Abstract
Objectives
Myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) is a complex chronic and debilitating multifactorial disease. Adequate patient care is challenged by poor knowledge among health care professionals and the historical misconception that the disease is psychological in nature. This study assessed the health-related challenges faced by patients with ME/CFS in Switzerland and examined whether they receive adequate health care.
Methods
Quantitative and qualitative data were collected through a self-administered questionnaire between June and September of 2021, among 169 patients with ME/CFS in Switzerland.
Results
The mean age at diagnosis was 38.8 years. Only one-third of ME/CFS affected children and youth were correctly diagnosed before their 18th birthday. The mean time from disease onset to diagnosis was 6.7 years, and patients had an average of 11.1 different appointments and 2.6 misdiagnoses. A poor diagnosis rate and insufficient disease knowledge among health professionals in Switzerland led 13.5% of the patients to travel abroad to seek a diagnosis. Most patients (90.5%) were told at least once that their symptoms were psychosomatic. Swiss patients expressed high dissatisfaction with the health system and indicated that physicians lacked knowledge regarding ME/CFS. Therapies prescribed by physicians or tried by patients, as well as their perceived efficacy, were described. Graded Exercise Therapy (GET) was perceived as harmful by patients, whereas pacing, complementary/alternative medicine, and dietary supplements and medications to alleviate symptoms were reported to be helpful to varying degrees.
Conclusion
This study highlights that poor disease knowledge among health care providers in Switzerland has led to high patient dissatisfaction, and delays in ME/CFS diagnoses and prescription of inappropriate therapies, thus adding to patient distress and disease burden.
Keywords: Diagnostic, Knowledge, Medical care, Myalgic encephalomyelitis/chronic fatigue syndrome, Switzerland, Therapies
الملخص
أهداف البحث
التهاب الدماغ والنخاع العضلي / متلازمة التعب المزمن هو مرض مزمن معقد متعدد العوامل. تواجه رعاية المرضى الكافية تحديا بسبب ضعف المعرفة بين المتخصصين في الرعاية الصحية والاعتقاد التاريخي الخاطئ بأن المرض له طبيعة نفسية. قيمت هذه الدراسة التحديات المتعلقة بالصحة التي يواجهها مرضى التهاب الدماغ والنخاع العضلي / متلازمة التعب المزمن في سويسرا، وما إذا كانوا يتلقون رعاية صحية كافية أم لا.
طريقة البحث
تم جمع البيانات الكمية والنوعية من استبيان تم إجراؤه ذاتيا بين يونيو وسبتمبر 2021 بين 169 مريضا بالتهاب الدماغ والنخاع العضلي / متلازمة التعب المزمن في سويسرا.
النتائج
كان متوسط العمر عند التشخيص 38.8 سنة. كان التشخيص في الأعمار دون 18 عاما سيئا للغاية. كان متوسط الوقت من بداية المرض إلى التشخيص 6.7 سنوات ، مع أخذ 11.1 موعدا مختلفا في المتوسط و 2.6 تشخيصا خاطئا. أدى ضعف معدل التشخيص والمعرفة بالأمراض بين المهنيين الصحيين في سويسرا إلى سفر 13.5٪ من المرضى إلى الخارج للبحث عن التشخيص. تم إخبار الغالبية العظمى من المرضى (90.5٪) مرة واحدة على الأقل أن أعراضهم نفسية جسدية. أعرب المرضى السويسريون عن مستوى عال من عدم الرضا عن النظام الصحي وأشاروا إلى نقص المعرفة بين الأطباء فيما يتعلق بالتهاب الدماغ والنخاع العضلي / متلازمة التعب المزمن. تم وصف العلاجات الموصوفة من قبل الأطباء أو التي جربها المرضى بالإضافة إلى فعاليتها المتصورة. اعتبر المرضى العلاج التدريجي التدريجي ضارا، في حين تم الإبلاغ عن فائدة (أثناء تنظيمه) الطب التكميلي / البديل، والمكملات الغذائية والأدوية لتخفيف الأعراض بدرجات متفاوتة.
الاستنتاجات
سلطت هذه الدراسة الضوء على ضعف المعرفة بالأمراض بين مقدمي الرعاية الصحية في سويسرا، مما أدى إلى ارتفاع مستوى عدم رضا المرضى، وتأخر تشخيص التهاب الدماغ والنخاع العضلي / متلازمة التعب المزمن ووصف العلاجات غير المناسبة، مما يزيد من ضائقة المريض وعبء المرض.
الكلمات المفتاحية: التشخيص, المعرفة, التهاب الدماغ والنخاع العضلي / متلازمة التعب المزمن, رعاية طبية, سويسرا, العلاجات
Introduction
Myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) is a multifactorial, complex, chronic, stigmatizing, physically debilitating disease whose etiology remains unknown.1 It simultaneously affects multiple systems of the body in response to triggers. Several factors have been associated with disease onset, such as infectious diseases, autoimmune dysfunction, extreme stress, underlying genetic predisposition, receptor dysfunction and toxins.2, 3, 4, 5, 6 The disease lasts more than 6 months but is often lifelong, and is characterized by severe debilitating fatigue, post-exertional-malaise, sleep disturbance, cognitive impairment, pains, and other immunological, neurological and endocrinological symptoms associated with the affected body system.7,8 Dysregulation of the immune system is a key feature of ME/CFS, as indicated by elevated inflammatory and immune processes and chronic neuro-inflammation.3,7,9,10 Other key features of the disease include decreased metabolism and impaired mitochondrial functions.7 Infectious diseases, particularly viral diseases, are believed to play important roles in disease onset and/or shaping the disease course.11 Symptoms are exacerbated by triggers, including physical and mental activities or stress, thus leading patients to experience so-called crashes, from which recovery is very slow.12,13
ME/CFS is often overlooked and historically was long misclassified by physicians as a psychosomatic or psychological disease.14 Consequently, patients were not provided with adequate care, help or support.15 Despite the large existing body of scientific evidence on the physical pathologies of ME/CFS, many health care workers still lack knowledge or the disease, and most still believe in a psychological underlying cause, as indicated by a recent audit performed in hospitals in the UK (Hng et al., 2019). In the US, more than 80% and 90% of adult and child patients, respectively, have been estimated to remain undiagnosed.16,17 Generally, patients report poor satisfaction with provided medical care.18,19 Several factors and barriers are regularly associated with poor health care provision for ME/CFS. First, few physicians are knowledgeable regarding the disease.20,21 Timbol and Baraniuk22 have reported that patients with ME/CFS admitted to the emergency department in the US for predominantly cardiovascular problems (e.g., orthostatic intolerance) have indicated that physicians attributed their symptoms to stress, anxiety or psychological issues, and had poor to no knowledge of ME/CFS. Patients must travel long distances to find specialists19,23 and usually attend several appointments before a diagnosis is made.20,21 Moreover, financial barriers exacerbate the lack of access to health care provision.19,23,24
To our knowledge, Switzerland currently has no scientific data on ME/CFS in its population. A meta-analysis by Lim et al.25 has estimated a pooled prevalence of ME/CFS of 0.4%, which would translate to at least 34,000 people with ME/CFS in Switzerland, which is comparable to the number of people with other recognized diseases, such as multiple sclerosis.26 In light of the globally poor disease knowledge among medical professionals, we asked whether the population with ME/CFS in Switzerland is receiving adequate health care. To answer this question, we conducted a preliminary epidemiological survey among patients with ME/CFS. Herein, we present the health care provision to patients with ME/CFS, on the basis of quantitative and qualitative data.
Materials and Methods
Study design and participants
This study was part of a larger cross-sectional study performed between June and September of 2021 in Switzerland, including 169 patients with ME/CFS who were 18 years of age or older, who were recruited through the largest Swiss ME/CFS association (Tschopp et al., submitted). Purposive sampling was used. All members of the national Swiss ME/CFS association were informed of the study (e.g., through mailing lists, newsletters, websites and direct information provided during association meetings). Detailed information on the study, the questionnaire and the consent form was sent individually by mail to all people willing to participate in the study. Written informed consent was requested from all participants. Completed questionnaires and consent forms were returned with a pre-stamped return envelope.
Questionnaire survey
A paper questionnaire survey was self-administered by participants, because of the ongoing COVID-19 pandemic. Respondents were able to answer questions at their own pace, given that responding to the questionnaire might potentially have been exhausting.
Each questionnaire was coded with a unique numerical ID number. The questionnaire was prepared in German and French, and was pre-tested with four patients with ME/CFS who did not participate in the survey, to ensure that the questions were well designed and well understood. Questions in the overall questionnaire included closed and open questions on the following topics: general demography, disease history, therapies, socio-economic impact of the disease, coping mechanisms and ME/CFS during the COVID-19 pandemic. Participants were invited to provide in depth, detailed or additional information about their disease that was not captured in the questionnaire.
Data management and statistical analysis
Questionnaire data were entered into Microsoft Access and analyzed in STATA software version 16.1 (StataCorp LLC, USA). Descriptive statistical analysis was used to analyze the study population data and perform group comparisons. A p-value <0.05 was considered statistically significant. Additional qualitative data collected in the questionnaire as open-ended questions were entered into Microsoft Excel and analyzed descriptively, and are reported as illustrative quotations. Qualitative data from the open-section were analyzed thematically.
Results
ME/CFS diagnosis
The mean age at diagnosis was 38.8 years (95% CI: 36.9–40.7; SE = 0.95). Half the patients were diagnosed between the ages of 25 and 44 years (51.6%). None of the young children (6–12 years) were diagnosed while being in their age category, and only one-third (N = 8/24; 33.3%) of all children and youths with ME/CFS were diagnosed before their 18th birthday (see Table 1).
Table 1.
Comparison of age groups at ME/CFS onset and at diagnosis (N = 155).
| Age category | ME/CFS onset | ME/CFS diagnosis |
|---|---|---|
| 6–12 years | 5 (3.2) | 0 (0.0) |
| 13–18 years | 19 (12.3) | 8 (5.2) |
| 19–24 years | 21 (13.6) | 12 (7.7) |
| 25–44 years | 89 (57.8) | 80 (51.6) |
| 45–64 years | 19 (12.3) | 53 (34.2) |
| ≥65 years | 2 (1.3) | 2 (1.3) |
The mean time from disease onset to final ME/CFS diagnosis by physicians was 6.7 years (95% CI: 5.5–7.9; SE = 0.60).
General practitioners (GPs) and specialists were both involved in diagnosis (often in combination). Among the 138 patients diagnosed by specialized physicians, the following top three medical specialties were described: physicians in clinics specializing in general medicine (N = 47; 34%), psychiatrists (N = 45; 32.6%) and neurologists (N = 35; 25.4%). Other specialties described were internists (N = 19; 13.8%), physicians in psychosomatic medicine (N = 16; 11.6%), psychologists (N = 15; 10.9%), immunologists (N = 14; 10.1%), cardiologists (N = 5; 3.6%), pediatricians (N = 2; 1.4%), physicians in tropical medicine (N = 1; 0.7%) and others (N = 22; 15.9%). No statistical differences were observed among patient genders or the types of specialists providing the diagnosis. Twenty-one patients (13.5%) sought treatment abroad (Germany, the Netherlands, United Kingdom, Austria, South Africa or USA) after no physician in Switzerland was able to provide a diagnosis. Therefore, these patients received an ME/CFS diagnosis from clinics and/or health specialists abroad.
Before receiving their ME/CFS diagnosis, patients visited an average of 11.1 physicians (95% CI: 9.4–12.9; SE = 0.86) and received a mean of 2.6 other diagnoses (95% CI: 2.1–3.1), primarily mental health conditions (e.g., depression or psychosomatic disease), burn-out or neurasthenia. A total of 90.5% of patients were told at least once before their final ME/CFS diagnosis that their symptoms were psychosomatic.
A total of 115 patients described how they felt when they received their ME/CFS diagnosis. Most of them experienced a sense of relief in finally knowing their diagnosis (N = 84; 73%), whereas 24 (20.9%) had negative feelings of sadness, hopelessness or anxiety because of the poor prognosis, and a loss of hope for healing, given the lack of available therapies and support. The diagnosis of ME/CFS led some patients to have less stress and anxiety (N = 3); to experience a feeling of finally being taken seriously (N = 2); and to stop fighting the symptoms, and learn to accept and cope with them (N = 4). Furthermore, the diagnosis enabled patients to focus on healing, to adjust to a new lifestyle (N = 1) and to be able to explain their condition to others. After having a name for the disease, patients were able to research the disease on the internet and in books.
Patients’ feelings toward provided medical care
Most patients found physicians understanding and supportive (N = 112; 66.3%), whereas 106 and 63 patients stated that physicians were arrogant and disparaging, respectively. Sixty-five described “other” behaviors, such as physicians being helpless and overwhelmed (N = 12), not believing patients (N = 4), having no knowledge of the disease (N = 8), being prejudiced (N = 1), being annoyed with patients (N = 3), showing no interest (N = 2), humiliating patients (N = 1) and categorizing (N = 1).
During the entire disease period, including pre-diagnosis, patients left medical appointments with negative feelings three times more often than with positive ones (e.g., feeling happy, hopeful or taken seriously). Negative feelings were reported by 113 patients who said that they were often not taken seriously, 92 who felt hopeless, 86 who felt desperate, 60 who felt humiliated and 59 who felt ignored.
Of 168 patients, only two described the medical service regarding ME/CFS as good, whereas 38 (22.6%) and 129 (76.8%) considered it bad and very bad, respectively. Only 16% (N = 27) of respondents thought that physicians had sufficient knowledge regarding ME/CFS. Table 2 highlights quotations from participants regarding their feelings toward the provided health care.
Table 2.
Illustrative quotations from qualitative data collected from participants regarding their diagnosis, and their feelings toward the provided medical care and prescribed therapies.
| Disease diagnostic | Patient's feelings toward provided medical care | Reported therapies |
|---|---|---|
| “The psychiatrist was the only one who knew ME/CFS.” (Female, 55, BE) | “I gave up going to doctors. I am so angry and frustrated. They have to learn to see patients as collaborators and not enemies.” (Female, 47, ZH) | “Before my disease, I have always been a big sportsman, so GET was attractive to me when prescribed. However, it worsened all my symptoms.” (Male, 53, BE) |
| “All doctors said I had depression, but the psychiatrist said I had no depression and that it was somatic.” (Female, 41, GR) | “If I had money, I would go abroad to a good doctor.” (Female, 59, LU) | “One of the first diagnoses was burn-out; doctors asked me then to do a lot of sports, which worsened my symptoms terribly. Then around ten doctors said it was psychosomatic, and I was sent to the psychiatrist, who said I was mentally healthy, and it was somatic, and I was sick. Then I was finally diagnosed with ME/CFS by a CFS specialist.” (Male, 38, ZH) |
| “I gave up going to physicians in Switzerland, I went to London, where I was diagnosed with ME/CFS. Back in Switzerland, I shared the report with my doctor, who said that CFS did not exist.” (Female, 51, AG) | “Being taken seriously by a doctor helped me to carry the burden caused by the disease.” (Male, 38, BE) | “Previously, I was mis-diagnosed as having psychosomatic issues and sent to a rehabilitation clinic. I had to do a lot of group activities to foster communication, as well as aggressive massages and lots of fitness. I had to stop, as I was getting sicker and sicker.” (Male, 51, VS) |
| “It was a relief to know what is wrong with me after 31 years, and understand my symptoms.” (Female, 46, LU). | “It is important for GPs to know the disease, because private clinics are too expensive for follow-ups.” (Female, 46, ZH) | “I was sent to a psychosomatic rehabilitation clinic. They made me do plenty of sports, which ultimately harmed me. I went into a three month crash afterwards. Nobody knew about ME/CFS there.” (Male, 44, AG) |
| “I was relieved to receive a diagnosis; after 10 years of being told it was all in my head, I really started believing I was mad.” (Female, 33, ZH) | “The specialist who diagnosed me is too far away; it is impossible to do follow-ups.” (Male, 69, AG) | “I went to a psychosomatic rehabilitation clinic, where I had to do a lot of sports. I left the clinic in a wheelchair. I went into a 1.5 month severe crash afterwards with fever and worsening of all symptoms; these clinics have to understand ME/CFS.” (female, 41, ZH) |
| “I am too weak to go the doctor and can often only do telephone appointments.” (Female, 54, AG) | “Many wrong diagnoses and therapies (anti-depressives and physical activities) worsened my condition over the years.” (Female, 57, SG) | |
| “There is no support; if my disease gets worse, I will register with EXIT.a” (Female, 46, LU) | “After the diagnosis of ME/CFS, I stopped the anti-depressants, and I feel so much better now.” (Female, 46, ZH) | |
| “Before my ME/CFS diagnosis, I received anti-depressants over many years. All my symptoms worsened.” (Male, 53, ZH) | ||
| “My worst experience was a doctor who forced me to do a lot of physical activities, which then worsened my symptoms a lot.” (Male, 38, BE). | ||
| “The wrong therapies I received, that led to severe worsening of the disease, make me now fearful to go to new doctors.” (Female, 31, AG) |
EXIT: Swiss Society dedicated to human self-determination, including physician-assisted suicide.
Reported therapies
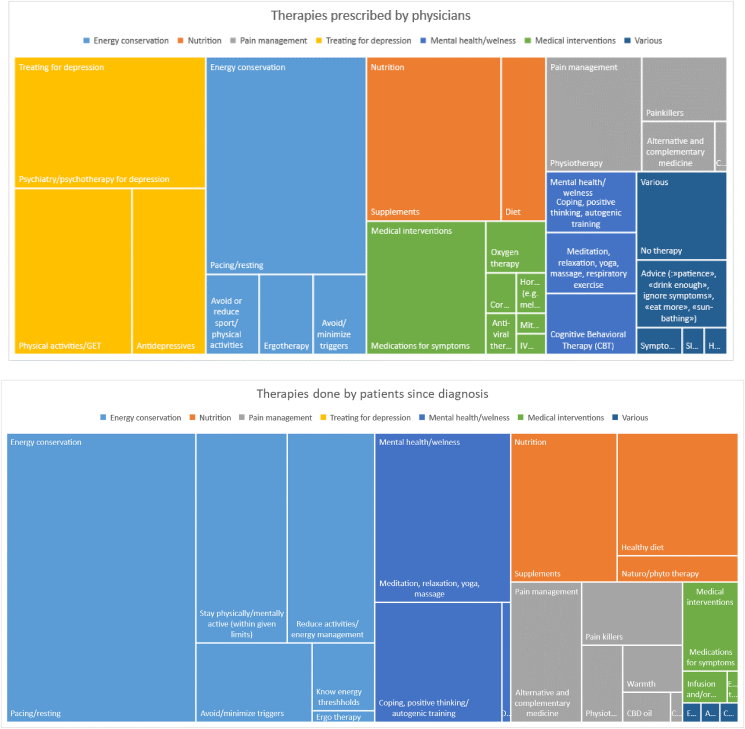
Before diagnosis, the most frequently prescribed intervention by medical professionals (N = 93) was treatment for depression (N = 93), regardless of patient gender (no statistical difference). In contrast, the top recommendations from physicians who had properly diagnosed patients with ME/CFS were primarily associated with energy conservation strategies (N = 78). The following strategies were also recommended by physicians (before and after diagnosis): nutrition (N = 48), medication to alleviate some of the symptoms (N = 39), pain management (N = 34) and a focus on mental health/wellness (N = 27). Patients, in contrast, prioritized energy conservation techniques (N = 301), a focus on mental health/wellbeing (N = 111), nutrition (N = 96) and pain management (N = 50).
Figure 1 shows a tree chart with the recommendations made by physicians during the entire duration of illness (pre and post diagnosis) and compares the strategies used by patients after diagnosis with ME/CFS.
Figure 1.
Tree chart comparing recommendations given by physicians (A) versus strategies that patients tried by themselves after diagnosis (open question) (B).
We investigated the efficiency of the reported treatment strategies. Table 3 shows the therapies tried by all participants at least once during the course of illness. The number of therapies tried by our patients with ME/CFS ranged from 1 to 11 (mean: 6.5; 95% CI: 6.2–6.9; SD: 2.2), and 82% of the participants reported trying multiple therapies.
Table 3.
Therapies tried by participants.
| Strategy | Number (%) |
|---|---|
| Pacing | 150 (89.8) |
| Supplements | 148 (88.1) |
| Alternative and complementary medicine | 143 (85.1) |
| Pharmacological symptom therapy | 130 (76.9) |
| Physiotherapy | 109 (64.9) |
| Graded Exercise Therapy (GET) | 98 (58.3) |
| Cognitive Behavioral Therapy (CBT) | 85 (50.6) |
| Microbiotics/probiotics/stool transplantation | 85 (50.6) |
| CBD oil | 69 (41.1) |
| Others | 40 (23.8) |
| Mitochondrial therapy | 35 (20.8) |
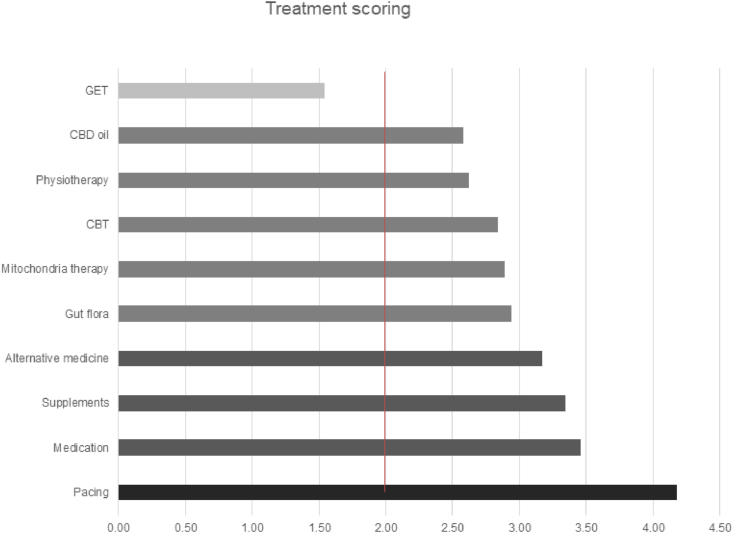
Figure 2 shows the average efficiency of each strategy, which was assigned a score with the following scale: 5 = helped a lot, 4 = helped moderately, 3 = helped a bit, 2 = did not help and 1 = was harmful. Only pacing, medication to alleviate symptoms, alternative medicine and dietary supplements helped, on average, whereas GET was reported to have been harmful.
Figure 2.
Average efficiency of prescribed treatments.
Participants reflected, in their own words, on the therapies that they received (Table 2).
Discussion
Although ME/CFS is diagnosed by exclusion of a broad spectrum of possible etiologies and follows international diagnostic guidelines, the lack for improved acknowledgement and expertise in ME/CFS among physicians in Switzerland is highlighted by the latency between the onset of disease and establishment of a diagnosis (approximately 7 years). Given that more than 80% of patients remain undiagnosed,27, 28, 29 this delay is likely to be an under-estimation. Our results suggest that children are at particular risk of remaining undiagnosed and consequently are exposed to mis-management. Pediatric ME/CFS, which has an estimated prevalence between 0.1% and 0.5% (Rowe et al., 2017) has long lasting effects on children, e.g., high dropout rates in school, limited access to education and entry into adulthood with chronic disease. The peak age of disease onset in pediatric ME/CFS is between 11 and 19 years.30, 31, 32 Hence, pediatricians' awareness of the negative effects of this disease must be addressed, and referral strategies with sensitization for early symptoms must be established.
In our study, only two pediatricians among the 24 pediatric patients provided an ME/CFS diagnosis. Overall, one-third of the diagnoses were made by general medicine physicians or psychiatrists, and one-quarter were made by neurologists. Only 10% of respondents were diagnosed by an immunologist, although the disease is known to have major immunological components.3,10,33 ME/CFS appears to remain poorly recognized among many medical experts and specialists in Switzerland; thus, better awareness among health professionals is urgently warranted. On average, more than 11 different appointments were required, and several misdiagnoses were made to establish a conformed diagnosis of ME/CFS—substantially more than previously reported in studies from other countries. Sunnquist et al.23 have observed an average of four or more appointments in the USA until diagnosis, and only 11.5% of the patients with ME/CFS being treated and managed by adequate specialists. Before the diagnosis, more than 90% of enrolled patients in our study were told at least once that their disease was of psychological nature and received inadequate management. This result is in line with findings from a recent audit performed in the UK showing that 91% of the health care providers considered ME/CFS at least partly psychological and demonstrated poor overall disease knowledge.34 Swiss patients with ME/CFS have therefore developed mistrust of, and resentment toward, psychologists/psychiatrists. Interestingly, however, one-third of the ME/CFS diagnoses were actually provided by psychiatrists who rejected the psychological etiology underlying the specialists’ referrals and instead identified a somatic health problem.
Health service provision was considered poor by patients: only two of 168 patients stated that they felt adequately managed, whereas 16% described that their physician displayed knowledge regarding ME/CFS. Overall, during their entire time of living with the disease (pre and post diagnosis), patients experienced negative feelings (desperation, not being taken seriously, and feeling hopeless, humiliated or ignored) three times more often than positive feelings after their medical appointments. The poor diagnosis success rate in Switzerland discouraged 13.5% of the study population from traveling abroad and seeking diagnosis elsewhere.
Others sought online medical support, such as therapy guidance with ME/CFS experts abroad, in countries where ME/CFS is recognized and better known among physicians. Patients with ME/CFS with high dissatisfaction with the provided medical care have also been reported from Germany, the UK and the US.19,21, 22, 23 Although experts in ME/CFS exist, they are few. Most physicians and health care providers in Switzerland have limited knowledge of the disease, as also reported in other studies.21,22,34 Some additional described barriers to heath care in our study, were the lack of house visits by GPs for house or bed-bound patients; the remoteness of specialized physicians, thereby making access difficult for patients with ME/CFS; and the difficulty in receiving follow-up care after diagnosis because of the high cost of private clinics. Poor overall disease knowledge was also reflected in the therapies recommended by physicians to patients. Before diagnosis, 93 patients received recommendations for treatment for depression (e.g., use of anti-depressants or psychiatrist support), which patients perceived as harmful. Post-exertional malaise and the pathology of ME/CFS, involving downregulation of the hypothalamic–pituitary–adrenal axis and impairment of the central and autonomous system, differ from pathologies causing depression35; Morris et al., 2007). Of particular concern, psychotropic medications prescribed for mood disorders and depression have shown detrimental effects on mitochondrial functions through, e.g., decreased carnitine availability, and impaired respiratory chain and ATP production.36, 37, 38 Mitochondrial impairment is a key feature among patients with ME/CFS. Such medications can therefore aggravate pre-existing impairments and worsen symptoms. Patients were encouraged to increase their physical activity or were sent to rehabilitation clinics in which promoting physical activity was among the core treatments. Those patients reported severe worsening of their illness, sometimes to dramatic levels (e.g., months-long crashes or being wheelchair bound). Of all types of therapy attempted by patients, GET was the only one reported to have harmful effects. Compelling scientific evidence has now indicated the pathways and pathological mechanisms underlying muscular fatigue and pains, as well as the neuro-immunological impairment triggered by physical activity; these findings support the counterproductive and harmful effects of GET in patients with ME/CFS.15,39,40 Until dissemination of the available robust evidence indicating that GET is detrimental and should no longer be used in treating patients with ME/CFS, the misconception of physical activity being beneficial in ME/CFS will remain anchored in the psychosomatic dogma widely held by health care providers.41,42
After being properly diagnosed with ME/CFS, patients received recommendations by their physicians to follow “energy-saving strategies.” Our study also indicated that pacing was perceived by the respondents to be the most effective strategy for alleviating some of the symptoms, beyond medications, complementary alternative medicine and diet. Nutritional supplements in ME/CFS have shown little evidence of benefit in the scientific literature,43 although the current evidence is insufficient to draw conclusions. The study participants’ views on the efficacy of supplements were also too heterogeneous to draw a conclusion; therefore, more research is clearly warranted in this field. No approved medical treatments are currently available for ME/CFS, and no cure exists.6 Our study showed that patients independently tried several approaches to improve their symptoms, with or without support from their physicians. Supplements were commonly used (e.g., NADH, coenzyme, vitamins and minerals) as well as approaches focusing on pain relief (medication and alternative/complementary medicine) and energy management (e.g., pacing and daily/weekly energy management plans). Antiviral agents were recommended in two patients, and one patient participated in a clinical trial of an experimental drug. Antiviral agents such as valaciclovir and valganciclovir have been described to elicit some improvements in a small number of patients, in whom EBV or HHP 6 were suspected as possible disease triggers.44, 45, 46 Therapeutic research and trials have focused mainly on targeting immune modulation through the use of antiviral agents, immunosuppressants, immunostimulation, and mitochondrial support.9,47, 48, 49, 50, 51, 52 To date, rintatolimod, a drug developed for cancer and aggressive viral infections, which increases natural killer cell function and acts as a TLR3 agonist, is the only drug that has been assessed specifically for ME/CFS; however, it has not yet been approved, except in Argentina.53
The limitations of this study were those inherent to self-administered questionnaire studies, including possible recall bias. This study indicated that patients with ME/CFS in Switzerland experience notable challenges in receiving prompt diagnosis, adequate health care and support. The disease may remain undiagnosed or mis-diagnosed for an often unacceptable latency, which is associated with incorrect and sometimes harmful treatments. Swiss patients reported high dissatisfaction with existing health care. The study highlighted the lack of disease knowledge among physicians. Because no diagnostic laboratory tests are currently available, and no cure currently exists, improving awareness and knowledge of ME/CFS among physicians and health care providers is of paramount importance. In Switzerland, an urgent need exists to improve the medical care of patients with ME/CFS, and achieve better understanding and acceptance of the disease, ideally through stronger early integration into the curriculum of human medicine training and continuing education.
If an adequate diagnosis can be made earlier in the disease course, (i) a more reliable prospective evidence base could be created to shape guidelines, inform healthcare policy and provide a foundation for greater investment in research, and (ii) adequate supportive treatment and correct management could be initiated in a higher proportion of patients with ME/CFS.
Source of funding
This research did not receive any specific grant from funding agencies in the public, commercial or not-for-profit sectors.
Conflict of interest
The authors have no conflict of interest to declare.
Ethical approval
This research received approval from the Ethics Committee of Northwestern and Central Switzerland (EKNZ, Switzerland), and copies were sent to all other relevant ethics committees in Switzerland (Basec nr. 2021-01098; July 2021).
Author's contributions
RT conceived and designed the study, and conducted the research. RT and DP provided the research material, and collected and organized the data. RT, RK, PR and DP analyzed and interpreted the data. RT, RK, PR and DP wrote the initial and final drafts of the article. All authors have critically reviewed and approved the final draft, and are responsible for the content and similarity index of the manuscript.
Acknowledgement
We thank the Swiss ME/CFS association for collaborating on this study and providing logistic support. We are greatly thankful to all participants with ME/CFS and their families for their time and effort, which made this study possible. We also thank Sophie Joy Mosko for help in editing the manuscript. This study was funded by Swiss TPH.
Footnotes
Peer review under responsibility of Taibah University.
References
- 1.Friedberg F., Bateman L., Bested A., Davenport T., Friedman K., Gurwitt A., et al. IACFS/ME; Chicago, IL: 2014. ME/CFS: A Primer for Clinical Practioners.https://iacfsme.org/portals/0/pdf/Primer_Post_2014_conference.pdf Available online at: [Google Scholar]
- 2.Glassford J.A.G. The neuroinflammatory etiopathology of myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) Front Physiol. 2017;8:88. doi: 10.3389/fphys.2017.00088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mandarano A.H., Maya J., Giloteaux L., Peterson D.L., Maynard M., et al. Myalgic encephalomyelitis/chronic fatigue syndrome patients exhibit altered T cell metabolism and cytokine associations. J Clin Invest. 2020 doi: 10.1172/JCI132185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marshall-Gradisnik S., Huth T., Anu C., Johnston S., Smith P., et al. Natural killer cells and single nucleotide polymorphisms of specific ion channels and receptor genes in Myalgic Encephalomyelitis/chronic fatigue syndrome. Appl Clin Genet. 2016;9:39–47. doi: 10.2147/TACG.S99405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wirth K., Scheibenbogen C. A unifying hypothesis of the pathophysiology of myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS): recognitions from the finding of autoantibodies against ß2-adrenergic receptors. Autoimmun Rev. 2020;19(6) doi: 10.1016/j.autrev.2020.102527. [DOI] [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention (CDC) 2022. Myalgic encephalomyelitis/chronic fatigue syndrome.https://www.cdc.gov/me-cfs/index.html [Google Scholar]
- 7.Sweetman E., Noble A., Edgar C., Mackay A., Helliwell A., Vallings R., et al. Current research provides insight into the biological basis and diagnostic potential for myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) Diagnostics. 2019;9:73. doi: 10.3390/diagnostics9030073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Poenaru S., Abdallah S.J., Corrales-Medina V., Cowan J. COVID-19 and post-infectious myalgic encephalomyelitis/chronic fatigue syndrome: a narrative review. Ther Adv Infect Dis. 2021;8 doi: 10.1177/20499361211009385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Montoya J.G., Holmes T.H., Anderson J.N., Maecker H.T., Rosenberg-Hasson Y., et al. Cytokine signature associated with disease severity in chronic fatigue syndrome patients. Proc Natl Acad Sci USA. 2017;114(34):E7150–E7158. doi: 10.1073/pnas.1710519114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Anderson G., Maes M. Mitochondria and immunity in chronic fatigue syndrome. Prog Neuro-Psychopharmacol Biol Psychiatry. 2020;103 doi: 10.1016/j.pnpbp.2020.109976. [DOI] [PubMed] [Google Scholar]
- 11.Albright F., Light K., Light A., Bateman L., Cannon-Albright L.A. Evidence for a heritable predisposition to chronic fatigue syndrome. BMC Neurol. 2011;11(1):62. doi: 10.1186/1471-2377-11-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Adamowicz J.L., Caikauskaite I., Friedberg F. Defining recovery in chronic fatigue syndrome: a critical review. Qual Life Res. 2014;23(9):2407–2416. doi: 10.1007/s11136-014-0705-9. [DOI] [PubMed] [Google Scholar]
- 13.Rowe P., Fontaine K., Lauver M., Jasion S.E., Marden C.L., et al. Neuromuscular strain increases symptom intensity in chronic fatigue syndrome. PLoS One. 2016;11 doi: 10.1371/journal.pone.0159386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Neu D., Mairesse O., Montana X., Gilson M., Corazza F., Lefevre N., et al. Dimensions of pure chronic fatigue: psychophysical, cognitive and biological correlates in the chronic fatigue syndrome. Eur J Appl Physiol. 2014;114:1841–1851. doi: 10.1007/s00421-014-2910-1. [DOI] [PubMed] [Google Scholar]
- 15.Friedman K.J. Advances in ME/CFS: past, present, and future. Front Pediat. 2019;7:131. doi: 10.3389/fped.2019.00131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Solomon L., Reeves W.C. Factors influencing the diagnosis of chronic fatigue syndrome. Arch Intern Med. 2004;164(20):2241–2245. doi: 10.1001/archinte.164.20.2241. [DOI] [PubMed] [Google Scholar]
- 17.Jason L.A., Katz B.Z., Sunnquist M., Torres C., Cotler J., Bhatia S. The prevalence of pediatric myalgic encephalomyelitis/chronic fatigue syndrome in a community-based sample. Child Youth Care Forum. 2020;49:563–579. doi: 10.1007/s10566-019-09543-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thanawala S., Taylor R.R. Service utilization, barriers to service access, and coping in adults with Chronic Fatigue Syndrome. J Chronic Fatigue Syndrome. 2007;14:5–21. doi: 10.1300/J092v14n01_02. [DOI] [Google Scholar]
- 19.Froehlich L., Hattesohl D.B.R., Jason L.A., Scheibenbogen C., Behrends U., Thoma M. Medical care situation of people with myalgic encephalomyelitis/chronic fatigue syndrome in Germany. Medicina. 2021;57(7):646. doi: 10.3390/medicina57070646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bowen J., Pheby D.F.H., Charlett A., McNulty C. Chronic Fatigue Syndrome: a survey of GPs' attitudes and knowledge. Fam Pract. 2005;22:389–393. doi: 10.1093/fampra/cmi019. [DOI] [PubMed] [Google Scholar]
- 21.Tidmore T., Jason L.A., Chapo-Kroger L., So S., Brown A., Silverman M. Lack of knowledgeable healthcare access for patients with neuro-endocrine-immune diseases. Front Clin Med. 2015;2:46–54. [Google Scholar]
- 22.Timbol C.R., Baraniuk J.N. Chronic fatigue syndrome in the emergency department. Open Access Emerg Med. 2019;11:15–28. doi: 10.2147/OAEM.S176843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sunnquist M., Nicholson L., Jason L.A., Friedman K.J. Access to medical care for individuals with Myalgic Encephalo-myelitis and Chronic Fatigue Syndrome: a call for centers of excellence. Mod. Clin. Med. Res. 2017;1:28–35. doi: 10.22606/mcmr.2017.11005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vanderbilt A.A., Dail M.D., Jaberi P. Reducing health disparities in underserved communities via interprofessional collaboration across health care professions. J Multidiscip Healthc. 2015;8:205–208. doi: 10.2147/JMDH.S74129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lim E.J., Ahn Y.C., Jang E.S., Lee S.W., Lee S.H., Son C.G. Systematic review and meta-analysis of the prevalence of chronic fatigue syndrome/myalgic encephalomyelitis (CFS/ME) J Transl Med. 2020;18(1):100. doi: 10.1186/s12967-020-02269-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kaufmann M., Puhan M.A., Kuhle J., Yaldizli Ö., Magnusson T., Kamm C.P., et al. A framework for estimating the burden of chronic diseases: design and application in the context of multiple sclerosis. Front Neurol. 2019;10:953. doi: 10.3389/fneur.2019.00953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reyes M., Nisenbaum R., Hoaglin D.C., Unger E.R., Emmons C., Randall B., et al. Prevalence and incidence of chronic fatigue syndrome in Wichita, Kansas. Arch Intern Med. 2003;163(13):1530–1536. doi: 10.1001/archinte.163.13.1530. [DOI] [PubMed] [Google Scholar]
- 28.Jason L.A., Richman J.A., Rademaker A.W., Jordan K.M., Plioplys A.V., Taylor R.R., et al. A community-based study of chronic fatigue syndrome. Arch Intern Med. 1999;159(18):2129–2137. doi: 10.1001/archinte.159.18.2129. [DOI] [PubMed] [Google Scholar]
- 29.Crawley E.M., Emond A.M., Sterne J.A.C. Unidentified chronic fatigue syndrome/myalgic encephalomyelitis (CFS/ME) is a major cause of school absence: surveillance outcomes from school-based clinics. BMJ Open. 2011;1(2) doi: 10.1136/bmjopen-2011-000252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bakken I.J., Tveito K., Gunnes N., Ghadori S., Stoltenberg C., et al. Two age peaks in the incidence of chronic fatigue syndrome/myalgic encephalomyelitis: a population-based registry study from Norway 2008-2012. BMC Med. 2014;12:167. doi: 10.1186/s12916-014-0167-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nijhof S.L., Maijer K., Bleijenberg G., Uiterwaal C., Kimpen J.L.L., van de Putte E.M. Adolescent chronic fatigue syndrome: prevalence, incidence, and morbidity. Pediatrics. 2011;127(5):E1169–E1175. doi: 10.1542/peds.2010-1147. [DOI] [PubMed] [Google Scholar]
- 32.Rimes K.A., Goodman R., Hotopf M., Wessely S., Meltzer H., Chalder T. Incidence, prognosis, and risk factors for fatigue and chronic fatigue syndrome in adolescents: a prospective community study. Pediatrics. 2007;119(3):e603–e609. doi: 10.1542/peds.2006-2231. [DOI] [PubMed] [Google Scholar]
- 33.Morris G., Anderson G., Maes M. Hypothalamic-Pituitary-adrenal hypofunction in Myalgic Encephalomyelitis (ME)/Chronic Fatigue Syndrome (CFS) as a consequence of activated immune-inflammatory and oxidative and nitrosative pathways. Mol Neurobiol. 2017;54:6806–6819. doi: 10.1007/s12035-016-0170-2. [DOI] [PubMed] [Google Scholar]
- 34.Hng K.N., Geraghty K., Pheby D.F.H. An audit of UK hospital doctors' knowledge and experience of myalgic encephalomyelitis. Medicina. 2021;57:885. doi: 10.3390/medicina57090885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cleare A.J. The neuroendocrinology of chronic fatigue syndrome. Endocr Rev. 2003;24:236–252. doi: 10.1210/er.2002-0014. PMID:12700181. [DOI] [PubMed] [Google Scholar]
- 36.Anglin R., Rosebush P., Mazurek M. Psychotropic medications and mitochondrial toxicity. Nat Rev Neurosci. 2012;13:650. doi: 10.1038/nrn3229-c1. [DOI] [PubMed] [Google Scholar]
- 37.Chan S.T., McCarthy M.J., Vawter M.P. Psychiatric drugs impact mitochondrial function in brain and other tissues. Schizophr Res. 2020;217:136–147. doi: 10.1016/j.schres.2019.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Emmerzaal T.L., Nijkamp G., Veldic M., Rahman S., Andreazza A.C., Morava E., et al. Effect of neuropsychiatric medications on mitochondrial function: for better or for worse. Neurosci Biobehav Rev. 2021;127:555–571. doi: 10.1016/j.neubiorev.2021.05.001. [DOI] [PubMed] [Google Scholar]
- 39.Gerwyn M., Maes M. Mechanisms explaining muscle fatigue and muscle pain in patients with myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS): a review of recent findings. Curr Rheumatol Rep. 2017;19(1) doi: 10.1007/s11926-017-0628-x. [DOI] [PubMed] [Google Scholar]
- 40.Twisk F.N., Maes M. A review on cognitive behavioral therapy (CBT) and graded exercise therapy (GET) in myalgic encephalomyelitis (ME)/chronic fatigue syndrome (CFS): CBT/GET is not only ineffective and not evidence-based, but also potentially harmful for many patients with ME/CFS. Neuroendocrinol Lett. 2009;30(3):284–299. [PubMed] [Google Scholar]
- 41.Vink M., Vink-Niese F. Graded exercise therapy does not restore the ability to work in ME/CFS – rethinking of a cochrane review. Work. 2020;20:283–308. doi: 10.3233/WOR-203174. [DOI] [PubMed] [Google Scholar]
- 42.Torjesen I. ME/CFS: exercise goals should be set by patients and not driven by treatment plan, says NICE. BMJ. 2021;375:n2643. doi: 10.1136/bmj.n2643. [DOI] [PubMed] [Google Scholar]
- 43.Castro-Marrero J., Saez-Francas N., Santillo D., Alegre J. Treatment and management of chronic fatigue syndrome/myalgic encephalomyelitis: all roads lead to Rome. Br J Pharmacol. 2017;174:345–369. doi: 10.1111/bph.13702. PMCID:5301046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lerner A.M., Beqaj S.H., Deeter R.G., Fitzgerald J.T. Valacyclovir treatment in Epstein-Barr virus subset chronic fatigue syndrome: thirty-six months follow-up. In Vivo. 2007;21:707–713. [PubMed] [Google Scholar]
- 45.Watt T., Oberfoell S., Balise R., Lunn M.R., Kar A.K., Merrihew L., et al. Response to valganciclovir in chronic fatigue syndrome patients with human herpesvirus 6 and Epstein-Barr virus IgG antibody titers. J Med Virol. 2012;84:1967–1974. doi: 10.1002/jmv.23411. [DOI] [PubMed] [Google Scholar]
- 46.Montoya J.G., Kogelnik A.M., Bhangoo M., Lunn M.R., Flamand L., Merrihew L.E., et al. Randomized clinical trial to evaluate the efficacy and safety of valganciclovir in a subset of patients with chronic fatigue syndrome. J Med Virol. 2013;85:2101–2109. doi: 10.1002/jmv.23713. [DOI] [PubMed] [Google Scholar]
- 47.Rowe K.S. Double-blind randomized controlled trial to assess the efficacy of intravenous gammaglobulin for the management of chronic fatigue syndrome in adolescents. J Psychiatr Res. 1997;31:133–147. doi: 10.1016/s0022-3956(96)00047-7. [DOI] [PubMed] [Google Scholar]
- 48.Fluge Ø., Risa K., Lunde S., Alme K., Rekeland I.G., Sapkota D., et al. B-lymphocyte depletion in myalgic encephalopathy/chronic fatigue syndrome. An open-label phase II study with rituximab maintenance treatment. PLoS One. 2015;10 doi: 10.1371/journal.pone.0129898. PMCID: 4488509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kaiser J.D. A prospective, proof-of-concept investigation of KPAX002 in chronic fatigue syndrome. Int J Clin Exp Med. 2015;8:11064–11074. [PMC free article] [PubMed] [Google Scholar]
- 50.Roerink M.E., Sebastian M.D., Bredie J.H., Heijnen M., Dinarello C.A., Knoop H., et al. Cytokine inhibition in patients with chronic fatigue syndrome. Ann Intern Med. 2017;166:557–564. doi: 10.7326/M16-2391. [DOI] [PubMed] [Google Scholar]
- 51.Rekeland I.G., Fossa A., Lande A., Ktoridou-Valen I., Sorland K., Holsen M., et al. Intravenous cyclophosphamide in myalgic encephalomyelitis/chronic fatigue syndrome. An open-label phase II study. Front Med. 2020;7:162. doi: 10.3389/fmed.2020.00162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Toogood P.L., Clauw D.J., Phadke S., Hoffman D. Myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS): where will the drugs come from? Pharmacol Res. 2021;165 doi: 10.1016/j.phrs.2021.105465. [DOI] [PubMed] [Google Scholar]
- 53.Mitchell W.M. Efficacy of rintatolimod in the treatment of chronic fatigue syndrome/myalgic encephalomyelitis (CFS/ME) Expet Rev Clin Pharmacol. 2016;9:755–770. doi: 10.1586/17512433.2016.1172960. [DOI] [PMC free article] [PubMed] [Google Scholar]