Abstract
Charcot–Marie–Tooth disease (CMT) and autosomal recessive spastic ataxia of Charlevoix–Saguenay type (ARSACS) are large heterogeneous groups of sensory, neurological genetic disorders characterized by sensory neuropathies, muscular atrophies, abnormal sensory conduction velocities, and ataxia. CMT2EE (OMIM: 618400) is caused by mutations in MPV17 (OMIM: 137960), CMT4F (OMIM: 614895) is caused by PRX (OMIM: 605725), CMTX1 (OMIM: 302800) is caused by mutations in GJB1 (OMIM: 304040), and ARSACS (OMIM: 270550) is caused by mutations in SACS (OMIM: 604490). In this study, we enrolled four families: DG-01, BD-06, MR-01, and ICP-RD11, with 16 affected individuals, for clinical and molecular diagnoses. One patient from each family was analyzed for whole exome sequencing and Sanger sequencing was done for the rest of the family members. Affected individuals of families BD-06 and MR-01 show complete CMT phenotypes and family ICP-RD11 shows ARSACS type. Family DG-01 shows complete phenotypes for both CMT and ARSACS types. The affected individuals have walking difficulties, ataxia, distal limb weakness, axonal sensorimotor neuropathies, delayed motor development, pes cavus, and speech articulations with minor variations. The WES analysis in an indexed patient of family DG-01 identified two novel variants: c.83G>T (p.Gly28Val) in MPV17 and c.4934G>C (p.Arg1645Pro) in SACS. In family ICP-RD11, a recurrent mutation that causes ARSACS, c.262C>T (p.Arg88Ter) in SACS, was identified. Another novel variant, c.231C>A (p.Arg77Ter) in PRX, which causes CMT4F, was identified in family BD-06. In family MR-01, a hemizygous missense variant c.61G>C (p.Gly21Arg) in GJB1 was identified in the indexed patient. To the best of our knowledge, there are very few reports on MPV17, SACS, PRX, and GJB1 causing CMT and ARSACS phenotypes in the Pakistani population. Our study cohort suggests that whole exome sequencing can be a useful tool in diagnosing complex multigenic and phenotypically overlapping genetic disorders such as Charcot–Marie–Tooth disease (CMT) and spastic ataxia of Charlevoix–Saguenay type.
Keywords: MPV17, PRX, GJB1, SACS, CMT, ARSACS, WES
1. Introduction
Charcot–Marie–Tooth disease (CMT), or hereditary motor and sensory neuropathy (HMSN), was first reported in 1886 by three neurologists, Jean-Martin Charcot, Pierre Marie, and Howard Henry Tooth, and was referred to as peroneal muscular atrophy (PMA) [1,2]. With an estimated prevalence of 1 in 2500 worldwide, it is one of the most common forms of a heterogeneous group of genetic neuropathies that are characterized by abnormalities in the peripheral and central nervous system [3,4,5]. It typically appears in the first two decades of life, and the majority of patients with CMT have an autosomal dominant (AD) inheritance, but many have autosomal recessive (AR) or X-linked inheritance [6]. The disease results in a slowly progressive and length-dependent degeneration of the peripheral nerves, causing muscle weakness, numbness, reduced tendon reflexes, atrophy in the feet and legs (which later extends to the hands), and slight to moderate distal sensory impairment [7]. Patients may also exhibit hip dysplasia, learning loss, and foot deformities such as pes cavus (high-arched foot deformity) [3,8,9]. In addition to these and other symptoms (Table 1) that vary depending on the different CMT subtypes, the disease can greatly decrease the quality of life of those affected [10,11].
Table 1.
Different phenotypes have been reported in literature related to three types of Charcot–Marie–Tooth disease and autosomal recessive spastic ataxia of Charlevoix–Saguenay type. These have been compared with the four gene mutations we have reported.
| S/No. | Phenotypes with References Respective to the Disorder | CMT 2EE | CMT 4F | CMT X1 | SACS | DG-01 | BD-06 | MR-01 | DG-01 | ICP_RD11 |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Sensorineural hearing loss [12,13] | + | - | + | - | + | + | - | + | - |
| 2 | Nystagmus [13] | - | - | + | + | - | - | - | - | - |
| 3 | Achilles tendon contractures [13] | - | - | + | - | - | - | + | - | - |
| 4 | Absent ankle jerk [13,14] | - | - | + | + | - | - | + | + | + |
| 5 | Distal upper limb weakness [13,14] | + | - | + | + | + | + | + | + | + |
| 6 | Hand tremor [15,16] | - | - | + | + | + | + | + | + | + |
| 7 | Clawed hands [17,18] | + | + | - | - | - | + | - | - | - |
| 8 | Muscle biopsy showed neurogenic changes [13] | - | - | + | - | n/a | n/a | n/a | n/a | n/a |
| 9 | Numbness of hands and legs [16] | - | - | + | - | - | - | + | - | - |
| 10 | Delayed motor development [13,19,20] | - | + | + | + | + | + | + | + | + |
| 11 | Distal limb muscle atrophy [19,21,22,23] | + | + | + | - | - | + | - | - | + |
| 12 | Axonal sensorimotor neuropathies [24,25,26] | + | + | + | + | + | + | + | + | + |
| 13 | Hyporeflexia [27,28] | + | - | + | - | + | - | + | + | - |
| 14 | Areflexia [29] | + | + | - | - | + | + | - | + | + |
| 15 | Ataxia [16,24,30] | + | + | + | + | + | + | + | + | + |
| 16 | Walking difficulties [24,25,27] | + | + | + | + | + | + | + | + | + |
| 17 | Toe walking [13] | - | - | + | - | - | + | + | - | - |
| 18 | Pes cavus [14,21,31] | + | + | + | + | + | + | + | + | + |
| 19 | Clawed toe [17] | + | - | - | - | - | + | - | - | - |
| 20 | Foot drop [17] | + | - | - | - | - | - | - | - | - |
| 21 | Scoliosis [14,19] | + | + | - | + | + | + | - | + | + |
| 22 | Intellectual disabilities [26,32] | + | + | + | + | + | - | + | + | + |
| 23 | Speech articulation [16,30] | + | + | + | + | + | + | + | + | + |
| 24 | Decreased COX reactivity [17] | + | - | - | - | n/a | n/a | n/a | n/a | n/a |
| 25 | Reported causative genes | MPV17 | PRX | GJB1 | SACS | MPV17 | PRX | GJB1 | SACS | SACS |
CMT is classified into six main types: CMT1, CMT2, CMT3, CMT4, CMTX, and I-CMT (DI-CMT and RI-CMT), which are further subdivided into subtypes (Supplementary Table S1). Molecular analysis has identified over 50 genes involved in these 76 subvarieties of the disease, which control specific functions in sub-neurons or Schwann cells, such as signaling, transduction, and nerve impulse propagation.
In our present clinical and molecular diagnosis study, we have identified four families from the Pakhtun ethnic group in Pakistan affected with CMT2EE, CMT4F, CMTX, and ARSACS.
2. Method
2.1. Samples
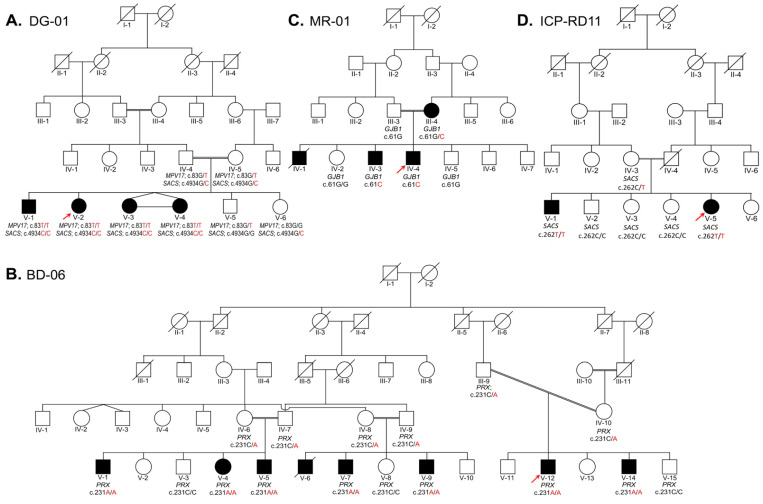
All four families were enrolled from the Pakhtun ethnic group of Khyber Pakhtunkhwa province of Pakistan. The pedigrees construction and analysis were conducted in light of standard protocol [33]. Family DG-01 (Figure 1A) segregates two conditions: autosomal recessive CMT and autosomal recessive ARSACS. Family BD-06 (Figure 1B) segregates autosomal recessive CMT. Family MR-01 (Figure 1C) segregates X-linked dominant CMT. Family ICP-RD11 (Figure 1D) segregates autosomal recessive ARSACS. Each study participant provided peripheral blood samples, which were collected and stored in 5 mL EDTA tubes.
Figure 1.
Four families designated as DG-01 (A), BD-06 (B), MR-01 (C), and ICP-RD11 (D) are presented here. Family (A) segregates two conditions: autosomal recessive CMT type 2EE and autosomal recessive ARSACS. Family (B) segregates autosomal recessive CMT Type 4F, while family (C) segregates X-linked dominant CMT. Family (D) segregates autosomal recessive ARSACS. In each family, the index patient is indicated with an arrow. The genotypes of the family members are written below their symbols, whose samples were available for Sanger sequencing. (A) DG-01: In the five-generation pedigree, the four affected siblings (V-1, V-2, V-3, and V-4) are homozygous mutants for both MPV17 c.83G>T and SACS c.4934G>C, while one unaffected sibling (V-5) is homozygous wild type for MPV17, and another unaffected sibling (V-6) is homozygous wild type for SACS. Their parents (IV-4 and IV-5) are heterozygous carriers for MPV17 c.83G>T and SACS c.4934G>C. (B) BD-06: A five-generation pedigree presents the disease in three different clades. Each clade was confirmed for the variant in PRX, and Sanger sequencing was carried out and confirmed that affected individuals (V-1, V-4, V-5, V-7, V-9, V-12, and V-14) are homozygous mutants, while parents (IV-6, IV-7, IV-8, IV-9, III-9, and IV-10) are heterozygous carriers, and normal siblings (V-3, V-7, V-15) are homozygous wild type for PRX c.231C>A. (C) MR-01: In a four-generation pedigree, the two affected siblings (IV-3 and IV-4) are hemizygous mutants, while one unaffected sibling (IV-5) and the father (III-3) are homozygous wild type, and the mother (III-4) is a heterozygous carrier for GJB1: c.61G>C variant. (D) ICP-RD11: In a five-generation pedigree, the two affected siblings (V-1, V-5) are homozygous for the mutant allele, while three unaffected siblings (V-2, V-3, and V-4) are homozygous for the wild type allele and their mother (IV-3) is heterozygous carrier for the SACS c.262C>T variant.
2.2. Genomic DNA Extraction and Genetic Analysis
Genomic DNA was extracted from all available family members’ blood using PureLinked® genomic extraction kits (Thermo Fisher, Waltham, MA, USA). DNA quantity and quality were analyzed using a Nanodrop 1000 spectrophotometer (Thermo Fisher, Waltham, USA). WES was planned for the index patients in each family for the genes involved in axonal motor sensory neuropathies (Supplementary Table S1). The exome, which covers about 22,000 disease-causing human genes, was captured using xGen Exome Research Panel v2 (Integrated DNA Technologies, Coralville, IA, USA) [34,35]. The captured exome was sequenced using NovaSeq 6000 (Illumina, San Diego, CA, USA) with a mean depth of coverage of 212.96. The sequencing data were aligned to the human genome database GRCh37 and the human mitochondrial genome reference database rCRS. The alignment covered approximately 99.3% of the RefSeq protein coding region. An in-house software, EVIDENCE, was used for variant interpretation and prioritization based on patient phenotypes, family history, and clinical diagnosis in accordance with the guidelines of the American College of Medical Genetics and Genomics (ACMG) and the Association for Molecular Pathology (AMP) [34,35,36]. The pathogenicity and segregation of the identified variants were then confirmed by sequencing selected affected and unaffected individuals in the families using forward and reverse primers (Table 2) from Macrogen Inc., Seoul, South Korea (https://dna.macrogen.com/ (accessed on 29 April 2022)).
Table 2.
Primer list with gene name, exon No., annealing temperature, and product size.
| S/No | Gene | Exon | Primers (5′-3′) | Temp | Product Size (bp) |
|---|---|---|---|---|---|
| 1 | MPV17 | Exon 3 | F 5′-AAGCCCTGGGGTTCAGAGTA-3′ | 56 °C | 320 bp |
| R 5′-TGTGAGAGTCCAAGGGAAGC-3′ | |||||
| 2 | SACS | Exon 5 | F 5′-CACATTGGTGACAATTCATGG-3′ | 56 °C | 373 bp |
| R 5′-AGCTTAGCGCATTTCTTTGC-3′ | |||||
| 2 | SACS | Exon 10 | F 5′-CAAATCCAATCCTGGGATCA-3′ | 57 °C | 339 bp |
| R 5′-AAACTGGGGTTGGTTTCCTC-3′ | |||||
| 3 | PRX | Exon 3 | F 5′-GGGACTAGCGTAACTGCGACT-3′ | 55 °C | 326 bp |
| R 5′-TGATCTCGTAGCCAGACACG-3′ | |||||
| 4 | GJB1 | Exon 1 | F 5′-GAGAAGCTGGCAAGGGAGAT-3′ | 56 °C | 773 bp |
| R 5′-AGGCAGCTAGCATGAAGACG-3′ |
For primer design, the genomic DNA sequences of the genes were retrieved from the Ensembl genome browser (https://asia.ensembl.org/ (accessed on 2 April 2022)). The following gene sequences were processed:
MPV17 with accession number ENSG00000115204 and transcript ID ENST00000380044.6;
SACS with accession number ENSG00000151835 and transcript ID ENST00000382292.9;
PRX with accession number ENSG00000105227 and transcript ID ENST00000324001.8;
GJB1 with accession number ENSG00000169562 and transcript ID ENST00000361726.7.
2.3. Protein Modeling of MPV17, GJB1, and SACS
As no known X-ray crystallography-based 3D structures were present, the artificial intelligence-based 3D structures of MPV17WT` (AlphaFold ID: AF-P39210-F1) and GJB1WT` (AlphaFold ID: AF-P39210-F1) were downloaded from the AlphaFold Protein Structure Database [37,38]. As no known X-ray crystallography-based 3D structure of SACSWT was present, its sequence (uniport ID: Q9NZJ4-1; 831-1740 AA) was subjected to I-TASSER [39,40] for 3D structure prediction. The structures were subjected to homology modeling as a template for predicting the 3D structure of MPV17GLY28VAL, GJB1GLY21ARG, and SACSARG1645PRO through MODELLER [41]. The mutated structures were subjected to energy minimization through AMBER ff99SB using UCSF Chimera 1.8.1 [42]. Rotamers and amino acid outliers were removed through WinCoot 0.9.8.1 [43]. The quality of the structure was assessed by MolProbity [44] and VERIFY3D [45]. Finally, the optimized design was visualized and analyzed through USCF Chimera 1.8.1 [42] and PyMOL (http://www.pymol.org/pymol (accessed on 10 January 2023)) for mutational and structural analysis. To assess the pathogenic/deleterious effect of mutations MPV17GLY28VAL, GJB1GLY21ARG, and SACSARG1645PRO MTBAN was employed [46].
3. Results
3.1. Clinical Features of the Patients
3.1.1. Family DG-01
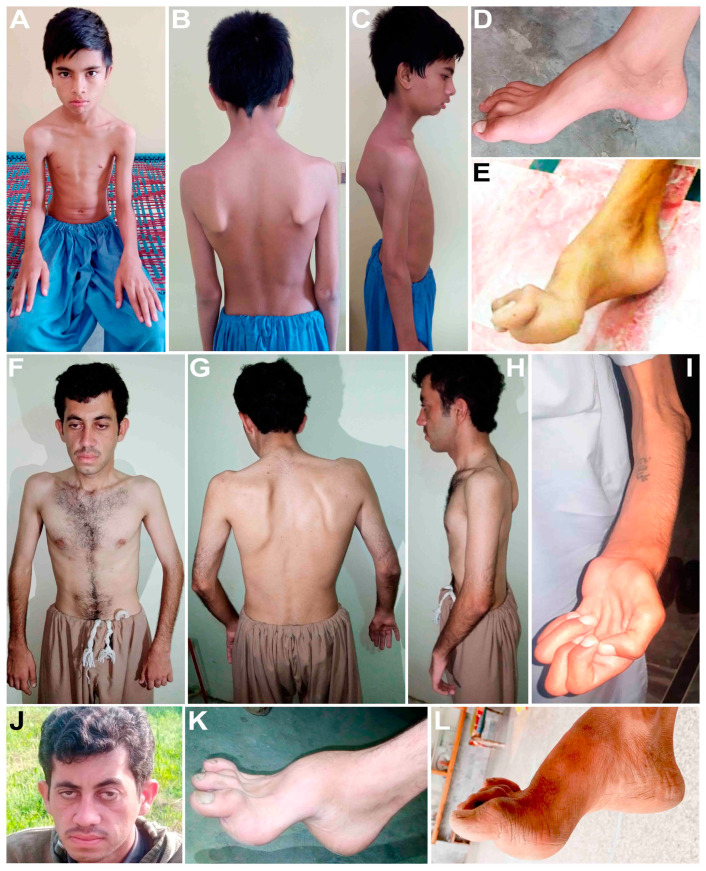
In the first family, DG-01 (Figure 1A), four affected siblings are presented: a 16-year-old boy (V-1) and three girls, a 14-year-old (V-2) and 9-year-old maternal twins (V-3, V-4), born to first cousin normal parents. They had a delayed onset of walking and later developed difficulty walking, gait ataxia, and spasticity of the lower limbs. They developed mild pes cavus at the ages of 16 and 14 years, have abnormal foot phalanges (Figure 2D), and walk sidewise with difficulty lifting their feet, and they frequently fall. They gradually lost their grip and cannot hold anything in their hands or stand without support. Their hands are affected by tremors and developed hand curvature. They are incredibly petite and have developed bilateral scapular dyskinesis and winged scapula (Figure 2A–C). They are intellectually dull compared to their normal siblings and cousins. They have squint strabismus eyes and dry skin that appears as white scales on their legs. The elder siblings presented more severe phenotypes, while the two younger siblings had not developed proper phenotypes. Electromyography (EMG) of V-1 and V-2 of bilateral lower and upper limbs shows spontaneous activities with neuropathic configurations of the myopathic motor unit potential (MUAPs). The electrodiagnostic studies developed for V-1 and V-2 show electrophysiological evidence of demyelinating polyneuropathy. Serum analysis performed for V-1 and V-2 shows severe vitamin D deficiency.
Figure 2.
Clinical observations of DG-01 (A–D), ICP-RD11 (E), BD-06 (F–K), and MR-01 (L) are presented here. (A) and (F) show extreme petite body in family DG-01 and BD-06. (B) and (G) demonstrate bilateral scapular dyskinesis and winged scapula in family DG-01 and BD-06. (F) and (J) present abnormal eye phenotypes such as ptosis or blepharoptosis in family DG-01 and BD-06. (I) shows clawed hands and muscular atrophy in family BD-06. (D,E, K,L) show pes cavus and abnormal phalanges in family DG-01, BD-06, MR-01, and ICP-RD1.
3.1.2. Family BD-06
The second family, BD-06 (Figure 1B), is a large consanguineous family with eight affected individuals (V-1, V-4, V-5, V-6, V-7, V-9, V-12, and V-14) ranging in age from 16 years (V-14) to 36 years (V-1), all born to first cousin marriages. The indexed patient for WES is V-12, a 19-year-old boy. They developed phenotypes at an early age, usually walking at an early age but later being unable to stand and walk properly. From the first decade of life, all affected members of this family developed pes cavus, bilateral steppage gait, and began toe walking. With age, they began to experience extreme difficulties in walking, standing, maintaining balance, areflexia, and sensory ataxia. Their hands, legs, and body are affected by tremors and developed hand curvature, bone deformities of the feet and hands, swollen joints, and abnormal phalanges. They are incredibly petite, have abnormal vertebral phenotypes, and developed bilateral scapular dyskinesis and winged scapula (Figure 2F–K). V-1, V-4, V-5, V-7, V-12, and V-14 were diagnosed with myoclonic seizure and epilepsy. With age, they also showed abnormal eye phenotypes such as ptosis or blepharoptosis, muscular atrophy, and steppage gait. However, mentally, all affected individuals are sound. The severity, signs, and symptoms within the family varied from patient to patient and clade to clade, with even siblings showing different phenotypes. Some members developed progressive sensory hearing loss early, and some are entirely deaf (V-1 and V-4).
3.1.3. Family MR-01
The third family presented here, MR-01 (Figure 1C), has two affected males born to a moderately affected mother (III-4) with no other family history. The index patient (IV-4) is a 28-year-old male, while another sibling, IV-3, is a 32-year-old male. They developed mild CMTX1 phenotypes in the second decade of their lives, becoming more prominent with advancing age. All three affected individuals (III-4, IV-3, IV-4) have developed pes cavus, cleaved toe, upper and lower limb numbness, tremor, weakness, and hyporeflexia (Figure 2L). From early childhood, they were diagnosed with epilepsy, especially when exposed to crowd and pressure. They cannot speak properly, spit while talking, have language delay, irregular and abnormal speech, mental stress, and non-progressive intellectual disability. For the indexed patient (IV-4), EEG was recorded many times, which showed a gross abnormal EEG pattern and suggested tonic–clonic seizure, myoclonic jerk, and epilepsy. Their mother (III-4) developed pes cavus and walking disabilities, upper and lower limb numbness, tremor, weakness, and hyporeflexia. With advancing age, the severity of the phenotypes increased.
3.1.4. Family ICP-RD11
The fourth family presented here (Figure 1D) has two affected siblings. The indexed patient (V-5) is a 26-year-old female born to third cousins with one 35-year-old affected brother. Both patients showed ARSACS phenotypes in the second decade of life, but at the time of blood collection, the affected brother, V-1, was completely paralyzed and unable to stand or walk. They developed pes cavus, astasia, difficulty walking, joint stiffness, joint contractures, and spasticity of the lower limbs. In addition, they have muscular atrophy of the lower limbs, and the upper limbs are comparatively less atrophic (Figure 2E).
3.2. Genetic Analysis
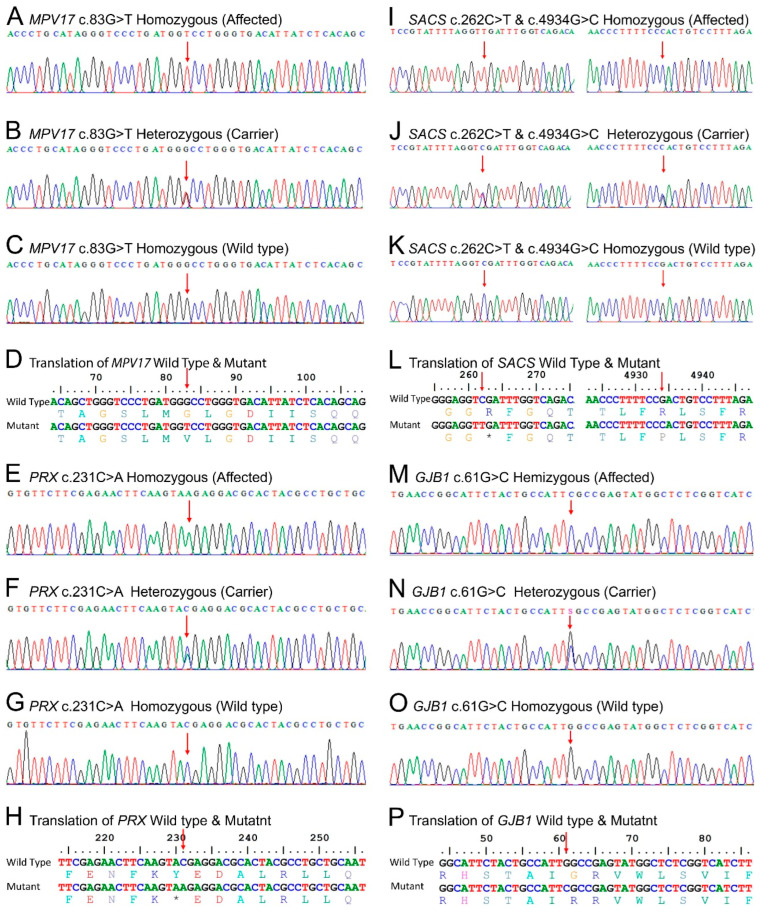
WES in family DG-01 identified two homozygous variants: in MPV17 (NM_002437.5: c.83G>T: NP_002428.1: p.Gly28Val) (Figure 3A–D) and SACS (NM_014363.6: c.4934G>C: NP_055178.3: p.Arg1645Pro) (Figure 3I–L right). In family BD-06, WES identified a biallelic variant (c.231C>A NM_181882.3: NP_870998.2: p.Tyr77Ter) (Figure 3E–H) in PRX. In family MR-01, WES identified a homozygous variant (NM_000166.6: c.61G>C: NP_000157.1: p.Gly21Arg) (Figure 3M–P) in GJB1. In family ICP-RD11, a variant (NM_014363.6: c.262C>T: NP_055178.3: p.Arg88Ter) (Figure 3I–L left) in SACS was identified through WES in the index patient. In families DG-01, BD-06, and ICP-RD11, Sanger validation confirmed autosomal recessive segregation of the identified variants with the disease phenotypes, while in family MR-01, Sanger sequencing validated the X-linked dominant mode of segregation. The variants were classified as MPV17 (c.83G>T) VUS, SACS (c.4934G>C) VUS, PRX (c.231C>A) pathogenic, SACS (c.262C>T) pathogenic, and GJB1 (c.61G>C) likely pathogenic, according to the ACMG/AMP guidelines. All the variants except ICP-RD11 c.262C>T in SACS were novel and absent from the ClinVar (https://www.ncbi.nlm.nih.gov/clinvar/ (accessed on 5 January 2022), gnomAD v3.1.2 (https://gnomad.broadinstitute.org/ (accessed on 5 January 2022)), and 1000 genomes (https://www.internationalgenome.org/ (accessed on 5 January 2022) databases. Population screening of 200 ethnically matched controls excluded the presence of these variants in healthy individuals.
Figure 3.
Sanger sequencing trace files were analyzed to identify homozygous mutated, hemizygous mutant, heterozygous carriers, and homozygous wild-type individuals for each gene. The point of alteration is indicated by an arrow above the point of mutation. Figures (A–C) show the mutation MPV17 c.83G>T, figures (E–G) show PRX c.231C>A, figures (I–K right) show SACS c.4934G>C, figures (I–K left) show SACS c.262C>T, and figures (M–O) show GJB1 c.61G>C. Figures (D,H,L) and (P) show the single letter translation of both the wild-type and mutant gene.
3.3. MPV17 c.83G>T: p.Gly28Val
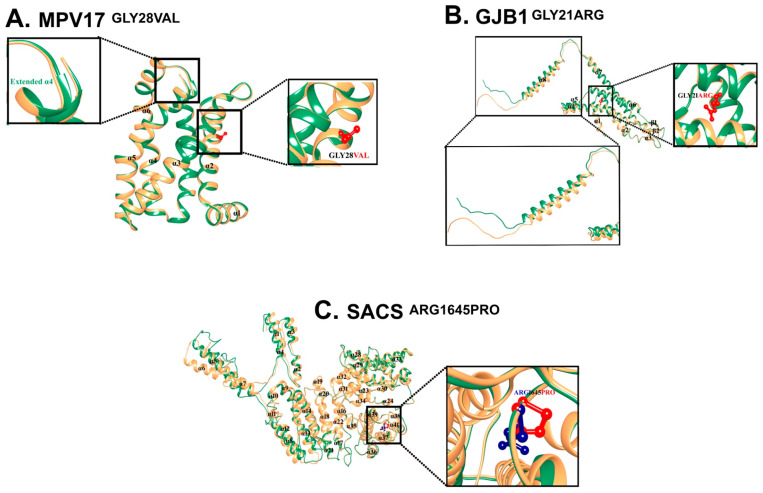
For most regions, the pLDDT value of the wild-type structure of MPV17 was between >= 90 and >= 70. The 3D-1D score of MPV17GLY28VAL was >= 0.2. MPV17WT contains six α-helices. Due to the induction of mutation in MPV17GLY28VAL, α4 extended to one amino acid (ASN112) compared to MPV17WT. MTB analysis (score: −8.091; Z-score: 0.2844; probability of deleteriousness: 0.6603) suggested the significant pathogenicity/deleteriousness of the mutation (Figure 4A).
Figure 4.
(A) Mutational and structural analysis of MPV17. Structural differences due to mutations in MPV17. Superimposition of MPV17WT (sandy brown) and MPV17GLY28VAL (sea green). GLY28 is labeled in black, and VAL28 is shown and labeled in red. Extended-α5 is labeled and shown in sea green. All the α-helices are marked in black. (B) Mutational and structural analysis of GJB1. Structural differences due to mutations in GJB1. Superimposition of GJB1WT (sandy brown) and GJB1GLY21ARG (sea green). GLY21 is labeled in black, and ARG21 is shown and labeled in red. The region signifying the structural changes is zoomed in on and displayed in the inset. (C) Structural differences due to mutation in SACS. Superimposition of SACSWT (sandy brown) and SACSARG1645PRO (sea green). ARG shown and labeled in blue, PRO shown and labeled in red. All the α-helices are labeled in black.
3.4. GJB1 c.61G>C: p.Gly21Arg
For most regions, the pLDDT value of the wild-type structure of GJB1 was between >= 90 to >= 70. The 3D-1D score of GJB1GLY21ARG was >= 0.2. GJB1WT contains eight α-helices and two β-sheets. Due to the induction of mutation in GJB1GLY21ARG, a slight upward movement of α4 and α5, along with a significant outward movement of the C-terminal (α8), was observed (Figure 4B).
3.5. SACS c.4934G>C: p.Arg1645Pro
The structural analysis of the 3D structure of the wild type and mutated SACS revealed that the N-terminal region of SACSARG1645PRO was significantly different from SACSWT. Specifically, inward movement of α1, α2, and α3 was observed, while outward movement of α5, α6, and α7 was observed. Moreover, α11 converted into a loop and a loop between α19 and α20 converted into a helical turn in SACSARG1645PRO as compared to SACSWT (Figure 4C).
4. Discussion
We reported a cohort of 16 patients from four unrelated Pakistani families of Pakhtun descent. All affected individuals showed clinical phenotypes of CMT and ARSACS. Patient DG-01 was diagnosed with a merger of autosomal recessive CMT2EE and ARSACS. Patient BD-06 was diagnosed with autosomal recessive CMT4F, and MR-01 was diagnosed with X-linked dominant CMTX1. While a few cases of CMTEE have been reported globally, this is the first case reported in the Pakistani population. Similarly, there are only a small number of reported cases of ARSACS, CMT4F, and CMTX1 in the literature from Pakistan.
The first diagnostic characteristics of axonal sensorimotor neuropathies are walking disabilities and ataxia [24,26]. Other reported phenotypes in the literature, as compared with our families, are tabulated in Table 1. In our study group, we reported distal upper limb weakness, hand tremor, delayed motor development, ataxia, pes cavus, intellectual disabilities, and speech articulation, with minor phenotypic variations and varying ages of onset, as previously documented [9,13,14,15,16,19,20,21,24,26,30,31,47].
In addition to the common phenotypes, in CMT2EE, sensorineural hearing loss, hyporeflexia, areflexia, and scoliosis are notable phenotypes [13,27,28,29] that were present in our family DG-01. In CMT4F, clawed hands [18], distal limb muscle atrophy [19], areflexia [29], and scoliosis [19] have also been reported, which were confirmed in our family BD-06. Additionally, we observed sensorineural hearing loss, a new phenotype not reported in CMT4F. In our family MR-01, we found Achilles tendon contractures, numbness of the hands and legs, hyporeflexia, areflexia, and toe walking, as reported previously [13,16,28,29].
Biallelic variants causing CMT2EE have been found in MPV17 (OMIM: 137960), a gene located on chromosome 2p23 that produces an inner mitochondrial membrane protein consisting of four transmembrane domains [48,49]. This protein is a non-selective channel responsible for maintaining the balance of nucleotides and mitochondrial DNA within the mitochondria. To date, 56 mutations have been reported in MPV17, the majority of which cause a condition called mitochondrial DNA depletion syndrome-6 (MTDPS6: 256810). Only two variants have been reported to cause CMT2EE, including a missense c.122G>A: Arg41Gln, and a splice site c.376-9T>G [21,27,50]. Our study identified a novel biallelic variant in MPV17 (NM_002437.5), c.83G>T, which changes a highly conserved glycine to valine at amino acid position 28 (NP_002428.1: p: Gly28Val). A missense alteration is a common mechanism for Charcot–Marie–Tooth disease, axonal, type 2EE, and the rate of benign missense variants is relatively low. In silico prediction tools, conservation analysis, and protein simulations predicted that this variant is damaging to the protein structure and function (REVEL: 0.787 >= 0.6, 3CNET: 0.983 >= 0.75) (Supplementary Table S2; Figure 4A).
ARSACS (OMIM: 270550) is caused by biallelic modifications in the SACS (OMIM: 604490) located on chromosome 13q12.12. This gene encodes for the large multidomain sacsin protein (Q9NZJ4), which acts as a regulator of the Hsp70 chaperone machinery and is responsible for processing ataxin-1 [51]. To date, 328 mutations have been reported in SACS, including 180 missense mutations, 3 splice substitutions, 91 small deletions, 37 insertions/duplications, 16 deletions, and 1 complex rearrangement. In our study, we have reported two variants in SACS. A recurrent variant c.262C>T (NM_014363.6) in family ICP-RD11, which lies in a highly conserved region of the sacsin protein (NP_055178.3) (Figure 5B left) and creates a premature termination codon (Figure 3I–L left), is expected to cause a loss of functional protein via non-sense-mediated mRNA decay. Additionally, we identified a novel biallelic variant in family DG-01 in SACS, c.4934G>C (Figure 3I–L right), at amino acid position 1645, which changes a large hydrophilic arginine to a neutral proline in a highly conserved region of the sacsin protein (Figure 5B right). In silico prediction tools and conservation analysis predicted that this variant is probably damaging to the protein structure/function (REVEL: 0.849 >= 0.6, 3CNET: 0.92 >= 0.75) (Supplementary Table S2; Figure 4C). The mutant sacsin protein is non-functional in large neurons, particularly within brain motor systems, including cerebellar Purkinje cells, which are associated with ARSACS.
Figure 5.
P rotein multiple sequence alignments of inner mitochondrial protein (MPV17: P39210), sacsin (SACS: Q9NZJ4), periaxin (PRX: Q9BXM0), and gap junction beta1 (GJB1: P08034) proteins with their respective members across species. The first column indicates the species and the second is its entry in the UniProtKB database (https://www.uniprot.org/uniprot/ (accessed on 5 January 2022)). The numbers before and after the sequences indicate the respective amino acids in proteins, while the mutated point is shown by an arrow above it. (A) In MPV17 (P39210) protein, the glycine at codon 21 is replaced with valine in a highly conserved region of the first transmembrane domain. (B) In sacsin (Q9NZJ4), (B Left) the arginine shown in blue at codon 88 is replaced with a termination codon in a highly conserved region, which truncates the protein. (B Right) The arginine (dark blue) at codon 1645 is replaced by proline in a highly conserved region. (C) In PRX (Q9BXM0) protein, the arginine (dark blue) at codon 77 is replaced with a termination codon in a highly conserved region of the first domain. (D) In GJB1 (P08034), the glycine (orange) located at codon 21 is replaced with arginine in a highly conserved region of the cytoplasmic topological domain.
CMT4F (OMIM: 614895) is caused by biallelic mutations in the PRX (OMIM: 605725) located on chromosome 19q13.2. The gene encodes for the Periaxin protein (Q9BXM0), which is necessary for maintaining the myelin sheath, normal nerve impulse, and conduction velocity. PRX has seven exons and encodes for two isoforms: L- and S-periaxin protein [31]. To date, a total of 116 mutations have been reported in PRX, of which only 10 variations—5 missense, 4 small deletions, and 1 insertion—cause CMT4F. Our analysis of family BD-06 revealed a novel biallelic pathogenic variant in PRX (NM_181882.3 c.231C>A) through WES analysis (Figure 3E–H). The c.231C>A variant leads to a premature termination of the peroxin protein at amino acid position 77 (NP_870998.2) (Figure 5C), which further leads to immature protein synthesis or loss of functional protein via nonsense-mediated mRNA decay. Furthermore, in silico prediction tools and conservation analysis predicted that the variant is damaging to the protein structure/function (Supplementary Table S2). The lack of periaxin protein in the affected family members can be related to their severe phenotypes.
CMTX1 (OMIM: 302800) is caused by mutations in the GJB1 (OMIM: 304040) located on the X-chromosome (Xq13.1). This gene encodes for the gap junction beta-1 connexin 32 (CX32) protein [52]. A clear genotype–phenotype correlation in GJB1 is not fully understood, but it is proposed that the altered protein is degraded quickly and trapped inside the cell, preventing it from reaching the cell membrane and forming gap junctions. The loss of functional gap junctions alters the activity of Schwann cells and the production of myelin. In addition to changes in the peripheral nervous system, GJB1 mutations are also associated with the loss of myelin in the brain and spinal cord [53,54]. Although the inheritance pattern is X-linked dominant, hemizygous males are more severely affected than heterozygous females [55]. In family MR-01, a hemizygous missense variant c.61G>C in GJB1 (NM_000166.6) (Figure 3M–P) lies in a highly conserved region of the gap junction beta 1 (P08034) protein (Figure 5D). GJB1 proteins form a closed cluster of six connexin proteins, which are believed to be responsible for the diffusion of low molecular weight materials between neighboring cells. The abnormal EEG pattern can be related to tonic–clonic seizures, myoclonic jerks, and epilepsy. The variant is in a well-established functional domain or exonic hotspot, where pathogenic variants have frequently been reported. A missense variant is a common mechanism associated with Charcot–Marie–Tooth neuropathy and X-linked dominant, 1, and the rate of benign missense variants is relatively low. In silico prediction tools and conservation analysis predicted that this variant was probably damaging to the protein structure/function (REVEL: 0.971 >= 0.6, 3CNET: 0.997 >= 0.75) (Supplementary Table S2; Figure 4B).
In conclusion, neuropathies are a diverse group of genetic disorders and have many overlapping phenotypes with many reported genes. Therefore, simple clinical and phenotypic investigation alone makes an exact diagnosis of the neuropathy types impossible. In such cases, WES analysis is the best, most precise, and most time-effective procedure for these diversified genetic neuropathies. In societies such as Pakistan, where around 60% of people practice cousin marriages, the segregation of mutant alleles is much higher [35]. The disease allele burden can be reduced, or at least attempted to be reduced, by premarital testing, newborn genetic disorder screening, and parental genetic screening [56,57]. The disease allele burden can also be reduced by carrier genetic screening, parental counseling, and neonatal work-up if the parents wish for future progeny [35].
Acknowledgments
The research work was funded by Institutional Fund Projects under grant No. IFPIP-843-141-1443. The authors’ gratefully acknowledge the technical and financial support provided by the Ministry of Education and King Abdulaziz University, DSR, Jeddah, Saudi Arabia.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/genes14020328/s1, Table S1: The classification of different types of Charcot-Marie-Tooth (CMT) disease, Table S2: In-silico predictions of the identified variants in their respective genes by online prediction tools.
Author Contributions
Q.Z. collected the samples, contributed to clinical and molecular diagnosis, performed in silico analysis, designed figures, and wrote the manuscript. M.A.K., K.S. and H.K. performed laboratory experiments. N. and M.S.F. performed protein modeling. G.R., M.R., I.A., M.T., A.A.A. and O.Y.M. helped with clinical analysis and experimentation. N.W., M.I.N., F.B. and M.J. contributed to study design and conceptualization and wrote and finalized the manuscript. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Review Board (or Ethics Committee) of Islamia College Peshawar with letter # 529/Oric/ICP dated 16-10-2019.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors without undue reservation. We have submitted the variants data to ClinVar, and the accession numbers VCV000559870.5, VCV001722539.1, VCV000958213.6, and VCV001722538.1 have been assigned to them.
Conflicts of Interest
The authors declare no conflict of interest.
Ethics Statement
Ethics statement consent was taken from the families’ elders and legal guardians for the study and provision of family history, clinical photographs, and blood samples. The study was approved by the Institutional Bioethical Committee (IBC) of Islamia College Peshawar, with reference # 529/Oric/ICP, according to the 2013 Declaration of Helsinki.
Funding Statement
The research work was funded by Institutional Fund Projects under grant No. IFPIP-843-141-1443.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Charcot J., Marie P. On a specific form of frequently familial progressive muscular atrophy beginning in the feet and legs and later affecting the hands. Rev. Med. 1886;6:97–138. [Google Scholar]
- 2.Tooth H.H. Recent observations on progressive muscular atrophy. Brain. 1887;10:243–253. doi: 10.1093/brain/10.2-3.243. [DOI] [Google Scholar]
- 3.Banchs I., Casasnovas C., Albertí A., De Jorge L., Povedano M., Montero J., Martínez-Matos J.A., Volpini V. Diagnosis of charcot-marie-tooth disease. J. Biomed. Biotechnol. 2009;35:239–245. doi: 10.1155/2009/985415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Martyn C., Hughes R. Epidemiology of peripheral neuropathy. J. Neurol. Neurosurg. Psychiatry. 1997;62:310. doi: 10.1136/jnnp.62.4.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Skre H. Genetic and clinical aspects of Charcot-Marie-Tooth's disease. Clin. Genet. 1974;6:98–118. doi: 10.1111/j.1399-0004.1974.tb00638.x. [DOI] [PubMed] [Google Scholar]
- 6.Pareyson D., Marchesi C. Diagnosis, natural history, and management of Charcot–Marie–Tooth disease. Lancet Neurol. 2009;8:654–667. doi: 10.1016/S1474-4422(09)70110-3. [DOI] [PubMed] [Google Scholar]
- 7.Timmerman V., Strickland A.V., Züchner S. Genetics of Charcot-Marie-Tooth (CMT) disease within the frame of the human genome project success. Genes. 2014;5:13–32. doi: 10.3390/genes5010013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barisic N., Claeys K., Sirotković-Skerlev M., Löfgren A., Nelis E., De Jonghe P., Timmerman V. Charcot-marie-tooth disease: A clinico-genetic confrontation. Ann. Hum. Genet. 2008;72:416–441. doi: 10.1111/j.1469-1809.2007.00412.x. [DOI] [PubMed] [Google Scholar]
- 9.Kennedy R.A., Carroll K., McGinley J.L. Gait in children and adolescents with Charcot-Marie-tooth disease: A systematic review. J. Peripher. Nerv. Syst. 2016;21:317–328. doi: 10.1111/jns.12183. [DOI] [PubMed] [Google Scholar]
- 10.Burns J., Ryan M.M., Ouvrier R.A. Quality of life in children with Charcot-Marie-Tooth disease. J. Child Neurol. 2010;25:343–347. doi: 10.1177/0883073809339877. [DOI] [PubMed] [Google Scholar]
- 11.Redmond A.C., Burns J., Ouvrier R.A. Factors that influence health-related quality of life in Australian adults with Charcot–Marie–Tooth disease. Neuromuscul. Disord. 2008;18:619–625. doi: 10.1016/j.nmd.2008.05.015. [DOI] [PubMed] [Google Scholar]
- 12.Meyer zum Gottesberge A.-M., Massing T., Hansen S. Missing mitochondrial Mpv17 gene function induces tissue-specific cell-death pathway in the degenerating inner ear. Cell Tissue Res. 2012;347:343–356. doi: 10.1007/s00441-012-1326-7. [DOI] [PubMed] [Google Scholar]
- 13.Yiu E., Geevasinga N., Nicholson G., Fagan E., Ryan M., Ouvrier R. A retrospective review of X-linked Charcot-Marie-Tooth disease in childhood. Neurology. 2011;76:461–466. doi: 10.1212/WNL.0b013e31820a0ceb. [DOI] [PubMed] [Google Scholar]
- 14.El Euch-Fayache G., Lalani I., Amouri R., Turki I., Ouahchi K., Hung W.-Y., Belal S., Siddique T., Hentati F. Phenotypic features and genetic findings in sacsin-related autosomal recessive ataxia in Tunisia. Arch. Neurol. 2003;60:982–988. doi: 10.1001/archneur.60.7.982. [DOI] [PubMed] [Google Scholar]
- 15.Fitzsimmons J., Watson A., Mellor D., Guilbert P. Familial spastic paraplegia, bilateral sensorineural deafness, and intellectual retardation associated with a progressive nephropathy. J. Med. Genet. 1988;25:168–172. doi: 10.1136/jmg.25.3.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Taylor R.A., Simon E.M., Marks H.G., Scherer S.S. The CNS phenotype of X-linked Charcot-Marie-Tooth disease: More than a peripheral problem. Neurology. 2003;61:1475–1478. doi: 10.1212/01.WNL.0000095960.48964.25. [DOI] [PubMed] [Google Scholar]
- 17.Blakely E.L., Butterworth A., Hadden R.D., Bodi I., He L., McFarland R., Taylor R.W. MPV17 mutation causes neuropathy and leukoencephalopathy with multiple mtDNA deletions in muscle. Neuromuscul. Disord. 2012;22:587–591. doi: 10.1016/j.nmd.2012.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tazir M., Bellatache M., Nouioua S., Vallat J.M. Autosomal recessive Charcot-Marie-Tooth disease: From genes to phenotypes. J. Peripher. Nerv. Syst. 2013;18:113–129. doi: 10.1111/jns5.12026. [DOI] [PubMed] [Google Scholar]
- 19.Marchesi C., Milani M., Morbin M., Cesani M., Lauria G., Scaioli V., Piccolo G., Fabrizi G., Cavallaro T., Taroni F. Four novel cases of periaxin-related neuropathy and review of the literature. Neurology. 2010;75:1830–1838. doi: 10.1212/WNL.0b013e3181fd6314. [DOI] [PubMed] [Google Scholar]
- 20.Muona M., Berkovic S.F., Dibbens L.M., Oliver K.L., Maljevic S., Bayly M.A., Joensuu T., Canafoglia L., Franceschetti S., Michelucci R. A recurrent de novo mutation in KCNC1 causes progressive myoclonus epilepsy. Nat. Genet. 2015;47:39–46. doi: 10.1038/ng.3144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baumann M., Schreiber H., Schlotter-Weigel B., Löscher W.N., Stucka R., Karall D., Strom T.M., Bauer P., Krabichler B., Fauth C. MPV17 mutations in juvenile-and adult-onset axonal sensorimotor polyneuropathy. Clin. Genet. 2019;95:182–186. doi: 10.1111/cge.13462. [DOI] [PubMed] [Google Scholar]
- 22.Bouchard J., Barbeau A., Bouchard R., Bouchard R. Autosomal recessive spastic ataxia of Charlevoix-Saguenay. Can. J. Neurol. Sci. 1978;5:61–69. doi: 10.1017/S0317167100024793. [DOI] [PubMed] [Google Scholar]
- 23.Spira P.J., McLeod J.G., Evans W.A. A spinocerebellar degeneration with X-linked inheritance. Brain. 1979;102:27–41. doi: 10.1093/brain/102.1.27. [DOI] [PubMed] [Google Scholar]
- 24.Arthur-Farraj P., Murphy S., Laura M., Lunn M., Manji H., Blake J., Ramdharry G., Fox Z., Reilly M. Hand weakness in Charcot-Marie-Tooth disease 1X. Neuromuscul. Disord. 2012;22:622–626. doi: 10.1016/j.nmd.2012.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kazuki K., Egi T., Okada M., Takaoka K. Anatomic variation—A bony canal for the median nerve at the distal humerus: A case report. J. Hand Surg. 2004;29:953–956. doi: 10.1016/j.jhsa.2004.05.018. [DOI] [PubMed] [Google Scholar]
- 26.Piekutowska-Abramczuk D., Pronicki M., Strawa K., Karkucińska-Więckowska A., Szymańska-Dębińska T., Fidziańska A., Więckowski M., Jurkiewicz D., Ciara E., Jankowska I. Novel c. 191C> G (p. Pro64Arg) MPV17 mutation identified in two pairs of unrelated Polish siblings with mitochondrial hepatoencephalopathy. Clin. Genet. 2014;85:573–577. doi: 10.1111/cge.12228. [DOI] [PubMed] [Google Scholar]
- 27.Choi Y.-R., Hong Y.B., Jung S.-C., Lee J.H., Kim Y.J., Park H.J., Lee J., Koo H., Lee J.-S., Jwa D.H. A novel homozygous MPV17 mutation in two families with axonal sensorimotor polyneuropathy. BMC Neurol. 2015;15:1–10. doi: 10.1186/s12883-015-0430-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fain P., Barker D., Chance P. Refined genetic mapping of X-linked Charcot-Marie-Tooth neuropathy. Am. J. Hum. Genet. 1994;54:229. [PMC free article] [PubMed] [Google Scholar]
- 29.Tokunaga S., Hashiguchi A., Yoshimura A., Maeda K., Suzuki T., Haruki H., Nakamura T., Okamoto Y., Takashima H. Late-onset Charcot–Marie–Tooth disease 4F caused by periaxin gene mutation. Neurogenetics. 2012;13:359–365. doi: 10.1007/s10048-012-0338-5. [DOI] [PubMed] [Google Scholar]
- 30.Valerie K., Brust D., Farnsworth J., Amir C., Taher M.M., Hershey C., Feden J. Improved radiosensitization of rat glioma cells with adenovirus-expressed mutant herpes simplex virus-thymidine kinase in combination with acyclovir. Cancer Gene Ther. 2000;7:879–884. doi: 10.1038/sj.cgt.7700185. [DOI] [PubMed] [Google Scholar]
- 31.Kabzińska D., Drac H., Sherman D., Kostera-Pruszczyk A., Brophy P., Kochański A., Hausmanowa-Petrusewicz I. Charcot–Marie–Tooth type 4F disease caused by S399fsx410 mutation in the PRX gene. Neurology. 2006;66:745–747. doi: 10.1212/01.wnl.0000201269.46071.35. [DOI] [PubMed] [Google Scholar]
- 32.Mrissa N., Belal S., Ben Hamida C., Amouri R., Turki I., Mrissa R., Ben Hamida M., Hentati F. Linkage to chromosome 13q11-12 of an autosomal recessive cerebellar ataxia in a Tunisian family. J. Peripher. Nerv. Syst. 2000;5:241–242. doi: 10.1111/j.1529-8027.2000.22-22.x. [DOI] [PubMed] [Google Scholar]
- 33.Bennett R.L., French K.S., Resta R.G., Austin J. Practice resource-focused revision: Standardized pedigree nomenclature update centered on sex and gender inclusivity: A practice resource of the National Society of Genetic Counselors. J. Genet. Couns. 2022;31:1238–1248. doi: 10.1002/jgc4.1621. [DOI] [PubMed] [Google Scholar]
- 34.Seo G.H., Kim T., Choi I.H., Park J.y., Lee J., Kim S., Won D.g., Oh A., Lee Y., Choi J. Diagnostic yield and clinical utility of whole exome sequencing using an automated variant prioritization system, EVIDENCE. Clin. Genet. 2020;98:562–570. doi: 10.1111/cge.13848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zaman Q., Sadeeda, Anas M., Rehman G., Khan Q., Iftikhar A., Ahmad M., Owais M., Ahmad I., Muthaffar O.Y., et al. Report of Hermansky-Pudlak Syndrome in Two Families with Novel Variants in HPS3 and HPS4 Genes. Genes. 2023;14:145. doi: 10.3390/genes14010145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Biesecker L.G., Harrison S.M. The ACMG/AMP reputable source criteria for the interpretation of sequence variants. Genet. Med. 2018;20:1687–1688. doi: 10.1038/gim.2018.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jumper J., Evans R., Pritzel A., Green T., Figurnov M., Ronneberger O., Tunyasuvunakool K., Bates R., Žídek A., Potapenko A. Highly accurate protein structure prediction with AlphaFold. Nature. 2021;596:583–589. doi: 10.1038/s41586-021-03819-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tunyasuvunakool K., Adler J., Wu Z., Green T., Zielinski M., Žídek A., Bridgland A., Cowie A., Meyer C., Laydon A. Highly accurate protein structure prediction for the human proteome. Nature. 2021;596:590–596. doi: 10.1038/s41586-021-03828-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yang J., Yan R., Roy A., Xu D., Poisson J., Zhang Y. The I-TASSER Suite: Protein structure and function prediction. Nat. Methods. 2015;12:7–8. doi: 10.1038/nmeth.3213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zheng W., Zhang C., Li Y., Pearce R., Bell E.W., Zhang Y. Folding non-homologous proteins by coupling deep-learning contact maps with I-TASSER assembly simulations. Cell Rep. Methods. 2021;1:14. doi: 10.1016/j.crmeth.2021.100014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Webb B., Sali A. Comparative protein structure modeling using MODELLER. Curr. Protoc. Bioinform. 2016;54:5.6.1–5.6.37. doi: 10.1002/cpbi.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pettersen E.F., Goddard T.D., Huang C.C., Couch G.S., Greenblatt D.M., Meng E.C., Ferrin T.E. UCSF Chimera—A visualization system for exploratory research and analysis. J. Comput. Chem. 2004;25:1605–1612. doi: 10.1002/jcc.20084. [DOI] [PubMed] [Google Scholar]
- 43.Emsley P., Cowtan K. Coot: Model-building tools for molecular graphics. Acta Crystallogr. Sect. D Biol. Crystallogr. 2004;60:2126–2132. doi: 10.1107/S0907444904019158. [DOI] [PubMed] [Google Scholar]
- 44.Williams C.J., Headd J.J., Moriarty N.W., Prisant M.G., Videau L.L., Deis L.N., Verma V., Keedy D.A., Hintze B.J., Chen V.B. MolProbity: More and better reference data for improved all-atom structure validation. Protein Sci. 2018;27:293–315. doi: 10.1002/pro.3330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Eisenberg D., Lüthy R., Bowie J.U. Methods in Enzymology. Volume 277. Elsevier; Amsterdam, The Netherlands: 1997. [20] VERIFY3D: Assessment of protein models with three-dimensional profiles; pp. 396–404. [DOI] [PubMed] [Google Scholar]
- 46.Kim H.Y., Jeon W., Kim D. MTBAN: An Enhanced Variant Effect Predictor Based on a Deep Generative Model and Born-Again Networks. Sci. Rep. 2021;11:19127. doi: 10.1038/s41598-021-98693-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hahn A.F., Bolton C.F., White C.M., Brown W.F., Tuuha S.E., Tan C.C., Ainsworth P.J. Genotype/phenotype correlations in X-linked dominant Charcot-Marie-Tooth disease. Ann. N. Y. Acad. Sci. 1999;883:366–382. doi: 10.1111/j.1749-6632.1999.tb08598.x. [DOI] [PubMed] [Google Scholar]
- 48.Antonenkov V.D., Isomursu A., Mennerich D., Vapola M.H., Weiher H., Kietzmann T., Hiltunen J.K. The human mitochondrial DNA depletion syndrome gene MPV17 encodes a non-selective channel that modulates membrane potential. J. Biol. Chem. 2015;290:13840–13861. doi: 10.1074/jbc.M114.608083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Karasawa M., Zwacka R., Reuter A., Fink T., Hsieh C., Lichter P., Francke U., Welher H. The human homolog of the glomerulosclerosis gene MPV17: Structure and genomic organization. Hum. Mol. Genet. 1993;2:1829–1834. doi: 10.1093/hmg/2.11.1829. [DOI] [PubMed] [Google Scholar]
- 50.Monies D., Abouelhoda M., Assoum M., Moghrabi N., Rafiullah R., Almontashiri N., Alowain M., Alzaidan H., Alsayed M., Subhani S. Lessons learned from large-scale, first-tier clinical exome sequencing in a highly consanguineous population. Am. J. Hum. Genet. 2019;104:1182–1201. doi: 10.1016/j.ajhg.2019.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Parfitt D.A., Michael G.J., Vermeulen E.G., Prodromou N.V., Webb T.R., Gallo J.M., Cheetham M.E., Nicoll W.S., Blatch G.L., Chapple J.P. The ataxia protein sacsin is a functional co-chaperone that protects against polyglutamine-expanded ataxin-1. Hum. Mol. Genet. 2009;18:1556–1565. doi: 10.1093/hmg/ddp067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bergoffen J., Scherer S.S., Wang S., Scott M.O., Bone L.J., Paul D.L., Chen K., Lensch M.W., Chance P.F., Fischbeck K.H. Connexin mutations in X-linked Charcot-Marie-Tooth disease. Science. 1993;262:2039–2042. doi: 10.1126/science.8266101. [DOI] [PubMed] [Google Scholar]
- 53.Scherer S.S., Deschênes S.M., Xu Y.T., Grinspan J.B., Fischbeck K.H., Paul D.L. Connexin32 is a myelin-related protein in the PNS and CNS. J. Neurosci. 1995;15:8281–8294. doi: 10.1523/JNEUROSCI.15-12-08281.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Scherer S.S., Kleopa K.A. X-linked Charcot-Marie-tooth disease. J. Peripher. Nerv. Syst. 2012;17:9–13. doi: 10.1111/j.1529-8027.2012.00424.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Casasnovas C., Banchs I., Corral J., Martínez-Matos J., Volpini V. Clinical and molecular analysis of X-linked Charcot-Marie-Tooth disease type 1 in Spanish population. Clin. Genet. 2006;70:516–523. doi: 10.1111/j.1399-0004.2006.00724.x. [DOI] [PubMed] [Google Scholar]
- 56.Alfadhel M., Umair M., Almuzzaini B., Alsaif S., AlMohaimeed S.A., Almashary M.A., Alharbi W., Alayyar L., Alasiri A., Ballow M., et al. Targeted SLC19A3 gene sequencing of 3000 Saudi newborn: A pilot study toward newborn screening. Ann. Clin. Transl. Neurol. 2019;6:2097–2103. doi: 10.1002/acn3.50898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Alyafee Y., Al Tuwaijri A., Umair M., Alharbi M., Haddad S., Ballow M., Alayyar L., Alam Q., Althenayyan S., Al Ghilan N. Non-invasive prenatal testing for autosomal recessive disorders: A new promising approach. Front. Genet. 2022;13:7474. doi: 10.3389/fgene.2022.1047474. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors without undue reservation. We have submitted the variants data to ClinVar, and the accession numbers VCV000559870.5, VCV001722539.1, VCV000958213.6, and VCV001722538.1 have been assigned to them.