Abstract
Small guanine nucleotide-binding proteins of the Ras and Rho (Rac, Cdc42, and Rho) families have been implicated in cardiac myocyte hypertrophy, and this may involve the extracellular signal-related kinase (ERK), c-Jun N-terminal kinase (JNK), and/or p38 mitogen-activated protein kinase (MAPK) cascades. In other systems, Rac and Cdc42 have been particularly implicated in the activation of JNKs and p38-MAPKs. We examined the activation of Rho family small G proteins and the regulation of MAPKs through Rac1 in cardiac myocytes. Endothelin 1 and phenylephrine (both hypertrophic agonists) induced rapid activation of endogenous Rac1, and endothelin 1 also promoted significant activation of RhoA. Toxin B (which inactivates Rho family proteins) attenuated the activation of JNKs by hyperosmotic shock or endothelin 1 but had no effect on p38-MAPK activation. Toxin B also inhibited the activation of the ERK cascade by these stimuli. In transfection experiments, dominant-negative N17Rac1 inhibited activation of ERK by endothelin 1, whereas activated V12Rac1 cooperated with c-Raf to activate ERK. Rac1 may stimulate the ERK cascade either by promoting the phosphorylation of c-Raf or by increasing MEK1 and/or -2 association with c-Raf to facilitate MEK1 and/or -2 activation. In cardiac myocytes, toxin B attenuated c-Raf(Ser-338) phosphorylation (50 to 70% inhibition), but this had no effect on c-Raf activity. However, toxin B decreased both the association of MEK1 and/or -2 with c-Raf and c-Raf-associated ERK-activating activity. V12Rac1 cooperated with c-Raf to increase expression of atrial natriuretic factor (ANF), whereas N17Rac1 inhibited endothelin 1-stimulated ANF expression, indicating that the synergy between Rac1 and c-Raf is potentially physiologically important. We conclude that activation of Rac1 by hypertrophic stimuli contributes to the hypertrophic response by modulating the ERK and/or possibly the JNK (but not the p38-MAPK) cascades.
Cardiac myocytes are terminally differentiated cells. However, agonists such as endothelin 1 (ET-1) or the α-adrenergic agonist phenylephrine (PE) stimulate hypertrophic growth of these cells in the absence of further cell division (55). This response is characterized by an increase in cell volume, increased myofibrillogenesis, and changes in gene expression (e.g, reexpression of fetal genes such as atrial natriuretic factor [ANF]). The signaling pathways utilized are probably manifold, but small (21-kDa) guanine nucleotide-binding proteins (G proteins) of both the Ras and Rho (Rho, Rac, and Cdc42) families have been strongly implicated in the regulation of this response (16). Many of the effects of these proteins are probably mediated through the mitogen-activated protein kinases (MAPKs) (2, 40, 62). These kinases are the final components of three-tiered cascades in which MAPK kinase kinases phosphorylate and activate MAPK kinases, which in turn phosphorylate and activate the MAPKs. Of the three best-characterized subfamilies, the extracellular signal-regulated kinases (ERKs) are generally implicated in the regulation of growth responses of the cell, whereas the c-Jun N-terminal kinases (JNKs) and p38-MAPKs are more usually associated with cellular responses to stresses (17, 26). We have previously shown that ET-1 and PE activate all three MAPK subfamilies in cardiac myocytes, with the activation of the ERK cascade being particularly powerful (8–10, 13, 15). All three MAPK subfamilies have been implicated in the regulation of cardiac myocyte hypertrophy, but there is considerable debate as to which are physiologically relevant in this response (55, 56).
Like all small G proteins, members of the Ras and Rho families act as molecular switches within the cell (2, 40, 62). In the GDP-bound form, they are inactive, and they are activated by the exchange of GDP for GTP, a reaction which is catalyzed by guanine nucleotide exchange factors (GEFs). GTPase-activating proteins enhance the innate GTPase activity of small G proteins, returning them to the inactive state. Ras is localized to the plasma membrane, and one of the effects of Ras-GTP is to bind to c-Raf, a MAPK kinase kinase for the ERK cascade, translocating it to the plasma membrane for activation. Full activation of c-Raf requires phosphorylation of Ser-338 and Tyr-341 (41). c-Raf phosphorylates and activates the MAPK kinases MEK1 and MEK2, which phosphorylate and activate the MAPKs ERK1 and ERK2. Other effectors of Ras include phosphatidylinositol 3-kinase (PI3K) and Ral-GDS (62). The Rho family is less well characterized. Rac1 and Cdc42 are both implicated in the activation of JNKs and p38-MAPKs (2, 40), an effect which may be mediated through p21-activated kinases (PAKs) (3, 19). PAKs may also regulate the ERK cascade by either increasing c-Raf(Ser-338) phosphorylation (37) or MEK1 and/or -2 association with c-Raf (22, 23). Consistent with this, transfection experiments in dividing cells have shown that Rac1 and Cdc42 can cooperate with Raf to activate ERKs and induce transformation (22, 23, 36, 57). Rho, Rac1, and Cdc42 all regulate cytoskeletal organization and cell shape in dividing cells (2, 40). Rho promotes stress fiber formation, Rac1 is necessary for the formation of lamellipodia, and Cdc42 is required for the formation of filopodia.
In cardiac myocytes, Ras, RhoA, and Rac1 have all been implicated in the hypertrophic growth response, mediating both the morphological changes and the changes in gene expression (16, 55). At least some of the effects of Ras on gene expression involve signaling through Raf (24, 25, 59), although other mediators are probably also involved (24). RhoA may act through the Rho-dependent kinase ROK (33). Here we have studied the activation of Rac1 in cardiac myocytes. We show that Rac1-GTP increased following stimulation with hypertrophic agonists and that this contributes to ERK activation in these cells. Furthermore, although Rho family proteins are involved in the stimulation of ERKs and JNKs, activation of p38-MAPK is mediated through an alternative pathway(s) in cardiac myocytes. We also show that although Rho family small G proteins regulate c-Raf(Ser-338) phosphorylation in cardiac myocytes, this has no effect on the overall activity of c-Raf, and that the principal input from Rac1 into the ERK cascade is at the level of MEK1 and/or -2.
MATERIALS AND METHODS
Myocyte culture.
Myocytes were dissociated from the ventricles of neonatal Sprague-Dawley rat hearts as previously described (7, 34) and were plated in 10% horse serum and 5% fetal calf serum for 18 h, at a density of 350 cells/mm2 for the transfection experiments involving ANF reporter gene expression or 1.4 × 103 cells/mm2 for other experiments.
Small G protein affinity binding assays.
Serum was withdrawn from myocyte cultures for 24 h before use. Myocytes were exposed to agonists with or without pretreatment (1 h) with toxin B (10 ng/ml). The affinity binding assays were performed as previously described (12) using glutathione S-transferase (GST) fusion proteins with the Cdc42 and Rac1 interactive binding (CRIB) domain from PAK1B (for the study of Rac1 or Cdc42) or the Ras-binding domain of c-Raf (for the study of Ras). Vectors for these proteins were gifts from J. G. Collard (The Netherlands Cancer Institute) and J. L. Bos (University of Utrecht). For the study of RhoA GTP loading, a GST fusion protein was prepared containing residues 7 to 89 of murine rhotekin. The proteins were expressed and the affinity binding assays were carried out as previously described for Ras-GTP (12). Samples were immunoblotted using antibodies for Ras or Rac1 (1/1,000; Transduction Laboratories), Cdc42, or RhoA (1/1,000 or 1/100, respectively; Santa Cruz Biotechnology), using polyclonal secondary and tertiary antibodies, with detection by enhanced chemiluminescence as described previously (12). Scanning densitometry was used for semiquantitative analysis of the data.
Phosphorylation of MAPKs.
Serum was withdrawn from myocyte cultures for 24 h before use. Myocytes were exposed to agonists with or without pretreatment (1 h) with toxin B (10 ng/ml). Extracts were prepared, and phosphorylated and total MAPKs were analyzed by immunoblotting as described previously for p38-MAPKs (15). Proteins were detected using MAPK and phospho-MAPK primary antibodies (1/1,000, New England Biolabs) and horseradish peroxidase-conjugated secondary antibodies and were visualized by enhanced chemiluminescence. Scanning densitometry was used for semiquantitative analysis of the data.
Transient transfection.
Myocytes were transfected overnight using the calcium phosphate technique (27). Plasmids encoding Rho family proteins were from J. Downward (Imperial Cancer Research Fund, London, United Kingdom) and A. Hall (University College, London, United Kingdom). For studies of inhibitory Rho family proteins and ERK phosphorylation, cells were transfected with c-Myc-tagged ERK2 (ERKMyc, 10 μg, in pEXV3) and N17Rac1 (10 μg, in pRK), N19RhoA (10 μg, in pcDNA3), N17Cdc42 (10 μg, in pRK) or, as a control, pRK (10 μg). For studies of constitutively activated Rho family proteins, myocytes were transfected with ERKMyc (10 μg, in pEXV3) and a total of 10 μg of two of the following: V12Rac1 (5 μg, in pEXV3), V14RhoA (5 μg, in pEXV3), ΔN-Raf (5 μg, in pEXV3), or pEXV3 (5 μg). Cells were washed, incubated for 24 h in serum-free medium, and exposed to ET-1 (100 nM, 5 min). They were washed twice in phosphate-buffered saline and extracted into immunoprecipitation buffer (14) containing 1% Triton X-100. Extracts were clarified (5 min, 10,000 × g), and the supernatants were incubated with 10 μl of 9E10 c-Myc antibody (Santa Cruz Biotechnology) (2 h, 4°C) and then with protein G-Sepharose (20 μl, 50% suspension in immunoprecipitation buffer, 1 h, 4°C). Immunoprecipitates were washed and analyzed by immunoblotting with antibodies to phosphorylated ERK. Parallel blots were performed and probed with rabbit anti-c-Myc antibodies (Santa Cruz Biotechnology) (1/1,000 dilution).
Luciferase expression vectors and the β-galactosidase expression vector pON249 were gifts from K. R. Chien, University of California, San Diego (39). For studies with V12Rac1, myocytes were transfected with the ANF-luciferase expression vector pANF(-638)LΔ5′ (3 μg) and pON249 (1 μg) and with a total of 4 μg of two of the following: V12Rac1 (2 μg), V14RhoA (2 μg), or pEXV3 backbone vector (2 μg). Cells were washed and incubated in the absence or presence of ET-1 (100 nM, 48 h) and were then extracted and assayed for luciferase and β-galactosidase (27).
Phosphorylation and activation of c-Raf and association with MEK.
Myocytes were deprived of serum for 24 h and exposed to ET-1 with or without pretreatment with toxin B (10 ng/ml, 1 h). Cells (4 × 106) were scraped into 150 μl of buffer A (20 mM Tris-HCl [pH 7.4], 2 mM EDTA, 100 mM KCl, 5 mM NaF, 0.2 mM Na3VO4, 2 μM microcystin, 10% [vol/vol] glycerol, 1% [vol/vol] Triton X-100, 0.5% [vol/vol] 2-mercaptoethanol, 10 mM benzamidine, 0.2 mM leupeptin, 0.01 mM trans-epoxy succinyl-l-leucylamido-[4-guanidino]butane, 0.3 mM phenylmethylsulfonyl fluoride) and were centrifuged (10,000 × g, 5 min). The supernatants were incubated (1 h, 4°C) with monoclonal c-Raf antibodies (1 μg; Transduction Laboratories) prebound to protein G-Sepharose (30 μl of a 1:1 slurry in buffer A). The supernatants were boiled with sample buffer (0.33 M Tris-HCl [pH 6.8], 10% [wt/vol] sodium dodecyl sulfate, 13% [vol/vol] glycerol, 133 mM dithiothreitol). Immunoprecipitates were washed with buffer A (3 times, 750 μl) and were boiled with sample buffer. To determine c-Raf(Ser-338) phosphorylation, samples were immunoblotted with antibodies that were for total c-Raf (1/1,000) or were selective for phospho(Ser-338)-c-Raf (41). To assess c-Raf association with MEK1 and/or -2, samples were immunoblotted with antibodies that recognize both isoforms. To determine c-Raf or c-Raf-associated ERK-activating activity, immunoprecipitates were washed with 300 μl of buffer B (30 mM Tris-HCl, 0.1 mM EGTA [pH 7.5] containing 0.1% [vol/vol] 2-mercaptoethanol, 0.03% Brij 35, 10 mM magnesium acetate, 20 mM n-octyl β-d-glucopyranoside, 200 μM ATP) and were resuspended in 30 μl of buffer B. Assays were initiated by the addition of 0.2 μg of GST-MEK1 or GST-ERK2 (11) for determination of c-Raf activity or c-Raf-associated ERK-activating activity, respectively. Following incubation (20 min, 30°C), assays were placed on ice and were terminated by the addition of sample buffer (12 μl). Samples (25 μl) were immunoblotted with antibodies selective for phosphorylated MEK or phosphorylated ERK.
RESULTS
Activation of Rac1 and RhoA by G protein-coupled receptor agonists in cardiac myocytes.
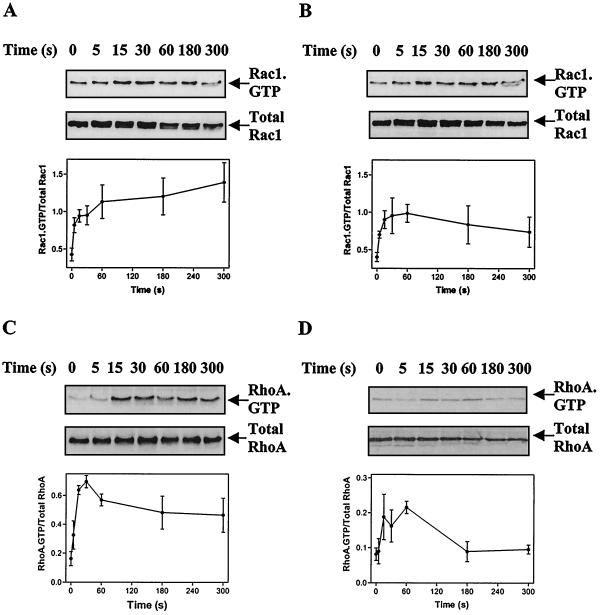
Using an affinity binding assay with the CRIB domain from PAK1B as a probe for Rac1-GTP, we investigated GTP loading of Rac1 in cardiac myocytes (Fig. 1A and B). A significant Rac1-GTP signal was detected in unstimulated cells, but 100 nM ET-1 (Fig. 1A) or 100 μM PE (Fig. 1B) induced a two- to threefold increase. Within 5 s, both agonists detectably increased Rac1-GTP loading, which was maximal by 15 s. We also investigated the GTP loading of RhoA using the Rho-binding domain from rhotekin to purify RhoA-GTP. ET-1 induced a significant (approximately fivefold) increase in RhoA-GTP in cardiac myocytes with maximal stimulation within 15 to 30 s (Fig. 1C). PE stimulation of RhoA-GTP was less than that induced by ET-1 (Fig. 1D).
FIG. 1.
Stimulation of Rac1-GTP and RhoA-GTP in cardiac myocytes. Myocytes were exposed to 100 nM ET-1 (A and C) or 100 μM PE (B and D) for the times indicated. Rac1-GTP (A and B) or RhoA-GTP (C and D) was isolated by affinity binding assays and detected by immunoblotting (upper panels). Total Rac1 or total RhoA was also immunoblotted to ensure comparable loading (middle panels). Rac1-GTP and RhoA-GTP were quantified by scanning densitometry and were expressed relative to total Rac1 and RhoA (lower panels). Results are means ± standard errors of the means (SEM) for three (RhoA-GTP) or four (Rac1-GTP) separate myocyte preparations.
Inhibition of MAPK activation by toxin B.
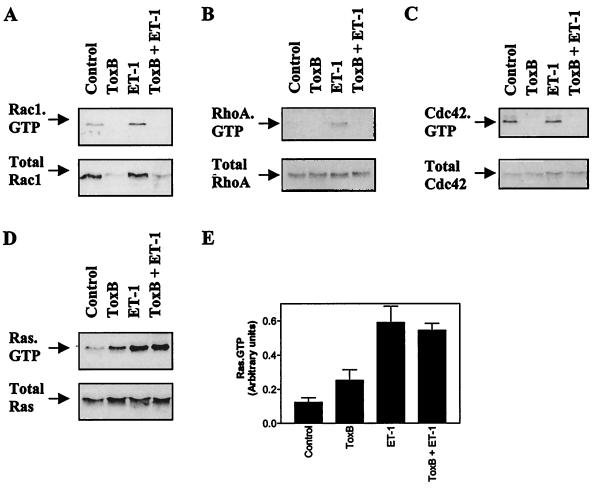
Toxin B glucosylates and inactivates Rho family proteins but not Ras family proteins (32, 52). We confirmed that this occurred in myocytes using affinity binding assays. Exposure of myocytes to 10 ng of toxin B/ml for up to 2 h had no effect on myocyte morphology or contractility (results not shown), and pretreatment with toxin B (10 ng/ml, 1 h) inhibited basal and ET-1-stimulated GTP loading of Rac1 and RhoA (Fig. 2A and B, upper panels). Basal Cdc42 GTP loading was also inhibited (Fig. 2C, upper panel). The signal for total Rac1 protein was essentially abolished by toxin B (Fig. 2A, lower panel), although total RhoA or Cdc42 was unaffected (Fig. 2B and C, lower panels). Whatever the reason for this difference, it is clear that Rac1, RhoA, and Cdc42 were all inactivated by toxin B. To confirm that this regimen selectively inhibited signaling through Rho family small G proteins and that any effects were not due to general toxicity, we examined the effects of toxin B on Ras-GTP-loading. Toxin B had no effect on ET-1-induced Ras-GTP, although there was some increase in basal Ras-GTP loading (Fig. 2D and E).
FIG. 2.
Toxin B inactivates Rac1, RhoA, and Cdc42 but not Ras. Myocytes were untreated (controls) or were exposed to 100 nM ET-1 for 15 s with or without pretreatment with toxin B (ToxB) (10 ng/ml, 1 h). GTP loading of Rac1 (A), RhoA (B), Cdc42 (C), or Ras (D) was determined by affinity binding assays and detected by immunoblotting (upper panels). Total Rac1, RhoA, Cdc42, or Ras was also immunoblotted (lower panels). GTP loading experiments for Rac1, RhoA, and Cdc42 were repeated with similar results. Ras-GTP loading was examined in five separate myocyte preparations. (E) Ras GTP loading was analyzed by scanning densitometry. Results are means ± SEM for five separate myocyte preparations.
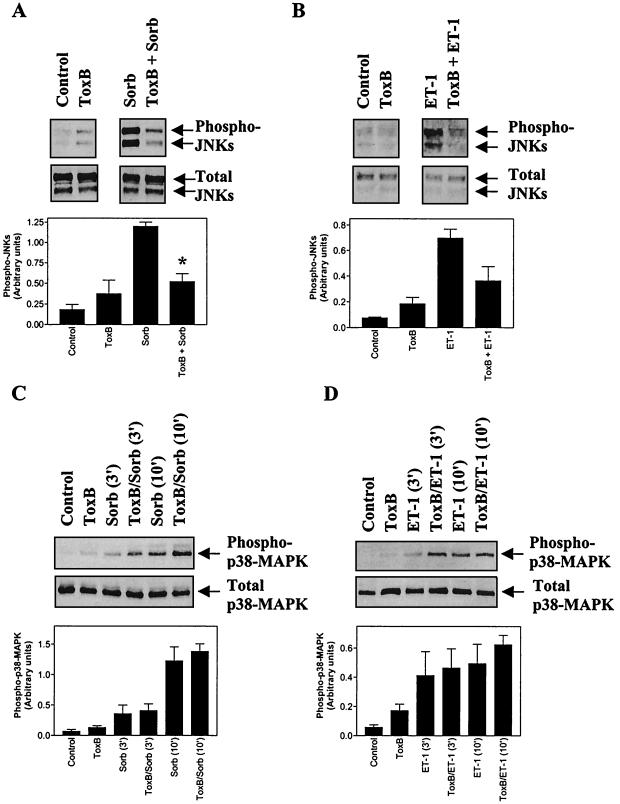
Rac1 and Cdc42 are implicated in the activation of JNKs and p38-MAPKs (2, 40). Although JNKs and p38-MAPKs are activated primarily by cellular stresses (e.g., hyperosmotic shock), we have shown that ET-1 also activates these MAPKs in cardiac myocytes (10, 15). We assessed the effects of toxin B on the activation of JNKs and p38-MAPKs by immunoblotting with antibodies selective for the dually phosphorylated (activated) forms of these kinases. The incubation times for these and subsequent experiments on MAPK activation are times at which they are maximally activated in cardiac myocytes (8, 10, 13, 15). Toxin B inhibited activation of JNKs in cardiac myocytes exposed to either hyperosmotic shock (0.5 M sorbitol) (Fig. 3A) or 10 nM ET-1 (Fig. 3B) but had no effect on the activation of p38-MAPKs (Fig. 3C and D). Thus, although JNK activation by these stimuli requires Rho family proteins, activation of p38-MAPKs in cardiac myocytes is mediated through alternative mechanisms.
FIG. 3.
Toxin B inhibits phosphorylation of JNKs but not of p38-MAPKs induced by ET-1 or hyperosmotic shock. Myocytes were unstimulated (controls) or were exposed to 0.5 M sorbitol (Sorb) (A and C) or 10 nM ET-1 (B and D) with or without pretreatment with toxin B (ToxB) (10 ng/ml, 1 h) for 0 or 30 min in the case of JNKs or for 0, 3, or 10 min in the case of p38-MAPKs. (A and B) Extracts were immunoblotted for phosphorylated (activated) JNKs (Phospho-JNKs) or total JNKs. The upper arrow on each blot indicates the 54-kDa JNKs, whereas the lower arrow indicates the 46-kDa JNKs. (C and D) Extracts were immunoblotted for phosphorylated (activated) p38-MAPKs (Phospho p38-MAPK) or total p38-MAPKs. Blots were analyzed by scanning densitometry (A to D, lower panels) Results are means ± SEM for three or four separate myocyte preparations. ∗, P < 0.001 relative to hyperosmotic shock in the absence of toxin B (unpaired two-tailed t test).
Regulation of ERK activation by ET-1 through small G proteins of the Rho family.
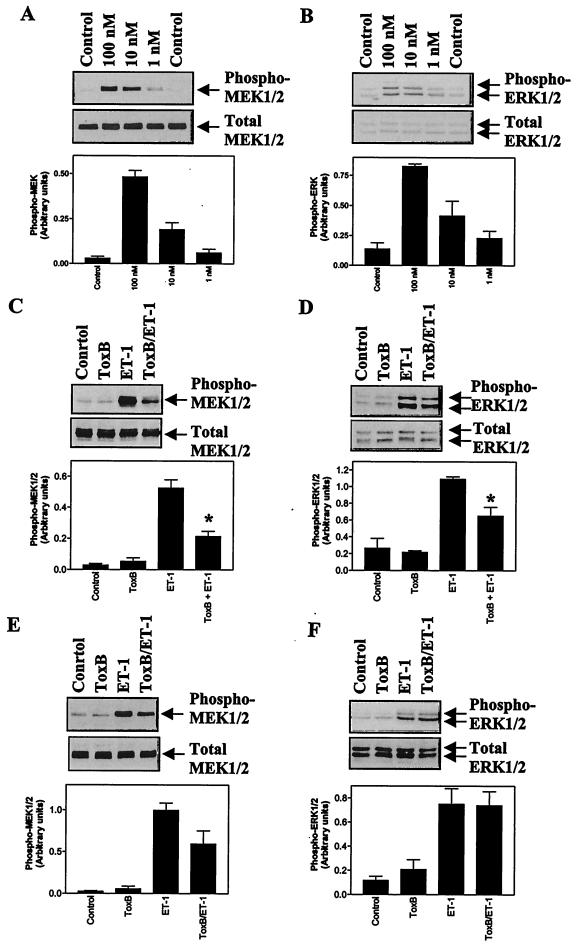
In cardiac myocytes, the ERK cascade is potently activated by ET-1 (8, 9, 13). We examined the effects of toxin B on the activation of the ERK cascade by ET-1 using antibodies selective for the phosphorylated forms of MEK1 and/or -2 and ERK1 and -2 for immunoblotting. ET-1 stimulated the activation of MEK1 and/or -2 and ERK1 and -2 in the concentration range of 1 to 100 nM (Fig. 4A and B). ET-1 (100 nM) stimulated maximal activation of ERK, as demonstrated by the complete shift of ERK2 to a band with reduced mobility (i.e., the phosphorylated form), whereas stimulation by 10 nM ET-1 was approximately 50% of the maximum (Fig. 4B, bottom panel). Toxin B significantly inhibited the activation of MEK1 and/or -2 and ERK1 and -2 induced by 10 nM ET-1 (Fig. 4C and D). However, in cardiac myocytes exposed to 100 nM ET-1, which promotes maximal activation of ERKs (Fig. 4B), the inhibition of MEK1 and/or -2 activity did not reach statistical significance (Fig. 4E) and there was no inhibition of ERK1 and -2 activation (Fig. 4F). These data indicate that Rho family proteins may contribute to stimulation of the ERK cascade by ET-1, particularly in the context of submaximal stimulation.
FIG. 4.
Toxin B inhibits activation of the ERK cascade induced by ET-1. (A and B) Myocytes were unstimulated (control) or were exposed to 1, 10, or 100 nM ET-1 for 5 min. (C to F) Myocytes were exposed to 10 nM ET-1 (C and D) or 100 nM ET-1 (E and F) for 0 or 3 min with or without pretreatment with toxin B (ToxB) (10 ng/ml, 1 h). (A, C, and E) Extracts were immunoblotted for phosphorylated MEK1 and -2 (Phospho-MEK1/2) or total MEK1 and -2. (B, D, and F) Extracts were immunoblotted for phosphorylated ERK1 and -2 (Phospho-ERK1/2) or total ERK1 and -2. The upper arrow on each blot indicates ERK1, whereas the lower arrow indicates ERK2. (A to F, lower panels) Blots were analyzed by scanning densitometry. Results are means ± SEM for three (A and B) or four (C to F) separate myocyte preparations. ∗, P < 0.01 relative to ET-1 in the absence of toxin B (unpaired two-tailed t test).
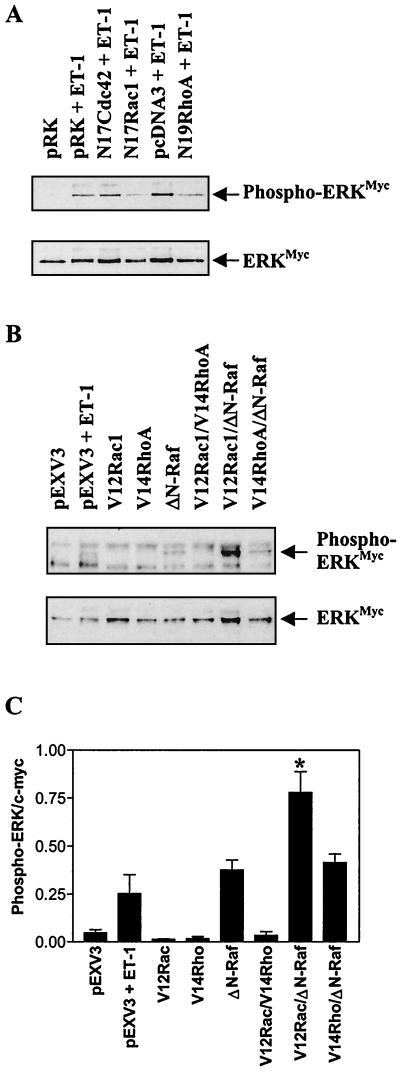
In further studies, cardiac myocytes were transfected with plasmids encoding c-Myc-tagged ERK2 (ERKMyc) and dominant-negative (N17Cdc42, N17Rac1, N19RhoA) or activating (V12Rac1, V14RhoA) mutants of Cdc42, Rac1, or RhoA. Following immunoprecipitation, the activation of ERKMyc was assessed by immunoblotting. Parallel blots were probed with antibodies to c-Myc to assess ERKMyc protein expression, and, following densitometric analysis, the amount of phospho-ERKMyc was corrected for total ERKMyc. ET-1 (100 nM) increased the activation of ERKMyc by >20-fold (Fig. 5A). N17Cdc42 had no effect on this response, but ERKMyc activation was suppressed by N17Rac1 or N19RhoA (>75%). Neither V12Rac1 nor V14RhoA alone nor in combination had any effect on ERKMyc activation, although ΔN-Raf (constitutively activated c-Raf) increased activation of ERKMyc to a degree similar to that caused by 100 nM ET-1 (Fig. 5B and C). V12Rac1 synergized with ΔN-Raf to increase ERKMyc activation, but V14RhoA did not enhance the response to ΔN-Raf (Fig. 5B and C). The effects of the inhibitory and activating mutants more clearly implicate Rac1 in the modulation of the ERK cascade than Cdc42 or RhoA. The inhibition of ET-1-stimulated ERK activation by N19RhoA suggests that RhoA may also be required for ERK activation. However, since V12RhoA had no effect, either alone or in combination with ΔN-Raf, the role of RhoA in the activation of the ERK cascade may be permissive.
FIG. 5.
Regulation of ERK phosphorylation by Rho family proteins. (A) Myocytes were transfected with ERKMyc and inhibitory mutants of Rho family proteins. Following exposure to ET-1 (100 nM, 5 min), ERKMyc was immunoprecipitated and immunoblotted with antibodies to phosphorylated ERK (upper panel). Parallel blots were stripped and probed with an antibody to the c-Myc tag (lower panel). The experiment was repeated on three separate occasions with similar results. (B) Myocytes were transfected with ERKMyc, ΔN-Raf, and/or activated mutants of Rho family proteins. When applicable, exposure to ET-1 (100 nM) was for 5 min. ERKMyc was immunoprecipitated and immunoblotted with antibodies to phosphorylated ERK (upper panel). Parallel blots were reprobed with an antibody to the c-Myc tag (lower panel). The experiment was repeated on four separate occasions with similar results. (C) Blots shown in panel B were analyzed by scanning densitometry, and the amount of phospho-ERKMyc was adjusted for total ERKMyc protein. Results are means ± SEM for four separate myocyte preparations. ∗, P < 0.05 relative to ΔN-Raf alone (unpaired two-tailed t test).
Toxin B inhibition of the ERK cascade is mediated at the level of MEK rather than c-Raf.
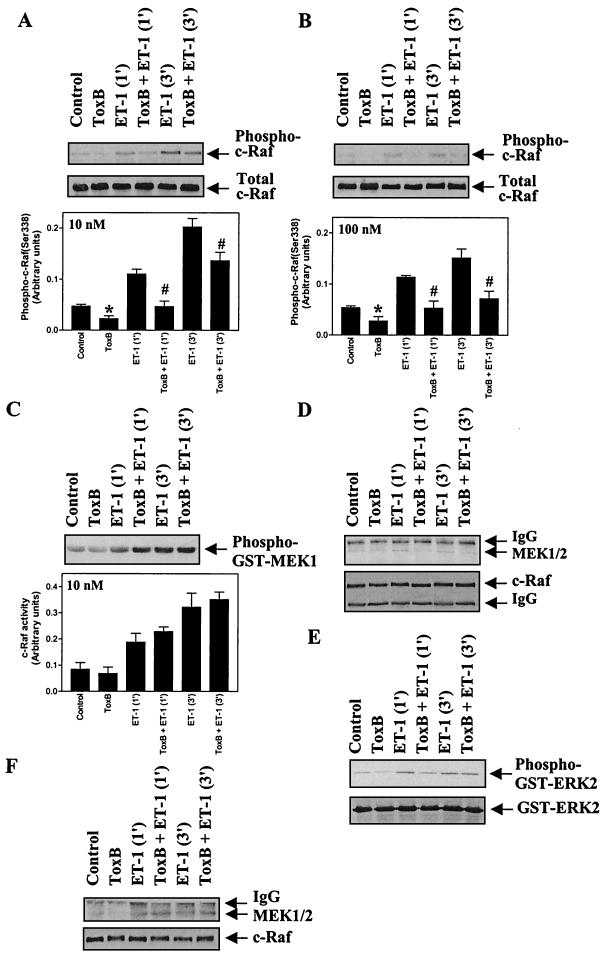
The transfection experiments clearly implicated Rac1 in the potentiation of ERK activation, whereas the effects of V14RhoA and N19RhoA were not consistent. Previous studies have indicated two potential mechanisms in which Rac1 and Cdc42 may promote activation of the ERK cascade. One possibility is that Rac1 and Cdc42 activate PAKs which can phosphorylate c-Raf on Ser-338 (37), one of two residues required for full activity (41). This was investigated following immunoprecipitation of c-Raf by immunoblotting with an antibody selective for the Ser-338 phosphorylated form of the protein and by assessing c-Raf activity using GST-MEK1 as a substrate. Phosphorylation of GST-MEK1 was measured by immunoblotting with phospho-MEK antibodies. Toxin B (10 ng/ml, 1 h) significantly inhibited the basal level of c-Raf (Ser-338) phosphorylation (51% ± 7%, n = 7, P < 0.001) and the increase in phospho(Ser-338)-c-Raf induced by 10 nM or 100 nM ET-1 (64% ± 5%, n = 4, P < 0.005, or 69% ± 14%, n = 4, P < 0.05, respectively, for myocytes exposed to ET-1 for 1 min) (Fig. 6A and B). Despite the inhibition of c-Raf(Ser-338) phosphorylation, there was no effect of toxin B on the stimulation of c-Raf activity by ET-1 (Fig. 6C), suggesting that phosphorylation of Ser-338 is not limiting for c-Raf activation in cardiac myocytes. These data indicate that the inhibitory effect of toxin B on the ERK cascade is not mediated at the level of c-Raf.
FIG. 6.
Toxin B inhibits c-Raf(Ser-338) phosphorylation but not c-Raf activity and attenuates MEK1 association with c-Raf in myocytes exposed to 10 nM ET-1. Myocytes were unstimulated or exposed to 10 nM ET-1 (A, C, D, and E) or 100 nM ET-1 (B and F) for the times indicated, and c-Raf was immunoprecipitated from myocyte extracts. (A and B) c-Raf immunoprecipitates were immunoblotted with antibodies selective for phospho(Ser-338)-c-Raf (upper blots) or total c-Raf (lower blots). Blots were analyzed by scanning densitometry (bar graphs). Results are means ± SEM for four separate myocyte preparations. ∗, P < 0.05, relative to unstimulated myocytes in the absence of toxin B (unpaired two-tailed t test). P < 0.05 relative to myocytes exposed to ET-1 in the absence of toxin B (unpaired two-tailed t test). (C) c-Raf activity was determined using GST-MEK1 as the substrate. Phosphorylation of GST-MEK1 was assessed by immunoblotting with antibodies selective for phospho-MEK1 and/or -2 (upper panel). Blots were analyzed by scanning densitometry (lower panel). Results are means ± SEM for three separate myocyte preparations. (D and F) c-Raf immunoprecipitates were immunoblotted for total MEK1/2 (upper panels) or for c-Raf (lower panels). (E) c-Raf immunoprecipitates were assayed for associated ERK-activating activity using GST-ERK2 as substrate. Phosphorylation of GST-ERK2 was assessed by immunoblotting with antibodies selective for phospho-ERK1 and -2 (upper panel). Parallel blots were probed with antibodies to total ERK1 and -2 (lower panel). The experiments were repeated with three separate preparations of myocytes with similar results. IgG, immunoglobulin G.
An alternative mechanism which may account for the input from Rac1 to the ERK cascade is through PAK-dependent phosphorylation of Ser-298 of MEK (22). This potentially increases its association with c-Raf, facilitating its activation. In cardiac myocytes exposed to 10 nM ET-1, toxin B attenuated the amount of MEK1 and/or -2 associated with c-Raf (Fig. 6D) and the c-Raf-associated ERK-activating activity (presumably MEK1 and/or -2) (Fig. 6E). In myocytes exposed to 100 nM ET-1, toxin B had no significant effect on the association of MEK1 and/or -2 with c-Raf (Fig. 6F). These data suggest that Rac1 promotes activation of the ERK cascade by increasing the association of MEK1 and/or -2 with c-Raf and are consistent with the lack of any effect of V12Rac1 alone on ERK activation and the synergistic effect of V12Rac1 with ΔN-Raf (Fig. 5C and D). This mechanism appears to be significant in the context of submaximal stimulation of the pathway, which may well be the situation in vivo.
Regulation of ANF expression by Rac1.
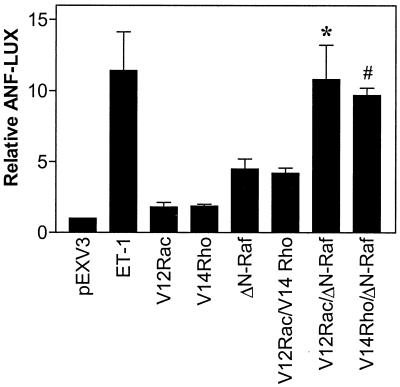
We examined whether the synergy observed between Rac1 and c-Raf with respect to the ERK cascade is reflected in a physiological response of the cardiac myocyte. One facet of cardiac myocyte hypertrophy is the reexpression of ANF (55). We therefore examined the expression of an ANF-luciferase reporter gene following transfection with V12Rac1 (Fig. 7). We also examined the effect of V12RhoA. ET-1 (100 nM) induced a large (>10-fold) increase in ANF expression, whereas ΔN-Raf stimulated a smaller (∼5-fold) increase, consistent with previous studies (25). V12Rac1 or V12RhoA alone increased ANF expression approximately twofold and, in combination, had an additive effect (four- to fivefold stimulation). However, V12Rac1 or V14RhoA had a synergistic effect on ANF expression when transfected in combination with ΔN-Raf, resulting in a response similar to that seen with 100 nM ET-1. These results are consistent with a role for Rac1 and RhoA in the upregulation of ANF expression.
FIG. 7.
Regulation of the ANF promoter by Rho family proteins and Raf. Myocytes were transfected with ANF-luciferase reporters (ANF-LUX). Cells were exposed to 100 nM ET-1 (48 h) or were cotransfected with backbone vector, V12Rac1, V14RhoA, or ΔN-Raf (in pEXV3) alone, or in combination. Extracts were assayed for luciferase activity. Results are expressed relative to backbone vector alone and are means ± SEM for five separate myocyte preparations.
DISCUSSION
Activation of Rac1.
Although experiments using transient overexpression have shown that Rho family proteins are recognized as key regulators of many intracellular systems, the recent development of affinity binding assays selective for the GTP-bound small G proteins has made it possible to study the activation of endogenous proteins. We used a GST fusion protein with the CRIB domain from PAK1B to assay Rac1-GTP and a GST fusion protein with the Rho-binding domain from rhotekin to assay RhoA-GTP. Such assays have been performed to demonstrate Rac-GTP loading in dividing cells (4, 50, 61) and during the chemotactic response of neutrophils (1, 5). Here we showed that Rac1-GTP is readily detected in unstimulated cardiac myocytes but that levels are rapidly (in <5 s) increased by the heterotrimeric G protein-coupled receptor agonists ET-1 and PE (Fig. 1A and B). Although the increase in Rac1-GTP was only two- to threefold, this is of the same order as that reported for other cell types (5, 61). ET-1 also stimulated a significant increase in RhoA-GTP, although the response to PE was less pronounced (Fig. 1C and D). The time course for the stimulation of RhoA-GTP appeared slower than that of Rac1-GTP, with maximal activation within 15 to 30 s.
The mechanisms involved in Rac1 activation are not clear. It is proposed that Ras activation of PI3K leads to production of phosphatidylinositol 3,4,5 trisphosphate, which is required for Rac1 activation (28, 47). Recent data further indicate that phosphatidylinositol 3,4,5 trisphosphate may recruit a complex containing Sos1 (which has GEF activity for both Ras and Rac) to the membrane where it facilitates exchange of GDP for GTP (45, 51). Our studies would not be inconsistent with this model, since ET-1 and PE stimulate similar increases in Ras-GTP in cardiac myocytes (12). However, it should be noted that Rac1-GTP loading is extremely rapid, and our results would not be incompatible with the alternative proposal that Gi βγ subunits may stimulate the exchange factor Ras-GRF to increase exchange of GDP for GTP on Rac1 directly (20, 38, 42). Other studies of Rac1 activation indicate that the chemotactic peptide, f-MetLeuPhe, which acts through Gi-coupled receptors, and bradykinin, which signals through Gq-coupled receptors, increase Rac-GTP in neutrophils and COS cells, respectively (1, 5, 61). ET-1 is known to signal through both Gq- and Gi-coupled receptors in cardiac myocytes (29), but it was not possible to determine whether Rac1-GTP was Gi dependent, since pretreatment with pertussis toxin increased basal Rac1-GTP levels (results not shown). The PI3K inhibitor LY294002 also increased levels of Rac1-GTP, and since we observed only a two- to threefold increase in Rac1-GTP (Fig. 1), it was not possible to determine whether PI3K was involved in this response.
Regulation of MAPK activation by Rac1.
Rac and Cdc42 are recognized as regulators of the JNK and p38-MAPK cascades (23, 43, 44), an effect which is probably mediated through PAK(s) (3, 19). However, transfection studies indicate that Rac1 can synergize with Raf to activate MEK and ERK and can induce transformation of dividing cells (22, 23, 36, 57). These effects are probably also mediated through PAKs. The majority of these conclusions have been derived from transfection experiments, and while such experiments are useful in establishing which signaling pathways are probably active in cells, overexpression of signaling intermediates may activate or inhibit pathways which would not occur with specific agonists and/or in specific cell types. We therefore used toxin B to examine the role of endogenous Rho family proteins in cardiac myocyte signal transduction. Although toxin B may have secondary or nonspecific effects, its effects on small G proteins were selective for the Rho family (Fig. 2A to C) and the stimulation of GTP loading of Ras by ET-1 was unaffected by toxin B pretreatment (Fig. 2D). Toxin B attenuated the phosphorylation (activation) of MEK1 and/or -2 and ERK1 and -2 induced by 10 nM ET-1 (Fig. 4C and D), consistent with a role for Rho family proteins in the potentiation of ERK signaling in these cells. The effects of toxin B were less apparent at higher concentrations of ET-1 (100 nM) (Fig. 4E and F), indicating that (as might be expected) as the degree of Raf activation increases, there is a reduced requirement to potentiate the response. This suggests that the potentiation of ERK signaling in cardiac myocytes through Rac1 is likely to be significant in the context of submaximal ERK stimulation, a situation which may be more representative of the physiological situation in the heart. It is probable that the lesser effect of toxin B on ERK phosphorylation is due to the amplification inherent in the MAPK cascades. Toxin B also attenuated MEK1 and/or -2 phosphorylation induced by hyperosmotic shock or by platelet-derived growth factor (results not shown), suggesting that Rho family small-G-protein signaling to the ERK cascade may be a universal mechanism operating within cardiac myocytes, rather than a receptor-specific effect.
Studies in other laboratories have identified two possible mechanisms whereby Rac1 or Cdc42 can promote activation of the ERK cascade through PAK. Full activation of c-Raf requires its recruitment to the membrane by binding to Ras-GTP (62) and phosphorylation of both Ser-338 and Tyr-341 (41). PAK can phosphorylate Ser-338 (37). Our experiments indicate that such a pathway may operate in cardiac myocytes, since toxin B significantly inhibited c-Raf(Ser-338) phosphorylation in unstimulated cells and following exposure to ET-1 (Fig. 6A and B). However, this had no effect on c-Raf activity (Fig. 6C), suggesting that in the context of the cardiac myocyte, phosphorylation of Tyr-341 may be the limiting factor and that any effect of PAK on Ser-338 phosphorylation has little impact on activation of the ERK cascade.
Alternatively, Rac1 signaling through PAK may not activate the ERK cascade directly. Rather, PAK-mediated phosphorylation of MEK1 in its c-Raf-binding domain (Ser-298) may promote the association of MEK1 with c-Raf, thus increasing the efficiency of MEK1 activation (22, 23). Our data are more consistent with this model. Our transfection experiments clearly show that Rac1 only potentiates ERK activation since, although N17Rac1 attenuated ERK activation by ET-1 (Fig. 5A), V12Rac1 alone had no effect on ERK activation and only promotes ERK activation in the context of cotransfected ΔN-Raf (Fig. 5B and C). Furthermore, toxin B suppressed the association of MEK1 and/or -2 with c-Raf in unstimulated myocytes and following stimulation with 10 nM ET-1 (Fig. 6D, upper panel), and this was associated with decreased ERK-activating activity (Fig. 6E, lower panel). However, in myocytes exposed to 100 nM ET-1, toxin B appeared to have no effect on MEK association with c-Raf (Fig. 6F). Presumably, with an increased proportion of total c-Raf having been activated, the interaction between MEK and c-Raf is no longer limiting. It is possible that phosphorylation of c-Raf(Ser-338) could affect its association with MEK. However, toxin B inhibited phosphorylation of c-Raf(Ser-338) in myocytes exposed to 100 nM ET-1 (Fig. 6B) but had no effect on MEK association with c-Raf at this concentration (Fig. 6F), suggesting that other factors (such as phosphorylation of Ser-298 of MEK1) are more important.
In addition to attenuating the ERK response, toxin B also inhibited JNK phosphorylation (Fig. 3A and B) but had no effect on the phosphorylation of p38-MAPKs (Fig. 3C and D) induced by either ET-1 or hyperosmotic shock. This contrasts with published transfection studies, which indicate that Rac or Cdc42 can activate either subfamily of the MAPKs (23, 43), and may reflect additional constraints within the cell (e.g., binding to scaffolding proteins) which are not apparent when studying overexpressed proteins.
Involvement of Rho family proteins in cardiac hypertrophy.
There is currently considerable debate regarding the intracellular signaling mechanisms which regulate the hypertrophic response in cardiac myocytes (54–56). Stimulation of ERKs was proposed to promote hypertrophy, since they are strongly activated by hypertrophic agonists (8, 9, 13), and activation of the ERK cascade stimulates the changes in gene expression characteristic of this response (27, 58). Furthermore, inhibition of the ERK cascade using the MEK-selective inhibitor U0126 suppresses the hypertrophic response induced by ET-1 in cardiac myocytes (63). ERK activation alone may be insufficient to promote the complete hypertrophic response, since p38-MAPKs and JNKs have also been implicated (55, 56). However, it should be noted that hypertrophic agonists activate JNKs and p38-MAPKs only relatively weakly, compared with their activation by cellular stresses (10, 15), and that cellular stresses (e.g., oxidative stress) induce apoptosis rather than hypertrophy (18).
The Rho family of small G proteins has become the focus of considerable attention in relation to cardiac myocyte hypertrophy because of their involvement in cytoskeletal reorganization (2, 40). RhoA and Rac1 both appear to be required for myocyte hypertrophy, since inhibitory mutants diminish the response to PE and activating mutations induce a hypertrophic pattern of gene expression (30, 33, 48, 49, 60). However, experiments with inhibitory mutants should be interpreted with care since these proteins may bind to and inhibit GEFs for other small G proteins (21). In the case of Rac1, it is generally assumed that at least some of the effects are mediated through JNKs and/or p38-MAPKs. Our data indicate that, particularly at the relatively low agonist concentrations that might be expected in vivo, some of the effects of Rac1 on hypertrophy may be attributable to an input into the ERK cascade (Fig. 4 to 6). In addition, toxin B inhibited phosphorylation of JNKs but not phosphorylation of p38-MAPKs (Fig. 3), indicating that while some of the hypertrophic effects of Rho family proteins may be mediated through JNKs, it is unlikely that p38-MAPKs are involved.
In our studies of ANF expression, we found that V14RhoA or V12Rac1 alone slightly increased ANF-luciferase and together induced a response similar to that induced by ΔN-Raf (Fig. 7). However, in combination with ΔN-Raf, V14RhoA or V12Rac1 synergistically stimulated ANF-luciferase (Fig. 7). The effects of V12Rac1 and ΔN-Raf are compatible with the synergistic effect of these proteins on ERK phosphorylation, although it must be considered that the effects may not be entirely due to this. In contrast to V12Rac1, V14RhoA had no effect on ERK phosphorylation either alone or in combination with V12Rac1 or ΔN-Raf, suggesting that its effects on ANF-luciferase may be mediated through a different mechanism. One of the effects of RhoA is to activate the transcription factor, SRF, although the mechanism(s) involved is not understood (2). Since the ANF promoter contains two high-affinity SRF binding sites (53) in addition to a low-affinity site (31), it is possible that RhoA regulates ANF through SRF.
It is of note that Rac1-GTP was readily detected in unstimulated myocytes (Fig. 1) and that prolonged exposure (up to 12 h) of cardiac myocytes to toxin B results in their detachment from each other and from the tissue culture surface (results not shown). These data suggest that, whether or not Rho family proteins are directly involved in cardiac hypertrophy, activated small G proteins may play a vital role in the maintenance of myocyte morphology. This would be consonant with other studies which indicate that activation of Rac is a key component of the survival response of fibroblasts exposed to insulin (6) and that Rac may function to suppress Ras-induced apoptosis (35, 46). The role of Rac in cardiac myocyte survival awaits further investigation.
ACKNOWLEDGMENTS
We thank Sharon M. Cole, Joanne G. Harrison, and Jatinder Kaur for their assistance.
This work was supported by the British Heart Foundation and the Medical Research Council. C.M. is a Cancer Research Campaign Gibb Life Research Fellow.
REFERENCES
- 1.Akasaki T, Koga H, Sumimoto H. Phosphoinositide 3-kinase-dependent and -independent activation of the small GTPase Rac2 in human neutrophils. J Biol Chem. 1999;274:18055–18059. doi: 10.1074/jbc.274.25.18055. [DOI] [PubMed] [Google Scholar]
- 2.Aspenström P. Effectors for the Rho GTPases. Curr Opin Cell Biol. 1999;11:95–102. doi: 10.1016/s0955-0674(99)80011-8. [DOI] [PubMed] [Google Scholar]
- 3.Bagrodia S, Cerione R A. PAK to the future. Trends Cell Biol. 1999;9:350–355. doi: 10.1016/s0962-8924(99)01618-9. [DOI] [PubMed] [Google Scholar]
- 4.Bagrodia S, Taylor S J, Jordan K A, van Aelst L, Cerione R A. A novel regulator of p21-activated kinases. J Biol Chem. 1998;273:23633–23636. doi: 10.1074/jbc.273.37.23633. [DOI] [PubMed] [Google Scholar]
- 5.Benard V, Bohl B P, Bokoch G M. Characterization of Rac and Cdc42 activation in chemoattractant-stimulated human neutrophils using a novel assay for active GTPases. J Biol Chem. 1999;274:13198–13204. doi: 10.1074/jbc.274.19.13198. [DOI] [PubMed] [Google Scholar]
- 6.Boehm J E, Chaika O V, Lewis R E. Rac-dependent anti-apoptotic signaling by the insulin receptor cytoplasmic domain. J Biol Chem. 1999;274:28632–28636. doi: 10.1074/jbc.274.40.28632. [DOI] [PubMed] [Google Scholar]
- 7.Bogoyevitch M A, Clerk A, Sugden P H. Activation of the mitogen-activated protein kinase cascade by pertussis toxin-sensitive and -insensitive pathways in cultured ventricular cardiomyocytes. Biochem J. 1995;309:437–443. doi: 10.1042/bj3090437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bogoyevitch M A, Glennon P E, Andersson M B, Clerk A, Lazou A, Marshall C J, Parker P J, Sugden P H. Endothelin-1 and fibroblast growth factors stimulate the mitogen-activated protein kinase signaling cascade in cardiac myocytes. The potential role of the cascade in the integration of two signaling pathways leading to myocyte hypertrophy. J Biol Chem. 1994;269:1110–1119. [PubMed] [Google Scholar]
- 9.Bogoyevitch M A, Glennon P E, Sugden P H. Endothelin-1, phorbol esters and phenylephrine stimulate MAP kinase activities in ventricular cardiomyocytes. FEBS Lett. 1993;317:271–275. doi: 10.1016/0014-5793(93)81291-7. [DOI] [PubMed] [Google Scholar]
- 10.Bogoyevitch M A, Ketterman A J, Sugden P H. Cellular stresses activate c-Jun N-terminal protein kinases (JNKs) in ventricular myocytes cultured from neonatal rat hearts. J Biol Chem. 1995;270:29710–29717. doi: 10.1074/jbc.270.50.29710. [DOI] [PubMed] [Google Scholar]
- 11.Bogoyevitch M A, Marshall C J, Sugden P H. Hypertrophic agonists stimulate the activities of the protein kinases c-Raf and A-Raf in cultured ventricular myocytes. J Biol Chem. 1995;270:26303–26310. doi: 10.1074/jbc.270.44.26303. [DOI] [PubMed] [Google Scholar]
- 12.Chiloeches A, Paterson H F, Marais R M, Clerk A, Marshall C J, Sugden P H. Regulation of Ras. GTP loading and Ras-Raf association in neonatal rat ventricular myocytes by G protein-coupled receptor agonists and phorbol esters. Activation of the ERK cascade by phorbol esters is mediated by Ras. J Biol Chem. 1999;274:19762–19770. doi: 10.1074/jbc.274.28.19762. [DOI] [PubMed] [Google Scholar]
- 13.Clerk A, Bogoyevitch M A, Andersson M B, Sugden P H. Differential activation of protein kinase C isoforms by endothelin-1 and phenylephrine, and subsequent stimulation of p42 and p44 mitogen-activated protein kinases in ventricular myocytes cultured from neonatal rat hearts. J Biol Chem. 1994;269:32848–32857. [PubMed] [Google Scholar]
- 14.Clerk A, Fuller S J, Michael A, Sugden P H. Stimulation of “stress-regulated” mitogen-activated protein kinases (SAPKs/JNKs and p38-MAPKs) in perfused rat hearts by oxidative and other stresses. J Biol Chem. 1998;273:7228–7234. doi: 10.1074/jbc.273.13.7228. [DOI] [PubMed] [Google Scholar]
- 15.Clerk A, Michael A, Sugden P H. Stimulation of the p38 mitogen-activated protein kinase pathway in neonatal rat ventricular myocytes by the G protein-coupled receptor agonists, endothelin-1 and phenylephrine; a role in cardiac myocyte hypertrophy? J Cell Biol. 1998;142:523–535. doi: 10.1083/jcb.142.2.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Clerk A, Sugden P H. Small guanine nucleotide-binding proteins and myocardial hypertrophy. Circ Res. 2000;86:1019–1023. doi: 10.1161/01.res.86.10.1019. [DOI] [PubMed] [Google Scholar]
- 17.Cohen P. The search for physiological substrates of MAP and SAP kinases in mammalian cells. Trends Cell Biol. 1997;7:353–361. doi: 10.1016/S0962-8924(97)01105-7. [DOI] [PubMed] [Google Scholar]
- 18.Cook S A, Sugden P H, Clerk A. Regulation of Bcl-2 family proteins during development and in response to oxidative stress in cardiac myocytes: association with changes in mitochondrial membrane potential. Circ Res. 1999;85:940–949. doi: 10.1161/01.res.85.10.940. [DOI] [PubMed] [Google Scholar]
- 19.Daniels R H, Bokoch G M. p21-activated protein kinase: a crucial component of morphological signaling? Trends Biochem Sci. 1999;24:350–355. doi: 10.1016/s0968-0004(99)01442-5. [DOI] [PubMed] [Google Scholar]
- 20.Fan W T, Koch C A, de Hoog C L, Fam N P, Moran M F. The exchange factor Ras-GRF2 activates Ras-dependent and Rac-dependent mitogen-activated protein kinase pathways. Curr Biol. 1998;8:935–938. doi: 10.1016/s0960-9822(07)00376-4. [DOI] [PubMed] [Google Scholar]
- 21.Feig L A. Tools of the trade: use of dominant-inhibitory mutants of Ras-family GTPases. Nat Cell Biol. 1999;1:E25–E27. doi: 10.1038/10018. [DOI] [PubMed] [Google Scholar]
- 22.Frost J A, Steen H, Shapiro P, Lewis T, Ahn N, Shaw P E, Cobb M H. Cross-cascade activation of ERKs and ternary complex factors by Rho family proteins. EMBO J. 1997;16:6426–6438. doi: 10.1093/emboj/16.21.6426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Frost J A, Xu S, Hutchison M R, Marcus S, Cobb M H. Actions of Rho family small G proteins and p21-activated kinases on mitogen-activated protein kinase family members. Mol Cell Biol. 1996;16:3707–3713. doi: 10.1128/mcb.16.7.3707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fuller S J, Finn S G, Downward J, Sugden P H. Stimulation of gene expression in neonatal rat ventricular myocytes by Ras is mediated by Ral.GDS and phosphatidylinositol 3-kinase in addition to Raf. Biochem J. 1998;335:241–246. doi: 10.1042/bj3350241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fuller S J, Gillespie-Brown J, Sugden P H. Oncogenic raf, src, and ras stimulate a hypertrophic pattern of gene expression and increase cell size in neonatal rat ventricular myocytes. J Biol Chem. 1998;273:18146–18152. doi: 10.1074/jbc.273.29.18146. [DOI] [PubMed] [Google Scholar]
- 26.Garrington T P, Johnson G L. Organization and regulation of mitogen-activated protein kinase pathways. Curr Opin Cell Biol. 1999;11:211–218. doi: 10.1016/s0955-0674(99)80028-3. [DOI] [PubMed] [Google Scholar]
- 27.Gillespie-Brown J, Fuller S J, Bogoyevitch M A, Cowley S, Sugden P H. The mitogen-activated protein kinase kinase MEK1 stimulates a pattern of gene expression typical of the hypertrophic phenotype in rat ventricular cardiomyocytes. J Biol Chem. 1995;270:28092–28096. doi: 10.1074/jbc.270.47.28092. [DOI] [PubMed] [Google Scholar]
- 28.Han J, Luby-Phelps K, Das B, Shu X, Mosteller R D, Krishna U M, Falck J R, White M A, Broek D. Role of substrates and products of PI 3-kinase in regulating activation of Rac-related guanosine triphosphatases by Vav. Science. 1998;279:558–560. doi: 10.1126/science.279.5350.558. [DOI] [PubMed] [Google Scholar]
- 29.Hilal-Dandan R, Merck D T, Lujan J P, Brunton L L. Coupling of the type A endothelin receptor to multiple responses in adult rat cardiac myocytes. Mol Pharmacol. 1994;45:1183–1190. [PubMed] [Google Scholar]
- 30.Hines W A, Thorburn A. Ras and Rho are required for Gαq-induced hypertrophic gene expression in neonatal rat cardiac myocytes. J Mol Cell Cardiol. 1998;30:485–494. doi: 10.1006/jmcc.1997.0613. [DOI] [PubMed] [Google Scholar]
- 31.Hines W A, Thorburn J, Thorburn A. A low-affinity serum response element allows other transcription factors to activate inducible gene expression in cardiac myocytes. Mol Cell Biol. 1999;19:1841–1852. doi: 10.1128/mcb.19.3.1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hofmann F, Busch C, Prepens U, Just I, Aktories K. Localization of the glucosyltransferase activity of Clostridium difficile toxin B to the N-terminal part of the holotoxin. J Biol Chem. 1997;272:11074–11078. doi: 10.1074/jbc.272.17.11074. [DOI] [PubMed] [Google Scholar]
- 33.Hoshijima M, Sah V P, Wang Y, Chien K R, Brown J H. The low molecular weight GTPase Rho regulates myofibril formation and organization in neonatal rat ventricular myocytes. Involvement of Rho kinase. J Biol Chem. 1998;273:7725–7730. doi: 10.1074/jbc.273.13.7725. [DOI] [PubMed] [Google Scholar]
- 34.Iwaki K, Sukhatme V P, Shubeita H E, Chien K R. α- and β-adrenergic stimulation induces distinct patterns of immediate early gene expression in neonatal rat myocardial cells. fos/jun expression is associated with sarcomere assembly; Egr-1 induction is primarily an α1-mediated response. J Biol Chem. 1990;265:13809–13817. [PubMed] [Google Scholar]
- 35.Joneson T, Bar-Sagi D. Suppression of Ras-induced apoptosis by the Rac GTPase. Mol Cell Biol. 1999;19:5892–5901. doi: 10.1128/mcb.19.9.5892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Joneson T, McDonough M, Bar-Sagi D, van Aelst L. RAC regulation of actin polymerization and proliferation by a pathway distinct from Jun kinase. Science. 1996;274:1374–1376. doi: 10.1126/science.274.5291.1374. [DOI] [PubMed] [Google Scholar]
- 37.King A J, Sum H, Diaz B, Barnard D, Miao W, Bagrodia S, Marshall M S. The protein kinase Pak3 positively regulates Raf-1 activity through phosphorylation of serine 338. Nature. 1998;396:180–183. doi: 10.1038/24184. [DOI] [PubMed] [Google Scholar]
- 38.Kiyono M, Satoh T, Kaziro Y. G protein βγ subunit-dependent Rac-guanine nucleotide exchange activity of Ras-GRF1/CDC25Mm. Proc Natl Acad Sci USA. 1999;96:4826–4831. doi: 10.1073/pnas.96.9.4826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Knowlton K U, Baracchini E, Ross R S, Harris A N, Henderson S A, Evans S M, Glembotski C C, Chien K R. Co-regulation of the atrial natriuretic factor and cardiac myosin light chain-2 genes during α-adrenergic stimulation of neonatal rat ventricular cells. Identification of cis sequences within an embryonic and a constitutive contractile protein gene which mediate inducible expression. J Biol Chem. 1991;266:7759–7768. [PubMed] [Google Scholar]
- 40.Mackay D J G, Hall A. Rho GTPases. J Biol Chem. 1998;273:20685–20688. doi: 10.1074/jbc.273.33.20685. [DOI] [PubMed] [Google Scholar]
- 41.Mason C S, Springer C J, Cooper R G, Superti-Furga G, Marshall C J, Marais R. Serine and tyrosine phosphorylations cooperate in Raf-1, but not B-Raf activation. EMBO J. 1999;18:2137–2148. doi: 10.1093/emboj/18.8.2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mattingly R R, Macara I G. Phosphorylation-dependent activation of the Ras.GRF/CDC25Mm exchange factor by muscarinic receptors and G protein βγ subunits. Nature. 1996;382:268–272. doi: 10.1038/382268a0. [DOI] [PubMed] [Google Scholar]
- 43.Minden A, Lin A, Claret F-X, Abo A, Karin M. Selective activation of the JNK signaling cascade and c-Jun transcriptional activity by the small GTPases Rac and Cdc42Hs. Cell. 1995;81:1147–1157. doi: 10.1016/s0092-8674(05)80019-4. [DOI] [PubMed] [Google Scholar]
- 44.Moriguchi T, Kawasaki H, Matsuda S, Gotoh Y, Nishida E. Evidence for multiple activators for stress-activated protein kinases/c-Jun amino-terminal kinases. Existence of novel activators. J Biol Chem. 1995;270:12969–12972. doi: 10.1074/jbc.270.22.12969. [DOI] [PubMed] [Google Scholar]
- 45.Nimnual A S, Yatsula B A, Bar-Sagi D. Coupling of Ras and Rac guanosine triphosphatases through the Ras exchanger Sos. Science. 1998;279:560–563. doi: 10.1126/science.279.5350.560. [DOI] [PubMed] [Google Scholar]
- 46.Nishida K, Kaziro Y, Satoh T. Anti-apoptotic function of Rac in hematopoietic cells. Oncogene. 1999;18:407–415. doi: 10.1038/sj.onc.1202301. [DOI] [PubMed] [Google Scholar]
- 47.Nobes C D, Hawkins P, Stephens L, Hall A. Activation of the small GTP-binding proteins rho and rac by growth factor receptors. J Cell Sci. 1995;108:225–233. doi: 10.1242/jcs.108.1.225. [DOI] [PubMed] [Google Scholar]
- 48.Pracyk J B, Tanaka K, Hegland D D, Kim K-S, Sethi R, Rovira I I, Blazina D R, Lee L, Bruder J T, Kovesdi I, Goldshmidt-Clermont P J, Irani K, Finkel T. A requirement for the rac1 GTPase in the signal transduction pathway leading to cardiac myocyte hypertrophy. J Clin Investig. 1998;102:929–937. doi: 10.1172/JCI2552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sah V P, Hoshijima M, Chien K R, Brown J H. Rho is required for Gαq and α1-adrenergic receptor signaling in cardiomyocytes. Dissociation of Ras and Rho pathways. J Biol Chem. 1996;271:31185–31190. doi: 10.1074/jbc.271.49.31185. [DOI] [PubMed] [Google Scholar]
- 50.Sander E E, van Delft S, ten Klooster J P, Reid T, van der Kammen R A, Michiels F, Collard J G. Matrix-dependent Tiam/Rac1 signaling in epithelial cells promotes either cell-cell adhesion or cell migration and is regulated by phosphatidylinositol 3-kinase. J Cell Biol. 1998;143:1385–1398. doi: 10.1083/jcb.143.5.1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Scita G, Nordstrom J, Carbone R, Tenca P, Giardina G, Gutkind S, Bjarnegard M, Betsholtz C, Di Fiore P P. EPS8 and E3B1 transduce signals from Ras to Rac. Nature. 1999;401:290–293. doi: 10.1038/45822. [DOI] [PubMed] [Google Scholar]
- 52.Sehr P, Joseph G, Genth H, Just I, Pick E, Aktories K. Glucosylation and ADP ribosylation of rho proteins: effects on nucleotide binding, GTPase activity, and effector coupling. Biochemistry. 1998;37:5296–5304. doi: 10.1021/bi972592c. [DOI] [PubMed] [Google Scholar]
- 53.Sprenkle A B, Murray S F, Glembotski C C. Involvement of multiple cis elements in basal and α-adrenergic agonist-inducible atrial natriuretic factor transcription. Roles for serum response elements and an SP-1-like element. Circ Res. 1995;77:1060–1069. doi: 10.1161/01.res.77.6.1060. [DOI] [PubMed] [Google Scholar]
- 54.Sugden P H. Signaling in myocardial hypertrophy: life after calcineurin? Circ Res. 1999;84:633–646. doi: 10.1161/01.res.84.6.633. [DOI] [PubMed] [Google Scholar]
- 55.Sugden P H, Clerk A. Cellular mechanisms of cardiac hypertrophy. J Mol Med. 1998;76:725–746. doi: 10.1007/s001090050275. [DOI] [PubMed] [Google Scholar]
- 56.Sugden P H, Clerk A. “Stress-responsive” mitogen-activated protein kinases in the myocardium. Circ Res. 1998;83:345–352. doi: 10.1161/01.res.83.4.345. [DOI] [PubMed] [Google Scholar]
- 57.Tang Y, Yu J, Field J. Signals from the Ras, Rac, and Rho GTPases converge on the Pak protein kinase in Rat-1 fibroblasts. Mol Cell Biol. 1999;19:1881–1891. doi: 10.1128/mcb.19.3.1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Thorburn J, Frost J A, Thorburn A. Mitogen-activated protein kinases mediate changes in gene expression, but not cytoskeletal organization associated with cardiac muscle hypertrophy. J Cell Biol. 1994;126:1565–1572. doi: 10.1083/jcb.126.6.1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Thorburn J, McMahon M, Thorburn A. Raf-1 kinase activity is necessary and sufficient for gene expression changes but not sufficient for cellular morphology changes associated with cardiac myocyte hypertrophy. J Biol Chem. 1994;269:30580–30586. [PubMed] [Google Scholar]
- 60.Thorburn J, Xu S, Thorburn A. MAP kinase and Rho-dependent signals interact to regulate gene expression but not actin morphology in cardiac muscle cells. EMBO J. 1997;16:1888–1900. doi: 10.1093/emboj/16.8.1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.van Leeuwen F N, van Delft S, Kain H E, van der Kammen R, Collard J G. Rac regulates phosphorylation of the myosin-II heavy chain, actinomyosin disassembly and cell spreading. Nat Cell Biol. 1999;1:242–248. doi: 10.1038/12068. [DOI] [PubMed] [Google Scholar]
- 62.Vojtek A B, Der C J. Increasing complexity of the Ras signaling pathway. J Biol Chem. 1998;273:19925–19928. doi: 10.1074/jbc.273.32.19925. [DOI] [PubMed] [Google Scholar]
- 63.Yue T L, Gu J-L, Wang C, Reith A D, Lee J C, Mirabile R C, Kreutz R, Wang Y, Maleeff B, Parsons A A, Ohlstein E H. Extracellular signal-regulated kinase plays an essential role in hypertrophic agonists, endothelin-1 and phenylephrine-induced cardiomyocyte hypertrophy. J Biol Chem. 2000;275:37895–37901. doi: 10.1074/jbc.M007037200. [DOI] [PubMed] [Google Scholar]