Abstract
Background
The COVID-19 pandemic had greatly and negatively impacted health services including the management of bone and soft tissue sarcoma. As disease progression is time-sensitive, decision taken by the oncology orthopedic surgeon on performing surgical treatment determines the patient outcome. On the other hand, as the world tried to control the spread of COVID-19 infection, treatment re-prioritization based on urgency level had to be done which consequently affect treatment provision for sarcoma patients. Patient and clinician's concern regarding the outbreak have also inflicted on treatment decision making. A systematic review was thought to be necessary to summarize the changes seen in managing primary malignant bone and soft tissue tumors.
Methods
We performed this systematic review in line with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2020 Statement. The review protocol had been registered on PROSPERO with submission number CRD42022329430. We included studies which reported primary malignant tumor diagnosis and its surgical intervention from March 11th, 2020 onwards. The main outcome is to report changes implemented by different centers around the world in managing primary malignant bone tumors surgically in response to the pandemic. Three electronic medical databases were scoured and by applying eligibility criteria. Individual authors evaluated the articles' quality and risk of bias using the Newcastle-Ottawa Quality Assessment Scale other instruments developed by JBI of the University of Adelaide. The overall quality assessment of this systematic review was self-evaluated using the AMSTAR (Assessing the Methodological Quality of Systematic Reviews) Checklist.
Results
There were 26 studies included in the review with various study designs, conveyed in almost all continents. The outcomes from this review are change in surgery time, change in surgery type, and change in surgery indication in patients with primary bone and soft tissue sarcoma. Surgery timing has been experiencing delay since the pandemic occurred, including delay in the multidisciplinary forum, which were all related to lockdown regulations and travel restrictions. For surgery type, limb amputation was preferred compared to limb-salvage procedures due to shorter duration and simpler reconstruction with better control of malignancy. Meanwhile, the indications for surgical management are still based on the patient's demographics and disease stages. However, some would stall surgery regardless of malignancy infiltration and fracture risks which are indication for amputation. As expected, our meta-analysis showed higher post-surgical mortality in patients with malignant bone and soft tissue sarcoma during the COVID-19 pandemic with odds ratio of 1.14.
Conclusion
Surgical management of patients with primary bone and soft tissue sarcoma has seriously been affected due to adjustments to the COVID-19 pandemic. Other than institutional restrictions to contain the infection, patient and clinician's decisions to postpone treatment due to COVID-19 transmission concern were also impactful in treatment course. Delay in surgery timing has caused higher risk of worse surgical outcome during the pandemic, which is aggravated if the patient is infected by COVID-19 as well. As we transition into a post-COVID-19 pandemic period, we expect patients to be more lenient in returning for their treatment but by then disease progression might have taken place, resulting in worse overall prognosis. Limitation to this study were few assumptions made in the synthesis of numerical data and meta-analysis only for changes in surgery time outcome and lack of intervention studies included.
Keywords: arcoma, Soft tissue, Bone, COVID-19, Change management
1. Introduction
The COVID-19 pandemic has been declared since March 11th, 2021 and negatively affected all aspects of life, especially within the health sector. Difficulties in accessing health services not only for patients infected with COVID-19 but also for patients requiring continuous medical treatment such as cancer patients.1,2 Cancer itself is a prognostic factor which can increase the risk of mortality of COVID-infected patients and a doubled case fatality rate up to more than 5%3 One of the most inflicted therapy provisions across different types of cancer is sarcoma, due to its multimodal approach requiring both locally-aggressive and systemic treatment.4
Currently, several countries have implemented changes regarding the management of bone and soft tissue sarcoma patients. The most basic change implemented had been the postponement of elective surgery and only continuing treatment for a limited number of urgent cases.5, 6, 7 Delays that have occurred were due to manpower limitation through reallocation of health workers and prioritization of medical equipment such as ventilators reserved for COVID-positive patients.8 Unfortunately, not all clinicians manifest the same understanding when classifying the urgency of a case where surgery must be performed regardless of the pandemic and this has caused differences in the management of bone and soft tissue sarcoma.9 A difficult consideration that oncology orthopedic surgeons may encounter is whether to delay and/or cancel surgery plans with possibility of worsening condition or to perform the surgery but risking of COVID-19 transmission.9
Based on these dilemmas, a systematic review was thought to be necessary to recap the challenges seen in managing primary malignant bone and soft tissue tumors, especially changes in their surgical management during the COVID-19 pandemic. This review is expected to provide insights on what to expect after the pandemic has subsided.
2. METHODS
This systematic review has followed the guidelines by Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. Our main objective was to report changes implemented by different centers around the world in surgically managing primary malignant bone and soft tissue tumors during the pandemic. The review protocol had been registered on PROSPERO with submission number CRD42022329430. A comprehensive and systematic search was initially performed on PubMed (MEDLINE), Scopus and Europe PMC on May 15th, 2022. The search strategy terms are listed in the Supplementary Material. We included both interventional and observational studies (randomized clinical trial, controlled clinical trial, case review, case report, case series, research studies, research article, follow up study or cohort study) that reported primary malignant tumor diagnosis and their surgical intervention after the COVID-19 pandemic was declared (March 11th, 2020 onwards). We exclude articles in languages other than English, commentary reviews or letters to editors. Electronically and printed journals were deemed acceptable. Three authors (Y.A.P., P.A.S, and A.F.H) independently assessed found titles and abstracts following the inclusion criteria. Disagreement regarding study exclusion between authors were with senior authors (F., R.M., H.B.).
Individual study demographics consisted of author, year, title, type of study, main objective and country of study origin along with their participant demographics (total patients, gender, median age) and surgical intervention were extracted and compared to their pre-pandemic practice. We further grouped the intervention changes into change in surgery timing, change in surgery type and change in surgery indication. Subgroup analysis was planned for mean time measured in days and descriptively for surgery type and indication. Unavailable data were reported as N/A. The extracted and synthesized data were descriptively analyzed and presented in a tabular format. Quality and risk of bias assessments utilized the Newcastle-Ottawa Quality Assessment Scale for non-randomized studies and other critical appraisal tools by JBI of University of Adelaide where appropriate. The overall quality assessment of this systematic review was self-evaluated using the AMSTAR (Assessing the Methodological Quality of Systematic Reviews) Checklist as a critical appraisal tool for systematic reviews.
3. Result
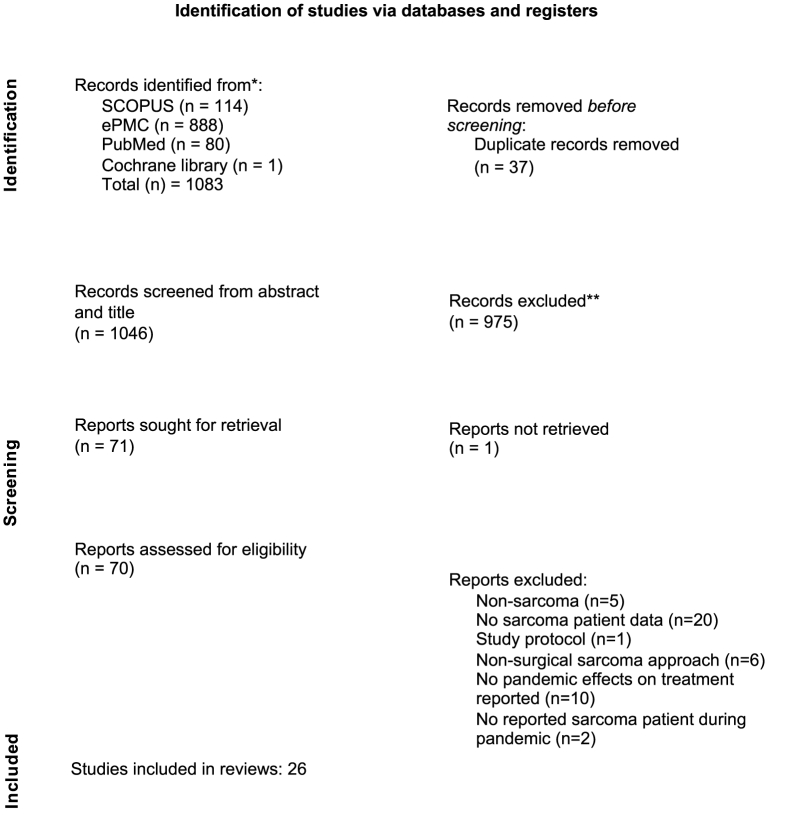
The PRISMA flowchart for study selection is presented in Fig. 1. The search across three databases resulted in a total of 1083 articles before duplicates were removed. A total of 26 studies were included in the review. The included studies have good quality and concluded to include all studies.
Fig. 1.
PRISMA Flowchart for study selection.
Demographic data for the included studies are listed inTable 1 (Supplementary Material). Study centers were distributed throughout the continents, with several involving international multicenter and conducted by collaborating authors.1,10,11 We provide geographical distribution of studies included in this review (Fig. 2). Surgical management changes from the included studies were divided into three subgroups (Supplementary material Table 2, 3 and 4). Numerical data of surgery timing changes were extracted from several studies and attempted for meta-analysis. As for changes in surgery type and indication, we descriptively extracted these data as planned along with their reasoning too.
Fig. 2.
Geographical distribution of included studies.
4. Change in surgery time
We defined surgery time as the period of surgery to be performed within the timeline of primary bone tumor management, which unfortunately few studies failed to report the delay in time units. Qureshi et al. reported a mean delay of performing surgery as cancer treatment for 25 days, leading to reduction in almost half of the total cases operated. Another included study reported a prolonged interval of two weeks between the multidisciplinary forum and the day of surgery.8 Musculoskeletal malignancy cases in India were facing surgical delay of almost two weeks compared to pre-pandemic,12 due to the constantly changing lockdown regulations throughout the pandemic. The COVID Surgery Collaborative1 compared the delay in surgery time during three different time points (with three different levels of restriction) during the pandemic. This study found a longer delay in surgical treatment from diagnosis of up to 12.7 weeks during the full lockdown than in light and moderate restrictions with mean of 2.4 and 5.5 weeks respectively.
Although no time delay was reported in details of which they encountered, Rajasekaran et al. described through 3 different studies regarding the system implemented during COVID-19 in the UK which allows delay from 24 h up to 12 weeks depending on the priority level.13, 14, 15 Similarly, prioritizations were vaguely described by Berjano et al. and Cardoso and Rodriguez-Pinto but specifically according to sarcoma diagnosis. High priority diagnoses were stratified according to metastasis probability and delays were deemed acceptable for diagnoses which are sensitive to non-surgical therapies.16,17 Interestingly, chondrosarcoma was categorized as high-priority by Cardoso and Rodriguez-Pinto but contradictively, surgery may be delayed according to Berjano et al.16,17
We also included some guideline-based articles in this review which most have recommended prioritizing surgical treatment.18,19 However, clinician and patient factors equally contributed to these recommendations unable to be implemented. Traveling restrictions, financial burden and fear of COVID-19 transmission were some patient rationale in delaying their surgical treatment.1,20 As for clinicians, some judgements were made based on the patients’ phase of chemotherapy treatment and maximizing its ability in shrinking the tumor to a resectable size while others were based on limited resources which disabled them to provide the holistic pre and post surgical care.3,5,8,9,18,21,22
We identified three cohort studies which provide numerical data of the number of patients having their surgery delayed and reported outcome of mortality rate.12,20,23 Meta-analysis of these data was performed, which pooled a total sample of 3826 patients. Data input was performed using the Review Manager (RevMan) Version 5.4. (The Cochrane Collaboration, 2020) which produced a forest plot displayed in Fig. 2 (Supplementary Material). The pooled data resulted in an odds ratio of 1.14 (95% CI 0.59–2.23), which favors better outcomes in the pre-pandemic group. However, as the confidence interval straddles the midline, the null hypothesis cannot be rejected. Heterogeneity analysis also showed low heterogeneity between studies and low importance of data differences (I2 = 4%) (P = 0.35). We speculate this result was due to unequal weight of data contributed by one of the comparator groups.23
5. Change in surgery type
Several studies have reported amputation to be preferred compared to limb-salvage procedure,9,20,24 while some still reported higher limb-salvage surgeries.7,12,25 Amputation was preferred in some centers due to shorter time spent in the operating theater, require simpler reconstruction and provide better control of malignancy18,20,24. As mentioned earlier, malignant primary bone and soft tissue tumors are prioritized to have surgical treatment as needed, setting aside elective surgeries such as arthroplasties or arthroscopies. This has resulted in availability of extra operating room time for high-priority orthopaedic surgeries as Kumar et al. reported.26 Compared to the pre-pandemic period, there seemed to be no difference, and rather, an increased number of malignant primary tumor resection performed since the COVID-19 outbreak.6,7,12 Research conducted by the Global Health Research Group on Children's Non-Communicable Disease Collaborative (2022) found that the number of pediatric cancer patients having their choice of operation changed was 8 times more frequent in low-middle income countries. These findings are describing possible underlying factors of variety changes seen in surgery types performed for sarcoma patients during the COVID-19 pandemic.
6. Change in surgical indication
Similar to changes in surgical type, surgical indications were rather vary according to patients’ demography and disease stages.8 In general, malignant tumor surgeries are prioritized and considered as indications for urgent surgical intervention.5,12,18,19,23,26 In a clinician-based survey by Thaler et al., almost 15% of surgeons would not perform sarcoma resection regardless of their risk of infiltration to surrounding tissues or risk of fractures.9 This has led us assuming these would have been the indication for amputation instead. The reluctance of exposing either patients or healthcare professionals to such an open environment (i.e. hospital) also became a possible rationale behind the deviation of indication between limb salvage and amputation.
Chemotherapy or radiotherapy are pivotal in determining surgical time points within the patients’ course of treatment.23 As mentioned before, chemotherapy potentially maximizes surgical outcome regardless of pandemic status. Recommendations and practices in surgical treatment of primary bone tumor heavily revolved around chemotherapy outcome ever since the COVID-19 outbreak.1,8,18,19,23,25 However practice, Gaston et al. recommended to postpone chemotherapy during the pandemic although they reported several cases of metastasis and poorer prognosis patients presenting as consequence. The authors deemed postponement of chemotherapy and surgery were necessary as risk of infection was higher in sarcoma patients. Similarly, Reddy et al. reported almost 80% of patients who contracted COVID-19 infection had their chemotherapy within 2 weeks after their treatment. And although there were no studies included directly compared patient surgical outcome in regards to their neoadjuvant treatment, the effects of radiotherapy in improving surgical outcome were somehow similar to chemotherapy.18 Indeed, chemotherapy and radiotherapy improved the outcome for patients prior to their surgeries but during the pandemic infection rate became the bigger obstacle in acquiring these treatments.
In India, Kumar et al. deferred pelvic and sacral tumor surgeries due to logistic limitations and prioritized to operate on malignant tumor than benign tumor or biopsy procedures.26 Difficulties were also faced by Gaston et al. in the Philippines where performing lengthy surgery for sarcoma patients while being enwrapped by the many layers of personal protection equipment (PPE) compromised surgeon's heat tolerability and face mask visibility.20 It was suggested that by changing the indication of surgery through prioritization, the absence of surgeries where it was pre-pandemically indicated may worsen the mortality with COVID-19. Hence, it is ever so important that malignant cancer surgeries should continually be performed even through the pandemic.
7. Discussion
7.1. Country based regulations
As the novel infection was initially a huge mystery to the community, the COVID- 19 pandemic setting has allowed much research to be undertaken, and discoveries regarding its multi-sectoral management were determined along the course. With varying study centers distributed throughout the continent, we aim to capture real-life situations within the orthopedic departments during the pandemic. Different countries implemented their individual approach and policies regarding prioritization of surgeries and lockdowns in their territory. European countries have started their lock-downs as early as March of 2020, which resulted in disruption of surgical care as many as 50% of their patient registries.27 In the Philippines, elective surgeries were deemed as non urgent and were recommended to be postponed since the pandemic was declared.20 Many sarcoma patients had to be redirected to other referral hospitals throughout the capital city in order to maintain their continuous cancer care. Similar regulation was reported by Bouche et al. in France, with elective surgeries were to be stopped in all to centralize medical manpower and supplies in providing comprehensive COVID-19 care.28 Meanwhile in Italy, medical personnel are reallocated to deal with COVID-19 cases instead, leading to non-urgent and non-cancer operations being stopped. This has resulted in a reduction of surgical activity and outpatient visits by up to 90%.8 The European Society for Medical Oncology has advocated to prioritize operative sarcoma cases when patients are cleared from COVID-19 screening, including chemotherapy phases,29 but individual reports and studies have discussed primary sarcoma patients still experiencing delays in their care.
Ban et al. reported a significantly longer period of hospital stay due to the requirement of performing CT scan prior to patient admissions and/or surgeries which have greatly impacted the Japanese economic footing.7 Although a similar recommendation is being imposed in France, CT scan was not as practical to be regularly performed in real life.28
Between the pandemic surge and one country's economic state, there is a vicious cycle which worsen the situation. Mainly through delay of treatment and centralization of resources, the pandemic have impacted the already-poor outcome of cancer patients especially in low-middle income countries and up to fifteen-fold worsened mortality rate compared to the pre-pandemic data.11 Regulations and policies made by the higher-ups of contributing sectors within a country should take into account the burden of co-morbidities and other diseases that may concur with sarcoma.1
7.2. Treatment changes
Restrictions set by the government and hospital themselves were not the only barrier between sarcoma patients and their required treatment. Thaler et al. conducted a survey of which orthopedic surgeons revealed their worry of the COVID-19 infections while practicing, reflected by changes in their treatment approach and indication.9 Up to five-percent of the respondents admit to have stopped surgeries entirely and one fifth of total respondents to have stopped or delayed surgeries for fatal cases of primary sarcoma. Other than surgeries, around 20% orthopedic surgeons from the same survey would delay chemotherapy and radiotherapy delivery had been postponed whether they were for therapeutic or palliative purposes.9 This clinician-level obstacle weighed down the possibility of performing intervention suited for the stage and type of sarcoma. The difficulty was further burdened by patient-level reluctance to seek their treatment. More than half of sarcoma patients decided to halt their routine management which was reflected by the number of outpatient admissions. Distance related to travel restrictions and fear of transmitting COVID-19 became some of the most common obstacles stood between sarcoma patients and their planned treatment.1,11,20,30
Most of the reasonings of these implemented changes revolve around the delivery of perisurgical treatment such as (neoadjuvant) chemotherapy and radiotherapy. As mentioned before, studies and recommendations preferred performing surgeries on patients who had completed their neoadjuvant chemotherapy to ensure better surgical outcome.3,18,22 In contrast to this, some studies actually recommended to avoid delays in treatment of primary malignant sarcoma cases due to the significant mortality and morbidity risks from immunosuppression and higher infection risk from visiting hospital.13,22 As for sarcoma with no distant metastasis, patients predicted with good tumor control after surgery and palliative patients with life-threatening bleeding should be highly considered for surgery.31 Other elements which should be taken into consideration when prioritizing primary malignant sarcoma surgeries include aggressiveness of cells, time interval from neoadjuvant therapy to planned surgery date, risk of tumor progressing to being unresectable, and availability for therapeutic alternatives.8
Use of surgical instruments such as electrocautery, drills, reamers, oscillating saws and pulse lavage systems should also be reconsidered and accompanied by suction devices to minimize floating droplets within the operating theater.5 Occasionally orthopedic surgeries would require vendor representatives to guide them in using certain instruments or implants which the surgeons may not be familiar with. Due to personnel limitation within the operating theater, this practice was unavailable which further restricted the type of surgery options.7 Furthermore, in attempting to control COVID-19 transmission, maximum surgery duration in some medical centers had been limited and had surgeons to re-evaluate their treatment approach for malignant sarcoma patients.5,20
7.3. Overall delayed care
New suspects of sarcoma patients presenting in the outpatient clinics were also affected by the diagnostic delays inflicted by the pandemic restrictions. As biopsy procedures were classified as elective intervention, thorough planning needs to be done. Although radiography examinations have also been limited in several instances with reduction around 15% compared to the pre-pandemic data,32 performing biopsy should be based on imaging result that indicates high-risk malignancy.22 Delay in diagnostic procedures were further exacerbated by prolonged periods between confirmed diagnosis and treatment delivery, as reported by Torzili et al. to have added two extra weeks to the average 3-week waiting time prior to the pandemic.8 A secondary analysis by Ban et al. showed an association between surgery delay longer than 6 weeks increases the likelihood of overall cancelation of surgery.7 Included studies in this review detailed their mean delay of treatment delivery from sarcoma and/or cancer diagnosis ranging from 12 days up to 12.7 weeks.1,8,12,21
7.4. Patient outcome
Consequences from treatment delays need to be borne in mind. The COVID Surg Collaborative reported new metastatic disease found in almost 2% of cancer patients who had their treatment delayed after a median time of 23 weeks follow up.1 From the same study, 185 tumors of patients who only just had their first cancer treatment surgery after at least 4 weeks from their initial diagnosis were deemed as unresectable. More than two-thirds of sarcoma patients presenting at the Philippine General Hospital during the COVID-19 pandemic have already metastasized to lungs on their initial visit.20 Whether delaying surgical treatment by giving chemotherapy/radiotherapy beforehand or by postponing treatment overall, malignant cells would rapidly invade and metastasise leaving the individual with worse prognosis.7
From complications related to the COVID-19 infection, Rajasekaran et al.14 reported COVID-19 infection rate of more than 7% of surgical sarcoma patients with mortality rate as high as 50% of it at the early start of pandemic. Similar rates were found by other studies in other global parts such as UK, India and by the COVID Surg Collaborative.3,8,9 Co-morbidities along with ASA grades were recommended to be the foundation of decision making prior to performing surgical treatment. Compared to pre-pandemic cohort, there is no significant difference in mortality rate of surgical osteosarcoma patients during the COVID-19 pandemic (p = 0.91) which Gaston et al. admitted the limitation of short follow-up period and the young median age of patients included which were less likely to have severe co-morbidities.15 Overall, the pandemic has affected the treatment outcome of primary sarcoma patients through the COVID-19 infection directly and synergistically with implications in treatment delivery.
8. CONCLUSION
Primary sarcoma progression is time-critical and its treatment is ever-so crucial to be delivered on time. However, during the pandemic many factors have caused delay in treatment delivery. Although the majority of hospitals would prioritize surgeries for high-grade malignant primary sarcoma, its implementation is affected by multi-factorial influence from patient, health system and even the government. Possible long-term consequences of deferring benign, low-grade and chemo/radiotherapy-controlled sarcoma patients during the pandemic may result in accumulation of patients presenting with worse prognosis after COVID-related bans are lifted.
The highlight of this systematic review result for orthopedic surgeons in raising their awareness for sarcoma treatments. As the COVID-19 pandemic slowly subsides, we reflect on how the sudden outbreak has severely delayed and compromised surgical treatment for musculoskeletal and sarcoma patients. It is important to anticipate the wave of worse presenting cases in the post-pandemic period and to aim for clinical reasonings behind making surgical decision in the patient's best interest. Due to the unpredictability of pandemic occurrence, we hope this study will be able to provide a review of different approaches in management of primary malignant sarcoma if another pandemic would happen in the future. We recognize our review hold several flaws. Due to limitation of quantitative data extracted, our meta-analysis result, which showed better surgical outcome in pre-pandemic cohort, has a low heterogeneity.
Author statement
Y.A. P. P: Conceptualization, Writing- Original Draft, Validation, Investigation.
R.M: Validation, Methodology, Writing- Review & editing, Supervision.
F.M: Validation, Format analysis, Writing -Review & editing, Supervision.
M.H B: Validation, Data curation, Writing-Review & editing, supervision.
P.A S: Methodology, Formal analysis, Writing- Original draft, Dara curation.
A. F. H: Methodology, Writing- original draft, visualization, formal analysis.
Disclosure
There was no external funding for this study. No competing interests are disclosed by the authors. On request, unprovided data are made available.
Funding statement
This review received no specific grant from any funding agency in the public, commercial, or not-for- profit sectors.
Declaration of competing interest
All author declare that they have no conflict of interest.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jor.2023.02.013.
Contributor Information
Yuni Artha Prabowo Putro, Email: yuniarthaprabowoputro@ugm.ac.id.
Rahadyan Magetsari, Email: magetsarir@yahoo.com.
Ferdiansyah Mahyudin, Email: ferdyortho@gmail.com.
Muhammad Hardian Basuki, Email: basukimh@gmail.com.
Paramita Ayu Saraswati, Email: paramita.ayu.s@mail.ugm.ac.id.
A. Faiz Huwaidi, Email: a.faiz.huwaidi@mail.ugm.ac.id.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.COVIDSurgCollaborative* andGlobalSurgCollaborative*NIHR Global Health Research Unit on Global Surgery BU Timing of surgery following SARS-CoV-2 infection: an international prospective cohort study. Anaesthesia. 2021;76:748–758. doi: 10.1111/anae.15458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ioannidis J.P.A. Global perspective of COVID-19 epidemiology for a full-cycle pandemic. Eur J Clin Invest. 2020;50 doi: 10.1111/eci.13423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pkr K., Elagandula J., Patel S., et al. The impact of COVID-19 pandemic on cancer care in a tertiary care facility. South Asian J Cancer. 2021;10:32–35. doi: 10.1055/s-0041-1731577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wagner M.J., Pollack S.M., Cranmer L.D., et al. Outcomes of patients with sarcoma and COVID-19 infection: a single institution cohort analysis. Cancer Invest. 2021;39:315–320. doi: 10.1080/07357907.2021.1903914. [DOI] [PubMed] [Google Scholar]
- 5.Öztürk K., Ünkar E.A., Öztürk A.A. Perioperative management recommendations to resume elective orthopaedic surgeries for post-covid-19 “new normal”: current vision of the Turkish society of orthopaedics and traumatology. Acta Orthop Traumatol Turcica. 2020;54:228–233. doi: 10.5152/j.aott.2020.20183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Iliadis A.D., Eastwood D.M., Bayliss L., et al. Vol. 1. 2020. (Follow Us @BoneJointOpen BJO on Behalf of Royal National Orthopaedic Hospital Paediatric COVID-19 Collaborative Providing a Paediatric Trauma and Orthopaedics Service during the Peak of the COVID-19 Pandemic ThE ROyal NaTiONal ORThOpaEDiC hOspiTal ExpERiENCE). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ban Y., Manabu H., Oebisu N., Shimatani A., Takada N., Nakamura H. The impact of COVID-19 pandemic on bone and soft tissue tumor treatment: a single institution study. 2022. [DOI] [PMC free article] [PubMed]
- 8.Torzilli G., Viganò L., Galvanin J., et al. A snapshot of elective oncological surgery in Italy during COVID-19 emergency: pearls, pitfalls, and perspectives. Ann Surg. 2020;272 doi: 10.1097/SLA.0000000000004081. e112–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thaler M., Khosravi I., Leithner A., Papagelopoulos P.J., Ruggieri P. Impact of the COVID-19 pandemic on patients suffering from musculoskeletal tumours. Int Orthop. 2020;44:1503–1509. doi: 10.1007/s00264-020-04636-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Glasbey J.C., Bhangu A. Elective cancer surgery in COVID-19-free surgical pathways during the SARS-CoV-2 pandemic: an international, multicenter, comparative cohort study. 2020. [DOI] [PMC free article] [PubMed]
- 11.Global Health Research Group on Children’s Non- Communicable Diseases Collaborative Impact of the COVID-19 pandemic on patients with paediatric cancer in low-income, middle-income and high-income countries: a multicentre, international, observational cohort study. BMJ Open. 2022;12 doi: 10.1136/bmjopen-2021-054690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Prajapati A., Gupta S., Nayak P., Gulia A., Puri A. The effect of COVID-19: adopted changes and their impact on management of musculoskeletal oncology care at a tertiary referral centre. J Clin Orthop Trauma. 2021;23 doi: 10.1016/j.jcot.2021.101651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rajasekaran R.B., Whitwell D., Cosker T.D.A., Gibbons C.L.M.H. Service delivery during the COVID-19 pandemic: experience from the oxford bone tumour and soft tissue sarcoma service. J Clin Orthop Trauma. 2020;11:S419–S422. doi: 10.1016/j.jcot.2020.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rajasekaran B., Kotecha S., Whitwell D., et al. Patient safety associated with the surgical treatment of bone and soft tissue tumours during the COVID-19 pandemic-results from an observational study at the Oxford Sarcoma Service. 2020. [DOI] [PMC free article] [PubMed]
- 15.Rajasekaran R.B., Ashford R.U., Cosker T.D.A., et al. What proportion of patients with bone and soft tissue tumors contracted coronavirus-19 and died from surgical procedures during the initial period of the Covid-19 pandemic? Results from the multicenter British Orthopaedic Oncology Society observational study. Clin Orthop Relat Res. 2021;479:1158–1166. doi: 10.1097/CORR.0000000000001568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Berjano P., Vanni D., Fariselli L., Cecchinato R., Boriani S. Strategy for the practice of spine oncological surgery during the covid-19 pandemic. Spine. 2020;45:1386–1394. doi: 10.1097/BRS.0000000000003623. [DOI] [PubMed] [Google Scholar]
- 17.Cardoso P., Rodrigues-Pinto R. Surgical management of bone and soft tissue sarcomas and skeletal metastases during the COVID-19 pandemic. Eur J Surg Oncol. 2020;46:1178–1179. doi: 10.1016/j.ejso.2020.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gulia A., Arora R.S., Panda P.K., et al. Adapting management of sarcomas in COVID-19: an evidence-based review. Indian J Orthop. 2021;55 doi: 10.1007/s43465-020-00143-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rossi B., Zoccali C., Baldi J., et al. Reorganization tips from a sarcoma unit at time of the COVID‐19 pandemic in Italy: early experience from a regional referral oncologic center. J Clin Med. 2020;9:1–10. doi: 10.3390/jcm9061868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gaston L., Yu H.V., Dacanay E., et al. 2021. Treatment of Osteosarcoma Patients in the Philippine General Hospital during the COVID-19 Outbreak. [Google Scholar]
- 21.Qureshi S.S., Ramraj D., Chinnaswamy G., et al. Assessment of outcomes of elective cancer surgeries in children during coronavirus disease 2019 pandemic: retrospective cohort study from a tertiary cancer center in India. Medicine. 2021;100 doi: 10.1097/MD.0000000000026752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Şahbat Y., Buyuktopcu O., Topkar O.M., Erol B. Management of orthopedic oncology patients during coronavirus pandemic. J Surg Oncol. 2020;122:594–601. doi: 10.1002/jso.26092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stevenson J.D., Evans S., Morris G., et al. Mortality of high-risk orthopaedic oncology patients during the COVID-19 pandemic: a prospective cohort study. J Surg Oncol. 2020;122:1027–1030. doi: 10.1002/jso.26127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gaston C.L.L., Pag-Ong J.P., Dacanay E., Quintos A.J. Radical change in osteosarcoma surgical plan due to COVID-19 pandemic. BMJ Case Rep. 2020;13 doi: 10.1136/bcr-2020-237197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tzeng C.W.D., Tran Cao H.S., Roland C.L., et al. Surgical decision-making and prioritization for cancer patients at the onset of the COVID-19 pandemic: a multidisciplinary approach. Surg Oncol. 2020;34:182–185. doi: 10.1016/j.suronc.2020.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kumar V.S., Banjara R., Thapa S., et al. Bone sarcoma surgery in times of COVID-19 pandemic lockdown-early experience from a tertiary centre in India. J Surg Oncol. 2020;122:825–830. doi: 10.1002/jso.26112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Neamţiu L., Martos C., Giusti F., et al. Impact of the first wave of the COVID-19 pandemic on cancer registration and cancer care: a European survey. Eur J Publ Health. 2022;32:311–315. doi: 10.1093/eurpub/ckab214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bouche P.-A., Valteau B., Dumaine V., et al. Were protective procedures against SARS-CoV-2 effective in an orthopaedic and trauma centre during the lockdown period? A retrospective study. 2020;44:2494–2496. doi: 10.1007/s00264-020-04729-0/Published. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.III A.K.C., Cassell L.T., Bague A.H. Management of cancer patients during the COVID-19 pandemic: a comprehensive review. WArtificial Intelligence in Cancer. 2020;1:8–18. doi: 10.35713/aic.v1.i1.8. [DOI] [Google Scholar]
- 30.Sharma J., Mahajan A., Bakhshi S., et al. The impact of COVID-19 pandemic on access to treatment for children with cancer in India and treating center practices. Cancer. 2022;128:579–586. doi: 10.1002/cncr.33945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Anwar S.L., Harahap W.A., Aryandono T. Perspectives on how to navigate cancer surgery in the breast, head and neck, skin, and soft tissue tumor in limited-resource countries during COVID-19 pandemic. Int J Surg. 2020;79:206–212. doi: 10.1016/j.ijsu.2020.05.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dabkeviciene D., Vincerzevskiene I., Urbonas V., et al. The impact of the COVID-19 pandemic on cancer patient's management—Lithuanian cancer center experience. Healthcare. 2021;9 doi: 10.3390/healthcare9111522. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.