Abstract
Improvements in early screening and treatment have contributed to the growth of the number of cancer survivors. Understanding and mitigating the adverse psychosocial, functional, and economic outcomes they experience is critical. Social wellbeing refers to the quality of the relationship with partners/spouses, children, or significant others. Close relationships contribute to quality of life and self-management; however, limited literature exists about social wellbeing during survivorship. This study examined positive and negative self-reported changes in a community sample of 505 cancer survivors. Fourteen items assessed changes in communication, closeness with partner/children, stability of the relationship, and caregiving burden. An exploratory factor analysis was conducted using a robust weighted least square procedure. Differences by sociodemographic and clinical characteristics were investigated. Respondents were mostly male, non-Hispanic white, and ≥4 years since diagnosis. Two factors, labeled Relationship Closeness and Ambivalence, emerged from the analysis. Women, younger survivors, individuals from minority groups, and those with lower income experienced greater negative changes in social wellbeing. Variations by treatment status, time since diagnosis, and institution were also reported. This contribution identifies groups of cancer survivors experiencing affected social wellbeing. Results emphasize the need to develop interventions sustaining the quality of interpersonal relationships to promote long-term outcomes.
Keywords: quality of life, close relationships, social wellbeing, cancer survivorship, psychosocial oncology, survivorship care, patient-reported outcomes
1. Introduction
The implementation of early cancer screening and detection, combined with advances in curative treatment options, have contributed to the continued growth of the number of cancer survivors living in the United States [1,2]. To date, estimates from the National Cancer Institute and the American Cancer Society indicate that there are 16.9 million cancer survivors in the country, accounting for 5% of the population [3]. Additionally, the number of cancer survivors is expected to increase to 26.1 million by 2040 [4]. Physical, emotional, and financial consequences are experienced well into survivorship [5,6,7,8,9,10]. About 60% of survivors report persistent distress and fear of recurrence [5,8], approximately 36.5% remain unable to work [11,12], and between 15% and 75% present cancer-related cognitive impairment [2,13,14]. As a result, it becomes imperative to better understand the experience of this heterogeneous group and to identify strategies and approaches to address their unmet needs and long-term issues, whether due to treatment side effects, disparities, or social determinants of health [7,15,16,17,18]. To this end, cancer survivorship research has emerged as a subset of efforts aimed at understanding the psychosocial sequelae associated with cancer treatment and to prevent and mitigate multifaceted adverse outcomes [9,19,20,21,22].
An extensive body of evidence has demonstrated the pervasive consequences of the illness for close relationships, in terms of mental health, communication, and relationship dissolution [7,23,24,25,26]. Elevated rates of psychological distress impair the quality of life of survivors and their partners [27,28,29]. Relationship satisfaction was reported among couples engaging in mutual constructive communication, expression of feelings, and negotiation [30]. On the contrary, avoidance, holding back, or disengagement have been linked to poorer relationship functioning, coping, and psychological wellbeing [30] Cancer-related distress and caregiving responsibilities may also negatively alter relationship stability, with greater odds of separation/divorce recorded among female survivors, young adults, and those experiencing greater distress and financial problems [31,32,33]. Although a recent systematic review documented that cancer is linked to a small decrease in divorce rate [34], Nalbant et al. (2021) found that cancer was the main reported cause for relationship dissolution among partners of cancer survivors [35].
While quality of life is a broad multidimensional concept—often resulting from the “individual’s perception of their position in life in the context of the culture and value system in which they live and in relation to their goals, expectations, standards, and concerns” [36], social wellbeing refers to the satisfaction the individual has in regard to the quality of the relationships with others [37,38,39]. Authors that investigated patient-reported outcomes in cancer survivorship found that close relationships are contributing factors for quality of life after diagnosis. Support from partners, family members, and the larger social network protects against physical morbidity and mortality, while also promoting psychological wellbeing and self-management [7,37,40,41,42,43,44]. Yet, adverse physical and psychosocial consequences of cancer have the potential to impair survivors’ social wellbeing [7,23,24,45]. Contributions have documented that cancer survivors tend to show decreased social functioning because of late treatment side-effects, impaired physical functioning, mental health symptomatology, perceived stigma, financial hardship, and access to and changes in their social networks [31,46,47,48].
Social wellbeing in cancer survivorship varies by gender, age, ethnic and cultural aspects, type/stage of cancer, and socioeconomic and personality characteristics [9,26,49,50,51]. Although most survivors are older than 65 [1], a substantial number of patients are diagnosed with cancer during young adulthood or adulthood, with differential effects on their psychosocial outcomes [6,41,52]. Studies have shown that older age is both an aggravating and a protective factor [40,41,52]. While older patients experience more comorbidities and social isolation, they appear to cope better with the impact of the disease on close relationships as they are more inclined to preserve or improve existing ones [40,52,53]. Younger survivors, on the contrary, are faced with the premature confrontation with mortality, disruption of educational and professional goals, financial difficulties, and reproductive and sexual health concerns, which lead to difficulties in maintaining or establishing romantic partnerships and intimacy [23,25]. While there is still scarce literature concentrating on cancer survivors from minoritized racial/ethnic groups and their social wellbeing, studies have shown that worse outcomes were reported, especially for Hispanic patients [54], and that culturally informed and contextual factors guide family interactions and coping [4,55].
Despite growing attention to social wellbeing after cancer and the development of intervention approaches that capitalize on the relationship with significant others to alleviate the burden of the illness [21,53,56], gaps remain in our understanding of patterns and quality of close relationships beyond active treatment, next to the inclusion of community-based samples able to illustrate the experience of survivors from different backgrounds, race/ethnicities, and receiving care in diverse oncology settings. The present study aims to examine positive and negative self-reported changes in social wellbeing by sociodemographic and clinical characteristics.
2. Materials and Methods
2.1. Procedure
This contribution is a secondary data analysis of the Survivorship Survey data collected between July and December 2015 from CancerCare, a leading US nonprofit organization providing professional supportive services including counseling, support groups, educational workshops, and financial assistance to cancer survivors and caregivers. Survivors were recruited through online panels; respondents were limited to individuals who were 25 years of age and older, and who had received a confirmed diagnosis of cancer from a physician/healthcare professional. Fifty percent of the sample included common cancers (lung, breast, colorectal, and prostate), and research vendors utilized specific criteria and filters so that approximately 25% of respondents were recruited from each region of the nation (Northeast, Midwest, Southeast, and Southwest/West) to increase sample representativeness. Approximately 3000 participants were invited by e-mail to reach the target sample of 500 respondents, and 505 answers were collected for the survivorship questionnaire. To minimize response biases, potential participants were not selected from cancer survivors who have used the services of the organization, online communities, or client database. Informed consent was obtained from all individual participants included in the original study. All procedures were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki declaration and its later amendments. The dataset inclusive of variables and measures of interest for the present work was shared with the research team after IRB approval (19 June 2018).
2.2. Measures
2.2.1. Impact of Cancer on Relationships
A total of 14 items were utilized to assess differences in the social wellbeing of the participants since the cancer diagnosis. Items were initially developed by social work counselors and subsequently reviewed by an advisory board inclusive of experts in survey development and patient care. Using dichotomous answer options (yes/no), cancer survivors were asked to ascertain whether the illness contributed to positive or negative changes in different aspects of their lives: communication (more meaningful conversation with loved ones, more likely/less likely to share their thoughts and feelings with loved ones), closeness with partner and children (time spent with partner, time spent with children, level of intimacy, sense of isolation, children becoming too attached or withdrawn/angry), stability of the relationship (divorce or relationship dissolution with spouse/partner), and caregiver burden (partner/spouse being exhausted because of extra responsibilities, having trouble being dependent on others).
2.2.2. Demographic and Clinical Information
Demographic characteristics such as age, sex, ethnicity/race, education, annual household income, and healthcare insurance were self-reported. Clinical factors assessed as part of the survey included cancer type, time since diagnosis, current cancer status, treatment type, and information about the institution where participants received care.
2.3. Data Analysis
Descriptive statistics were obtained to summarize the sample’s characteristics. Means and standard deviations were calculated for continuous variables, while frequency and percentages have been used for categorical variables. An exploratory factor analysis (EFA) was conducted on the 14 items that asked participants to rate positive and negative changes in their relationships with partners/spouses, family members, and children. Then, the resulting pseudo-factors obtained by summing items loading on the two-factor solution were compared by socio-demographic and clinical characteristics using chi-square tests for nominal variables and ANOVAs for continuous variables. We also calculated post hoc Tukey’s test for comparison between individual groups as well as Cohen’s d effect size when appropriate. Bonferroni correction was conducted for all analyses. Mplus version 7.31 was utilized for data cleaning, management, and analysis [57]. The level of significance was set at p < 0.05.
3. Results
3.1. Sample
The characteristics of the sample are presented in Table 1. A total of 505 participants were included. Half of the respondents identified as male (52.9%) and non-Hispanic white (65.9%), and they were young adult cancer survivors below the age of 44 years (39.2%). Most of the participants were college graduates (60.8%), declared an annual income of over 50,000 USD (68.3%), and had health insurance (97.2%). The most reported cancer types were prostate (13.7%), early-stage breast (13.1%), colorectal (8.9%), and gynecological (7.3%). Participants had received multiple forms of treatment (56.2%), and they were not undergoing maintenance therapy when the survey was completed (40.4%). Respondents were diagnosed more than 4 years earlier (long-term survivorship 34.9%), with one-fourth of the sample been diagnosed within the previous 2 years (short-term survivorship, 25.0%). Most cancer survivors received care at academic cancer centers (29.7%) and in community hospitals (30.8%).
Table 1.
Sociodemographic and clinical characteristics of the sample (N = 505).
| Variable | N | % |
|---|---|---|
| Sex | ||
| Female | 238 | 47.1% |
| Male | 267 | 52.9% |
| Age (recoded in 3 groups) | ||
| ≤44 | 198 | 39.2% |
| 45–65 | 179 | 35.4% |
| ≥66 | 128 | 25.3% |
| Race/Ethnicity | ||
| Asian | 10 | 2.0% |
| Black/African American | 115 | 22.8% |
| Hispanic | 38 | 7.5% |
| Non-Hispanic white | 333 | 65.9% |
| Other | 9 | 1.8% |
| Income (USD) | ||
| ≤49,999 | 150 | 31.6% |
| 50,000–99,999 | 204 | 43.0% |
| ≥100,000 | 120 | 25.3% |
| Education | ||
| Less than high school | 6 | 1.2% |
| High-school graduate | 63 | 12.5% |
| Some college | 129 | 25.5% |
| Associate degree | 62 | 12.3% |
| Bachelor’s degree | 147 | 29.1% |
| Master’s degree | 78 | 15.4% |
| Doctorate degree | 20 | 4.0% |
| Insurance | ||
| Insurance coverage | 491 | 97.2% |
| Lack of insurance coverage | 14 | 2.8% |
| Region in the US | ||
| Midwest | 116 | 23% |
| Northeast | 129 | 25.5% |
| Southeast | 133 | 26.3% |
| Southwest/West | 127 | 25.1% |
| Cancer Type | ||
| Prostate cancer | 69 | 13.7% |
| Breast cancer (early stage) | 66 | 13.1% |
| Colorectal cancer | 41 | 8.1% |
| Endometrial, cervical, or ovarian cancer | 37 | 7.3% |
| Thyroid | 26 | 5.1% |
| Breast cancer (metastatic) | 24 | 4.8% |
| Bladder | 22 | 4.4% |
| Head and neck | 21 | 4.2% |
| Kidney cancer | 21 | 4.2% |
| Lymphoma | 19 | 3.8% |
| Leukemia | 18 | 3.6% |
| Brain tumor | 17 | 3.4% |
| Liver cancer | 16 | 3.2% |
| Melanoma | 16 | 3.2% |
| Pancreatic | 9 | 1.8% |
| Myeloma | 8 | 1.6% |
| Stomach | 5 | 1.0% |
| Other | 45 | 8.9% |
| Treatment | ||
| No treatment | 19 | 3.8% |
| Single treatment | 202 | 40.0% |
| Multiple treatment | 284 | 56.2% |
| Time Since Diagnosis | ||
| ≤12 months | 89 | 17.6% |
| 13–24 months | 126 | 25.0% |
| >2–4 years | 114 | 22.6% |
| >4 years | 176 | 34.9% |
| Treatment Facility | ||
| Academic medical center | 146 | 29.7% |
| Community cancer center | 84 | 17.1% |
| Community hospital | 151 | 30.8% |
| Private physician practice | 77 | 15.7% |
| US Department of Veterans Affairs medical lefts | 19 | 3.9% |
| Unsure | 14 | 2.9% |
| Current Cancer Status | ||
| Diagnosed but not yet treatment | 36 | 7.1% |
| Active treatment | 110 | 21.8% |
| Completed treatment and maintenance therapy | 137 | 27.1% |
| Completed treatment and not on maintenance therapy | 204 | 40.4% |
| Comfort care | 8 | 1.6% |
| Other | 10 | 2.0% |
Note: Not all groups of n values and % add up to the reported sample size because of missing data.
Examination of the demographic variables by age category revealed that the Black/African American and Hispanic categories tended to be mostly present in the younger age group, with older participants being mostly non-Hispanic white (χ2(6) = 118.98; p < 0.0001). Males were under-represented in the middle-age group, while females were over-represented among adults (χ2(2) = 17.49; p < 0.01). Significant differences were identified by treatment status (χ2(4) = 18.68; p = 0.0009), with multiple treatments more frequently reported by younger patients (χ2(1) = 14.39; p = 0.00015). Additionally, insurance status varied by age group (χ2(2) = 7.65; p = 0.02), and younger survivors were more likely to be lacking coverage. No significant differences were detected for income (p = 0.7) and education (p = 0.06).
3.2. Exploratory Factor Analysis
As an initial step, an exploratory factor analysis (EFA) was performed on the 14 items assessing self-reported changes in close relationships. As the items were dichotomous, a robust weighted least square procedure was used, and the initial factor solution was rotated using the GEOMIN oblique method [57]. Up to four factors were extracted, according to the previously hypothesized domains (communication, closeness with partner/children/family members, stability of the relationship, and caregiver burden). An overview of the items, different models, and the two-factor structure loadings is available in Supplementary Materials Tables S1 and S2. Model 1, using a single factor, resulted in a poor fit (χ2(77) = 469.22; p < 0.0001). Four items (item 4, 11, 12, and 14) were not significantly related to the single factor. Model 2 utilized a two-factor solution and was a significant improvement over the one factor model (χ2(13) = 213.53; p < 0.0001). Items 2, 4, 5, 7, 12, and 14 had significant loadings on both factors; however, in all cases, one loading was negative and/or substantially smaller than the other. The three-factor solution (Model 3) showed only a minimal improvement (χ2(52) = 120.47; p < 0.0001), and all but four of the items (items 7, 11, 13, and 14) had significant cross loadings. Lastly, a four-factor solution (Model 4) was only minimally better than the three-factor option (χ2(41) = 89.78; p < 0.0001) with six items (items 4, 5, 6, 7, 8, and 10) indicating significant cross loadings and similar loadings on two factors. Despite the best model fit, the four-factor solution did not match the previously hypothesized domains. Because of the sensitivity of the chi-square test to the large sample size, it was decided to utilize a two-factor solution considering the best conceptual model and the empirical model fit. To provide a simple description that could easily be replicated by other studies, the two factors were created by summing the items with the highest loadings on each factor (≥0.45), which were then labeled Relationship Closeness and Relationship Ambivalence. The correlation between the two factors was examined (r = 0.23, p < 0.05), and internal consistency was investigated (Cronbach’s alpha for relationship closeness, α = 0.65; Cronbach’s alpha for relationship ambivalence, α= 0.57). Mean scores (ranging from 0 to 2) were then compared by variables of interest, with results presented below.
3.3. Differences in Relationship Closeness and Ambivalence by Sociodemographic Characteristics
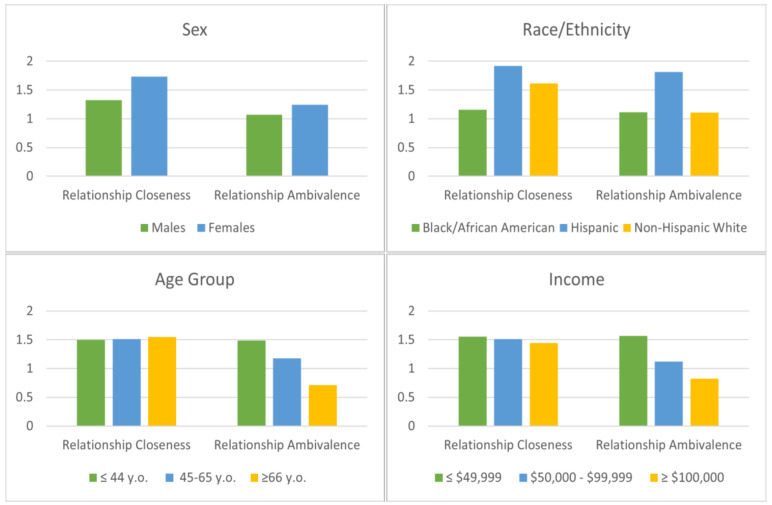
Figure 1 illustrates mean scores for relationship closeness and ambivalence compared by sociodemographic variables. Significant differences were identified between male and female respondents for both closeness (F (1, 503) = 10.04; p = 0.0016; R2 = 0.02) and ambivalence (F (1, 503) = 4.04; p = 0.0451, R2 = 0.008), with female survivors reporting higher levels of positive (Cohen’s d = 0.28) and negative changes in social wellbeing (Cohen’s d = 0.18) than males. Race was also significantly correlated with both positive (F (4, 500) = 3.32; p = 0.0106; R2 = 0.026) and negative effects (F (4, 500) = 3.54; p = 0.007; R2 = 0.027). Hispanic cancer survivors and non-Hispanic whites reported significantly greater closeness than Black/African American participants (Cohen’s d = 0.53, Cohen’s d = 0.32, respectively). Relationship ambivalence was more elevated among survivors who identified as Hispanic than Black/African American (Cohen’s d = 0.59) and non-Hispanic white respondents (Cohen’s d = 0.58).
Figure 1.
Mean scores for relationship closeness and ambivalence by key sociodemographic variables.
Although there were no differences in positive changes by age group (F (2, 502) = 0.04 p = 0.9597, R2 = 0.0001), variations existed in terms of relationship ambivalence in the illness aftermath (F (2, 502) = 11.01; p < 0.0001, R2 = 0.042), suggesting greater vulnerability for survivors diagnosed at a younger age. The oldest age group (≥66 years) had fewer negative changes than either young adult survivors (Cohen’s d = 0.53) or the middle-age group (Cohen’s d = 0.33), but these two groups did not differ significantly (Cohen’s d = 0.20).
Lastly, while relationship closeness did not differ by income (F (2, 471) =0.20; p = 0.821, R2 = 0.001), cancer survivors from low socioeconomic backgrounds were significantly more likely to report negative consequences (F (2, 471) = 8.65; p = 0.0002; R2 = 0.035). Those with the lowest income (≤49,999 USD) had more elevated ambivalence than the middle (50,000–99,999 USD; Cohen’s d = 0.26) and higher income (≥100,000 USD; Cohen’s d = 0.51) groups. No significant differences were registered for education level, insurance coverage, and geographical locations.
3.4. Differences in Relationship Closeness and Ambivalence by Clinical Characteristics
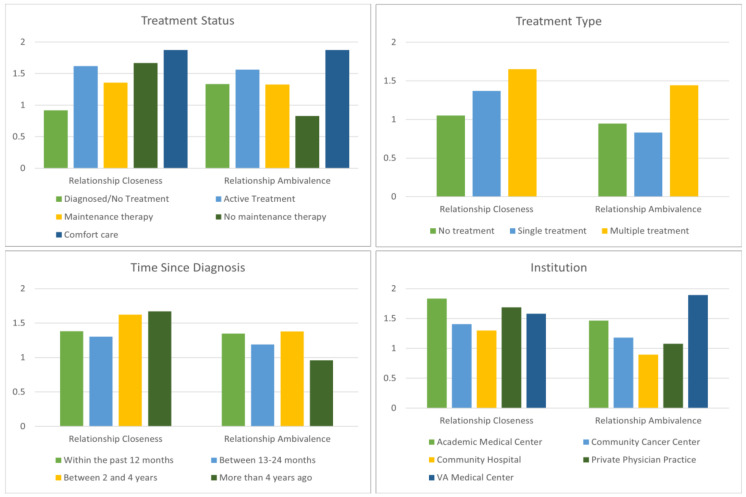
Figure 2 illustrates mean scores for relationship closeness and ambivalence by clinical variables. No differences were detected by cancer type; a result that may be due to the large number of cancers included. When cancer status was examined, significant differences for positive changes in social wellbeing (F (5, 499) = 2.25; p = 0.0481, R2 = 0.018) were identified. Post hoc analysis revealed that individuals who had completed treatment and were not on maintenance therapy presented greater closeness than those who were diagnosed but not yet receiving treatment (mean difference = 0.75, p < 0.05). Similar results emerged when ambivalence was investigated; significant variations existed between those on active treatment and cancer survivors who completed treatment, as well as between survivors on maintenance therapy and those who were not (F (5, 499) = 5.312; p < 0.001, R2 = 0.023).
Figure 2.
Mean scores for relationship closeness and ambivalence by key clinical characteristics.
Significant differences for positive (F (2, 502) = 3.25; p = 0.0394, R2 = 0.013) and negative changes existed by treatment category (F (2, 502) = 13.53; p < 0.0001; R2 = 0.051); respondents who received multiple treatments tended to report greater closeness than those who had received a single treatment approach (Cohen’s d = 0.19), yet the multiple treatment group also had significantly higher rates of ambivalence (Cohen’s d = 0.47). More negative effects were found according to time since diagnosis (F (3, 501) = 2.682; p = 0.046, R2 = 0.009), with a trend toward significance for the difference between short (2–4 years) and long-term survivorship (mean difference = 0.417, p = 0.061). Lastly, variations were assessed by institution (F (5, 485) = 3.686; p = 0.003, R2 = 0.075); fewer negative outcomes were identified for those treated at community hospitals when compared to those treated at academic medical centers (mean difference = 0.571, p = 0.005) and Veteran’s Administration agencies (mean difference = 1.001, p = 0.035).
4. Discussion
The present work extends current literature on social wellbeing in cancer survivorship, by investigating self-reported positive and negative changes in the context of close relationships. Two factors, Relationship Closeness and Relationship Ambivalence were identified via exploratory factor analysis. Then, differences by sociodemographic and clinical characteristics were examined. Results indicate that women, younger survivors, Black and Hispanic survivors, and those with lower income presented more impaired social wellbeing. Additionally, variations were registered by treatment status, time since diagnosis, and institution.
The study confirms existing literature investigating social outcomes in cancer survivors. Female participants reported both greater negative and positive changes in social wellbeing. This finding can be linked to reported sex and gender differences in morbidity and adjustment [10], as well as to emerging application of theoretical frameworks that contribute to describe gender-related differences [58]. For instance, Social Role Theory can characterize this finding as resulting from perceived role and caregiving responsibilities [58], while transactional approaches may relate this to differential appraisals [59]. Although no differences for closeness were detected by age group, greater ambivalence among younger survivors confirms the profound psychosocial impact of facing cancer as a young adult. Studies have consistently documented the clinical decrement of social functioning over time, especially for young survivors experiencing greater symptomatology, limited social support [48,60,61], and higher distress in their relationships [23,48,62,63,64,65]. Three recent systematic reviews identified that this group continues to experience difficulties establishing and maintaining relationships with peers, family members, and partners [64,65,66].
In addition to sex and age, members of minoritized groups and socioeconomically vulnerable individuals experienced higher levels of ambivalence. These findings can contribute to illustrate the differential impact of the illness for those who experience cancer from a position of vulnerability. Financial hardship [67,68,69,70,71,72] can affect psychological distress, quality of life, and social relations [73]. Worse outcomes, in the form of lower closeness and higher ambivalence, were reported by Black/African American and Hispanic respondents, respectively. These results reflect the intersection of social determinants of health [17] and culturally informed expectations for family interaction and provision of support [54,55], which require greater understanding by the literature and multilevel interventions [16,56].
The transition to survivorship is confirmed to be a delicate moment for the social wellbeing of the individual, as evidenced by significant variations in relationship ambivalence between those on active treatment and cancer survivors who completed treatment, as well as between survivors on maintenance therapy and those who were not. Previous evidence has revealed cancer survivors and their partners’ tendency to withdraw from each other in the period immediately following the end of active treatment [24]. Differences in negative consequences by treatment modality, status, and time since diagnosis can help in identifying moments of potential susceptibility for the social wellbeing of survivors; in this sample, active treatment, maintenance therapy, and the early survivorship phase were characterized by greater ambivalence. This result can assist future efforts to identify and intervene on psychosocial resources that contribute to the wellbeing of both patients and caregivers [21,22,56]. As greater survival rates have been reported for those treated at NCI-designated comprehensive cancer centers [74,75], it was unexpected to register fewer negative outcomes for those that received care at community hospitals. While this finding may be due to this sample’s characteristics, Zebrack et al. [76] found that providers at community cancer programs presented greater institutional capacity for continuity in the delivery of psychosocial care over time. Future contributions investigating the implementation and outcome evaluation of comprehensive psychosocial support services across cancer care settings are needed.
The cross-sectional design, the utilization of self-reported dichotomous items, and the lack of a comparison group of healthy peers are important limitations that affect the present work. Positive and negative variations in social wellbeing were evaluated using a list of dichotomous items created for the purpose of the survey by providers. Hence, it was not possible to discriminate among the different domains affected by the illness, nor to elaborate on the amount of change participants experienced since diagnosis. The inclusion of standardized and validated questionnaires is, therefore, recommended for future studies. Furthermore, SEM model fit indices were acceptable but lower than ideal, suggesting future research to consider alternative models when additional measures are available to describe social wellbeing in the cancer aftermath. The lack of a comparison group of healthy peers prevented the authors from inferring whether these changes occurred due to illness, aging process, or other sample characteristics. Furthermore, the survey was cross-sectional, and it was not possible to elaborate on causation nor on trajectories of positive and negative changes over time and at critical turning points of the cancer continuum. Similarly, the association with mental health data should be further investigated to clarify whether variations in social wellbeing were linked to affected mood or distress. While the inclusion of a large, national, and diverse sample is an aspect of strength of the present analysis, recruitment via online panels led to the overrepresentation and underrepresentation of certain groups of survivors, which may have influenced some of the current results.
5. Conclusions
The present study revealed that there are groups of cancer survivors experiencing more affected social wellbeing: women, young adults, individuals from minoritized groups, and those with lower financial resources. Furthermore, variations by treatment status, time since diagnosis, and institution suggest that social wellbeing may be influenced by the interaction with the healthcare system. Specifically, our findings indicate that there may be settings not fully equipped to provide models of care encompassing the psychosocial needs of patients, which can ultimately affect their social relationships. This work also has implications for oncology social workers and healthcare teams involved in direct care delivery. Results emphasize the need to enhance providers’ capacity for addressing psychosocial issues related to the relationship with partners, family members, and the larger social network. At the same time, this contribution unveiled the necessity to develop interventions able to sustain the quality of survivors’ interpersonal relationships and overall social wellbeing, with a particular emphasis on the experience of certain groups and for the differential burden that accompanies active treatment, early vs. long-term survivorship. Future research should expand both qualitatively and quantitatively current understanding of the experience of survivors reporting more affected social wellbeing and investigate the development and implementation of supportive care services alleviating stressors impairing the quality of close relationships.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/curroncol30020133/s1, Table S1. Exploratory factor analysis (EFA): comparison of fit indices; Table S2. Exploratory factor analysis (EFA): factor loadings.
Author Contributions
Conceptualization, C.A. and E.M.-S.; methodology, A.Z.; software, A.Z.; formal analysis, C.A. and A.Z.; investigation, E.M.-S. and C.A.; resources, E.M.-S.; data curation, E.M.-S.; writing—original draft preparation, C.A.; writing—review and editing, C.A., E.M.-S., A.Z., and E.I.; project administration, E.M.-S. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board of University of Houston (STUDY00001071, 06/19/2018).
Informed Consent Statement
This was a secondary data analysis. Informed consent was obtained from all subjects involved in the original study.
Data Availability Statement
Primary data for this secondary analysis article were collected by CancerCare as part of the Patient Access and Engagement Report. The datasets analyzed during the current study are available from the corresponding author on reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Siegel R.L., Miller K.D., Fuchs H.E., Jemal A. Cancer Statistics, 2021. CA Cancer J. Clin. 2021;71:7–33. doi: 10.3322/caac.21654. [DOI] [PubMed] [Google Scholar]
- 2.Miller K.D., Nogueira L., Mariotto A.B., Rowland J.H., Yabroff K.R., Alfano C.M., Jemal A., Kramer J.L., Siegel R.L. Cancer treatment and survivorship statistics, 2019. CA Cancer J. Clin. 2019;69:363–385. doi: 10.3322/caac.21565. [DOI] [PubMed] [Google Scholar]
- 3.American Cancer Society . Cancer Treatment & Survivorship Facts & Figures 2019–2021. American Cancer Society; Atlanta, GA, USA: 2019. [Google Scholar]
- 4.AACR . Disparities in Cancer Survivorship. AACR; Orlando, FL, USA: 2020. [Google Scholar]
- 5.Fitch M.I., Nicoll I., Lockwood G. Exploring the impact of physical, emotional, and practical changes following treatment on the daily lives of cancer survivors. J. Psychosoc. Oncol. 2020;39:219–232. doi: 10.1080/07347332.2020.1848967. [DOI] [PubMed] [Google Scholar]
- 6.Leach C.R., Weaver K.E., Aziz N.M., Alfano C.M., Bellizzi K.M., Kent E.E., Forsythe L.P., Rowland J.R. The complex health profile of long-term cancer survivors: Prevalence and predictors of comorbid conditions. J. Cancer Surviv. 2015;9:239–251. doi: 10.1007/s11764-014-0403-1. [DOI] [PubMed] [Google Scholar]
- 7.Jacobsen P.B., Rowland J.H., Paskett E.D., Van Leeuwen F., Moskowitz C., Wollins S.K., Robison L.L. Identification of key gaps in cancer survivorship research: Findings from the American Society of Clinical Oncology Survey. J. Oncol. Pract. 2016;12:190–193. doi: 10.1200/JOP.2015.009258. [DOI] [PubMed] [Google Scholar]
- 8.Meeker C.R., Wong Y.-N., Egleston B.L., Hall M.J., Plimack E.R., Martin L.P., von Mehren M., Lewis B.R., Geynisman D.M. Distress and financial distress in adults with cancer: An age-based analysis. J. Natl. Compr. Cancer Netw. 2017;15:1224. doi: 10.6004/jnccn.2017.0161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vrontaras N. Cancer patients’ views on the family changes and the family social support. J. Eur. Psychol. Stud. 2018;9:16–27. doi: 10.5334/jeps.403. [DOI] [Google Scholar]
- 10.Linden W., Vodermaier A., Mackenzie R., Greig D. Anxiety and depression after cancer diagnosis: Prevalence rates by cancer type, gender, and age. J. Affect. Disord. 2012;141:343–351. doi: 10.1016/j.jad.2012.03.025. [DOI] [PubMed] [Google Scholar]
- 11.Mehnert A. Employment and work-related issues in cancer survivors. Crit. Rev. Oncol. Hematol. 2011;77:109–130. doi: 10.1016/j.critrevonc.2010.01.004. [DOI] [PubMed] [Google Scholar]
- 12.Mehnert A., de Boer A., Feurstein M. Employment challenges for cancer survivors. Am. Cancer Soc. 2013;119:2151–2159. doi: 10.1002/cncr.28067. [DOI] [PubMed] [Google Scholar]
- 13.Lange M., Licaj I., Clarisse B., Humbert X., Grellard J.M., Tron L., Joly F. Cognitive complaints in cancer survivors and expectations for support: Results from a web–based survey. Cancer Med. 2019;8:2654–2663. doi: 10.1002/cam4.2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Országhová Z., Mego M., Chovanec M. Long-term cognitive dysfunction in cancer survivors. Front. Mol. Biosci. 2021;8:770413. doi: 10.3389/fmolb.2021.770413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chan R.J., Hollingdrake O., Bui U., Nekhlyudov L., Hart N.H., Lui C.-W., Feuerstein M. Evolving landscape of cancer survivorship research: An analysis of the Journal of Cancer Survivorship, 2007–2020. J. Cancer Surviv. 2021;15:651–658. doi: 10.1007/s11764-021-01042-6. [DOI] [PubMed] [Google Scholar]
- 16.Alcaraz K.I., Wiedt T.L., Daniels E.C., Yabroff K.R., Guerra C.E., Wender R.C. Understanding and addressing social determinants to advance cancer health equity in the United States: A blueprint for practice, research, and policy. CA Cancer J. Clin. 2020;70:31–46. doi: 10.3322/caac.21586. [DOI] [PubMed] [Google Scholar]
- 17.Ellis L., Canchola A.J., Spiegel D., Ladabaum U., Haile R., Gomez S.L. Racial and ethnic disparities in cancer survival: The Contribution of tumor, sociodemographic, institutional, and neighborhood characteristics. J. Clin. Oncol. 2018;36:25–33. doi: 10.1200/JCO.2017.74.2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Howell D., Mayer D.K., Fielding R., Eicher M., Verdonck-de Leeuw I.M., Johansen C., Soto-Perez-de-Celis E., Foster C., Chan R., Alfano C.M., et al. Management of cancer and health after the clinic visit: A call to action for self-management in cancer care. JNCI J. Natl. Cancer Inst. 2020;113:523–531. doi: 10.1093/jnci/djaa083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lisy K., Kent J., Piper A., Jefford M. Facilitators and barriers to shared primary and specialist cancer care: A systematic review. Support. Care in Cancer. 2020;29:85–96. doi: 10.1007/s00520-020-05624-5. [DOI] [PubMed] [Google Scholar]
- 20.Nekhlyudov L., Mollica M.A., Jacobsen P.B., Mayer D.K., Shulman L.N., Geiger A.M. Developing a Quality of Cancer Survivorship Care Framework: Implications for clinical care, research, and policy. J. Natl. Cancer Inst. 2019;111:1120–1130. doi: 10.1093/jnci/djz089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kent E.E., Rowland J.H., Northouse L., Litzelman K., Chou W.-Y.S., Shelburne N., Timura C., O’Mara A., Huss K. Caring for caregivers and patients: Research and clinical priorities for informal cancer caregiving. Cancer. 2016;122:1987–1995. doi: 10.1002/cncr.29939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alfano C.M., Leach C.R., Smith T.G., Miller K.D., Alcaraz K.I., Cannady R.S., Wender R.C., Brawley O.W. Equitably improving outcomes for cancer survivors and supporting caregivers: A blueprint for care delivery, research, education, and policy. CA Cancer J. Clin. 2019;69:35–49. doi: 10.3322/caac.21548. [DOI] [PubMed] [Google Scholar]
- 23.Bellizzi K.M., Smith A., Schmit S., Keegan T.H.M., Zebrack B., Lynch C.F., Deapen D., Shnorhavorian M., Tompkins B.J., Simon M. Positive and negative psychosocial impact of being diagnosed with cancer as an adolescent or young adult. Am. Cancer Soc. 2012;118:5155–5162. doi: 10.1002/cncr.27512. [DOI] [PubMed] [Google Scholar]
- 24.Keesing S., Rosenwax L., McNamara B. A dyadic approach to understanding the impact of breast cancer on relationships between partners during early survivorship. BMC Women’s Health. 2016;16:57. doi: 10.1186/s12905-016-0337-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Acquati C., Kayser K. Dyadic coping across the lifespan: A comparison between younger and middle-aged couples with breast cancer. Front. Psychol. 2019;10:404. doi: 10.3389/fpsyg.2019.00404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Manne S., Kashy D., Virtue S.M., Zaider T. Relationship communication and the course of psychological outcomes among couples coping with localised prostate cancer. Eur. J. Cancer Care. 2021;30:e13401. doi: 10.1111/ecc.13401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Weaver K.E., Forsythe L.P., Reeve B.B., Alfano C.M., Rodriguez J., Sabatino S.A., Hawkins N.A., Rowland J.H. Mental and physical health-related quality of life among U.S. cancer survivors: Population estimates from the 2010 National Health Interview Survey. Cancer Empidemiol. Biomark. Prev. 2012;21:2108–2117. doi: 10.1158/1055-9965.EPI-12-0740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Woźniak K., Iżycki D. Cancer: A family at risk. Menopause Rev. 2014;13:253–261. doi: 10.5114/pm.2014.45002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Litzelman K., Yabroff K.R. How are spousal depressed mood, distress and quality of life associated with risk of depressed mood in cancer survivors? Longitudinal findings from a national sample. Cancer Epidemiol. Biomark. Prev. 2016;24:969–977. doi: 10.1158/1055-9965.EPI-14-1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hawkey A.J., Ussher J.M., Perz J., Parton C., Patterson P., Bateson D., Hobbs K., Kirsten L. The impact of cancer-related fertility concerns on current and future couple relationships: People with cancer and partner perspectives. Eur. J. Cancer Care. 2020;30:e13348. doi: 10.1111/ecc.13348. [DOI] [PubMed] [Google Scholar]
- 31.Kirchhoff A.C., Yi J., Wright J., Warner E.L., Smith K.R. Marriage and divorce among young adult cancer survivors. J. Cancer Surviv. 2012;6:441–450. doi: 10.1007/s11764-012-0238-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Song H.Y., Kwon J.A., Choi J.W., Kim S.J., Park E.C. Gender differences in marital disruption among patients with cancer: Results from the Korean National Health and Nutrition Examination Survey (KNHANES) Asian Pac. J. Cancer Prev. 2014;15:6547–6552. doi: 10.7314/APJCP.2014.15.16.6547. [DOI] [PubMed] [Google Scholar]
- 33.Stephens C., Westmaas J.L., Kim J., Cannady R., Stein K. Gender differences in associations between cancer-related problems and relationship dissolution among cancer survivors. J. Cancer Surviv. 2016;10:865–873. doi: 10.1007/s11764-016-0532-9. [DOI] [PubMed] [Google Scholar]
- 34.Fugmann D., Boeker M., Holsteg S., Steiner N., Prins J., Karger A. A systematic review: The effect of cancer on the divorce rate. Front. Psychol. 2022;13:828656. doi: 10.3389/fpsyg.2022.828656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nalbant B., Karger A., Zimmermann T. Cancer and relationship dissolution: Perspective of partners of cancer patients. Front. Psychol. 2021;12:624902. doi: 10.3389/fpsyg.2021.624902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bowling A. Measuring Disease. A Review of Disease-Specific Quality of Life Measurement Scales. Open University Press; Buckingham, UK: 2001. [Google Scholar]
- 37.Kraemer L.M., Stanton A.L., Meyerowitz B.E., Rowland J.H., Ganz P.A. A longitudinal examination of couples’ coping strategies as predictors of adjustment to breast cancer. J. Fam. Psychol. 2011;25:963–972. doi: 10.1037/a0025551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McDowell I., Newell C. Measuring Health: A Guide to Rating Scales and Questionnaires. Oxford University Press; New York, NY, USA: 1987. [Google Scholar]
- 39.Cicognani E. Social Well-Being. In: Michalos A.C., editor. Encyclopedia of Quality of Life and Well-Being Research. Springer; Dordrecht, The Netherlands: 2014. pp. 6193–6197. [Google Scholar]
- 40.Lo C., Lin J., Gagliese L., Zimmermann C., Mikulincer M., Rodin G. Age and depression in patients with metastatic cancer: The protective effects of attachment security and spiritual wellbeing. Ageing Soc. 2009;30:325–336. doi: 10.1017/S0144686X09990201. [DOI] [Google Scholar]
- 41.Kadambi S., Soto-Perez-de-Celis E., Garg T., Loh K.P.L., Krok-Schoen J.L., Battisti N.M.L., Moffat G.T., Gil L.J., Jr., Mohile S., Hsuh T. Social support for older adults with cancer: Young international society of geriatric oncology review paper. J. Geriatr. Oncol. 2020;11:217–224. doi: 10.1016/j.jgo.2019.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Farrell A.K., Stanton S.C.E. Toward a mechanistic understanding of links between close relationships and physical Health. Curr. Dir. Psychol. Sci. 2019;28:483–489. doi: 10.1177/0963721419855657. [DOI] [Google Scholar]
- 43.Regan T., Acquati C., Zimmermann T. Handbook of Cancer Survivorship. Springer; Berlin/Heidelberg, Germany: 2018. Interpersonal Relationships; pp. 265–284. [Google Scholar]
- 44.Manne S., Badr H. Intimacy and relationship processes in couples’ psychosocial adaptation to cancer. Cancer. 2008;112:2541–2555. doi: 10.1002/cncr.23450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kim Y., Carver C.S., Ting A. Family caregivers’ unmet needs in long-term cancer survivorship. Semin. Oncol. Nurs. 2019;35:380–383. doi: 10.1016/j.soncn.2019.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Husson O., Mols F., Fransen M.P., Poll-Franse L.V., Ezendam N.P.M. Low subjective health literacy is associated with adverse health behaviors and worse health-related quality of life among colorectal cancer survivors: Results from the profiles registry. Psychooncology. 2015;24:478–486. doi: 10.1002/pon.3678. [DOI] [PubMed] [Google Scholar]
- 47.Manne S., Kashy D.A., Siegel S., Virtue S.M., Heckman C., Ryan D. Unsupportive partner behaviors, social-cognitive processing, and psychological outcomes in couples coping with early stage breast cancer. J. Fam. Psychol. 2014;28:214–224. doi: 10.1037/a0036053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schilstra C.E., Fardell J.E., Burns M.A., Ellis S.J., Anazodo A.C., Trahair T.N., Sansom-Daly U.M. Determinants of social functioning among adolescents and young adults with cancer: A systematic review. Psychooncology. 2021;30:1626–1642. doi: 10.1002/pon.5740. [DOI] [PubMed] [Google Scholar]
- 49.Henshall L.C., Greenfield M.S., Gale K.N. Typologies for restructuring relationships in cancer survivorship: Temporal changes in social support and engagement with self-management practices. Cancer Nurs. 2018;41:E32–E40. doi: 10.1097/NCC.0000000000000538. [DOI] [PubMed] [Google Scholar]
- 50.Sinnott S.M., Park C.L. Social well-being in Adolescent and Young Adult cancer survivors. J. Adolesc. Young Adult Oncol. 2019;8:32–39. doi: 10.1089/jayao.2018.0043. [DOI] [PubMed] [Google Scholar]
- 51.Kim Y., Kashy D.A., Spillers R.L., Evans T.V. Needs assessment of family caregivers of cancer survivors: Three cohorts comparison. Psycho Oncol. 2010;19:573–582. doi: 10.1002/pon.1597. [DOI] [PubMed] [Google Scholar]
- 52.Bluethmann S.M., Mariotto A.B., Rowland J.H. Anticipating the “Silver Tsunami”: Prevalence Trajectories and comorbidity burden among older cancer survivors in the United States. Cancer Epidemiol. Biomark. Prev. 2016;25:1029–1036. doi: 10.1158/1055-9965.EPI-16-0133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Badr H., Bakhshaie J., Chhabria K. Dyadic Interventions for cancer survivors and caregivers: State of the science and new directions. Semin. Oncol. Nurs. 2019;35:337–341. doi: 10.1016/j.soncn.2019.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Luckett T., Goldstein D., Butow P.N., Gebski V., Aldridge L.J., McGrane J., Ng W., King M.T. Psychological morbidity and quality of life of ethnic minority patients with cancer: A systematic review and meta-analysis. Lancet Oncol. 2011;12:1240–1248. doi: 10.1016/S1470-2045(11)70212-1. [DOI] [PubMed] [Google Scholar]
- 55.Huang Y.-J., Acquati C., Cheung M. Family communication and coping among racial-ethnic minority cancer patients: A systematic review. Health Soc. Care Community. 2022;30:e605–e620. doi: 10.1111/hsc.13623. [DOI] [PubMed] [Google Scholar]
- 56.Chan R.J., Nekhlyudov L., Duijts S.F.A., Hudson S.V., Jones J.M., Keogh J., Love B., Lustberg M.B., Mehnert-Theuerkauf A., Nathan P., et al. Future research in cancer survivorship. J. Cancer Surviv. 2021;15:659–667. doi: 10.1007/s11764-021-01102-x. [DOI] [PubMed] [Google Scholar]
- 57.Muthén L.K., Muthén B. Mplus User’s G. 1998–2012. [(accessed on 6 February 2019)]. Available online: file:///C:/Users/MDPI/Downloads/Mplus%20user%20guide%20Ver_7_r6_web.pdf.
- 58.Kim Y., Mitchell H.-R., Ting A. Application of psychological theories on the role of gender in caregiving to psycho-oncology research. Psycho Oncol. 2019;28:228–254. doi: 10.1002/pon.4953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tamres L.K., Janicki D., Helgeson V.S. Sex differences in coping behavior: A meta-analytic review and an examination of relative coping. Personal. Soc. Psychol. Rev. 2002;6:2–30. doi: 10.1207/S15327957PSPR0601_1. [DOI] [Google Scholar]
- 60.Walsh C.A., Yi J.C., Rosenberg A.R., Crouch M.V., Leisenring W.M., Syrjala K.L. Factors associated with social functioning among long-term cancer survivors treated with hematopoietic stem cell transplantation as adolescents or young adults. Psychooncology. 2020;29:1579–1586. doi: 10.1002/pon.5460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Husson O., Zebrack B.J., Aguilar C., Hayes-Lattin B., Cole S. Cancer in adolescents and young adults: Who remains at risk of poor social functioning over time? Cancer. 2017;123:2743–2751. doi: 10.1002/cncr.30656. [DOI] [PubMed] [Google Scholar]
- 62.Acquati C., Zebrack B.J., Faul A.C., Embry L., Aguilar C., Block R., Hayes-Lattin B., Freyer D.R., Cole S. Sexual functioning among young adult cancer patients: A 2-year longitudinal study. Cancer. 2018;124:398–405. doi: 10.1002/cncr.31030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sopfe J., Gupta A., Appiah L.C., Eric J.C., Peterson P.N. Sexual dysfunction in Adolescent and Young Adult survivors of childhood cancer: Presentation, risk factors, and evaluation of an underdiagnosed late effect. A Narrative Review. J. Adolesc. Young Adult Oncol. 2020;9:549–560. doi: 10.1089/jayao.2020.0025. [DOI] [PubMed] [Google Scholar]
- 64.Robin C. Impact of Cancer on Romantic Relationships Among Young Adults: A Systematic Review. J. Clin. Psychol. Med. Settings. 2019;26:1–12. doi: 10.1007/s10880-018-9566-7. [DOI] [PubMed] [Google Scholar]
- 65.Barnett M., McDonnell G., DeRosa A., Schuler T., Philip E., Peterson L., Touza K., Jhanwar S., Atkinson T.M., Ford J.S. Psychosocial outcomes and interventions among cancer survivors diagnosed during adolescence and young adulthood (AYA): A systematic review. J. Cancer Surviv. 2016;10:814–831. doi: 10.1007/s11764-016-0527-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Warner E.L., Kent E.E., Trevino K.M., Parsons H.M., Zebrack B.J., Kirchhoff A.C. Social well-being among adolescents and young adults with cancer: A systematic review. Cancer. 2016;122:1029–1037. doi: 10.1002/cncr.29866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wheeler S.B., Spencer J.C., Pinheiro L.C., Carey L.A., Olshan A.F., Reeder-Hayes K.E. Financial impact of breast cancer in Black versus White women. J. Clin. Oncol. 2018;36:1695–1701. doi: 10.1200/JCO.2017.77.6310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Politi M.C., Yen R.W., Elwyn G., O’Malley A.J., Saunders C.H., Schubbe D., Forcino R., Durand M.A. Women who are young, non-white, and with lower socioeconomic status report higher financial toxicity up to 1 year after breast cancer surgery: A mixed-effects regression analysis. Oncologist. 2021;26:e142–e152. doi: 10.1002/onco.13544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yabroff K.R., Dowling E.C., Guy G.P., Jr., Banegas M.P., Davidoff A., Han X., Virgo K.S., McNeel T.S., Chawla N., Blanch-Hartigan D., et al. Financial hardship associated with cancer in the United States: Findings from a population-based sample of adult cancer survivors. J. Clin. Oncol. 2016;34:259–267. doi: 10.1200/JCO.2015.62.0468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Shankaran V., Jolly S., Blough D., Ramsey S.D. Risk factors for financial hardship in patients receiving adjuvant chemotherapy for colon cancer: A population-based exploratory analysis. J. Clin. Oncol. 2012;30:1608–1614. doi: 10.1200/JCO.2011.37.9511. [DOI] [PubMed] [Google Scholar]
- 71.Chino F., Peppercorn J.M., Rushing C., Kamal A.H., Altomare I., Samsa G., Zafar S.Y. Out-of-pocket costs, financial distress, and underinsurance in cancer care. JAMA Oncol. 2017;3:1582–1584. doi: 10.1001/jamaoncol.2017.2148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Smith G.L., Lopez-Olivo M.A., Advani P.G., Ning M.S., Geng Y., Giordano S.H., Volk R.J. Financial burdens of cancer treatment: A systematic review of risk factors and outcomes. J. Natl. Compr. Cancer Netw. JNCCN. 2019;17:1184–1192. doi: 10.6004/jnccn.2019.7305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ver Hoeve E.S., Ali-Akbarian L., Price S.N., Lothfi N.M., Hamann H.A. Patient-reported financial toxicity, quality of life, and health behaviors in insured US cancer survivors. Support. Care Cancer. 2021;29:349–358. doi: 10.1007/s00520-020-05468-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Boffa D.J., Mallin K., Herrin J., Resio B., Salazar M.C., Palis B., Facktor M., McCabe R., Nelson H., Shulman L.N. Survival after cancer treatment at top-ranked US cancer hospitals vs. affiliates of top-ranked cancer hospitals. J. Am. Med. Assoc. Oncol. 2020;3:e203942. doi: 10.1001/jamanetworkopen.2020.3942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wolfson J., Sun C.-L., Wyatt L., Hurria A., Bhatia S. Impact of care at comprehensive cancer centers on outcome—Results from a population-based study. Cancer. 2016;121:3885–3893. doi: 10.1002/cncr.29576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zebrack B., Kayser K., Padgett L., Sundstrom L., Jobin C., Nelson K., Fineberg I.C. Institutional capacity to provide psychosocial oncology support services: A report from the Association of Oncology Social Work. Cancer. 2016;122:1937–1945. doi: 10.1002/cncr.30016. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Primary data for this secondary analysis article were collected by CancerCare as part of the Patient Access and Engagement Report. The datasets analyzed during the current study are available from the corresponding author on reasonable request.