Abstract
Both fission yeast and mammalian cells require the function of the checkpoint kinase CHK1 for G2 arrest after DNA damage. The tumor suppressor p53, a well-studied stress response factor, has also been shown to play a role in DNA damage G2 arrest, although in a manner that is probably independent of CHK1. p53, however, can be phosphorylated and regulated by both CHK1 as well as another checkpoint kinase, hCds1 (also called CHK2). It was therefore of interest to determine whether reciprocally, p53 affects either CHK1 or CHK2. We found that induction of p53 either by diverse stress signals or ectopically using a tetracycline-regulated promoter causes a marked reduction in CHK1 protein levels. CHK1 downregulation by p53 occurs as a result of reduced CHK1 RNA accumulation, indicating that repression occurs at the level of transcription. Repression of CHK1 by p53 requires p21, since p21 alone is sufficient for this to occur and cells lacking p21 cannot downregulate CHK1. Interestingly, pRB is also required for CHK1 downregulation, suggesting the possible involvement of E2F-dependent transcription in the regulation of CHK1. Our results identify a new repression target of p53 and suggest that p53 and CHK1 play interdependent and complementary roles in regulating both the arrest and resumption of G2 after DNA damage.
Detection of DNA damage results in the activation of signaling pathways that trigger cell cycle checkpoints (15, 25, 31, 52). Cell cycle arrest prevents DNA replication and mitosis in the presence of unrepaired chromosomal alterations. The p53 tumor suppressor protein and its transcriptional targets play an important role in both G1 and G2 checkpoints in mammalian cells (1, 13, 41). While G1 arrest relies extensively on the synthesis of p21 which is induced by p53 (11, 12, 24, 69, 70), it has been postulated that p53-mediated G2 arrest involves not only induction of p21 but other p53 targets such as 14-3-3-ς (17, 32) and GADD45 (38). Arrest in the G2 phase of the cell cycle in p53−/− cells is the consequence of the activation of the CHK-Cdc25-Cdc2 pathway (51, 54, 55), although it has been demonstrated that a prolonged G2-M arrest requires p53 as well (13).
Mitotic cell cycle checkpoints are conserved between yeasts and mammals. After genotoxic stress such as DNA damage or stalled DNA replication, fission yeast CHK1 and Cds1 effector kinases phosphorylate Cdc25 (25, 71, 74, 78), leading to its inactivation as a result of its association with 14-3-3 proteins (54, 78). The activity of yeast CHK1 is controlled by the upstream kinase Rad3, a phosphatidylinositol 3-kinase family member that has homology with mammalian ATM and ATR genes (42, 46, 72). The mammalian homologues of CHK1 (26, 57) and hCds1 (also called CHK2) (8, 10, 47) have been identified, and their activation has been shown to require ATM and ATR as well. Arrest in the G2 phase of the cell cycle in mammals has been shown to require CHK proteins (33, 45, 64). However, despite the existence of a conserved CHK-Cdc25-Cdc2 pathway, there is increasing evidence that there are differences in the CHK1 and CHK2 activating pathways in mammals. It has been demonstrated that CHK2 requires ATM for its activation in response to γ irradiation (10, 47). On the other hand, CHK1 is active in ATM-deficient cells (40), and ATR is likely to be upstream of CHK1 (45). It is not yet clear if these two pathways (ATM-CHK2 and ATR-CHK1) cross-regulate each other, although coregulation between these two kinases has been reported in Schizosaccharomyces pombe (9). Although both CHK1 and CHK2 can regulate Cdc25 activity, they may have evolved to perform other nonoverlapping roles in mammals. In line with this, human cancers with mutations in each of these kinases have been reported (6, 7). Another line of evidence that supports the specialization of these kinases in mammals is the different phenotypes observed in mouse cells deficient for CHK1 and CHK2. While CHK2 is not required at least for T-cell development, CHK1 deficiency results in early embryonic lethality, suggesting different activities of these kinases during development (33, 45, 64).
Cdc25 is not the only substrate of CHK kinases in mammals. Both kinases have been shown to phosphorylate p53 at multiple sites in vitro, including at S20, phosphorylation of which is correlated with disruption of the p53–Hdm-2 interaction (19), and stabilization of p53 after stress signals such as γ irradiation (18, 58, 60, 68). There is mounting evidence that both CHK1 and CHK2 can regulate the stability of p53 after DNA damage: CHK2 activity is required for p53 activation in vivo, since γ irradiation of CHK2−/− cells results in defective p53 stabilization and p53-dependent transcription (33). Additionally, when CHK2 activity is compromised in transfected cells, p53 cannot be stabilized efficiently after DNA damage and fails to become phosphorylated at S20 (18). Whether CHK1 is required for phosphorylation of p53 in vivo is not yet established, at least partly because CHK1 deficiency causes early embryonic lethality. Nevertheless, modulation of CHK1 in transfected cells by either antisense or overexpression has a proportional impact on p53 protein levels (58).
While CHK1 can activate a p53-independent checkpoint through its negative regulation of Cdc25, and thus the G2 cyclin-dependent kinase complex (Cdc2-cyclin B), it is interesting that the role of p53 in the G2 checkpoint also involves multiple modes by which this complex is downregulated. Targets of p53 transactivation including p21, GADD45, and 14-3-3ς, are each involved in the inactivation of Cdc2-cyclin B (11, 16, 27, 75, 79). Moreover, p53 is able to transcriptionaly repress Cdc2 and cyclin B mRNA levels (27, 36, 65). Yet another means by which p53 negatively affects the Cdc2-cyclin B complex is by interfering with its nuclear localization (65). Although p53 can repress the Cdc2-cyclin B complex at many levels, it cannot inhibit modifications on Cdc2 required for its activation (65, 75), a pathway that is regulated by the CHK kinases (8, 10, 47, 57).
To gain further insight into the relationship between p53 and CHK kinases, we have examined the relative levels of CHK1 and CHK2 protein as a function of endogenously and ectopically expressed p53. We have discovered that CHK1 expression is negatively affected by p53 and have investigated the mechanism by which this downregulation occurs.
MATERIALS AND METHODS
Cell cultures and regulation of p53 and p21 expression.
HCT116 cells (human colorectal cancer) containing (+/+; clone 40.16) or lacking (−/−; clone 379.2) wild-type p53, as well as HCT116 parental cells (p21+/+) and its derivative lacking p21 (p21−/−) (13) were generously provided by B. Vogelstein and maintained in McCoy's medium supplemented with 10% fetal calf serum (FCS). H1299 cells expressing tetracycline-regulated wild-type p53 or the transcriptionally inactive mutant p53 L22Q/W23S (p53[22–23]) (43, 44) or p21 were generated using the two-step tetracycline-regulated system and were previously described (50). These cells were grown and maintained in RPMI medium supplemented with 10% fetal bovine serum, puromycin (2 μg/ml; Sigma), G418 (300 μg/ml; Gibco), and tetracycline (4.5 μg/ml). To induce p53 or p21, cells were plated and then maintained in the above medium lacking tetracycline. RKO cell lines stably expressing human papillomavirus (HPV) E6; RKO-E6, clones 10.1 and 10.2), E7 (RKO-E7; clones 7.6 and 7.14), or an empty vector (RKO-NEO), generously provided by K. Cho (University of Michigan, Ann Arbor, Mich.), were cultured in McCoy's medium supplemented with G418 (500 μg/ml). RB+/+ and RB−/− mouse embryonic fibroblasts were generously provided by L. Yamasaki (Columbia University) and grown in Dulbecco's modified Eagle's medium containing 10% FCS.
For p53 induction in HCT116 cells, exponentially growing cells were treated either with daunorubicin (0.22 μM; Oncogene Research Products), actinomycin D (5 nM; Calbiochem). γ irradiation (10 Gy), N-phosphoracetyl-l-aspartate (PALA; 500 μM; kindly provided by G. Stark), hydroxyurea (1.5 mM; Sigma), aphidicolin (5 μg/ml; Calbiochem), (camptothecin (CPT; 3 μM; Sigma), and deferoxamine mesylate (DFX; 250 μM; Sigma). For proteosome inhibition, the following compounds were added before lysis: LLnL (50 μM) and MG132 (30 μM) (both from Calbiochem).
Protein analysis.
Cell extracts were prepared by incubating phosphate-buffered saline (PBS)-washed cell cultures in buffer containing 10 mM Tris (pH 7.5), 1 mM EDTA, 400 mM NaCl, 10% glycerol, 0.5% NP-40, 5 mM NaF, 0.5 mM sodium orthovanadate, 1 mM dithiothreitol, 0.1 mM phenylmethylsulfonyl fluoride, and protease inhibitors for 20 min at 4°C. Proteins were then separated by sodium dodecyl sulfate (SDS)–10% polyacrylamide gel electrophoresis (PAGE) and transferred to nitrocellulose. Monoclonal antibodies used for immunoblotting were PAb 1801 and PAb DO-1 for human p53; PAb 240 for mouse p53; WAF-1 for human p21 (Calbiochem); anti-p21 (Pharmingen) for mouse p21; anti-CHK1 (G-4) for CHK1 (Santa Cruz); and anti-CHK2 (H-300; Santa Cruz) and an affinity-purified anti-CHK2 polyclonal antibody produced in this laboratory for CHK2. For detection of p53 phosphorylation at S15, the phospho-p53 (S15) antibody from New England Biolabs was used according to the manufacturer's instructions or generously provided by Y. Taya.
Northern blotting.
HCT116 and inducible H1299 cell lines were treated as described in the Results section and then washed three times with PBS. RNA was isolated using Trizol reagent (Gibco), resolved on agarose gels, transferred to nylon, and hybridized with [32P]dCTP-labeled CHK1 and p21 plasmid probes. To assess RNA loading, blots were stripped and then rehybrydized with a [32P]dCTP-labeled glyceraldehyde-3-phosphate dehydrogenase (GAPDH) probe.
Cell cycle analysis.
Cells were trypsinized and centrifuged at 1,500 rpm, and the pellets were suspended in 300 μl of PBS and fixed with 5 ml of ice-cold methanol for at least 1 h. For fluorescence-activated cell sorting (FACS) analysis, fixed cells were centrifuged and suspended in 0.9 ml of PBS containing RNase I (50 μg/ml) and propidium iodide (25 μg/ml; Sigma). The stained cells were analyzed in a fluorescence-activated cell sorter (FACSCalibur; Becton Dickinson), and their cell cycle stage was analyzed using the ModFit LT program.
RESULTS
CHK1 protein levels are downregulated as a consequence of p53 stabilization by stress signals.
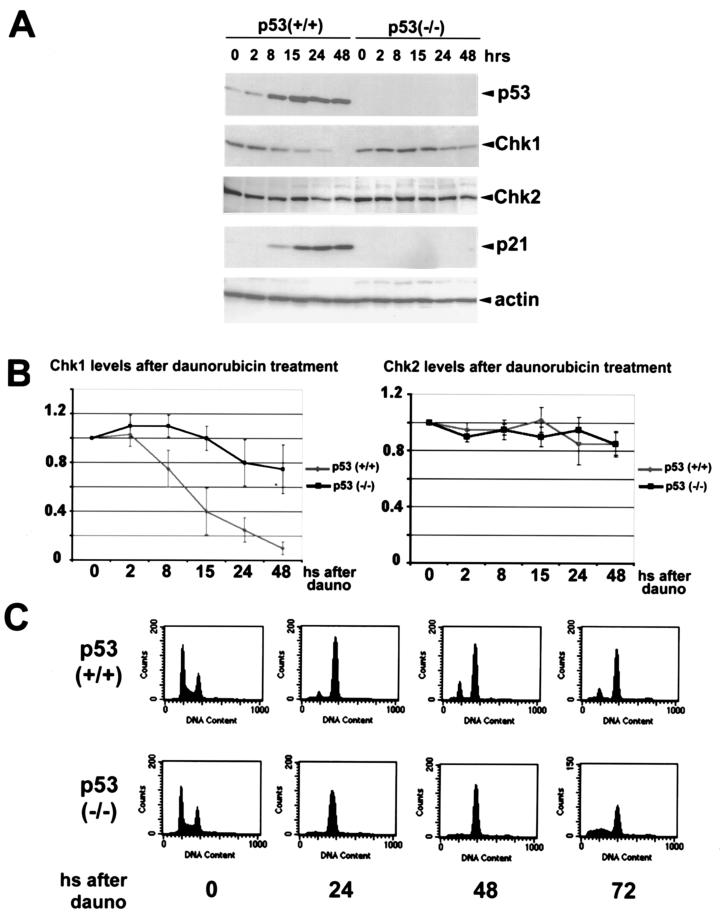
Since CHK1 and CHK2 kinases have been shown to regulate the stability of p53, it was of interest to examine whether a reciprocal relationship exists such that p53 might affect the levels of CHK1 and CHK2. HCT116 (p53+/+) and its derivative HC116 (p53−/−) cells (13) were treated with daunorubicin, a topoisomerase II inhibitor and an intercalating agent (29, 49), and cells were extracted at various time points thereafter. Levels of p53, CHK1, CHK2, and p21 were examined by direct Western blotting of cell extracts using antibodies directed against these proteins (Fig. 1). As expected, p53 and p21 protein levels were increased only in the p53+/+ cells. In the p53+/+ cells, CHK1 protein levels were markedly reduced in a time-dependent fashion. The reduction of CHK1 was first detected at the 8-h time point in the p53+/+ cells, and less than 10% of the CHK1 protein was detectable by 48 h. These experiments were performed with an anti-CHK1 mouse monoclonal antibody, and similar results were obtained with an anti-CHK1 rabbit polyclonal antiserum (not shown). Under these conditions, there was only a slight decrease in the levels of CHK2. Fig. 1B shows a quantitative analysis of the levels of these two kinases as an average of three different experiments. CHK1 levels were slightly reduced in p53−/− cells, although by 48 h, p53−/− cells contained approximately eight times more CHK1 protein than the corresponding p53+/+ cells at the same time point. CHK1 levels were markedly decreased in RKO colorectal cancer cells and WI38 normal human fibroblasts, both of which contain wild-type p53, after treatment with daunorubicin and other agents as described below (data not shown). Based on the fact that CHK1 levels were decreased significantly only in the p53+/+ HCT116 cells, it is likely that in these other cell lines the repression of CHK1 is also p53 dependent.
FIG. 1.
DNA damage-induced p53 downregulates CHK1 protein levels. (A) HCT116 colorectal cancer cells (p53+/+) and its derivative p53−/− cells were treated with daunorubicin (0.22 μM) and harvested at the indicated times after treatment. Cell lysates were then subjected to Western blot analysis with antibodies specific for p53, CHK1, CHK2, and p21 as described in the text. Actin levels were probed as a loading control. (B) Densitrometric analysis of CHK1 and CHK2 protein levels after treatment of HCT116 (p53+/+) and its derivative (p53−/−) with daunorubicin. The data represent the reduction with respect to the value obtained in three independent experiments for the untreated controls (taken as 1). All samples were normalized to their respective actin levels. (C) Cells were treated as described for panel A, and at the indicated times (hours [hs]) were subjected to cell cycle analysis as described in the text.
It was reported that CHK1 levels are regulated in a cell cycle-dependent manner, peaking in the S to G2 phases of the cell cycle and decreasing in G1 phase (40). To assess the cell cycle stage of daunorubicin-treated cells, HCT116 cells were fixed, stained with propidium iodide, and analyzed by FACS (Fig. 1C). Daunorubicin treatment caused cells to accumulate in the G2 phase of the cell cycle of both p53+/+ and p53−/− HCT116 lines (Fig. 1C). The p53−/− cells, however, were not capable of sustaining the arrest in G2, as is the case after other treatments, such as γ irradiation (13), while the p53+/+ cells were irreversibly arrested mainly in G2. Since CHK1 levels are maximal in G2, the fact that daunorubicin treatment causes both CHK1 downregulation and G2 arrest indicates that the reduced CHK1 is not an indirect consequence of cell cycle regulation by p53.
Numerous forms of stress lead to induction of p53, which can occur as a result of distinct signaling pathways. To determine if CHK1 downregulation occurs after different stress signals, we treated HCT116 p53+/+ and p53−/− cells with different agents known to induce accumulation of p53. The results are summarized in Table 1. Not only daunorubicin but also actinomycin D an inhibitor of RNA polymerase II (61), γ irradiation, and PALA, an inhibitor of carbamyl-P-synthetase–aspartate transcarbamylase–dihydroprotase that disrupts CTP and UTP nucleotide pools (21), were able to induce a p53-dependent downregulation of CHK1. With each of these treatments, we observed a much less dramatic reduction of CHK1 in the p53−/− cells. Other agents such as hydroxyurea, a ribonucleotide reductase inhibitor (66), and aphidicolin, an inhibitor of DNA polymerase α (35), were incapable of significantly reducing CHK1 levels more than the reduction observed in p53−/− cells. CPT, an inhibitor of topoisomerase I (34), strongly downregulated CHK1 but did so even in the absence of p53. Finally, DFX, an activator of the hypoxia-inducible factor 1α (3), caused CHK1 downregulation independently of p53 status. In agreement with recent reports, we observed detectable gel mobility shifts of CHK1 after treatment with hydroxyurea (45); additionally, aphidicolin and DFX treatment also resulted in reduced gel mobility of CHK1 (unpublished results).
TABLE 1.
CHK1 downregulation correlates with p53 transcriptional activity and not with its N-terminal phosphorylationa
| Inducer | Phosphorylation at S15 | Phosphorylation at S20 | CHK1 downregulation | p53 dependence | p21 induction |
|---|---|---|---|---|---|
| Daunorubicin | + | ND | + | + | + |
| Actinomycin D | − | −a | + | + | + |
| γ-Irradiation | + | +b,c | + | + | + |
| PALA | − | ND | + | + | + |
| Hydroxyurea | + | +d | − | NA | − |
| Aphidiolin | + | ND | − | NA | − |
| CPT | + | +a | + | − | + |
| DFX | + | −a | + | − | − |
HCT116 (p53+/+) and (p53−/−) cells were treated with different agents as described in the text. Cell lysates were analyzed by Western blotting for levels of p53, phosphorylation at S15, CHK1, and p21. Whether CHK1 downregulation requires p53 is indicated in the p53 dependence column. The efficient accumulation of p21 in p53+/+ cells is indicated in the p21 induction column. Note that no p53-independent induction of p21 was observed after any treatment. The superscript letters in the phosphorylation at S20 column refer to the sources of the information: a, Ashcroft et al. (4); b, Shieh et al. (60); c, Chehab et al. (18); d, Gottifredi and Prives (29a); ND, not determined; NA, not applicable.
Based on these results, it is proposed that repression of CHK1 by p53 is not necessarily related to the upstream pathways that target p53 accumulation. This conclusion is highlighted by comparing results obtained with daunorubicin, γ irradiation, actinomycin D, and PALA. Each of these treatments results in different patterns of phosphorylated p53, most likely as a consequence of the activation of different upstream pathways (4, 19, 29a, 59, 60). Another realization emerging from this analysis is that there is a correlation between the induction of p21 accumulation and the ability of p53 to repress CHK1. Additionally, the results with CPT and DFX point to the possibility of a p53-independent mechanism(s) for CHK1 repression. These issues will be revisited later in this paper.
p53 repression of CHK1 does not require genotoxic stress.
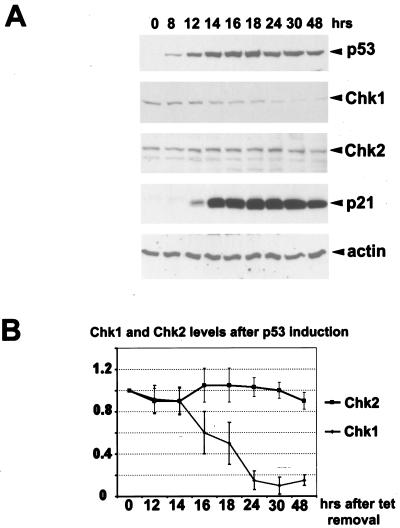
To confirm and extend our findings, we tested a cell line derived from H1299 cells which contain tetracycline-regulated wild-type p53 (20) to determine whether CHK1 levels would be decreased upon induction of p53 without treatment of cells with stress-inducing agents (Fig. 2). In these cells, the removal of tetracycline results in p53 accumulation, and p21 levels rise as a consequence of p53 stabilization (Fig. 2A). Here again there was a striking reduction of CHK1 protein that correlated with both p53 and p21 accumulation. CHK2 protein levels were not significantly affected. Figure 2B shows quantitative analyses of CHK1 and CHK2 protein levels as a function of time after the induction of p53. Thus, in several cell lines and after diverse treatments leading to induction of p53 protein, CHK1 protein is selectively reduced. It should be mentioned that the failure of p53 to repress CHK2 contradicts a previous report by Tominaga et al. (67), in which a correlation between p53 expression in tumor cell lines and CHK2 downregulation was reported. We are at this point unable to explain such a discrepancy, although different cell lines and experimental approaches were used in their study and ours. The polyclonal antibody used in our experiments was raised against and validated using purified recombinant CHK2 protein (data not shown), and we are confident that the polypeptide it recognizes is human CHK2. Moreover, we also immunoprecipitated CHK2 from total cell extracts after daunorubicin and actinomycin D treatments with a commercial polyclonal antibody. After SDS-PAGE and transfer to nitrocellulose, we performed Western blot analysis with our and the commercial antibody and again observed no p53-dependent changes in the levels of CHK2 after these treatments (not shown). We cannot, however, exclude changes in CHK2 mRNA levels, as had been reported (67), and more experiments are required to elucidate possible differences between the two studies.
FIG. 2.
p53 repression of CHK1 does not require genotoxic stress. (A) Exponentially growing H1299 cells expressing inducible wild-type p53 were extensively washed to remove tetracycline. Cells were harvested at the indicated time points after p53 induction, and Western blot analysis was performed with antibodies specific for the indicated proteins. Actin was used as a loading control. (B) Densitrometric analysis of CHK1 and CHK2 proteins levels after p53 induction in H1299 cells. The data represent the average reduction with respect to the tetracycline-treated controls (taken as 1) in three independent experiments. All samples were normalized to their respective actin levels.
p53 causes a decrease in CHK1 mRNA levels.
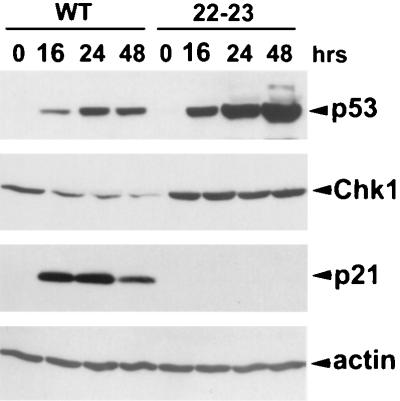
To gain further insight into features of p53 which are necessary for repression of CHK1 protein, we tested a line of H1299 cells with an inducible version of mutant p53 L22Q/W23S (p53[22/23]) that is transcriptionally impaired (43). This mutant has been well characterized to be defective for transactivation, presumably due to its inability to interact with TATA binding protein-associated factors (44). As previously shown (20), after induction in H1299 cells, p53[22/23] was unable to induce p21 (Fig. 3). Moreover, induction of p53[22/23] at levels higher than those in wild-type p53-expressing H1299 cells caused no detectable changes in the levels of CHK1. This result indicates that some aspect of the transcriptional regulatory activity of p53 is necessary for CHK1 downregulation.
FIG. 3.
Transcriptionally impaired p53 does not downregulate CHK1. Exponentially growing H1299 cells expressing inducible wild-type p53 (WT) or the p53[22–23] mutant (22–23) were extensively washed to remove tetracycline. After the indicated hours, cells were harvested and subjected to Western blot analysis with antibodies specific for the indicated proteins. Actin was used as a normalizing control.
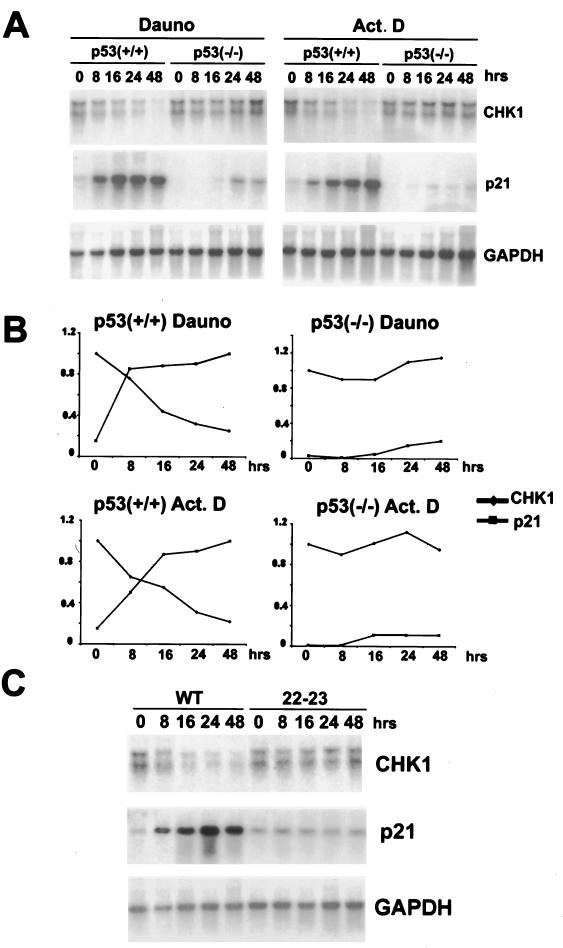
To determine whether CHK1 mRNA levels are decreased when p53 is induced, Northern blotting analysis was performed. HCT116 p53+/+ and its derivative p53−/− cells were treated with daunorubicin and actinomycin D, and RNA was prepared at different time points after application of these compounds. The p21 RNA levels rose sharply after induction of p53 in the wild-type cells, with only a modest and delayed increase in the p53−/− cells. CHK1 RNA levels, by contrast, were dramatically reduced after these treatments only in the p53+/+ cells and were unaffected in the p53−/− cells (Fig. 4A). A quantitative representation of this experiment in Fig. 4B shows the correlation between p21 accumulation and CHK1 repression. Similar results were obtained in another experiment in which the time course was slightly different (not shown). In both cases, CHK1 mRNA levels were reduced as a consequence of these treatments, and by 48 h there was only about 20% of the starting amount of RNA.
FIG. 4.
p53 downregulates CHK1 RNA levels. (A) Exponentially growing HCT116 (p53+/+) and their derivative null cells (p53−/−) were treated with daunorubicin (Dauno, 0.22 μM) or actinomycin D (Act. D, 5 nM), and total RNA samples were prepared at the indicated time points. Northern blots were performed using the indicated 32P-labeled plasmid probes. The GAPDH plasmid probe was used as a normalizing control. (B) Quantitative representation of the experiment reported in panel A. For CHK1, the initial mRNA levels were taken as 1. For p21, the maximal p21 mRNA levels were also taken as 1. (C) Exponentially growing H1299 expressing inducible wild-type p53 (WT) or p53 [22–23] mutant (22–23) cells were extensively washed to remove tetracycline. After the indicated hours, total RNA samples were prepared and Northern blots were performed using the indicated 32P-labeled plasmid probes. The GAPDH plasmid probe was used as a normalizing control.
In cells expressing the transcriptionally defective mutant p53[22/23], p21 mRNA was not increased and CHK1 mRNA was not reduced (Fig. 4C). We cannot at this point distinguish between an effect of p53 on transcription initiation and a reduction of the half-life of the CHK1 mRNA; however, our data identify CHK1 as a new repression target of p53.
Support for the likelihood that the effect of p53 on CHK1 is exclusively at the level of RNA came from an experiment in which HCT116 cells were treated with two proteosome inhibitors (LLnL and MG132) in the presence and absence of daunorubicin. Although the characteristic reduction in CHK1 levels occurred when p53 was induced, there was no further impact on CHK1 levels by the proteasome inhibitors either before or after daunorubicin treatment of HCT116 cells (data not shown).
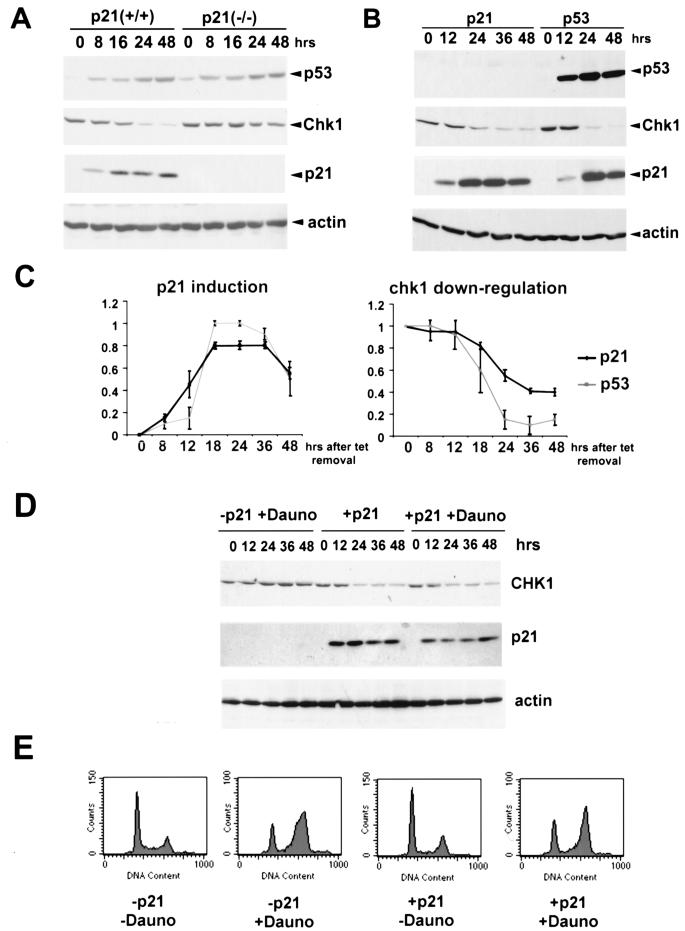
p21 is necessary and sufficient for CHK1 downregulation.
As mentioned above, we noted a correlation between p21 induction by p53 and CHK1 repression, and thus it was decided to test whether p21 might be responsible for the downregulation of CHK1. We first tested the effects of inducing p53 under circumstances in which p21 cannot be synthesized. For this we used an HCT116 wild-type cell line and its p21−/− derivative, in which p21 has been disrupted by homologous recombination (13). In the experiment summarized in Fig. 5A, both cell lines were treated with daunorubicin. Strikingly, even though p53 levels were induced to similar extents in wild-type and p21−/− cells after treatment, in the p21-null cells there was only a modest reduction in CHK1 protein levels of approximately the same extent as seen in the p53−/− cells in Fig. 1A. This demonstrates that p21 is an essential factor required for p53-dependent CHK1 repression. Similar results were obtained with treatment of these two cell lines with actinomycin D, γ irradiation, and PALA (data not shown).
FIG. 5.
p21 induction is sufficient for p53-dependent repression of CHK1. (A) Exponentially growing HCT116 (p53+/+) and their derivative p21−/− cells (p21−/−) were treated with daunorubicin (Dauno, 0.22 μM) and harvested at the indicated time points after treatment. Total lysates were then subjected to Western blot analysis with antibodies specific for p53, CHK1, and p21 as described in the text. Actin was used as a loading control. (B) Exponentially growing H1299 cells expressing inducible wild-type p53 or p21 were extensively washed to remove tetracycline. After the indicated hours, protein samples were collected and Western blot analysis was performed with antibodies specific for the indicated proteins. (C) Densitometric analysis of p21 and CHK1 protein levels after ectopic p21 and p53 induction. For p21, the data represent the accumulation of the protein in three independent experiments in which the maximal p21 levels were taken as 1. For CHK1, the data represent the reduction of the protein levels in three independent experiments in which the initial CHK1 level was also taken as 1. All the samples were normalized to their respective actin levels. (D) Exponentially growing H1299 cells expressing inducible p21 were either treated with daunorubicin (Dauno, 0.22 μM) in the presence of tetracycline and extensively washed to remove tetracycline or both washed and treated with daunorubicin (0.22 μM). After the indicated hours, protein samples were collected and Western blot analysis was performed with antibodies specific for the indicated proteins. (E) Exponentially growing H1299 expressing inducible p21 were treated as in panel D, and after 48 h they were collected and subjected to FACS analysis as described in the text.
If p21 accrual is sufficient for CHK1 downregulation, then induction of p21 in a p53-null background should produce essentially the same effect. To test this, we used an H1299 cell line containing tetracycline-regulated p21 (50). As shown in Fig. 5B, accumulation of p21 in H1299 cells resulted in significant reduction of CHK1. Note, however, that while the p21-mediated repression of CHK1 was significant, it was not as pronounced as when p53 is induced in these cell lines (see quantification of data in Fig. 5C). CHK1 levels were reduced to approximately 40% of the original level at the last time point checked. CHK1 mRNA levels were also reduced (not shown). Thus, p21 is a major component of CHK1 downregulation, even if it cannot be excluded that other factors can cooperate with it to repress synthesis of CHK1.
Since p21 induces accumulation of cells preferentially in the G1 phase of the cell cycle, we wondered if p21 expression can also affect CHK1 when cells are arrested in G2. For this, the H1299 cells with inducible p21 were treated with daunorubicin in the presence and absence of tetracycline (Fig. 5D). As expected, daunorubicin treatment resulted in a cell cycle distribution where the majority of the cells were in G2. Nevertheless, only in the presence of p21 was there a significant repression of CHK1 (Fig. 5D and E). This experiment thus strongly supports the likelihood that p21 repression of CHK1 can occur in G2.
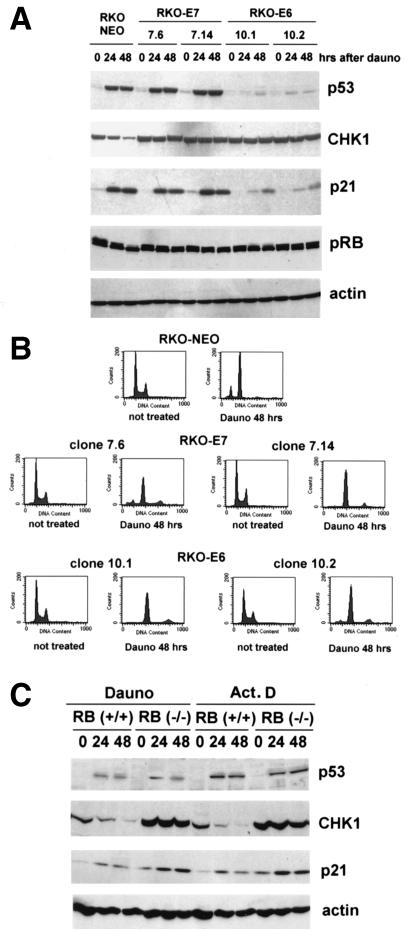
pRB activity is required for CHK1 downregulation.
To further explore the mechanisms by which p21 might repress CHK1 expression, we considered the possible involvement of a p21 target, pRB. Unphosphorylated pRB is well documented to serve as an active repressor for E2F family members, and p21 inhibits CDK phosphorylation of pRB (39). Indeed, both cdc2 and cyclin B have been shown to be repressed by p53 through a similar process (27). This prompted us to analyze the involvement of pRB in the downregulation of CHK1. For this we used RKO colorectal cancer cells stably expressing HPV-16 E7 protein (RKO-E7) and HPV E6 protein (RKO-E6), which can inactivate pRB and trigger p53 degradation, respectively. RKO cells stably expressing the vector alone were used as a control (RKO-NEO). We treated these cell lines with daunorubicin and performed comparative protein and cell cycle analyses (Fig. 6). In the control cell line (RKO-NEO), we observed a dephosphorylation of pRB that correlated with CHK1 downregulation (Fig. 6A). As expected, in the cell lines stably expressing HPV E7 (7.6 and 7.14), we observed defects in the dephosphorylation of pRB. This was correlated with defects in G2 arrest, resulting in endoreduplication (Fig. 6B), as was previously reported (27). We observed no deficiency in the induction of p53 or p21 in these cell lines compared with the control RKO-NEO cell line. However, CHK1 downregulation was greatly reduced, implying a direct involvement of pRB activity in CHK1 repression. As expected, the cell lines expressing HPV E6 showed defects in p21 induction and in the downregulation of CHK1. There was a partial dephosphorylation of pRB in one of these two cell lines (10.1) that could be related to a p53-independent induction of p21. However, this was not sufficient to induce detectable repression of CHK1 in these cells.
FIG. 6.
pRB activity is required for CHK1 downregulation. (A) RKO cells stably expressing an empty vector (RKO-NEO) or either HPV E7 (RKO-E7, clones 7.6 and 7.14) or HPV E6 (RKO-E6, clones 10.1 and 10.2) were treated with daunorubicin (Dauno, 0.22 μM) for the indicated periods. After lysis, samples were collected and Western blot analysis was performed with antibodies specific for the indicated proteins. (B) RKO cell lines were treated as in panel A and subjected to FACS analysis as described in the text after 48 h from the beginning of the treatment. (C) pRB+/+ and pRB−/− mouse embryo fibroblasts were treated with daunorubicin (Dauno, 0.44 μM) or actinomycin D (Act. D, 5 nM). After the indicated hours, protein samples were collected and Western blot analysis was performed with antibodies specific for the indicated proteins.
Another line of evidence for the involvement of pRB in repression of CHK1 expression was derived from pRB+/+ and pRB−/− mouse embryo fibroblasts. As shown in Fig. 6C, when these cells were treated with either daunorubicin or actinomycin D, CHK1 repression was observed only with RB+/+ cells, while in the RB−/− cells, CHK1 failed to be repressed after these treatments. Interestingly, we not only observed defects in the downregulation of CHK1, but there were actually higher levels of CHK1 in untreated RB−/− cells when compared to the RB+/+ fibroblasts. Taken together, our results show that pRB activity is required for CHK1 downregulation and thus provide further insight into the mechanism by which p53 and p21 are causing this phenomenon.
DISCUSSION
p53-dependent downregulation of CHK1 expression occurs as a result of diverse stress signals and in multiple cell types. This repression requires the transactivation function of p53 and is mediated, at least in part, by p21 and pRB. Before discussing the ramifications of these findings, it is important to stress again that our experiments argue strongly that CHK1 reduction is not an indirect consequence of cell cycle redistribution in G1 enforced by p53 via p21. CHK1 levels were reported to be reduced in G1 and increased again in the S to G2 phases of a normal cell cycle (40). One of the sources of genotoxic stress tested during this study, daunorubicin, induces accumulation of cells in the G2 phase of the cell cycle regardless of p53 status (Fig. 1C), at which point the level of CHK1 should be maximal. Thus, cell cycle and p53 regulation of CHK1 does not necessarily overlap.
Our observations suggest an interesting relationship between the levels and kinetics of accumulation and/or degradation of two important checkpoint molecules, p53 and CHK1. They also have implications for the relationship between CHK1 and CHK2. It has recently been reported that CHK2 is not sufficient to enforce the G2 checkpoint when CHK1 has been selectively inactivated (14, 37). Our results, however, reveal a situation in which G2 arrest does not require normal levels of CHK1. Moreover, although CHK1 disruption is incompatible with embryonic development (45, 64), our experiments show that normal levels of CHK1 are not required for cell survival. This implies that in some cases essential molecules become dispensable when p53 is stabilized. This could be the consequence of the ability of p53 to perform roles similar to those of CHK1 at least in a cellular context, such as maintenance of G2 arrest.
It has been recently reported that many different pathways lead to p53 stabilization after genotoxic stress (for a review, see reference 4). Since CHK1 could contribute to upstream processes that signal to p53, we compared the ability of different mechanisms of p53 stabilization to repress CHK1 (Table 1). Notably, this led to the realization that the p53-induced repression of CHK1 is not the consequence of the activation of a specific p53-stabilizing pathway but rather requires the accumulation of transcriptionally active p53. Both γ irradiation and daunorubicin treatments result in p53 phosphorylation at sites within its N terminus, thus attenuating its interaction with Hdm-2, while actinomycin D does not induce phosphorylation of p53 and stabilizes it by redirecting Hdm-2 to the nucleolus (4). It is still unclear how PALA stabilizes p53, although we detected no phosphorylation at S15 as a result of this treatment (data not shown). Despite the differences in these varied inducers of p53, all of the above induce a p53-dependent repression of CHK1. On the other hand, agents such us hydroxyurea and aphidicolin that trigger p53 phosphorylation at the N terminus but compromise its transcriptional activity (Gottifredi and Prives, submitted) do not activate p53-dependent repression of CHK1. Thus, the mechanism of CHK1 downregulation is not affected by any specific signal upstream of p53; rather, it appears to be exclusively dependent on pathways downstream of p53 stabilization.
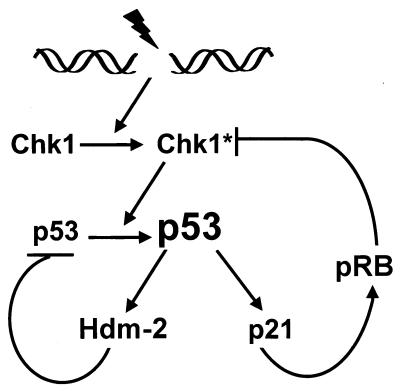
Why has p53 evolved to downregulate CHK1? Speculative scenarios can be proposed, taking into account activities of CHK1, including its role as a checkpoint effector of G2 arrest and ability to modulate p53 protein levels. It is possible that under some conditions there exists a feedback loop between p53 and CHK1 which CHK1 stabilizes p53, which in turn decreases CHK1. This would be analogous to another proposed feedback loop existing between p53 and ARF (62). Here, while ARF is required for stabilization of p53 after inappropriate hyperproliferative signals, p53 in turn represses the expression of ARF in normal cells. The best-characterized regulatory circuit involves the relationship between p53 and MDM-2, although here the reciprocal relationship exists in that p53 induces MDM-2, which then targets p53 for degradation (for reviews, see references 5, 28, and 53). In the same manner, CHK1 could also participate in a stressed-induced autoregulatory loop. This is diagrammed in the model shown in Fig. 7. After p53 stabilization, the consequential downregulation of CHK1 would result in a reduction of p53 in the next cycle. Indeed, when CHK1 levels were lowest, we detected a small reduction of p53 and p21 levels in H1299 cells (see Fig. 2A, 3, and 5B). However, such a reduction was not evident in HCT116 cells, possibly related to the fact that induction of p53 in HCT116 cells requires genotoxic stress, implying that other pathways can mitigate the effect of CHK1 on p53. More extended kinetic analysis of p53 and CHK1 (and MDM-2) levels in a variety of cell types will hopefully reveal whether and when p53-CHK1 circuitry exists.
FIG. 7.
Model depicting a potential regulatory loop involving p53 and CHK1. Genotoxic stress activates CHK1 (and other factors), leading to the accumulation of p53 protein. Stabilized p53 transcriptionaly activates the Hdm-2 and p21 genes. Hdm-2 negatively affects p53 by targeting it to the ubiquitination machinery, while p21 through pRB transcriptionally represses CHK1, thus both controlling p53 levels and partially releasing the G2 checkpoint. CHK1∗ represents the activated form of the kinase.
Another intriguing possibility is that while p53 functions as a checkpoint factor, it may also be required in some cases for the eventual disabling of checkpoint pathways. The initiation of the G2 checkpoint requires CHK1-dependent inactivation of the Cdc2-cyclin B complex (14, 30, 37, 63, 73). After this initial stage, however, Cdc2 and cyclin B levels, localization, and activity may be regulated by p53 and its transcriptional targets. Thus, distinct but overlapping functions of both p53 and CHK1 are required to maintain the G2 checkpoint. Moreover, a quantitative analysis of the kinetics of this checkpoint leads to the prediction that it would be impossible to produce sufficient active Cdc2-cyclin B complex to resume cell cycle progression if both p53 and CHK1 pathways are active (2). In the potential event of successful repair of damaged DNA, p53 levels would become reduced and a small amount of Cdc2-cyclin B complex would be available. Since CHK1 has been reported to be active also in a normal cell cycle during G2 phase (40), once DNA repair had occurred, CHK1 would interfere with the reentrance into the cell cycle of a G2-arrested cell. Only with decreased CHK1 would low levels of Cdc2-cylin B complex be able to direct the cell back to the cycle. In this context, CHK1 repression by p53 may be required to prevent an excessively prolonged arrest that might eventually trigger apoptosis.
It is now well documented that the cellular transcriptional program initiated by p53 involves not only many targets that are induced as a result of the sequence-specific transactivation function of p53, but also genes whose expression is repressed when p53 accumulates (77 and 80 and references therein). Transcriptional repression by p53 is less well understood mechanistically than transactivation. As yet, a consensus site defining a p53-specific transcriptional repression element has not been identified, and there is still much to learn about how this phenomenon takes place. The ability of p53 to repress two genes, MAP4 and stathmin, involves its interaction with m-Sin-3A (48). Our data postulate a different sort of mechanism in which the function of p21 is critical for repression of CHK1 by p53. This is of interest since p21 ((13) and references therein) and pRB (56, 76) play an essential role in sustained arrest in G2 after genotoxic stress. Moreover, p21 is necessary for the repression of Cdc2 and cyclin B by p53 (27, 65). In line with our findings, not only p21 but also pRB was shown to be required for this process (27). Here the presumptive role of p21 is postulated to be through prevention of Rb inhibition of E2F-dependent transcription (27). As in the case of Cdc2 and cyclin B, CHK1 transcription is normally upregulated in the G2 phase of the cell cycle (40). In the presence of hyphophosphorylated pRB, transcription of these genes is reduced regardless of the cell cycle phase. The mechanism of this repression however could be very indirect. In the case of Cdc2, its promoter has been shown to be regulated by E2F transcription factors directly (22, 23). However, in the case of cyclin B, this repression may result from E2F sites in its promoter or alternatively because of an indirect effect caused by the downregulation of cyclin A, which is itself regulated by E2F transcription factors (27). The possibility that CHK1 may be regulated by E2F awaits analysis of the CHK1 gene promoter.
Interestingly, even if p21 expression leads to CHK1 downregulation, our data suggest that other factors may produce CHK1 repression. First, the kinetics and efficiency of CHK1 repression are more enhanced in the presence of both p53 and p21 than with p21 alone (Fig. 5B and C). Second, two different compounds, CPT and DFX, caused significant downregulation of Chk1 in p53−/− cells. In fact, CPT induces p53-dependent accumulation of p21, and this correlates with a stronger downregulation of CHK1 in p53+/+ cells than in the p53−/− cell line (data not shown). As reported recently, DFX-induced p53 fails to induce p21 (4). Thus, at least one condition can be identified where downregulation of CHK1 occurs independently of p21 or p53. Taken together, these results suggest the possibility that a p53- or p21-independent mechanism(s) cooperates with the novel repression pathway that we have identified in this study.
ACKNOWLEDGMENTS
We are extremely grateful to Nicole Baptiste for crucial suggestions and discussions. We thank Ella Freulich and Chris Cain for help in the purification of antibodies against CHK2 as well as the members of the Prives laboratory for helpful suggestions.
This work was supported by NIH grant CA 58316.
REFERENCES
- 1.Agarwal M L, Agarwal A, Taylor W R, Stark G R. p53 controls both the G2/M and the G1 cell cycle checkpoints and mediates reversible growth arrest in human fibroblasts. Proc Natl Acad Sci USA. 1995;92:8493–8497. doi: 10.1073/pnas.92.18.8493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aguda B D. A quantitative analysis of the kinetics of the G(2) DNA damage checkpoint system. Proc Natl Acad Sci USA. 1999;96:11352–11357. doi: 10.1073/pnas.96.20.11352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.An W G, Kanekal M, Simon M C, Maltepe E, Blagosklonny M V, Neckers L M. Stabilization of wild-type p53 by hypoxia-inducible factor 1alpha. Nature. 1998;392:405–408. doi: 10.1038/32925. [DOI] [PubMed] [Google Scholar]
- 4.Ashcroft M, Taya Y, Vousden K H. Stress signals utilize multiple pathways to stabilize p53. Mol Cell Biol. 2000;20:3224–3233. doi: 10.1128/mcb.20.9.3224-3233.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ashcroft M, Vousden K H. Regulation of p53 stability. Oncogene. 1999;18:7637–7643. doi: 10.1038/sj.onc.1203012. [DOI] [PubMed] [Google Scholar]
- 6.Bell D W, Varley J M, Szydlo T E, Kang D H, Wahrer D C, Shannon K E, Lubratovich M, Verselis S J, Isselbacher K J, Fraumeni J F, Birch J M, Li F P, Garber J E, Haber D A. Heterozygous germ line hCHK2 mutations in Li-Fraumeni syndrome. Science. 1999;286:2528–2531. doi: 10.1126/science.286.5449.2528. [DOI] [PubMed] [Google Scholar]
- 7.Bertoni F, Codegoni A M, Furlan D, Tibiletti M G, Capella C, Broggini M. CHK1 frameshift mutations in genetically unstable colorectal and endometrial cancers. Genes Chromosomes Cancer. 1999;26:176–180. [PubMed] [Google Scholar]
- 8.Blasina A, de Weyer I V, Laus M C, Luyten W H, Parker A E, McGowan C H. A human homologue of the checkpoint kinase Cds1 directly inhibits Cdc25 phosphatase. Curr Biol. 1999;9:1–10. doi: 10.1016/s0960-9822(99)80041-4. [DOI] [PubMed] [Google Scholar]
- 9.Brondello J M, Boddy M N, Furnari B, Russell P. Basis for the checkpoint signal specificity that regulates Chk1 and Cds1 protein kinases. Mol Cell Biol. 1999;19:4262–4269. doi: 10.1128/mcb.19.6.4262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brown A L, Lee C H, Schwarz J K, Mitiku N, Piwnica-Worms H, Chung J H. A human Cds1-related kinase that functions downstream of ATM protein in the cellular response to DNA damage. Proc Natl Acad Sci USA. 1999;96:3745–3750. doi: 10.1073/pnas.96.7.3745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brown J P, Wei W, Sedivy J M. Bypass of senescence after disruption of p21CIP1/WAF1 gene in normal diploid human fibroblasts. Science. 1997;277:831–834. doi: 10.1126/science.277.5327.831. [DOI] [PubMed] [Google Scholar]
- 12.Brugarolas J, Chandrasekaran C, Gordon J I, Beach D, Jacks T, Hannon G J. Radiation-induced cell cycle arrest compromised by p21 deficiency. Nature. 1995;377:552–557. doi: 10.1038/377552a0. [DOI] [PubMed] [Google Scholar]
- 13.Bunz F, Dutriaux A, Lengauer C, Waldman T, Zhou S, Brown J P, Sedivy J M, Kinzler K W, Vogelstein B. Requirement for p53 and p21 to sustain G2 arrest after DNA damage. Science. 1998;282:1497–1501. doi: 10.1126/science.282.5393.1497. [DOI] [PubMed] [Google Scholar]
- 14.Busby E C, Leistritz D F, Abraham R T, Karnitz L M, Sarkaria J N. The radiosensitizing agent 7-hydroxystaurosporine (UCN-01) inhibits the DNA damage checkpoint kinase hChk1. Cancer Res. 2000;60:2108–2112. [PubMed] [Google Scholar]
- 15.Carr A M. Checkpoints take the next step. Science. 1996;271:314–315. doi: 10.1126/science.271.5247.314. [DOI] [PubMed] [Google Scholar]
- 16.Chan T A, Hermeking H, Lengauer C, Kinzler K W, Vogelstein B. 14-3-3Sigma is required to prevent mitotic catastrophe after DNA damage. Nature. 1999;401:616–620. doi: 10.1038/44188. [DOI] [PubMed] [Google Scholar]
- 17.Chan T A, Hwang P M, Hermeking H, Kinzler K W, Vogelstein B. Cooperative effects of genes controlling the G(2)/M checkpoint. Genes Dev. 2000;14:1584–1588. [PMC free article] [PubMed] [Google Scholar]
- 18.Chehab N H, Malikzay A, Appel M, Halazonetis T D. Chk2/hCds1 functions as a DNA damage checkpoint in G(1) by stabilizing p53. Genes Dev. 2000;14:278–288. [PMC free article] [PubMed] [Google Scholar]
- 19.Chehab N H, Malikzay A, Stavridi E S, Halazonetis T D. Phosphorylation of Ser-20 mediates stabilization of human p53 in response to NA damage. Proc Natl Acad Sci USA. 1999;96:13777–13782. doi: 10.1073/pnas.96.24.13777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen X, Ko L J, Jayaraman L, Prives C. p53 levels, functional domains, and DNA damage determine the extent of the apoptotic response of tumor cells. Genes Dev. 1996;10:2438–2451. doi: 10.1101/gad.10.19.2438. [DOI] [PubMed] [Google Scholar]
- 21.Collins K D, Stark G R. Aspartate transcarbamylase: interaction with the transition state analogue N-(phosphonacetyl)-l-aspartate. J Biol Chem. 1971;246:6599–6605. [PubMed] [Google Scholar]
- 22.Dalton S. Cell cycle regulation of the human cdc2 gene. EMBO J. 1992;11:1797–1804. doi: 10.1002/j.1460-2075.1992.tb05231.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Degregori J, Kowalik T, Nevins J R. Cellular targets for activation by the E2F1 transcription factor include DNA synthesis- and G1/S regulatory genes. Mol Cell Biol. 1999;15:5846–5847. doi: 10.1128/mcb.15.8.4215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Deng C, Zhang P, Harper J W, Elledge S J, Leder P. Mice lacking p21CIP1/WAF1 undergo normal development, but are defective in G1 checkpoint control. Cell. 1995;82:675–684. doi: 10.1016/0092-8674(95)90039-x. [DOI] [PubMed] [Google Scholar]
- 25.Elledge S J. Cell cycle checkpoints: preventing an identity crisis. Science. 1996;274:1664–1672. doi: 10.1126/science.274.5293.1664. [DOI] [PubMed] [Google Scholar]
- 26.Flaggs G, Plug A W, Dunks K M, Mundt K E, Ford J C, Quiggle M R, Taylor E M, Westphal C H, Ashley T, Hoekstra M F, Carr A M. Atm-dependent interactions of a mammalian chk1 homolog with meiotic chromosomes. Curr Biol. 1997;7:977–986. doi: 10.1016/s0960-9822(06)00417-9. [DOI] [PubMed] [Google Scholar]
- 27.Flatt P M, Tang L J, Scatena C D, Szak S T, Pietenpol J A. p53 regulation of G2 checkpoint is retinoblastoma protein dependent. Mol Cell Biol. 2000;20:4210–4223. doi: 10.1128/mcb.20.12.4210-4223.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Freedman D A, Wu L, Levine A J. Functions of the MDM2 oncoprotein. Cell Mol Life Sci. 1999;55:96–107. doi: 10.1007/s000180050273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gewirtz D A. A critical evaluation of the mechanisms of action proposed for the antitumor effects of the anthracycline antibiotics adriamycin and daunorubicin. Biochem Pharmacol. 1999;57:727–741. doi: 10.1016/s0006-2952(98)00307-4. [DOI] [PubMed] [Google Scholar]
- 29a.Gottifredi, V., S. Y. Shieh, Y. Taya, and C. Prives. p53 accumulates but is functionally impaired when DNA synthesis is blocked. Proc. Natl. Acad. Sci. USA, in press. [DOI] [PMC free article] [PubMed]
- 30.Graves P R, Yu L, Schwarz J K, Gales J, Sausville E A, O'Connor P M, Piwnica-Worms H. The Chk1 protein kinase and the Cdc25C regulatory pathways are targets of the anticancer agent UCN-01. J Biol Chem. 2000;275:5600–5605. doi: 10.1074/jbc.275.8.5600. [DOI] [PubMed] [Google Scholar]
- 31.Hartwell L H, Weinert T A. Checkpoints: controls that ensure the order of cell cycle events. Science. 1989;246:629–634. doi: 10.1126/science.2683079. [DOI] [PubMed] [Google Scholar]
- 32.Hermeking H, Lengauer C, Polyak K, He T C, Zhang L, Thiagalingam S, Kinzler K W, Vogelstein B. 14-3-3 sigma is a p53-regulated inhibitor of G2/M progression. Mol Cell. 1997;1:3–11. doi: 10.1016/s1097-2765(00)80002-7. [DOI] [PubMed] [Google Scholar]
- 33.Hirao A, Kong Y Y, Matsuoka S, Wakeham A, Ruland J, Yoshida H, Liu D, Elledge S J, Mak T W. DNA damage-induced activation of p53 by the checkpoint kinase Chk2. Science. 2000;287:1824–1827. doi: 10.1126/science.287.5459.1824. [DOI] [PubMed] [Google Scholar]
- 34.Hsiang Y H, Hertzberg R, Hecht S, Liu L F. Camptothecin induces protein-linked DNA breaks via mammalian DNA topoisomerase I. J Biol Chem. 1985;260:14873–14878. [PubMed] [Google Scholar]
- 35.Ikegami S, Taguchi T, Ohashi M, Oguro M, Nagano H, Mano Y. Aphidicolin prevents mitotic cell division by interfering with the activity of DNA polymerase-alpha. Nature. 1978;275:458–460. doi: 10.1038/275458a0. [DOI] [PubMed] [Google Scholar]
- 36.Innocente S A, Abrahamson J L, Cogswell J P, Lee J M. p53 regulates a G2 checkpoint through cyclin B1. Proc Natl Acad Sci USA. 1999;96:2147–2152. doi: 10.1073/pnas.96.5.2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jackson J R, Gilmartin A, Imburgia C, Winkler J D, Marshall L A, Roshak A. An indolocarbazole inhibitor of human checkpoint kinase (Chk1) abrogates cell cycle arrest caused by DNA damage. Cancer Res. 2000;60:566–572. [PubMed] [Google Scholar]
- 38.Jin S, Antinore M J, Lung F D, Dong X, Zhao H, Fan F, Colchagie A B, Blanck P, Roller P P, Fornace A J, Jr, Zhan Q. The GADD45 inhibition of Cdc2 kinase correlates with GADD45-mediated growth suppression. J Biol Chem. 2000;275:16602–16608. doi: 10.1074/jbc.M000284200. [DOI] [PubMed] [Google Scholar]
- 39.Kaelin W G., Jr Functions of the retinoblastoma protein. Bioessays. 1999;21:950–958. doi: 10.1002/(SICI)1521-1878(199911)21:11<950::AID-BIES7>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 40.Kaneko Y S, Watanabe N, Morisaki H, Akita H, Fujimoto A, Tominaga K, Terasawa M, Tachibana A, Ikeda K, Nakanishi M, Kaneko Y. Cell-cycle-dependent and ATM-independent expression of human Chk1 kinase. Oncogene. 1999;18:3673–3681. doi: 10.1038/sj.onc.1202706. [DOI] [PubMed] [Google Scholar]
- 41.Kastan M B, Onyekwere O, Sidransky D, Vogelstein B, Craig R W. Participation of p53 protein in the cellular response to DNA damage. Cancer Res. 1991;51:6304–6311. [PubMed] [Google Scholar]
- 42.Lavin M F, Shiloh Y. The genetic defect in ataxia telangiectasia. Annu Rev Immunol. 1997;15:177–202. doi: 10.1146/annurev.immunol.15.1.177. [DOI] [PubMed] [Google Scholar]
- 43.Lin J, Chen J, Elenbaas B, Levine A J. Several hydrophobic amino acids in the p53 amino-terminal domain are required for transcriptional activation, binding to mdm-2 and the adenovirus 5 E1B 55-kD protein. Genes Dev. 1994;8:1235–1246. doi: 10.1101/gad.8.10.1235. [DOI] [PubMed] [Google Scholar]
- 44.Lin J, Teresky A K, Levine A J. Two critical hydrophobic amino acids in the N-terminal domain of the p53 protein are required for the gain of function phenotypes of human p53 mutants. Oncogene. 1995;10:2387–2390. [PubMed] [Google Scholar]
- 45.Liu Q, Guntuku S, Cui X S, Matsuoka S, Cortez D, Tamai K, Luo G, Carattini-Rivera S, DeMayo F, Bradley A, Donehower L A, Elledge S J. Chk1 is an essential kinase that is regulated by atr and required for the G(2)/M DNA damage checkpoint. Genes Dev. 2000;14:1448–1459. [PMC free article] [PubMed] [Google Scholar]
- 46.Martinho R G, Lindsay H D, Flaggs G, DeMaggio A J, Hoekstra M F, Carr A M, Bentley N J. Analysis of Rad3 and Chk1 protein kinases defines different checkpoint responses. EMBO J. 1998;17:7239–7249. doi: 10.1093/emboj/17.24.7239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Matsuoka S, Huang M, Elledge S J. Linkage of ATM to cell cycle regulation by the Chk2 protein kinase. Science. 1998;282:1893–1897. doi: 10.1126/science.282.5395.1893. [DOI] [PubMed] [Google Scholar]
- 48.Murphy M, Ahn J, Walker K K, Hoffman W H, Evans R M, Levine A J, George D L. Transcriptional repression by wild-type p53 utilizes histone deacetylases, mediated by interaction with mSin3a. Genes Dev. 1999;13:2490–2501. doi: 10.1101/gad.13.19.2490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Myers C E, Chabner B A. Anthracyclines: cancer therapy: principles and practice. New York, N.Y: J. B. Lippincott; 1990. pp. 356–381. [Google Scholar]
- 50.Niculescu A B, 3rd, Chen X, Smeets M, Hengst L, Prives C, Reed S I. Effects of p21(Cip1/Waf1) at both the G1/S and the G2/M cell cycle transitions: pRb is a critical determinant in blocking DNA replication and in preventing endoreduplication. Mol Cell Biol. 1998;18:629–643. doi: 10.1128/mcb.18.1.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nurse P. Checkpoint pathways come of age. Cell. 1997;91:865–867. doi: 10.1016/s0092-8674(00)80476-6. [DOI] [PubMed] [Google Scholar]
- 52.Nurse P. Ordering S phase and M phase in the cell cycle. Cell. 1994;79:547–550. doi: 10.1016/0092-8674(94)90539-8. [DOI] [PubMed] [Google Scholar]
- 53.Oren M. Regulation of the p53 tumor suppressor protein. J Biol Chem. 1999;274:36031–36034. doi: 10.1074/jbc.274.51.36031. [DOI] [PubMed] [Google Scholar]
- 54.Peng C Y, Graves P R, Thoma R S, Wu Z, Shaw A S, Piwnica-Worms H. Mitotic and G2 checkpoint control: regulation of 14-3-3 protein binding by phosphorylation of Cdc25C on serine-216. Science. 1997;277:1501–1505. doi: 10.1126/science.277.5331.1501. [DOI] [PubMed] [Google Scholar]
- 55.Poon R Y, Chau M S, Yamashita K, Hunter T. The role of Cdc2 feedback loop control in the DNA damage checkpoint in mammalian cells. Cancer Res. 1997;57:5168–5178. [PubMed] [Google Scholar]
- 56.Rigberg D, Kim F S, Sebastian J L, Kazanjian K K, McFadden D W. Hypophosphorylated retinoblastoma protein is associated with G2 arrest in esophageal squamous cell carcinoma. J Surg Res. 1999;84:101–105. doi: 10.1006/jsre.1999.5617. [DOI] [PubMed] [Google Scholar]
- 57.Sanchez Y, Wong C, Thoma R S, Richman R, Wu Z, Piwnica-Worms H, Elledge S J. Conservation of the Chk1 checkpoint pathway in mammals: linkage of DNA damage to Cdk regulation through Cdc25. Science. 1997;277:1497–1501. doi: 10.1126/science.277.5331.1497. [DOI] [PubMed] [Google Scholar]
- 58.Shieh S Y, Ahn J, Tamai K, Taya Y, Prives C. The human homologs of checkpoint kinases Chk1 and Cds1 (Chk2) phosphorylate p53 at multiple DNA damage-inducible sites. Genes Dev. 2000;14:289–300. [PMC free article] [PubMed] [Google Scholar]
- 59.Shieh S Y, Ikeda M, Taya Y, Prives C. DNA damage-induced phosphorylation of p53 alleviates inhibition by MDM2. Cell. 1997;91:325–334. doi: 10.1016/s0092-8674(00)80416-x. [DOI] [PubMed] [Google Scholar]
- 60.Shieh S Y, Taya Y, Prives C. DNA damage-inducible phosphorylation of p53 at N-terminal sites including a novel site, Ser20, requires tetramerization. EMBO J. 1999;18:1815–1823. doi: 10.1093/emboj/18.7.1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sobell H M. Actinomycin and DNA transcription. Proc Natl Acad Sci USA. 1985;82:5328–5331. doi: 10.1073/pnas.82.16.5328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Stott F J, Bates S, James M C, McConnell B B, Starborg M, Brookes S, Palmero I, Ryan K, Hara E, Vousden K H, Peters G. The alternative product from the human CDKN2A locus, p14(ARF), participates in a regulatory feedback loop with p53 and MDM2. EMBO J. 1998;17:5001–5014. doi: 10.1093/emboj/17.17.5001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Suganuma M, Kawabe T, Hori H, Funabiki T, Okamoto T. Sensitization of cancer cells to DNA damage-induced cell death by specific cell cycle G2 checkpoint abrogation. Cancer Res. 1999;59:5887–5891. [PubMed] [Google Scholar]
- 64.Takai H, Tominaga K, Motoyama N, Minamishima Y A, Nagahama H, Tsukiyama T, Ikeda K, Nakayama K, Nakanishi M, Nakayama K. Aberrant cell cycle checkpoint function and early embryonic death in Chk1(−/−) mice. Genes Dev. 2000;14:1439–1447. [PMC free article] [PubMed] [Google Scholar]
- 65.Taylor W R, DePrimo S E, Agarwal A, Agarwal M L, Schonthal A H, Katula K S, Stark G R. Mechanisms of G2 arrest in response to overexpression of p53. Mol Biol Cell. 1999;10:3607–3622. doi: 10.1091/mbc.10.11.3607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Timson J. Hydroxyurea. Mutat Res. 1975;32:115–132. doi: 10.1016/0165-1110(75)90002-0. [DOI] [PubMed] [Google Scholar]
- 67.Tominaga K, Morisaki H, Kaneko Y, Fujimoto A, Tanaka T, Ohtsubo M, Hirai M, Okayama H, Ikeda K, Nakanishi M. Role of human Cds1 (Chk2) kinase in DNA damage checkpoint and its regulation by p53. J Biol Chem. 1999;274:31463–31467. doi: 10.1074/jbc.274.44.31463. [DOI] [PubMed] [Google Scholar]
- 68.Unger T, Juven-Gershon T, Moallem E, Berger M, Vogt Sionov R, Lozano G, Oren M, Haupt Y. Critical role for Ser20 of human p53 in the negative regulation of p53 by Mdm2. EMBO J. 1999;18:1805–1814. doi: 10.1093/emboj/18.7.1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Waldman T, Kinzler K W, Vogelstein B. p21 is necessary for the p53-mediated G1 arrest in human cancer cells. Cancer Res. 1995;55:5187–5190. [PubMed] [Google Scholar]
- 70.Waldman T, Lengauer C, Kinzler K W, Vogelstein B. Uncoupling of S phase and mitosis induced by anticancer agents in cells lacking p21. Nature. 1996;381:713–716. doi: 10.1038/381713a0. [DOI] [PubMed] [Google Scholar]
- 71.Walworth N, Davey S, Beach D. Fission yeast chk1 protein kinase links the rad checkpoint pathway to cdc2. Nature. 1993;363:368–371. doi: 10.1038/363368a0. [DOI] [PubMed] [Google Scholar]
- 72.Walworth N C, Bernards R. rad-dependent response of the chk1-encoded protein kinase at the DNA damage checkpoint. Science. 1996;271:353–356. doi: 10.1126/science.271.5247.353. [DOI] [PubMed] [Google Scholar]
- 73.Wang Q, Fan S, Eastman A, Worland P J, Sausville E A, O'Connor P M. UCN-01: a potent abrogator of G2 checkpoint function in cancer cells with disrupted p53. J Natl Cancer Inst. 1996;88:956–965. doi: 10.1093/jnci/88.14.956. [DOI] [PubMed] [Google Scholar]
- 74.Weinert T. DNA damage checkpoints update: getting molecular. Curr Opin Genet Dev. 1998;8:185–193. doi: 10.1016/s0959-437x(98)80140-8. [DOI] [PubMed] [Google Scholar]
- 75.Winters Z E, Ongkeko W M, Harris A L, Norbury C J. p53 regulates Cdc2 independently of inhibitory phosphorylation to reinforce radiation-induced G2 arrest in human cells. Oncogene. 1998;17:673–684. doi: 10.1038/sj.onc.1201991. [DOI] [PubMed] [Google Scholar]
- 76.Yen A, Sturgill R. Hypophosphorylation of the Rb protein in S and G2 as well as G1 during growth arrest. Exp Cell Res. 1998;241:324–331. doi: 10.1006/excr.1998.4007. [DOI] [PubMed] [Google Scholar]
- 77.Yu J, Zhang L, Hwang P M, Rago C, Kinzler K W, Vogelstein B. Identification and classification of p53-regulated genes. Proc Natl Acad Sci USA. 1999;96:14517–14522. doi: 10.1073/pnas.96.25.14517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zeng Y, Forbes K C, Wu Z, Moreno S, Piwnica-Worms H, Enoch T. Replication checkpoint requires phosphorylation of the phosphatase Cdc25 by Cds1 or Chk1. Nature. 1998;395:507–510. doi: 10.1038/26766. [DOI] [PubMed] [Google Scholar]
- 79.Zhan Q, Antinore M J, Wang X W, Carrier F, Smith M L, Harris C C, Fornace A J., Jr Association with Cdc2 and inhibition of Cdc2/Cyclin B1 kinase activity by the p53-regulated protein Gadd45. Oncogene. 1999;18:2892–2900. doi: 10.1038/sj.onc.1202667. [DOI] [PubMed] [Google Scholar]
- 80.Zhao R, Gish K, Murphy M, Yin Y, Notterman D, Hoffman W H, Tom E, Mack D H, Levine A J. Analysis of p53-regulated gene expression patterns using oligonucleotide arrays. Genes Dev. 2000;14:981–993. [PMC free article] [PubMed] [Google Scholar]