Abstract
Retroviral infection induces integrase-dependent apoptosis in DNA-PK-deficient murine scid lymphocytes. Furthermore, the efficiency of stable transduction of reporter genes is reduced in adherent cell lines that are deficient in cellular DNA-repair proteins known to mediate nonhomologous end joining (NHEJ), such as DNA-PK and XRCC4 (R. Daniel, R. A. Katz, and A. M. Skalka, Science 284:644–647, 1999). Here we report that wortmannin, an irreversible inhibitor of phosphatidylinositol 3-kinase (PI-3K)-related PKs, including the catalytic subunit of DNA-dependent protein kinase (DNA-PKCS) and ATM, sensitizes normal murine lymphocytes to retrovirus-mediated cell killing. We also show that the efficiency of stable transduction of reporter genes in human (HeLa) cells, mediated by either an avian sarcoma virus or a human immune deficiency virus type 1 vector, is reduced in the presence of wortmannin. The dose dependence of such reduction correlates with that for inhibition of PI-3K-related protein kinase activity in these cells. Results from wortmannin treatment of a panel of cell lines confirms that formation and/or survival of transductants is dependent on components of the NHEJ pathway. However, stable transduction is virtually abolished by wortmannin treatment of cells that lack ATM. These results suggest that ATM activity is required for the residual transduction observed in the NHEJ-deficient cells. Our studies support the hypothesis that DNA repair proteins of the NHEJ pathway and, in their absence, ATM are required to avoid integrase-mediated 2killing and allow stable retroviral DNA transduction. The studies also suggest that cells can be sensitized to such killing and stable retroviral DNA integration blocked by drugs that inhibit cellular DNA repair pathways.
The catalytic subunit of DNA-PK, DNA-PKCS, is a member of a family of large, presumably multifunctional, phosphatidylinositol 3-kinase (PI-3K)-related protein kinases (19, 25). DNA-PKCS is a component of the cellular, nonhomologous end-joining pathway (NHEJ) and plays an important role in monitoring and repairing DNA damage (23, 40). A second member of this family, the ATM kinase, is also involved in monitoring and repair of DNA damage (24). ATM is the gene mutated in patients with ataxia teleangiectasia (A-T), a disorder characterized by cerebellar degeneration with resulting ataxia, oculocutaneous teleangiectasia, immunodeficiency, premature aging, and increased sensitivity to ionizing radiation. A-T patients also have a high risk of cancer, particularly lymphoid malignancies (22, 24). Cell lines derived from A-T patients are hypersensitive to ionizing radiation and show high radioresistant DNA synthesis, a high level of chromosomal instability, and defects in DNA repair (22, 24).
PI-3K-related protein kinases are inhibited irreversibly by the small sterol-like fungal metabolite wortmannin (21, 32, 33, 34). Wortmannin binds covalently to a critical lysine residue in the conserved C-terminal kinase domains of these proteins (41). Treatment with wortmannin renders cells hypersensitive to ionizing radiation and certain DNA-damaging drugs. Such effects have been attributed primarily to inhibition of DNA-PKCS and ATM kinase activities which exhibit similar 50% inhibitory concentration (IC50) values for this drug in vivo (21, 34). However, the specific concentration of wortmannin required to sensitize cells to DNA damage differs among cell types, ranging from 1 to 50 μM (8, 21, 32, 33, 34).
The joining of retroviral DNA 3′ ends to host DNA is catalyzed by the retroviral integrase protein (IN). This reaction forms an integration intermediate in which the 5′ ends of each strand are not joined (cf. Fig. 6 and reference 16). We have reported that DNA-PKCS-deficient mouse pre-B scid cells undergo apoptotic cell death in response to retroviral infection and that this response is dependent on IN activity (12). Our initial studies also measured the ability of retroviral vectors to stably transduce a selectable marker. In our studies, stable transduction is defined as complete covalent integration of viral DNA, followed by continued cell division of the infected cells. The observed 80 to 90% reduction in stable transduction in NHEJ-deficient cells was interpreted to be the result either of cell death prior to the first division, triggered by unrepaired integration intermediates (DNA damage), or of some other effect of DNA integration (12). It was reported subsequently that Ty1 retrotransposition is reduced to a similar degree in Ku− yeast cells (14), suggesting a similar requirement in this system. We have hypothesized that the residual, 10 to 20% stable transduction observed in NHEJ-deficient cells could be mediated by the compensating activity of another cellular DNA repair pathway. Alternatively, such residual transduction could be due to the residual activity of the NHEJ pathway.
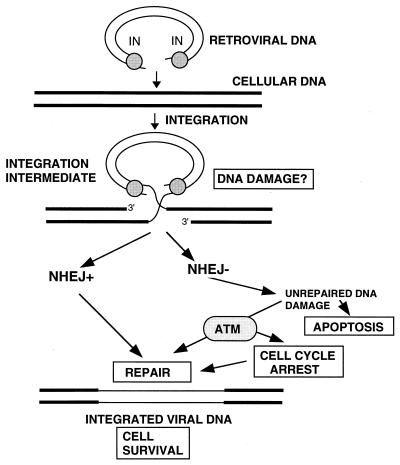
FIG. 6.
Cellular response to IN-mediated DNA damage. As noted in the text, possible IN-mediated damage signals include discontinuities in viral DNA or changes in cellular DNA or chromatin structure introduced during integration of the retroviral DNA. We propose that such damage is normally sensed (either directly or indirectly) by DNA-PK, together with other components of the NHEJ DNA repair pathway. The exact manner in which the NHEJ pathway mediates repair is still unknown. Activities might include signaling to other proteins and/or the recruitment of repair proteins. A direct interaction of NHEJ with IN can also not be excluded. Our results indicate that ATM can also respond to IN-mediated damage in the absence of DNA-PK. The manner in which ATM contributes to repair of the DNA damage is also unknown. It may also signal to other proteins and/or recruit repair proteins, or it may block the cell cycle until repair can be affected by another pathway.
In this report we make use of the inhibitor wortmannin to verify that DNA-PKCS activity is required to spare cells from integrase-mediated death and to allow efficient retrovirus-mediated transduction. We also investigate whether ATM activity allows the residual transduction observed in cells that are defective in NHEJ.
MATERIALS AND METHODS
Cell lines and retroviruses.
Cells were maintained in a humidified incubator at 37°C and 5% CO2. HeLa cells were grown in Dulbecco modified Eagle medium (DMEM) medium supplemented with 10% fetal bovine serum (FBS) and penicillin-streptomycin. CHO-K1 and XR-1 cells were grown in DMEM medium supplemented with 2× concentrations of amino acids and vitamins, 10% FBS, and penicillin-streptomycin. AT2SF and AT5BI cell lines were grown in DMEM supplemented with 10% FBS and penicillin-streptomycin. The scid pre-B cell line, S33, and a DNA-PK-proficient control pre-B line, N2 (35), were grown in RPMI supplemented with 10% FBS, penicillin-streptomycin, and 5 × 10−6 M 2-mercaptoethanol. AT22IJE-T cells were grown in DMEM supplemented with 10% FBS, penicillin-streptomycin, and 100 μg of hygromycin per ml.
IN+ virus is an avian sarcoma-leukosis virus (ASV) with an amphotropic envelope, which was described previously (12). The human immunodeficiency virus type 1 (HIV-1)-based vector was also described previously (31) and either was prepared in our laboratory or was obtained as a gift from Muhammad Mukhtar in R. Pomerantz's laboratory at The Thomas Jefferson University School of Medicine. For HIV-1 vector preparation, 293T cells were transfected using the Profection Kit (Promega E1200), with the three plasmids that contribute distinct viral components, at a ratio of 50 μg of transfer vector to 50 μg of packaging construct to 5 μg of vesicular stomatitis virus (VSV) G-expressing plasmid per 100-mm dish. Virus was harvested at 48 h posttransfection.
Infections and drug treatment.
In the viability assays, nonadherent pre-B S33 and N2 cell lines were infected as previously described (12). Briefly, cells were plated at 5 × 105 cells per ml per well in 24-well plates. Virus (IN+) (12) was added to a multiplicity of infection (MOI) of 4 infectious units/cell and DEAE-dextran at 5 μg/ml. Wortmannin was added at the time of infection, and viability was measured by trypan-blue dye exclusion, with two plates counted for each time point. To determine the effect of the drug on retroviral DNA integration in HeLa cells and other adherent cell lines (CHO-K1, XR-1, MO59J, MO59K, AT5BI, AT2SF, and AT22IJE-T), cells were plated at a concentration 105 per 60-mm dish, and the indicated concentration of wortmannin was added at the time of plating. Virus (1 ml of a 10−3 dilution of IN+ virus per dish) was added the following day, along with 10 μg of DEAE-dextran per ml and the appropriate concentration of wortmannin. After 2 h, the virus-containing medium was removed and fresh medium with the same concentration of wortmannin was added. After 16 h, wortmannin-containing medium was removed and replaced with medium containing 1 mg of G418 per ml to select resistant cells.
For HIV-1 infections, adherent cells were treated as above. The cells were stained 7 days postinfection using a β-galactosidase assay according to the Transfection MBS Mammalian Transfection Kit protocol (Stratagene) to detect expression of the virally transduced reporter gene in individual colonies.
Treatment with antisense oligonucleotides.
Antisense oligonucleotides were complementary to codons 2 to 7 of mouse ATM and the first exons of DNA-PK. The ATM antisense sequence was 5′-ATC ATT GAG TGC TAG ACT-3′/ and that of the control sense oligonucleotide was 5′-AGT CTA GCA CTC AAT GAT-3′. The sequence of the DNA-PK antisense oligonucleotide was 5′-GCC GGT TCC CTC CTC CGC-3′, and that of the control sense oligonucleotide was 5′-GCG GAG GAG GGA ACC GGC-3′. The pre-B cells were infected at an MOI of 4 as described above (Fig. 1), except that the oligonucleotides (final concentration, 10 μM) were added in place of wortmannin.
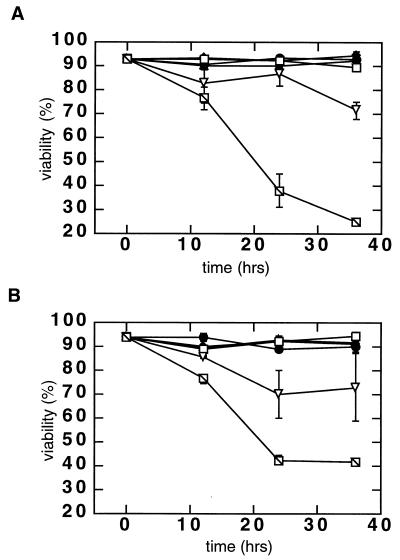
FIG. 1.
Effect of infection on the viability of wortmannin-treated, DNA repair-competent pre-B N2 cells. (A) Viability of N2 cells after infection with the IN+ ASV vector. Cells were infected at an MOI of 4 transducing units/cell in the absence of wortmannin (□), in the presence of 0.5 μM wortmannin (▿), or in the presence of 1 μM wortmannin ( ). As a control, cells were mock infected in the absence of wortmannin (●), in the presence of 0.5 μM wortmannin (▴), or in the presence of 1 μM wortmannin (⧫). Cells were harvested at the indicated time points, and viability was measured by trypan blue dye exclusion. An average of two independent counts is shown. (B) Viability of wortmannin-treated N2 cells after infection with the integrase-defective (IN−) virus. Cells were infected with the IN− ASV vector at an MOI of 4 in the absence of wortmannin (●), in the presence of 0.5 μM wortmannin (▴), or in the presence of 1 μM wortmannin (⧫). As a positive control, cells were also infected with the IN+ virus (conditions and symbols as in Fig. 1A). Viability was again measured by trypan blue dye exclusion; two independent counts were made at each time point.
). As a control, cells were mock infected in the absence of wortmannin (●), in the presence of 0.5 μM wortmannin (▴), or in the presence of 1 μM wortmannin (⧫). Cells were harvested at the indicated time points, and viability was measured by trypan blue dye exclusion. An average of two independent counts is shown. (B) Viability of wortmannin-treated N2 cells after infection with the integrase-defective (IN−) virus. Cells were infected with the IN− ASV vector at an MOI of 4 in the absence of wortmannin (●), in the presence of 0.5 μM wortmannin (▴), or in the presence of 1 μM wortmannin (⧫). As a positive control, cells were also infected with the IN+ virus (conditions and symbols as in Fig. 1A). Viability was again measured by trypan blue dye exclusion; two independent counts were made at each time point.
DNA-dependent protein kinase assay.
HeLa cell nuclei were isolated and lysed in 30 μl of nuclear lysis buffer per sample as described elsewhere (3). The p53-related peptide Glu-Pro-Pro-Leu-Ser-Gln-Glu-Ala-Phe-Ala-Asp-Leu-Trp-Lys-Lys (Promega) was used as a substrate. The kinase assay was performed as described previously (3), except that 1 μl of nuclear lysate was used per 30 μl of reaction volume instead of purified DNA-PK. Sheared salmon sperm DNA (100 ng per sample) was added to activate DNA-dependent kinase. The reactions were incubated for 30 min at 30°C.
Immunoblot analysis.
Clarified whole-cell lysates from approximately 106 cells/sample were incubated with anti-AT1.8 (antibody 1 from reference 17) at an 8-μg/ml final dilution, and the resulting immunoprecipitates were then analyzed by immunoblotting using the same antibody.
RESULTS
Wortmannin sensitizes normal murine pre-B lymphocytes to retrovirus-mediated cell killing.
We have shown that DNA-PKCS-deficient pre-B lymphocyte lines derived from severe combined immune-deficient (scid) mice undergo apoptosis after infection with retroviruses (12). This response is independent of the expression of any viral genes, as the vectors used are either defective in viral gene expression in mammalian cells (an amphotropic ASV vector [5]) or carry no viral genes (HIV-1 vector [31]). However, the response is dependent on an active integrase brought into the cell with the infecting vector. If scid cell killing is due to DNA-PK deficiency, then treatment of a matched normal cell line (N2) with a drug that inhibits DNA-PKCS should render these cells sensitive to retroviral killing. To test this prediction, we infected N2 cells with the amphotropic ASV vector (denoted IN+) at a MOI of 4 infectious units/cell in the presence of wortmannin. The results showed rapid cell killing in the infected cultures in the presence of 1.0 μM wortmannin (Fig. 1). The time course of cell death was similar to that observed after infection of DNA-PKCS-deficient scid pre-B cell lines (12). A slight loss in the viability of infected cells was also detected in the presence of 0.5 μM wortmannin. In contrast, neither 0.5 nor 1.0 μM wortmannin had any effect on the viability of uninfected N2 cells.
To determine if sensitization of wortmannin-treated N2 cells to retroviral infection is dependent on an active integrase, cells were infected with an integrase-defective (IN−) virus that differs from the ASV IN+ vector by a single D64E substitution in the active site of this enzyme. As we have reported previously (12), this mutant virus is competent for all of the early steps in infection (viral DNA is synthesized and enters the cell nucleus as normal), but its DNA cannot be integrated. The results showed that infection with the IN− virus had no effect on the viability of the wortmannin-treated N2 cells (Fig. 1B). Therefore, as reported previously for scid cells, active integrase is required for retrovirus-mediated killing of the wortmannin-treated N2 cells. These data indicate that wortmannin sensitizes N2 cells to killing in a manner similar to that observed with scid cells and is consistent with the interpretation that such sensitization is a consequence of DNA-PKCS inhibition.
Wortmannin enhances retrovirus-mediated killing of DNA-PK-deficient murine scid cells.
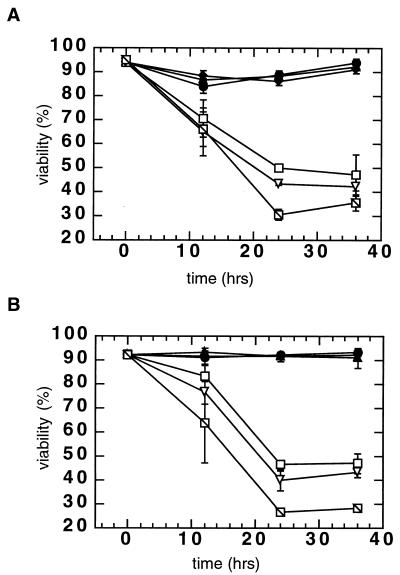
We hypothesized that incomplete scid cell killing might be due to residual DNA-PKCS activity or another wortmannin-sensitive enzyme. Therefore, we sought to determine if wortmannin could enhance killing of scid cells. For these experiments, scid (S33) cells were infected with the IN+ virus in the absence or presence of 1 or 2 μM wortmannin. The expected decrease in viability of IN+ virus-infected scid cells was observed in the absence of the drug, with only ∼50% viability at 24 h after infection (Fig. 2). However, when infected scid cells were treated with 2 μM wortmannin, their viability was further reduced to only ∼30% after 24 h. In contrast, the addition of 1 or 2 μM wortmannin had no effect on the viability of uninfected scid cells. These results suggest either that an additional wortmannin-sensitive enzyme(s) or residual DNA-PK activity can contribute to the survival of scid cells after viral infection. As with the N2 cells, no reduction in viability of wortmannin-treated cells was observed after infection with the IN− virus (Fig. 2B). Therefore, active retroviral integrase is required for the reduced survival of the scid cells infected in the presence of this drug. As DNA-PK-deficient cells can express ATM (2, 10), these results suggested that the activity of this protein kinase may be required for the residual survival of infected scid cells. To test this hypothesis, we used antisense oligonucleotides to block ATM expression in these scid cells.
FIG. 2.
Effect of infection on viability of wortmannin-treated scid (S33) cells. (A) Viability of scid cells after infection with the IN+ virus. Cells were infected at an MOI of 4 in the absence of wortmannin (□), in the presence of 1 μM wortmannin (▵), or in the presence of 2 μM wortmannin ( ). As a control, cells were mock infected in the absence of wortmannin (●), in the presence of 1 μM wortmannin (▴), or in the presence of 2 μM wortmannin (⧫). Cells were harvested at the indicated times, and viability was measured by trypan blue dye exclusion. An average of two independent counts is shown. (B) Viability of wortmannin-treated S33 cells after infection with the integrase-defective, IN− virus. Cells were infected with the IN− virus at an MOI of 4 in the absence of wortmannin (●), in the presence of 1 μM wortmannin (▴), or in the presence of 2 μM wortmannin (⧫). In addition, cells were infected with the IN+ virus (controls and symbols as in Fig. 2A). Viability was again measured by trypan blue dye exclusion; two independent counts were made at each time point.
). As a control, cells were mock infected in the absence of wortmannin (●), in the presence of 1 μM wortmannin (▴), or in the presence of 2 μM wortmannin (⧫). Cells were harvested at the indicated times, and viability was measured by trypan blue dye exclusion. An average of two independent counts is shown. (B) Viability of wortmannin-treated S33 cells after infection with the integrase-defective, IN− virus. Cells were infected with the IN− virus at an MOI of 4 in the absence of wortmannin (●), in the presence of 1 μM wortmannin (▴), or in the presence of 2 μM wortmannin (⧫). In addition, cells were infected with the IN+ virus (controls and symbols as in Fig. 2A). Viability was again measured by trypan blue dye exclusion; two independent counts were made at each time point.
Antisense oligonucleotides against ATM potentiate retrovirus-mediated scid cell killing.
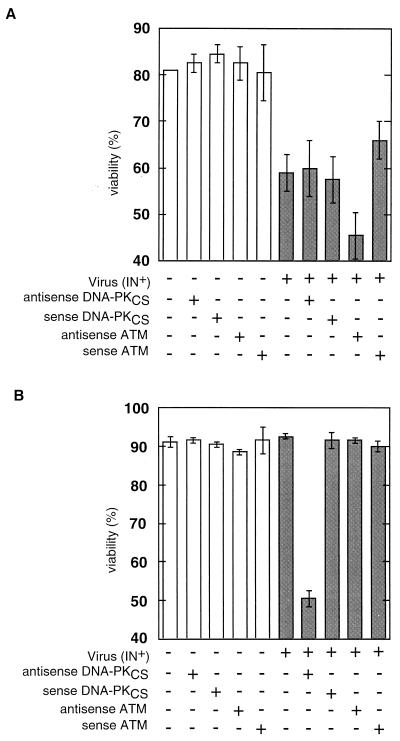
In the experiment summarized in Fig. 3, scid S33 and control N2 cells were treated with sense or antisense oligonucleotides against DNA-PKCS or ATM and then either mock infected or infected with the IN+ virus. Consistent with previous results, viability of untreated scid cells was reduced approximately 25% at 18 h postinfection (compare bars 1 and 6 in Fig. 3A). As scid cells may still possess some DNA-PKCS activity (13), we next investigated whether residual DNA-PKCS activity might account for the incomplete cell killing. The results showed that neither sense nor antisense DNA-PK oligonucleotides had a significant effect on cell killing postinfection (compare bars 1 to 3 with bars 6 to 8 in Fig. 3A). Thus, residual DNA-PKCS activity is unlikely to account for the incomplete killing of scid cells. In contrast to the results with DNA-PKCS antisense oligonucleotides, we observed an augmentation of cell killing to approximately 44% by treatment of infected scid cells with ATM antisense but not ATM sense oligonucleotides (Fig. 3A, bars 9 and 10). These results suggest that ATM contributes to the survival of scid cells after retroviral infection. As additional controls, we tested oligonucleotides in which the ATM sense or antisense sequence was scrambled, and we observed no additional killing of infected scid cells. We also tested another ATM oligonucleotide sequence and found the expected augmentation of infected cell killing with the antisense but not the sense oligonucleotide (data not included).
FIG. 3.
Effect of antisense oligonucleotides on viability of control N2 and scid S33 cells infected with IN+ virus. Cells were mock infected or infected at an MOI of 4 and simultaneously treated with antisense oligonucleotides (final concentration, 10 μM) as indicated. Cells were then counted at 18 h postinfection. (A) Effect of antisense oligonucleotides on the viability of S33 cells. (B) Effect of antisense oligonucleotides on the viability of N2 cells. Open bars, mock-infected cells; shaded bars, IN+ virus-infected cells.
We next wanted to confirm that the DNA-PKCS antisense oligonucleotides were biologically active and to determine independently if normal cells can be sensitized to cell killing by inhibition of DNA-PKCS or ATM. Accordingly, the control N2 cells were treated with DNA-PKCS antisense oligonucleotides and infected with the retroviral vector. Figure 3B (bar 7) shows a ∼45% reduction in viability after 24 h with such a treatment. This result is consistent with the data in Fig. 1, 2, and 3A and indicates that these oligonucleotides are effective inhibitors of DNA-PKCS expression. The control DNA-PKCS sense oligonucleotide (Fig. 3B, bar 8) had no detectable effect. ATM antisense oligonucleotides also had no detectable effect on the viability of N2 cells after infection (Fig. 3B, bar 9). The latter result can be interpreted in at least two ways. Either the ATM pathway is active only in the absence of DNA-PKCS or the contribution of ATM is small and cannot be measured in the presence of DNA-PKCS activity.
The results in Fig. 3 are relevant to our interpretation of the experiments with wortmannin as follows. If the sensitization of normal cells to killing is a direct effect of wortmannin inhibition of both DNA-PK and ATM, similar sensitization should be observed using ATM and DNA-PKCS antisense oligonucleotides. The results show that sensitization can, indeed, be achieved with both ATM and DNA-PKCS antisense oligonucleotides. In particular, further sensitization of scid cells using ATM antisense oligonucleotides is consistent with the ability to further sensitize scid cells with wortmannin. These results are consistent with our interpretation that the relevant targets of wortmannin in the viability assays are DNA-PKCS and ATM and that the results do not reflect wortmannin inhibition of PI-3K or interference with other cellular functions.
Wortmannin reduces the efficiency of retroviral transduction in human (HeLa) cells.
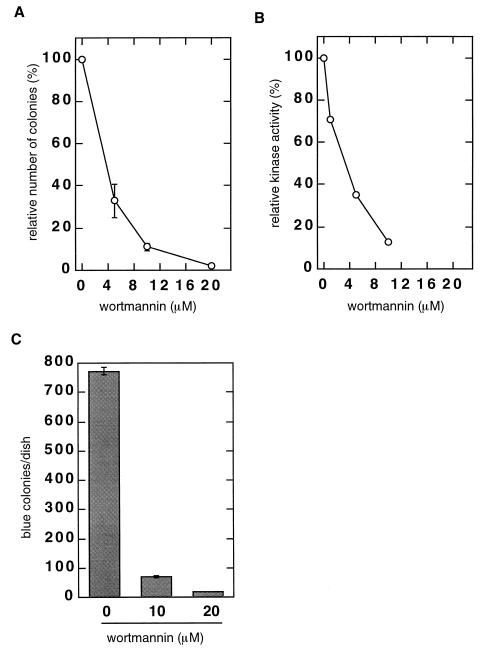
An effect of deficiency in components of the NHEJ repair pathway can also be observed using a colony-forming assay for stable transduction. In this assay, the survival of adherent cells is dependent on the stable retroviral transduction of the Neor marker. We have shown previously that the efficiency of such colony formation is only ∼10% of control values in murine scid fibroblasts, or in Ku− and XRCC4-null hamster cell lines (12). In the studies summarized here, we used the colony assay to determine if wortmannin can reduce the efficiency of retrovirus-mediated transduction of human (HeLa) cells that express both DNA-PKCS and ATM (17). The cells were infected with the same dilution of the IN+ vector in the presence of increasing concentrations of wortmannin. The following day, wortmannin-containing medium was removed and G418-containing medium was added to select for Neor colonies. The results (Fig. 4A) showed a dose-dependent reduction in the number of G418-resistant colonies, with only 10% remaining after treatment with 10 μM wortmannin. A PCR-based assay verified that the wortmannin did not inhibit synthesis or nuclear import of the viral DNA (data not shown). The IC50 calculated for wortmannin in this transduction assay was 3.6 μM. In a separate experiment (Fig. 4B), we analyzed DNA-dependent protein kinase activity in nuclear extracts of uninfected HeLa cells treated with increasing concentrations of wortmannin. This assay, which measures the DNA-dependent transfer of 32P from ATP to a p53-related peptide substrate, is used routinely to quantitate DNA-PKCS activity. However, the assay also detects ATM activity, as ATM can also phosphorylate the relevant p53 residue in vitro (4, 9, 26). We observed a dose-dependent reduction of DNA-dependent phosphorylation of the p53 peptide in lysates from the wortmannin-treated HeLa cells that correlated closely with the reduction in G418-resistant colony formation (compare Fig. 4A and B), with an IC50 calculated to be 4 μM. In control experiments, we observed no adverse effect of wortmannin on the growth rate (not shown) or colony formation (Table 1) by uninfected HeLa cells at up to a 10 μM concentration. At 20 μM, colony formation was reduced by 30%, with a similar reduction in growth rate.
FIG. 4.
(A) Retrovirus-mediated transduction in wortmannin-treated HeLa cells. HeLa cells were treated with 0 to 20 μM wortmannin (two dishes with 105 cells/dish for each point) and infected with a dilution of the IN+ virus to an MOI of ∼0.01. On the following day, wortmannin was removed and medium containing G418 at final concentration of 1 mg/ml was added. Resistant colonies were counted 2 weeks postinfection. The colony numbers were 329.5 ± 39 per dish in the absence of wortmannin, 110 ± 25 per dish at 5 μM wortmannin, 38 ± 6 per dish at 10 μM, and 6 ± 1 per dish at 20 μwortmannin. The results were plotted as a percentage of the number of colonies in the absence of wortmannin. (B) DNA-dependent protein kinase activity in wortmannin-treated HeLa cells. Cells were treated overnight with 0 to 10 μM wortmannin; nuclei were then isolated and lysed, and the DNA-dependent kinase activity was measured in the nuclear extracts as described (see Materials and Methods). The kinase activity was stimulated with sheared salmon sperm DNA, and the activity in the absence of salmon sperm DNA was subtracted from each datum point. The results were plotted as a percentage of the activity in the absence of wortmannin which was taken as 100%. (C) Transduction mediated by the HIV-1-based virus. HeLa cells (at 105 per dish) were treated with 0, 10, or 20 μM wortmannin and infected with the HIV-1-based virus vector. Cells were stained for β-galactosidase activity at 1 week postinfection, and stained (blue) colonies were counted on two dishes for each point.
TABLE 1.
Colony formation by uninfected cells in the presence of wortmannin
| Cell line | % Colonies formed with a wortmannin concn (μM) of:
|
|||
|---|---|---|---|---|
| 0 | 1 | 5 | 10 | |
| HeLa | 100 (79)b | 95 | 100 | 96 |
| AT2SF | 100 (58) | 107 | 106 | 105 |
| M059K | 100 (98) | 90 | 105 | 67 |
| M059J | 100 (54) | 90 | 102 | 53 |
Cells were plated at a density 300 cells per 60-mm dish, two dishes for each point, in the presence of wortmannin. Wortmannin was kept on cells for 48 h with and then removed. Colonies were counted 9 to 14 days after plating.
The numbers in parentheses are average colony counts in the absence of wortmannin.
To determine if wortmannin affected stable transduction by another retrovirus, HeLa cells were infected with a VSV G protein-pseudotyped HIV-1 retrovirus vector (31) in the absence or presence of wortmannin. The vector genome encodes no viral proteins and DNA integration is detected by expression of the β-galactosidase reporter protein that is encoded in its genome. We again observed an approximately 10-fold reduction in stable transduction with 10 μM wortmannin (Fig. 4C). Thus, we conclude that treatment with wortmannin can reduce the efficiency of transduction by both ASV- and HIV-1-derived vectors.
Wortmannin reduces the efficiency of retrovirus-mediated transduction in mutant human and hamster cell lines deficient in DNA-PK or ATM.
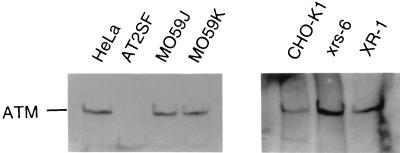
To distinguish between the contributions of the DNA-PKCS and ATM kinases to retrovirus-mediated transduction, we compared the effects of wortmannin on a panel of mutant adherent cell lines that lack either components of the NHEJ pathway or ATM (Table 2). In these experiments, cells were infected with the same dilution of the ASV IN+ virus, and stable transduction was again detected using the colony assay (Neor transduction). All cell lines except AT2SF express ATM, and AT5BI expresses a very low level of mutated ATM (Fig. 5 and references 17 and 18). Both AT2SF and AT5BI are compound heterozygotes carrying a different mutation in each allele of ATM (18). One cell line, MO59J, is DNA-PKCS null, and another, XR-1, is XRCC4 null; both are defective in NHEJ (27, 28). We observed the expected 80 to 90% reduction in the number of G418-resistant colonies with the MO59J cell line, compared to the HeLa cell control, and 76% reduction compared to MO59K, which is a widely used matched control cell line (Table 2). We attribute this smaller reduction to the slower growth rate of MO59K cells; the growth rate of HeLa cells is close to that of MO59J cells. Treatment of MO59J cells with 1 and 5 μM wortmannin resulted in a further reduction in G418-resistant colony formation. At 5 μM wortmannin, which is close to the IC50 for colony formation with HeLa cells (Fig. 4), residual colony formation with MO59J cells was 53% of that seen in the absence of drug (Table 2). As with the analyses of scid-cell killing (Fig. 2), these results indicate that a wortmannin-sensitive pathway other than DNA-PKCS is responsible for the residual retrovirus-mediated transduction observed with these cells. Furthermore, as MO59J cells lack any detectable DNA-PKCS, this wortmannin-sensitive residual colony formation cannot be the result of residual DNA-PKCS activity. Because similar results were observed after treatment of the XRCC4-null rodent XR-1 cells with wortmannin, we conclude that these components of the NHEJ DNA repair pathway are not included in the residual, wortmannin-sensitive pathway (Table 2).
TABLE 2.
Infection of cell lines deficient in NHEJ or ATMa
| Cell line | Relative % G418-resistant colonies at a wortmannin concn (μM) of:
|
||
|---|---|---|---|
| 0 | 1 | 5 | |
| HeLa | 100 (304) | 56 | 45 |
| AT2SF (ATM−) | 100 (288) | 11 | 5 |
| ATSBI (ATM−) | 100 (293) | 4 | 1 |
| M059J (DNA-PKCS−) | 100 (155) | 80 | 50 |
| M059J (DNA-PKCS−) | 100 (38) | 80 | 53 |
| CHO-K1 | 100 (235) | 75 | 32 |
| XR-1 (XRCC4−) | 100 (24) | 69 | 29 |
Cells were infected as described in Materials and Methods. Colony numbers are in parentheses; numbers from two plates were averaged for each datum point.
FIG. 5.
ATM expression in human and hamster (CHO) lines. Cells were lysed, and either whole-cell lysates (human cells) or immunoprecipitates obtained with ATM antibodies (CHO lines) were probed for ATM expression as described (see Materials and Methods). Cell line names are indicated.
In contrast to results with the DNA-PKCS- and XRCC4-deficient cells, in the absence of wortmannin the number of G418-resistant colonies formed by the two A-T-null cell lines (AT2SF and AT5BI) was very similar to that seen with control HeLa cells. Thus, when a functioning NHEJ pathway is present, ATM appears to contribute little to the efficiency of retrovirus-mediated transduction. This is consistent with results described in Fig. 3B. However, the number of G418-resistant colonies was reduced dramatically when these A-T cells were treated with as little as 1 μM wortmannin (Table 2). In the presence of 5 μM wortmannin, colony formation by the AT2SF and AT5BI cells was almost obliterated (Table 2). Similar results were obtained with the HIV-1 vector (Table 3). As shown in Table 1, wortmannin at 1 to 5 μM had no significant effect on colony formation by uninfected control, NHEJ-deficient, or ATM-deficient cells. Thus, the effects observed with infected cells cannot be due to the nonspecific inhibition of colony formation by wortmannin.
TABLE 3.
Infection of wortmannin-treated cells with the HIV-1-based vectora
| Cell line | Wortmannin (1 μM)b | No. of colonies per dish |
|---|---|---|
| HeLa | − | 136 |
| + | 103 | |
| AT2SF | − | 165 |
| + | 21 |
Cells were infected as described in Materials and Methods. At 1 week postinfection, cells were stained for β-galactosidase activity, and positive colonies were averaged from two dishes for each datum point.
Absence (−) or presence (+) of wortmannin at 1 μM.
Expression of ATM rescues stable retrovirus-mediated transduction in A-T cells in the presence of wortmannin.
To verify that the observed hypersensitivity of retrovirus-mediated transduction in A-T cells in the presence of wortmannin is due to a lack of ATM and not some other deficiency of A-T cell lines, A-T cells (line A-T 22IJE) that have been complemented with a vector that expresses ATM cDNA (11) were infected with the ASV IN+ virus (Table 4). Such cells exhibited sensitivity similar to that observed with the ATM-positive cell lines shown in Table 2. In contrast, colony formation was dramatically reduced in infected, wortmannin-treated A-T cells that express the empty vector. From these results we conclude that ATM contributes significantly to the efficiency of retrovirus-mediated transduction in the absence of DNA-PK.
TABLE 4.
Infection of A-T cells transduced with a vector encoding ATMa
| Cell type | No. of G418-resistant colonies at a wortmannin concn (μM) of:
|
||
|---|---|---|---|
| 0 | 1 | 5 | |
| AT22IJE/empty vector | 225 | 9 | 2 |
| AT22IJE-T/ATM | 224 | 97 | 22 |
Cells were infected with the IN+ virus as described in Materials and Methods; colony counts from two plates were averaged for each datum point.
DISCUSSION
In this report we show that wortmannin, an irreversible inhibitor of ATM and DNA-PKCS protein kinases, sensitizes a normal murine pre-B lymphocyte cell line to integrase-dependent retroviral killing (Fig. 1). The kinetics of such killing are similar to those observed with DNA-PKCS-deficient pre-B lymphocyte lines derived from scid mice. These results are consistent with the interpretation that the viability of infected normal lymphocytes is partially dependent on the activity of DNA-PKCS. We show further that wortmannin can also increase the sensitivity of scid pre-B cells to integrase-dependent retroviral killing, suggesting that an additional wortmannin-sensitive protein(s) contributes to survival of these cells (Fig. 2). As a similar increase in sensitivity was observed after treatment of scid cells with ATM antisense oligonucleotides (Fig. 3), we propose that ATM can compensate partially for the loss of DNA-PKCS in such cells.
Using a colony assay in which cell survival is dependent on the expression of a stably transduced, virus-encoded reporter (Neor) gene, we showed previously that the efficiency of such transduction is reduced by 80 to 90% in scid cells compared to normal murine fibroblasts (12). Here we describe a similar loss in retrovirus-mediated transduction of human (HeLa) cells that are treated with wortmannin (Fig. 4). We observed a dose-dependent reduction in the number of ASV-transduced colonies, with an IC50 value virtually identical to that determined for inhibition of DNA-dependent protein kinase activity in these cells (3.6 versus 4.0 μM). These decreases were observed at concentrations of the drug that had no effect on HeLa cell viability or colony-forming ability. Similar results were obtained with an HIV-1 vector in which transduction was monitored by an independent method, the expression of β-galactosidase activity in individual cells (Fig. 4). These results suggest that some function(s) of the cellular protein targets of wortmannin is required to avoid integrase-dependent cell killing and to allow stable retroviral DNA transduction. The simplest interpretation of these data is that the reduced efficiency of stable transduction is a consequence of IN-mediated cell killing. However, other possibilities, such as reduced growth rate or cell cycle arrest of these adherent cells, cannot be excluded.
The colony assay was also used to determine the effect of wortmannin on the efficiency of retroviral transduction with a panel of mutant human and rodent cell lines that lack either ATM or components of the NHEJ pathway (Table 2). In the absence of wortmannin, we observed the expected low level of transduction in cells that lacked the NHEJ components, DNA-PKCS or XRCC4. As these cells are null for expression of these two components, this low level of transduction cannot be due to residual NHEJ activity. However, this transduction was reduced even further in the presence of wortmannin. These results are consistent with our observation of increased scid cell killing in the presence of the drug. The results also indicate that the activity of a second wortmannin-sensitive protein, likely ATM, contributes to the residual transduction observed in these cells. This was confirmed by analyses of two A-T cell lines and the demonstration that the observed hypersensitivity of A-T cells was reversed by expression of ATM cDNA (Table 4). We also observed that although there was no significant reduction in colony formation in A-T cells in the absence of wortmannin, transduction was reduced by ca. 90 to 95% with only 1 μM wortmannin, and it was virtually abolished with 5 μM wortmannin (Table 2). In the absence of infection, the viability of the A-T cell lines was unaffected by these concentrations of drug. As these A-T cells have no ATM kinase, the relevant target in this case is likely DNA-PKCS. Thus, these results suggest that DNA-PK is essential for the survival of stably transduced cells that lack ATM.
Retrovirus-mediated killing of lymphocyte lines is observed as early as 12 h postinfection (12) (Fig. 1). Therefore, an early event in retroviral life cycle or the proteins mediating this event seems to be inducing scid cell death. However, the integrase-inactivated virus (IN−) does not kill scid cells (Fig. 1). Because the IN− virus can perform all early steps of the retroviral life cycle except integration, none of these steps can account for the scid cell death. Likewise, the expression of viral proteins cannot be responsible for cell killing because the ASV vector is defective for such expression in mammalian cells, and scid cells are also killed by an HIV-based vector that expresses no viral proteins but only a β-galactosidase reporter (12). Thus, we conclude that scid cell killing is dependent on the presence of an active integrase.
Integration into the host cell genome is an essential step in the replication cycle of retroviruses and retrotransposons. In the first two steps of integration, denoted processing and joining, two nucleotides are removed from the 3′ ends of the viral DNA, and these newly created 3′ ends are then joined to staggered phosphates in the complementary strands of host cell DNA (Fig. 6) (16). In the resulting integration intermediate, 5′ ends of the viral DNA are separated by single-strand gaps of four to six nucleotides from the 3′ ends of the flanking host DNA. The processing and joining reactions have been reconstituted in vitro with purified integrase and model DNA substrates. In vivo, repair of the gaps in the host DNA results in the generation of 4- to 6-bp repeats of host DNA flanking each proviral end, and the final covalent joining of the 5′ ends of the viral DNA to the host DNA. The proteins that catalyze this final step in integration have not yet been identified, but host cell repair enzymes are generally assumed to contribute to the reaction.
Our results indicate that the activities of NHEJ pathway or, in their absence, ATM are required for the formation or survival of stable retroviral transductants. As these cellular functions are implicated in DNA damage monitoring and repair, a plausible explanation for our findings is that the integration of viral DNA into the host genome is sensed as DNA damage in infected cells (Fig. 6). If NHEJ components are absent or inhibited, apoptotic cell death or growth arrest may occur due to the inability to repair such damage. As predicted from our previous studies (12), the inhibition of DNA-PKCS by wortmannin sensitizes normal cells to retrovirus-mediated cell killing. ATM appears to compensate partially for absence of the NHEJ pathway. However, the exact nature of the DNA damage produced by retroviral infection is unknown. In addition to the short gaps in host DNA introduced during IN-mediated joining of viral and host sequences, the viral DNA itself may contain single-strand interruptions. It seems possible that these discontinuities (30), or double-strand breaks produced when these regions are replicated, are the relevant signals of DNA damage. Other possible signals are changes in host DNA conformation and/or chromatin structure that may occur as a consequence of viral DNA integration. For example, there is evidence that other components of the NHEJ pathway are involved in chromatin silencing (7). Finally, the lymphocytic cell killing and transduced colony reduction we observed in wortmannin-treated (and repair-deficient cells) could be due to DNA-damaging activity of free integrase rather than to the integration reaction per se. The latter interpretation seems unlikely, as integrase presumably remains associated with the viral DNA until host target DNA is encountered. It is also inconsistent with the results of our computational analyses of retrovirus-induced scid cell death (R. Daniel, S. Litwin, R. A. Katz, and A. M. Skalka, unpublished data). However, further studies will be required to address this issue.
The exact manner in which the NHEJ pathway mediates repair of DNA damage is still unknown. A direct role has been proposed, in which the DNA-binding Ku heterodimer subunits attach the DNA-PK complex to the site of damage and allow the recruitment of other necessary components (40). The protein kinase activity of DNA-PK may be needed for signaling to other proteins or for modification of those recruited to site of damage. In V(D)J recombination, DNA-PK is also thought to interact with the RAG proteins to complete the joining of immunoglobulin coding strands (6, 39). As the mechanism of V(D)J recombination and retroviral integration seem to be related evolutionarily (1, 20), it is possible that DNA-PK also interacts with the viral integrase or other viral proteins during integration.
The hypersensitivity of A-T cells to DNA damage is due to a dual defect in DNA repair and checkpoint control (24). Either one or both of these activities could be relevant to the establishment of a stably integrated provirus. The ATM kinase is required for phosphorylation and activation of p53 and Chk2 proteins, which are implicated in cell cycle arrest and apoptosis (4, 9, 15, 26, 29). A-T cells are unable to arrest at the G1/S and G2/M checkpoints in response to DNA damage, and they also exhibit radiation-resistant DNA synthesis. It is possible that, in the absence of DNA-PK, ATM-mediated growth arrest allows cells to repair the potentially lethal damage introduced by retroviral DNA integration via an alternative, DNA-PK-independent pathway(s). It is also possible that ATM participates directly in the repair of such DNA damage (11, 36–38).
The finding that NHEJ and, in its absence, ATM are required for stable retrovirus-mediated transduction lends further credence to our proposal that the integration intermediate is sensed as DNA damage by the cell (12). This type of “damage” can be titrated, and its molecular aspects can be studied using the viral sequences as a probe. Further experiments with this system should help us to determine which activities of DNA-PK and ATM are critical and to identify other cellular proteins that may play a role in integration damage repair. An understanding of the mechanisms by which cellular repair proteins contribute to this process could have a practical application. We show here that a drug which blocks cellular repair pathways can inhibit stable transduction by HIV-1, most likely because infected cells cannot survive IN-mediated DNA damage. These results suggest a strategy for antiretroviral (AIDS) therapy that targets cellular proteins rather than viral proteins. Such a strategy would have the advantage of minimizing the potential of selecting for viral escape mutants.
ACKNOWLEDGMENTS
The work was supported by National Institutes of Health grants AI40385, CA71515, PO1 CA75138, and CA06927 and also by an appropriation from the Commonwealth of Pennsylvania. R.D. is the recipient of a fellowship from the Fox Chase Cancer Center Board of Associates.
We thank D. Cortez and Y. Shiloh for the AT22IJE-T cells; A. Bellacosa, Y. Matsumoto, and C. Seeger for critical review of the manuscript; and M. Estes for its preparation.
REFERENCES
- 1.Agrawal A, Eastman Q M, Schatz D G. Transposition mediated by RAG1 and RAG2 and its implications for the evolution of the immune system. Nature. 1998;394:744–751. doi: 10.1038/29457. [DOI] [PubMed] [Google Scholar]
- 2.Araki R, Fukumura R, Fujimori A, Taya Y, Shiloh Y, Kurimasa A, Burma S, Li G C, Chen D J, Sato K, Hoki Y, Tatsumi K, Abe M. Enhanced phosphorylation of p53 serine 18 following DNA damage in DNA-dependent protein kinase catalytic subunit-deficient cells. Cancer Res. 1999;59:3543–3546. [PubMed] [Google Scholar]
- 3.Bandyopadhyay D, Mandal M, Adam L, Mendelsohn J, Kumar R. Physical interaction between epidermal growth factor receptor and DNA-dependent protein kinase in mammalian cells. J Biol Chem. 1998;273:1568–1573. doi: 10.1074/jbc.273.3.1568. [DOI] [PubMed] [Google Scholar]
- 4.Banin S, Moyal L, Shieh S-Y, Taya Y, Anderson C W, Chessa L, Smorodinsky N I, Prives C, Reiss Y, Shiloh Y, Ziv Y. Enhanced phosphorylation of p53 by ATM in response to DNA damage. Science. 1998;281:1674–1677. doi: 10.1126/science.281.5383.1674. [DOI] [PubMed] [Google Scholar]
- 5.Barsov E V, Hughes S H. Gene transfer into mammalian cells by a Rous sarcoma virus-based retroviral vector with the host range of the amphotropic murine leukemia virus. J Virol. 1996;70:3922–3929. doi: 10.1128/jvi.70.6.3922-3929.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Besmer E, Mansilla-Soto J, Cassard S, Sawchuk D J, Brown G, Sadofsky M, Lewis S M, Nussenzweig M C, Cortes P. Hairpin coding end opening is mediated by RAG1 and RAG2 proteins. Mol Cell. 1998;2:817–828. doi: 10.1016/s1097-2765(00)80296-8. [DOI] [PubMed] [Google Scholar]
- 7.Boulton S J, Jackson S P. Components of the Ku-dependent non-homologous end-joining pathway are involved in telomeric length maintenance and telomeric silencing. EMBO J. 1998;17:1819–1828. doi: 10.1093/emboj/17.6.1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boulton S, Kyle S, Yalcintepe L, Durkacz B W. Wortmannin is a potent inhibitor of DNA double strand break but not single strand break repair in Chinese hamster ovary cells. Carcinogenesis. 1996;17:2285–2290. doi: 10.1093/carcin/17.11.2285. [DOI] [PubMed] [Google Scholar]
- 9.Canman C E, Lim D-S, Cimprich K A, Taya Y, Tamai K, Sakaguchi K, Appella E, Kastan M B, Siciliano J D. Activation of the ATM kinase by ionizing radiation and phosphorylation of p53. Science. 1998;281:1677–1679. doi: 10.1126/science.281.5383.1677. [DOI] [PubMed] [Google Scholar]
- 10.Chan D W, Gately D P, Urban S, Galloway A M, Lees-Miller S P, Yen T, Allalunis-Turner J. Lack of correlation between ATM protein expression and tumor cell radiosensitivity. Int J Radiat Biol. 1998;74:217–224. doi: 10.1080/095530098141591. [DOI] [PubMed] [Google Scholar]
- 11.Cortez D, Wang Y, Qin J, Elledge S J. Requirement of ATM-dependent phosphorylation of Brca1 in the DNA damage response to double-strand breaks. Science. 1999;286:1162–1166. doi: 10.1126/science.286.5442.1162. [DOI] [PubMed] [Google Scholar]
- 12.Daniel R, Katz R A, Skalka A M. A role for DNA-PK in retroviral DNA integration. Science. 1999;284:644–647. doi: 10.1126/science.284.5414.644. [DOI] [PubMed] [Google Scholar]
- 13.Danska J S, Holland D P, Mariathasan S, Williams K M, Guidos C J. Biochemical and genetic defects in the DNA-dependent protein kinase in murine scid lymphocytes. Mol Cell Biol. 1996;16:5507–5517. doi: 10.1128/mcb.16.10.5507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Downs J A, Jackson S P. Involvement of DNA-binding protein Ku in Ty element retrotransposition. Mol Cell Biol. 1999;19:6260–6268. doi: 10.1128/mcb.19.9.6260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Elledge S J. Cell cycle checkpoints: preventing an identity crisis. Science. 1996;274:1664–1672. doi: 10.1126/science.274.5293.1664. [DOI] [PubMed] [Google Scholar]
- 16.Flint S J, Enquist L W, Krug R M, Racaniello V R, Skalka A M. Principles of virology: molecular biology, pathogenesis, and control. Washington, D.C.: ASM Press; 1999. [Google Scholar]
- 17.Gately D P, Hittle J C, Chan G K T, Yen T J. Characterization of ATM expression, localization, and associated DNA-dependent protein kinase activity. Mol Biol Cell. 1998;9:2361–2374. doi: 10.1091/mbc.9.9.2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gilad S, Khosravi R, Shkedy D, Uziel T, Ziv Y, Savitsky K, Rotman G, Smith S, Chessa L, Jorgensen T J, Harnik R, Frydman M, Sanal O, Portnoi S, Goldwicz Z, Jaspers N G, Gatti R A, Lenoir G, Lavin M F, Tatsumi K, Wegner R D, Shiloh Y, Bar-Shira A. Predominance of null mutations in ataxia-telangiectasia. Hum Mol Genet. 1996;5:433–439. doi: 10.1093/hmg/5.4.433. [DOI] [PubMed] [Google Scholar]
- 19.Hartley K O, Gell D, Smith G C M, Zhang H, Divecha N, Connelly M A, Admon A, Lees-Miller S P, Anderson C W, Jackson S P. DNA-dependent protein kinase catalytic subunit: a relative of phosphatidylinositol 3-kinase and the ataxia telangiectasia gene product. Cell. 1995;82:849–856. doi: 10.1016/0092-8674(95)90482-4. [DOI] [PubMed] [Google Scholar]
- 20.Hiom K, Melek M, Gellert M. DNA transposition by the RAG1 and RAG2 proteins: a possible source of oncogenic translocations. Cell. 1998;94:463–470. doi: 10.1016/s0092-8674(00)81587-1. [DOI] [PubMed] [Google Scholar]
- 21.Hosoi Y, Miyachi H, Matsumoto Y, Ikehata H, Komura J, Ishii K, Zhao H-J, Yoshida M, Takai Y, Yamada S, Suzuki N, Ono T. A phosphatidylinositol 3-kinase inhibitor wortmannin induces radioresistant DNA synthesis and sensitizes cells to bleomycin and ionizing radiation. Int J Cancer. 1998;78:642–647. doi: 10.1002/(sici)1097-0215(19981123)78:5<642::aid-ijc19>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 22.Jackson S P. Ataxia-telangiectasia at the crossroads. Curr Biol. 1995;11:1210–1215. doi: 10.1016/s0960-9822(95)00238-7. [DOI] [PubMed] [Google Scholar]
- 23.Jeggo P A. Identification of genes involved in repair of DNA double-strand breaks in mammalian cells. Radiat Res. 1998;150(Suppl.):S80–S91. [PubMed] [Google Scholar]
- 24.Jeggo P A, Carr A M, Lehmann A R. Splitting the ATM: distinct repair and checkpoint defects in ataxia-telangiectasia. Trends Genet. 1998;14:312–316. doi: 10.1016/s0168-9525(98)01511-x. [DOI] [PubMed] [Google Scholar]
- 25.Keith C T, Schreiber S L. PIK-related kinases: DNA repair, recombination, and cell cycle checkpoints. Science. 1995;270:50–51. doi: 10.1126/science.270.5233.50. [DOI] [PubMed] [Google Scholar]
- 26.Khanna K K, Keating K E, Kozlov S, Scott S, Gatei M, Hobson K, Taya Y, Gabrielli B, Chan D, Lees-Miller S P, Lavin M F. ATM associates with and phosphorylates p53: mapping the region of interaction. Nat Genet. 1998;20:398–400. doi: 10.1038/3882. [DOI] [PubMed] [Google Scholar]
- 27.Lees-Miller S P, Godbout R, Chan D W, Weinfield M, Day III R S, Barron G M, Allalunis-Turner J. Absence of p350 subunit of DNA-activated protein kinase from a radiosensitive human cell line. Science. 1995;267:1183–1185. doi: 10.1126/science.7855602. [DOI] [PubMed] [Google Scholar]
- 28.Li Z, Otevrel T, Gao Y, Cheng H-L, Seed B, Stamato T D, Taccioli G E, Alt F W. The XRCC4 gene encodes a novel protein involved in DNA double-strand break repair and V(D)J recombination. Cell. 1995;83:1079–1089. doi: 10.1016/0092-8674(95)90135-3. [DOI] [PubMed] [Google Scholar]
- 29.Matsuoka S, Huang M, Elledge S J. Linkage of ATM to cell cycle regulation by the Chk2 protein kinase. Science. 1998;282:1893–1897. doi: 10.1126/science.282.5395.1893. [DOI] [PubMed] [Google Scholar]
- 30.Morozov V E, Falzon M, Anderson C W, Kuff E L. DNA-dependent protein kinase is activated by nicks and larger single-stranded gaps. J Biol Chem. 1994;269:16684–16688. [PubMed] [Google Scholar]
- 31.Naldini L, Blomer U, Gallay P, Ory D, Mulligan R, Gage F H, Verma I M, Trono D. In vivo gene delivery and stable transduction of nondividing cells by a lentiviral vector. Science. 1996;272:263–267. doi: 10.1126/science.272.5259.263. [DOI] [PubMed] [Google Scholar]
- 32.Powis G, Bonjouklian R, Berggren M M, Gallegos A, Abraham R, Ashendel C, Zalkow L, Matter W F, Dodge J, Grindey G, Vlahos C J. Wortmannin, a potent and selective inhibitor of phosphatidylinositol-3-kinase. Cancer Res. 1994;54:2419–2423. [PubMed] [Google Scholar]
- 33.Rosenzweig K E, Youmell M B, Palayoor S T, Price B D. Radiosensitization of human tumor cells by the phosphatidylinositol 3-kinase inhibitors wortmannin and LY294002 correlates with inhibition of DNA-dependent protein kinase and prolonged G2-M delay. Clin Cancer Res. 1997;3:1149–1156. [PubMed] [Google Scholar]
- 34.Sarkaria J N, Tibbetts R S, Busby E C, Kennedy A P, Hill D E, Abraham R T. Inhibition of phosphoinositide 3-kinase related kinases by the radiosensitizing agent wortmannin. Cancer Res. 1998;58:4375–4382. [PubMed] [Google Scholar]
- 35.Schuler W, Weiler I J, Schuler A, Phillips R A, Rosenberg N, Mak T W, Kearney J F, Perry R P, Bosma M J. Rearrangement of antigen receptor genes is defective in mice with severe combined immune deficiency. Cell. 1986;46:963–972. doi: 10.1016/0092-8674(86)90695-1. [DOI] [PubMed] [Google Scholar]
- 36.Scully R, Chen J, Ochs R L, Keegan K, Hoekstra M, Feunteun J, Livingston D M. Dynamic changes of Brca1 subnuclear location and phosphorylation state are initiated by DNA damage. Cell. 1997;90:425–435. doi: 10.1016/s0092-8674(00)80503-6. [DOI] [PubMed] [Google Scholar]
- 37.Scully R, Chen J, Plug A, Xiao Y, Weaver D, Feunteun J, Ashley T, Livingston D M. Association of Brca1 with Rad51 in mitotic and meiotic cells. Cell. 1997;88:265–275. doi: 10.1016/s0092-8674(00)81847-4. [DOI] [PubMed] [Google Scholar]
- 38.Sharan S K, Morimatsu M, Albrecht U, Lim D-S, Regel E, Dinh C, Sands A, Eichele G, Hasty P, Bradley A. Embryonic lethality and radiation hypersensitivity mediated by Rad51 in mice lacking Brca2. Nature. 1997;386:804–810. doi: 10.1038/386804a0. [DOI] [PubMed] [Google Scholar]
- 39.Shockett P E, Schatz D G. DNA hairpin opening mediated by the RAG1 and RAG2 proteins. Mol Cell Biol. 1999;19:4159–4166. doi: 10.1128/mcb.19.6.4159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Smith G C M, Jackson S P. The DNA-dependent protein kinase. Genes Dev. 1999;13:916–934. doi: 10.1101/gad.13.8.916. [DOI] [PubMed] [Google Scholar]
- 41.Wymann M P, Bulgarelli-Leva G, Zvelebil M J, Pirola L, Vanhaesebroeck B, Waterfield M D, Panayotou G. Wortmannin inactivates phosphoinositide 3-kinase by covalent modification of Lys-802, a residue involved in the phosphate transfer reaction. Mol Cell Biol. 1996;16:1722–1733. doi: 10.1128/mcb.16.4.1722. [DOI] [PMC free article] [PubMed] [Google Scholar]