Summary
Background
Obesity and related metabolic disturbances including diabetes, hypertension and hyperlipidemia predict future cognitive decline. Asia has a high prevalence of both obesity and metabolic disease, potentially amplifying the future burden of dementia in the region. We aimed to investigate the impact of adiposity and metabolic risk on cognitive function in Asian populations, using an epidemiological analysis and a two-sample Mendelian Randomization (MR) study.
Methods
The Health for Life in Singapore (HELIOS) Study is a population-based cohort of South-East-Asian men and women in Singapore, aged 30–84 years. We analyzed 8769 participants with metabolic and cognitive data collected between 2018 and 2021. Whole-body fat mass was quantified with Dual X-Ray Absorptiometry (DEXA). Cognition was assessed using a computerized cognitive battery. An index of general cognition ‘g’ was derived through factor analysis. We tested the relationship of fat mass indices and metabolic measures with ‘g’ using regression approaches. We then performed inverse-variance-weighted MR of adiposity and metabolic risk factors on ‘g’, using summary statistics for genome-wide association studies of BMI, visceral adipose tissue (VAT), waist-hip-ratio (WHR), blood pressure, HDL cholesterol, triglycerides, fasting glucose, HbA1c, and general cognition.
Findings
Participants were 58.9% female, and aged 51.4 (11.3) years. In univariate analysis, all 29 adiposity and metabolic measures assessed were associated with ‘g’ at P < 0.05. In multivariable analyses, reduced ‘g’ was consistently associated with increased visceral fat mass index and lower HDL cholesterol (P < 0.001), but not with blood pressure, triglycerides, or glycemic indices. The reduction in ‘g’ associated with 1SD higher visceral fat, or 1SD lower HDL cholesterol, was equivalent to a 0.7 and 0.9-year increase in chronological age respectively (P < 0.001). Inverse variance MR analyses showed that reduced ‘g’ is associated with genetically determined elevation of VAT, BMI and WHR (all P < 0.001). In contrast, MR did not support a causal role for blood pressure, lipid, or glycemic indices on cognition.
Interpretation
We show an independent relationship between adiposity and cognition in a multi-ethnic Asian population. MR analyses suggest that both visceral adiposity and raised BMI are likely to be causally linked to cognition. Our findings have important implications for preservation of cognitive health, including further motivation for action to reverse the rising burden of obesity in the Asia–Pacific region.
Funding
The Nanyang Technological University—the Lee Kong Chian School of Medicine, National Healthcare Group, National Medical Research Council, Ministry of Education, Singapore.
Keywords: Adiposity, Visceral adiposity, DEXA, General cognition, Cognitive function, Aging, Mendelian Randomization, Asia, Cardiovascular risk factors, Metabolic syndrome
Research in context.
Evidence before this study
We searched Embase & MEDLINE for “Cognition” [MeSH] and “metabolic syndrome” [MeSH] and alternate MeSH terms from 1946 till 23rd August 2019 and found 673 relevant studies, including a meta-analysis. Altogether with the 2020 report of Lancet Commission for Dementia prevention, there is sufficient evidence for clustering of metabolic disturbances as risk factors for cognitive function. However, the evidence for excess adiposity as risk factor for cognition is more modest, owing to the inconsistency of the indices used (BMI vs. waist circumference), with few studies using imaging-based indices. Most epidemiological studies were also performed in the older individuals of Caucasian ancestry, few considered individuals of South-East-Asian ancestry and <60 years old. We also searched PubMed from inception till end-of-2021 for “Mendelian Randomization” [MeSH] AND “cognit∗” OR “Alzheimer's” OR “dementia” AND “adiposity” and identified only 3 relevant studies. These studies, together with 1 multiple Mendelian Randomization (MR) analysis from the UK Biobank reported no significant causal effect of genetically predicted metabolic risk factors with cognitive performance. In summary, it remains unclear which metabolic risk factors, particularly excess adiposity, have independent adverse effect on cognition.
Added value of this study
Using both observational and two-sample MR analysis, we present causal evidence linking excess adiposity as key metabolic risk factor for poorer cognitive function, such that for each 1 SD increase (0.27 Kg) of excess visceral fat, there was reduced cognitive performance equivalent to 0.7 years of cognitive aging. Our population cohort (n = 8769) is based in South-East-Asia and includes 6381 individuals <60 years old recruited from the general population. In our observational analysis, we used the Dual X-Ray Absorptiometry-based quantification of visceral fat mass, providing clarity in assessing the contribution of visceral fat relative to other fat depots or overall body mass. In the MR analysis, we also used the novel summary statistics of genetically predicted visceral fat as instrument-exposure. Our findings support that visceral fat may have specific etiological role on cognition beyond overall adiposity.
Implications of all the available evidence
Altogether, both overall and excess adiposity have independent adverse effect on cognition and managing excess adiposity may maintain and/or improve cognition.
Introduction
Maintaining cognitive function is a key goal in healthy aging. Loss of cognitive function may lead to dementia, a progressive chronic disease that can have a devastating impact on the quality of life for the affected individual and caregivers. Dementia is already the 7th leading cause of death globally, and the number of affected people is predicted to double from 2030 to 2050.1 Understanding the risk factors for cognitive decline is of major global importance.
Observational studies amongst European, North American, East Asian and African populations report that obesity and related metabolic disturbances are risk factors for both cognitive impairment and dementia, as comprehensively reviewed.2 Raised body mass index (BMI), central adiposity, elevated blood pressure, abnormal lipid profile and perturbed glucose metabolism have individually, and in aggregate been reported to be associated with reduced cognitive function in both cross-sectional and prospective analyses.2,3 Although this raises the possibility that interventions which improve metabolic risk factors may preserve cognitive function, these benefits have not been consistently observed in intervention studies focused on control of obesity, blood pressure, cholesterol, or glucose levels.4,5 The causal relationships of adiposity and metabolic risk factors with the development of cognitive impairment remain unclear.
Both obesity and metabolic disease are highly prevalent in the Asia–Pacific region, rendering the potential impact of these disturbances on cognitive health of high importance to Asian populations. In this study, we therefore set out to quantify the relationship of adiposity and related metabolic disturbances with cognitive function in a population cohort comprising people of East-Asian, South-East-Asian, and South-Asian ancestry living in Singapore. We then use Mendelian Randomization (MR) as an alternative approach to traditional regression analysis, to explore the potential causal pathways involved.
Methods
Study parameters for epidemiological analysis
HELIOS is a population-based cohort comprising 10,004 Asian men and women living in Singapore (ethics approval from the Nanyang Technological University's Institutional Review Board: 2016-11-030). HELIOS includes Singapore citizens or Permanent Residents aged 30–84 years old and excludes pregnant and breastfeeding women, those with major illness requiring hospitalization/surgery, cancer treatment in the past year or participating in drug trials within the past month. Participants were recruited from the general population and through community outreach programs to ensure diversity in ethnicity and socio-economic background. All consenting study participants underwent extensive physiological assessments and biological sample collections and self-completed comprehensive health and lifestyle questionnaires (www.healthforlife.sg). Classification of ethnicity was based on self-report and relates closely to ancestry assessed objectively by genetic information.6 The three primary ethnic groups are: i. Chinese and other East Asian (Chinese), ii. Malay and other South-East-Asian (Malay) and iii. South Asian (India and other countries from Indian subcontinent).
Cognitive function was evaluated using a computerized test adopted from the UK Biobank,7 covering four major cognitive domains: memory, executive function, processing speed and attention (Supplementary-Method). The reliability, validity and longitudinal stability of the UK Biobank cognitive test battery have been reported.8 We derived a single proxy of general cognition ‘g’ by dimensionally reducing z-normalized cognitive test raw scores using a confirmatory factor analysis, similar to the approach in the GWAS of general cognition7; ‘g’ is the first un-rotated principal component with eigen value ≥ 1 (Supplementary-Method). Unlike the dementia screener designed for older individuals in prior systematic reviews,3,9 ‘g’ evaluates population variance of general cognitive function, addresses the general challenges of study comparability, and facilitates future meta-analysis.7
Body fat composition was quantified by DEXA whole-body scans (Horizon™ W densitometer, S/N 30052M, Hologic, Massachusetts, USA, QDR software version 13.6.0.5). DEXA machine was calibrated daily with QDR anthropomorphic spine phantom (Hologic™), and weekly with radiographic uniformity and whole-body phantom scan. Fat mass from different regions was expressed in grams. As persons of different height and/or nutritional state may have similar fat mass, we derived Fat Mass Index (FMI, Kg/m2) by dividing fat mass with height2,10 across body compartment. BMI is weight/height.2 Both waist (at midpoint between the iliac crest and the lowest rib) and hip (at the level of the largest posterior protrusion of the buttocks) circumferences were measured 3 times and averaged. WHR is waist divided by hip circumference.
For the current analysis we excluded participants who were not of Chinese, Malay or other South Asian background (n = 125), who did not complete the cognitive tests (n = 597) or the physiological and biochemical assessments (n = 513). This left 8769 participants of Chinese (East-Asian), Malay (South-East-Asian) or South-Asian ethnicity for whom baseline cognitive test, DEXA scans, physiological, and biochemical assessments were complete. Excluded participants were older by 7.7 (0.4) years (P < 0.001), more likely to be female (65.4 vs. 58.9%, P < 0.001) and less likely to be Chinese (30.4 vs 38.2% P < 0.001).
Epidemiological analysis in multi-ethnic Asian population
R version 4.0.5 (R, Vienna, Austria) was used for statistical analysis and illustrations. Descriptive statistics were performed using Chi-squared test and T-test for categorical [n (%)] and continuous variables [mean (SD)], respectively. HDL, triglycerides, and glucose were Ln-transformed for further analysis. To compare the effect of each metabolic risk factor on general cognition, we performed univariate linear regressions on the 30 body fat measures and metabolic parameters and 3 demographic and lifestyle factors (Total Years of Education, smoking status and alcohol intake, Supplementary-Method) against ‘g’, adjusting for sex, age, and ethnicity.
We derived 16 body fat indices to evaluate the impact of various fat deposition on cognition: i) 9 DEXA-based fat mass indices: total, visceral, arm, leg, limb, android, gynoid, limbs and trunk FMI; ii) 3 conventional anthropometric indices (BMI, waist, and hip circumference); and iii) 4 fat distribution ratios: waist/hip, android/gynoid, trunk/limb, and visceral/total adiposity. We also considered 14 metabolic parameters: i) 8 individual measures including systolic and diastolic blood pressure (SBP and DBP), total, HDL- and LDL-cholesterol, triglycerides, fasting glucose, and HbA1c (Supplementary-Method); ii) 3 disease diagnoses (hypertension, Type-2 diabetes, and hypercholesterolemia), and iii) 3 composite metabolic measures. The first composite measure is a binary status of metabolic syndrome (MetS) as per the International Diabetes Federation - American Heart Association 2009 guideline: having three out of the five following criteria: 1) SBP and DBP ≥130 mmHg or ≥85 mmHg, respectively or treatment for hypertension; 2) waist circumference ≥90 cm and ≥80 cm for male and female, respectively; 3) HDL <1 mmol/L or <1.3 mmol/L for male and female, respectively; 4) triglyceride ≥1.7 mmol/L or treatment for hypertriglyceridemia; and 5) glucose ≥5.6 mmol/L or treatment for diabetes.11 The second composite measure is MetS_sum, a severity index based on counts of criteria ranging from 0 to 5. The third measure is MetS_continuous, a continuous severity score based on the dimensional reduction methods. The factor loading for MetS_continuous range from |0.33| for SBP to |0.69| for HDL.
We conducted multivariable linear regression to determine the independent contributions of adiposity and metabolic risk factors on ‘g’, adjusting for sex, age, and ethnicity. Since adiposity and metabolic measures are highly correlated, we identified a parsimonious set of adiposity and metabolic markers for inclusion in the models. Specifically, we first ranked measures of adiposity and metabolism based on their P value for association with ‘g’. We then identified the ‘lead’ marker most closely associated with ‘g’, and excluded any other marker correlated at r > 0.8 with the lead marker. We then identified the next highest-ranking marker, and repeated the exercise, until there were no markers remaining. Bivariate correlations were determined with adjustment for age, sex, and ethnicity. To obtain cognitive ‘aging’, we divided the β of a risk factor to ‘g’ with that of 1 year of increased age to ‘g’. We then compared the cognitive ‘aging’ derived from this Asian population with those of other ancestry using the UK Biobank dataset (https://www.ukbiobank.ac.uk/, approved research ID 43769). We extracted shared cognitive test scores, anthropometry and cardiometabolic measures, and bioimpedance variables to calculate visceral fat using the UK Biobank's validated, DEXA-based predictive equation (Supplementary-Table S1).12
As sensitivity analyses, regression analyses were repeated amongst participants who i) completed the cognitive test in English only (to account for the administration of test in non-English language), ii) did not have any medications for Type-2 diabetes, hypertension, and hyperlipidemia, and iii) in all participants adjusted for smoking, alcohol, and education levels. We evaluated heterogeneity of effect between ethnic groups, and between sexes, using i) Higgin's index I2 and ii) comparison of effect sizes between stratified analyses.
Mendelian Randomization (MR) to assess causal relationships for cognition
MR is an analytical method to investigate the causality between the exposure and outcome using germline genetic variants which are assigned randomly at conception, and therefore are less affected by confounding and reverse causation, provided that the MR assumptions are satisfied.13 MR assumes that genetic variants are i) associated with exposures; ii) not associated with the confounders of the exposure-outcome association; and iii) influence outcomes only through exposures.14 Two-sample MR uses summary statistics of Single Nucleotide Polymorphism (SNP)s from 2 separate datasets of instrument-exposure and instrument-outcome.14 To obtain causal evidence linking metabolic disturbances and general cognition, we used the Genome-Wide Association Studies (GWAS) summary statistics of BMI,15 BMI-adjusted-waist circumference and -WHR,16 visceral adipose tissue (VAT),12 SBP and DBP,17 HDL and triglycerides,18 fasting glucose and HbA1c19 as instrument-exposure, and cognitive performance20 as instrument-outcome (Table 1). We used the GWAS summary statistics of years of schooling20 and coronary heart disease21 as positive controls for instrument-exposure and instrument-outcome, respectively. Changes of phenotypes are in SD unit across instruments (Table 1). VAT was predicted using a bioimpedance-based fat measurements adjusted by demographics factors, and calibrated by DEXA measurement in a subset of participants.12 The UK Biobank datasets were used across the VAT,12 SBP and DBP17 GWAS; and were ∼60%15 and ∼85%20 of the total sample in the BMI and cognitive performance meta-analysis, respectively. We calculated F-statistics for each instrument to evaluate weak instrument bias,22 and the lower limit of F-statistics to assess potential bias due to participant overlap.23
Table 1.
Summary of the instrument variables for two-sample Mendelian Randomization.
| Type | MRC-IEU ID | Phenotype | unit | N | Year | Ethnicity | Consortia | SNPs after clumping and harmonization |
|---|---|---|---|---|---|---|---|
| Exposure | – | BMI | 1 SD | ∼700,000 | 2018 | European | GIANT, UKB | 323 |
| Exposure | ieu-a-67 | BMI-adjusted Waist circumference | 1 SD (cm) = 12.52 | 231,353 | 2015 | Mixed | GIANT | 62 |
| Exposure | ieu-a-78 | BMI-adjusted WHR | 1 SD = 0.08 | 224,452 | 2015 | Mixed | GIANT | 36 |
| Exposure | GWAS catalog: GCST008744 | VAT | 1 SD (Kg) 0.68 and 1.12 in male and female | 325,153 | 2019 | European | UKB | 219 (107 non-BMI) |
| Exposure | ukb-b-20175 | SBP | 1 SD (mmHg) 19.4 and 21.2 in male and female | 436,419 | 2018 | European | UKB | 260 |
| Exposure | ukb-b-7992 | DBP | 1 SD (mmHg) 11 and 11.1 in male and female | 241 | ||||
| Exposure | ieu-a-299 | HDL | 1 SD = 15.51 mg/dl | 187,167 | 2013 | Mixed | GLGC | 86 |
| Exposure | ieu-a-302 | Triglycerides | 1 SD = 90.72 mg/dl | 177,861 | 2013 | Mixed | GLGC | 54 |
| Exposure | – | Glucose (fasting) | 1SD | 281,416 | 2021 | Mixed | MAGIC | 153 |
| Exposure | HbA1c | 1SD | 166 | |||||
| Exposure (+control) | ieu-a-1239 | Years of schooling | years, SD = 4.2 | 766,345 | 2018 | European | SSGAC | 306 |
| Outcome | ebi-a-GCST006572 | Cognitive performance | 1 SD | 257,841 | 2018 | European | COGENT, UKB | – |
| Outcome (+control) | ieu-a-7 | Coronary heart disease | log odds | control = 123,504 case = 60,801 |
2015 | Mixed | CARDIoGRAMplusC4D | – |
Phenotype (top-bottom): BMI = body-mass-index; WHR = waist-hip-ratio; VAT = visceral adipose tissue; SBP and DBP = systolic and diastolic blood pressure. SD = standard deviation. Consortia (top-bottom): UKB = UK Biobank; GIANT = Genetic Investigation of Anthropometric Traits; GLGC = Global Lipids Genetics Consortium; MAGIC = the Meta-Analyses of Glucose and Insulin-related traits Consortium; SSGACc = cSocial Science Genetic Association Consortium; COGENT = Cognitive Genomic Consortium; CARDIoGRAMplusC4D = Coronary ARtery DIsease Genome-wide Replication and Meta-analysis (CARDIoGRAM) plus The Coronary Artery Disease (C4D) Genetics) consortium.
Two-sample MR was next performed with TwoSampleMR R package to call GWAS summary statistics from https://gwas.mrcieu.ac.uk/datasets/ (Table 1), or to manually upload external summary statistics.12 We applied the default TwoSampleMR clumping procedure to all exposure instruments. The summary statistics at genome-wide significance for both exposure and outcome were then harmonized to the forward strand, which is inferred using allele frequency information, including for palindromic SNPs. Ambiguous SNPs which forward strand cannot be inferred were excluded. Table 1 and Supplementary-Table S2 summarize available SNPs after clumping and harmonization. We conducted inverse-variance-weighted MR, where Wald ratio is estimated for each SNP and meta-analyzed; the weight of each ratio is the inverse of the variance of SNP-outcome association. To account for multiple testing, we used Bonferroni significance threshold for 0.05/11 = P < 0.005.24 To distinguish the effect of visceral fat from overall body fat, we repeated the MR for VAT after removing SNPs which also predict BMI12 (107 out of 219, Table 1, Supplementary-Table S2).
For sensitivity testing, we performed weighted-median MR, which provides valid estimates assuming 50% of the instrument is non-pleiotropic. We then repeated the MR for each instrument-exposure after removing SNPs predicting alternative phenotypes by verifying each SNP against the GWAS catalog (https://www.ebi.ac.uk/gwas/) using gwasrapidd package (Supplementary-Table S2). We also performed multivariable MR (Supplementary Methods). To test for horizontal pleiotropy, we performed MR-Egger, leave-one-out and single-SNP MR analysis. We also performed MR with cognitive performance as instrument-exposure with any significant metabolic risk factors as instrument-outcome to evaluate reverse causality.
Role of funding sources
The funders were not involved in the design and conduct of this study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
Results
Characteristics of HELIOS participants
Participants were 51.4 ± 11.3 years old and 58.9% female. The ethnic distribution was 69% Chinese, 13% Malay and 18% South Asian (Table 2). The average years of education was 14.1 ± 3.2 years. The prevalence of cigarette smoking was 8.5%, and of regular alcohol consumption 4.5%. Adiposity and metabolic characteristics for participants are summarized in Table 1, Supplementary-Fig. S1, and Supplementary Table S3. There were 23.3% participants with obesity (BMI>27.5 kg/m2, 12% with BMI>30 kg/m2). Mean DEXA-derived visceral FMI was 0.24 ± 0.10 kg/m2, representing 0.63 ± 0.27 Kg of VAT (Table 1 and Supplementary Table S3). The prevalence of hypertension, hypercholesterolemia, and Type-2-diabetes in the study population was 33.3%, 29.5%, and 14.1%, respectively. Sociodemographic and biological characteristics varied across ethnic groups; in particular, the prevalence of obesity, diabetes and hypertension were higher in Malay and South Asian people compared to Chinese (Supplementary Table S4).
Table 2.
Socio-demographic factors, metabolic profile, and cognitive performance in the HELIOS Study.
| Total (n = 8769) | Correlations with ‘g’ | p | |
|---|---|---|---|
| Socio-demography, continuous variables mean ± SD, categorical variables n (%) | |||
| Age (years) | 51.39 ± 11.32 | −0.649 | 0.000 |
| Sex | |||
| Female | 5167 (58.9) | −0.096 | 2 × 10−6 |
| Ethnicity | |||
| Chinese | 6099 (69.6) | (Reference) | 2.6 × 10−95 |
| Malay | 1092 (12.5) | −0.168 | |
| South Asian | 1578 (18.0) | −0.537 | |
| Total years of education | 14.10 ± 3.17 | 0.347 | 3 × 10−247 |
| Active smoker | 747 (8.5) | 0.081 | 2.2 × 10−14 |
| Alcohol intake | |||
| Never or on special occasion | 6967 (79.5) | 0.113 | 1.9 × 10−26 |
| 1-2 times/month or 1–2/week | 1407 (16.0) | ||
| 3-4 times/week or daily | 395 (4.5) | ||
| Adiposity measures, mean ± SD | |||
| BMI (Kg/m2) | 24.77 ± 4.52 | −0.066 | 5.6 × 10−10 |
| Waist circumference (cm) | 82.91 ± 11.59 | −0.134 | 2.6 × 10−36 |
| Hip circumference (cm) | 97.29 ± 8.87 | −0.020 | 0.065 |
| Fat Mass Index (FMI, Kg/m2) | 9.39 ± 3.11 | −0.147 | 1.2 × 10−43 |
| Visceral FMI | 0.24 ± 0.1 | −0.297 | 1.5 × 10−177 |
| Android FMI | 0.81 ± 0.33 | −0.193 | 1.4 × 10−74 |
| Gynoid FMI | 1.58 ± 0.52 | −0.067 | 4.5 × 10−10 |
| Limb FMIa | 4.3 ± 1.59 | −0.101 | 2 × 10−21 |
| Trunk FMI | 4.62 ± 1.63 | −0.180 | 5.5 × 10−65 |
| Waist/hip ratio | 0.85 ± 0.08 | −0.191 | 9.8 × 10−73 |
| Android/Gynoid ratio | 1.02 ± 0.17 | −0.123 | 8.1 × 10−31 |
| Trunk/limb ratio | 1.1 ± 0.23 | −0.161 | 4.4 × 10−52 |
| vFMI/FMI ratio | 0.03 ± 0.01 | −0.310 | 2.4 × 10−194 |
| Other metabolic risk factors, mean ± SD | |||
| Systolic blood pressure (mmHg) | 120.62 ± 17.67 | −0.267 | 1.5 × 10−143 |
| Diastolic blood pressure (mmHg) | 68.65 ± 10.49 | −0.101 | 1.6 × 10−21 |
| HDL (mmol/L) | 1.53 ± 0.42 | 0.046 | 1.4 × 10−5 |
| Triglycerides (mmol/L) | 1.26 ± 0.82 | −0.069 | 1.2 × 10−10 |
| Glucose (mmol/L) | 5.07 ± 1.23 | −0.180 | 4.5 × 10−65 |
| HbA1c (%) | 5.7 ± 0.86 | −0.242 | 2.9 × 10−117 |
| MetS_sum, 0:1:2:3:4:5 | 33: 24: 18: 13: 8: 4 | −0.309 | 1 × 10−193 |
| MetS_continuous | −0.02 ± 0.87 | −0.178 | 1.45 × 10−63 |
| Cognitive Test, mean ± SD | |||
| Pairing test; total incorrect guess | 8.81 ± 5.97 | −0.328 | 1.4 × 10−218 |
| Total digits recalled | 8.22 ± 1.61 | 0.434 | 0.000 |
| Executive functioning: total correct answers | 3.2 ± 1.56 | 0.412 | 0.000 |
| Reaction time, average (ms) | 746.7 ± 314.19 | −0.618 | 0.000 |
| Box test, average time (ms) | 1405.21 ± 312.79 | −0.902 | 0.000 |
| Ink test, average time (ms) | 1796.03 ± 476.26 | −0.711 | 0.000 |
| General cognition, ‘g’ | 0.01 ± 0.93 | – | – |
FMI = Fat Mass Index. MetS_sum = a severity index based on symptom counts (range:0–5). MetS_continuous = a continuous severity score based on the dimensional reduction.
Derived from the left and right arms and legs.
Cognitive testing
Most participants (84.5%) completed the cognitive test in English. The scores across cognitive domains were inter-correlated (|0.08| to |0.65| (all P ≤ 0.001) (Supplementary-Fig. S1). General cognition ‘g’ explained 38.1% variance of the sample, with principal components ranging from |0.31| for pairing test (episodic memory) to |0.91| for Stroop box. Participants' ‘g’ was normally distributed (Supplementary-Fig. S1), positively correlated with total years of education (r = 0.35, P < 0.001), and inversely correlated with age (r = −0.65, P < 0.001) (Table 2, Supplementary-Fig. S1).
Univariate predictors of general cognition in Asian populations
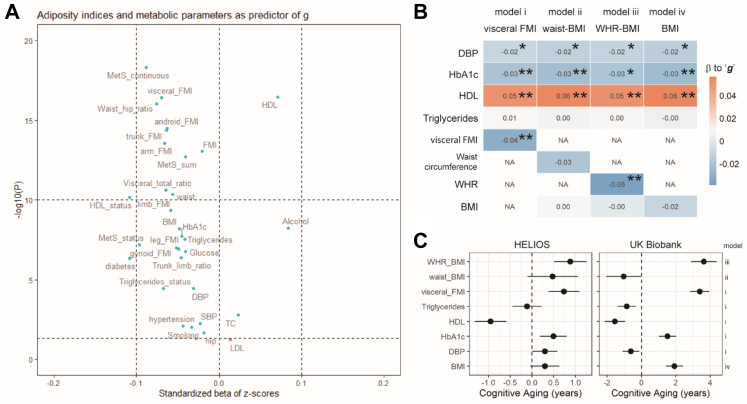
In univariate analyses, ‘g’ was associated with gender, ethnic group, and education (P < 0.001, Table 2). Twenty-nine available measures of adiposity and metabolism were also associated with ‘g’ at P < 0.05 (Fig. 1A). The strongest associations were observed for MetS_continuous (β = −0.09; P = 4.9 × 10−19), DEXA-derived visceral FMI (β = −0.07; P = 3.9 × 10−17), HDL cholesterol (β = 0.07; P = 3.4 × 10−17), and BMI (β = −0.05; P = 2.9 × 10−9).
Fig. 1.
Univariate and multivariate associations of visceral adiposity and metabolic phenotypes to general cognition. All variables were z-normalized, and all associations were adjusted for sex, age, and ethnicity. A) A volcano plot summarizing multiple univariate associations. X-axis = standardized beta of z-scores; Y-axis = −10log(p). Dotted horizontal lines were to signify p ≤ 0.05 and p ≤ 10−10. FMI = Fat Mass Index; MetS_status = status of metabolic syndrome (having 3 or more out of 5 symptoms); MetS_sum = metabolic syndrome severity index based on counts of symptoms ranging from 0 to 5. MetS_continuous = metabolic syndrome continuous scores. SBP and DBP = systolic and diastolic blood pressure, respectively. TC = total cholesterol. LDL and HDL = Low- and High-density lipoproteins, respectively. B) A heatmap depicting 4 multivariate regression models comparing i) visceral BMI with alternative adiposity indices ii) BMI-adjusted waist circumference; iii) BMI-adjusted waist-hip-ratio; and iv) BMI. The number within each cell indicates beta coefficient. White NA cell indicates excluded factors. ∗∗p ≤ 0.001; ∗p ≤ 0.05, >0.001. In model iv), the p value for BMI = 0.08. C) A forest plot summarizing cognitive aging of adiposity and metabolic risk factors calculated from the multivariate regression models in sub-Figure B), and a replication of the same multivariate regression models using the UK Biobank dataset (n = 48,135). Cognitive aging = β of a risk factor to ‘g’/β of 1 year of increased age to ‘g’.
Multivariate predictors of general cognition in Asian populations
Our variable selection strategy prioritized DBP, HbA1c, HDL, triglycerides, and visceral FMI as predictors of general cognition for inclusion in the primary model (Supplementary-Fig. S2). As sensitivity analyses, we also evaluated alternative models that replaced visceral FMI with BMI, BMI-adjusted-waist circumference and BMI-adjusted-WHR (Supplementary-Fig. S2). Across the multivariable models evaluated, we found that reduced HDL and increased visceral FMI and WHR were consistently associated with reduced ‘g’ (P < 0.05, Fig. 1B). In contrast, the relationships of DBP, HbA1c and BMI with ‘g’ were weaker, while triglyceride levels and BMI-adjusted-waist circumference were not associated with ‘g’ (Fig. 1B). There was no evidence for heterogeneity of effect between the ethnic groups in the associations of visceral FMI, HbA1c, HDL cholesterol or triglycerides with ‘g’ (all p > 0.05, Supplementary-Fig. S3). There was weak evidence for heterogeneity in the associations of DBP (I2 = 63.6%, p = 0.02) with ‘g’ between ethnic groups (Supplementary-Fig. S3).
Numerically, the reduction in ‘g’ with 1SD higher DEXA-derived visceral FMI, BMI-adjusted WHR, or 1SD lower HDL cholesterol, was equivalent to a 0.74 (95%CI 0.38 to 1.1), 0.88 (95%CI 0.51 to 1.25), or 0.95 (95%CI 0.59 to 1.31) increase in chronological age in Asian populations, respectively (Fig. 1C). In the replication analysis using the UK Biobank dataset comprising people of European, African, and South-Asian ancestry (Fig. 1C, n = 48,135, Supplementary-Table S1), we also found that a 1SD change of bioimpedance-derived visceral FMI, BMI-adjusted WHR, and HDL cholesterol were associated with a reduction of 3.40 (95%CI 2.84 to 3.95), 3.64 (95%CI 2.90 to 4.38), and 1.55 (95%CI 0.95 to 2.14) years of cognitive aging, respectively (Fig. 1C).
Further sensitivity analyses amongst people who completed the test in English, and in people not on treatment for chronic diseases, or when additionally adjusting for smoking, alcohol intake and education levels, confirmed the findings of both univariate (Supplementary-Fig. S4) and multivariate regression models (Supplementary-Fig. S5).
MR analysis of the relationships between adiposity, metabolic disturbances, and cognition
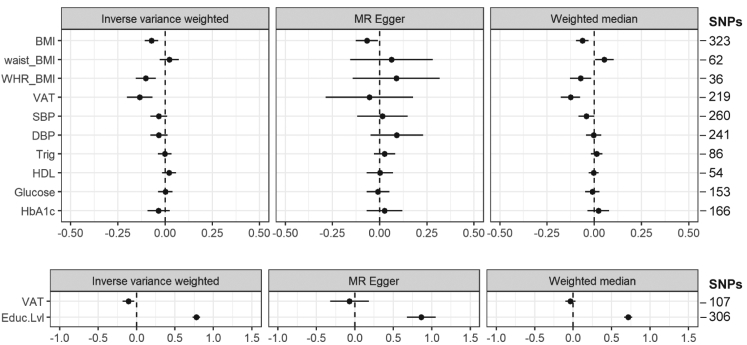
Inverse-variance-weighted MR identified 3 risk factors with potential causal effects on genetically predicted general cognition: VAT, BMI and BMI-adjusted-WHR. Genetic variants for 1 SD increase of VAT (1 SD = 0.68 Kg and 1.12 Kg in male and female respectively, Table 1, Supplementary-Data) had the greatest causal effect on reduced general cognition (β[95%CI] = −0.13 [−0.20; −0.07]), followed by increased BMI (β = −0.09 [−0.14; −0.04]) and BMI-adjusted-WHR (β = −0.10 [−0.16; −0.05]) (Fig. 2, Supplementary-Table S5). In contrast, genetic variants of higher BMI-adjusted-waist circumference, blood pressure, triglycerides, glycemic indices, and lower HDL were not associated with cognitive function (Fig. 2, Supplementary-Table S5).
Fig. 2.
Two-sample Mendelian Randomization reveals causal evidence for visceral adiposity in influencing general cognition. The forest plots illustrate standardized beta (95% Confidence Interval) for each two-sample MR in inverse variance weighted, MR Egger and weighted median. BMI = Body Mass Index; waist_BMI = Waist Circumference adjusted for BMI; WHR_BMI = Waist Hip Circumference adjusted for BMI; VAT = Visceral Adipose Tissue; SBP = Systolic Blood Pressure; DBP = Diastolic Blood Pressure; Trig = Triglycerides; HDL = High-density lipoproteins; Educ.Lvl = Education levels. VAT_no_BMI refers to two-sample MR performed without SNPs that are also genetic variants for BMI.
The causal effect of VAT on general cognition was independent of BMI (Fig. 2). The MR-weighted-median analyses revealed similar findings, with β for VAT, BMI and BMI-adjusted-WHR = −0.14 [−0.20; −0.07], −0.06 [−0.10; −0.02] and −0.07 [−0.13; −0.02], respectively (Fig. 2, Supplementary-Table S5). The β for VAT and BMI remained significant (−0.13 [−0.21; −0.05] and −0.08 [−0.12; −0.05]) in the repeat inverse-variance-weighted MR after removing SNPs predicting alternative phenotypes (Supplementary-Figs. S6 and S7, Supplementary-Table S6). The β for VAT and BMI also remained significant (−0.15 [−0.27; −0.03] and −0.10 [−0.16; −0.04]) in the multivariable MR (Supplementary-Table S7). The β for WHR is no longer significant, though this is likely to due to the loss of statistical power (Supplementary-Table S6). We did not observe weak instrument bias; the F-statistics for all instrument variables range from 47.4 to 924.9 with close lower limit (Supplementary-Table S8), and all instrument-exposure variables presented expected results in the positive control MR analysis (Supplementary-Fig. S8), and likewise for the total year of schooling (Fig. 2). Genetically predicted unadjusted waist circumference was causally linked to reduced cognition, but not after adjusting for BMI (Supplementary-Table S9). To additionally account for potential VAT SNPs that are in linkage-disequilibrium with BMI SNPs but not identified in the initial clumping, we performed joint-clumping of BMI and VAT, and repeated the MR of VAT on general cognition (Supplementary Methods). The β for VAT remained significant (−0.14 [−0.22; −0.07]) in this repeat MR (Supplementary-Table S10).
The MR-Egger intercept for each metabolic risk factor did not significantly deviate from 0 (Supplementary-Table S5, Supplementary-Table S6). The leave-one-out (Supplementary-Figs. S9–S11) and single-SNP analyses (Supplementary-Figs. S12–S14) for BMI, WHR and VAT also did not reveal outlying pleiotropic variants. In the reverse MR with ‘g’ as instrument-exposure, genetically predicted ‘g’ was associated with lower BMI through 3 SNPs (rs11079849, rs13107325, rs1862451) (Supplementary-Table S11).
Discussion
We show that adiposity is as an independent predictor of cognitive function in a multi-ethnic Asian population, comprising people of East-Asian, South-East-Asian, and South-Asian ancestry. Our MR analyses suggest that the relationships of visceral adiposity, and of raised BMI, with cognition are causal. In contrast, despite strong cross–sectional associations, our MR analyses did not reveal causal evidence for an effect of raised blood pressure, lipid, and poorer glycemic indices on cognitive performance. Our findings provide strong evidence for the importance of healthy weight to protect cognitive function amongst Asians, who represent ∼60% of the global population.
The global burden of dementia is shifting from the western hemisphere to the Asia–Pacific region. Currently, 4 million Asians develop dementia annually (40% of new global cases); this number is expected to double by 2030.25 Understanding and addressing the determinants of cognitive function is a major public health priority for Asia and globally, as dementia not only affects patients and their caregivers, but also has tremendous social and economic impacts with estimated societal cost surpassing US$ 2.8 trillion by 2030.1 Importantly, there is increasing opportunity to detect early-onset dementia, as the UK Biobank participants diagnosed with Alzheimer's Disease were shown to have lower cognitive test scores at baseline 9 years prior.26 Our epidemiological study confirms the relationship of adiposity with cognitive function in Asians, and shows that it is independent of blood pressure, lipid, and glycemic indices. Numerically, a 0.27 Kg increase in visceral or 4.52 kg/m2 general adiposity is associated with a 0.7 years reduction in cognitive age. We note that the prevalence of obesity is ∼7% higher in the Asia–Pacific region than the global average, and rising.27 Prevention and control of obesity in Asian populations may thus play a critical role in maintaining cognitive function, and protecting against the future risk of dementia, and other obesity related chronic diseases.
We pursued two-sample MR to assess the causal relationships of adiposity and related metabolic disturbances with cognitive function. For visceral adiposity, we used genetic instruments from recently reported GWAS for DEXA-based VAT.12 We show that a genetically predicted increase in visceral adiposity is associated with reduced cognition performance as assessed by ‘g’. Our approach extends previous MR studies of cognitive health, which have used genetic instruments from GWAS on waist circumference, a proxy measure of visceral adiposity.28, 29, 30, 31 We also found significant causal effect of elevated BMI on cognitive function. This contrasts previous studies which reported no causal effect of excess BMI on 'g’,28,32 and may reflect our use of more recent GWAS summary statistics for BMI with larger sample size,15 and a higher number of adiposity-related SNPs in both the main and sensitivity analyses enabling better power to detect significant causal effect. MR-Egger analysis did illustrate significant causal effect in the BMI associations with cognition, which may reflect shared genetic variants with VAT,12 although we note plausible reverse causation bias from general cognition towards raised BMI, potentially through rs11079849, rs13107325, rs1862451. Collectively, the selected GWAS instruments and our sensitivity analyses including MR Egger, multivariable MR, and many others have addressed common sources of assumption violations in MR such heterogeneity, pleiotropy, bidirectionality, and power.13 Together, our MR studies provide strong support for the hypothesis that visceral and generalised adiposity may play a causal role in determining cognitive health. The potential mechanisms underlying an effect of excess adiposity on cognition are uncertain. Adipocyte turnover in visceral cavity influences circulating levels of fatty-acids, lipoproteins, and adipocytokines,33 which could cross the blood brain barrier and disrupt the homeostasis of cellular signaling, synaptic plasticity and memory, and maintenance of various neuronal cells.34
Our MR investigation showed no evidence for blood pressure, lipid trait or glycemic indices, as determinants of cognitive function. This agrees with prior MR exercises using single metabolic phenotype as instrument-exposure.29,30 The absence of causal relationship of blood pressure, cholesterol, or glycemic indices on cognitive function may help explain the inconsistent results of interventions studies targeting these risk factors for maintenance of cognitive performance.4,5 We do note a previous MR study that reports a potential causal relationship between raised blood pressure and cognition31; however, the genetically predicted cognition in this study was derived from domain-specific cognitive assessments instead of general cognition ‘g’. This may merit further analysis of causal relationship between adiposity, metabolic risk factors and specific cognitive domains.
Our study has some limitations. The associations of metabolic phenotypes and cognition could be attributed as a byproduct of metabolic diseases, although the sensitivity analyses in a subset of participants not on any medication argued against confounding by disease treatment. The cross-sectional nature of our epidemiological analysis limited the extrapolation on cognitive decline; nevertheless, the associations of composite metabolic measures with reduced cognitive function remained congruent with prior meta-analysis reporting dose-dependent effect of risk factors for cognitive decline.3,9 The use of computerized cognitive test, whilst suitable for a large epidemiological study, might have disadvantaged those with lower digital proficiency, who were likely to be older, female, and ethnic minority. Given that these subgroups had historically had less opportunities for higher education,35 our findings might have understated the full potential cognitive health burden. We also did not perform one-sample MR using multi-ethnic Asian dataset, although we used trans-ancestry GWAS summary statistics where available. Further, no significant trans-ancestry heterogeneity was observed in 80% of 242 glycemic trait loci,36 75% of major lipid loci,37 and 505 blood pressure loci,38 suggesting that, at least for these phenotypes, findings from the GWAS summary statistics could be applicable to the non-European population. The use of SNPs obtained from GWAS adjusted for heritable covariate, as in the BMI-adjusted-waist and -WHR GWAS, might introduce bias39 but here we also used the summary statistics of imaging-based visceral fat. As we used publicly available summary statistics, we were unable to prevent participant overlaps to reduce bias,14 but we did calculate potential bias due to participant overlap,23 and repeated the MR for each instrument-exposure after removing SNPs predicting alternative phenotype as part of sensitivity analyses. Finally, MR inferences are also limited in temporality and linearity as it provides linear, lifetime estimates of risk.13
Despite the limitations, our study has notable strengths. We used both epidemiological and genetic evidence to test our hypothesis to triangulate evidence and observed consistent results. Our population cohort comprises the under-investigated individuals of Asian ancestry. Unlike many cognitive studies which tend to recruit older participants from healthcare settings, we recruited from the general population and included relatively younger participants, who are otherwise healthy and are therefore less likely to have developed cognitive decline, thus reducing the likelihood to over-estimate the associations of risk factors on ‘g’. Additionally, the inclusion of working-age population highlights the early impact of excess adiposity on cognition and present an opportunity to prevent future cognitive decline. Finally, we employed DEXA-based whole-body quantification of adiposity in both the epidemiological and the two-sample MR. DEXA more accurately quantifies VAT and other fat depots than conventional anthropometry measures,40 enabling us to distinguish the effect of VAT from other fat depot and unravel unique etiology of visceral adiposity in metabolic health.41
Conclusion
Our study provides evidence that excess adiposity is an independent and causal determinant of cognitive function in Asian populations. Prevention of excess adiposity could help maintain or improve cognitive function and reduce the risk of future dementia. Given the rapidly rising burden of obesity across the Asia Pacific region, our findings have important implications for global health.
Data sharing statement
Any data access request proposals should be directed to helios_science@ntu.edu.sg for the consideration of the HELIOS Study's principal investigators.
Declaration of interests
J.C receives support for attending meetings and travel from Lee Kong Chian School of Medicine Strategic Academic Initiative and/or National Medical Research Council Singapore Translational Research Investigator Award and/or President's Chair in Cardiovascular Epidemiology. J.C. is Programme Director for Population and Global Health Programme at Lee Kong Chian School of Medicine, and Chief Scientific Officer at Precision Health Research, Singapore. J.N. receives research and educational grant from Astra Zeneca for cancer research unrelated to this work. All other authors declare no conflict of interest.
Acknowledgements
We are very grateful to the outstanding support of past and present members of the HELIOS study steering committee, operational study team, and administrative staff for driving the study and assisting in the data collection. Many thanks to Rob Elliott for sharing the Airwave study protocol and assisting in the installation of the cognitive tests; Dr. P. Kumarasan Roystonn and Dr. Anitha Jeyagurunathan (Institute of Mental Health, Singapore) for guidance on cognitive interviewing and in conducting the cognitive interview of the HELIOS cognitive test in Tamil; Dr. Ramiro Magno for guidance in troubleshooting the interfacing with GWAS catalog through gwasrapidd.
This work was supported by intramural funding from Nanyang Technological University, Lee Kong Chian School of Medicine, and the National Healthcare Group. J.C. is supported by: the Singapore Ministry of Health and National Medical Research Council STaR funding scheme [NMRC/StaR/0028/2017], LCG funding [MOH-000271], and Phase 2 National Precision Medicine Programme (Research Platform and Data Enablers) [NMRC/PRECISE/2020]; and the Singapore Agency for Science Technology and Research IAF-PP National Precision Medicine Program Phase 1A (A Population Level Genomic Infrastructure) [H17/01/a0/007] and IAF-PP Asian Skin Microbiome Programme [H18/01/a0/016]. T.M. received postdoctoral fellowship from the Singapore Ministry of Education – Research Scholarship Block and Lee Kong Chian School of Medicine. T.M. and J.C. had full access to all the data in this study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.lanwpc.2023.100710.
Appendix A. Supplementary data
References
- 1.World Health Organization . 2020. Dementia.https://www.who.int/news-room/fact-sheets/detail/dementia [Google Scholar]
- 2.Livingston G., Huntley J., Sommerlad A., et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet. 2020;396:413–446. doi: 10.1016/S0140-6736(20)30367-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Siervo M., Harrison S.L., Jagger C., Robinson L. Metabolic syndrome and longitudinal changes in cognitive function: a systematic review and meta-analysis. J Alzheim Dis. 2014;41:151–161. doi: 10.3233/JAD-132279. [DOI] [PubMed] [Google Scholar]
- 4.Rapp S.R., Gaussoin S.A., Sachs B.C., et al. Effects of intensive versus standard blood pressure control on domain-specific cognitive function: a substudy of the SPRINT randomised controlled trial. Lancet Neurol. 2020;19:899–907. doi: 10.1016/S1474-4422(20)30319-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mcguinness B., Craig D., Bullock R., Passmore P. Statins for the prevention of dementia. Cochrane Database Syst Rev. 2016;2016 doi: 10.1002/14651858.CD003160.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chan S.H., Bylstra Y., Teo J.X., et al. Analysis of human disease variants from ancestrally diverse Asian genomes. Nat Commun. 2022;13(1):6694. doi: 10.1038/s41467-022-34116-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Davies G., Lam M., Harris S.E., et al. Study of 300,486 individuals identifies 148 independent genetic loci influencing general cognitive function. Nat Commun. 2018;9:1–16. doi: 10.1038/s41467-018-04362-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fawns-Ritchie C., Deary I.J. Reliability and validity of the UK Biobank cognitive tests. PLoS One. 2020;15:1–24. doi: 10.1371/journal.pone.0231627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lipnicki D.M., Makkar S.R., Crawford J.D., et al. Determinants of cognitive performance and decline in 20 diverse ethno-regional groups: a COSMIC collaboration cohort study. PLoS Med. 2019;16:1–27. doi: 10.1371/journal.pmed.1002853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.VanItallie T.B., Yang M.U., Heymsfield S.B., Funk R.C., Boileau R.A. Height-normalized indices of the body's fat-free mass and fat mass: potentially useful indicators of nutritional status. Am J Clin Nutr. 1990;52:953–959. doi: 10.1093/ajcn/52.6.953. [DOI] [PubMed] [Google Scholar]
- 11.Alberti K.G.M.M., Eckel R.H., Grundy S.M., et al. Harmonizing the metabolic syndrome: a joint interim statement of the International diabetes Federation Task Force on Epidemiology and prevention; National heart, Lung, and blood Institute; American heart association; World heart Federation; International. Circulation. 2009;120:1640–1645. doi: 10.1161/CIRCULATIONAHA.109.192644. [DOI] [PubMed] [Google Scholar]
- 12.Karlsson T., Rask-Andersen M., Pan G., et al. Contribution of genetics to visceral adiposity and its relation to cardiovascular and metabolic disease. Nat Med. 2019;25:1390–1395. doi: 10.1038/s41591-019-0563-7. [DOI] [PubMed] [Google Scholar]
- 13.Wootton R.E., Jones H.J., Sallis H.M. Mendelian randomisation for psychiatry: how does it work, and what can it tell us? Mol Psychiatr. 2022;27:53–57. doi: 10.1038/s41380-021-01173-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hartwig F.P., Davies N.M., Hemani G., Smith G.D. Two-sample Mendelian randomization: avoiding the downsides of a powerful, widely applicable but potentially fallible technique. Int J Epidemiol. 2016;45:1717–1726. doi: 10.1093/ije/dyx028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yengo L., Sidorenko J., Kemper K.E., et al. Meta-analysis of genome-wide association studies for height and body mass index in ∼700 000 individuals of European ancestry. Hum Mol Genet. 2018;27:3641–3649. doi: 10.1093/hmg/ddy271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shungin D., Winkler T., Croteau-Chonka D.C., et al. New genetic loci link adipose and insulin biology to body fat distribution. Nature. 2015;518:187–196. doi: 10.1038/nature14132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mitchell R., Hemani G., Dudding T., Corbin L., Harrison S., Paternoster L. 2019. MRC IEU UK Biobank GWAS pipeline version 2. [DOI] [Google Scholar]
- 18.Willer C.J., Schmidt E.M., Sengupta S., et al. Discovery and refinement of loci associated with lipid levels. Nat Genet. 2013;45:1274–1285. doi: 10.1038/ng.2797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen J., Spracklen C.N., Marenne G., et al. The trans-ancestral genomic architecture of glycemic traits. Nat Genet. 2021;53:840–860. doi: 10.1038/s41588-021-00852-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee J.J., Wedow R., Okbay A., et al. Gene discovery and polygenic prediction from a genome-wide association study of educational attainment in 1.1 million individuals. Nat Genet. 2018;50:1112–1121. doi: 10.1038/s41588-018-0147-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nikpay M., Goel A., Won H.H., et al. A comprehensive 1000 Genomes-based genome-wide association meta-analysis of coronary artery disease. Nat Genet. 2015;47:1121–1130. doi: 10.1038/ng.3396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Burgess S., Thompson S.G. Avoiding bias from weak instruments in mendelian randomization studies. Int J Epidemiol. 2011;40:755–764. doi: 10.1093/ije/dyr036. [DOI] [PubMed] [Google Scholar]
- 23.Burgess S., Davies N.M., Thompson S.G. Bias due to participant overlap in two-sample Mendelian randomization. Genet Epidemiol. 2016;40:597–608. doi: 10.1002/gepi.21998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Larsson S.C., Traylor M., Malik R., Dichgans M., Burgess S., Markus H.S. Modifiable pathways in Alzheimer's disease: Mendelian randomisation analysis. BMJ. 2017;359:j5375. doi: 10.1136/bmj.j5375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Prince M., Wimo A., Guerchet M., Ali G.-C., Wu Y.-T., Prina M. Alzheimer's Disease International; 2015. World Alzheimer Report 2015: The Global Impact of Dementia; pp. 1–87. World Alzheimer’s Report. [Google Scholar]
- 26.Swaddiwudhipong N., Whiteside D.J., Hezemans F.H., Street D., Rowe J.B., Rittman T. Pre-diagnostic Cognitive and functional impairment in multiple sporadic Neurodegenerative diseases. Alzheimer's Dementia. 2022:1–22. doi: 10.1002/alz.12802. [DOI] [PubMed] [Google Scholar]
- 27.Global Health Observatory . World Health Organization; 2016. Prevalence of obesity among adults, BMI ≥ 30 (age-standardized estimate) (%)https://www.who.int/data/gho/data/indicators/indicator-details/GHO/prevalence-of-obesity-among-adults-bmi-=-30-(age-standardized-estimate)-(-) [Google Scholar]
- 28.Wang S.H., Su M.H., Chen C.Y., et al. Causality of abdominal obesity on cognition: a trans-ethnic Mendelian randomization study. Int J Obes. 2022 doi: 10.1038/s41366-022-01138-8. [DOI] [PubMed] [Google Scholar]
- 29.Fu M., Bakulski K.M., Higgins C., Ware E.B. Mendelian randomization of dyslipidemia on cognitive impairment among older Americans. Front Neurol. 2021;12 doi: 10.3389/fneur.2021.660212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Garfield V., Farmaki A.-E., Fatemifar G., et al. Relationship between glycemia and cognitive function, structural brain outcomes, and dementia: a Mendelian randomization study in the UK Biobank. Diabetes. 2021;70:2313–2321. doi: 10.2337/db20-0895. [DOI] [PubMed] [Google Scholar]
- 31.Sun D., Thomas E.A., Launer L.J., Sidney S., Yaffe K., Fornage M. Association of blood pressure with cognitive function at midlife: a Mendelian randomization study. BMC Med Genom. 2020;13:1–9. doi: 10.1186/s12920-020-00769-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hagenaars S.P., Gale C.R., Deary I.J., Harris S.E. Cognitive ability and physical health: a Mendelian randomization study. Sci Rep. 2017;7:1–7. doi: 10.1038/s41598-017-02837-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Morys F., Dadar M., Dagher A. Association between midlife obesity and its metabolic consequences, cerebrovascular disease, and cognitive decline. J Clin Endocrinol Metab. 2021;106:E4260–E4274. doi: 10.1210/clinem/dgab135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vitali C., Wellington C.L., Calabresi L. HDL and cholesterol handling in the brain. Cardiovasc Res. 2014;103:405–413. doi: 10.1093/cvr/cvu148. [DOI] [PubMed] [Google Scholar]
- 35.Ong J. S’pore population better educated across age, ethnicity; women make greater strides. The Strait Times; 2021. S’pore population better educated across age, ethnicity; women make greater strides. published online June 16. [Google Scholar]
- 36.Scott R.A., Lagou V., Welch R.P., et al. Large-scale association analyses identify new loci influencing glycemic traits and provide insight into the underlying biological pathways. Nat Genet. 2012;44:991–1005. doi: 10.1038/ng.2385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kuchenbaecker K., Telkar N., Reiker T., et al. The transferability of lipid loci across African, Asian and European cohorts. Nat Commun. 2019;10 doi: 10.1038/s41467-019-12026-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Giri A., Hellwege J.N., Keaton J.M., et al. Trans-ethnic association study of blood pressure determinants in over 750,000 individuals. Nat Genet. 2019;51:51–62. doi: 10.1038/s41588-018-0303-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Aschard H., Vilhjálmsson B.J., Joshi A.D., Price A.L., Kraft P. Adjusting for heritable covariates can bias effect estimates in genome-wide association studies. Am J Hum Genet. 2015;96:329–339. doi: 10.1016/j.ajhg.2014.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vasan S.K., Osmond C., Canoy D., et al. Comparison of regional fat measurements by dual-energy X-ray absorptiometry and conventional anthropometry and their association with markers of diabetes and cardiovascular disease risk. Int J Obes. 2018;42:850–857. doi: 10.1038/ijo.2017.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Karpe F., Pinnick K.E. Biology of upper-body and lower-body adipose tissue - link to whole-body phenotypes. Nat Rev Endocrinol. 2015;11:90–100. doi: 10.1038/nrendo.2014.185. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.