Abstract
Alterations in homocysteine, methionine, folate, and/or B12 homeostasis have been associated with neural tube defects, cardiovascular disease, and cancer. Methionine synthase, one of only two mammalian enzymes known to require vitamin B12 as a cofactor, lies at the intersection of these metabolic pathways. This enzyme catalyzes the transfer of a methyl group from 5-methyl-tetrahydrofolate to homocysteine, generating tetrahydrofolate and methionine. Human patients with methionine synthase deficiency exhibit homocysteinemia, homocysteinuria, and hypomethioninemia. They suffer from megaloblastic anemia with or without some degree of neural dysfunction and mental retardation. To better study the pathophysiology of methionine synthase deficiency, we utilized gene-targeting technology to inactivate the methionine synthase gene in mice. On average, heterozygous knockout mice from an outbred background have slightly elevated plasma homocysteine and methionine compared to wild-type mice but seem to be otherwise indistinguishable. Homozygous knockout embryos survive through implantation but die soon thereafter. Nutritional supplementation during pregnancy was unable to rescue embryos that were completely deficient in methionine synthase. Whether any human patients with methionine synthase deficiency have a complete absence of enzyme activity is unclear. These results demonstrate the importance of this enzyme for early development in mice and suggest either that methionine synthase-deficient patients have residual methionine synthase activity or that humans have a compensatory mechanism that is absent in mice.
Methionine synthase (MS; EC 2.1.1.13), one of two B12-dependent mammalian enzymes, catalyzes the remethylation of homocysteine to methionine and the concurrent demethylation of 5-methyltetrahydrofolate (5-Me-THF) to tetrahydrofolate (THF). Methylcobalamin, a derivative of vitamin B12, is the cofactor for this reaction. MSs are highly conserved, large (about 140-kDa) monomeric Zn metalloproteins and consist of three domains: a catalytic domain that contains the binding sites for 5-Me-THF and homocysteine; a B12 domain, where the methylcobalamin cofactor is tightly bound; and an activation domain. The cob(I)alamin form of the cofactor is methylated by 5-Me-THF, generating enzyme-bound methylcob(III)alamin and THF. Then methylcob(III)alamin transfers its methyl group to homocysteine to produce methionine and returns to the cob(I)alamin form. Occasionally, the highly reactive cob(I)alamin cofactor is oxidized to the nonfunctional cob(II)alamin form during catalysis. The enzyme is reactivated by an S-adenosylmethionine (AdoMet) and NADPH-dependent reductive methylation of enzyme-bound cob(II)alamin to methylcob(III)alamin. The enzyme(s) involved in this reductive methylation has not been well characterized, and two pathways have been proposed. The first pathway involves a single protein, MS reductase, a soluable P450-reductase-like protein that contains binding sites for NADPH, flavin adenine dinucleotide, and flavin mononucleotide (14). The second pathway is dependent on two proteins: P450 reductase and soluble cytochrome b5 (5).
The human MS gene, located on chromosome 1q42.3-q43, produces two major transcripts of roughly 8 and 10 kb, which encode a 1,265-amino-acid protein that has been highly conserved through evolution and shares ∼64% and 55% identity with the Caenorhabditis elegans and Escherichia coli proteins, respectively (4, 13, 15). Mutations in the MS gene are responsible for the rare autosomal recessive disease of cobalamin metabolism known as cblG (8, 13). Patients with this disorder have homocysteinemia, homocysteinuria, and hypomethioninemia, suffer from megaloblastic anemia similar to that of folate or B12 deficiency, and may manifest some degree of neural dysfunction and mental retardation.
The intracellular synthesis of 5-Me-THF from 5, 10-methylene-THF, catalyzed by methylenetetrahydrofolate reductase (MTHFR), is irreversible under physiological conditions, and MS activity is absolutely required for the further metabolism of 5-Me-THF. Under conditions of B12 depletion, such as pernicious anemia, loss of MS activity leads to a methyl folate trap. The depletion of other folate coenzymes results in defective thymidylate and purine synthesis and impaired DNA synthesis with the development of megaloblastic anemia (22). In addition, 5-Me-THF, which is the major circulating form of folate, is a very poor substrate for folylpolyglutamate synthetase, and the inability to convert entering folates to the polyglutamate species leads to the inability of tissues to retain folate.
Homocysteine is a product of AdoMet-dependent transmethylation reactions. The ubiquitously distributed MS reaction is the sole mechanism, with the exception of hepatic (and renal in some species) betaine homocysteine methyltransferase (BHMT), for the regeneration of methionine from homocysteine. In the liver, but not in peripheral tissues, remethylation of homocysteine to methionine by MS is regulated by dietary intake of methionine. High levels of hepatic AdoMet allosterically inhibit MTHFR and the formation of 5-Me-THF and stimulate cystathionine β-synthase (CBS). CBS catalyzes the committing step in the transulfuration pathway in which homocysteine is converted to cysteine.
Elevated plasma homocysteine has been identified as an independent risk factor for the development of cardiovascular disease (20), and there has been recent evidence suggesting that pregnant women with elevated homocysteine levels are at an increased risk of bearing children with neural tube defects (17, 25, 26). In addition to the megaloblastic anemia associated with both folate and B12 deficiencies and the neuropathy associated with B12 deficiency, a low folate status is an established risk factor for neural tube defects (6, 10, 12, 18, 23, 24). Low B12 status is also reported to be a risk factor for neural tube defects (NTDs) (11). In order to gain further insights into the physiological roles of MS and the effects of impairment of MS activity and potentially to generate an animal model for B12 defiency, NTDs and/or cardiovascular disease, we have utilized a gene-targeting strategy to create MS-deficient mice.
MATERIALS AND METHODS
Targeting construct and MS gene targeting.
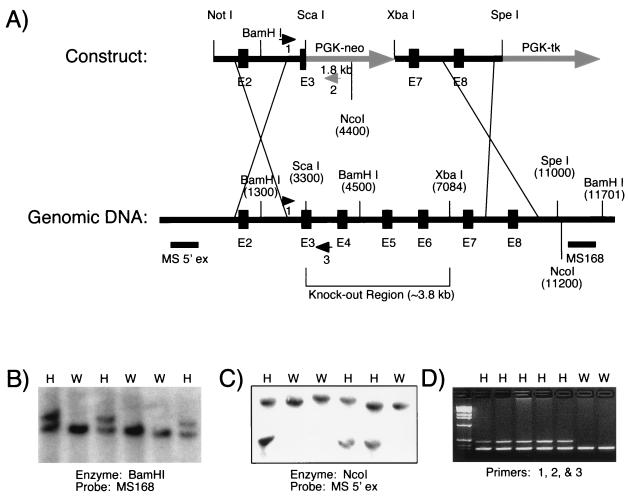
A 1.7-kb mouse MS cDNA fragment, corresponding to human MS cDNA sequence +539 to +2230 (+1 translation start site), was obtained by PCR amplification of a mouse brain cDNA library (Stratagene, La Jolla, Calif.) using nested primers (primary, 5′ GCTTGTTGAAGCATACCAAGAGCAG; secondary, 5′ CCAAAGGACTTCTGGATGGCGGGG) corresponding to the human MS cDNA sequences from +513 to +537 and from +539 to +562 and T3 or T7 primers to the phagemid region in the Lambda ZAP II vector. This mouse cDNA fragment was used as a probe to isolate genomic clones spanning the mouse MS gene from a mouse lambda genomic library 129 SV/J (Stratagene). Clones corresponding to various regions of the human MS cDNA were verified by Southern blot analysis. One clone (∼13 kb) starting in intron 1 and extending to intron 9 was characterized extensively (Fig. 1). An MS homologous recombination construct, in which a 3.8-kb genomic sequence spanning from the middle of exon 3 to the middle of intron 6 was replaced with a 1.8-kb PGK-neomycin resistance (PGK-neo) cassette, was generated using the pPNT vector as the backbone (28). This construct contained a 3.3-kb fragment (from intron 1 to exon 3) and a 4.2-kb fragment (from intron 6 to intron 8) of the MS gene flanking the PGK-neo cassette and a PGK-tk cassette at the 3′ end when linearized by NotI digestion (Fig. 1).
FIG. 1.
Targeted disruption of the MS gene. (A) A knockout construct was made by replacing 3.8 kb of the MS gene (part of exon 3 through part of intron 6) with a 1.8-kb PGK-neo cassette. The linearized knockout construct and genomic DNA with various restriction sites are shown. Probes and primers used for genotyping are indicated. Numbers by restriction sites indicate distance, in base pairs, from the 5′ end of the linearized knockout construct. (B) Southern blots of BamHI-digested DNAs of offspring from heterozygous matings have been probed with MS168. This probe detects an ∼7.2-kb endogenous 129 mouse fragment and an ∼8.4-kb fragment from the targeted allele. H, heterozygote; W, wild type. (C) Southern blots of NcoI-digested DNAs of offspring from heterozygous matings have been probed with MS 5′ ex. This probe detects an ∼13.8-kb fragment from the endogenous 129 mouse allele and an ∼7-kb fragment from the targeted allele. (The middle two lanes are from 129 mice; the flanking lanes are from BS mice. The endogenous BS NcoI fragment is smaller than the 129 mouse fragment, indicating an NcoI restriction fragment length polymorphism between the two strains.) (D) A PCR-based genotyping assay amplifies an ∼350-bp fragment from the endogenous allele and an ∼500-bp fragment from the targeted allele.
Electroporation of the construct into 129SV/Ev mouse embryonic stem (ES) cells and selection for homologous recombinants were performed according to standard procedures. BamHI genomic Southern blots of neomycin-resistant clones were probed with MS168 (see Fig. 1) and with a portion of the NEO gene. The one positive homologous recombinant was used to generate chimeric mice, which were then crossed either with 129SV/Ev129 mice, generating the MS knock out allele on a pure 129SV/Ev129 (129) background, or with mice from the outbred Black Swiss (BS) strain.
Genotyping.
Mice were genotyped by Southern blot (BamHI genomic digests probed with MS168 or NcoI digests probed with MS 5′ ex [see Fig. 1]) or by PCR with a three-primer assay. The forward primer, MSKO 5′ 1 (5′ AAGATGTGGTTGGCTGTTAGTGAC 3′), lies upstream of the NEO insertion and will anneal to both the endogenous and targeted alleles. There are two reverse primers. MSKO 3′ ko 1 (5′ TCCATTGCTCAGCGGTGCTGTC 3′) is specific for the targeted allele and MSKO 3′ wt 1 (5′ CACACTGTTGTTATATGACTCTTGC 3′) is specific for the endogenous allele (Fig. 1). Products from the targeted and endogenous alleles are ∼500 bp and 350 bp, respectively. Reactions were done in 50 μl with 60 ng of MSKO 5′ 1, 10 ng of MSKO 3′ ko 1, and 120 ng of MSKO 3′ wt 1 per reaction: 1 cycle of 7 min at 95°C; 30 cycles of 1 min 15 s, at 64°C, 2 min at 72°C, and 1 min at 95°C; and 1 cycle of 1 min 15 s at 64°C and 10 min at 72°C.
Embryo analyses.
Pregnant females were sacrificed at embryonic day (E) 12.5 to 13.5, and viable embryos were counted. Additional pregnancies were sacrificed at E10.5 or E9.5, reabsorption sites were counted, and viable embryos were harvested for genotyping following successive rounds of washing in fresh 1× phosphate-buffered saline in order to avoid contamination from maternal cells.
Dietary supplementations.
Unless otherwise specified, animals were fed standard laboratory chow (Lab Animal) ad libitum. In initial studies, the diet of BS females used in matings was supplemented beginning at least 1 week before matings were set up and continued throughout gestation or until the females were sacrificed. Folic acid supplementation was achieved by substituting a 1% folic acid (FA)–1% sodium bicarbonate solution for the animals' drinking water. In additional studies to rescue embryos nullizygous for the MS knockout, breeding pairs of heterozygous BS mice were maintained on an AIN-93G (Dyets) diet for 3 weeks prior to pregnancy and throughout gestation. The diet and/or drinking water was supplemented with elevated folate or folinate, lipotropes such as methionine, choline, and betaine, and other products of one-carbon metabolism such as thymidine and purines (Table 1). The AIN-93G diet is the recommended daily allowance (RDA) diet for the rodent and contains ample folate as well as other nutrients to allow optimal growth, including approximately 3.3 g of methionine per kg, 2.5 g of choline per kg, and 2 mg of folate per kg. Supplements were tried singly and in various combinations, including administration of all supplements at once. In some studies, animals were placed on a complete amino-acid-based diet (29).
TABLE 1.
Supplements to the AIN-93G diet
| Diet addition | Amt (g/kg) | Water addition | Amt (g/liter) |
|---|---|---|---|
| Thymidine | 10 or 30 | Folic acid | 0.2 |
| Folinate | 0.1 | Folinate | 0.1 |
| Methionine | 3.0 | Methionine | 3.0 |
| Choline | 3.0 | Choline | 3.0 |
| Hypoxanthine | 50 | ||
| Betaine | 3.0 | ||
| Inositol | 1.0 | ||
| Threonine | 8.0 |
RNA analyses.
Total RNA was isolated from E9.5 embryos and from adult kidneys and livers using RNAzol (Tel-Test, Inc., Friendswood, Tex.). For Northern analysis, 40 μg per sample was loaded on a 1% formaldehyde-agarose gel, electrophoresed, and blotted using standard techniques. MS mRNA was detected using a 520-bp fragment from a mouse MS expressed sequence tag [EST] (AA387056). BHMT mRNA was detected using the entire insert (∼1 kb) of a mouse BHMT EST (AA530640). For reverse transcription-PCR (RT-PCR) analysis, an MS-specific primer (MS R15: 5′ GGCCGAGGGTATTTTTCCCCG 3′) was used for reverse transcription from 5 μg of total kidney or E9.5 embryo RNA. Ten percent of the product was then amplified by PCR, using sense primer MS F12 and antisense primer MS R15 (MS F12: 5′ CCGAGGGATGGAAGCCATTCG 3′).
MS enzyme assay.
MS activity in adult, age-matched mouse +/+ and +/− liver tissue was determined as previously described (3). Assays were performed in triplicate. CBS assays were also performed as a control (27). All samples were coded, and the person performing the assays was blinded to the genotype of the animal being tested.
Hematocrit and plasma amino acid analyses.
Blood was collected from animals via the retro-orbital sinus using heparinized capillary tubes. A portion of the blood sample was used for hematocrit measurement; the remainder was spun for 5 min at 16,000 × g (4°C), and the plasma was drawn off. Plasma amino acid concentrations were determined by ion exchange chromatography followed by detection with ninhydrin on a Beckman model 6300 amino acid analyzer (laboratory of George Thomas, Kennedy Krieger Institute, Johns Hopkins University, Baltimore, Md.). For more sensitive determination of homocysteine levels, total plasma homocysteine was measured by reducing oxidized derivatives in plasma with tri-n-butylphosphine, and the homocysteine (and other free sulfhydryls) was derivatized with 7-fluoro-benzo-2-oxa-1,3-diazole-4-sulfonate. The derivatized samples were separated by reverse-phase high-pressure liquid chromatography and quantitated by fluorescence as previously described (2).
Statistical analyses.
All statistical analyses were performed using GraphPad Prism (GraphPad Software, San Diego, Calif.).
RESULTS
Generation of MS-deficient mice.
An MS knockout construct was designed in which 3.5 exons of the gene were replaced by a PGK promoter/neomycin resistance cassette (see Fig. 1A). In humans, the region corresponding to the deletion encodes amino acids 101 to 254 which, by analogy with the E. coli protein, reside within the region of the protein responsible for homocysteine binding (7). Consequently, if a stable protein were synthesized from the targeted allele, it would be predicted to be nonfunctional. The targeting construct was introduced into mouse 129SV/Ev (129) ES cells. We screened 184 neomycin-resistant colonies by Southern blot analysis and identified one colony (0.54%) that demonstrated the appropriate integration. This colony was used to generate chimeric mice which were crossed with both 129 and BS mice to generate heterozygotes for the knockout allele (six of seven chimeras transmitted the knockout allele). We then crossed heterozygotes: 129-derived mice with 129-derived mice, and BS-derived mice with BS-derived mice. Figure 1B to D depict the genotyping assays utilized. Sex ratios for the offspring of the heterozygous matings were as expected, with approximately equal numbers of males and females. The ratio of homozygous wild-type (WT) offspring to heterozygous offspring to homozygous knockout offspring was approximately 1:2:0, suggesting that homozygosity for the knockout allele results in embryonic lethality (Table 2). An exception to the 1:2:0 ratio is seen in BS females, which have a genotype ratio of approximately 1:1.5:0 (different from BS males [P = 0.019 by the chi-square test]).
TABLE 2.
Genotype distribution of offspring from heterozygous matings
| Mouse strain | Gender | Genotype
|
||
|---|---|---|---|---|
| +/+ | +/− | −/− | ||
| 129 | Male | 36 | 80 | 0 |
| Female | 39 | 85 | 0 | |
| Total | 75 | 165 | 0 | |
| Black Swiss | Male | 119 | 259 | 0 |
| Female | 149 | 227 | 0 | |
| Total | 268 | 486 | 0 | |
In utero development of MS−/− mice.
In order to determine the developmental stage at which the MS−/− mice are lost, we set up heterozygous matings, sacrificed the pregnant females at specific times, and analyzed the embryos. At E12.5 to E13.5, there was a significantly higher reabsorption frequency in pregnancies of heterozygous matings compared to matings in which at least one parent was WT (Table 3), suggesting that the MS −/− embryos die before this time. Consequently, we then harvested embryos from heterozygous matings at E9.5 for genotyping. No MS−/− embryos were found, although roughly a quarter of the embryos were being reabsorbed at this stage (Table 4). We were not able to reliably genotype embryos from earlier stages.
TABLE 3.
E12.5 to E13.5 reabsorptions in various mouse crosses
| Crossa (M × F) | No. of pregnancies | Viable embryos | Reabsorptions | Total implantations | Implantations/ pregnancy | Reabsorptions/ implantation |
|---|---|---|---|---|---|---|
| het × het | 15 | 121 | 32 | 153 | 10.20 | 0.21b |
| wt × het | 15 | 151 | 7 | 158 | 10.53 | 0.04 |
| wt × wt | 15 | 145 | 13 | 158 | 10.53 | 0.08 |
| het × wt | 15 | 150 | 16 | 166 | 11.07 | 0.10 |
| het × hetc | 10 | 77 | 30 | 107 | 10.70 | 0.28d |
M, male; F, female; het, heterozygous; wt, wild type.
Statistically significantly different (wt × het, p < 0.0001; wt × wt, p = 0.002; het × wt, p = 0.0072; Fisher's exact test).
FA-supplemented pregnancies.
Not different from unsupplemented het × het pregnancies (P = 0.1877, Fisher's exact test).
TABLE 4.
Genotype distribution of E9.5 embryos from heterozygous matings
| Mouse strain | Genotype
|
Reabsorptions | ||
|---|---|---|---|---|
| +/+ | +/− | −/− | ||
| 129 | 7 | 16 | 0 | 6 |
| Black Swiss | 10 | 18 | 0 | 16 |
Embryonic expression of MS.
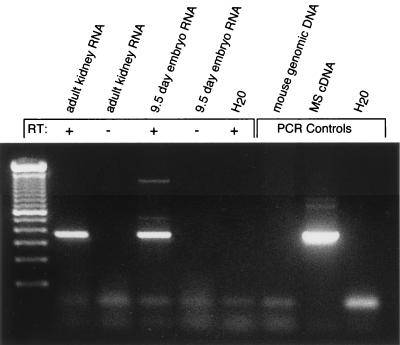
In order to determine how early in development MS is expressed, we performed RT-PCR analysis from whole-embryo RNA. We were able to isolate embryos and detect expression as early as E9.5 (Fig. 2).
FIG. 2.
Embryonic expression of MS. MS antisense oligomer MuMS15 was used to prime reverse transcription from total kidney or E9.5 RNA. This product was amplified using sense primer MuMS12 and antisense primer MuMS15. The three right lanes are controls for the PCR.
Supplementation of pregnancies.
In initial efforts to bring MS−/− mice to term, we supplemented the diet of pregnant females with pharmacologic doses of FA in an attempt to overcome the functional folate deficiency expected to be induced by the “methyl trap.” No MS−/− pups were born to females receiving the FA supplementation (three litters, 18 pups). We then sacrificed FA-supplemented pregnant females from heterozygous matings at E12.5 to determine if the supplementation enabled mouse −/− embryos to progress farther along in development. No reduction in the number of reabsorptions in the supplemented pregnancies was observed at E12.5 (see Table 3).
Animals were then placed on an AIN-93G diet, the RDA diet for the growing rodent. As the folate transporter in some tissues is specific for reduced folates, we supplemented the diet with pharmacological doses of folinate [(6S)-5-formyl-THF] at 50 times the RDA level, but this failed to rescue the nullizygous embryos. We then further supplemented the diets with lipotropes such as methionine, choline, and betaine, because interruption of the MS gene would be expected to decrease methyl group availability. Because excess methionine is toxic, these supplements were added at more modest levels and approximately doubled the intake of these nutrients by the mice. The diet was also supplemented in some cases by the other major products of folate-mediated one-carbon metabolism, purines and thymidine, but none of these supplements, either singly or in combination, rescued the nullizygotes. Finally, inositol and threonine supplements, attempted because unbalanced dietary methionine intake leads to increased threonine and inositol requirements by unknown mechanisms, failed to rescue the knockout mice. In all cases, WT and heterozygote mice were born at the expected frequencies and appeared to be healthy (the ratio of WT/heterozygote/nullizygote was a composite of 42:85:0 for these studies).
Analysis of MS expression in heterozygous knockout mice.
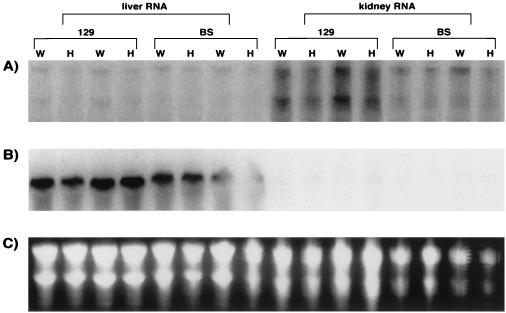
We harvested RNA from the livers and kidneys of MS heterozygous knockout and WT littermates and performed Northern blot analysis. In WT and heterozygous mice, MS mRNA levels were higher in kidney tissue than in liver tissue (Fig. 3A). We observed a modest decrease in MS expression in the heterozygotes compared to WT mice. Expression levels of BHMT, a liver-specific enzyme that converts homocysteine to methionine using betaine as the methyl donor, are similar in MS+/+ and MS+/− mice (Fig. 3B).
FIG. 3.
Northern blot analysis of MS expression in adult liver and kidney. A Northern blot (40 μg of total RNA per lane) was probed with MS cDNA (A) or BHMT cDNA (B). (C) A photo of the ethidium bromide stain of the gel is shown. W, wild type; H, heterozygote.
MS enzyme activity in heterozygous deficient mice.
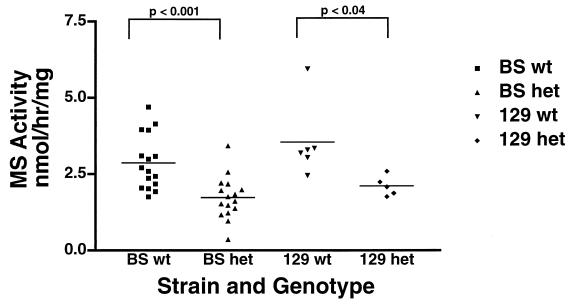
To assess the effect of the gene knockout on enzyme activity, we assayed liver tissue MS in 16 MS+/− and 16 MS+/+ mice (BS). Average MS activity in heterozygous MS knockout mice (1.7 nmol/h/mg) was ∼60% of the average observed in WT animals (2.9 nmol/h/mg; P = 0.0004 by the unpaired two-tailed t test [Fig. 4]). Among these 32 mice, there were seven sib pairs. When data from sib pairs alone are analyzed, the average activity in MS+/− heterozygotes (1.6 nmol/h/mg) was ∼54% of WT (3.0 nmol/h/mg; P = 0.0059 by the two-tailed paired t test). Additionally, we assayed MS activity in liver tissue from five MS+/− and six MS+/+ mice with the 129 background. The average activity in liver tissue from these MS-deficient mice (2.1 nmol/h/mg) was ∼60% of the activity in WT mice (3.5 nmol/h/mg; p = 0.0320 by unpaired t test; Fig. 4). As expected, CBS activity in liver tissue was indistinguishable in the heterozygote versus WT mice (data not shown).
FIG. 4.
Liver MS enzyme activity. MS enzyme activity in adult liver tissue was measured from heterozygous and WT mice. For BS mice, the average activity in heterozygotes was 60% of the average observed in WT animals (1.7 versus 2.9 nmol/h/mg; P = 0.0004, by unpaired two-tailed t test). For cell line 129 mice, the average activity in heterozygotes was 59% of the average observed in WT animals (2.1 versus 3.5 nmol/h/mg; P = 0.0320 by the unpaired, two-tailed t test).
Hematocrits.
Because megaloblastic anemia is a manifestation of MS deficiency in humans, we measured the hematocrits of WT and heterozygous MS-deficient mice. The hematocrit is a measurement of red blood cell concentration in the blood and thus can be used to detect anemia. There was no difference between the hematocrits of BS MS+/+ (average, 49.6%; n = 20) and BS MS+/− mice (average, 50.3%; n = 20). Similarly, we observed no difference between the hematocrits of strain 129 MS+/+ (average 47.2%; n = 8) and strain 129 MS+/− mice (average, 49.2%; n = 7).
Plasma amino acid profiles.
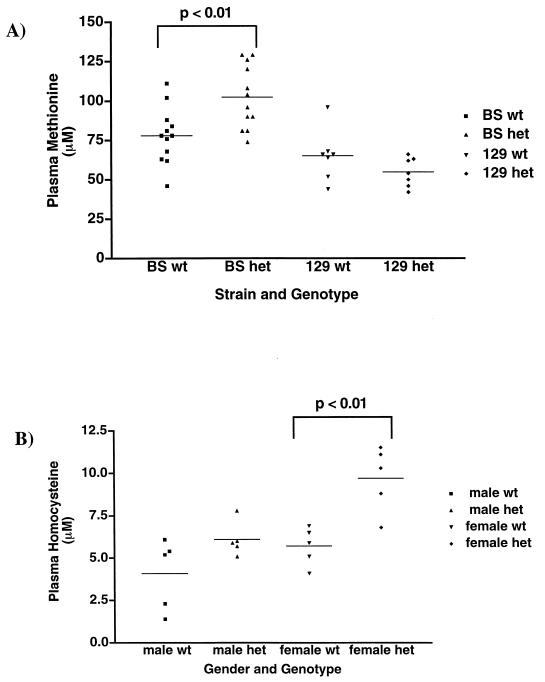
Human cblG patients with very low MS activity have hyperhomocysteinemia and hypomethioninemia. We measured plasma amino acid concentrations of 12 BS-derived mice and 7 of the 129-derived MS+/+ and MS+/− male mice. No differences were observed between 129-derived MS+/+ and MS+/− mice for any of the 31 amino acids measured. However, a difference in methionine levels was observed when the BS +/+ and +/− mice were compared (Fig. 5A). MS-deficient mice had an elevated plasma methionine (102 μM) compared to WT mice (78 μM; P = 0.0046 by the two-tailed unpaired t test). Six littermate pairs were included in this set. Analysis of those six pairs alone also revealed a statistically significant difference between the average plasma methionine concentration in heterozygous (105.8 μM) and WT mice (87.3 μM; P = 0.04 by the two-tailed paired t test).
FIG. 5.
Plasma methionine and homocysteine levels in MS-deficient mice. (A) Plasma methionine levels were measured in WT and heterozygous male mice from each genetic background. No difference was observed in average plasma methionine on the 129 mouse background. The average plasma methionine of heterozygotes on the BS mouse background was elevated compared to the WT average (102 μM versus 73 μM; P = 0.0046 by unpaired, two-tailed t test). (B) Plasma homocysteine was measured in males and females on the BS background. The average plasma homocysteine was not statistically significantly different between heterozygous and WT BS males (6.1 versus 4.1 μM; P = 0.0874 by unpaired, two-tailed t test). The heterozygous BS females have elevated plasma homocysteine levels (average 9.7 versus 5.7 μM; P = 0.0039 by unpaired, two-tailed t test).
The initial plasma amino acid analysis was capable of detecting only gross alterations in homocysteine concentrations. Since the differences in homocysteine concentrations that are associated with an increased risk for cardiovascular disease and NTDs are only mild to moderate elevations, we performed a more sensitive assay for homocysteine concentration (see Materials and Methods). Plasma homocysteine levels were measured in five BS animals of each genotype and gender that had been maintained on an amino-acid-based diet equivalent to the RDA for the mouse (Fig. 5B). The average plasma homocysteine in heterozygous females (9.7 μM) was elevated compared to that of WT females (5.7 μM; P = 0.0039 by the two-tailed unpaired t test). There was also a slight elevation of the-average plasma homocysteine in heterozygous males compared to that of WT males, although the difference did not reach statistical significance (P = 0.0874 by the two-tailed unpaired t test).
DISCUSSION
We have utilized homologous recombination to create an MS-deficient mouse. The approximately 50 to 60% decrease in enzyme activity in heterozygous knockout mice compared to WT littermates provides evidence of a successful knockout of the MS gene. Southern blot analyses with 5′ and 3′ external probes, as well as a neomycin probe, reveal restriction fragments of the expected sizes, indicating no additional rearrangements have occurred. However, we cannot rule out the possibility that our knockout disrupts the expression of a neighboring gene.
Complete MS deficiency results in embryonic lethality in both 129 and BS mice, assuming neighboring genes have been unaffected. This is in contrast to what has been reported in humans. Three cblG patients have been found to have virtually undetectable fibroblast MS RNA (30) although two of the three have been reported to have measurable but low levels of MS activity in fibroblasts (8). The mutations identified in the MS structural genes of these patients result in the production of aberrant transcripts with premature stop codons (30). It is possible that a small amount of properly processed MS mRNA is produced in these patients, which results in sufficient normal MS protein being made to surpass some critical threshold of enzyme activity required for viability. From our results and those previously published, we conclude that these patients probably do have some small amount of residual MS activity that is sufficient for embryonic and fetal development, although we cannot rule out the possibility that humans have a mechanism to compensate for absolute MS deficiency that is lacking in mice.
The difference between reabsorption rates in females from heterozygous matings and those in females from matings in which at least one parent is WT indicates that the nullizygous embryos survive at least until implantation, which occurs around E4.5, although our genotyping data suggest that the embryos die sometime before E9.5. Examination of serial sections through uteri from heterozygous matings at E8.5 and E7.5 suggests that the −/− embryos are undergoing reabsorption by E8.5 and mostly likely are undergoing reabsorption by E7.5 (data not shown). We were able to demonstrate that MS is expressed at least as early as E9.5 and the mouse EST database (http://www.ncbi.nlm.nih.gov/dbEST/) contains an MS EST (AA415486) isolated from two-cell embryos. Together, these data indicate that MS has a crucial role in early embryonic mouse development.
There are several possible mechanisms by which MS deficiency in mice results in embryonic lethality. These include precursor toxicity (homocysteine buildup), product deficit (methionine shortage), and folate trapping (i.e., folate becoming trapped in the 5-Me-THF form) with a commensurate decrease in all folate-dependent one-carbon metabolism including DNA precursor synthesis. Folate is normally used catalytically, and active coenzyme forms are regenerated for use in the various cycles of one-carbon metabolism; a complete lack of MS would block this regeneration. We attempted to circumvent these possible problems by supplementing the diet of pregnant females with various nutrients.
Lipotropes such as choline and methionine can spare the requirement for each other, and methionine at elevated levels could slow down the trapping of folate as 5-Me-THF in liver tissue. Additionally, choline, an immediate precursor of betaine, can potentially shunt homocysteine into methionine via the action of BHMT, which would simultaneously lower homocysteine and raise methionine levels. However, in rats, BHMT is not expressed until the liver is formed, and the MS knockout embryos die prior to this point. The diet of pregnant mothers was also supplemented with methionine to alleviate a potential methionine deficit in the embryos. However, since high doses of methionine are toxic to the mothers, it is possible that the administered dose was insufficient to support the developing embryos.
MS is the only enzyme known to convert 5-Me-THF into another form of folate. Supplementations with FA or folinic acid were aimed at circumventing the 5-Me-THF trap by providing additional folate. In pigs, FA fed to dams crosses from the maternal circulation to that of their embryos (16), and the work of Piedrahita and coworkers (19) suggests that the same is true in mice as well. Piedrahita et al. rescued the embryonic lethal phenotype of folate-binding protein 1 knockout embryos by supplementing the diet of pregnant dams with folinic acid. Supplementations with hypoxanthine and thymidine were included to avert a potential shortage of precursors for DNA synthesis that could result from folate trapping. There are a number of possible reasons why the supplementation failed. The first and most likely is that inadequate amounts of the supplemented compounds were reaching the −/− embryos. All supplemented compounds should be able to cross the placenta (9, 16, 19). However, placentation does not begin in mice until E8.5 (21), a time at which the homozygous knockout embryos already appeared to be dead. Nutrients from the mother can reach the embryos prior to placentation, but the levels of supplements that the embryos received may have been insufficient. Second, the combined supplements may not have completely addressed the need for products of one-carbon metabolism in the developing mouse. Although the supplements used are sufficient to allow normal growth of cultured cells under folate-free conditions, it is possible that folate may be required for a development-specific reaction or process. Third, even if pharmacological doses of folate did reach the developing embryos, the methyl trap would result in the accumulation of very high levels of 5-Me-THF, and it is possible that this might inhibit some essential function.
An MTHFR knockout mouse has recently been developed. MTHFR immediately precedes MS in the folate pathway and produces 5-Me-THF from 5,10-methylene tetrahydrofolate. MTHFR null mutants suffer from various developmental defects, but many do survive gestation. Increased folate intake ameliorates some of the abnormal pathologies in these animals and increases survival (R. Rozen, personal communication). Both the MTHFR and MS knockouts would be impaired similarly in their abilities to remethylate homocysteine because both defects would eliminate the folate-dependent remethylation pathway. They differ in that the MS knockout would trap folate as 5-Me-THF and would lead to a functional and actual folate deficiency, while the MTHFR animal would not be impaired in folate accumulation or in other folate-dependent cycles of one-carbon metabolism. This strongly suggests that the embryonic lethality of the MS knockout is due to the methyl trap rather than an impairment in methyl group status.
The MS heterozygous deficient mice are virtually indistinguishable from WT mice despite a 40 to 50% decrease in MS activity. Plasma homocysteine was modestly increased in BS heterozygotes on a complete diet, and this elevation in the heterozygote has been observed under a number of dietary conditions that modulate homocysteine levels, such as changes in folate, B12, and/or methionine status (Liu and Shane, unpublished data). In this regard, the MS heterozygote is similar to the CBS knockout, which produces greatly elevated circulating homocysteine levels in the null animal but only slightly elevated homocysteine in the heterozygote animal. Other differences we have observed occur only on the BS background. It is unclear whether the decrease in the heterozygote/WT female offspring ratio from heterozygous matings is directly related to MS deficiency. There is at least one other instance of this phenomenon seen with a pGK-neo knockout construct on a BS background but not a 129 background (L. Bei, personal communication). The elevation in plasma methionine concentration in the BS MS heterozygotes may be physiologically relevant. While most patients with folate or vitamin B12 deficiency have low or low-normal plasma methionine, there is a subset of patients with elevated plasma methionine that is similar to what we see in the BS MS heterozygous mice (1). The fact that we see this only on the mixed genetic background and not on the inbred 129 background suggests that genetic modifiers other than MS are involved.
This study has demonstrated that MS activity is essential for early embryonic development in mice. Although the MS knockout mouse has not provided an immediately obvious animal model of human disease, heterozygotes with ∼50% MS activity may prove to be useful. It is likely that MS heterozygote knockouts are more susceptible to dietary deficiencies than WT mice and thus would have merit as a model in which interactions between genetic status and nutritional status can be studied. For example, homocysteine levels are more responsive to inadequate folate intake in the heterozygotes than in WT mice (Liu and Shane, unpublished data). Additionally, in ongoing experiments, other enzymes in the homocysteine-methionine pathway as well as enzymes involved in folate metabolism are being knocked out. Crosses between the MS heterozygote mice and mice deficient in other pathway components may uncover a threshold of MS activity that is important but not otherwise recognizable for conditions such as NTD development or cancer risk.
ACKNOWLEDGMENTS
We thank Celina Chang (University of California—Berkeley) for her assistance in some of the studies and Amy Chen, Theresa Hernandez, and Anthony Wynshaw-Boris for advice and technical assistance (National Human Genome Research Institute). We are indebted to T. Swanson-Linville for his patience.
This work was supported by NIH grant HL58984 to R.B. and DK42033 and HL58991 to B.S. R.B. is an Established Investigator of the American Heart Association.
REFERENCES
- 1.Allen R H, Stabler S P, Savage D G, Lindenbaum J. Metabolic abnormalities in cobalamin (vitamin B12) and folate deficiency. FASEB J. 1993;7:1344–1353. doi: 10.1096/fasebj.7.14.7901104. [DOI] [PubMed] [Google Scholar]
- 2.Araki A, Sako Y. Determination of free and total homocysteine in human plasma by high-performance liquid chromatography with fluorescence detection. J Chromatogr. 1987;422:43–52. doi: 10.1016/0378-4347(87)80438-3. [DOI] [PubMed] [Google Scholar]
- 3.Banerjee R, Chen Z, Gulati S. Methionine synthase from pig liver. Methods Enzymol. 1997;281:189–196. doi: 10.1016/s0076-6879(97)81025-7. [DOI] [PubMed] [Google Scholar]
- 4.Chen L H, Liu M L, Hwang H Y, Chen L S, Korenberg J, Shane B. Human methionine synthase: cDNA cloning, gene localization, and expression. J Biol Chem. 1997;272:3628–3634. [PubMed] [Google Scholar]
- 5.Chen Z, Banerjee R. Purification of soluble cytochrome b5 as a component of the reductive activation of porcine methionine synthase. J Biol Chem. 1998;273:26248–26255. doi: 10.1074/jbc.273.40.26248. [DOI] [PubMed] [Google Scholar]
- 6.Czeizel A E, Dudas I. Prevention of the first occurrence of neural-tube defects by periconceptional vitamin supplementation. N Engl J Med. 1992;327:1832–1835. doi: 10.1056/NEJM199212243272602. [DOI] [PubMed] [Google Scholar]
- 7.Goulding C W, Postigo D, Matthews R G. Cobalamin-dependent methionine synthase is a modular protein with distinct regions for binding homocysteine, methyltetrahydrofolate, cobalamin, and adenosylmethionine. Biochemistry. 1997;36:8082–8091. doi: 10.1021/bi9705164. [DOI] [PubMed] [Google Scholar]
- 8.Gulati S, Baker P, Li Y N, Fowler B, Kruger W, Brody L C, Banerjee R. Defects in human methionine synthase in cblG patients. Hum Mol Genet. 1996;5:1859–1865. doi: 10.1093/hmg/5.12.1859. [DOI] [PubMed] [Google Scholar]
- 9.Hayashi T T, Garvey B I. Transplacental passage of nucleotides, nucleotides, and bases. Am J Obstet Gynecol. 1968;102:1154–1161. doi: 10.1016/0002-9378(68)90406-7. [DOI] [PubMed] [Google Scholar]
- 10.Kirke P N, Daly L E, Elwood J H. A randomised trial of low dose folic acid to prevent neural tube defects. The Irish Vitamin Study Group. Arch Dis Child. 1992;67:1442–1446. doi: 10.1136/adc.67.12.1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kirke P N, Molloy A M, Daly L E, Burke H, Weir D G, Scott J M. Maternal plasma folate and vitamin B12 are independent risk factors for neural tube defects. Q J Med. 1993;86:703–708. [PubMed] [Google Scholar]
- 12.Laurence K M, James N, Miller M H, Tennant G B, Campbell H. Double-blind randomised controlled trial of folate treatment before conception to prevent recurrence of neural-tube defects. Br Med J. 1981;282:1509–1511. doi: 10.1136/bmj.282.6275.1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leclerc D, Campeau E, Goyette P, Adjalla C E, Christensen B, Ross M, Eydoux P, Rosenblatt D S, Rozen R, Gravel R A. Human methionine synthase: cDNA cloning and identification of mutations in patients of the cblG complementation group of folate/cobalamin disorders. Hum Mol Genet. 1996;5:1867–1874. doi: 10.1093/hmg/5.12.1867. [DOI] [PubMed] [Google Scholar]
- 14.Leclerc D, Wilson A, Dumas R, Gafuik C, Song D, Watkins D, Heng H H, Rommens J M, Scherer S W, Rosenblatt D S, Gravel R A. Cloning and mapping of a cDNA for methionine synthase reductase, a flavoprotein defective in patients with homocystinuria. Proc Natl Acad Sci USA. 1998;95:3059–3064. doi: 10.1073/pnas.95.6.3059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li Y N, Gulati S, Baker P J, Brody L C, Banerjee R, Kruger W D. Cloning, mapping and RNA analysis of the human methionine synthase gene. Hum Mol Genet. 1996;5:1851–1858. doi: 10.1093/hmg/5.12.1851. [DOI] [PubMed] [Google Scholar]
- 16.Matte J J, Girard C L, Tremblay G F. Effect of long-term addition of folic acid on folate status, growth performance, puberty attainment, and reproductive capacity of gilts. J Anim Sci. 1993;71:151–157. doi: 10.2527/1993.711151x. [DOI] [PubMed] [Google Scholar]
- 17.Mills J L, McPartlin J M, Kirke P N, Lee Y J, Conley M R, Weir D G, Scott J M. Homocysteine metabolism in pregnancies complicated by neural-tube defects. Lancet. 1995;345:149–151. doi: 10.1016/s0140-6736(95)90165-5. [DOI] [PubMed] [Google Scholar]
- 18.MRC Vitamin Study Research Group. Prevention of neural tube defects: results of the Medical Research Council Vitamin Study. Lancet. 1991;338:131–137. [PubMed] [Google Scholar]
- 19.Piedrahita J A, Oetama B, Bennett G D, van Waes J, Kamen B A, Richardson J, Lacey S W, Anderson R G, Finnell R H. Mice lacking the folic acid-binding protein Folbp1 are defective in early embryonic development. Nat Genet. 1999;23:228–232. doi: 10.1038/13861. [DOI] [PubMed] [Google Scholar]
- 20.Refsum H, Ueland P M, Nygard O, Vollset S E. Homocysteine and cardiovascular disease. Annu Rev Med. 1998;49:31–62. doi: 10.1146/annurev.med.49.1.31. [DOI] [PubMed] [Google Scholar]
- 21.Rugh R. The mouse: its reproduction and development. New York, N.Y: Oxford University Press; 1990. [Google Scholar]
- 22.Shane B, Stokstad E L. Vitamin B12-folate interrelationships. Annu Rev Nutr. 1985;5:115–141. doi: 10.1146/annurev.nu.05.070185.000555. [DOI] [PubMed] [Google Scholar]
- 23.Smithells R. Vitamin deficiencies and neural tube defects. Arch Dis Child. 1976;51:944–950. doi: 10.1136/adc.51.12.944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Smithells R W, Nevin N C, Seller M J, Sheppard S, Harris R, Read A P, Fielding D W, Walker S, Schorah C J, Wild J. Further experience of vitamin supplementation for prevention of neural tube defect recurrences. Lancet. 1983;i:1027–1031. doi: 10.1016/s0140-6736(83)92654-5. [DOI] [PubMed] [Google Scholar]
- 25.Steegers-Theunissen R P, Boers G H, Blom H J, Nijhuis J G, Thomas C M, Borm G F, Eskes T K. Neural tube defects and elevated homocysteine levels in amniotic fluid. Am J Obstet Gynecol. 1995;172:1436–1441. doi: 10.1016/0002-9378(95)90474-3. [DOI] [PubMed] [Google Scholar]
- 26.Steegers-Theunissen R P, Boers G H, Trijbels F J, Finkelstein J D, Blom H J, Thomas C M, Borm G F, Wouters M G, Eskes T K. Maternal hyperhomocysteinemia: a risk factor for neural-tube defects? Metabolism. 1994;43:1475–1480. doi: 10.1016/0026-0495(94)90004-3. [DOI] [PubMed] [Google Scholar]
- 27.Taoka S, Ohja S, Shan X, Kruger W D, Banerjee R. Evidence for heme-mediated redox regulation of human cystathionine beta-synthase activity. J Biol Chem. 1998;273:25179–25184. doi: 10.1074/jbc.273.39.25179. [DOI] [PubMed] [Google Scholar]
- 28.Tybulewicz V L, Crawford C E, Jackson P K, Bronson R T, Mulligan R C. Neonatal lethality and lymphopenia in mice with a homozygous disruption of the c-abl proto-oncogene. Cell. 1991;28:1153–1163. doi: 10.1016/0092-8674(91)90011-m. [DOI] [PubMed] [Google Scholar]
- 29.Walzem R L, Clifford A J. Folate deficiency in rats fed diets containing free amino acids or intact proteins. J Nutr. 1988;118:1089–1096. doi: 10.1093/jn/118.9.1089. [DOI] [PubMed] [Google Scholar]
- 30.Wilson A, Leclerc D, Saberi F, Campeau E, Hwang H Y, Shane B, Phillips III J A, Rosenblatt D S, Gravel R A. Functionally null mutations in patients with the cblG-variant form of methionine synthase deficiency. Am J Hum Genet. 1998;63:409–414. doi: 10.1086/301976. [DOI] [PMC free article] [PubMed] [Google Scholar]