Abstract
Plant height is an important characteristic, the modification of which can improve the ability of stress adaptation as well as the yield. In this study, genome-wide association analysis was performed for plant height traits in 370 potato cultivars using the tetraploid potato genome as a reference. A total of 92 significant single nucleotide polymorphism (SNP) loci for plant height were obtained, which were particularly significant in haplotypes A3 and A4 on chromosome 1 and A1, A2, and A4 on chromosome 5. Thirty-five candidate genes were identified that were mainly involved in the gibberellin and brassinolide signal transduction pathways, including the FAR1 gene, methyltransferase, ethylene response factor, and ubiquitin protein ligase. Among them, PIF3 and GID1a were only present on chromosome 1, with PIF3 in all four haplotypes and GID1a in haplotype A3. This could lead to more effective genetic loci for molecular marker-assisted selection breeding as well as more precise localization and cloning of genes for plant height traits in potatoes.
Keywords: autotetraploid potato, plant height, whole genome re-sequencing (WGRS), genome-wide association analysis (GWAS), significant SNP
1. Introduction
The potato (Solanum tuberosum L.), a staple food for 1.3 billion people, was cultivated on 17.5 million hectares and yielded 370.5 million tons in 2019 worldwide [1]. Two thirds of the annual yield is marketed fresh, while the remainder is processed for snack and other industrial food products, including animal feed, adhesives, pharmaceuticals, wood, and textile commodities [2,3]. It contains abundant starch, proteins, sugars, vitamins, minerals, ascorbic acid, carotenoids, and other trace elements required by humans, thereby making it “the most affordable nutritious food.” It originated in the Andes and evolved into a tuber-forming crop with a short sunlight photoperiod, mainly for asexual reproduction [4]. It was introduced to Europe as early as the 15th century and has been cultivated since the 17th century. The major potato-producing countries include China, Russia, India, Ukraine, and the United States. Nowadays, the area of land in developing countries has surpassed that of developed countries. Global potato production is continuously and gradually growing, with multiple countries becoming highly dependent on potatoes for food production.
Plant height is one of the important agronomic traits targeted in crop breeding and cultivation management. Adequately dwarfed plants have greater lodging resistance and can improve the light energy utilization of the crop population, thereby increasing the crop yield [5]. Since the Green Revolution in the 1950s, phenomenal success has been achieved in reducing plant height to consequently increase the actual crop yield [6]. Currently, some genes regulating plant height have been expressed in crop species, including bread wheat [7], rice [8], and other crops. Brassinosteroids (BRs) and gibberellins (GAs) are the known plant height-promoting regulators with the most direct impact, the fewest side effects, and the greatest agronomic potential [9].
GAs, one of the most important plant hormones, is a class of tetracyclic diterpene phytohormones that play important roles in seed germination, root crown elongation, stem elongation, leaf growth, and flowering and fruiting in plants. Currently, the erythromycin synthesis and metabolic pathway of the model plant Arabidopsis thaliana have been studied in depth, with its related genes having been cloned and identified [10,11]. The Green Revolution in crops is closely related to GAs metabolic pathway-related genes, which promote stem elongation and plant height. Researchers localized the QTL (MTD1) that regulates plant height and tiller number in a 66-kb interval on chromosome 9 and clarified that LOC_Os09g02650 depends on the GAs biosynthetic pathway to regulate the rice plant height [12]. A previous study found that the DELLA proteins (Dwarf8 and Dwarf9) regulated maize plant height by regulating gibberellin signal transduction [13].
The sixth major phytohormone, BRs, is a class of steroid phytohormones that is vital for plant growth regulation. It is mainly involved in cell wall remodeling and also promotes cell elongation by regulating the expression of genes, thus promoting the growth of plant organs and regulating plant growth and development [14,15]. Consistent with the primary function of BRs to promote cell elongation, mutants defective in BRs biosynthesis or signal transduction display a dwarf phenotype. Conversely, increasing the BRs levels or activity increased the plant size, biomass, and seed yield [9]. Identification of br-deficient dwarf mutants helped identify several BRs biosynthesis-related enzymes in rice, including OsDwarf2, OsDwarf11, and br-deficient dwarf mutant-1 (brd1) and brd2 [16,17]. Reduced expression of ZmDWF1 (the maize homolog of DIM1/DWF1) resulted in dwarf maize plants [18]. Additionally, hormones, including auxin [19] and strigolactone [20], can affect plant stalk development via different processes, including sugar metabolism [21], root hair growth [22], and transcription factor regulation [23].
Genome-wide association study (GWAS) is a genome-wide analysis method to decipher the relationship between phenotypic traits and single nucleotide polymorphism (SNP) markers based on the linkage disequilibrium principle [24]. This can identify candidate genes associated with target traits with high resolution and sensitivity [25,26]. GWAS has been widely used in recent years to study the genetic mechanisms of complex traits in various crops. In this study, we subjected 370 cultivars of tetraploid potatoes to whole genome sequencing (WGS) and performed the GWAS analysis for plant height traits to identify significant molecular markers and candidate genes, while also providing molecular markers and genetic resources for potato plant improvement.
2. Materials and Methods
2.1. Experimental Material Planting and Sources
A total of 370 potato cultivars were collected from the Institute of Biotechnology, Qinghai Academy of Agricultural and Forestry Sciences, China. Among them, 152 were from Peru, 126 from China, 25 from Israel, Russia, Canada, Australia, the USA, the Netherlands, and New Zealand, and 67 from unknown regions (Table S1).
According to the current guidelines for testing plant cultivars for specificity, consistency, and stability in potatoes (GB/T 19557.28-2018), two independent growth cycles (2019 and 2020, planted at the end of April and harvested at the beginning of October) were set up for observation. The test location was set in Huangyuan County, Qinghai Province (36°680′ N, 101°260′ E). It not only has a cool climate and is extremely suitable for potato cultivar breeding and germplasm conservation but also supports trait collection and identification for maintaining and characterizing the potato material and restoring its native habitat conditions. The propagation material for this study was potato tubers, which were 35–50 mm in diameter, unsprouted, healthy in appearance, and free of pests and diseases. The field trials were divided into three plots, with 6–10 potato plants of each variety planted 30 cm apart from each other and 90 cm apart in rows, with a protected row every 15 rows. A randomized complete block design (RCBD) with three repetitions was used to grow the 370 cultivars.
2.2. Identification of Plant Height
The plant height data were collected by observing 370 potato plants with reference to the requirements of trait observation in the national standard for potatoes (GB/T 19557.28-2018). Before the potato was mature, we measured the height of the plant, compared it with the height of the standard cultivars (Table 1), and recorded the height index.
Table 1.
Plant height index standard in potato germplasms.
| Agronomic Trait | The Name of Standard Cultivar | Height Index | |
|---|---|---|---|
| Plant height | Extremely short | A-6 | 1 |
| Very short to short | - | 2 | |
| Short | Dongnong303 | 3 | |
| Short to medium | - | 4 | |
| Medium | Kexin2 | 5 | |
| Medium to high | - | 6 | |
| High | Hongtudou | 7 | |
| High to very high | - | 8 | |
| Very high | Qingshu9 (Q9) | 9 | |
2.3. Potato DNA Extraction and Sequencing
A month after the potatoes started growing, the young leaves were removed for DNA extraction. The Plant DNA Extraction Kit (QIAGEN) was used to extract DNA from potato cubes. The DNA purity (OD260/280 ratio) was measured using a spectrophotometer, whereas the degree of DNA degradation and RNA/protein contamination was analyzed by agarose gel electrophoresis, and DNA concentration was accurately quantified by Qubit.
Whole genome re-sequencing (WGRS) was performed for DNA libraries using DNBSEQ-T7.
2.4. Variable Loci Extraction and Statistics
Raw data were tested by the FastQC software (https://github.com/s-andrews/FastQC/ (accessed on 22 March 2022)), while clean reads were obtained using the fastp software by filtering the raw data that passed the test. Then the BWA software [27] was not only used to establish the index file of the tetraploid potato reference genome Q9 (https://ngdc.cncb.ac.cn/biosample/browse/SAMC490813 (accessed on 22 March 2022)) but was also used for sequence comparison of the clean reads of each sample to get the SAM file. Samtools was used [28] to reorder and convert the SAM file into a BAM file, followed by another sorting and the final generation of the index file. Picard was used to mark the duplicates in the Java environment, which were then used to create citations for the new BAM files.
Then the HaplotypeCaller application of the GATK4 [29] software was used to generate each sample GVCF file (GVCF format includes all variant types, including SNPs and Indel, which need to be further filtered). Finally, the GVCF was merged using CombineGVCFs to obtain the total merged GVCF file, followed by GenotypeGVCFs being used to extract genotypes. The filtered VCF files were then obtained using VCFtools [30] with MAF > 0.05 (minimum allele frequency of 0.05, post removal of rare alleles) and HWE > 0.001 (removal of loci that do not satisfy the Hardy-Weinberg equilibrium (p < 0.001)). The VCF tools were further used to remove the variant loci with a >50% deletion rate of all materials to obtain the final filtered, high-quality variant loci. The marker density of SNPs was visualized for mapping using CMplot in R.
2.5. Genome-Wide Asociation Analysis
GWAS analysis was performed using the high-quality SNPs and indels obtained. Association analysis was performed on the height of the plants using the compressed mixed linear model (MLM) of the R package GAPIT. Manhattan and QQ plots were plotted using the R package (CMplot) to screen the significant SNP loci with a threshold of p = 10−4, while the type and location of SNPs were identified using the reference genome of Q9.
2.6. Candidate Gene Mining and KEGG Enrichment Analysis
Upstream and downstream genes were searched for significant SNP loci, followed by the screening and identification of the final candidate genes. To determine the functions of the candidate genes and their metabolic pathways, the KEGG database was used for functional annotation to (1) systematically analyze the genetic basis of potato plant height development, (2) mine for superior genes for providing candidate genes and analytical ideas for potato plant morphology formation, and (3) serve potato breeding practice. Meanwhile, to further understand the expression pattern of candidate genes, different parts (including flower, leaf, stem, and root) of the highest plant height of potato Q9 were selected for transcriptome sequencing, with the tetraploid potato Q9 being the reference genome to calculate its expression.
The sampling period for RNA-seq sequencing was the flowering stage. After obtaining the raw data of the transcriptome, we used fastqc software to detect the quality and fastp software to filter the qualified raw data. Furthermore, we compared filtered clean data alignment with reference genome using hisat2 software (http://daehwankimlab.github.io/hisat2/ (accessed on 22 March 2022)), then got and converted Sam files to Bam files, and sorted them using Samtools. Finally, we used the Stringtie [31] software to obtain the expression file (FPKM value).
3. Results
3.1. Analysis of the Diversity of Potato Plant Height
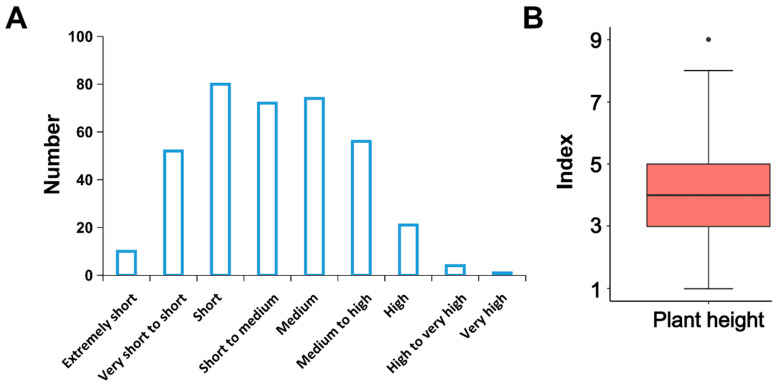
Since the variation was small across environments and years, we took the mean values of the observations from 2019 to 2020 to plot the frequency of their trait distribution (Figure 1A, Table S1). Plant height was dominated by “short”, “dwarf to medium”, and “medium”, with 80 (21.62%), 72 (19.46%), and 74 (20.00%) materials, respectively. The upper, median, and lower limits of plant height were 8, 4, and 1, respectively (Figure 1B), with Q9 being the only plant height index of 9, which was our reference genome cultivar. All plant traits covered all phenotypic states, and all phenotypes conformed to the normal distribution, thus indicating that the population was suitable for GWAS analysis.
Figure 1.
Distribution of the height and boxplot of the potato plant height. (A) Plant height distribution of 370 potato cultivars; (B) Boxplot of plant height index.
3.2. Variable Loci Marker Density and Statistical Analysis
The whole genome sequencing of 370 tetraploid potatoes yielded ~9.88 Tb of raw data with an average sequencing depth of ~10 X, ultimately generating 232,581,777 SNPs and Indels. VCFtools helped filter the original VCF file and we obtained 4,986,690 high-quality mutation sites (including 4,535,735 SNPs and 450,955 Indels) (Table 2).
Table 2.
Summary of SNPs and indels among the 370 tetraploid potato cultivars.
| SNPs | Indels | |||||||
|---|---|---|---|---|---|---|---|---|
| A1 | A2 | A3 | A4 | A1 | A2 | A3 | A4 | |
| Chr01 | 138,006 | 194,142 | 129,323 | 127,535 | 15,004 | 17,111 | 10,826 | 14,762 |
| Chr02 | 102,837 | 101,023 | 117,660 | 85,563 | 10,583 | 9856 | 12,222 | 9275 |
| Chr03 | 55,117 | 76,170 | 85,021 | 68,587 | 6313 | 7950 | 6530 | 9677 |
| Chr04 | 57,575 | 57,327 | 86,486 | 58,568 | 7218 | 6798 | 8429 | 6109 |
| Chr05 | 44,400 | 197,492 | 249,222 | 136,165 | 6255 | 14,701 | 22,302 | 11,520 |
| Chr06 | 47,830 | 91,815 | 98,615 | 72,776 | 6701 | 8380 | 10,860 | 7075 |
| Chr07 | 186,990 | 158,514 | 140,408 | 105,042 | 15,751 | 12,262 | 11,911 | 11,757 |
| Chr08 | 99,099 | 101,120 | 73,798 | 116,424 | 8699 | 11,011 | 7217 | 10,402 |
| Chr09 | 142,118 | 70,221 | 150,275 | 47,334 | 12,861 | 8067 | 13,384 | 5101 |
| Chr10 | 29,802 | 26,892 | 21,862 | 28,140 | 3480 | 3489 | 2049 | 2904 |
| Chr11 | 53,935 | 81,915 | 96,253 | 48,863 | 6439 | 10,028 | 10,263 | 6931 |
| Chr12 | 61,094 | 38,639 | 97,251 | 80,491 | 5612 | 4649 | 11,682 | 8549 |
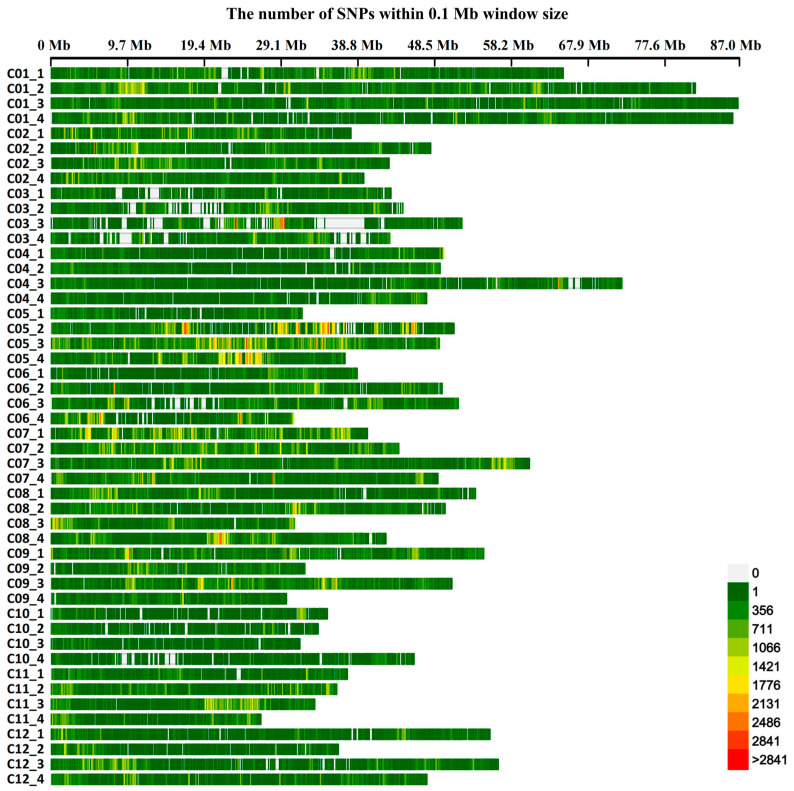
We used a total of 4,535,735 high-quality SNPs for a GWAS of the plant height trait, with an average marker density of 2031 SNP/Mb at the genome-wide scale (Table S2). The lowest marker density (608 SNP/Mb) was found on Chr10A4, whereas the highest marker density (5061 SNP/Mb) was found on Chr05A3. Thus, the markers were unevenly distributed on the tetraploid potato Q9 genome (Figure 2).
Figure 2.
SNP distributions on the 48 chromosomes of potato. The horizontal axis displays the chromosome length; the 0–2841 legend insert indicates the SNP density.
3.3. Genome-Wide Association Analysis of Plant Height
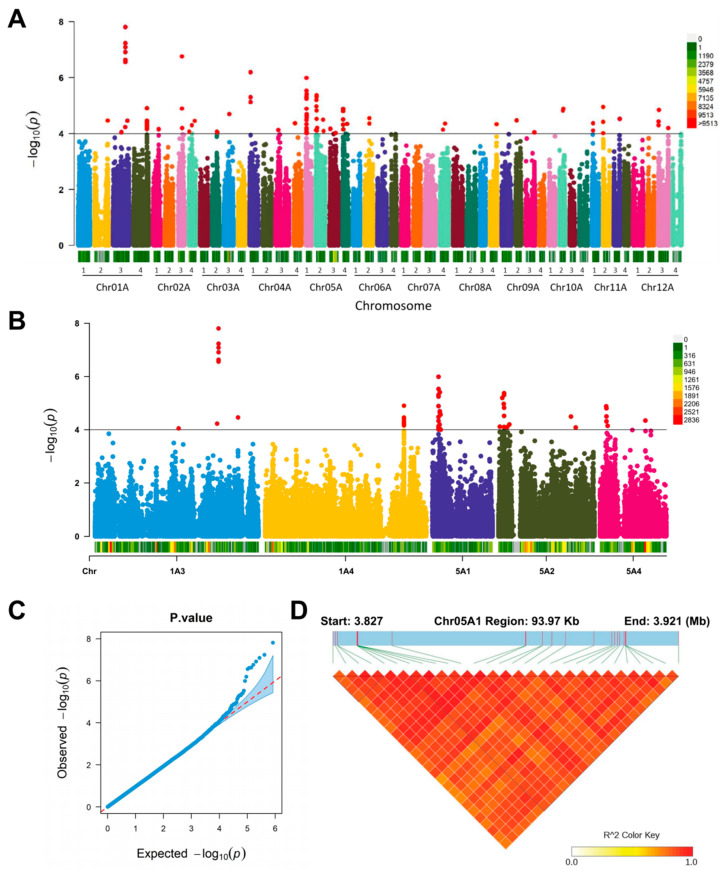
We used GAPIT to perform GWAS analysis on the phenotypic and genotypic data of the potato plant height using an MLM and plotted the Manhattan and Q-Q plots. Subsequently, we obtained a total of 92 significant SNP loci (Table S3) and found that the significant SNPs had a certain distribution pattern on chromosomes 1, 2, 3, 4, 5, 11, and 12 (Figure 3A), with A3 and A4 on chromosome 1 and A1, A2, and A4 on chromosome 5 being particularly significant (Figure 3B). Since we speculated that there might be important molecular markers associated with plant height on these two chromosomes, Q-Q plots helped check the reliability of our markers. It found that the model fit was good and the significant SNP loci were significantly higher than the fitted curve (Figure 3C). To further determine the linkage disequilibrium within the association region, we plotted the LD heat map of the A1 haplotype association region of chromosome 5 using PopLDdecay and LDBlockShow and found a very strong association of SNPs in the 38.827–38.921 Mb region (Figure 3D).
Figure 3.
The genome-wide association analysis of plant height. (A,B) The Manhattan plot of the plant height. Horizontal axis displays the chromosome: 0–9513 legend insert indicates the SNP density (A) and 0–2836 legend insert indicates the SNP density (B); (C) Quantile–quantile plot for the plant height; (D) the LD heat map of the plant height associated region on Chr05A1; Red and white color legend insert indicate high and low degree of linkage disequilibrium, respectively.
3.4. Identification and Analysis of Candidate Genes
We identified the upstream and downstream genes as candidate genes and obtained a total of 35 candidate genes via screening and identification. Among them, five were transcription factors (bHLH, MYB, TALE, ERF, and B3) (Table S4). To better understand the functions of the candidate genes, we performed their KEGG enrichment analysis and found that “plant hormone signal transduction” had the most candidates, followed by “metabolic pathways” (Figure S1). Additionally, they were also enriched in “zeatin biosynthesis”, “starch and sucrose metabolism”, and the “MAPK (mitogen-activated protein kinase) signaling pathway.” Candidate genes include the FAR1 gene, methyltransferase, ethylene response factor, and ubiquitin protein ligase, which may be related to potato plant height regulation.
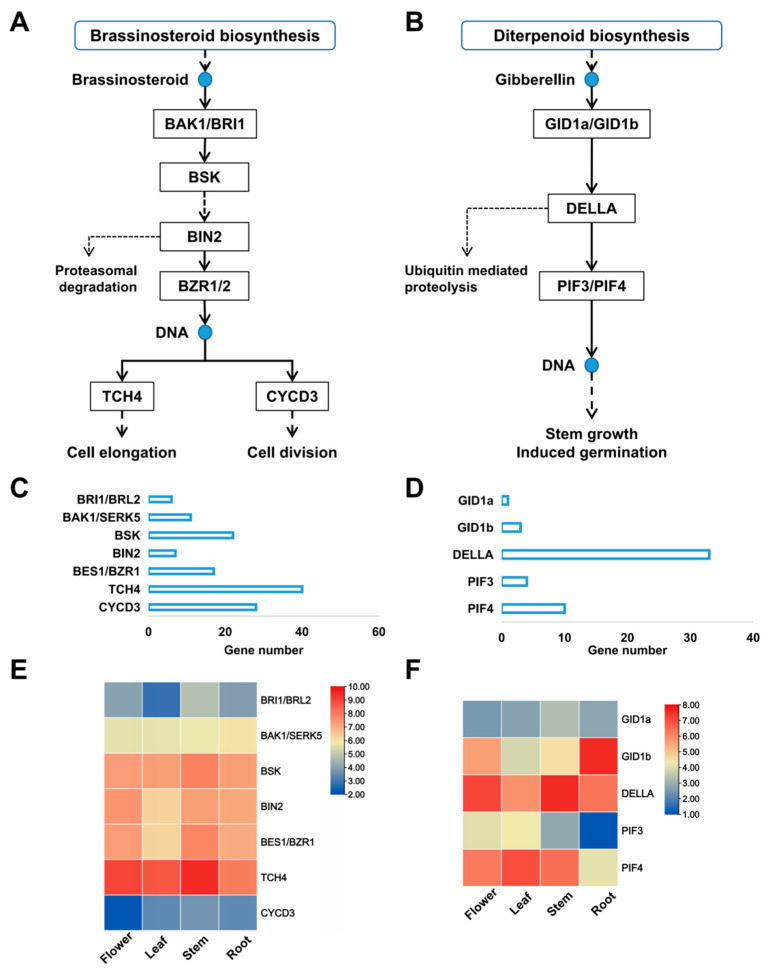
Upon further analysis, we identified six genes in the phytohormone signal transduction pathway, mainly gibberellin receptor 1 (GID1) and transcription factor PIF3 of the GA signaling pathway and BR-signaling kinase (BSK) and D-type cyclin family 3 (CYCD3) of the BR signaling pathway. To further resolve the genes related to gibberellin and brassinolide in potatoes, we identified the genes related to the tetraploid potato genome and mapped their regulatory pathways (Figure 4A,B). After counting, we found that there were 131 genes in the BRs signaling pathway, of which xyloglucosyl transferase (TCH4) and CYCD3 were the most abundant (Figure 4C) and widely distributed in the genome (Table S5). Additionally, they may be related to regulating cell elongation and division processes. The gibberellin signaling pathway has 51 genes, among which DELLA is the most abundant, followed by PIF4, PIF3, and GID1 (Figure 4D). We found that the DELLA, PIF3 (Phytochrome interacting factor 3), and GID1 are distributed in chromosome 1, with PIF3 especially being present only in chromosome 1 and GID1a being present only in the A3 haplotype of chromosome 1 (Table S6, Figure S2).
Figure 4.
The candidate genes in potato brassinolide and gibberellin pathways. (A) potato-brassinolide pathway. (B) potato gibberellin pathway. (C,D) The copy number of candidate genes in potato brassinolide (C) and gibberellin (D) pathways. (E,F) Expression profiles of associated, differentially expressed candidate genes among different tissues in potatoes.
We also analyzed the gene expression profile in the flowers, stems, leaves, and roots of the tetraploid potato cultivar Q9. The results showed that the expression of TCH4, BSK, and CYCD3 in BR signaling was significantly higher in the stems than in the other tissues (Figure 4E). The GID1a/GID1b expression in the gibberellin signaling pathway was highest in the roots, with DELLA being the highest in the stems and flowers. Moreover, PIF3 and PIF4 were the most highly expressed in the leaves.
4. Discussion
The genetic gain in potatoes has been small as compared to other major crops, with the tetraploid inheritance complexity being a key factor hindering the genetic improvement of cultivated potatoes [32]. With the rapid development of sequencing technology, the genomes of different potato varieties and their ploidy levels were gradually deciphered, especially the assembly of the genomes of tetraploid cultivated potatoes Otava [33], C88 [34], and Q9 [35]. This laid the groundwork for understanding the genetic mechanism of tetraploid potatoes, which is crucial in breeding common tetraploid cultivated potato varieties. In this study, we re-sequenced 370 varieties or strains of tetraploid potatoes, performed the GWAS analysis for the plant height traits using the tetraploid potato genome as a reference, mined for significant molecular markers, and analyzed the candidate genes.
Plant height is an important component of its varietal structure, and its alteration can not only improve the plant’s stress adaptation ability but also improve its yields. Since the Green Revolution in the 1950s, great success has been achieved in reducing plant height to improve actual crop yields [5]. In this study, GWAS analysis of plant height traits revealed the presence of significant markers on multiple chromosomes that may be associated with the regulation of plant height. Among them, especially the A3 haplotype of chromosome 1, we found PIF3 and GID1 at the significant locus attachment. In active GA signaling in plants, the gibberellin-insensitive dwarf gene GID1 (gibberellin insensitive dwarf 1) encodes a gibberellin-binding receptor that causes the DELLA protein to aggregate into the SCFSLY1 complex, thus resulting in ubiquitin-mediated degradation of DELLA protein, which activates its downstream signaling and affects the plant growth and development [36]. The phytochrome-interacting factors (PIFs) are a class of bHLH transcription factors that were first identified and shown to regulate photomorphogenesis [37]. PIFs are thought to be hubs that integrate both external environmental factors and internal signals during plant development, thereby regulating the expression of their downstream genes [38]. Thus, these are essential genes in the GAs transduction pathway, which finely regulates the entire transcriptome network. Research shows that GA metabolism and signaling are both critical for controlling plant height, which is consistent with the results of this study [9].
Additionally, we identified one BSK gene and three CYCD3 genes on the A3 haplotype of chromosomes 1 and 2. BSK and CYCD3 are important components of the BR signal pathway. Studies have shown that BSK plays a crucial role in plant growth, development, and stress regulation [39]. CYCD3 can promote plant growth by controlling cell division [40,41]. We speculate that BSK and CYCD3 can further control the establishment of plant height by regulating BRs. BRs also interact with other plant signaling pathways, notably those of light and the hormones auxin, GA, abscisic acid (ABA), and ethylene [42]. We found that the far-red impaired response 1 (FAR1) may be involved in the regulation of plant height [43]. The protein encoded contains a DNA-binding domain and belongs to a transposase-derived class of transcription factors that directly activate the expression of the far-red light gene FHY1/FHL, an important regulatory player in the plant starch anabolism and energy deprivation processes triggered by carbon starvation [43]. Sugars play an important role in plant height development by providing the necessary raw materials for cell division and elongation, like the cloned plant height trait regulator gene SXD1. Its mutant leaves do not export sucrose properly, thereby resulting in dwarf plants [21].
Furthermore, some transcription factors may be involved in plant height development, and candidate genes include MYB and ERF. The MYB transcription factors are a powerful superfamily of transcription factors in plants, and recent studies have shown that they play an important regulatory role in plant growth and development [44,45]. Overexpression of MYB has been found to alter the plant structure, as evident from the increased lateral branching and reduced plant height [46]. The genes GmGAMYB [47] and GmLHY [48] regulate plant height via the gibberellin pathway, where GmGAMYB is induced by gibberellin to up-regulate GmGA20ox expression, which increases the plant height. Moreover, GmLHY regulates plant height by directly or indirectly increasing the expression levels of GA synthesis-related and GA reaction-related genes.
In this study, a GWAS of plant height traits in 370 cultivars of tetraploid potatoes revealed that the most significant molecular markers associated with potato plant height were located on chromosome 1, while the more significant molecular markers were also present on chromosomes 2, 4, and 5. Additionally, we identified 35 candidate genes, six of which were related to phytohormone signaling, especially GAs and BRs. Therefore, these molecular markers and candidate genes will provide good markers and genetic resources for potato plant height regulation, thus enhancing its breeding process.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/genes14020507/s1. Table S1: Identification of 370 tetraploid potatoes’ origin and their plant heights; Table S2: Density statistics of SNPs on the tetraploid potato chromosomes; Table S3: Potato plant height association analysis of the significant SNP sites; Table S4: Candidate genes for the significant association markers; Table S5: The number of genes involved in the BR signal transduction pathway and their host chromosomes; Table S6: The number of genes involved in the GAs signal transduction pathway and their host chromosomes; Figure S1: KEGG enrichment analysis of the candidate genes; Figure S2: Genes associated with the gibberellin signaling transduction pathway and their chromosomal distribution.
Author Contributions
L.Z., Z.X. and F.W. conceived and managed the experiments. L.Z., S.J., X.D., T.N. and K.D. conducted the experiments. L.Z., F.W., S.J. and Z.X. analyzed the experimental results. L.Z. and M.Z. wrote the manuscript. Z.X., F.W. and J.W. reviewed and improved it, and they revised the final version of the manuscript. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Supporting data can be found at the National Genomics Data Center and are accessible at http://bigd.big.ac.cn/ (accessed on 22 March 2022) under BioProject numbers PRJCA011806.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research was funded by Genome wide association analysis of main agronomic traits of cultivated potato (2023-HZ-807), China Agriculture Research System of MOF and MARA (NO.CARS-9), Developing Bioinformatics Platform in Hainan Yazhou Bay Seed Lab (B21HJ0001) and Hainan University Startup Fund (KYQD(ZR)-20101).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Del Mar Martínez-Prada M., Curtin S.J., Gutiérrez-González J.J. Potato improvement through genetic engineering. GM Crops Food. 2021;12:479–496. doi: 10.1080/21645698.2021.1993688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Halterman D., Guenthner J., Collinge S., Butler N., Douches D. Biotech potatoes in the 21st century: 20 years since the first biotech potato. Am. J. Potato Res. 2015;93:1–20. doi: 10.1007/s12230-015-9485-1. [DOI] [Google Scholar]
- 3.Hameed A., Zaidi S.S.-E.-A., Shakir S., Mansoor S. Applications of new breeding technologies for potato improvement. Front. Plant Sci. 2018;9:925. doi: 10.3389/fpls.2018.00925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kloosterman B., Abelenda J.A., Gomez M.d.M.C., Oortwijn M., de Boer J.M., Kowitwanich K., Horvath B.M., van Eck H.J., Smaczniak C., Prat S., et al. Naturally occurring allele diversity allows potato cultivation in northern latitudes. Nature. 2013;495:246–250. doi: 10.1038/nature11912. [DOI] [PubMed] [Google Scholar]
- 5.Wang Y., Zhao J., Lu W., Deng D. Gibberellin in plant height control: Old player, new story. Plant Cell Rep. 2017;36:391–398. doi: 10.1007/s00299-017-2104-5. [DOI] [PubMed] [Google Scholar]
- 6.Wang B., Wang H. IPA1: A New “Green Revolution” Gene? Mol. Plant. 2017;10:779–781. doi: 10.1016/j.molp.2017.04.011. [DOI] [PubMed] [Google Scholar]
- 7.Tian X., Wen W., Xie L., Fu L., Xu D., Fu C., Wang D., Chen X., Xia X., Chen Q., et al. Molecular Mapping of Reduced Plant Height Gene Rht24 in Bread Wheat. Front. Plant Sci. 2017;8:1379. doi: 10.3389/fpls.2017.01379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liao Z., Yu H., Duan J., Yuan K., Yu C., Meng X., Kou L., Chen M., Jing Y., Liu G., et al. SLR1 inhibits MOC1 degradation to coordinate tiller number and plant height in rice. Nat. Commun. 2019;10:2738. doi: 10.1038/s41467-019-10667-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Salas Fernandez M.G., Becraft P.W., Yin Y., Lübberstedt T. From dwarves to giants? Plant height manipulation for biomass yield. Trends Plant Sci. 2009;14:454–461. doi: 10.1016/j.tplants.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 10.Han F., Zhu B. Evolutionary analysis of three gibberellin oxidase genes in rice, Arabidopsis, and soybean. Gene. 2011;473:23–35. doi: 10.1016/j.gene.2010.10.010. [DOI] [PubMed] [Google Scholar]
- 11.Rieu I., Ruiz-Rivero O., Fernandez-Garcia N., Griffiths J., Powers S.J., Gong F., Linhartova T., Eriksson S., Nilsson O., Thomas S.G., et al. The gibberellin biosynthetic genes AtGA20ox1 and AtGA20ox2 act, partially redundantly, to promote growth and development throughout the Arabidopsis life cycle. Plant J. 2008;53:488–504. doi: 10.1111/j.1365-313X.2007.03356.x. [DOI] [PubMed] [Google Scholar]
- 12.Yu H., Ren D., Zhu Y., Xu J., Wang Y., Liu R., Wang Y., Liu R., Fang Y., Shi Z., et al. MULTI-TILLERING DWARF1, a new allele of BRITTLE CULM 12, affects plant height and tiller in rice. Sci. Bull. 2016;61:1810–1817. doi: 10.1007/s11434-015-0981-y. [DOI] [Google Scholar]
- 13.Lawit S.J., Wych H.M., Xu D., Kundu S., Tomes D.T. Maize DELLA proteins dwarf plant8 and dwarf plant9 as modulators of plant development. Plant Cell Physiol. 2010;51:1854–1868. doi: 10.1093/pcp/pcq153. [DOI] [PubMed] [Google Scholar]
- 14.Hartwig T., Chuck G.S., Fujioka S., Klempien A., Weizbauer R., Potluri D.P., Choe S., Johal G.S., Schulz B. Brassinosteroid control of sex determination in maize. Proc. Natl. Acad. Sci. USA. 2011;108:19814–19819. doi: 10.1073/pnas.1108359108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhiponova M.K., Vanhoutte I., Boudolf V., Betti C., Dhondt S., Coppens F., Mylle E., Maes S., González-García M.P., Caño-Delgado A.I., et al. Brassinosteroid production and signaling differentially control cell division and expansion in the leaf. New Phytol. 2013;197:490–502. doi: 10.1111/nph.12036. [DOI] [PubMed] [Google Scholar]
- 16.Tanabe S., Ashikari M., Fujioka S., Takatsuto S., Yoshida S., Yano M., Yoshimura A., Kitano H., Matsuoka M., Fujisawa Y., et al. A novel cytochrome P450 is implicated in brassinosteroid biosynthesis via the characterization of a rice dwarf mutant, dwarf11, with reduced seed length. Plant Cell. 2005;17:776–790. doi: 10.1105/tpc.104.024950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hong Z., Ueguchi-Tanaka M., Fujioka S., Takatsuto S., Yoshida S., Hasegawa Y., Ashikari M., Kitano H., Matsuoka M. The Rice brassinosteroid-deficient dwarf2 mutant, defective in the rice homolog of Arabidopsis DIMINUTO/DWARF1, is rescued by the endogenously accumulated alternative bioactive brassinosteroid, dolichosterone. Plant Cell. 2005;17:2243–2254. doi: 10.1105/tpc.105.030973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tao Y., Zheng J., Xu Z., Zhang X., Zhang K., Wang G. Functional analysis of ZmDWF1, a maize homolog of the Arabidopsis brassinosteroids biosynthetic DWF1/DIM gene. Plant Sci. 2004;167:743–751. doi: 10.1016/j.plantsci.2004.05.012. [DOI] [Google Scholar]
- 19.Phillips K.A., Skirpan A.L., Liu X., Christensen A., Slewinski T.L., Hudson C., Barazesh S., Cohen J.D., Malcomber S., McSteen P. vanishing tassel2 encodes a grass-specific tryptophan aminotransferase required for vegetative and reproductive development in maize. Plant Cell. 2011;23:550–566. doi: 10.1105/tpc.110.075267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guan J.C., Koch K.E., Suzuki M., Wu S., Latshaw S., Petruff T., Goulet C., Klee H.J., McCarty D.R. Diverse roles of strigolactone signaling in maize architecture and the uncoupling of a branching-specific subnetwork. Plant Physiol. 2012;160:1303–1317. doi: 10.1104/pp.112.204503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Russin W.A., Evert R.F., Vanderveer P.J., Sharkey T.D., Briggs S.P. Modification of a Specific Class of Plasmodesmata and Loss of Sucrose Export Ability in the sucrose export defective1 Maize Mutant. Plant Cell. 1996;8:645–658. doi: 10.2307/3870341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wen T., Schnable P.S. Analyses of mutants of three genes that influence root hair development in Zea mays (Gramineae) suggest that root hairs are dispensable. Am. J. Bot. 1994;81:833–842. doi: 10.1002/j.1537-2197.1994.tb15564.x. [DOI] [Google Scholar]
- 23.Jiang F., Guo M., Yang F., Duncan K., Jackson D., Rafalski A., Wang S., Li B. Mutations in an AP2 transcription factor-like gene affect internode length and leaf shape in maize. PLoS ONE. 2012;7:e37040. doi: 10.1371/journal.pone.0037040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yu J., Buckler E.S. Genetic association mapping and genome organization of maize. Curr. Opin. Biotechnol. 2006;17:155–160. doi: 10.1016/j.copbio.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 25.Nordborg M., Weigel D. Next-generation genetics in plants. Nature. 2008;456:720–723. doi: 10.1038/nature07629. [DOI] [PubMed] [Google Scholar]
- 26.Gilad Y., Pritchard J.K., Thornton K. Characterizing natural variation using next-generation sequencing technologies. Trends Genet. 2009;25:463–471. doi: 10.1016/j.tig.2009.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li H., Durbin R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics. 2009;25:1754–1760. doi: 10.1093/bioinformatics/btp324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li H. A statistical framework for SNP calling, mutation discovery, association mapping and population genetical parameter estimation from sequencing data. Bioinformatics. 2011;27:2987–2993. doi: 10.1093/bioinformatics/btr509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McKenna A., Hanna M., Banks E., Sivachenko A., Cibulskis K., Kernytsky A., Garimella K., Altshuler D., Gabriel S., Daly M., et al. The Genome Analysis Toolkit: A MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res. 2010;20:1297–1303. doi: 10.1101/gr.107524.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Danecek P., Auton A., Abecasis G., Albers C.A., Banks E., DePristo M.A., Handsaker R.E., Lunter G., Marth G.T., Sherry S.T., et al. The variant call format and VCFtools. Bioinformatics. 2011;27:2156–2158. doi: 10.1093/bioinformatics/btr330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pertea M., Kim D., Pertea G.M., Leek J.T., Salzberg S.L. Transcript-level expression analysis of RNA-seq experiments with HISAT, StringTie and Ballgown. Nat. Protoc. 2016;11:1650–1667. doi: 10.1038/nprot.2016.095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang C., Yang Z., Tang D., Zhu Y., Wang P., Li D., Zhu G., Xiong X., Shang Y., Li C., et al. Genome design of hybrid potato. Cell. 2021;184:3873–3883.e12. doi: 10.1016/j.cell.2021.06.006. [DOI] [PubMed] [Google Scholar]
- 33.Sun H., Jiao W.B., Krause K., Campoy J.A., Goel M., Folz-Donahue K., Kukat C., Huettel B., Schneeberger K. Chromosome-scale and haplotype-resolved genome assembly of a tetraploid potato cultivar. Nat. Genet. 2022;54:342–348. doi: 10.1038/s41588-022-01015-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bao Z., Li C., Li G., Wang P., Peng Z., Cheng L., Li H., Zhang Z., Li Y., Huang W., et al. Genome architecture and tetrasomic inheritance of autotetraploid potato. Mol. Plant. 2022;15:1211–1226. doi: 10.1016/j.molp.2022.06.009. [DOI] [PubMed] [Google Scholar]
- 35.Wang F., Xia Z., Zou M., Zhao L., Jiang S., Zhou Y., Zhang C., Ma Y., Bao Y., Sun H., et al. The autotetraploid potato genome provides insights into highly heterozygous species. Plant Biotechnol. J. 2022;20:1996–2005. doi: 10.1111/pbi.13883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xu P., Chen H., Li T., Xu F., Mao Z., Cao X., Miao L., Du S., Hua J., Zhao J., et al. Blue light-dependent interactions of CRY1 with GID1 and DELLA proteins regulate gibberellin signaling and photomorphogenesis in Arabidopsis. Plant Cell. 2021;33:2375–2394. doi: 10.1093/plcell/koab124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Choi H., Jeong S., Kim D.S., Na H.J., Ryu J.S., Lee S.S., Nam H.G., Lim P.O., Woo H.R. The homeodomain-leucine zipper ATHB23, a phytochrome B-interacting protein, is important for phytochrome B-mediated red light signaling. Physiol. Plant. 2014;150:308–320. doi: 10.1111/ppl.12087. [DOI] [PubMed] [Google Scholar]
- 38.Leivar P., Quail P.H. PIFs: Pivotal components in a cellular signaling hub. Trends Plant Sci. 2011;16:19–28. doi: 10.1016/j.tplants.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang S., Hu X., Dong J., Du M., Song J., Xu S., Zhao C. Identification, evolution, and expression analysis of OsBSK gene family in Oryza sativa Japonica. BMC Plant Biol. 2022;22:565. doi: 10.1186/s12870-022-03905-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Guo J., Wang M.H. Transgenic tobacco plants overexpressing the Nicta; CycD3; 4 gene demonstrate accelerated growth rates. BMB Rep. 2008;41:542–547. doi: 10.5483/BMBRep.2008.41.7.542. [DOI] [PubMed] [Google Scholar]
- 41.Guan C., Xue Y., Jiang P., He C., Zhuge X., Lan T., Yang H. Overexpression of PtoCYCD3;3 Promotes Growth and Causes Leaf Wrinkle and Branch Appearance in Populus. Int. J. Mol. Sci. 2021;22:1288. doi: 10.3390/ijms22031288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ephritikhine G., Fellner M., Vannini C., Lapous D., Barbier-Brygoo H. The sax1 dwarf mutant of Arabidopsis thaliana shows altered sensitivity of growth responses to abscisic acid, auxin, gibberellins and ethylene and is partially rescued by exogenous brassinosteroid. Plant J. 1999;18:303–314. doi: 10.1046/j.1365-313X.1999.00454.x. [DOI] [PubMed] [Google Scholar]
- 43.Casal J.J. Phytochromes, cryptochromes, phototropin: Photoreceptor interactions in plants. Photochem. Photobiol. 2000;71:1–11. doi: 10.1562/0031-8655(2000)071<0001:PCPPII>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 44.Dubos C., Stracke R., Grotewold E., Weisshaar B., Martin C., Lepiniec L. MYB transcription factors in Arabidopsis. Trends Plant Sci. 2010;15:573–581. doi: 10.1016/j.tplants.2010.06.005. [DOI] [PubMed] [Google Scholar]
- 45.Li J., Han G., Sun C., Sui N. Research advances of MYB transcription factors in plant stress resistance and breeding. Plant Signal. Behav. 2019;14:1613131. doi: 10.1080/15592324.2019.1613131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yang H., Xue Q., Zhang Z., Du J., Yu D., Huang F. GmMYB181, a Soybean R2R3-MYB Protein, Increases Branch Number in Transgenic Arabidopsis. Front. Plant Sci. 2018;9:1027. doi: 10.3389/fpls.2018.01027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yang X., Li X., Shan J., Li Y., Zhang Y., Wang Y., Li W., Zhao L. Overexpression of GmGAMYB Accelerates the Transition to Flowering and Increases Plant Height in Soybean. Front. Plant Sci. 2021;12:667242. doi: 10.3389/fpls.2021.667242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cheng Q., Dong L., Su T., Li T., Gan Z., Nan H., Lu S., Fang C., Kong L., Li H., et al. CRISPR/Cas9-mediated targeted mutagenesis of GmLHY genes alters plant height and internode length in soybean. BMC Plant Biol. 2019;19:562. doi: 10.1186/s12870-019-2145-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Supporting data can be found at the National Genomics Data Center and are accessible at http://bigd.big.ac.cn/ (accessed on 22 March 2022) under BioProject numbers PRJCA011806.