Abstract
The protein tyrosine kinase (PTK) Csk is a potent negative regulator of several signal transduction processes, as a consequence of its exquisite ability to inactivate Src-related PTKs. This function requires not only the kinase domain of Csk, but also its Src homology 3 (SH3) and SH2 regions. We showed previously that the Csk SH3 domain mediates highly specific associations with two members of the PEP family of nonreceptor protein tyrosine phosphatases (PTPs), PEP and PTP-PEST. In comparison, the Csk SH2 domain interacts with several tyrosine phosphorylated molecules, presumed to allow targetting of Csk to sites of Src family kinase activation. Herein, we attempted to understand better the regulation of Csk by identifying ligands for its SH2 domain. Using a modified yeast two-hybrid screen, we uncovered the fact that Csk associates with PTP-HSCF, the third member of the PEP family of PTPs. This association was documented not only in yeast cells but also in a heterologous mammalian cell system and in cytokine-dependent hemopoietic cells. Surprisingly, the Csk–PTP-HSCF interaction was found to be mediated by the Csk SH2 domain and two putative sites of tyrosine phosphorylation in the noncatalytic portion of PTP-HSCF. Transfection experiments indicated that Csk and PTP-HSCF synergized to inhibit signal transduction by Src family kinases and that this cooperativity was dependent on the domains mediating their association. Finally, we obtained evidence that PTP-HSCF inactivated Src-related PTKs by selectively dephosphorylating the positive regulatory tyrosine in their kinase domain. Taken together, these results demonstrate that part of the function of the Csk SH2 domain is to mediate an inducible association with a PTP, thereby engineering a more efficient inhibitory mechanism for Src-related PTKs. Coupled with previously published observations, these data also establish that Csk forms complexes with all three known members of the PEP family.
The Src family of cytoplasmic protein tyrosine kinases (PTKs) has been linked to a wide variety of signal transduction pathways (2, 9, 31, 39). First and foremost, there is firm genetic and biochemical evidence that these enzymes play a pivotal role in the initiation of immunoreceptor (i.e., antigen receptor and Fc receptor) signaling in hemopoietic cells. In addition, Src-related kinases have been implicated in the modulation of signaling through cytokine receptors, receptor PTKs, integrins and G protein-coupled receptors. Some indication also suggests that they may be involved in the progression through mitosis and in secretion. Lastly, several members of the Src family have been demonstrated to carry the potential to cause malignant cellular transformation, when deregulated by mutation or overexpression.
Given the biological importance of Src-related PTKs, significant efforts have been directed towards understanding their regulation. Current data indicate that their function is principally regulated by tyrosine phosphorylation (9, 36). Their catalytic activity is augmented by phosphorylation of a tyrosine (Y) positioned in the kinase domain (Y394 for Lck; Y417 for FynT). This phosphorylation occurs through autophosphorylation and provokes a conformational alteration in the catalytic domain that favors enzymatic activity. Conversely, Src kinases are repressed by phosphorylation of another tyrosine located near their carboxy terminus (Y505 for Lck; Y528 for FynT). This inhibitory effect results from the ability of the phosphorylated carboxy terminus to bind intramolecularly to the Src homology 2 (SH2) domain of Src kinases, therby causing a change of structure in the kinase domain. Phosphorylation of the carboxy-terminal tyrosine is not the result of autophosphorylation but rather is mediated by another group of cytoplasmic PTKs, the Csk family.
The Csk family of inhibitory PTKs comprises two members named Csk and Chk (9). They share a common primary structure, including, from the amino terminus to the carboxy terminus, (i) an SH3 domain, capable of interactions with proline-rich polypeptides; (ii) an SH2 domain involved in associations with tyrosine phosphorylated molecules; and (iii) a catalytic domain. Unlike Src-related enzymes, which are anchored to the inner aspect of the plasma membrane through lipid modifications at their amino terminus, Csk family kinases are primarily located in the cytosol. On the basis of this distinction, it is postulated that Csk and Chk need to associate with the plasma membrane to inactivate Src kinases, presumably as a result of reversible SH2 domain-mediated interactions.
Whereas little is known of the biological role of Chk, there is mounting evidence that Csk is a potent negative regulator of intracellular processes induced by Src family kinases. Most notably, Csk is a key repressor of antigen receptor-mediated signal transduction in T lymphocytes (8, 35). Furthermore, Csk was demonstrated to be essential for normal embryonic development in the mouse (24, 29). Structure-function analyses revealed that, in addition to its kinase domain, the SH3 and SH2 regions of Csk are necessary for its inhibitory function (10, 22). Interestingly, the Csk SH3 domain was found to allow constitutive association of Csk with two proline-enriched cytoplasmic protein tyrosine phosphatases (PTPs) belonging to the PEP family, PEP and PTP-PEST (11, 13, 21). Further studies revealed that PEP cooperates with Csk to inactivate Src family kinases, through its capacity to dephosphorylate the positive regulatory tyrosine of Src-related enzymes (12). In contrast, the Csk SH2 domain was observed to allow binding to tyrosine phosphorylated molecules such as the transmembrane adapter PAG (also named CREB binding protein), members of the Dok family of adapters, and the focal adhesion-associated molecules paxillin and tensin (4, 5, 10, 25, 30, 34). It is presumed that these polypeptides allow recruitment of Csk to diverse sites of Src family kinase activation at the membrane.
In this manuscript, we attempted to understand further the regulation of Csk. Through a modified yeast two-hybrid screen, we found that Csk physically interacts with PTP-HSCF, the third known member of the PEP family of PTPs. Contrary to the Csk-PEP and Csk–PTP-PEST interactions, this association was revealed to be mediated by the SH2 region of Csk and by sites of tyrosine phosphorylation on PTP-HSCF. Like Csk and PEP, however, Csk and PTP-HSCF were shown to cooperate to inactivate signaling events triggered by Src kinases.
MATERIALS AND METHODS
Yeast two-hybrid screen.
A full-length rat csk cDNA was inserted in the SaII site of the Saccharomyces cerevisiae expression vector pBTM116/Src (provided by M. Lioubin and L. Rohrschneider, Fred Hutchinson Cancer Center, Seattle, Wash.). In addition to the DNA-binding domain of LexA, this vector contains the Src kinase (with tyrosine-to-phenylalanine mutations at positions 416 and 527), which allows tyrosine phosphorylation of potential targets in yeast cells. The construct was stably expressed in the yeast strain L40, according to standard protocols (11). After confirming adequate expression of Csk-LexA (“bait”) and Src (data not shown), yeast cells were transformed with a mouse primitive hemopoietic cell (EML) cDNA library cloned in the expression vector pVP16 (28, 40). This vector bears the transactivation region of VP16. Transformants were selected for their ability to grow in medium lacking histidine, and for the presence of β-galactosidase activity (data not shown). Over 100 independent clones were subsequently subjected to elimination of the bait, and those showing concomitant loss of β-galactosidase activity were kept for further analyses. Plasmids were rescued from these yeast cells according to standard protocols and analyzed by sequencing, restriction enzyme digestions, or both (data not shown). To determine whether binding to Csk in the yeast two-hybrid system required the presence of Src, mating assays were carried out using derivatives of AMR70 expressing either pBTM116-Csk or pBTM116/Src-Csk (data not shown) (11).
Cells.
Cos-1 cells were propagated in α-minimal essential medium supplemented with 10% fetal calf serum (FCS) and antibiotics. The interleukin-3 (IL-3)-dependent mouse myeloid cell line 32D was grown in RPMI 1640 medium with 10% FCS, antibiotics and 5% WEHI-3B conditioned medium (as a source of IL-3). To inhibit PTP activity in 32D cells, cells were incubated for 10 min in growth medium containing PIC (bPV; 20 μM), a synthetic analog of pervanadate (provided by B. Posner, McGill University, Montréal, Québec, Canada).
cDNAs and antibodies.
A full-length mouse ptp-hscf cDNA (6) was cloned by PCR from IL-3-dependent Ba/F3 pro-B cells. The entire cDNA was sequenced and found to contain no mutations (data not shown). cDNAs encoding variants of PTP-HSCF with mutations of critical residues in the phosphatase domain (cysteine 229-to-serine [C229S] and aspartate 197-to-alanine [D197A] PTP-HSCF), or of tyrosines in the carboxy-terminal noncatalytic domain of PTP-HSCF (tyrosine 354-to-phenylalanine [Y354F], Y381F, and Y419F PTP-HSCF), were produced by PCR. All mutants were fully resequenced to ensure that no unwanted mutation was introduced in the process of their creation (data not shown). For expression in Cos-1 cells, ptp-hscf cDNAs were inserted in the expression vector pXM139, which possesses the origin of replication of simian virus 40 and the adenovirus major late promoter. cDNAs coding for the various forms of Csk were reported elsewhere (8, 10). Those encoding activated (Y505F) Lck, Chk, wild-type FynT, Tac-ζ, and kinase-inactive (K295R) Zap-70 were also described previously (1, 7, 12). Antibodies against PTP-HSCF were produced in rabbits using a bacterial fusion protein (TrpE) encompassing amino acids 283 to 453 of mouse PTP-HSCF, which correspond to the carboxy-terminal noncatalytic segment of PTP-HSCF (6). These antibodies recognized efficiently PTP-HSCF, but failed to react with PEP and PTP-PEST (data not shown). Antibodies directed against Csk, Lck, Fyn, Chk, Syk, phospholipase C-γ1, Cbl, Vav, p85, Shc, glutathione-S-transferase (GST) or phosphotyrosine were described elsewhere.
Transfections.
To study protein associations, Cos-1 cells were transiently transfected by the DEAE-dextran method (19). To analyze the effect of PTP-HSCF on protein tyrosine phosphorylation, Cos-1 cells were transfected through lipofection, using the Lipofectamine-Plus reagent (12).
Immunoprecipitations and immunoblots.
Cells were lysed in modified 1× TNE buffer (50 mM Tris [pH 8.0], 1% Nonidet P-40, 2 mM EDTA [pH 8.0], and 150 mM NaCl) supplemented with protease and phosphatase inhibitors, as detailed previously (14). Immunoprecipitations and immunoblots were performed according to protocols detailed elsewhere (41). For quantitation, data were analyzed with a PhosphorImager (BAS2000; Fuji).
In vitro binding assays.
GST fusion proteins were produced in bacteria and purified on agarose-glutathione beads as described elsewhere (32). In vitro binding assays were performed using 100 μg of lysates from Cos-1 cells transfected with the indicated cDNAs and 0.2 μg of GST fusion proteins. After several washes, bound proteins were eluted in sample buffer and detected by immunoblotting with anti-PTP-HSCF or antiphosphotyrosine antibodies.
Immune-complex phosphatase assays.
Wild-type and mutated versions of the PTP-HSCF proteins were expressed in Cos-1 cells by transient transfection. Proteins were then recovered by immunoprecipitation with anti-PTP-HSCF antibodies and assayed for phosphatase activity using the PTP assay system (New England Biolabs, Inc.), as described elsewhere (12). The expression of the various PTP-HSCF mutants was verified by immunoblotting of parallel immunoprecipitates with anti-PTP-HSCF antibodies. All experiments were conducted under linear assay conditions (data not shown).
Metabolic labeling and peptide mapping.
Transfected Cos-1 cells were labeled for 2 h in phosphate-free Dulbecco minimal essential medium containing 32Pi (1.0 mCi/ml; carrier free; New England Nuclear Research Products) and 2% dialyzed FCS. They were subsequently lysed in 1X TNE buffer containing protease and phosphatase inhibitors. FynT molecules were isolated by immunoprecipitation and separated in 8% sodium dodecyl sulfate (SDS)-polyacrylamide gels. Cleavage with cyanogen bromide was performed as explained previously (42). Cyanogen bromide-produced fragments of FynT were resolved by electrophoresis in 18% SDS-polyacrylamide gels and detected by autoradiography.
RESULTS
Identification of potential Csk-binding proteins by a modified yeast two-hybrid screen.
To comprehend further the regulation of Csk, we attempted to identify additional ligands for its noncatalytic domains using the yeast two-hybrid system. In these experiments, a modified version of the two-hybrid system was used, in which the bait (full-length Csk) was coexpressed with the Src kinase in the yeast. Since yeast cells contain little or no endogenous PTK activity, this modification encourages the detection of ligands for SH2 domains. Yeasts were subsequently transformed with a mouse primitive hemopoietic cell cDNA library and screened for interacting proteins as detailed in Materials and Methods (data not shown).
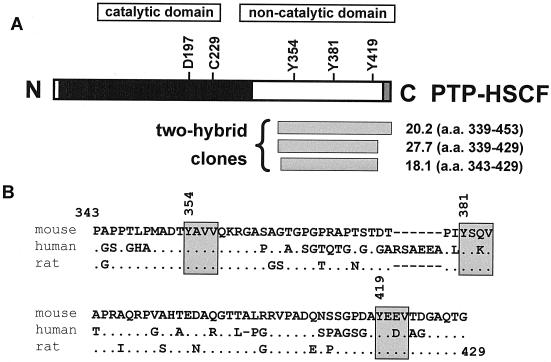
Through this approach, we were able to identify the already-known Csk-binding partners, Dok-3 and paxillin (data not shown) (4, 27). Moreover, Dok-2, another Dok-related molecule (16), was uncovered. In all cases, these associations were dependent on coexpression of Src in the yeast (data not shown), thereby implying that they were mediated by the Csk SH2 domain. We also identified three independent cDNA clones encoding PTP-HSCF (also named FLP-1, PTP-K1, BDP1, and PTP20) (Fig. 1A), a third member of the PEP/PTP-PEST family of PTPs expressed exclusively in primitive hemopoietic cells (3, 6, 18, 23, 26). No other PTP was found (data not shown). All three ptp-hscf clones encoded sequences contained within the carboxy-terminal noncatalytic domain of PTP-HSCF (Fig. 1A). The smallest clone (18.1) corresponded to amino acids 343 to 429 of the mouse PTP-HSCF protein (Fig. 1) (6). While the precise function of this region of the molecule is not known, its sequence is significantly conserved in mouse, human, and rat PTP-HSCF (Fig. 1B).
FIG. 1.
Identification of PTP-HSCF as a potential Csk-binding protein in the yeast two-hybrid system. (A) The primary structure of PTP-HSCF is shown. The positions of conserved critical residues in the phosphatase domain (aspartate 197 and cysteine 229 in the mouse protein) and of three tyrosines in the carboxy-terminal noncatalytic domain (tyrosines 354, 381, and 419) are indicated. The locations and boundaries of the three independent ptp-hscf cDNA clones identified in the yeast two-hybrid screen are shown at the bottom. a. a., amino acid. (B) Comparison of the carboxy-terminal sequences of mouse, human, and rat PTP-HSCF. The amino acid sequences (residues 343 to 429) corresponding to the smallest cDNA clone identified in the yeast two-hybrid screen (clone 18.1) are compared for mouse, human, and rat PTP-HSCF. In the case of the human protein sequence, part of the data is derived from expressed sequence tag database analyses. Identical amino acids are shown as dots, while gaps in the sequence alignment are revealed by hyphens. The three conserved tyrosine-based motifs are boxed.
Reconstitution of Csk-PTP-HSCF association in mammalian cells.
Csk associates with PEP and PTP-PEST via a direct interaction involving the SH3 region of Csk and a conserved proline-rich motif in the carboxy-terminal noncatalytic domain of PEP and PTP-PEST (PPPLPERTPESFIVVEE in PEP and PPPLPERTPESFVLADM in PTP-PEST [core prolines are underlined]) (11, 13, 21). It is notable, however, that the noncatalytic domain of PTP-HSCF is markedly shorter than that of PEP and PTP-PEST and that it does not contain a related proline-enriched sequence. Hence, the mechanism underlying the interaction between Csk and PTP-HCSF may be different. This idea was also supported by the observation that the association of Csk with the carboxy-terminal fragment of PTP-HSCF in yeast required coexpression of Src (data not shown). Hence, the Csk–PTP-HSCF interaction may actually involve the Csk SH2 domain and sites of tyrosine phosphorylation on PTP-HSCF. In further agreement with this notion, it was reported that Src-related PTKs can cause tyrosine phosphorylation of PTP-HSCF in transfected cells (38).
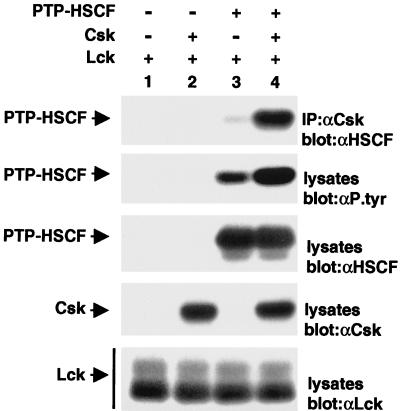
We wanted to examine whether the association between Csk and PTP-HSCF also occurred in mammalian cells. To this end, Cos-1 cells were transiently transfected with cDNAs coding for PTP-HSCF and the Src kinase Lck (to permit tyrosine phosphorylation of PTP-HSCF) in the absence or in the presence of a csk cDNA. Since others had demonstrated that PTP-HSCF is capable of autodephosphorylation (38), an inactive version of the phosphatase (C229S PTP-HSCF) was employed in these assays, to favor PTP-HSCF tyrosine phosphorylation. After 40 h, cells were lysed in nonionic detergent-containing buffer, and the ability of Csk to associate with PTP-HSCF was monitored by immunoblotting of anti-Csk immunoprecipitates with antibodies directed against PTP-HSCF (Fig. 2, first panel).
FIG. 2.
Association of Csk with PTP-HSCF in mammalian cells. Cos-1 cells were transfected (+) or not (−) with cDNAs coding for a phosphatase-inactive version of PTP-HSCF (C229S PTP-HSCF) and wild-type Csk, in the presence of a constitutively activated version of Lck (Y505F Lck). After 40 h, the association of Csk with PTP-HSCF was assessed by immunoblotting of anti-Csk immunoprecipitates (IP) with anti-PTP-HSCF (αHSCF) antibodies (first panel). Tyrosine phosphorylation of PTP-HSCF was determined by immunoblotting of total cell lysates with antiphosphotyrosine (αP.tyr) antibodies (second panel). Expression of PTP-HSCF (third panel), Csk (fourth panel), and Lck (fifth panel) was verified by immunoblotting of total cell lysates with the appropriate antibodies. The positions of PTP-HSCF, Csk, and Lck are indicated on the left. Exposures in all panels except the second panel were 2 h, and that in the second panel was 4 h.
This experiment demonstrated that, in cells transfected with both the ptp-hscf and the csk cDNAs (Fig. 2, lane 4), large amounts of a 48-kDa product consistent with PTP-HSCF were present in anti-Csk immunoprecipitates. Small quantities of PTP-HSCF were also found in these immunoprecipitates in the absence of Csk overexpression (Fig. 2, lane 3), most likely due to the presence of endogenous Csk molecules in Cos-1 cells (data not shown). No coimmunoprecipitation was detected in cells lacking PTP-HSCF (Fig. 2, lanes 1 and 2). A concomitant immunoblot of total cell lysates with antiphosphotyrosine antibodies (second panel) confirmed that C229S PTP-HSCF was detectably tyrosine phosphorylated in this system (Fig. 2, lanes 3 and 4), in keeping with an earlier report (38). While tyrosine phosphorylation of PTP-HSCF occurred in the presence of Lck alone (Fig. 2, lane 3), it is noteworthy that this phosphorylation was further increased by coexpression of Csk (Fig. 2, lane 4). This observation will be further addressed in Fig. 5. Parallel immunoblots with anti-PTP-HSCF, anti-Csk, and anti-Lck (Fig. 2, third to fifth panel, respectively) sera demonstrated that all polypeptides were appropriately expressed in this study. No tyrosine phosphorylation of PTP-HSCF occurred in the absence of Lck and Csk, or in the presence of other PTKs such as Syk, Pyk2, or Chk (data not shown).
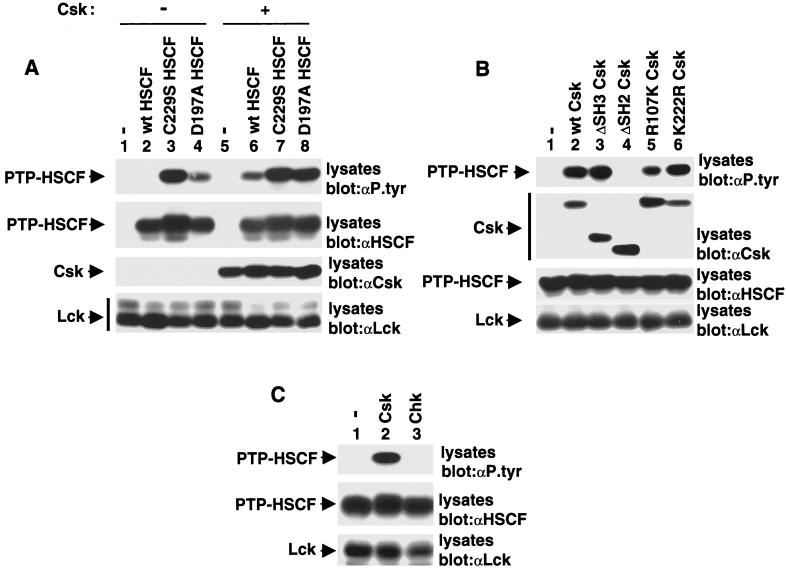
FIG. 5.
The Csk SH2 domain protects PTP-HSCF from dephosphorylation. (A) Cos-1 cells were transfected with cDNAs coding for various forms of PTP-HSCF, in the absence (lanes 1 to 4) or presence (lanes 5 to 8) of Csk. All transfections contained Y505F Lck. Tyrosine phosphorylation of PTP-HSCF was determined by immunoblotting of total cell lysates with antiphosphotyrosine (αP.tyr) antibodies (first panel). The positions of PTP-HSCF, Csk, and Lck are shown on the left. wt, wild type. Exposures in the first and second panels were 14 and 3 h, respectively, and those in the third and fourth panels were 6 h. (B) Structural requirements for induction of PTP-HSCF tyrosine phosphorylation by Csk. Cells were transfected and tested as detailed for panel A, with the exception that wild-type PTP-HSCF and various forms of Csk were utilized. The positions of PTP-HSCF, Csk, and Lck are shown on the left. Exposures in the first to fourth panels were 14, 4, 7, and 4 h, respectively. (C) Differential ability of Csk and Chk to provoke tyrosine phosphorylation of PTP-HSCF. Cells were transfected and tested as detailed for panel A, except that Csk and Chk were compared. Expression of Csk and Chk was confirmed by immunoblotting of total cell lysates with the appropriate antibodies (data not shown). The positions of PTP-HSCF and Lck are shown on the left. Exposure in the top panel was 14 h, and those in the middle and bottom panels were 6 h.
The interaction between Csk and PTP-HSCF is mediated by the Csk SH2 domain.
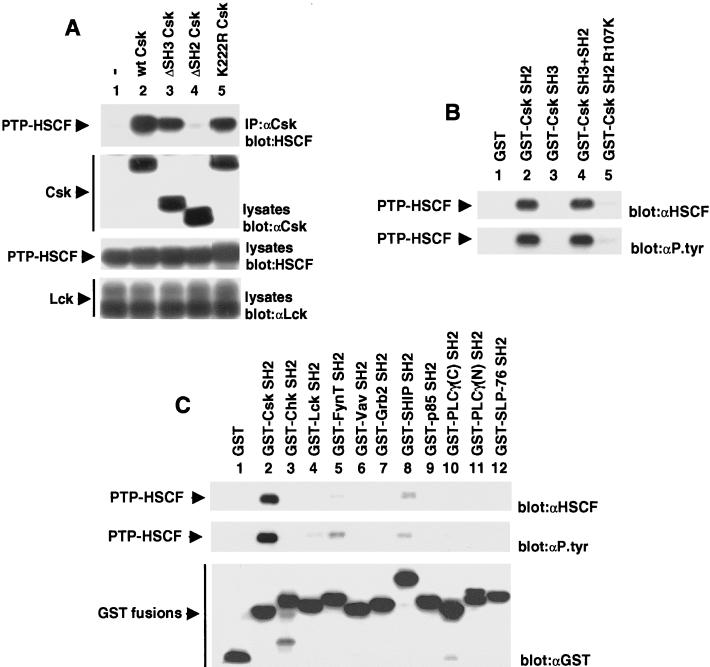
The structural domains mediating the association between Csk and PTP-HSCF were subsequently examined (Fig. 3). Transient transfection assays were performed as detailed above, except that variants of Csk carrying a deletion in the SH3 (ΔSH3 Csk) or SH2 motif (ΔSH2 Csk) or a point mutation abolishing kinase activity (K222R Csk) were tested (Fig. 3A). We found that, as was the case for wild-type Csk (Fig. 3A, top panel, lane 2), the SH3 domain-lacking variant of Csk (lane 3) and kinase-inactive Csk (lane 5) interacted strongly with PTP-HSCF. By opposition, ΔSH2 Csk (Fig. 3A, top panel, lane 4) failed to associate with the phosphatase. Importantly, a parallel immunoblot of total cell lysates with anti-Csk antibodies (middle panel) confirmed that all Csk variants were expressed in equivalent amounts in the transfected cells. Hence, the results of this experiment indicated that the SH2 domain but not the SH3 region or the kinase domain was required for the association between Csk and PTP-HSCF.
FIG. 3.
The SH2 domain of Csk is necessary and sufficient to mediate binding to PTP-HSCF. (A) Structure-function analyses of Csk. Cos-1 cells were transfected and assayed as detailed in the legend of Fig. 2, except that various forms of Csk were used. The migrations of PTP-HSCF, Csk, and Lck are shown on the left. Exposures in the first to fourth panels were 7, 4, 7, and 4 h, respectively. (B) In vitro binding assays. Cos-1 cells were transfected with cDNAs coding for phosphatase-inactive PTP-HSCF (C229S PTP-HSCF) and activated Lck (Y505F Lck). After 40 h, cells were lysed and postnuclear lysates were incubated with equivalent quantities of the indicated bacterial fusion proteins. After several washes, binding of PTP-HSCF was revealed by immunoblotting with anti-PTP-HSCF (αHSCF) or antiphosphotyrosine (αP.tyr) antibodies. The position of PTP-HSCF is indicated on the left. (C) Differential ability of various SH2 domains to bind PTP-HSCF in vitro. As detailed in the legend of Fig. 3B, except that various recombinant SH2 domains were used in the binding assay. The amounts of GST fusion proteins utilized in the assays were verified by reprobing the immunoblot membrane with anti-GST antibodies (bottom panel). The positions of PTP-HSCF and of the GST fusion proteins are shown on the left. Exposures in the top, middle, and bottom panels were 16, 26, and 16 h, respectively.
In light of these findings, we tested whether the SH2 domain of Csk alone was sufficient to mediate the association with PTP-HSCF (Fig. 3B). Tyrosine phosphorylated PTP-HSCF was first produced in Cos-1 cells by expressing C229S PTP-HSCF with Lck. Cell lysates were then incubated with immobilized GST fusion proteins encompassing either the Csk SH3 domain, the Csk SH2 domain, or both. After several washes, associated PTP-HSCF polypeptides were detected by immunoblotting with either anti-PTP-HSCF (top panel) or antiphosphotyrosine (bottom panel) antibodies. This analysis demonstrated that the SH2 domain of Csk (Fig. 3B, lane 2), but not the SH3 region (lane 3) or GST alone (lane 1), could interact with PTP-HSCF. The association of the Csk SH2 domain with PTP-HSCF was not further augmented by the additional presence of the SH3 domain (lane 4). A mutant version of the Csk SH2 domain (R107K Csk [lane 5]), in which a critical arginine (arginine 107) participating in phosphotyrosine-binding in other SH2 domains was replaced by lysine, showed reduced binding to PTP-HSCF. All GST fusion proteins were present in equivalent quantities in this assay (data not shown).
The specificity of the interaction between the Csk SH2 domain and PTP-HSCF was ascertained next, by testing additional SH2 domains derived from other molecules (Fig. 3C). We found that most other SH2 domains evaluated (top and middle panels), including those of the Csk-related enzyme Chk (lane 3), Vav (lane 6), Grb2 (lane 7), p85 (lane 9), phospholipase C-γ1 (lanes 10 and 11), and SLP-76 (lane 12), did not bind to tyrosine phosphorylated PTP-HSCF. Small amounts of binding were detected with the SH2 domain of the Src-related PTKs Lck (lane 4) and FynT (lane 5) and the inositol phosphatase SHIP (lane 8). Nonetheless, titration experiments with serial dilutions of fusion proteins revealed that these SH2 regions bound to PTP-HSCF approximately 10 to 20 times less strongly than the Csk SH2 domain (data not shown). Reprobing of the immunoblot membrane with anti-GST antibodies (bottom panel) demonstrated that all fusion proteins were expressed in comparable amounts.
Binding of the Csk SH2 domain requires two conserved tyrosines in the carboxy-terminal noncatalytic domain of PTP-HSCF.
After that, we wanted to identify the binding site(s) for Csk in PTP-HSCF. Since the ability of Csk to associate with PTP-HSCF was mediated by the Csk SH2 domain, we focused our attention on potential sites of tyrosine phosphorylation in the carboxy-terminal noncatalytic segment of PTP-HSCF. Sequences analyses revealed that three conserved tyrosines were present within the smallest fragment of PTP-HSCF identified in the yeast two-hybrid screen (Fig. 1B): Y354 (Y354 AVV), Y381 (Y381SQV), and Y419 (Y419EEV). In order to identify which one(s) of these residues was recognized by the Csk SH2 region, the three tyrosines were mutated either individually or in combination to phenylalanines.
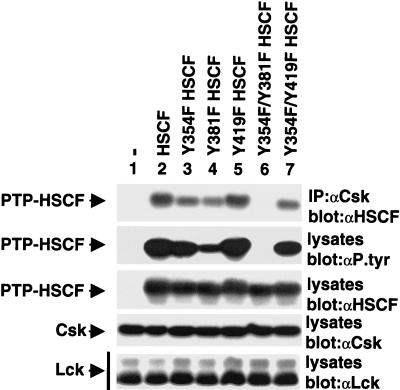
The resulting mutants were tested for their ability to associate with Csk using the cotransfection system described in Fig. 2. The analysis depicted in Fig. 4 (first panel) demonstrated that the PTP-HSCF variants in which either Y354 (lane 3) or Y381 (lane 4) was substituted by phenylalanine exhibited slightly reduced binding to Csk, by comparison to control PTP-HSCF (lane 2). Y419F PTP-HSCF (lane 5) had unaltered Csk-binding capacity. Interestingly, mutation of both Y354 and Y381 (lane 6) totally abolished the Csk-PTP-HSCF interaction. By opposition, alteration of both Y354 and Y419 (lane 7) had the same effect as mutation of Y354 alone (lane 3). In keeping with these data, an antiphosphotyrosine immunoblot (second panel) also showed that the phosphotyrosine content of PTP-HSCF was reduced by mutation of either Y354 (∼30%) (lane 3) or, to a greater extent, Y381 (∼75%) (lane 4). It was fully abrogated by replacement of both tyrosines (lane 6). Therefore, the results of this experiment suggested that both Y354 and Y381 were sites of phosphorylation in PTP-HSCF, and that these two tyrosines were involved in mediating binding to Csk. The importance of these residues for binding to the Csk SH2 domain was confirmed by in vitro binding assays (data not shown).
FIG. 4.
Two tyrosines in the carboxy-terminal region of PTP-HSCF are required for PTP-HSCF tyrosine phosphorylation and binding to Csk. Cells were transfected and tested as outlined in the legend of Fig. 2, with the exception that a series of PTP-HSCF mutants was used. All PTP-HSCF variants also carried the C229S mutation. The migrations of PTP-HSCF, Csk, and Lck are shown on the left. Exposures in the first and second panels were 2 and 12 h, respectively, and those in the third to fifth panels were 5 h.
The SH2 domain of Csk can protect PTP-HSCF from dephosphorylation.
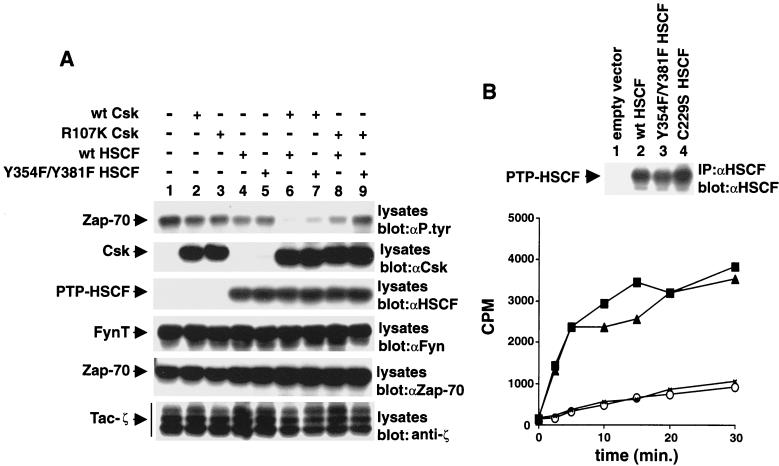
The results depicted in Fig. 2 suggested that expression of Csk also influenced the phosphotyrosine content of PTP-HSCF. In order to test this possibility more rigorously, Cos-1 cells were transfected with cDNAs coding for PTP-HSCF and Lck, in the absence or presence of wild-type Csk, as outlined above. In addition to C229S PTP-HSCF, two other PTP-HSCF variants were tested in this experiment: wild-type PTP-HSCF and D197A PTP-HSCF, another inactive version of PTP-HSCF. The extent of tyrosine phosphorylation of the various forms of PTP-HSCF was determined by immunoblotting with antiphosphotyrosine antibodies (Fig. 5A, first panel). In the absence of Csk (lanes 1 to 4), no tyrosine phosphorylation of wild-type PTP-HSCF (lane 2) could be noted, presumably due to rapid autodephosphorylation of the phosphatase (38). However, in keeping with the results presented above, tyrosine phosphorylation of C229S (lane 3) and D197A (lane 4) PTP-HSCF was readily detected. In cells cotransfected with the csk cDNA (lanes 5 to 8), there was clear tyrosine phosphorylation of wild-type PTP-HSCF (lane 6). The phosphotyrosine content of the two inactive PTP-HSCF variants (lanes 7 and 8) was either unchanged or moderately augmented. Thus, these results showed that Csk expression augmented the extent of tyrosine phosphorylation of PTP-HSCF, in particular wild-type PTP-HSCF.
Two possibilities could underlie the impact of Csk on PTP-HSCF tyrosine phosphorylation. First, Csk could phosphorylate PTP-HSCF directly. Alternatively, through binding of its SH2 domain, Csk could protect PTP-HSCF from autodephosphorylation and/or dephosphorylation by other cellular PTPs. To distinguish between these two propositions, the impact of various mutations in Csk on its capacity to promote tyrosine phosphorylation of wild-type PTP-HSCF was determined (Fig. 5B). Like wild-type Csk (first panel, lane 2), we observed that ΔSH3 Csk (lane 3) and kinase-inactive Csk (lane 6) were apt at inducing PTP-HSCF tyrosine phosphorylation in this system. In comparison, ΔSH2 Csk (lane 4) was incapable of augmenting the phosphotyrosine content of PTP-HSCF. R107K Csk (lane 5), which carries a point mutation in the SH2 region, also had a reduced effect compared to wild-type Csk (lane 2).
In addition, we contrasted the ability of Csk to enhance PTP-HSCF tyrosine phosphorylation with that of Chk, the other Csk family member (Fig. 5C). In accord with the inability of the Chk SH2 domain to bind tyrosine phosphorylated PTP-HSCF (Fig. 3C), we found that Chk (Fig. 5C, top panel, lane 3) was incapable of increasing the phosphotyrosine content of PTP-HSCF. Hence, in combination, the findings in Fig. 5 supported the idea that Csk augmented the tyrosine phosphorylation of PTP-HSCF via an SH2 domain-dependent, kinase activity-independent, mechanism. Presumably, the Csk SH2 domain protected PTP-HSCF from autodephosphorylation and/or dephosphorylation by other cellular PTPs, through binding and shielding of the tyrosine phosphorylated residues.
Association of Csk with PTP-HSCF in primitive hemopoietic cells.
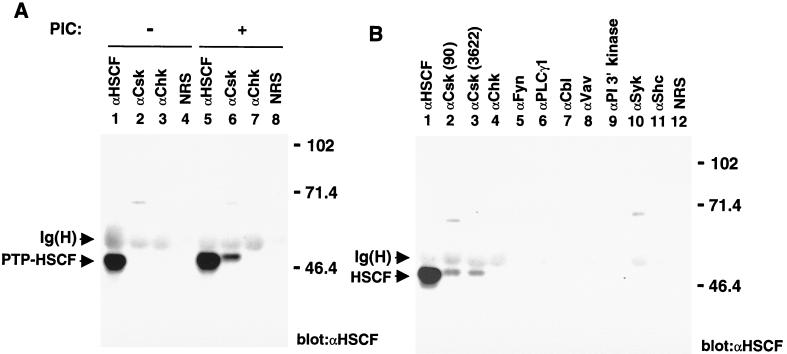
All the experiments reported above were conducted either in yeast cells or in transfected Cos-1 cells. Obviously, we needed to obtain evidence that endogenous Csk and PTP-HSCF proteins also interacted in primitive hemopoietic cells. For this purpose, we chose the IL-3-dependent mouse myeloid cell line 32D, which is known to express PTP-HSCF. Unfortunately, though, the physiological stimuli leading to activation of Src family kinases and/or tyrosine phosphorylation of PTP-HSCF in early hemopoietic cells are not identified. To circumvent this problem, protein tyrosine phosphorylation was induced in 32D cells by treatment with PIC (bPV), a pharmacological inhibitor of PTPs known to activate Src-related enzymes in other systems (43). After PIC treatment, Csk was immunoprecipitated from cell lysates, and the presence of PTP-HSCF in these immunoprecipitates was detected by immunoblotting with anti-PTP-HSCF antibodies (Fig. 6A). As expected, we found that no PTP-HSCF was associated with Csk in unstimulated cells (lane 2). Following stimulation with PIC, however, significant quantities of PTP-HSCF became complexed with Csk (lane 6). By comparison, PTP-HSCF was not associated with the Csk-related enzyme Chk, which is also expressed in 32D cells, in either unstimulated (lane 3) or stimulated (lane 7) cells. No PTP-HSCF was observed in immunoprecipitates obtained with normal rabbit serum (lanes 4 and 8). Taking into consideration the total amount of PTP-HSCF present in 32D cells (lanes 1 and 5), it was estimated that approximately 10% of PTP-HSCF became associated with Csk in PIC-treated cells.
FIG. 6.
Association of Csk with PTP-HSCF in primitive hemopoietic cells. (A) Association of Csk with PTP-HSCF in factor-dependent mouse myeloid cells. IL-3-dependent 32D cells were treated or not for 10 min with bPV (PIC), an analog of pervanadate. After cell lysis, postnuclear supernatants were immunoprecipitated with the indicated antibodies, and the presence of PTP-HSCF in these immunoprecipitates was determined by immunoblotting with anti-PTP-HSCF (αHSCF) antibodies. (B) Specificity of the association between Csk and PTP-HSCF in mouse myeloid cells. As described for panel A, except that, in all cases, cells were pretreated with bPV (PIC). PI, phosppatidyl inositol; NRS, normal rabbit serum. For both panels, the positions of PTP-HSCF and of the heavy chain of immunoglobulin [Ig(H)] are shown on the left; those of prestained molecular weight markers (in kilodaltons) are indicated on the right. Exposure, 14 h.
The ability of PTP-HSCF to associate with other signaling molecules expressed in 32D cells was also ascertained (Fig. 6B). Whereas PTP-HSCF was clearly detected in immunoprecipitates obtained with two distinct anti-Csk sera (lanes 2 and 3), none was found to be associated with the kinases Chk (lane 4), Fyn (lane 5) and Syk (lane 10). Similarly, no PTP-HSCF was bound to the SH2 domain-containing molecules phospholipase C-γ1 (lane 6), Cbl (lane 7), Vav (lane 8), phosphatidylinositol 3′ kinase (p85 subunit) (lane 9), and Shc (lane 11). Therefore, we concluded that Csk and PTP-HSCF could inducibly associate in primitive hemopoietic cells and that their association was highly specific.
Csk and PTP-HSCF cooperate to inhibit signalling initiated by Src family protein tyrosine kinases.
Finally, we wished to evaluate the impact of the Csk-PTP-HSCF interaction on cell signaling. On the one hand, Csk and PTP-HSCF might cooperate to inhibit intracellular signaling initiated by Src family kinases, as described for Csk and PEP (12). However, in light of the distinct structural basis for the Csk-PTP-HSCF interaction, it is conceivable that the purpose of this association is different. For example, Csk and PTP-HSCF might antagonize each other's activity by having opposite actions on the same substrate, possibly Src family kinases. To test these hypotheses, the effect of Csk and PTP-HSCF on a signaling mechanism dependent on Src kinases was studied. Because little is known of the role of Src-related PTKs in primitive hemopoietic cells, these experiments were performed with the help of a previously described heterologous system in which a typical Src kinase-mediated signaling event is recapitulated (12).
Briefly, Cos-1 cells were transfected with cDNAs encoding a chimeric receptor containing the cytoplasmic domain of the ζ chain of the T-cell antigen receptor complex (Tac-ζ), the Src family kinase FynT, and the protein tyrosine kinase Zap-70. A kinase-inactive variant of Zap-70 (lysine 295-to-arginine Zap-70) was used in these assays, to ensure that baseline tyrosine phosphorylation of the substrate Zap-70 was caused exclusively by FynT. Cells were also transfected with cDNAs encoding Csk alone (either wild-type or R107K), PTP-HSCF alone (either wild-type or Y359F-Y381F), or both, and tyrosine phosphorylation of Zap-70 was monitored by immunoblotting of total cell lysates with antiphosphotyrosine antibodies (Fig. 7A, first panel). This analysis showed that wild-type Csk alone (lane 2) or wild-type PTP-HSCF alone (lane 4) provoked only a small reduction in substrate tyrosine phosphorylation. Similar results were obtained with expression of R107K Csk (lane 3) or Y359F-Y381F PTP-HSCF (lane 5). By contrast, in cells expressing both wild-type Csk and wild-type PTP-HSCF (lane 6), there was a marked decrease in the phosphotyrosine content of Zap-70. The inhibitory impact of Csk and PTP-HSCF combined was partially alleviated by mutation of either the SH2 domain of Csk (lane 8) or the two sites of tyrosine phosphorylation of PTP-HSCF (lane 7). More strikingly, it was nearly eliminated when both molecules were mutated (lane 9). Parallel immunoblots with antibodies directed against Csk (second panel), PTP-HSCF (third panel), Fyn (fourth panel), Zap-70 (fifth panel), and ζ (sixth panel) confirmed that all polypeptides were adequately expressed in this experiment.
FIG. 7.
Cooperative inhibition of Src kinase-mediated signaling by Csk and PTP-HSCF. (A) Cos-1 cells were transfected with cDNAs coding for Tac-ζ, FynT, and kinase-inactive Zap-70 (K295R Zap-70), in the absence (−) or presence (+) of the indicated cDNAs. Tyrosine phosphorylation of Zap-70 was monitored by immunoblotting of total cell lysates with antiphosphotyrosine (αP. tyr) antibodies (first panel). Similar results were obtained when Zap-70 was isolated from cell lysates by immunoprecipitation (data not shown). The positions of Zap-70, Csk, PTP-HSCF, FynT, and Tac-ζ are shown on the left. wt, wild type. Exposures in the first to sixth panels were 48, 15, 5, 10, 15, and 10 h, respectively. (B) Immune complex phosphatase assays. The activities of various PTP-HSCF polypeptides were measured in an immune complex kinase assay, as outlined in Materials and Methods. The reactions were conducted for various periods of time (abscissa), and 32P-labeled myelin basic protein was used as substrate. The amount of radioactivity released in the medium is shown on the ordinate (in counts per minute [CPM]). The abundance of the PTP-HSCF proteins was verified by immunoblotting of parallel PTP-HSCF immunoprecipitates with anti-PTP-HSCF antibodies (top). Exposure, 3 h. Symbols: ○, empty vector; ×, C229S PTP-HSCF; ■, Y354F-Y381F PTP-HSCF; ▴, wild-type PTP-HSCF.
For proper interpretation of this experiment, it was important to ensure that the enzymatic activity of Csk and PTP-HSCF was not affected by these mutations. Along these lines, previous studies had shown that the kinase activity of Csk was not altered by the R107K mutation (10, 34). However, we also had to verify that the Y354F-Y381F mutations did not reduce the phosphatase activity of PTP-HSCF. To eliminate this possibility, immune complex phosphatase assays were performed as detailed in Materials and Methods, using myelin basic protein as an exogenous substrate (Fig. 7B). This study showed that the catalytic activity of Y354F-Y381F PTP-HSCF was similar to that of wild-type PTP-HSCF. In comparison, the enzymatic function of C229S PTP-HSCF was abrogated. Therefore, we could deduce that Csk and PTP-HSCF cooperated to inhibit signalling events triggered by Src-related PTKs. While some small degree of cooperation between these two molecules could take place in the absence of the domains mediating their association (Fig. 7, first panel, compare lane 9 with lane 1), it was clear that their synergism was largely dependent on their capacity to associate physically (lane 6).
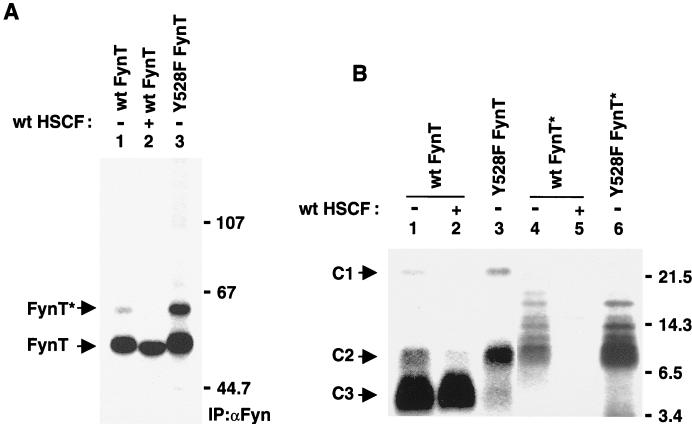
In order to comprehend the mechanism by which PTP-HSCF synergized with Csk, the possibility that it regulated the state of tyrosine phosphorylation of Src-related enzymes was tested (Fig. 8). Cos-1 cells were transfected as outlined for Fig. 7A, in the absence or in the presence of a wild-type ptp-hscf cDNA. Later, they were metabolically labeled with 32Pi, and lysed, and FynT polypeptides were recovered by immunoprecipitation with anti-Fyn antibodies. The state of phosphorylation of FynT was examined by gel electrophoresis (Fig. 8A). Although overexpression of PTP-HSCF did not reduce the overall phosphorylation of FynT (compare lane 2 with lane 1), it provoked a reproducible increase in the electrophoretic mobility of FynT. Additionally, PTP-HSCF caused the disappearance of FynT∗, an activated version of FynT previously found to be phosphorylated at the positive regulatory site, Y417 (12).
FIG. 8.
Impact of PTP-HSCF on FynT phosphorylation. (A) Overall FynT phosphorylation. Cos-1 cells were transfected as outlined in the legend of Fig. 7A, in the absence or in the presence of wild-type (wt) PTP-HSCF. Cells were subsequently metabolically labeled with 32Pi, and the extent of FynT phosphorylation was evaluated by immunoprecipitation with anti-Fyn antibodies and gel electrophoresis. Cells transfected with a constitutively activated form of FynT (Y528F FynT) were used as control. This mutant is highly phosphorylated at the positive regulatory site, tyrosine 417, while lacking phosphorylation at the inhibitory site, tyrosine 528. The migrations of FynT and FynT∗ are indicated on the left, whereas those of prestained molecular mass markers (in kilodaltons) are shown on the right. Exposure, 3 h. (B) Cyanogen bromide cleavage. The phosphorylated polypeptides from panel A were subjected to cleavage with cyanogen bromide. The products of these reactions were then separated in 18% SDS-polyacrylamide gels and detected by autoradiography. The positions of C1, C2, and C3 are indicated on the left; those of prestained molecular mass markers (in kilodaltons) are shown on the right. Exposure: lanes 1 to 3, 8 h; lanes 4 to 6, 60 h.
In light of these observations, FynT phosphorylation was investigated in greater detail through peptide mapping studies (Fig. 8B). The phosphorylated products detected in Fig. 8A were gel purified, cleaved with cyanogen bromide, and resolved in 18% SDS-polyacrylamide gels. This analysis demonstrated that PTP-HSCF had no appreciable impact on phosphorylation of the C3 fragment of FynT (compare lane 2 with lane 1). This peptide bears Y528, the inhibitory carboxy-terminal tyrosine phosphorylated by Csk. Nonetheless, PTP-HSCF provoked a significant reduction in the extent of phosphorylation of the C2 fragment, which contains the positive regulatory site Y417. A decrease in the phosphate content of the amino-terminal C1 fragment of FynT was also noted in this experiment. However, it was not observed in other experiments (data not shown). The significance of this finding is not clear. Lastly, we observed that FynT∗ polypeptides isolated from cells lacking PTP-HSCF (Fig. 8A, lane 4) were predominantly phosphorylated within the C2 fragment. This phosphorylation was absent in cells expressing PTP-HSCF (lane 5). On the basis of these results, we concluded that PTP-HSCF caused dephosphorylation of Y417, but not Y528, of FynT in cells.
DISCUSSION
Earlier studies established that the ability of Csk to inhibit Src-related PTKs in vivo requires intact Csk SH3 and SH2 domains (10, 22). We subsequently reported that the SH3 domain of Csk mediates highly specific and constitutive interactions with the nonreceptor PTPs PEP and PTP-PEST (11, 13, 21). In the case of Csk-PEP, this association was shown to augment the capacity of Csk to inactivate Src kinases, by way of the ability of associated PEP to dephosphorylate the activating tyrosine and, possibly, substrates of Src-related enzymes (12). Several groups also documented that the SH2 domain of Csk can interact with various tyrosine phosphorylated proteins, including Dok-related molecules, PAG, paxillin, and tensin (5, 10, 25, 30, 34). While the functions of these associations have not been firmly established, they likely mediate the recruitment of Csk to foci of Src-related kinase activation in the cell.
Herein, we found that Csk also associates with PTP-HSCF, the third known member of the PEP family (3, 6, 18, 23, 26). This interaction was documented in yeast, in a heterologous mammalian cell system, and in primitive hemopoietic cells. Unlike the previously described Csk-PEP and Csk-PTP-PEST interactions, the association between Csk and PTP-HSCF involved the Csk SH2 domain and conserved tyrosines (Y354 and Y381) in the carboxy-terminal noncatalytic region of PTP-HSCF. Transfection studies revealed that Csk and PTP-HSCF cooperated to inhibit signaling events initiated by Src-related PTKs, and that this synergy was greatly facilitated by the domains mediating their association. Finally, evidence was adduced that the inhibitory impact of PTP-HSCF was due at least in part to its capacity to prevent phosphorylation of the positive regulatory tyrosine of Src family kinases.
These findings led to the identification of a novel function for the Csk SH2 domain, distinct from its aforementioned role in recruiting Csk to sites of Src-related PTK activation. By binding the PTP-HSCF, the SH2 region enhanced the capacity of Csk to inhibit Src-related PTKs. This synergism was seemingly consequent to the ability of PTP-HSCF to dephosphorylate the positive regulatory site of Src kinases, thereby ideally complementing the capacity of Csk to phosphorylate their inhibitory carboxy-terminal tyrosine. Such a mechanism does not exclude the possibility that PTP-HSCF also served the purpose of recruiting Csk near activated Src-related molecules. In fact, this is likely to be the case, as tyrosine phosphorylation of PTP-HSCF is triggered by activated Src kinases (this report) (38). Hence, binding of Csk to tyrosine phosphorylated PTP-HSCF probably fulfils two complementary goals: juxtaposition of Csk near activated Src kinases by physical recruitment and enhancement of the inhibitory potential of Csk by a catalytic mechanism.
Obviously, the interaction of Csk with PTP-HSCF is evocative of its association with PEP and PTP-PEST. One important difference, however, is that the binding to PEP and PTP-PEST is mediated by the SH3 domain of Csk and is constitutive (11, 13, 21). By opposition, the association of Csk with PTP-HSCF was found to be SH2 domain mediated and reversible. It could be implied from this distinction that the inhibitory signal transduced by Csk-PTP-HSCF is qualitatively different from that triggered by Csk-PEP and Csk-PTP-PEST. Whereas this notion remains plausible, our studies failed to produce any evidence supporting this notion. Rather, the SH2 domain- and SH3 domain-dependent associations of Csk with PTPs seem to result in functionally analogous inhibitory mechanisms. The capacity of PTP-HSCF (this report) and PEP (12) to inactivate Src kinases through a similar biochemical effect is in keeping with this proposition. The phosphotyrosine-dependence of the Csk-PTP-HSCF association may simply indicate that it is of shorter duration, as the complex would dissociate once the activity of the Src kinases is repressed and tyrosine phosphorylation of PTP-HSCF has subsided. Consequently, inhibition by Csk-PTP-HSCF may be more finely tuned to the degree of activation of Src family PTKs.
Our data revealed that the presence of Csk also favored the tyrosine phosphorylation of PTP-HSCF. Interestingly, this effect was found to be independent of the catalytic activity of Csk. Rather, it required the presence of an intact Csk SH2 domain. Binding of the Csk SH2 domain probably stabilized tyrosine phosphorylation of PTP-HSCF, by shielding the phosphotyrosines from dephosphorylation by PTP-HSCF and/or other cellular PTPs. A similar situation has been documented for other SH2 domains (20, 33). Through this mechanism, the association of Csk with PTP-HSCF would trigger an amplifying loop that augments Csk recruitment and allows a more sustained inhibitory response to take place. It is likely that this feature is a common and advantageous consequence of SH2 domain-mediated interactions.
Since tyrosine phosphorylation of PTP-HSCF was difficult to detect in the absence of PTP inhibition, one could argue that such a phosphorylation does not occur in a physiologically meaningful way. However, several findings indicated that this is unlikely to be the case. First, tyrosine phosphorylation of PTP-HSCF was highly specific, as we were unable to detect a similar modification of PEP or PTP-PEST (our unpublished results). Second, tyrosine phosphorylation of PTP-HSCF could be detected in the absence of PTP inhibition, such as when the levels of Csk were increased in the cell (Fig. 5). Importantly, this phosphorylation occurred at the sites that were also phosphorylated under conditions of PTP inhibition (our unpublished results). And third, the putative sites of tyrosine phosphorylation of PTP-HSCF were essential for the ability of wild-type PTP-HSCF and Csk to cooperate towards inhibiting Src-related PTKs. Thus, tyrosine phosphorylation of PTP-HSCF is very likely to occur in cells, albeit transiently, and to be a biologically significant event.
The results of our studies allowed the identification of two potential binding motifs for the Csk SH2 domain: Y354AVV and Y381SQV of PTP-HSCF. It was previously demonstrated that the sequence Y314SSV in PAG was also recognized by the SH2 domain of Csk (5, 25). These three sequences are in clear agreement with the motif pY(T/A/S)X(M/I/V) (where pY is phosphotyrosine and X is any residue), which has been selected as the preferential Csk SH2 domain-binding sequence in a peptide library (37). Hence, it is probable that the peptide library-derived motif represents a predominant Csk SH2 domain-binding sequence in vivo. Obviously, it will be of interest to see whether other Csk SH2 region-binding proteins, such as Dok-related molecules, paxillin, tensin, and Fak, associate with Csk through a similar decoy.
Chk is a Csk-related molecule selectively expressed in hemopoietic cells and brain (9). Interestingly, we found herein that Chk did not bind to PTP-HSCF. Likewise, it was reported earlier that Chk was incapable of associating with PEP and PTP-PEST (11, 13, 21). Therefore, the ability to interact physically with the PEP family of PTPs appears to be restricted to Csk. Even though the biological significance of this distinction remains to be fully elucidated, it is noteworthy that Csk and Chk do seem to have dissimilar biological roles. In support of this idea, it was found that Chk was an inefficient negative regulator of antigen receptor signaling in T-cells, in striking contrast to Csk (15). The inability of Chk to interact with PEP-related phosphatases may explain at least part of this functional difference.
In combination with previously published findings (11, 13, 21), the results reported here reveal that Csk associates with all three members of the PEP family. This is probably more than a coincidence. As there is no conclusive evidence that Csk interacts with other PTPs (our unpublished results), this observation strongly argues for a unique functional affinity between the two classes of molecules. Very possibly, their alliance has evolved from their shared capacity to inhibit Src-related PTKs. As a corollary, the selective nature of the association of Csk with PEP family members suggests that PEP-related PTPs play a significant role in the regulation of Src-related PTKs in vivo. Along these lines, it should be mentioned that, with the exception of the receptor-like PTP CD45 and members of the PEP family (12, 17; this report), little is known of the PTPs responsible for inactivating Src kinases in mammalian cells. In light of our data, it seems probable that PEP-related molecules play a pivotal role in this process. If this is the case, it will be interesting to determine whether the three distinct types of Csk-PTP complexes have independent functions. Our studies to date suggest that the Csk-PEP and Csk-PTP-HSCF complexes are capable of similar inhibitory effects on Src kinases. However, taking into consideration the reported differences in the intracellular localization of PEP, PTP-PEST, and, possibly, PTP-HSCF (11, 13, 18, 23), these three complexes may be aimed at inhibiting separate cellular pools of Src-related kinases. Future studies will be needed to address this possibility.
ACKNOWLEDGMENTS
We thank the members of our laboratory for useful discussions. We also acknowledge M. Lioubin, L. Rohrschneider, and T. Pawson for gifts of reagents.
This work was supported by grants from the National Cancer Institute of Canada and the Canadian Institutes of Health Research to A.V. S.L. was supported by a fellowship from the Kidney Foundation of Canada and by a Joseph Kaufmann Fellowship from the Faculty of Medicine, McGill University, while A.V. is a Senior Scientist of the Canadian Institutes of Health Research.
REFERENCES
- 1.Abraham N, Miceli M C, Parnes J R, Veillette A. Enhancement of T-cell responsiveness by the lymphocyte-specific tyrosine protein kinase p56lck. Nature. 1991;350:62–66. doi: 10.1038/350062a0. [DOI] [PubMed] [Google Scholar]
- 2.Abram C L, Courtneidge S A. Src family tyrosine kinases and growth factor signaling. Exp Cell Res. 2000;254:1–13. doi: 10.1006/excr.1999.4732. [DOI] [PubMed] [Google Scholar]
- 3.Aoki N, Yamaguchi-Aoki Y, Ullrich A. The novel protein-tyrosine phosphatase PTP20 is a positive regulator of PC12 cell neuronal differentiation. J Biol Chem. 1996;271:29422–29426. doi: 10.1074/jbc.271.46.29422. [DOI] [PubMed] [Google Scholar]
- 4.Bergman M, Joukov V, Virtanen I, Alitalo K. Overexpressed Csk tyrosine kinase is localized in focal adhesions, causes reorganization of alpha v beta 5 integrin, and interferes with HeLa cell spreading. Mol Cell Biol. 1995;15:711–722. doi: 10.1128/mcb.15.2.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brdicka T, Pavlistova D, Leo A, Bruyns E, Korinek V, Angelisova P, Scherer J, Shevchenko A, Hilgert I, Cerny J, Drbal K, Kuramitsu Y, Kornacker B, Horejsi V, Schraven B. Phosphoprotein associated with glycosphingolipid-enriched microdomains (PAG), a novel ubiquitously expressed transmembrane adaptor protein, binds the protein tyrosine kinase csk and is involved in regulation of T cell activation. J Exp Med. 2000;191:1591–1604. doi: 10.1084/jem.191.9.1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cheng J, Daimaru L, Fennie C, Lasky L A. A novel protein tyrosine phosphatase expressed in lin(lo)CD34(hi)Sca(hi) hematopoietic progenitor cells. Blood. 1996;88:1156–1167. [PubMed] [Google Scholar]
- 7.Chow L M, Davidson D, Fournel M, Gosselin P, Lemieux S, Lyu M S, Kozak C A, Matis L A, Veillette A. Two distinct protein isoforms are encoded by ntk, a csk-related tyrosine protein kinase gene. Oncogene. 1994;9:3437–3448. [PubMed] [Google Scholar]
- 8.Chow L M, Fournel M, Davidson D, Veillette A. Negative regulation of T-cell receptor signalling by tyrosine protein kinase p50csk. Nature. 1993;365:156–160. doi: 10.1038/365156a0. [DOI] [PubMed] [Google Scholar]
- 9.Chow L M, Veillette A. The Src and Csk families of tyrosine protein kinases in hemopoietic cells. Semin Immunol. 1995;7:207–226. doi: 10.1006/smim.1995.0026. [DOI] [PubMed] [Google Scholar]
- 10.Cloutier J F, Chow L M, Veillette A. Requirement of the SH3 and SH2 domains for the inhibitory function of tyrosine protein kinase p50csk in T lymphocytes. Mol Cell Biol. 1995;15:5937–5944. doi: 10.1128/mcb.15.11.5937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cloutier J F, Veillette A. Association of inhibitory tyrosine protein kinase p50csk with protein tyrosine phosphatase PEP in T cells and other hemopoietic cells. EMBO J. 1996;15:4909–4918. [PMC free article] [PubMed] [Google Scholar]
- 12.Cloutier J F, Veillette A. Cooperative inhibition of T-cell antigen receptor signaling by a complex between a kinase and a phosphatase. J Exp Med. 1999;189:111–121. doi: 10.1084/jem.189.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Davidson D, Cloutier J F, Gregorieff A, Veillette A. Inhibitory tyrosine protein kinase p50csk is associated with protein-tyrosine phosphatase PTP-PEST in hemopoietic and non-hemopoietic cells. J Biol Chem. 1997;272:23455–23462. doi: 10.1074/jbc.272.37.23455. [DOI] [PubMed] [Google Scholar]
- 14.Davidson D, Chow L M, Fournel M, Veillette A. Differential regulation of T cell antigen responsiveness by isoforms of the src-related tyrosine protein kinase p59fyn. J Exp Med. 1992;175:1483–1492. doi: 10.1084/jem.175.6.1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Davidson D, Chow L M, Veillette A. Chk, a Csk family tyrosine protein kinase, exhibits Csk-like activity in fibroblasts, but not in an antigen-specific T-cell line. J Biol Chem. 1997;272:1355–1362. doi: 10.1074/jbc.272.2.1355. [DOI] [PubMed] [Google Scholar]
- 16.Di Cristofano A, Carpino N, Dunant N, Friedland G, Kobayashi R, Strife A, Wisniewski D, Clarkson B, Pandolfi P P, Resh M D. Molecular cloning and characterization of p56dok-2 defines a new family of RasGAP-binding proteins. J Biol Chem. 1998;273:4827–4830. doi: 10.1074/jbc.273.9.4827. [DOI] [PubMed] [Google Scholar]
- 17.D'Oro U, Sakaguchi K, Appella E, Ashwell J D. Mutational analysis of Lck in CD45-negative T cells: dominant role of tyrosine 394 phosphorylation in kinase activity. Mol Cell Biol. 1996;16:4996–5003. doi: 10.1128/mcb.16.9.4996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dosil M, Leibman N, Lemischka I R. Cloning and characterization of fetal liver phosphatase 1, a nuclear protein tyrosine phosphatase isolated from hematopoietic stem cells. Blood. 1996;88:4510–4525. [PubMed] [Google Scholar]
- 19.Fournel M, Davidson D, Weil R, Veillette A. Association of tyrosine protein kinase Zap-70 with the protooncogene product p120c-cbl in T lymphocytes. J Exp Med. 1996;183:301–306. doi: 10.1084/jem.183.1.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gervais F G, Chow L M, Lee J M, Branton P E, Veillette A. The SH2 domain is required for stable phosphorylation of p56lck at tyrosine 505, the negative regulatory site. Mol Cell Biol. 1993;13:7112–7121. doi: 10.1128/mcb.13.11.7112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gregorieff A, Cloutier J F, Veillette A. Sequence requirements for association of protein-tyrosine phosphatase PEP with the Src homology 3 domain of inhibitory tyrosine protein kinase p50csk. J Biol Chem. 1998;273:13217–13222. doi: 10.1074/jbc.273.21.13217. [DOI] [PubMed] [Google Scholar]
- 22.Howell B W, Cooper J A. Csk suppression of Src involves movement of Csk to sites of Src activity. Mol Cell Biol. 1994;14:5402–5411. doi: 10.1128/mcb.14.8.5402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huang K, Sommers C L, Grinberg A, Kozak C A, Love P E. Cloning and characterization of PTP-K1, a novel nonreceptor protein tyrosine phosphatase highly expressed in bone marrow. Oncogene. 1996;13:1567–1573. [PubMed] [Google Scholar]
- 24.Imamoto A, Soriano P. Disruption of the csk gene, encoding a negative regulator of Src family tyrosine kinases, leads to neural tube defects and embryonic lethality in mice. Cell. 1993;73:1117–1124. doi: 10.1016/0092-8674(93)90641-3. [DOI] [PubMed] [Google Scholar]
- 25.Kawabuchi M, Satomi Y, Takao T, Shimonishi Y, Nada S, Nagai K, Tarakhovsky A, Okada M. Transmembrane phosphoprotein Cbp regulates the activities of Src-family tyrosine kinases. Nature. 2000;404:999–1003. doi: 10.1038/35010121. [DOI] [PubMed] [Google Scholar]
- 26.Kim Y W, Wang H, Sures I, Lammers R, Martell K J, Ullrich A. Characterization of the PEST family protein tyrosine phosphatase BDP1. Oncogene. 1996;13:2275–2279. [PubMed] [Google Scholar]
- 27.Lemay S, Davidson D, Latour S, Veillette A. Dok-3, a novel adapter molecule involved in the negative regulation of immunoreceptor signaling. Mol Cell Biol. 2000;20:2743–2754. doi: 10.1128/mcb.20.8.2743-2754.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lioubin M N, Algate P A, Tsai S, Carlberg K, Aebersold A, Rohrschneider L R. p150Ship, a signal transduction molecule with inositol polyphosphate-5-phosphatase activity. Genes Dev. 1996;10:1084–1095. doi: 10.1101/gad.10.9.1084. [DOI] [PubMed] [Google Scholar]
- 29.Nada S, Yagi T, Takeda H, Tokunaga T, Nakagawa H, Ikawa Y, Okada M, Aizawa S. Constitutive activation of Src family kinases in mouse embryos that lack Csk. Cell. 1993;73:1125–1135. doi: 10.1016/0092-8674(93)90642-4. [DOI] [PubMed] [Google Scholar]
- 30.Neet K, Hunter T. The nonreceptor protein-tyrosine kinase CSK complexes directly with the GTPase-activating protein-associated p62 protein in cells expressing v-Src or activated c-Src. Mol Cell Biol. 1995;15:4908–4920. doi: 10.1128/mcb.15.9.4908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Parsons J T, Parsons S J. Src family protein tyrosine kinases: cooperating with growth factor and adhesion signaling pathways. Curr Opin Cell Biol. 1997;9:187–192. doi: 10.1016/s0955-0674(97)80062-2. [DOI] [PubMed] [Google Scholar]
- 32.Peri K G, Gervais F G, Weil R, Davidson D, Gish G D, Veillette A. Interactions of the SH2 domain of lymphocyte-specific tyrosine protein kinase p56lck with phosphotyrosine-containing proteins. Oncogene. 1993;8:2765–2772. [PubMed] [Google Scholar]
- 33.Rotin D, Margolis B, Mohammadi M, Daly R J, Daum G, Li N, Fischer E H, Burgess W H, Ullrich A, Schlessinger J. SH2 domains prevent tyrosine dephosphorylation of the EGF receptor: identification of Tyr992 as the high-affinity binding site for SH2 domains of phospholipase C gamma. EMBO J. 1992;11:559–567. doi: 10.1002/j.1460-2075.1992.tb05087.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sabe H, Hata A, Okada M, Nakagawa H, Hanafusa H. Analysis of the binding of the Src homology 2 domain of Csk to tyrosine-phosphorylated proteins in the suppression and mitotic activation of c-Src. Proc Natl Acad Sci USA. 1994;91:3984–3988. doi: 10.1073/pnas.91.9.3984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schmedt C, Saijo K, Niidome T, Kuhn R, Aizawa S, Tarakhovsky A. Csk controls antigen receptor-mediated development and selection of T-lineage cells. Nature. 1998;394:901–904. doi: 10.1038/29802. [DOI] [PubMed] [Google Scholar]
- 36.Sicheri F, Kuriyan J. Structures of Src-family tyrosine kinases. Curr Opin Struct Biol. 1997;7:777–785. doi: 10.1016/s0959-440x(97)80146-7. [DOI] [PubMed] [Google Scholar]
- 37.Songyang Z, Shoelson S E, McGlade J, Olivier P, Pawson T, Bustelo X R, Barbacid M, Sabe H, Hanafusa H, Yi T. Specific motifs recognized by the SH2 domains of Csk, 3BP2, fps/fes, GRB-2, HCP, SHC, Syk, and Vav. Mol Cell Biol. 1994;14:2777–2785. doi: 10.1128/mcb.14.4.2777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Spencer S, Dowbenko D, Cheng J, Li W, Brush J, Utzig S, Simanis V, Lasky L A. PSTPIP: a tyrosine phosphorylated cleavage furrow-associated protein that is a substrate for a PEST tyrosine phosphatase. J Cell Biol. 1997;138:845–860. doi: 10.1083/jcb.138.4.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thomas S M, Brugge J S. Cellular functions regulated by Src family kinases. Annu Rev Cell Dev Biol. 1997;13:513–609. doi: 10.1146/annurev.cellbio.13.1.513. [DOI] [PubMed] [Google Scholar]
- 40.Tsai S, Bartelmez S, Sitnicka E, Collins S. Lymphohematopoietic progenitors immortalized by a retroviral vector harboring a dominant-negative retinoic acid receptor can recapitulate lymphoid, myeloid, and erythroid development. Genes Dev. 1994;8:2831–2841. doi: 10.1101/gad.8.23.2831. [DOI] [PubMed] [Google Scholar]
- 41.Veillette A, Bookman M A, Horak E M, Bolen J B. The CD4 and CD8 T cell surface antigens are associated with the internal membrane tyrosine-protein kinase p56lck. Cell. 1988;55:301–308. doi: 10.1016/0092-8674(88)90053-0. [DOI] [PubMed] [Google Scholar]
- 42.Veillette A, Horak I D, Bolen J B. Post-translational alterations of the tyrosine kinase p56lck in response to activators of protein kinase C. Oncogene Res. 1988;2:385–401. [PubMed] [Google Scholar]
- 43.Veillette A, Thibaudeau E, Latour S. High expression of inhibitory receptor SHPS-1 and its association with protein-tyrosine phosphatase SHP-1 in macrophages. J Biol Chem. 1998;273:22719–22728. doi: 10.1074/jbc.273.35.22719. [DOI] [PubMed] [Google Scholar]