Abstract
The low-density lipoprotein (LDL) receptor-related protein (LRP) is a multiligand endocytic receptor that belongs to the LDL receptor family. Recently, studies have revealed new roles of LDL receptor family members as transducers of extracellular signals. Our previous studies have demonstrated LRP phosphorylation within its cytoplasmic tail, but the nature of LRP phosphorylation and its potential function was unknown. In the present study using both in vivo and in vitro analysis, we found that LRP phosphorylation is mediated by the cAMP-dependent protein kinase A (PKA). Using site-directed mutagenesis and LRP minireceptor constructs, we further identified the predominant LRP phosphorylation site at serine 76 of its cytoplasmic tail. Finally, we demonstrated that mutations of serine 76, which abolish LRP phosphorylation by PKA, result in a decrease in the initial endocytosis rate of LRP and a lower efficiency in delivery of ligand for degradation. Thus, the role of PKA phosphorylation of LRP in receptor-mediated endocytosis may provide a mechanism by which the endocytic function of LRP can be regulated by external signals.
The low-density lipoprotein (LDL) receptor-related protein (LRP) is a member of the LDL receptor (LDLR) gene family, which also include in mammals: LDLR itself, the very-low-density lipoprotein receptor (VLDLR), megalin–LRP-2, apolipoprotein E receptor-2 (apoER2)–LR8B, and LR11–sorLA-1 (30, 44, 54, 55). LRP is synthesized as a 600-kDa single-chain precursor, which undergoes posttranslational proteolytic processing within the trans-Golgi compartment by the endopeptidase furin (21, 53). This posttranslational processing results in a formation of mature LRP as a noncovalently associated heterodimer, consisting of the extracellular 515-kDa chain and the transmembrane 85-kDa chain (21). The 515-kDa subunit contains all the putative ligand-binding domains including 31 copies of complement-type ligand-binding repeats arranged in four clusters of 2, 8, 10, and 11. In addition, there are 22 copies of the cysteine-rich epidermal growth factor (EGF)-type repeat flanking the ligand-binding clusters (30, 44). The multiple domain structure of LRP provides potential binding sites for many structurally and functionally diverse ligands including apolipoprotein E-lipoproteins, α2-macroglobulin, plasminogen activators, and β-amyloid precursor protein (7, 30, 44). Ligand interactions with LRP can be antagonized by a 39-kDa receptor-associated protein (RAP), a unique LRP ligand frequently used as a tool in the study of ligand-receptor interaction. RAP also functions intracellularly as a molecular chaperone for LRP and facilitates LRP folding and trafficking within the secretory pathway (7). Increasing evidence has shown that LRP plays important roles in lipoprotein remnant catabolism (52), protease regulation (49), cell migration (50, 51), neuronal process outgrowth (23), and the pathogenesis of Alzheimer's disease (29, 48).
Despite extensive studies on the extracellular domains of the LDLR members in ligand binding, information regarding the structural and functional elements within their cytoplasmic tails has just recently begun to emerge. It has been demonstrated that cytoplasmic adaptor proteins, FE65 and mammalian Disabled proteins, interact with the NPXY motifs in the cytoplasmic tails of LRP, LDLR, VLDLR, apoER2, and megalin (24, 40, 46, 47). In addition, it was shown that VLDLR and apoER2 function as obligate components in the Reelin/Disabled-mediated signal transduction during neuronal development (10, 22, 47). More recently, we have demonstrated that the YXXL motif, but not the two NPXY sequences, within the 100-amino-acid cytoplasmic tail of LRP serves as the dominant signal for receptor-mediated endocytosis (33). Potential signaling functions for the lipoprotein receptors have also been suggested from other observations. For example, Goretzki and Mueller (16) have shown that the LRP tail interacts with a GTP-binding protein and that ligand binding to LRP induces cyclic AMP (cAMP)-dependent protein kinase A (PKA) activity. Our previous studies have shown that the cytoplasmic tail of LRP is phosphorylated (8). However, the nature of this phosphorylation and its potential function are unclear. In the present study, we report that LRP phosphorylation is mediated by PKA at residue serine 76 of its cytoplasmic tail and that this phosphorylation contributes to receptor-mediated endocytosis.
MATERIALS AND METHODS
Materials.
Human recombinant RAP was expressed in a glutathione S-transferase (GST) expression vector and isolated as described previously (3). Single-chain urokinase (scuPA) was kindly provided by G. F. Vovis of Collaborative Research (35). Rabbit polyclonal anti-LRP (generated against purified human LRP) and monoclonal antihemagglutinin (anti-HA) antibodies have been described before (39). Goat anti-mouse immunoglobulin-fluorescein isothiocyanate (FITC) was from Becton Dickinson. Quantum Simply Cellular Microbead Standard was from Flow Cytometry Standards Corporation, San Juan, Puerto Rico. Peroxidase labeled anti-mouse antibody and the ECL System were from Amersham Life Science. All tissue culture media, sera, and plastic ware were from Life Technologies, Inc. Immobilon-P transfer membrane was from Millipore. Rainbow molecular weight markers were from Bio-Rad. [35S]cysteine and [32P]orthophosphate were obtained from ICN (Costa Mesa, Calif.). Carrier-free Na125I was purchased from NEN Life Science Products. IODO-GEN was from Pierce. Proteins were iodinated by using the IODO-GEN method as described previously (2). The proteinase inhibitor cocktail Complete was from Boehringer Mannheim. The protein kinase inhibitors genistein, H-89, K-252a, lavendustin A, staurosporine, and PKA inhibitor peptide (PKI) were from Biomol (Plymouth Meeting, Pa.). Forskolin was from Research Biochemicals Internatinal (Natik, Mass.). The catalytic subunit of PKA, protein kinase C (PKC; a mixture of α, β, and γ isoforms), the PKA assay kit, and the PKC assay kit were from Upstate (Lake Placid, N.Y.). The QuickChange site-directed mutagenesis kit was from Strategene.
Cell culture and transfection.
Human glioblastoma U87 cells were cultured in Dulbecco minimum essential medium supplemented with 10% fetal bovine serum, 2 mM l-glutamine, 100 U of penicillin per ml, 100 μg of streptomycin per ml, and 1 mM sodium pyruvate and maintained at 37°C in humidified air containing 5% CO2 (4). The LRP-null Chinese hamster ovary (CHO) cell line (kindly provided by David FitzGerald [see reference 13]) was cultured in Ham's F-12 medium containing 10% fetal bovine serum. For transient transfection, U87 cells were transfected with various plasmids at 40 to 60% confluence using a calcium phosphate precipitation method (38). For each well of six-well plates, 6 μg of DNA for each LRP minireceptor was cotransfected with 8 μg of pcDNA-RAP in a total volume of 4 ml of medium. At 16 h after the start of transfection, cells were washed with medium and cultured continuously for an additional 24 h before being used in experiments. Stable transfection into LRP-null CHO cells was achieved by transfection of 30 μg of plasmid DNA in 10-cm dishes. Stable transfectants were selected using 700 μg of G418 per ml and maintained with 400 μg of G418 per ml.
Construction of LRP minireceptor and site-directed mutagenesis.
The construction of the membrane-containing minireceptor of LRP (see Fig. 3) via PCR was performed essentially as described previously (6, 39). Site-directed mutagenesis was carried out using QuickChange kit from Strategene according to manufactory's instructions. All oligonucleotides were synthesized at Washington University School of Medicine Protein Chemistry Laboratory.
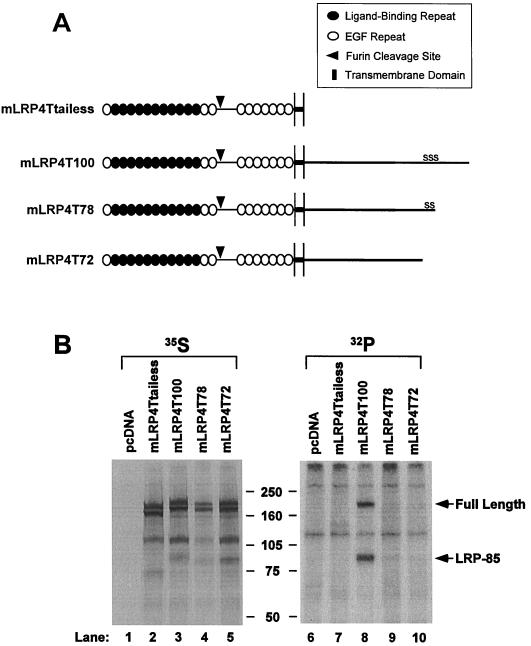
FIG. 3.
Deletion of the three serine residues within the LRP tail eliminates LRP phosphorylation. (A) Schematic representation of the structures of LRP4T100 and its three deletion variants. Note that the lengths of minireceptor tails are not drawn to proper scale compared to their extracellular domains. (B) U87 cells were cotransfected with cDNAs for RAP and pcDNA, mLRP4Ttailess, mLRP4T100, mLRP4T78, or mLRP4T72 and were labeled with [35S]cysteine or [32P]orthophosphate for 4 h. LRP minireceptors were immunoprecipitated with anti-HA antibody and analyzed via SDS–6% PAGE under reducing conditions as described in Materials and Methods. The data represent the results from one of the three separate experiments performed with similar results.
Expression and purification of recombinant GST–LRP-tail.
cDNA that encodes the entire LRP tail (100 amino acids) was generated via PCR and subcloned into the GST expression vector pGEX-2T (Amersham Life Science). GST–LRP-tail fusion protein was expressed in Escherichia coli and purified as previously described (3).
Metabolic labeling, immunoprecipitation, and SDS-PAGE.
Metabolic labeling with [35S]cysteine and immunoprecipitation were performed essentially as described previously (3, 5). Protein A-agarose beads were used to precipitate protein-immunoglobulin G (IgG) complexes. The immunoprecipitated material was released from the beads under reducing conditions by boiling each sample for 5 min in Laemmli sample buffer (62.5 mM Tris-HCl, pH 6.8; 2% [wt/vol] sodium dodecyl sulfate [SDS], 10% [vol/vol] glycerol) containing 5% β-mecaptoethanol and then analyzed by SDS–6% polyacrylamide gel electrophoresis (PAGE).
Phosphorylation of LRP in intact cells.
Stably transfected CHO cells were plated in six-well plates. Cells were washed and incubated twice for 20 min with phosphate-free minimal essential medium, followed by the addition of 200 μCi of [32P]orthophosphate per ml in 0.7 ml of medium. After 1 to 4 h of labeling at 37°C, cells were washed three times with phosphate-buffered saline (PBS) and then solubilized for 30 min at 4°C in 500 μl of lysis buffer (PBS containing 1% Triton X-100, 1 mM glycerophosphate, 1 mM sodium orthovanadate, 5 mM sodium fluoride, 1 mM phenylmethylsulfonyl fluoride, and 1× Complete). Following immunoprecipitation with either anti-LRP or anti-HA antibody, samples were examined via SDS-PAGE.
In vitro phosphorylation of GST–LRP-tail.
In vitro phosphorylation assays for GST and GST–LRP-tail by PKA or PKC were carried out at 30°C. The PKA assay was performed according to the manufacturer's instructions, with each assay containing 100 ng of catalytic subunit of PKA and 0.6 μg of individual substrate. The experiments were performed in parallel with or without the addition of a PKI. The PKC assay was also performed according to the manufacturer's instructions with 100 ng of PKC used in each assay. After 10-min incubations for both the PKA and the PKC assays, GST or GST–LRP-tail was precipitated by using trichloroacetic acid (TCA; final concentration, 20%) containing 0.5% SDS. The precipitates were then dissolved in Laemmli sample buffer and separated by using SDS–12% polyacrylamide gels, and phosphorylated proteins were detected by autoradiography.
Flow cytometric analysis of cell surface LRP minireceptors.
For cell surface LRP minireceptor analysis, living cells were used (33). Briefly, CHO cells were detached by incubation with nonenzymatic cell dissociation solution. Successive incubations with affinity-purified anti-HA IgG (25 μg/ml) and goat anti-mouse immunoglobulin-FITC were carried out at 4°C for 45 min. The background fluorescence intensity was assessed in the absence of primary monoclonal antibody. The antibody binding capacities were evaluated from the standardized Quantum Simply Cellular bead calibration plot (56). The bead standards consist of four populations of microbeads coated with goat anti-mouse antibody which bind different numbers of mouse IgG monoclonal antibody molecules (5686, 18,329, 50,908, and 150,477 molecule-binding capacities) in addition to a blank population. The beads were stained in the same way as the CHO cells.
Saturation-binding analysis.
RAP saturation-binding analysis was performed essentially as described earlier (25). Cells were cultured in 12-well plates to approximately 106 cells per well. Cell monolayers were rinsed twice in ice-cold ligand-binding buffer (minimal Eagle medium containing 0.6% bovine serum albumin [BSA]). 125I-RAP at various concentrations, either in the absence or in the presence of 500 nM RAP, was added in cold ligand-binding buffer (0.6 ml/well), and the incubation was carried out at 4°C for 60 min with gentle rocking. Thereafter, overlying buffer containing unbound ligand was removed, and the cells were washed and lysed in low-SDS lysis buffer (62.5 mM Tris-HCl, pH 6.8; 0.2% SDS; 10% [vol/vol] glycerol) and counted.
Kinetic analysis of endocytosis.
Stably transfected CHO cells were plated in 12-well plates at a density of 2 × 105 cells/well and used after overnight culture. Cells were rinsed twice in ice-cold ligand-binding buffer, and 125I-RAP was added at a 5 nM final concentration in cold ligand-binding buffer (0.5 ml/well). The binding of 125I-RAP was carried out at 4°C for 30 min with gentle rocking. Binding of 125I-RAP was specific, i.e., the addition of 100-fold excess unlabeled RAP inhibited binding by 90 to 95%. Unbound ligand was removed by washing cell monolayers three times with cold binding buffer. Ice-cold stop-strip solution (0.2 M acetic acid, pH 2.6; 0.1 M NaCl) was added to one set of plates without warming up, and then the cells were kept on ice. The remaining plates were then placed in a 37°C water bath, and 0.5 ml of ligand-binding buffer prewarmed to 37°C was quickly added to the well monolayers to initiate internalization. After each time point, the plates were quickly placed on ice and the ligand-binding buffer was replaced with cold stop-strip solution. Ligand that remained on the cell surface was stripped by incubation of cell monolayers with cold stop-strip solution for a total of 20 min (0.75 ml for 10 min, twice) and counted. Cell monolayers were then solubilized with low-SDS lysis buffer and counted. The sum of ligand that was internalized plus those remained on the cell surface after each assay was used as the maximum potential internalization. The fraction of internalized ligand after each time point was calculated and plotted.
Ligand degradation efficiency.
Ligand degradation efficiency was performed by using the methods described elsewhere (9, 31, 33). Briefly, 2 × 105 cells are seeded into 12-well dishes 1 day prior to the assays. Assay buffer (minimal Eagle medium containing 0.6% BSA with 5 nM radioligand at 0.6 ml/well) was added to cell monolayers, in the absence or in the presence of unlabeled 500 nM RAP, and followed with incubation for 2 h at 37°C. Thereafter, the medium overlying the cell monolayers was removed, and proteins were precipitated by the addition of BSA to 10 mg/ml and TCA to 20%. Degradation of radioligand was defined as the appearance of radioactive fragments in the overlying medium that were soluble in 20% TCA. The cell numbers of each well were counted in parallel plates that did not contain LRP ligands. The ligand degradation efficiency is the value of degraded ligand (counts per minute per 106 cells) divided by the number of cell surface LRP minireceptors (determined by flow cytometry [see above]) and calculated relative to wild-type mLRP4T100.
RESULTS
LRP phosphorylation is inhibited by wide-spectrum serine-threonine kinase inhibitors and by specific PKA inhibitors.
Our previous studies have shown that LRP is phosphorylated in a neuronal cell line (8). To investigate whether LRP phosphorylation is a general feature of this endocytic receptor, we tested basal levels of LRP phosphorylation in several mammalian cell lines including human hepatoma HepG2, CHO, and U87 cells. We found that LRP is phosphorylated in all of the cell lines examined, with the highest phosphorylation seen in the human glioblastoma U87 cells (data not shown; see references 4 and 5). The LRP tail contains 100 amino acid residues, including four tyrosine, six threonine, and three serine residues. The most noticeable sequence elements within LRP tails are the two NPXY signals, which are also found in the cytoplasmic tails of other members of the LDL receptor gene family and interact with the cytoplasmic adaptor proteins (40, 46, 47). In the EGF receptor, the tyrosine residue within the NPXY motif is phosphorylated (34). In order to assess the possibility of tyrosine phosphorylation of the LRP tail, we analyzed the effect of two potent inhibitors of tyrosine kinases, genistein and lavendustin A, on LRP phosphorylation in U87 cells. Figure 1A shows that neither genistein nor lavendustin A has any significant effect on the level of LRP phosphorylation in U87 cells, suggesting that tyrosine sites are unlikely to be involved in LRP phosphorylation. In contrast, broad-spectrum kinase inhibitors staurosporine and K-252a, which inhibit serine-threonine kinases, markedly decreased the level of LRP phosphorylation (Fig. 1A), indicating that LRP phosphorylation may occur on serine and/or threonine residue.
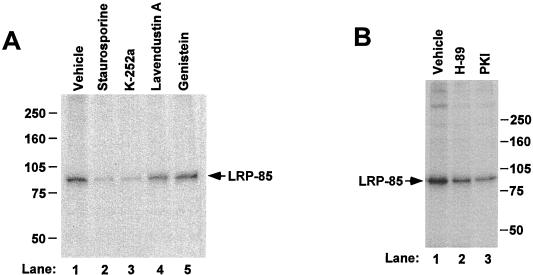
FIG. 1.
Effect of protein kinase inhibitors on LRP phosphorylation. U87 cells were labeled with [32P]orthophosphate for 60 min, with protein kinase inhibitors added during the last 30 min as indicated. LRP was immunoprecipitated from the same amount of protein lysate for each condition and analyzed via SDS–6% PAGE under reducing conditions. (A) Broad-spectrum inhibitor staurosporine and K-252a were used at 3 and 15 μM, respectively. The tyrosine kinase inhibitor lavendustin A was used at 30 μM, and genistein was used at 50 μM. Dimethyl sulfoxide was used as the vehicle control. (B) Specific PKA inhibitor H-89 was used at 5 μM, and myristoylated PKA specific inhibitor peptide (PKI) (peptide sequence 14–22) was used at 40 μM. The position of the LRP-85 subunit that is phosphorylated is labeled. The molecular size markers in this and subsequent figures are given in kilodaltons. These data are representative of two separate experiments with identical results.
Further examination of the LRP tail sequence identified a consensus sequence (RXS) for PKA. To examine whether PKA contributes to LRP phosphorylation, we assessed the effect of PKA specific inhibitors on LRP phosphorylation (Fig. 1B). We found that LRP phosphorylation in U87 cells is significantly decreased in the presence of either H-89, a relatively specific inhibitor of PKA, or the myriostylated pseudosubstrate for PKA, PKI. The decreases of LRP-85 phosphorylation from several such experiments were 61% ± 8% (i.e., the standard error [SE] for this and subsequent statistics; n = 3) for H-89, and 60% ± 10% (n = 3) for PKI compared to the vehicle controls. These results suggest that PKA is likely a major kinase that phosphorylates LRP on serine residue(s).
GST–LRP-tail is phosphorylated by PKA.
Since the RHS sequence within the LRP tail is also a potential phosphorylation site for PKC (41), we examined whether PKA and/or PKC are capable of phosphorylating the LRP tail in vitro. For these studies, we generated a GST–LRP-tail fusion protein, which includes the entire tail sequence of LRP. The fusion protein was purified via glutathione-agarose affinity chromatography. A Coomassie blue-stained gel of the purified GST–LRP-tail is shown in Fig. 2A. As shown in the figure, the GST–LRP-tail exhibits an expected molecular size of ∼37 kDa, whereas GST alone is ∼27 kDa. Western blotting analyses confirmed that the 37-kDa GST–LRP-tail band is immunoreactive with antibody to LRP (data not shown).
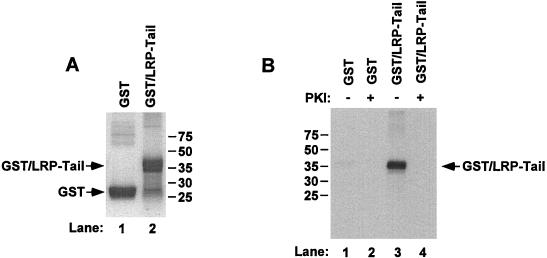
FIG. 2.
Phosphorylation of purified GST–LRP-tail by PKA in vitro. (A) Purified GST or purified GST–LRP-tail was analyzed via SDS–12.5% PAGE under nonreducing conditions and then stained with Coomassie blue. (B) GST or GST–LRP-tail was phosphorylated by PKA catalytic subunit and [γ-32P]ATP in vitro as described in Materials and Methods. As an additional control for PKA specificity, the PKI peptide was added in parallel samples (lanes 2 and 4).
We then analyzed whether the GST–LRP-tail can be phosphorylated by PKA. As shown in Fig. 2B, the purified GST–LRP-tail was specifically phosphorylated by the purified PKA catalytic subunit. The specificity of PKA phosphorylation is further confirmed by the inhibition of PKI. In a similar assay, we examined the effect of PKC on GST–LRP-tail phosphorylation. We found that while a PKC substrate was readily phosphorylated in this assay, the GST–LRP-tail fusion protein was not phosphorylated by purified PKC. These results together further suggest that PKA, but not PKC, is capable of phosphorylating the LRP tail in vitro.
LRP is phosphorylated on serine 76 of its cytoplasmic tail.
The very large size of LRP (∼600 kDa) limits molecular manipulations at the cDNA level and the expression of this protein via transfection. Therefore, we generated an LRP minireceptor that mimics the function and trafficking of LRP. This LRP minireceptor encodes residues 3274 to 4525 of the full-length LRP (20), i.e., the fourth cluster of ligand binding repeats through the carboxyl terminus of the receptor (designated mLRP4T100, with “m” indicating that it is membrane containing, “4” representing the fourth cluster of ligand-binding repeats, “T” representing the cytoplasmic tail, and “100” representing the 100 amino acid residues within the LRP tail (see Fig. 3A and reference 39). To facilitate immunoprecipitation and Western blot analysis, an HA epitope was included near the amino-terminal end of mLRP4T100.
Since both our in vitro and in vivo results suggested PKA phosphorylation of the LRP tail (Fig. 1 and 2) and that the cytoplasmic tail of the LDL receptor may be phosphorylated on a serine residue (27), we targeted the three serine residues within the LRP tail for deletion and mutagenesis analyses. The three serine residues within the LRP tail are closely localized at residues 73, 76, and 79 (the first amino acid following the transmembrane domain is numbered 1). In order to assess the possibility of serine residue phosphorylation, we made three deletion constructs of LRP minireceptors (Fig. 3A). As illustrated in the figure, mLRP4T78 is a truncated LRP minireceptor with 78 amino acid residues in its tail and lacks the third serine residue, whereas mLRP4T72 is a truncated LRP minireceptor with 72 amino acid residues in its tail and lacks all three serine residues. mLRP4Ttailess contains only the fourth ligand-binding domain and the transmembrane domain with no cytoplasmic residues (Fig. 3A). Our previous studies using soluble and membrane-containing LRP minireceptors have shown that proper folding of these minireceptors is facilitated by the coexpression of RAP (6, 38, 39). Thus, we transiently transfected cDNAs for mLRP4 constructs into U87 cells with cotransfection of RAP. Metabolic labeling with [35S]cysteine followed with immunoprecipitation showed that the four minireceptors are expressed and processed similarly (Fig. 3B, lanes 2 to 5). For mLRP4T100, four distinct bands are seen on the SDS–6% PAGE gel under reducing conditions. The 85-kDa band and the 120-kDa band represent the furin-processed minireceptor forms that correspond to the LRP-85 and LRP ligand-binding domain 4 (LRP-LBD4), respectively (39). The uppermost band at ca. 205 kDa represents the full-length minireceptor which was not cleaved in the trans-Golgi, probably due to a saturation of furin cleavage in transiently transfected cells. The band that migrates slightly faster than the full-length form represents the endoplasmic reticulum (ER) form devoid of complex sugar modification (lane 3 [39]). mLRP4Ttailess (lane 2), mLRP4T78 (lane 4), and mLRP4T72 (lane 5) exhibit similar banding patterns, except that LRP-85 bands migrate faster than that of mLRP4T100 due to the tail truncations.
[32P]orthophosphate labeling of the same transfections showed that only mLRP4T100 was phosphorylated (Fig. 3B, lane 8). It was noted that not only LRP-85 but also the full-length minireceptor was phosphorylated, suggesting that at least part of the full-length form of the minireceptor was presented on the cell surface. The presence of nonprocessed mLRP4T100 on the cell surface was confirmed by cell surface iodination and immunoprecipitation (data not shown). As expected, mLRP4Ttailess was not phosphorylated (lane 7). However, the fact that mLRP4T72 and mLRP4T78 are not phosphorylated (lanes 9 and 10) suggests that LRP phosphorylation occurs within the last 28 residues of the LRP tail, most likely on the three serine residues.
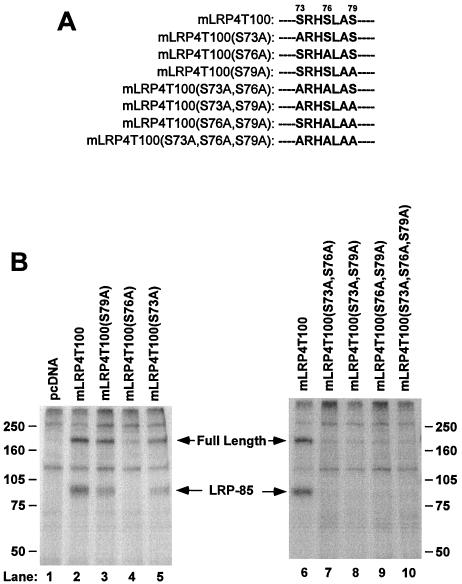
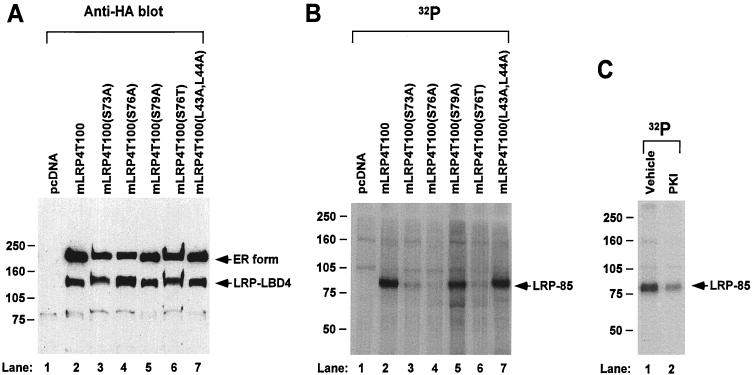
Based on these results, we generated single, double, or triple serine-to-alanine mutations using site-directed mutagenesis techniques and mLRP4T100 as the template (Fig. 4A). Compared to the wild-type mLRP4T100, the level of LRP tail phosphorylation was decreased to 57.8% ± 6.9% (n = 4 for all the constructs) for mLRP4T100(S73A), 4.5% ± 3.2% for mLRP4T100(S76A), and 48.8% ± 7.5% for mLRP4TS79A. These results strongly suggest that the serine 76 was the major, if not the only, phosphorylation site within the LRP tail. As seen in the figure, all of the double or triple mutations resulted in little or no phosphorylation of the minireceptor (Fig. 4B, lanes 7 to 10).
FIG. 4.
LRP phosphorylation occurs on serine 76 of its cytoplasmic tail. (A) Cytoplasmic tail sequences of LRP4T100 and its site-directed mutants from serine 73 to serine 79. (B) U87 cells were cotransfected with cDNAs for RAP and one of the minireceptor constructs as indicated, labeled with [32P]orthophosphate for 4 h, immunoprecipitated with anti-HA antibody, and analyzed via SDS–6% PAGE under reducing conditions. The data represent the results from one of the four separate experiments performed with similar results.
In Fig. 3B, we have shown that mLRP4T78 is not phosphorylated (Fig. 3B, lane 9). It is particularly interesting that the level of mLRP4T100(S73A,S79A) phosphorylation was very low compared to their single mutants, which agrees well with the result of Fig. 3B that mLRP4T78 is not phosphorylated, suggesting that the serine 73 and the serine 79 might function in a coordinated fashion for the docking of the kinase and are part of the phosphorylation motif of LRP tail. These results together allow us to conclude that serine 76 within the LRP tail is the major phosphorylation site for LRP, which is consistent with PKA-mediated phosphorylation of LRP as demonstrated above.
To confirm these results, we generated stably transfected cell lines expressing mLRP4T100 or its serine mutants in an LRP-null CHO cell line (13). Because a threonine residue can also potentially be phosphorylated by PKA, we also mutated serine 76 to threonine. Figure 5A shows that mLRP4T100 and its mutants were expressed at similar levels as revealed by Western blot analyses. Since the HA epitope was included near the amino terminus of the LRP minreceptors, Western blot analyses with HA antibody did not detect the LRP-85 band. However, the presence of this band was confirmed by metabolic labeling with [35S]cysteine (data not shown). [32P]orthophosphate labeling showed that mLRP4T100 was strongly phosphorylated (Fig. 5B, lane 2), whereas neither mLRP4T100(S76A) nor mLRP4T100(S76T) was phosphorylated (lanes 4 and 6). Similar to the data obtained from the transient transfection, the level of LRP tail phosphorylation was significantly decreased for mLRP4T100(S73A) in stably transfected CHO cells (lane 3), while mLRP4T100(S79A) exhibits a nearly normal level of LRP cytoplasm tail phosphorylation (lane 5).
FIG. 5.
Expression and phosphorylation of LRP minireceptors in stably transfected LRP-null CHO cells. (A) Equal amounts of cell lysates from LRP-null CHO cells stably transfected with pcDNA, mLRP4T100, or its various mutants were separated via SDS–6% PAGE under reducing conditions and the Western blotted with anti-HA antibody. The positions of the ER form and the extracellular subunits following furin cleavage (LRP-LBD4) are labeled. (B) Stably transfected cell lines were labeled with [32P]orthophosphate for 60 min. LRP minireceptors were immunoprecipitated from the same amount of lysates with anti-HA antibody and analyzed via SDS–6% PAGE under reducing conditions. The position of phosphorylated LRP-85 subunit is labeled. (C) LRP-null CHO cells stably transfected with mLRP4T100 were labeled with [32P]orthophosphate for 60 min, with either vehicle or 40 μM myristoylated PKA specific inhibitor peptide (PKI) (peptide sequence 14–22) added during the last 30 min. mLRP4T100 was immunoprecipitated from the same amount of lysates with anti-HA antibody and analyzed via SDS–6% PAGE under reducing conditions.
To confirm the role of PKA on LRP phosphorylation, we assessed the effect of the specific PKA inhibitor on wild-type mLRP4T100 phosphorylation. As shown in Fig. 5C, the specific PKA inhibitor PKI markedly decreased the phosphorylation of mLRP4T100 to 30% ± 8% of the control level (Fig. 5C, n = 3). These results together further indicate that LRP phosphorylation is mediated by PKA on the serine 76 residue within its cytoplasmic tail and that the phosphorylation is specific for serine but not for theronine.
By using flow cytometry, we found that the number of mLRP4T100 on the surface of stably transfected CHO cells is ∼36,700 sites per cell. In our previous studies using quantitative immunoelectron microscopy, we have shown that there are about 28% of mature LRP localized on the cell surface (4). To analyze the percentage of mature LRP being phosphorylated at steady state, we quantitated the number of phosphate groups following 4 h of metabolic 32P labeling with a known number of total matured LRP minireceptors using methods described previously (12). We found that about 15% of mature mLRP4T100 was phosphorylated under these conditions (data not shown). This percentage of LRP phosphorylation resembles that of another endocytic receptor, the asialoglycoprotein receptor (12). These results suggest that at steady state only a certain population of LRP is phosphorylated and that this phosphorylation may be regulated under some conditions.
Forskolin enhances LRP phosphorylation.
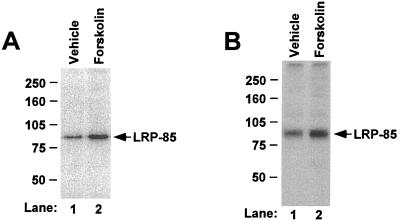
To further analyze the role of PKA in LRP phosphorylation, we assessed the effect of a specific cAMP-PKA stimulator forskolin. As seen in Fig. 6, forskolin markedly increased the phosphorylation of the endogenous LRP in U87 cells (Fig. 6A), as well as the phosphorylation of mLRP4T100 in CHO cells (Fig. 6B). The increases of LRP phosphorylation in the presence of forskolin were 162% ± 8% (n = 3) of the control level for endogenous LRP and 141% ± 9% (n = 3) of the control level for mLRP4T100.
FIG. 6.
Effect of forskolin on LRP phosphorylation. U87 cells (A) or LRP-null CHO cells stably transfected with mLRP4T100 (B) were cultured in serum-free medium for 24 h, followed by metabolic labeling with [32P]orthophosphate for 60 min. Either dimethyl sulfoxide vehicle control or 50 μM forskolin was added during the last 15 min of labeling. LRP was immunoprecipitated from the same amount of lysates with either anti-LRP antibody (A) or anti-HA antibody (B) and analyzed via SDS–6% PAGE under reducing conditions.
Binding affinity of RAP is not affected by phosphorylation mutation.
Having established that LRP phosphorylation occurs on serine 76 by PKA, we then investigated potential function of LRP phosphorylation. Our previous studies have shown that the fourth ligand-binding domain of LRP binds RAP with high affinity (6, 33, 38, 39). Using RAP saturation binding, we then compared RAP binding affinity of wild-type mLRP4T100 with its phosphorylation mutant expressed in stably transfected CHO cells. Scatchard analysis of RAP binding is consistent with a single homogeneous population of binding sites (data not shown). It was found that mLRP4T100 and mLRP4T100(S76A) exhibited similar RAP binding affinity, with equilibrium dissociation constant (Kd) values of ∼2.2 and ∼2.4 nM, respectively. These affinities of RAP for LRP minireceptors are comparable to that of endogenous full-length LRP, which on hepatoma cells exhibited a Kd of ∼3.3 nM (25). These results together indicate that endogenous LRP, mLRP4T100, and mLRP4T100(S76A) have a similar RAP binding affinity.
LRP phosphorylation contributes to receptor-mediated endocytosis.
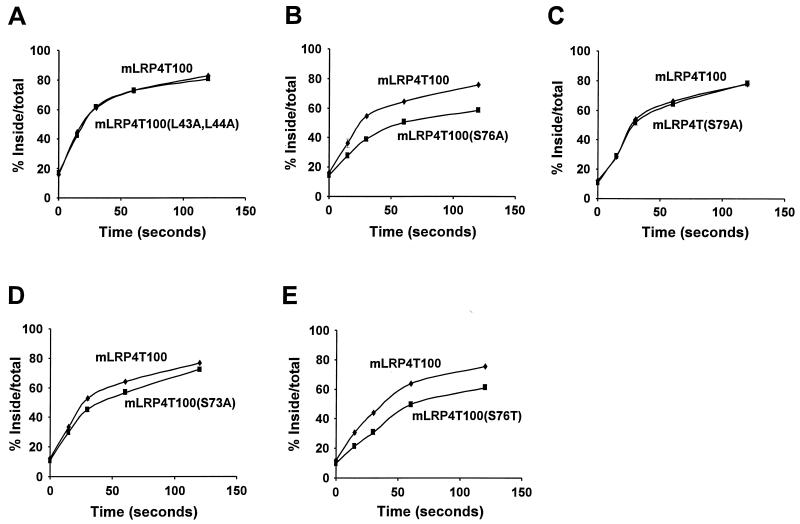
LRP is a cell surface endocytic receptor that undergoes constitutive endocytosis in the presence or absence of its ligands. In order to further assess the function of the receptor phosphorylation, we compared the endocytosis rates of mLRP4T100 and its mutants. Our recent studies have shown that the majority of LRP endocytosis is mediated by the YXXL and the distal dileucine motifs and that the two NPXY motifs and the proximal dileucine motif do not contribute to initial endocytosis (33). Thus, we utilized the proximal dileucine mutant, mLRP4T100(L43A,L44A), as our negative control since our previous experiments have shown that the endocytosis rate of mLRP4T100(L43A,L44A) is identical to that of wild-type mLRP4T100 (33). The level of mLRP4T100(L43A,L44A) phosphorylation is similar to that of wild-type mLRP4T100 (Fig. 5B, lane 7). As expected, the endocytosis rate of mLRP4T100(L43A,L44A) was indistinguishable from that of mLRP4T100 (Fig. 7A). However, the endocytosis rate for the phosphorylation mutant, mLRP4T100(S76A), is reduced compare to that of the wild-type mLRP4T100 (Fig. 7B). This reduction of initial endocytosis rate by phosphorylation mutation was also observed in several other mLRP4T100(S76A) stable cell lines examined (data not shown).
FIG. 7.
Endocytosis of 125I-RAP by LRP minireceptor-transfected CHO cells. LRP-null CHO cells stably transfected with mLRP4T100 or its various mutants were incubated with 5 nM 125I-RAP at 4°C for 30 min, washed on ice, and then shifted to 37°C for the indicated times. The amounts of ligand internalized as the fraction of the maximum possible internalized ligand (the sum of the internalized ligand plus the ligand remaining on the cell surface at the end of the assay) are plotted against time. Values are the average of triple determinations with the SE values indicated by error bars. This experiment is representative of at least two such experiments performed with similar data. (A) Endocytosis of 125I-RAP by mLRP4T100 and mLRP4T100(L43A,L44A). (B) Endocytosis of 125I-RAP by mLRP4T100 and mLRP4T100(S76A). (C) Endocytosis of 125I-RAP by mLRP4T100 and mLRP4T100(S79A). (D) Endocytosis of 125I-RAP by mLRP4T100 and mLRP4T100(S73A). (E) Endocytosis of 125I-RAP by mLRP4T100 and mLRP4T100(S76T).
To confirm the role of LRP phosphorylation on receptor endocytosis, we compared the endocytosis rate of wild-type mLRP4T100 to that of other LRP minireceptor mutants. mLRP4T100(S79A), which possesses a high level of cytoplasmic tail phosphorylation, exhibits an endocytosis rate identical to that of mLRP4T100 (Fig. 7C). On the other hand, mLRP4T100(S73A), with a reduced level of cytoplasmic tail phosphorylation, exhibits an impaired endocytosis rate (Fig. 7D), while the phosphorylation mutant mLRP4T100(S76T) exhibits a significantly lower level of endocytosis (Fig. 7E).
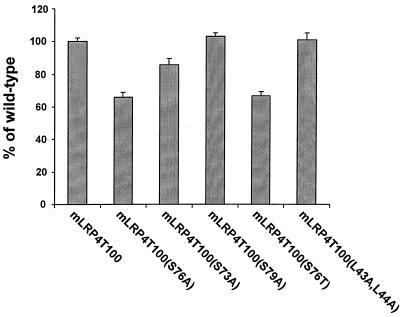
Based on the above results, we analyzed the percentage of total initial bound 125I-RAP that has been internalized by different LRP minireceptors after the medium was warmed up at 37°C for 30 s. As shown in Fig. 8, mLRP4T100, mLRP4T100(S79A), and mLRP4T100(L43A,L44A) exhibit similar levels of 125I-RAP internalization. On the other hand, mLRP4T100(S76A) and mLRP4T100(S76T) exhibit significantly lower levels of 125I-RAP internalization. Taken together, these results strongly indicate that LRP phosphorylation is involved in receptor endocytosis.
FIG. 8.
Internalization of 125I-RAP by LRP minreceptor-transfected CHO cells. LRP-null CHO cells stably transfected with mLRP4T100 or its various mutants were incubated with 5 nM 125I-RAP at 4°C for 30 min, washed on ice to remove unbound ligand, and then warmed up to 37°C for 30 s. The amounts of ligand internalized as the fraction of the maximum possible internalized ligand were measured and were calculated relative to wild-type mLRP4T100. This experiment is representative of two such experiments performed with similar data.
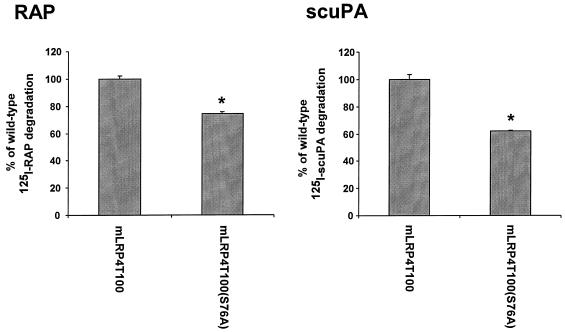
To further confirm the role of LRP phosphorylation on receptor-mediated endocytosis, we then investigated the efficiency of LRP ligand degradation for wild-type mLRP4T100 and its phosphorylation mutant mLRP4T100(S76A) (Fig. 9). By using flow cytometry, we found that the numbers of LRP minireceptor on the surface of CHO cells stably transfected with mLRP4T100 and mLRP4T100(S76A) are 36,700 and 51,700 sites per cell, respectively. Consistent with the numbers of the minireceptors on the cell surface, the levels of 4 nM 125I-RAP binding to cell surface mLRP4T100 and mLRP4T100(S76A) after incubation at 4°C for 1 h were 1.6 and 2.5 fmol/μg of cell protein, respectively. After we normalized the degradation by using the receptor numbers, we found that 125I-RAP degradation efficiency by mLRP4T100(S76A) stable cells was decreased to ∼74% compared to that of wild-type mLRP4T100 cells (Fig. 9). Our recent studies showed that mLRP4T100 is able to degrade scuPA efficiently (L. M. Obermoeller and G. Bu, unpublished data). Unlike RAP, scuPA is a physiological ligand for LRP (28). Thus, we utilized scuPA to measure the efficiency of ligand degradation by LRP minireceptor cell lines. Similar to that seen with 125I-RAP, LRP phosphorylation mutant cells exhibited slower degradation of 125I-scuPA (62% compared to that of wild-type mLRP4T100 cells; see Fig. 9). Taken together, these data clearly demonstrate that LRP phosphorylation regulates its endocytosis rate and ligand delivery for degradation.
FIG. 9.
mLRP4T100 phosphorylation mutant exhibits lower ligand degradation efficiency. LRP-null CHO cells stably transfected with mLRP4T100 or mLRP4T100(S76A) were incubated with 5 nM 125I-RAP or 125I-scuPA at 37°C for 2 h in the presence or absence of 500 nM RAP. The ligand degradation efficiency was determined as described in Materials and Methods. Values are the average of triple determinations with the SE values indicated by error bars. This experiment is representative of two such experiments performed with similar data. ∗, P < 0.01 versus wild-type mLRP4T100.
DISCUSSION
Phosphorylation of cell surface receptors is one of the most important mechanisms by which receptor trafficking and/or signal transduction is regulated (19, 32, 42). Until recently, little was known regarding phosphorylation and its function for members of the LDLR gene family. Studies by Kishimoto et al. (27) demonstrated that the cytoplasmic tail of the LDLR could be phosphorylated on a serine residue by an LDLR kinase which was purified from the cytosol of bovine adrenal cortex and shared several properties with casein kinase II. However, this phosphorylation event occurs only in vitro, since neither cultured human fibroblasts nor A431 carcinoma cells were able to incorporate [32P]orthophosphate into the LDLR (27). Our previous studies have demonstrated LRP phosphorylation within its cytoplasmic tail (8). However, the nature of LRP phosphorylation and its potential function was unknown. By using specific protein kinase inhibitors, truncated LRP minireceptors, and site-directed mutagenesis techniques, we now provide direct evidence that serine 76 within the LRP cytoplasmic tail is the major phosphorylation site. In addition, our in vitro and in vivo phosphorylation analyses demonstrate that LRP phosphorylation is mediated by PKA. Furthermore, we show that phosphorylation of LRP contributes to receptor-mediated endocytosis. The mutations of serine 76 to alanine or theronine, which abolish LRP phosphorylation by PKA, result in a decrease in both the initial endocytosis rate of LRP and the efficiency of ligand delivery for degradation.
The two neighboring serine residues (serine 73 and serine 79) may also contribute to LRP phosphorylation. In our transient-transfection experiments, both mLRP4T100(S73A) and mLRP4T100(S79A) show lower levels of cytoplasmic tail phosphorylation compared to that of wild-type mLRP4T100. However, the level of mLRP4T100(S79A) phosphorylation is nearly normal in stably transfected CHO cells, while the level of mLRP4T100(S73A) phosphorylation is significantly decreased. Nevertheless, our results strongly indicate that serine 76 is the major site of LRP phosphorylation. Thus, the serine 73 and the serine 79 might function in a coordinated fashion for the docking of the kinase and are part of the phosphorylation motif of the LRP tail.
LRP belongs to the class of receptors that undergo constitutive endocytosis in the presence or absence of ligands. This feature may be determined by the constant exposure of its endocytosis signals and is highlighted by its concentrated distribution within clathrin-coated pits on the cell surface (4, 33). We recently reported that the YXXL motif within the cytoplasmic tail of LRP serves as the dominant signal for LRP endocytosis and that the distal dileucine motif within the LRP tail also contributes to its endocytosis (33). In the present study, we demonstrate that LRP-mediated endocytosis is further regulated by PKA phosphorylation of the LRP cytoplasmic tail. Our results are consistent with a previous report demonstrating that LRP-mediated endocytosis is inhibited by specific PKA inhibitors H-89 and PKI (15).
Goretzki and Mueller have reported that the cytoplasmic tail of LRP interacts with a GTP-binding protein, suggesting that LRP may be coupled to a heterotrimeric G protein (16). Furthermore, they demonstrated that treatment of LRP-expressing cell lines with LRP ligands lactoferrin and urokinase-plasminogen activator inhibitor complex resulted in a significant increase in the intracellular cAMP level and PKA activity (16). LRP, like other members of the LDLR family, contains only a single transmembrane domain, and thus its membrane topology differs from those of classical heptahelical G protein-coupled receptors. However, it has been shown previously that single transmembrane receptors, such as the insulin receptor, the mannose 6-phosphate–insulin-like growth factor II receptor, and the EGF receptor, are coupled to heterotrimeric G proteins (26, 36, 45). For several members of the G protein-coupled receptor family, in particular the β2-adrenergic receptor, ligand-induced phosphorylation of serine residues in the carboxyl-terminal domain of the molecule leads to recruitment of nonvisual arrestins that both uncouple associated heterotrimeric G proteins and act as adaptors to recruit the receptor into clathrin-coated pits (14). Since our present results demonstrate that the LRP tail is phosphorylated by PKA and that this phosphorylation facilitates endocytosis, it is possible that ligand binding to LRP regulates receptor-mediated endocytosis via enhanced PKA phosphorylation of LRP. Taken together, it is likely that the binding of certain LRP ligands induces a dissociation of the stimulatory heterotrimeric G protein subunit, which leads to activation of the adenylate cyclase and a subsequent rise in intracellular cAMP. This, in turn, results in an enhanced PKA activity that increases LRP phosphorylation and its endocytosis. Alternatively, other signal transduction pathways that influence PKA activity can also regulate LRP phosphorylation and endocytosis.
Recently, studies have revealed new roles of LDLR family members as transducers of extracellular signals (17, 22, 24, 43, 46, 47). It has been demonstrated that the lipoprotein receptors VLDLR and apoER2 function as obligate components in the Reelin/Disabled-mediated neuronal migration pathway (10, 22, 46, 47). A signaling pathway involving the extracellular protein Reelin and the intracellular adaptor protein Disabled-1 is involved in the control of cell position during mammalian brain development. Disabled-1 interacts with NPXY motifs in the tails of the lipoprotein receptors (24, 46, 47). After binding to the lipoprotein receptors VLDLR and apoER2, Reelin is internalized into vesicles and induces tyrosine phosphorylation of Disabled-1 (10, 22). In addition, mice which lack the genes for both VLDLR and apoER2 demonstrate a neurological and neuroanatomical phenotype that is indistinguishable from animals deficient in either Reelin or Disabled-1 (47). Potential signaling functions for the lipoprotein receptors have also been suggested from other observations. First, several studies have shown that apoE3, a ligand of LRP, increases neurite extension via LRP (1, 11, 23, 37). Second, our previous studies have shown that negative feedback regulation of tissue plasminogen activator gene expression in colon fibroblasts is mediated via cell surface LRP (18). Third, studies have shown that the LRP tail interacts with a GTP-binding protein induces cAMP-dependent protein kinase activity (16). In the present study, we demonstrated that the cytoplasmic tail of LRP is phosphorylated by PKA. Although, the potential signaling pathway(s) downstream from LRP is still not clear at present, it is possible that the signaling event may be regulated by LRP phosphorylation.
In summary, our present study provides the first evidence that PKA phosphorylation of a member of the LDLR family participates in receptor-mediated endocytosis. Since protein phosphorylation is one of the major mechanisms by which cells convert extracellular signals into intracellular responses, it will be interesting to examine in future studies potential signaling events downstream from LRP phosphorylation.
ACKNOWLEDGMENTS
We are grateful to Alan Schwartz and David Holtzman for their critical readings and suggestions for the manuscript. We also thank David FitzGerald (NIH) for providing the LRP-null CHO cell line.
This work was supported by NIH grants NS37525 and HL59150 (to G.B.) and a grant from Fondecyt 1990600 (to M.P.M.).
REFERENCES
- 1.Bellosta S, Nathan B P, Orth M, Dong L M, Mahley R M, Pitas R E. Stable expression and secretion of apolipoproteins E3 and E4 in mouse neuroblastoma cells produces differential effects on neurite outgrowth. J Biol Chem. 1995;270:27063–27071. doi: 10.1074/jbc.270.45.27063. [DOI] [PubMed] [Google Scholar]
- 2.Bu G, Morton P A, Schwartz A L. Identification and partial characterization by chemical cross-linking of a binding protein for tissue-type plasminogen activator (t-PA) on rat hepatoma cells. A plasminogen activator inhibitor type 1-independent t-PA receptor. J Biol Chem. 1992;267:15595–15602. [PubMed] [Google Scholar]
- 3.Bu G, Maksymovitch E A, Schwartz A L. Receptor-mediated endocytosis of tissue-type plasminogen activator by low density lipoprotein receptor-related protein on human hepatoma HepG2 cells. J Biol Chem. 1993;268:13002–13009. [PubMed] [Google Scholar]
- 4.Bu G, Maksymovitch E A, Geuze H, Schwartz A L. Subcellular localization and endocytic function of low density lipoprotein receptor-related protein in human glioblastoma cells. J Biol Chem. 1994;269:29874–29882. [PubMed] [Google Scholar]
- 5.Bu G, Geuze H J, Strous G J, Schwartz A L. 39 kDa receptor-associated protein is an ER resident protein and molecular chaperone for LDL receptor-related protein. EMBO J. 1995;14:2269–2280. doi: 10.1002/j.1460-2075.1995.tb07221.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bu G, Rennke S. Receptor-associated protein is a folding chaperone for low density lipoprotein receptor-related protein. J Biol Chem. 1996;271:22218–22224. doi: 10.1074/jbc.271.36.22218. [DOI] [PubMed] [Google Scholar]
- 7.Bu G. Receptor-associated protein: a specialized chaperone and antagonist for members of the LDL receptor gene family. Curr Opin Lipidol. 1998;9:149–155. doi: 10.1097/00041433-199804000-00012. [DOI] [PubMed] [Google Scholar]
- 8.Bu G, Sun Y, Schwartz A L, Holtzman D M. Nerve growth factor induces rapid increases in functional cell surface low density lipoprotein receptor-related protein. J Biol Chem. 1998;273:13359–13365. doi: 10.1074/jbc.273.21.13359. [DOI] [PubMed] [Google Scholar]
- 9.Chen W J, Goldstein J L, Brown M S. NPXY, a sequence often found in cytoplasmic tails, is required for coated pit-mediated internalization of the low density lipoprotein receptor. J Biol Chem. 1990;265:3116–3123. [PubMed] [Google Scholar]
- 10.D'Arcangelo G, Homayouni R, Keshvara L, Rice D S, Sheldon M, Curran T. Reelin is a ligand for lipoprotein receptors. Neuron. 1999;24:471–479. doi: 10.1016/s0896-6273(00)80860-0. [DOI] [PubMed] [Google Scholar]
- 11.DeMattos R B, Curtiss L K, Williams D L. A minimally lipidated form of cell-derived apolipoprotein E exhibits isoform-specific stimulation of neurite outgrowth in the absence of exogenous lipids or lipoproteins. J Biol Chem. 1998;273:4206–4212. doi: 10.1074/jbc.273.7.4206. [DOI] [PubMed] [Google Scholar]
- 12.Fallon R J, Schwartz A L. Asialoglycoprotein receptor phosphorylation and receptor-mediated endocytosis in hepatoma cells. Effect of phorbol esters. J Biol Chem. 1988;263:13159–13166. [PubMed] [Google Scholar]
- 13.FitzGerald D J, Fryling C M, Zdanovsky A, Saelinger C B, Kounnas M, Winkles J A, Strickland D, Leppla S. Pseudomonas exotoxin-mediated selection yields cells with altered expression of low-density lipoprotein receptor-related protein. J Cell Biol. 1995;129:1533–1541. doi: 10.1083/jcb.129.6.1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goodman O B, Jr, Krupnick J G, Santini F, Gurevich V V, Penn R B, Gagnon A W, Keen J H, Benovic J L. β-Arrestin acts as a clathrin adaptor in endocytosis of the β2-adrenergic receptor. Nature. 1996;383:447–450. doi: 10.1038/383447a0. [DOI] [PubMed] [Google Scholar]
- 15.Goretzki L, Mueller B M. Receptor-mediated endocytosis of urokinase-type plasminogen activator is regulated by cAMP-dependent protein kinase. J Cell Sci. 1997;110:1395–1402. doi: 10.1242/jcs.110.12.1395. [DOI] [PubMed] [Google Scholar]
- 16.Goretzki L, Mueller B M. Low-density-lipoprotein-receptor-related protein (LRP) interacts with a GTP-binding protein. Biochem J. 1998;336:381–386. doi: 10.1042/bj3360381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gotthardt M, Trommsdorff M, Nevitt M F, Shelton J, Richardson J A, Stockinger W, Nimpf J, Herz J. Interactions of the low density lipoprotein receptor gene family with cytosolic adaptor and scaffold proteins suggest diverse biological functions in cellular communication and signal transduction. J Biol Chem. 2000;275:25616–25624. doi: 10.1074/jbc.M000955200. [DOI] [PubMed] [Google Scholar]
- 18.Hardy M M, Feder J, Wolfe R A, Bu G. Low density lipoprotein receptor-related protein modulates the expression of tissue-type plasminogen activator in human colon fibroblasts. J Biol Chem. 1997;272:6812–6817. doi: 10.1074/jbc.272.10.6812. [DOI] [PubMed] [Google Scholar]
- 19.Hausdorff W P, Caron M G, Lefkowitz R J. Turning off the signal:desensitization of beta-adrenergic receptor function. FASEB J. 1990;4:2881–2889. [PubMed] [Google Scholar]
- 20.Herz J, Hamann U, Rogne S, Myklebost O, Gausepohl H, Stanley K K. Surface location and high affinity for calcium of a 500-kd liver membrane protein closely related to the LDL-receptor suggest a physiological role as lipoprotein receptor. EMBO J. 1988;7:4119–4127. doi: 10.1002/j.1460-2075.1988.tb03306.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Herz J, Kowal R C, Goldstein J L, Brown M S. Proteolytic processing of the 600 kd low density lipoprotein receptor-related protein (LRP) occurs in a trans-Golgi compartment. EMBO J. 1990;9:1769–1776. doi: 10.1002/j.1460-2075.1990.tb08301.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hiesberger T, Trommsdorff M, Howell B W, Goffinet A, Mumby M C, Cooper J A, Herz J. Direct binding of Reelin to VLDL receptor and ApoE receptor 2 induces tyrosine phosphorylation of disabled-1 and modulates tau phosphorylation. Neuron. 1999;24:481–489. doi: 10.1016/s0896-6273(00)80861-2. [DOI] [PubMed] [Google Scholar]
- 23.Holtzman D M, Pitas R E, Kilbridge J, Nathan B, Mahley R W, Bu G, Schwartz A L. Low density lipoprotein receptor-related protein mediates apolipoprotein E-dependent neurite outgrowth in a central nervous system-derived neuronal cell line. Proc Natl Acad Sci USA. 1995;92:9480–9484. doi: 10.1073/pnas.92.21.9480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Howell B W, Lanier L M, Frank R, Gertler F B, Cooper J A. The disabled 1 phosphotyrosine-binding domain binds to the internalization signals of transmembrane glycoproteins and to phospholipids. Mol Cell Biol. 1999;19:5179–588. doi: 10.1128/mcb.19.7.5179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Iadonato S P, Bu G, Maksymovitch E A, Schwartz A L. Interaction of a 39 kDa protein with the low-density-lipoprotein-receptor-related protein (LRP) on rat hepatoma cells. Biochem J. 1993;296:867–875. doi: 10.1042/bj2960867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jo H, Radding W, Anantharamaiah G M, McDonald J. An insulin receptor peptide (1135–1156) stimulates guanosine 5′-[γ-thio]triphosphate binding to the 67 kDa G-protein associated with the insulin receptor. Biochem J. 1993;294:19–24. doi: 10.1042/bj2940019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kishimoto A, Brown M S, Slaughter C A, Goldstein J L. Phosphorylation of serine 833 in cytoplasmic domain of low density lipoprotein receptor by a high molecular weight enzyme resembling casein kinase II. J Biol Chem. 1987;262:1344–1351. [PubMed] [Google Scholar]
- 28.Kounnas M Z, Henkin J, Argraves W S, Strickland D K. Low density lipoprotein receptor-related protein/α2-macroglobulin receptor mediates cellular uptake of pro-urokinase. J Biol Chem. 1993;268:21862–21867. [PubMed] [Google Scholar]
- 29.Kounnas M Z, Moir R D, Rebeck G W, Bush A I, Argraves W S, Tanzi R E, Hyman B T, Strickland D K. LDL receptor-related protein, a multifunctional ApoE receptor, binds secreted beta-amyloid precursor protein and mediates its degradation. Cell. 1995;82:331–340. doi: 10.1016/0092-8674(95)90320-8. [DOI] [PubMed] [Google Scholar]
- 30.Krieger M, Herz J. Structures and functions of multiligand lipoprotein receptors: macrophage scavenger receptors and LDL receptor-related protein (LRP) Annu Rev Biochem. 1994;63:601–637. doi: 10.1146/annurev.bi.63.070194.003125. [DOI] [PubMed] [Google Scholar]
- 31.Lai A, Sisodia S S, Trowbridge I S. Characterization of sorting signals in the beta-amyloid precursor protein cytoplasmic domain. J Biol Chem. 1995;270:3565–3573. [PubMed] [Google Scholar]
- 32.Lefkowitz R J. G protein-coupled receptors. III. New roles for receptor kinases and β-arrestins in receptor signaling and desensitization. J Biol Chem. 1998;273:18677–18680. doi: 10.1074/jbc.273.30.18677. [DOI] [PubMed] [Google Scholar]
- 33.Li Y, Marzolo M P, van Kerkhof P, Strous G J, Bu G. The YXXL motif, but not the two NPXY motifs, serves as the dominant endocytosis signal for LDL receptor-related protein (LRP) J Biol Chem. 2000;275:17187–17194. doi: 10.1074/jbc.M000490200. [DOI] [PubMed] [Google Scholar]
- 34.Margolis B L, Lax I, Kris R, Dombalagian M, Honegger A M, Howk R, Givol D, Ullrich A, Schlessinger J. All autophosphorylation sites of epidermal growth factor (EGF) receptor and HER2/neu are located in their carboxyl-terminal tails. Identification of a novel site in EGF receptor. J Biol Chem. 1989;264:10667–10671. [PubMed] [Google Scholar]
- 35.Morton P A, Owensby D A, Wun T C, Billadello J J, Schwartz A L. Identification of determinants involved in binding of tissue-type plasminogen activator-plasminogen activator inhibitor type 1 complexes to HepG2 cells. J Biol Chem. 1990;265:14093–14099. [PubMed] [Google Scholar]
- 36.Murayama Y, Okamoto T, Ogata E, Asano T, Iiri T, Katada T, Ui M, Grubb J H, Sly W S, Nishimoto I. Distinctive regulation of the functional linkage between the human cation-independent mannose 6-phosphate receptor and GTP-binding proteins by insulin-like growth factor II and mannose 6-phosphate. J Biol Chem. 1990;265:17456–17462. [PubMed] [Google Scholar]
- 37.Narita M, Bu G, Holtzman D M, Schwartz A L. The low-density lipoprotein receptor-related protein, a multifunctional apolipoprotein E receptor, modulates hippocampal neurite development. J Neurochem. 1997;68:587–595. doi: 10.1046/j.1471-4159.1997.68020587.x. [DOI] [PubMed] [Google Scholar]
- 38.Obermoeller L M, Warshawsky I, Wardell M R, Bu G. Differential functions of triplicated repeats suggest two independent roles for the receptor-associated protein as a molecular chaperone. J Biol Chem. 1997;272:10761–10768. doi: 10.1074/jbc.272.16.10761. [DOI] [PubMed] [Google Scholar]
- 39.Obermoeller L M, Chen Z, Schwartz A L, Bu G. Ca2+ and receptor-associated protein are independently required for proper folding and disulfide bond formation of the low density lipoprotein receptor-related protein. J Biol Chem. 1998;273:22374–22381. doi: 10.1074/jbc.273.35.22374. [DOI] [PubMed] [Google Scholar]
- 40.Oleinikov A V, Zhao J, Makker S P. Cytosolic adaptor protein Dab2 is an intracellular ligand of endocytic receptor gp600/megalin. Biochem J. 2000;347:613–621. [PMC free article] [PubMed] [Google Scholar]
- 41.Pearson R B, Kemp B E. Protein kinase phosphorylation site sequences and consensus specificity motifs: tabulations. In: Sefton B M, Hunter T, editors. Protein phosphorylation. San Diego, Calif: Academic Press, Inc.; 1998. pp. 65–83. [DOI] [PubMed] [Google Scholar]
- 42.Sibley D R, Benovic J L, Caron M G, Lefkowitz R J. Regulation of transmembrane signaling by receptor phosphorylation. Cell. 1987;48:913–922. doi: 10.1016/0092-8674(87)90700-8. [DOI] [PubMed] [Google Scholar]
- 43.Stockinger W, Brandes C, Fasching D, Hermann M, Gotthardt M, Herz J, Schneider W J, Nimpf J. The reelin receptor ApoER2 recruits JNK-interacting proteins-1 and -2. J Biol Chem. 2000;275:25625–25632. doi: 10.1074/jbc.M004119200. [DOI] [PubMed] [Google Scholar]
- 44.Strickland D K, Kounnas M Z, Argraves W S. LDL receptor-related protein: a multiligand receptor for lipoprotein and proteinase catabolism. FASEB J. 1995;9:890–898. doi: 10.1096/fasebj.9.10.7615159. [DOI] [PubMed] [Google Scholar]
- 45.Sun H, Chen Z, Poppleton H, Scholich K, Mullenix J, Weipz G J, Fulgham D L, Bertics P J, Patel T B. The juxtamembrane, cytosolic region of the epidermal growth factor receptor is involved in association with α-subunit of Gs. J Biol Chem. 1997;272:5413–5420. [PubMed] [Google Scholar]
- 46.Trommsdorff M, Borg J P, Margolis B, Herz J. Interaction of cytosolic adaptor proteins with neuronal apolipoprotein E receptors and the amyloid precursor protein. J Biol Chem. 1998;273:33556–33560. doi: 10.1074/jbc.273.50.33556. [DOI] [PubMed] [Google Scholar]
- 47.Trommsdorff M, Gotthardt M, Hiesberger T, Shelton J, Stockinger W, Nimpf J, Hammer R E, Richardson J A, Herz J. Reeler/Disabled-like disruption of neuronal migration in knockout mice lacking the VLDL receptor and ApoE receptor 2. Cell. 1999;97:689–701. doi: 10.1016/s0092-8674(00)80782-5. [DOI] [PubMed] [Google Scholar]
- 48.Ulery P G, Beers J, Mikhailenko I, Tanzi R E, Rebeck G W, Hyman B T, Strickland D K. Modulation of beta-amyloid precursor protein processing by the low density lipoprotein receptor-related protein (LRP). Evidence that LRP contributes to the pathogenesis of Alzheimer's disease. J Biol Chem. 2000;275:7410–7415. doi: 10.1074/jbc.275.10.7410. [DOI] [PubMed] [Google Scholar]
- 49.Warshawsky I, Bu G, Schwartz A L. 39-kD protein inhibits tissue-type plasminogen activator clearance in vivo. J Clin Investig. 1993;92:937–944. doi: 10.1172/JCI116669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Webb D J, Nguyen D H, Gonias S L. Extracellular signal-regulated kinase functions in the urokinase receptor-dependent pathway by which neutralization of low density lipoprotein receptor-related protein promotes fibrosarcoma cell migration and matrigel invasion. J Cell Sci. 2000;113:123–134. doi: 10.1242/jcs.113.1.123. [DOI] [PubMed] [Google Scholar]
- 51.Weaver A M, Hussaini I M, Mazar A, Henkin J, Gonias S L. Embryonic fibroblasts that are genetically deficient in low density lipoprotein receptor-related protein demonstrate increased activity of the urokinase receptor system and accelerated migration on vitronectin. J Biol Chem. 1997;272:14372–14379. doi: 10.1074/jbc.272.22.14372. [DOI] [PubMed] [Google Scholar]
- 52.Willnow T E, Sheng Z, Ishibashi S, Herz J. Inhibition of hepatic chylomicron remnant uptake by gene transfer of a receptor antagonist. Science. 1994;264:1471–1474. doi: 10.1126/science.7515194. [DOI] [PubMed] [Google Scholar]
- 53.Willnow T E, Moehring J M, Inocencio N M, Moehring T J, Herz J. The low-density-lipoprotein receptor-related protein (LRP) is processed by furin in vivo and in vitro. Biochem J. 1996;313:71–76. doi: 10.1042/bj3130071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Willnow T E, Nykjaer A, Herz J. Lipoprotein receptors: new roles for ancient proteins. Nat Cell Biol. 1999;1:E157–E162. doi: 10.1038/14109. [DOI] [PubMed] [Google Scholar]
- 55.Yamazaki H, Bujo H, Kusunoki J, Seimiya K, Kanaki T, Morisaki N, Schneider W J, Saito Y. Elements of neural adhesion molecules and a yeast vacuolar protein sorting receptor are present in a novel mammalian low density lipoprotein receptor family member. J Biol Chem. 1996;271:24761–24768. doi: 10.1074/jbc.271.40.24761. [DOI] [PubMed] [Google Scholar]
- 56.Zagursky R J, Sharp D, Solomon K A, Schwartz A. Quantitation of cellular receptors by a new immunocytochemical flow cytometry technique. BioTechniques. 1995;18:504–509. [PubMed] [Google Scholar]