Abstract
We investigated the role of the cyclin-dependent kinase inhibitors p21Cip1 and p27Kip1 in cell cycle regulation during hypoxia and reoxygenation. While moderate hypoxia (1 or 0.1% oxygen) does not significantly impair bromodeoxyuridine incorporation, at very low oxygen tensions (0.01% oxygen) DNA replication is rapidly shut down in immortalized mouse embryo fibroblasts. This S-phase arrest is intact in fibroblasts lacking the cyclin kinase inhibitors p21Cip1 and p27Kip1, indicating that these molecules are not essential elements of the arrest pathway. Hypoxia-induced arrest is accompanied by dephosphorylation of pRb and inhibition of cyclin-dependent kinase 2, which results in part from inhibitory phosphorylation. Interestingly, cells lacking the retinoblastoma tumor suppressor protein also display arrest under hypoxia, suggesting that pRb is not an essential mediator of this response. Upon reoxygenation, DNA synthesis resumes by 3.5 h and reaches aerobic levels by 6 h. Cells lacking p21, however, resume DNA synthesis more rapidly upon reoxygenation than wild-type cells, suggesting that this inhibitor may play a role in preventing premature reentry into the cell cycle upon cessation of the hypoxic stress. While p27 null cells did not exhibit rapid reentry into the cell cycle, cells lacking both p21 and p27 entered S phase even more aggressively than those lacking p21 alone, revealing a possible secondary role for p27 in this response. Cdk2 activity is also restored more rapidly in the double-knockout cells when returned to normoxia. These studies reveal that restoration of DNA synthesis after hypoxic stress, but not the S phase arrest itself, is regulated by p21 and p27.
Tumor hypoxia is considered a limiting factor in response to therapy, particularly radiation and chemotherapy. The radioresistance of hypoxic cells due to dose modification is well documented (35). Since hypoxic cells are poorly perfused, they are likely to receive an inadequate dosage of chemotherapeutic drugs delivered via the bloodstream. Even if these cells do receive sufficient drug, their slowly cycling nature has been proposed to make them resistant to chemotherapeutic drugs, which in most cases are potent only against rapidly dividing cells. Therefore, understanding the pathway by which cells arrest their cycle in response to oxygen deprivation could potentially help circumvent this resistance.
DNA synthesis has been found to diminish upon the removal of oxygen from cell culture systems, and a substantial body of work addresses this apparent requirement for oxygen in DNA replication (reviewed in reference 13). Explanations for these findings have focused on two critical enzymes in nucleoside biosynthesis, ribonucleotide reductase and dihydroorotate dehydrogenase, which require molecular oxygen for their function. Mammalian ribonucleotide reductase contains a tyrosyl radical in its active site that requires oxygen and iron for its regeneration; in vitro studies have found that the radical has a half-life of approximately 10 min under anaerobic conditions (36). A second oxygen sensitive enzyme, dihydroorotate dehydrogenase, resides in the mitochondrial membrane, and its activity is coupled to the respiratory chain (21, 23). It has been hypothesized that inactivation of these enzymes under hypoxia would lead to a shortage of deoxynucleotide precursors for DNA synthesis and a consequent shutdown of DNA replication. Thus, these oxygen-dependent enzymes could potentially act as sensors to halt DNA replication under adverse conditions (23).
Studies using alkaline sedimentation analysis and fiber autoradiography have revealed that replicon initiation is suppressed by severe oxygen deprivation, but chain elongation and maturation continue normally for at least a few hours (11, 29, 32). While the persistence of chain elongation under hypoxic conditions implies that the lack of precursors is not directly responsible for the failure to synthesize DNA, it has been proposed that the nucleotide imbalance caused by hypoxia can act as a signal leading to cessation of replication (32). Indeed, drugs such as N-phosphonoacetyl-l-aspartate (PALA), which inhibits uridine biosynthesis, have been found to cause a G1/S, checkpoint-type arrest in cells with wild-type p53 but S-phase arrest in cells lacking functional p53 (19). Attempts to test the hypothesis that nucleotide shortage is a causative factor in hypoxia-induced arrest have gotten mixed results; while addition of exogenous nucleosides and/or deoxynucleosides can cause some entry into S phase and replicon initiation in hypoxic cells, it does not allow for normal progression through the cell cycle (20–22, 31). Thus, while nucleotide imbalance may play a role in inhibition of replication under hypoxia, it is not the only barrier to cell cycling in the absence of oxygen.
Though early studies on Ehrlich ascites cells and NHIK cells suggested that nucleotide availability was the limiting factor in progression through S phase under hypoxia, recent studies have suggested that this is not the case in all cell types. Human T-47D breast carcinoma cells, which unlike NHIK cells contain functional pRb, appeared to have a secondary arrest pathway that prevented cellular DNA synthesis under hypoxia even in the presence of exogenous deoxynucleosides. Amellem et al. suggest that nucleotide shortage is still the primary cause of onset of arrest and that pRb is involved in the maintenance of arrest (1).
Research on more moderate hypoxia (∼1% O2 as opposed to <0.1%; normal atmospheric oxygen is ∼21%), under which nucleotide biosynthesis is unlikely to be severely impaired, has supported the hypothesis that hypoxia leads to a cyclin/Cdk-dependent, checkpoint-type arrest. Krtolica et al. (16, 17) have observed decreased Cdk4 and Cdk2 activity in CV-1P monkey kidney cells treated with ∼1% O2, and this kinase inhibition was associated with an increase in p27Kip1 and a decrease in Cdk4, cyclin D, and cyclin E protein levels. They propose that this decreased kinase activity, coupled with increased PP-1 phosphatase activity, leads to dephosphorylation of pRb and inhibition of cell cycle progression (16). Similar results were obtained in ovarian carcinoma cells, suggesting a possible common pathway (17).
Cell cycle arrest induced by hypoxia is reversible, though the extent of reversibility is reduced with longer or more stringent hypoxic treatments (30). In fact, most cells are able to resume DNA synthesis within 10 min to 3 h after reoxygenation (38). However, little information is known about the genetic determinants that govern the reentry of hypoxic cells into the cell cycle except that recovery of DNA synthesis is blocked by cycloheximide addition in Ehrlich ascites cells (11, 32) and is associated with the appearance of hyperphosphorylated pRb in T-47D breast cancer cells (2).
Earlier work in our lab revealed that hypoxia induces arrest in a variety of transformed and nontransformed cell types and that this response is independent of p53 status (12). Here, we further examine the oxygen-dependency, kinetics, and reversibility of the cell cycle response to hypoxia in nontransformed, immortalized mouse embryo fibroblasts (MEFs). We find that only stringent hypoxia (<0.01% O2) elicits a robust cell cycle arrest response; DNA synthesis ceases within 5 to 10 h and recovers within a few hours after reoxygenation. Using genetically matched cell lines we further demonstrate that neither p21Cip1, p27Kip1, nor pRb is necessary for cell cycle arrest under hypoxia. Hypoxia causes a 50 to 75% decrease in Cdk2 activity that is independent of p21 and p27 and is not associated with appreciable changes in cyclin E, cyclin A, or Cdk2 protein or in Thr160 or Tyr15 phosphorylation of Cdk2; however, dephosphorylation of Thr14 and Tyr15 with CDC25B can partially restore activity to Cdk2 complexes from hypoxic cells. While not necessary for arrest under hypoxia, p21 and p27 do play a role in inhibiting cell cycle reentry upon reoxygenation; both DNA synthesis and Cdk2 activity are restored more rapidly in cells lacking both p21 and p27. These results suggest that p21 and p27 act as important modulators of cellular reentry into S phase.
MATERIALS AND METHODS
Tissue culture and hypoxia treatment.
Immortalized wild type, p21−/−, p27−/−, and p21−/− p27−/− MEFs were generously provided by James Roberts (7). All were found to be wild type for p53 by cDNA sequence analysis (data not shown). Rb−/− and wild-type MEFs were generously provided by Scott Lowe and immortalized in our lab. All cells were grown at 37°C in Dulbecco modified Eagle medium plus 10% fetal bovine serum in the presence of 5% CO2. Cells were plated at a density of 9 × 105 cells per 150-mm glass dish 18 to 24 h prior to hypoxic treatment; the medium was changed ∼1 h before treatment to assure an adequate nutrient and growth factor supply. Cells were rendered hypoxic by placement in a Bactron IV anaerobic chamber (Sheldon Manufacturing) filled with a defined gas mixture. For moderate hypoxia experiments, this gas mixture was 5% CO2, 1 or 0.1% O2, and the remainder nitrogen. For stringent hypoxia, the chamber was filled with a mixture of 90% nitrogen, 5% CO2, and 5% hydrogen, and a palladium catalyst was used to scavenge any contaminating oxygen by combining it with hydrogen, bringing the oxygen concentration to <0.01%. Oxygen concentrations of between 0.01 and 0.1% were found to be experimentally infeasible; without the use of the oxygen-scavenging catalyst, which reduces oxygen essentially to zero, oxygen concentration rapidly rises in this semisealed chamber such that frequent purging is necessary to maintain even a 0.1% oxygen level. Oxygen concentration was monitored using an oxygen electrode (OS1000; Oxygen Sensors, Inc., Frazer, Pa.), whose threshold of detection is 0.01% oxygen, or oxygen-sensitive chemical strips (Becton Dickinson, Cockeysville, Md.).
Flow cytometry.
During the last half hour of treatment (2 h for the experiment with Rb−/− and p130−/− cells), S-phase cells were labeled with 10 μM bromodeoxyuridine (BrdU; a thymidine analog) added directly to the medium. Cells were then removed from the dishes by trypsinization, collected in ice-cold phosphate-buffered saline (PBS), and centrifuged 5 min at 500 × g to pellet them. For hypoxic samples, trypsinization was carried out under hypoxia to avoid BrdU incorporation upon reoxygenation. Cells were then washed in cold PBS, fixed in 80% ethanol, and stored at −20°C until stained. Staining with fluorescein isothiocyanate (FITC)-conjugated anti-BrdU antibody (Becton Dickinson, San Jose, Calif.) and propidium iodide was carried out essentially according to the manufacturer's instructions. Briefly, cells were treated with 2 N hydrochloric acid plus 0.5% Triton X-100 to denature the DNA, washed in 0.1 M sodium tetraborate to neutralize the acid, and then stained with anti-BrdU antibody diluted 1:50 in PBS containing 0.5% Tween 20 and 1% bovine serum albumin (BSA). Following anti-BrdU staining, cells were washed in the same solution and resuspended in 5 μg of propidium iodide per ml in PBS. After at least 1 h on ice, cells were analyzed on a FACScalibur flow cytometer (Becton Dickinson).
[3H]thymidine incorporation.
[3H]thymidine was added to dishes at a concentration of 2 μCi/ml 1 h prior to lysis. Cells were lysed by adding 200 μl of lysis buffer (25 mM Tris, pH 7.5; 25 mM EDTA; 0.5% sodium dodecyl sulfate [SDS]) directly to the dish. Lysate was collected and precipitated with 12% trichloroacetic acid (TCA), and precipitated counts were collected on Whatman GF-C filters. Filters were washed extensively with 20 mM sodium pyrophosphate–5% TCA followed by 70% ethanol, dried, immersed in scintillation fluid, and counted.
Cell extracts, IPs, and kinase assays.
Cells were removed from dishes by trypsinization. For hypoxic samples, this was generally done after removal from hypoxia; carrying out this step within the hypoxic environment was found to have no effect on final results. Cells were collected in cold PBS and washed in PBS, and the pellet was resuspended in immunoprecipitation (IP) buffer (50 mM HEPES, pH 7.5; 150 mM NaCl; 1 mM EDTA; 0.1% Tween 20) plus 10 mM sodium fluoride, 1 mM sodium orthovanadate, 20 mM β-glycerophosphate, 5 μg of leupeptin per ml, 5 μg of aprotinin per ml, 1 μg of pepstatin per ml, and 1 mM phenylmethylsulfonyl fluoride. Extracts were sonicated on ice twice for 10 s and centrifuged 10 min at 15,000 rpm in a microcentrifuge to remove debris. The protein concentration was measured using the BCA assay (Pierce, Rockford, Ill.).
For IP reactions, 100 to 300 μg (for kinase assays) or 0.5 to 1 mg (for Western blotting) of cell extract was incubated with 1 to 5 μg of antibody in a 0.4- to 1-ml total volume 1× IP buffer with inhibitors for 4 to 12 h. To this reaction, 20 to 60 μl of a 1:4 slurry of protein A/G PLUS Agarose (Santa Cruz) was added, and the reaction was incubated for 1 h. The beads were then collected by centrifugation and washed once in IP buffer and then three times in kinase reaction buffer (20 mM Tris, pH 7.4; 7.5 mM MgCl2; 1 mM dithiothreitol [DTT]). For Western blots, the beads were then resuspended in 20 μl of kinase reaction buffer plus 20 μl of 2× SDS dissociation buffer and stored at −80°C. For kinase assays, the beads were resuspended in 50 μl of kinase reaction mixture (kinase reaction buffer plus 20 μM ATP, 40 μg of Histone H1 [Sigma] per ml, and 5 μCi of [γ-32P]ATP [Amersham]) and incubated for 30 min at 37°C. The kinase reaction was stopped by the addition of 50 μl of 2× SDS dissociation buffer and boiling at 95°C for 5 min. Beads were then removed by centrifugation, and 50 μl of the reaction was run on a SDS–12.5% polyacrylamide gel electrophoresis (PAGE) gel. The gel was dried and exposed to a screen that was scanned on a Molecular Dynamics Storm Imager. For Cdk2 reactivation experiments, IPs were carried out as described above with 300 μg of cell extract and 3 μg of anti-Cdk2 antibody (Santa Cruz). Beads were then washed once in IP buffer and three times in phosphatase buffer (100 mM Tris, pH 7.4; 250 mM NaCl; 1 mM DTT). The reaction mixture was divided into three tubes, and CDC25B or an equal volume of glutathione elution buffer was added. This reaction was then incubated for 30 min at 30°C; the beads were then washed three times in kinase reaction buffer.
Antibodies and Western blotting.
Rabbit polyclonal antibody to p21Cip1 was a generous gift from Greg Hannon. Mouse monoclonal antibodies to p27Kip1, Cdk2, phosphotyrosine (PY20), and p130Rb2 were from Transduction Laboratories; rabbit polyclonal antibodies to cyclin A, p57Kip2, and p107 (C-18) and goat polyclonal antibody to Cdk2 (used for IPs) were from Santa Cruz Biotechnology. Polyclonal antibody to cyclin E was generously provided by James Roberts.
Samples (35 μg of cell extract or total IP supernatant) were loaded onto an SDS-PAGE gel and run at 30 mA/gel. Proteins were transferred to polyvinylidene difluoride (PVDF) membrane using a semidry transfer apparatus. Membranes were blocked in Tris-buffered saline (TBS) containing 5% nonfat milk and 0.2% Tween 20 for 1 h at room temperature (except for the antiphosphotyrosine blots, in which BSA was used instead of milk) and then incubated overnight in primary antibody diluted in the same solution at 4°C. They were then subjected to three 15-min washes in TBST (TBS plus 1% BSA, 0.1% Tween 20) and incubated in alkaline phosphatase-conjugated anti-mouse or anti-rabbit secondary antibody (Vector Labs) diluted 1:2,500 in TBST for 1 h. After three 15-min washes in TBST, the membranes were incubated in AttoPhos reagent (Vistra, Amersham, and Molecular Dynamics) for 5 min, dried, and scanned on a Storm Imager (Molecular Dynamics).
CDC25B expression and purification.
Glutathione S-transferase (GST)-CDC25B bacterial expression construct was kindly provided by Tony Hunter (University of California at San Diego). The protein was expressed in BL21-Gold(DE3)pLysS-competent cells (Stratagene). Bacteria were grown to mid-log phase (optical density at 60 nm of 0.6 to 0.7) and induced with 0.6 mM IPTG for 2 h at room temperature. They were then harvested, washed in TD buffer (150 mM NaCl; 10 mM Tris-HCl, pH 7.5) and lysed in TD plus 1% Tween 20 and 1% Triton X-100 by sonication three times for 10 s each time. Extracts were clarified by centrifugation at 15,000 rpm in a microcentrifuge for 10 min. Then, 100 μl of glutathione-Sepharose was added, and the reaction was incubated overnight at 4°C. Beads were then washed four times in wash buffer (500 mM NaCl; 10 mM HEPES, pH 8.0; 10 mM DTT) and eluted in glutathione elution buffer (100 mM Tris-HCl, pH 8.0; 10 mM DTT; 5 mM glutathione) for 3 h at 4°C. Beads were centrifuged out, and the supernatant was used fresh for Cdk2 reactivation.
RESULTS
The majority of previous studies investigating the molecular factors contributing to hypoxia-induced cell cycle arrest relied on transformed cells that were not genetically matched. In order to study the molecules necessary for arrest in normal cells, we used matched immortalized MEFs that contain deletions in defined cell cycle regulatory genes. We initially determined the level of oxygen at which cellular growth was inhibited. While normal atmospheric oxygen contains ca. 21% oxygen, tissues typically experience a lower oxygen concentration in the range of 3 to 9% (3, 15, 37). Thus, mild reductions in oxygen concentration often improve cell growth compared to growth in air; higher-than-normal oxygen (hyperoxia) appears to limit cell growth due to oxidative stress. At some level of hypoxia, however, oxygen availability becomes limiting and cell proliferation is inhibited. Oxygen concentrations as low as 1% have been found to enhance cell growth; however, other reports have found this oxygen level to be growth inhibiting (3, 17).
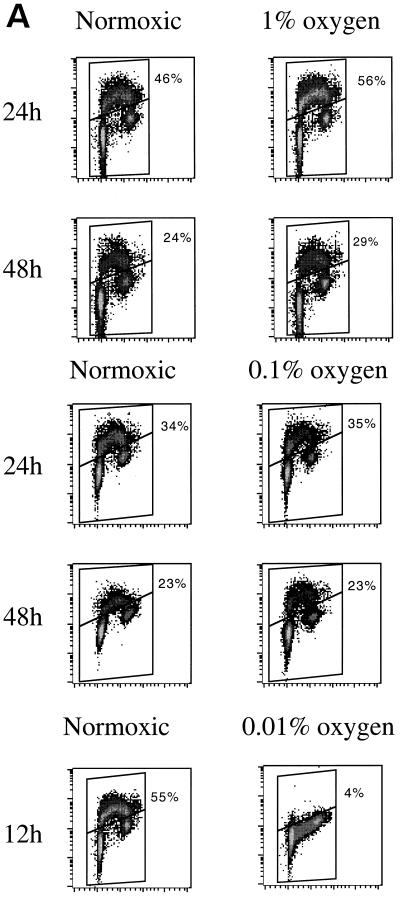
We exposed immortalized MEFs to 1% oxygen to determine whether this degree of hypoxia would lead to a cell cycle arrest. After 24 or 48 h, no cell cycle inhibition was apparent in 1% O2-treated cultures. At 24 h, hypoxic cells appeared to show a somewhat larger S phase (BrdU-positive) population than normoxic controls (56 versus 46%; Fig. 1). At 48 h, both aerobic and hypoxic cultures exhibited a slight decrease in the S phase compared to the situation at 24 h, a finding presumably resulting from the depletion of nutrients and/or growth factors from the medium; there was no significant difference in cell cycle distribution between control and hypoxic cells.
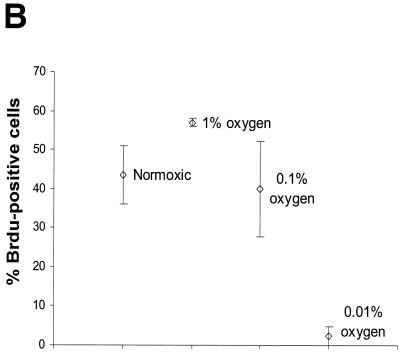
FIG. 1.
Cell cycle response to moderate and stringent hypoxia in immortalized MEFs. (A) 2D cell cycle diagrams of cells treated with 1, 0.1, or 0.01% oxygen compared to time-matched normoxic controls. The x axis is red propidium iodide fluorescence; the y axis is green fluorescence from FITC-conjugated anti-BrdU antibody. The percentage of cells that are BrdU positive is indicated. (B) Composite data from multiple experiments, indicating the percentage of cells that are BrdU positive after 24 h (12 h in the case of 0.01% oxygen treatment).
Similar experiments at 0.1% oxygen revealed that even this rather profound decrease in oxygen tension did not appear to inhibit cell cycling. Cell cycle profiles of cells incubated in either 21 or 0.1% oxygen for 24 or 48 h reveal virtually no changes in BrdU incorporation in hypoxic samples compared with time-matched controls (Fig. 1A). These experiments were repeated several times, and composite results are graphed in Fig. 1B. After 24 h of treatment, the number of BrdU-positive cells was consistently higher in 1% oxygen-treated samples and unchanged in 0.1% oxygen-treated samples. Stringent hypoxia (<0.01% O2), in contrast, led to complete growth arrest within 12 h (Fig. 1).
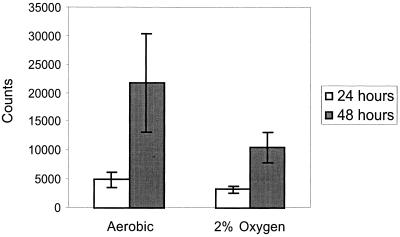
These results are at odds with recent reports that have found oxygen concentrations of 0.5 to 1.5% oxygen to be capable of inducing cell cycle arrest (16–18). This difference may be due, in part, to differences in cell type; this previous work was done with ovarian carcinoma cells and CV-1P monkey kidney cells. It may also be due to differences in methodology, including both the methods used to achieve hypoxia (described in detail in Materials and Methods) and the methods used to measure DNA synthesis, specifically [3H]thymidine incorporation versus BrdU incorporation. While these two methods might seem very similar, two-dimensional (2D) fluorescence-activated cell sorter (FACS) analysis measures DNA synthesis on a cell-by-cell basis, while [3H]thymidine incorporation measures the total incorporation in a dish of cells. As a result, [3H]thymidine incorporation, unlike 2D FACS analysis, is influenced by cell number as well as by the percentage of cells in S phase and the rate of DNA synthesis. Therefore, during a prolonged hypoxic treatment, a subtle decrease in proliferation rate could lead to a pronounced reduction in [3H]thymidine incorporation. To test the possibility that these two measures would lead to different conclusions, we incubated cells in 2% oxygen for 24 and 48 h and measured [3H]thymidine incorporation during a 1-h pulse-label at the conclusion of the experiment (Fig. 2). Using this method, we found that hypoxic samples did exhibit reduced [3H]thymidine incorporation compared to aerobic conditions; in this experiment, the difference was only borderline statistically significant. However, a comparison between the 24- and 48-h samples revealed two interesting facts. First, incorporation increased more than fourfold in the aerobic samples at 48 h; this difference is presumably due entirely to the increase in cell number, since the fraction of cells in S phase would not be expected to increase. Second, incorporation increased threefold in the hypoxic samples, suggesting that significant cellular proliferation is able to take place under these conditions, albeit at a slightly reduced rate compared to the aerobic conditions. Therefore, we believe that the reductions in [3H]thymidine incorporation under moderate hypoxia are largely due to the accumulated effects of a mild deceleration of progress through the cell cycle and not a true “arrest.”
FIG. 2.
Effect of mild hypoxia on tritiated-thymidine incorporation. Raw counts are plotted for dishes incubated in 2 or 21% oxygen for 24 or 48 h.
In order to study the genetics and biochemistry of cell cycle arrest under hypoxia, we considered it necessary to use conditions capable of eliciting a robust arrest response. Therefore, the remaining experiments were performed using stringent hypoxic treatments in which the oxygen concentration was kept as low as was experimentally achievable using an oxygen-free gas mixture and an oxygen-scavenging catalyst. Some cancer cell lines are able to continue BrdU incorporation for as long as 24 to 48 h under these conditions (10); therefore, we believe the arrest observed in MEFs is the result of an active signaling pathway rather than nucleotide pool depletion or other energetic or metabolic limitations.
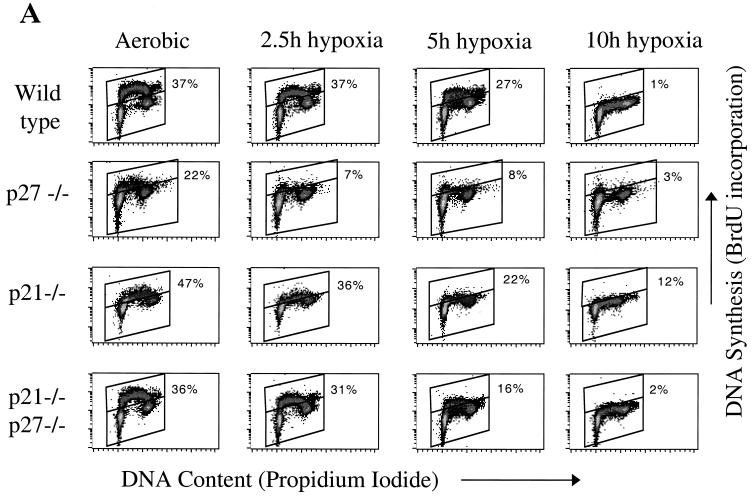
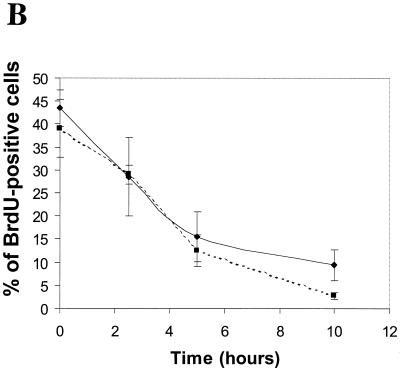
A closer examination of the kinetics of arrest showed that immortalized wild-type MEFs exhibit a gradual decrease in DNA synthesis under stringent hypoxic conditions. In these cells, a slight reduction in BrdU incorporation is apparent by as early as 2.5 h, and by 10 h there is virtually no incorporation (Fig. 3). Interestingly, essentially no change is observed in the DNA histograms of these populations; analysis with ModFit reveals the G1, S, and G2 populations to vary by no more than 5% of the total number of cells, and no consistent trends are observed. Therefore, the decrease in DNA synthesis observed in these cells appears to be the result of cells arresting in mid-S phase rather than accumulating in another phase of the cell cycle.
FIG. 3.
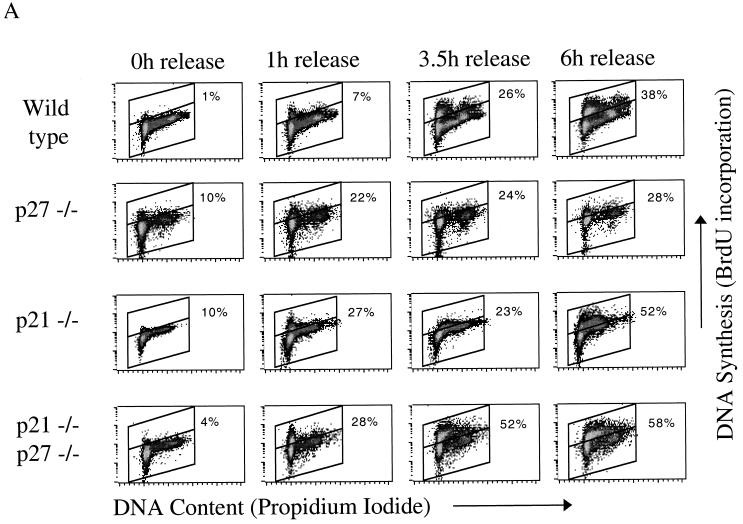
Cell cycle behavior of matched immortalized MEFs during hypoxia. (A) 2D cell cycle diagrams of all four cells lines from a representative experiment. (B) Composite data for wild-type (⧫) and p21−/− p27−/− double-knockout (■) cell lines from all experiments; error bars represent the standard error. Cells were placed in an anaerobic chamber for the times indicated, pulse-labeled for 30 min with BrdU under continuous hypoxia, and then collected and prepared for analysis as described in Materials and Methods.
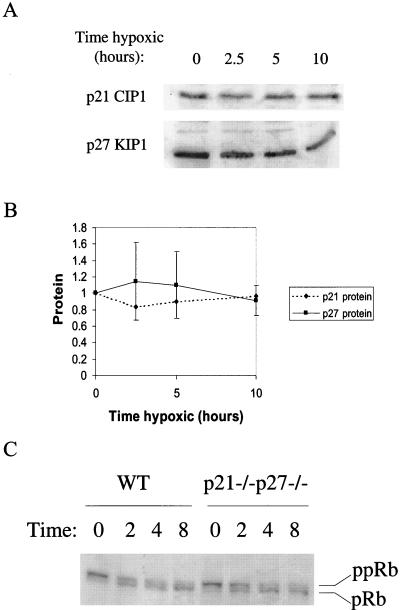
Two molecules that have been implicated in a variety of stress-induced cell cycle arrest pathways are the cyclin kinase inhibitors (CKIs) p21Cip1 and p27Kip1 (6, 9, 33). p27Kip1 has been found to be upregulated by hypoxia and has been suggested as a mediator of hypoxia-induced arrest (5, 16). To more rigorously test the role of these proteins in hypoxia-induced arrest, we obtained immortalized MEFs lacking p21 and p27 individually, as well as cells inactivated in both, and examined their cell cycle behavior under hypoxia. The control aerobic cell cycle distributions differ somewhat among cell lines, which may be a result of the immortalization pathways, as well as the CKI deletions (7). However, all four cells lines arrest with similar kinetics under hypoxia: a reduction in DNA synthesis beginning at 2.5 h leading to total arrest by 10 h (Fig. 3). Furthermore, p21 and p27 levels in the cell are unchanged, as determined by Western blot (Fig. 4A and B). These observations reveal that something other than these CKIs is responsible for blocking DNA synthesis under these severely hypoxic conditions.
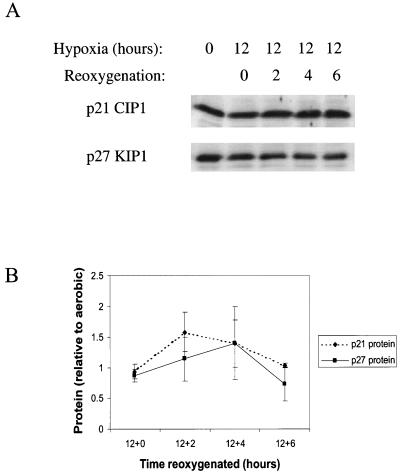
FIG. 4.
(A) Representative Western blots of p21 and p27 in hypoxic cell extracts. (B) Composite Western blot data from multiple experiments. Blots were scanned on a Storm Imager and analyzed with ImageQuant; the numbers given are ratios relative to the control. (C) Western blot of pRb in hypoxia-treated cells; the higher mobility band represents unphosphorylated or hypophosphorylated pRb (pRb) while the lower mobility band contains hyperphosphorylated pRb (ppRb). WT, wild type.
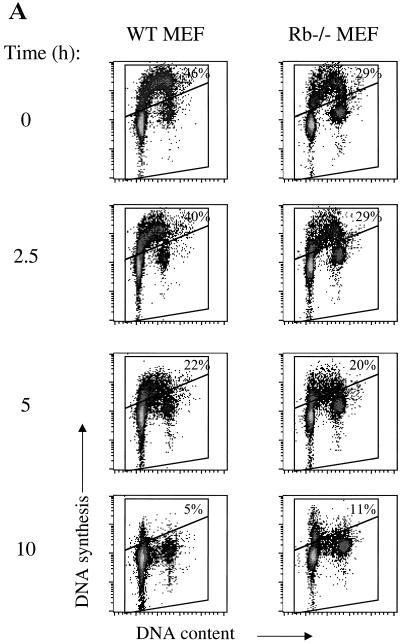
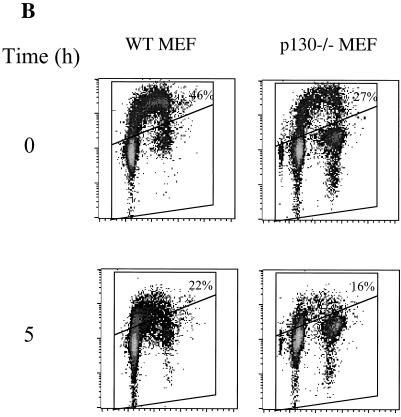
Since p21 and p27 were found not to be essential in induction of arrest under hypoxia, it remained in question whether the lack of DNA synthesis under hypoxia was due to a cyclin-Cdk-dependent arrest pathway or some basic metabolic constraint. To address this question, we examined the phosphorylation state of pRb, an important regulator of the G1/S-phase transition. As early as 2 h after initiation of the hypoxic treatment, pRb begins to appear in the hypophosphorylated, active form, suggesting that pRb could be an upstream element of the hypoxia-induced arrest, as has been proposed previously (16) (Fig. 4C). However, cell cycle analysis of hypoxia-treated Rb knockout cells in comparison to matched immortalized MEFs reveals that they exhibit a normal hypoxia-induced arrest (Fig. 5). Thus, pRb is not a vital component of the arrest pathway. We also assessed the arrest competency of MEFs deleted in p130Rb2, an Rb homolog, and found that they, too, exhibit cell cycle arrest when subjected to hypoxia (Fig. 5B).
FIG. 5.
(A) Cell cycle profiles of Rb−/− and wild-type fibroblasts during hypoxia. (B) Cell cycle profiles of p130−/− cells under hypoxia compared to the wild type. Cells were labeled with BrdU for the final 2 h of treatment. WT, wild type.
These results were surprising, given the importance attributed to pRb in previous studies of hypoxia-induced arrest; however, pRb phosphorylation is not the only important element of the G1/S transition. In fact, while cyclin D1 has been found to be dispensable in cells lacking Rb, cyclin E is still required for S-phase entry in these cells (27). This and other evidence suggests that Cdk2, which forms complexes with cyclins E and A, has targets besides pRb whose phosphorylation is necessary for entry into and progression through S phase. We therefore assayed the activity of Cdk2 in extracts from hypoxia-treated cells. We found that Cdk2 activity decreases dramatically in both wild-type and mutant cells after 4 to 12 h of hypoxia, though to a slightly lower degree in the mutants (Fig 6A and B). Therefore, the activity of this kinase is being downregulated under hypoxia by some means independent of p21 and p27. Replication inhibitors such as hydroxyurea or aphidicolin, in contrast, do not affect Cdk2 activity (reference 25 and unpublished observations), so these data suggest that hypoxia's effects on the cell cycle are not solely the result of direct DNA synthesis inhibition.
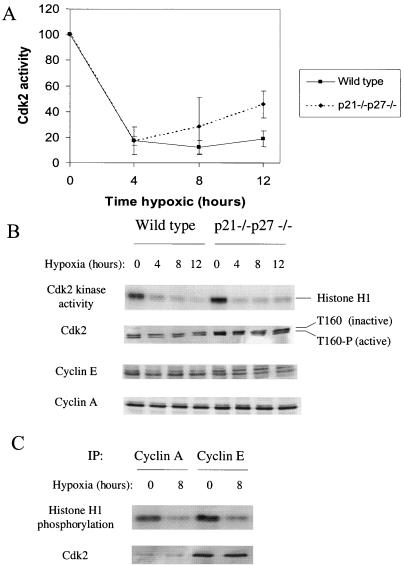
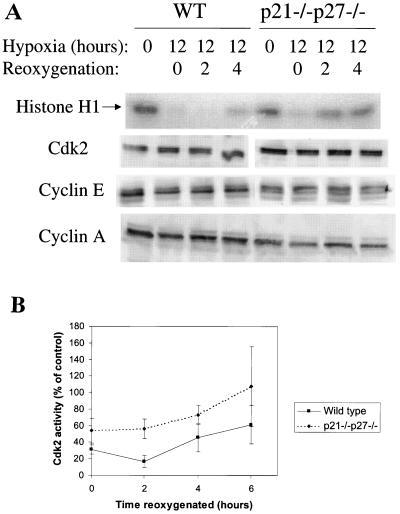
FIG. 6.
Cdk2 activity regulation in hypoxic cells. (A) Cdk2 activity in hypoxia-treated cells, measured by immune-complex kinase assay. Autoradiograms of phosphorylated Histone H1 were scanned and then analyzed by using ImageQuant, and 32P incorporation was plotted as a percentage of the control. (B) Histone H1 autoradiogram and Western blots of Cdk2, cyclin E, and cyclin A from a representative time course. (C) Kinase activity and Cdk2 content of cyclin A and cyclin E immunoprecipitates from control and hypoxic cell extracts.
In order to form an active kinase complex, Cdk2 must first bind to cyclin E or cyclin A. Since hypoxia is known to generally downregulate macromolecular synthesis, it was important to determine whether these subunits were present in adequate amounts in hypoxic cells. By Western blotting, we showed that the levels of Cdk2, cyclin E, and cyclin A remain stable during a 12-h hypoxic treatment (Fig. 6B); thus, the lack of Cdk2 activity in hypoxic extracts is not the result of a decrease in either Cdk2 protein or its cyclin binding partners. After binding to cyclin, Cdk2 must be phosphorylated on Thr160 by the cyclin-activating kinase to become fully active; this phosphorylation leads to an increase in mobility on SDS-PAGE. The relative intensity of the two bands in the Cdk2 doublet on also remains unchanged with hypoxia (Fig. 6B), suggesting that dephosphorylation of this residue is not responsible for Cdk2 inactivation. This analysis was also performed on the p21−/− p27−/− cells to ensure that the behavior of this cell line was the same.
Though Western blots showed that Cdk2 and cyclin E and A levels are essentially unchanged by hypoxic treatment, it was not clear that they actually remain associated in complexes. It was therefore possible that the Cdk2 in hypoxic cells is rendered inactive by sequestration that prevents cyclin association. We tested this hypothesis by measuring the level of cyclin-associated Cdk2. Complexes were immunoprecipitated from control or hypoxic cell extracts using antibodies to cyclin E or cyclin A; the reaction was then divided and assayed for kinase activity or for associated Cdk2 by Western blot. As shown in Fig. 6C, both cyclin A- and cyclin E-associated activities are inhibited by hypoxia, but the amount of bound Cdk2 is unchanged.
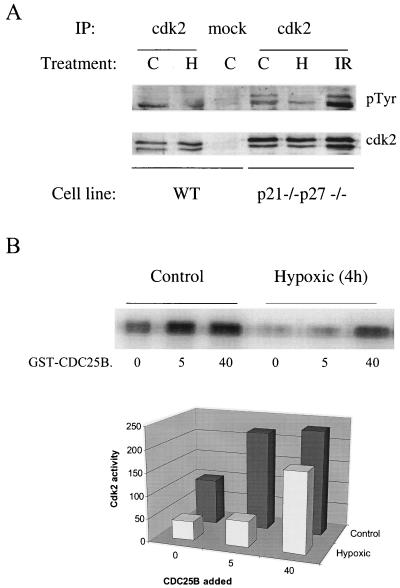
Cdks can also be inactivated by inhibitory phosphorylation; in the case of mouse Cdk2, the inhibitory residues are Thr14 and Tyr15. Most reports have indicated that, of these two inhibitory phosphorylation sites, Tyr15 is the more important and the more frequently phosphorylated in vivo. In fact, Thr14 is seldom if ever phosphorylated in the absence of Tyr15 phosphorylation, at least in normally cycling cells (14). To determine whether Tyr15 phosphorylation was increased by hypoxia, we used an antiphosphotyrosine antibody to probe a Western blot of immunoprecipitated Cdk2. While antiphosphotyrosine immunoreactivity is low, there is a distinct pair of bands present in anti-Cdk2 IPs that is absent in a mock IP (Fig. 7A). Most of the phosphotyrosine appears to be associated with the higher-mobility, Thr160-phosphorylated form of Cdk2, as has been observed previously. However, while phosphotyrosine immunoreactivity is increased in irradiated cells, as reported (8), Cdk2 complexes from hypoxic cells do not demonstrate increased phosphotyrosine staining. Reprobing of this blot with an anti-Cdk2 antibody revealed equal amounts of Cdk2 in control and hypoxic samples (Fig. 6A).
FIG. 7.
Inhibitory phosphorylation under hypoxia. (A) Antiphosphotyrosine and anti-Cdk2 Western blots of anti-Cdk2 or mock IPs. (B) Histone H1 kinase activity of Cdk2 complexes treated with GST-CDC25B.
We further tested whether dephosphorylation of Thr14 and Tyr15 could restore activity to Cdk2 complexes from hypoxic cells. Removal of the inhibitory phosphates from Cdks is carried out by the CDC25 family of dual-specificity phosphatases; a member of this family, CDC25B, can dephosphorylate and reactivate both Cdk2 and Cdc2 in vitro (34). We treated the Cdk2 immunoprecipitates from control and hypoxic cells with GST-tagged CDC25B expressed in bacteria and purified on glutathione agarose. CDC25B treatment leads to significant activation of Cdk2 complexes from hypoxic cells; however, complexes from control cells are activated to a similar degree (Fig. 7B). Cdk2 immunoprecipitates from confluent and serum-starved cells, in contrast, remain inactive upon CDC25B treatment (data not shown). This suggests that inhibitory phosphorylation plays some role in Cdk2 inactivation under hypoxia but probably cannot entirely account for the lack of Cdk2 activity in hypoxic cell extracts.
A few other proteins are known to be capable of inhibiting Cdk2 in vitro or in vivo; these include the Cip/Kip family member p57Kip2 and the Rb-related pocket proteins p130 and p107. Western blot analysis of these proteins did not reveal any significant induction by hypoxia (data not shown); moreover, p130 null cells arrest normally, as shown in Fig. 5B. Thus, Cdk2 inhibition under hypoxia may result in part from a novel mechanism or from changes in complex formation or phosphorylation that are too subtle to be detected by the methods used here.
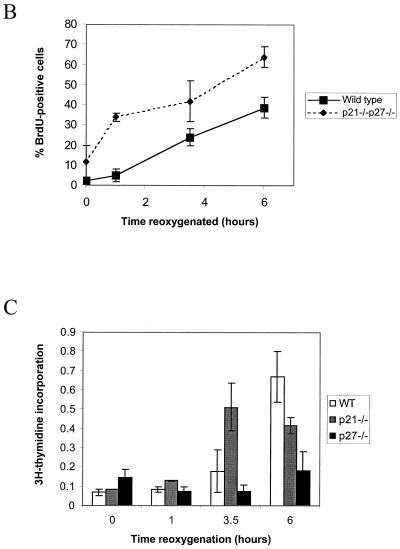
Although p21 and p27 play no significant role in the onset of hypoxia-induced arrest, we hypothesized that these CKIs could regulate the kinetics of cell cycle reentry upon reoxygenation. After a 12-h hypoxic treatment, wild-type cells are able to resume DNA synthesis within 3 h of reoxygenation. A group of G1 DNA content cells synchronously enters S phase after roughly 3.5 h, and by 6 h the number of BrdU-positive cells is comparable to that of aerobic samples (Fig. 8). Approximately 5 to 10% of cells remain arrested in S phase at this point. The p21 knockout cells, on the other hand, initiate DNA synthesis within an hour after release from hypoxia, substantially earlier than the wild type (Fig. 8A). The p27−/− fibroblasts do not exhibit rapid cell cycle reentry, though these results were difficult to interpret due to the slowly cycling nature of this cell line (Fig. 8A). Deletion of p27 in a p21 knockout background, however, results in even greater DNA synthesis posthypoxia; by 6 h the majority of cells are BrdU positive, even more than in aerobic control cells (Fig. 8).
FIG. 8.
Cell cycle profiles of matched immortalized MEFs after reoxygenation. (A) 2D cell cycle diagrams from a representative experiment. (B) Composite BrdU incorporation data for wild-type and double-knockout cell lines from all experiments. (C) [3H]thymidine incorporation. After a 12-h hypoxic treatment, cells were returned to atmospheric oxygen and allowed to reenter the cell cycle. Incorporation for each cell line was normalized to a 12-h aerobic control.
Since these differences in timing are fairly subtle, we reconfirmed the results with the single knockout cells using [3H]thymidine incorporation to assess DNA synthesis (Fig. 8C). As expected, a 12-h hypoxic treatment reduces incorporation to background levels; synthesis recovers upon reoxygenation, but total incorporation remains less than for the time-matched controls because of the reduced number of cells in hypoxia-treated dishes. Cells lacking p21 alone exhibit significantly greater DNA synthesis at 3.5 h of reoxygenation, reconfirming the results obtained by 2D FACS analysis. At 6 h the incorporation in the p21 knockouts is reduced compared to that at 3.5 h, a finding which we believe to be the result of cell death; these cells appear to be hypersensitive to hypoxic stress (data not shown). The p27 knockout cells again show poor incorporation but clearly seem to be recovering at 6 h postreoxygenation.
To further investigate the roles of p21 and p27 in regulating the cell cycle during reoxygenation, we examined the levels of these cyclin kinase inhibitors both under hypoxia and at multiple times after reoxygenation. Both p21 and p27 levels are largely unchanged during a 12-h hypoxic treatment and are only mildly induced upon subsequent reoxygenation (Fig. 9). Although this may seem surprising in light of the cell cycle data, some evidence suggests that redistribution of inhibitory molecules in the absence of major protein induction can have a significant effect on cell cycling (28). Differences in association are not readily apparent by coimmunoprecipitation and Western blotting, but this is not unexpected given the subtlety of the effects observed and the crude nature of this assay.
FIG. 9.
Western blots of p21 and p27 from reoxygenated cells (A), along with a graph of average values over all experiments (B). Cells were exposed to hypoxia for 12 h prior to being returned to atmospheric oxygen.
After reoxygenation, changes in Cdk2 activity in the wild-type and double-knockout cells parallel changes in DNA synthesis (Fig. 10). In the wild-type cells, Cdk2 activity decreases slightly at 2 h of reoxygenation and then begins to recover by 4 h. In the double p21 and p27 knockout cells, kinase activity is consistently higher than that in the wild-type cells, but the kinetics of recovery are variable between experiments (Fig. 10). The changes in activity are not associated with changes in protein levels, since Cdk2, cyclin A, and cyclin E levels remain stable under all of these conditions (Fig. 10A). The close correlation between Cdk2 activity, which is required for S-phase entry, and DNA synthesis implies that kinase activity is a limiting factor for S-phase entry under these conditions.
FIG. 10.
Cdk2 activity regulation in reoxygenated cells. (A) Autoradiograms of phosphorylated Histone H1 and Western blots of Cdk2, cyclin E, and cyclin A from a representative reoxygenation time course. (B) [32P]phosphate incorporation into Histone H1 averaged over all experiments. WT, wild type.
DISCUSSION
In this study, we have observed a cessation of DNA synthesis in immortalized MEFs under low-oxygen conditions similar to that observed in other cell types. This arrest is accompanied by a significant downregulation of Cdk2 kinase activity and therefore is unlikely to result from a direct block to DNA synthesis such as deoxynucleotide shortage. Interestingly, the hypoxia-induced arrest is intact in cells lacking pRb, implying that other Cdk2 targets are mediating this response. We found that the kinase inhibition is not due to association with the inhibitory molecules p27Kip1 or p21Cip1; both the arrest and the Cdk2 activity downregulation under hypoxia are normal in cells lacking both p21 and p27. Cdk2, cyclin E, and cyclin A protein levels are largely unaffected by hypoxia, so the lack of kinase activity is also not due to lack of the Cdk2 protein itself or its cyclin-binding partners. Activating phosphorylation on Thr160, inhibitory phosphorylation on Tyr15, and the binding of cyclins by Cdk2 also appear unchanged, though Cdk2 complexes from hypoxic cells can be partially reactivated by treatment with CDC25B. The mechanism by which Cdk2 is inactivated under hypoxia is still under investigation; while inhibitory phosphorylation appears responsible in part, a further means of inhibition exists which could act through a novel mechanism or through changes too subtle to be detected by the methods used here.
We have also found that both the arrest and the kinase inhibition under hypoxia are reversible and regulated by p21 and p27 upon reoxygenation. In wild-type cells, Cdk2 activity and DNA synthesis are restored within 6 h after the return to normoxia, while this recovery is much more rapid in the p21 p27 double-knockout cells. This may be due to p21 and p27 representing an inhibitory “threshold” which must be overcome in the wild-type cells. Also, a moderate (∼50%) induction of these CKIs after reoxygenation may contribute to the prolonged Cdk2 inhibition in the wild-type cells. p21−/− p27−/− cells also appear to be hypersensitive to hypoxia in a clonogenic survival assay (data not shown); while a variety of factors may influence the colony-forming potential in immortalized cells, it is interesting to note that premature reentry into the cell cycle may impair viability.
These results suggest that hypoxia-induced Cdk2 inhibition occurs through a pathway distinct from known stress-activated cell cycle arrest pathways, many of which appear to involve p21 and/or p27. These include both genotoxic stresses, such as ionizing or UV radiation (4, 28), and nongenotoxic stresses, such as transforming growth factor β treatment or serum deprivation (6, 33). Interestingly, oxidative stresses such as peroxide treatment and hyperoxia are among the stimuli known to upregulate p21 (26, 39). This could explain the p21 induction caused by reoxygenation, which is known to lead to free radical production and can be considered a stress in itself. In addition, the p21 message, but not protein, is induced in a p53-independent manner under hypoxia in a variety of cell lines (10), and reoxygenation may allow translation of this accumulated mRNA.
Our finding that pRb is not essential to the arrest in response to hypoxia appears to be in conflict with previously published work. However, this work has been mostly correlative, demonstrating that arrest under hypoxia is associated with the appearance of hypophosphorylated pRb (1, 24). Amellem et al. did find that T-47D breast cancer cells, unlike two Rb-null cancer cell lines, could not be stimulated to enter S phase upon deoxynucleotide addition under hypoxia, and these authors attributed this to a secondary arrest pathway dependent on functional Rb. However, no matched cell lines that differed in Rb status were compared (1). Our results are therefore consistent with previously published reports in that we, too, observed pRb hypophosphorylation under hypoxia but differ from them in that we found that this modification is not a critical component of the arrest pathway. Therefore, other Cdk2 targets are likely to be involved in mediating the arrest in response to hypoxia.
ACKNOWLEDGMENTS
This work was supported by NIH grants PO1 CA67166 and CA64489 to A.J.G. S.L.G. was supported in part by a Lucille P. Markey fellowship in Molecular Mechanisms of Disease.
We thank James Roberts for the wild-type, p21−/−, p27−/−, and p21−/− p27−/− fibroblasts and the cyclin E antibody; Greg Hannon for the p21 antibody; and Scott Lowe for the wild-type, Rb−/−, and p130−/− fibroblasts. We also thank members of the Giaccia and Roberts labs for useful advice and assistance.
REFERENCES
- 1.Amellem O, Sandvik J A, Stokke T, Pettersen E O. The retinoblastoma protein-associated cell cycle arrest in S-phase under moderate hypoxia is disrupted in cells expressing HPV18 E7 oncoprotein. Br J Cancer. 1998;77:862–872. doi: 10.1038/bjc.1998.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amellem O, Stokke T, Sandvik J, Pettersen E. The retinoblastoma gene product is reversibly dephosphorylated and bound in the nucleus in S and G2 phases during hypoxic stress. Exp Cell Res. 1996;227:106–115. doi: 10.1006/excr.1996.0255. [DOI] [PubMed] [Google Scholar]
- 3.Balin A K, Fisher A J, Carter D M. Oxygen modulates growth of human cells at physiologic partial pressures. J Exp Med. 1984;160:152–166. doi: 10.1084/jem.160.1.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brugarolas J, Moberg K, Boyd S D, Taya Y, Jacks T, Lees J A. Inhibition of cyclin-dependent kinase 2 by p21 is necessary for retinoblastoma protein-mediated G1 arrest after gamma-irradiation. Proc Natl Acad Sci USA. 1999;96:1002–1007. doi: 10.1073/pnas.96.3.1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carmeliet P, Dor Y, Herbert J M, Fukumura D, Brusselmans K, Dewerchin M, Neeman M, Bono F, Abramovitch R, Maxwell P, Koch C J, Ratcliffe P, Moons L, Jain R K, Collen D, Keshert E. Role of HIF-1alpha in hypoxia-mediated apoptosis, cell proliferation and tumour angiogenesis. Nature. 1998;394:485–490. doi: 10.1038/28867. . (Erratum, 395:525.) [DOI] [PubMed] [Google Scholar]
- 6.Coats S, Flanagan W M, Nourse J, Roberts J M. Requirement of p27Kip1 for restriction point control of the fibroblast cell cycle. Science. 1996;272:877–880. doi: 10.1126/science.272.5263.877. [DOI] [PubMed] [Google Scholar]
- 7.Coats S, Whyte P, Fero M L, Lacy S, Chung G, Randel E, Firpo E, Roberts J M. A new pathway for mitogen-dependent cdk2 regulation uncovered in p27(Kip1)-deficient cells. Curr Biol. 1999;9:163–173. doi: 10.1016/s0960-9822(99)80086-4. [DOI] [PubMed] [Google Scholar]
- 8.D'Anna J A, Valdez J G, Habbersett R C, Crissman H A. Association of G1/S-phase and late S-phase checkpoints with regulation of cyclin-dependent kinases in Chinese hamster ovary cells. Radiat Res. 1997;148:260–271. [PubMed] [Google Scholar]
- 9.Deng C, Zhang P, Harper J W, Elledge S J, Leder P. Mice lacking p21CIP1/WAF1 undergo normal development, but are defective in G1 checkpoint control. Cell. 1995;82:675–684. doi: 10.1016/0092-8674(95)90039-x. [DOI] [PubMed] [Google Scholar]
- 10.Denko N C, Green S L, Edwards D, Giaccia A J. p53 checkpoint-defective cells are sensitive to X rays, but not hypoxia. Exp Cell Res. 2000;258:82–91. doi: 10.1006/excr.2000.4928. [DOI] [PubMed] [Google Scholar]
- 11.Gekeler V, Epple J, Kleymann G, Probst H. Selective and synchronous activation of early-S-phase replicons of Ehrlich ascites cells. Mol Cell Biol. 1993;13:5020–5033. doi: 10.1128/mcb.13.8.5020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Graeber T G, Peterson J F, Tsai M, Monica K, Fornace A J, Jr, Giaccia A J. Hypoxia induces accumulation of p53 protein, but activation of a G1-phase checkpoint by low-oxygen conditions is independent of p53 status. Mol Cell Biol. 1994;14:6264–6277. doi: 10.1128/mcb.14.9.6264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Green S L, Giaccia A J. Tumor hypoxia and the cell cycle: implications for malignant progression and response to therapy. Cancer J Sci Am. 1998;4:218–223. [PubMed] [Google Scholar]
- 14.Gu Y, Rosenblatt J, Morgan D O. Cell cycle regulation of CDK2 activity by phosphorylation of Thr160 and Tyr15. EMBO J. 1992;11:3995–4005. doi: 10.1002/j.1460-2075.1992.tb05493.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kallinowski F, Zander R, Hoeckel M, Vaupel P. Tumor tissue oxygenation as evaluated by computerized-pO2-histography. Int J Radiat Oncol Biol Phys. 1990;19:953–961. doi: 10.1016/0360-3016(90)90018-f. [DOI] [PubMed] [Google Scholar]
- 16.Krtolica A, Krucher N A, Ludlow J W. Hypoxia-induced pRB hypophosphorylation results from downregulation of CDK and upregulation of PP1 activities. Oncogene. 1998;17:2295–304. doi: 10.1038/sj.onc.1202159. [DOI] [PubMed] [Google Scholar]
- 17.Krtolica A, Krucher N A, Ludlow J W. Molecular analysis of selected cell cycle regulatory proteins during aerobic and hypoxic maintenance of human ovarian carcinoma cells. Br J Cancer. 1999;80:1875–1883. doi: 10.1038/sj.bjc.6690615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Krtolica A, Ludlow J W. Hypoxia arrests ovarian carcinoma cell cycle progression, but invasion is unaffected. Cancer Res. 1996;56:1168–1173. [PubMed] [Google Scholar]
- 19.Linke S P, Clarkin K C, Di Leonardo A D, Tsou A, Wahl G M. A reversible, p53-dependent G0/G1 cell cycle arrest induced by ribonucleotide depletion in the absence of detectable DNA damage. Genes Dev. 1996;10:934–947. doi: 10.1101/gad.10.8.934. [DOI] [PubMed] [Google Scholar]
- 20.Loffler M. Characterization of the deoxynucleoside-dependent reversal of hypoxia-induced inhibition of cell cycle progression in Ehrlich ascites tumor cells. Eur J Cell Biol. 1985;39:198–204. [PubMed] [Google Scholar]
- 21.Loffler M. A cytokinetic approach to determine the range of O2-dependence of pyrimidine(deoxy)nucleotide biosynthesis relevant for cell proliferation. Cell Prolif. 1992;25:169–179. doi: 10.1111/j.1365-2184.1992.tb01392.x. [DOI] [PubMed] [Google Scholar]
- 22.Loffler M. Restimulation of cell cycle progression by hypoxic tumour cells with deoxynucleosides requires ppm oxygen tension. Exp Cell Res. 1987;169:255–261. doi: 10.1016/0014-4827(87)90243-6. [DOI] [PubMed] [Google Scholar]
- 23.Loffler M, Jockel J, Schuster G, Becker C. Dihydroorotat-ubiquinone oxidoreductase links mitochondria in the biosynthesis of pyrimidine nucleotides. Mol Cell Biochem. 1997;174:125–129. [PubMed] [Google Scholar]
- 24.Ludlow J W, Howell R L, Smith H C. Hypoxic stress induces reversible hypophosphorylation of pRB and reduction in cyclin A abundance independent of cell cycle progression. Oncogene. 1993;8:331–339. [PubMed] [Google Scholar]
- 25.Matsumoto Y, Hayashi K, Nishida E. Cyclin-dependent kinase 2 (Cdk2) is required for centrosome duplication in mammalian cells. Curr Biol. 1999;9:429–432. doi: 10.1016/s0960-9822(99)80191-2. [DOI] [PubMed] [Google Scholar]
- 26.McGrath S A. Induction of p21WAF/CIP1 during hyperoxia. Am J Respir Cell Mol Biol. 1998;18:179–187. doi: 10.1165/ajrcmb.18.2.2964m. [DOI] [PubMed] [Google Scholar]
- 27.Ohtsubo M, Theodoras A M, Schumacher J, Roberts J M, Pagano M. Human cyclin E, a nuclear protein essential for the G1-to-S phase transition. Mol Cell Biol. 1995;15:2612–2624. doi: 10.1128/mcb.15.5.2612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Poon R Y, Toyoshima H, Hunter T. Redistribution of the CDK inhibitor p27 between different cyclin-CDK complexes in the mouse fibroblast cell cycle and in cells arrested with lovastatin or ultraviolet irradiation. Mol Biol Cell. 1995;6:1197–1213. doi: 10.1091/mbc.6.9.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Probst G, Riedinger H J, Martin P, Engelcke M, Probst H. Fast control of DNA replication in response to hypoxia and to inhibited protein synthesis in CCRF-CEM and HeLa cells. Biol Chem. 1999;380:1371–1382. doi: 10.1515/BC.1999.177. [DOI] [PubMed] [Google Scholar]
- 30.Probst H, Schiffer H, Gekeler V, Kienzle-Pfeilsticker H, Stropp U, Stotzer K, Frenzel-Stotzer I. Oxygen dependent regulation of DNA synthesis and growth of Ehrlich ascites tumor cells in vitro and in vivo. Cancer Res. 1988;48:2053–2060. [PubMed] [Google Scholar]
- 31.Probst H, Schiffer H, Gekeler V, Scheffler K. Oxygen-dependent regulation of mammalian ribonucleotide reductase in vivo and possible significance for replicon initiation. Biochem Biophys Res Commun. 1989;163:334–340. doi: 10.1016/0006-291x(89)92140-2. [DOI] [PubMed] [Google Scholar]
- 32.Riedinger H J, Gekeler V, Probst H. Reversible shutdown of replicon initiation by transient hypoxia in Ehrlich ascites cells. Dependence of initiation on short-lived protein. Eur J Biochem. 1992;210:389–398. doi: 10.1111/j.1432-1033.1992.tb17433.x. [DOI] [PubMed] [Google Scholar]
- 33.Robson C N, Gnanapragasam V, Byrne R L, Collins A T, Neal D E. Transforming growth factor-beta1 up-regulates p15, p21 and p27 and blocks cell cycling in G1 in human prostate epithelium. J Endocrinol. 1999;160:257–266. doi: 10.1677/joe.0.1600257. [DOI] [PubMed] [Google Scholar]
- 34.Sebastian B, Kakizuka A, Hunter T. Cdc25M2 activation of cyclin-dependent kinases by dephosphorylation of threonine-14 and tyrosine-15. Proc Natl Acad Sci USA. 1993;90:3521–3524. doi: 10.1073/pnas.90.8.3521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tannock I F, Hill R P, editors. The basic science of oncology. 2nd ed. New York, N.Y: McGraw-Hill, Inc.; 1992. [Google Scholar]
- 36.Thelander L, Graslund A, Thelander M. Continual presence of oxygen and iron required for mammalian ribonucleotide reduction: possible regulation mechanism. Biochem Biophys Res Commun. 1983;110:859–865. doi: 10.1016/0006-291x(83)91040-9. [DOI] [PubMed] [Google Scholar]
- 37.Vaupel P, Schlenger K, Knoop C, Hockel M. Oxygenation of human tumors: evaluation of tissue oxygen distribution in breast cancers by computerized O2 tension measurements. Cancer Res. 1991;51:3316–3322. [PubMed] [Google Scholar]
- 38.Wilson R E, Keng P C, Sutherland R M. Changes in growth characteristics and macromolecular synthesis on recovery from severe hypoxia. Br J Cancer. 1990;61:14–21. doi: 10.1038/bjc.1990.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yakes F M, Van Houten B. Mitochondrial DNA damage is more extensive and persists longer than nuclear DNA damage in human cells following oxidative stress. Proc Natl Acad Sci USA. 1997;94:514–519. doi: 10.1073/pnas.94.2.514. [DOI] [PMC free article] [PubMed] [Google Scholar]