Abstract
Transcriptional regulation plays an important role in Drosophila melanogaster circadian rhythms. The period promoter has been well studied, but the timeless promoter has not been analyzed in detail. Mutagenesis of the canonical E box in the timeless promoter reduces but does not eliminate timeless mRNA cycling or locomotor activity rhythms. This is because there are at least two other cis-acting elements close to the canonical E box, which can also be transactivated by the circadian transcription factor dCLOCK. These E-box-like sequences cooperate with the canonical E-box element to promote high-amplitude transcription, which is necessary for wild-type rhythmicity.
Many eukaryotic and some prokaryotic organisms regulate their metabolisms, physiology, and behavior with a circadian (∼24-h) period. These rhythms have been shown to involve complex feedback circuits involving transcriptional regulation in a diverse set of organisms, including fungi, plants, cyanobacteria, insects, and higher mammals such as mice and humans. These feedback loops appear inextricably linked to the oscillation of central pacemaker components with circadian periodicity.
The current model for the circadian pacemaker of Drosophila melanogaster (10, 11, 44) posits that two basic helix-loop-helix PAS (for Period-Arnt-Sim) domain-containing transcription factors, CLOCK (dCLK) and CYCLE (CYC), activate transcription of the period (per) and timeless (tim) genes by binding to E-box elements (CACGTG) in the promoter regions of these genes (1, 3, 9, 42). Levels of per and tim mRNA increase throughout the day and peak in the early evening (19, 45). PER and TIM proteins reach their peak some 4 to 6 h later (12, 56). PER levels accumulate slowly, due to destabilization through phosphorylation by the doubletime protein kinase (DBT), a D. melanogaster homolog of mammalian casein kinase 1ε (25, 31, 38). Increasing amounts of TIM lead to PER stabilization through dimerization (39). PER-TIM dimers then translocate to the nucleus (43, 53), where they then interact with dCLK and CYC (2, 28). Formation of this complex decreases dCLK-CYC-mediated transcription and invokes the negative limb of the pacemaker feedback loop by preventing dCLK-CYC binding to the E box (9, 29). This negative feedback is abrogated by light-mediated TIM degradation, which restarts the next cycle (22, 30, 34, 50, 55, 56). There might also be positive feedback, in which PER and TIM lead to an increase in transcription factor synthesis (16a, 28).
Strong genetic evidence from mammals supports the notion that the mCLOCK (dCLK orthologue) and BMAL1 (CYCLE orthologue) family of transcription factors is central to pacemaker transcriptional regulation. mCLOCK-BMAL1-dependent transcriptional cycling has been shown previously for mper1, mper2, mper3, mcry1, mcry2, dbp, and vasopressin transcripts, which fail to oscillate in mCLOCK mutant mice (23, 24, 26, 35, 40). In cell culture reporter assays, mCLOCK-BMAL1 has been shown to activate transcription from E boxes in the mper1, vasopressin, and dbp promoters (16, 23, 26, 40). In Drosophila, the importance of dCLK-CYC-mediated transcription is readily observable in the clock mutants jrk and cyc0, which are arrhythmic and lack oscillations in both per and tim mRNA (1, 42). Furthermore, S2 cell transfection assays demonstrate that dCLK-CYC activates transcription through E boxes in the period, timeless, and vrille promoters (7, 9).
The most extensive transcriptional studies in Drosophila circadian biology have centered on the period gene promoter. A promoterless per transgene, 7.2, was found to partially rescue arrhythmic per0 flies (15). The weak molecular cycling of the 7.2 transcript was attributed to posttranscriptional mechanisms (46). It was also demonstrated previously that per requires at least two sequences, one in the promoter and one in the 5′ coding sequence, to fully rescue wild-type per-like cycling and PER expression (48). Hao et al. (18) discovered that a per promoter 69-bp clock regulatory sequence (CRS) containing an E box was sufficient to generate 5- to 10-fold oscillations of a lacZ reporter RNA. Deletions in either the 5′ or 3′ half site of the period E box along with surrounding nucleotides led to decreases in reporter levels and lower amplitudes of cycling, indicating the importance of the E-box region but also suggesting that sequences other than the E box within the 69-bp CRS contribute to cycling. These sequences have yet to be identified. In S2 cells, dCLK and CYC were shown to activate transcription from a luciferase reporter fused to a tetramerized 14-mer centered around the per E box. The activation was blocked by a central 2-bp inversion of the E-box sequence (9). Subsequent experiments have shown that dCLK and CYC physically bind this E-box sequence and that the binding is abrogated by the same central 2-bp inversion in the E-box core sequence (29). These experiments firmly establish the per E box as a central component of circadian transcriptional regulation.
In contrast, there are no published reports investigating the importance of the timeless promoter to the circadian pacemaker. The following study presents a detailed analysis of the elements controlling tim transcription. We distinguish between the contributions of two canonical E boxes and two noncanonical E boxes within the tim promoter. In addition, we describe a role for a novel sequence element that might contribute to Drosophila circadian transcription.
MATERIALS AND METHODS
Constructs.
All constructs involving PCR-amplified fragments were confirmed by sequencing. All tim promoter-tim cDNA constructs originated from a tim+ rescue transgene (see Fig. 1 in reference 41) referred to here as 4.5-kb tim. The 4.5-kb tim construct was truncated to a 2.5-kb construct using the XbaI site at position 2512. The 1.5-kb construct was made by truncating the construct to the AatII site at position 1587. The constructs that contain 1,082 bp or less of promoter were made by PCR, introducing a NotI site at the 5′ end (except for timpDEAD, in which an NsiI site was used). These fragments were ligated back into the 4.5-kb tim parent construct utilizing the 5′ NotI site (see Fig. 1 in reference 41). The truncation constructs used as reporters in S2 cell transfection experiments were created by PCR using timpFL as a template and introducing a SacII site at the 5′ end and a XhoI site at +26. Three-way ligations were performed among these fragments, a SalI-BamHI luciferase cDNA fragment (pJD261 plasmid [32]), and the pMECA vector (a kind gift from Wayne Parrot, University of Georgia) cut with SacII-BamHI. Substitution constructs were created using PCR, and mainly transversion mutations were created. All mutated DNA elements contain a BglII site. To make the transformation vectors for luciferase-bearing flies, the S2 cell constructs were digested with SacII-BamHI and cloned into those sites of the pCaSpeR 4 transformation vector (52). The per constructs were made using PCR with PLO-LUC (8) as a template. These PCR products contain SacII sites at their 5′ end (−713) and SalI sites at their 3′ end (+9) and were cloned with luciferase into pMECA as described above. In S2 cell transfections, the pAC-dCLK construct used was created by introducing a NotI site at position 3029 in pBS-SK(−) clock by site-directed mutagenesis. An EagI digest of this cDNA clone was then ligated into the NotI site of Invitrogen's pAc5.1-V5/HIS vector.
Transgenic flies.
y w virgin females (luciferase constructs) and y w;tim0 virgin females (rescue constructs) were crossed to y w0; KiPP Δ(2–3) and y w;tim0; KiPP Δ(2–3) males, respectively. Flies were kept in bottles for 2 days and then transferred to a collecting cage for 2 days. Embryos were collected every hour and dechorionated with forceps. Embryos were fixed to double-sided tape on a glass slide and grouped by 2-min intervals. After being dried for about 10 min, embryos were covered with a thin layer of halocarbon oil (series 700; Halocarbon Production Corp.). Embryos were injected with Qiagen Midiprep DNA at a concentration of 0.5 μg/μl in 5 mM KCl–0.1 mM PO4 (pH 7.8) buffer containing 10% green food coloring. Injected embryos were kept in a humidified chamber in a 25°C incubator overnight, and surviving larvae were picked into fresh vials the next day. Adults were crossed to y w virgin females or y w;tim0 virgins (rescue constructs), and transformants were judged by eye color. Transformants were balanced, and then lines were established. The lines analyzed biochemically and behaviorally are as follows: timpFL.1 (756–43.1), timpFLmut.1 (756–15.1), timpFLmut.2 (756–23.1), timpMIN.1 (624–39.1), and timpDEAD.1 (522/Y). When requesting lines, please request the numerical identifier for the line.
Transfection assays.
S2 cells were maintained at high density in HyQ-SFX insect medium (HyClone Laboratories, Inc.) supplemented with 10% fetal bovine serum (GIBCO) and 10 mg of penicillin-streptomycin (Sigma) per ml. S2 cells at a density of 106 per ml in HyQ-SFX-lacking serum were plated in six-well tissue culture plates (Costar Corp.). Twenty-four hours later, the medium was aspirated and replaced with 1 ml of transfection mix. This mix consists of HyQ-SFX-lacking serum containing 20 μl of Geneporter reagent (Gene Therapy Systems), 500 ng of reporter DNA, 25 ng of Renilla reniformis luciferase reporter under the control of the copia promoter, and either 100 ng of pAC-clockV5 or empty vector incubated at room temperature for 45 min. Transfection was stopped by adding 1 ml of serum containing HyQ-SFX medium. Cell lysates were prepared with Promega's dual luciferase reporter assay system per the manufacturer's instructions. Photinus pyralis luciferase activity was measured with a Wallace, Inc., TD20/20 luminometer and normalized to the Renilla luciferase activity.
Fly head Western blots.
Flies were collected at the appropriate time point, and protein extracts were prepared from the homogenization of 30 heads in 30 μl of extraction buffer (12). PER and TIM Western blots were performed as described in reference 56. Western blots were quantified using the Molecular Analyst program (Bio-Rad).
RNase protection assays (RPAs).
Fifty heads were homogenized with a motorized pestle in 500 μl of TRIZOL reagent (GIBCO). RNA was precipitated according to the manufacturer's suggestions. RNA was resuspended in 50 μl of diethyl pyrocarbonate-treated water–50 μl of DNA digestion mix (40 mM Tris [pH 8], 6 mM MgCl2, 5 mM NaCl, 5 mM dithiothreitol, 0.05 mg of bovine serum albumin per ml, 0.4 U of RNasin [Promega] per μl, and 0.05 U of RQ1 DNase [Promega] per μl). The mixture was incubated at 37°C for 1 h and then phenol-chloroform extracted twice and precipitated overnight at −80°C. The mRNA was resuspended in 50 μl of hybridization buffer (13). The period 2–3, tim, and rp49 probes have been described previously (33, 49). Probes (1 × 106 cpm of per2/3 and tim probes and 2.5 × 105 cpm of rp49 probe) were added to each reaction mixture and heated to 95°C for 5 min and then at 50°C overnight. RNA was digested with 15 U of RNase I (Promega) for 1 h at 37°C and then phenol-chloroform extracted twice and precipitated for 1 h at −80°C. The samples were resuspended in 10 μl of loading dye (80% formamide, 0.1% xylene cyanol, 0.1% bromphenol blue) and resolved on a 5% sequencing gel. Quantification was carried out using a Bio-Rad GS-363 phosphorimager and Molecular Analyst software.
In vivo luciferase monitoring.
The assays were performed essentially as described in reference 48. Monitoring of luciferase activity was performed every half hour for 7 days. The first 2 days' data were discarded, and the remaining data were analyzed using the I-and-A program (36). Lines timpFL-LUC.1 and timpFL-LUC/PERR.3 each displayed levels that were about twofold higher than those of their respective sister lines and were reproducible in all flies tested in those lines. The enhanced levels in these lines are most likely due to position effects. In our analysis, these were the only cases of significant variations between different lines in the same genotype.
Behavioral analysis.
Fly circadian behavior was analyzed as described previously (41).
RESULTS
The tim E-box region is dispensable for rhythmic locomotor activity.
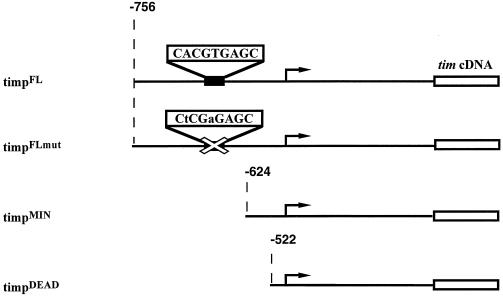
To begin a study of tim transcriptional regulation, we generated several different tim promoter-tim cDNA transgenic lines in a tim0 background. In the initial behavioral characterization of these lines, constructs harboring 756 bp or more of tim promoter displayed only small differences from a construct bearing 2.5 kb of tim promoter (Table 1). Within this 756-bp region of the tim promoter, there is a 9-bp canonical E-box sequence, CACGTGAGC, which is conserved in the 69-bp CRS of the period gene promoter. Flies with the 756-bp promoter construct had 26-h periods and were named timpFL, for tim promoter full-length. Flies that carry a timpFL transgene with a mutated E box, CTCGAG, termed timpFLmut (Fig. 1), displayed substantially longer periods, from 28 to 29.5 h for the three lines tested (Table 1). Lines that contain the minimal amount of tim promoter rescuing behavior, timpMIN, lack this E box and surrounding region but still manifest periods of ∼29 to 30 h, similar to the three timpFLmut lines (Table 1). Lines possessing a further truncated promoter, timpDEAD, are arrhythmic (Fig. 1). These data allow us to conclude that sequences other than this E box can contribute to tim transcription and that there is some important promoter element necessary to achieve behavioral rhythmicity in the region present in timpMIN but absent in timpDEAD, between −624 and −522 (Fig. 1).
TABLE 1.
Rescue of circadian behavior in tim0 flies with truncated and mutated tim promotersa
| Genetic background | Transgenic line | n | % AR | τ |
|---|---|---|---|---|
| y w | 39 | 18 | 23.9 ± 0.3 | |
| y w;tim0 | 49 | 96 | 23.2 ± 1.0 | |
| y w;tim0 | 2512.1 | 11 | 0 | 25.3 ± 0.4 |
| y w;tim0 | 2512.2 | 18 | 17 | 26.1 ± 0.5 |
| y w;tim0 | 2512.3 | 13 | 0 | 25.2 ± 0.4 |
| y w;tim0 | 2512.4 | 19 | 0 | 25.9 ± 0.5 |
| y w;tim0 | 1587.1 | 12 | 8 | 26.5 ± 0.5 |
| y w;tim0 | 1587.2 | 11 | 18 | 25.9 ± 0.7 |
| y w;tim0 | 1587.3 | 14 | 0 | 26.2 ± 0.5 |
| y w;tim0 | 1587.4 | 16 | 0 | 26.6 ± 0.3 |
| y w;tim0 | 1082.1 | 13 | 0 | 26.3 ± 0.3 |
| y w;tim0 | 1082.2 | 13 | 15 | 25.8 ± 0.4 |
| y w;tim0 | timpFL.1 (756) | 12 | 8 | 26.6 ± 0.5 |
| y w;tim0 | timpFL.2 | 11 | 27 | 26.6 ± 0.5 |
| y w;tim0 | timpFLmut.1/Y | 29 | 3 | 28.2 ± 0.6 |
| y w;tim0 | timpFLmut.2 | 8 | 12 | 29.0 ± 0.8 |
| y w;tim0 | timpFLmut.3 | 8 | 0 | 29.5 ± 0.5 |
| y w;tim0 | timpMIN.1 (624) | 25 | 20 | 29.3 ± 1.0 |
| y w;tim0 | timpMIN.2/Y | 12 | 50 | 29.9 ± 1.3 |
| y w;tim0 | timpMIN.3/+ | 5 | 0 | 29.5 ± 1.6 |
| y w;tim0 | timpDEAD.1/Y (522) | 40 | 83 | 26.9 ± 1.2 |
| y w;tim0 | timpDEAD.2/+ | 31 | 100 | |
| y w;tim0 | timpDEAD.3/+ | 35 | 100 | |
| y w;tim0 | timpDEAD.4 | 35 | 100 |
n represents the number of flies analyzed, % AR represents the percentage of arrhythmic flies (cutoff: power, <10; width, <2; see reference 14 for power and width definitions), and τ represents the period of the behavioral circadian rhythms. The names of the lines 2512, 1587, 1082, 756, 624, and 522 correspond to the length (in base pairs) of the tim promoter present in the transgene used to create these lines. “/+” indicates lines tested as heterozygotes (transgene over wild type; “/Y” indicates lines tested as males and containing transgene insertions on the X chromosome. All other lines were tested as homozygotes.
FIG. 1.
Diagram of tim promoter-tim transgenes. Shown are the tim promoter-tim constructs (right) used to obtain the lines analyzed (left). The genomic sequence reaches 3′ to the SalI site at +2206 and then is fused to the tim cDNA. timpFL and timpFLmut begin at −756, timpMIN begins at −624, and timpDEAD begins at −522. The E-box and mutant E-box sequences are shown. The transcription start site is marked by the arrow.
tim mRNA levels strongly influence TIM levels and the strength of behavioral rescue.
To assay tim mRNA levels in these transgenic flies, heads were collected from entrained flies, sacrificed at peak and trough times, and subjected to mRNA analysis by RPA (Fig. 2). These times were selected based on an extended time point analysis for each strain (data not shown). Biochemical analysis was performed on a representative line from each genotype, but two timpFLmut lines were analyzed because they showed a 1-h period difference.
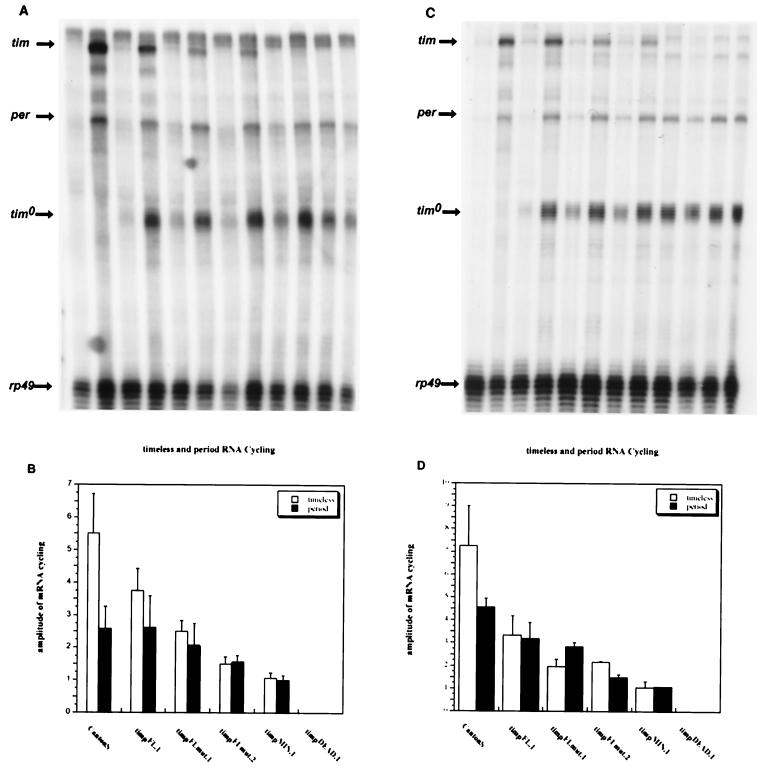
FIG. 2.
LD and DD tim and per mRNA profiles of tim promoter-tim transgenic lines. The mRNA levels for per, tim, tim0, and rp49 were measured in tim promoter-tim transgenic lines and Canton S flies by RPA in LD (12-h light; 12-h dark) and DD (constant darkness) conditions. “tim” denotes the transgene mRNA, “per” and “tim0” are endogenous per and tim0 mRNAs, and “rp49” is the normalization control. The genotypes are indicated. (A) A representative RPA of fly heads from flies sacrificed at high (ZT15) and low (ZT3) zeitgeber time points in LD cycles. (B) Quantitation of per and transgenic tim mRNAs from four LD experiments. (C) A representative RPA of fly heads from flies sacrificed at high (CT15) and low (CT3) circadian time points in the first day of DD after entraining for 3 days of 12:12 LD cycles. (D) Quantitation of per and transgenic tim mRNAs from two DD experiments.
In wild-type flies, tim mRNA cycles 5.5-fold in 12-h-light–12-h-dark conditions (LD) and 7.25-fold in constant-dark conditions (DD) (Fig. 2). These values are lower than previously published data (20, 45, 46), probably due to relatively high background levels and consequent difficulties in accurately quantifying mRNA at trough time points. Cycling amplitudes and peak tim mRNA levels progressively diminish from line timpFL.1 to timpFLmut.1, timpFLmut.2, timpMIN.1, and timpDEAD.1, in both LD and DD. The residual tim mRNA cycling in lines timpFLmut.1 and timpFLmut.2 (Fig. 2) indicates that sequences other than this E box contribute to circadian transcription. Line timpMIN.1 has barely detectable tim mRNA levels (visible on the autoradiograms but not in Fig. 2) that do not appear to cycle, suggesting that low levels of noncycling tim mRNA are adequate to rescue behavior (Fig. 2). tim mRNA in line timpDEAD.1 was not detectable, indicating that a critical region of the promoter is present in timpMIN.1 but absent in timpDEAD.1 (Fig. 2A and C), i.e., between −624 and −522.
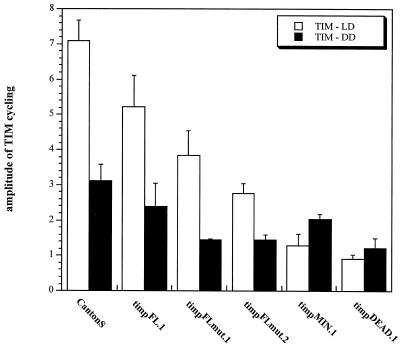
We also assayed cycling from the per and tim0 genes in these transgenic strains. Both mRNAs cycle with similar amplitudes in CS, timpFL.1, timpFLmut.1, and timpFLmut.2 (except tim0 in CS, because there is no tim0 gene in this strain) (Fig. 2). The amplitude is reduced in lines timpMIN.1 and timpDEAD.1, principally due to an increase in mRNA levels at trough time points (Fig. 2A and C). This is probably a consequence of decreased negative feedback on these promoters due to low TIM levels (Fig. 3A and B). Western blots show that TIM oscillations closely follow the tim mRNA profiles, both in cycling amplitude and in levels (Fig. 2A and C, 3A and B, and Fig. 4). The transgenic lines show progressively lower amounts of TIM, from timpFL.1 through timpMIN.1. This correlates well with progressively weaker behavioral rescue, both in period length and in percentage of arrhythmic flies. Line timpDEAD.1, which is arrhythmic, has barely discernible levels of TIM (Fig. 3A and B, Fig. 4, and Table 1).
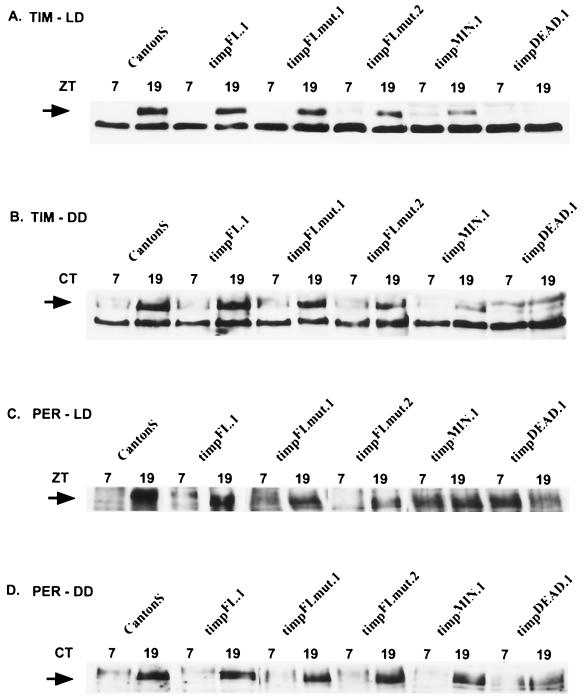
FIG. 3.
PER and TIM protein levels in tim promoter-tim transgenic lines. Representative LD and DD Western blots for PER and TIM in the tim promoter-tim transgenic lines. Protein extracts were made from heads of flies collected at high (ZT19 and CT19) and low (ZT7 and CT7) time points. Arrows denote the band of interest. Western blots from LD experiments were probed for TIM (A) and then stripped and reprobed for PER (C). Western blots from flies sacrificed on the first day of DD entrainment were probed for TIM (B) and then stripped and reprobed for PER (D).
FIG. 4.
Quantification of TIM Western blots. Shown are the results of quantitation of TIM protein levels from the different tim promoter-tim transgenic lines under LD and DD conditions (three experiments). The graph represents the amplitude of TIM cycling in head extracts between high (ZT19 and CT19) and low (ZT7 and CT7) time points.
The cycling amplitude and levels of PER were moderately affected in lines timpFL.1, timpFLmut.1, and timpFLmut.2 compared to CS (Fig. 3C and D). In line timpMIN.1, PER does not cycle in LD but does cycle in DD (Fig. 3C and D). An extended time point analysis in this strain confirmed that PER levels in LD are moderately high and stable and do not undergo cyclic phosphorylation, whereas PER levels in DD cycle both in amplitude and in phosphorylation state and have a lengthened period as expected (data not shown). In summary, tim mRNA levels largely determine TIM levels, and these determine the strength of behavioral rescue and period length.
The intronic E box does not significantly contribute to tim transcription in S2 cells.
The murine dbp and per1 promoters, as well as the human per1 promoter, have multiple E boxes that contribute to transcriptional activation (16, 40, 51, 54). In Drosophila, period and vrille also contain multiple E boxes in their promoters, although functional roles for all of these E boxes have not been confirmed (7; M. J. McDonald and M. Rosbash, unpublished observations). Through sequence searching, we found an additional canonical E box in the first intron of timeless at bp +1810. To assess whether this E box also contributes to tim transcription, we generated tim promoter-luciferase fusions with the upstream and intronic E-box regions mutated in various combinations (Fig. 5A) and compared the transcriptional activities of the constructs with an S2 cell-dCLK cotransfection assay (9).
FIG. 5.
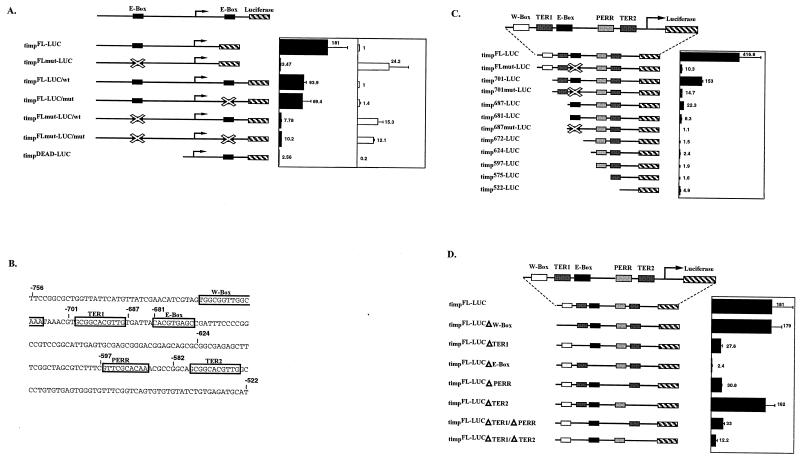
Truncation and substitutional mutagenesis of tim promoter. Black bars represent the induction of luciferase activity from tim-luciferase transgenes in S2 cells cotransfected with 100 ng of pAC-dCLK (see Materials and Methods). Standard deviations are indicated. (A) The contributions of the upstream and intronic E boxes were measured by mutating the E boxes (mutated E boxes are crossed out). In the far right panel, white bars represent the values of luciferase activity measured from reporters in non-dCLK-cotransfected cells (baseline values). The baseline value of the timpFLmut-LUC construct was normalized to the baseline value obtained from the timpFL-LUC construct. The baseline values for all intron-containing constructs were normalized to the baseline value obtained for the timpFL-LUC/wt intron construct. (B) Sequence of the tim promoter region −756 to −522. The sequence elements that were mutated are boxed, and their names as referred to in the text are shown above the boxes. (C) Truncation constructs of the tim promoter. The elements are outlined by boxes with the name of the element above the box. (D) Substitutional mutagenesis of the elements within the tim promoter, as shown in panel C. The timpFL-LUCΔE-box construct contains an AGATCT substitution for the E box (CACGTG), whereas timpFLmut-LUC contains the sequence CTCGAG.
In these S2 cell experiments, an intronic E-box mutation had little effect on dCLK-mediated transcriptional activation of constructs with either an intact or mutated upstream E box. In fact, addition of the intronic E-box region decreased transcription twofold (Fig. 5A, compare timpFL-LUC to timpFL-LUC/wt), suggesting that the intronic E box does not play an important role in tim transcription. This is consistent with the fact that timpDEAD.1 flies do not produce detectable levels of tim mRNA or rescue behavior and is consistent with the very weak activity of the timpDEAD-LUC construct in S2 cells (Table 1, Fig. 2A and C, and Fig. 5B).
Although consistent with previously published observations (7, 9), the very weak activation of the timpFLmut-LUC reporter by dCLK is misleading; luciferase activity from this construct in dCLK-transfected cells is about one-half to two-thirds of the activity of the intact E box, the timpFL-LUC construct (Fig. 5A and data not shown). This parallels the data for flies, in which peak tim mRNA levels in timpFLmut flies are 50 to 60% of the levels in timpFL flies (Fig. 2 and data not shown). The weak fold activation in S2 cells is due to the baseline luciferase activity of the timpFLmut-LUC being ∼24-fold higher than that of the timpFL-LUC reporter in non-dCLK-transfected cells (Fig. 5A, far right panel). All constructs tested with a mutated upstream E box exhibit dramatically enhanced reporter activity in non-dCLK-transfected cells (Fig. 5A, far right panel). This might reflect a transcriptional repressor that recognizes an intact E box in S2 cells. In contrast, there are not large increases in tim mRNA levels at the trough time point ZT3 or CT3 in timpFLmut.1 or timpFLmut.2 flies (Fig. 2A and C) compared to levels for timpFL flies. This suggests that the elevated baseline transcription is an S2 cell phenomenon and that other mechanisms exist in flies to suppress tim transcription in the absence of a functional E box. This phenomenon was specific to the tim promoter and was seen only with a mutation in the E box. In short, the data indicate that activation of tim reporter constructs with mutated E boxes by dCLK-CYC in S2 cells is substantially underestimated. They also indicate that cis-acting regions other than the canonical E box contribute to tim transcriptional activity.
The region 5′ of the tim E box contributes to transcriptional activity.
To verify that other regions contribute to tim transcription, we truncated timpFL-LUC from the 5′ end and measured the luciferase reporter activity when cotransfected with dCLK into S2 cells (Fig. 5C). We had previously identified the W box, an NF-1-like binding site which might possibly contribute to activity of the tim promoter (47). Searching the tim promoter sequence with the TransFac database for other possible transcription factor binding sites near the E box yielded no significant similarities to known binding sites. Scanning the sequence manually revealed two 11-bp Tim E-box-like repeats, TER1 and TER2 (Fig. 5B). Additionally, a sequence comparison between the per and tim promoters detected what we term the PER repeat, PERR, a conserved 10-bp sequence.
timp701-LUC lacks the W-box element and is activated by dCLK 2.7 times less well than is timpFL-LUC. This suggests that the W box or another nearby sequence element plays a small role in tim transcription (Fig. 5C). Truncating the promoter to timp687-LUC causes a dramatic 20-fold decrease in reporter activity compared to that of timpFL-LUC (Fig. 5C). This decrease is most likely due to deletion of TER1 and not to a disruption of the E box itself (see below). Removal of six additional nucleotides up to the 5′ border of the E box, timp681-LUC, causes a further decrease in transcriptional activation, suggesting that some of these six upstream nucleotides are also important for dCLK-mediated transcription (Fig. 5C). This construct still shows an eightfold level of activation. Construct timp672-LUC, which completely removes the E-box sequence, eliminates transcriptional activation by dCLK, indicating that the E box itself is still functional in the absence of any upstream tim sequence (Fig. 5C). Further truncations have smaller effects, suggesting that most of the regulation goes through the E box and TER1.
TER1 and PERR play major roles in tim transcription in S2 cells.
Because results from deletions can be incomplete or misleading, we eliminated these putative sequence elements primarily with transversion mutations that did not create known transcription factor binding sites. Similar sequences were used in the mutagenesis of each element in order to minimize artifacts. These mutated tim promoter constructs were cotransfected with dCLK into S2 cells.
Mutating the W-box element demonstrated that it plays little if any role in tim transcription. However, transcriptional activation decreased about eightfold with a mutation in either TER1 or PERR (Fig. 5D). For TER1, the results correlate well with the truncation of that region, which caused a 20-fold decrease (Fig. 5C, compare constructs timp701-LUC and timp687-LUC). Mutating the TER2 element had little or no effect on dCLK-mediated transcription (Fig. 5D). The double mutant TER1/TER2 led to a further threefold decrease, indicating that weak effects of TER2 may be masked by TER1 (Fig. 5D).
The PERR box plays a role in transcriptional activation of the period promoter in S2 cells.
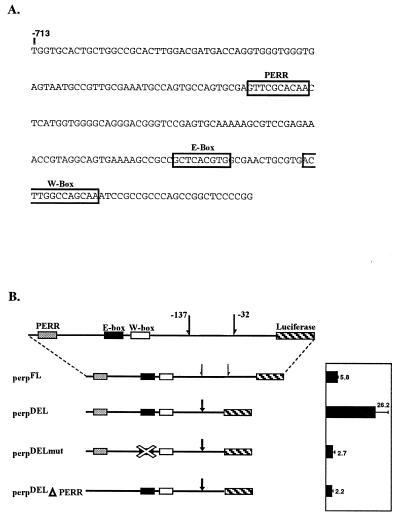
We also assayed the role of the PERR box in transcriptional activation by substitutional mutagenesis in the period promoter. The PERR box is upstream of the E box in per and downstream of the E box in tim but nearly the same distance from both E boxes (Fig. 5B and 6A). We examined four constructs in cotransfection assays with dCLK in S2 cells. A full-length construct, perpFL, gives rise to a 5.8-fold activation when cotransfected with dCLK (Fig. 6B). perpDEL, created by deleting the region −137 bp to −32 bp in perpFL, increased activation to 26-fold. A similar deletion enhanced transcriptional activity from a period-LacZ fusion protein in flies (18) and presumably reflects the absence of an inhibitory sequence element. Mutation of the PERR box in the context of perpDEL decreased transcriptional activation 10-fold (Fig. 6B), similar to the approximately eightfold decrease in reporter activity observed for the tim promoter containing a mutated PERR box (Fig. 5D). Mutation of the E box in perpDEL caused a nearly identical 10-fold decrease in transcriptional activation of the per promoter (Fig. 6B).
FIG. 6.
Mutagenesis of the PERR box in the per promoter in S2 cell assays. The role of the PERR box in the context of the period promoter in the S2 cell transfection assay was analyzed. (A) Sequence map of the per promoter. Elements are outlined by boxes with names above the boxes. (B) Within these constructs, the region between −137 and −32 (bounded by half arrows) was deleted in the constructs indicated (denoted by the single whole arrow). Mutant E boxes are crossed out and have the sequence CTCGAG. The PERR box, E box, and W box are shown. Black bars represent the induction of luciferase activity from tim-luciferase transgenes in S2 cells cotransfected with 100 ng of pAC-dCLK.
In vivo promoter element analysis indicates that TER1 and TER2 play a major role in tim transcription.
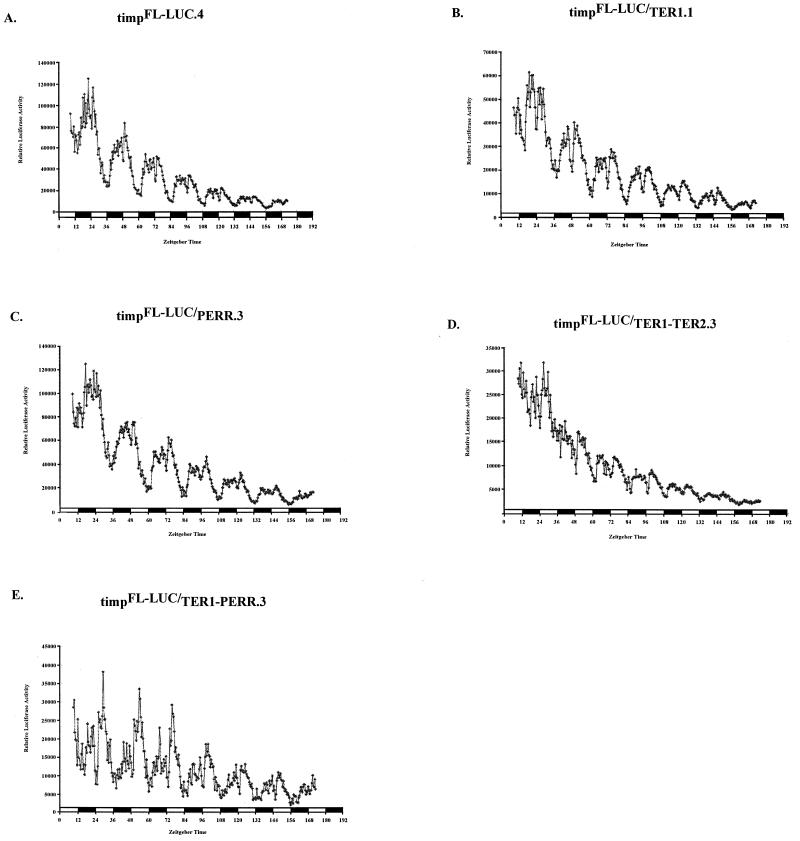
To assay the in vivo role of the PERR and TER elements, we analyzed four timpFL-LUC transgenic lines and TER1 (four lines), PERR (three lines), TER1/PER (three lines), and TER1/TER2 (three lines) mutants. Figure 7 shows the average luciferase activity for each genotype from a group of flies within a representative line. In all luciferase recordings, there is a second peak. This peak has been previously described by others (4, 8, 48) and is a function of the assay conditions (8, 48).
FIG. 7.
In vivo functional analysis of tim promoter elements. Flies contained tim promoter-luciferase fusions. Each graph shows the average luciferase activity (y axis) of flies (n = 12) from a representative line for the genotype indicated. All experiments were done under LD conditions (12 h of light [white boxes]; 12 h of dark [black boxes]); zeitgeber times are denoted on the x axis. Mutations in the tim promoter are the same as those used in S2 cell assays. (A) Line containing a normal 756-bp tim promoter, designated timpFL-LUC. (B) Line containing a mutagenized TER1 box. (C) Line containing a mutagenized PERR box. (D) TER1/TER2 double mutant line. (E) TER1/PERR double mutant line.
In the timpFL-LUC lines, luciferase activity cycles robustly with a periodicity of 24 h. The TER1 mutants manifest about 50% of the total luciferase activity of timpFL-LUC flies (Fig. 7A and B). In TER1/TER2 double mutants, luciferase activity is even lower, approximately 20 to 30% of that of timpFL-LUC flies (Fig. 7D). In these strains, cycling is nearly abolished, indicating that TER2 makes significant contributions to cycling and transcriptional activation in the absence of TER1. The PERR mutant lines were almost indistinguishable from timpFL-LUC flies (Fig. 7C). The TER1/PERR double mutants exhibit a slight reduction in luciferase activity levels compared to those for TER1 mutants, but cycling amplitude appears unaffected, suggesting that PERR functions differently in the adult fly than in S2 cells (Fig. 7E; also see the Discussion). The in vivo analysis validates the importance of the TER1 and TER2 boxes as well as the canonical E box in tim transcriptional regulation.
DISCUSSION
Noncanonical E-box-dependent transcription has been described extensively for the Myc family of binding proteins, whose canonical high-affinity binding site has been determined as CACGTG by sequential selection and amplification of binding sites (5, 6, 37). These studies led to the identification of lower-affinity, noncanonical MYC-MAX binding sites, such as CATGTG, CACGCG, CATGCG, CACGAC, and CAACGTG. Grandori et al. (17) also found that MYC-MAX dimers were able to bind a similar set of sequences in vivo, in a tumorigenic cell line. A similar observation was recently reported for mCLOCK/CYC (40). Four E boxes were identified in the first and second introns of the mammalian dbp gene, whose gene product is important in generating the cycling transcription of several circadian genes in the liver (27). All four E boxes were shown to activate a luciferase reporter in cell culture assays upon transfection with mCLOCK and BMAL1, but only two of the E-box regions showed circadian differences in DNase I-hypersensitive sites: one with a canonical CACGTG motif and the other with a noncanonical CACATG motif. Our study provides definitive evidence that a noncanonical E box contributes to circadian transcription in Drosophila.
We have also reaffirmed the idea that multiple E boxes contribute to circadian transcription. Previously, the study of the mper1 promoter had demonstrated a role for multiple canonical E boxes (16, 54). However, the tim promoter is unique, as it possesses three functional E boxes within a short distance, about 150 bp. This combination of multiple elements likely allows important protein-protein interactions, which contribute to the stronger amplitude of tim than of per transcriptional cycling (46). The observed direct correlation between behavioral period length and tim mRNA levels suggests that this more robust tim transcription amplitude is crucial for proper 24-h behavioral rhythmicity. These interactions could also accelerate the kinetics of transcription factors binding to the tim promoter and be responsible for tim's earlier peak of transcription (46). This also has important implications for the phase of PER accumulation, as TIM counteracts the effects of DBT on PER stability (V. Suri and M. Rosbash, unpublished results).
The TER boxes each have the identical sequence GCGGCACGTTG. In S2 cells, the TER1 mutations, CAAGTTG and CTCGAAG, cause the same eightfold deficit in transcription as does deletion of the entire sequence by transversion mutations (data not shown), demonstrating the necessity of an intact noncanonical E box for proper function. These data suggest that TER1 and probably TER2 serve as additional binding sites for the dCLK-CYC transcription factor complex. It has been shown previously that the mammalian homolog of CYC (BMAL1 or MOP3) can bind multiple members of the PAS domain family (21). Therefore, dCLK or CYC could also dimerize with another partner to bind the TER1 element with high affinity. Alternatively or in addition, another protein could bind to the 5′ side of the TER motif (GCGG) and stabilize the dCLK-CYC heterodimer on the 3′ adjacent noncanonical E box.
We also identified an E box in the first intron of the tim gene. We were unable to detect any activity of this sequence in S2 cells, and a transgenic construct lacking the whole TER E-box region but containing this intronic E box failed to generate detectable tim mRNA levels. This indicates that the intronic E box is probably nonfunctional, for unknown reasons. Nevertheless, we still cannot rule out the possibility that the TERs and the E box collaborate with other sequence elements. However, transgenic flies with only the region of the tim promoter containing TER1 and the E box fused to luciferase exhibited robust transcriptional cycling (data not shown). These two elements can therefore work independently of any other tim promoter sequence. TER1 alone may even be able to drive transcriptional cycling, as a mutation in the E box did not completely eliminate circadian oscillations of luciferase activity (data not shown).
Do the two TERs have different functions? TER1 appears to be more important than TER2. Both in vivo and in S2 cells, disrupting TER1 leads to strong effects on the transcriptional activity of the tim promoter. In addition, examination of the TER1/TER2 double mutant suggests that TER2 is somewhat redundant in regard to TER1. This could be due to a distance effect: TER1 is closer to the E box, and factors bound to these motifs could be interacting more strongly. Disruption of TER1 has a substantial effect on expression levels (decrease of ∼50%) but little or no effect on cycling amplitude. In contrast, mutagenizing both TERs has a detrimental effect on cycling amplitude as well as expression levels. We therefore propose that both TERs are important determinants of tim mRNA levels and their oscillations and thus contribute to proper rhythmicity. The observation that transgenic flies lacking an E box but containing TER1 have less arrhythmicity than do flies without both elements further supports this notion (Table 1).
We noticed another element conserved within the timeless and period promoters. This PERR box has the sequence GTTCGCACAA, which does not correspond to any known transcription factor binding site described in the literature. Mutating this element in the context of the tim promoter-S2 cell assay led to decreases in transcription comparable to those caused by a mutation in the TER1 box. Activation of the double TER1/PERR mutant was equal to that of the TER1 and PERR single mutants, suggesting that these two elements might collaborate to enhance tim transcription in S2 cells. Importantly, mutation of the PERR box led to a similar ∼10-fold decrease in transcription in the context of the period promoter.
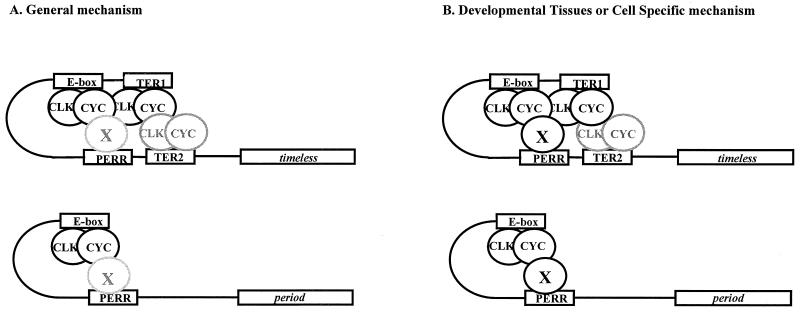
These data strongly suggest that the PERR box plays a role in per as well as tim transcription. However, PERR box mutant flies have at most subtle differences in luciferase activity compared to wild-type, timpFL-LUC flies. As S2 cells are derived from embryos, the PERR box might play a role during development rather than in adult flies (Fig. 8). Alternatively, PERR could contribute to tissue-specific transcriptional regulation of per and tim (Fig. 8). If this element were active in only a small subset of cells, it would explain why mutations have no observable effect in the luciferase assays.
FIG. 8.
Molecular model of per and tim transcription. (A) It may be in vivo that the majority of cells undergo a general process in which the TERs and E-box elements serve as the primary loading sites for dCLK and CYC and the PERR box plays a minor role in transcription. (B) If expression of the protein or protein complex that binds the PERR box were tissue specific or developmentally restricted, then that protein would interact with the proteins binding to the TERs and the E box, probably dCLK and CYC, and increase transcription of per and tim.
Our study therefore shows the importance of tim regulation for proper 24-h rhythmicity. Circadian period is very sensitive to tim mRNA levels, with more tim mRNA generating periods closer to 24 h. per mRNA cycling follows the same trend, as per mRNA trough levels are progressively lowered with increasing tim mRNA. Near-wild-type TIM levels are therefore required for accurate negative feedback on PER transcription. Lower TIM levels probably delay PER stabilization and nuclear entry and therefore lengthen the period. However, the amount of tim mRNA required for rhythmicity is rather low, and the levels generated by the minimal promoter are probably very close to this threshold. It is somewhat surprising that these periods are not longer than ∼29 to 30 h, suggesting that tim levels can affect period only within a narrow range. tim missense mutations can have a much larger effect on period (40a). In the timpMIN line, it is also surprising that PER is not cycling under LD, whereas it cycles in DD. Perhaps TIM levels fall just below threshold in LD, because TIM is degraded by light. PER cycling in timpMIN.1 would therefore be due to the increased amount of TIM in DD (data not shown), which is sufficient to generate PER stabilization and accumulation and yield a quasinormal cycling profile.
It will be of considerable interest to monitor precisely the kinetics of PER and TIM nuclear entry in lines with very limited amounts of TIM, both in peripheral oscillators and in the circadian pacemaker cells (the ventral lateral neurons). This could solidify the relationship between PER and TIM relocalization on the one hand and period length on the other. It will also be important to verify that expression levels and cycling of PER and TIM in the lateral neurons, the cells responsible for behavioral rhythmicity, parallel what is observed in the biochemical assays.
ACKNOWLEDGMENTS
We thank Ravi Allada for providing critical comments on the manuscript. We also thank Ed Dougherty for help with figures, Heather Felton for administrative assistance, and members of the Rosbash lab for stimulating discussions.
P.E. was supported by the Swiss National Science Foundation (fellowships 81GE-048176 and 823A050335). This work was also supported by NIH grant POI GM 33205.
REFERENCES
- 1.Allada R, White N E, So W V, Hall J C, Rosbash M. A mutant Drosophila homolog of mammalian Clock disrupts circadian rhythms and transcription of period and timeless. Cell. 1998;93:791–804. doi: 10.1016/s0092-8674(00)81440-3. [DOI] [PubMed] [Google Scholar]
- 2.Bae K, Lee C, Hardin P E, Edery I. dCLOCK is present in limiting amounts and likely mediates daily interactions between the dCLOCK-CYC transcription factor and the PER-TIM complex. J Neurosci. 2000;20:1746–1753. doi: 10.1523/JNEUROSCI.20-05-01746.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bae K, Lee C, Sidote D, Chuang K Y, Edery I. Circadian regulation of a Drosophila homolog of the mammalian Clock gene: PER and TIM function as positive regulators. Mol Cell Biol. 1998;18:6142–6151. doi: 10.1128/mcb.18.10.6142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Belvin M P, Zhou H, Yin J C. The Drosophila dCREB2 gene affects the circadian clock. Neuron. 1999;22:777–787. doi: 10.1016/s0896-6273(00)80736-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blackwell T K, Huang J, Ma A, Kretzner L, Alt F W, Eisenman R N, Weintraub H. Binding of Myc proteins to canonical and noncanonical DNA sequences. Mol Cell Biol. 1993;13:5216–5224. doi: 10.1128/mcb.13.9.5216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blackwell T K, Kretzner L, Blackwood E M, Eisenman R N, Weintraub H. Sequence-specific DNA binding by the c-Myc protein. Science. 1990;250:1149–1151. doi: 10.1126/science.2251503. [DOI] [PubMed] [Google Scholar]
- 7.Blau J, Young M W. Cycling vrille expression is required for a functional Drosophila clock. Cell. 1999;99:661–671. doi: 10.1016/s0092-8674(00)81554-8. [DOI] [PubMed] [Google Scholar]
- 8.Brandes C, Plautz J D, Stanewsky R, Jamison C F, Straume M, Wood K V, Kay S A, Hall J C. Novel features of Drosophila period transcription revealed by real-time luciferase reporting. Neuron. 1996;16:687–692. doi: 10.1016/s0896-6273(00)80088-4. [DOI] [PubMed] [Google Scholar]
- 9.Darlington T K, Wager-Smith K, Ceriani M F, Staknis D, Gekakis N, Steeves T D L, Weitz C J, Takahashi J S, Kay S A. Closing the circadian loop: CLOCK-induced transcription of its own inhibitors per and tim. Science. 1998;280:1599–1603. doi: 10.1126/science.280.5369.1599. [DOI] [PubMed] [Google Scholar]
- 10.Dunlap J C. Molecular bases for circadian clocks. Cell. 1999;96:271–290. doi: 10.1016/s0092-8674(00)80566-8. [DOI] [PubMed] [Google Scholar]
- 11.Edery I. Role of post-transcriptional regulation in circadian clocks: lessons from Drosophila. Chronobiol Int. 1999;16:377–414. doi: 10.3109/07420529908998716. [DOI] [PubMed] [Google Scholar]
- 12.Edery I, Zwiebel L J, Dembinska M E, Rosbash M. Temporal phosphorylation of the Drosophila period protein. Proc Natl Acad Sci USA. 1994;91:2260–2264. doi: 10.1073/pnas.91.6.2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Emery P, So W V, Kaneko M, Hall J C, Rosbash M. CRY, a Drosophila clock and light-regulated cryptochrome, is a major contributor to circadian rhythm resetting and photosensitivity. Cell. 1998;95:669–679. doi: 10.1016/s0092-8674(00)81637-2. [DOI] [PubMed] [Google Scholar]
- 14.Ewer J, Frisch B, Hamblen-Coyle M J, Rosbash M, Hall J C. Expression of the period clock gene within different cell types in the brain of Drosophila adults and mosaic analysis of these cells' influence on circadian behavioral rhythms. J Neurosci. 1992;12:3321–3349. doi: 10.1523/JNEUROSCI.12-09-03321.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Frisch B, Hardin P E, Hamblen-Coyle M J, Rosbash M, Hall J C. A promoterless DNA fragment from the period locus rescues behavioral rhythmicity and mediates cyclical gene expression in a restricted subset of the Drosophila nervous system. Neuron. 1994;12:555–570. doi: 10.1016/0896-6273(94)90212-7. [DOI] [PubMed] [Google Scholar]
- 16.Gekakis N, Staknis D, Nguyen H B, Davis C F, Wilsbacher L D, King D P, Takahashi J S, Weitz C J. Role of the CLOCK protein in the mammalian circadian mechanism. Science. 1998;280:1564–1569. doi: 10.1126/science.280.5369.1564. [DOI] [PubMed] [Google Scholar]
- 16a.Glossop N R, Lyons L C, Hardin P E. Interlocked feedback loops within the Drosophila circadian pacemaker. Science. 1999;286:766–768. doi: 10.1126/science.286.5440.766. [DOI] [PubMed] [Google Scholar]
- 17.Grandori C, Mac J, Siebelt F, Ayer D E, Eisenman R N. Myc-Max heterodimers activate a DEAD box gene and interact with multiple E box-related sites in vivo. EMBO J. 1996;15:4344–4357. [PMC free article] [PubMed] [Google Scholar]
- 18.Hao H, Allen D L, Hardin P E. A circadian enhancer mediates PER-dependent mRNA cycling in Drosophila melanogaster. Mol Cell Biol. 1997;17:3687–3693. doi: 10.1128/mcb.17.7.3687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hardin P E, Hall J C, Rosbash M. Feedback of the Drosophila period gene product on circadian cycling of its messenger RNA levels. Nature. 1990;343:536–540. doi: 10.1038/343536a0. [DOI] [PubMed] [Google Scholar]
- 20.Hardin P E, Hall J C, Rosbash M. Circadian oscillations in period gene mRNA levels are transcriptionally regulated. Proc Natl Acad Sci USA. 1992;89:11711–11715. doi: 10.1073/pnas.89.24.11711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hogenesch J B, Gu Y-Z, Jain S, Bradfield C A. The basic-helix-loop-helix-PAS orphan MOP3 forms transcriptionally active complexes with circadian and hypoxia factors. Proc Natl Acad Sci USA. 1998;95:5474–5479. doi: 10.1073/pnas.95.10.5474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hunter-Ensor M, Ousley A, Sehgal A. Regulation of the Drosophila protein timeless suggests a mechanism for resetting the circadian clock by light. Cell. 1996;84:677–685. doi: 10.1016/s0092-8674(00)81046-6. [DOI] [PubMed] [Google Scholar]
- 23.Jin X, Shearman L P, Weaver D R, Zylka M J, de Vries G J, Reppert S M. A molecular mechanism regulating rhythmic output from the suprachiasmatic circadian clock. Cell. 1999;96:57–68. doi: 10.1016/s0092-8674(00)80959-9. [DOI] [PubMed] [Google Scholar]
- 24.King D P, Zhao Y, Sangoram A M, Wilsbacher L D, Tanaka M, Antoch M P, Steeves T D L, Vitaterna M H, Kornhauser J M, Lowrey P L, Turek F W, Takahashi J S. Positional cloning of the mouse circadian clock gene. Cell. 1997;89:641–653. doi: 10.1016/s0092-8674(00)80245-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kloss B, Price J L, Saez L, Blau J, Rothenfluh-Hilfiker A, Wesley C S, Young M W. The Drosophila clock gene double-time encodes a protein closely related to human casein kinase I. Cell. 1998;94:97–107. doi: 10.1016/s0092-8674(00)81225-8. [DOI] [PubMed] [Google Scholar]
- 26.Kume K, Zylka M J, Sriram S, Shearman L P, Weaver D R, Jin X, Maywood E S, Hastings M H, Reppert S M. mCRY1 and mCRY2 are essential components of the negative limb of the circadian clock feedback loop. Cell. 1999;98:193–205. doi: 10.1016/s0092-8674(00)81014-4. [DOI] [PubMed] [Google Scholar]
- 27.Lavery D J, Lopez-Molina L, Margueron R, Fleury-Olela F, Conquet F, Schibler U, Bonfils C. Circadian expression of the steroid 15 α-hydroxylase (Cyp2a4) and coumarin 7-hydroxylase (Cyp2a5) genes in mouse liver is regulated by the PAR leucine zipper transcription factor DBP. Mol Cell Biol. 1999;19:6488–6499. doi: 10.1128/mcb.19.10.6488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee C, Bae K, Edery I. The Drosophila CLOCK protein undergoes daily rhythms in abundance, phosphorylation and interactions with the PER-TIM complex. Neuron. 1998;4:857–867. doi: 10.1016/s0896-6273(00)80601-7. [DOI] [PubMed] [Google Scholar]
- 29.Lee C, Bae K, Edery I. PER and TIM inhibit the DNA binding activity of a Drosophila CLOCK-CYC/dBMAL1 heterodimer without disrupting formation of the heterodimer: a basis for circadian transcription. Mol Cell Biol. 1999;19:5316–5325. doi: 10.1128/mcb.19.8.5316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee C, Parikh V, Itsukaichi T, Bae K, Edery I. Resetting the Drosophila clock by photic regulation of PER and a PER-TIM complex. Science. 1996;271:1740–1744. doi: 10.1126/science.271.5256.1740. [DOI] [PubMed] [Google Scholar]
- 31.Lowrey P L, Shimomura K, Antoch M P, Yamazaki S, Zemenides P D, Ralph M R, Menaker M, Takahashi J S. Positional syntenic cloning and functional characterization of the mammalian circadian mutation tau. Science. 2000;288:483–492. doi: 10.1126/science.288.5465.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Luehrsen K R, de Wet J R, Walbot V. Transient expression analysis in plants using firefly luciferase reporter gene. Methods Enzymol. 1992;216:397–414. doi: 10.1016/0076-6879(92)16037-k. [DOI] [PubMed] [Google Scholar]
- 33.Marrus S B, Zeng H, Rosbash M. Effect of constant light and circadian entrainment of pers flies: evidence for light-mediated delay of the negative feedback loop in Drosophila. EMBO J. 1996;15:6877–6886. [PMC free article] [PubMed] [Google Scholar]
- 34.Myers M P, Wager-Smith K, Rothenfluh-Hilfiker A, Young M W. Light-induced degradation of TIMELESS and entrainment of the Drosophila circadian clock. Science. 1996;271:1736–1740. doi: 10.1126/science.271.5256.1736. [DOI] [PubMed] [Google Scholar]
- 35.Okamura H, Miyake S, Sumi Y, Yamaguchi S, Yasui A, Muijtjens M, Hoeijmakers J H, van der Horst G T. Photic induction of mPer1 and mPer2 in cry-deficient mice lacking a biological clock. Science. 1999;286:2531–2534. doi: 10.1126/science.286.5449.2531. [DOI] [PubMed] [Google Scholar]
- 36.Plautz J D, Kaneko M, Hall J C, Kay S A. Independent photoreceptive circadian clocks throughout Drosophila. Science. 1997;278:1632–1635. doi: 10.1126/science.278.5343.1632. [DOI] [PubMed] [Google Scholar]
- 37.Prendergast G C, Ziff E B. DNA-binding motif. Nature. 1989;341:392. doi: 10.1038/341392a0. [DOI] [PubMed] [Google Scholar]
- 38.Price J L, Blau J, Rothenfluh-Hilfiker A, Abodeely M, Kloss B, Young M W. double-time is a novel Drosophila clock gene that regulates PERIOD protein accumulation. Cell. 1998;94:83–95. doi: 10.1016/s0092-8674(00)81224-6. [DOI] [PubMed] [Google Scholar]
- 39.Price J L, Dembinska M E, Young M W, Rosbash M. Suppression of PERIOD protein abundance and circadian cycling by the Drosophila clock mutation timeless. EMBO J. 1995;14:4044–4049. doi: 10.1002/j.1460-2075.1995.tb00075.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ripperger J A, Shearman L P, Reppert S M, Schibler U. CLOCK, an essential pacemaker component, controls expression of the circadian transcription factor DBP. Genes Dev. 2000;14:679–689. [PMC free article] [PubMed] [Google Scholar]
- 40a.Rothenfluh A, Young M W, Saez L. A TIMELESS-independent function for PERIOD proteins in the Drosophila clock. Neuron. 2000;26:505–514. doi: 10.1016/s0896-6273(00)81182-4. [DOI] [PubMed] [Google Scholar]
- 41.Rutila J E, Maltseva O, Rosbash M. The timSL mutant affects a restricted portion of the Drosophila melanogaster circadian cycle. J Biol Rhythms. 1998;13:380–392. doi: 10.1177/074873098129000200. [DOI] [PubMed] [Google Scholar]
- 42.Rutila J E, Suri V, Le M, So W V, Rosbash M, Hall J C. CYCLE is a second bHLH-PAS protein essential for circadian transcription of Drosophila period and timeless. Cell. 1998;93:805–814. doi: 10.1016/s0092-8674(00)81441-5. [DOI] [PubMed] [Google Scholar]
- 43.Saez L, Young M W. Regulation of nuclear entry of the Drosophila clock proteins PERIOD and TIMELESS. Neuron. 1996;17:911–920. doi: 10.1016/s0896-6273(00)80222-6. [DOI] [PubMed] [Google Scholar]
- 44.Scully A L, Kay S A. Time flies for Drosophila. Cell. 2000;100:297–300. doi: 10.1016/s0092-8674(00)80665-0. [DOI] [PubMed] [Google Scholar]
- 45.Sehgal A, Rothenfluh-Hilfiker A, Hunter-Ensor M, Chen Y, Myers M, Young M W. Circadian oscillations and autoregulation of timeless RNA. Science. 1995;270:808–810. doi: 10.1126/science.270.5237.808. [DOI] [PubMed] [Google Scholar]
- 46.So W V, Rosbash M. Post-transcriptional regulation contributes to Drosophila clock gene mRNA cycling. EMBO J. 1997;16:7146–7155. doi: 10.1093/emboj/16.23.7146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.So W V, Sarov-Blat L, Kotarski C, McDonald M J, Rosbash M. takeout: a clock-regulated gene and the transcriptional regulation for novel cycling phase. Mol Cell Biol. 2000;20:6935–6944. doi: 10.1128/mcb.20.18.6935-6944.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stanewsky R, Jamison C F, Plautz J D, Kay S A, Hall J C. Multiple circadian-regulated elements contribute to cycling period gene expression in Drosophila. EMBO J. 1997;16:5006–5018. doi: 10.1093/emboj/16.16.5006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Suri V, Lanjuin A, Rosbash M. TIMELESS-dependent positive and negative autoregulation in the Drosophila circadian clock. EMBO J. 1999;18:675–686. doi: 10.1093/emboj/18.3.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Suri V, Qian Z, Hall J C, Rosbash M. Evidence that the TIM light response is relevant to light-induced phase shifts in Drosophila melanogaster. Neuron. 1998;21:225–234. doi: 10.1016/s0896-6273(00)80529-2. [DOI] [PubMed] [Google Scholar]
- 51.Taruscio D, Zoraqi G K, Falchi M, Iosi F, Paradisi S, Di Fiore B, Lavia P, Falbo V. The human per1 gene: genomic organization and promoter analysis of the first human orthologue of the Drosophila period gene. Gene. 2000;253:161–170. doi: 10.1016/s0378-1119(00)00248-1. [DOI] [PubMed] [Google Scholar]
- 52.Thummel C S, Boulet A M, Lipshitz H D. Vectors for Drosophila P-element-mediated transformation and tissue culture transfection. Gene. 1988;74:445–456. doi: 10.1016/0378-1119(88)90177-1. [DOI] [PubMed] [Google Scholar]
- 53.Vosshall L B, Price J L, Sehgal A, Saez L, Young M W. Specific block in nuclear localization of period protein by a second clock mutation, timeless. Science. 1994;263:1606–1609. doi: 10.1126/science.8128247. [DOI] [PubMed] [Google Scholar]
- 54.Yamaguchi S, Mitsui S, Miyake S, Yan L, Onishi H, Yagita K, Suzuki M, Shibata S, Kobayashi M, Okamura H. The 5′ upstream region of mPer1 gene contains two promoters and is responsible for circadian oscillation. Curr Biol. 2000;10:873–876. doi: 10.1016/s0960-9822(00)00602-3. [DOI] [PubMed] [Google Scholar]
- 55.Yang Z, Emerson M, Su H S, Sehgal A. Response of the timeless protein to light correlates with behavioral entrainment and suggests a nonvisual pathway for circadian photoreception. Neuron. 1998;21:215–223. doi: 10.1016/s0896-6273(00)80528-0. [DOI] [PubMed] [Google Scholar]
- 56.Zeng H, Qian Z, Myers M P, Rosbash M. A light-entrainment mechanism for the Drosophila circadian clock. Nature. 1996;380:129–135. doi: 10.1038/380129a0. [DOI] [PubMed] [Google Scholar]