Abstract
Oligosaccharides derived from natural resources are attracting increasing attention as both food and nutraceutical products because of their beneficial health effects and lack of toxicity. During the past few decades, many studies have focused on the potential health benefits of fucoidan. Recently, new interest has emerged in fucoidan, partially hydrolysed into fuco-oligosaccharides (FOSs) or low-molecular weight fucoidan, owing to their superior solubility and biological activities compared with fucoidan. There is considerable interest in their development for use in the functional food, cosmetic, and pharmaceutical industries. Therefore, this review summarises and discusses the preparation of FOSs from fucoidan using mild acid hydrolysis, enzymatic depolymerisation, and radical degradation methods, and discusses the advantages and disadvantages of hydrolysis methods. Several purification steps performed to obtain FOSs (according to the latest reports) are also reviewed. Moreover, the biological activities of FOS that are beneficial to human health are summarised based on evidence from in vitro and in vivo studies, and the possible mechanisms for the prevention or treatment of various diseases are discussed.
Keywords: fuco-oligosaccharides, brown algae, preparation, biological activities, prebiotics
1. Introduction
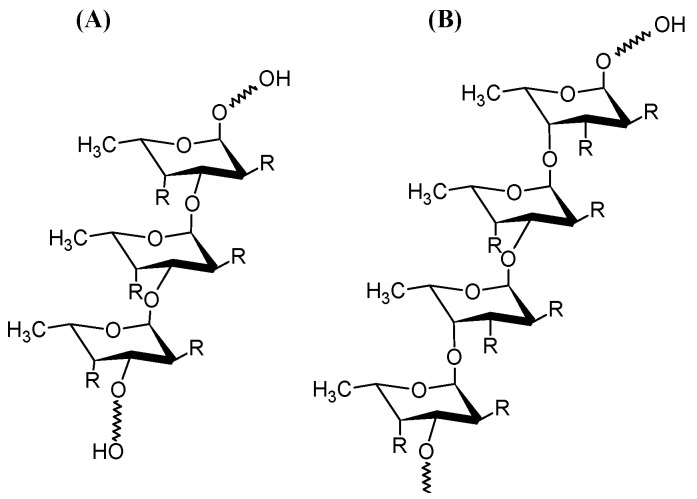
Fucoidan is a polysaccharide mainly composed of fucose, with a high sulphate content (more than 20%). This carbohydrate moiety is found in marine resources, generally in brown algae, sea cucumbers, and sea urchin eggs (as indicated in Table 1). The chemical structure of fucoidans consists of a backbone of α-(1→3)-L-fucopyranose residues, or alternating α-(1→3)-linked and α-(1→4)-linked L-fucopyranose or α-(1→2)-L-fucopyranose residues (Figure 1), with different substitutions, such as fucose, sulphate, acetate, and uronic acid [1,2]. Fucoidan presents a wide range of pharmaceutical activities, including antioxidant, anticancer, immunomodulatory, anticoagulant, and antimicrobial activities [3,4].
Table 1.
Sources of fucoidan derived from brown algae, sea cucumber, and sea urchin species.
| Sources | Species |
|---|---|
| Brown algae | Adenocystis utricularis, Ascophyllum nodosum [5], Bifurcaria bifurcate [6], Cladosiphon okamuranus [6], Coccophora langsdorfii [7], Cystoseira sedoides [7], Desmarestia intermedia [6], Dictyosiphon foeniculaceus [6], Dictyota dichotoma [6], Ecklonia kurome [6], Eisenia bicyclis [6], Fucus vesiculosus [5], F. serratus [6], F. spiralis [6], Laminaria digitate [5], L. saccharina [5], Saundersella simplex [6], Sargassum siliquosum [6], Scytosiphon lomentaria [6], Undaria pinnatifida [6] |
| Sea cucumber | Acaudina leucoprocta [2], A. molpadioides [2], Holothuria fuscopunctata [2], H. polii [2], Isostichopus badionotus [2], Ludwigothurea grisea [2], Stichopus horrens [2], Thelenota ananas [2] |
| Sea urchin | Arbacia lixula [6], Lytechinus variegatus [6], Strongylocentrotus droebachiensis [8], S. franciscanus [6], S. intermedius [6], S. pallidus [8], S. purpuratus [6] |
Figure 1.
The chemical structure of fucoidan. (A) backbone of α-(1→3)-L-fucopyranose residues; (B) backbone alternating α-(1→3)-linked and α-(1→4)-linked L-fucopyranose residues. R = OH or OSO3−.
In fact, the preparation and structural characterisation of fucoidan seem to be well elucidated. However, haemorrhagic risk, high molecular weight (MW), high viscosity of fucoidan, and a poor dissolution rate in solution may limit their successful application in functional foods and pharmaceuticals [9,10]. Fucoidans often differ in terms of structural diversity and complexity; therefore, standardisation and quality assurance of fucoidans are challenging. Therefore, there has been a recent increase in interest in degrading fucoidan into fuco-oligosaccharides (FOSs) or low-MW fucoidan (LMWF) to broaden potential applications.
Oligosaccharides consist of a mixture of 2–20 monosaccharides, with a variable extent of polymerisation. They have attracted increasing interest owing to their nutritional and biological properties [11]. Many functional oligosaccharides, such as fructo-oligosaccharides, galacto-oligosaccharides, xylo-oligosaccharides, manno-oligosaccharides, and isomalto-oligosaccharides, have been widely used as prebiotics in food application [12,13,14]. Commercial prebiotics are derived from terrestrial plant oligosaccharides. However, research is underway to explore new prebiotics from natural resources, especially from renewable sources. Marine algae have unparalleled advantages as easily renewable and extremely abundant resources, as they grow in seawater and avoid the demand for freshwater. FOSs, also called LMWF, is an oligomer of fucoidan, which is normally obtained from brown algae. FOSs have a degree of polymerisation (DP) < 20 and an average MW of less than 10 kDa. FOSs also show a prebiotic ability and can be fermented by the gut microbiota, exert a range of physiological effects on body functions and improve human health [15]. Therefore, FOSs and other marine algae oligosaccharides are considered promising prebiotics.
This review provides a comprehensive background to FOSs, and evidence of their functional effects on human health. To this end, a detailed review of the current state of research on the preparation, purification, and characterisation of FOSs is presented. Thereafter, the potential health benefits of FOSs are discussed explicitly.
2. Preparation of Fucoidan and/or Raw Materials for Fuco-Oligosaccharide Production
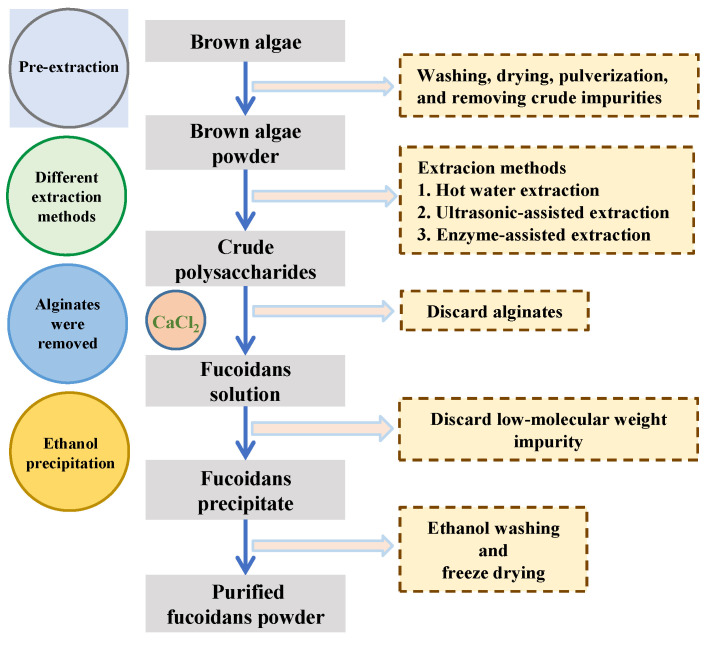
The advantages of FOSs include high water solubility, low viscosity, and good biological activity. However, FOSs are seldom found in natural resources. Therefore, the degradation of fucodain is necessary for the production of FOSs. Figure 2 shows a singular diagram of the process of preparing fucoidan from brown algae. The extraction processes are based on aqueous (water, acidic, or alkaline solution) extraction; the polysaccharides are precipitated by ethanol; proteins are removed by the Sevag or enzymatic method, and the low-molecular-weight impurity is discarded by diafiltration [16]. The Sevag method is widely used to discard protein. It was first exploited by Sevag et al., as they used an organic solvent, including chloroform and n-butanol, to precipitate protein from sample solution [17]. An acid or calcium chloride solution added to the polysaccharide mixture has been commonly used to separate alginic acid from fucoidan. Béress et al. precipitated the alginic acid by adding glacial acetic acid, and the process was completed after 12 h at 4 °C [18]. Furthermore, column chromatography, such as ion-exchange and size-exclusion methods, can be used to obtain fucoidan [19]. Currently, three major processes exist to obtain FOSs from fucoidan: degradation by mild acids, enzymes, and radical methods. The mild acids and radical degradation methods are considered to be random and non-specific in degrading fucosidic linkages, while enzymatic method recognizes the specific substrate to cleave fucosidic linkages.
Figure 2.
Schematic diagram of the extraction and preparation of fucoidan from brown algae.
3. Preparation of Fuco-Oligosaccharides
3.1. Mild Acid Hydrolysis
Mild acid hydrolysis usually uses dilute acid solutions, such as sulphuric acid, hydrochloric acid, and trifluoroacetic acid, at suitable heating temperatures and times. FOSs mixtures are obtained after hydrolysis and subsequent neutralisation. Mild acid hydrolysis seems to offer a simple, low-cost production, and an easy operation, especially to fulfil requirements in the laboratory. Therefore, the mild acid hydrolysis method is always adopted by laboratories to screen for potential biological activities of FOSs. An aliquot of 0.05 mol/L sulphuric acid solution was used to degrade Nemacystus decipiens fucoidan at 80 °C for 2 h to obtain FOSs with a DP ranging from 2 to 9, and the FOSs showed antithrombotic activity [20]. A high-MW fucoidan, derived from S. hemiphyllum, was degraded by 0.01 mol/L hydrochloric acid at 85 °C for 15 min to obtain FOSs with a MW of 800 Da. The result showed skin-protective effects against ultraviolet B damage [21].
However, the degradation method is non-specific and may produce a series of FOSs, which increases the challenge of purifying FOSs. Moreover, the sulphated group in fucoidan is acid-labile and easily hydrolysed during acid hydrolysis. Mild acid hydrolysis (parameters used 0.005–0.01 mol/L trifluoroacetic acid at 60–100 °C for 2–4 h) was conducted on fucan sulphate derived from S. herrmanni, to obtain a number of FOSs. They contained two series of FOSs: 2-desulphated and 2-sulphated residues at the reducing ends of FOSs [22]. Pomin et al. found that in the dilute acid hydrolysis of fucoidan, the 2-sulphate ester group is initially cleaved, subsequently degrading the glycosidic bonds between the non-sulphated residue and the 4-sulphate group [23]. The kinetic constant of mild acid hydrolysis on sulphated fucan derived from three sea cucumber species, I. badionotus, H. floridana, and L. variegatus, with core α (1→3)-linked fucose, degraded the sulphate group substitution at the 2-O and 4-O-positions. The mild acid hydrolysis progress was under 0.05 mol/L sulphuric acid at 60 °C for 9 h, and selective 2-desulphation in sulphated fucan and tetrasaccharides FOSs were obtained [24].
Mild acid hydrolysis is suitable for a large-scale production of FOSs. Future studies on the kinetics and mechanism of mild acid hydrolysis should focus on providing fundamental data for industrial application in the large-scale production of FOSs.
3.2. Enzymatic Hydrolysis
Several hydrolytic enzymes that catalyse the hydrolysis of polysaccharides have been characterised. Normally, the breakdown of fucoidans involves the degradation of glycosidic linkages by fucoidanases (EC 3.2.1.44) and α-L-fucosidases (EC 3.2.1.51, EC 3.2.1.63, EC 3.2.1.111, and EC 3.2.1.127), which converts fucoidans into FOSs [25]. The enzymes that produce FOSs are produced by marine microorganisms and include endo- and exo-enzymes [26]. The well characterized endo-enzymes for fucoidan hydrolysis include endo-fucanases FcnA, FFA1, FFA2, Fp273, Fp277, Fp279, FWf1, FWf2, Fhf1, Fhf2 of the GH107 family and endo-fucanase FunA of the GH168 family, while the α-L-fucosidase is exo-enzymes and described for the families of glycoside hydrolases (GH) 29, 95, and 141 [25,27,28]. Enzymatic depolymerisation is highly selective for specific substrates and efficient for producing the desired end-product under selective method conditions. Therefore, with the enzymatic method, unlike the mild acid hydrolysis method, it is possible to achieve hydrolysate of crude fucoidan yielding FOSs.
The extent of enzymatic action is strongly dependent on operating conditions, including temperature and pH. Fucoidan with a MW of approximately 400 kDa from F. distichus was incubated together with endo-α-(1,4)-fucoidanase from the marine bacterium Formosa haliotis (Fhf1Δ470), in Tris-HCl buffer (pH 8) at 37 °C for 1 d, yielding FOSs with a MW of 2 kDa [29]. The study demonstrated the use of the enzymatic technique to screen out the tailored fucoidan functional group (FOSs) to specifically improve bone regeneration [29]. Enzymatic depolymerisation of L. japonica fucoidan by fucoidanase—which is produced by Flavobacteriaceae RC2-3—yielded FOSs with a MW < 2 kDa (96.3%) after incubation at 37 °C for 10 h. The FOSs products showed an excellent radical scavenging activity and inhibition of tyrosinase activity, rendering them suitable for application as cosmetic skin-whitening additives [30]. Another advantage of the enzymatic method is that the sulphated groups in FOSs may be preserved during the depolymerisation process. A fucoidanase obtained from the digestive glands of mollusc Lambis sp., specifically, degraded (1→3) and (1→4)-α-L-fucans derived from F. evanescens and F. vesiculosus, yielding sulphated FOSs, without any effect on the sulphate group [31].
To overcome problems of using the enzyme, including those owing to its high cost and poor stability, the immobilised enzyme technique is preferred. The immobilised enzyme technique has the advantages of maintaining enzymatic activity, ease of recovery after coating monolithic substrates, and easy scaling up of processes for industrial application [32].
The enzymatic method is considered a good choice for producing FOSs for applications in the food, cosmetic, and pharmaceutical industries. Therefore, screening for new enzymes that can degrade fucoidan has become an increasingly important focus of scientists and industrial engineers; in particular, searching for new endo-fucoidanases and their subsequent kinetic analysis, molecular cloning, and immobilisation techniques to provide valuable fundamental knowledge to produce highly active FOSs.
3.3. Radical Depolymerisation
Radical depolymerisation is a process that uses radicals to attack glyosidic linkages of polysaccharides to form oligosaccharides. The radicals, including HOO−•, •O2−, and HO•, can be generated by hydrogen peroxide (H2O2) and a catalytic metal system. Depolymerisation by H2O2 involves radicals (HOO•, •O2−, and HO•) attacking the glycosidic linkages [33]. This method has the advantages of good reproducibility and controllability. The main parameters that influence the efficacy of depolymerisation include the temperature, concentration of H2O2, reaction time, and substrate concentration.
Radical depolymerisation of fucan sulphate from S. herrmanni was performed by adding H2O2 and copper (II) acetate monohydrate. On average, the MW of fucan sulfate was degraded from 790.8 kDa to 8.32–22.11 kDa. The resulting products exhibited good anticoagulant activity [34]. Moreover, a combination of ascorbic acid and H2O2 was used to degrade fucoidan at 25 °C for 16 h, to obtain a fraction with the MW of approximately 3 kDa, which exhibited anti-lung-cancer activity in vitro [35]. Qi et al. reported that radical depolymerisation can maintain the sulphate content or other functional units in the degradation process [36]. Qi et al. degraded U. pinnatifida fucoidan using a photocatalytic method, which processes the reaction in H2O2 and TiO2 under xenon light for 3 h. While the MW of 190 kDa was degraded to 3 kDa, the resulting product exhibited anticoagulant activity [36].
Moreover, photoirradiation techniques for the degradation of polysaccharides have been attracting increasing attention. These techniques provide efficient degradation methods for the production of FOSs from fucoidan, because photoirradiation can be achieved at room temperature and under normal pressure. Ultraviolet and gamma radiation are typically used to degrade fucoidan. They are an energetic form of electromagnetic radiation, with a short wavelength and high energy. Water radiolysis is the major effect of gamma irradiation of solutions. Higher gamma irradiation doses may result in a lower MW of fucoidan: the MW of F. vesiculosus fucoidan degraded from 210 kDa to 85 kDa and 7 kDa following irradiation doses of 8 kGy and 100 kGy, respectively, while the latter degraded product showed the highest antioxidant activity [37]. In addition, photoirradiation combined with radical depolymerisation increased the efficiency of FOSs production. The average MW of fucoidan decreased to 6.7 kDa at an irradiation dose of 20 kGy, while the degradation efficiency increased by 5% when a 10% H2O2 concentration was used in combination with gamma radiation. The hydroxyl radical scavenging abilities of degraded product of FOSs were more effective than fucoidan [38]. Park and Choi also used a combination of gamma radiation at a dose of 100 kGy and H2O2 to degrade U. pinnatifida fucoidan from MW 378 kDa to 6 kDa. Park and Choi found that degraded fucoidan has a higher inhibitory activity against tyrosinase and antioxidant activity than fucoidan [39].
4. Purification of Fuco-Oligosaccharides
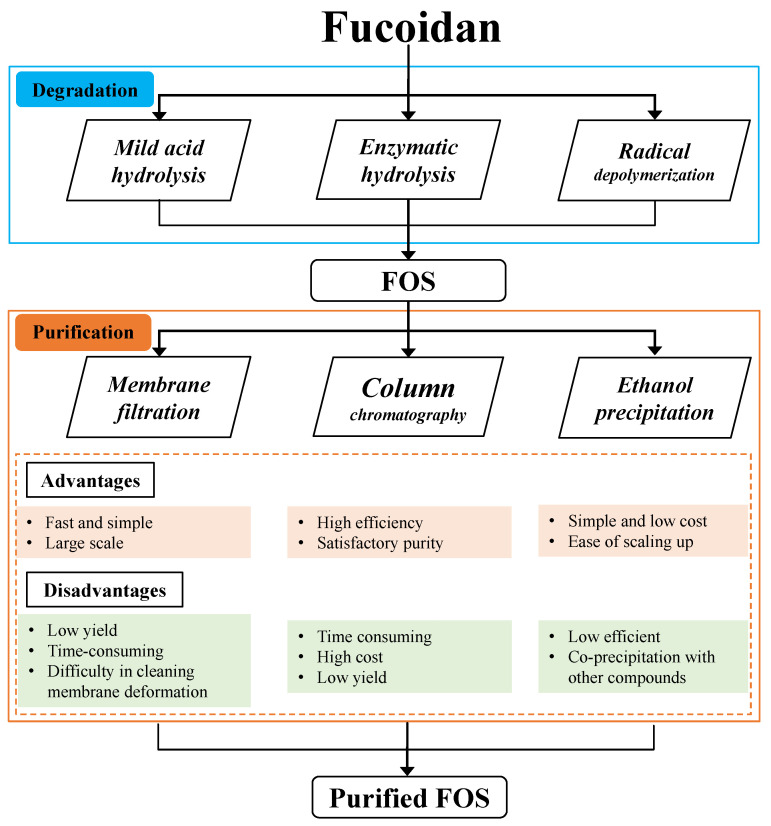
The mixtures of FOSs produced by degradation methods include a variety of compounds, i.e., monosaccharides, FOSs ranges of different DPs, and large MW fucoidan. To obtain high-purity FOSs (for use in studies of the structure–function relationship and mechanism, and for applications in functional food or pharmaceutical areas), some suitable purification steps should be performed. Figure 3 shows a schematic diagram of the production and purification of FOSs.
Figure 3.
Schematic diagram of FOS degradation and purification processes.
Step-gradient ethanol precipitation is a convenient and time-saving method for the large-scale production of FOSs. Different MWs can be prepared by adding an ethanol gradient at a final concentration of 10–80%. However, this method yields a broad MW distribution of FOSs, and it is difficult to obtain high-purity FOSs. For example, Fernando et al. performed a step-gradient ethanol precipitation to exclude high-MW fucoidan, and a broad MW distribution of FOSs, ranging from 8 to 25 kDa, was achieved [40]. Thus, ethanol precipitation is useful as a preliminary step for discarding high-MW fucoidans or proteins.
Membrane filtration is another green technique for the purification of FOSs, and its performances are quite similar to gradient ethanol precipitation, which obtained a broad MW distribution of FOSs. Zhao et al. purified a degraded fucoidan mixture, using an ultrafiltration membrane (10 kDa and 2.5 kDa MW cut-off) to obtain the FOSs fraction, and the fraction range 2.5–10.0 kDa was collected [9]. The degraded fucoidan samples passed through a 30-kDa-MW cut-off membrane and subsequently through a 1-kDa membrane, obtaining an FOSs fraction MW range of 1–30 kDa [41]. Dialysis bags with different MW cut-off membranes have been used to obtain MW values of lower than 10 kDa fraction [42].
Column chromatography, including ion-exchange chromatography and size-exclusion chromatography, is widely used for fractionation oligosaccharides during purification. Size-exclusion chromatography has been used to fractionate oligosaccharides according to their MW, and this is suitable for the purification of FOSs and achieving high purity. Commercially available size-exclusion chromatography resins have been used to purify FOSs. The purified FOSs fractions are suitable for structural identification and pharmaceutical activity investigation. After mild acid hydrolysis the fucoidan hydrolysate sample was purified using a Bio-Gel P-2 column, and a high-purity fucotrioligosaccharide was obtained [43]. A mixture of oligosaccharides, produced from acid hydrolysis, was further purified using Bio-Gel P-4 and P-2, to obtain a high-purity yield of 6–9% of monosulphated fucobiose and monosulphated fucotriose [44]. Ion-exchange chromatography for FOSs purification proceeds according to the sulphate content of the FOSs, which leads to different ionic interactions with the resins. The FOSs mixture produced from fucoidan was degraded by the enzymatic method and further purified by ion-exchange chromatography on Q-Sepharose, to yield DP 4–10 FOSs with different sulphate contents [45].
5. Biological Activities of Fuco-Oligosaccharides
5.1. Antioxidant Activity
Numerous studies have reported the importance of antioxidants in human health systems. Antioxidants counteract oxidative damage to nucleic acids, lipids, peptides, and proteins that causes diseases including cancers, chronic inflammation, cardiovascular diseases, neurodegenerative disorders, and metabolic diseases [46,47]. The search for new and safe antioxidant components obtainable from natural sources, and the investigation of their antioxidant properties has attracted substantial interest worldwide.
The in vitro antioxidant activity of components of interest are normally tested in more than one assay model, as different antioxidant methods vary and are established based on their various scavenging mechanisms [48]. Previous studies have shown that antioxidant activities are affected by the MW of oligosaccharides, and several reports have demonstrated that antioxidant activities increase with a decrease in the MW of oligosaccharides [49]. Hou et al. compared different LWMF, which were degraded from Saccharina japonica fucoidans; they revealed that the hydroxyl scavenging ability was the highest in 1.5–4.0 kDa and 80 kDa fractions, while the reducing power increased as the MW decreased below 13 kDa [50].
The antioxidant activity of FOSs is also influenced by its degree of polymerisation and sulphate content. Different degrees of polymerisation (DP2, DP4, and DP6) of oligosaccharides were obtained from S. thunbergii fucoidan by acid degradation. The DP 2 oligosaccharides had lower hydroxyl radical scavenging activity, 1,1-diphenyl-2-picrylhydrazyl radical scavenging and reducing power than DP 4 and DP 6. The DP2–6 oligosaccharides had a lower reducing power when compared with sulphated oligosaccharides [51].
On the contrary, FOSs with antioxidant activities also play important roles in the antioxidant defence system in vivo. FOSs act as a frontline defence by suppressing the formation of potentially harmful metabolic by-products (reactive oxygen and nitrogen species) that can have deleterious effects on the body and cause damage to host biological structures [52]. A crude LWMF, obtained from S. autumnale by protamex-assisted hydrolysis, showed significant prevention against H2O2-induced oxidative stress in a zebrafish model, and demonstrated that it improved the heart rate, decreased the generation of ROS, and suppressed lipid peroxidation in zebrafish embryos [53]. Notably, FOSs have also been demonstrated to improve the skin antioxidant network. The UVB-irradiated skin-damaged mouse models showed an increase in glutathione and decreased myeloperoxidase, malondialdehyde, and superoxide after treatment with FOSs. FOSs treatment suppresses the progression to oxidative stress and improves innate antioxidant-related enzyme activities [54].
In short, FOSs have promising potential applications as naturally derived antioxidant or radical scavenging agents in the pharmaceutical, nutraceutical, cosmetic, and food industries.
5.2. Anticancer Activity
Cancer is a major health problem and the second leading cause of death worldwide. More than half of all cancer cases and tumour development processes in the body are considered preventable [55]. As anticancer drugs have adverse side effects on several tissues, including the brain, heart, and gastrointestinal tract, doctors and biologists have been interested in the application of compounds derived from natural products to prevent cancer development in the body. Numerous studies have demonstrated a relationship between anticancer activities and highly functional compounds derived from natural resources [56,57]. Marine algae are among the most abundant resources and remain under-exploited. They contain a variety of nutrients, among which, marine algae polysaccharides and their derived oligosaccharides have attracted considerable interest. In our previous review, we summarised a variety of direct and indirect anticancer activities of marine algal polysaccharides that inhibit or affect cancer and tumour cells [58]. Marine algae polysaccharides exhibit anticancer activities with different reported signalling pathways, including mitogen-activated protein kinase, nuclear factor-kappa B, phosphoinositide 3-kinase/protein kinase B, and Wnt/β-catenin signalling pathways, which have been summarised and discussed in a previous review [58]. The increasing recognition of bioactive oligosaccharides has accelerated research into their potential anticancer activity. We believe that FOSs may have promising potential applications in cancer therapy.
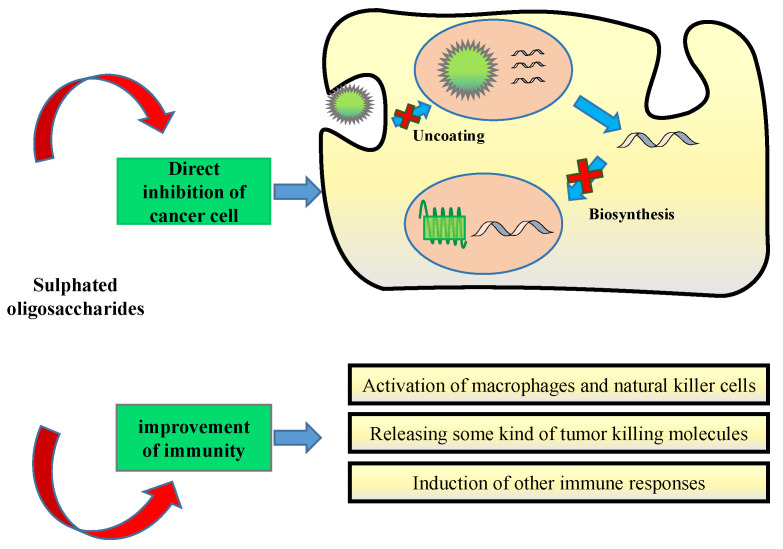
Recently, anticancer activities of FOSs have been demonstrated through two common mechanisms: direct and indirect anticancer activities that affect both tumour cells and their surrounding microenvironment (Figure 4). In the former, FOSs directly inhibit cancer cells or induce tumour-cell apoptosis. In the latter, FOSs stimulate the host immune defence system (including activating macrophages, lymphocytes, and natural killer cells) and regulate the gut microbiota.
Figure 4.
Anticancer activities of FOSs through two common mechanisms: direct and indirect anticancer activities that affect both tumor cells and their surrounding microenvironment.
Apoptosis is a form of programmed cell death that plays a critical role in eliminating unnecessary or unhealthy cells. Numerous studies have found that sulphation present on carbohydrate moieties shows anti-proliferative activity in tumour cells by causing a cell cycle arrest and apoptosis [59]. FOSs with DP 2–6, with approximately five sulphate groups per sugar, showed significantly inhibited growth of the human malignant melanoma cell line SK-MEL-28, and better activity than fucoidan [60]. Another report on LWMF also showed higher inhibitory activity against breast cancer cells (MCF-7), human stomach cancer cells (AGS), and human liver cancer cells (HepG-2) than fucoidan [61]. A unique low-MW FOSs obtained from New Zealand U. pinnatifida inhibited the growth of MCF-7 and MDA-MB-231 cell lines (breast cancer cells) in a concentration-dependent manner, and induced apoptotic cell death through a caspase-dependent pathway [62]. Similarly, FOSs (containing approximately 72% and MW < 500 Da) induced MDA-MB-231 cell apoptosis, which is related to the activation of caspases and mitochondrial dysfunction, while decreasing the level of the Bc1-2 family [63].
In contrast, another anticancer approach is to use FOSs to enhance host immune defence by enhancing macrophages and natural killer cell functions. Macrophages are phagocytic cells, which have been considered for cancer immunotherapy in recent years, as they play an important role in host immune defence systems by killing tumour cells via phagocytosis, and releasing tumour-killing molecules, such as nitric oxide (NO), tumour necrosis factor (TNF-α), reactive oxygen species (ROS), interleukin (IL)-1β, and IL-6 [64]. FOSs with a MW < 10 kDa were degraded from U. pinnatifida and effectively triggered NO release, enhanced iNOS levels, and induced TNF-α and IL-6 secretion in RAW264.7 macrophages. The results also demonstrated that the mechanism of macrophage expression is related to the MAPK and NF-KB signalling pathways, which are the major regulators of inflammation and apoptosis in cancer cells [42]. Hwang et al. reported that an FOSs with a MW < 3000 Da significantly activated the immune defence system by improving NK cell activity, phagocytic activity, lymphocyte proliferation, and cytokine production [65].
Furthermore, mainstream cancer treatment sometimes incorporates the complementary therapy approach, which is a broad set of non-mainstream practices for the use of natural products together with conventional medicine. A previous study indicated that FOSs may serve as an efficient complementary therapy for cancer. Huang et al. demonstrated in vitro that FOSs and fluoropyrimidine combination chemotherapy improved therapeutic efficiency by inhibiting cancer cells. FOSs enhanced the suppressive effects of 5-FU on cancer cell migration through the c-mesenchymal–epithelial transition/matrix metalloproteinase-2 signalling pathway in HCT116 and Caco-2 cells [66]. The application of FOSs as a complementary therapy for cancer treatment has been investigated in clinical studies. Tsai et al. reported that FOSs with an average MW of 800 Da and sulphate content of approximately 39% (w/w) were used as a supplemental therapy combined with chemo-target agents in patients with metastatic colorectal cancer. They found that FOSs are an auxiliary therapy for metastatic colorectal cancer and improve the rate of disease control [67].
In summary, FOSs showed direct and indirect anticancer activity and could be developed as an anticancer agent. It would be notable to study the interactions between FOSs and conventional chemotherapeutic agents in the treatment or management of cancer. Clinical practices can provide large amounts of data and more convincing evidence; therefore, we only investigated clinical practices and obtained data to fully understand the therapeutic effect of FOSs.
5.3. Prebiotic Activity
Prebiotics are well known as health-promoting functional foods and nutraceuticals. Prebiotic oligosaccharides are usually indigestible by the stomach and upper gastrointestinal tract, and when they reach the large intestine, they are fermented and utilised by the gut microbiota [68,69]. This process transmits numerous health benefits to humans by producing numerous functional metabolites and modulating the intestinal ecosystem.
Maintaining a balance in the intestinal ecosystem is important to host health, as gut microbiota dysbiosis may increase the risk of some diseases, such as inflammatory bowel diseases, central nervous system disorders, cardiovascular diseases, and many kinds of cancers [70,71]. Several studies have reported that prebiotic oligosaccharides effectively maintain a favourable balance in the gut microbiota. Currently, fructo-oligosaccharides, galacto-oligosaccharides, xylo-oligosaccharides, and isomalto-oligosaccharides with prebiotic properties are commercially available [72]. Although FOSs are neither commercially available nor approved for use in functional food production, their role in the improvement of the gut microbiota is gaining increasing attention.
The ability of the gut microbiota to utilise poly/oligosaccharides is dictated by the repertoire of carbohydrate-active enzymes (CAZymes) encoded by their genomes, which normally include GH, polysaccharide lyases, and carbohydrate esterases (CE) [73]. The CAZyme-encoding genes act on fucoidan, and associated with the degradation of FOSs, belong to the families of CE4, GH29, GH107, S1_17 and S1_25 [27,28]. The diversity of CAZymes in the gut microbiota is linked to the complexity of the dietary fibre. Therefore, the dominant bacterial population in the gut is related to the oligosaccharides that are consumed by the host. In our previous study of in vitro fermentation of FOSs using a mixed culture of human faecal bacteria, the FOSs, prepared from S. japonica polysaccharides by mild acid hydrolysis, modulated the gut microbiota composition to increase the relative abundance of the phylum Bacteroidetes, and decrease the phylum Proteobacteria. Moreover, the production of SCFAs increased [74]. Such evidence was also found in vivo. The FOSs obtained from two species of sea cucumber, Pearsonothuria graeffei and I. badionotus, alleviated disordered intestinal bacteria in high-fat-diet-induced mice, especially by increasing the relative abundance of Bacteroidetes and decreasing of Firmicutes [75].
Bacteroidetes are one of the most abundant bacterial phyla in the human gut, and are considered primary utilisers of polysaccharides because they contains CAZyme-encoding genes that are necessary for the hydrolysis of poly-/oligosaccharide linkages [76]. Some species, such as Bacteroides thetaiotaomicron and B. ovatus, act as generalists able to cleave a wide range of glycosidic linkages of oligosaccharides, whereas others prefer to utilise specific glycosidic bonds or mono-/oligosaccharides which are released from poly-/oligosaccharides [77]. Bacteroidetes not only degrade the large MW of carbohydrates into smaller sugars, but also produce metabolites that may influence the formation of complex ecological networks and the diversity of the gut microbiota in the large intestine. The gut microbiotas exchange their metabolites with other bacteria, and these cross-feeding interactions play critical roles in the formation of diverse complex microbiota. Beneficial bacteria, such as Lactobacillus and Bifidobacterium species, are responsible for the cross-feeding behaviour of Bacteroidetes on oligosaccharides, because beneficial bacteria can utilise the smaller carbohydrates and metabolites produced by Bacteroidetes [78,79]. Some evidence has been obtained from previous studies on xylan-type polysaccharides. Co-fermentation of Bifidobacterium animalis with Bacteroides species, using xylan as a carbohydrate source, can enhance the growth of B. animalis. The results demonstrate that the prebiotic activity of xylans may be related to the cross-feeding of Bacteroides species, degrading xylan into smaller oligosaccharides and producing metabolites that are favourable to the Bifidobacterium species for growth [80]. Furthermore, the cross-feeding B. ovatus is also present on galactomannas, which share degraded products and metabolites together with Bifidobacterium adolescentis and Lactiplantibacillus plantarum, promoting their growth and increasing their production of SCFA [81]. FOSs prepared from S. hemiphyllum fucoidan showed a better prebiotic effect than their fucoidan, which promoted the growth of Bifidobacterium lactis [82]. Therefore, we believe that FOSs can support other beneficial bacterial growth and the diversity of the microbial community through cross-feeding interactions.
Prebiotic-derived functional metabolites, mainly SCFAs, exist and host a wide range of pharmacological effects, such as maintaining gut integrity, regulating glucose homeostasis, altering lipid metabolism, modulating the immune system, and protecting the cardiovascular system [83,84]. The major products of SCFAs include acetate, propionate, and butyrate, which are generated in the large intestine by beneficial bacterial fermentation of FOSs. SCFAs address their functions by activating specific G protein-coupled receptors, GPR43 and GPR41, which are also known as free fatty acid receptors 2 (FFR2) and 3 (FFR3), respectively, and are present in a variety of tissues [85]. Acetate normally stimulates the FFR2 at the cell surface, whereas butyrate binds and activates FFR3 [86]. Deng et al. reported that LWMF fraction LF2 ameliorated gut microbiota dysbiosis induced by a high-fat diet, by increasing the relative abundance of Akkermansia muciniphila and the production of SCFAs [87]. Recently, A. muciniphila, regarded as a probiotic, has been shown to degrade mucins or oligosaccharides as energy sources to produce SCFAs. The relative abundances of A. muciniphila and its derived SCFA are closely associated with the thickness of the colonic mucus layers and mucosal barrier integrity [88]. Acetate and propionate, the metabolites of oligosaccharide degradation by A. muciniphila, specifically stimulate GPR43, leading to modulation during inflammation and protection in high-fat-diet-induced mice [89].
In short, FOSs have potential prebiotic ability because they are indigestible in the upper gastrointestinal tract, possesse cross-feeding interaction with the gut microbiota and produce functional metabolites [15,90]. However, further studies are needed to determine the mechanisms by which specific gut bacteria utilise FOSs, and the isolation and purification of bacterial strains are necessary. Meanwhile, to investigate how specific microbiotas share their metabolites with other beneficial bacteria, metabolomics studies need to be conducted.
5.4. Antiviral Activity
Viral infections are a global public health problem that threaten human life and health. Although several vaccines have been explored for protection against specific viruses, they do not provide permanent protection. Moreover, some clinically available antiretrovirals have serious side effects, such as mitochondrial toxicity, hypersensitivity, and lipodystrophy [91]. However, effective and low-toxicity antiviral components have been identified from natural sources. Several polysaccharides and oligosaccharides derived from natural resources have shown excellent antiviral activity against several viruses [92].
FOSs prepared from L. japonica with a MW of 3700–7600 Da presented good anti-influenza viral activities both in vitro and in vivo. FOSs showed inhibitory effects on I-type influenza virus, adenovirus, and parainfluenza virus infection in Hep-2, HeLa, and MDCK cell lines, respectively. In vivo, intragastric administration of FOSs (5–10 mg/kg dosage) increased the survival time of mice exposed to the influenza virus, and also increased the organ indices of the thymus, spleen, and lung [93]. The antivirus mechanism of sulphated FOSs may associate with their ability in interfering the virus life cycle, including transcription, replication, and invasion [94].
Hantaan orthohantavirus is a global public health threat and may cause haemorrhagic fever with cardiopulmonary syndrome after infection. The mixture of FOSs fractions obtained from F. evanescens fucoidan, degraded by fucanases (GH107 family), showed anti-orthohantaviral activity. This FOSs mixture contains oligosaccharides with DPs ranging from 4–16, and with a high concentration of fuco-tetrasaccharide, comprising the repeating units of →3)-α-L-Fucp2S-(1→4)-α-L-Fucp2S(1→ [95]. The anti-orthohantaviral mechanism of FOSs was observed by molecular docking, which showed that 2O-sulphated fuco-tetrasaccharide is able to bind with AMRV and block the viral glycoproteins Gn/Gc and integrin β3 binding site of the host cells, to prevent synthesis of new viral proteins [95].
Currently, COVID-19, caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has a high mortality rate and has spread worldwide [96]. Patients infected with SARS-CoV-2 can develop pneumonia, severe symptoms of acute respiratory distress syndrome, neurological disorders, and multiple organ complications [97]. Emerging evidence suggests that some highly sulphated polysaccharides, such as heparin, heparan sulphates, glycosaminoglycans, and fucoidan, have potential antiviral activity against COVID-19 [98].
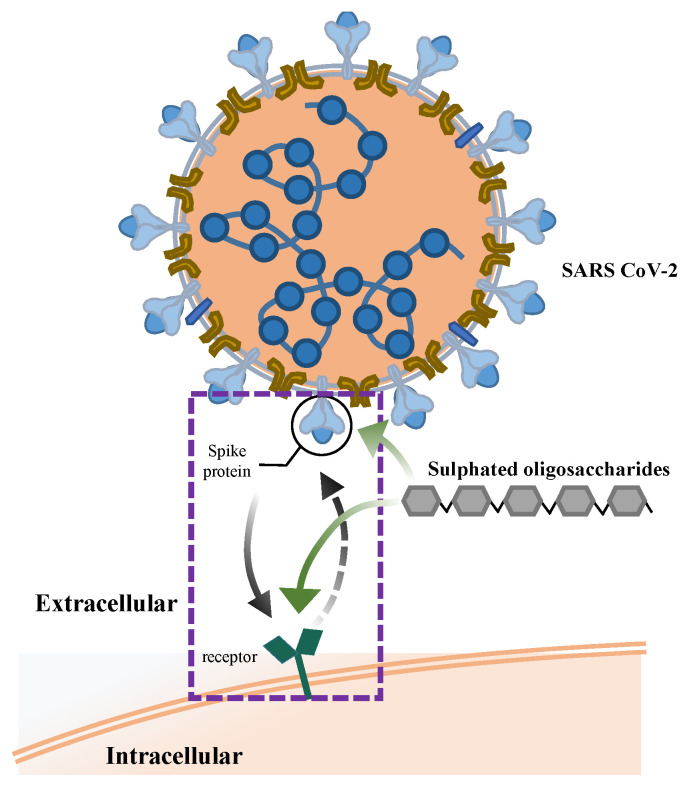
Understanding the structural activity relationship and related mechanisms of the antiviral activities of sulphated polysaccharides against COVID-19 remains challenging and these are not yet fully understood. However, the key potential features can be identified from previous reports. First and foremost, it has been accepted by researchers that fucoidan and its degradation products, FOSs, contain negatively charged sulphate components, which can interfere with electrostatic interactions between the charged ions of the COVID-19 virus spike glycoprotein. Kwon et al. demonstrated that a specific sulphated polysaccharide binds tightly to the spike protein of COVID-19 in in vitro Vero cells infected experimentally, and demonstrated antiviral properties in host tissues by interfering with spike proteins binding to the heparin sulphate co-receptor to inhibit COVID-19 infection [98]. A similar finding was reported by Salih et al.: marine algae sulphated polysaccharides bind with an affinity for the receptor-binding domain of the COVID-19 spike protein and human angiotensin-converting enzyme-2, preventing the virus from affecting host cells [99] (Figure 5). LWMF derivatives, which were mainly composed of repeating units of α(1,4)-linked L-fucopyranosyl with different sulphation patterns, were synthesised by Koike et al. They found that compound 10 showed inhibitory activities against the interaction of heparin with a couple of mutant COVID-19 spike proteins, while no inhibitory activity was observed against factor Xa, which is a key serine protease of the coagulation cascade, that means without intrinsic anticoagulant [100].
Figure 5.
Schematic representation of the FOSs binding to the COVID-19 spike protein. FOSs (green arrow) suppress the viral attachment to receptor and subsequently its entry inside the host cell (black arrow).
Second, FOSs are known to modulate the immune system activity and may be capable of reducing COVID-19 infection. Several studies have shown that FOSs can stimulate the immune defence cells, such as macrophages, dendritic cell, T cells, and B cells, and the production of pro-inflammatory cytokines; these processes are able to induce the cytolytic killing of virus-infected cells [101].
Finally, FOSs are good prebiotics for modulating the gut microbiota and increasing the production of short-chain fatty acids (SCFAs), which are beneficial for preventing or reducing COVID-19 infection. Gut dysbiosis has been associated with infection by various viruses, and emerging evidence includes COVID-19; and the gut microbiota in COVID-19 patients shows an increasing abundance of opportunistic pathogens and decreasing abundance of beneficial bacteria [102]. Gut-microbiota-derived functional metabolites, such as SCFAs, play an important role in maintaining a good intestinal barrier and related immune functions, so they could potentially alleviate the disease in COVID-19 patients [103]. The current findings propose that the functions of FOSs in the intestinal barrier and modulation of the gut microbiota may potentially contribute to reducing the risk of COVID-19 infection and relieving the severity of COVID-19 symptoms.
The FOSs derived from fucoidan, which is derived from brown algae, shows low cytotoxicity and promising antiviral activities, although most results obtained are from in vitro experiments and still need more testing in vivo in future. We suggest that FOSs may have promising clinical applications in future.
6. Concluding Remarks and Prospects
Oligosaccharides have gained wide attention owing to their prebiotic properties and potential beneficial health implications. FOSs are derived from marine resources, which are abundant and renewable, have promise for many biological activities and are useful for treating various diseases. It is necessary to review the research into FOSs so far, to point out the potential for their application and exploitation. For example, FOSs can be potentially used in food ingredients or food preservatives due to their antioxidant activities. The use of FOSs in food preservatives preserves the quality of food and enhances the shelf life. In addition, FOSs can be potential applications in biomedical fields, such as drug carriers for efficient chemotherapy and anticancer agents.
Several reports have concentrated on the production of FOSs, and the enzymatic degradation of fucoidan into FOSs is a promising method; however, it has only been investigated in the laboratory. Further research should focus on the design and development of novel preparation methods that are suitable for FOSs in industrial scale-up processes. In addition, because FOSs can vary in their glycosidic linkages, MW, and sulphate content, pharmaceutical activities may also vary. Therefore, research on the structure–function relationship of FOSs will become a main focus for the application of FOSs in various areas. This review and the data summarized and discussed here can encourage innovative research on FOSs, and future research should focus on the preparation, biological activities, and regulatory mechanisms of FOSs, thus providing more fundamental data for their future application.
Author Contributions
Conceptualization, S.Z. and K.-L.C.; writing—original draft preparation, M.W.; funding acquisition, S.Z. and K.-L.C.; writing—review and editing, M.W., S.V., S.Z. and K.-L.C. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This work was supported in part by the Key-Area Research and Development Program of Guangdong Province (2020B1111030004), The Innovative Team Program of High Education of Guangdong Province (2021KCXTD021), and The Program for Scientific Research Start-up Funds of Guangdong Ocean University (2023).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Ale M.T., Meyer A.S. Fucoidans from brown seaweeds: An update on structures, extraction techniques and use of enzymes as tools for structural elucidation. RSC Adv. 2013;3:8131–8141. doi: 10.1039/C3RA23373A. [DOI] [Google Scholar]
- 2.He W., Sun H., Su L., Zhou D., Zhang X., Shanggui D., Chen Y. Structure and anticoagulant activity of a sulfated fucan from the sea cucumber Acaudina leucoprocta. Int. J. Biol. Macromol. 2020;164:87–94. doi: 10.1016/j.ijbiomac.2020.07.080. [DOI] [PubMed] [Google Scholar]
- 3.Yao Y., Yim E.K.F. Fucoidan for cardiovascular application and the factors mediating its activities. Carbohydr. Polym. 2021;270:118347. doi: 10.1016/j.carbpol.2021.118347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lin Z., Tan X., Zhang Y., Li F., Luo P., Liu H. Molecular targets and related biologic activities of fucoidan: A review. Mar. Drugs. 2020;18:376. doi: 10.3390/md18080376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chollet L., Saboural P., Chauvierre C., Villemin J.-N., Letourneur D., Chaubet F. Fucoidans in Nanomedicine. Mar. Drugs. 2016;14:145. doi: 10.3390/md14080145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berteau O., Mulloy B. Sulfated fucans, fresh perspectives: Structures, functions, and biological properties of sulfated fucans and an overview of enzymes active toward this class of polysaccharide. Glycobiology. 2003;13:29R–40R. doi: 10.1093/glycob/cwg058. [DOI] [PubMed] [Google Scholar]
- 7.Xu S.-Y., Huang X., Cheong K.-L. Recent advances in marine algae polysaccharides: Isolation, structure, and activities. Mar. Drugs. 2017;15:388. doi: 10.3390/md15120388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vilela-Silva A.-C.E., Castro M.O., Valente A.-P., Biermann C.H., Mourao P.A. Sulfated fucans from the egg jellies of the closely related sea urchins Strongylocentrotus droebachiensis and Strongylocentrotus pallidus ensure species-specific fertilization. J. Biol. Chem. 2002;277:379–387. doi: 10.1074/jbc.M108496200. [DOI] [PubMed] [Google Scholar]
- 9.Zhao X., Guo F., Hu J., Zhang L., Xue C., Zhang Z., Li B. Antithrombotic activity of oral administered low molecular weight fucoidan from Laminaria Japonica. Thromb. Res. 2016;144:46–52. doi: 10.1016/j.thromres.2016.03.008. [DOI] [PubMed] [Google Scholar]
- 10.Xie X.-T., Cheong K.-L. Recent advances in marine algae oligosaccharides: Structure, analysis, and potential prebiotic activities. Crit. Rev. Food Sci. Nutr. 2022;62:7703–7717. doi: 10.1080/10408398.2021.1916736. [DOI] [PubMed] [Google Scholar]
- 11.Delzenne N.M. Oligosaccharides: State of the art. Proc. Nutr. Soc. 2007;62:177–182. doi: 10.1079/PNS2002225. [DOI] [PubMed] [Google Scholar]
- 12.Nobre C., Teixeira J.A., Rodrigues L.R. New trends and technological challenges in the industrial production and purification of fructo-oligosaccharides. Crit. Rev. Food Sci. Nutr. 2015;55:1444–1455. doi: 10.1080/10408398.2012.697082. [DOI] [PubMed] [Google Scholar]
- 13.Vera C., Illanes A., Guerrero C. Enzymatic production of prebiotic oligosaccharides. Curr. Opin. Food Sci. 2021;37:160–170. doi: 10.1016/j.cofs.2020.10.013. [DOI] [Google Scholar]
- 14.Davani-Davari D., Negahdaripour M., Karimzadeh I., Seifan M., Mohkam M., Masoumi S.J., Berenjian A., Ghasemi Y. Prebiotics: Definition, types, sources, mechanisms, and clinical applications. Foods. 2019;8:92. doi: 10.3390/foods8030092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zheng L.-X., Liu Y., Tang S., Zhang W., Cheong K.-L. Preparation methods, biological activities, and potential applications of marine algae oligosaccharides: A review. Food Sci. Hum. Well. 2023;12:359–370. doi: 10.1016/j.fshw.2022.07.038. [DOI] [Google Scholar]
- 16.Zayed A., Ulber R. Fucoidan production: Approval key challenges and opportunities. Carbohydr. Polym. 2019;211:289–297. doi: 10.1016/j.carbpol.2019.01.105. [DOI] [PubMed] [Google Scholar]
- 17.Sevag M., Lackman D.B., Smolens J. The isolation of the components of streptoeoeeal nueleoproteins in serologieally active form. J. Biol. Chem. 1938;124:425–436. doi: 10.1016/S0021-9258(18)74048-9. [DOI] [Google Scholar]
- 18.Béress A., Wassermann O., Bruhn T., Béress L., Kraiselburd E.N., Gonzalez L.V., de Motta G.E., Chavez P.I. A new procedure for the isolation of anti-HIV compounds (polysaccharides and polyphenols) from the marine alga Fucus vesiculosus. J. Nat. Prod. 1993;56:478–488. doi: 10.1021/np50094a005. [DOI] [PubMed] [Google Scholar]
- 19.Zhang R., Zhang X., Tang Y., Mao J. Composition, isolation, purification and biological activities of Sargassum fusiforme polysaccharides: A review. Carbohydr. Polym. 2020;228:115381. doi: 10.1016/j.carbpol.2019.115381. [DOI] [PubMed] [Google Scholar]
- 20.Cui K., Tai W., Shan X., Hao J., Li G., Yu G. Structural characterization and anti-thrombotic properties of fucoidan from Nemacystus decipiens. Int. J. Biol. Macromol. 2018;120:1817–1822. doi: 10.1016/j.ijbiomac.2018.09.079. [DOI] [PubMed] [Google Scholar]
- 21.Hwang P.-A., Yan M.-D., Kuo K.-L., Phan N.N., Lin Y.-C. A mechanism of low molecular weight fucoidans degraded by enzymatic and acidic hydrolysis for the prevention of UVB damage. J. Appl. Phycol. 2017;29:521–529. doi: 10.1007/s10811-016-0929-x. [DOI] [Google Scholar]
- 22.Li X., Sun H., Ning Z., Yang W., Cai Y., Yin R., Zhao J. Mild acid hydrolysis on Fucan sulfate from Stichopus herrmanni: Structures, depolymerization mechanism and anticoagulant activity. Food Chem. 2022;395:133559. doi: 10.1016/j.foodchem.2022.133559. [DOI] [PubMed] [Google Scholar]
- 23.Pomin V.H., Pereira M.S., Valente A.-P., Tollefsen D.M., Pavão M.S.G., Mourão P.A.S. Selective cleavage and anticoagulant activity of a sulfated fucan: Stereospecific removal of a 2-sulfate ester from the polysaccharide by mild acid hydrolysis, preparation of oligosaccharides, and heparin cofactor II–dependent anticoagulant activity. Glycobiology. 2004;15:369–381. doi: 10.1093/glycob/cwi021. [DOI] [PubMed] [Google Scholar]
- 24.Kim S.B., Farrag M., Mishra S.K., Misra S.K., Sharp J.S., Doerksen R.J., Pomin V.H. Selective 2-desulfation of tetrasaccharide-repeating sulfated fucans during oligosaccharide production by mild acid hydrolysis. Carbohydr. Polym. 2023;301:120316. doi: 10.1016/j.carbpol.2022.120316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Silchenko A.S., Rubtsov N.K., Zueva A.O., Kusaykin M.I., Rasin A.B., Ermakova S.P. Fucoidan-active α-L-fucosidases of the GH29 and GH95 families from a fucoidan degrading cluster of the marine bacterium Wenyingzhuangia fucanilytica. Arch. Biochem. Biophys. 2022;728:109373. doi: 10.1016/j.abb.2022.109373. [DOI] [PubMed] [Google Scholar]
- 26.Trincone A. Update on marine carbohydrate hydrolyzing enzymes: Biotechnological applications. Molecules. 2018;23:901. doi: 10.3390/molecules23040901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vickers C., Liu F., Abe K., Salama-Alber O., Jenkins M., Springate C.M.K., Burke J.E., Withers S.G., Boraston A.B. Endo-fucoidan hydrolases from glycoside hydrolase family 107 (GH107) display structural and mechanistic similarities to α-l-fucosidases from GH29. J. Biol. Chem. 2018;293:18296–18308. doi: 10.1074/jbc.RA118.005134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Silchenko A.S., Rasin A.B., Zueva A.O., Kusaykin M.I., Zvyagintseva T.N., Kalinovsky A.I., Kurilenko V.V., Ermakova S.P. Fucoidan sulfatases from marine bacterium Wenyingzhuangia fucanilytica CZ1127T. Biomolecules. 2018;8:98. doi: 10.3390/biom8040098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ohmes J., Mikkelsen M.D., Nguyen T.T., Tran V.H.N., Meier S., Nielsen M.S., Ding M., Seekamp A., Meyer A.S., Fuchs S. Depolymerization of fucoidan with endo-fucoidanase changes bioactivity in processes relevant for bone regeneration. Carbohydr. Polym. 2022;286:119286. doi: 10.1016/j.carbpol.2022.119286. [DOI] [PubMed] [Google Scholar]
- 30.Chen Q., Kou L., Wang F., Wang Y. Size-dependent whitening activity of enzyme-degraded fucoidan from Laminaria japonica. Carbohydr. Polym. 2019;225:115211. doi: 10.1016/j.carbpol.2019.115211. [DOI] [PubMed] [Google Scholar]
- 31.Silchenko A.S., Kusaykin M.I., Zakharenko A.M., Menshova R.V., Khanh H.H.N., Dmitrenok P.S., Isakov V.V., Zvyagintseva T.N. Endo-1,4-fucoidanase from Vietnamese marine mollusk Lambis sp. which producing sulphated fucooligosaccharides. J. Mol. Catal. B: Enzym. 2014;102:154–160. doi: 10.1016/j.molcatb.2014.02.007. [DOI] [Google Scholar]
- 32.Basso A., Serban S. Industrial applications of immobilized enzymes—A review. Mol. Catal. 2019;479:110607. doi: 10.1016/j.mcat.2019.110607. [DOI] [Google Scholar]
- 33.Chen X., Sun-Waterhouse D., Yao W., Li X., Zhao M., You L. Free radical-mediated degradation of polysaccharides: Mechanism of free radical formation and degradation, influence factors and product properties. Food Chem. 2021;365:130524. doi: 10.1016/j.foodchem.2021.130524. [DOI] [PubMed] [Google Scholar]
- 34.Li X., Li S., Liu J., Lin L., Sun H., Yang W., Cai Y., Gao N., Zhou L., Qin H., et al. A regular fucan sulfate from Stichopus herrmanni and its peroxide depolymerization: Structure and anticoagulant activity. Carbohydr. Polym. 2021;256:117513. doi: 10.1016/j.carbpol.2020.117513. [DOI] [PubMed] [Google Scholar]
- 35.Wu T.-C., Hong Y.-H., Tsai Y.-H., Hsieh S.-L., Huang R.-H., Kuo C.-H., Huang C.-Y. Degradation of Sargassum crassifolium fucoidan by ascorbic acid and hydrogen peroxide, and compositional, structural, and in vitro anti-lung cancer analyses of the degradation products. Mar. Drugs. 2020;18:334. doi: 10.3390/md18060334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Qi Y., Wang L., You Y., Sun X., Wen C., Fu Y., Song S. Preparation of low-molecular-weight fucoidan with anticoagulant activity by photocatalytic degradation method. Foods. 2022;11:822. doi: 10.3390/foods11060822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lim S., Choi J.-i., Park H. Antioxidant activities of fucoidan degraded by gamma irradiation and acidic hydrolysis. Radiat. Phys. Chem. 2015;109:23–26. doi: 10.1016/j.radphyschem.2014.12.008. [DOI] [Google Scholar]
- 38.Jeong G.-W., Choi Y.-S. Physicochemical properties and antioxidant effects of fucoidans degraded by hydrogen peroxide under electron beam at various irradiation doses. Appl. Chem. Eng. 2022;33:322–327. doi: 10.14478/ACE.2022.1023. [DOI] [Google Scholar]
- 39.Park E.-J., Choi J.-i. Melanogenesis inhibitory effect of low molecular weight fucoidan from Undaria pinnatifida. J. Appl. Phycol. 2017;29:2213–2217. doi: 10.1007/s10811-016-1048-4. [DOI] [Google Scholar]
- 40.Fernando I.P.S., Dias M.K.H.M., Madusanka D.M.D., Han E.J., Kim M.J., Jeon Y.-J., Ahn G. Step gradient alcohol precipitation for the purification of low molecular weight fucoidan from Sargassum siliquastrum and its UVB protective effects. Int. J. Biol. Macromol. 2020;163:26–35. doi: 10.1016/j.ijbiomac.2020.06.232. [DOI] [PubMed] [Google Scholar]
- 41.Hwang P.-A., Hung Y.-L., Phan N.N., Hieu B.-T.-N., Chang P.-M., Li K.-L., Lin Y.-C. The in vitro and in vivo effects of the low molecular weight fucoidan on the bone osteogenic differentiation properties. Cytotechnology. 2016;68:1349–1359. doi: 10.1007/s10616-015-9894-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bi D., Yu B., Han Q., Lu J., White W.L., Lai Q., Cai N., Luo W., Gu L., Li S., et al. Immune activation of RAW264.7 macrophages by low molecular weight fucoidan extracted from New Zealand Undaria pinnatifida. J. Agric. Food. Chem. 2018;66:10721–10728. doi: 10.1021/acs.jafc.8b03698. [DOI] [PubMed] [Google Scholar]
- 43.Cong Q., Chen H., Liao W., Xiao F., Wang P., Qin Y., Dong Q., Ding K. Structural characterization and effect on anti-angiogenic activity of a fucoidan from Sargassum fusiforme. Carbohydr. Polym. 2016;136:899–907. doi: 10.1016/j.carbpol.2015.09.087. [DOI] [PubMed] [Google Scholar]
- 44.Usoltseva R.V., Anastyuk S.D., Shevchenko N.M., Zvyagintseva T.N., Ermakova S.P. The comparison of structure and anticancer activity in vitro of polysaccharides from brown algae Alaria marginata and A. angusta. Carbohydr. Polym. 2016;153:258–265. doi: 10.1016/j.carbpol.2016.07.103. [DOI] [PubMed] [Google Scholar]
- 45.Silchenko A.S., Rasin A.B., Kusaykin M.I., Kalinovsky A.I., Miansong Z., Changheng L., Malyarenko O., Zueva A.O., Zvyagintseva T.N., Ermakova S.P. Structure, enzymatic transformation, anticancer activity of fucoidan and sulphated fucooligosaccharides from Sargassum horneri. Carbohydr. Polym. 2017;175:654–660. doi: 10.1016/j.carbpol.2017.08.043. [DOI] [PubMed] [Google Scholar]
- 46.Luo J., Mills K., le Cessie S., Noordam R., van Heemst D. Ageing, age-related diseases and oxidative stress: What to do next? Ageing Res. Rev. 2020;57:100982. doi: 10.1016/j.arr.2019.100982. [DOI] [PubMed] [Google Scholar]
- 47.Wang M., Cheong K.-L. Preparation, structural characterisation, and bioactivities of fructans: A review. Molecules. 2023;28:1613. doi: 10.3390/molecules28041613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hu Y.-C., Hu J.-L., Li J., Wang J., Zhang X.-Y., Wu X.-Y., Li X., Guo Z.-B., Zou L., Wu D.-T. Physicochemical characteristics and biological activities of soluble dietary fibers isolated from the leaves of different quinoa cultivars. Food Res. Int. 2023;163:112166. doi: 10.1016/j.foodres.2022.112166. [DOI] [PubMed] [Google Scholar]
- 49.Naveed M., Phil L., Sohail M., Hasnat M., Baig M.M.F.A., Ihsan A.U., Shumzaid M., Kakar M.U., Mehmood Khan T., Akabar M.D., et al. Chitosan oligosaccharide (COS): An overview. Int. J. Biol. Macromol. 2019;129:827–843. doi: 10.1016/j.ijbiomac.2019.01.192. [DOI] [PubMed] [Google Scholar]
- 50.Hou Y., Wang J., Jin W., Zhang H., Zhang Q. Degradation of Laminaria japonica fucoidan by hydrogen peroxide and antioxidant activities of the degradation products of different molecular weights. Carbohydr. Polym. 2012;87:153–159. doi: 10.1016/j.carbpol.2011.07.031. [DOI] [PubMed] [Google Scholar]
- 51.Jin W., Ren L., Liu B., Zhang Q., Zhong W. Structural features of sulfated glucuronomannan oligosaccharides and their antioxidant activity. Mar. Drugs. 2018;16:291. doi: 10.3390/md16090291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Weidinger A., Kozlov A.V. Biological activities of reactive oxygen and nitrogen species: Oxidative stress versus signal transduction. Biomolecules. 2015;5:472–484. doi: 10.3390/biom5020472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Geun Lee H., Jayawardena T.U., Liyanage N.M., Song K.-M., Choi Y.-S., Jeon Y.-J., Kang M.-C. Antioxidant potential of low molecular weight fucoidans from Sargassum autumnale against H2O2-induced oxidative stress in vitro and in zebrafish models based on molecular weight changes. Food Chem. 2022;384:132591. doi: 10.1016/j.foodchem.2022.132591. [DOI] [PubMed] [Google Scholar]
- 54.Kim Y.-I., Oh W.-S., Song P.H., Yun S., Kwon Y.-S., Lee Y.J., Ku S.-K., Song C.-H., Oh T.-H. Anti-photoaging effects of low molecular-weight fucoidan on ultraviolet B-irradiated mice. Mar. Drugs. 2018;16:286. doi: 10.3390/md16080286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.López-Gómez M., Malmierca E., de Górgolas M., Casado E. Cancer in developing countries: The next most preventable pandemic. The global problem of cancer. Crit. Rev. Oncol. Hematol. 2013;88:117–122. doi: 10.1016/j.critrevonc.2013.03.011. [DOI] [PubMed] [Google Scholar]
- 56.Lichota A., Gwozdzinski K. Anticancer activity of natural compounds from plant and marine environment. Int. J. Mol. Sci. 2018;19:3533. doi: 10.3390/ijms19113533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Patel S., Goyal A. Recent developments in mushrooms as anti-cancer therapeutics: A review. 3 Biotech. 2012;2:1–15. doi: 10.1007/s13205-011-0036-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yao W., Qiu H.-M., Cheong K.-L., Zhong S. Advances in anti-cancer effects and underlying mechanisms of marine algae polysaccharides. Int. J. Biol. Macromol. 2022;221:472–485. doi: 10.1016/j.ijbiomac.2022.09.055. [DOI] [PubMed] [Google Scholar]
- 59.Xu L., He D., Zhang C., Bai Y., Zhang C. The regulate function of polysaccharides and oligosaccharides that with sulfate group on immune-related disease. J. Funct. Food. 2022;88:104870. doi: 10.1016/j.jff.2021.104870. [DOI] [Google Scholar]
- 60.Anastyuk S.D., Shevchenko N.M., Ermakova S.P., Vishchuk O.S., Nazarenko E.L., Dmitrenok P.S., Zvyagintseva T.N. Anticancer activity in vitro of a fucoidan from the brown alga Fucus evanescens and its low-molecular fragments, structurally characterized by tandem mass-spectrometry. Carbohydr. Polym. 2012;87:186–194. doi: 10.1016/j.carbpol.2011.07.036. [DOI] [PubMed] [Google Scholar]
- 61.Choi J.-i., Kim H.-J. Preparation of low molecular weight fucoidan by gamma-irradiation and its anticancer activity. Carbohydr. Polym. 2013;97:358–362. doi: 10.1016/j.carbpol.2013.05.002. [DOI] [PubMed] [Google Scholar]
- 62.Lu J., Shi K.K., Chen S., Wang J., Hassouna A., White L.N., Merien F., Xie M., Kong Q., Li J., et al. Fucoidan extracted from the New Zealand Undaria pinnatifida—Physicochemical comparison against five other fucoidans: Unique low molecular weight fraction bioactivity in breast cancer cell lines. Mar. Drugs. 2018;16:461. doi: 10.3390/md16120461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhang Z., Teruya K., Eto H., Shirahata S. Induction of apoptosis by low-molecular-weight fucoidan through calcium- and caspase-dependent mitochondrial pathways in MDA-MB-231 breast cancer cells. Biosci. Biotechnol. Biochem. 2013;77:235–242. doi: 10.1271/bbb.120631. [DOI] [PubMed] [Google Scholar]
- 64.Duan Z., Luo Y. Targeting macrophages in cancer immunotherapy. Signal Transduct. Tar. 2021;6:127. doi: 10.1038/s41392-021-00506-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hwang P.-A., Lin H.-T.V., Lin H.-Y., Lo S.-K. Dietary supplementation with low-molecular-weight fucoidan enhances innate and adaptive immune responses and protects against Mycoplasma pneumoniae antigen stimulation. Mar. Drugs. 2019;17:175. doi: 10.3390/md17030175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Huang C.-W., Chen Y.-C., Yin T.-C., Chen P.-J., Chang T.-K., Su W.-C., Ma C.-J., Li C.-C., Tsai H.-L., Wang J.-Y. Low-molecular-weight fucoidan as complementary therapy of fluoropyrimidine-based chemotherapy in colorectal cancer. Int. J. Mol. Sci. 2021;22:8041. doi: 10.3390/ijms22158041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tsai H.-L., Tai C.-J., Huang C.-W., Chang F.-R., Wang J.-Y. Efficacy of low-molecular-weight fucoidan as a supplemental therapy in metastatic colorectal cancer patients: A Double-blind randomized controlled trial. Mar. Drugs. 2017;15:122. doi: 10.3390/md15040122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zheng L.-X., Chen X.-Q., Cheong K.-L. Current trends in marine algae polysaccharides: The digestive tract, microbial catabolism, and prebiotic potential. Int. J. Biol. Macromol. 2020;151:344–354. doi: 10.1016/j.ijbiomac.2020.02.168. [DOI] [PubMed] [Google Scholar]
- 69.Yao W., Gong Y., Li L., Hu X., You L. The effects of dietary fibers from rice bran and wheat bran on gut microbiota: An overview. Food Chem. X. 2022;13:100252. doi: 10.1016/j.fochx.2022.100252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cheong K.-L., Yu B., Chen J., Zhong S. A comprehensive review of the cardioprotective effect of marine algae polysaccharide on the gut microbiota. Foods. 2022;11:3550. doi: 10.3390/foods11223550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ni J., Wu G.D., Albenberg L., Tomov V.T. Gut microbiota and IBD: Causation or correlation? Nat. Rev. Gastro. Hepat. 2017;14:573–584. doi: 10.1038/nrgastro.2017.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ambrogi V., Bottacini F., Cao L., Kuipers B., Schoterman M., van Sinderen D. Galacto-oligosaccharides as infant prebiotics: Production, application, bioactive activities and future perspectives. Crit. Rev. Food Sci. Nutr. 2021;63:753–766. doi: 10.1080/10408398.2021.1953437. [DOI] [PubMed] [Google Scholar]
- 73.Wardman J.F., Bains R.K., Rahfeld P., Withers S.G. Carbohydrate-active enzymes (CAZymes) in the gut microbiome. Nat. Rev. Microbiol. 2022;20:542–556. doi: 10.1038/s41579-022-00712-1. [DOI] [PubMed] [Google Scholar]
- 74.Zhang X., Liu Y., Chen X.-Q., Aweya J.J., Cheong K.-L. Catabolism of Saccharina japonica polysaccharides and oligosaccharides by human fecal microbiota. LWT. 2020;130:109635. doi: 10.1016/j.lwt.2020.109635. [DOI] [Google Scholar]
- 75.Li S., Li J., Mao G., Yan L., Hu Y., Ye X., Tian D., Linhardt R.J., Chen S. Effect of the sulfation pattern of sea cucumber-derived fucoidan oligosaccharides on modulating metabolic syndromes and gut microbiota dysbiosis caused by HFD in mice. J. Funct. Food. 2019;55:193–210. doi: 10.1016/j.jff.2019.02.001. [DOI] [Google Scholar]
- 76.Lapébie P., Lombard V., Drula E., Terrapon N., Henrissat B. Bacteroidetes use thousands of enzyme combinations to break down glycans. Nat. Commun. 2019;10:2043. doi: 10.1038/s41467-019-10068-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ye M., Yu J., Shi X., Zhu J., Gao X., Liu W. Polysaccharides catabolism by the human gut bacterium—Bacteroides thetaiotaomicron: Advances and perspectives. Crit. Rev. Food Sci. Nutr. 2021;61:3569–3588. doi: 10.1080/10408398.2020.1803198. [DOI] [PubMed] [Google Scholar]
- 78.Luo Y., Xiao Y., Zhao J., Zhang H., Chen W., Zhai Q. The role of mucin and oligosaccharides via cross-feeding activities by Bifidobacterium: A review. Int. J. Biol. Macromol. 2021;167:1329–1337. doi: 10.1016/j.ijbiomac.2020.11.087. [DOI] [PubMed] [Google Scholar]
- 79.Bornet E., Westermann A.J. The ambivalent role of Bacteroides in enteric infections. Trends Microbiol. 2022;30:104–108. doi: 10.1016/j.tim.2021.11.009. [DOI] [PubMed] [Google Scholar]
- 80.Zeybek N., Rastall R.A., Buyukkileci A.O. Utilization of xylan-type polysaccharides in co-culture fermentations of Bifidobacterium and Bacteroides species. Carbohydr. Polym. 2020;236:116076. doi: 10.1016/j.carbpol.2020.116076. [DOI] [PubMed] [Google Scholar]
- 81.Mary P.R., Kapoor M. Co-culture fermentations suggest cross-feeding among Bacteroides ovatus DSMZ 1896, Lactiplantibacillus plantarum WCFS1 and Bifidobacterium adolescentis DSMZ 20083 for utilizing dietary galactomannans. Food Res. Int. 2022;162:111942. doi: 10.1016/j.foodres.2022.111942. [DOI] [PubMed] [Google Scholar]
- 82.Hwang P.-A., Phan N.N., Lu W.-J., Hieu B.T.N., Lin Y.-C. Low-molecular-weight fucoidan and high-stability fucoxanthin from brown seaweed exert prebiotics and anti-inflammatory activities in Caco-2 cells. Food Nutr. Res. 2016;60:32033. doi: 10.3402/fnr.v60.32033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Morrison D.J., Preston T. Formation of short chain fatty acids by the gut microbiota and their impact on human metabolism. Gut Microbes. 2016;7:189–200. doi: 10.1080/19490976.2015.1134082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Dalile B., Van Oudenhove L., Vervliet B., Verbeke K. The role of short-chain fatty acids in microbiota–gut–brain communication. Nat. Rev. Gastro. Hepat. 2019;16:461–478. doi: 10.1038/s41575-019-0157-3. [DOI] [PubMed] [Google Scholar]
- 85.Tang C., Ahmed K., Gille A., Lu S., Gröne H.-J., Tunaru S., Offermanns S. Loss of FFA2 and FFA3 increases insulin secretion and improves glucose tolerance in type 2 diabetes. Nat. Med. 2015;21:173–177. doi: 10.1038/nm.3779. [DOI] [PubMed] [Google Scholar]
- 86.Bolognini D., Tobin A.B., Milligan G., Moss C.E. The pharmacology and function of receptors for short-chain fatty acids. Mol. Pharmacol. 2016;89:388. doi: 10.1124/mol.115.102301. [DOI] [PubMed] [Google Scholar]
- 87.Deng Z., Wu N., Wang J., Geng L., Yue Y., Wang F., Zhang Q. Low molecular weight fucoidan fraction LF2 improves metabolic syndrome via up-regulating PI3K-AKT-mTOR axis and increasing the abundance of Akkermansia muciniphila in the gut microbiota. Int. J. Biol. Macromol. 2021;193:789–798. doi: 10.1016/j.ijbiomac.2021.10.188. [DOI] [PubMed] [Google Scholar]
- 88.Dao M.C., Everard A., Aron-Wisnewsky J., Sokolovska N., Prifti E., Verger E.O., Kayser B.D., Levenez F., Chilloux J., Hoyles L., et al. Akkermansia muciniphila and improved metabolic health during a dietary intervention in obesity: Relationship with gut microbiome richness and ecology. Gut. 2016;65:426. doi: 10.1136/gutjnl-2014-308778. [DOI] [PubMed] [Google Scholar]
- 89.Ang Z., Ding J.L. GPR41 and GPR43 in obesity and inflammation – Protective or causative? Front. Immunol. 2016;7:28. doi: 10.3389/fimmu.2016.00028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Luthuli S., Wu S., Cheng Y., Zheng X., Wu M., Tong H. Therapeutic effects of fucoidan: A review on recent studies. Mar. Drugs. 2019;17:487. doi: 10.3390/md17090487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Carr A., Cooper D.A. Adverse effects of antiretroviral therapy. Lancet. 2000;356:1423–1430. doi: 10.1016/S0140-6736(00)02854-3. [DOI] [PubMed] [Google Scholar]
- 92.Chen L., Huang G. The antiviral activity of polysaccharides and their derivatives. Int. J. Biol. Macromol. 2018;115:77–82. doi: 10.1016/j.ijbiomac.2018.04.056. [DOI] [PubMed] [Google Scholar]
- 93.Sun T., Zhang X., Miao Y., Zhou Y., Shi J., Yan M., Chen A. Studies on antiviral and immuno-regulation activity of low molecular weight fucoidan from Laminaria japonica. J. Ocean Univ. China. 2018;17:705–711. doi: 10.1007/s11802-018-3794-1. [DOI] [Google Scholar]
- 94.Lu W., Yang Z., Chen J., Wang D., Zhang Y. Recent advances in antiviral activities and potential mechanisms of sulfated polysaccharides. Carbohydr. Polym. 2021;272:118526. doi: 10.1016/j.carbpol.2021.118526. [DOI] [PubMed] [Google Scholar]
- 95.Krylova N.V., Silchenko A.S., Pott A.B., Ermakova S.P., Iunikhina O.V., Rasin A.B., Kompanets G.G., Likhatskaya G.N., Shchelkanov M.Y. In vitro anti-orthohantavirus activity of the high-and low-molecular-weight fractions of fucoidan from the brown alga Fucus evanescens. Mar. Drugs. 2021;19:577. doi: 10.3390/md19100577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zhu N., Zhang D., Wang W., Li X., Yang B., Song J., Zhao X., Huang B., Shi W., Lu R., et al. A novel coronavirus from patients with pneumonia in China, 2019. N. Engl. J. Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Yang L., Liu S., Liu J., Zhang Z., Wan X., Huang B., Chen Y., Zhang Y. COVID-19: Immunopathogenesis and immunotherapeutics. Signal Transduct. Tar. 2020;5:128. doi: 10.1038/s41392-020-00243-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kwon P.S., Oh H., Kwon S.-J., Jin W., Zhang F., Fraser K., Hong J.J., Linhardt R.J., Dordick J.S. Sulfated polysaccharides effectively inhibit SARS-CoV-2 in vitro. Cell Discov. 2020;6:50. doi: 10.1038/s41421-020-00192-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Salih A.E.M., Thissera B., Yaseen M., Hassane A.S.I., El-Seedi H.R., Sayed A.M., Rateb M.E. Marine sulfated polysaccharides as promising antiviral agents: A comprehensive report and modeling study focusing on SARS CoV-2. Mar. Drugs. 2021;19:406. doi: 10.3390/md19080406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Koike T., Sugimoto A., Kosono S., Komaba S., Kanno Y., Kitamura T., Anzai I., Watanabe T., Takahashi D., Toshima K. Synthesis of low-molecular weight fucoidan derivatives and their binding abilities to SARS-CoV-2 spike proteins. RSC Med. Chem. 2021;12:2016–2021. doi: 10.1039/D1MD00264C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Barbosa J.R., de Carvalho Junior R.N. Polysaccharides obtained from natural edible sources and their role in modulating the immune system: Biologically active potential that can be exploited against COVID-19. Trends Food Sci. Technol. 2021;108:223–235. doi: 10.1016/j.tifs.2020.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zuo T., Zhan H., Zhang F., Liu Q., Tso E.Y.K., Lui G.C.Y., Chen N., Li A., Lu W., Chan F.K.L., et al. Alterations in fecal fungal microbiome of patients with COVID-19 during time of hospitalization until discharge. Gastroenterology. 2020;159:1302–1310.e1305. doi: 10.1053/j.gastro.2020.06.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Włodarczyk J., Czerwiński B., Fichna J. Short-chain fatty acids–microbiota crosstalk in the coronavirus disease (COVID-19) Pharmacol. Rep. 2022;74:1198–1207. doi: 10.1007/s43440-022-00415-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.