capripoxvirus DNA and infectivity in blood
The kinetics of viremia in capripoxvirus-infected sheep and goats was assessed initially using real-time PCR to detect viral genomes and, subsequently, by virus isolation in lamb testis cell monolayers to confirm the presence of infectious virus. Using DNA extracted from whole blood, capripoxvirus genomes were first detected in one of nine goats on DPI 4 and three of eight sheep on DPI 6 (Table 2 ). By DPI 8, the proportion of qPCR-positive animals had increased substantially, with the viral genome copies per ml of blood in 5 of the 12 animals ranging between 103.8 and 105.7. The viral genetic load in blood peaked between DPI 10 and 12 in sheep, and between DPI 10 and 14 in goats. Viral genetic loads during these periods were also elevated in a greater proportion of goats than sheep. Whereas infectious virus was only isolated in blood from one of seven sheep on DPI 8 and three of six sheep on DPI 10, viremia was confirmed by virus isolation in all but two goat samples between DPI 8 and 14. The infectivity in virus-positive sheep and goat blood samples was, however, uniformly low in that none of them generated sufficient viral plaques in lamb testis cell monolayers to enable titration to be undertaken (data not shown). Although one surviving goat had viral genomes detectable by qPCR at DPI 22 and 28, virus could not be detected by qPCR or virus isolation beyond DPI 14 in sheep or DPI 28 in goats.
Table 2.
Detection of capripoxviruses in blood by qPCR and virus isolationa
| Animal no. | Day post inoculation |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2 | 4 | 6 | 8 | 10 | 12 | 14 | 21b | 28 | 42b | 56 | 64b | ||
| Sheep | 1 | −,− | −,− | nc | n | n | n | n | n | n | n | n | n |
| 2 | −,− | −,− | +,− | n | n | n | n | n | n | n | n | n | |
| 3 | −,− | −,− | −,− | 3.8,− | n | n | n | n | n | n | n | n | |
| 4 | −,− | −,− | +,− | +,− | 5.6,+ | n | n | n | n | n | n | n | |
| 5 | −,− | −,− | −,− | +,− | 4.3,− | +,− | n | n | n | n | n | n | |
| 6 | −,− | −,− | +,− | +,+ | 4.2,+ | 5.9,− | 4.5,− | n | n | n | n | n | |
| 7 | −,− | −,− | −,− | 4.4,− | −,− | +,− | +,− | −,− | −,− | −,− | −,− | −,− | |
| 8 | −,− | −,− | −,− | +,− | +,− | +,− | −,− | −,− | −,− | −,− | −,− | −,− | |
| 9 | −,− | −,− | −,− | −,− | +,+ | 3.6,− | +,− | −,− | −,− | −,− | −,− | −,− | |
| Goat | 1 | −,− | −,− | n | n | n | n | n | n | n | n | n | n |
| 2 | −,− | −,− | +,− | n | n | n | n | n | n | n | n | n | |
| 3 | −,− | −,− | −,− | 4.4,+ | n | n | n | n | n | n | n | n | |
| 4 | −,− | −,− | −,− | +,+ | 4.8,+ | n | n | n | n | n | n | n | |
| 5 | −,− | −,− | +,− | 5.7,+ | 5.3,+ | n | n | n | n | n | n | n | |
| 6 | −,− | −,− | +,− | +,+ | 6.2,+ | 5.4,+ | n | n | n | n | n | n | |
| 7 | −,− | +,− | +,− | 4.8,+ | 5.6,+ | 6.0,+ | 6.3,+ | n | n | n | n | n | |
| 8 | −,− | −,− | +,− | −,+ | +,+ | 4.8,+ | 5.2,+ | n | n | n | n | n | |
| 9 | −,− | −,− | −,− | +,+ | 4.2,+ | 5.1,− | 4.4,− | +,− | +,− | −,− | −,− | −,− | |
On any given day, qPCR and virus isolation data, respectively, are shown for each sample. Where quantifiable, viral genome copies (log10) per ml are also shown; “−” indicates the absence of detectable DNA or infectious virus; “+" indicates that the genome copy number was below 103.6/ml or that infectious virus was isolated in lamb testis cell monolayers (note that titration of infectivity was not undertaken on blood samples).
The goat was sampled on DPI 22, 41 and 61, while the sheep were sampled on DPI 21, 42 and either 63 (Sheep 7) or 64 (Sheep 8 and 9).
No sample collected.
Capripoxvirus tropism in solid tissues
The manifestation of pox lesions in skin has long been recognized as a key feature of disease in sheep and goats caused by capripoxviruses. Nevertheless, comprehensive studies investigating the underlying pathogenesis of these diseases have not been previously reported. To gain a better understanding of which tissues facilitate virus replication and dissemination in vivo, we used real-time PCR and virus isolation to identify sites of virus production in capripoxvirus-infected sheep and goats that were necropsied at regular intervals between days 4 and 15 post intradermal inoculation. DNA extracted from tissue homogenates was first quantified and then its concentration adjusted such that 100 ng of DNA from each sample was assayed.
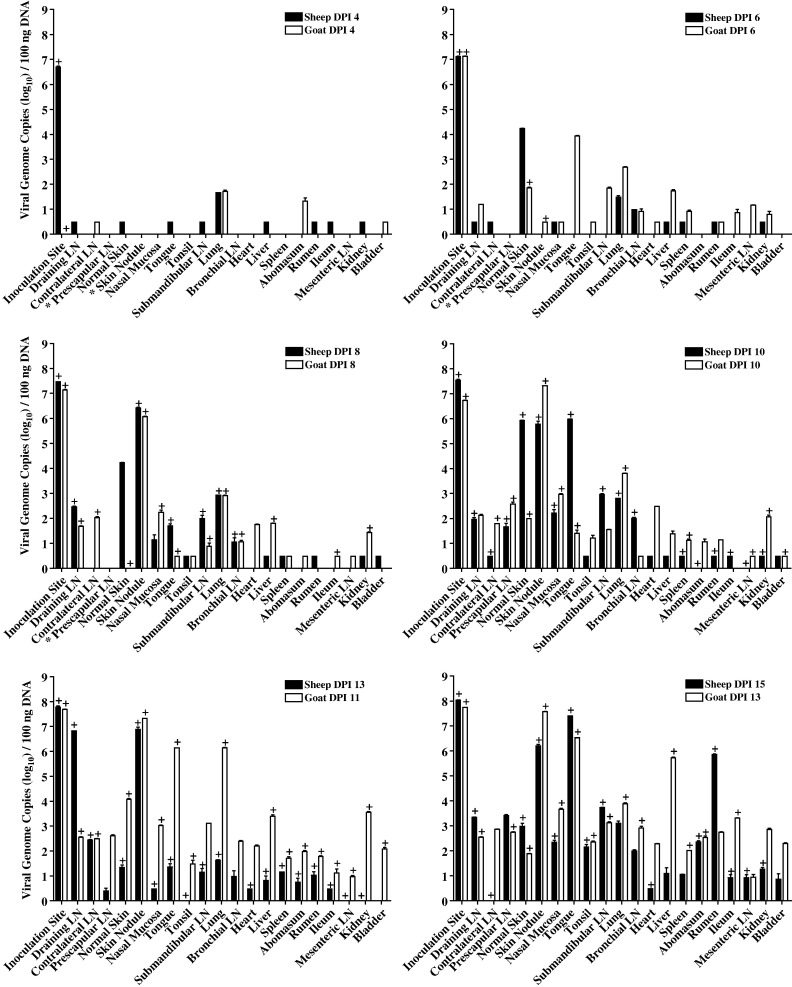
Sampling of the primary lesion that developed at the site of inoculation revealed substantial concentrations of viral genome in sheep and goats by DPI 4 to 6 (approximately 107 viral genomes per 100 ng of extracted DNA), which increased gradually to 108 genomes per 100 ng of extracted DNA by DPI 15 in sheep or DPI 13 in goats (Fig. 3 ). Infectious virus was also readily isolated from the skin inoculation site on all days sampled. On DPI 4, low concentrations of viral DNA (fewer than 102 genomes per 100 ng of extracted DNA) were detected in sheep and goat lung, as well as in a small number of other tissues of either sheep or goat origin. By DPI 6, viral DNA was detected in multiple organs with highest concentrations occurring in apparently normal sheep skin and goat tongue (approximately 104 genomes per 100 ng of extracted DNA). In contrast, infectious virus was only recovered from goat skin on DPI 6, with positive samples including those that were either grossly normal or exhibiting macular lesions. From DPI 8, viral DNA and infectious virus were recovered from an increasing range of tissues, including those from the respiratory and gastrointestinal tracts as well as lymph nodes. However, the highest concentrations of viral DNA were consistently detected at the inoculation site (107 to 108 genomes per 100 ng of extracted DNA) and in secondary skin lesions (106 to 108 genomes per 100 ng of extracted DNA) and, to a lesser extent, in lung (102 to 106 genomes per 100 ng of extracted DNA). Although infectious virus was recovered from most other tissues beyond DPI 8, the viral DNA concentration in these tissues was generally below 102 to 103 genomes per 100 ng of extracted DNA. Several tissues had concentrations of viral DNA greater than or approximately equal to 106 genomes per 100 ng of extracted DNA on one or more days, including tongue on DPI 10 and 15 (sheep) and DPI 11 and 13 (goat), draining lymph node on DPI 13 (sheep), liver on DPI 13 (goat) and rumen on DPI 15 (sheep). With the exception of the draining lymph node, these samples were from discrete lesions such as those depicted in Fig. 1.
Fig. 3.
Viral DNA concentrations in tissues collected from capripoxvirus-infected sheep and goats. Viral DNA concentrations were determined by qPCR and are expressed as log10 viral genome copies per 100 ng of extracted tissue DNA; data are the mean of duplicate reactions with error bars indicating standard deviations. Tissues that were positive by virus isolation are indicated by a plus (+) symbol and an asterisk (⁎) in x-axis labels denotes that a sample was not collected. Note that the goat DPI 4 inoculation site and nasal mucosa, the goat DPI 6 abomasum and the sheep DPI 8 and 15 contralateral lymph node samples were not assayed by qPCR. Note in addition that all qPCR positive samples with fewer than 10 viral genome copies per 100 ng of extracted DNA could not be accurately quantified and were arbitrarily assigned a value of 0.5 on the y-axis. Abbreviations: LN (lymph node); Normal Skin (skin with no apparent gross pathology); Kidney (kidney cortex).
Selected goat tissues were subsequently homogenized and titrated on replicate lamb testis cell monolayers to determine viral titers (Table 3 ). The titer at the inoculation site increased from DPI 4 (104.9 TCID50/g) to DPI 8 (greater than 107.2 TCID50/g). Similarly, moderate to high viral titers were detected consistently in apparently normal skin (102.7 to 104.4 TCID50/g) and in secondary skin lesions (less than 102.7 to greater than 107.2 TCID50/g) from DPI 6, in lung (103.2 to 105.2 TCID50/g) and tongue (103.7 to 105.7 TCID50/g) from DPI 8, and, to a lesser extent, in nasal mucosa and ileum on DPI 11 and 13. Infectious virus was detectable in spleen on DPI 10, 11 and 13, but it was not of a sufficiently high titer to permit quantitation. In contrast, moderate titers of virus were present in liver on DPI 8 and 13. Of note, even though there were moderate to high viral titers in skin, nasal mucosa, lung, tongue and ileum on two or more days, infectious virus was only detected inconsistently at low to moderate levels in the lymph nodes that drained these regions.
Table 3.
Quantitation of viral infectivity in goat tissuesa
| Tissue | Day post inoculation |
|||||
|---|---|---|---|---|---|---|
| 4 | 6 | 8 | 10 | 11 | 13 | |
| Inoculation site | 4.9 | 6.7 | > 7.2 | > 7.2 | > 7.2 | > 7.2 |
| Draining LNb | − | − | 4.2 | − | + | + |
| Liver | − | − | 3.2 | − | + | 4.7 |
| Spleen | − | − | − | + | + | + |
| Normal skin | − | 2.7 | 3.0 | 4.2 | 4.4 | 3.2 |
| Macule | nc | + | n | n | n | n |
| Papule | n | n | 6.0 | 7.0 | 5.2 | > 7.2 |
| Nasal mucosa | − | − | + | + | 3.2 | 2.7 |
| Lung | − | − | 3.2 | 5.2 | 3.7 | 5.2 |
| Bronchial LN | − | − | 2.7 | − | − | + |
| Ileum | − | − | + | − | 3.2 | 3.2 |
| Mesenteric LN | − | − | − | + | 2.7 | − |
| Tongue | − | − | 5.2 | 3.7 | 4.4 | 5.7 |
| Submandibular LN | − | − | + | − | − | + |
| Rumen | − | − | − | − | + | − |
On any given day, the viral infectivity in each tissue is shown. Where quantifiable, viral titers (log10 TCID50/g) are indicated; “−” indicates the absence of infectious virus; “+” indicates that the titer was below102.7 TCID50/g.
Lymph node.
No sample collected.
Kinetics of capripoxvirus shedding from mucosal surfaces
To investigate virus shedding from mucosal surfaces, various swabs and urine, collected at different stages throughout the study, were screened firstly by qPCR, to quantify viral genomes, and, subsequently, by titration of infectivity in replicate lamb testis cell monolayers. DNA extracted from standardized volumes of each swab or urine sample was used as template for qPCR. The accumulation of viral DNA or infectious virus in nasal, conjunctival and oral secretions was first detected in most animals by DPI 6 to 8, peaked between DPI 10 and 14 and declined rapidly thereafter (Table 4 ). Of particular note, infectious virus was detected in nasal secretions of every sheep sampled between DPI 8 and 64, albeit at low levels (less than 101.7 TCID50/ml of viral transport medium) from DPI 21. Low level viral shedding in nasal secretions was also observed in the only goat to survive beyond DPI 15, with infectious virus in this animal detectable from DPI 12 through to DPI 41. A similar pattern of extended shedding was observed in conjunctival and oral samples, with infectious virus detectable in conjunctival swabs from three of three sheep until DPI 42 and from one goat until DPI 22, and in oral swabs from one of three sheep until DPI 28 and from one goat until DPI 22.
Table 4.
Kinetics of virus shedding from mucosal surfaces measured by qPCR and virus isolationa
| Swab | Animal no. | Day post inoculation |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2 | 4 | 6 | 8 | 10 | 12 | 14 | 21b | 28 | 42b | 56 | 64b | |||
| Nasal | Sheep | 1 | −,− | −,− | nc | n | n | n | n | n | n | n | n | n |
| 2 | −,− | −,− | −,− | n | n | n | n | n | n | n | n | n | ||
| 3 | −,− | −,− | +,− | 5.1,+ | n | n | n | n | n | n | n | n | ||
| 4 | −,− | −,− | +,− | +,+ | 6.2,3.0 | n | n | n | n | n | n | n | ||
| 5 | −,− | −,− | +,− | +,+ | 4.4,2.2 | +,+ | n | n | n | n | n | n | ||
| 6 | −,− | −,− | −,− | +,+ | 5.0,2.2 | 4.6,+ | 4.5,3.2 | n | n | n | n | n | ||
| 7 | −,− | −,− | −,− | +,+ | 3.5,1.7 | +,+ | +,1.7 | 3.6,2.0 | 3.5,+ | 3.7,+ | +,+ | −,+ | ||
| 8 | −,− | −,− | −,− | −,+ | +,2.2 | +,+ | 5.1,+ | −,+ | +,+ | 3.4,+ | +,+ | +,+ | ||
| 9 | −,− | −,− | −,− | +,+ | +,1.7 | +,+ | +,+ | 3.6,+ | +,+ | +,+ | +,+ | −,+ | ||
| Goat | 1 | −,− | −,− | n | n | n | n | n | n | n | n | n | n | |
| 2 | −,− | −,− | +,− | n | n | n | n | n | n | n | n | n | ||
| 3 | −,− | −,− | +,− | +,− | n | n | n | n | n | n | n | n | ||
| 4 | −,− | −,− | −,− | −,− | 5.3,− | n | n | n | n | n | n | n | ||
| 5 | −,− | −,− | −,− | +,− | 5.7,− | n | n | n | n | n | n | n | ||
| 6 | −,− | −,− | −,− | +,− | 6.0,4.2 | 4.9,3.0 | n | n | n | n | n | n | ||
| 7 | −,− | −,− | −,− | +,− | 4.2,− | 5.0,− | 6.4,2.7 | n | n | n | n | n | ||
| 8 | −,− | −,− | −,− | +,− | n,+ | 6.2,+ | 5.6,+ | n | n | n | n | n | ||
| 9 | −,− | −,− | −,− | −,− | −,− | +,+ | +,+ | +,+ | +,+ | +,+ | +,− | −,− | ||
| Cond | Sheep | 1 | −,− | −,− | n | n | n | n | n | n | n | n | n | n |
| 2 | −,− | −,− | −,− | n | n | n | n | n | n | n | n | n | ||
| 3 | −,− | −,− | −,− | +,+ | n | n | n | n | n | n | n | n | ||
| 4 | −,− | −,− | −,− | 3.6,− | 5.1,+ | n | n | n | n | n | n | n | ||
| 5 | −,− | −,− | −,− | −,− | 4.4,+ | −,+ | n | n | n | n | n | n | ||
| 6 | −,− | −,− | −,− | 4.2,− | 3.8,+ | 4.4,+ | 4.3,+ | n | n | n | n | n | ||
| 7 | −,− | −,− | −,− | −,− | +,+ | +,+ | +,− | +,3.2 | 3.8,+ | +,+ | +,− | −,− | ||
| 8 | −,− | −,− | −,− | −,− | −,− | +,+ | 4.7,− | +,+ | +,+ | 3.9,+ | +,− | −,− | ||
| 9 | −,− | −,− | −,− | −,− | +,+ | −,+ | +,− | +,+ | +,+ | +,+ | +,− | −,− | ||
| Goat | 1 | −,− | −,− | n | n | n | n | n | n | n | n | n | n | |
| 2 | −,− | −,− | −,− | n | n | n | n | n | n | n | n | n | ||
| 3 | −,− | −,− | −,− | −,− | n | n | n | n | n | n | n | n | ||
| 4 | −,− | −,− | −,− | +,− | 4.7,2.5 | n | n | n | n | n | n | n | ||
| 5 | −,− | −,− | −,− | +,− | 4.2,− | n | n | n | n | n | n | n | ||
| 6 | −,− | −,− | −,+ | 3.8,+ | 6.0,3.0 | 6.4,4.2 | n | n | n | n | n | n | ||
| 7 | −,− | −,− | −,− | −,− | 5.3,3.2 | 6.0,4.4 | 7.0,5.0 | n | n | n | n | n | ||
| 8 | −,− | −,− | −,− | −,− | n,− | +,− | +,− | n | n | n | n | n | ||
| 9 | −,− | −,− | −,− | −,− | +,− | 5.5,− | +,+ | +,+ | −,− | +,− | +,− | +,− | ||
| Oral | Sheep | 1 | −,− | −,− | n | n | n | n | n | n | n | n | n | n |
| 2 | −,− | −,− | −,− | n | n | n | n | n | n | n | n | n | ||
| 3 | −,− | −,− | +,− | 4.3,+ | n | n | n | n | n | n | n | n | ||
| 4 | −,− | −,− | +,+ | 4.2,+ | 5.5,2.4 | n | n | n | n | n | n | n | ||
| 5 | −,− | −,− | −,− | +,− | +,+ | 4.7,1.7 | n | n | n | n | n | n | ||
| 6 | −,− | −,− | −,− | +,+ | +,+ | 3.7,+ | 3.4,− | n | n | n | n | n | ||
| 7 | −,− | −,− | −,− | 3.6,− | 5.3,+ | 4.1,− | 3.7,1.7 | 4.9,+ | 4.2,+ | +,− | −,− | −,− | ||
| 8 | −,− | −,− | +,− | +,− | +,+ | 5.2,+ | 5.8,1.7 | 3.6,− | +,− | +,− | −,− | −,− | ||
| 9 | −,− | −,− | −,− | +,− | 3.5,1.7 | 3.7,+ | 3.5,− | 3.5,− | −,− | +,− | −,− | −,− | ||
| Goat | 1 | −,− | −,− | n | n | n | n | n | n | n | n | n | n | |
| 2 | −,− | −,− | −,− | n | n | n | n | n | n | n | n | n | ||
| 3 | −,− | −,− | −,− | +,− | n | n | n | n | n | n | n | n | ||
| 4 | −,− | −,− | +,− | +,− | 3.4,− | n | n | n | n | n | n | n | ||
| 5 | −,− | −,− | −,− | −,− | 5.3,2.4 | n | n | n | n | n | n | n | ||
| 6 | −,− | −,− | −,− | +,− | 5.1,2.4 | 3.8,+ | n | n | n | n | n | n | ||
| 7 | −,− | −,− | −,− | +,− | 5.1,+ | 5.1,2.2 | 6.2,3.7 | n | n | n | n | n | ||
| 8 | −,− | −,− | −,− | +,− | n,− | 3.7,+ | 4.9,+ | n | n | n | n | n | ||
| 9 | −,− | −,− | −,− | −,− | 3.5,− | +,1.7 | 3.8,+ | 4.4,+ | 4.8,− | 4.1,− | 3.7,− | +,− | ||
On any given day, the viral genetic load and viral infectivity in each sample, determined by qPCR and virus isolation, respectively, are shown. Where quantifiable, viral genome copies (log10) and viral titers (log10 TCID50) per ml of viral transport medium, into which swabs were collected, are also shown; “−” indicates the absence of detectable DNA or infectious virus; “+” indicates either that the genome copy number was below 103.4/ml or that the titer was below 101.7 TCID50/ml.
The goat was sampled on DPI 22, 41 and 61, whereas the sheep were sampled on DPI 21, 42 and either 63 (Sheep 7) or 64 (Sheep 8 and 9).
No sample collected.
Conjunctival.
The recovery of viral DNA and infectious virus from rectal swabs and urine, collected during necropsy, was less consistent (Table 5 ). For rectal swabs, infectious virus was recovered from goats that were necropsied between DPI 8 and 15, whereas viral DNA was detected as early as DPI 4 and as late as DPI 61 by qPCR. For sheep, infectious virus was only recovered from rectal swabs on DPI 8, with samples collected on DPI 10 and 15 positive by qPCR. No viral DNA or infectious virus was recovered from the urine of any goat. However, viral DNA was detected in sheep urine on DPI 10 and 15, with the later sample also positive by virus isolation. Nevertheless, the infectivity of all virus-positive rectal swabs and urine specimens was low (below 101.7 TCID50/ml of viral transport medium or urine, respectively).
Table 5.
Detection of virus in rectal swabs and urine at necropsya
| Sample | Animal | Day post inoculation |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 4 | 6 | 8 | 10 | 11 | 13 | 15b | 61 | 63 | 64b | ||
| Rectal swab | Sheep | −,− | −,nc | −,+ | 3.5,− | n,n | n,− | 3.4,− | n,n | −,− | −,− |
| −,− | |||||||||||
| Goat | +,n | +,− | −,+ | +,+ | +,+ | −,+ | 7.4,+ | +,− | n,n | n,n | |
| 4.1,+ | |||||||||||
| Urine | Sheep | −,− | −,− | −,− | 3.4,− | n,n | −,− | 3.9,+ | n,n | −,− | n,− |
| n,− | |||||||||||
| Goat | −,n | n,n | n,− | −,− | −,− | −,− | −,− | −,− | n,n | n,n | |
| −,− | |||||||||||
On any given day, the viral genetic load and viral infectivity in each sample, determined by qPCR and virus isolation, respectively, are shown. Where quantifiable, viral genome copies (log10) and viral titers (log10 TCID50) per ml of viral transport medium, into which swabs were collected, or urine, respectively, are also shown; “−” indicates the absence of detectable DNA or infectious virus; “+” indicates either that the genome copy number was below 103.4/ml or that the titer was below 101.7 TCID50/ml of each respective sample type.
Two goats or two sheep were sampled on DPI 15 and 64, respectively.
No sample tested.
Discussion
This study confirmed the relative ease of producing systemic capripoxvirus infections in sheep and goats, by intradermal inoculation, using tissue culture passaged virus. Two distinct virus isolates were used, specifically Nigerian SPPV and Indian GTPV, which were selected on the basis that each has a clear specificity for causing disease in sheep or goats, respectively (Kitching and Taylor, 1985a). The clinical signs and gross pathology that developed following experimental infection of sheep and goats were comparable, and were also consistent with previous descriptions of sheeppox and goatpox caused by these, and other, capripoxvirus isolates (Kitching et al., 1986, Kitching et al., 1987b, Kitching et al., 1989, Kitching and Taylor, 1985a). Notably, the morbidity in goats infected with Indian GTPV was more severe than that in sheep infected with Nigerian SPPV. In this regard, the increased severity of disease in goats was characterized by: fevers of greater duration and magnitude; more consistent and longer lasting viremia; development of profuse, mucopurulent nasal discharges; excessive salivation; development of moderate to marked depression; the presence, later in the disease course, of gross lesions in a greater range of viscera at necropsy; and the unscheduled euthanasia, on welfare grounds, of additional goats between DPI 11 and 15. An explanation for the apparent differences in pathogenicity of each isolate was not investigated as part of this study, but could be attributed to one or more factors including the passage history of each inoculum, the intrinsic virulence of the virus isolates, the genetic susceptibility of the animals and the route of inoculation. Although we were unable to determine mortality rates for either capripoxvirus isolate, previous experiments using Nigerian SPPV or Indian GTPV resulted in mortalities of 75 to 100% depending on the host breed and dose of inoculum (Kitching and Taylor, 1985a). Nevertheless, the disease in sheep and goats was clearly systemic, which enabled a detailed, quantitative analysis of capripoxvirus dissemination and shedding to be undertaken using real-time PCR to determine viral DNA concentrations in clinical specimens, followed by virus isolation to confirm sample infectivity.
Although transmission of sheeppox and goatpox is believed to occur primarily via respiratory aerosol, the difficulty in precisely synchronizing the experimental infection of sheep and goats by this method (Kitching and Taylor, 1985b) led us to inoculate animals intradermally instead. Inoculation of capripoxviruses into the dermis is recognized as an efficient means by which to experimentally infect sheep and goats (Davies, 1976, Kitching et al., 1986, Kitching et al., 1987b, Kitching et al., 1989, Kitching and Taylor, 1985a) and, apart from a shorter incubation period, results in clinical disease that closely resembles that seen following successful infection by aerosol or by spread from infected to susceptible, in-contact animals (Kitching and Taylor, 1985a, Kitching and Taylor, 1985b). The incubation period which, in this study, we defined as the interval from intradermal inoculation to onset of fever, was 5 to 6 days in sheep and goats. The end of the incubation period coincided with the onset of measurable viremia, for which the presence of viral DNA, first detected by qPCR on DPI 4 in goats or DPI 6 in sheep, preceded the isolation of infectious virus in sheep and goats by 1 to 2 days. However, although virus clearly replicated at the inoculation site and in multiple tissues remote to the primary lesion that developed at this site, the tissues which contributed principally to the development of viremia could not be identified. Even though very low concentrations of viral DNA could be detected in the lymph node draining the inoculation site in animals that were necropsied on DPI 4 and 6, infectious virus could not be recovered from this node until DPI 8 in either sheep or goats. Furthermore, no infectious virus was recovered from any organ distant to the inoculation site on DPI 4, despite the fact that viral DNA could be detected at low concentrations (fewer than 10 genome copies per 100 ng of extracted DNA) in multiple organs, as well as in lung (approximately 102 genome copies per 100 ng of extracted DNA). A slightly higher viral DNA concentration in sheep and goat lung on DPI 4 suggests that this might be one of multiple organs which were infected during the primary viremia that must have occurred prior to DPI 4. Nevertheless, infectious virus could not be recovered in tissues, other than skin, until DPI 8. Beyond DPI 8, viral DNA and infectious virus could be detected in most tissues, with highest concentrations of DNA present in skin (exceeding 106 genome copies per 100 ng of extracted DNA) and, to a lesser extent, lung (approximately 103 to 106 genome copies per 100 ng of extracted DNA). Several other tissues had concentrations of viral DNA that were comparable to that seen in skin but, apart from the draining lymph node collected from a sheep on DPI 13, these were invariably associated with discrete pox lesions in tongue, rumen or liver. The low concentrations of viral DNA (fewer than 102 genome copies per 100 ng of extracted DNA) that were consistently detected between DPI 8 to 15 in spleen, which is a well-perfused organ, probably reflect the maximum viral genetic load that could be attributed to viremia alone. Accordingly, the slightly higher viral DNA concentrations (approximately 103 genome copies per 100 ng of extracted DNA) in various tissues, including those of lymphoid origin, suggest that they might also be target sites for virus replication. Of particular note, however, was the conspicuous absence of histological lesions in liver and spleen prior to DPI 10 (data not shown), as well as the low viral genetic loads and infectivity in these organs. These observations demonstrate that the pathogenesis of sheeppox and goatpox is substantially different to that of mousepox, in which there is extensive replication and necrosis in liver and spleen prior to widespread seeding of virus to the skin (Fenner, 1948a, Fenner, 1948b). Nevertheless, our data confirm the tropism of SPPV and GTPV for skin and lung, as well as discrete sites within mucosal surfaces of oronasal tissues and the gastrointestinal tract, and, to a lesser extent, lymphoid tissues.
Although we did not investigate the nature of the viremia in this study, the previously reported infectivity of buffy coats (Kitching and Taylor, 1985a) suggests that it is cell-associated. More recently, immunohistochemical studies in skin and lung of naturally infected lambs have confirmed that the majority of virus-infected cells in the inflammatory infiltrates are in fact virus-infected monocytes and macrophages (Gulbahar et al., 2006). By analogy with the monocyte-associated viremia that has been observed in monkeys infected with either monkeypox virus (Zaucha et al., 2001) or variola virus (Jahrling et al., 2004), we suggest that dissemination of SPPV and GTPV from the primary lesion to the draining lymph node, and then to the systemic circulation, with localization to skin and other tissues, is therefore also likely to occur through infected monocytes and macrophages. It is clearly of interest to examine all tissues histologically to further define the cellular tropism of these viruses, both during and after the incubation period, and these studies are currently in progress.
The onset of virus shedding in nasal, conjunctival and oral secretions occurred in most animals between DPI 6 to 10 and correlated with the appearance of ulcerated lesions on associated mucosal surfaces, most probably resulting from hematogenous spread to these tissues. Peak shedding of viral DNA and infectious virus in each sample type occurred between DPI 10 and 14 and declined rapidly thereafter. In all cases, the highest infectious titers in nasal, conjunctival and oral swabs were observed in goats, and correlated with the greater severity of clinical signs in these animals. Nevertheless, despite the development of virus neutralizing antibodies in sheep and goat sera by DPI 10 to 14 (data not shown), virus was shed at low concentrations in nasal secretions until DPI 64 in sheep or 41 in goats, conjunctival secretions until DPI 42 in sheep or 22 in goats, and oral secretions until DPI 28 in sheep or 22 in goats. Furthermore, virus shedding was also detected at low levels in urine and feces between DPI 8 and 15, with the higher proportion of positive goat rectal swabs presumably also a reflection of the more severe disease in these animals. Conceivably, all such secretions and excretions could contribute to virus transmission either directly by aerosol, in the case of nasal and oral secretions, or indirectly by contamination of the environment. Although such extended shedding has not been reported previously for SPPV or GTPV isolates, shedding in semen has been observed for as long as 42 days following inoculation of bulls with LSDV (Irons et al., 2005). In addition, shedding of ectromelia virus in mice has been observed from tail lesions or in feces for up to 116 days following oral inoculation (Gledhill, 1962), and in feces for up to 47 days or from skin for up to 60 days following intragastric inoculation (Wallace and Buller, 1985). Similar extended periods of virus shedding have not been reported for other poxviruses. Even so, our findings contrast with a previous report by Kitching and Taylor (1985b), who were unable to isolate infectious virus from nasal swabs beyond the twelfth day after the onset of fever in sheep that had been infected by aerosol or contact with a capripoxvirus isolate from Yemen. Based on these apparently conflicting observations, it would be of considerable interest to extend these investigations in sheep and goats that have been inoculated either intradermally or by inhalation. Although the source of virus for the extended shedding from mucosal surfaces was not identified in our studies, it is plausible that replication and shedding might occur at specific mucosal sites without marked lesion development, as is thought to take place in the upper respiratory tract of some human vaccinees who are subsequently exposed to variola virus (Fenner, 1988). Evidence for this hypothesis might be obtained by detailed histopathological and immunohistochemical examination of these sites, and this work is in progress.
Sheeppox and goatpox are classically considered to be transmitted by contact and inhalation (Kitching and Taylor, 1985b). However, in view of the very high viral titers in skin, the importance of mechanical transmission by biting insects may well be greater than previously suspected. For lumpy skin disease, direct contact between cattle is not considered an important mode of viral transmission (Carn and Kitching, 1995a). Instead, biting flies and mosquitoes are considered responsible for disease spread (Carn and Kitching, 1995a, Chihota et al., 2001, Woods, 1988), and good epidemiological evidence exists to support this notion (Woods, 1988, Yeruham et al., 1995). Myxoma virus (Day et al., 1956, Fenner et al., 1952) and avipoxviruses (Damon, 2007, Murphy et al., 1999, Tripathy and Reed, 2003) are transmitted mechanically by biting insects and mosquitoes, and experimental transmission of SPPV and GTPV by biting flies has been documented (Kitching and Mellor, 1986, Mellor et al., 1987). Nevertheless, further experimental and epidemiological investigations in sheep and goats will be required to clarify the involvement of insect vectors in the spread of SPPV and GTPV.
The pathogenesis and tissue tropism of capripoxvirus infections in sheep and goats observed here are in marked contrast to that seen with ectromelia virus in mice, which is the model most often used in pathogenesis and immunological studies of acute poxvirus diseases of animals (Buller and Palumbo, 1991). The tropism of SPPV and GTPV for the skin, as well as the apparent minor involvement of liver and spleen, instead suggests a pathogenesis which more closely resembles that of smallpox and monkeypox (Fenner, 1988, Jahrling et al., 2004, Zaucha et al., 2001). Although only detected in one goat, the less pronounced second temperature spike which occurred beyond DPI 17 also resembles that seen in humans with smallpox, especially in patients with secondary bacterial infections (Breman and Henderson, 2002). A striking feature of sheeppox and goatpox, however, is the generalized and prominent lymphadenopathy that develops at or shortly after the onset of clinical disease, and which is analogous to that seen with monkeypox virus infections in humans (Di Giulio and Eckburg, 2004, Huhn et al., 2005, Jezek and Fenner, 1988) as well as lumpy skin disease in cattle (Carn and Kitching, 1995b). In sheeppox and goatpox, this appears to occur in the absence of substantial virus replication, which suggests unique underlying viral or immunological mechanisms might be involved.
While vaccines and control strategies used for eradication of smallpox were effective for their time, the vaccines no longer appear acceptable for large scale emergency vaccination. Furthermore, although there are now several candidate antiviral drugs that appear suitable for mass protection against a possible global smallpox pandemic arising from an act of bioterrorism (Bailey et al., 2007, Painter and Hostetler, 2004, Quenelle et al., 2004, Quenelle et al., 2007, Sbrana et al., 2007, Yang et al., 2005), regulatory approval for their use in humans has not yet been completed. Since none of the surrogate animal models currently in use faithfully reproduces all aspects of smallpox disease in humans, there is a need for additional model systems to test alternative vaccine and therapeutic strategies (Jordan and Hruby, 2006, McFadden, 2004). Non-human primates are potentially useful models for variola virus infection in humans (Jahrling et al., 2004). However, limited access to animals, welfare concerns and the greater costs of housing and containment suggest that poxviruses of other species should also be considered for initial concept development. Conceivably, it would be possible to introduce drug target genes from orthopoxviruses into the capripoxvirus genome by way of recombinant virus construction (Romero et al., 1993) and thus directly evaluate, in sheep or goats, the potential efficacy of therapeutic drug candidates. Additionally, the identification of potential capripoxvirus antigens for subunit vaccine development may provide guidance to suitable candidates for evaluation and development of orthopoxvirus vaccines. Other intervention strategies targeting the cytokine storm precipitated during acute poxvirus disease (Stanford et al., 2007) or specific poxvirus-encoded immunomodulatory proteins (Johnston and McFadden, 2003, Seet et al., 2003, Smith and Kotwal, 2002) might also be explored using capripoxvirus infections in sheep and goats.
Our studies have revealed novel insights regarding the replication, dissemination and shedding of capripoxviruses in sheep and goats, the pathogenesis of which has more in common with smallpox and monkeypox in humans than ectromelia in mice. The extensive involvement of skin, with its associated high infectivity, suggests that mechanical transmission by biting insects might be more significant in disease spread than previously thought. Furthermore, long-term shedding of virus from mucosal surfaces, particularly in nasal and conjunctival secretions, illustrates the potential for some animals, at least, to remain infectious for up to 2 months after the onset of fever. These findings should enable the development of improved strategies for capripoxvirus detection and disease control. The simple reproduction of acute disease in sheep and goats, together with the reduced hazards associated with handling SPPV or GTPV over variola virus or monkeypox virus, also support the potential application of capripoxviruses to the study of novel poxvirus therapeutic and vaccine strategies.
Materials and methods
Animal housing and husbandry
Ten adult Suffolk-cross sheep and ten adult Saanen or Alpine goats were housed in microbiologically secure (Biosecurity level 3) animal rooms at the National Centre for Foreign Animal Disease (NCFAD), Winnipeg, Canada. Following the euthanasia and necropsy of one sheep and one goat for collection of uninfected control swab and tissue samples, sheep and goats were housed in groups of three until such time as additional animals were sacrificed for necropsy and sample collection. Animals were fed a complete balanced diet and water ad libitum, and all experimental procedures, which complied with Canadian Council on Animal Care guidelines, were approved by the Animal Care Committee of the Canadian Science Centre for Human and Animal Health.
Virus isolates
Capripoxviruses were obtained from the Institute for Animal Health (Pirbright Laboratory, Pirbright, Surrey, UK). The passage history of each isolate prior to inoculation of experimental animals was as follows: Nigerian SPPV was isolated in 1977 (Asagba and Nawathe, 1981) and was subsequently passaged three times in lamb testis cells at the Institute for Animal Health (Kitching and Taylor, 1985a) and twice in lamb testis cells at NCFAD; Indian GTPV was isolated in 1983 and was subsequently passaged once in lamb testis cells at the Institute for Animal Health (Kitching and Taylor, 1985a) and twice in lamb testis cells at NCFAD. Neither isolate was plaque-purified.
Inoculation of animals, monitoring and sample collection
Animals were inoculated intradermally, in a shaved area on the right chest, with either 104.9 50% tissue culture infectious doses (TCID50) of Nigerian SPPV (sheep) or 104.4 TCID50 of Indian GTPV (goats). Each day rectal temperatures were recorded and animals were examined for clinical signs, including the development of lesions at the inoculation site and elsewhere on the body.
On DPI 2, 4, 6, 8, 10, 12, 14, 21, 28, 42 and 56, and at the conclusion of the experiment (DPI 61 for goats or DPI 63 to 64 for sheep), whole blood was collected into EDTA Vacutainer tubes (Becton-Dickenson, Franklin Lakes, NJ, USA) and nasal, conjunctival and oral swabs were collected and placed into 1.25 ml of viral transport medium (phosphate buffered saline containing 1% (w/v) bovine serum albumin, 200 U/ml penicillin, 200 μg/ml streptomycin, 50 μg/ml gentamicin and 5 μg/ml amphotericin B). These samples were stored immediately on wet ice (4 °C) while sampling was in progress. Subsequently, aliquots of EDTA blood (50 μl) and swabs (100 μl) were pipetted into 300 μl of Lysis/Binding buffer (Roche, Laval, QC, Canada) and stored at − 20 °C prior to extraction of DNA, whereas the remainder of each sample was transferred to − 80 °C prior to virus isolation.
On DPI 4, 6, 8 and 10, one sheep and one goat were euthanized by barbiturate overdose and necropsied. Thereafter one additional sheep on DPI 13 and 15, one additional goat on DPI 11 and 13, and two additional goats on DPI 15 were also euthanized and necropsied. Prior to euthanasia, animals were routinely anaesthetized by intramuscular administration of atropine sulfate (0.05 mg/kg; Atropine Injection; MTC Pharmaceuticals, Cambridge, ON, Canada) followed by intravenous administration of xylazine HCl (0.05–0.1 mg/kg; Rompun; Bayer, Toronto, ON, Canada) and ketamine HCl (5.0–10 mg/kg; Ketaset; Ayerst Veterinary Laboratories, Guelph, ON, Canada), and partially exsanguinated by jugular catheterization.
To eliminate risks of cross contamination between necropsy samples, substantial precautions were taken during specimen collection and processing. These included the use of separate sterile scissors and forceps to access all tissues sampled, and the harvesting of tissues onto individual sterile disposable petri dishes for further processing with separate sterile disposable forceps and scalpels. Urine, collected by cystocentesis, and rectal swabs were also taken at necropsy. Tissue samples for virus isolation were collected in sterile 2 ml screw cap tubes (Sarstedt, Nümbrecht, Germany). Tissue samples for DNA extraction (no bigger than 125 mm3) were stored in 1.25 ml of RNAlater (Ambion, Austin, TX, USA), initially at 4 °C overnight, followed by − 20 °C thereafter, whereas aliquots of urine (100 μl) and rectal swabs (100 μl) were pipetted into 300 μl of Lysis/Binding buffer (Roche, Laval, QC, Canada) and stored at − 20 °C prior to extraction of DNA. To ensure sample integrity, all specimens were collected onto wet ice during the necropsy and replicate samples for virus isolation were subsequently transferred to − 80 °C for long-term storage. All samples for DNA extraction were inactivated by gamma irradiation (20 kGy) before shipment to the Australian Animal Health Laboratory, Geelong, Victoria, Australia.
DNA extraction
DNA from all clinical materials was extracted using the MagNA Pure LC System (Roche, Melbourne, Victoria, Australia), an automated nucleic acid isolation and purification platform, according to the recommendations of the manufacturer. DNA from blood, urine or swab material, mixed with MagNa Pure Lysis/Binding buffer, was extracted using a MagNA Pure DNA I kit and eluted in 100 μl of the provided Elution buffer. DNA from RNAlater stored tissues was extracted using the MagNA Pure DNA II kit following an overnight proteinase K digestion. Briefly, 20 to 30 mg of each tissue was homogenized in 240 μl of MagNA Pure DNA II kit Tissue Lysis buffer using the MagNA Lyser instrument (Roche, Melbourne, Victoria, Australia). Samples were subjected to two 25 s periods of homogenization at 6500 rpm with 90 s cooling between cycles. Proteinase K (Roche, Melbourne, Victoria, Australia) was added to a final concentration of 3 mg/ml and the samples were incubated at 56 °C overnight on an orbital shaker. RNase A (Roche, Melbourne, Victoria, Australia) was added to a final concentration of 3.5 mg/ml and the samples were further incubated for 15 min at 65 °C. Samples were clarified in a bench top microcentrifuge at 13,000 rpm for 1 min, after which DNA was extracted from 110 μl of supernatant using the MagNA Pure DNA II kit external lysis protocol and eluted in 200 μl of Elution buffer. DNA concentrations of each eluate were determined using the GeneQuant II RNA/DNA Calculator (GE Healthcare Life Sciences, Castle Hill, NSW, Australia) and adjusted to 20 ng/μl with nuclease free water (Promega, Madison, WI, USA) prior to viral genome quantitation by real-time PCR.
Quantitative real-time PCR
A quantitative real-time PCR (qPCR) TaqMan assay that amplified and detected an 89 bp region within SPPV, GTPV and LSDV ORF074 (Tulman et al., 2001, Tulman et al., 2002), which encodes the intracellular mature virion envelope protein P32 (vaccinia virus H3L homolog), was used to determine viral genetic loads in clinical samples. Duplexed detection of either an endogenous control (TaqMan rRNA Control Reagents, Applied Biosystems, Scorseby, Victoria, Australia), for solid tissues, or an exogenous control (TaqMan Exogenous IPC Reagents, Applied Biosystems), for blood, swabs and urine, provided a means of verifying the absence of PCR inhibitors in samples tested. For amplification of target sequences, the TaqMan Universal PCR Master Mix Kit (Applied Biosystems) was used. The capripoxvirus-specific primers and probe were designed using the Applied Biosystems Primer Express software (version 2) using the default design parameters. Primers CaPV-074F1 5′-AAA ACG GTA TAT GGA ATA GAG TTG GAA-3′ and CaPV-074R1 5′-AAA TGA AAC CAA TGG ATG GGA TA-3′ were used in conjunction with the minor groove binder (MGB) TaqMan probe CaPV-074P1 5′-6FAM-TGG CTC ATA GAT TTC CT-MGBNFQ-3′. The reactions were performed in 96-well Optical Reaction Plates (Applied Biosystems) and, for extracted solid tissues, contained 12.5 μl TaqMan Universal PCR Master Mix, 900 nM of each capripoxvirus primer, 250 nM of capripoxvirus probe, 50 nM of each 18S rRNA primer, 200 nM of 18S rRNA probe, 5 μl (100 ng) of template and water to 25 μl. For extracted blood, urine and swab specimens which, in contrast to templates derived from solid tissues, contained very low concentrations of DNA, reactions were set up similarly except that the rRNA primers and probe were replaced with 0.5 μl 50× IPC DNA (a synthetic plasmid template) and 2.5 μl 10× Exo IPC mix (containing the corresponding primers and probe). Rather than amplification of the endogenous 18S rRNA gene, such reactions relied on the multiplexed quantitation of an exogenous, synthetic DNA target for confirming the absence of inhibitors. The reactions were run on the ABI PRISM 7700 Sequence Detection System (Applied Biosystems) using the following amplification program: 50 °C for 2 min; 95 °C for 10 min; and 45 cycles of 95 °C for 15 s and 60 °C for 1 min. Results were generated by determination of the threshold cycle (C T), the fractional cycle number at which the change in the fluorescence (ΔR n) of each reporter dye passed a fixed threshold value set in the log (exponential) phase of amplification. For all reactions, the baseline was routinely set between cycles 3 and 15, while the threshold ΔR n values were set at 0.15 for capripoxvirus-specific products and either 0.1 or 0.05 for products derived from the endogenous or exogenous controls, respectively.
For absolute quantitation of viral genetic loads in samples tested, a standard curve was developed by assaying 10-fold serial dilutions, ranging from 106 to 10 copies, of a pDONR221 plasmid (Invitrogen, Mount Waverley, Victoria, Australia) containing the full open reading frame of gene 074. The plasmid (pDONR221-S074) was linearized by digestion with PvuI (New England Biolabs, Beverly, MA, USA), gel-purified and diluted in herring sperm DNA (Promega, Madison, WI, USA) at 5 ng/μl to ensure a uniform background of DNA at all template concentrations used. Quantitative standards were always included in triplicate on each reaction plate, whereas sample reactions were routinely conducted in duplicate. Viral genome copies per 100 ng of extracted tissue DNA were calculated with reference to the plasmid standard curve, and tissue samples containing very high genome copies were diluted before assay if necessary. For blood, urine and swabs, the genome copy numbers were calculated and expressed as copies per milliliter of each respective sample type. The lower limit of the standard curve linear dynamic range was 10 copies of pDONR221-S074, which equated approximately to a C T value of 37. Sample templates with C T values greater than 37 and less than 45 were considered positive (containing fewer than 10 genome copies per well) but could not be precisely quantified. All such samples corresponded to viral genome copy numbers < 10 per 100 ng of extracted DNA (solid tissues), < 103.6 per ml of blood and < 103.4 per ml of urine or viral transport medium (swabs).
Virus isolation and titration
Isolation of infectious virus was routinely attempted from swab specimens, urine, blood (diluted 1:10 (v/v) in phosphate buffered saline) and solid tissues (as 10% (w/v) homogenates in viral transport medium, prepared using a Mini-BeadBeater-8 (Biospec Products, AB, Canada)) by inoculation of samples, in duplicate, onto 80–90% confluent ovine testis cell monolayers (OA3.Ts; ATCC CRL-6546, Manassas, VA, USA) (Babiuk et al., 2007) grown in 12-well plates (ICN Biomedicals Canada Ltd., Mississauga, ON, Canada). Development of viral-induced cytopathic effect on OA3.Ts cells was monitored for a period of 10 to 14 days. Capripoxvirus-positive specimens were subsequently titrated on replicate OA3.Ts cell monolayers (Babiuk et al., 2007), and viral titers (TCID50/ml or TCID50/g) were estimated using the method of Reed and Muench (1938).
Acknowledgments
We thank Mary-Ann Anderson for her technical support, Christine DeGraff, Michelle French, Stacey Halayko, Marlee Ritchie, Kevin Tierney and Shannon Toback for assistance with animal care and sample collection, Dr. Marta Sabara and June Lawrence for preparation and maintenance of OA3.Ts cell cultures, and Dr. Jef Hammond and Dr. Ken McColl for critical review of the manuscript. The valuable discussions and input by Dr. R Paul Kitching to the project are also gratefully acknowledged.
This work was supported by the Australian Woolgrowers and the Australian Government through Australian Wool Innovation Limited Project No. EC145, as well as by Canadian Food and Inspection Agency (CFIA) Quickstart grants QSW0505 and QSW0503, and CFIA RPS W0309.
References
- Altschul S.F., Madden T.L., Schaffer A.A., Zhang J., Zhang Z., Miller W., Lipman D.J. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asagba M.O., Nawathe D.R. Evidence of sheep pox in Nigeria. Trop. Anim. Health Prod. 1981;13:61. doi: 10.1007/BF02237892. [DOI] [PubMed] [Google Scholar]
- Babiuk S., Parkyn G., Copps J., Larence J.E., Sabara M.I., Bowden T.R., Boyle D.B., Kitching R.P. Evaluation of an ovine testis cell line (OA3.Ts) for propagation of capripoxvirus isolates and development of an immunostaining technique for viral plaque visualization. J. Vet. Diagn. Invest. 2007;19:486–491. doi: 10.1177/104063870701900505. [DOI] [PubMed] [Google Scholar]
- Bailey T.R., Rippin S.R., Opsitnick E., Burns C.J., Pevear D.C., Collett M.S., Rhodes G., Tohan S., Huggins J.W., Baker R.O., Kern E.R., Keith K.A., Dai D., Yang G., Hruby D., Jordan R. N-(3,3a,4,4a,5,5a,6,6a-Octahydro-1,3-dioxo-4,6-ethenocycloprop[f]isoindol-2-(1H)-yl)carboxamides: identification of novel orthopoxvirus egress inhibitors. J. Med. Chem. 2007;50:1442–1444. doi: 10.1021/jm061484y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belwal L.M., Nivsarkar A.E., Mathur P.B., Singh R.N. Epidemiology of sheep pox. Trop. Anim. Health Prod. 1982;14:229–233. doi: 10.1007/BF02242164. [DOI] [PubMed] [Google Scholar]
- Bhanuprakash V., Indrani B.K., Hegde R., Kumar M.M., Moorthy A.R. A classical live attenuated vaccine for sheep pox. Trop. Anim. Health Prod. 2004;36:307–320. doi: 10.1023/b:trop.0000026661.88631.50. [DOI] [PubMed] [Google Scholar]
- Bhanuprakash V., Moorthy A.R., Krishnappa G., Srinivasa Gowda R.N., Indrani B.K. An epidemiological study of sheep pox infection in Karnataka State. Indian Rev. Sci. Tech. 2005;24:909–920. [PubMed] [Google Scholar]
- Bhanuprakash V., Indrani B.K., Hosamani M., Singh R.K. The current status of sheep pox disease. Comp. Immunol. Microbiol. Infect. Dis. 2006;29:27–60. doi: 10.1016/j.cimid.2005.12.001. [DOI] [PubMed] [Google Scholar]
- Black D.N., Hammond J.M., Kitching R.P. Genomic relationship between capripoxviruses. Virus Res. 1986;5:277–292. doi: 10.1016/0168-1702(86)90024-9. [DOI] [PubMed] [Google Scholar]
- Breman J.G., Henderson D.A. Diagnosis and management of smallpox. N. Engl. J. Med. 2002;346:1300–1308. doi: 10.1056/NEJMra020025. [DOI] [PubMed] [Google Scholar]
- Buller R.M., Palumbo G.J. Poxvirus pathogenesis. Microbiol. Rev. 1991;55:80–122. doi: 10.1128/mr.55.1.80-122.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buller R.M., Arif B.M., Black D.N., Dumbell K.R., Esposito J.J., Lefkowitz E.J., McFadden G., Moss B., Mercer A.A., Moyer R.W., Skinner M.A., Tripathy D.N. In: Virus Taxonomy: Classification and Nomenclature of Viruses. Eighth Report of the International Committee on Taxonomy of Viruses. Fauquet C.M., Mayo M.A., Maniloff J., Desselberger U., Ball L.A., editors. Elsevier Academic Press; San Diego: 2005. Family Poxviridae; pp. 117–133. [Google Scholar]
- Carn V.M., Kitching R.P. An investigation of possible routes of transmission of lumpy skin disease virus (Neethling) Epidemiol. Infect. 1995;114:219–226. doi: 10.1017/s0950268800052067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carn V.M., Kitching R.P. The clinical response of cattle experimentally infected with lumpy skin disease (Neethling) virus. Arch. Virol. 1995;140:503–513. doi: 10.1007/BF01718427. [DOI] [PubMed] [Google Scholar]
- Chihota C.M., Rennie L.F., Kitching R.P., Mellor P.S. Mechanical transmission of lumpy skin disease virus by Aedes aegypti (Diptera: Culicidae) Epidemiol. Infect. 2001;126:317–321. doi: 10.1017/s0950268801005179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damon I.K. In: Fields Virology. Knipe D.M., Howley P.M., Griffin D.E., Lamb R.A., Martin M.A., Roizman B., Straus S.E., editors. Lippincott Williams & Wilkins; Philadelphia: 2007. Poxviruses; pp. 2947–2975. [Google Scholar]
- Davies F.G. Characteristics of a virus causing a pox disease in sheep and goats in Kenya, with observation on the epidemiology and control. J. Hyg. (Lond.) 1976;76:163–171. doi: 10.1017/s0022172400055066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies F.G., Otema C. Relationships of capripox viruses found in Kenya with two Middle Eastern strains and some orthopox viruses. Res. Vet. Sci. 1981;31:253–255. [PubMed] [Google Scholar]
- Day M.F., Fenner F., Woodroofe G.M., McIntyre G.A. Further studies on the mechanism of mosquito transmission of myxomatosis in the European rabbit. J. Hyg. (Lond.) 1956;54:258–283. doi: 10.1017/s002217240004451x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Giulio D.B., Eckburg P.B. Human monkeypox: an emerging zoonosis. Lancet, Infect. Dis. 2004;4:15–25. doi: 10.1016/S1473-3099(03)00856-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenner F. The clinical features and pathogenesis of mouse-pox (infectious ectromelia of mice) J. Pathol. Bacteriol. 1948;60:529–552. [Google Scholar]
- Fenner F. The pathogenesis of the acute exanthems: an interpretation based on experimental investigations with mousepox (infectious ectromelia of mice) Lancet. 1948;2:915–920. doi: 10.1016/s0140-6736(48)91599-2. [DOI] [PubMed] [Google Scholar]
- Fenner F. In: Smallpox and its Eradication. Fenner F., Henderson D.A., Arita I., Jezek Z., Ladnyi I.D., editors. World Health Organization; Geneva: 1988. The pathogenesis, pathology and immunology of smallpox and vaccinia; pp. 121–168. [Google Scholar]
- Fenner F., Day M.F., Woodroofe G.M. The mechanism of the transmission of myxomatosis in the European rabbit (Oryctolagus cuniculus) by the mosquito Aedes aegypti. Aust. J. Exp. Biol. Med. Sci. 1952;30:139–152. doi: 10.1038/icb.1952.13. [DOI] [PubMed] [Google Scholar]
- Garner M.G., Lack M.B. Modelling the potential impact of exotic diseases on regional Australia. Aust. Vet. J. 1995;72:81–87. doi: 10.1111/j.1751-0813.1995.tb15013.x. [DOI] [PubMed] [Google Scholar]
- Garner M.G., Sawarkar S.D., Brett E.K., Edwards J.R., Kulkarni V.B., Boyle D.B., Singh S.N. The extent and impact of sheep pox and goat pox in the state of Maharashtra, India. Trop. Anim. Health Prod. 2000;32:205–223. doi: 10.1023/a:1005263601964. [DOI] [PubMed] [Google Scholar]
- Gershon P.D., Black D.N. A comparison of the genomes of capripoxvirus isolates of sheep, goats, and cattle. Virology. 1988;164:341–349. doi: 10.1016/0042-6822(88)90547-8. [DOI] [PubMed] [Google Scholar]
- Gledhill A.W. Latent ectromelia. Nature. 1962;196:298. doi: 10.1038/196298a0. [DOI] [PubMed] [Google Scholar]
- Gulbahar M.Y., Davis W.C., Yuksel H., Cabalar M. Immunohistochemical evaluation of inflammatory infiltrate in the skin and lung of lambs naturally infected with sheeppox virus. Vet. Pathol. 2006;43:67–75. doi: 10.1354/vp.43-1-67. [DOI] [PubMed] [Google Scholar]
- Hosamani M., Mondal B., Tembhurne P.A., Bandyopadhyay S.K., Singh R.K., Rasool T.J. Differentiation of sheep pox and goat poxviruses by sequence analysis and PCR-RFLP of P32 gene. Virus Genes. 2004;29:73–80. doi: 10.1023/B:VIRU.0000032790.16751.13. [DOI] [PubMed] [Google Scholar]
- Hosamani M., Nandi S., Mondal B., Singh R.K., Rasool T.J., Bandyopadhyay S.K. A Vero cell-attenuated goatpox virus provides protection against virulent virus challenge. Acta Virol. 2004;48:15–21. [PubMed] [Google Scholar]
- Huhn G.D., Bauer A.M., Yorita K., Graham M.B., Sejvar J., Likos A., Damon I.K., Reynolds M.G., Kuehnert M.J. Clinical characteristics of human monkeypox, and risk factors for severe disease. Clin. Infect. Dis. 2005;41:1742–1751. doi: 10.1086/498115. [DOI] [PubMed] [Google Scholar]
- Irons P.C., Tuppurainen E.S., Venter E.H. Excretion of lumpy skin disease virus in bull semen. Theriogenology. 2005;63:1290–1297. doi: 10.1016/j.theriogenology.2004.06.013. [DOI] [PubMed] [Google Scholar]
- Jahrling P.B., Hensley L.E., Martinez M.J., Leduc J.W., Rubins K.H., Relman D.A., Huggins J.W. Exploring the potential of variola virus infection of cynomolgus macaques as a model for human smallpox. Proc. Natl. Acad. Sci. U. S. A. 2004;101:15196–15200. doi: 10.1073/pnas.0405954101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jezek Z., Fenner F. Human monkeypox. Monogr. Virol. 1988;17:1–135. [Google Scholar]
- Johnston J.B., McFadden G. Poxvirus immunomodulatory strategies: current perspectives. J. Virol. 2003;77:6093–6100. doi: 10.1128/JVI.77.11.6093-6100.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan R., Hruby D. Smallpox antiviral drug development: satisfying the animal efficacy rule. Expert. Rev. Anti. Infect. Ther. 2006;4:277–289. doi: 10.1586/14787210.4.2.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitching R.P. Passive protection of sheep against capripoxvirus. Res. Vet. Sci. 1986;41:247–250. [PubMed] [Google Scholar]
- Kitching R.P. Vaccines for lumpy skin disease, sheep pox and goat pox. Dev. Biol. (Basel) 2003;114:161–167. [PubMed] [Google Scholar]
- Kitching R.P. In: Infectious Diseases of Livestock. Coetzer J.A.W., Tustin R.C., editors. Oxford University Press; 2004. Sheeppox and goatpox; pp. 1277–1281. [Google Scholar]
- Kitching R.P., Carn V.M. Office International des Epizooties Manual of Diagnostic Tests and Vaccines for Terrestrial Animals (mammals, birds and bees) OIE; Paris: 2004. Sheep pox and goat pox; pp. 211–220. [Google Scholar]
- Kitching R.P., Mellor P.S. Insect transmission of capripoxvirus. Res. Vet. Sci. 1986;40:255–258. [PubMed] [Google Scholar]
- Kitching R.P., Taylor W.P. Clinical and antigenic relationship between isolates of sheep and goat pox viruses. Trop. Anim. Health Prod. 1985;17:64–74. doi: 10.1007/BF02360774. [DOI] [PubMed] [Google Scholar]
- Kitching R.P., Taylor W.P. Transmission of capripoxvirus. Res. Vet. Sci. 1985;39:196–199. [PubMed] [Google Scholar]
- Kitching R.P., McGrane J.J., Taylor W.P. Capripox in the Yemen Arab Republic and the Sultanate of Oman. Trop. Anim. Health Prod. 1986;18:115–122. doi: 10.1007/BF02359725. [DOI] [PubMed] [Google Scholar]
- Kitching R.P., Hammond J.M., Taylor W.P. A single vaccine for the control of capripox infection in sheep and goats. Res. Vet. Sci. 1987;42:53–60. [PubMed] [Google Scholar]
- Kitching R.P., McGrane J.J., Hammond J.M., Miah A.H., Mustafa A.H., Majumder J.R. Capripox in Bangladesh. Trop. Anim. Health Prod. 1987;19:203–208. doi: 10.1007/BF02242117. [DOI] [PubMed] [Google Scholar]
- Kitching R.P., Bhat P.P., Black D.N. The characterization of African strains of capripoxvirus. Epidemiol. Infect. 1989;102:335–343. doi: 10.1017/s0950268800030016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan R. Pathogenesis of sheep pox. Indian Vet. J. 1968;45:297–302. [PubMed] [Google Scholar]
- McFadden G. Smallpox: an ancient disease enters the modern era of virogenomics. Proc. Natl. Acad. Sci. U. S. A. 2004;101:14994–14995. doi: 10.1073/pnas.0406207101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellor P.S., Kitching R.P., Wilkinson P.J. Mechanical transmission of capripox virus and African swine fever virus by Stomoxys calcitrans. Res. Vet. Sci. 1987;43:109–112. [PubMed] [Google Scholar]
- Murphy F.A., Gibbs E.P.J., Horzinek M.C., Studdert M.J. Veterinary Virology. Academic Press; San Diego: 1999. Poxviridae; pp. 277–291. [Google Scholar]
- Murray M., Martin W.B., Koylu A. Experimental sheep pox. A histological and ultrastructural study. Res. Vet. Sci. 1973;15:201–208. [PubMed] [Google Scholar]
- Painter G.R., Hostetler K.Y. Design and development of oral drugs for the prophylaxis and treatment of smallpox infection. Trends Biotechnol. 2004;22:423–427. doi: 10.1016/j.tibtech.2004.06.008. [DOI] [PubMed] [Google Scholar]
- Plowright W., MacLeod W.G., Ferris R.D. The pathogenesis of sheep pox in the skin of sheep. J. Comp. Pathol. 1959;69:400–413. doi: 10.1016/s0368-1742(59)80039-4. [DOI] [PubMed] [Google Scholar]
- Quenelle D.C., Collins D.J., Wan W.B., Beadle J.R., Hostetler K.Y., Kern E.R. Oral treatment of cowpox and vaccinia virus infections in mice with ether lipid esters of cidofovir. Antimicrob. Agents Chemother. 2004;48:404–412. doi: 10.1128/AAC.48.2.404-412.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quenelle D.C., Buller R.M., Parker S., Keith K.A., Hruby D.E., Jordan R., Kern E.R. Efficacy of delayed treatment with ST-246 given orally against systemic orthopoxvirus infections in mice. Antimicrob. Agents Chemother. 2007;51:689–695. doi: 10.1128/AAC.00879-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed L.J., Muench H. A simple method for estimating fifty percent endpoints. Am. J. Hyg. 1938;27:493–497. [Google Scholar]
- Romero C.H., Barrett T., Evans S.A., Kitching R.P., Gershon P.D., Bostock C., Black D.N. Single capripoxvirus recombinant vaccine for the protection of cattle against rinderpest and lumpy skin disease. Vaccine. 1993;11:737–742. doi: 10.1016/0264-410x(93)90258-y. [DOI] [PubMed] [Google Scholar]
- Roth J.A., Spickler A.R. A survey of vaccines produced for OIE list A diseases in OIE member countries. Dev. Biol. (Basel) 2003;114:5–25. [PubMed] [Google Scholar]
- Sbrana E., Jordan R., Hruby D.E., Mateo R.I., Xiao S.Y., Siirin M., Newman P.C., da Rosa A.P., Tesh R.B. Efficacy of the antipoxvirus compound ST-246 for treatment of severe orthopoxvirus infection. Am. J. Trop. Med. Hyg. 2007;76:768–773. [PubMed] [Google Scholar]
- Seet B.T., Johnston J.B., Brunetti C.R., Barrett J.W., Everett H., Cameron C., Sypula J., Nazarian S.H., Lucas A., McFadden G. Poxviruses and immune evasion. Annu. Rev. Immunol. 2003;21:377–423. doi: 10.1146/annurev.immunol.21.120601.141049. [DOI] [PubMed] [Google Scholar]
- Sharma S.N., Nilakantan P.R., Dhanda M.R. A preliminary note on pathogenicity and antigenicity of sheep and goat pox viruses. Indian Vet. J. 1966;43:673–678. [PubMed] [Google Scholar]
- Smith S.A., Kotwal G.J. Immune response to poxvirus infections in various animals. Crit. Rev. Microbiol. 2002;28:149–185. doi: 10.1080/1040-840291046722. [DOI] [PubMed] [Google Scholar]
- Stanford M.M., McFadden G., Karupiah G., Chaudhri G. Immunopathogenesis of poxvirus infections: forecasting the impending storm. Immunol. Cell Biol. 2007;85:93–102. doi: 10.1038/sj.icb.7100033. [DOI] [PubMed] [Google Scholar]
- Tantawi H.H., Awad M.M., Shony M.O., Alwan A.H., Hassan F.K. Preliminary characterisation of the Sersenk strain of goat pox virus. Trop. Anim. Health Prod. 1980;12:30–34. doi: 10.1007/BF02242628. [DOI] [PubMed] [Google Scholar]
- Tripathy D.N., Reed W.M. In: Diseases of Poultry. Saif Y.M., Barnes H.J., Glisson J.R., Fadly A.M., McDougald L.R., Swayne D.E., editors. Blackwell Publishing; Ames, Iowa: 2003. Pox; pp. 253–269. [Google Scholar]
- Tulman E.R., Afonso C.L., Lu Z., Zsak L., Kutish G.F., Rock D.L. Genome of lumpy skin disease virus. J. Virol. 2001;75:7122–7130. doi: 10.1128/JVI.75.15.7122-7130.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tulman E.R., Afonso C.L., Lu Z., Zsak L., Sur J.H., Sandybaev N.T., Kerembekova U.Z., Zaitsev V.L., Kutish G.F., Rock D.L. The genomes of sheeppox and goatpox viruses. J. Virol. 2002;76:6054–6061. doi: 10.1128/JVI.76.12.6054-6061.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace G.D., Buller R.M. Kinetics of ectromelia virus (mousepox) transmission and clinical response in C57BL/6j, BALB/cByj and AKR/J inbred mice. Lab. Anim. Sci. 1985;35:41–46. [PubMed] [Google Scholar]
- Woods J.A. Lumpy skin disease—a review. Trop. Anim. Health Prod. 1988;20:11–17. doi: 10.1007/BF02239636. [DOI] [PubMed] [Google Scholar]
- Yang G., Pevear D.C., Davies M.H., Collett M.S., Bailey T., Rippen S., Barone L., Burns C., Rhodes G., Tohan S., Huggins J.W., Baker R.O., Buller R.L., Touchette E., Waller K., Schriewer J., Neyts J., DeClercq E., Jones K., Hruby D., Jordan R. An orally bioavailable antipoxvirus compound (ST-246) inhibits extracellular virus formation and protects mice from lethal orthopoxvirus challenge. J. Virol. 2005;79:13139–13149. doi: 10.1128/JVI.79.20.13139-13149.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeruham I., Nir O., Braverman Y., Davidson M., Grinstein H., Haymovitch M., Zamir O. Spread of lumpy skin disease in Israeli dairy herds. Vet. Rec. 1995;137:91–93. doi: 10.1136/vr.137.4.91. [DOI] [PubMed] [Google Scholar]
- Zaucha G.M., Jahrling P.B., Geisbert T.W., Swearengen J.R., Hensley L. The pathology of experimental aerosolized monkeypox virus infection in cynomolgus monkeys (Macaca fascicularis) Lab. Invest. 2001;81:1581–1600. doi: 10.1038/labinvest.3780373. [DOI] [PMC free article] [PubMed] [Google Scholar]