Abstract
Introduction and importance
Uterine fibroids, benign tumors of the myometrium, can cause pelvic pain. Obesity and diabetes mellitus can increase the risk of developing fibroid. We present two cases of uterine fibroid, diabetes mellitus, and obesity with moderate-to-severe chronic pain.
Case presentation
The first case is a 37-year-old woman with pelvic pain and a subserosal uterine fibroid, primary infertility, grade 2 obesity, and diabetes mellitus. Pathologic examination revealed smooth muscle cells with degeneration sites. The second case is a 35-year-old nulliparous woman with abdominal enlargement, lower abdominal pain, diabetes mellitus, and morbid obesity. Ultrasonography showed a large uterus with a hyperechoic mass and cystic degeneration. Histopathological examination revealed leiomyoma.
Clinical discussion
Our patient's chronic pelvic pain may be caused by its large size. Excess adipose tissue in obesity may result in the formation of estrone, causing the proliferation of fibroids. A subserous fibroid is less likely to cause infertility; thus, a myomectomy was performed to relieve pain. Obesity and diabetes could interfere with patients' periods. Higher levels of insulin and fat tissue induce androgen production. Increased estrogen levels lead to alteration of gonadotropin production, menstrual abnormalities, and ovulatory dysfunction.
Conclusion
Cystic degeneration of the subserous uterine fibroid could induce pain though it rarely affects fertility. A myomectomy was conducted to relieve pain. Comorbid diseases such as diabetes mellitus and obesity can lead to cystic degeneration of the uterine fibroid.
Abbreviations: SCARE, Surgical CAse REport; VAS, visual analog scale; BMI, body mass index; HSG, hysterosalpingography; ROS, reactive oxygen species; ART, assisted reproduction technology; HPO, hypothalamus-pituitary-ovarian
Keywords: Subserosal uterine fibroid, Cystic degeneration, Pelvic pain, Type 2 diabetes, Obesity, Case report
Highlights
-
•
Uterine fibroid is the most common benign tumor of myometrium.
-
•
Uterine fibroid may be associated with obesity and diabetes mellitus.
-
•
Subserosal fibroid may induce pain and is less likely to cause infertility.
-
•
Comorbids such as obesity and diabetes mellitus could play a role in infertility.
1. Introduction
The most prevalent tumor in women of reproductive age is uterine fibroid. Although half of cases are asymptomatic, the rest can cause significant symptoms that affect daily activities. The symptoms are menstrual abnormalities, noncyclic pelvic pain, pressure symptoms, and reproductive function disturbances [1]. Uterine fibroids are estrogen and progesterone-dependent. Several factors are associated with a higher risk of developing fibroids, including obesity, polycystic ovarian syndrome, and diabetes mellitus [2]. We present two cases of women suffering from uterine fibroid, type 2 diabetes mellitus, and obesity with chronic moderate-severe pain and abdominal mass. This work has been reported in line with the Surgical CAse REport (SCARE) 2020 criteria [3].
2. Presentation of case
2.1. Case 1
A 37-year-old woman came to our institute with chief complaints of abdominal mass and noncyclic pelvic pain on a 7–8 Visual Analog Scale (VAS) in the last four months before admission. The patient had menarche at 13 with a regular menstrual cycle, except in March–June 2020, when she missed the period. She had primary infertility for five years, while her husband had two children from the previous marriage. She had type 2 diabetes mellitus with the current medication of metformin 500 mg three times daily.
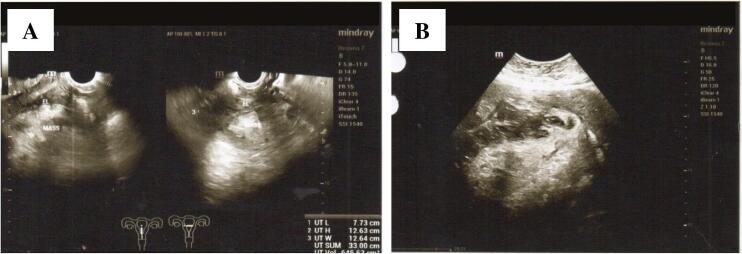
On physical examination, a solid abdominal mass was palpable one finger below the navel. Her body mass index (BMI) was 32.1 kg/m2. Laboratory results are shown in Table 1. A transvaginal sonogram showed an enlarged uterus with a hyperechoic-hypoechoic mass sized 88 × 99 × 96 mm and no vascularization, which was suspected to be a subserous myoma. Hysterosalpingography (HSG) showed bilateral tubal patency with an arcuate-shaped uterus (Fig. 1). Semen analysis results of the husband revealed evidence of oligoasthenozoospermia.
Table 1.
Laboratory profile of the patient.
| Value | |
|---|---|
| Hemoglobin (g/dL) | 14,3 |
| Leucocyte (103/μL) | 12.98 |
| Neutrophile (%) | 55,8 |
| Lymphocyte count (%) | 34,1 |
| Random blood glucose (mg/dL) | 310 |
| Fasting blood glucose (mg/dL) | 165 |
| Two hours post-meal blood glucose (mg/dL) | 314 |
| Glucose tolerance test (mg/dL) | 165/314 |
| HbA1c (%) | 9,2 |
| Fasting insulin (mIU) | 20,5 |
| Total cholesterol (mg/dL) | 233 |
| Triglyceride (mg/dL) | 88 |
| High density lipoprotein (mg/dL) | 35 |
| Low density lipoprotein (mg/dL) | 175 |
| Aspartate transaminase (U/L) | 18 |
| Alanine transaminase (U/L) | 28 |
| Ureum (mg/dL) | 24 |
| Creatinine (mg/dL) | 0,50 |
| Total protein (g/dL) | 8,1 |
| Albumin (g/dL) | 4,71 |
| Globulin (g/dL) | 3,39 |
| Procalcitonin (ng/mL) | 0,02 |
| C-reactive protein (mg/mL) | 6,8 |
| Estradiol (pg/mL) | 81,9 |
| Follicle stimulating hormone (mIU/mL) | 2,8 |
| Luteinizing hormone (mIU/mL) | 4,8 |
| Prolactin (ng/mL) | 11,2 |
| Thyroid stimulating hormone (IU/mL) | 1,8 |
| Free T4 (ng/mL) | 0,9 |
| Anti-Mullerian Hormone (ng/mL) | 2,8 |
| Urinalysis | Macroscopic: cloudy Microscopic: leucocyte 3–4, erythrocyte 1–2, bacteria (+), albumin +2 |
Fig. 1.
Radiology examination of the patient. (A) Transvaginal sonogram showed a hyperechoic-hypoechoic mass in the serous layer of the uterus corresponding to a subserous myoma. (B) Hysterosalpingography indicated an arcuate-shaped uterus and bilateral tubal patency. The uterus position was located slightly on the left side.
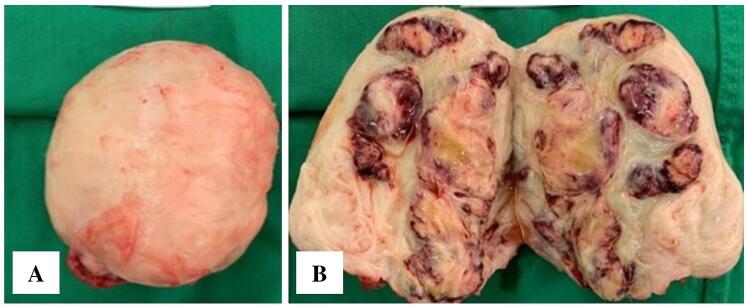
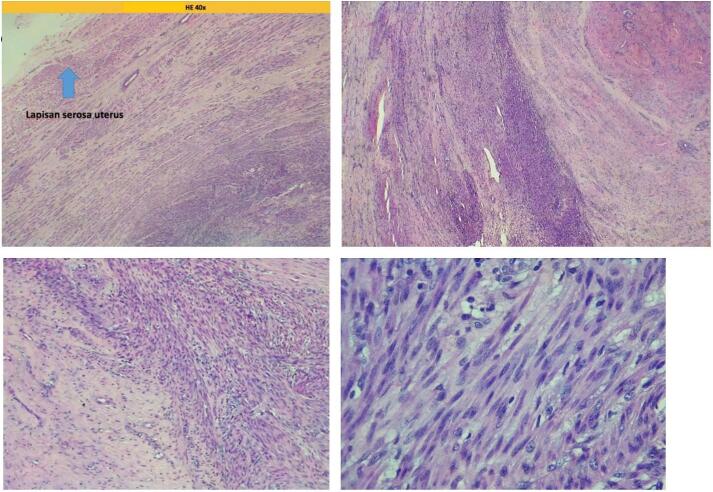
The patient was diagnosed with a subserosal uterine fibroid with five years of primary infertility, obesity grade 2, type 2 diabetes mellitus, and pelvic pain. Laparotomy, myomectomy, and chromotubation were performed by a team of experienced obstetricians/gynecologists. During the chromotubation procedure, the right tube was found to be non-patent. The specimen taken is shown in Fig. 2. Pathologic examination revealed irregular and interlaced smooth muscle cells with hemorrhagic and degenerative sites and scanty lymphocytes. No mitotic division was found in the specimen (Fig. 3).
Fig. 2.
(A) Gross morphology of the fibroid specimen taken out from the patient. (B) Macroscopic appearance of cut surface showing fibroid mass with cystic degeneration.
Fig. 3.
Pathologic examination revealed irregular and interlacing smooth muscle cells appearance with hemorrhagic and degeneration sites as well as scanty lymphocytes.
2.2. Case 2
A 35-year-old nulliparous woman complained of abdominal enlargement for four years with lower abdominal pain that radiated to the legs. She had a regular menstrual cycle and no dysmenorrhea. She had a history of diabetes mellitus and hypertension. She was also morbidly obese, with a BMI of 40.1 kg/m2. Medications used include glimepiride, metformin, bisoprolol, candesartan, and amlodipine. The patient has yet to marry. An abdominal examination revealed a massive, mobile mass that was palpable until halfway between the umbilical and xiphoid process, with no tenderness. The external genitalia were normal. The laboratory results were good, with controlled blood glucose.
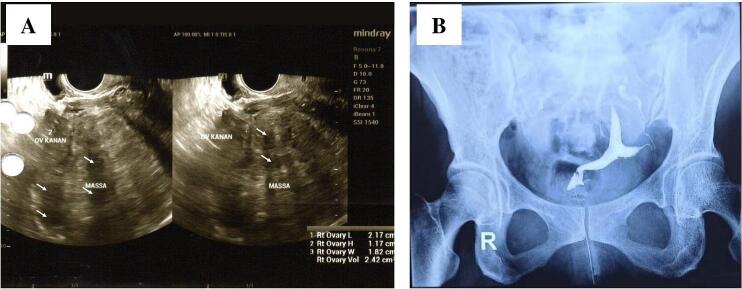
Ultrasonography showed a large uterus sized 15.5 × 20.24 × 31 cm with a hyperechoic, well-defined mass on the posterior corpus sized 14.05 × 10.51 × 21.46 cm. We also discovered cystic degeneration with a necrotic area and ring of color appearance. The endometrial line was within the normal limit. The left ovary cannot be visualized. Multiloculated right ovarian cyst sized 4.13 × 3.58 × 6.21 cm without solid part (Fig. 4).
Fig. 4.
(A) Transvaginal sonogram showed a hyperechoic-hypoechoic mass. (B) Transabdominal sonogram showed cystic degeneration with necrotic area and ring of color appearance.
A hysteroscopic diagnostic examination revealed a thin and regular endometrium with no mass or hypervascularization. The pathology revealed endometrial tissue with hormonal imbalance towards progesterone and polypoid growth—no visible signs of malignancy. Abdominal CT with contrast revealed a calcified solid mass at the fundal-posterosuperior part of the uterus. The mass pushed the intestine laterally, the left lobe liver to the right, and obliterated the inferior cava vein. The differential diagnosis was a subserous fibroid.
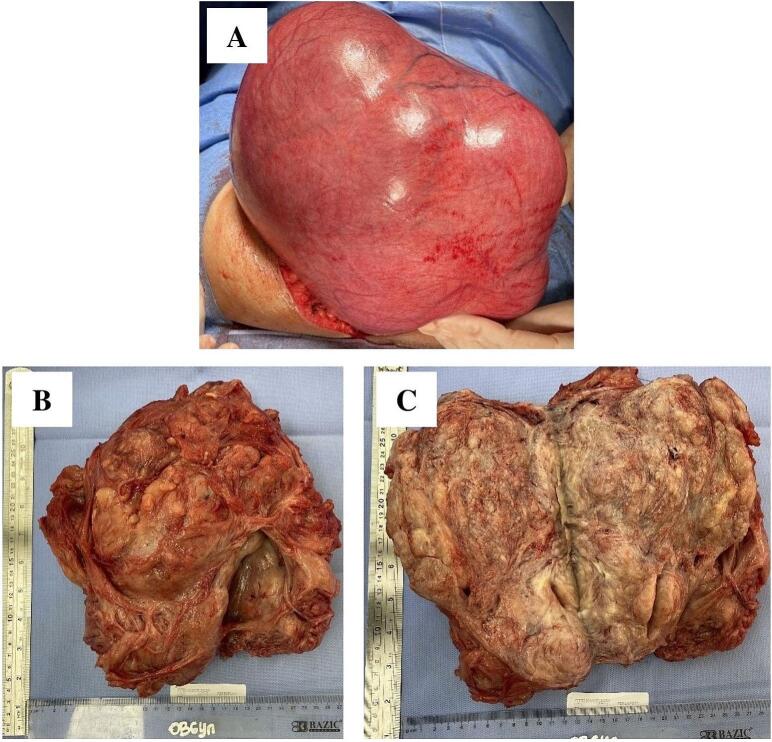
She underwent a laparotomy with a midline incision. There was a lobulated mass sized 28 × 20 × 15 cm arising from the uterus with cystic degeneration (Fig. 5). Ovaries and adnexa on both sides were visualized and found to be normal. There was no adhesion or ascites. A tourniquet was applied around the uterine vessel on the lower part of the uterus. Vasopressin was given, followed by myomectomy. The blood loss during surgery has been estimated at 800 ml, and two units of packed red blood cells were transfused. The patient was discharged three days after the operation in good condition. Histopathological examination revealed a uterine leiomyoma.
Fig. 5.
(A) Lobulated enlarged uterus. (B) and (C) Macroscopic appearance of cut surface showing fibroid mass also showed a cystic degeneration.
3. Discussion
The first patient had primary infertility for five years and chronic moderate-to-severe noncyclic pelvic pain due to a subserous fibroid. Both patients had obesity grade 2 and type 2 diabetes mellitus. Women with fibroids tend to have a higher BMI [4]. A study showed that women with fibroids are more likely to suffer moderate to severe pain, dyspareunia and noncyclic pelvic pain. Compared to the submucous and subserous types, the intramural fibroid tends to cause moderate to severe pain, primarily when it is located in the fundus area [5]. In subserous fibroid, severe pain is mostly caused by torsion of the pedunculated fibroids. The surrounding organs also play a role in fixing the position of the fibroid and may cause ischemia, which leads to abdominal pain [6]. In our patient, chronic pelvic pain was considered to be caused by the fibroid's large size, which had a mechanical effect as it pushed the surrounding structures [1]. The initial size of the fibroid might serve as a predictor for its growth rate. Interestingly, the fibroid growth rate is not constant but depends on hormonal changes [7]. In the first case, the results of HSG and chromotubation on tubal patency were contradictory. A study revealed that intravasation of the contrast to the veins could happen in the HSG, resulting in a false negative reading [8]. Although chromotubation is the current gold standard for assessing tubal patency, a false positive result may occur due to the cornual spasm [9].
Uterine fibroid can develop because of metabolic syndrome issues. Changes in the metabolism of sex hormones in obese women can occur due to excess adipose tissue that plays a role in the formation of estrone from androgens. Uterine fibroids can proliferate because of excess estrogen production. The production of inflammatory cytokines and adipokines increases, causing reactive oxygen species (ROS) formation. ROS cause uterine fibroids through excessive cell growth and inhibition of cell death [10]. A higher risk of developing uterine fibroids was found in women with hyperglycaemia, according to a study in Korea [11].
Whether a fibroid could cause infertility depends mainly on its location throughout the uterus. Patients with a subserous fibroid have similar implantation, pregnancy, and live births rates as those without fibroids [12]. Even in the assisted reproduction technology (ART) setting, only the submucosal and intramural fibroids affect the pregnancy rate. It is thought that both submucosal and intramural fibroids change myometrial contractility and endometrial vascularization through dysregulation of the secretion of either growth or angiogenic factors, thus leading to implantation failure [13]. Hence, myomectomy, in this case, aimed to relieve the pain due to mechanical pressure.
However, to preserve fertility function and avoid surgery, medical therapy remains the first choice in managing uterine fibroids [14]. Medical therapy for uterine fibroids includes estroprogestestogens, tranexamic acid, levonorgestrel-releasing intrauterine system (LNG-IUS), selective progesterone receptor modulators (SPRMs), mifepristones, gonadotropin-releasing hormone (GnRH) agonists and antagonists. Each of these drugs has its advantages and disadvantages. GnRH agonists (leuprolide, goserelin, triptorelin) can be given for 3–6 months to reduce the size of fibroids before surgery. However, menopausal symptoms such as hot flushes and decreased bone density may happen with long-term use of more than six months. While the use of SPRMs does not have a menopausal effect, monitoring liver enzymes is necessary due to a history of liver failure after using ulipristal acetate (UPA) reviewed by European Medicines Agency (EMA). Its use is limited in which intermittent therapy of uterine fibroids with UPA at a dose of 5 mg may be reserved for women with moderate to severe symptoms if surgical treatment is not feasible [15].
Although menstrual bleeding is one of the most common symptoms of uterine fibroids, our patient had no period since six months before admission. There are two possible arguments. First, menstrual bleeding is a common symptom of a submucous fibroid, not a subserous one [16]. The second and most likely reason is that obesity and diabetes could interfere with her periods. According to the International Diabetes Federation criteria, the patient could also be diagnosed with metabolic syndrome [17]. Either obesity, diabetes mellitus, or metabolic syndrome could lead to amenorrhea by interfering with the hypothalamus-pituitary-ovarian (HPO) axis. The higher level of circulating insulin and the excess peripheral fat tissue induces androgen production in the ovaries. The increased estrogen level, as a result of androgen aromatization, gives negative-feedback to the HPO axis, leading to altered gonadotropin production, menstrual abnormalities, and ovulatory dysfunction [18].
To recover the ovulatory menstrual cycle and achieve pregnancy, the first important step is weight loss. Studies have shown that some patients became pregnant six months after completing a weight loss program and successfully delivered the babies at term; some patients also received clomiphene citrate [19]. Furthermore, the utilization of ART does not simply solve infertility caused by obesity because obesity reduces the live birth rates in the ART cycle [20]. Hence, a weight loss program is a must for this patient to achieve pregnancy.
4. Conclusion
Cystic degeneration and mechanical effect of the subserous uterine fibroid may cause chronic pain, but it rarely affects fertility. Myomectomy was conducted to relieve pain. Other comorbid diseases such as diabetes mellitus, obesity, and metabolic syndrome may be the leading cause of infertility for this patient due to the alteration of the HPO axis. Thus, before starting any fertility treatment, it is best to advise the patient to lose weight.
Consent
Written informed consent was obtained from the patient for publication of this case series and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.
Ethical approval
This study is exempt from ethical approval.
Funding
N/A.
Guarantor
Mila Maidarti, MD, PhD.
Research registration number
NA.
CRediT authorship contribution statement
Mila Maidarti: Conceptualization, Methodology, Formal analysis, Investigation, Writing – original draft, Writing – review & editing. Beta Andewi Resti Anggraheny: Formal analysis, Investigation, Writing – original draft. Sarah Safira Umarghanies: Formal analysis, Investigation, Writing – original draft. Prini Diandara Garinasih: Formal analysis, Investigation, Writing – original draft. Achmad Kemal Harzif: Methodology. Octaviyana Nadia Nitasari Simatupang: Formal analysis, Investigation, Writing – original draft.
Declaration of competing interest
None.
References
- 1.Gupta S., Jose J., Manyonda I. Clinical presentation of fibroids. Best Pract. Res. Clin. Obstet. Gynaecol. 2008;22(4):615–626. doi: 10.1016/j.bpobgyn.2008.01.008. [DOI] [PubMed] [Google Scholar]
- 2.Okolo S. Incidence, aetiology and epidemiology of uterine fibroids. Best Pract. Res. Clin. Obstet. Gynaecol. 2008;22(4):571–588. doi: 10.1016/j.bpobgyn.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 3.Agha R.A., Franchi T., Sohrabi C., Mathew G., Kerwan A., Group S The SCARE 2020 guideline: updating consensus Surgical CAse REport (SCARE) guidelines. Int J Surg. 2020;84:226–230. doi: 10.1016/j.ijsu.2020.10.034. [DOI] [PubMed] [Google Scholar]
- 4.Bizjak T., Turkanovic A., But I. Prevalence and risk factors of uterine fibroids in north-East Slovenia. Gynecol. Obstet. 2016;6(350) [Google Scholar]
- 5.Lippman S.A., Warner M., Samuels S., Olive D., Vercellini P., Eskenazi B. Uterine fibroids and gynecologic pain symptoms in a population-based study. Fertil. Steril. 2003;80(6):1488–1494. doi: 10.1016/s0015-0282(03)02207-6. [DOI] [PubMed] [Google Scholar]
- 6.Tsai Y.-J., Yeat S.-K., Jeng C.-J., Chen S.-C. Torsion of a uterine leiomyoma. Taiwan J. Obstet. Gynecol. 2006;45(4):333–335. doi: 10.1016/S1028-4559(09)60254-0. [DOI] [PubMed] [Google Scholar]
- 7.Mavrelos D., Ben-Nagi J., Holland T., Hoo W., Naftalin J., Jurkovic D. The natural history of fibroids. Ultrasound Obstet. Gynecol. 2010;35(2):238–242. doi: 10.1002/uog.7482. [DOI] [PubMed] [Google Scholar]
- 8.Dutta S., Mazumder P., Mishra D., Saha J.K. Study on comparative diagnostic efficacy of HSG & laparoscopy in infertility. J. Evol. Med. Dent. Sci. 2020;9(12):937–942. [Google Scholar]
- 9.Dun E.C., Nezhat C.H. Tubal factor infertility: diagnosis and management in the era of assisted reproductive technology. Obstet. Gynecol. Clin. N. Am. 2012;39(4):551–566. doi: 10.1016/j.ogc.2012.09.006. [DOI] [PubMed] [Google Scholar]
- 10.Ilaria S., Marci R. From obesity to uterine fibroids: an intricate network. Curr. Med. Res. Opin. 2018;34(11):1877–1879. doi: 10.1080/03007995.2018.1505606. [DOI] [PubMed] [Google Scholar]
- 11.Tak Y.J., Lee S.Y., Park S.K., Kim Y.J., Lee J.G., Jeong D.W., et al. Association between uterine leiomyoma and metabolic syndrome in parous premenopausal women: a case-control study. Medicine (Baltimore) 2016;95(46) doi: 10.1097/MD.0000000000005325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pritts E.A., Parker W.H., Olive D.L. Fibroids and infertility: an updated systematic review of the evidence. Fertil. Steril. 2009;91(4):1215–1223. doi: 10.1016/j.fertnstert.2008.01.051. [DOI] [PubMed] [Google Scholar]
- 13.Eldar-Geva T., Meagher S., Healy D.L., MacLachlan V., Breheny S., Wood C. Effect of intramural, subserosal, and submucosal uterine fibroids on the outcome of assisted reproductive technology treatment. Fertil. Steril. 1998;70(4):687–691. doi: 10.1016/s0015-0282(98)00265-9. [DOI] [PubMed] [Google Scholar]
- 14.Angioni S., D'Alterio M.N., Daniilidis A. Highlights on medical treatment of uterine fibroids. Curr. Pharm. Des. 2021;27(36):3821–3832. doi: 10.2174/1381612826666210101152820. [DOI] [PubMed] [Google Scholar]
- 15.Dolmans M.M., Cacciottola L., Donnez J. Conservative management of uterine fibroid-related heavy menstrual bleeding and infertility: time for a deeper mechanistic understanding and an individualized approach. J. Clin. Med. 2021;10(19) doi: 10.3390/jcm10194389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Puri K., Famuyide A.O., Erwin P.J., Stewart E.A., Laughlin-Tommaso S.K. Submucosal fibroids and the relation to heavy menstrual bleeding and anemia. Am. J. Obstet. Gynecol. 2014;210(1):38.e1-.e7 doi: 10.1016/j.ajog.2013.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alberti K.G., Zimmet P., Shaw J. Metabolic syndrome–a new world-wide definition. A consensus statement from the International Diabetes Federation. Diabetic Med. 2006;23(5):469–480. doi: 10.1111/j.1464-5491.2006.01858.x. [DOI] [PubMed] [Google Scholar]
- 18.Broughton D.E., Moley K.H. Obesity and female infertility: potential mediators of obesity's impact. Fertil. Steril. 2017;107(4):840–847. doi: 10.1016/j.fertnstert.2017.01.017. [DOI] [PubMed] [Google Scholar]
- 19.Rothberg A., Lanham M., Randolph J., Fowler C., Miller N., Smith Y. Feasibility of a brief, intensive weight loss intervention to improve reproductive outcomes in obese, subfertile women: a pilot study. Fertil. Steril. 2016;106(5):1212–1220. doi: 10.1016/j.fertnstert.2016.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Poston L., Caleyachetty R., Cnattingius S., Corvalán C., Uauy R., Herring S., et al. Preconceptional and maternal obesity: epidemiology and health consequences. Lancet Diabetes Endocrinol. 2016;4(12):1025–1036. doi: 10.1016/S2213-8587(16)30217-0. [DOI] [PubMed] [Google Scholar]