Abstract
Antigen (ag)-specific T cell analysis is an important step for investigation of cellular immunity in many settings, such as infectious diseases, cancer and vaccines. Multiparameter flow cytometry has advantages in studying both the rarity and heterogeneity of these cells. In the cellular immunologist's toolbox, the expression of activation-induced markers (AIM) following antigen exposure has made possible the study and sorting of ag-specific T cells without using human leukocyte antigen (HLA)-multimers. In parallel, assessing the cytokine profile of responding T cells would support a more comprehensive description of the ongoing immune response by providing information related to cell function, such as polarization and effector activity. Here, a method and flow cytometry panel were optimized to combine the detection of activated CD4+ and CD8+ T cells in a TCR-dependent manner with the evaluation of cytokine production by intracellular staining, without affecting the positivity of activation markers. In particular, the expression of CD134 (OX40) and CD69 have been tested in conjunction with intracellular (ic) CD137 (4-1BB) to detect SARS-CoV-2 Spike protein-specific activated T cells. In our setting, CD134 provided minimal contribution to detect the pool of AIM+ T cells, whereas a key role was described for ic-CD69 which was co-expressed with ic-CD137 in both CD4+ and CD8+ lymphocytes. Moreover, the analysis of TCR-triggered cytokine-producing T cells (IFNγ, TNFα and IL-2 were assessed) further confirmed the capacity of ic-CD69 to identify functionally responsive antigen-specific T cells which were often largely negative or weakly positive for CD134 expression. In parallel, the use of CD45RA, CCR7 and CXCR5 allowed us to describe the T cell matuarion curve and detect T follicular helper (Tfh) CD4+ cells, including the antigen specific activated subsets.
In conclusion, we optimized a method and flow cytometry panel combining assessment of activation induced markers and intracellular cytokines that will be useful for measuring TCR stimulation-dependent activation of CD4+ and CD8+ T cells.
Keywords: SARS-Cov-2 vaccine, T cell mediated immunity, Flow cytometry
1. Introduction
The identification and study of ag-specific T cells is key for a broad range of immunological research, including infectious diseases and vaccine responses (Appay et al., 2008). T cell mediated immunity against a pathogen or following vaccine treatment is a consequence of the coordinated contribution of two arms: cytotoxic CD8+ T cells mainly responsible for the clearance of target cells (René, 2003) and CD4+ T cells that interact with B cells and CD8+ T cells for optimal development, maturation, and maintenance of immunity against pathogens or vaccines (Borst et al., 2018). Therefore, monitoring both CD4+ and CD8+ responses is crucial for immunological research. Peptide/HLA (pHLA) multimers (Altman et al., 1996) accurately detect ag-specific T cells, but the knowledge of the immunogenic peptide, its HLA allele restriction, and the availability of the reagents are limiting factors, in particular for the CD4+ T cell population, and rarely sufficient to appreciate the breadth of ag-specific T cells in relation to functional capabilities. Deep analysis of the ag-specific T cell repertoire requires assays that focus on specific functions such as production of a particular cytokine or degranulation in response to antigen (Mosmann et al., 1997; Sallusto et al., 2004). However, such functional assays often reflect only a subset of specifically activated cells, as the in vivo T-cell response is composed of functionally diverse cells that cannot be identified using a single cytokine or the detection of degranulation (Mosmann et al., 1997) (Sallusto et al., 2004). To overcome the limitations of these approaches and discriminate simultaneously both TCR-stimulated CD4+ and CD8+ T cells, the detection of activation-induced markers (AIMs) in response to antigen has facilitated the assessment of the complete antigen-specific T cell response.
Favorable characteristics of such surrogate markers would include specific surface expression after activation over a transient but sufficiently prolonged time period to allow reliable detection, corroborated by absent or low molecule expression if unstimulated and during resting phases. Several markers have been shown to be specifically induced in a TCR stimulation-dependent manner, such as CD154, CD107, CD137 and CD134 (Betts et al., 2003) (Zaunders et al., 2009). CD154 (CD40L) molecule is mainly expressed on activated CD4+ T cells, being in fact proposed to monitor and isolate antigen-specific T helper cells (Chattopadhyay et al., 2006; Meier et al., 2008; Yellin et al., 1994). CD107A is an endosomal component and translocates to the cell surface during degranulation of ag-specific T cells triggered by TCR stimulation, therefore it is considered the most significant marker to define the cytotoxic potential (Betts et al., 2003) (Rubio et al., 2003). CD137 (4-1BB) is a member of the TNF Receptor family that mediates costimulatory function, and it has been identified as an up-regulated molecule expressed on activated human CD8+ and CD4+ T cells from 12 h to up to 5 days after stimulation depending on the stimulus (Vinay and Kwon, 1998) (Dawicki and Watts, 2004). CD134 (OX-40) also belongs to the TNF Receptor superfamily and is a marker of antigen-specific CD4+ T cells (Zaunders et al., 2009). More recently, the CD134 and CD137 combined expression has been used to identify antigen specific CD4+ T cells (Reiss et al., 2017; Dan, 2017).
Moreover, early activation markers such as CD25 and CD69 have been used to identify antigen responsive CD4+ T cells. Even if these markers assessed alone may lack specificity (Bremser et al., 2015; Kmieciak et al., 2009), their adoption in conjunction with TCR stimulation dependent markers may increase the sensitivity and discrimination of rare cells in flow cytometry data.
Among the TCR stimulation dependent markers, CD137 appears to be particularly interesting given its kinetics of expression after antigen recognition and its biologic role to provide a survival signal to activated T cells, both CD4+ and CD8+ T lymphocytes (Watts, 2005) (Wen et al., 2002). In parallel, emerging evidence are pointing to the value of assessing the cytokine profile of AIM+ T cells to better describe the intensity and features of ongoing immune response (Painter, 2021; Yu et al., 2021; Braun et al., 2020).
In the present study, we optimized a straightforward protocol and related flow cytometry panel for detection of ag-specific CD4+ and CD8+ T cells. This has been developed using Spike reactive samples and further tested using CMV antigen. The study resulted in a method relying on intracellular evaluation of activation induced CD137 and CD69 expression, in combination with Th1 cytokine profiling. That allows describing the antigen specific T cell maturation curve in addition to the T helper follicular cell sub-population.
2. Results
To recover the maximum number of TCR-dependent activated T cells, and in parallel evaluate their cytokine profile, three points have been addressed in optimizing the protocol: i) the impact of cytokine release inhibitors on AIM expression, ii) the combination of AIM markers, and iii) the flow cytometry staining protocol in parallel with the panel design and data analysis approach.
2.1. Determining conditions for resolving activation induced markers and cytokines
A key consideration to optimize a reliable AIM test combined with cytokine detection is whether AIM marker staining should be performed on the cell surface (s) or intracellular (ic), since inhibitors of endosomal trafficking (Brefeldin-A BFA and monensin) are known to prevent secretion of both cytokines and surface markers (Nylander and Kalies, 1999) (O'Neil-Andersen and Lawrence, 2002). Our previous experience (not shown), in agreement with published literature, suggests that ic-CD137 staining can be performed successfully, in parallel with ic-cytokine evaluation, to detect both CD4+ and CD8+ T cell activation. This can be done after overnight incubation in the presence of stimuli and inhibitor of endosomal trafficking (BFA) (Braun et al., 2020; Yan et al., 2017).
As the activation molecules CD69 (expressed by CD4+ and CD8+ cells) and CD134 (expressed by CD4+) have been among the first published markers, used in conjunction with CD137, to detect antigen specific T cells in the context of anti SARS-Cov-2 immune response (Grifoni et al., 2020), we tested the intracellular versus surface staining of these markers with the goal of defining which approach is most reliable for the detection of antigen specific T cell activation, in conjunction with intracellular cytokine assessment. For this evaluation 106 cells/sample were seeded per well in a 96-well round bottom plate, with or without Spike peptide pool stimulation (see methods), in the presence of anti-CD28 monoclonal antibody at 1 μg/ml. After 4 h of incubation at 37 °C, 5% CO2, the protein transport inhibitor Golgi-Plug (Brefeldin A, 1 μl/ml) was added for a further incubation of 20 h in all the samples.
As shown in Fig. 1A, staining of ic-CD69 strongly correlated with ic-CD137 expression for both CD4+ (row I) and CD8+ cells (row II) and allowed clear resolution of ic-CD137+ elements able to produce cytokines. In contrast, the use of ic/s-CD134 to stain CD4+ cells (row III and IV) or s-CD69 to stain CD8+ cells (row V) displayed no co-expression (ic/sCD134) or much lower co-expression level (s-CD69) with ic-CD137 and failed to capture most of the ic-CD137+ cytokine producing cells.
Fig. 1.
ic-CD69 co-stains with ic-CD137 thus facilitating the measurement of cytokine+ ic-CD137bright CD4+ and CD8+ T lymphocytes.
A Exemplary plots showing patterns of ic-CD137, ic/s-CD69, ic/s-CD134, and cytokines on stimulated CD4+ or CD8+ T cells. Rows I and II show ic-CD137+ CD4+ and CD8+ cells co-stained with ic-CD69: in this approach almost all the CD4+ or CD8+ cytokine producing cells are ic-CD69+. Rows III and IV show ic-CD137+ CD4+ cells co-stained with ic/s-CD134 respectively: in this approach the majority of cytokine producing CD4+ cells are ic/s-CD134-. Rows V shows ic-CD137+ CD8+ cells co-stained with s-CD69: in this approach many of the cytokine producing CD8+ cells are s-CD69-. Large dots represent ic-CD137bright cells.
B Analysis of concordance of s/ic-CD69 and s/ic-CD134 staining with total ic-CD137bright CD4+ and CD8+ events and their cytokine producing subset. Ic-CD69 is the only marker showing full concordance (Trendline regression slopes =1). Each dot represents a sample: blue dots refer to CD69 staining, orange dots refer to CD134 staining. Four donors have been assessed at three different incubation times.
Abbreviations: ic = intracellular; s = surface.
Next, we explored the concordance of the three activation-induced markers and cytokines for different conditions. Specifically, the co-expression of CD134 or CD69 with ic-CD137bright was assessed on four donors at three time points (16–20-24 h of incubation in the presence of Brefeldin A), revealing a clear pattern shown in Fig. 1B (graphs I to VIII). Ic-CD69 staining perfectly correlated with ic-CD137bright staining on both CD4+ and CD8+ cells. Linear trendlines showed a slope of 1.00 and the correlation coefficient R2 was equal to 0.999 in both cases (Graphs I and V). Conversely, ic-CD134 staining was negative on most of ic-CD137bright CD4+ events having a slope of just 0.21 and R2 of 0.85 (Graph I). Interestingly, s-CD69 staining also showed reduced capacity to co-stain with ic-CD137bright on CD4+ and CD8+ cells, with slopes of 0.69 and 0.35 and R2 of 0.94 and 0.67, respectively (Graphs II and VI). Notably s-CD134 staining was almost completely negative on ic-CD137bright events on CD4+ subset displaying a slope of 0.04 and R2 of 0.04 (Graph II). Then, we evaluated the capacity of CD69 and CD134 to co-stain with cytokine producing ic-CD137bright CD4+ and CD8+ events. Once again, only ic-CD69 showed a perfect correlation with these events resulting in slopes equal to 1 and R2 of 0.999 (Graphs III and VII). S-CD69 on CD4+ or CD8+ correlated to a lower extent with cytokine producing ic-CD137bright elements and s-CD134 did not correlate at all (Graphs IV and VIII).
To rule out that the poor performance of ic-CD134 to detect AIM+ CD4+ T cells was not clone dependent, we compared the previously used Clone L106 with Clone Act35, which is also referenced in the literature as suitable for AIM testing (Grifoni et al., 2020). We activated PBMCs from three healthy donors vaccinated with BNT162b2 and analyzed the percentages of co-expression for ic-CD134 and ic-CD137. As shown in Supplemental Fig. 1, even if ic-CD134 clone Act35 displays higher level of co-expression with ic-CD137 (ranging from 14 to 24% versus 9.9 to 19.4% of clone L106) its performance was far from showing the full concordance observed by using ic-CD69.
2.2. Analysis of antigen responding T cells
In this study, we defined as AIM+ T cells the T elements staining bright for ic-CD137 and being positive for the second activation marker (ic-CD69 or ic-CD134). We also defined as Cytokine + AIM+ T cells the subset of AIM+ T events producing any of the assessed cytokines. Additionally, we referred to as Antigen Responding (AR) T cells the percentage of AIM+ T cells calculated as the difference between stimulated and unstimulated samples, and as Cytokine producing Antigen Responding (Cytokine + AR) T cells the AR T cells further defined as producing any of the assessed cytokine. Unstimulated samples were always treated with co-stimulus/i only.
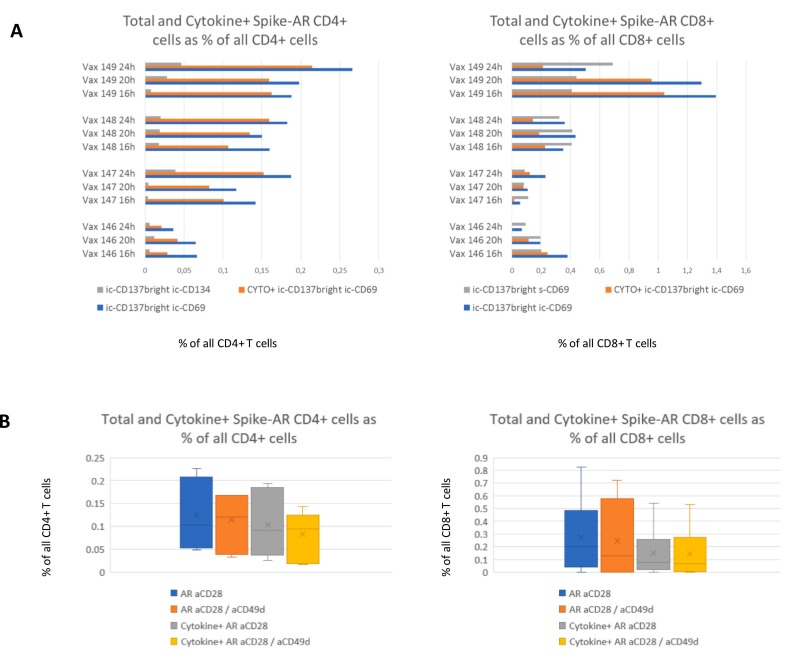
We assessed the effectiveness of s/ic-CD134 and s/ic-CD69 to detect AR T cells in conjunction with ic-CD137. As displayed in Fig. 2A, ic-CD69 in conjunction with ic-CD137 allowed a higher counting of AR CD4+ cells in comparison to ic-CD134. In parallel, ic-CD69 performed better than s-CD69 when either was used in conjunction with ic-CD137 to quantify CD8+ AR cells. In the same experiment the effect of different incubation times was explored on the percentages of AR T cells, (total or cytokine producing fraction) without displaying a clear time-dependent trend. For this reason, we selected the intermediate time of 20 h of incubation with BFA, and ic-CD69 was indeed designated as the best companion activation marker to be used in conjunction with ic-CD137 for defining AR CD4+ or CD8+ T cells. The choice was further confirmed looking at cell viability which remained stable over time ranging from 92.8% after 16 h, to 91.3% after 20 hors and and to 91.8% after 24 h of incubation (not shown).
Fig. 2.
Spike-AR CD4+ and CD8+ assessment based on incubation time and co-stimuli.
A Graphs represent the percentages of Total and Cytokine+ Spike-AR CD4+ and CD8+ T cells calculated as the difference between stimulated and unstimulated samples. Spike-AR cells have been evaluated based on different activation phenotypes being for CD4+ cells: ic-CD137bright and ic-CD134+ (gray bars, left graph); ic-CD137bright and ic-CD69+ (blue bars, left graph); Cytokine+ ic-CD137bright and ic-CD69+ (orange bar left graph). With regard to CD8+ cells, activation phenotypes were: ic-CD137bright and s-CD69+ (gray bars, right graph); ic-CD137bright and ic-CD69+ (blue bars, right graph), Cytokine+ ic-CD137bright and ic-CD69+ (orange bars, right graph). Cytokine positivity includes Spike-AR cells producing at least one of the three evaluated cytokines: IFNγ, TNFα or IL-2. The use of ic-CD137 and ic-CD69 results in higher percentage of Spike-AR retrieval for both CD4+ and CD8+. Variation in incubation times in the presence of Brefeldin A from 16 to 24 h did not lead to clear trends in the percentage of Spike-AR cells.
B Graphs represent the percentage of total and cytokine producing Spike-AR T cells out of CD4+ and CD8+ populations to compare the costimulatory affect of aCD28 only versus aCD28 and aCD49d. The addition of aCD49d does not result in significant changes of Spike-AR events detection, with or without cytokines. Evaluation was performed on four donors, three of which were assessed in duplicate.
Abbreviations: AR = Antigen Responding, ic = intracellular; s = surface.
With the aim of optimizing T-cell co-stimulation, we verified if the use of anti-CD49d in conjunction with anti-CD28 monoclonal antibodies (mAbs) may help to quantify higher percentages of detectable AR T cells and their cytokine producing subset. We perfomed the comparison on the same four COVID-19 reactive samples previously used in the kinetic experiments, assessing three out of four in duplicate in independent experiments. We found that adding CD49d did not result in substantial change, and thus it was excluded from further evaluation (Fig. 2B).
2.3. Panel and protocol optimization
Having finetuned the above-described variables, we next improved the design of the flow cytometry panel and staining protocol.
The non-informative CD134 marker was eliminated and replaced with CXCR5 to detect T helper follicular cells (Tfh), defined as CD4+ CD45RA- CXCR5+ events ((Reiss et al., 2017). For this purpose, the panel was slightly redesigned as illustrated in Supplemental Table 1. The BV421 channel was assigned to CXCR5 for maximum sensitivity, the former CD4 (V450) reagent in this channel was moved in place of CD45RA APC-H7, which was in turn assigned to the BV786 detector, previously dedicated to CD134. These changes left the activation markers and cytokine fluorochromes unmodified and allowed better resolution of the T cell maturation curve through increased brightness of CD45RA.
The staining protocol was also furter optimized, as careful data analysis during the setup phase highlighted that activated ic-CD137bright T cells underwent a 60% statistically significant decrease of CD3 Mean Fluorescent Intensity (MFI) (p = 7.6−19). This was clearly detectable in the CD3 vs ic-CD137 dot plot (Supplemental fig. 2A, plot 1) and raised the question of whether CD3 downmodulation would either include non-T-cells or miss some T-cells in the expanded gate. For this reason, the re-designed panel was preliminarily acquired on five different donors in an extended form which included CD19, CD16, CD56 and CD14. Data revealed that the CD3dim cells were completely negative for CD19 and CD14, and partially showed a dim positivity for CD56 and CD16, which is compatible with the NKT phenotype. Interestingly, the ic-CD137bright CD3neg population was only partially positive for the exclusion markers. Gating of lineage negative cells allowed to capture subsets of CD69+ cytokine-producing CD4+ and CD8+ elements, which may be due to either functional T cells - characterized by complete downmodulation of surface CD3 antigen - or by innate lymphoid cells (ILCs), which are ‘lineage negative’ and known to produce these cytokines (some ILC1s are CD4+, whereas others are CD8+ or CD4-CD8-). (Supplemental fig. 2A). Based on these findings, we performed further evaluations using ic-CD3 staining, and found that an ic-CD137bright CD69+ subset negative for ic- CD3 and surface CD19/CD16/CD56/CD14 was still present, yet CD4 and CD8 expression, as well as cytokine production, were almost completely abrogated (Supplemental fig. 2B). As such ic-CD3 staining was established as the best condition to detect AIM+ T cells in our context.
Notably, CD8 and CD4 staining were also affected by a statistically significant 32% and 6% MFI decrease respectively (p = 8.8–15 and p = 0.003), which did not interfere with correct subset identification.
We also assessed whether Fluorescence Minus Two (FMT) controls -consisting of the full panel without ic-CD137 and ic-CD69- could help for gate positioning. Evaluation on three donors clearly highlighted that the bright signal for both activation markers correlated with activation and cytokine production (Supplemental Fig. 2C). Positioning of gates based on FMT controls would thus include excessive background in the analysis and was not further used. The decision of testing fluorescence minus two samples instead of classic Fluorescence Minus One control was made based on scarce sample availability and because the two involved dyes (PE-Cy7 and APC) were not strongly interfering.
2.4. Evaluation of the optimized panel
We finally used the optimized panel to characterize ten additional donors, four of which were evaluated in triplicate. CMV peptide stimulation was also included to ensure that the protocol could detect responses to an antigen different than Spike, and to verify that diverse unrelated antigens generate different pattern of responses in the evaluated donors.
For these experiments we pushed the sample acquisition to the limit of available cells by recording 110,000 live singlet lymphocytes, corresponding to an average of 84,200 CD3+, 48,700 CD4+ and 28,300 CD8+ events.
We arbitrary defined as “responders” samples showing a percentage of AR CD4+ or CD8+ ≥ 0.08%. This condition allowed us to collect >50 AIM+ CD4+ or AIM+ CD8+ events in all the stimulated samples and ensured that all the differences between stimulated and unstimulated samples accounted for >20 events, which can be used in defining the limit of detection in flow cytometry (Palmieri et al., 2020).
This ensured a consistent classification of all the replicates as responders or not, with the single exception for Spike-AR CD8+ of Vax 214, which being close to the threshold was classified twice as responder and once as not responder (%CV = 44). (Supplemental Table 2).
By applying this threshold, we classified for Spike stimulation nine CD4+ responsers and four CD8+ responders out of ten. We also classified for CMV stimulation five CD4+ responders and four CD8+ responders out of ten. (Fig. 3A and Supplemental Table 2). We also verified that the AIM+ event counts of the stimulated samples from responders were significantly different from the counts of the unstimulated ones, treated with anti-CD28 mAb only (Fig. 3B). Here we observed a relevant background for AIM+ CD8+ cells in unstimulated samples that impacted the Spike response assesment much more than CMV. Notably this background did not impair the statistically significant difference in AIM+ CD8+ events between Spike stimulated and unstimulated samples. We hypothesize that the increased background for the CD8+ T cells could depend on a greater in vivo basal activation of this compartment, dependent on the repeated stimulation following vaccine boosts and natural Sars-CoV-2 infections.
Fig. 3.
Assessment of Spike and CMV-AR T cells and quantification of unstimulated background.
A Graphs represent the evaluation of Spike and CMV-AR T cells in a cohort of ten donors, four of which evaluated in triplicate (Vax 201–202–204-214). AR T cells are calculated as the difference in the percentage of AIM+ elements between stimulated and not stimulated samples. The variations observed in the evaluation of Spike-AR CD8+ T cells are likely due to the limited number of acquired events and by small differences in background and activation status of each evaluation.
B Graphs show the consolidated percentages of activated CD4+ and CD8+ (AIM+ and Cytokine+ AIM+) in Spike and CMV stimulated samples versus the unstimulated (aCD28 only) ones for responder donors. All the differences were statistically significant.
Cytokine positivity includes cells resulting positive for at least one of the three evaluated cytokines: IFNγ, TNFα or IL-2.
Abbreviations: AIM = Activation Induced Markers; AR = Antigen Responding.
We also observed some variability in the replicates of our assay, with % CVs ranging from 5% to 44% (Fig. 2A, Supplemental Table 2) and we speculated that this can be attributed to two parallel factors:
-
i.
the relatively low number of activated events collected by recording 110,000 live singlet lymphocytes.
-
ii.
the need to estimate AR events as the result of a difference between stimulated and not stimulated samples: both being affected by random variation, stronger random variations can at times be observed in the difference between them.
In accordance with previous literature, we confirmed that the amount of cytokine producing AIM+ CD4+ and CD8+ T cells was lower in comparison to total AIM+ events. (Fig. 4 ). Looking at the pool of stimulated samples only, cytokine+ AIM+ CD4+ cells were nearly 81% and 83% of total AIM+ CD4+ events following Spike and CMV stimulation, respectively (Fig. 4 upper left graphs in A and B panels). In parallel, cytokine+ AIM+ CD8+ were 30% and 81% of total AIM+ CD8+, following Spike and CMV stimulation, respectively (Fig. 4 upper right graphs in A and B panels). Notably, when the percentage of AR elements was correctly calculated as the difference between stimulated and non-stimulated samples, the percentage of cytokine-producing AIM+ events among total AIM+ events was increased to 87% and 92% for Spike- and CMV- stimulated CD4+ cells, respecitively, and to 52% and 90% for Spike- and CMV- stimulated CD8+ cells, respectively (Fig. 4 lower graphs in A and B panels). This is due to lower background affecting cytokine+ AIM+ T cell events in unstimulated samples, and
Fig. 4.
Quantification of Cytokine production by AIM+ and AR cells.
A-B Graphs represent the comparison between AIM+ and AR CD4+ and CD8+ with their cytokine producing counterpart for Spike (A) and CMV (B) stimulation in responder donors. AIM+ CD4+ or CD8+ T cells are reported based on stimulated samples only, whereas Spike-AR T cells and cytokine producing counterpart are calculated as the difference between stimulated and non stimulated samples. Y axes refer to percentage of all CD4+ or CD8+ cells, whereas numbers displayed within the graph bars refer to the percentage of cytokine producing cells among total activated cells.
Cytokine positivity includes cells positive for at least one of the three evaluated cytokines: IFNγ, TNFα or IL-2.
Abbreviations: AIM = Activation Induced Markers; AR = Antigen Responding.
suggests that ic-cytokine based detection of antigen specific T cell activation (compared to AIM+) may be less sensitive but more specific. Of course, the hypothesis that AIM+ cells negative for the assessed soluble factors may produce other cytokines should be also considered. Lower background of Cytokine+ AIM+ events in our cohort was particularly evident in the case of Spike-AR CD8+ cell evaluation (Fig. 3B upper right graph). As expected, the pattern of ic-cytokine staining showed some differences between helper and cytotoxic compartments and among the Spike versus CMV stimulation. Cytokine producing AIM+ CD4+ T cells were 69.5% IFNγ+, 62.9% TNFα+, and 78.5% IL-2+ following Spike stimulation, and 79.4% IFNγ+, 59.5% TNFα+, 58.4% IL-2+ following CMV stimulation. Conversely, cytokine producing AIM+ CD8+ T were 89.1% IFNγ+, 59.9% TNFα+, 20.9% IL-2+ following Spike stimulation, and were 91.1% IFNγ+, 68.4% TNFα+, 14.7% IL-2+ following CMV stimulation. The proportion of CD4+ AIM+ IFNγ and TNFα double positive events was 51.3% and 38.3% following Spike and CMV stimulation respectively,and was 47.8% and 49.5% in the CD8+ compartment. Finally, 34.8% and 22.2% of cytokine producing AIM+ CD4+ T cells were positive for all three cytokines following Spike and CMV stimulation, whereas the same percentage were just 10.3% and 7.9% in the CD8+ subset, due to much lower IL-2 production (not shown).
2.5. T cell maturation
We then evaluated the maturation pattern of total and AIM+ CD4+ and CD8+ T cells by means of CD45RA and CCR7 staining, as shown in Fig. 5 , panel B. The most advanced maturation stage is evident for AIM+ T cells, whereby AIM+ CD4+ T cells were mainly effector memory and AIM+ CD8+ T cells were mainly terminally differentiated (Fig. 5, panel A. Data refers to stimulated samples of donors showing a percentage of Spike-AR CD4+ and CD8+ > 0.08%) As expected, a clear shift in the maturation curve is observed comparing total CD4+ or CD8+ subsets with their matched Spike-activated counterpart. AIM+ CD4+ were enriched for effector memory and CD8+ cells were enriched for terminally differentiated CD45RA+. Interestingly few naïve cells were also detectable, particularly in the CD4+ population, which may be due to bystander activation. These clearly showed a lower level of CCR7 expression in comparison to total naïve counterparts (Supplemental Fig. 3). Lower CCR7 expression was also consistently observed in central memory (CM) activated CD4+ and CD8+ T cells in comparison to total parent populations. CMV activated AIM+ events displayed fully consistent pattern (not shown).
Fig. 5.
Maturation curve of CD4+ and CD8+ T cells and their AIM+ counterparts.
A Graphs represents the percentages of CD4+ and CD8+ T cell subsets defined by surface staining of CD45RA and CCR7. Naïve = CD45RA+, CCR7+; Central Memory (CM) = CD45RA-, CCR7+; Effector Memory (EM) = CD45RA-, CCR7-; Terminally differentiated effector memory (TEMRA) = CD45RA+, CCR7-. The most advanced maturation stage is evident for AIM+ T cells, being mainly effector memory for AIM+ CD4+ T cells and mainly terminally differentiated for AIM+ CD8+ T cells. Data refer to responder donors.
B Exemplary plots from two responder donors, showing total and AIM+ CD4+ and CD8+ maturation curve and gate positioning.
Abbreviations: AIM = Activation Induced Markers.
2.6. Evaluation of T follicular helper cells
Responding donors to Spike and CMV were assessed for the increase of AIM+ Tfh in stimulated samples versus unstimulated ones. Eight out of nine Spike CD4+ responders showed a clear increase of Tfh cells, yet only two CMV responders out of five showed an increase (Fig. 6A). Considering the rarity of this subset, the quantification was barely significant at single donor level, given that only four out of fourteen evaluated samples had >20 acquired events as the difference between stimulated and unstimulated samples. Nonetheless this difference was statistically significant at the group level, with particular reference to Spike Tfh AIM+ cells showing a p value of 8 × 10−5 when the stimulated and unstimulated samples were compared. (Fig. 6B).
Fig. 6.
Quantification of Tfh in stimulated and unstimulated responder samples to Spike and CMV.
A Increase of Tfh AIM+ CD4+ cells as % of all CD4+ cells in stimulated and unstimulated samples from responder donors: single donor view. Eight Spike stimulated samples out of nine, and two CMV stimulated samples out of five are showing a clear increase.
B Increase of Tfh AIM+ CD4+ cells as % of all CD4+ cells in stimulated and unstimulated samples from responder donors: consolidated view. The differences are statistically significant.
Abbreviations: AIM = Activation Induced Markers; Tfh = T follicular helper.
3. Discussion
Multiple methodologies have been described to investigate antigen-specific T cells ex vivo or after in vitro expansion, by direct or indirect interrogation of the T cell repertoire and in combination or not with cell phenotype evaluation. Approaches for ex vivo detection of antigen specific T cells should rely on minimal in vitro manipulation, so that during short-term stimulation TCR repertoire of antigen-responsive cells is not biased and accurately reflects the clonal dominance hierarchy of the repertoire in vivo.
In the last decade, multiple surface proteins have been established as cytokine-independent surrogates of T cell activation and proven useful to identify and isolate antigen-responsive T cells after TCR engagement by peptide-HLA ligands. These activation markers upregulated on activated T cells thus allowing the detection of the repertoire of antigen-responsive T elements with minimal in vitro manipulation, short time of antigen stimulation, and regardless of the functional heterogeneity of reactive T cell themselves.
Based on these premises, the measure of inducible markers on the cell surface in a TCR.
stimulation-dependent manner—the AIM test—is a practical approach to study and monitor antigen-specific T cells. Recently, it has been proposed that AIM testing can be performed in conjunction with intracellular cytokine detection (Braun et al., 2020). In this context, it should be considered that the use of protein secretion inhibitors could negatively impact the expression of activation markers on the cell surface, resulting in an underestimation of the frequency of antigen-responsive T cells. For these reasons, optimizing appropriate conditions for simultaneous AIM testing and cytokine detection is critical.
Among the TCR-dependent activation markers to identify antigen-responsive T cells with high specificity, 4-1BB (CD137) is reported to be selectively expressed on all activated CD4+ and CD8+ T cells, and its upregulation is independent of cytokine secretion profile or cell differentiation stage. CD137 expression reaches a maximal level of expression 24 h after in vitro stimulation on both CD4+ and CD8+ T cells (Wolfl et al., 2007) (Wehler et al., 2008). In the design of AIM test assays, preferably different activation markers are used in association to facilitate gating and improve specificity, and several optimized combinations have been reported in the literature to identify preferably antigen specific CD4 + or CD8 + T cells (Grifoni et al., 2020) (Painter, 2021).
Here, we tested ic-CD137 upregulation in association with OX40 (CD134) and CD69. The first is suggested as an inducible marker for CD4+ T cells that has a role in regulating the development of effector and memory stages (Grifoni et al., 2020), while the second is an early activation marker that is frequently linked with cytokine production in intracellular staining (Grifoni et al., 2020). Although it is known that CD69 can be upregulated in a TCR-independent manner (Bremser et al., 2015) it has been also proposed that its use in association with other TCR-dependent activation markers like CD137 may improve sensitivity for the discrimination of TCR-triggered cells (Bacher and Scheffold, 2013). Indeed, CD69 being an early activation marker, its positivity ensures that no pre-existing activation are measured during the execution of the AIM test.
In our hands, intracellular assessment of CD137 and CD69 allowed clear detection of an activated T cell population, composed of both CD4+ and CD8+ T cells, which was evidently increased upon in vitro stimulation with Spike or CMV peptide pools. Importantly, both ic-CD137 and ic-CD69 were equally efficient in detecting activated cytokine producing cells (Fig. 1 panels A-B). In contrast, ic- CD134 in conjunction with ic- CD137 was much less effective in both detecting the pool of activated T cells and capturing the cytokine producing T cell fraction (Fig. 1).
Surface staining of either CD69 or CD134 clearly showed worse results than intracellular staining, as expected due to the protein transport inhibition which was required to allow intracellular cytokine accumulation. In particular, a much lower amount of cytokine producing ic-CD137+ T cells co-stained with surface CD134 or CD69 thus breaking the key concordance between activation and production of cytokines, which was particularly evident in the case of CD134.
Synergic use of CD137 and CD69 markers for AIM testing is reported in the literature although not in conjunction with intracellular cytokine detection (Geers et al., 2021). In the AIM protocol without evaluation of cytokine production, a better correlation between CD69 and CD134 has been described, which we did not observe here likely due to different experimental conditions.
Interestingly, we also detected in our experiments that the fraction of activated cytokine producing T cells is characterized by higher ic-CD137 brighteness, thus further emphasizing the good correlation between this marker and T cell activation (Supplemental Fig. 4).
The optimized panel and protocol were tested on a cohort of ten donors and responses to Spike and CMV were assessed. As expected, the two antigens generated completely uncorrelated response patterns in terms of number of responders and intensity of responses (Fig. 3A). This is consistent with the fact of measuring two different and independent antigen specific T cell responses.
Three replicates have been performed for each test in four donors, showing %CV of AR cells ranging from 5% to 44%. Reasonably, this variation is influenced by the relatively low number of AIM+ events collected. In our setting, we decided to limit the starting number of cells per sample to 106 PBMCs, with the goal of facilitating routine assay execution. To rely on a sufficient number of AIM+ recorded events, we arbitrarily defined as “responders” samples showing a percentage of AR CD4+ or CD8+ ≥ 0.08%. Under such limitation, all stimulated samples from responder donors accounted for >50 AIM+ CD4+ or CD8+ events, and all the differences between stimulated and unstimulated samples accounted for >20 events, which is considered the limit of detection in flow cytometry (Palmieri et al., 2020).
Indeed, this setting allowed us to detect statistically significant differences from stimulated and unstimulated samples on responder donors. While this approach is sufficiently reliable in a cohort study, whenever data is reported on single individuals, the number of cells may be increased proportionally to the required level of sensitivity.
Antigen Responding cells were always evaluated by subtracting the background of unstimulated samples from the signal of the stimulated ones. We found this background higher for AIM+ CD8+ cells, which could be because many of the donors had contracted SARS-CoV-2 infection after the second vaccine booster. Nevertheless, a statistically significant difference was detected between signal and background of responder samples. Lower background was affecting the cytokine producing AIM+ CD4+ and CD8+ subsets which were also numerically less represented. We argue that this can be due to some cells needing longer time to produce cytokine and/or producing cytokines different from the three assessed.
The use of CD45RA and CCR7 markers allowed describing the maturation curve of total and activated CD4+ and CD8+ cells. Data clearly indicate a more advanced maturation stage for ic-CD137+ ic-CD69+ T cells which were enriched for effector memory (CD4+) and terminally differentiated (CD8+) subsets. Interestingly, a subset of naïve ic-CD137+ ic-CD69+ CD4+ T cells was also detectable, likely due to bystander activation. Further investigation would be required to properly classify this specific subgroup.
The possibility to detect CD4+ and CD8+ activation with CD137 and CD69 markers, and remove the less informative CD134, allowed addition of CXCR5 to our 12-color flow cytometry panel, thus adding specificity for detecting T follicular helper cells and their antigen specific subset. Since Tfh cells support antigen-specific B cells in antibody production, this simple addition enables the method to provide insights on the relationship between antigen specific T cell activation and humoral immunity (Reiss et al., 2017) (Aiello, 2021). Whereas the number of assessed cells was very limited for this cluster, their presence in the stimulated samples of Antigen Responding donors was significant, with particular reference to Spike Tfh AIM+ cells showing a p value of 8 × 10−5 when the stimulated and unstimulated samples were compared. (Fig. 6B).
The main limitation of the current study is related to the relatively low numbers of the assessed donors: a larger and more homogeneous cohort would be required to precisely define the limit of detection for this approach. This is particularly difficult for the Spike antigen, as currently most of the subjects are characterized by hybrid immunity, with very heterogeneous histories of antigen exposure between individuals.
Cell availability per sample has also been a limitation in our case, which was why we limited to 106 the number of PBMCs treated in each condition. Whenever an unequivocal result is required at the single donor level, we would recommend running multiple replicates, possibly more than a single timepoint, and increase the number of processed cells.
4. Conclusions
AIM testing by flow cytometry is a powerful and relatively straightforward technique that relies on surface protein detection to obtain an ex vivo portrait of the frequency of antigen responding T cells; nevertheless, it does not provide indications regarding the functional features of the specific T response in terms of cytokines and effector functions. On the other hand, cytokine profiling by intracellular staining normally does not reflect the totality of antigen responding T cells. The method introduced here allows AIM testing in conjunction with intracellular cytokine evaluation and T cell maturation to provide a more detailed description of the different facets of the specific T response. The method has been applied for studying responses during SARS-CoV-2 infection or following vaccination or infection, and responses to CMV, and is potentially applicable to any other setting requiring investigation of antigen specific T cell activation.
5. Materials and methods
Specimen collection. Blood samples have been obtained after informed consent from BNT162b2 fully vaccinated healthy volunteers within several weeks after the second booster dose and after SARS-CoV-2 infection (Supplemental Table 3). Peripheral blood mononuclear cells (PBMCs) were isolated by density gradient cell separation media (Cedarlane) from whole blood collected in heparin tube (Vacutainer Becton Dickinson, BD) and used after cryopreservation in fetal bovine serum (FBS, Euroclone) supplemented with 10% dimethyl sulfoxide (DMSO, MERCK).
Materials. Antibodies for flow cytometry staining (Supplemental Table 1 Panels A-B-C), purified monoclonal antibodies (mAbs) against CD3, CD28 and CD49d costimulatory molecules, Golgi-Plug (Brefeldin A, BFA), Fc Block Receptor, Stain Buffer supplemented with BSA, and Cytofix/Cytoperm solution kit, were provided by Becton Dickinson (BD).
PepTivator® SARS-CoV-2 S, S1 and S+ as well as CMV pp65 and CMV IE-1 for antigen specific T cell stimulation were provided by Miltenyi Biotec and used at the concentration recommended by manufacturer. Literature reports for Spike PepTivator® reagents a sensitivity of 77% and specificity of 96% which have been assessed by IFNg release assay (QuantiFERON)((Aiello, 2021) PepTivator® CMV pp65 and CMV IE-1 for antigenic stimulation were provided by Miltenyi Biotec and used at the concentration recommended by manufacturer.
Cell culture and activation. Cryopreserved PBMCs were quickly thawed, washed and resuspended at 10 (Betts et al., 2003)/ml in RPMI 1640 complete medium supplemented with 10% FBS and 1× Antibiotic/Antimycotic (Gibco). 106 cells were seeded per well in a 96 round bottom plate and stimulated or not with 1 μg/ml of PepTivator® SARS-CoV-2 Prot_S, Prot_S1, Prot_S+ or with 1 μg/ml CMV pp65 and CMV IE-1 (Miltenyi Biotech). Monoclonal antibodies anti-CD28 and/or anti-CD49d at 1 μg/ml were added as co-stimulus. After 4 h of incubation at 37 °C, 5% CO2, the protein transport inhibitor Golgi-Plug (BFA, 1 μl/ml) was added in each condition for a further incubation of 16, 20 or 24 h. Positive controls were stimulated with immobilized mAbs against CD3 and CD28 molecules at at 1 μg/ml.
Cell staining. After incubation with Golgi-Plug, cells were washed with 100 ul of Stain Buffer to remove culture medium. Staining was performed in 96 round bottom plates. Cells were blocked using Fc Block Receptor for 10 min at room temperature (RT) and afterwards stained 15 min RT with the surface antibody cocktail and viability dye (Supplemental Table 1). For subsequent intracellular protocol, samples were washed using Stain Buffer and fixed with 200 μl of Cytofix/Cytoperm Kit for 20 min at RT. After washing with 200 μl BD Perm/Wash buffer, cells were incubated with intracellular antibodies for 20 min at RT (Supplemental Table 1). Samples were finally washed with Stain buffer and re-suspended in 400 μl of PBS in 5 ml polystyrene tubes for acquisition.
Flow cytometry and data analysis. Cells were acquired with Blue, Violet and Red lasers equipped BD FACSCelesta™ or with Blue, UV, Violet, Yellow Green and Red lasers equipped BD FACSymphony™ A3 flow cytometer and analyzed using BD FACSDiva™ software (v9.0). Compensation was calculated using single-stained anti-mouse or anti rat Ig,κ Comp Beads (BD Biosciences). Doublets were excluded by plotting forward scatter area versus forward scatter height. Viable lymphocytes were defined as fixable viability stain neg-low cells.
T cell maturation curve was analyzed as shown in Fig. 5 based on CD45RA and CCR7 staining. Subsets were defined as follows: Naïve = CD45RA+, CCR7+; Central Memory (CM) = CD45RA-, CCR7+; Effector Memory (EM) = CD45RA-, CCR7-; Terminally differentiated effector memory (TEMRA) = CD45RA+, CCR7-.
Combinations of cells expressing AIM, maturation markers, and cytokines were determined using appropriate gating strategy (Supplemental Fig. 5).
Calculations. Antigen responding cells are calculated by subtracting the percentage of AIM+ events in unstimulated samples from stimulated samples. Parametric data were analyzed by paired Student's t-test (one tail). P values are summarized as follow: * = p < 0.05; ** = p < 0.005; *** = p < 0.0005.
Funding
Reagents used for this study have been provided free of charge by BD Switzerland Srl, under collaborative research agreement with IRCCS Ospedale Policlinico San Martino.
CRediT authorship contribution statement
Tiziana Altosole: Investigation, Writing - original draft. Gianluca Rotta: Investigation, Conceptualization, Formal analysis, Writing - original draft. Chiara R.M. Uras: Investigation. Scott J. Bornheimer: Conceptualization, Writing – review & editing. Daniela Fenoglio: Conceptualization, Supervision, Writing - original draft.
Declaration of Competing Interest
Gianluca Rotta and Scott J. Bornheimer are employees of Becton, Dickinson and Company.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jim.2023.113443.
Appendix A. Supplementary data
Supplemental figures
References
- Aiello A., et al. Spike is the most recognized antigen in the whole-blood platform in both acute and convalescent COVID-19 patients. Int. J. Infect. Dis. 2021;106:338–347. doi: 10.1016/j.ijid.2021.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altman J.D., et al. Phenotypic analysis of antigen-specific T lymphocytes. Science (80-.) 1996;274:94–96. [PubMed] [Google Scholar]
- Appay V., Douek D.C., Price D.A. CD8+ T-cell differentiation in response to viruses. Nat. Med. 2008;14 doi: 10.1038/nm.f.1774. [DOI] [PubMed] [Google Scholar]
- Bacher P., Scheffold A. Flow-cytometric analysis of rare antigen-specific T cells. Cytom. A. 2013;83:692–701. doi: 10.1002/cyto.a.22317. [DOI] [PubMed] [Google Scholar]
- Betts M.R., et al. Sensitive and viable identification of antigen-specific CD8+ T cells by a flow cytometric assay for degranulation. J. Immunol. Methods. 2003;281:65–78. doi: 10.1016/s0022-1759(03)00265-5. [DOI] [PubMed] [Google Scholar]
- Borst J., Ahrends T., Bąbała N., Melief C.J.M., Kastenmüller W. CD4+ T cell help in cancer immunology and immunotherapy. Nat. Rev. Immunol. 2018;18:635–647. doi: 10.1038/s41577-018-0044-0. [DOI] [PubMed] [Google Scholar]
- Braun J., et al. SARS-CoV-2-reactive T cells in healthy donors and patients with COVID-19. Nature. 2020;587:270–274. doi: 10.1038/s41586-020-2598-9. [DOI] [PubMed] [Google Scholar]
- Bremser A., Brack M., Izcue A. Higher sensitivity of Foxp3+ Treg compared to Foxp3- conventional T cells to TCR-independent signals for CD69 induction. PLoS One. 2015;10:1–15. doi: 10.1371/journal.pone.0137393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chattopadhyay P.K., Yu J., Roederer M. Live-cell assay to detect antigen-specific CD4+ T-cell responses by CD154 expression. Nat. Protoc. 2006;1:1–6. doi: 10.1038/nprot.2006.1. [DOI] [PubMed] [Google Scholar]
- Dan J.M. A cytokine-independent approach to identify antigen-specific human germinal center Tfh cells and rare antigen-specific CD4+ T cells in blood. Physiol. Behav. 2017;176:139–148. [Google Scholar]
- Dawicki W., Watts T.H. Expression and function of 4-IBB during CD4 versus CD8 T cell responses in vivo. Eur. J. Immunol. 2004;34:743–751. doi: 10.1002/eji.200324278. [DOI] [PubMed] [Google Scholar]
- Geers D., et al. SARS-CoV-2 variants of concern partially escape humoral but not T-cell responses in COVID-19 convalescent donors and vaccinees. Sci. Immunol. 2021;6:1–22. doi: 10.1126/sciimmunol.abj1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grifoni A., et al. Targets of T cell responses to SARS-CoV-2 coronavirus in humans with COVID-19 disease and unexposed individuals. Cell. 2020;181:1489–1501.e15. doi: 10.1016/j.cell.2020.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kmieciak M., et al. Human T cells express CD25 and Foxp3 upon activation and exhibit effector/memory phenotypes without any regulatory/suppressor function. J. Transl. Med. 2009;7:1–7. doi: 10.1186/1479-5876-7-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier S., Stark R., Frentsch M., Thiel A. The influence of different stimulation conditions on the assessment of antigen-induced CD154 expression on CD4+ T cells. Cytom. Part A. 2008;73:1035–1042. doi: 10.1002/cyto.a.20640. [DOI] [PubMed] [Google Scholar]
- Mosmann T.R., Li L., Sad S. Functions of CD8 T-cell subsets secreting different cytokine patterns. Semin. Immunol. 1997;9:87–92. doi: 10.1006/smim.1997.0065. [DOI] [PubMed] [Google Scholar]
- Nylander S., Kalies I. Brefeldin a, but not monensin, completely blocks CD69 expression on mouse lymphocytes: efficacy of inhibitors of protein secretion in protocols for intracellular cytokine staining by flow cytometry. J. Immunol. Methods. 1999;224:69–76. doi: 10.1016/s0022-1759(99)00010-1. [DOI] [PubMed] [Google Scholar]
- O’Neil-Andersen N.J., Lawrence D.A. Differential modulation of surface and intracellular protein expression by T cells after stimulation in the presence of monensin or brefeldin a. Clin. Diagn. Lab. Immunol. 2002;9:243–250. doi: 10.1128/CDLI.9.2.243-250.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Painter M.M. Rapid induction of antigen-specific CD4+ T cells is associated with coordinated humoral and cellular immunity to SARS-CoV-2 mRNA vaccination. Immunity. 2021;2133–2142 doi: 10.1016/j.immuni.2021.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmieri R., et al. Clinical relevance of- limit of detection (LOD) - limit of quantification (LOQ) - based flow cytometry approach for measurable residual disease (MRD) assessment in acute myeloid leukemia (AML) Blood. 2020;136:37–38. [Google Scholar]
- Reiss S., et al. Comparative analysis of activation induced marker (AIM) assays for sensitive identification of antigen-specific CD4 T cells. PLoS One. 2017 doi: 10.1371/journal.pone.0186998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- René Van Lier. CD8+ T-cell differentiation in response to viruses. Nat. Rev. Immunol. 2003;3:913–918. doi: 10.1038/nri1254. [DOI] [PubMed] [Google Scholar]
- Rubio V., et al. Ex vivo identification, isolation and analysis of tumor-cytolytic T cells. Nat. Med. 2003;9:1377–1382. doi: 10.1038/nm942. [DOI] [PubMed] [Google Scholar]
- Sallusto F., Geginat J., Lanzavecchia A. Central memory and effector memory T cell subsets: function, generation, and maintenance. Annu. Rev. Immunol. 2004;22:745–763. doi: 10.1146/annurev.immunol.22.012703.104702. [DOI] [PubMed] [Google Scholar]
- Vinay D.S., Kwon B.S. Role of 4-1BB in immune responses. Semin. Immunol. 1998;10:481–489. doi: 10.1006/smim.1998.0157. [DOI] [PubMed] [Google Scholar]
- Watts T.H. TNF/TNFR family members in costimulation of T cell responses. Annu. Rev. Immunol. 2005;23:23–68. doi: 10.1146/annurev.immunol.23.021704.115839. [DOI] [PubMed] [Google Scholar]
- Wehler T.C., et al. Rapid identification and sorting of viable virus-reactive CD4+ and CD8+ T cells based on antigen-triggered CD137 expression. J. Immunol. Methods. 2008;339:23–37. doi: 10.1016/j.jim.2008.07.017. [DOI] [PubMed] [Google Scholar]
- Wen T., Bukczynski J., Watts T.H. 4-1BB ligand-mediated Costimulation of human T cells induces CD4 and CD8 T cell expansion, cytokine production, and the development of Cytolytic effector function. J. Immunol. 2002;168:4897–4906. doi: 10.4049/jimmunol.168.10.4897. [DOI] [PubMed] [Google Scholar]
- Wolfl M., et al. Activation-induced expression of CD137 permits detection, isolation, and expansion of the full repertoire of CD8+ T cells responding to antigen without requiring knowledge of epitope specificities. Blood. 2007;110:201–210. doi: 10.1182/blood-2006-11-056168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Z.H., et al. CD137 is a useful marker for identifying CD4+ T cell responses to mycobacterium tuberculosis. Scand. J. Immunol. 2017;85:372–380. doi: 10.1111/sji.12541. [DOI] [PubMed] [Google Scholar]
- Yellin M.J., et al. CD40 molecules induce down-modulation and endocytosis of T cell surface T cell-B cell activating molecule/CD40-L. J.Immunol. 1994;152:598–608. [PubMed] [Google Scholar]
- Yu E.D., et al. Balanced cellular and humoral immune responses targeting multiple antigens in adults receiving a quadrivalent inactivated influenza vaccine. Vaccines. 2021;9:1–17. doi: 10.3390/vaccines9050426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaunders J.J., et al. High levels of human antigen-specific CD4 + T cells in peripheral blood revealed by stimulated Coexpression of CD25 and CD134 (OX40) J. Immunol. 2009;183:2827–2836. doi: 10.4049/jimmunol.0803548. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental figures