Abstract
Cabbage (Brassica oleracea var. capitata) is a vegetable rich in glucosinolates (GSLs) that have proven health benefits. To gain insights into the synthesis of GSLs in cabbage, we systematically analyzed GSLs biosynthetic genes (GBGs) in the entire cabbage genome. In total, 193 cabbage GBGs were identified, which were homologous to 106 GBGs in Arabidopsis thaliana. Most GBGs in cabbage have undergone negative selection. Many homologous GBGs in cabbage and Chinese cabbage differed in expression patterns indicating the unique functions of these homologous GBGs. Spraying five exogenous hormones significantly altered expression levels of GBGs in cabbage. For example, MeJA significantly upregulated side chain extension genes BoIPMILSU1-1 and BoBCAT-3-1, and the expression of core structure construction genes BoCYP83A1 and BoST5C-1, while ETH significantly repressed the expression of side chain extension genes such as BoIPMILSU1-1, BoCYP79B2-1, and BoMAMI-1, and some transcription factors, namely BoMYB28-1, BoMYB34-1, BoMYB76-1, BoCYP79B2-1, and BoMAMI-1. Phylogenetically, the CYP83 family and CYP79B and CYP79F subfamilies may only be involved in GSL synthesis in cruciferous plants. Our unprecedented identification and analysis of GBGs in cabbage at the genome-wide level lays a foundation for the regulation of GSLs synthesis through gene editing and overexpression.
Keywords: glucosinolate biosynthetic gene, cabbage, exogenous hormone treatment, gene expression, phylogeny
1. Introduction
Glucosinolates (GSLs) are an important class of plant secondary metabolites. Currently, about 200 GSLs are known, which are distributed in 16 families of dicotyledonous plants, especially in Brassicaceae [1,2,3]. The GSL hydrolysis products display diverse bioactivities, function both in defence and as an attractant in plants, play a role in cancer prevention in humans, and act as flavour compounds. Due to their effects on both plants and humans, GSLs are a current biology research focus [4,5,6,7,8,9]. Indeed, rapeseed breeders focus on harmful GSLs in rapeseed cakes, vegetable breeders are more interested in anticancer GSLs that are beneficial to humans, and medical scientists focus on GSLs that are inhibitory to tumor cells [10,11,12,13,14]. Sulforaphane, a secondary metabolite, relieves neuropathic pain caused by chemotherapy and inhibits prostate, colon, breast, pancreatic, and bladder cancers [15,16,17,18,19].
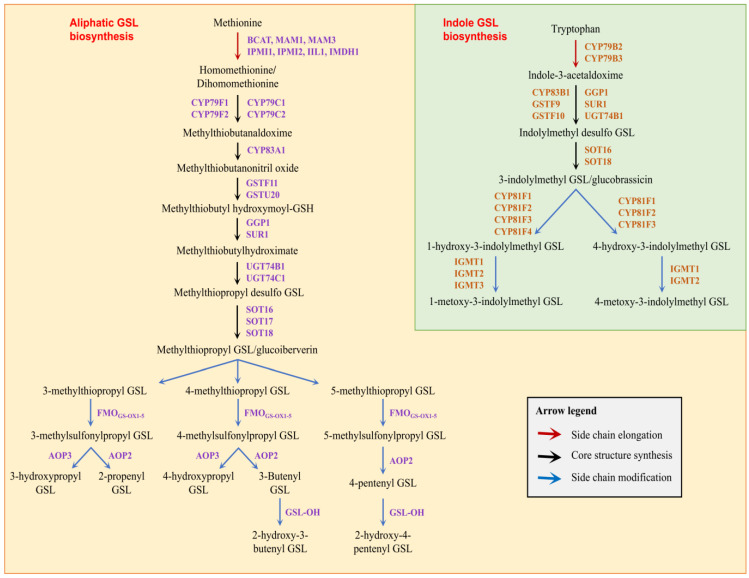
Structurally, all GSLs comprise a β-D-glucopyranose residue linked through a sulfur atom to a (Z)-N-hydroximinosulfate ester plus an R side-chain group derived from an amino acid (AA). Based on different AA sources of R groups, GSLs can be divided into three categories: aliphatic GSLs (R side chains are derived from methionine, alanine, valine, leucine, and isoleucine), aromatic GSLs (R side chains are derived from amino acids containing aromatic rings, tyrosine, and phenylalanine), and indole GSLs (R side chains are derived from tryptophan). The GSL synthetic pathway has been thoroughly studied in the model plant Arabidopsis thaliana [20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38]. The biosynthesis of glucosinolates can be summarized into three stages: extension of the R side chain, synthesis of the core structure, and modification of the side chain [39] (Figure 1) Figure 1 is quoted from references [40,41].
Figure 1.
Roadmap for glucosinolate biosynthesis. The compound beside the direction line is the catalytic enzyme, and the compound that the direction line points to is the reaction product.
Cabbage (Brassica oleracea L. var. capitata L.) is an important cruciferous vegetable that is rich in GSLs and widely grown globally. Although the whole-genome sequence of cabbage is available, there is a paucity of studies on cabbage GSL biosynthetic genes (GBGs). Therefore, to gain an in-depth understanding of the regulatory mechanism of GSL synthesis in cabbage, we conducted genome-wide identification, evolution, and expression analyses of cabbage GBGs using the Arabidopsis GSL synthesis pathway as a reference. This study establishes a reference for improving the composition and content of cabbage GSLs through molecular breeding technologies.
2. Materials and Methods
2.1. Data Sources
The sequence and annotation data of Arabidopsis GBGs were retrieved from the TAIR database (https://www.arabidopsis.org (accessed on 18 May 2022)). The genomic and annotation data of both cabbage and Chinese cabbage (B. rapa pekinensis) were retrieved from the BRAD database (http://brassicadb.cn (accessed on 21 May 2022)).
2.2. Identification of GBGs in Cabbage
To identify cabbage GBGs, we queried the cabbage proteins in the BRAD database with the protein sequences of Arabidopsis GBGs using BLASTP with E-value ≤ 10−10 and coverage ≥ 0.75 cutoffs. Syntenic orthologous GBGs between Arabidopsis and cabbage were identified according to the sequence similarity (E-value ≤ 10−20) of flanking genes. The specific distribution of cabbage GBGs on chromosomes was analyzed using MG2C (http://mg2c.iask (accessed on 5 June 2022)).
2.3. Non-Synonymous/Synonymous Substitution (Ka/Ks) Analysis
To evaluate the selection pattern of GBGs between Arabidopsis and both cabbage and Chinese cabbage, we used MEGA 7 software [42] to calculate the value of Ka/Ks of the orthologous gene pairs. A value of Ka/Ks greater than one represents positive selection, a value of Ka/Ks equal to one represents neutral selection, and a value of Ka/Ks less than one represents negative selection.
2.4. Expression Analysis of GBGs
The expression levels of GBGs in seven different organs (root, callus, leaf, stem, bud, flower, and silique) of cabbage and Chinese cabbage were determined using RNA-seq data in the Gene Expression Omnibus database under accession number GSE42891 and GSE43245, respectively.
To investigate the effect of different phytohormones on the expression of GBGs in cabbage, we sprayed leaves with five exogenous phytohormones: ethylene (ETH), abscisic acid (ABA), salicylic acid (SA), strigolactone (SL), and methyl jasmonate (MeJA) at concentrations of 100 mg/L, 50 mg/L, 100 µM, 5 µM, and 100 µM, respectively. As a negative control, a set of leaves were sprayed with double distilled water. The control group and five treatment groups were sprinkled with 200 mL solution with a spray kettle. Leaves were sampled at 2, 4, 6, 12, and 24 h after spraying. The cabbage were grown in a climate-controlled greenhouse with 20/15 °C (12/12 h) day/night temperature and 70% relative humidity for 20–30 days.
Six tissues, including the root, leaf, stem, flower, callus, and silique of B. oleracea accession ‘02-12’ were used for RNA extraction. Samples were rapidly obtained and placed in liquid nitrogen, and all collected samples were stored in a cryogenic freezer at −80 °C. RNA was extracted using the RNAprep Pure Plant Kit. Residual genomic DNA was removed with DNase 1, RNase-free and the quality of RNA was detected by agarose gel electrophoresis. The cDNA was synthesized using a FastKing RT Kit (Tiangen Biotech CO., Ltd., Beijing, China) and the reaction conditions were as follows 25 °C for 5 min, 37 °C for 45 min, 85 °C for 5 s. The relative expressions of glucosinolate genes were determined using qRT-PCR. qRT-PCR was performed using the ChamQ™ Universal SYBR® qPCR Master Mix (Vazyme Biotech CO., LTD, Nanjing, China) on a Bio-Rad CFX96TM Real-Time System (Bio-Rad, Hercules, CA, USA). Each reaction has a total volume of 10 μL, which includes 10 ng cDNA, 0.01 nmol forward and reverse primers, 5 μL of SYBR qPCR Master Mix, and 0.2 μL of 50 × ROX reference dye. Predenaturation was carried out at 95 °C for 30 s, 40 cycle reactions: 95 °C for 10 s, 60 °C for 30 s, and melting curve: 95 °C for 15 s, 60 °C for 1 min, 95 °C for 15 s. Relative expression was calculated using the 2−ΔΔCT method. All reactions for qPCR were performed with three biological and technical replicates.
2.5. Phylogenetic Analysis
P450 amino acid sequences from seven plant species were obtained from the cytochrome P450 database (https://drnelson.uthsc.edu/plants/ (accessed on 16 June 2022)). These protein sequences were aligned by MAFFT (v7.037) [43] with default parameters. The phylogenetic tree was constructed using FastTree with WAG + CAT model [44].
3. Results
3.1. Identification of Glucosinolate Biosynthetic Genes in B. oleracea
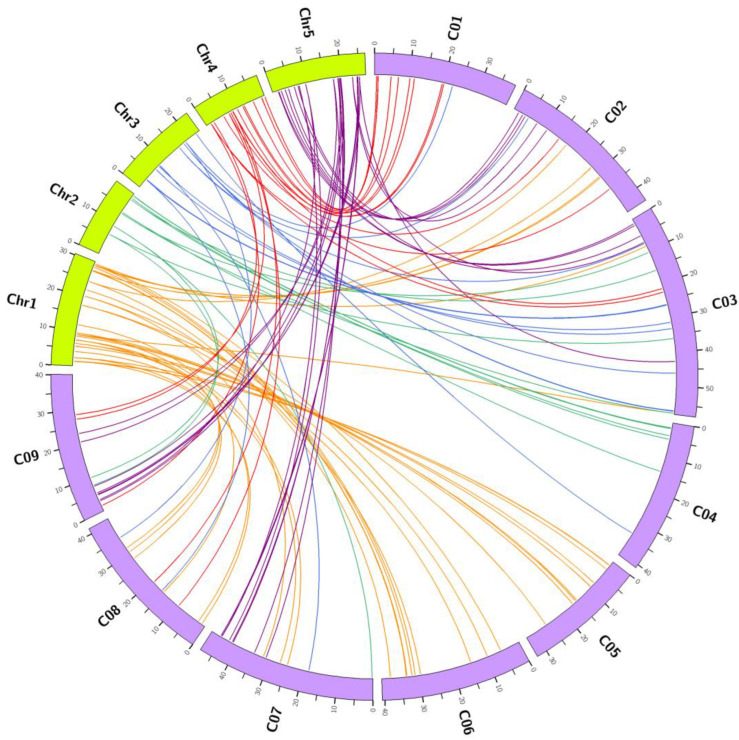
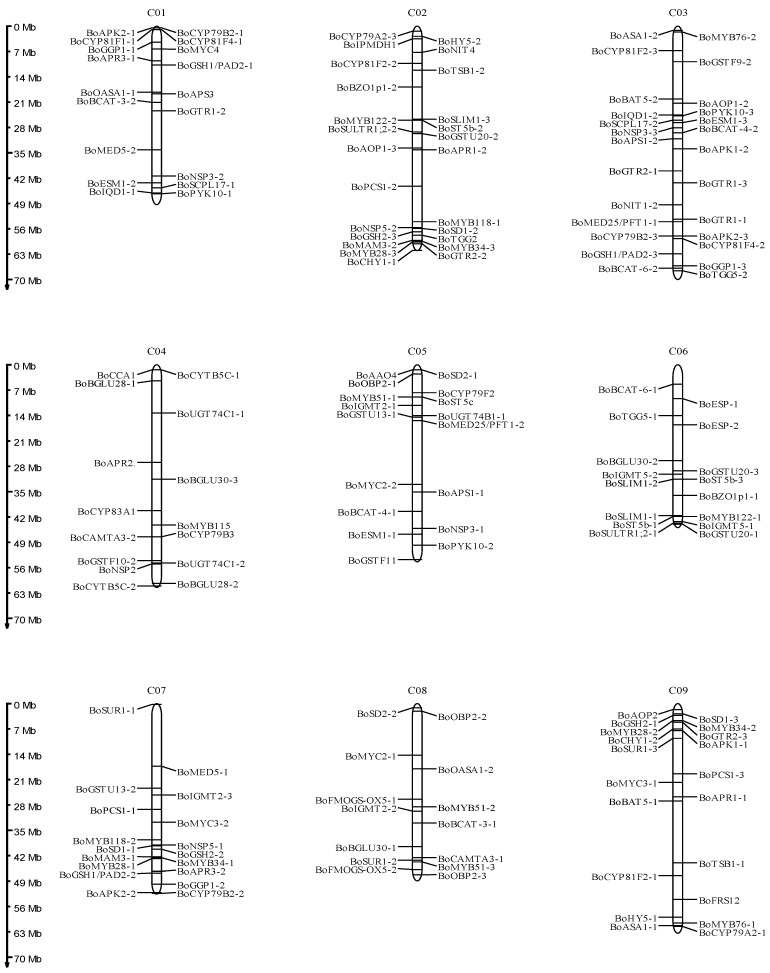
Glucosinolate biosynthetic genes from A. thaliana (AtGBSs) were used to identify B. oleracea glucosinolate biosynthetic genes (BoGBGs). A total of 193 BoGBGs were identified (Table S1), of which 169 BoGBGs were syntenic orthologs of the 106 AtGBGs (Figure 2). Based on the genome annotation file, 169 BoGBGs were mapped to nine chromosomes. As shown in Figure 3, there were 18, 25, 26, 15, 17, 16, 18, 14, and 20 BoGBGs located on chromosomes C01-C09, respectively.
Figure 2.
Circos diagram of glucosinolate biosynthetic genes in Brassica oleracea and Arabidopsis thaliana. C01 to C09 denote Brassica oleracea chromosomes, and Chr1 to Chr5 denote Arabidopsis thaliana chromosomes.
Figure 3.
Genomic localization diagrams of the BoGBGs on nine chromosomes of Brassica oleracea. C01-C09 denote Brassica oleracea chromosomes.
3.2. Comparative Evolutionary Analyses of Orthologous Gene Pairs of Glucosinolate Biosynthetic Genes
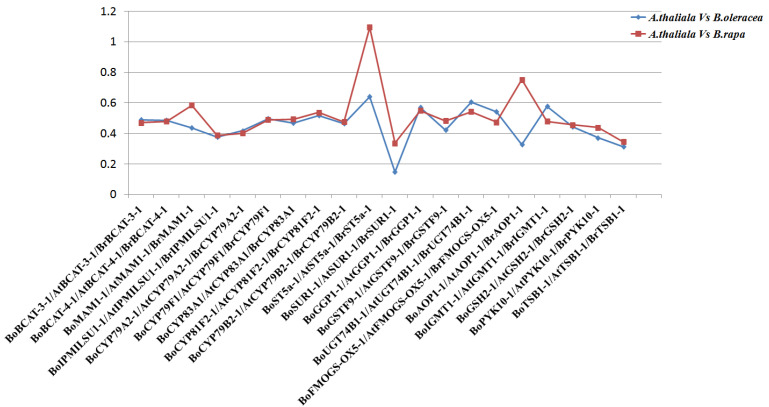
To explore the selection pressure acting on GBGs in B. oleracea, A. thaliana and B. rapa, Ka/Ks values for BoGBGs, BrGBGs and AtGBGs were comparatively analyzed. Ultimately, 20 homologous gene pairs of GBGs were selected (Figure 4). The Ka/Ks values of GBGs in B. oleracea, B. rapa, and A. thaliana were similar. However, the Ka/Ks ratios of BoMAM1-1, BoST5a-1, BoSUR1-1, and BoAOP1-1 were slightly lower in B. oleracea than in B. rapa genomes. Except for BoST5a, Ka/Ks values were all less than one, indicating that most genes were under negative selection (Figure 4).
Figure 4.
Scatter plot of Ka/Ks values of orthologous gene pairs for GBGs among Arabidopsis thaliana, Brassica oleracea and Brassica rapa.
3.3. Expression Analysis of Glucosinolate Biosynthetic Genes Orthologs from B. oleracea, B. rapa, and A. thaliana genomes
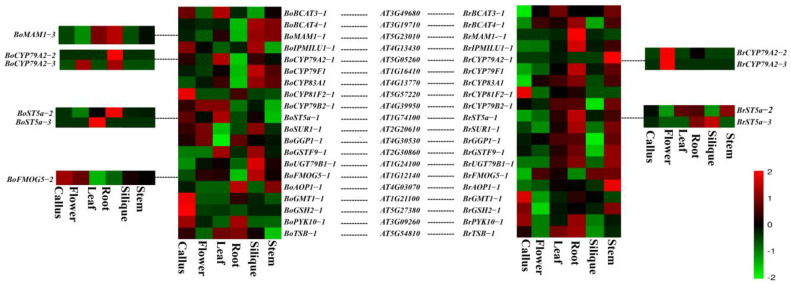
The expression levels of BoGBGs and BrGBGs in different tissues (callus, flower, leaf, root, silique, and stem) were analyzed based on a publicly available transcriptomic dataset. The transcript levels of 133 glucosinolate biosynthetic genes were assessed (Table S2). From the heatmap visualization, 20 BoGBGs and BrGBGs, which were important for glucosinolate synthesis, were expressed (Figure 5). Seven of these genes had the highest and lowest expression levels in the siliques and roots, respectively: BoUGT79B1-1, BoFMOG5-1, BoSUPR1, BoBCAT-4-1, BoMAM1-1, BoCYP83A1-1, and BoCYP79F1. By contrast, the expression levels of BoAOP1-1, BoPYK10-,1 and BoTBS-1 were highest in the roots and lowest in the siliques. Some genes were highly expressed in cabbage leaves, such as BoBCAT-3-1, BoCYP79A2-1, BoCYP79B2-1, BoST5a-1, and BoGSTF9-1. BoCYP81F2-1, BoGMT1-1, and BoGSH2-1 were barely expressed, except in the callus. BoST5a-1 had the lowest and highest expression levels in the stems and leaves, respectively. BoST5a-2 was expressed in all tissues and had the highest and lowest expression levels in the roots and flowers, respectively. BoAOP1-1 had the highest expression levels in the roots and stems.
Figure 5.
Heat map of homologous gene pairs of glucosinolate biosynthetic genes of Brassica oleracea and Brassica rapa. The color scale bar represents the FPKM value after the log2 transformation.
The different expression patterns of orthologous gene pairs indicate possible functional differentiation. We found that BoMAM1-1 displayed the highest expression levels in the siliques and stems, whereas BrMAM1-1 displayed the highest expression levels in the roots. BoCYP79A2-1 was highly expressed in the leaves, whereas the expression levels of BoCYP79A2-2 and BoCYP79A2-3 were the highest in the roots. However, BrCYP79A2-1 was highly expressed in the stems and BrCYP79A2-2 and BrCYP79A2-3 were highly expressed in the flowers. Our results showed that some homologous genes in B. oleracea and B. rapa have different expression patterns, indicating that the functions of these genes possibly diverged with speciation.
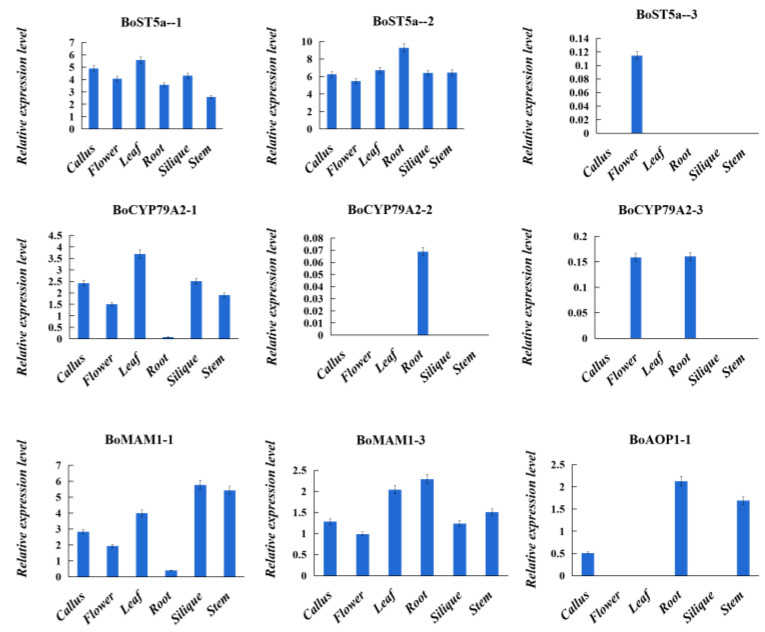
The tissue expression of important genes in the glucosinolate biosynthetic pathway was analyzed to further understand the expression of GBGs in B. oleracea (Figure 6). The results showed that BoST5a-1 and BoST5a-2 were expressed in all organs, including the calluses, flowers, leaves, roots, siliques, and stems, while BoST5a-3 was only expressed in the flowers. The expression of BoCYP79A2-1 was highest in the leaves, calluses, and siliques, followed by the stems, flowers, and roots. Almost no expression of BoCYP79A2-1 was detected in the roots. Meanwhile, the expression of BoCYP79A2-2 was only expressed in the roots. BoCYP79A2-3 was expressed in the flowers and roots. BoMAM1-1 and BoMAM1-3 were expressed in all tissues. The expression of BoAOP1-1 was in the roots, stems, and calluses, and no expression or weak expression was observed in other tissues. Gene expression analysis revealed an extensive variance between paralogs of each GBGs.
Figure 6.
Relative expression levels of nine key glucosinolate biosynthetic genes in different tissues of cabbage.
3.4. Twelve Glucosinolate Biosynthetic Genes Respond to Exogenous Phytohormone Treatments
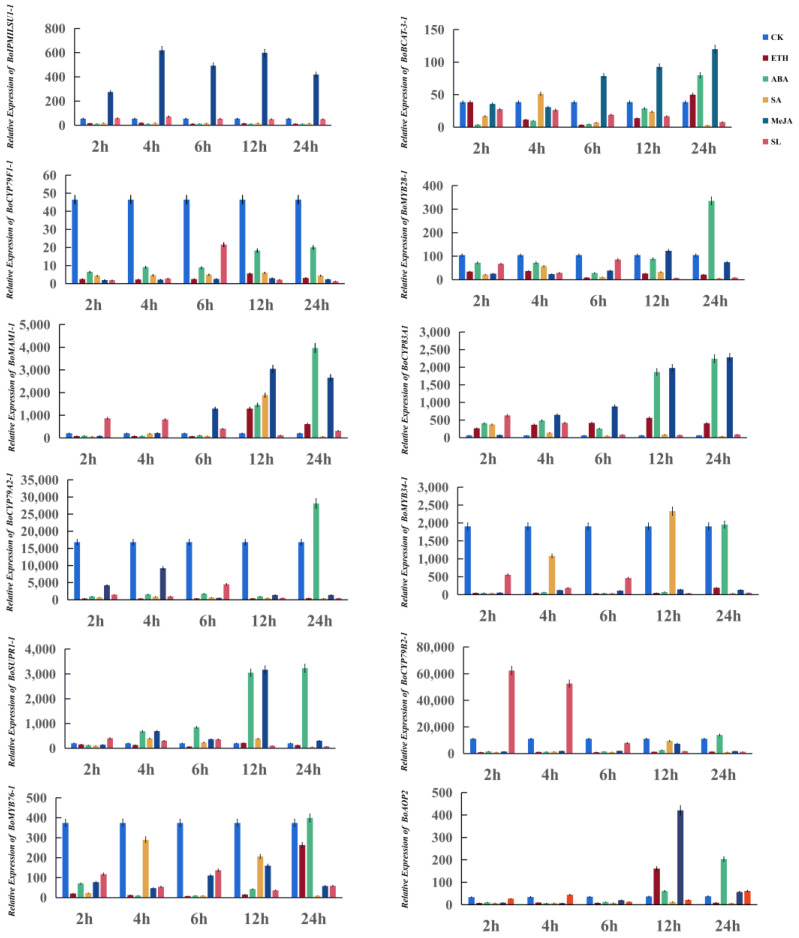
To investigate the effects of different exogenous phytohormones on GBG expression, we sprayed cabbages with ETH, ABA, SA, MeJA, and SL (Figure 7).
Figure 7.
The expression of glucosinolate biosynthetic genes after treatment with exogenous phytohormones. The Y and X axes represent the relative expression level and time course of exogenous phytohormone treatment, respectively. Leaves were sampled 2, 4, 6, 12, and 24 h after H2O(CK), ethylene (ETH), abscisic acid (ABA), salicylic acid (SA), strigolactone (SL), and methyl jasmonate (MeJA) application. The values represent the mean ± SD of three technical replications.
At 100 mg/L, ETH promoted the expression of two genes (BoCYP83A1 and BoMAM1-1) 2 h after treatment. The expression of BoBCAT-3-1 decreased 4 h after treatment. The expression levels of the remaining genes (BoIPMILSU1-1, BoSUPR1-1, BoST5C-1, BoCYP79A2-1, BoCYP79B2-1, BoMYB34-1, BoMYB28-1, BoMYB76-1, and BoCYP79F1-1) were not affected.
At 50 mg/L, ABA promoted the expression of 10 genes (BoIPMILSU1-1, BoBCAT-3-1, BoMYB28-1, BoCYP79B2-1, BoMYB34-1, BoMYB76-1, BoCYP83A1, BoST5C-1, BoMAM1-1 and BoSUPR1-1). Two other genes (BoCYP79A2-1, BoCYP79F1-1) were significantly repressed within 24 h of treatment.
Treatment with 100 µmol/L of SA promoted the expression of five genes (BoIPMILSU1-1-1, BoBCAT-3-1, BoMAM1-1, BoMYB34-1, and BoSUPR1-1). Specifically, the expression of BoIPMILSU1-1-1 and BoBCAT-3-1 was promoted 4 h after treatment and that of BoMAM1 and BoMYB34-1 12 h after treatment. The expression of BoSUPR1-1 was promoted between 4 h to 12 h after treatment. Six genes were completely repressed: BoCYP79A2-1, BoCYP79F1-1, BoMYB28-1, BoCYP79B2-1, BoMYB76-1, and BoST5C-1. BoCYP83A1 was promoted up to 4 h after treatment and repressed thereafter.
The expression of five genes (BoCYP79A2-1, BoCYP79B2-1, BoMYB34-1, BoMYB76-1, and BoCYP79F1-1) was repressed by treatment with MeJA at 100 µmol/L. Two genes (BoIPMILSU1-1 and BoMYB28-1) were initially promoted and then repressed. The expression of BoST5C-1 was not promoted within 24 h of treatment.
Treatment with 5 µmol/L SL promoted the expression of BoMAM1-1 and BoCYP83A1 12 h and 2 h after treatment, respectively. The expression levels of other selected genes were unaffected by SL treatments.
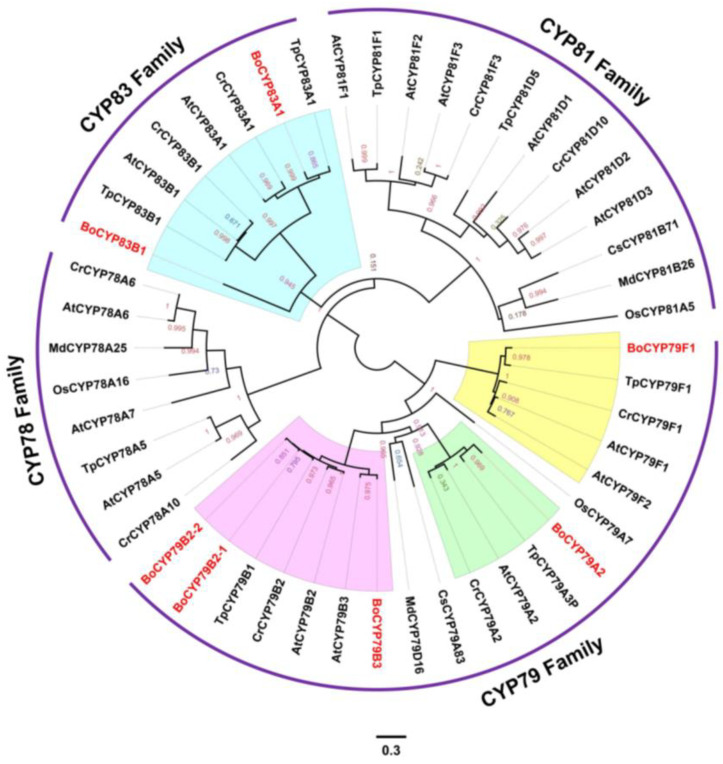
3.5. Evolutionary History of P450 Genes among Seven Plant Species
The cytochrome P450 gene family is important for plant development as it is largely involved in the synthesis and metabolism of plant hormones, pigments, and fatty acids. In this study, P450 genes were identified in B. oleracea and six other representative plant species. As shown in Figure 8, 48 genes clustered into four clades (CYP78, CYP79, CYP81, and CYP83). BoCYP83A1 and BoCYP83B1 clustered with the CYP83 family (blue area), whereas BoCYP79F1, BoCYP79A2, BoCYP79B3, BoCYP79B2-1, and BoCYP79B2-1 clustered with the CYP79 family (yellow, green, and pink area).
Figure 8.
Phylogenetic tree and conserved motif analysis of P450 genes. Protein sequences were aligned by MAFFT (v7.037). The phylogenetic tree was constructed using FastTree with WAG + CAT model. Bo: Brassica oleracea; At: Arabidopsis thaliana; Tp: Thellungiella parvula; Cs: Cucumis sativus; Cr: Capsella rubella; Md: Malus domestica; Os: Oryza sativa.
4. Discussion
4.1. Characterization of Glucosinolate Biosynthetic Genes in B. oleracea
Whole genome duplication provides genetic material for the expansion of gene families and the evolution of new genes [45]. Three rounds of whole genome duplication occurred in the evolution of B. oleracea. We identified 193 GBGs in B. oleracea, suggesting their possible evolution from a duplicated gene. A total of 169 GBGs were syntenic orthologs of A. thaliana, whereas 24 GBGs had no syntenic relationships. However, 19 AtGBGs (for example, AT3G44320, AT3G16390, and AT5G40360) had no B. oleracea orthologs, suggesting their possible loss during the evolution of B. oleracea.
To analyze the evolutionary relationships of glucosinolate biosynthesis homologous gene pairs among A. thaliana, B. oleracea, and B. rapa, we compared the non-synonymous/synonymous (Ka/Ks) ratios of GBGs (Figure 4). The Ka/Ks ratio expresses the selective pressure on the evolution of genes [46]. Two genes (BoST5a and BoAOP1) significantly differed in orthologous gene pairs between A. thaliana–B. oleracea and A. thaliana–B. rapa. The KA/KS ratio of BoST5a in B. rapa was above one, indicating that it had undergone positive selection. Conversely, the KA/KS ratio of BoST5a in B. oleracea was below one, indicating that it underwent negative selection during evolution. For both BoAOP1 and BrAOP1, the ratio was below one. However, more negative selection occurred in B. rapa. The Ka/Ks ratios of BoGUT74B1, BoFMOGS-OX5, and BoGTM1 were all below one, there are more positive selection occurred in B. oleracea compared to B. rapa. The remaining homologous genes such as BoBACT-3 and BrBACT-3, BoCYP83A1, and BrCYP83A1 are closely underwent negative selection during evolution. Therefore, we speculated that BoGBGs and BrGBGs underwent different selective pressures.
4.2. Exogenous Plant Hormones Regulate the Expression of Glucosinolate Biosynthetic Genes
The biosynthesis of glucosinolate in B. oleracea is a complex process regulated by multiple genes and transcription factors. Relatedly, exogenous plant hormones affect the accumulation of glucosinolate. In this study, different concentrations of five exogenous phytohormones (ETH, ABA, MeJA, SA, and SL) were sprayed on cabbage leaves to investigate their effects on the expression of GBGs.
In A. thaliana, the MAM locus has three duplicate genes, including MAM1, MAM2, and MAM3. They are involved in the elongation of the R chain during the biosynthesis of GSLs [47]. For example, MAM2 catalyzes the first elongation only, whereas MAM1 and MAM3 catalyze C3-C5 and C3-C8 reactions, respectively [48]. The expression of BoMAM1-1 in cabbage leaves significantly increased after ETH and ABA treatments. Therefore, the application of ETH and ABA may be beneficial to the synthesis of short-chain aliphatic thiosinolates. Moreover, overexpression of the MAM1 gene in Brassica napus significantly increased the content of aliphatic thiosinolates [49]. We speculate that spraying cabbage leaves with ETH and ABA increases aliphatic thiosinolates. The next step is the production of elongated 2-oxo acid catalyzed by isopropionate isomerases, which can participate in the core structure of GSL synthesis. IPMI, one of isomerases, can create chain-elongated derivatives of methionine such as dihomomethionine, trihomomethionine, tetrahomomethionine, pentahomomethionine, and hexahomomethionine. In Arabidopsis thaliana, isopropylmate isomerases (IPMI) consists of a single large (IPMI LSU1) and one of three different small subunits (IPMI SSU1 to SSU3). IPM1 and transcription factor MYB28 are co-expressed to positively regulate the synthesis of GLS. MeJA significantly upregulated the expression of BoIPMILSU1-1. Thus, it may promote amino acid side chain to extension in cabbage. Interestingly, MeJA inhibits the expression of BoMYB28. We speculate that MeJA is more beneficial in promoting the synthesis of long-chain methionine. The next side chain extension process is the transamination process involving BCAT3, BCAT4, or BCAT6. The chain-lengthened 2-oxoacid is catalyzed to the corresponding amino acid while the production of homomethionine from methionine. ETH, ABA, and MeJA can increase the expression level of BoBCAT3-1. This may promote the synthesis of aliphatic thioglycosides.
Glucosinolate biosynthesis is a complex process regulated by many genes and transcription factors. Cytochrome P450 from CYP79 and CYP83 families are involved in synthesizing the core structure of glucosinolates. AtCYP83A1 converts the aliphonic aldehyde group to an activated acylnitro compound. Indeed, the lipid content in the CYP83A1 deletion mutant in Arabidopsis was significantly lower than that of the wild type [50,51,52]. Spraying cabbage leaves with MeJA, ETH, and ABA upregulated BoCYP83A1 expression. Therefore, we speculate that spraying these three hormones increases the content of aliphatic thiosinolates in cabbage leaves. CYP79B2/B3, important enzymes in the glucoside biosynthesis pathway, convert tryptophan to indole-3-acetylaldoxime (a precursor of Indole-3-aceticacid). The expression level of CYP79B2-1 in B. oleracea increased shortly after the application of SL, which possibly temporarily increased indole-glucosinolide and auxin content in cabbage leaves. CYP79F1 is involved in the biosynthesis of short and long-chain aliphatic glucosinolates, while CYP79F2 only catalyzes the production of long-chain aliphatic glucosinolates [53,54,55]. In CYP79F1 knockout mutants of A. thaliana, the content of short-chain aliphatic glucosinolates significantly decreased while levels of auxins and cytokinins increased. After spraying cabbage leaves with ABA, BoCYP79F1-1 expression significantly decreased and this repression decreased with time. Spraying cabbage leaves with ABA possibly reduced their content of short-chain aliphatic glucosinolates, auxins, and cytokinins.
MYB34 is an important transcription factor of indole glucosinolate biosynthesis. In A. thaliana, MYB34 positively regulates the transcription of CYP79B2/B3 genes for the biosynthesis of indole glucosinolates [56,57]. Additionally, in Arabidopsis, the expression of AtMYB34 is inhibited through ABA and MejA signaling pathways, thereby negatively regulating the synthesis of glucoside. The expression level of BoMYB34 significantly decreased in cabbage leaves within 24 h after ABA and MejA treatments, which was similar to Arabidopsis. Therefore, BoMYB34-1 had a positive regulatory effect on indoles glucosinolate biosynthesis in cabbage. MYB28 and MYB76 transcription factors were highly associated and co-expressed with structural aliphatic glucosinolates biosynthetic genes, such as BCAT4, MAM1, CYP79F2, and CYP83A1. Essentially, MYB28 and MYB76 are closely related to the regulation of aliphatic thioside biosynthesis. The aliphatic thioside content in MYB28-overexpressed plants was altered compared to wild-type Arabidopsis. Metabolic analysis showed 3C-5C glucosinolates were increased significantly. However, the content of long-chain aliphatic glucosinolates remained unchanged or decreased slightly. Practically, while ABA promotes MYB28 expression, the expression levels of BCAT4, MAM1, CYP79F2, and CYP83A1 are also significantly upregulated. It is well illustrated that BoMYB28 may have a co-expression relationship with BoBCAT4, BoMAM1, BoCYP79F2, and BoCYP83A1 in cabbage. Compared with wild-type Arabidopsis, levels of both short-chain and long-chain aliphatic glucosinolates in MYB76 overexpressed plants were increased. However, the promotion effect of MYB76 is weak compared to the MYB28 regulator. For example, the content of 4C glucosinolates is 3–4 times higher than that of wild type, and the transcription level of MYB76 needs to be increased by 100 times, while the transcription level of MYB28 only needs to be increased by 14 times. Interestingly, overexpression of MYB76 not only leads to the accumulation of aliphatic glucosinolates, but also to an increase in the content of indole glucosinolates. After spraying ABA, the expression of MYB76 was significantly inhibited within 12 h. Therefore, it might be detrimental to the biosynthesis of indole glucosinolates in cabbage leaves.
In the future, gene editing will be used to verify the biological function of some significant BoGBGs in cabbage. Thus, the materials with a high content of the beneficial thioglycoside glucosinolates are obtained.
4.3. Role of CYP450 in Glucosinolate Biosynthesis in Members of Cruciferae
Plant cytochrome P450 is an important oxygenating enzyme in the synthesis of glucosinolates in cruciferous plants [58,59]. Specifically, P450 is widely involved in the core structure synthesis of glucosinolates. During the synthesis of aliphatic thiosinolates in A. thaliana, CYP79F1 converts short-chain amino acids into long-chain amino acids. CYP79F2 then oxidizes long-chain amino acids to aldehyde groups. The aldehyde group is activated to an acyl compound by CYP83A1. The genes that encode the aldehyde conversion of indole glucosinolates are CYP79B2 and CYP79B3. CYP81B1 converts the aldehyde group to butyl cyanide, whereas CYP81F2 and CYP81F3 participate in the side chain modification of indole glucosinolates. In this study, we analyzed 11 P450 gene families in seven plant species. These 11 gene families are clustered into CYP78, CYP79, CYP81, and CYP83 clades. The number of genes varied greatly with species. The P450 genes of cabbage in the CYP81 and CYP78 clades were partly lost due to various evolutionary selection pressures. Comparative genomic analyses revealed that some P450 genes of the CYP79F, CYP81F, CYP79B, CYP83B, CYP83A, and CYP78B families existed only in Cruciferae. We speculate that this is related to the fact that glucosinolates are special cruciferous metabolites.
5. Conclusions
In this study, 193 glucosinolates biosynthetic genes were identified in cabbage. Some BoGBGs underwent negative selection similar to those in B. rapa. RNA-Seq data analysis of six organs in B. oleracea and B. rapa indicated that many BoGBGs exhibit both organ-specific expression and functional differentiation. Spraying cabbages with five exogenous hormones affected the expression of BoGBGs, and this potentially aids the improvement of the cabbage glucosinolates content by phytohormones application. Overall, this study facilitates unraveling metabolic mechanisms of glucosinolates in B. oleracea, providing valuable insights for the development of cabbage varieties with high glucosinolate content through some significative BoGBG editing such as BoCYP83A1, BoCYP79F1, and BoMAM1.
Acknowledgments
The authors are grateful to all lab members for their useful suggestions, support, and encouragement.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/genes14020476/s1, Table S1: Glucosinolate biosynthetic genes identified in cabbage. Table S2: Expression of BoGBG genes in different organs of bud, callus, flower, leaf, silique, and stem in homozygous cabbage line 02-12.
Author Contributions
Conceptualization, J.J. and S.C.; methodology, S.C. and P.W.; software, J.J. and P.W.; validation, P.W., J.J. and W.C.; formal analysis, H.L.; investigation, L.Y.; resources, Z.F. and Y.Z.; data curation, W.C. and J.J.; writing—original draft preparation, P.W. and W.C.; writing—review and editing, J.J. and S.C.; visualization, J.J. and W.C.; supervision, H.L.; project administration, M.Z. and Y.W.; funding acquisition, J.J. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research was funded by the National Key Research and Development Program (2022YFD1200502), National Natural Science Foundation of China (32002034), the Science and Technology Innovation Program of the Chinese Academy of Agricultural Sciences (CAAS-ASTIP-2013-IVFCAAS), and the Modern Agro-Industry Technology Research System (CARS-25-B-01), and State Key Laboratory of Vegetable Biobreeding, Institute of Vegetables and Flowers, Chinese Academy of Agricultural Sciences, Beijing 100081, China.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Fahey J.W., Zalcmann A.T., Talalay P. The Chemical Diversity and Distribution of Glucosinolates and Isothiocyanates among Plants. Phytochemistry. 2001;56:5–51. doi: 10.1016/S0031-9422(00)00316-2. [DOI] [PubMed] [Google Scholar]
- 2.Clarke D.B. Glucosinolates Structures and Analysis in Food. Anal. Methods. 2010;2:310–325. doi: 10.1039/b9ay00280d. [DOI] [Google Scholar]
- 3.Agerbirk N., Olsen C.E. Glucosinolate Structures in Evolution. Phytochemistry. 2012;77:16–45. doi: 10.1016/j.phytochem.2012.02.005. [DOI] [PubMed] [Google Scholar]
- 4.Hare J.D. Ecological Role of Volatiles Produced by Plants in Response to Damage by Herbivorous Insects. Annu. Rev. Entomol. 2011;56:161–180. doi: 10.1146/annurev-ento-120709-144753. [DOI] [PubMed] [Google Scholar]
- 5.Sarai Q.M., Paula G.I., Francisco Q.M., Débora V., Diego A.M. The Role of Brassica Bioactives on Human Health: Are We Studying It the Right Way? Molecules. 2020;25:1591. doi: 10.3390/molecules25071591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mawson R., Heaney R.K., Zdunczyk Z., Kozlowska H. Rapeseed Meal-glucosinolates and Their Antinutritional Effects Part 6. Taint in End-products. Food/Nahrung. 2010;39:21–31. doi: 10.1002/food.19950390103. [DOI] [PubMed] [Google Scholar]
- 7.Herr I., Büchler M.W. Dietary Constituents of Broccoli and Other Cruciferous Vegetables: Implications for Prevention and Therapy of Cancer. Cancer Treat. Rev. 2010;36:377–383. doi: 10.1016/j.ctrv.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 8.Abuyusuf M., Robin A., Lee J. Glucosinolate Profiling and Expression Analysis of Glucosinolate Biosynthesis Genes Differentiate White Mold Resistant and Susceptible Cabbage Lines. Int. J. Mol. Sci. 2018;19:4037. doi: 10.3390/ijms19124037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aires A., Carvalho R., Barbosa M.D.C. Suppressing Potato Cyst Nematode, Globodera Rostochiensis with Extracts of Brassicacea Plants. Am. J. Pot. Res. 2009;86:327–333. doi: 10.1007/s12230-009-9086-y. [DOI] [Google Scholar]
- 10.Salem A.Z., Medhat D., Fathy S.A., Mohamed M.R., ElKhayat Z., ElDaly S.M. Indole Glucosinolates Exhibit Anti-inflammatory Effects on Ehrlich Ascites Carcinoma CellsThrough Modulation of Inflammatory Markers and MiRNAs. Mol. Biol. Rep. 2021;48:10. doi: 10.1007/s11033-021-06683-5. [DOI] [PubMed] [Google Scholar]
- 11.Punetha H., Saif N.S., Dinesh P. Glucosinolates in Oilseed Brassica: Neutraceuticals with Tremendous Health Benefits. Int. J. Biol. Sci. 2020;9:26–32. doi: 10.5958/2322-0996.2019.00016.4. [DOI] [Google Scholar]
- 12.Lucarini E., Micheli L., Trallori E. Effect of Glucoraphanin and Sulforaphane Against Chemotherapy-induced Neuropathic Pain:Kv7 Potassium Channels Modulation by H2S Release in Vivo. Phytother. Res. 2018;32:2226–2234. doi: 10.1002/ptr.6159. [DOI] [PubMed] [Google Scholar]
- 13.Tsaur I., Thomas A., Taskiran E., Rutz J., Chun Felix K.H., Haferkamp A.J., Eva B., Roman A. Concomitant Use of Sulforaphane Enhances Antitumor Efficacy of Sunitinib in Renal Cell Carcinoma In Vitro. Cancers. 2022;14:4643. doi: 10.3390/cancers14194643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xie H., Rutz J., Maxeiner S., Grein T., Thomas A., Juengel E., Chun Felix K.H., Cinatl J., Haferkamp A., Tsaur I., et al. Plant-Derived Sulforaphane Suppresses Growth and Proliferation of Drug-Sensitive and Drug-Resistant Bladder Cancer Cell Lines In Vitro. Cancers. 2022;14:4682. doi: 10.3390/cancers14194682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu B., Mao Q.Q., Cao M. Cruciferous Vegetables Intake and Risk of Prostate Cancer: A Meta-analysis. Int. J. Urol. 2012;19:134–141. doi: 10.1111/j.1442-2042.2011.02906.x. [DOI] [PubMed] [Google Scholar]
- 16.Wu Q.J., Yang Y., Vogtmann E. Cruciferous Vegetables Intake and The Risk of Colorectal Cancer:A Meta-analysis of Observational Studies. Ann. Oncol. 2013;24:1079–1087. doi: 10.1093/annonc/mds601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu X., Lü K. CruciferousVegetables Intakeis Inversely Associated with Risk of Breast Cancer:A Meta-analysis. Breast. 2013;22:309–313. doi: 10.1016/j.breast.2012.07.013. [DOI] [PubMed] [Google Scholar]
- 18.Citi V., Piragine E., Pagnotta E. Anticancer Properties of Erucin, an H2S-releasing Isothiocyanate on Human Pancreatic Adenocarcinoma Cells (AsPC-1) Phytother. Res. 2019;33:845–855. doi: 10.1002/ptr.6278. [DOI] [PubMed] [Google Scholar]
- 19.Abbaoui B., Lucas C., Riedl K.M. Cruciferous Vegetables, Isothiocyanates and Bladder Cancer Prevention. Mol. Nutr. Food Res. 2018;62:e1800079. doi: 10.1002/mnfr.201800079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Halkier B.A., Du L. The Biosynthesis of Glucosinolates. Trends Plant Sci. 1997;2:425–431. doi: 10.1016/S1360-1385(97)90026-1. [DOI] [PubMed] [Google Scholar]
- 21.Padilla G., Cartea M.E., Velasco P. Variation of Glucosinolates in Vegetable Crops of Brassica rapa. Phytochemistry. 2007;68:536–545. doi: 10.1016/j.phytochem.2006.11.017. [DOI] [PubMed] [Google Scholar]
- 22.Schonhof I., Krumbein A., Brückner B. Genotypic Effects on Glucosinolates and Sensory Properties of Broccoli and Cauliflower. Food/Nahrung. 2010;48:25–33. doi: 10.1002/food.200300329. [DOI] [PubMed] [Google Scholar]
- 23.Augustine R., Bisht N.C. Biofortification of Oilseed Brassica juncea with the Anti-cancer Compound Glucoraphanin by Suppressing GSL-ALK Gene Family. Sci. Rep. 2015;5:18005. doi: 10.1038/srep18005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang J., Wang H., Liu Z. A Naturally Occurring Variationinthe BrMAM-3 Geneis Associated with Aliphatic Glucosinolate Accumulationin Brassica Rapa Leaves. Hort. Res. 2018;5:69. doi: 10.1038/s41438-018-0074-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tamara G., Bettina B., Hans-peter M. The Transcription Factor Hig1/Myb51 Regulates Indolic Glucosinolate Biosynthesis in Arabidopsis Thaliana. Plant J. 2010;50:886–901. doi: 10.1111/j.1365-313X.2007.03099.x. [DOI] [PubMed] [Google Scholar]
- 26.Gigolashvili T., Berger B., Flügge U.I. Specific and Coordinated Control of Indolic and Aliphatic Glucosinolate Biosynthesis by r2r3-Myb Transcription Factors in Arabidopsis thaliana. Phytochem.Rev. 2009;8:3–13. doi: 10.1007/s11101-008-9112-6. [DOI] [Google Scholar]
- 27.Cai C., Yuan W., Miao H. Functional Characterization of BoaMYB51 Sas Central RegulatorsofIndole Glucosinolate Biosynthesis in Brassica oleracea var. Alboglabra bailey. Front. Plant Sci. 2018;9:1599. doi: 10.3389/fpls.2018.01599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yu Q., Hao G., Zhou J. Identification and Expression Pattern Analysis of Bomyb51 Involvedin Indolic Glucosinolate Biosynthesis from Broccoli (Brassica Oleracea var. Italica) Biochem. Bioph. Res. Commun. 2018;501:598–604. doi: 10.1016/j.bbrc.2018.05.058. [DOI] [PubMed] [Google Scholar]
- 29.Sonderby I.E., Hansen B.G., Bjarnholt N. A Systems Biology Approach Identifies A r2r3 Myb Gene Subfamily with Distinct and Overlapping Functions in Regulation of Aliphatic Glucosinolates. PLoS ONE. 2007;2:e1322. doi: 10.1371/journal.pone.0001322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sonderby I.E., Burow M., Rowe H.C. A Complex Interplay of Three r2r3Myb Transcription Factors Determinesthe Profile of Aliphatic Glucosinolates in Arabidopsis. Plant Physiol. 2010;153:348–363. doi: 10.1104/pp.109.149286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sergey M., Eyal B., Hadar L. The Transcript and Metabolite Networks Affected by the Two Clades of Arabidopsis Glucosinolate Biosynthesis Regulators. Plant Physiol. 2008;148:2021–2049. doi: 10.1104/pp.108.124784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schuster J., Knill T., Reichelt M., Gershenzon J., Binder S. Branched Chain Aminotransferase 4 is Part of The Chain Elongation Pathway in The Biosynthesis of Methionnie-derived Glucosinolates in Arabidopsis. Plant Cell. 2006;18:2664–2679. doi: 10.1105/tpc.105.039339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miao H., Wei J., Zhao Y., Yan H., Sun B., Huang J., Wang Q. Glucose Signalling Positively Regulates Aliphatic Glucosinolate Biosynthesis. J. Exp. Bot. 2013;64:1097–1109. doi: 10.1093/jxb/ers399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang A., Luo R., Li J., Miao R., An H., Yan X., Pang Q. Arabidopsis Glutathione-S-Transferases GSTF11 and GSTU20 Function in Aliphatic Glucosinolate Biosynthesis. Front. Plant Sci. 2022;12:816233. doi: 10.3389/fpls.2021.816233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Glutathione S-Transferases in The Biosynthesis of Sulfur-containing Secondary Metabolites in Brassicaceae Plants. Front. Plant Sci. 2018;9:1639. doi: 10.3389/fpls.2018.01639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kong W., Li j., Yu Q., Cang W., Xu R., Wang Y., Ji W. Two Novel Flavin-containing Monooxygenases Involved in Biosynthesis of Aliphatic Glucosinolates. Front. Plant Sci. 2016;7:1292. doi: 10.3389/fpls.2016.01292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang J.F., Liu Z.Y., Liang J.I., Wu J., Cheng F., Wang X.W. Three Genes Encoding AOP2, A Protein Involved in Aliphatic Glucosinolate Biosynthesis, Are Differentially Expressed in Brassica rapa. J. Exp. Bot. 2015;66:20. doi: 10.1093/jxb/erv331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Araki R., Hasumi A., Nishizawa O.I. Novel Bioresources for Studies of Brassica Oleracea: Identification of A Kale Myb Transcription Factor Responsible for Glucosinolate Production. Plant Biotechnol. J. 2013;11:1017–1027. doi: 10.1111/pbi.12095. [DOI] [PubMed] [Google Scholar]
- 39.Mitreiter S., Gigolashvili T. Regulation of Glucosinolate Biosynthesis. J. Exp. Bot. 2020;72:70–91. doi: 10.1093/jxb/eraa479. [DOI] [PubMed] [Google Scholar]
- 40.Harun S., Abdullah-Zawawi M.R., Goh H.H. A Comprehensive Gene Inventory for Glucosinolate Biosynthetic Pathway in Arabidopsis thaliana. J. Agric. Food Chem. 2020;68:7281–7297. doi: 10.1021/acs.jafc.0c01916. [DOI] [PubMed] [Google Scholar]
- 41.Lei J.J., Chen C.M., Chen G.J. Progress in Glucosinolates and Its Molecular Mechanism of Biosynthesis. J. South China Agric. Univ. 2019;40:59–70. [Google Scholar]
- 42.Kumar S., Stecher G., Tamura K. MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for Bigger Datasets. Mol. Biol. Evol. 2016;33:1870–1874. doi: 10.1093/molbev/msw054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Katoh K., Standley D.M. Mafft Multiple Sequence Alignment Software Version 7: Improvements in Performance and Usability. Mol. Biol. Evol. 2013;30:772–780. doi: 10.1093/molbev/mst010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Price M.N., Dehal P.S., Arkin A.P. Fasttree: Computing large Minimum Evolution Trees with Profiles Instead of A Distance Matrix. Mol. Biol. Evol. 2009;26:1641–1650. doi: 10.1093/molbev/msp077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kazuki S., Fumio M. Metabolomics for Functional Genomics, Systems Biology, and Biotechnology. Annu. Rev. Plant Biol. 2010;61:1. doi: 10.1146/annurev.arplant.043008.092035. [DOI] [PubMed] [Google Scholar]
- 46.Zhang Z. KaKs-calculator 3.0: Calculating Selective Pressure on Coding and Non-coding Sequences. Genom. Proteom. Bioinform. 2022;20:536–540. doi: 10.1016/j.gpb.2021.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Das Bidyadhar. Glucosinolate Biosynthesis: Role of MAM Synthase and Its Perspectives. Biosci. Rep. 2021;41:BSR20211634. doi: 10.1042/BSR20211634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Abrahams R.S., Pires J.C., Schranz M.E. Genomic Origin and Diversification of the Glucosinolate MAM Locus. Front. Plant Sci. 2020;11:111. doi: 10.3389/fpls.2020.00711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang P.L., Chang T., Zhang Q.Z., Cai Y.L., Zhou Z.Y., Hu M.F., Zhang J.Y. Identification of MAM1s in Regulation of 3C Glucosinolates Accumulation in Allopolyploid Brassica juncea. Crit. Rev. Biotechnol. 2020;6:409–418. [Google Scholar]
- 50.Meenu A.R., Majee M., Pradhan A.K., Bisht N.C. Genomic Origin, Expression Differentiation and Regulation of Multiple Genes Encoding CYP83A1, A Key Enzyme for Core Glucosinolate Biosynthesis, from The Allotetraploid Brassica juncea. Planta. 2015;241:3. doi: 10.1007/s00425-014-2205-0. [DOI] [PubMed] [Google Scholar]
- 51.Biao Z., Wang Z.Z., Yang Y., Zhu Z.J., Wang H.S. Isolation and Expression of Glucosinolate Synthesis Genes CYP83A1 and CYP83B1 in Pak Choi ( Brassica rapa L. ssp. chinensis var. communis (N. Tsen & S.H. Lee) Hanelt) IJMS. 2012;13:5. doi: 10.3390/ijms13055832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bak S., Feyereisen R. The Involvement of Two P450 Enzymes, CYP83B1 and CYP83A1, in Auxin Homeostasis and Glucosinolate Biosynthesis. Plant Physiol. 2001;127:1. doi: 10.1104/pp.127.1.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kiarash J.G., Ali Riahi-Madvar F.R., Rambod P., Fereshteh J.B., Mahshid G.A. Effect of Salinity Stress on Enzymes’ Activity, Ions Concentration, Oxidative Stress Parameters, Biochemical Traits, Content of Sulforaphane, and CYP79F1 Gene Expression Level in Lepidium draba Plant. J. Plant Growth Regul. 2019;39:1075–1094. [Google Scholar]
- 54.Chen S.X. CYP79F1 and CYP79F2 Have Distinct Functions in The Biosynthesis of Aliphatic Glucosinolates in Arabidopsis. Plant J. 2003;33:923–937. doi: 10.1046/j.1365-313X.2003.01679.x. [DOI] [PubMed] [Google Scholar]
- 55.Sharma M., Mukhopadhyay A., Gupta V., Pental D., Pradhan A.K. CYP79F1 Regulates Synthesis of Propyl Fraction of Aliphatic Glucosinolates in Oilseed Mustard Brassica juncea: Functional Validation through Genetic and Transgenic. PLoS ONE. 2016;11:e0150060. doi: 10.1371/journal.pone.0150060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Czerniawski P., PiślewskaBednarek M., Piasecka A., Kułak K., Bednarek P. Loss of MYB34 transcription Factor Supports Backward Evolution of Indole Glucosinolate Biosynthesis in A Subclade of Camelineae tribe and Releases Feedback Loop in This Pathway in Arabidopsis. Plant Cell Physiol. 2022;12:pcac142. doi: 10.1093/pcp/pcac142. [DOI] [PubMed] [Google Scholar]
- 57.Henninge F., Eriche G., Tamarae G. The Role of MYB34, MYB51 and MYB122 in The Regulation of Camalexin Biosynthesis in Arabidopsis thaliana. Front. Plant Sci. 2015;6:654. doi: 10.3389/fpls.2015.00654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hansen C.C., Nelson D.R., Møller B.L., WerckReichhart D. Plant Cytochrome P450 Plasticity and Evolution. Mol. Plant. 2021;14:1772. doi: 10.1016/j.molp.2021.09.013. [DOI] [PubMed] [Google Scholar]
- 59.Dimaano N.G., Iwakami S. Cytochrome P450-mediated Herbicide Metabolism in Plants: Current Understanding and Prospects. Pest Manag. Sci. 2021;77:22–32. doi: 10.1002/ps.6040. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not applicable.