Abstract
The increasing number and high prices of orphan drugs have triggered concern among patients, payers, and policymakers about the affordability of new drugs approved using the incentives set by the Orphan Drug Act (ODA) of 1983. This study evaluated the factors associated to the differences in the treatment cost of new orphan and non-orphan drugs approved by the FDA from 2017 to 2021. A generalized linear model (GLM) with the Gamma log-link analysis was used to ascertain the association of drug characteristics with the treatment costs of orphan and non-orphan drugs. The results of the study showed that the median and interquartile range (IQR) drug cost was USD 218,872 (IQR = USD 23,105) for orphan drugs and USD 12,798 (IQR = USD 57,940) for non-orphan drugs (p < 0.001). Higher market entry prices were associated with biologics (108%; p < 0.001), orphan status (177%; p < 0.001), US sponsor companies (48%; p = 0.035), chronic use (1083%; p < 0.001), treatment intent (163%; p = 0.004), and indications for oncology (624%; p < 0.001) or genetic disorders (624%; p < 0.001). Higher market entry treatment cost for newly approved drugs were associated with biologics, orphan status, US sponsor companies, chronic use, therapeutic intent, and indications for oncology or genetic disorders.
Keywords: orphan drugs, non-orphan drugs, price, market entry
1. Introduction
In the United States (US), orphan drugs are indicated for the treatment of rare diseases and conditions affecting fewer than 200,000 patients [1]. With an estimated 7000 orphan diseases, 1 out of every 10 Americans live with a rare condition [2]. The Orphan Drug Act (ODA) was introduced in 1983 to encourage the development of new drugs for such conditions. The orphan designation introduced by the Orphan Drug Act of 1983 allows drug manufacturers to benefit from several incentives, such as market exclusivity, fee waivers, direct funding for research and development (R&D), and tax credits that aim to boost returns on investment in orphan drug research and development [3,4].
The Increase in the demand for orphan drugs to address a growing number of rare diseases coupled with the steady increase in prices has raised concerns about the affordability of orphan drugs [5,6,7,8]. New drugs are expensive and contribute to rising healthcare costs for public and private patients [9,10,11], and the FDA orphan designation is associated with higher prices and out-of-pocket expenditures [12,13].
However, studies assessing the factors behind differences in the costs of orphan and non-orphan drugs in the US are lacking. This study evaluated the factors associated to the differences in the treatment cost of new orphan and non-orphan drugs approved by the FDA from 2017 to 2021.
2. Material and Methods
2.1. Data Sources
We extracted the list of new molecular entities and therapeutic biologics approved and marketed in the US in 2017–2021 from the FDA website [14]. Vaccines, allergenic products, and blood and blood products were excluded from the study. We collected the first wholesale acquisition costs (WACs) from the IBM Micromedex RED BOOK and used the WACs at market entry as proxies for the actual acquisition costs by private payers. Pharmaceutical companies use the WACs to set the initial reference price in the Medicaid outpatient pharmacy, 340B Drug Pricing Program, and Federal Supply Schedule programs [15,16]. The Medicare Part B program also uses the WACs to set the initial prices for reimbursement of drugs used in physician offices. We collected price data at the national drug code (NDC) level and selected the lowest NDC cost per unit at market entry whenever several NDCs were available for the same active ingredient, dosage form, and strength. We selected the unit (tablet, capsule, vial, etc.,) closest to the FDA-recommended strength when a drug had several strengths. We classified the approved drugs in the following therapeutic categories [17]: genetic disorders, HIV and related comorbidities, other infectious diseases, oncology, transplants, and other areas.
We collected each drug’s recommended dose and treatment duration from the first FDA approved label. When the FDA-approved label did not indicate the treatment duration, we used the median treatment duration from pivotal clinical trials listed on the label. We assumed an average patient weight of 70 kg and a body surface area of 1.75 m2 to calculate the daily dose for adult patients, and 40 kg was used to calculate the daily dose for pediatric patients if any adjustment was needed (Appendix A, Table A1).
We calculated the treatment cost for single-use, use for less than one year, and use for one year or longer. We inflated the prices to USD 2021 using consumer price index (CPI) non-seasonally adjusted data for all US city average items and all urban consumers from the US Bureau of Labor Statistics [18].
2.2. Data Analysis
We conducted descriptive statistics for each variable included in the analysis. Then, we studied the correlations between the treatment cost of newly approved drugs at market entry and the variables, as well as between the variables themselves. We used the Chi-squared test or Fisher’s exact test in combination with the Phi-coefficient or Cramer’s V considered in cases where both variables were categorical. If both variables were continuous, scatter plots were depicted, and Spearman or Bravais–Pearson correlation coefficients were calculated. We used the point–biserial correlation to check for correlations between categorical and continuous variables. Kruskal–Wallis test is also conducted to check for a significant difference between the means of the ordinal variables’ groups (Appendix A, Table A2).
2.3. Study Outcome: The Treatment Costs of New Approved Drugs at Market Entry
We used a generalized linear model (GLM) with the Gamma log-link to assess the association between the treatment costs of newly approved drugs at market entry and potential variables: the date of first market entry, application type (New Drug Applications (NDAs), Biologic License Applications (BLAs)), country of incorporation of the sponsor company (US vs. non-US), a binary indicator for first-in-class, a binary indicator for orphan drugs, FDA review type (standard vs. priority), therapeutic intent (diagnosis, prevention, or treatment), therapeutic area (genetic disorders, HIV and related comorbidities, other infectious diseases, oncology, transplants, and other areas), age group (adult, pediatric and adult, or pediatric), and treatment duration (single-use, less than one year, or one year or longer) while addressing the right-skewed distribution of our data. We included all statistically significant variables (p < 0.05) from the bivariate analysis in the GLM. We tested for multicollinearity among independent variables in the GLM using the variance inflation factor (Appendix A, Table A3).
We used the train-test split procedure to estimate our model’s performance and prevented the model from overfitting by using root-mean-square error (RMSE). We used two-tailed statistical tests and a p value of 0.05 as the significance threshold. We conducted all analyses using RStudio statistical software (version 4.0.3).
3. Results
The FDA approved 257 new drugs, including 127 (49.4%) orphan and 130 (50.6%) non-orphan drugs in 2017–2021. We excluded 15 drugs that were not marketed in the US as of March 31, 2022; thus, the analytical sample included 242 drugs, including 118 (48.8%) orphan drugs and 124 (51.2%) non-orphan (Table 1).
Table 1.
Characteristics and median cost of new drugs and biological products approved by the US Food and Drug Administration, 2017–2021.
| Drugs Characteristics | Non-Orphan | Orphan | ||||
|---|---|---|---|---|---|---|
| No. (%) | Median Cost (USD 2021) | p Value | No. (%) | Median Cost (USD 2021) | p Value | |
| Total | 124 (51.2%) | $12,798.36 | 118 (48.8%) | $218,871.51 | ||
| Application type | ||||||
| NDA | 95 (53.1%) | $8701.27 | <0.001 | 84 (46.9%) | $206,176.28 | 0.051 |
| BLA | 29 (46.8%) | $61,468.75 | 34 (54.0%) | $264,007.88 | ||
| Combination | ||||||
| Fixed-dose combination | 12 (85.7%) | $30,895.32 | 0.010 | 2 (14.3%) | $100,177.88 | <0.001 |
| Single active ingredient | 112 (49.1%) | $12,111.33 | 116 (50.9%) | $223,076.48 | ||
| Country of Incorporation | ||||||
| US | 88 (49.7%) | $15,834.14 | 0.666 | 89 (50.3%) | $237,264.66 | 0.005 |
| Other Countries | 36 (55.4%) | $9483.82 | 29 (44.6%) | $128,579.61 | ||
| First in class | ||||||
| Yes | 40 (40.8%) | $19,252.87 | 0.041 | 58 (59.2%) | $239,593.23 | 0.322 |
| No | 84 (58.3%) | $9483.82 | 60 (41.7%) | $206,176.28 | ||
| FDA review | ||||||
| Priority review | 56 (36.6%) | $29,093.35 | 0.003 | 97 (63.4%) | $233,934.14 | 0.053 |
| Standard review | 68 (76.4%) | $7383.70 | 21 (23.6%) | $142,195.27 | ||
| FDA Designations and Pathways | ||||||
| Accelerated approval | 12 (26.1%) | $163,239.32 | <0.001 | 34 (73.9%) | $209,306.88 | 0.656 |
| Breakthrough therapy | 20 (25.6%) | $102,425.22 | 0.002 | 58 (74.4%) | $242,091.05 | 0.073 |
| Fast track | 35 (40.2%) | $28,677.06 | 0.325 | 52 (59.8%) | $232,237.26 | 0.667 |
| Therapeutic Intent | ||||||
| Diagnosis | 4 (57.1%) | $1274.04 | 0.123 | 3 (42.9%) | $2527.44 | 0.085 |
| Prevention | 11 (64.7%) | $2311.92 | 6 (35.3%) | $71,503.98 | ||
| Treatment | 108 (49.8%) | $18,486.88 | 109 (50.2%) | $230,768.11 | ||
| Therapeutic Area | ||||||
| Genetic disorders | 2 (4.9%) | $290,279.77 | <0.001 | 39 (95.1%) | $274,515.15 | 0.002 |
| HIV | 4 (80.0%) | $37,825.76 | 1 (20.0%) | $36,982.36 | ||
| Infectious diseases | 17 (77.3%) | $3152.25 | 5 (22.7%) | $3207.95 | ||
| Oncology | 27 (36.5%) | $199,370.90 | 47 (63.5%) | $156,126.94 | ||
| Transplant | 0 (0.0%) | $0.00 | 2 (100.0%) | $25,790.23 | ||
| Other | 74 (75.5%) | $9557.37 | 24 (24.5%) | $8411.00 | ||
| Age Group | ||||||
| Adult | 111 (59.7%) | $77,064.00 | 0.019 | 75 (40.3%) | $15,834.14 | <0.001 |
| Pediatric/Adult | 10 (26.3%) | $212,437.49 | 28 (73.7%) | $1067.40 | ||
| Pediatrics | 3 (16.7%) | $211,046.32 | 15 (83.3%) | $35,684.60 | ||
| Treatment Duration | ||||||
| Single use | 15 (75.0%) | $727.85 | 0.011 | 5 (25.0%) | $715.47 | 0.001 |
| Less than one year | 43 (52.4%) | $92,438.35 | 39 (47.6%) | $12,069.44 | ||
| One year or longer | 65 (46.8%) | $130,151.75 | 74 (53.2%) | $23,174.91 | ||
BLA, biologics license application; NDA, new drug application.
The percentages for orphan drugs versus non-orphan drugs were as follows: therapeutic biologics (54.0% vs. 46.8%), US country of incorporation of the sponsor company (50.3% vs. 49.7%), first-in-class (59.2% vs. 40.8%), and intended for treatment (50.2% vs. 49.8%).
Orphan drugs also had higher percentages of approvals for FDA-expedited review processes and other regulatory designations (62.4% vs. 37.6%), priority review designations (63.4% vs. 36.6%), accelerated approvals (73.9% vs. 26.1%), breakthrough therapy designations (74.4% vs. 25.6%), and fast-track designations (59.8% vs. 40.2%). Similarly, orphan drugs accounted for higher percentages of approved new oncology drugs (63.5% vs. 36.5%) and genetic disorder drugs (95.1% vs. 4.9%; Table 1). The most frequently approved new drugs for pediatric patients were orphan drugs (83.3% vs. 16.7%), adult and pediatric drugs (73.7% vs. 26.3%), and adult drugs (40.3% vs. 59.7%; Table 1).
3.1. Treatment Cost of New Approved Drugs at Market Entry
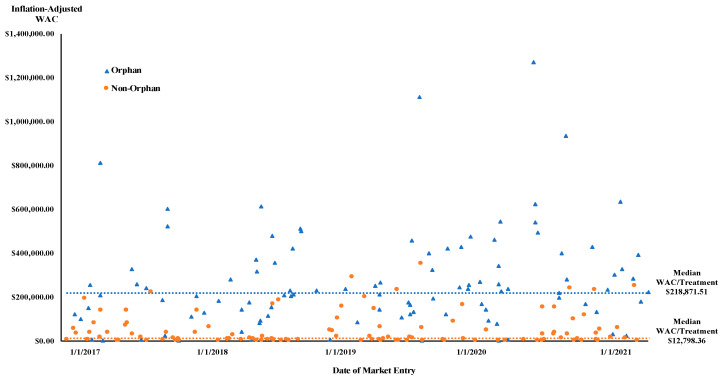
The median treatment cost was USD 218,872 for orphan drugs (IQR = USD 231,057, range USD 237–USD 1,272,021) and USD 12,798 for non-orphan drugs (IQR = USD 57,940, range USD 44–USD 382,866, p < 0.001; Figure 1; Appendix A, Table A1).
Figure 1.
New drugs treatment cost at US market entry (USD 2021) and median WAC.
Compared with non-orphan biologics drugs, the median treatment cost was 4.3 times higher for orphan therapeutic biologics (USD 264,007.88 vs. USD 61,468.75, p < 0.001) and 3.2 times higher for orphan fixed drug combinations (USD 100,177.88 vs. USD 30,895.32, p < 0.001; Table 1).
The median treatment cost was higher for orphan drugs marketed by US companies than for companies from other countries (USD 237,265 vs. USD 128,580, p = 0.005; Table 1; Appendix A, Figure A1).
However, the difference in the median treatment cost for non-orphan drugs marketed by US companies and those marketed by companies from other countries was insignificant (USD 9483.82 vs. USD 15,834.14, p = 0.262; Table 1).
The median treatment cost for first-in-class approved orphan drugs was not statistically significant relative to the median treatment cost for other orphan drugs (USD 239,593.23 vs. USD 206,176.28, p = 0.322). The median treatment cost for orphan drugs that received a priority review was not significantly different from the cost for orphan drugs with standard reviews (USD 233,934.14 vs. USD 142,195.27, p = 0.053).
Although orphan drugs intended for treatment had a median treatment cost three times higher than drugs for preventive use, the difference was not statistically significant (USD 230,768.11 vs. USD 71,503.98, p = 0.190). The median treatment cost for non-orphan drugs intended for treatment indication was significantly higher than for drugs for the preventive indication (USD 18,486.88 vs. USD 2311.92, p = 0.047).
For the therapeutic areas, we identified a significant difference in the median treatment cost for oncology orphan drugs compared to non-orphan drugs (USD 220,832.30 vs. USD 156,126.94, p = 0.002; Appendix A, Figure A2). Finally, the median treatment cost across patient age groups was significantly higher for orphan drugs targeting both adult and pediatric populations than for non-orphan drugs (USD 280,152.74 vs. USD 1067.40, p < 0.001).
3.2. Factors Explaining Treatment Cost of New Approved Drugs at Market Entry
The date of market entry, priority review, and approval as first-in-class drugs were not statistically significantly associated with the mean treatment cost for newly approved drugs at market entry. However, the mean treatment cost at market entry was positively associated with biologics (110%; p < 0.001) and orphan drugs (177%; p < 0.001). Higher market entry treatment costs were also associated with drugs sponsored by US pharmaceutical companies (67%; p = 0.035), drugs intended for treatment rather than prevention (163%; p = 0.004), and treatments with a duration of one year or longer compared to single use (1092%; p < 0.001; Table 2).
Table 2.
Factors explaining the treatment cost of new drugs at market entry, 2017–2021.
| Independent Variable | Treatment Cost of New Drugs at Market Entry | ||
|---|---|---|---|
| Ratio of Means a | 95% CI | p Value | |
| Date of Market Entry | 1.02 | 0.91–1.15 | 0.722 |
| Application Type (Reference: NDA) | |||
| BLA | 2.10 | 1.39–3.24 | <0.001 |
| Orphan drug (Reference: non-orphan) | |||
| Yes | 2.77 | 1.85–4.17 | <0.001 |
| Country of Incorporation (Reference: US) | |||
| Other Countries | −0.67 | 0.46–0.99 | 0.035 |
| FDA Regulatory Review Approval Pathway (Reference: Standard review) | |||
| Priority review | 1.34 | 0.86–2.07 | 0.188 |
| First in Class Drugs (Reference: non-First in class drug) | |||
| Yes | 1.28 | 0.88–1.87 | 0.187 |
| Intent (Reference: Treatment) | |||
| Diagnosis | −0.30 | 0.09–1.12 | 0.049 |
| Prevention | −0.39 | 0.21–0.80 | 0.005 |
| Therapeutic Area (Reference: Infectious diseases) | |||
| Genetic disorders | 7.08 | 2.86–17.15 | <0.001 |
| HIV | 2.48 | 0.74–10.89 | 0.164 |
| Oncology | 7.98 | 3.81–16.22 | <0.001 |
| Transplant | 1.05 | 0.21–12.55 | 0.961 |
| Other | 2.06 | 0.95–4.29 | 0.047 |
| Patient Population Indication (Reference: Pediatrics) | |||
| Adults | −0.86 | 0.38–1.79 | 0.653 |
| Pediatric/Adult | −0.73 | 0.33–1.54 | 0.402 |
| Treatment Duration (Reference: one year or longer) | |||
| Less than 1 year | −0.77 | 0.51–1.18 | 0.189 |
| Single use | −0.08 | 0.04–0.20 | <0.001 |
a: exponentiated coefficients, a percentage increase in the mean treatment drug cost per unit increase in the covariate. BLA, biologics license application; NDA, new drug application.
Among the therapeutic areas, the higher market entry treatment costs were significantly associated with drugs indicated for oncology (698%; p < 0.001) and genetic disorders (608%; p < 0.001) compared to infectious diseases (Table 2). An RMSE was obtained for each train model and test model (RMSE-train model = 3.0 ≈ REMS-test model = 2.8), indicating a good model fit.
4. Discussion
This novel study assessing US treatment costs of newly approved drugs at market entry from 2017 to 2021 found that the median treatment cost was 17 times higher for orphan than non-orphan drugs. However, after controlling for the characteristics of the drug, date of market entry, therapeutic class, FDA review designation, country of the sponsor company, therapeutic intent, and treatment duration, the treatment drug cost was 2.8 times higher for orphan than non-orphan drugs.
The median treatment costs for orphan drugs exceeded USD 200,000 at market entry. Over the past 20 years, drug expenditures in the US market have increasingly shifted toward drugs that treat relatively few people [7], and the rapid growth of orphan drug approvals has raised concerns about their pricing and affordability [5]. The high costs of orphan drugs are also associated with large out-of-pocket expenditures [12,13].
The findings that the cost of new drugs is associated with orphan drugs, therapeutic biologics, therapeutic class, therapeutic intent, and long treatment duration align with prior research that found that launch prices of new drugs in the US increased faster for biologics and drugs treating rare diseases [11]. In fact, the financial burden on patients and healthcare payers results in high profits for pharmaceutical companies in marketing orphan drugs, even for a small patient populations [12,19,20].
Previous studies have pointed out that drug development is less costly for orphan than for non-orphan drugs due to smaller and fewer efficacy and safety trials, shorter FDA review time, higher marketing approval success rates, and lower marketing prices [6,12,13,21]. Since rare diseases are often serious or life-threatening, most orphan drugs qualify for designations and regulatory pathways established by Congress to expedite new drug development and FDA review and approvals [22,23]. Our study confirmed that a higher percentage of orphan than non-orphan drugs benefited from FDA-expedited designations and approval pathways.
The pharmaceutical industry has been criticized for high prices and profits from orphan drug incentives in situations that do not meet the Orphan Drug Act’s original intent [24,25]. Orphan designations for marketed drugs and the division of diseases into sub-types to apply for multiple orphan designations have also been associated with delays in generics entry [26]. Moreover, results showed that drugs sponsored by US pharmaceutical companies were significantly associated with higher drug treatment costs at market entry than non-US pharmaceutical companies. The differences in prices of drugs at market entry between US and non-US pharmaceutical companies could be explained by different factors than the country of the sponsored company, such as disease severity, additional non-orphan indications, or route of administration.
Previous studies found that the year of market entry was associated with increased drug prices at US market entry [11,27]. However, our analysis showed no statistically significant association between the date of market entry and treatment drug cost, possibly due to the relatively short period evaluated in our study.
To mitigate the high price of drugs for vulnerable populations, Congress created the 340B program in 1992 that requires pharmaceutical manufacturers to provide front-end discounts (typically 30–50%) for outpatient prescription medicines that serve high numbers of uninsured and poor patients [28]. More than 40% of hospitals in the US are eligible to participate in the 340B program [29]. In 2010, The Affordable Care Act (ACA) of 2010 excluded all sales of drugs that obtained orphan drug approval from the discounts offered by the 340B program to safety-net healthcare providers [30]. Manufacturers of frequently utilized drugs, such as the best-selling drug adalimumab, may identify a new use that meets the definition of a rare disease and obtain FDA approval for an orphan drug indication, thus effectively ceasing the provision of 340B discounts for sales of the drug [31,32].
This study evaluated the cost of drug treatment but not the effectiveness of new drugs approved in the US. However, coverage and reimbursement decisions consider both factors (cost and effectiveness). From an economic perspective, orphan drugs should be subject to the same clinical effectiveness, cost-effectiveness, and budget impact analyses as non-orphan drugs [33,34]. However, healthcare organizations and insurers use special criteria when making orphan drug reimbursement decisions [33]. The economic incentive and ethical imperatives remain unresolved for ensuring access to safe, effective, and affordable treatments for patients with rare diseases [35]. Moreover, balancing the economic incentives to develop and market orphan drugs against the overall benefits and improvements in health outcomes remains critically important [36,37].
A potential reason explaining the high cost of orphan drugs is that R&D expenses for orphan drugs must be recouped from a small number of patients, resulting in high drug treatment costs per patient [21].
A previous study found that the prices of an orphan drugs in Europe were higher for conditions with low prevalence [38]. However, another study concluded that the prices of orphan drugs in the US are unlikely to be driven by the prevalence of the target disease [39]. Further studies are needed to associate disease prevalence and drug treatment costs.
The Orphan Drug Act, enacted 40 years ago, has been credited to have an important role in the development and approval of drugs for rare diseases [36]. As a result, there has been a substantial increase in the number of orphan drugs approved by the FDA, providing therapeutic options for patients with unmet medical needs. However, the high cost of these drugs creates significant financial barriers to patient access and highlights the need for a more sustainable and equitable pricing structure to ensure patients’ access to affordable treatments.
5. Limitations
This study used the wholesale acquisition cost (WAC) at market entry as a proxy of the actual acquisition cost by private payers. Companies typically use the WAC to set the initial reference price in the Medicaid outpatient pharmacy, the 340B Drug Pricing Program, and the Federal Supply Schedule programs. As price increases in those programs are limited by the rise in the consumer price index, pharmaceutical companies do not have incentives to reduce the market entry price below the WAC. The Medicare Part B program also used the WAC to set new drugs’ initial price. Public and private payers also use the WAC to estimate drug product reimbursement to pharmacies and providers.
The sample included new molecular entities and new therapeutic biologics approved by the FDA in 2017–2021. The study excluded non-therapeutic biologics and approvals of already marketed drugs. However, these exclusions do not affect the validity of the results for the stated period, although future studies on more extensive and inclusive datasets could further extend the validity of our findings. We also did not consider the potential number of users for each drug. Follow-up studies could evaluate the effect of patient population size on drug prices. We used median treatment costs for the FDA-recommended dose and treatment duration. Future studies could use doses and treatment durations observed in clinical practices and average prices weighted by the number of users to better estimate the societal impact of high-cost drugs.
6. Conclusions
Orphan drugs were priced significantly higher than non-orphan drugs at market entry. Higher market entry treatment costs were associated with biologics, orphan status, US sponsor companies, chronic use, therapeutic intent, and indications for oncology or genetic disorders. Future research should assess whether the clinical benefits of orphan drugs justify their high costs.
Appendix A
Table A1.
The treatment costs of new molecular entities and new biologics at market entry, 2017–2021.
| Product Name (Non-Proprietary Name) | Approval Date | Therapeutic Area | Posology Type | Age Group | Units/Treatment | Adjusted WAC Cost Per Year/Treatment at Market Entry |
|---|---|---|---|---|---|---|
| Non-Orphan Drugs New Therapeutic Biologics | ||||||
| aducanumab-avwa | 7 Jun 2021 | Other | Chronic Use (1 year) | Adult | 9125 mg | $52,540.51 |
| amivantamab-vmjw | 21 May 2021 | Oncology | Cycles | Adult | 26,985 mg | $233,668.03 |
| anifrolumab-fnia | 30 Jul 2021 | Other | Cycles | Adult | 3900 mg | $60,644.32 |
| benralizumab | 14 Nov 2017 | Other | Chronic Use (1 year) | Pediatric | 195 mg | $35,684.60 |
| brodalumab | 15 Feb 2017 | Other | Cycles | Adult | 5460 mg | $33,131.85 |
| brolucizumab-dbll | 7 Oct 2019 | Genetic Disorders | Chronic Use (1 year) | Adult | 39 mg | $233,934.14 |
| cemiplimab-rwlc | 28 Sep 2018 | Oncology | Cycles | Adult | 6067 mg | $168,790.78 |
| dasiglucagon | 22 Mar 2021 | Other | Single-Use | Adult & Pediatric | 0.6 mg | $522.21 |
| dostarlimab-gxly | 22 Apr 2021 | Oncology | Cycles | Adult | 8667 mg | $117,686.30 |
| dupilumab | 28 Mar 2017 | Other | Short treatment course | Adult | 2400 mg | $6217.48 |
| durvalumab | 1 May 2017 | Oncology | Cycles | Adult | 18,200 mg | $138,311.22 |
| efgartigimod alfa-fcab | 17 Dec 2021 | Genetic Disorders | Cycles | Adult | 18,200 mg | $274,515.15 |
| enfortumab vedotin-ejfv | 18 Dec 2019 | Oncology | Cycles | Adult | 3150 mg | $350,189.92 |
| eptinezumab-jjmr | 21 Feb 2020 | Other | Cycles | Adult | 400 mg | $6148.56 |
| erenumab-aooe | 17 May 2018 | Other | Chronic Use (1 year) | Adult | 840 mg | $7383.70 |
| fam-trastuzumab deruxtecan-nxki | 20 Dec 2019 | Oncology | Cycles | Adult | 6552 mg | $158,518.55 |
| fremanezumab-vfrm | 14 Sep 2018 | Other | Chronic Use (1 year) | Adult | 2700 mg | $4922.47 |
| galcanezumab-gnlm | 27 Sep 2018 | Other | Chronic Use (1 year) | Adult | 1440 mg | $7383.70 |
| guselkumab | 13 Jul 2017 | Other | Cycles | Adult | 650 mg | $68,753.32 |
| margetuximab (anti-HER2 mAb) | 16 Dec 2020 | Oncology | Cycles | Adult | 18,249 mg | $153,735.33 |
| ocrelizumab | 28 Mar 2017 | Other | Cycles | Adult | 1200 mg | $70,996.82 |
| risankizumab-rzaa | 23 Apr 2019 | Other | Cycles | Adult | 750 mg | $155,429.21 |
| romosozumab-aqqg | 9 Apr 2019 | Other | Short treatment course | Adult | 2520 mg | $19,724.18 |
| sacituzumab govitecan-hziy | 22 Apr 2020 | Oncology | Cycles | Adult | 14,200 mg | $163,239.32 |
| sarilumab | 22 May 2017 | Other | Chronic Use (1 year) | Adult | 5200 mg | $37,366.74 |
| tezepelumab-ekko | 27 Dec 2021 | Other | Chronic Use (1 year) | Adult & Pediatric | 2730 mg | $23,808.22 |
| tildrakizumab-asmn | 20 Mar 2018 | Other | Cycles | Adult | 433 mg | $61,468.75 |
| tisotumab vedotin-tftv | 20 Sep 2021 | Oncology | Cycles | Adult | 1680 mg | $251,117.72 |
| tralokinumab-ldrm | 27 Dec 2021 | Other | Chronic Use (1 year) | Adult | 3000 mg | $16,121.70 |
| New Molecular Entities | ||||||
| abaloparatide | 28 Apr 2017 | Other | Chronic Use (1 year) | Adult | 28,800 mg | $16,383.88 |
| abemaciclib | 28 Sep 2017 | Oncology | Chronic Use (1 year) | Adult | 103,200 mg | $220,369.75 |
| air polymer-type A | 7 Nov 2019 | Other | Single-Use | Adult | 217.4 mg | $740.22 |
| alpelisib | 24 May 2019 | Oncology | Cycles | Adult | 99,000 mg | $288,748.34 |
| amisulpride | 26 Feb 2020 | Other | Single-use | Adult | 5 mg | $43.70 |
| angiotensin ii | 21 Dec 2017 | Other | Single use | Adult | 1 mg | $1605.15 |
| apalutamide | 14 Feb 2018 | Oncology | Chronic Use (1 year) | Adult | 86,400 mg | $140,226.19 |
| atogepant | 28 Sep 2021 | Other | Chronic Use (1 year) | Adult | 3600 mg | $2009.75 |
| avatrombopag | 21 May 2018 | Other | Short treatment course | Adult | 200 mg | $9630.92 |
| baloxavir marboxil | 24 Oct 2018 | Infectious disease | Single-Use | Adult & Pediatric | 80 mg | $165.33 |
| baricitinib | 31 May 2018 | Other | Chronic Use (1 year) | Adult | 720 mg | $26,384.87 |
| bempedoic acid | 21 Feb 2020 | Other | Chronic use (1 year) | Adult | 64,800 mg | $3392.97 |
| betrixaban | 23 Jun 2017 | Other | Short treatment course | Adult | 3360 mg | $688.12 |
| bictegravir, embitcitabine, tenofovir alafenamide | 7 Feb 2018 | HIV | Chronic Use (1 year) | Adult | 18,000 mg | $37,825.76 |
| bremelanotide | 21 Jun 2019 | Other | Single-use | Adult | 2 mg | $759.66 |
| brexanolone | 19 Mar 2019 | Other | Short treatment course | Adult | 126,000 mg | $49,458.10 |
| cabotegravir and rilpivirine | 21 Jan 2021 | HIV | Chronic Use (1 year) | Adult | 7200 mg | $36,138.96 |
| cefiderocol | 14 Nov 2019 | Infectious disease | Short treatment course | Adult | 84 mg | $15,834.14 |
| cenobamate | 21 Nov 2019 | Other | Chronic Use (1 year) | Adult | 72,000 mg | $12,153.21 |
| clascoterone | 26 Aug 2020 | Other | Short treatment course | Adult & Pediatric | 180 mg | $1394.25 |
| darolutamide | 30 Jul 2019 | Oncology | Chronic Use (1 year) | Adult | 432,000 mg | $146,050.77 |
| delafloxacin | 19 Jun 2017 | Infectious disease | Short treatment course | Adult | 12,600 mg | $3096.55 |
| difelikefalin | 23 Aug 2021 | Other | Chronic Use (1 year) | Adult | 5475 mg | $12,164.38 |
| doravirine | 30 Aug 2018 | HIV | Chronic Use (1 year) | Adult | 36,000 mg | $17,720.89 |
| drospirenone and estetrol | 15 Apr 2021 | Other | Chronic Use (1 year) | Adult | 365 mg | $2311.92 |
| elagolix sodium | 23 Jul 2018 | Other | Chronic Use (1 year) | Adult | 54,000 mg | $11,624.06 |
| eravacycline | 27 Aug 2018 | Infectious disease | Short treatment course | Adult | 1960 mg | $1835.23 |
| erdafitinib | 12 Apr 2019 | Oncology | Cycles | Adult | 1080 mg | $102,425.22 |
| ertugliflozin | 19 Dec 2017 | Other | Chronic Use (1 year) | Adult | 5400 mg | $10,332.05 |
| etelcalcetide | 7 Feb 2017 | Other | Chronic Use (1 year) | Adult | 780 mg | $55,718.30 |
| ferric maltol | 25 Jul 2019 | Other | Chronic use (1 year) | Adult | 21,600 mg | $6084.00 |
| finerenone | 9 Jul 2021 | Other | Chronic Use (1 year) | Adult | 7300 mg | $14,041.97 |
| flortaucipir F18 | 28 May 2020 | Oncology | Single use | Adult | 1.5ml | $1407.33 |
| fosnetupitant and palonosetron | 19 Apr 2018 | Other | Single-use | Adult | 235 mg | $545.75 |
| fostemsavir | 2 Jul 2020 | HIV | Chronic Use (1 year) | Adult | 432,000 mg | $94,387.79 |
| Gallium 68 PSMA-11 | 1 Dec 2020 | Oncology | Single-use | Adult | 6 mCi | $1140.75 |
| glecaprevir and pibrentasvir | 3 Aug 2017 | Infectious disease | Short treatment course | Adult | 18,000 mg | $30,895.32 |
| ibrexafungerp | 1 Jun 2021 | Infectious disease | Single-Use | Adult & Pediatric | 600 mg | $481.65 |
| imipenem, cilastatin, relebactam | 16 Jul 2019 | Infectious disease | Short treatment course | Adult | 70 mg | $19,252.87 |
| istradefylline | 27 Aug 2019 | Other | Chronic Use (1 year) | Adult | 7200 mg | $9483.82 |
| lasmiditan | 11 Oct 2019 | Other | Single-Use | Adult | 50 mg | $715.47 |
| latanoprostene bunod | 2 Nov 2017 | Other | Chronic Use (1 year) | Adult | 360 mg | $3774.85 |
| lefamulin | 19 Aug 2019 | Infectious disease | Short treatment course | Adult | 6000 mg | $1448.92 |
| lemborexant | 20 Dec 2019 | Other | Chronic Use (1 year) | Adult | 1800 mg | $1696.51 |
| lofexidine hydrochloride | 16 May 2018 | Other | Short treatment course | Adult | 30 mg | $3719.59 |
| lumateperone | 20 Dec 2019 | Other | Chronic Use (1 year) | Adult | 15,120 mg | $16,286.54 |
| lusutrombopag | 31 Jul 2018 | Other | Chronic Use (1 year) | Adult | 21 mg | $9095.87 |
| meropenem and vaborbactam | 29 Aug 2017 | Infectious disease | Short treatment course | Adult | 84 mg | $14,831.62 |
| naldemedine | 23 Mar 2017 | Other | Chronic Use (1 year) | Adult | 72 mg | $4541.48 |
| neratinib maleate | 17 Jul 2017 | Oncology | Cycles | Adult | 86,400 mg | $137,624.60 |
| netarsudil | 18 Dec 2017 | Other | Chronic Use (1 year) | Adult | 360 mg | $3528.77 |
| olanzapine and samidor- phan | 28 May 2021 | Other | Chronic use (1 year) | Adult | 3650 mg | $34,296.86 |
| oliceridine | 7 Aug 2020 | Other | Short treatment course | Adult | 27 mg | $485.82 |
| omadacycline | 2 Oct 2018 | Infectious disease | Short treatment course | Adult | 4200 mg | $5917.66 |
| opicapone | 24 Apr 2020 | Other | Chronic Use (1 year) | Adult | 18,000 mg | $7279.58 |
| ozanimod | 25 Mar 2020 | Other | Chronic Use (1 year) | Adult | 331 mg | $87,213.00 |
| ozenoxacin | 11 Dec 2017 | Infectious disease | Short treatment course | Adult & Pediatric | 50 mg | $524.40 |
| piflufolastat f 18 | 26 May 2021 | Oncology | Single-use | Adult | 9 mCi | $4498.92 |
| plazomicin | 25 Jun 2018 | infectious disease | Short treatment course | Adult | 7350 mg | $4955.11 |
| plecanatide | 19 Jan 2017 | Other | Chronic Use (1 year) | Adult | 1080 mg | $5385.55 |
| ponesimod | 18 Mar 2021 | Other | Chronic Use (1 year) | Adult | 7300 mg | $99,807.53 |
| pretomanid | 14 Aug 2019 | Infectious disease | Short treatment course | Adult | 35,400 mg | $3689.29 |
| prucalopride succinate | 14 Dec 2018 | Other | Chronic use (1 year) | Adult | 720 mg | $5360.25 |
| relugolix | 18 Dec 2020 | Oncology | Chronic Use (1 year) | Adult | 44,040 mg | $29,093.35 |
| remdesivir | 22 Oct 2020 | Infectious disease | Short treatment course | Adult & Pediatric | 600 mg | $3207.95 |
| remimazolam | 2 Jul 2020 | Other | Single Use | Adult | 5 mg | $395.46 |
| revefenacin | 9 Nov 2018 | Other | Chronic Use (1 year) | Adult | 63,000 mg | $4408.82 |
| ribociclib | 13 Mar 2017 | Oncology | Cycles | Adult | 168,000 mg | $191,363.72 |
| rifamycin SV MMX | 16 Nov 2018 | Infectious disease | Short treatment course | Adult | 2328 mg | $154.09 |
| rimegepant | 27 Feb 2020 | Other | Short treatment course | Adult | 1125 mg | $1638.67 |
| safinamide | 21 Mar 2017 | Other | Chronic Use (1 year) | Adult | 18,000 mg | $4390.22 |
| sarecycline | 1 Oct 2018 | Other | Short treatment course | Adult & Pediatric | 9000 mg | $2718.69 |
| secnidazole | 15 Sep 2017 | Infectious disease | Short treatment course | Adult | 2 gm/1 packet | $284.94 |
| segesterone acetate and ethinyl estradiol | 10 Aug 2018 | Other | Single-use | Adult | 0.013 mg | $2107.51 |
| selinexor | 3 Jul 2019 | Oncology | Cycles | Adult | 5504 mg | $199,370.90 |
| semaglutide | 5 Dec 2017 | Other | Chronic Use (1 year) | Adult | 52 mg | $12,798.36 |
| serdexmethylphenidate and dexmethylphenidate | 2 Mar 2021 | Other | Chronic Use (1 year) | Adult & Pediatric | 18,828 mg | $31,574.56 |
| siponimod | 26 Mar 2019 | Other | Chronic Use (1 year) | Adult | 363 mg | $46,405.16 |
| sodium zirconium cyclosilicate | 18 May 2018 | Other | Chronic Use (1 year) | Adult | 3600 mg | $8411.00 |
| sofosbuvir, velpatasvir and voxilaprevir | 18 Jul 2017 | Infectious disease | Short treatment course | Adult | 33,600 mg | $81,657.26 |
| talazoparib | 16 Oct 2018 | Oncology | Cycles | Adult | 360 mg | $187,225.07 |
| tenapanor | 12 Sep 2019 | Other | Chronic Use (1 year) | Adult | 36,000 mg | $17,331.04 |
| tirbanibulin | 14 Dec 2020 | Other | Short treatment course | Adult | 5 mg | $1003.86 |
| tivozanib | 10 Mar 2021 | Oncology | Cycles | Adult | 276 mg | $239,272.43 |
| trifarotene | 4 Oct 2019 | Other | Short treatment course | Adult & Pediatric | 45 gm | $740.55 |
| trilaciclib | 12 Feb 2021 | Oncology | Cycles | Adult | 2520 mg | $12,069.44 |
| ubrogepant | 23 Dec 2019 | Other | Single-use | Adult | 50 mg | $87.40 |
| upadacitinib | 16 Aug 2019 | Other | Chronic Use (1 year) | Adult | 5400 mg | $62,171.69 |
| valbenazine | 11 Apr 2017 | Other | Chronic Use (1 year) | Adult | 28,800 mg | $81,591.72 |
| vericiguat | 19 Jan 2021 | Other | Chronic Use (1 year) | Adult | 3650 mg | $28,677.06 |
| vibegron | 23 Dec 2020 | Other | Chronic Use (1 year) | Adult | 27,000 mg | $5577.81 |
| viloxazine | 2 Apr 2021 | Other | Chronic use (1 year) | Pediatric | 67,200 mg | $7198.85 |
| voclosporin | 22 Jan 2021 | Other | Chronic Use (1 year) | Adult | 17,064 mg | $154,284.16 |
| vosoritide | 19 Nov 2021 | Genetic Disorders | Chronic Use (1 year) | Pediatric | 168 mg | $382,866.12 |
| Orphan drugs New Biologics | ||||||
| asparaginase erwinia chrysanthemi (recombi- nant)-rywn |
30 Jun 2021 | Oncology | Short treatment course | Adult & Pediatric | 262.5 mg | $233,701.65 |
| avalglucosidase alfa-ngpt | 6 Aug 2021 | Oncology | Chronic Use (1 year) | Adult & Pediatric | 36,498 mg | $634,666.86 |
| avelumab | 23 Mar 2017 | Cycles | Adult & Pediatric | 18,200 mg | $149,490.89 | |
| belantamab mafodotin-blmf | 5 Aug 2020 | Oncology | Cycles | adult | 3041.5 mg | $258,737.92 |
| brexucabtagene autoleucel | 24 Jul 2020 | Oncology | Single-use | adult | 1 × 106 CAR-positive viable T cells/kg body weight | $460,217.51 |
| burosumab-twza | 17 Apr 2018 | Genetic Disorders | Cycles | Adult & Pediatric | 770 mg | $280,152.74 |
| calaspargase pegol-mknl | 20 Dec 2018 | Oncology | Cycles | Adult & Pediatric | 76,042 mg | $511,422.87 |
| caplacizumab-yhdp | 6 Feb 2019 | Genetic Disorders | Short treatment course | Adult | 330 mg | $230,772.87 |
| cenegermin-bkbj | 22 Aug 2018 | Genetic Disorders | Short treatment course | adult | 360 mg | $92,771.30 |
| cerliponase alfa | 27 Apr 2017 | Genetic Disorders | Chronic Use (1 year) | Pedatric | 7800 mg | $810,995.23 |
| crizanlizumab-tmca | 15 Nov 2019 | Genetic Disorders | Cycles | Adult & Pediatric | 4900 mg | $121,708.92 |
| elapegademase-lvlr | 5 Oct 2018 | Genetic Disorders | Short treatment course | Adult & Pediatric | 336 mg | $393,751.93 |
| emapalumab-lzsg | 20 Nov 2018 | Genetic Disorders | Cycles | Adult & Pediatric | 6720 mg | $230,768.11 |
| emicizumab | 16 Nov 2017 | Genetic Disorders | Chronic Use (1 year) | Adult & Pediatric | 4830 mg | $523,352.19 |
| evinacumab-dgnb | 11 Feb 2021 | Genetic Disorders | Chronic Use (1 year) | Adult & Pediatric | 12,600 mg | $399,262.34 |
| ibalizumab-uiyk | 6 Mar 2018 | HIV | Chronic Use (1 year) | adult | 21,120 mg | $128,579.61 |
| inebilizumab-cdon | 11 Jun 2020 | Genetic Disorders | Cycles | adult | 600 mg | $269,277.83 |
| inotuzumab ozogamicin | 17 Aug 2017 | Oncology | Cycles | adult | 11.4 mg | $258,152.31 |
| lanadelumab | 23 Aug 2018 | Other | Cycles | Adult & Pediatric | 7800 mg | $614,046.02 |
| loncastuximab tesirine-lpyl | 23 Apr 2021 | Oncology | Cycles | adult | 73.5 mg | $168,218.08 |
| luspatercept-aamt | 8 Nov 2019 | Genetic Disorders | Cycles | Adult | 1213 mg | $175,989.95 |
| mogamulizumab-kpkc | 8 Aug 2018 | Genetic Disorders | Cycles | adult | 1820 mg | $369,067.53 |
| moxetumomab pasudotox-tdfk | 13 Sep 2018 | Oncology | Cycles | adult | 50.4 mg | $114,368.37 |
| naxitamab-gqgk | 25 Nov 2020 | Oncology | Cycles | Adult & Pediatric | 3780 mg | $1,005,223.62 |
| pegvaliase-pqpz | 24 May 2018 | Genetic Disorders | Chronic Use (1 year) | adult | 1344 mg | $280,740.01 |
| polatuzumab vedotin-piiq | 10 Jun 2019 | Oncology | Cycles | Adult | 756 mg | $85,354.35 |
| ravulizumab-cwvz | 21 Dec 2018 | Genetic Disorders | Chronic Use (1 year) | adult | 21,450 mg | $470,793.46 |
| ropeginterferon alfa-2b-njft | 12 Nov 2021 | Other | Chronic Use (1 year) | adult | 2607 mg | $36,945.53 |
| satralizumab-mwge | 14 Aug 2020 | Genetic Disorders | Chronic Use (1 year) | adult | 1800 mg | $225,320.67 |
| tafasitamab-cxix | 31 Jul 2020 | Oncology | Cycles | adult | 12,618 mg | $77,811.02 |
| tagraxofusp-erzs | 21 Dec 2018 | Oncology | Cycles | Adult & Pediatric | 50,400 mg | $527,034.73 |
| teprotumumab-trbw | 21 Jan 2020 | Other | Short treatment course | adult | 10,500 mg | $321,591.73 |
| vestronidase alfa-vjbk | 15 Nov 2017 | Genetic Disorders | Chronic Use (1 year) | Adult & Pediatric | 2600 mg | $600,633.06 |
| New Molecular Entities | ||||||
| acalabrutinib | 31 Oct 2017 | Oncology | Cycles | adult | 72,000 mg | $184,338.32 |
| amifampridine phosphate | 28 Nov 2018 | Genetic Disorders | Chronic use (1 year) | adult | 7200 mg | $131,934.10 |
| asciminib | 29 Oct 2021 | Oncology | Chronic use (1 year) | adult | 29,200 mg | $220,832.30 |
| avacopan | 7 Oct 2021 | Other | Chronic use (1 year) | adult | 21,900 mg | $178,244.98 |
| avapritinib | 9 Jan 2020 | Oncology | Chronic use (1 year) | adult | 109,500 mg | $400,148.23 |
| belumosudil | 16 Jul 2021 | transplant | Cycles | Adult & Pediatric | 11,560 mg | $30,281.42 |
| belzutifan | 13 Aug 2021 | Genetic Disorders | Chronic use (1 year) | adult | 43,800 mg | $325,696.80 |
| benznidazole | 29 Aug 2017 | Infectious disease | Short treatment course | Pediatric | 15,600 mg | $3604.42 |
| berotralstat | 3 Dec 2020 | Genetic Disorders | Chronic use (1 year) | Adult & Pediatric | 54,000 mg | $492,998.67 |
| binimetinib | 27 Jun 2018 | Oncology | Chronic use (1 year) | adult | 32,400 mg | $140,958.14 |
| brigatinib | 28 Apr 2017 | Oncology | Chronic use (1 year) | adult | 32,400 mg | $171,550.75 |
| brilliant blue g | 20 Dec 2019 | Other | Single-Use | adult | 0.5 mL | $236.60 |
| cannabidiol | 25 Jun 2018 | Genetic Disorders | Chronic Use (1 year) | Pediatric | 288,000 mg | $40,256.90 |
| capmatinib | 6 May 2020 | Oncology | Chronic use (1 year) | adult | 288,000 mg | $237,196.48 |
| casimersen | 25 Feb 2021 | Genetic Disorders | Chronic use (1 year) | Pediatric | 57,600 mg | $934,502.40 |
| copanlisib | 14 Sep 2017 | Oncology | Cycles | adult | 3120 mg | $238,549.30 |
| copper Cu 64 dotatate injection | 3 Sep 2020 | Other | Single-Use | adult | 4 mCi | $3597.22 |
| dacomitinib | 27 Sep 2018 | Oncology | Chronic use (1 year) | adult | 16,200 mg | $477,693.60 |
| decitabine and cedazuridine | 7 Jul 2020 | Oncology | Cycles | adult | 8100 mg | $92,438.35 |
| deflazacort | 9 Feb 2017 | Genetic Disorders | Chronic use (1 year) | Pediatric | 12,960 mg | $122,273.13 |
| deutetrabenazine | 3 Apr 2017 | Genetic Disorders | Short treatment course | adult | 1008 mg | $5484.97 |
| duvelisib | 24 Sep 2018 | Oncology | Cycles | adult | 16,800 mg | $151,526.48 |
| edaravone | 5 May 2017 | Genetic Disorders | Cycles | adult | 7200 mg | $1423.43 |
| elexacaftor, tezacaftor, ivacaftor | 21 Oct 2019 | Genetic Disorders | Chronic use (1 year) | Adult & Pediatric | 36,000 mg | $107,917.40 |
| enasidenib | 1 Aug 2017 | Oncology | Chronic use (1 year) | adult | 36,000 mg | $325,999.90 |
| encorafenib | 27 Jun 2018 | Oncology | Chronic Use (1 year) | adult | 162,000 mg | $140,958.14 |
| entrectinib | 15 Aug 2019 | Oncology | Chronic use (1 year) | Adult & Pediatric | 216,000 mg | $212,437.49 |
| fedratinib | 16 Aug 2019 | Other | Cycles | Adult | 144,000 mg | $265,546.86 |
| fish oil triglycerides | 27 Jul 2018 | Other | Short treatment course | Pediatric | 560 mg | $4563.54 |
| fosdenopterin | 26 Feb 2021 | Genetic Disorders | Chronic use (1 year) | Pediatric | 3285 mg | $480,314.73 |
| fostamatinib | 17 Apr 2018 | Other | Chronic use (1 year) | adult | 72,000 mg | $80,899.72 |
| gilteritinib | 28 Nov 2018 | Oncology | Chronic use (1 year) | adult | 43,200 mg | $288,927.58 |
| givosiran | 20 Nov 2019 | Genetic Disorders | Chronic use (1 year) | adult | 2100 mg | $456,627.56 |
| glasdegib | 21 Nov 2018 | Oncology | Cycles | adult | 33,600 mg | $202,848.56 |
| golodirsen | 12 Dec 2019 | Genetic Disorders | Chronic use (1 year) | Pediatric | 62,400 mg | $1,112,756.85 |
| inclisiran | 22 Dec 2021 | Genetic Disorders | Chronic use (1 year) | adult | 852 mg | $9903.94 |
| infigratinib | 28 May 2021 | Oncology | Cycles | adult | 31,500 mg | $81,753.72 |
| inotersen | 5 Oct 2018 | Genetic Disorders | Chronic use (1 year) | adult | 14,768 mg | $320,887.96 |
| isatuximab | 2 Mar 2020 | Oncology | Cycles | adult | 18,200 mg | $121,634.21 |
| ivosidenib | 20 Jul 2018 | Oncology | Chronic use (1 year) | adult | 180,000 mg | $347,577.05 |
| larotrectinib | 26 Nov 2018 | Oncology | Chronic use (1 year) | Adult & Pediatric | 67,200 mg | $421,192.21 |
| letermovir | 8 Nov 2017 | transplant | Short treatment course | adult | 48,000 mg | $21,299.04 |
| lonafarnib | 20 Nov 2020 | Genetic Disorders | Chronic use (1 year) | Pediatric | 40,800 mg | $1,272,021.04 |
| lonapegsomatropin-tcgd | 25 Aug 2021 | Other | Chronic use (1 year) | Pediatric | 473.2 mg | $24,194.24 |
| lorlatinib | 2 Nov 2018 | Oncology | Chronic use (1 year) | adult | 36,000 mg | $206,176.28 |
| lumasiran | 23 Nov 2020 | Genetic Disorders | Cycles | Adult & Pediatric | 450 mg | $538,575.32 |
| lurbinectedin | 15 Jun 2020 | Oncology | Cycles | adult | 97.3 mg | $165,886.45 |
| lutetium Lu 177 dotatate | 26 Jan 2018 | Oncology | Cycles | adult | 800 mg | $109,902.38 |
| macimorelin acetate | 20 Dec 2017 | Other | Single-Use | adult | 35 mg | $2527.44 |
| maralixibat | 29 Sep 2021 | Genetic Disorders | Chronic use (1 year) | Pediatric | 2357.9 mg | $390,095.94 |
| maribavir | 23 Nov 2021 | infectious disease | Short treatment course | adult | 48,000 mg | $54,103.80 |
| melphalan flufenamide | 26 Feb 2021 | Oncology | Cycles | Adult | 160 mg | $77,064.00 |
| midostaurin | 28 Apr 2017 | Oncology | Cycles | adult | 42,000 mg | $245,594.36 |
| migalastat | 10 Aug 2018 | Genetic Disorders | Chronic use (1 year) | adult | 20,664 mg | $259,499.77 |
| mobocertinib | 15 Sep 2021 | Oncology | Chronic use (1 year) | adult | 53,760 mg | $283,920.00 |
| nifurtimox | 6 Aug 2020 | Infectious disease | Short treatment course | Pediatric | 180 mg | $587.01 |
| niraparib | 27 Mar 2017 | Oncology | Chronic use (1 year) | adult | 108,000 mg | $253,639.89 |
| odevixibat | 20 Jul 2021 | Genetic Disorders | Chronic use (1 year) | Pediatric | 268,800 mg | $299,819.52 |
| osilodrostat | 6 Mar 2020 | Other | Chronic use (1 year) | adult | 5110 mg | $420,155.64 |
| patisiran | 10 Aug 2018 | Genetic Disorders | Cycles | adult | 364 mg | $370,041.32 |
| pegcetacoplan | 14 May 2021 | Genetic Disorders | Chronic use (1 year) | adult | 103,680 mg | $428,687.40 |
| pemigatinib | 17 Apr 2020 | Oncology | Cycles | adult | 2268 mg | $629,000.12 |
| pexidartinib | 2 Aug 2019 | Oncology | Cycles | Adult | 28,8000 mg | $250,372.75 |
| pitolisant | 14 Aug 2019 | Other | Chronic use (1 year) | Adult | 12,816 mg | $143,774.66 |
| pralsetinib | 4 Sep 2020 | Oncology | Chronic use (1 year) | adult | 144,000 mg | $237,332.85 |
| ripretinib | 15 May 2020 | Oncology | Chronic use (1 year) | adult | 54,000 mg | $473,789.61 |
| risdiplam | 7 Aug 2020 | Genetic Disorders | Chronic use (1 year) | Pediatric | 1800 mg | $339,668.66 |
| selpercatinib | 8 May 2020 | Oncology | Chronic use (1 year) | Adult & Pediatric | 115,200 mg | $254,066.72 |
| selumetinib | 10 Apr 2020 | Genetic Disorders | Cycles | Adult & Pediatric | 32,400 mg | $242,091.05 |
| setmelanotide | 25 Nov 2020 | Other | Chronic use (1 year) | Adult & Pediatric | 720 mg | $244,200.05 |
| solriamfetol | 20 Mar 2019 | Other | Chronic use (1 year) | Adult | 27,000 mg | $4172.88 |
| sotorasib | 28 May 2021 | Oncology | Chronic use (1 year) | adult | 292,032 mg | $180,003.02 |
| stiripentol | 20 Aug 2018 | Genetic Disorders | Chronic Use (1 year) | Pediatric | 720,000 mg | $81,491.71 |
| tafamidis meglumine | 3 May 2019 | Other | Chronic use (1 year) | Adult | 28,800 mg | $237,095.41 |
| tafenoquine | 20 Jul 2018 | Infectious disease | Single-Use | adult | 300 mg | $34.24 |
| tucatinib | 17 Apr 2020 | Oncology | Chronic use (1 year) | adult | 216,000 mg | $228,166.71 |
| umbralisib | 5 Feb 2021 | Oncology | Chronic use (1 year) | adult | 292,000 mg | $196,158.30 |
| viltolarsen | 12 Aug 2020 | Genetic Disorders | Chronic use (1 year) | Adult & Pediatric | 268,800 mg | $544,028.87 |
| voxelotor | 25 Nov 2019 | Genetic Disorders | Chronic use (1 year) | Adult & Pediatric | 540,000 mg | $131,723.88 |
| zanubrutinib | 14 Nov 2019 | Oncology | Chronic use (1 year) | Adult | 115,200 mg | $163,564.22 |
mCi = millicurie (radioactivity units).
Table A2.
Correlation matrix.
| Treatment Cost of New Drugs at Market Entry | Date of Market Entry | Application Type | Orphan | Priority Review | First in Class Drugs | Country of Incorporation | Therapeutic Intent | Treatment Duration | Therapeutic Area | Age Group | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Treatment Cost of New Drugs at Market Entry | 1 | 0.023 | 0.28 | 0.548 | 0.378 | 0.21 | −0.14 | 0.452 | 0.452 | 0.452 | 0.452 |
| Date of Market Entry | 1 | 0.045 | −0.007 | 0.116 | −0.146 | −0.036 | 0.852 | 0.08 | 0.83 | 0.535 | |
| Application Type | 1 | 0.05 | 0.007 | 0.186 | 0.198 | 0.103 | 0.133 | 0.238 | 0.179 | ||
| Orphan | 1 | 0.381 | 0.202 | 0.053 | 0.079 | 0.154 | 0.623 | 0.159 | |||
| Priority Review | 1 | 0.307 | 0.122 | 0.098 | 0.226 | 0.582 | 0.159 | ||||
| First in Class Drugs | 1 | 0.008 | 0.065 | 0.053 | 0.277 | 0.1 | |||||
| Country of Incorporation | 1 | 0.065 | 0.047 | 0.113 | 0.102 | ||||||
| Therapeutic Intent | 1 | 0.428 | 0.186 | 0.077 | |||||||
| Treatment Duration | 1 | 0.351 | 0.108 | ||||||||
| Therapeutic Area | 1 | 0.332 | |||||||||
| Age Group | 1 |
Cramer’s V, point-biserial, Kruskal. Test and Spearman correlation coefficients were used.
Table A3.
Multicollinearity.
| Independent Variable | VIF | Increased SE | Tolerance |
|---|---|---|---|
| Year of Market Entry | 1.15 | 1.07 | 0.87 |
| Application Type | 1.24 | 1.11 | 0.81 |
| Orphan | 1.78 | 1.33 | 0.56 |
| Country of Incorporation | 1.08 | 1.04 | 0.92 |
| Priority.Review | 1.84 | 1.35 | 0.54 |
| First in Class Drugs | 1.34 | 1.16 | 0.75 |
| Intent | 1.81 | 1.34 | 0.55 |
| Therapeutic Area | 4.23 | 2.06 | 0.24 |
| Age Group | 1.47 | 1.21 | 0.68 |
| Treatment Duration | 2.24 | 1.5 | 0.45 |
Variance inflation factor (VIF), standard error (SE). We checked the multicollinearity of independent variables by using VIF for each independent variable in the set of multiple regression variables. The higher the value of VIF, the higher the correlation between this variable and the rest. If the VIF value is higher than 5, it is usually considered to have a high correlation with other independent variables. However, the value of VIF for each independent variable included in our model was less than 5. Our data show low multicollinearity; it is not severe enough to warrant corrective measures.
Figure A1.
New drugs treatment cost at US market entry (USD 2021) and median WAC.
Figure A2.
New drugs treatment cost at US market entry (USD 2021) and median WAC.
Author Contributions
H.A. and E.S.-V. data extraction, and analyses. H.A., E.S.-V. and R.R.-M. study conception and design. H.A., E.S.-V. and R.R.-M., data analysis and interpretation, and drafted manuscript. H.A., E.S.-V., R.R.-M., L.M.B. and M.L.F. participated in the interpretation of the study findings and revised it critically for intellectual content. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data used to support the findings of this study will be available upon request.
Conflicts of Interest
The authors declare that they have no competing interests.
Funding Statement
The work of Enrique Seoane-Vazquez and Rosa Rodriguez-Monguio was funded by a grant from Arnold Ventures. Arnold Ventures had no role in the design and conduct of the study, approval of the manuscript, and decision to submit the manuscript for publication.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Waxman H.A. H.R.5238-97th Congress (1981-1982): Orphan Drug Act. [(accessed on 2 November 2021)];1983 Available online: https://www.congress.gov/bill/97th-congress/house-bill/5238.
- 2.FAQs about Rare Diseases|Genetic and Rare Diseases Information Center (GARD)—An NCATS Program. [(accessed on 2 November 2021)]; Available online: https://rarediseases.info.nih.gov/diseases/pages/31/faqs-about-rare-diseases.
- 3.US Food and Drug Administration Orphan Drug Act-Relevant Excerpts. [(accessed on 5 November 2021)]; Available online: https://www.fda.gov/industry/designating-orphan-product-drugs-and-biological-products/orphan-drug-act-relevant-excerpts.
- 4.Szydlo R. Office of Orphan Products Development: Financial Incentives for CDER Medical Products. [(accessed on 11 March 2021)]; Available online: https://www.fda.gov/media/135236/download.
- 5.Aitken M., Kleinrock M., Muñoz E., Porwal U. Orphan Drugs in the United States: Rare Disease Innovation and Cost Trends Through 2019. IQVIA. [(accessed on 5 November 2021)]. Available online: https://rarediseases.org/wp-content/uploads/2021/03/orphan-drugs-in-theunited-states-NRD-2020.pdf.
- 6.Jayasundara K., Hollis A., Krahn M., Mamdani M., Hoch J.S., Grootendorst P. Estimating the clinical cost of drug development for orphan versus non-orphan drugs. Orphanet J. Rare Dis. 2019;14:12. doi: 10.1186/s13023-018-0990-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Orphan Drugs in the United States (Part Two) [(accessed on 14 November 2021)]. Available online: https://www.iqvia.com/insights/the-iqvia-institute/reports/orphan-drugs-in-the-united-states-exclusivity-pricing-and-treated-populations.
- 8.Côté A., Keating B. What Is Wrong with Orphan Drug Policies? Value Health. 2012;15:1185–1191. doi: 10.1016/j.jval.2012.09.004. [DOI] [PubMed] [Google Scholar]
- 9.DeMartino P.C., Miljković M.D., Prasad V. Potential Cost Implications for All US Food and Drug Administration Oncology Drug Approvals in 2018. JAMA Intern. Med. 2021;181:162–167. doi: 10.1001/jamainternmed.2020.5921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kesselheim A.S., Avorn J., Sarpatwari A. The High Cost of Prescription Drugs in the United States: Origins and Prospects for Reform. JAMA. 2016;316:858–871. doi: 10.1001/jama.2016.11237. [DOI] [PubMed] [Google Scholar]
- 11.Rome B.N., Egilman A.C., Kesselheim A.S. Trends in Prescription Drug Launch Prices, 2008-2021. JAMA. 2022;327:2145–2147. doi: 10.1001/jama.2022.5542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chua K.P., Conti R.M. Out-of-pocket Spending on Orphan Drug Prescriptions among Commercially Insured Adults in 2014. J. Gen. Intern. Med. 2019;34:338–340. doi: 10.1007/s11606-018-4694-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chua K.P., Conti R.M. Trends in Orphan Drug Spending and Out-Of-Pocket Spending Among US Children, 2013–2018. Health Aff. 2020;39:1811A–1811I. doi: 10.1377/hlthaff.2020.00595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.US Food and Drug Administration Drugs@FDA: FDA-Approved Drugs. [(accessed on 10 November 2021)]; Available online: https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm.
- 15.Curtiss F.R., Lettrich P., Fairman K.A. What is the price benchmark to replace average wholesale price (AWP)? J. Manag. Care Pharm. 2010;16:492–501. doi: 10.18553/jmcp.2010.16.7.492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee C.H., Chang J., McCombs J. Specialty Drug Price Trends in the Federal 340B Drug Discount Program. J. Manag. Care Spec. Pharm. 2019;25:178–187. doi: 10.18553/jmcp.2019.25.2.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miller K.L., Fermaglich L.J., Maynard J. Using four decades of FDA orphan drug designations to describe trends in rare disease drug development: Substantial growth seen in development of drugs for rare oncologic, neurologic, and pediatric-onset diseases. Orphanet J. Rare Dis. 2021;16:265. doi: 10.1186/s13023-021-01901-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Consumer Price Index (CPI) Databases: US Bureau of Labor Statistics. [(accessed on 10 November 2021)]; Available online: https://www.bls.gov/cpi/data.htm.
- 19.Pomeranz K. Pharma Orphan Drug Report 2019. Evaluate.com. [(accessed on 2 March 2021)]. Available online: https://www.evaluate.com/sites/default/files/media/download-files/EvaluatePharma_Orphan_Drug_Report_2019.pdf.
- 20.Karas L., Lu C.Y., Agrawal P.B., Asgari M.M. The Impact of the Orphan Drug Act on FDA-Approved Therapies for Rare Skin Diseases and Skin-Related Cancers. J. Am. Acad. Dermatol. 2019;81:867–877. doi: 10.1016/j.jaad.2019.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Drummond M.F., Wilson D.A., Kanavos P., Ubel P., Rovira J. Assessing the economic challenges posed by orphan drugs. Int. J. Technol. Assess Health Care. 2007;23:36–42. doi: 10.1017/S0266462307051550. [DOI] [PubMed] [Google Scholar]
- 22.Darrow J.J., Avorn J., Kesselheim A.S. FDA Approval and Regulation of Pharmaceuticals, 1983–2018. JAMA. 2020;323:164–176. doi: 10.1001/jama.2019.20288. [DOI] [PubMed] [Google Scholar]
- 23.Kesselheim A.S., Wang B., Franklin J.M., Darrow J.J. Trends in utilization of FDA expedited drug development and approval programs, 1987-2014: Cohort study. BMJ. 2015;351:h4633. doi: 10.1136/bmj.h4633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Orphan Drug Utilization and Pricing Patterns (2012–2014). AHIP. [(accessed on 8 November 2021)]. Available online: https://www.ahip.org/resources/orphan-drug-utilization-and-pricing-patterns-2012-2014.
- 25.Divino V., DeKoven M., Kleinrock M., Wade R.L., Kaura S. Orphan Drug Expenditures In The United States: A Historical And Prospective Analysis, 2007–2018. Health Aff. 2016;35:1588–1594. doi: 10.1377/hlthaff.2016.0030. [DOI] [PubMed] [Google Scholar]
- 26.Murphy S.M., Puwanant A., Griggs R.C. Unintended Effects of Orphan Product Designation for Rare Neurological Diseases. Ann. Neurol. 2012;72:481–490. doi: 10.1002/ana.23672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bin Sawad A., Seoane-Vazquez E., Rodriguez-Monguio R., Turkistani F. Price analysis of multiple sclerosis disease-modifying therapies marketed in the United States. Curr. Med. Res. Opin. 2016;32:1783–1788. doi: 10.1080/03007995.2016.1208644. [DOI] [PubMed] [Google Scholar]
- 28.Conti R.M., Bach P.B. The 340B Drug Discount Program: Hospitals Generate Profits By Expanding To Reach More Affluent Communities. Health Aff. Proj. Hope. 2014;33:1786–1792. doi: 10.1377/hlthaff.2014.0540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Office USGA Medicare Part B Drugs: Action Needed to Reduce Financial Incentives to Prescribe 340B Drugs at Participating Hospitals. [(accessed on 9 November 2021)]; Available online: https://www.gao.gov/products/gao-15-442.
- 30.Yang Y.T., Chen B., Bennett C.L. Federal 340B Program Payment Scheme for Drugs Designated As Orphan Products: Congressional Clarification Needed to Close the Government-Industry Revolving Door. J. Clin. Oncol. 2016;34:4320–4322. doi: 10.1200/JCO.2016.68.2989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lupkin SJT Sydney Drugmakers Manipulate Orphan Drug Rules To Create Prized Monopolies. Kaiser Health News. 2017. [(accessed on 9 November 2021)]. Available online: https://khn.org/news/drugmakers-manipulate-orphan-drug-rules-to-create-prized-monopolies/
- 32.Lupkin S., Elizabeth L. Interactive: How Orphan Drugs Win The ‘Monopoly’ Game. Kaiser Health News. 2017. [(accessed on 9 November 2021)]. Available online: https://khn.org.
- 33.Cohen J.P., Felix A. Are payers treating orphan drugs differently? J. Mark Access Health Policy. 2014;2:23513. doi: 10.3402/jmahp.v2.23513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Danzon P.M. Affordability Challenges to Value-Based Pricing: Mass Diseases, Orphan Diseases, and Cures. Value Health. 2018;21:252–257. doi: 10.1016/j.jval.2017.12.018. [DOI] [PubMed] [Google Scholar]
- 35.Rodriguez-Monguio R., Spargo T., Seoane-Vazquez E. Ethical imperatives of timely access to orphan drugs: Is possible to reconcile economic incentives and patients’ health needs? Orphanet J. Rare Dis. 2017;12:1. doi: 10.1186/s13023-016-0551-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Seoane-Vazquez E., Rodriguez-Monguio R., Szeinbach S.L., Visaria J. Incentives for orphan drug research and development in the United States. Orphanet J. Rare Dis. 2008;3:33. doi: 10.1186/1750-1172-3-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Grabowski H.G., DiMasi J.A., Long G. The Roles Of Patents And Research And Development Incentives In Biopharmaceutical Innovation. Health Aff. 2015;34:302–310. doi: 10.1377/hlthaff.2014.1047. [DOI] [PubMed] [Google Scholar]
- 38.Worm F., Dintsios C.M. Determinants of Orphan Drug Prices in Germany. PharmacoEconomics. 2020;38:397–411. doi: 10.1007/s40273-019-00872-8. [DOI] [PubMed] [Google Scholar]
- 39.Jarosławski S., Auquier P., Toumi M. No correlation between the prices of oncology orphan drugs in the US and their patient population sizes. J. Cancer Policy. 2017;14:1–4. doi: 10.1016/j.jcpo.2017.09.005. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used to support the findings of this study will be available upon request.