Graphical abstract
Keywords: IVUS, OCT, Intravascular, Imaging, PCI, Myocardial infarction, Trend
Abbreviations: aOR, Adjusted odds ratio; BMS, Bare-metal stent; CI, Confidence interval; cOR, Crude odds ratio; DES, Drug-eluting stent; HCUP, Healthcare Cost and Utilization Project; ICD-9-CM, International Classification of Diseases, 9th Revision, Clinical Modification; ICD-10-CM, International Classification of Diseases, 10th Revision, Clinical Modification; ICD-9-PCS, International Classification of Diseases, 9th Revision, Procedural Coding System; ICD-10-PCS, International Classification of Diseases, 10th Revision, Procedural Coding System; IVUS, Intravascular ultrasound; MI, Myocardial infarction; NIS, National Inpatient Sample; NSTEMI, Non-ST-elevation myocardial infarction; OCT, Optical coherence tomography; PCI, Percutaneous coronary intervention; STEMI, ST-elevation myocardial infarction; U.S, United States
Abstract
Background
Intravascular imaging with either intravascular ultrasound (IVUS) or optical coherence tomography (OCT) during percutaneous coronary intervention (PCI) is associated with improved outcomes, but these techniques have previously been underutilized in the real world. We aimed to examine the change in utilization of intravascular imaging-guided PCI over the past decade in the United States and assess the association between intravascular imaging and clinical outcomes following PCI for myocardial infarction (MI).
Methods
We surveyed the National Inpatient Sample from 2008 to 2019 to calculate the number of PCIs for MI guided by IVUS or OCT. Temporal trends were analyzed using Cochran-Armitage trend test or simple linear regression for categorical or continuous outcomes, respectively. Multivariable logistic regression was used to compare outcomes following PCI with and without intravascular imaging.
Results
A total of 2,881,746 PCIs were performed for MI. The number of IVUS-guided PCIs increased by 309.9 % from 6,180 in 2008 to 25,330 in 2019 (P-trend < 0.001). The percentage of IVUS use in PCIs increased from 3.4 % in 2008 to 8.7 % in 2019 (P-trend < 0.001). The number of OCT-guided PCIs increased 548.4 % from 246 in 2011 to 1,595 in 2019 (P-trend < 0.001). The percentage of OCT guidance in all PCIs increased from 0.0 % in 2008 to 0.6 % in 2019 (P-trend < 0.001). Intravascular imaging-guided PCI was associated with lower odds of in-hospital mortality (adjusted odds ratio 0.66, 95 % confidence interval 0.60–0.72, p < 0.001).
Conclusions
Although the number of intravascular imaging-guided PCIs have been increasing, adoption of intravascular imaging remains poor despite an association with lower mortality.
1. Introduction
Intravascular imaging-guided percutaneous coronary intervention (PCI) is associated with lower in-hospital mortality, myocardial infarction (MI), and target-lesion revascularization when compared with conventional angiography-guided PCI [1], [2]). Guidance with intravascular imaging can complement angiographic data by assisting in the assessment of lesion severity, plaque characteristics, and stent optimization [3]. In fact, patients with acute MI may derive the greatest benefit from PCI guided with intravascular imaging [4]. Adoption of intravascular imaging [intravascular ultrasound (IVUS) or optical coherence tomography (OCT)] to guide PCI has remained slow despite consistent evidence of improved outcomes [5], [6]. While patients with acute MI have been underrepresented in prior studies [7], [8], limited data on IVUS-guided PCI in this subset has demonstrated an association with lower in-hospital mortality but limited uptake [9], [10]. As the evidence supporting the use of intravascular imaging to guide PCI in patients with MI has accumulated [11], [12], the longitudinal trajectory of adoption in contemporary practice remains unknown. Furthermore, whether increased utilization of intravascular imaging has translated into improved outcomes in real-world practice is unresolved. Thus, we set out to examine trends in the uptake of intravascular imaging for patients presenting with MI from 2008 to 2019 and undergoing PCI using the largest inpatient database in the United States (U.S.). We also evaluated the association between use of intravascular imaging and in-hospital mortality.
2. Methods
2.1. Data source
Developed by the collaboration of the Healthcare Cost and Utilization Project (HCUP) and the Agency for Healthcare Research and Quality, the National Inpatient Sample (NIS) is the largest inpatient healthcare database consisting of sampled discharges from community hospitals in the U.S., excluding long-term acute care and rehabilitation hospitals [13]. After application of weights, it approximates more than 35 million admissions, representing 97 % of the national population. Beginning in the year 2012, the former Nationwide Inpatient Sample was renamed NIS for sampling all HCUP-participating hospitals in contrast to the previous method of sampling hospitals that retained all their discharges. The NIS protects patient confidentiality by anonymizing all entries, so the database strictly contains de-identified patient information. Therefore, our study was exempt from approval by our institutional review board as only openly available data from the NIS were used. The data that support the findings of this study are readily available on the public website of the HCUP [13].
2.2. Study population and variables
We used International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) and International Classification of Diseases, Tenth Revision, Clinical Modification (ICD-10-CM) who identify all hospital admissions with the primary diagnosis of myocardial infarction from NIS 2008 to 2019. We then used International Classification of Diseases, Ninth Revision, Procedural Coding System (ICD-9-PCS) and International Classification of Diseases, Tenth Revision, Procedural Coding System (ICD-10-PCS) to leave only those in which PCI, defined by the deployment of either a drug-eluting stent (DES) or bare-metal stent (BMS), was performed. ICD-9-CM and ICD-9-PCS codes were used up to September 30, 2015, after which ICD-10-CM and ICD-10-PCS codes were used in accordance with the updated NIS. We additionally used procedural codes to identify PCIs that were guided by intravascular imaging, consisting of IVUS and OCT. To delineate patient characteristics, we extracted information on demographics (sex, age, race), comorbidities (smoking, hypertension, diabetes mellitus, hyperlipidemia, obesity, heart failure, chronic ischemic heart disease, atrial fibrillation, valvular heart disease, peripheral artery disease, previous stroke, previous coronary artery bypass graft, previous pacemaker, chronic pulmonary disease, pulmonary hypertension, chronic kidney disease, end-stage renal disease, liver cirrhosis, history of malignancy, deficiency anemia, malnutrition, dementia, major depression), hospital characteristics (region, bed size, urban location), primary payer, median income by ZIP code, and clinical presentation. We also collected procedural data regarding the use of mechanical thrombectomy, intra-aortic balloon pump, extracorporeal membranous oxygenation, percutaneous left ventricular assist device, durable left ventricular assist device, renal replacement therapy, and mechanical ventilation. A summary of the ICD-9 and ICD-10 codes can be found in Table S1.
2.3. Study outcomes
Primary outcomes of interest included the annual trends in the number and percentage of IVUS-guided PCIs and OCT-guided PCIs from 2008 to 2019. Secondary outcomes included the trend of in-hospital mortality, length of stay, and total hospital cost over the same period. In comparing PCIs with and without intravascular imaging, identical outcomes of in-hospital mortality, length of stay, and total hospital cost were analyzed.
2.4. Statistical analysis
Survey analysis methodology using weights of hospital-level discharge from the NIS was used to calculate nationally representative estimates [14]. The survey weight, DISCWT, was used from NIS 2012 to 2019, and TRENDWT from NIS 2008 to 2011 to provide national estimates for trend analyses that are consistent throughout the entire period [15]. Continuous variables in patient and procedural characteristics were presented as means with standard deviations while categorical variables were summarized as percentages. The annual number of PCIs for myocardial infarction was divided by the total projected U.S. population from the Bureau of the Census to provide the number of PCIs per 100,000 persons [16]. Age-standardized in-hospital mortality rate was calculated based on direct age-standardization method using the projected U.S. population at year 2000 [11], [17], [18]. Cost-to-Charge Ratio files, provided by HCUP, were used to calculate total hospital costs from total hospital charges. Total hospital costs were then adjusted for inflation to 2014 U.S. dollars based on the medical care component of the U.S. Consumer Price Index.
Temporal trends in patient and procedural characteristics were examined used Cochran-Armitage trend test for categorical variables and simple linear regression test for continuous variables. Trends of continuous outcomes, including number of PCIs, length of stay, and total hospital cost, were analyzed using simple linear regression test. Poisson regression was used to assess the trend of in-hospital mortality from 2008 to 2019. The frequency of intravascular imaging was examined after stratification into different races, hospital regions, hospital bed sizes, hospital urban locations, primary payers, and median income levels. When comparing in-hospital mortalities after PCIs with and without intravascular imaging, we used simple logistic regression to generate the crude odds ratio (cOR) with 95 % confidence interval (CI) and multivariable logistic regression to generate adjusted odds ratio (aOR) with 95 % CI. Mean differences in length of hospital stay and total hospital cost were each calculated using linear regression models. Covariates used to adjust included sex, age, race, comorbidities, hospital characteristics, primary payer, median income, stent type, and procedures. Sensitivity analyses stratified to STEMI and non-ST-elevation myocardial infarction (NSTEMI) were conducted. We considered P-value < 0.05 as significant, and all tests were 2-sided. All data curation and analyses were conducted using SAS, version 9.4 (SAS Institute, Cary, NC).
3. Results
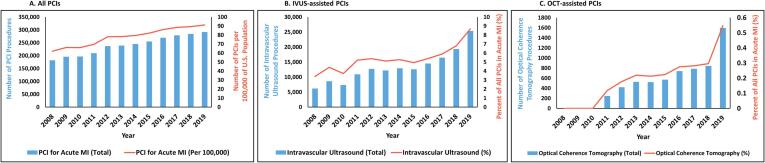
From 2008 to 2019, a total of 2,881,746 PCIs with either DES or BMS were performed for myocardial infarction (Figure S1). The number of PCIs increased 60.7 % from 181,629 in 2008 to 291,855 in 2019 (P-trend < 0.001) (Fig. 1A). The number of PCIs significantly increased even when U.S. population growth was taken into consideration: 62 per 100,000 in 2008 to 91 per 100,000 in 2019 (P-trend < 0.001). The mean age of patients who underwent PCI increased from 62.7 years in 2008 to 64.6 years in 2019 (P-trend < 0.001) (Table S2). The proportion of African American, Hispanic, and Asian patients in yearly PCIs each increased from 2008 to 2019 (all P-trend < 0.001) while that of Caucasian patients decreased (P-trend < 0.001). The prevalence of most comorbidities, including diabetes, hyperlipidemia, obesity, heart failure, atrial fibrillation, and chronic kidney disease, increased over the same period (all P-trend < 0.001). The proportion of STEMIs decreased from 53.2 % in 2008 to 43.4 % in 2019 (P-trend < 0.001). The use of DES demonstrated significant growth from 56.9 % in 2008 to 96.3 % in 2019 (P-trend < 0.001) in contrast to the dwindling usage of BMS (46.5 % in 2008 to 4.1 % in 2019, P-trend < 0.001) (Table S3). In-hospital mortality after PCI slightly increased from 2.7 % in 2008 to 3.1 % in 2019 (P-trend < 0.001), a trend that was consistent even after age-standardization (Table S4).
Fig. 1.
Trend in the number of percutaneous coronary interventions for myocardial infarction from 2008 to 2019, The bar graphs illustrate the number of PCIs performed for myocardial infarction from 2008 to 2019. Fig. 1A represents all PCIs, and the red line depicts the number of PCIs per 100,000 persons in the U.S. population. Fig. 1B represents IVUS-guided PCIs, and the red line shows the percent of IVUS-guided PCIs in all PCIs. Fig. 1C represents OCT-guided PCIs, and the red line shows the percent of OCT-guided PCIs in all PCIs. Abbreviations: IVUS = intravascular ultrasound; OCT = optical coherence tomography; PCI = percutaneous coronary intervention; U.S. = United States.
Overall, most (94.3 %) of the PCIs for myocardial infarction did not have intravascular imaging. However, the number of IVUS-guided PCIs increased by 309.9 % from 6,180 in 2008 to 25,330 in 2019 (P-trend < 0.001) (Fig. 1B). The percentage of PCIs for MI in which IVUS was utilized also increased from 3.4 % in 2008 to 8.7 % in 2019 (P-trend < 0.001) (Table S2). Similar increasing adoption was seen with OCT-guided PCI. No OCT-guided PCI was performed from 2008 to 2010, but beginning in 2011, the number of OCT-guided PCIs increased 548.4 % from 246 to 1,595 in 2019 (P-trend < 0.001) (Fig. 1C). The percentage of PCIs in which OCT was performed also increased from 0.0 % in 2008 to 0.6 % in 2019 (P-trend < 0.001) (Table S2). The baseline and procedural characteristics of IVUS- and OCT-guided PCIs are separately shown in Tables S5-S8.
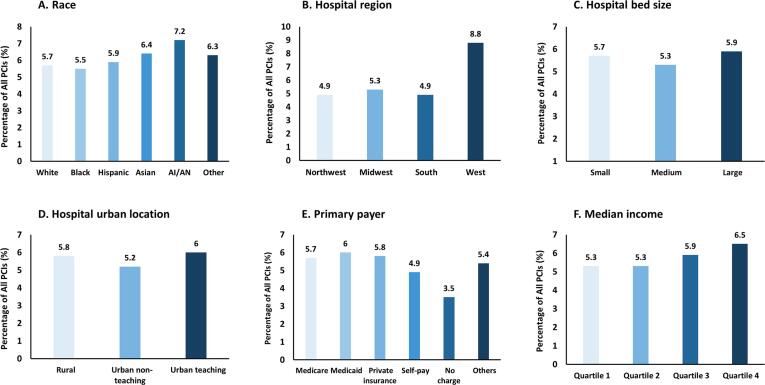
The use of intravascular imaging did not substantially differ by patient race, except for moderately higher use (7.2 %) in American Indians or Alaskan Natives (Fig. 2A). Intravascular imaging was almost twice as frequently adopted in hospitals geographically located in the West (8.8 %) compared with those in the Northwest (4.9 %), Midwest (5.3 %), and the South (4.9 %) (Fig. 2B). No substantial differences were found across different hospital sizes (Fig. 2C) and hospital urban locations (Fig. 2D). Less intravascular imaging was used in PCIs that were self-pay (4.9 %) or uncharged (3.5 %) compared with those primarily paid by Medicare (5.7 %), Medicaid (6.0 %), or private insurance (5.8 %) (Fig. 2E). A marginally increasing trend (P-trend = 0.056) in the use of intravascular imaging-guided PCIs was seen with increasing quartile of median income (Fig. 2F and Table S9).
Fig. 2.
Use of intravascular imaging across different races, hospitals, primary payer, and income, The bar graphs show the frequency of intravascular imaging, consisting of either intravascular ultrasound or optical coherence tomography, across different races (Fig. 2A), hospital regions (Fig. 2B), hospital bed sizes (Fig. 2C), hospital urban locations (Fig. 2D), primary payers (Fig. 2E), and median income quartiles (Fig. 2F).
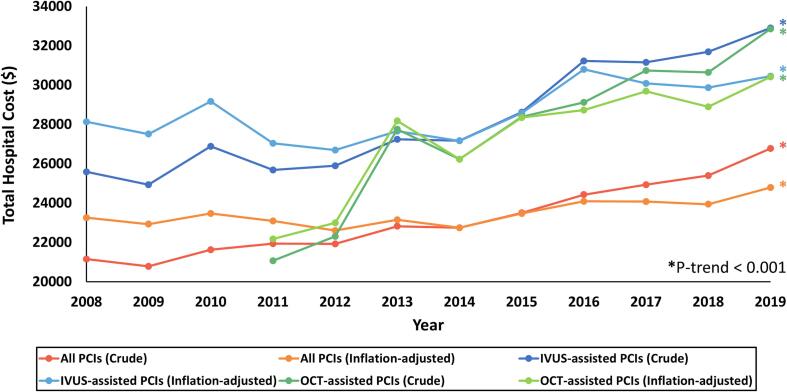
After stratifying all PCIs for myocardial infarction to those with and without intravascular imaging, 164,660 and 2,717,086 PCIs were allocated to the former and latter, respectively. Patient and procedural characteristics of both groups are described in Table S10. Intravascular imaging-guided PCIs had a mean in-hospital mortality rate of 2.1 % compared with 2.8 % in PCIs without intravascular imaging (Table 1). The former was associated with lower odds of in-hospital mortality (cOR 0.75, 95 % CI 0.70–0.81, p < 0.001), which was consistent even after adjustment for potential confounders (aOR 0.66, 95 % CI 0.60–0.72, p < 0.001). The difference was largely preserved even after stratifying intravascular imaging to IVUS-guided and OCT-guided PCIs (Table S11). Intravascular imaging-guided PCI was associated with lower odds of in-hospital mortality in sensitivity analysis of STEMI (aOR 0.70, 95 % CI 0.62–0.79, p < 0.001) and NSTEMI (aOR 0.71, 95 % CI 0.61–0.82, p < 0.001). However, they were associated with slightly longer length of hospital stay (adjusted mean difference 0.19, 95 % CI 0.16–0.23, p < 0.001) and moderately higher inflation-adjusted total hospital cost (adjusted mean difference $3,998, 95 % CI 3,839–4,157, p < 0.001). Additional trend analysis of total hospital cost revealed increasing trends for all PCIs, IVUS-guided PCIs, and OCT-guided PCIs, both inflation-adjusted and unadjusted (all P-trend < 0.001) (Fig. 3). However, IVUS-guided PCIs had higher total hospital cost than all PCIs throughout all the years while OCT-guided PCIs had higher total hospital cost than all PCIs since the year following its first adoption.
Table 1.
Comparison of outcomes after percutaneous coronary intervention with and without intravascular imaging.
| Outcome | Intravascular Imaging (+) | Intravascular Imaging (-) | Crude Odds Ratio | P-value | Adjusted Odds Ratioa | P-value | |
|---|---|---|---|---|---|---|---|
| All MI | In-hospital mortality (%) | 2.1 | 2.8 | 0.75 (0.70–0.81) | <0.001 | 0.66 (0.60–0.72) | <0.001 |
| Length of stay (days) | 4.0 | 3.7 | 0.28 (0.23–0.32)b | <0.001 | 0.19 (0.16–0.23)b | <0.001 | |
| Total hospital costc ($) | 28,853 | 23,211 | 5,642 (5,448–5,836)b | <0.001 | 3,998 (3,839–4,157)b | <0.001 | |
| STEMI | In-hospital mortality ($) | 3.2 | 4.4 | 0.72 (0.66–0.80) | <0.001 | 0.70 (0.62–0.79) | <0.001 |
| Length of stay (days) | 3.8 | 3.7 | 0.10 (0.03–0.18)b | 0.007 | 0.13 (0.07–0.19)b | <0.001 | |
| Total hospital costc ($) | 29,725 | 24,123 | 5,602 (5,267–5,937)b | <0.001 | 4,295 (4,022–4,567)b | <0.001 | |
| NSTEMI | In-hospital mortality (%) | 1.3 | 1.4 | 0.97 (0.86–1.10) | 0.643 | 0.71 (0.61–0.82) | <0.001 |
| Length of stay (days) | 4.0 | 3.6 | 0.41 (0.36–0.47)b | <0.001 | 0.22 (0.17–0.27)b | <0.001 | |
| Total hospital costc ($) | 28,238 | 22,406 | 5,832 (5,607–6,057)b | <0.001 | 3,761 (3,576–3,946)b | <0.001 |
Abbreviations: MI = myocardial infarction; NSTEMI = non-ST-elevation myocardial infarction; STEMI = ST-elevation myocardial infarction.
Adjusted for sex, age, comorbidities, hospital characteristics, primary payer, median income, and procedural characteristics.
Mean difference with 95% confidence interval.
Inflation-adjusted total hospital cost, rounded to the nearest United States dollar.
Fig. 3.
Total hospital cost of percutaneous coronary intervention for acute myocardial infarction, The line graphs show the total hospital costs for all PCIs, unadjusted (red) and adjusted for inflation (orange); IVUS-guided PCIs, unadjusted (blue) and adjusted for inflation (sky blue); and OCT-guided PCIs, unadjusted (green) and adjusted for inflation (yellow green) from 2008 to 2019. Asterisks (*) at the end of the graphs denote significant P-trend less than 0.001. Abbreviations: IVUS = intravascular ultrasound; OCT = optical coherence tomography; PCI = percutaneous coronary intervention.
4. Discussion
In the largest study to date of intravascular imaging to guide PCI for MI in the United States, we identified several key findings: (a) the use of intravascular imaging for PCI is rapidly increasing throughout the U.S., though overall usage remains a small proportion of all PCIs performed; (b) PCI for MI did increase from 2008 to 2019, but the proportion of IVUS-guided and OCT-guided PCIs increased at a greater rate; (c) usage differed by hospital systems and region; and (d) after adjustment, use of intravascular imaging was associated with lower in-hospital mortality in patients undergoing PCI for MI.
Our findings add to the growing body of evidence that favor the use of intravascular imaging in PCI guidance, which is also reflected in the recent global guidelines. The 2021 ACC/AHA/SCAI Guideline for Coronary Artery Revascularization provided a 2a class of recommendation for intravascular imaging in patients undergoing stent implantation based on evidence from RCTs as well as meta-analyses [11]. The 2018 ESC/EACTS/EAPCI Guidelines on Myocardial Revascularization also provide 2a class of recommendation of IVUS or OCT to optimize stent implantation, and IVUS in optimizing the treatment of unprotected left main lesions [12]. Notably, the ULTIMATE (Intravascular Ultrasound Guided Drug Eluting Stents Implantation in “All-Comers” Coronary Lesions) trial, which was the largest trial of routine IVUS-guided PCI, demonstrated a lower rate of target-vessel failure with IVUS-guided PCI compared with angiography-guided PCI at 12 months and lower rates of target vessel failure at 3 years [7], [15]. However, more studies investigating the impact of intravascular imaging on PCI of STEMI patients are needed [12], and our study, albeit retrospective in nature, suggests a potential benefit in both STEMI and NSTEMI.
In contrast to most previous studies which showed a benefit of intravascular imaging after longer-term follow-up [7], [15], our findings suggest a potential improvement in in-hospital mortality even in the short-term, including the index hospitalization. This may be due to stent optimization, reduction of early stent thrombosis, and accurate detection as well as management of post-PCI complications, all of which are facilitated by intravascular imaging [19], [20]. As the importance of MI with no obstructive coronary disease increases with the aging population [21], the use of IVUS or OCT can allow more accurate understanding of the underlying pathology [22], providing significant diagnostic and prognostic value which may potentially translate into improved early outcomes [23]. A smaller registry study has similarly demonstrated that early clinical events can be improved using IVUS [24]. However, operators who utilize intravascular imaging may be more experienced and working in more resource-abundant and high-volume settings, which may also be contributing to the benefit seen in our study. Despite the multiple potential benefits of routine intravascular imaging, some observational studies have reported inharmonious results [25]. Thus, further studies, more standardized approaches, and dissemination of expert knowledge regarding intravascular imaging are necessary. In addition, whether lesion-guided use of IVUS versus OCT in specific sub-populations can improve outcomes represents a key area for future investigation. For instance, greater stent expansion and significant reduction in calcium thickness was reported with OCT-guided PCI in patients with calcified lesions [26]. It remains to be seen if these characteristics can be translated into differential clinical outcomes.
In a recent analysis using the National Readmissions Database, Belakrishna et al studied the utilization of OCT-guided and IVUS-guided PCI and differences in their in-hospital mortality and 30 and 90-day readmission rates and found that intravascular imaging has been steadily increasing in the U.S., a finding consistent with our study [27]. Furthermore, they showed that the overall 30-day readmission rates of patients who underwent OCT-guided PCI were lower compared with those who underwent IVUS-guided PCI [27]. In comparison, our study builds on the findings from Belakrishna et al in several ways. First, our study compares outcomes specifically in patients with MI (STEMI & NSTEMI) undergoing PCI with and without intravascular imaging, thus providing a control group, larger sample size, and more accurate inpatient estimates. Belakrishna et al included patients who underwent balloon angioplasty in their cohort whereas we focused on patients who received either DES or BMS in order to avoid confounding from patients who undergo POBA alone, as they may differ in key ways from the general population of patients with MI. Their study included patients with unstable angina and stable ischemic heart disease which impacted the number of procedures since increasing numbers of PCI for stable disease were performed in the outpatient setting along the course of their study [28]. We also utilized the NIS, which includes data from more participating hospitals and U.S. States and is primarily designed to calculate national estimates of total hospitalizations [29]. Our study confirms a clear association between the use of intravascular-imaging to guide PCI in patients with MI and improved in-hospital mortality, demonstrating that the benefits demonstrated in the early years of intravascular imaging have been maintained as the technology gained more traction and broader uptake over the years [30], [31], [32].
Despite these clear benefits, intravascular imaging is still not used routinely. In a recent survey, the most common reasons for reluctance to use intravascular imaging include high cost, uncertainty whether it provides additional clinical benefit, and concerns about receiving adequate training [33]. These findings were consistent with prior surveys among interventional cardiologists and interventional cardiology fellows [34]. Device-related complications, such as dissection, perforation, arrhythmia, thrombosis, and vasospasm, may also be contributing to the slow reception of intravascular imaging [35]. We did identify higher cost associated with the use of intravascular imaging, but with significantly lower in-hospital mortality offsetting that cost. We also believe that the lowered risk of post-discharge all-cause mortality, myocardial infarction, and target lesion revascularization after guidance with intravascular imaging may lead to better cost-efficiency in the long run by reducing additional hospitalizations and procedures [2]. Nevertheless, potential solutions to these barriers include widely available sessions for additional training or proctoring which can increase operator comfort and expertise, leading to improved uptake of intravascular imaging.
Although intravascular imaging use remained relatively infrequent at the end of the study period, there were certain factors that were associated with increased use. Hospitals located in the West and large and urban teaching hospitals had higher use of intravascular imaging. The influence of inter-hospital variability demonstrated here is consistent with prior studies [6]. Further efforts to mitigate the differences across different geographical areas and hospitals are urgently needed to provide the highest level of care regardless of where a patient is hospitalized.
4.1. Limitations
Our study has some limitations. First, it is a retrospective observational study over 12 years, which limits any conclusions regarding causality between intravascular imaging use and in-hospital outcomes. Second, data are derived from procedure codes and are therefore subject to coding errors and/or reporting bias. Third, there are no-prespecified criteria on intravascular imaging-based management and no detailed criteria to guide treatment decisions based on intravascular imaging findings, which can potentially lead to differences across operators. Fourth, information on medications, complexity of PCI, coronary anatomy, and location of target lesion was not available in the dataset. Fifth, there are a plethora of specific indications for IVUS in PCI (i.e. left main intervention), but more granular data with this information were not available. Sixth, there are no long-term follow-up data available in the NIS at the patient level. Seventh, our analysis was confined to patients with myocardial infarction, so our findings may not be generalizable to those with stable ischemic heart disease. Eighth, additional studies are needed to assess the cost-effectiveness of intravascular imaging, its contemporary trend, and benefits associated with differential use of IVUS and OCT.
4.2. Conclusion
The adoption of intravascular imaging is rapidly increasing throughout the U.S., though overall usage remains low, with differences across hospital systems and regions. After adjustment, the use of intravascular imaging was associated with lower in-hospital mortality in patients undergoing PCI for MI (Graphical Abstract).
CRediT authorship contribution statement
Dae Yong Park: Data curation, Formal analysis, Investigation, Validation, Methodology, Resources, Software, Visualization, Writing – original draft, Writing – review & editing. Evangelia Vemmou: Investigation, Validation, Methodology, Writing – original draft, Writing – review & editing. Seokyung An: Data curation, Formal analysis, Investigation, Methodology, Resources, Software. Ilias Nikolakopoulos: Validation, Writing – review & editing. Christopher J. Regan: Validation, Writing – review & editing. Brian C. Cambi: Validation, Writing – review & editing. Jennifer Frampton: Validation, Writing – review & editing. Aviral Vij: Validation, Writing – review & editing. Emmanouil Brilakis: Validation, Writing – review & editing. Michael G. Nanna: Conceptualization, Project administration, Supervision, Validation, Writing – original draft, Writing – review & editing.
Declaration of Competing Interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Brilakis E: consulting/speaker honoraria from Abbott Vascular, American Heart Association (Associate Editor of Circulation), Amgen, Asahi Intecc, Biotronik, Boston Scientific, Cardiovascular Innovations Foundation (Board of Directors), CSI, Elsevier, GE Healthcare, IMDS, Medicure, Medtronic, Siemens, and Teleflex; research support: Boston Scientific, GE Healthcare; owner, Hippocrates LLC; shareholder: MHI Ventures, Cleerly Health, Stallion Medical. Nanna MG: Dr. Nanna reports funding from the American College of Cardiology Foundation supported by the George F. and Ann Harris Bellows Foundation and from the National Institute on Aging/National Institutes of Health from R03AG074067 (GEMSSTAR award).
Acknowledgments
Acknowledgements
Disclosures.
Park D: None.
Vemmou E: None.
An S: None.
Nikolakopoulos I: None.
Regan CJ: None.
Cambi BC: None.
Frampton J: None.
Vij A: None.
Brilakis E: consulting/speaker honoraria from Abbott Vascular, American Heart Association (associate editor Circulation), Amgen, Asahi Intecc, Biotronik, Boston Scientific, Cardiovascular Innovations Foundation (Board of Directors), CSI, Elsevier, GE Healthcare, IMDS, Medicure, Medtronic, Siemens, and Teleflex; research support: Boston Scientific, GE Healthcare; owner, Hippocrates LLC; shareholder: MHI Ventures, Cleerly Health, Stallion Medical.
Nanna MG: Dr. Nanna reports current research support from the American College of Cardiology Foundation supported by the George F. and Ann Harris Bellows Foundation, the Patient-Centered Outcomes Research Institute (PCORI), the Yale Claude D. Pepper Older Americans Independence Center (P30AG021342), and the National Institute on Aging/National Institutes of Health from R03AG074067 (GEMSSTAR award).
Sponsor’s Role
N/A. No funding was received in conducting this study.
Footnotes
This author takes responsibility for all aspects of the reliability and freedom from bias of the data presented and their discussed interpretation.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ijcha.2023.101186.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- 1.Darmoch F., Alraies M.C., Al-Khadra Y., Moussa Pacha H., Pinto D.S., Osborn E.A. Intravascular Ultrasound Imaging-Guided Versus Coronary Angiography-Guided Percutaneous Coronary Intervention: A Systematic Review and Meta-Analysis. J. Am. Heart Assoc. 2020;9:e013678. doi: 10.1161/JAHA.119.013678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Buccheri S., Franchina G., Romano S., Puglisi S., Venuti G., D'Arrigo P., Francaviglia B., Scalia M., Condorelli A., Barbanti M., Capranzano P., Tamburino C., Capodanno D. Clinical Outcomes Following Intravascular Imaging-Guided Versus Coronary Angiography-Guided Percutaneous Coronary Intervention With Stent Implantation: A Systematic Review and Bayesian Network Meta-Analysis of 31 Studies and 17,882 Patients. JACC Cardiovasc. Interv. 2017;10:2488–2498. doi: 10.1016/j.jcin.2017.08.051. [DOI] [PubMed] [Google Scholar]
- 3.Groenland F.T.W., Neleman T., Kakar H., Scoccia A., Ziedses des Plantes A.C., Clephas P.R.D., Chatterjee S., Zhu M., den Dekker W.K., Diletti R., Zijlstra F., Mahmoud K.D., Van Mieghem N.M., Daemen J. Intravascular ultrasound-guided versus coronary angiography-guided percutaneous coronary intervention in patients with acute myocardial infarction: A systematic review and meta-analysis. Int. J. Cardiol. 2022;353:35–42. doi: 10.1016/j.ijcard.2022.01.021. [DOI] [PubMed] [Google Scholar]
- 4.Witzenbichler B., Maehara A., Weisz G., Neumann F.J., Rinaldi M.J., Metzger D.C., Henry T.D., Cox D.A., Duffy P.L., Brodie B.R., Stuckey T.D., Mazzaferri E.L., Jr., Xu K., Parise H., Mehran R., Mintz G.S., Stone G.W. Relationship between intravascular ultrasound guidance and clinical outcomes after drug-eluting stents: the assessment of dual antiplatelet therapy with drug-eluting stents (ADAPT-DES) study. Circulation. 2014;129:463–470. doi: 10.1161/CIRCULATIONAHA.113.003942. [DOI] [PubMed] [Google Scholar]
- 5.Smilowitz N.R., Mohananey D., Razzouk L., Weisz G., Slater J.N. Impact and trends of intravascular imaging in diagnostic coronary angiography and percutaneous coronary intervention in inpatients in the United States. Catheter Cardiovasc. Interv. 2018;92:E410–E415. doi: 10.1002/ccd.27673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.E.L. Hannan, Y. Zhong, P. Reddy, A.K. Jacobs, F.S.K. Ling, S.B. King, 3rd, P.B. Berger, F.J. Venditti, G. Walford, J. Tamis-Holland Percutaneous Coronary Intervention With and Without Intravascular Ultrasound for Patients With Complex Lesions: Utilization, Mortality, and Target Vessel Revascularization. Circ. Cardiovasc. Interv. 2022: 101161CIRCINTERVENTIONS121011687. [DOI] [PubMed]
- 7.Zhang J., Gao X., Kan J., Ge Z., Han L., Lu S., Tian N., Lin S., Lu Q., Wu X., Li Q., Liu Z., Chen Y., Qian X., Wang J., Chai D., Chen C., Li X., Gogas B.D., Pan T., Shan S., Ye F., Chen S.L. Intravascular Ultrasound Versus Angiography-Guided Drug-Eluting Stent Implantation: The ULTIMATE Trial. J. Am. Coll. Cardiol. 2018;72:3126–3137. doi: 10.1016/j.jacc.2018.09.013. [DOI] [PubMed] [Google Scholar]
- 8.Ali Z.A., Maehara A., Genereux P., Shlofmitz R.A., Fabbiocchi F., Nazif T.M., Guagliumi G., Meraj P.M., Alfonso F., Samady H., Akasaka T., Carlson E.B., Leesar M.A., Matsumura M., Ozan M.O., Mintz G.S., Ben-Yehuda O., Stone G.W., Investigators I.I.O.P. Optical coherence tomography compared with intravascular ultrasound and with angiography to guide coronary stent implantation (ILUMIEN III: OPTIMIZE PCI): a randomised controlled trial. Lancet. 2016;388:2618–2628. doi: 10.1016/S0140-6736(16)31922-5. [DOI] [PubMed] [Google Scholar]
- 9.Singh V., Badheka A.O., Arora S., Panaich S.S., Patel N.J., Patel N., Pant S., Thakkar B., Chothani A., Deshmukh A., Manvar S., Lahewala S., Patel J., Patel S., Jhamnani S., Bhinder J., Patel P., Savani G.T., Patel A., Mohamad T., Gidwani U.K., Brown M., Forrest J.K., Cleman M., Schreiber T., Grines C. Comparison of inhospital mortality, length of hospitalization, costs, and vascular complications of percutaneous coronary interventions guided by ultrasound versus angiography. Am. J. Cardiol. 2015;115:1357–1366. doi: 10.1016/j.amjcard.2015.02.037. [DOI] [PubMed] [Google Scholar]
- 10.Kim N., Lee J.H., Jang S.Y., Bae M.H., Yang D.H., Park H.S., Cho Y., Jeong M.H., Park J.S., Kim H.S., Hur S.H., Seong I.W., Cho M.C., Kim C.J., Chae S.C. Korea Acute Myocardial Infarction Registry - National Institute of Health I. Intravascular modality-guided versus angiography-guided percutaneous coronary intervention in acute myocardial infarction. Catheter Cardiovasc. Interv. 2020;95:696–703. doi: 10.1002/ccd.28359. [DOI] [PubMed] [Google Scholar]
- 11.Lawton J.S., Tamis-Holland J.E., Bangalore S., Bates E.R., Beckie T.M., Bischoff J.M., Bittl J.A., Cohen M.G., DiMaio J.M., Don C.W., Fremes S.E., Gaudino M.F., Goldberger Z.D., Grant M.C., Jaswal J.B., Kurlansky P.A., Mehran R., Metkus T.S., Jr., Nnacheta L.C., Rao S.V., Sellke F.W., Sharma G., Yong C.M., Zwischenberger B.A. 2021 ACC/AHA/SCAI Guideline for Coronary Artery Revascularization: Executive Summary: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2022;145:e4–e17. doi: 10.1161/CIR.0000000000001039. [DOI] [PubMed] [Google Scholar]
- 12.F.J. Neumann, M. Sousa-Uva, A. Ahlsson, F. Alfonso, A.P. Banning, U. Benedetto, R.A. Byrne, J.P. Collet, V. Falk, S.J. Head, P. Juni, A. Kastrati, A. Koller, S.D. Kristensen, J. Niebauer, D.J. Richter, P.M. Seferovic, D. Sibbing, G.G. Stefanini, S. Windecker, R. Yadav, M.O. Zembala, Group ESCSD, 2018 ESC/EACTS Guidelines on myocardial revascularization, Eur. Heart J. 40 (2019) 87–165.
- 13.Overview of the National (Nationwide) Inpatient Sample (NIS). Rockville, MD: Healthcare Cost and Utilization Project (HCUP), 2021.
- 14.Khera R., Angraal S., Couch T., Welsh J.W., Nallamothu B.K., Girotra S., Chan P.S., Krumholz H.M. Adherence to Methodological Standards in Research Using the National Inpatient Sample. J. Am. Med. Assoc. 2017;318:2011–2018. doi: 10.1001/jama.2017.17653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gao X.F., Ge Z., Kong X.Q., Kan J., Han L., Lu S., Tian N.L., Lin S., Lu Q.H., Wang X.Y., Li Q.H., Liu Z.Z., Chen Y., Qian X.S., Wang J., Chai D.Y., Chen C.H., Pan T., Ye F., Zhang J.J., Chen S.L., Investigators U. 3-Year Outcomes of the ULTIMATE Trial Comparing Intravascular Ultrasound Versus Angiography-Guided Drug-Eluting Stent Implantation. JACC Cardiovasc Interv. 2021;14:247–257. doi: 10.1016/j.jcin.2020.10.001. [DOI] [PubMed] [Google Scholar]
- 16.Population Projections of the United States by Age, Sex, Race, and Hispanic Origin: 1995- 2050. United States Census Bureau: U.S. Department of Commerce, 1996.
- 17.R.J. Klein, C.A. Schoenborn, Age adjustment using the 2000 projected U.S. population. Healthy People 2010 Stat Notes 2001:1-10. [PubMed]
- 18.Naing N.N. Easy way to learn standardization : direct and indirect methods. Malays J. Med. Sci. 2000;7:10–15. [PMC free article] [PubMed] [Google Scholar]
- 19.Jegere S., Narbute I., Erglis A. Use of intravascular imaging in managing coronary artery disease. World J. Cardiol. 2014;6:393–404. doi: 10.4330/wjc.v6.i6.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maehara A., Mintz G.S., Weissman N.J. Advances in Intravascular Imaging. Circ. Cardiovasc. Interv. 2009;2:482–490. doi: 10.1161/CIRCINTERVENTIONS.109.868398. [DOI] [PubMed] [Google Scholar]
- 21.Ishii M., Kaikita K., Sakamoto K., Seki T., Kawakami K., Nakai M., Sumita Y., Nishimura K., Miyamoto Y., Noguchi T., Yasuda S., Tsutsui H., Komuro I., Saito Y., Ogawa H., Tsujita K. Characteristics and in-hospital mortality of patients with myocardial infarction in the absence of obstructive coronary artery disease in super-aging society. Int. J. Cardiol. 2020;301:108–113. doi: 10.1016/j.ijcard.2019.09.037. [DOI] [PubMed] [Google Scholar]
- 22.Tsujita K., Yamanaga K., Komura N., Sakamoto K., Miyazaki T., Oimatsu Y., Ishii M., Tabata N., Akasaka T., Sueta D., Yamamoto E., Yamamuro M., Izumiya Y., Kojima S., Nakamura S., Kaikita K., Hokimoto S., Ogawa H. Clinical and morphological presentations of acute coronary syndrome without coronary plaque rupture - An intravascular ultrasound study. Int. J. Cardiol. 2016;220:112–115. doi: 10.1016/j.ijcard.2016.06.191. [DOI] [PubMed] [Google Scholar]
- 23.Alasnag M., Q-u-a J., Johnson T.W., Parapid B., Balghaith M., Al-Shaibi K. The Role of Imaging for MINOCA (Myocardial Infarction with No Obstructive Coronary Artery Disease): a Review of Literature and Current Perspectives. Curr. Cardiovasc. Imaging Reports. 2020;13:21. [Google Scholar]
- 24.Claessen B.E., Mehran R., Mintz G.S., Weisz G., Leon M.B., Dogan O., Costa J.R., Stone G.W., Apostolidou I., Morales A., Chantziara V., Syros G., Sanidas E., Xu K., Tijssen J.G.P., Henriques J.P.S., Piek J.J., Moses J.W., Maehara A., Dangas G.D. Impact of Intravascular Ultrasound Imaging on Early and Late Clinical Outcomes Following Percutaneous Coronary Intervention With Drug-Eluting Stents. J. Am. Coll. Cardiol. Intv. 2011;4:974–981. doi: 10.1016/j.jcin.2011.07.005. [DOI] [PubMed] [Google Scholar]
- 25.Kaziród-Wolski K., Sielski J., Gąsior M., Bujak K., Hawranek M., Pyka Ł., Gierlotka M., Pawłowski T., Siudak Z. Factors affecting short- and long-term survival of patients with acute coronary syndrome treated invasively using intravascular ultrasound and fractional flow reserve: Analysis of data from the Polish Registry of Acute Coronary Syndromes 2017–2020. Kardiol Pol. 2022 doi: 10.33963/KP.a2022.0261. [DOI] [PubMed] [Google Scholar]
- 26.Kurogi K., Ishii M., Ikebe S., Kaichi R., Mori T., Komaki S., Yamamoto N., Yamanaga K., Arima Y., Yamamoto E., Kaikita K., Matsushita K., Tsujita K. Optical coherence tomography-versus intravascular ultrasound-guided stent expansion in calcified lesions. Cardiovasc. Interv. Ther. 2022;37:312–323. doi: 10.1007/s12928-021-00790-7. [DOI] [PubMed] [Google Scholar]
- 27.Balakrishna A.M., Ismayl M., Walters R.W., Aboeata A., Gowda R.M., Vallabhajosyula S., Goldsweig A.M., Dahal K. Comparing Optical Coherence Tomography and Intravascular Ultrasound Guidance for Percutaneous Coronary Intervention: Trends and Outcomes 2010–2019. Curr. Probl. Cardiol. 2022;101270 doi: 10.1016/j.cpcardiol.2022.101270. [DOI] [PubMed] [Google Scholar]
- 28.Inohara T., Kohsaka S., Spertus J.A., Masoudi F.A., Rumsfeld J.S., Kennedy K.F., Wang T.Y., Yamaji K., Amano T., Nakamura M. Comparative Trends in Percutaneous Coronary Intervention in Japan and the United States, 2013 to 2017. J. Am. Coll. Cardiol. 2020;76:1328–1340. doi: 10.1016/j.jacc.2020.07.037. [DOI] [PubMed] [Google Scholar]
- 29.HCUP Central Distributor Availability of Databases . Agency for Healthcare Research and Quality; Rockville, MD: 2022. Healthcare Cost and Utilization Project (HCUP) [Google Scholar]
- 30.Megaly M., Pershad A., Glogoza M., Elbadawi A., Omer M., Saad M., Mentias A., Elgendy I., Burke M.N., Capodanno D., Brilakis E.S. Use of Intravascular Imaging in Patients With ST-Segment Elevation Acute Myocardial Infarction. Cardiovasc. Revasc. Med. 2021;30:59–64. doi: 10.1016/j.carrev.2020.09.032. [DOI] [PubMed] [Google Scholar]
- 31.Yamashita T., Sakamoto K., Tabata N., Ishii M., Sato R., Nagamatsu S., Motozato K., Yamanaga K., Sueta D., Araki S., Arima Y., Yamamoto E., Takashio S., Fujisue K., Fujimoto K., Shimomura H., Tsunoda R., Maruyama H., Nakamura N., Sakaino N., Nakamura S., Yamamoto N., Matsumura T., Kajiwara I., Tayama S., Sakamoto T., Nakao K., Oshima S., Kaikita K., Hokimoto S., Tsujita K., Kumamoto Intervention Conference Study I Imaging-guided PCI for event suppression in Japanese acute coronary syndrome patients: community-based observational cohort registry. Cardiovasc. Interv. Ther. 2021;36:81–90. doi: 10.1007/s12928-020-00649-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vallabhajosyula S., El Hajj S.C., Bell M.R., Prasad A., Lerman A., Rihal C.S., Holmes D.R., Jr., Barsness G.W. Intravascular ultrasound, optical coherence tomography, and fractional flow reserve use in acute myocardial infarction. Catheter Cardiovasc. Interv. 2020;96:E59–E66. doi: 10.1002/ccd.28543. [DOI] [PubMed] [Google Scholar]
- 33.Vemmou E., Nikolakopoulos I., Xenogiannis I., Karacsonyi J., Rangan B.V., Garcia S., Burke M.N., Jneid H., Croce K.J., Bergmark B.A., Brilakis E.S. Learning and innovation among interventional cardiologists: Insights from an international survey. Catheter Cardiovasc. Interv. 2022;99:11–16. doi: 10.1002/ccd.29548. [DOI] [PubMed] [Google Scholar]
- 34.Koskinas K.C., Nakamura M., Raber L., Colleran R., Kadota K., Capodanno D., Wijns W., Akasaka T., Valgimigli M., Guagliumi G., Windecker S., Byrne R.A. Current use of intracoronary imaging in interventional practice - Results of a European Association of Percutaneous Cardiovascular Interventions (EAPCI) and Japanese Association of Cardiovascular Interventions and Therapeutics (CVIT) Clinical Practice Survey. EuroIntervention. 2018;14:e475–e484. doi: 10.4244/EIJY18M03_01. [DOI] [PubMed] [Google Scholar]
- 35.Hausmann D., Erbel R., Alibelli-Chemarin M.J., Boksch W., Caracciolo E., Cohn J.M., Culp S.C., Daniel W.G., De Scheerder I., DiMario C., et al. The safety of intracoronary ultrasound. A multicenter survey of 2207 examinations. Circulation. 1995;91:623–630. doi: 10.1161/01.cir.91.3.623. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.