Abstract
Caffeine is the most consumed drug in the world, and it is commonly used by children. Despite being considered relatively safe, caffeine can have marked effects on sleep. Studies in adults suggest that genetic variants in the adenosine A2A receptor (ADORA2A, rs5751876) and cytochrome P450 1A (CYP1A, rs2472297, rs762551) loci are correlated with caffeine-associated sleep disturbances and caffeine intake (dose), but these associations have not been assessed in children. We examined the independent and interaction effects of daily caffeine dose and candidate variants in ADORA2A and CYP1A on the sleep quality and duration in 6112 children aged 9–10 years who used caffeine and were enrolled in the Adolescent Brain Cognitive Development (ABCD) study. We found that children with higher daily caffeine doses had lower odds of reporting > 9 h of sleep per night (OR = 0.81, 95% CI = 0.74–0.88, and p = 1.2 × 10−6). For every mg/kg/day of caffeine consumed, there was a 19% (95% CI = 12–26%) decrease in the odds of children reporting > 9 h of sleep. However, neither ADORA2A nor CYP1A genetic variants were associated with sleep quality, duration, or caffeine dose. Likewise, genotype by caffeine dose interactions were not detected. Our findings suggest that a daily caffeine dose has a clear negative correlation with sleep duration in children, but this association is not moderated by the ADORA2A or CYP1A genetic variation.
Keywords: caffeine, sleep, genotype, dose–response
1. Introduction
Caffeine use by children is common and considered relatively safe [1]. However, caffeine has physiological side effects among which, its ability to disturb sleep quality and duration are the most recognized and reported in children [2,3]. Given the importance of sleep to brain development [4], recommendations for caffeine consumption in children have been published by the American Academy of Child and Adolescent Psychiatry (AACAP) [5], Health Canada [6], and the European Food Safety Authority (EFSA) [7]. According to the AACAP, Health Canada, and EFSA, children and adolescents under the age of 18 years should consume no more than 100 mg/day, 2.5 mg/kg/day, and 3.0 mg/kg/day, respectively. The AACAP further recommends that caffeine use should be completely avoided for children under the age of 12. However, a safe dose of caffeine for children has yet to be established and it is unclear whether the current recommendations are sufficient for guarding against disruptions to sleep quality and duration for all children.
Sensitivity to caffeine, like any other drug, can vary from person-to-person and may, in part, result from interindividual variations in genes involved in caffeine pharmacodynamics [8] and pharmacokinetics [9]. Genome-wide association studies have found that genetic variants in the adenosine A2A receptor (ADORA2A) and the cytochrome P450 1A (CYP1A) loci are correlated with caffeine-associated sleep disturbance [10] and caffeine metabolism [11], respectively. For ADORA2A, rs5751876 C homozygotes were more likely to experience sleep disturbances when consuming caffeine [10], concurring with numerous candidate gene studies [12,13,14,15,16]. For the CYP1A locus, carriers of the T allele in the rs2472297 promoter region variant, located upstream of CYP1A1 and CYP1A2, reported increased caffeine consumption and had lower plasma caffeine concentrations compared to their C allele carrying counterparts [17,18,19]. Likewise, a meta-analysis of eight adult studies found that CYP1A2 rs762551 A carriers (also known as *1F) had a significantly higher CYP1A2 enzyme activity, as measured by caffeine metabolism, than individuals who did not carry the *1F variant [20]. These associations have potential clinical implications for children but the evidence to date has been exclusively generated in adult populations.
In the current study, we examined the independent and interaction effects of caffeine intake and candidate variants in ADORA2A and CYP1A on the sleep quality and duration in children. We hypothesized that a higher caffeine intake would be associated with greater odds of disruptions in sleep quality and duration. We also hypothesized that ADORA2A rs5751876 CC, CYP1A rs2472297 CC, and CYP1A2 rs762551 CC carriers would have greater odds of reporting caffeine-associated disturbances in sleep quality and duration, compared to children that carried the ADORA2A rs5751876 T, CYP1A rs2472297 T, and CYP1A2 rs762551 A (*1F) alleles.
2. Materials and Methods
2.1. Participants
Participants were drawn from the 11,875 children enrolled in the Adolescent Brain Cognitive Development (ABCD) study [21]. The ABCD study began in 2015 and is the largest, prospective, longitudinal, multi-site project designed to study brain and cognitive development in youth, as they transition into adolescence and young adulthood, across the United States [21]. At baseline, only children aged 9–10 years old were enrolled into the ABCD study. Anonymized baseline data (release 3.0) collected from enrolled children were downloaded following approval from the National Institute of Mental Health Data Archive Data Access Committee (eRSO# 1052319). All procedures were conducted in compliance with the Declaration of Helsinki and its subsequent revisions. All participants provided written informed consent prior to participation.
For the current study, only children aged 9–10 years that reported use of caffeine in the past six months and had genomic data available were included. To mitigate the potential impact of concomitant drugs with significant effects on sleep, children that reported use of melatonin, benzodiazepines, or antihistamines in the past six months were excluded. Commonly used psychotropic medications that could impact sleep (i.e., selective serotonin reuptake inhibitors, antipsychotics, and stimulants) were not an exclusion criterion, but they were accounted for in our analysis when appropriate. We also excluded children that had a T-score greater than 65 on the Child Behavior Checklist ADHD scale [22], given the strong association between ADHD symptomatology and sleep problems [23], as well as the notion that caffeine consumption may be increased in children with ADHD [24].
2.2. Measures
2.2.1. Caffeine Intake
Youth in the ABCD study reported caffeine use via the modified Supplemental Beverage Questions administered by a trained interviewer. The modified Supplemental Beverage Questions were previously validated and showed high correlation with urine caffeine concentrations [25]. Caffeine intake from coffee, espresso drinks, tea, soda, and energy drinks were assessed. Total caffeine intake was calculated based on the number of beverages consumed per day for the previous six months. Serving size of each type of drink was defined as 8 oz for coffee, 1 oz for espresso, 8 oz for tea, 12 oz for soda, and 8 oz for energy drinks. For children who reported consuming a drink below the defined serving size, the drink amount was coded as the fraction of the serving size. The caffeine content present in each drink was estimated using standardized values obtained from published sources (Supplementary Table S1). Caffeine intake per day (mg/day) was calculated by multiplying the number of daily servings of each type of drink by the calculated caffeine content of each drink type and summing across all reported drinks. Caffeine intake per day was then divided by the child’s weight (kg) to calculate caffeine intake per kilogram per day (mg/kg/day).
2.2.2. Sleep Quality and Duration
Parents of participants administered the Sleep Disturbance Scale for Children (SDSC) [26] to assess their sleep quality and duration over the past six months. The SDSC comprised 26 questions representing the most common areas of sleep disorders in childhood and adolescence, including disorders of initiating and maintaining sleep, sleep breathing disorders, disorders of arousal, sleep-wake transition disorders, disorders of excessive somnolence, and sleep hyperhidrosis. The scale has a total score ranging from 26 to 130. Previous research has shown that scores greater than 39 correspond to the upper quartile of the normal range, and give a sensitivity of 89% and specificity of 74% for identifying children with disturbed sleep quality [26].
Sleep duration was measured by a single item on the SDSC that asked, “How many hours of sleep does your child get on most nights?” with five possible response choices: 9–11 h, 8–9 h, 7–8 h, 5–7 h, or fewer than 5 h. The American Academy of Sleep Medicine consensus recommendation suggests that children aged 6 to 12 years should sleep 9 to 12 h per 24 h for optimal health [27]. As such, we merged participants reporting 8–9 h, 7–8 h, 5–7 h, or fewer than 5 h, and compared them to those reporting 9–11 h.
2.3. Genotyping, Imputation, and Quality Control Procedures
ABCD participants were genotyped for 503,856 genome-wide markers using the Affymetrix SmokeScreen Array (Affymetrix, Santa Clara, CA, USA) [28,29]. PLINK v1.9 was used to perform genotyping quality control. We excluded SNPs and individuals with more than 10% missing genotype calls as well as SNPs out of Hardy–Weinberg equilibrium (p-value < 10−6) or with a minor allele frequency less than 5%. Phasing and imputation were conducted via the Michigan imputation server using the Haplotype Reference Consortium (Version r1.1 2016) reference panel with mixed ancestry option. Imputed genotypes with quality scores (r2) > 0.3 (Nsnps = 32,634,646) underwent post-imputation quality control using the same procedures performed on the pre-imputed data. To assess cryptic relatedness and population stratification, linkage disequilibrium (LD) pruning was performed with a threshold of 50 SNPs, with a 5 SNPs window, and r2 = 0.5, followed by calculation of the identical-by-descent metric (pi_ha). Samples with pi_hat > 0.2 were excluded. To account for population stratification, principal component analysis was conducted using the smartpca module of the EIGENSOFT package [30]. Each participant’s ancestry was assigned using genomic probabilities for four biogeographical groups (African, European, East Asian, and American) available in the ABCD post stratification weights database. If a clear maximum ancestry probability (>60%) was not present for an individual, they were assigned to an ‘admixed’ ancestry group.
2.4. Candidate Gene and Variant Selection
Two genetic variants (CYP1A rs2472297 and ADORA2A rs5751876) identified by genome-wide association studies of caffeine metabolism [11] and caffeine sensitivity to sleep disruptions [10], as well as one variant (CYP1A2 rs762551) identified by meta-analysis of caffeine metabolism [20], were extracted from the imputed ABCD dataset.
2.5. Statistical Analysis
All analyses were performed with jamovi (version 2.3.18) [31]. Receiver operating characteristic (ROC) curve analysis was conducted to estimate the area under the curve for caffeine intake, and the Youden J-index (sensitivity + specificity − 1) was calculated to identify the optimal caffeine intake threshold for undesirable sleep outcomes [32]. Binomial logistic regression models were used to examine the effect of caffeine intake, ADORA2A rs5751876, CYP1A rs2472297, and CYP1A2 rs762551 on sleep quality (normal versus disturbed based on a SDSC cut-off of 39) and duration (9–11 h versus <9 h). Odds ratios and 95% confidence intervals were calculated and adjusted for the effects of common concomitant psychotropic medications (i.e., selective serotonin reuptake inhibitors, antipsychotics, and stimulants), gender, and population stratification (principal components 1–10). For models examining the effects of ADORA2A and CYP1A variants on sleep quality and duration, odds ratios were also adjusted for caffeine use and interaction terms between caffeine use and the included variants. Exploratory models including self-reported ethnicity were also fitted.
3. Results
A total of 6112 children aged 9–10 years met the criteria for inclusion in this study (Table 1). More boys than girls were included in the study cohort (54.2% versus 45.8%). The mean weight of the cohort was 38.3 kg, which is between the 85th and 95th percentile for 9–10 year-old boys and girls according to the World Health Organization’s weight-for-age reference table [33]. The study population was diverse, but most (62.7%) were of European ancestry and identified as White (48.2%) (see Supplementary Table S2 for concordance between ancestry and self-reported ethnicity). The average caffeine intake was 0.58 mg/kg/day. Under half (44.9%) of the cohort reported routinely getting the recommended sleep duration and about three-quarters (76.1%) reported normal sleep quality. None of the participants reported the use of CYP1A2 inhibitors (e.g., fluvoxamine) or inducers (e.g., tobacco) included in the Flockhart Drug Interactions Table [34].
Table 1.
Participant characteristics (n = 6112).
| Characteristic | |
|---|---|
| Gender, % (N) girl | 45.8 (2801) |
| Ancestry, % (N) | |
| European | 62.7 (3835) |
| African | 14.9 (914) |
| East Asian | 0.9 (60) |
| American | 0.8 (52) |
| Admixed | 13.2 (810) |
| Not available | 7.2 (441) |
| Ethnicity (self-reported), % N | |
| White | 48.2 (2948) |
| Black | 14.7 (897) |
| Asian | 1.4 (83) |
| Hispanic | 19.1 (1168) |
| Other | 9.4 (574) |
| Not available | 7.2 (442) |
| Weight, mean (sd) kg | 38.3 (11.1) |
| Concomitant psychotropic use, % (N) | |
| SSRI 1 | 0.8 (47) |
| Antipsychotic 2 | 0.3 (17) |
| Stimulant 3 | 4.9 (298) |
| Caffeine use, mean (sd) mg/kg/day | 0.58 (0.008) |
| Sleep Duration, % (N) | |
| 9–11 h | 44.9 (2742) |
| 8–9 h | 38.4 (2349) |
| 7–8 h | 13.4 (816) |
| 5–7 h | 3.2 (193) |
| <5 h | 0.2 (12) |
| SDSC total score, mean (sd) | 35.8 (0.09) |
| SDSC sleep quality, % (N) disturbed | 23.9 (1461) |
SDSC, Sleep Disturbance Scale for Children; SSRI, selective serotonin reuptake inhibitors. 1 Fluoxetine, citalopram, escitalopram, paroxetine, sertraline, fluvoxamine, and vortioxetine; 2 risperidone, aripiprazole, quetiapine, and olanzapine; 3 amphetmaine, dextroamphetamine, and methylphenidate, lisdexamfetamine. sd = standard deviation, N = number of participants.
3.1. Effect of Caffeine on Sleep Quality and Duration
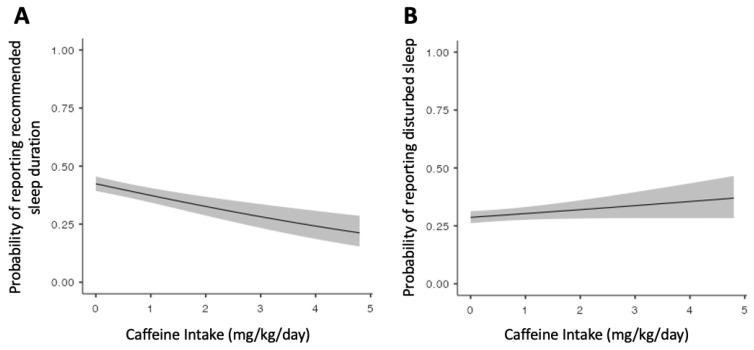
Caffeine intake was not associated with disturbed sleep quality (OR = 1.08, 95% CI = 0.99–1.18, and p = 0.072) but it was associated with sleep duration (OR = 0.81, 95% CI = 0.75–0.88, and p = 1.4 × 10−6) (Figure 1). The inclusion of self-reported ethnicity in these models did not meaningfully change the effect estimates. The ROC curve analysis of the caffeine intake’s ability to distinguish sleep duration above or below the recommended 9 h of sleep revealed an area under the curve of 0.57 (95% CI = 0.56–0.59, p < 0.001). A caffeine intake threshold of 0.10 mg/kg/day was identified as the optimal threshold (Youden J-index = 0.11) for differentiating children who did from those that did not report > 9 h of sleep per night (Table 2).
Figure 1.
Predicted probability of reporting (A) recommended sleep duration (>9 h) and (B) disturbed sleep by caffeine intake (mg/kg/day) adjusted for psychotropic use (i.e., SSRIs, antipsychotics, and stimulants), gender, and population stratification (principal components 1–10). Shading represents 95% confidence intervals.
Table 2.
Sensitivity and specificity at various thresholds of caffeine intake for detection of children reporting fewer than 9 h of sleep.
| Caffeine Threshold (mg/kg/day) | Detection of Children Reporting < 9 h of Sleep Per Night |
||
|---|---|---|---|
| Sensitivity | Specificity | Youden J-Index * | |
| 0.01 | 0.99 | 0.01 | 0.00 |
| 0.05 | 0.79 | 0.30 | 0.09 |
| 0.10 | 0.66 | 0.45 | 0.11 |
| 0.25 | 0.41 | 0.68 | 0.09 |
| 0.50 | 0.26 | 0.82 | 0.08 |
| 1.00 | 0.14 | 0.91 | 0.05 |
| 1.50 | 0.08 | 0.95 | 0.02 |
| 2.00 | 0.05 | 0.97 | 0.01 |
| 2.50 a | 0.03 | 0.98 | 0.01 |
| 3.00 b | 0.02 | 0.99 | 0.01 |
a = Health Canada recommended threshold; b = European Food Safety Authority recommended threshold; * J = (sensitivity + specificity − 1).
3.2. Effect of CYP1A and ADORA2A Genotypes on Caffeine Intake
Genotype frequencies for CYP1A rs2472297 and ADORA2A rs5751876 in the current study and those reported in the five 1000 Genomes Project populations [35] are shown in Table 3. As expected, the genotype frequencies were most closely aligned with those reported in European populations. Neither rs2472297 (p = 0.509), rs762551 (p = 0.864), nor rs5751876 (p = 0.159) were associated with caffeine intake (Supplementary Figure S1).
Table 3.
CYP1A and ADORA2A genotype frequencies in the current study and 1000 genomes populations.
| 1000 Genomes Project | ||||||||
|---|---|---|---|---|---|---|---|---|
| SNP | Genotype | Current Study (n = 6112) | ALL (n = 2504) | AFR (n = 661) | AMR (n = 347) | EAS (n = 504) | EUR (n = 503) | SAS (n = 489) |
| rs2472297 | C/C | 70.3 | 88.0 | 97.1 | 83.0 | 100.0 | 62.0 | 93.5 |
| (CYP1A) | C/T | 26.5 | 10.9 | 2.9 | 16.1 | 0.0 | 33.0 | 6.5 |
| T/T | 3.2 | 1.1 | 0.0 | 0.9 | 0.0 | 5.0 | 0.0 | |
| rs762551 | C/C | 10.2 | 15.2 | 19.8 | 7.5 | 10.9 | 11.5 | 22.7 |
| (CYP1A2) | C/A or A/A | 89.8 | 84.8 | 80.2 | 92.5 | 89.1 | 88.5 | 77.3 |
| rs5751876 | C/C | 31.8 | 20.7 | 9.4 | 28.0 | 22.8 | 37.4 | 11.7 |
| (ADORA2A) | C/T | 46.6 | 47.0 | 44.9 | 52.4 | 48.8 | 47.1 | 44.0 |
| T/T | 21.7 | 32.3 | 45.7 | 19.6 | 28.4 | 15.5 | 44.4 | |
ALL, full 1000 Genomes Project sample; AFR, African; AMR, American; EAS, East Asian; EUR, European; SAS, South Asian.
3.3. Effect of CYP1A and ADORA2A Genotypes on Sleep Quality and Duration
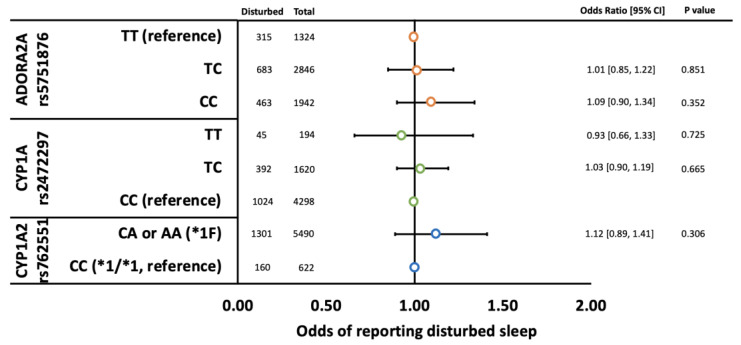
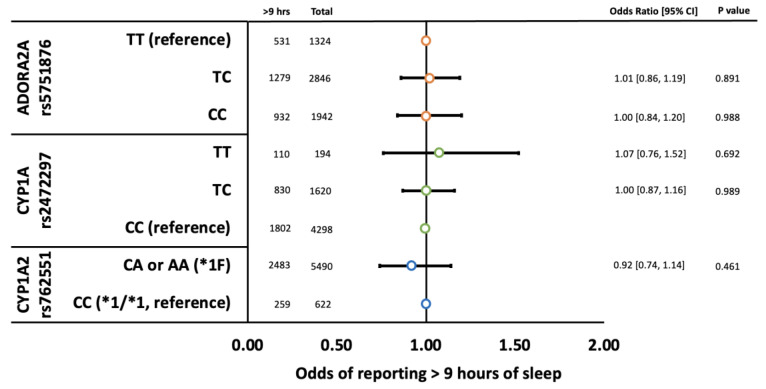
Neither rs2472297, rs762551, nor rs5751876 were associated with disturbed sleep quality (Figure 2) or sleep duration (Figure 3). Likewise, genotype by caffeine intake interactions were not detected (Supplementary Table S3).
Figure 2.
Odds of children reporting disturbed sleep by ADORA2A rs5751876 (orange circles), CYP1A rs2472297 (green circles), and CYP1A2 rs762551 (blue circles) genotypes adjusted for psychotropic use (i.e., SSRIs, antipsychotics, and stimulants), gender, caffeine use (mg/kg/day), and population stratification (principal components 1–10). Error bars represent 95% confidence intervals.
Figure 3.
Odds of children reporting > 9 h of sleep by ADORA2A rs5751876 (orange circles), CYP1A rs2472297 (green circles), and CYP1A2 rs762551 (blue circles) genotypes adjusted for psychotropic use (i.e., SSRIs, antipsychotics, and stimulants), gender, caffeine use (mg/kg/day), and population stratification (principal components 1–10). Error bars represent 95% confidence intervals.
4. Discussion
Our findings showed the mean caffeine intake among children in this study was 0.58 mg/kg/day, which is equivalent to 22 mg or approximately 7 ounces (207 mL) of cola (e.g., Pepsi) per day for the average 38 kg child in the ABCD cohort. This average daily dose is below the recommended thresholds published by Health Canada (2.5 mg/kg/day) [6] and the EFSA (3.0 mg/kg/day) [7], but it is higher than previously reported daily intakes [36]. In a representative survey of US children aged 6–11 from 1994 to 1998 [36], mean caffeine intake estimates of 0.40 mg/kg/day were reported, supporting the notion that caffeine intake trends over time have increased in children [1].
In partial support of our hypothesis, children with a higher caffeine intake had lower odds of reporting > 9 h of sleep per night. For every mg/kg/day of caffeine consumed, there was a 19% (95% CI = 12–26%) decrease in the odds of children reporting > 9 h of sleep, in concordance with the findings in adults [37]. We further explored the sensitivity and specificity of various caffeine intake thresholds to determine whether the current recommended intake thresholds set by Health Canada (2.5 mg/kg/day) and the EFSA (3.0 mg/kg/day) were sufficient for guarding against a lower than recommend sleep duration. Our results suggested that the current recommended thresholds have a limited ability to distinguish children above or below the recommended 9 h of sleep per night. Only 1.5% and 2.4% of the cohort consumed caffeine above the Health Canada and EFSA recommended thresholds, respectively, but more than half (55.1%) of the cohort reported getting fewer than the recommended 9 h of sleep per night. Our findings suggest a significantly lower threshold of 0.10 mg/kg/day would be the most appropriate for children aged 9–10. However, the sensitivity (0.66) and specificity (0.45) of this threshold was modest, and may have limited clinical utility as indicated by a Youden J-index less than 0.50 [32].
Our findings did not support an association between caffeine intake and disturbed sleep quality. The absence of an association could be attributed to the relatively low mean caffeine intake in the cohort, the sensitivity of the SDSC measure to detect caffeine-induced disturbed sleep quality, or the presence of moderating factors in which only certain subgroups are susceptible to caffeine-induced disturbed sleep quality. This latter explanation is supported by previous work in the full ABCD cohort that reported a modest association (Spearman’s rho = 0.06) between caffeine intake and SDSC measured sleep disturbance [38]. Unlike this previous study, we excluded children that were presumed to have a higher propensity for sleep disturbance (i.e., those with high ADHD symptomatology and those taking melatonin or antihistamines), which may explain why we were unable to detect a similar association between caffeine and disturbed sleep quality. Regardless, the association between caffeine intake and disturbed sleep quality measured by the SDSC, if present, appears modest, and may have minimal clinical significance.
Our results also did not support an association between genetic variation in ADORA2A or CYP1A and sleep quality or duration. This contrasts with several adult studies that have reported that ADORA2A rs5751876 C homozygotes were more likely to experience sleep disturbances when consuming caffeine [10,12,13,14,15,16]. However, these adult studies included participants with significantly higher mean daily intakes of caffeine (range: 2.1 mg/kg/day–4.8 mg/kg/day) than was reported in the current study (0.58 mg/kg/day), suggesting that the moderating effect of ADORA2A rs5751876 on caffeine-induced sleep phenotypes may be dose-dependent. Unlike ADORA2A, the rs2472297 promoter region variant in the CYP1A locus has not been previously associated with caffeine-induced sleep phenotypes, but adult T allele carriers at this locus have been shown to consume greater amounts of caffeine and have lower caffeine plasma concentrations [17,18,19]. We did not have access to plasma concentrations and did not detect a difference in caffeine consumption by the CYP1A genotype, nor did genotype correlate with sleep duration or quality. The failure to detect an association with consumption could be a result of a gatekeeping effect by which parents of the participating children limit their access to caffeine, inhibiting consumption among children that might otherwise consume more caffeine. In addition, the developmental trajectory or ontogeny of CYP1A may explain the lack of association in the current study. In early childhood, CYP1A2 is expressed at 1.5 times that of adults before regressing to adult levels in early adolescence [39]. The precise level of CYP1A2 expression in children aged 9–10 is uncertain, with modeling studies suggesting that expression could be increased or equivalent to adult levels [39,40]. If CYP1A2 expression is higher among children aged 9–10, it is possible that the increased enzyme activity associated with the CYP1A rs2472297 T allele may have a lesser impact on caffeine plasma concentrations, consumption, and sleep phenotypes. Future works exploring whether the CYP1A rs2472297 variant is associated with caffeine consumption and sleep phenotypes in an age-dependent manner are warranted.
Our findings should be interpreted in the context of several caveats. First, caffeine intake was collected using a validated approach but was reliant on the recall of caffeine consumed per day for the previous six months. Second, the time of day at which caffeine was typically consumed by participating children was not collected and could modify the magnitude of caffeine’s effects on sleep phenotypes. Third, caffeine consumption was based exclusively on the intake of caffeinated beverages. The consumption of other sources of caffeine (e.g., chocolate) was not included, and as such, our caffeine consumption was likely underestimated in this study. Fourth, our results are based on cross-sectional data, prohibiting temporal relationships being established between caffeine intake and sleep duration or quality. Fifth, the causes of caffeine-associated sleep disturbances are multifactorial. We did not examine several lifestyle (e.g., physical activity, diet), medical (e.g., health conditions), psychological (e.g., trauma), and familial (e.g., family history of psychiatric conditions) factors that may impact caffeine use and sleep disturbances. The future exploration of these factors is warranted. Finally, sleep phenotypes were measured subjectively via self-report. Objective measures of sleep duration and quality via wearable devices should be considered in future studies.
In summary, the current study represents the largest examination of caffeine’s association with sleep duration and quality in children. The findings show a clear association between increased caffeine intake and reduced sleep duration and suggest that the current recommended caffeine thresholds for children may be inadequate to ensure that they obtain the recommended hours of sleep. Despite robust findings in adults that suggest CYP1A and ADORA2A genetic variations moderate caffeine intake and caffeine-induced sleep disturbance, the current study could not replicate these findings in children. As such, additional work to identify the appropriate markers of caffeine sensitivity in children is warranted.
Acknowledgments
Data used in the preparation of this article were obtained from the Adolescent Brain Cognitive DevelopmentSM (ABCD) Study (https://abcdstudy.org (accessed on 11 May 2021)), held in the NIMH Data Archive (NDA). This is a multisite, longitudinal study designed to recruit more than 10,000 children aged 9–10 years, and to follow them over 10 years into early adulthood. The ABCD Study® is supported by the National Institutes of Health and additional federal partners under award numbers U01DA041048, U01DA050989, U01DA051016, U01DA041022, U01DA051018, U01DA051037, U01DA050987, U01DA041174, U01DA041106, U01DA041117, U01DA041028, U01DA041134, U01DA050988, U01DA051039, U01DA041156, U01DA041025, U01DA041120, U01DA051038, U01DA041148, U01DA041093, U01DA041089, U24DA041123, and U24DA041147. The full list of supporters is available at https://abcdstudy.org/federal-partners.html (accessed on 11 May 2021). The listing of participating sites and the complete listing of the study investigators can be found at https://abcdstudy.org/consortium_members/ (accessed on 11 May 2021). ABCD consortium investigators designed and implemented the study and/or provided data but did not necessarily participate in the analysis or writing of this report. This manuscript reflects the views of the authors and may not reflect the opinions or views of the NIH or ABCD consortium investigators.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/genes14020289/s1, Table S1: Standardized caffeine content by type of beverage; Table S2: Concordance between self-reported ethnicity and genomic-derived ancestry; Table S3: Binomial logistic regression models assessing the impact of ADORA2A and CYP1A genotypes on sleep duration and quality; Figure S1: mean caffeine intake by CYP1A and ADORA2A genotype adjusted for gender and population stratification.
Author Contributions
Conceptualization, C.A.B.; methodology, C.D.J. and C.A.B.; formal analysis, C.D.J., A.N. and C.A.B.; data curation, C.D.J., A.N. and R.Z.; writing—original draft preparation, C.D.J. and C.A.B.; writing—review and editing, C.D.J., A.N., R.Z. and C.A.B.; supervision, C.A.B.; project administration, C.A.B.; funding acquisition, C.D.J. and C.A.B. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study was exempt from ethics review. The study was conducted in accordance with the Declaration of Helsinki.
Informed Consent Statement
Informed consent was obtained from all subjects.
Data Availability Statement
The ABCD data used in this report came from http://dx.doi.org/10.15154/1519007 (accessed on 11 May 2021).
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research was funded by an Alberta Innovates Summer Research Studentship.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Temple J.L. Review: Trends, Safety, and Recommendations for Caffeine Use in Children and Adolescents. J. Am. Acad. Child Adolesc. Psychiatry. 2018;58:36–45. doi: 10.1016/j.jaac.2018.06.030. [DOI] [PubMed] [Google Scholar]
- 2.Lodato F., Araújo J., Barros H., Lopes C., Agodi A., Barchitta M., Ramos E. Caffeine intake reduces sleep duration in adolescents. Nutr. Res. 2013;33:726–732. doi: 10.1016/j.nutres.2013.06.005. [DOI] [PubMed] [Google Scholar]
- 3.Owens J.A., Mindell J., Baylor A. Effect of energy drink and caffeinated beverage consumption on sleep, mood, and performance in children and adolescents. Nutr. Rev. 2014;72:65–71. doi: 10.1111/nure.12150. [DOI] [PubMed] [Google Scholar]
- 4.Temple J.L. Caffeine use in children: What we know, what we have left to learn, and why we should worry. Neurosci. Biobehav. Rev. 2009;33:793–806. doi: 10.1016/j.neubiorev.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.American Academy of Child and Adolescent Psychiatry Caffeine and Children. No. 131. 2020. [(accessed on 1 September 2022)]. Available online: https://www.aacap.org/AACAP/Families_and_Youth/Facts_for_Families/FFF-Guide/Caffeine_and_Children-131.aspx#:~:text=At%20this%20time%2C%20pediatricians%20advise,those%2012%2D18%20years%20old.
- 6.Health Canada Caffeine in Foods. 2022. [(accessed on 1 September 2022)]. Available online: https://www.canada.ca/en/health-canada/services/food-nutrition/food-safety/food-additives/caffeine-foods.html.
- 7.European Food Safety Authority (EFSA) Outcome of a Public Consultation on the Draft Scientific Opinion of the EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA) on the Safety of Caffeine. Volume 12. EFSA; Parma, Italy: 2015. pp. 1–101. [Google Scholar]
- 8.Nehlig A. Interindividual Differences in Caffeine Metabolism and Factors Driving Caffeine Consumption. Pharmacol. Rev. 2018;70:384–411. doi: 10.1124/pr.117.014407. [DOI] [PubMed] [Google Scholar]
- 9.Thorn C.F., Aklillu E., McDonagh E.M., Klein T.E., Altman R.B. PharmGKB summary: Caffeine pathway. Pharmacogenet. Genom. 2012;22:389–395. doi: 10.1097/FPC.0b013e3283505d5e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Byrne E.M., Johnson J., McRae A.F., Nyholt D.R., Medland S.E., Gehrman P.R., Heath A.C., Madden P.A., Montgomery G.W., Chenevix-Trench G., et al. A Genome-Wide Association Study of Caffeine-Related Sleep Disturbance: Confirmation of a Role for a Common Variant in the Adenosine Receptor. Sleep. 2012;35:967–975. doi: 10.5665/sleep.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cornelis M.C., Kacprowski T., Menni C., Gustafsson S., Pivin E., Adamski J., Artati A., Eap C.B., Ehret G., Friedrich N., et al. Genome-wide association study of caffeine metabolites provides new insights to caffeine metabolism and dietary caffeine-consumption behavior. Hum. Mol. Genet. 2016;25:5472–5482. doi: 10.1093/hmg/ddw334. [DOI] [PubMed] [Google Scholar]
- 12.Nunes R.A., Mazzotti D.R., Hirotsu C., Andersen M.L., Tufik S., Bittencourt L. The association between caffeine consumption and objective sleep variables is dependent on ADORA2A c.1083T>C genotypes. Sleep Med. 2017;30:210–215. doi: 10.1016/j.sleep.2016.06.038. [DOI] [PubMed] [Google Scholar]
- 13.Rétey J.V., Adam M., Khatami R., Luhmann U.F.O., Jung H.H., Berger W., Landolt H.-P. A genetic variation in the adenosine A2A receptor gene (ADORA2A) contributes to individual sensitivity to caffeine effects on sleep. Clin. Pharmacol. Ther. 2007;81:692–698. doi: 10.1038/sj.clpt.6100102. [DOI] [PubMed] [Google Scholar]
- 14.Bodenmann S., Hohoff C., Freitag C., Deckert J., Rétey J.V., Bachmann V., Landolt H.-P. Polymorphisms of ADORA2A modulate psychomotor vigilance and the effects of caffeine on neurobehavioural performance and sleep EEG after sleep deprivation. Br. J. Pharmacol. 2012;165:1904–1913. doi: 10.1111/j.1476-5381.2011.01689.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baur D.M., Lange D., Elmenhorst E.-M., Elmenhorst D., Bauer A., Aeschbach D., Landolt H.-P. Coffee effectively attenuates impaired attention in ADORA2A C/C-allele carriers during chronic sleep restriction. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2021;109:110232. doi: 10.1016/j.pnpbp.2020.110232. [DOI] [PubMed] [Google Scholar]
- 16.Erblang M., Drogou C., Gomez-Merino D., Metlaine A., Boland A., Deleuze J.F., Thomas C., Sauvet F., Chennaoui M. The Impact of Genetic Variations in ADORA2A in the Association between Caffeine Consumption and Sleep. Genes. 2019;10:1021. doi: 10.3390/genes10121021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shin S.-Y., The Multiple Tissue Human Expression Resource (MuTHER) Consortium. Fauman E.B., Petersen A.-K., Krumsiek J., Santos R., Huang J., Arnold M., Erte I., Forgetta V., et al. An atlas of genetic influences on human blood metabolites. Nat. Genet. 2014;46:543–550. doi: 10.1038/ng.2982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sulem P., Gudbjartsson D., Geller F., Prokopenko I., Feenstra B., Aben K.K., Franke B., Heijer M.D., Kovacs P., Stumvoll M., et al. Sequence variants at CYP1A1–CYP1A2 and AHR associate with coffee consumption. Hum. Mol. Genet. 2011;20:2071–2077. doi: 10.1093/hmg/ddr086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.The Coffee and Caffeine Genetics Consortium. Cornelis M.C., Byrne E.M., Esko T., Nalls M.A., Ganna A., Paynter N., Monda K.L., Amin N., Fischer K., et al. Genome-wide meta-analysis identifies six novel loci associated with habitual coffee consumption. Mol. Psychiatry. 2014;20:647–656. doi: 10.1038/mp.2014.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Koonrungsesomboon N., Khatsri R., Wongchompoo P., Teekachunhatean S. The impact of genetic polymorphisms on CYP1A2 activity in humans: A systematic review and meta-analysis. Pharm. J. 2017;18:760–768. doi: 10.1038/s41397-017-0011-3. [DOI] [PubMed] [Google Scholar]
- 21.Karcher N.R., Barch D.M. The ABCD study: Understanding the development of risk for mental and physical health outcomes. Neuropsychopharmacology. 2020;46:131–142. doi: 10.1038/s41386-020-0736-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Achenbach T. The Child Behavior Checklist and related instruments. In: Maruish M.E., editor. The Use of Psychological Testing for Treatment Planning and Outcomes Assessment. Lawrence Erlbaum Associates Publishers; Hillsdale, NJ, USA: 1999. pp. 429–466. [Google Scholar]
- 23.Wajszilber D., Santisteban J.A., Gruber R. Sleep disorders in patients with ADHD: Impact and management challenges. Nat. Sci. Sleep. 2018;10:453–480. doi: 10.2147/NSS.S163074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marmorstein N.R. Energy Drink and Coffee Consumption and Psychopathology Symptoms Among Early Adolescents: Cross-Sectional and Longitudinal Associations. J. Caffeine Res. 2016;6:64–72. doi: 10.1089/jcr.2015.0018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vanderlee L., Reid J.L., White C.M., Acton R.B., Kirkpatrick S.I., Pao C.-I., Rybak M.E., Hammond D. Evaluation of a 24-Hour Caffeine Intake Assessment Compared with Urinary Biomarkers of Caffeine Intake among Young Adults in Canada. J. Acad. Nutr. Diet. 2018;118:2245–2253.e1. doi: 10.1016/j.jand.2018.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bruni O., Ottaviano S., Guidetti V., Romoli M., Innocenzi M., Cortesi F., Giannotti F. The Sleep Disturbance Scale for Children (SDSC). Construction and validation of an instrument to evaluate sleep disturbances in childhood and adolescence. J. Sleep Res. 1996;5:251–261. doi: 10.1111/j.1365-2869.1996.00251.x. [DOI] [PubMed] [Google Scholar]
- 27.Paruthi S., Brooks L.J., D’Ambrosio C., Hall W.A., Kotagal S., Lloyd R.M., Malow B.A., Maski K., Nichols C., Quan S.F. Recommended Amount of Sleep for Pediatric Populations: A Consensus Statement of the American Academy of Sleep Medicine. J. Clin. Sleep Med. 2016;12:785–786. doi: 10.5664/jcsm.5866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Baurley J.W., Edlund C.K., Pardamean C.I., Conti D.V., Bergen A.W. Smokescreen: A targeted genotyping array for addiction research. BMC Genom. 2016;17:145. doi: 10.1186/s12864-016-2495-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Uban K.A., Horton M.K., Jacobus J., Heyser C., Thompson W.K., Tapert S.F., Madden P.A., Sowell E.R. Biospecimens and the ABCD study: Rationale, methods of collection, measurement and early data. Dev. Cogn. Neurosci. 2018;32:97–106. doi: 10.1016/j.dcn.2018.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Price A.L., Patterson N.J., Plenge R.M., Weinblatt M.E., Shadick N.A., Reich D. Principal components analysis corrects for stratification in genome-wide association studies. Nat. Genet. 2006;38:904–909. doi: 10.1038/ng1847. [DOI] [PubMed] [Google Scholar]
- 31.Jamovi. The Jamovi Project. 2019. [(accessed on 11 May 2021)]. Available online: https://www.jamovi.org.
- 32.Youden W.J. Index for rating diagnostic tests. Cancer. 1950;3:32–35. doi: 10.1002/1097-0142(1950)3:1<32::AID-CNCR2820030106>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 33.World Health Organization Weight-for-Age (5–10 Years) [(accessed on 1 September 2022)]. Available online: https://www.who.int/tools/growth-reference-data-for-5to19-years/indicators/weight-for-age-5to10-years.
- 34.Flockhart D. Drug Interactions: Cytochrome P450 Drug Interaction Table 2007. [(accessed on 11 May 2021)]. Available online: https://drug-interactions.medicine.iu.edu.
- 35.Fairley S., Lowy-Gallego E., Perry E., Flicek P. The International Genome Sample Resource (IGSR) collection of open human genomic variation resources. Nucleic Acids Res. 2019;48:D941–D947. doi: 10.1093/nar/gkz836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Frary C.D., Johnson R.K., Wang M.Q. Food sources and intakes of caffeine in the diets of persons in the United States. J. Am. Diet. Assoc. 2005;105:110–113. doi: 10.1016/j.jada.2004.10.027. [DOI] [PubMed] [Google Scholar]
- 37.Chaudhary N.S., Grandner M., Jackson N.J., Chakravorty S. Caffeine consumption, insomnia, and sleep duration: Results from a nationally representative sample. Nutrition. 2016;32:1193–1199. doi: 10.1016/j.nut.2016.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang H., Lee Z.X., Qiu A. Caffeine intake and cognitive functions in children. Psychopharmacology. 2020;237:3109–3116. doi: 10.1007/s00213-020-05596-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Upreti V.V., Wahlstrom J.L. Meta-analysis of hepatic cytochrome P450 ontogeny to underwrite the prediction of pediatric pharmacokinetics using physiologically based pharmacokinetic modeling. J. Clin. Pharmacol. 2015;56:266–283. doi: 10.1002/jcph.585. [DOI] [PubMed] [Google Scholar]
- 40.Salem F., Johnson T.N., Abduljalil K., Tucker G.T., Rostami-Hodjegan A. A Re-evaluation and Validation of Ontogeny Functions for Cytochrome P450 1A2 and 3A4 Based on In Vivo Data. Clin. Pharmacokinet. 2014;53:625–636. doi: 10.1007/s40262-014-0140-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The ABCD data used in this report came from http://dx.doi.org/10.15154/1519007 (accessed on 11 May 2021).