Abstract
In this era of sporadic advancement in science and technology, a substantial amount of intervention is being set in motion to reduce health-related diseases. Discoveries from researchers have pinpointed the usefulness of heterocyclic compounds, amongst which benzothiazepine (BTZ) derivatives have been synthesized for their various pharmacological activities. This also contributes to their undeniable application in therapeutic medicine for the development of efficacious drugs. BTZs are compounds with a benzene ring fused with a thiazepine ring. This work contains several methods that have been used to synthesize 1,3-, 1,4-, 1,5-, and 4–1-benzothiazepine derivatives. In addition, up-to-date information about the crucial pharmacological activities of BTZ derivatives has been reviewed in this present study to appreciate their druggable potential in therapeutic medicine for drug development. Drug design and development have further been simplified with the implementation of computer aided approaches to predict biological interactions which can help in the design of several derivatives. Hence, the structural activity relationship (SAR), ADMET and the molecular docking studies of BTZ derivatives were discussed to further establish their interactions and safety in biological systems. This present work aims to expound on the reported chemistry and pharmacological propensity of BTZ moiety in relation to other relevant moieties to validate their potential as excellent pharmacophores in drug design and development.
Keywords: ADMET, Drug design, Heterocyclic compounds, Medicinal properties, Molecular docking, Synthesis
Graphical Abstact

1. Introduction
Several organic compounds have been synthesized with the sole aim of developing a novel alternative that can help to treat various diseases.1–3 The search for new alternatives is not just important but is a necessity as the problem of drug resistance becomes more prominent. Due to abuse, incomplete dosage, and application of antibiotics on agricultural produce over a long duration, some microbes become resistant after much exposure as a result of gene mutation.4,5 Over the years, some of the research carried out has shown the rise of different drug-resistant species like P. falciparum in malaria, Candida auris in fungal infection, Staphylococcus aureus, Klebsiella pneumonia in bacterial infections, Mycobacterium tuberculosis strains in tuberculosis, and strain of N. gonorrhoeae in gonorrhea.6–9 The level of resistance from these microbes has further increased the mortality rate of infants and adults. Malaria, Tuberculosis, and diarrhea are part of the causes of death worldwide this past year. It was estimated in 2018 that about 1.5 million people died as a result of tuberculosis and in 2020 about 627 000 people died due malaria worldwide,10–12 while in 2016 about 1.6 million deaths were recorded for diarrheal disease globally.13,14
It has been established that heterocyclics possess properties that make them relevant in drug design and synthesis.2,15–17 More so, the presence of different heteroatoms within the heterocycle confers unique properties on them. The common heteroatoms include sulphur, nitrogen, oxygen, and phosphorus (S, N, O, P respectively) but are not limited to this.18–20 Amongst the numerous heterocyclic scaffolds, those containing nitrogen, and sulphur molecules have gained attention as they are applied for their medicinal properties. They continue to remain relevant not only in medicinal chemistry but in pharmaceuticals, agriculture, and industry.2,3,19 Some of these heterocyclic compounds are applied in medicine and relevant fields such as antibiotics, alkaloids, vitamins, amino acids, hemoglobin, and pigments. Benzothiazepine is a thiazepine derivative that contains a benzene ring and a seven-membered heterocyclic scaffold that is fused.21–24 Benzothiazepines are classified under calcium channel blockers as they are used as cardiovascular agents which are useful in the treatment of hypertension. Various classes of benzothiazepine exist namely: 1,2-, 1,3-, 1,4-, 1,5-, and 4,1-benzothiazepines based on the positions of their heteroatoms (sulphur and nitrogen) on the thiazepine ring.25–27 More so, benzothiazepines (i) can also exist as a dihydrobenzothiazepine (ii) or tetrahydrobenzothiazepine (iii) when the thiazepine ring is deficient in one or two double bonds (Figure 1).26
Figure 1:

Different forms of the benzothiazepine ring
Over time, the radar has moved from the synthesis of benzothiazepine moiety to the synthesis of substituted benzothiazepine. This substituted benzothiazepine are mainly achieved by incorporating functional groups or moieties with known activity to further increase the overall effectiveness when applied. They are usually solids observed as white, yellow, or green in colour when synthesized. The therapeutic functions of benzothiazepine and its derivatives have made them a potential anticancer, antidiabetic, anti-inflammatory, antiviral agent, antimalarial agent, CNS depressant, antiplatelet aggregator, anti-cholinesterases inhibitor, anticonvulsant agent, Ca2+ channel antagonist, and vasodilator.25,26,28 They are also applicable as corrosion inhibitors,29 and antiviral agents in agriculture.17 Some of the common examples include diltiazem, tianeptine, thiazesim, quetiapine, and clothiapine. This review aims to expound on the reported chemistry and pharmacological propensity of benzothiazepine derivatives to validate their potential as excellent pharmacophores in drug design and development.
2. Chemistry
2.1. Synthesis of Benzothiazepine derivatives
Several methods have been employed in the synthesis of benzothiazepine derivatives which are evolving from conventional methods to greener methods that uses mild reaction conditions and green solvents or exclude solvents. One or more spectroscopic method (1H and 13C-NMR, FT-IR, Ultraviolet-Visible (UV-Vis), X-ray crystallography and Mass Spectroscopy) were used in ascertaining the purity and formation of the synthesized compounds.30,31
2.2.1. Heterogeneous catalysis
(a) In the synthesis of 1,4-benzothiazepine derivatives (4), coumarin derivatives (1), 2-aminothiophenol (2), and alkyl isocyanides (3) were reacted together using a nano-biocatalyst (NBC) made from a lipase extracted from Aspergillus niger bonded on Fe3O4 nanoparticle (Table 1). The appropriate catalyst was selected by employing different catalyst and varying the amount used. The overall product yield for the reaction was 95% (Table 1). The biocatalyst was preferred because it affords a large suface area for the reaction to be carried out upon and its easily recoverable using magnet.32
Table 1:
Heterogeneous catalyzed reaction for 1,4-benzothiazepine derivative

| |||||
|---|---|---|---|---|---|
| Entry | Catalyst | Catalyst amount (g) | Time (h) | Yield of 8 (%) | Yield of 13 (%) |
| 1 | Baker’s yeast | 0.5 | 24 | 20 | 25 |
| 2 | Fe3O4 | 0.025 | 24 | 30 | 45 |
| 3 | Free lipase (ANL) | 0.1 | 5 | 65 | 75 |
| 4 | Fe3O4 NPs@lipase | 0.01 | 1 | 60 | 60 |
| 5 | Fe3O4 NPs@lipase | 0.015 | 1 | 72 | 75 |
| 6 | Fe3O4 NPs@lipase | 0.025 | 1 | 95 | 95 |
| 7 | Fe3O4 NPs@lipase | 0.030 | 1 | 95 | 95 |
| 8 | Fe3O4 NPs@lipase | 0.04 | 1 | 95 | 95 |
(b) Fang and co-workers reported the reaction between α-bromoenal (5) and 2-aminobenzenethiol (6) in the presence of an organocatalyst (7e) which gave an enantioselective N-H- free 1,5-benzothiazepine derivative (8) (Table 2). This synthesis proceeded through a one-step reaction unlike the common methods with two steps. The catalyst and solvents were varied to afford the optimal conditions for the reaction with a 63% yield and 94% entantiomeric excess (ee) (Table 2). The product (8) had a R configuration which was influenced by the stereochemistry of compound (5) and the product was further explored for the synthesis of (R)-thiazesim which is an antidepressant.33
Table 2:
Asymmetric synthesis of 1,5-benzothiazepines derivative

| |||||
|---|---|---|---|---|---|
| Entry | Catalyst | Solvent | Base | Yield (%)a | ee (%)b |
| 1 | 7a | PhMe | NaOAc | 40 | 78 |
| 2 | 7b | PhMe | NaOAc | 22 | 90 |
| 3 | 7c | PhMe | NaOAc | 18 | 79 |
| 4 | 7d | PhMe | NaOAc | trace | - |
| 5 | 7e | PhMe | NaOAc | 43 | 94 |
| 6 | 7e | Mesitylene | NaOAc | 30 | 92 |
| 7 | 7e | DCM | NaOAc | trace | - |
| 8 | 7e | THF | NaOAc | trace | - |
| 9 | 7e | PhMe | DIPEA | trace | - |
| 10 | 7e | PhMe | TBuOK | trace | - |
| 11 | 7e | PhMe | CsOAc | 37 | 93 |
| 12 | 7e | PhMe | LiOAc | 31 | 93 |
| 13 c | 7e | PhMe | NaOAc | 63 | 94 |
Isolated yields based on 5.
Ee values were determined by HPLC analysis.
20 mol % 7e was used.
Mes = 2,4,6-(CH3)3C6H2; ee = entantiomeric excess; DIPEA = N,N-diisopropylethylamine.
(c) In other studies, Meninno and co-workers synthesized 1,5-benzothiazepine derivatives (11) from α,β-unsaturated N-acyl pyrazoles (9), and 2-aminothiophenol (10) using a minute quantity of takemoto’s catalyst (VII) (Table 3). The reaction was regioselective, this was made possible by the effectiveness of compound (9). After optimization, 97% yield with 80% ee was reported and the product in this reaction can also be employed as a precursor for synthesizing (R)-thiazesim.34
Table 3:
Asymmetric synthesis of 1,5-benzothiazepine scaffold

| |||||
|---|---|---|---|---|---|
| Entry | Catalyst | T [°C]/Additive | t1(t2) [h] | Yield [%]a | ee [%]b |
| 1 | I | rt | 92 | 85 | 52 |
| 2 | I | 40 °C | 14(11) | 81 | 66 |
| 3 | I | rt/silica gel | 1.5(4) | 82 | 76 |
| 4 | - | rt | 72 | 30 | - |
| 5 | - | rt/silica gel | 5 | 85 | - |
| 6 | II | rt/silica gel | 1.5(4) | 85 | −61 |
| 7 | III | rt/silica gel | 1.5(4) | 81 | −61 |
| 8 | IV | rt/silica gel | 2(4) | 80 | −81 |
| 9 | V | rt/silica gel | 2.5(4) | 75 | −76 |
| 10 | VI | rt/silica gel | 14(4) | 95 | −64 |
| 11 | VII | rt/silica gel | 0.5(4) | 97 | −80 |
| 12 | VIII | rt/silica gel | 4.5(4) | 71 | 66 |
Determined by 1H NMR spectroscopy using 1,3,5-(MeO)3C6H3 as an internal standard.
Determined by HPLC on a chiral stationary phase. Negative sign indicates the enantiomeric excess (ee) for the opposite enantiomer.
2.2.2. Microwave-aided approach
(a) Kendre and co-workers described the synthesis of 1,4-benzothiazepine analog (14) in the presence of acetic acid in water by microwave irradiation using 2-aminothiophenol and 1,3-diketone (12) with N,N-dimethylformamide dimethylacetal (13) (Scheme 1). The product yield was 85%, and it showed better activity against Bacillus subtilis, Pseudomonas aeruginosa, than when the thiazepine ring was replaced with an oxazepine/diazepine ring.35
Scheme 1:

Microwave synthesis of 1,4 benzothiazepine derivative
(b) In another microwave synthesized reaction between cycloheptanone derivative (15) and oaminothiophenol (16) using AcOH as a catalyst for 20 minutes, a 1,4-benzothiazepine analog (17) was derived (Scheme 2). The reaction was carried out under thermal heat and microwave heat. It was observed that the microwave method was more promising with a shorter reaction time of 20 mins and a higher yield of 89% as compared to the thermal method with 5 hours reaction time and 73% yield.36
Scheme 2:

Microwave synthesis of 1,4-benzothiazepine derivative
(c) Therapeutically active compounds from 1,5-benzothiazepine scaffold (19) and (20) were synthesized from 6-oxocholest-4-ene (18) and 2-aminobenzenethiol under microwave irradiation (Scheme 3). Density functional theory was employed to determine the theoretical spectroscopic results for the products and when compared to the experimental results it was similar making it easy to design compounds similar to the precursor.37
Scheme 3:

Microwave synthesis of 1,5-benzothiazepine
2.2.3. Ultrasound-aided synthesis
Amongst the green methods of synthesis, the use of ultrasound is gaining recognition as it offers a short reaction time with a better product yield and purity when compared to conventional methods.
(a) A 1,5-benzothiazepine derivatives (23a-i) were synthesized by Pawar et al., (2018) using a combination of 2-aminothiophenol (22) and substituted chalcones (21) with ferrous sulphate as a catalyst at 40 °C (Scheme 4). The various substituted benzothiazepine derived had varied yields and reaction times with p-methyl benzaldehyde (d) giving an excellent yield.38
Scheme 4:

Ultrasonic synthesis of 1,5-benzothiazepine
(b) Also, Ghodke and co-workers prepared twelve novel 1,5-benzothiazepine derivatives (26j-u) by dissolving chalcones (24) and 2-aminobenzenethiol (25) into dimethyl formamide (DMF) at room temperature under ultrasonic irradiation (Scheme 5). The chalcones were made from aldehydes and different ketones while zirconium oxychloride was employed as the catalyst and the yield ranged from 60–90% for various derivatives.31
Scheme 5:

Ultrasonic synthesis of 2,4-substituted 1,5-benzothiazepine
(c) Similarly, the synthesis of 1,5-benzothiazepine derivatives (29v-b’) were carried out by condensing a series of chalcones (27) on 2-aminobenzenethiol (28) using different solvents under ultrasound irradiation and PEG-400 performed better with excellent yield (Scheme 6). The reaction proceeded at room temperature with a yield ranging from 86–93% for the various derivatives.39
Scheme 6:

Ultrasonic synthesis of 1,5-benzothiazepine
2.2.4. Solvent-free system
(a) Farghaly and co-workers synthesized new 1,5-benzothiazepine derivatives (32a-f) from 1,3-diaryl-2-propenones (30a-f) and 2-amino-thiophenol (31) under a solvent-free system employing H-ferrierite zeolite as catalyst (Scheme 7). The final stage involved a ring closure with the elimination of water and the product was of high purity as well as good yield.30
Scheme 7:

Solvent-free synthesis of 1,5-benzothiazepine derivatives
(b) The synthesis of 2, 4-substituted-1,5-benzothiazepine (35) was achieved by reacting 2-amino-thiophenol (33) with 1, 3-substituted- prop-2-en-1-one (34) using a catalytic amount of zinc acetate (Scheme 8). The mixture was then placed in the microwave for 2–3 mins to afford a yield of 60–88% for the various derivatives.40
Scheme 8:

Synthesis of 1,5-benzothiazepine derivatives
2.2.5. Combinatorial method
(a) An interesting combination of 2-acetylbenzofuranes (38) and different benzaldehydes (39) yielded α,β-unsaturated carbonyl compounds (40). This intermediate further reacted with ATP through thia-Michael addition and cyclo condensation reaction to give 22 substituted 1,5-benzothiazepine derivatives with a benzofuran part (41) (Scheme 9). It was also observed that the benzofuran moiety increased the pharmacological properties of the synthesized compound. The intermediate (38) was a product of a ring closure reaction of chloroacetone (37) and salicylaldehyde (36). Several reaction steps gave different compounds with their various derivatives with yields ranging from 60–90%.41
Scheme 9:

Combinatorial synthetic route for 1,5-benzothiazepine derivatives
2.2.6. Synthesis via intramolecular cyclization
(a) The formation of 1,3-benzothiazepine derivative (44) was achieved by reacting diphenylphosphoryl thioamide (42) and fluoro-hydroxyl(phenyl)iodotosylate (4-F-HTIB) (43) under room temperature at a very short reaction time (Scheme 10). The reaction proceeded through direct intra-molecular dehydrogenative C−S bond formation to give a product yield of 85%.27
Scheme 10:

Synthesis of 1,3-Benzothiazepines derivative
(b) A new compound with a 4,1-benzothiazepine template (48) was synthesized using Boc-protected p-chloroaniline (45) which underwent ortho-lithiation to give an intermediate (46) which was further reacted with (47) in an intermolecular nucleophilic reaction (Scheme 11).42
Scheme 11:

Synthesis of 4,1-Benzothiazepines derivative
2.2.7. Complex catalysized reaction
(a) Yin and co-workers carried out asymmetric hydrogenation using Rh/Zhaophos complex as the catalyst to synthesize 1,5-benzothiazepines (50a-o) from a sulpur-coordinated medium-sized ring of unsaturated NH lactams (49) (Scheme 12). When the activity of the sulphur atoms was compared to bisphosphine ligand which is commercially used it was discovered that the sulfur atoms did not cause inhibition.43
Scheme 12:

Rh-catalyzed reaction route of 1,5-Benzothiazepine derivatives
(b) Wen and co-workers successfully synthesized 1,2-benzothiazepine 1-oxides (53) from phenyl vinyl ketone (52) and [4 + 3] cyclization of S-methyl-S-phenylsulfoximine (51) employing rhodium as a catalyst to give 89% product yield (Scheme 13).44
Scheme 13:

Synthesis of the 1,2-Benzothiazepine 1-Oxides derivative using Rh-catalyst
2.2.8. Other synthesis routes
(a) Li and co-workers reported the synthesis of pyridine incorporated into 1,5-benzothiazepine derivatives (58a-z) through a three-step 1,3-dipolar cycloaddition reaction. The first intermediate (55a-z) was derived by reacting aromatic aldehyde and hydroxyacetophenone (54), then it was further reacted with 2-aminothiophenol to produce the second intermediate (56a-z). The second intermediate was finally reacted with a pyridine moiety (57) to give the final product (Scheme 14).17
Scheme 14:

A 1,3 dipolar cycloaddition synthetic route for 1,5-benzothiazepine derivatives
(b) In another reaction, Afzal and co-workers synthesized a chloropyrazine conjugated 1,5-benzothiazepine derivatives (62a-t) by first preparing a chalcone intermediate (61) from chloropyrazine-based chalcones (59) and heteroaryl aldehyde (60). The intermediates (61a-t) were then reacted with 2-aminothiophenol under given conditions to give products with good yields (Scheme 15).16
Scheme 15:

Synthetic route for chloropyrazine conjugated 1,5-benzothiazepine derivatives
(c) Lokeshwari and co-workers reported the synthesis of furan attached to 1,4 benzothiazepine ring (66a-h) from chalcones intermediates (65a-h) and 2-aminobenzenethiol in acidic conditions (Scheme 16). The chalcones were produced from 1-(furan-2-yl)ethanone (63), and various aromatic aldehydes (64a-h).15
Scheme 16:

Synthetic route for furan attached to 1,4 benzothiazepine ring
3. Pharmacology properties of Benzothiazepine Scaffolds
3.1. Biological activities
3.1.1. Antitumor activity
A synthesized 1,4-benzothiazepine derivative (67) was screened and results showed a level of inhibition on the cancer cell lines at IC50 = 1.48 μM for HeLa, 1.94 μM for A549, 1.52 μM for HT29, and 1.47 μM for MCF-7 when compared to Colchicine. It was also preferred due to its low cytotoxicity22. The anti-tumor activity of dioxoles conjugated 1,4-benzothiazepines was described and results showed that compound (68) exhibited strong activity with IC50 = 8.23 mM and 16.22 mM against the two human cancer cells Ec9706 and Eca109 respectively, better than 5-Fluorouracil with IC50 of 23.26.45
3.1.2. Anti-malarial activity
In search of a better alternative for the treatment of malaria which has increased the mortality rate in children and adults, the results from discoveries have been reported. A carbazole-based 1,4-benzothiazepine (69) had inhibitory activity on the Plasmodium falciparum strain with an IC50 of 0.75 μg/mL.46
3.1.3. Antibacterial activity
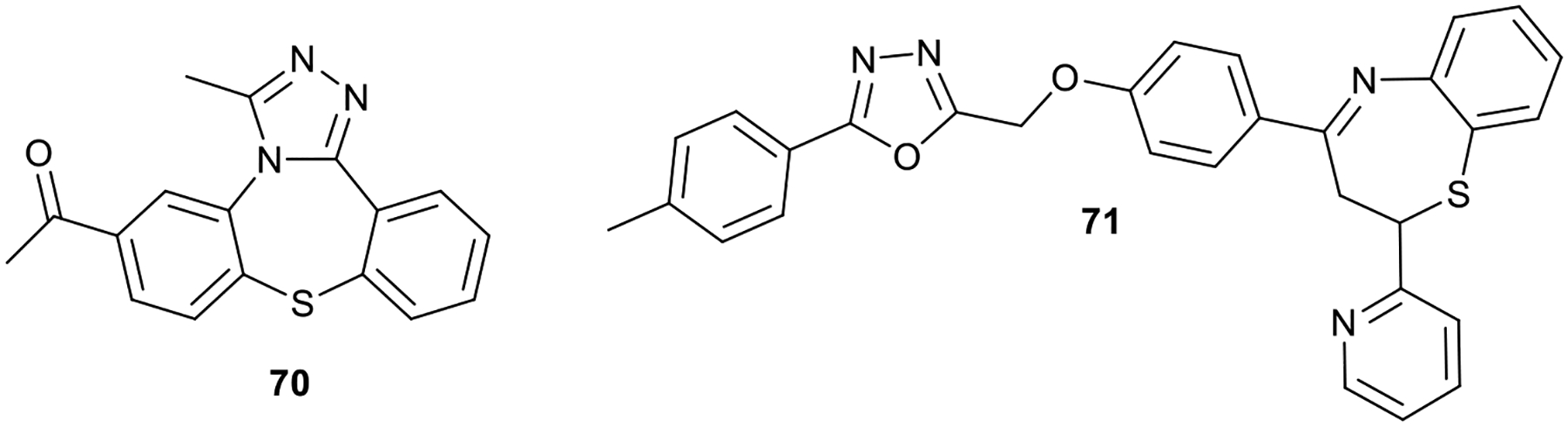
The efficacy of benzothiazepine in inhibiting bacterial strain is no longer farfetched. Several studies have reported the potency of benzothiazepine in inhibiting the growth of microbes. Studies showed that triazolo[1,5]benzothiazepine derivative (70) exhibited notable inhibitory properties against Enterobacter cloacae, Escherichia coli, and Staphylococcus aureus.47 The antimicrobial activity of 1,4-benzothiazepine containing 1,3,4-oxadiazoles moiety (71) was found to inhibit the growth of Pseudomonas aeruginosa at MIC = 62.5 μg/mL.48
3.1.4. Antifungal activity
The antifungal property of 1,4-benzothiazepine derivatives (72) bearing an aryl sulfonate moiety was tested against Aspergillus flavus, Aspergillus niger, and Fusarium oxysporium amongst other synthesized compounds and was found to have the highest zone of inhibition of 18, 22, 18 mm respectively.35 Several synthesized 2,3/2,5-dihydro-1,5-benzothiazepines (73) were tested against Candida albicans, and from observations, the 2,5-dihydro-1,5-benzothiazepines derivatives were twice better in activity than the reference drug used.49 Also, it was reported that a 1,5-benzothiazepine (74) containing benzofuran moiety possessed a strong activity against Aspergillus brasiliensis and A. niger with an inhibition zone of 20 and 23 mm respectively as compared to the standard drug.41
3.1.5. Anti-cancer activity
In recent times, research efforts have been made to discover novel drugs for cancer treatment. Amongst these findings includes piperazine conjugated 1,4 benzothiazepine derivatives (75) reported to inhibit the growth of colon carcinoma cells, leukemia cells, and breast cancer cells at IC50 of 10, 12, 39 μM. A proposed mechanism will be through the inhibition of sirtuin protein.50 Similarly, a 1,5-Benzothiazepine derivative (76) was effective against MCF-7, DU-145 and HT-29 cell lines with IC50 27 μg/mL, 16 μg/mL and 28 μg/mL.51
3.1.6. Anti-protozoal activity
Studies have shown that 1,5-benzothiazepine derivatives make impressive scaffolds for diverse compounds. The amoebicidal activity of a synthesized ferrocenylthiazepine derivative (77) was investigated using trophozoites of the strain HM1: IMSS. The compound was found to have an IC50 = 7.5 μM while that of metronidazole which is mostly recommended for humans had IC50 = 6.8 μM.50 A 1,4-benzothiazepine derivative (78) was tested against Giardia lamblia which causes diarrheal disease in humans and it was inhibited with an IC50 = 2.74 μg/mL.48
3.1.7. Anti-diabetic activity
Efforts to reduce elevated blood glucose levels as further propelled researchers in this field to source effective alternatives. From recent research, 1,4-benzothiazepine derivative (79) displayed impressive activity comparable to metformin and CGP37157 and the activity was traced to the electronegative substitution of benzothiazepine nucleus at the 7’ position and that phenyl ring at the 2’ position.28
3.1.8. Antioxidant activity
Antioxidants help to inhibit oxidation or scavenge free radicals like oxygen, halogens, hydrogen peroxide, and nitric acid that may cause damage to the cells. Diltiazem (80) was employed in reducing oxidative stress in human umbilicus vein endothelial cells.52
3.1.9. Anti-inflammatory and analgesic activity
Anti-inflammatory agents are important in the body, as they have pain-relieving abilities. A new [1,4] benzothiazepine incorporated furan analogs (81) showed appreciable anti-inflammatory and ATPase inhibitory activities, making it a potential anti-ulcer drug.15
3.1.10. Anti-leishmanial activity
The leishmanicidal activity of oxadiazoles conjugated 1,4-benzothiazepine (82) against Leishmania mexicana was reported and the results (IC50 of 0.65 μg/mL) were comparable to miltefosine.48
3.2. Structure-activity relationship study
The SAR study was done on anti-viral agent 1,5-benzothiazepine derivatives (83) and it was deduced that when the R group was 3-Br-Ph and 3,4-di-Cl-Ph groups it was more effective and has a protective activity than ningnanmycin. While when R was 4-OCH3-Ph, 2-Br-Ph, and 3-OCH3-Ph, the activity was poor. In summary, the electron-withdrawing group present in the phenyl group of the target compound made it highly effective against the tobacco mosaic virus.17
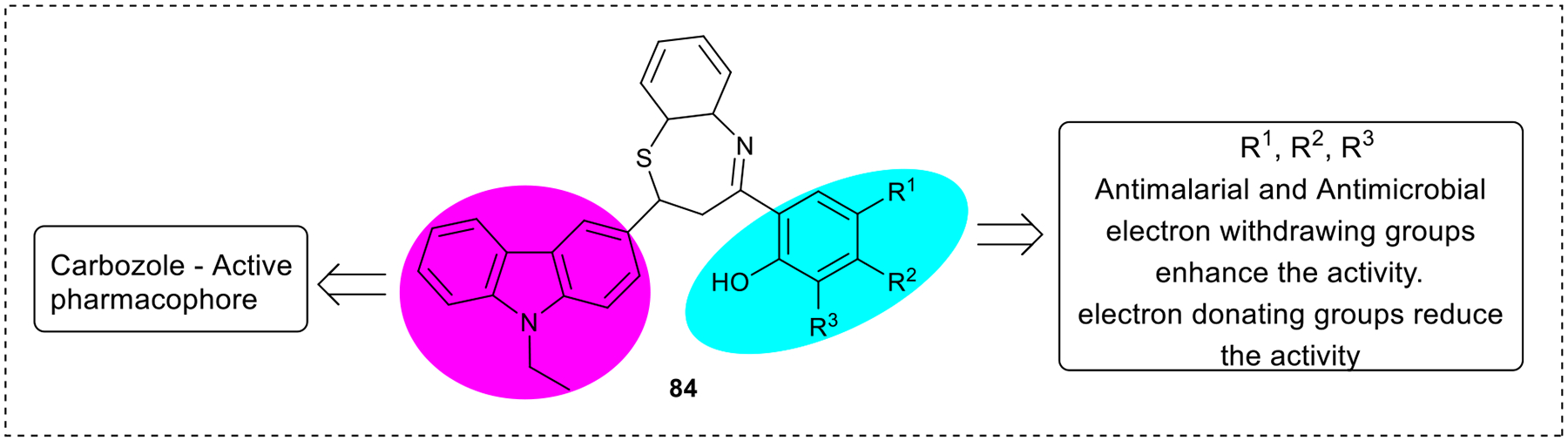
A similar observation was reported by Kadnor & Skelke, (2019) for the antimalarial and antimicrobial activities of the 1,5-benzothiazepine derivatives (84). In the presence of electron-withdrawing groups such as Cl and Br substituents on the aromatic ring, there was an increase in the various activities. Also, the carbazole group attached to the compound increases its antimicrobial activity. In other studies, it was observed that the substitution with electron-withdrawing groups confers stronger activities on the benzothiazepine derivatives than electron-donating groups.16
3.3. ADMET studies on benzothiazepine derivatives
The need to ascertain the safety and drug-likeness of a synthesized compound has made it necessary to carry out several tests to confirm it compatibility as a drug candidate.
In different studies, a novel 1,5 derivative (76) and 1,4-benzothiazepine-2-one derivative (79) were synthesized and tested as an anti-cancer agent51 and anti-diabetic agent.28 The compounds meet the lipinski’s rule of five with molecular weight less than 500, hydrogen bond donor less than 5, and octanol/water partition coefficient logP less than 5. Hence the compounds were easily soluble and effective as a drug candidate. The in silico ADME studies carried out predicted that the compounds have no level of carcinogenicity nor tumorgenicity28,51.
Diltiazem (80) is a calcium blocker that is administered orally in an extended-release and intravenously. It is sold under the brand name Cardizem, absorption takes place in the gastrointestinal tract.52,53 Diltiazem is distributed to the liver, plasma, and intestine for further metabolism to take place. The metabolism of diltiazem takes place in the body when administered orally, it was reported that conversion of the drug to N-demethylation, O-demethylation, and deacetylation takes place in the liver, plasma, and intestine by the appropriate enzymes.53,54 Diltiazem has a plasma elimination half-life ranging from 3–4.5 h for oral and intravenous administration, there might be an increase in half-life if the dose increases or hepatic impairment.53,54 Similarly, extended-release pills if administered once or multiple times might have a half-life between 6–9 h, the drug can be eliminated through the bile and kidney. Clinical tests were conducted for 24 months on rats using a dose of 100 mg/kg per day, reports showed no level of carcinogenicity, no mutagenic response in mammalian cells nor signs of impaired fertility.53,55 Overdose of this drug can cause several diseases like hypotension, bradycardia, heart block, and cardiac failure with signs like lightheadedness and fatigue which can lead to death at its extreme.53 Also, it was observed that there were possibilities of skeletal abnormalities, risk of stillbirths, embryo, and fetal lethality when the diltiazem dose is more than the daily prescribed intake for humans in experimental animals.53
3.4. Molecular modeling and binding study
Docking studies help in the prediction of the binding affinity of drug-like molecules to a specific protein. Some of the advantages include the cost-effectiveness, short time, and little energy required when compared to experimental determined biological activity.
Frimayanti, Nasution, & Etavianti (2021) carried out molecular docking studies of benzothiazepine-chalcone derivatives. It was predicted that compounds 85 and 86 were active inhibitors against helicase of Zika virus protein (PDB ID: 5GJB) with binding affinities of −4.9291 and −4.6490 kcal/mol, respectively. The derivatives were reported to be stable and they formed hydrogen bonds with Glu573, and Arg367, through van der Waals bonds. The compounds had the lowest binding energy making them more stable and efficient.56
In another studies, Prasada and co-workers designed novel 1,5-benzothiazepine derivatives and carried out a docking studies to ascertain their anti-cancer potential. The epidermal growth factor receptor (EGFR) kinase domain was the target protein as it aids the growth of many tumor which includes cancer. Results predicted showed that compound 87 was more active in inhibiting the activity of receptor with binding energy of −9.71 kcal/mol and this resonated with the experimental values done on three cancer cell lines.51
Similarly, the anti-tumor potential of some 1,4-benzothiazepine bearing diaryl derivatives were predicted through the docking studies and using Tubulin (PDB ID: 4O2B) as the target protein. Tubulin gives rise to microtubules through polymerization and this microtubules plays a role in mitosis. Compound 88 was predicted to be a more potent inhibitor for the target and when the experimental tests were carried out on various cancer cell lines it inhibited their growth appreciably.22
4. Conclusion
The various benzothiazepine derivatives reported in this work have proven to be pharmacologically active and efficient which makes them potential drug candidates as various synthetic routes can be employed in synthesizing these molecules. Greener synthetic routes are encouraged alongside optimization of reaction conditions like the substrate, substituent, enzymes, solvent, and temperature to reduce costs, reduce pollution, and save energy. The SAR studies of the benzothiazepine analogs revealed that the positions of the substituents on the analogs had appreciable effects on their activities and this can help to design more active derivatives. The drug-likeness of some the derivatives were also reported to give an insight into their pharmacokinetic properties. Further research can be carried out to discover potential drug candidates with huge benefits that can remedy challenges like drug resistance faced in the pharmaceutical sector.
Figure 2:

Structures of benzothiazepine with anti-tumor properties
Figure 3:

Structures of benzothiazepine with anti-malaria properties
Figure 4:
Structures of benzothiazepine with anti-bacterial properties
Figure 5:

Structures of benzothiazepine with anti-fungal properties
Figure 6:

Structures of benzothiazepine with anti-cancer properties
Figure 7:

Structures of benzothiazepine with anti-protozoal properties
Figure 8:

Structures of benzothiazepine with anti-diabetic properties
Figure 9:

Structures of benzothiazepine with anti-oxidant properties
Figure 10:

Structures of benzothiazepine with anti-anti-inflammatory properties
Figure 11:

Structures of benzothiazepine with anti-leishmanial properties
Figure 12:

SAR of 1,5-benzothiazepine derivatives containing pyridine moiety the antiviral activity
Figure 13:
SAR of carbazole incorporated 1,5-benzothiazepine the antimalarial and antimicrobial activity
Figure 14:

2D ligand interaction of compounds (a) 85 and (b) 86 against helicase of Zika virus protein.56
Figure 15:

2D ligand interaction (c) of compound 87 against EGFR kinase domain.51
Figure 16:

2D ligand interaction (d) of compound 88 against Tubulin.
What does the manuscript add to existing knowledge? (Review highlights).
Identification of various suitable synthetic routes and pharmacological activities of benzothiazepine derivatives,
Considers the SAR studies of active benzothiazepine motifs to further explain their activity,
Bring to fore the ADMET studies for various benzothiazepine derivatives,
Exploration of molecular docking studies as a viable means of predicting pharmacological activity of benzothiazepine motifs
Funding:
This work was supported by the Fogarty National Institutes of Health Common Fund (Grant No: U2RTW010679).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest: The authors declare no potential conflict of interest concerning the research, authorship, and/or publication of this article.
REFERENCES
- 1.Cabrele C, Reiser O. The Modern Face of Synthetic Heterocyclic Chemistry. J Org Chem. 2016;81(21):10109–10125. doi: 10.1021/acs.joc.6b02034 [DOI] [PubMed] [Google Scholar]
- 2.Zlotin SG, Churakov AM, Dalinger IL, et al. Recent advances in synthesis of organic nitrogen–oxygen systems for medicine and materials science. Mendeleev Commun. 2017;27(6):535–546. doi: 10.1016/J.MENCOM.2017.11.001 [DOI] [Google Scholar]
- 3.Yet L. Introduction. In: Privileged Structures in Drug Discovery: Medicinal Chemistry and Synthesis. First. Jonh Wiley & Sons, Inc.; 2018:1–14. [Google Scholar]
- 4.Nsanzabana C, Djalle D, Guérin PJ, Ménard D, González IJ. Tools for surveillance of anti-malarial drug resistance: An assessment of the current landscape. Malar J. 2018;17(1):1–16. doi: 10.1186/s12936-018-2185-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Michael CA, Dominey-Howes D, Labbate M. The Antimicrobial Resistance Crisis: Causes, Consequences, and Management. Front Public Heal. 2014;2(SEP):1–8. doi: 10.3389/fpubh.2014.00145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Olszewska MA, Gędas A, Simões M. Antimicrobial polyphenol-rich extracts : Applications and limitations in the food industry. Food Res Int. 2020;134(August 2019):109214. doi: 10.1016/j.foodres.2020.109214 [DOI] [PubMed] [Google Scholar]
- 7.White NJ. Antimalarial drug resistance. J Clin Invest. 2004;113(8):1084–1092. doi: 10.1172/jci200421682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Farha AK, Yang QQ, Kim G, et al. Inhibition of multidrug-resistant foodborne Staphylococcus aureus biofilms by a natural terpenoid (+)-nootkatone and related molecular mechanism. Food Control. 2020;112(January):107154. doi: 10.1016/j.foodcont.2020.107154 [DOI] [Google Scholar]
- 9.Muthupandian S, Ramachandran B, Barabadi H. The prevalence and drug resistance pattern of extended spectrum β – lactamases (ESBLs) producing Enterobacteriaceae in Africa. Microb Pathog. 2018;114:180–192. doi: 10.1016/j.micpath.2017.11.061 [DOI] [PubMed] [Google Scholar]
- 10.Gebreselassie N, Hutubessy R, Vekemans J, den Boon S, Kasaeva T, Zignol M. The case for assessing the full value of new tuberculosis vaccines. Eur Respir J. 2020;55(3):1902414. doi: 10.1183/13993003.02414-2019 [DOI] [PubMed] [Google Scholar]
- 11.Abbafati C, Abbas KM, Abbasi-Kangevari M, et al. Global burden of 87 risk factors in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. 2020;396(10258):1223–1249. doi: 10.1016/S0140-6736(20)30752-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Monroe A, Williams NA, Ogoma S, Karema C, Okumu F. Reflections on the 2021 World Malaria Report and the future of malaria control. Malar J. 2022;21(1):1–6. doi: 10.1186/s12936-022-04178-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wolf J, Hunter PR, Freeman MC, et al. Impact of drinking water, sanitation and handwashing with soap on childhood diarrhoeal disease: updated meta-analysis and meta-regression. Trop Med Int Heal. 2018;23(5):508–525. doi: 10.1111/tmi.13051 [DOI] [PubMed] [Google Scholar]
- 14.Prüss-Ustün A, Wolf J, Bartram J, et al. Burden of disease from inadequate water, sanitation and hygiene for selected adverse health outcomes: An updated analysis with a focus on low- and middle-income countries. Int J Hyg Environ Health. 2019;222(5):765–777. doi: 10.1016/j.ijheh.2019.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lokeshwari DM, Rekha ND, Srinivasan B, Vivek HK, Kariyappa AK. Design, synthesis of novel furan appended benzothiazepine derivatives and in vitro biological evaluation as potent VRV-PL-8a and H+/K+ ATPase inhibitors. Bioorganic Med Chem Lett. 2017;27(14):3048–3054. doi: 10.1016/j.bmcl.2017.05.059 [DOI] [PubMed] [Google Scholar]
- 16.Shaik AB, Bhandare RR, Nissankararao S, Lokesh BVS, Shahanaaz S, Mukhlesur Rahman M. Synthesis, and biological screening of chloropyrazine conjugated benzothiazepine derivatives as potential antimicrobial, antitubercular and cytotoxic agents. Arab J Chem. 2021;14(2):102915. doi: 10.1016/j.arabjc.2020.102915 [DOI] [Google Scholar]
- 17.Li T, Zhang J, Pan J, Wu Z, Hu D, Song B. Design, synthesis, and antiviral activities of 1,5-benzothiazepine derivatives containing pyridine moiety. Eur J Med Chem. 2017;125:657–662. doi: 10.1016/j.ejmech.2016.09.069 [DOI] [PubMed] [Google Scholar]
- 18.Arya CG, Chandrakanth M, Fabitha K, et al. Coumarin – benzimidazole hybrids: A review on diverse synthetic strategies. Results Chem. 2022;4(September):100631. doi: 10.1016/j.rechem.2022.100631 [DOI] [Google Scholar]
- 19.Hooshmand SE, Halimehjani AZ. Nitroalkenes in diverse synthesis of heterocyclic compounds with two or three heteroatoms: Recent advances. Targets Heterocycl Syst. 2018;22:119–137. doi: 10.17374/targets.2019.22.119 [DOI] [Google Scholar]
- 20.Guha AK, Sarmah S, Phukan AK. Effect of substituents at the heteroatom on the structure and ligating properties of heterocyclic carbene, silylene, germylene and abnormal carbene: A theoretical study. Dalt Trans. 2010;39:7374–7383. doi: 10.1039/c003266b [DOI] [PubMed] [Google Scholar]
- 21.Vatankhah E, Akbarzadeh M, Jabbari A, Saadat K, Shiri A. Synthesis and Characterization of Various Novel Derivatives of Dipyrimido[4,5-b:4’,5’-e][1,4]thiazepine and Their Theoretical Evaluation as 15-Lipoxygenase Inhibitor. Polycycl Aromat Compd. 2023;43(1):288–301. doi: 10.1080/10406638.2021.2014536 [DOI] [Google Scholar]
- 22.Wang B, Wang LR, Liu LL, et al. A novel series of benzothiazepine derivatives as tubulin polymerization inhibitors with anti-tumor potency. Bioorg Chem. 2021;108(December 2020):104585. doi: 10.1016/j.bioorg.2020.104585 [DOI] [PubMed] [Google Scholar]
- 23.García-Casas P, Arias-Del-Val J, Alvarez-Illera P, et al. The Neuroprotector Benzothiazepine CGP37157 Extends Lifespan in C. elegans Worms Published online 2019. doi: 10.3389/fnagi.2018.00440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gutteridge CE, Sadowski BW, Hughes SM, et al. Synthesis of Substituted Benzothiazepine Compounds with Medicinal Potential. Int J Org Chem. 2020;10(03):123–134. doi: 10.4236/ijoc.2020.103009 [DOI] [Google Scholar]
- 25.Devi V, Singh G, Monga V. Recent advances in the synthetic chemistry of 1, 5-benzothiazepines : A minireview. J Heterocycl Chem. 2020;57(9):3255–3270. doi: 10.1002/jhet.4062 [DOI] [Google Scholar]
- 26.El-Bayouki KAM. Benzo[1,5]thiazepine: Synthesis, Reactions, Spectroscopy, and Applications. Org Chem Int. 2013;2013:1–71. doi: 10.1155/2013/210474 [DOI] [Google Scholar]
- 27.Guo W si, Gong H, Zhang Y an, Wen L rong, Li M. Fast Construction of 1,3-Benzothiazepines by Direct Intramolecular Dehydrogenative C − S Bond Formation of Thioamides under Metal-Free Conditions. Org Lett. 2018;20(20):6394–6397. doi: 10.1021/acs.orglett.8b02697 [DOI] [PubMed] [Google Scholar]
- 28.Srivastav VK, Tiwari M. Synthesis and evaluation of antihyperglycemic activity of 1,4-benzothiazepine-2-one derivatives in alloxan induced diabetic rats. Indian J Pharm Sci. 2021;83(5):1033–1043. doi: 10.36468/pharmaceutical-sciences.857 [DOI] [Google Scholar]
- 29.Sasikala T, Parameswari K, Chitra S. Synthesis and corrosion inhibition study of benzothiazepine derivatives on mild steel in acid medium. Orient J Chem. 2016;32(2):1215–1222. doi: 10.13005/ojc/320248 [DOI] [Google Scholar]
- 30.Farghaly TA, Hassaneen HME. H-ferrierite zeolite: As an effective and reusable heterogeneous catalyst for synthesis of 1,5-benzothiazepine under solvent free condition and 1,3-dipolar cycloaddition in water. Arab J Chem. 2017;10:S3255–S3262. doi: 10.1016/j.arabjc.2013.12.024 [DOI] [Google Scholar]
- 31.Ghodke M, Wagh S, Nikalje AP, Seijas Vázquez J. Ultrasound assisted synthesis of 2, 4-substituted 1, 5-benzothiazepine derivatives. In: Proceedings of The 23rd International Electronic Conference on Synthetic Organic Chemistry. Vol 3. MDPI; 2019:6704. doi: 10.3390/ecsoc-23-06704 [DOI] [Google Scholar]
- 32.Baharfar R, Mohajer S. Synthesis and Characterization of Immobilized Lipase on Fe3O4 Nanoparticles as Nano biocatalyst for the Synthesis of Benzothiazepine and Spirobenzothiazine Chroman Derivatives. Catal Letters. 2016;146(9):1729–1742. doi: 10.1007/s10562-016-1797-3 [DOI] [Google Scholar]
- 33.Fang C, Lu T, Zhu J, Sun K, Du D. Formal [3 + 4] Annulation of α, β-Unsaturated Acyl Azoliums: Access to Enantioenriched N − H - Free 1,5-Benzothiazepines. Org Lett. 2017;19(13):3470–3473. doi: 10.1021/acs.orglett.7b01457 [DOI] [PubMed] [Google Scholar]
- 34.Meninno S, Volpe C, Lattanzi A. Catalytic Enantioselective Synthesis of Protecting-Group-Free 1, 5-Benzothiazepines. Chem A Eur J. 2017;23(19):4547–4550. doi: 10.1002/chem.201700837 [DOI] [PubMed] [Google Scholar]
- 35.Kendre BV, Landge MG, Bhusare SR. Synthesis and biological evaluation of some novel pyrazole, isoxazole, benzoxazepine, benzothiazepine and benzodiazepine derivatives bearing an aryl sulfonate moiety as antimicrobial and anti-inflammatory agents. Arab J Chem. 2019;12(8):2091–2097. doi: 10.1016/j.arabjc.2015.01.007 [DOI] [Google Scholar]
- 36.Kassem AF, Abbas EMH, Al-Qurashi NT, Farghaly TA. New azoloazine derivatives as antimicrobial agents: Synthesis under microwave irradiations, structure elucidation, and antimicrobial activity. J Heterocycl Chem. 2020;57(2):611–620. doi: 10.1002/jhet.3792 [DOI] [Google Scholar]
- 37.Alam MJ, Khan AU, Alam M, Ahmad S. Spectroscopic (FTIR, FT-Raman, 1H NMR and UV–Vis) and DFT/TD-DFT studies on cholesteno [4,6-b,c]-2′,5′-dihydro-1′,5′-benzothiazepine. J Mol Struct. 2019;1178:570–582. doi: 10.1016/j.molstruc.2018.10.063 [DOI] [Google Scholar]
- 38.Pawar P, Shivankar VS, Gaikwad YA, Patil SS, Wadhawa GC. ferrous-sulphate-catalysed-synthesis-of-1–5benzothiazepines-using-sonication-method. J Biol Chem Chron. 2018;4(2):95–98. https://www.eresearchco.com/articles/ferrous-sulphate-catalysed-synthesis-of-1-5benzothiazepines-using-sonication-method.pdf [Google Scholar]
- 39.Devkate CG, Kola SS, Gaikwad DD, Siddique MIM. Ultrasound Promoted One Pot Synthesis of 1,5-Benzothiazepines Using Polyethylene Glycol (Peg-400). Int Res J Pharm. 2018;9(11):182–185. doi: 10.7897/2230-8407.0911280 [DOI] [Google Scholar]
- 40.Malik RK. Synthesis of Novel 1, 5 - Benzothiazepines As CNS Agents. Acta Cienc Indica. 2017;XLIII(2):235–240. http://www.acta.co.in/acta-bo/files/published_general/5cd904c76cde3-134-C017.pdf [Google Scholar]
- 41.Mostofi M, Mohammadi Ziarani G, Lashgari N. Design, synthesis and biological evaluation of benzofuran appended benzothiazepine derivatives as inhibitors of butyrylcholinesterase and antimicrobial agents. Bioorganic Med Chem. 2018;26(12):3076–3095. doi: 10.1016/j.bmc.2018.02.049 [DOI] [PubMed] [Google Scholar]
- 42.Viejo L, Rubio-Alarcón M, Arribas RL, et al. Synthesis and biological assessment of 4,1-benzothiazepines with neuroprotective activity on the Ca2+ overload for the treatment of neurodegenerative diseases and stroke. Molecules. 2021;26:4473. doi: 10.3390/molecules26154473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yin C, Yang T, Pan Y, Wen J, Zhang X. Rh-Catalyzed Asymmetric Hydrogenation of Unsaturated Medium-Ring NH Lactams: Highly Enantioselective Synthesis of N-Unprotected 2,3-Dihydro-1,5-benzothiazepinones. Org Lett. 2020;22(3):920–923. doi: 10.1021/acs.orglett.9b04478 [DOI] [PubMed] [Google Scholar]
- 44.Wen J, Cheng H, Raabe G, Bolm C. Rhodium-Catalyzed [4 + 3] Annulations of Sulfoximines with α,β-Unsaturated Ketones Leading to 1,2-Benzothiazepine 1-Oxides. Org Lett. 2017;19(21):6020–6023. doi: 10.1021/acs.orglett.7b03106 [DOI] [PubMed] [Google Scholar]
- 45.Wu L, Yang X, Peng Q, Sun G. Synthesis and anti-proliferative activity evaluation of novel benzo[d][1,3] dioxoles-fused 1,4-thiazepines. Eur J Med Chem. 2017;127:599–605. doi: 10.1016/j.ejmech.2017.01.021 [DOI] [PubMed] [Google Scholar]
- 46.Kadnor VA, Shelke SN. Synthesis, antimicrobial and antimalarial activity of 1, 4-benzothiazepine and pyrazoline derivatives incorporating carbazole moiety. Bulg Chem Commun. 2019;51(2):234–241. doi: 10.34049/bcc.51.2.4921 [DOI] [Google Scholar]
- 47.Sharma A, Kishore D, Singh B. An Expedient Method for the Synthesis of 1,2,4-Triazolo-fused 1,5-Benzodiazepine, 1,5-Benzoxazepine, and 1,5-Benzothiazepine Scaffolds: A Novel Seven-membered Ring System of Biological Interest. J Heterocycl Chem. 2018;55(3):586–592. doi: 10.1002/jhet.3060 [DOI] [Google Scholar]
- 48.Navin P, Sarvil P, Amit P, et al. Synthesis and biological evaluation of newer 1,3,4-oxadiazoles incorporated with benzothiazepine and benzodiazepine moieties. Zeitschrift fur Naturforsch - Sect C J Biosci. 2017;72(3–4):133–146. doi: 10.1515/znc-2016-0129 [DOI] [PubMed] [Google Scholar]
- 49.Pant S, Godwal P, Sanju K. Syntheses and Studies of 8-Substituted-2-(2-Chlorophenyl/3-chlorophenyl)-4-(4-hydroxyphenyl/phenyl)-2,3/2,5-dihydro-1,5-benzothiazepines. Asian J Chem. 2021;33(11):2653–2656. doi: 10.14233/ajchem.2021.23369 [DOI] [Google Scholar]
- 50.Sánchez García JJ, Toledano-Magaña Y, Flores-Alamo M, et al. Polycyclic ferrocenyl(dihydro)thiazepine derivatives: Diastereo-selective synthesis, characterization, electrochemical behavior, theoretical and biological investigation. J Inorg Biochem. 2017;166:141–149. doi: 10.1016/j.jinorgbio.2016.09.002 [DOI] [PubMed] [Google Scholar]
- 51.Prasad CMMR, Yejella RP, Rehman S, Rajeswari C, Basha SH. Novel series of 1, 5 Benzothiazepine skeleton based compounds as anti-cancer agents – In silico and MTT assay based study. J PeerScientist. 2018;1(2):1–10. doi: 10.5281/zenodo.3372436 [DOI] [Google Scholar]
- 52.Zhou L, Yang M, Zuo S, et al. Dlitiazem inhibits the oxidative stress induced by angiotensin II through growth hormone secretagogue receptor type 1a in human umbilicus vein endothelial cells. Biomed Pharmacother. 2017;89:76–82. doi: 10.1016/j.biopha.2017.02.022 [DOI] [PubMed] [Google Scholar]
- 53.Rodríguez Padial L, Barón-Esquivias G, Hernández Madrid A, Marzal Martín D, Pallarés-Carratalá V, de la Sierra A. Clinical Experience with Diltiazem in the Treatment of Cardiovascular Diseases. Cardiol Ther. 2016;5(1):75–82. doi: 10.1007/S40119-016-0059-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Manitpisitkul P, Curtin CR, Shalayda K, Wang SS, Ford L, Heald D. Pharmacokinetic interactions between topiramate and diltiazem, hydrochlorothiazide, or propranolol. Clin Pharmacol Drug Dev. 2014;3(5):378–387. doi: 10.1002/cpdd.107 [DOI] [PubMed] [Google Scholar]
- 55.Yan XQ, Chen ZG, Wang RL, et al. Pharmacokinetics of diltiazem hydrochloride delay-onset sustained-release pellet capsules in healthy volunteers. Brazilian J Pharm Sci. 2013;49(1):29–38. doi: 10.1590/S1984-82502013000100004 [DOI] [Google Scholar]
- 56.Frimayanti N, Nasution MR, Etavianti E. Molecular Docking and Molecular Dynamic Simulation of 1, 5-Benzothiazepine Chalcone Derivative Compounds as Potential Inhibitors for Zika Virus Helicase. J Ris Kim. 2021;12(1):44–52. [Google Scholar]


