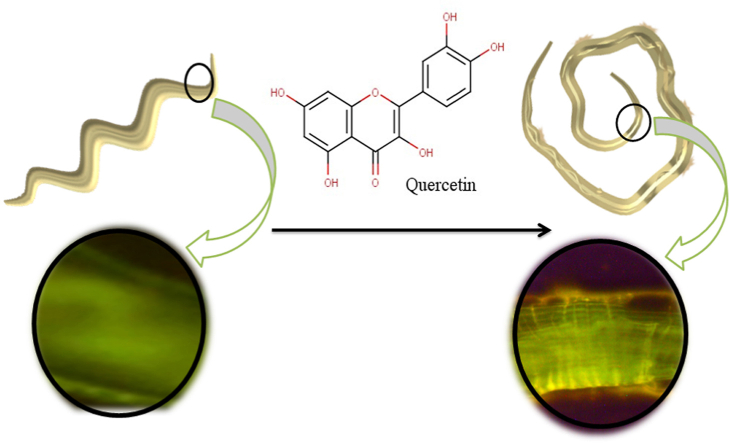
Abstract
Prevalence of infection, limited choice of drugs, and emerging resistance against contemporary medications lead to a pressing need to develop new anthelmintic drugs and drug targets. However, little understanding of worms' physiology has substantially delayed the process. Here, we are reporting the tissue morphology of Haemonchus contortus, intestinal parasitic helminths found in small ruminants, and targeting its nervous system with quercetin, a naturally occurring flavonoid. Quercetin showed anthelmintic activity against all of the developmental stages of H. contortus. Further, histological analysis demonstrated damage to various body parts, including isthmus, brut, pseudocoele, and other organs. Mechanistic studies revealed the generation of oxidative stress and alterations in the activities of the stress response enzymes, such as catalase, superoxide dismutase, and glutathione peroxidase. Moreover, the time-dependent imaging of reactive oxygen species (ROS) generated due to quercetin treatment disclosed neuropils as the primary targets of quercetin in adult worms, which eventually lead to the paralysis and death of the worms. Thus, this work demonstrates that the nervous system of the parasitic helminth, H. contortus, is a novel target of the drug quercetin.
Keywords: Haemonchus contortus, Quercetin, ROS, Nervous system, Parasitic nematodes, Catalase, Superoxide dismutase, Glutathione peroxidase, Oxidative stress
Graphical abstract
1. Introduction
Gastrointestinal infections of parasitic nematodes are prevalent worldwide, infecting humans and animals and costing billions of U.S. Dollars per year. According to the WHO estimates of 2020, 24% of the world's human population, including the most vulnerable group, infants and school-age children, and pregnant women, is infected with parasitic infections [1]. Globally, 3.5 billion people are affected, with around 200,000 deaths yearly due to parasitic intestinal infections. In many developing countries, many children are infected, leading to 39 million disability-adjusted life years, higher susceptibility to diseases, and low economic status [2,3]. The global diversity of the H. contortus worm was studied based on the genome-wide diversity and showed three broad genetic clusters, including subtropical Africa, Atlantic, and Mediterranean-Oceania regions [4]. Moreover, these infections can easily be transferred from animals to humans, with a few reports of human infection from Iran, Brazil, Sudan, Australia, etc. [[5], [6], [7]]. H. contortus is one of the most economically main nematodes responsible for the loss of livestock, particularly ruminants [8,9]. The blood feeder nematode causes acute anemia, hemorrhagic gastroenteritis, weight loss, etc., which may lead to the death of ruminants [10]. H. contortus can also infect humans and cause similar health hazards [5]. Despite the colossal infection rate and the prevalence of animal-to-human transmission, the choices of medicines are mainly limited to a handful of drugs, including benzimidazole, ivermectin, levamisole, pyrantel, and avermectins [11]. Moreover, the increased prevalence of resistance against the existing drugs leads to an urgent need to develop new antihelminthic drugs and drug targets [[12], [13], [14], [15]]. The nervous system, an essential communication system, vastly differs between helminths and mammals. Unlike human neurons, neurons in nematodes are non-myelinated and nematodes have fewer neurons (up to a few hundred) with neurons to glial ratio of 6:1. In contrast, most mammals, including humans, have billions of neurons with a neuron to glial cell ratio of 1:1 [16,17]. Therefore, targeting the nervous system of the pathogen for infection control is a novel approach and may lead to the development of new classes of drugs with lesser side effects. But, despite the high prevalence of infection of H. contortus, extensive physicochemical studies are lacking for this pathogen, just like for the majority of the nematodes. Although, due to the lesser complexity and conservation of the structure, the majority of the nematodes share similar physiological features.
The structural elements of the Caenorhabditis elegans (C. elegans) reveal the nervous system of nematodes that consists of the nerve ring as the central nervous system at the anterior end of the body, the ganglia, the ventral cord that runs longitudinally throughout the body, and the commissural connections [16,[18], [19], [20]]. Also, the similarity in the total number of neurons and their anatomic correspondence is remarkable among the nematodes [16]. Therefore, we hypothesized that studying tissue architecture, including the nervous system of H. contortus, is possible by comparing it to the C. elegans system. Further, we have also hypothesized to target the nervous system of H. contortus with natural polyphenols to develop an effective antihelminthic drug. Nature is abundant in polyphenolic compounds with medical benefits, including anticancer, antimicrobial, and anthelminthic properties [9,21], with better biocompatibility and lesser side effects.
Quercetin, a naturally occurring flavonoid present in onions, green tea, apples, garlic, etc., is well recognized for its anticancer, anti-inflammatory, antioxidant, and neuroprotective activities in mammals, including humans [[22], [23], [24]]. Earlier Borges, D. G. L et al., in 2004, reported that quercetin, in combination with ivermectin, induces mortality in ivermectin-resistant larvae of H. contortus [25]. But the molecular mechanism of quercetin activity and the target site of its action has not been reported. We tried to target H. contours with quercetin and studied its molecular mechanism based on our hypothesis. While doing that, we have also investigated the histopathology of the adult H. contortus and studied tissue-specific damage of the worms, primarily focusing on the neuronal system.
2. Methods
2.1. Materials
Quercetin hydrate (≥95%) and 2′,7′-dichlorodihydrofluoresceindiacetate (DCFDA, ≥97%) were procured from Merck, India. Roswell Park Memorial Institute (RPMI-1640) medium, Dulbecco’s Modified Eagle Medium (DMEM), antibiotics, fetal bovine serum, hematoxylin, and eosin were purchased from Himedia Ltd., India. All other reagents, including Sodium chloride, Potassium chloride, Disodium hydrogen phosphate, and Potassium dihydrogen phosphate, were purchased from Loba Chemie, India, and were of analytical grade.
2.2. Experimental model and subject details
2.2.1. Collection of Haemonchus contortus from the ruminants
The abomasa of a goat infected with adult H. contortus worms were collected from the local slaughterhouses and dissected to collect the internal content. H. contortus adults were handpicked and identified using a fine brush and were readily transferred to 1× phosphate buffer saline (PBS) at pH 7.4 [9,26].
2.2.2. Isolation of eggs from the adult female worms
The egg isolation was carried out from the adult female worms using particular guidelines with some modifications previously used by us [9,26]. At first, adult worms were identified and cleaned; eggs were extracted by centrifugation at 11,000×g for 15 min in the freshly prepared, sterile, 0.8% saline. Finally, the egg count was taken using the McMaster technique [27] and stored at 4°C after adjusting 200 eggs/ml concentration.
2.2.3. Collection of larval stage (L3)
Infected fecal samples were incubated for a week at room temperature (25°C) in dark and humidified conditions. After incubation, the L3 larva was collected from the mixed fecal culture and stored at 4°C for future tests [9,26].
2.3. Method details
2.3.1. Adult paralysis and mortality assay
Adult male and female H. contortus worms (15 worms each) were distributed in a 35 mm culture plate containing 2 ml RPMI in the presence of different concentrations of quercetin (0, 0.125, 0.25, 0.50, 1, and 2 mM) and albendazole (Alb, 0.2 mM) for 24 h at room temperature (37°C). After the incubation, the mortality, morphology, and responses to the physical stimuli were assessed directly under the microscope at 40 × magnification. Paralysis and death times were calculated at regular intervals (0, 1, 3, 6, 12, and 24 h). Paralysis was confirmed in the absence of movement for 60 S at moderate agitation. The morbidity was established after the lack of action for 1 min at heat shock at 50 °C [9,26]. Treated samples were stained with Lugol's stain to observe the morphological changes under an optical microscope.
2.3.2. Larval mortality assay
The L3 larvae contained in 200 μl of RPMI medium having about 25–30 larvae were incubated in quercetin at different concentrations (0, 0.125, 0.25, 0.50, 1, and 2 mM) and albendazole (0.2 mM) for 24 h. After that, a microscopic examination (at 40× magnification) was performed to check for mobility (for 20 s) to the physical stimuli. The morphology of the larvae was also monitored. Larvae, other than the motile ones, were considered as dead. Lugol's staining was performed to check the morphological changes [28]. The percentage of Larval Mortality (% ML3) was calculated using the formula:
| (1) |
2.3.3. Egg hatching assay
Approximately 200 eggs were taken in RPMI medium as control and treated with quercetin and albendazole for 48 h at 28°C. After 48 h of the treatments, Lugol's iodine drop (10 μl) was added to each well to discontinue the inhibition process. The final counts of eggs hatched and inhibited were obtained under the microscope. The percentage of Inhibition to Egg Hatching (% EHI) was calculated using the formula:
| (2) |
2.3.4. Scanning electron microscopy studies for monitoring physical damage to the worms
Scanning electron microscopy (SEM) was performed to monitor the drug-induced physical damage in adult worms. Worms were treated with 1 mM of quercetin and 0.2 mM of albendazole for 12 h and then washed with 1 × PBS. After the PBS wash, treated and control worms were dehydrated using an ethanol gradient from 50% to 100%, followed by gold coating (5 nm). The gold-coated specimens were then observed in the SEM (JSM-6490LV Scanning Electron Microscope, JEOL, USA) at an electron accelerating voltage of 15 KeV [9,26].
2.3.5. Histopathological investigation in Haemonchus contortus
To study drug-induced histopathological changes Hematoxylin and Eosin (H&E) staining was done on the female worms treated for 12 h with 1 mM of Quercetin and Alb (0.2 mM). Parafilm blocks were prepared with the control and treated worms after fixation with 4% glutaraldehyde and dehydration from an alcohol gradient from 50% to 100%. The sections of 100 μm were cut using a Rotary microtome, readily transferred on a glass slide, and fixed after incubating on a hot plate at 65°C. Staining was done using Hematoxylin and Eosin stain, and images were taken at 40× and 100× magnification [29,30].
2.3.6. Detection of reactive oxygen species
To check the production of reactive oxygen species (ROS) in treated worms after quercetin (1 mM) exposure for 3 h, these were incubated with 100 nM of DCFDA (2′,7′-dichlorodihydrofluoresceindiacetate) for 1, 3, 5, and 7 min in the dark at 37°C. After incubation, samples were washed with distilled water to remove the excess DCFDA and fixed in the slide for fluorescent imaging. ROS generation by quercetin was observed at the anterior and posterior ends and body regions and was compared with the albendazole-treated (0.2 mM) controls [9,26].
2.3.7. Measurements of antioxidant enzyme activities
The highly generated free radicals react with the cellular molecules during normal metabolic functions, which induce oxidative damage defense mechanisms. The following mechanisms which include the damage are CAT, SOD, GSH-Px, LPO, and thiol-specific antioxidants (GSH). To begin with, 250 mg of the treated and control helminth worm tissues were homogenized using 500 μl of RIPA buffer (Radioimmunoprecipitation assay). Total protein concentrations in the treated and control worms were calculated using the Bradford method after centrifugation at 10,000 rpm for 15 min, using the bovine serum albumin (BSA) standard curve. The superoxide dismutase (SOD) activity was determined using the spectrophotometric method of Kong et al. [9,26,31]; Catalase activity (CAT) was determined following the method of Hadwan and Abed [9,26,32]; and Glutathione peroxidase activity (GSH-Px) with the method reported by Antunes and Cadenas [9,26,33]. Lipid peroxidation activity (LPO) was determined using the method of Zhang et al. [34], and the total glutathione concentration (GSH) was determined using the method described by Jain and Soni et al. [35]. Total enzyme activity was determined by using the following equation (iii).
| (3) |
2.4. Statistical analysis
All the experiments were performed in independent triplicate, and data were presented as mean ± SD. A one-way ANOVA and Tukey's HSD posthoc tests were performed to understand the reliability of the larval mortality, egg hatching, and enzymatic activities. For ROS intensity analysis, a 10 × 10 pixel area was selected within the nerve ring portions of the images (captured at multiple time points). ANOVA test was performed between the control and treated samples. Two measurements were considered statistically significant if the corresponding p-value was <0.01. Statistical data analysis was conducted using SPSS, and images were prepared using Matlab and Microsoft PowerPoint.
3. Results
3.1. Adult paralysis and mortality assay
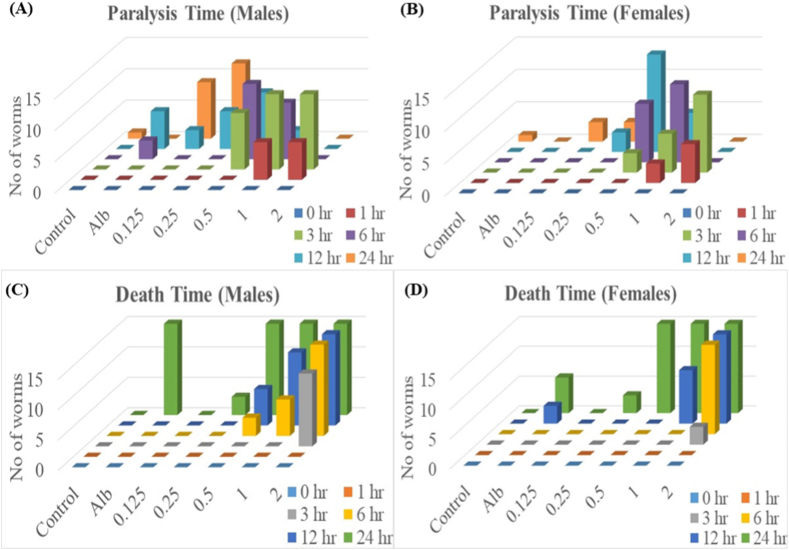
In the male adult worms, quercetin was most active at the concentration of 1 mM, at which 40% of the worms were paralyzed after 1 h, and 80% were paralyzed after 3 h of exposure. At 6 h, 40% were dead, and after 12 h, we found 80% mortality in the adult worms (Fig. 1). Furthermore, in adult males, quercetin at 1 mM concentration caused 100% mortality within 24 h after the treatment. In the case of adult females, quercetin showed a slower effect than the males as 40% paralysis of the adults took place after 3 h, and only at 6 h 80% of the worms were paralyzed. Mortality in the females was only visible at 12 h (60%); however, 100% mortality was found after 24 h of quercetin treatment (Table S1). The LD50 values for adult mortality calculated for the adult male were 0.62 mM (95% lower confidence limit 0.49, 95% higher confidence limit 0.78) and for female worms were 0.88 mM (95% lower confidence limit 0.71, 95% higher confidence limit 1.1) at 12 h of quercetin exposure. Physical damage under the bright field has also been demonstrated in control, albendazole (0.2 mM), and quercetin (1 mM) treated worms, respectively (Figs. S1A–C).
Fig. 1.
Paralysis and death time analysis in the adult H. contortus. Adult H. contortus worms were exposed to different doses of quercetin (0.125, 0.25, 0.5, 1, and 2 mM), 0.2 mM albendazole, and RPMI media (control condition) for 24 h. In male worms, quercetin was most active in 1 mM, where approx 80% of the worms were paralyzed in 12 h, and nearly 100% of the worms died within 24 h of Quercetin treatment. Whereas with quercetin in 1 mM till 12 h of treatment, only 60% of the female worms got paralyzed, and 100% died in 24 h of quercetin treatment.
3.2. Larval mortality assay
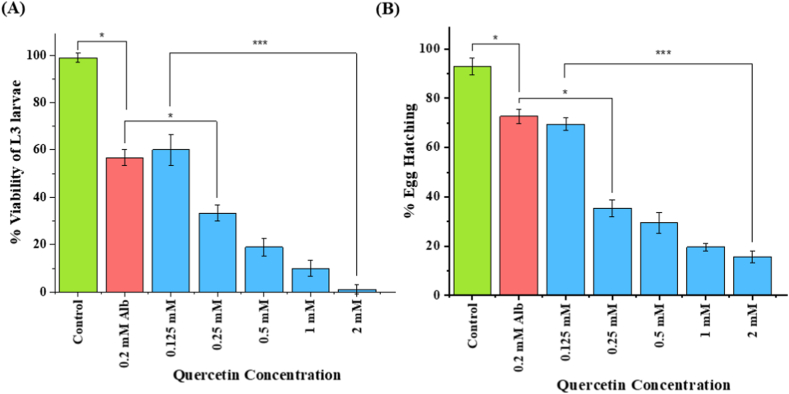
Spindling shrinkage and tissue damage morphology of L3 larvae were observed in the case of quercetin treatment, whereas no such change was found in the albendazole-treated samples. The reduction in the percentage of larval survival with the increase in quercetin concentration was seen: 60.0 ± 6.6, 33.3 ± 3.3, 18.8 ± 3.8, 10.0 ± 3.3, and 1.1 ± 1.9% survival respectively for 0.125, 0.25, 0.50, 1, and 2 mM of quercetin. In contrast, albendazole (Alb) showed 56.6 ± 3.3% survival of the L3 larvae at 0.2 mM (Fig. 2A). The LD50 value of 0.16 mM (95% lower confidence limit 0.09, 95% higher confidence limit 0.22) for the larval mortality for 24 h of quercetin treatment was obtained. Detailed statistical analysis is presented in the supplementary materials section in Table S2).
Fig. 2.
Percent viability of L3 larval stage and percentage of eggs hatched of H. contortus after treatments with different concentrations of quercetin and Alb. The blue bars are showing the effect after 24 h of treatment at different concentrations of quercetin (0.125, 0.25, 0.50, 1, and 2 mM). The green and red-colored bars are representing the treatment of the control set (RPMI media) and albendazole (0.2 mM). Quercetin treatment showed concentration-dependent effects on (A) the survival of the L3 larvae and higher mortality than Alb and (B) the reduced hatching of eggs. The results of the three experiments are plotted as mean ± SD. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
3.3. Egg hatch assay
The egg hatch assay results, demonstrating the toxic effect of quercetin, showed reduced egg hatching with the increase in quercetin concentration: 69.5 ± 2.5, 35.3 ± 3.3, 29.5 ± 4.2, 19.5 ± 1.5, and 15.6 ± 2.3% of egg hatching (presence of larvae L1) respectively at the concentrations of 0.125, 0.25, 0.50, 1, and 2 mM (Fig. 2B). Contrastingly, albendazole (0.2 mM) showed 72.6 ± 3.01% egg hatching. LD50 measured for the inhibition of egg hatching was 0.19 mM (95% lower confidence limit 0.04, 95% higher confidence limit 0.37) for 48 h. A one-way ANOVA test showed a significant interaction between the egg hatching and concentration of quercetin (F(5,12) = 177.78, p < 0.00001) has confirmed the dose-response effect for quercetin. Detailed statistical analysis is presented in the supplementary materials section in Table S3).
3.4. Morphological damage inflicted by quercetin
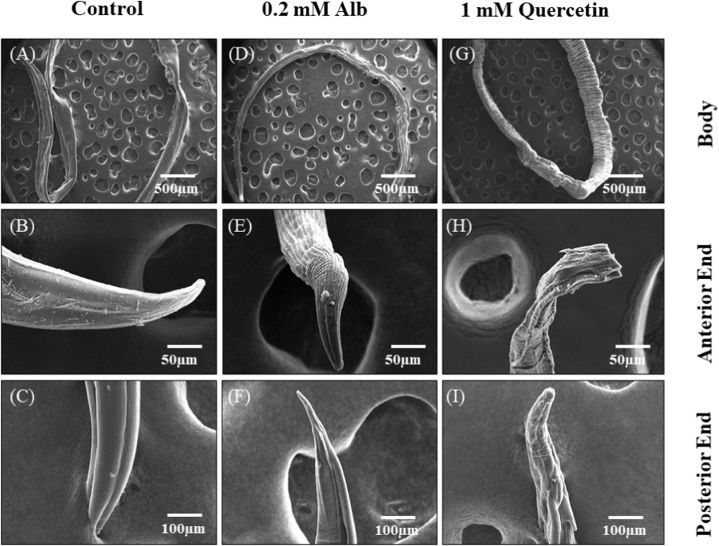
Scanning electron microscopy was performed on the adult worms to understand the possible morphological changes caused by albendazole and quercetin exposure. In the control group, smooth cuticles with well-developed body regions were seen in the adult female worm (Fig. 3A). An intact sharp end and blood-sucking mouth region were identified in the anterior portion with no disruption (Fig. 3B). In the posterior end of the worm, an intact tail region showing anal pore were identified (Fig. 3C). In the case of Alb-treated (0.2 mM for 3 h) worm, folds, partial shrinkage, and disorganization of the epicuticle were observed throughout the body (Fig. 3D) as well as at the anterior buccal cavity (Fig. 3E) and posterior tail region (Fig. 3F) ends. However, in the quercetin-treated worm (1 mM for 3 h), complete disorganizations and disruptions of body regions and the loss of the epicuticle were observed (Fig. 3G). In addition, shrinkage of the cervical papillae, a complete loss of the buccal capsule, and rupturing of the epicuticle at the tail region were observed (Fig. 3H and I).
Fig. 3.
Study of morphological damage in the adult H. contortus due to the treatment with quercetin. Scanning electron microscopic images are showing the morphology of adult H. contortus A, B, and C are showing the whole body, anterior and posterior ends in the control group, respectively. D, E, and F are showing partial disruptions of the body ends and cuticle due to the treatment with 0.2 mM Alb of the same body parts. G, H, and I are showing the same for 1 mM quercetin-treated worms.
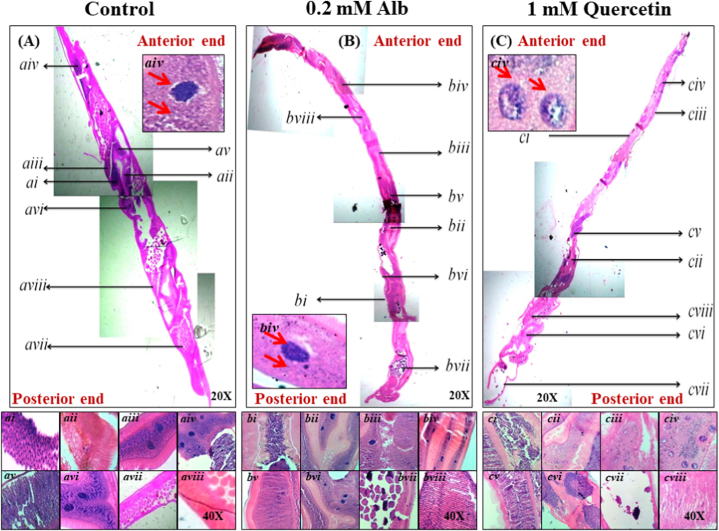
3.5. Histopathology caused by quercetin treatment
Massive changes in the morphology, caused by quercetin treatment, were observed under the light microscope (Fig. 4C), whereas Alb (Fig. 4B) showed little to no change in the morphology in comparison to the control (Fig. 4A). Detailed magnified image analysis revealed intact anterior and posterior ends with unblemished (ai) isthmus, (aii) brut, (aiii) pseudocoele, (aiv) globular leukocytes (surrounded by small-sized cells), (av) muscle cells, (avi) intestinal epithelial region, (avii) ovum and growth zone of the ovary, and (aviii) intact skin tissue in control adult worm (Fig. 4A). In the 0.2 mM Alb-treated worms (Fig. 4B), moderate disruptions were visible at the (bi) isthmus and (biii) pseudocoele along with the (bvi) partially-punctured muscle cells, (bv) globular leukocytes with less number of surrounding cells, and a (bviii) ruptured ovary. On the other hand, quercetin treatment (Fig. 4C) has caused complete disruption of the anterior end, a near-total disruption of the ovary, and loss of eggs, splitting of the pseudocoele, punctured muscle cells, and damaged globular leukocytes with limited counts of surrounding-cells. Fig. 4ci–cviii shows the histopathology of different body parts in the adult female H. contortus treated with quercetin.
Fig. 4.
Histopathological changes in the adult H. contortus after the treatment with quercetin. A shows the tissue morphology (H&E) of the control adult female H. contortus at 20×. Higher magnified (100×) images are showing detailed structures of the isthmus, brut, pseudocoele, globular leukocytes, muscle cells, intestinal epithelial region, ovary, and intact skin tissue in ai–aviii, respectively. Due to the treatment with Alb, partial disorganizations in some of these body parts were observed (B). Treatment with 1 mM of quercetin resulted in extensive damage to the body part, such as loss of egg count, wrecked globular leukocytes, etc. . (C) bi–bviii and ci–cviii are showing magnified images of the body parts as before; the same for Alb (0.2 mM) and Quercetin (0.1 mM) treated worms, respectively.
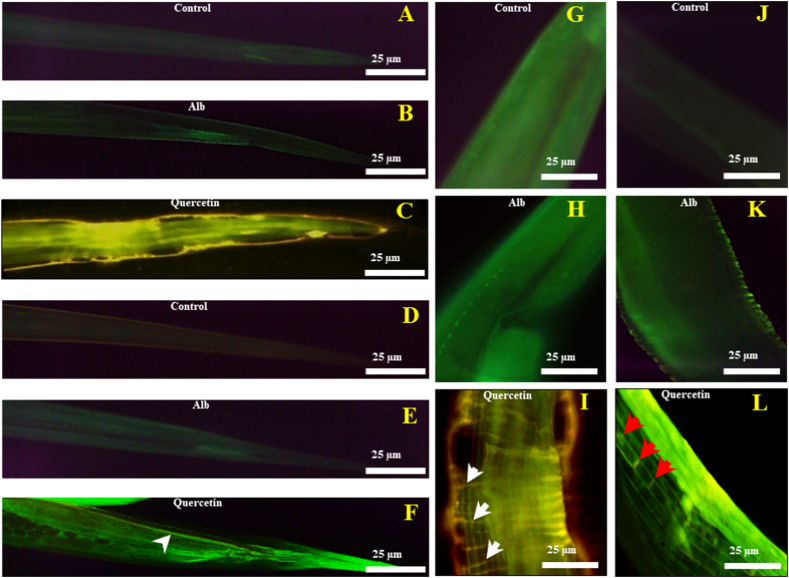
3.6. Generation of reactive oxygen species in the nervous system due to quercetin treatment
The mechanism underlying the toxicity elicited by quercetin was the generation of reactive oxygen species or ROS (Fig. 5C, F, I, and L). In contrast, ROS was not detected in control (Fig. 5A, D, G, and J) and Alb (Fig. 5B, E, H, and K) groups. The oxidative stress produced due to quercetin treatment in the adult worms showed more significant fluorescence in the anterior part of the body (Fig. 5C), ventral cord and tail ganglia (at the posterior end) (Fig. 5F), nerve ring (at the anterior end) (Fig. 5I), and commissural connections (middle of body region) (Fig. 5L). Alteration in ROS generation in the nerve ring of the adult worm was measured by repeated-measures ANOVA at different time points (after 1, 3, 5, and 7 min of DCFDA treatment) and found a significant increase in pixel intensity (indicating increased staining with the ROS-detecting dye, DCFDA) with a significant time effect (F(3,80) = 875.69, p < 0.0001). Detailed statistical analysis is given in the supplementary materials (Fig. S4).
Fig. 5.
Generation of the reactive oxygen species (ROS) in the adult worms' nervous system due to quercetin exposure. Adult worms were treated for 3 h separately with Alb and quercetin and processed with DCFDA (100 nM) to detect the ROS. The images from A-C are showing the differences in staining (10× magnification) in the anterior end, after 7 min of DCFDA treatment (due to differences in ROS generation) respectively in control (treated with RPMI media), Alb (0.2 mM) and quercetin (1 mM)-treated worms. The following three images (D–F) are showing the differences in staining (10× magnification), at the posterior end, in the same three experimental groups. In subfigure F, the blue arrowhead indicates the eggs of the quercetin-treated worm. Generations of ROS respectively in the nerve ring (I), marked with white arrowhead; (40×), commissural connections (L), marked with red arrowheads; (40×) and ventral cord, marked with yellow arrowhead; (40×) were detected in the adult worms treated with quercetin. No such structural discrimination and elevation of ROS were observed in the control (G, H) and Alb-treated (J, K) worms. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
3.7. Alterations in catalase, superoxide dismutase, and glutathione peroxidase activity after quercetin treatment
To confirm the ROS-induced stress in the worms' tissue, oxidative stress-responsive enzymes, including catalase (CAT), superoxide dismutase (SOD), and glutathione peroxidase (GSH-Px) activities, were measured (Table 1). An increase in the catalase activity and quercetin concentration were observed. The activity levels of CAT calculated respectively were 107.86 ± 0.64, 123.97 ± 1.11, 149.6 ± 0.42, 332.26 ± 0.66, and 342.08 ± 0.72 U/mg proteins at the concentrations of quercetin, 0.125, 0.25, 0.5, 1.0, and 2.0 mM after 3 h of treatment. A one-way ANOVA test found an increase in the activity level of CAT depending on the concentration of quercetin; significant interaction between the CAT-activity and quercetin-concentration (F(5,12) = 88,765, p < 0.0001). A similar dose-response effect was also found for superoxide dismutase (SOD) with a significant SOD-activity × quercetin-concentration effect (F(5,12) = 1398.3, p < 0.0001) and 18.36 ± 0.08, 26.40 ± 2.26, 34.63 ± 0.08, 57.49 ± 0.3, and 63.30 ± 0.19 U/mg protein respectively for the same set of concentrations of quercetin after 3 h of treatment. We also found the increasing activity of glutathione peroxidase, with increasing concentration of quercetin. The activity levels of GSH-Px were found to be 183.61 ± 0.97, 272.76 ± 2.56, 436.47 ± 1.68, 503.47 ± 0.48, and 518.33 ± 1.75 U/mg protein, respectively, concentrations of quercetin, 0.125–2.0 mM after 3 h of treatment with a significant GSH-Px-activity × quercetin-concentration effect (F(5,12) = 25,181, p < 0.0001). Tables S4, S5, and S6 for CAT, SOD, and GSH-Px are detailed statistical analyses.
Table 1.
Increase in the activity levels of enzymes involved in coping with oxidative stress induced by quercetin treatment. The oxidative stress caused by quercetin treatment in the adult worm has increased the activities of the following enzymes, catalase (CAT), superoxide dismutase (SOD), glutathione peroxidase (GSH-Px), Lipid peroxidation (LPO) and reduced glutathione (GSH), as measured by biochemical and spectrophotometric assays. All three enzymes showed an increase in activities with an increase in quercetin concentration (0.125, 0.25, 0.5, 1.0, 2.0, and 5.0 mM). Higher activities of these enzymes are also found in the Alb-treated worms compared to the controls. The enzymatic activity for CAT, SOD, and GSH-Px was measured as the enzyme unit per mg of protein (U/mg protein), for LPO (μM per mg protein), and GSH as mM per mg of protein.
| Experimental Groups | U/mg protein |
μM/mg protein |
mM/mg protein |
||
|---|---|---|---|---|---|
| CAT | SOD | GSH-Px | LPO | GSH | |
| Control | 8.85 ± 0.46 | 1.65 ± 0.11 | 19.9 ± 1.75 | 3.08 ± 0.07 | 0.14 ± 0.01 |
| 0.2 mM Alb | 134.48 ± 8.4 | 24.89 ± 1.53 | 319.58 ± 22.25 | 3.19 ± 0.24 | 0.314 ± 0.04 |
| 0.125 mM | 107.86 ± 0.64 | 18.36 ± 0.08 | 183.61 ± 0.97 | 2.94 ± 0.07 | 1.56 ± 0.01 |
| 0.25 mM | 123.97 ± 1.11 | 26.40 ± 2.26 | 272.76 ± 2.56 | 3.05 ± 0.08 | 2.00 ± 0.01 |
| 0.50 mM | 149.6 ± 0.42 | 34.63 ± 0.08 | 436.47 ± 1.68 | 3.19 ± 0.10 | 3.35 ± 0.07 |
| 1 mM | 332.26 ± 0.66 | 57.49 ± 0.3 | 503.47 ± 0.48 | 3.24 ± 0.10 | 4.68 ± 0.16 |
| 2 mM | 342.08 ± 0.72 | 63.30 ± 0.19 | 518.33 ± 1.75 | 3.43 ± 0.07 | 5.22 ± 0.02 |
3.8. Cellular combat with ROS by increasing the reduced glutathione concentration
In combat against the ROS, GSH also induces a cellular response, which causes oxidative stress in helminths when exposed to quercetin. A significant increase in the amount of GSH produced was measured in quercetin-treated worms (Table 1). The increase in GSH was observed along with the increase of the dose of quercetin, from 1.56 ± 0.01, 2.00 ± 0.01, 3.35 ± 0.07, 4.68 ± 0.16, and 5.22 ± 0.02 mM/mg protein. Detailed statistical analyses are given in Table S7. The lipid peroxidation levels were analyzed using MDA content according to the thiobarbituric acid (TBA) assay. They were found to be 2.94 ± 0.07, 3.05 ± 0.08, 3.19 ± 0.10, 3.24 ± 0.10, and 3.43 ± 0.07 μM/mg protein after 3 h treatments with the following quercetin concentrations (0.125, 0.25, 0.5, 1.0 and. 2.0 mM).
4. Discussion
Helminth infections worldwide result in an estimated death of 135,000 infants worldwide [28]. The choices of medicines for anthelminthic activity are limited to a few drug categories, like benzimidazole, ivermectin, levamisole, pyrantel, and avermectins. Moreover, the increased cases in the prevalence of resistance against existing drugs lead to an urgent need to develop new anthelminthic drugs and drug targets [[14], [36]]. This leads to major problems in veterinary medicine and affects both agricultural income and animal welfare. Therefore it is very important to either use the anthelmintics in a way that minimizes the development of anthelminthic resistance in the parasitic nematodes or to find novel alternate drugs that will reduce the dependence on these already resistant drugs [37,38]. In the quest for alternative medicines against parasitic nematodes, research efforts were invested in assessing the effectiveness of plant metabolites, including flavonoids [39]. Many flavonoids have been used as traditional medicines like from extracts of Justicia adhatoda, Vernonia amygdalina, Mikania micrantha, Momordica charantia, Lippia javanica, Newbouldia laevis, and Zanthoxylum zanthoxyloides have shown significant anthelminthic activity by inducing paralysis and death of helminthic parasites [[40], [41], [42]]. Quercetin is non-toxic to animals, with an LD50 value of 16 g/kg body weight [43]. Here we show quercetin, an anti-inflammatory and neuroprotective agent in mammals, has excellent antihelminthic properties against all three stages of the parasitic nematode, H. contortus. Quercetin exerts a nematicidal effect with the most effective concentration of 1 mM. At this concentration, adult male worms manifested 80% paralysis after 3 h of treatment, and 100% were found dead after 24 h. Females showed more resistance to 1 mM quercetin than the males as the paralysis of 80% of the adult females took 6 h; however, 100% death of the females was observed at 24 h of exposure, like the males. Likewise, the LD50 value for the adult females was higher than that of the males for quercetin. The higher susceptibility of the males compared to the females could be due to the smaller size of the males than the females [44]. Alternatively, the genetic constitution of the females has probably contributed, as it is reported that female H. contortus showed increased resistance to benzimidazole and thiabendazole [45].
The primary response of quercetin activity is the generation of reactive oxygen species (ROS). Although the drug-induced ROS mainly affected the whole body of the organism, the primary target was the neuronal system, evidenced by time-dependent DCFDA staining. Deformations of the body parts like the cuticular structure, anterior mouth part, cervical papillae, and posterior tail region due to exposure to quercetin for 3 h, were found to be associated with the generation of the high amount of ROS in the nerve ring, ventral cord, and commissural connections in the adult worms. Besides, we found that quercetin treatment produces a higher amount of ROS than treatment with albendazole. The nerve ring area showed a significant increase in ROS generation with time due to quercetin exposure. The finding leads to establishing the helminthic neuronal system as a novel anthelminthic drug target and quercetin as a drug that primarily damages the target. The quercetin shows early paralysis in adult worms which may be explained by higher ROS generation in the neuronal system of the parasite. The differences in the structural and functional architectures of the nervous system in worms and mammals may be the reason for the non-toxic effect of quercetin in mammals but the toxic impact on parasites. This unique advantage and the other differences between the mammalian and helminthic neuronal systems make the helminthic neuronal system an attractive target for anthelminthic drug targeting.
Although the in vitro results look promising, in vivo studies are required to understand better the pharmacological possibilities for quercetin as an antihelminthic drug. Further, new formulations are also necessary to increase the potency of the compounds. The effect of quercetin can also be evaluated for its anthelminthic properties against other parasitic nematodes.
5. Conclusion
Quercetin is a naturally occurring flavonoid and is found in grapes, berries, cherries, apples, citrus fruits, onion, tomato, red wine, and black tea and was used particularly because of its therapeutic properties like anti-diabetic, anti-inflammatory, antimicrobial, anti-alzheimer's, cardiovascular, and wound-healing effects [46]. In the present study, quercetin has shown a significant effect on adult H. contortus parasitic worms. The histopathological detail of parasitic nematodes upon quercetin treatment and ROS studies showed that quercetin establishes as nematodes' neuronal system as a drug target. It showed the best activity at 1 mM concentration, where 80% of male and 60% of female worms were paralyzed within 12 h, and all the worms were dead by 24 h. The larval motility and viability assay and egg hatch assay also confirmed the toxicity of quercetin at various stages of the worm. These results showed significant dose-dependent free radical scavenging and in vitro anthelminthic activity against H. contortus. These results repurpose the drug quercetin and create a foundation for exploring new molecules to develop a novel class of anthelminthic drugs targeting the parasitic nervous system.
Author contribution statement
Diptiman Choudhury and Lachhman Das Singla: Conceived and designed the experiments; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Vanshita Goel and Sunidhi Sharma: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Neloy Kumar Chakroborty: Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
Diptiman Choudhury was supproted by Indian Council of Medical Research (17× (3)/Adhoc/63/2022-ITR) and Thapar Institute of Engineering and Technology (TIET-VT-CEEMS TOF Project).
Data availability statement
Data included in article/supp. material/referenced in article.
Declaration of interest's statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
DC is thankful to TIET-VT-CEEMS (CEEMS-TOF/2020) for the instrumental facility, and NKC is grateful for the Director's Discretionary Fund, TSLAS, TIET. VG, and SS are thankful to TIET for fellowship. VG and SS are grateful to TIET for their fellowships.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2023.e13699.
Appendix A. Supplementary data
The following is the Supplementary data to this article.
References
- 1.Soil-transmitted helminth infections, https://www.who.int/news-room/fact-sheets/detail/soil-transmitted-helminth-infections.
- 2.Hajare S.T., Gobena R.K., Chauhan N.M., Eriso F. Prevalence of intestinal parasite infections and their associated factors among food handlers working in selected catering establishments from Bule hora, Ethiopia. BioMed Res. Int. 2021;2021 doi: 10.1155/2021/6669742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Elmonir W., Elaadli H., Amer A., El-Sharkawy H., Bessat M., Mahmoud S.F., Shukry Atta M., El-Tras W.F. Prevalence of intestinal parasitic infections and their associated risk factors among preschool and school children in Egypt. PLoS One. 2021;16 doi: 10.1371/JOURNAL.PONE.0258037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sallé G., Doyle S.R., Cortet J., Cabaret J., Berriman M., Holroyd N., Cotton J.A. The global diversity of Haemonchus contortus is shaped by human intervention and climate. Nat. Commun. 2019;10:1–14. doi: 10.1038/s41467-019-12695-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ghadirian E., Arfaa F. First report of human infection with Haemonchus contortus, Ostertagia ostertagi, and Marshallagia marshalli (family trichostrongylidae) in Iran. J. Parasitol. 1973;59:1144–1145. doi: 10.2307/3278661. [DOI] [PubMed] [Google Scholar]
- 6.Mohammedsalih K.M., Krücken J., Bashar A., Juma F.R., Abdalmalaik A.A.H., Khalafalla A., Abakar A., Coles G., von Samson-Himmelstjerna G. Susceptible trichostrongyloid species mask presence of benzimidazole-resistant Haemonchus contortus in cattle. Parasites Vectors. 2021;14 doi: 10.1186/S13071-021-04593-W. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sanders J., Xie Y., Gazzola D., Li H., Abraham A., Flanagan K., Rus F., Miller M., Hu Y., Guynn S., Draper A., Vakalapudi S., Petersson K.H., Zarlenga D., Li R.W., Urban J.F., Ostroff G.R., Zajac A., Aroian R.V. A new paraprobiotic-based treatment for control of Haemonchus contortus in sheep. Int. J. Parasitol. Drugs Drug Resist. 2020;14:230–236. doi: 10.1016/J.IJPDDR.2020.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sutherland I.A., Leathwick D.M. Anthelmintic resistance in nematode parasites of cattle: a global issue? Trends Parasitol. 2011;27:176–181. doi: 10.1016/j.pt.2010.11.008. [DOI] [PubMed] [Google Scholar]
- 9.Goel V., Das Singla L., Choudhury D. Cuminaldehyde induces oxidative stress-mediated physical damage and death of Haemonchus contortus. Biomed. Pharmacother. 2020;130 doi: 10.1016/j.biopha.2020.110411. [DOI] [PubMed] [Google Scholar]
- 10.Wolstenholme A.J. Ion channels and receptor as targets for the control of parasitic nematodes. Int. J. Parasitol. Drugs Drug Resist. 2011;1:2–13. doi: 10.1016/j.ijpddr.2011.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Holden-Dye L., Walker R.J. Anthelmintic drugs. WormBook. 2007;44:1–13. doi: 10.1895/wormbook.1.143.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kaplan R.M., Vidyashankar A.N. An inconvenient truth: global worming and anthelmintic resistance. Vet. Parasitol. 2012;186:70–78. doi: 10.1016/j.vetpar.2011.11.048. [DOI] [PubMed] [Google Scholar]
- 13.Morrison A.A., Chaudhry U., Andrews L., Melville L., Doyle S.R., Sargison N.D., Bartley D.J. Phenotypic and genotypic analysis of benzimidazole resistance in reciprocal genetic crosses of Haemonchus contortus. Int. J. Parasitol. Drugs Drug Resist. 2022;18:1–11. doi: 10.1016/J.IJPDDR.2021.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fissiha W., Kinde M.Z. Anthelmintic resistance and its mechanism: a review. Infect. Drug Resist. 2021;14:5403–5410. doi: 10.2147/IDR.S332378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wolstenholme A.J., Fairweather I., Prichard R., Von Samson-Himmelstjerna G., Sangster N.C. Drug resistance in veterinary helminths. Trends Parasitol. 2004;20:469–476. doi: 10.1016/j.pt.2004.07.010. [DOI] [PubMed] [Google Scholar]
- 16.Schafer W. Nematode nervous systems. Curr. Biol. 2016;26:R955–R959. doi: 10.1016/j.cub.2016.07.044. [DOI] [PubMed] [Google Scholar]
- 17.Herculano-Houzel S., Lent R. Isotropic fractionator: a simple, rapid method for the quantification of total cell and neuron numbers in the Brain. J. Neurosci. 2005;25:2518–2521. doi: 10.1523/JNEUROSCI.4526-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Burns A.R., Luciani G.M., Musso G., Bagg R., Yeo M., Zhang Y., Rajendran L., Glavin J., Hunter R., Redman E., Stasiuk S., Schertzberg M., Angus McQuibban G., Caffrey C.R., Cutler S.R., Tyers M., Giaever G., Nislow C., Fraser A.G., MacRae C.A., Gilleard J., Roy P.J. Caenorhabditis elegans is a useful model for anthelmintic discovery. Nat. Commun. 2015;6:1–11. doi: 10.1038/ncomms8485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alexander A.G., Marfil V., Li C. Use of C. elegans as a model to study Alzheimer's disease and other neurodegenerative diseases. Front. Genet. 2014;5:1–21. doi: 10.3389/fgene.2014.00279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pereira L., Kratsios P., Serrano-Saiz E., Sheftel H., Mayo A.E., Hall D.H., White J.G., LeBoeuf B., Garcia L.R., Alon U., Hobert O. A cellular and regulatory map of the cholinergic nervous system of C. Elegans. Elife. 2015;4:1–46. doi: 10.7554/eLife.12432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Choudhury D., Ganguli A., Dastidar D.G., Acharya B.R., Das A., Chakrabarti G. Apigenin shows synergistic anticancer activity with curcumin by binding at different sites of tubulin. Biochimie. 2013;95:1297–1309. doi: 10.1016/J.BIOCHI.2013.02.010. [DOI] [PubMed] [Google Scholar]
- 22.Ulusoy H.G., Sanlier N. A minireview of quercetin: from its metabolism to possible mechanisms of its biological activities. Crit. Rev. Food Sci. Nutr. 2020;60:3290–3303. doi: 10.1080/10408398.2019.1683810. [DOI] [PubMed] [Google Scholar]
- 23.Yi L., Ma S., Ren D. Phytochemistry and bioactivity of Citrus flavonoids: a focus on antioxidant, anti-inflammatory, anticancer and cardiovascular protection activities. Phytochemistry Rev. 2017;16:479–511. doi: 10.1007/s11101-017-9497-1. [DOI] [Google Scholar]
- 24.Anand David A.V., Arulmoli R., Parasuraman S. Overviews of biological importance of quercetin: a bioactive flavonoid. Pharmacogn. Rev. 2016;10:84–89. doi: 10.4103/0973-7847.194044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Borges D.G.L., de Araújo M.A., Carollo C.A., Carollo A.R.H., Lifschitz A., Conde M.H., de Freitas M.G., dos Santos Freire Z., Tutija J.F., Nakatani M.T.M., Borges F. de A. Combination of quercetin and ivermectin: in vitro and in vivo effects against Haemonchus contortus. Acta Trop. 2020;201 doi: 10.1016/j.actatropica.2019.105213. [DOI] [PubMed] [Google Scholar]
- 26.Goel V., Kaur P., Das Singla L., Choudhury D. Biomedical evaluation of lansium parasiticum extract-protected silver nanoparticles against Haemonchus contortus, a parasitic worm. Front. Mol. Biosci. 2020;7 doi: 10.3389/fmolb.2020.595646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pal A., Chakravarty A.K. Disease-resistant traits—analytic tools and indicator traits. Genet. Breed. Dis. Resist. Livest. 2020:85–94. doi: 10.1016/b978-0-12-816406-8.00003-6. [DOI] [Google Scholar]
- 28.EL Requisito Para Obtener Grado De C., DE Jesús Martínez X. Universidad Autónoma de Guerrero (México); 2019. Actividad Nematicida de los Frutos de la Leguminosa Tesis por Artículo Científico Presenta.http://ri.uagro.mx/handle/uagro/1381 (accessed April 1, 2021) [Google Scholar]
- 29.Cheng R., Billet S., Liu C., Haldar S., Choudhury D., Tripathi M., Hav M., Merchant A., Hu T., Huang H., Zhou H., Bhowmick N.A. Periodontal inflammation recruits distant metastatic breast cancer cells by increasing myeloid-derived suppressor cells. Oncogene. 2019;39:1543–1556. doi: 10.1038/s41388-019-1084-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu C., Billet S., Choudhury D., Cheng R., Haldar S., Fernandez A., Biondi S., Liu Z., Zhou H., Bhowmick N.A. Bone marrow mesenchymal stem cells interact with head and neck squamous cell carcinoma cells to promote cancer progression and drug resistance. Neoplasia. 2021;23:118–128. doi: 10.1016/J.NEO.2020.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kong W., Zhao Y., Liu F., He Y., Tian T., Zhou W. Fast analysis of superoxide dismutase (SOD) activity in barley leaves using visible and near infrared spectroscopy. Sensors. 2012;12 doi: 10.3390/s120810871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hadwan M.H., Abed H.N. Data supporting the spectrophotometric method for the estimation of catalase activity. Data Br. 2016;6:194–199. doi: 10.1016/j.dib.2015.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Antunes F., Cadenas E. Estimation of H 2 O 2 gradients across biomembranes. FEBS Lett. 2000;475:121–126. doi: 10.1016/S0014-5793(00)01638-0. [DOI] [PubMed] [Google Scholar]
- 34.Zhang Q., Zhu L., Wang J., Xie H., Wang J., Han Y., Yang J. Oxidative stress and lipid peroxidation in the earthworm Eisenia fetida induced by low doses of fomesafen. Environ. Sci. Pollut. Res. 2013;20:201–208. doi: 10.1007/S11356-012-0962-5/FIGURES/5. [DOI] [PubMed] [Google Scholar]
- 35.Jain A., Soni M., Deb L., Jain A., Rout S.P., Gupta V.B., Krishna K.L. Antioxidant and hepatoprotective activity of ethanolic and aqueous extracts of Momordica dioica Roxb. leaves. J. Ethnopharmacol. 2008;115:61–66. doi: 10.1016/J.JEP.2007.09.009. [DOI] [PubMed] [Google Scholar]
- 36.Ngwese M.M., Manouana G.P., Moure P.A.N., Ramharter M., Esen M., Adégnika A.A. Diagnostic techniques of soil-transmitted helminths: impact on control measures. Trop. Med. Infect. Dis. 2020;5 doi: 10.3390/TROPICALMED5020093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Waller P.J. From discovery to development: current industry perspectives for the development of novel methods of helminth control in livestock. Vet. Parasitol. 2006;139:1–14. doi: 10.1016/j.vetpar.2006.02.036. [DOI] [PubMed] [Google Scholar]
- 38.Baudinette E., O'Handley R., Trengove C. Anthelmintic resistance of gastrointestinal nematodes in goats: a systematic review and meta-analysis. Vet. Parasitol. 2022;312 doi: 10.1016/J.VETPAR.2022.109809. [DOI] [PubMed] [Google Scholar]
- 39.Huffman M.A. Animal self-medication and ethno-medicine: exploration and exploitation of the medicinal properties of plants. Proc. Nutr. Soc. 2003;62:371–381. doi: 10.1079/pns2003257. [DOI] [PubMed] [Google Scholar]
- 40.Swargiary A., Daimari A., Daimari M., Basumatary N., Narzary E. Phytochemicals, antioxidant, and anthelmintic activity of selected traditional wild edible plants of lower Assam. Indian J. Pharmacol. 2016;48:418. doi: 10.4103/0253-7613.186212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Azando E.V.B., Hounzangbé-Adoté M.S., Olounladé P.A., Brunet S., Fabre N., Valentin A., Hoste H. Involvement of tannins and flavonoids in the in vitro effects of Newbouldia laevis and Zanthoxylum zanthoxyloïdes extracts on the exsheathment of third-stage infective larvae of gastrointestinal nematodes. Vet. Parasitol. 2011;180:292–297. doi: 10.1016/J.VETPAR.2011.03.010. [DOI] [PubMed] [Google Scholar]
- 42.Ikbal A.M.A., Rajkhowa A., Chinglemba Singh P., Choudhury P.D., Sahu R.K. Assessment of phytochemical and anthelmintic activity of some selected ethnomedicinal plants from Barak Valley region of Assam. Biomed. Pharmacol. J. 2020;13:1825–1831. doi: 10.13005/BPJ/2057. [DOI] [Google Scholar]
- 43.Lucida H., Primadini Y., Suhatri A study on the acute toxicity of quercetin solid dispersion as a potential nephron-protector. Rasayan J. Chem. 2019;12:727–732. doi: 10.31788/RJC.2019.1224068. [DOI] [Google Scholar]
- 44.Soulsby E.J.L. Bailliere Tindall; London: 1986. Helminths, Arthopods, and Protozoa of Domesticated Animal Seventh Editon. [Google Scholar]
- 45.Mohammedsalih K.M., Krücken J., Khalafalla A., Bashar A., Juma F.R., Abakar A., Abdalmalaik A.A.H., Coles G., Von Samson-Himmelstjerna G. 2020. New Codon 198 β-tubulin Polymorphisms in Highly Benzimidazole Resistant Haemonchus contortus from Goats in Three Different States in Sudan, Parasites and Vectors; pp. 1–15. 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Salehi B., Machin L., Monzote L., Sharifi-Rad J., Ezzat S.M., Salem M.A., Merghany R.M., El Mahdy N.M., Klllç C.S., Sytar O., Sharifi-Rad M., Sharopov F., Martins N., Martorell M., Cho W.C. Therapeutic potential of quercetin: new insights and perspectives for human health. ACS Omega. 2020;5:11849–11872. doi: 10.1021/acsomega.0c01818. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data included in article/supp. material/referenced in article.