Abstract
Euphorbia humifusa is a plant species with medicinal and food characteristics used to treat diarrhea and other intestinal diseases. This study investigated the prebiotic effects of E. humifusa-derived polysaccharides (EHPs) on human colonic microbiota and their regulatory effects on ulcerative colitis (UC). Structural characterization showed that EHPs mainly consisted of galactose, glucose, and glucuronic acid and were heteropolysaccharides having molecular weights of 7.70 × 103 and 1.76 × 102 kDa, respectively. EHPs were identified as poorly absorbed macromolecules, verified by the apparent permeability coefficient values (Papp < 1.0 × 10−6 cm/s) and cellular uptake by Caco-2 cell monolayers. During in vitro fermentation studies, the contents of acetic, propionic, and valeric acids increased significantly in EHP-supplemented samples after 24 h compared to that in the control sample. Moreover, EHPs could alter the intestinal microbiota composition by increasing the relative abundance of Bifidobacterium and Holdemanella and reducing that of Escherichia-Shigella, Tyzzerella, and Parasutterella at the genus level. In a dextran sulfate sodium (DSS)-induced UC mouse model, EHPs alleviated UC symptoms by increasing the colon length, reversing the colon tissue damage and inhibiting pro-inflammatory cytokines. Overall, these results suggest that EHPs could be utilized as a potential prebiotic or a promising nutritional strategy for UC management.
Keywords: Euphorbia humifusa polysaccharides, absorption, fermentation, ulcerative colitis
1. Introduction
Ulcerative colitis (UC) is a chronic and recurrent inflammatory bowel disease. Most UC lesions start appearing in the rectum and can spread throughout the colon, and the characteristic symptoms include abdominal pain, bloody stools, and weight loss [1,2]. Although UC pathogenesis is still not fully elucidated, several recent studies have indicated a relationship between intestinal microbiota and UC. The intestinal microbiota is an important frontier in understanding the development and progression of UC and other diseases [3]. Gut microbiota usually attaches to the intestinal mucosal layer and plays crucial functions in maintaining host health, such as regulating the body’s immunity, enhancing nutrient absorption, preserving the integrity of the intestinal barrier, and increasing pathogen defense ability [4]. In the past decade, accumulating studies have shown that manipulating the intestinal flora could alleviate various chronic diseases, such as inflammatory bowel disease, obesity, hypertension, diabetes, and liver disorders. Thus, increasing efforts have been made to explore microbiologically-guided interventions to improve human health [5,6].
Polysaccharides have attracted impressive attention as regulators of intestinal microbiota. Because of specific digestive enzymes lacking in the human body, most polysaccharides can pass through the gastrointestinal tract to the colon, where they can be fermented by intestinal microbiota [7,8]. Notably, the fermentation of polysaccharides exerts a positive effect against UC, which could be attributed to its influence on inflammatory cytokines and intestinal microbiota [9]. Additionally, metabolites produced during colonic fermentation, including short-chain fatty acids (SCFAs), could promote the growth of probiotic organisms and prevent intestinal disorders [3]. Exploring the potential health benefits of polysaccharides has become one of the hot issues in current functional food and medical research. The plant species Euphorbia humifusa is widely distributed across Eastern Asia and has been used as a folk medicine in China and a health assistance food in Korea [10,11] (Figure 1). According to the Chinese Pharmacopeia (2020 edition), E. humifusa has been utilized in the treatment of dysentery and enteritis, with a suggestive role in maintaining gut ecosystem stability [10,11]. Considering these findings, we speculated that E. humifusa might be applied in UC treatment via regulating intestinal microbiota.
Figure 1.
A picture of fresh Euphorbia humifusa.
The effect of natural plants on intestinal bacteria is firmly known to be related to their active polysaccharides; however, little information is presently available on E. humifusa-derived polysaccharides (EHPs). Therefore, this study aims to evaluate the potential therapeutic effects of EHPs on UC. Although most polysaccharides can transit into the large intestine with few physicochemical changes in the upper digestive tract of humans, the absorption mechanism of EHPs remains unclear. Firstly, we employed a Caco-2 cell monolayer model to investigate the absorption behavior of EHPs. Secondly, the effects of EHPs on intestinal microbiota composition and SCFAs were studied via in vitro fermentation. Lastly, the anti-inflammatory and beneficial effects of EHPs were evaluated in UC mouse models. These findings could provide an alternative therapy for UC and promote the development and utilization of EHPs.
2. Materials and Methods
2.1. Materials
E. humifusa was harvested from the Anguo traditional Chinese medicine market (Baoding, Hebei Province, China). All monosaccharide standards were purchased from Solarbio Science and Technology Co. (Beijing, China), and the dextran standard was procured from Aladin company (Guangzhou, Guangdong Province, China). Other reagents used were of analytical grade.
2.2. Extraction and Purification of EHPs
According to a previous method [12], 20 g of E. humifusa ultrafine powder was extracted with 800 mL deionized water at 95 °C for 2 h. The crude polysaccharides were then concentrated and precipitated using 80% ethanol (v/v), followed by centrifugation at 4000 rpm for 10 min, and the precipitation was dissolved in distilled water and deproteinized by the Sevag method. The protein-removed sample was dialyzed against distilled water for 24 h (8–14 kDa molecular mass cutoff) and precipitated again in 80% ethanol (v/v). After centrifugation, the precipitate was freeze-dried.
2.3. Structural Characterization of EHPs
2.3.1. Chemical Composition
The total carbohydrate content of EHPs was determined by the phenol-sulfuric acid method [13], and the amount of protein was measured using Coomassie brilliant blue G250 [14].
2.3.2. Molecular Weight (Mw) Distribution
The Mw of polysaccharides was determined using high-performance size exclusion chromatography, as described previously by Xia et al. [15]. The instrument was equipped with a multi-angle laser light scattering system (DAWN, HELEOS, Wyatt Technology Co., Santa Barbara, CA, USA), connected with a refractive index detector (Waters-2414, Milford, MA, USA) and the MALLS system (Waters 2695, Milford, MA, USA), equipped with columns of TSK-gel G-3000 PW XL (300 × 7.8 mm, Tokyo, Japan) and TSK-gel G5000PWXL columns (300 × 7.8 mm, Tokyo, Japan). Briefly, the EHP solution (1 mg/mL) was passed through a 0.22 μm filter, and 20 μL of the sample was injected into the system. The experimental conditions were as follows: the mobile phase consisted of a 0.9% NaCl solution at a flow rate of 0.5 mL/min and 35 °C.
2.3.3. Monosaccharide Composition
EHPs (5 mg) were hydrolyzed by trifluoroacetic acid (TFA, 3.0 M, 2 mL) at 120 °C for 4 h in an ampoule bottle. After removing excess TFA, the resulting products were derivatized with 1-phenyl-3-methyl-5-pyrazolone (PMP). The PMP-labeled samples were analyzed using high-performance liquid chromatography (HPLC, L-20A, Shimadzu, Japan) with an XDB-C18 chromatographic column (250 mm × 4.6 mm, 5 μm, Agilent Technologies, Santa Clara, CA, USA). Glucose (Glc), galactose (Gal), galacturonic acid (GalA), glucuronic acid (GlcA), rhamnose (Rha), arabinose (Ara), xylose (Xyl), and mannose (Man) standards were combined for use as a mixed standard. The mobile phase was the mixture of acetonitrile and phosphate buffer (17:83, v/v). Samples (20 μL) were applied to the HPLC system (1.0 mL/min, 25 °C), and detected at 250 nm by a UV detector [16].
2.3.4. Fourier Transform Infrared (FTIR) Spectroscopic Analysis
The polysaccharide powder was mixed with spectroscopic potassium bromide (KBr) powder (200 mg), then pressed into pellets, and the spectra were recorded using an FTIR spectrometer (Thermo Fisher Scientific, Waltham, WA, USA) in the 400–4000 cm−1 region [17].
2.4. In Vitro Absorption of EHPs
2.4.1. Fluorescent Labeling of Polysaccharides
To understand the absorption characteristics and cellular uptake of EHPs by the Caco-2 cell (American Type Culture Collection, ATCC, HTB037) monolayers, EHPs were covalently labeled with fluorescein isothiocyanate (FITC), named FITC-EHPs and the fluorescence substitution degree was calculated to be 1.76% by UV-visible spectroscopy [18].
2.4.2. Caco-2 Cell-Based Intestinal Absorption Model
The cellular intestinal model was established based on a previously reported method [18]. Briefly, a 0.5 mL cell suspension (1 × 105 cells/mL) was seeded into a 12-well polycarbonate transwell chamber and incubated at 37 °C in an atmosphere of 5% CO2 for 21 days to allow cell monolayer formation. The integrity of the Caco-2 cell monolayers was evaluated by measuring values of transepithelial electrical resistance (TEER). Only the cells with TEER values higher than 500 Ω·cm2 were selected for transport studies. Furthermore, we explored the transport of FITC-EHPs (200 μg/mL) from the apical to basolateral side across Caco-2 cell monolayers. The fluorescence absorption was measured to estimate the concentration of EHPs, and the apparent permeability coefficient (Papp) was also determined. The cytotoxicity of FITC-EHPs to Caco-2 cells was assayed using the cell counting kit-8 (CCK-8) assay (Beyotime, Shanghai, China). The Caco-2 cells were prepared and dispersed in 96-well cell culture plates at a cellular density of 1.0 × 105 cells per well. After incubating with different concentrations of EHPs for 24 h, 10 μL of CCK-8 solution in PBS was added to each well and incubated at 37 °C for 2 h. In the end, the optical density of each well was measured by using a microplate reader at 450 nm.
2.4.3. EHP Uptake by Caco-2 Cells
Following a previously reported method by Wang et al. [18], Caco-2 cells with a density of 1 × 105/mL were seeded in a 48-well plate at 37 °C for 5 days, followed by incubation with a 1 mL solution of FITC-EHPs (1000 μg/mL) for 4 h. After incubation, the cellular uptake of EHP was observed by laser scanning confocal microscopy (LSCM, Olympus, Japan).
2.5. In Vitro Fermentation of EHPs
2.5.1. Fermentation
Fecal samples were provided by three healthy volunteers (age: 20–25) who had not taken antibiotics or probiotics in the past three months. All samples were mixed with phosphate buffer saline to obtain fecal suspensions (20%, w/v), which were then filtered through two layers of sterile gauze sponges and immediately transferred into an anaerobic jar. The growth medium was prepared according to Wu et al. [8]. The final fermentation liquid contained 10 mL growth medium, 9 mL fecal inoculum, and 100 mg EHPs. A blank control was set using the autoclaved ultrapure water, replacing the EHPs. All samples were incubated in anaerobic sealed tubes for 24 h at 37 °C.
2.5.2. Determination of SCFA Concentration
The SCFA contents at the fermentation time of 12 h and 24 h were determined by gas chromatography (GC) using the Agilent 7890 series GC system equipped with an HP-FFAP column (30 m × 0.25 mm × 0.25 μm, Agilent Technologies, USA) [8].
2.5.3. DNA Extraction and Sequence Analysis
After fermentation, the DNA of fermented samples was extracted using the E.Z.N.A.® Stool DNA Kit (Omega Bio-tek, Norcross, GA, USA). Next, the V3−V4 region of the bacterial 16S rRNA gene was PCR amplified with universal primers (338F and 806R) on a thermocycler PCR system (ABI GeneAmp 9700, Foster City, CA, USA). The PCR products were then purified using the AxyPrep DNA Gel Extraction Kit (Axygen Biosciences, Union City, CA, USA), and sequenced using the IlluminaHiSeq 2500 platform (San Diego, CA, USA) following the protocols developed/provided by MajorBio Bio-Pharm Technology Co., Ltd. (Shanghai, China).
2.6. Animal Studies
2.6.1. Experimental Design
4-week-old male mice were purchased from GuangDong Medical Laboratory Animal Center (Guangdong, China). Experimental mice were housed under a standard feeding environment and supplied ad libitum food and pure water with free access for one week to adapt them to laboratory conditions. All experiments involving animals were conducted in accordance with the National Institutional Animal Care and Medical Ethics Committee of GuangDong Medical Laboratory Animal Center (C202301-5). Mice were administered with 3% dextran sulfate sodium (DSS) dissolved in drinking water for 7 consecutive days to induce colitis, followed by DSS replacement with drinking water for 3 days. To examine the effects of EHPs on DSS-induced colitis, the mice were divided into five groups (n = 10 per group): (1) Control group: water (2) DSS group: 3% DSS + water; (3) LEHPs: 3% DSS + 100 mg/kg EHPs; (4) MEHPs: 3% DSS + 200 mg/kg EHPs; (5) HEHPs: 3% DSS + 300 mg/kg EHPs. The EHPs were dissolved in water and administered by gavage feeding every day. The total experimental and EHP administration period was 10 days. The mice were weighed and monitored for stool consistency daily [19]. The mice colons were fixed in a 4% paraformaldehyde stationary solution for dehydration, embedded in paraffin, cut into 5 μm sections, and subjected to hematoxylin and eosin (H&E) staining for histopathological examinations [20].
2.6.2. qRT-PCR
Total RNA from colitis tissue was extracted and purified using the Total RNA Extraction Kit (Foregen, Sichuan, China) and quantified by a Trace Ultraviolet-Visible Spectrophotometer. RNA was reverse transcribed to cDNA using the RNA PrimeScript™ RT Master Mix (Perfect Real Time, Takara Biomedical Technology Co., Ltd., Beijing, China). qRT-PCR was carried out using the SYBR Premix ExTaqTM (Takara). Fold changes from PCR analysis were determined using the ΔΔCT method [21]. The primers of RT-qPCR were listed in Table 1.
Table 1.
Primer Sequences for RT-PCR.
| Genes | Forward Primer | Reverse Primer |
|---|---|---|
| IL-6 | 5′-AGCGATGATGCACTGTCAGA-3′ | 5′-GGAACTCCAGAAGACCAGAGC-3′ |
| IL-17 | 5′-TTCTTTCAAACAAAGGACCAGC-3′ | 5′-GCAACCCAAGTAACCCTTAAAG-3′ |
| IL-10 | 5′-GACTTCACCATGGAACCCGT-3′ | 5′-GGAGACTGCCCATTCTCGAC-3′ |
2.7. Statistical Data Analysis
All data are expressed as the mean ± standard deviation (SD), and the statistical analysis was carried out using SPSS 26.0 software (SPSS Inc., Chicago, IL, USA). The analysis methods included one-way analysis of variance (ANOVA) and Tukey’s test for two independent samples.
3. Results
3.1. Structural Characterization of EHPs
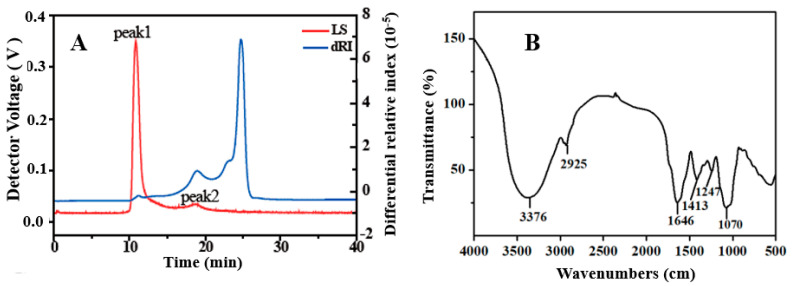
EHPs were prepared through the conventional heating method, containing carbohydrate and protein contents of 75.30% and 2.02%, respectively (Table 2). This result revealed that EHPs comprised polysaccharide transport chains and protein residues or residual free proteins [22]. Moreover, EHPs consisted of galactose (Gal), glucose (Glu), glucuronic acid (GluA), arabinose (Ara), and mannose (Man) with the respective molar ratio percentages of 55.34:21.71:8.30:6.30:6.12, indicating Gal and Glu as the major monosaccharide components. The HPSEC chromatograms of EHPs exhibited two peaks (Figure 2A) at a ratio of 15.8:84.2, with the corresponding Mw values of 7.70 × 103 kDa and 1.76 × 102 kDa, further indicating that EHPs are heteropolysaccharides. In a previous report, three polysaccharides extracted from Sagittaria sagittifolia L. contained Glu and Gal with varying molar percentages and were classified as heteropolysaccharides by Gu et al. [22].
Table 2.
Chemical properties and monosaccharide composition of EHPs.
| Items | Content |
|---|---|
| Composition (w%) | |
| Carbohydrate | 75.30 ± 0.04 |
| Protein | 2.02 ± 0.41 |
| Monosaccharide composition (molar ratio %) | |
| Mannose (Man) | 6.12 |
| Rhamnose (Rha) | 1.47 |
| Glucuronic acid (GluA) | 8.30 |
| Galacturonic acid (GalA) | 0.77 |
| Glucose (Glu) | 21.71 |
| Galactose (Gal) | 55.34 |
| Arabinose (Ara) | 6.30 |
Figure 2.
The molecular weight (A) and FITR spectrum (B) of EHPs.
The FTIR spectra were recorded for structural insights into EHPs (Figure 2B). A broad absorption peak at 3376 cm−1 could be attributed to the O-H stretching vibration and the rise at 2925 cm−1 to the C-H stretching vibration. The absorption peaks at 1646 cm−1 and 1413 cm−1 originated from the asymmetric and symmetric vibrations of the COO- group, indicating the presence of uronic acid [22], which is consistent with the monosaccharide composition of EHPs. The absorption peak of the methyl-ester around 1700 cm−1 was not observed, while the C-O stretching of the acetyl group can be seen at 1247 cm−1. These results indicate that EHPs are a type of acetylated and non-methyl-esterified polysaccharides [23]. In addition, the peak at 1070 cm−1 suggests the presence of pyranosyl glycoside bonds in the EHPs.
3.2. In Vitro Absorption Characteristics of Polysaccharides
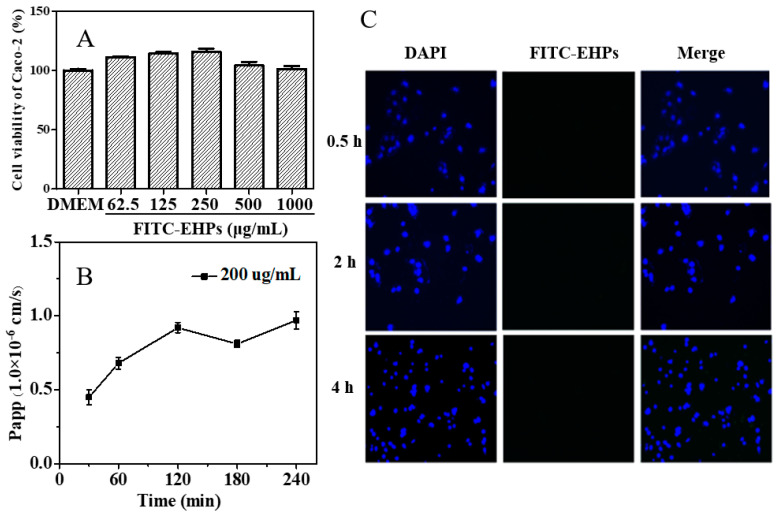
Caco-2 cells can slowly differentiate into a monolayer exhibiting many properties similar to the small intestinal villus epithelium and, thus, have been used to study the mechanism of nutrient absorption and transport [24,25]. To explore the intestinal absorption of EHPs by Caco-2 cell monolayers, the cytotoxic effect of FTIC-EHPs on Caco-2 cells was first examined by the CCK-8 assay (Figure 3A). The Caco-2 cell viability exceeded 100%, even when the concentration of FTIC-EHPs reached 1000 μg/mL, suggesting that FTIC-EHPs had no toxicity effects on Caco-2 cells. Furthermore, Caco-2 cells were grown on a polycarbonate film for 21 days to obtain the monolayers to study EHP transport. The TEER value is a strong indicator of the epithelial barrier integrity of cell monolayers; a high TEER value signifies tight junctions between the cell monolayers [24,25]. In this study, the TEER values of monolayers exceeded 500 Ω·cm2 after 21 days of culture, satisfying the experimental requirement.
Figure 3.
Toxicity of FITC-EHPs on Caco-2 cells (A), Papp value (B), and uptake of FITC-EHPs in Caoco-2 cells (C).
The paracellular and transcellular pathways are the two main pathways reported for macromolecule transport through the intestinal epithelial layer [26,27]. The paracellular pathway is restricted due to the tight junctions; typically, the gaps between the epithelial cells do not allow the passage of high Mw polysaccharides (Mw > 3.5 kDa) [28]. To support the smooth passing of these polysaccharides, the tight junctions must be relaxed or open; however, this may cause a decrease in the TEER value [24,25]. In this study, no apparent drop in TEER value was observed at any time, indicating the probable transport of EHPs across the Caco-2 cell monolayers through the transcellular pathway. The transport process of this pathway is mediated by crossing both the apical and basolateral membranes through passive diffusion or carrier vesicle-mediated processes.
The Caco-2 cell monolayer-associated Papp value is closely related to the intestinal absorption ability of a substance [29]. Generally, a Papp value higher than 1.0 × 10−6 cm/s denotes ~70–100% absorptivity of a drug. On the contrary, if the Papp value is lower than 1.0 × 10−6 cm/s, the drug can be regarded as poorly absorbed (~0–20%) [18,25]. The Papp values of FITC-EHPs detected at various time points within 4 h were observed in the range of 0.45 × 10−6 cm/s to 0.97 × 10−6 cm/s (Figure 3B), suggesting that FITC-EHPs are poorly-absorbed biological macromolecules. The cellular uptake of FITC-EHPs further confirmed this result, as shown in Figure 3C. A slight green fluorescence was observed in Caco-2 cells, even after incubation with a high concentration of FITC-EHPs (1000 ug/mL), indicating the uptake of only a few EHP molecules. Consequently, the unabsorbed EHPs could enter the colon as a source of carbohydrates and be fermented by the intestinal microbiota to regulate colon health.
3.3. In Vitro Fermentation of EHPs
3.3.1. Changes in Intestinal Microecology
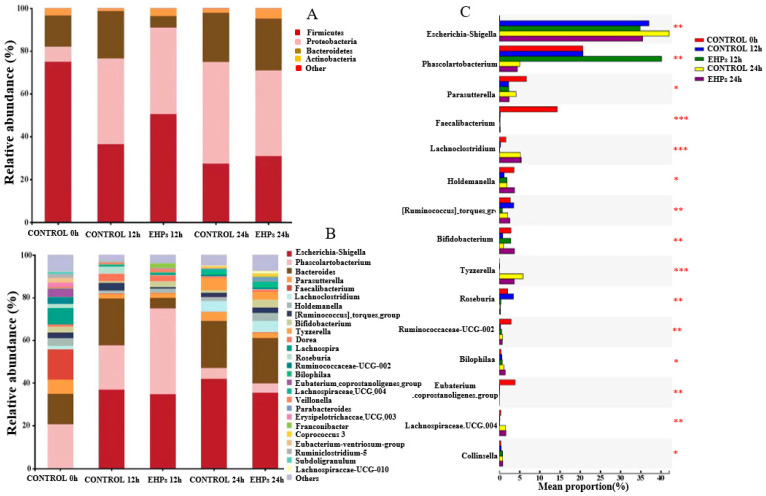
The effect of EHPs on intestinal microbiota composition was investigated by in vitro fermentation followed by bacterial 16S rRNA sequencing. The three fermentation samples were analyzed at the phylum level to determine microbiota composition (Figure 4A). Firmicutes, Bacteroidetes, Actinobacteria, and Proteobacteria were observed as the major bacterial phyla, while other microflora accounted for only 0.01–0.03%, which is consistent with previous reports [8,16,30]. Compared with the control 24 h group, the relative abundance of Firmicutes and Actinobacteria increased, and that of Proteobacteria decreased in the EHP-supplemented fermentation groups, suggesting that the presence of EHPs significantly boosted the growth of Firmicutes and Actinobacteria, while inhibiting Proteobacteria. Generally, acidic conditions can stimulate Firmicutes growth during fecal fermentation [8]. Firmicutes are dominant intestinal flora that can ferment dietary indigestible carbohydrates into SCFAs, thus promoting host health [31]. Actinobacteria are pivotal in the maintenance of gut homeostasis and are widely used as probiotics, especially Bifidobacteria [30,31]. Although Proteobacteria is the most abundant phylum in healthy humans, it comprises some well-known opportunistic pathogens, such as Escherichia-Shigella, enterohepatic Helicobacter species, and Campylobacter concisus, which may cause intestinal flora imbalance, inflammation, and even chronic colitis [31].
Figure 4.
Relative abundance levels of the gut microbial community at the phylum (A), genus (B), and variance analysis of genus level (C). * p < 0.05, ** p < 0.01, *** p < 0.001 versus the control group.
At the genus level (Figure 4B,C), the relative abundance of Bifidobacterium (0.91% vs. 3.86%) and Holdemanella (1.81% vs. 3.64%) enhanced markedly with EHP intervention compared with the control 24 h group. Lachnospiraceae UCG 004 (0.14% vs. 1.12%) also presented increasing trends, though not significantly. In addition, an evident decreasing tendency was observed for Escherichia-Shigella (42.08% vs. 35.46%), Tyzzerella (5.91% vs. 3.54%), and Parasutterella (4.09% vs. 2.39%). Notably, Bifidobacterium is recognized as a key member of the gut microbiota and has been used as a commercial probiotic. In addition, Bifidobacterium can produce various glycosidases to hydrolyze macromolecular carbohydrates, such as EHPs [8]. Escherichia-Shigella can utilize low Mw carbon sources to maintain growth but cannot feed on polysaccharides due to the lack of carbohydrate-active enzymes [8]. Hence, the relative abundance of Bifidobacterium increased, and that of Escherichia-Shigella decreased in EHP-containing groups. These results indicate that EHPs could regulate the composition of intestinal microflora by stimulating the growth of beneficial microbiota and inhibiting potential pathogens.
3.3.2. Changes in SCFA Content
SCFAs are primary metabolites of dietary carbohydrates, which have been shown to exert multiple favorable effects on health maintenance [32,33]. In this study, the type and concentration of SCFAs were determined in the fermentation broth after an interval of 4, 8, 12, and 24 h (Table 3). The difference in total SCFA concentration between EHP-supplemented and control groups was insignificant during the early fermentation stage. However, after 24 h of fermentation, the concentration of total SCFAs in the EHP-supplemented group (32.75 ± 1.10 mmol/L) was remarkably higher than that in the control group (26.46 ± 2.55 mmol/L). The main types of SCFAs were found to be acetic acid, propionic acid, and butyric acid. Acetic acid content reached 8.82 ± 0.32 mmol/L in the EHP-supplemented groups at 24 h, which was moderately higher compared with the control group (6.10 ± 0.46 mmol/L). Being the major fermentation product of Bacteroidetes, acetic acid plays a crucial role in the metabolism of carbohydrates and fats [34]. Furthermore, the concentration of propionic acid was also elevated in the EHP-supplemented groups (9.92 ± 0.98 mmol/L) than in the blank group (7.01 ± 0.30 mmol/L). There is a good correlation between propionic acid and intestinal immune cells because propionic acid can alleviate intestinal immune stress and promote intestinal environmental homeostasis [16]. Likewise, the valeric acid concentration increased in groups with EHPs (0.90 ± 0.05 mmol/L) compared to that in the control group (0.41 ± 0.02 mmol/L). Valeric acid has been reported to promote the growth of intestinal epithelium with a beneficial effect on colitis. At the genus level, the concentration of valeric acid positively correlated with Lachnospiraceae [35]. However, the present data revealed no considerable change in the concentrations of butyric, isobutyric, and isovaleric acids between the EHP-supplemented and control groups during the entire fermentation period. Notably, the contents of acetic and propionic acids in the EHP-containing group were higher than those in the control group at different fermentation stages, which is in accordance with previous studies [36]. Thus, EHPs show a good potential to produce more SCFAs and regulate host health. The potential protective effects of EHP supplementation on digestive tract diseases were further confirmed using DSS-induced UC mouse models.
Table 3.
Production changes of SCFAs during fermentation in vitro.
| Sample | Time (h) | SCFAs(mmol/L) | ||||||
|---|---|---|---|---|---|---|---|---|
| Acetic Acid | Propionic Acid | Butyric Acid | Isobutyric Acid | Valeric Acid | Isovaleric Acid | Total | ||
| Control | 0 | ND | ND | ND | ND | ND | ND | ND |
| 4 | 1.21 ± 0.21 f | 1.51 ± 0.17 d | 0.18 ± 0.01 e | 0.07 ± 0.00 c | 0.07 ± 0.00 d | 0.05 ± 0.00 b | 3.09 ± 0.11 f | |
| 8 | 2.61 ± 0.09 e | 2.43 ± 0.29 cd | 1.53 ± 0.16 cd | 0.10 ± 0.01 c | 0.11 ± 0.01 cd | 0.05 ± 0.00 b | 6.63 ± 0.10 de | |
| 12 | 5.62 ± 0.26 c | 6.20 ± 0.35 b | 5.80 ± 0.67 b | 1.70 ± 0.18 b | 0.25 ± 0.01 bc | 0.16 ± 0.01 b | 19.73 ± 1.84 c | |
| 24 | 6.10 ± 0.46 c | 7.01 ± 0.30 b | 7.34 ± 0.27 a | 3.46 ± 0.39 a | 0.41 ± 0.02 b | 2.14 ± 0.12 a | 26.46 ± 2.55 b | |
| EHPs | 0 | ND | ND | ND | ND | ND | ND | ND |
| 4 | 1.64 ± 0.08 ef | 1.85 ± 0.09 d | 0.24 ± 0.01 de | 0.07 ± 0.01 c | 0.06 ± 0.00 d | 0.04 ± 0.00 b | 3.90 ± 0.67 ef | |
| 8 | 3.70 ± 0.42 d | 3.48 ± 0.35 c | 2.12 ± 0.42 c | 0.13 ± 0.01 c | 0.14 ± 0.01 cd | 0.07 ± 0.00 b | 9.64 ± 0.35 d | |
| 12 | 7. 50 ± 0.50 b | 7.20 ± 0.24 b | 5.43 ± 0.46 b | 1.81 ± 0.17 b | 0.35 ± 0.01 bc | 0.22 ± 0.02 b | 22.51 ± 1.81 c | |
| 24 | 8.82 ± 0.32 a | 9.92 ± 0.98 a | 7.62 ± 0.39 a | 3.24 ± 0.40 a | 0.90 ± 0.05 a | 2.25 ± 0.19 a | 32.75 ± 1.10 a | |
Values are expressed as mean ± SD (n = 3). Different letters indicate significant differences under different fermentation conditions; ND: not detected.
3.4. Therapeutic Effects of EHP Treatment in DSS-Induced Colitis Mice
3.4.1. EHP Supplementation Ameliorated Colitis Symptoms
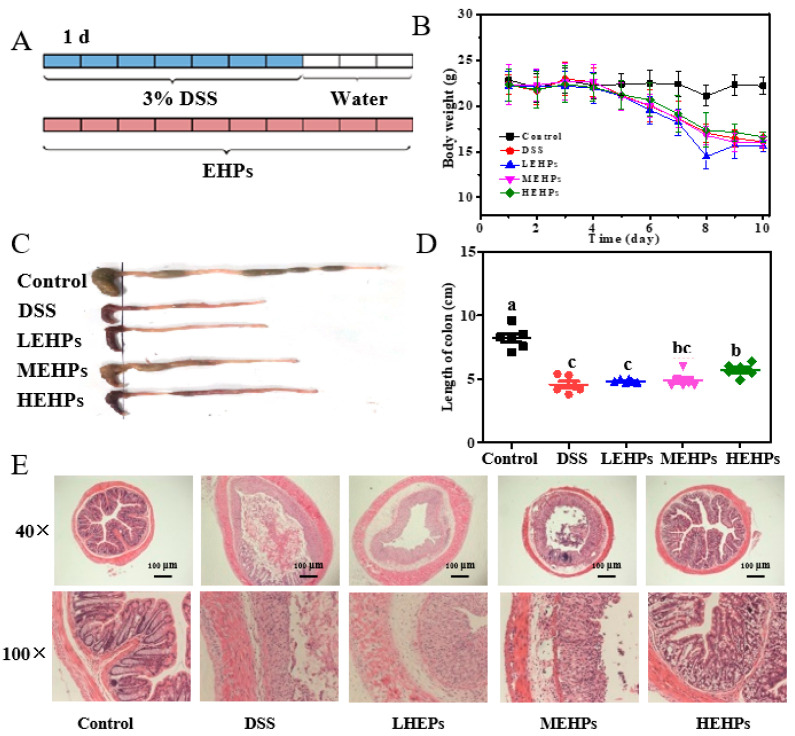
DSS-induced acute colitis could directly destroy the integrity of the colonic epithelial barrier, causing infiltration of inflammatory cells and intestinal microenvironment imbalance [37]. In this study, the effect of EHPs on intestinal functions was investigated in a DSS-induced colitis mouse model and the intervention schedule is shown in Figure 5A. The DSS model shows similar symptoms of human UC, such as body weight loss, diarrhea, and rectal bleeding [37]. The EHP treatment alleviated these adverse changes (Figure 5B–D). Especially, the colon length in the HEHPs group increased (6.15 cm), with a significant difference from the DSS group (Figure 5C,D). These results confirmed the successful establishment of the UC model and alleviation of the typical colon atrophy induced by DSS after EHP treatment.
Figure 5.
Therapeutic effects of EHPs on DSS-induced colitis. The mice were supplied with water or 3% DSS in drinking water for 10 d (A). Body weight loss during the DSS treatment (B). Images of mouse colons of different groups (C). Histogram statistics of colon length (D). H&E staining of colonic sections (40×, 100×) (E). The letters (a–c) indicate the significant differences of all treatment groups (p < 0.05).
For further verification, H&E staining of the colon was adopted as the most direct method to assess the extent of colonic injuries. The crypt deformation, goblet cell disappearance, epithelial damage, and inflammatory cell infiltration were significantly alleviated in EHP-supplemented groups, especially in the HEHPs group, compared to that in the DSS group (Figure 5E). Thus, treatment with EHPs significantly reversed the colon tissue damage (p < 0.01).
3.4.2. EHP Treatment Inhibited Colonic Inflammation
The imbalance between pro-inflammatory and anti-inflammatory factors plays an important role in UC pathogenesis [38]. Accumulating evidence showed IL-6 and IL-17 as the primary cytokines in experimental colitis, and the average numbers of IL-6 and IL-17 were significantly increased in UC patients [37]. In inflammatory bowel disease, the anti-inflammatory cytokine IL-10 is pivotal for controlling the inflammatory responses to enteric organisms. Additionally, higher IL-10 levels have been shown to provide maximum protection against in vivo colitis [39]. Compared with the control group, the mRNA expression of inflammatory mediators (IL-6, IL-17) was remarkably elevated in the DSS group. However, EHP treatment significantly downregulated the expression levels of IL-6 and IL-17, particularly in the HEHPs group (Figure 6A,B). The expression of IL-10 was dramatically declined in the DSS-induced mice, while EHP treatment upregulated IL-10 levels (Figure 6C). Therefore, EHP supplementation inhibited the UC-associated pathological inflammation by reducing the expressions levels of pro-inflammatory factors and enhancing those of anti-inflammatory factors.
Figure 6.
Effect of EHPs on the pro-inflammatory and anti-inflammatory cytokines in colonic tissue. (A,B) Pro-inflammatory cytokines (IL-6 and IL-17) in colonic tissue. (C) Anti-inflammatory cytokines (IL-10) in colonic tissue. The letters (a–c) indicate the significant differences of all treatment groups (p < 0.05).
4. Discussion
UC is a refractory chronic bowel disease gradually increasing worldwide. Current treatment options, including corticosteroids, aminosalicylate, immunomodulators, and monoclonal antibodies, often lack clinical efficacy with multiple harmful side effects [37]. Therefore, the development of new treatment methods and management strategies to treat patients according to UC severity is necessary. Numerous studies have shown that dietary polysaccharides could reduce UC-associated inflammation and symptoms [40]. In this study, the in vitro utilization of EHPs by intestinal microbiota could modulate microbiota growth. Furthermore, EHP supplementation showed beneficial effects on DSS-induced UC mice, alleviating body weight loss and colon shortening and suppressing the inflammatory responses in the colon.
The efficacy of Bifidobacterium in UC treatment has been reported previously [39,40,41]. Bifidobacterium strains proved beneficial in inducing and maintaining UC remission, exhibiting regulatory activities that contributed to controlling intestinal inflammation. In this study, EHP treatment increased the relative abundance of Bifidobacterium, which could reduce the inflammatory reaction associated with UC. In inflammatory bowel disease gut microbiota, the number of Proteobacteria was significantly elevated, especially Escherichia-Shigella, which has been considered a signature characteristic of dysbiosis in gut microbiota [42]. Thus, lower levels of Proteobacteria are likely to be beneficial to host health. Here, the relative abundance of Proteobacteria at the phylum level and Escherichia-Shigella at the genus level markedly declined after EHP fermentation. These findings indicate that EHPs might exhibit a therapeutic potential against UC, mediated by promoting the growth of Bifidobacterium and suppressing proteobacterial growth, especially Escherichia-Shigella. Overall, the effectiveness of EHP treatment in UC patients has been demonstrated.
5. Conclusions
This study presented the absorption and fermentation characteristics of EHPs. The indigestible and poorly absorbable EHPs were degraded by intestinal microbiota, promoting the production of acetic, propionic, and valeric acids. Additionally, EHP fermentation altered the diversity of intestinal microbiota, significantly increasing the relative abundance of Bifidobacteria and reducing that of Escherichia-Shigella at the genus level. Moreover, EHP treatment effectively suppressed the colitis symptoms in mouse models, which manifested as improvements in body weight loss, increasing colon length, reducing inflammatory cell infiltration and restoring intestinal epithelial barrier integrity. The current study suggests that EHP supplementation could be a promising nutritional therapeutic strategy for inflammatory bowel diseases. However, the current research has a limitation of not collecting and analyzing the feces of DSS-induced mice. In the future, the study will focus on the diversity, composition, and metabolites of microbiota in mouse colonic feces, to reveal the causality between intestinal microbiome changes and UC development during EHP treatment.
Abbreviations
| Papp | Apparent permeability coefficient |
| Ara | Arabinose |
| DSS | Dextran sulfate sodium |
| EHPs | Euphorbia humifusa-derived polysaccharides |
| FTIR | Fourier transform infrared spectroscopy |
| FITC | Fluorescein isothiocyanate |
| HPSEC | High-performance size exclusion chromatography |
| H&E | Hematoxylin-eosin |
| Gal | Galactose |
| Glu | Glucose |
| GluA | Glucuronic acid |
| Man | Mannose |
| SCFAs | Short-chain fatty acids |
| TEER | Transepithelial electrical resistance |
| UC | Ulcerative colitis |
Author Contributions
N.X., data curation, writing original draft; J.Z., formal analysis, investigation; S.C., writing—original draft preparation; S.L. (Shasha Li), software, investigation; S.L. (Shuwen Liu), writing—review and editing; C.W., resources, formal analysis, supervision. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
All experiments involving animals were conducted in accordance with the National Institutional Animal Care and Medical Ethics Committee of Southern Medical University (Guangdong Medical Laboratory Animal Center C202301-5).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Data is contained within the article.
Conflicts of Interest
The authors declare that they have no known competing financial interest or personal relationships that could have appeared to influence the work reported in this paper.
Funding Statement
This research was funded by the financial support received from the National Natural Science Foundation of China (32202220); the Science and Technology Programs of Guangzhou (202102020544); and the Scientific Research Project of High Level Talents of Southern Medical University (G622310045).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Chang J.T. Pathophysiology of inflammatory bowel diseases. N. Engl. J Med. 2020;383:2652–2664. doi: 10.1056/NEJMra2002697. [DOI] [PubMed] [Google Scholar]
- 2.Kayal M., Shah S. Ulcerative colitis: Current and emerging treatment strategies. J. Clin. Med. 2019;9:94. doi: 10.3390/jcm9010094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fan Y., Pedersen O. Gut microbiota in human metabolic health and disease. Nat. Rev. Microbiol. 2021;19:55–71. doi: 10.1038/s41579-020-0433-9. [DOI] [PubMed] [Google Scholar]
- 4.Wang X., Zhang A., Miao J., Sun H., Yan G., Wu F., Wang X. Gut microbiota as important modulator of metabolism in health and disease. RSC Adv. 2018;8:42380–42389. doi: 10.1039/C8RA08094A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fei Y., Chen Z., Han S., Zhang S., Zhang T., Lu Y., Berglund B., Xiao H., Li L., Yao M. Role of prebiotics in enhancing the function of next-generation probiotics in gut microbiota. Crit. Rev. Food Sci. Nutr. 2021 doi: 10.1080/10408398.2021.1958744. [DOI] [PubMed] [Google Scholar]
- 6.Patnode M.L., Beller Z.W., Han N.D., Cheng J., Peters S.L., Terrapon N., Henrissat B., Gall S.L., Saulnier L., Hayashi D.K., et al. Interspecies competition impacts targeted manipulation of human gut bacteria by fiber-derived glycans. Cell. 2019;179:59–73. doi: 10.1016/j.cell.2019.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Han R., Pang D., Wen L., You L., Huang R., Kulikouskaya V. In vitro digestibility and prebiotic activities of a sulfated polysaccharide from Gracilaria Lemaneiformis. J. Funct. Foods. 2020;64:103652. doi: 10.1016/j.jff.2019.103652. [DOI] [Google Scholar]
- 8.Wu D.T., Nie X.R., Gan R.Y., Guo H., Fu Y., Yuan Q., Zhang Q., Qin W. In vitro digestion and fecal fermentation behaviors of a pectic polysaccharide from okra (Abelmoschus esculentus) and its impacts on human gut microbiota. Food Hydrocolloids. 2021;114:106577. doi: 10.1016/j.foodhyd.2020.106577. [DOI] [Google Scholar]
- 9.Yuan D., Li C., Huang Q., Fu X., Dong H. Current advances in the anti-inflammatory effects and mechanisms of natural polysaccharides. Crit. Rev. Food Sci. Nutr. 2022 doi: 10.1080/10408398.2022.2025535. [DOI] [PubMed] [Google Scholar]
- 10.Shin S.Y., Kim C.G., Jung Y.J., Jung Y., Jung H., Im J., Lim Y., Lee Y.H. Euphorbia humifusa Willd exerts inhibition of breast cancer cell invasion and metastasis through inhibition of TNFα-induced MMP-9 expression. BMC Complem. Altern. Med. 2016;16:413. doi: 10.1186/s12906-016-1404-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rakotondrabe T.F., Fan M., Guo M. Exploring potential antidiabetic and anti-inflammatory flavonoids from Euphorbia humifusa with an integrated strategy. Front. Pharmacol. 2022;13:980945. doi: 10.3389/fphar.2022.980945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ding Q., Nie S., Hu J., Zong X., Li Q., Xie M. In vitro and in vivo gastrointestinal digestion and fermentation of the polysaccharide from Ganoderma atrum. Food Hydrocolloids. 2017;63:646–655. doi: 10.1016/j.foodhyd.2016.10.018. [DOI] [Google Scholar]
- 13.DuBois M., Gilles K., Hamilton J., Rebers P., Smith F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956;28:350–356. doi: 10.1021/ac60111a017. [DOI] [Google Scholar]
- 14.Chen F., Huang G., Yang Z., Hou Y. Antioxidant activity of Momordica charantia polysaccharide and its derivatives. Int. J. Biol. Macromol. 2019;10:673–680. doi: 10.1016/j.ijbiomac.2019.07.129. [DOI] [PubMed] [Google Scholar]
- 15.Xia Y.G., Yu L.S., Liang J., Yang B.Y., Kuang H.X. Chromatography and mass spectrometry-based approaches for perception of polysaccharides in wild and cultured fruit bodies of Auricularia auricular-judae. Int. J. Biol. Macromol. 2019;137:1232–1244. doi: 10.1016/j.ijbiomac.2019.06.176. [DOI] [PubMed] [Google Scholar]
- 16.Hu Y.C., Hu J.L., Li J., Wang J., Zhang X.Y., Wu X.Y., Li X., Guo Z.N., Zou L., Wu D.T. Physicochemical characteristics and biological activities of soluble dietary fibers isolated from the leaves of different quinoa cultivars. Food. Res. Int. 2023;163:112166. doi: 10.1016/j.foodres.2022.112166. [DOI] [PubMed] [Google Scholar]
- 17.Wu D.T., An L.Y., Liu W., Hu Y.C., Wang S.P., Zou L. In vitro fecal fermentation properties of polysaccharides from Tremella fuciformis and related modulation effects on gut microbiota. Food. Res. Int. 2022;156:111185. doi: 10.1016/j.foodres.2022.111185. [DOI] [PubMed] [Google Scholar]
- 18.Wang Z., Zhang H., Shen Y., Zhao X., Wang X., Wang J., Fan K., Zhan X. Characterization of a novel polysaccharide from Ganoderma lucidum and its absorption mechanism in caco-2 cells and mice model. Int. J. Biol. Macromol. 2018;118:320–326. doi: 10.1016/j.ijbiomac.2018.06.078. [DOI] [PubMed] [Google Scholar]
- 19.Chen S., Chen Z., Wang Y., Hao W., Yuan Q., Zhou H., Gao C., Wang Y., Wu X., Wang S. Targeted delivery of Chinese herb pair-based berberine/tannin acid self-assemblies for the treatment of ulcerative colitis. J. Adv. Res. 2022;40:263–267. doi: 10.1016/j.jare.2021.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ren B., Yuan T., Zhang X., Wang L., Pan J., Liu Y., Zhao B., Zhao W., Liu Z., Liu X. Protective effects of sesamol on systemic inflammation and cognitive impairment in aging mice. J. Agric. Food. Chem. 2020;68:3099–3111. doi: 10.1021/acs.jafc.9b07598. [DOI] [PubMed] [Google Scholar]
- 21.Wang J., Geng T., Zou Q., Yang N., Zhao W., Li Y., Tan X., Yuan T., Xuebo L., Liu Z. Lycopene prevents lipid accumulation in hepatocytes by stimulating PPARα and improving mitochondrial function. J. Funct. Foods. 2020;67:103857. doi: 10.1016/j.jff.2020.103857. [DOI] [Google Scholar]
- 22.Gu J.Y., Zhang H., Yao H., Zhou J., Duan Y.Q., Ma H.L. Comparison of characterization, antioxidant and immunological activities of three polysaccharides from Sagittaria sagittifolia L. Carbohydr. Polym. 2020;235:115939. doi: 10.1016/j.carbpol.2020.115939. [DOI] [PubMed] [Google Scholar]
- 23.Olawuyi I.F., Lee W.Y. Structural characterization, functional properties and antioxidant activities of polysaccharide extract obtained from okra leaves (Abelmoschus esculentus) Food Chem. 2021;354:129437. doi: 10.1016/j.foodchem.2021.129437. [DOI] [PubMed] [Google Scholar]
- 24.Fu Q., Wang H., Xia M., Deng B., Shen H., Ji G., Li G., Xie Y. The effect of phytic acid on tight junctions in the human intestinal Caco-2 cell line and its mechanism. Eur. J. Pharm. Sci. 2015;80:1–8. doi: 10.1016/j.ejps.2015.09.009. [DOI] [PubMed] [Google Scholar]
- 25.Xiang Q.F., Zhang W.J., Li Q., Zhao J., Feng W., Zhao T., Mao G., Chen Y., Xu X., Yang L., et al. Investigation of the uptake and transport of polysaccharide from Se-enriched Grifola frondosa in Caco-2 cells model. Int. J. Biol. Macromol. 2020;158:1330–1341. doi: 10.1016/j.ijbiomac.2020.04.160. [DOI] [PubMed] [Google Scholar]
- 26.Liu C., Kou Y., Zhang X., Cheng H., Chen X., Mao S. Strategies and industrial perspectives to improve oral absorption of biological macromolecules. Expert Opin. Drug Deliv. 2018;15:223–233. doi: 10.1080/17425247.2017.1395853. [DOI] [PubMed] [Google Scholar]
- 27.Zhang Y., Liu J., Dou P., Wu Z., Zheng Z., Pan X., Zhou T., Wang K.P. Oral absorption characteristics and mechanisms of a pectin-type polysaccharide from Smilax China L. across the intestinal epithelium. Carbohydr. Polym. 2021;270:118383. doi: 10.1016/j.carbpol.2021.118383. [DOI] [PubMed] [Google Scholar]
- 28.Zheng Z., Pan X., Luo L., Zhang Q., Huang X., Liu Y., Wang K., Zhang Y. Advances in oral absorption of polysaccharides: Mechanism, affecting factors, and improvement strategies. Carbohydr. Polym. 2022;282:119110. doi: 10.1016/j.carbpol.2022.119110. [DOI] [PubMed] [Google Scholar]
- 29.Wang K., Cheng F., Pan X., Zhou T., Liu X., Zheng Z., Luo L., Zhang Y. Investigation of the transport and absorption of Angelica sinensis polysaccharide through gastrointestinal tract both in vitro and in vivo. Drug Deliv. 2017;24:1360–1371. doi: 10.1080/10717544.2017.1375576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wu D.T., He Y., Yuan Q., Wang S.P., Gan R.Y., Hu Y.C., Zou L. Effects of molecular weight and degree of branching on microbial fermentation characteristics of okra pectic-polysaccharide and its selective impact on gut microbial composition. Food Hydrocolloids. 2022;132:107897. doi: 10.1016/j.foodhyd.2022.107897. [DOI] [Google Scholar]
- 31.Wang M., Wichienchot S., He X., Fu X., Huang Q., Zhang B. In vitro colonic fermentation of dietary fibers: Fermentation rate, short-chain fatty acid production and changes in microbiota. Trends Food Sci. Tech. 2019;88:1–9. doi: 10.1016/j.tifs.2019.03.005. [DOI] [Google Scholar]
- 32.Annunziata G., Arnone A., Ciampaglia R., Tenore G.C., Novellino E. Fermentation of foods and beverages as a tool for increasing availability of bioactive compounds. Focus on short-chain fatty acids. Foods. 2020;8:999. doi: 10.3390/foods9080999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Do M.H., Seo Y.S., Park H.Y. Polysaccharides: Bowel health and gut microbiota. Crit. Rev. Food Sci. Nutr. 2020;61:1212–1224. doi: 10.1080/10408398.2020.1755949. [DOI] [PubMed] [Google Scholar]
- 34.Wang L., Cen S., Wang G., Lee Y.K., Zhao J., Zhang H., Chen W. Acetic acid and butyric acid released in large intestine play different roles in the alleviation of constipation. J. Funct. Foods. 2020;69:103953. doi: 10.1016/j.jff.2020.103953. [DOI] [Google Scholar]
- 35.Qing Y., Xie H., Su C., Wang Y., Yu Q., Pang Q., Cui F. Gut microbiome, short-chain fatty acids, and mucosa injury in young adults with human immunodeficiency virus infection. Dig. Dis. Sci. 2019;64:1830–1843. doi: 10.1007/s10620-018-5428-2. [DOI] [PubMed] [Google Scholar]
- 36.Liu Y., Duan X., Duan S., Li C., Hu B., Liu A., Wu Y., Wu H., Chen H., Wu W. Effects of in vitro digestion and fecal fermentation on stability and metabolic behavior of polysaccharides from Craterellus cornucopioides. Food Funct. 2020;11:6899–6910. doi: 10.1039/D0FO01430C. [DOI] [PubMed] [Google Scholar]
- 37.Hao W., Chen Z.J., Yuan Q., Ma M.L., Gao C.F., Zhou Y.Y., Zhou H.F., Wu X., Wu D.T., Farag M.A. Ginger polysaccharides relieve ulcerative colitis via maintaining intestinal barrier integrity and gut microbiota modulation. Int. J. Biol. Macromol. 2022;219:730–739. doi: 10.1016/j.ijbiomac.2022.08.032. [DOI] [PubMed] [Google Scholar]
- 38.Nitima T., Waranya C., Kanokwan J. Immune response and inflammatory pathway of ulcerative colitis. J. Basic Clin. Physiol. Pharmacol. 2019;30:1–10. doi: 10.1515/jbcpp-2018-0036. [DOI] [PubMed] [Google Scholar]
- 39.Imaoka A., Shima T., Kato K., Mizuno S., Uehara T., Matsumoto S., Setoyama H., Hara T., Umesaki Y. Anti-inflammatory activity of probiotic Bifidobacterium: Enhancement of IL-10 production in peripheral blood mononuclear cells from ulcerative colitis patients and inhibition of IL-8 secretion in HT-29 cells. World J. Gastroenterol. 2008;14:2511–2516. doi: 10.3748/wjg.14.2511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pan X., Yin M.Y., Guo M.Z., Niu X.Y., Han L.R. The latest progress of natural food polysaccharides preventing ulcerative colitis by regulating intestinal microbiota. J. Funct. Foods. 2022;96:105201. doi: 10.1016/j.jff.2022.105201. [DOI] [Google Scholar]
- 41.Wang Y., Zhu H., Wang X., Yu Y., Xie J. Natural food polysaccharides ameliorate inflammatory bowel disease and its mechanisms. Foods. 2021;10:1288. doi: 10.3390/foods10061288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen G.J., Wang M.J., Zeng Z.Q., Xie M.H., Xu W.Q., Peng Y.J., Zhou W.T., Sun Y., Zeng X.X., Liu Z.H. Fuzhuan brick tea polysaccharides serve as a promising candidate for remodeling the gut microbiota from colitis subjects in vitro: Fermentation characteristic and anti-inflammatory activity. Food Chem. 2022;391:133203. doi: 10.1016/j.foodchem.2022.133203. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data is contained within the article.