Abstract
Objectives
Baroreflex sensitivity (BRS) is an estimate of autonomic control of cardiovascular system via the baroreflex arc. It has been suggested that exercise pressure reflex and muscle metaboreflex override baroreflex during exercise to decrease baroreflex gain, which facilitates the simultaneous rise in blood pressure (BP) and heart rate during the exercise. This study investigated the effects of isometric handgrip exercise (IHE) on baroreflex gain and frequency dependence of baroreflex sensitivity while fluctuations in arterial BP were generated.
Methods
Thirteen healthy men performed IHE at 20% and 30% of their maximum voluntary contraction (MVC), while oscillatory lower body negative pressure (OLBNP) of 40 mmHg was applied in 0.1 and 0.25 Hz frequencies.
Results
Compared to the OLBNP at 0.25 Hz frequency alone, the baroreflex gain for diastolic BP (DBP) was significantly reduced with the addition of IHE at 20% and 30% of MVC in the high frequency band. At rest (without IHE and OLBNP) the baroreflex gain was significantly more in the high frequency band for DBP, but the baroreflex gain for DBP was not significantly different when IHE + OLBNP were applied at 20% and 30% of MVC in both frequencies.
Conclusions
The significant reduction of DBP baroreflex gain with the addition of graded IHE might indicate that exercise pressure reflex and muscle metaboreflex override baroreflex during exercise to decrease baroreflex gain at a high frequency band (0.25 Hz). The frequency-dependent phenomenon of BRS was altered when IHE and OLBNP were applied, meaning that the frequency dependence of BRS was nullified during IHE.
Keywords: Baroreflex gain, Baroreflex sensitivity, Isometric handgrip exercise, Maximum voluntary contraction, Oscillatory lower body negative pressure
المخلص
أهداف البحث
حساسية باروريفلكس هي تقدير للتحكم اللاإرادي في الجهاز القلبي الوعائي عبر قوس منعكس الضغط. لقد تم اقتراح أن منعكس ضغط التمرين وعضلة ميتابوريفلكس يتجاوزان منعكس الضغط أثناء التمرين لتقليل اكتساب منعكس الضغط ، مما يسهل الارتفاع المتزامن في ضغط الدم ومعدل ضربات القلب أثناء التمرين. بحثت هذه الدراسة في تأثير تمرين قبضة اليد متساوي القياس على اكتساب منعكس الضغط والاعتماد على التردد لحساسية منعكس الضغط أثناء حدوث تقلبات في ضغط الدم الشرياني.
طرق البحث
أجرى ثلاثة عشر رجلا بصحة جيدة تمارين قبضة اليد متساوية القياس بنسبة 20٪ و 30٪ من أقصى تقلص طوعي بينما تم تطبيق الضغط السلبي السفلي المتذبذب لأسفل الجسم البالغ 40 مم زئبق بترددات 0.1 و 0.25 هرتز.
النتائج
مقارنة بالضغط السلبي التذبذب لأسفل الجسم عند تردد 0.25 هرتز لوحده ، تم تقليل مكاسب منعكس الضغط لضغط الدم الانبساطي بشكل كبير مع إضافة تمرين قبضة اليد متساوي القياس بنسبة 20٪ و 30٪ من الانكماش الطوعي الأقصى في نطاق التردد العالي. في حالة الراحة (بدون تمرين قبضة اليد متساوي القياس والضغط السلبي المتذبذب للجزء السفلي من الجسم) كان كسب منعكس الضغط أكثر بشكل ملحوظ في نطاق التردد العالي لضغط الدم الانبساطي. لكن اكتساب منعكس الضغط لضغط الدم الانبساطي لم يكن مختلفًا بشكل كبير عندما تم تطبيق تمرين قبضة اليد متساوي القياس + ضغط سلبي متذبذب لأسفل الجسم عند 20٪ و 30٪ من الانكماش الطوعي الأقصى في كلا الترددين.
الاستنتاجات
قد يشير الانخفاض الملحوظ في كسب ضغط الدم الانبساطي مع إضافة تمرين قبضة اليد المتدرجة إلى أن انعكاس ضغط التمرين وعضلة ميتابوريفلكس تتجاوز انعكاس الضغط أثناء التمرين لتقليل اكتساب انعكاس الضغط في نطاق التردد العالي (0.25 هرتز). تم تغيير الظاهرة المعتمدة على التردد لحساسية الضغط المنعكس عندما تم تطبيق تمرين قبضة اليد متساوي القياس والضغط السلبي المتذبذب لأسفل الجسم ، وهذا يعني أن الاعتماد على التردد لحساسية منعكس الضغط قد تم إبطاله أثناء تمرين قبضة اليد متساوي القياس.
الكلمات المفتاحية: كسب منعكس الضغط, حساسية منعكس الضغط, تمرين قبضة اليد متساوي القياس, أقصى تقلص طوعي, ضغط سلبي تذبذب للجزء السفلي من الجسم
Introduction
Short-term autonomic regulation of arterial blood pressure (BP) at rest and exercise are mainly done by baroreflex, which also participates in long-term regulation apart from neurohormonal control; baroreflex sensitivity (BRS) is the measure of this feedback mechanism.1, 2, 3 BRS is composed of vagal cholinergic and sympathetic adrenergic components, although the adrenergic component is not quantifiable in routine autonomic laboratories due to the unavailability of microneurographic techniques.4,5 BRS can be quantified as a change in R–R interval (interbeat interval) in milliseconds in electrocardiogram (ECG) with respect to unit change in BP in mmHg with the help of various non-invasive techniques such as the valsalva maneuver, head-up tilt, neck suction technique, and lower body negative pressure (LBNP) application6,7; this value is denoted as BRS gain.8 In addition to the amplitude of BP fluctuations, the frequency at which the fluctuations occur also determine BRS. BRS is reportedly maximum at low frequency (0.1 Hz) oscillations in arterial BP.9 The frequency dependence of BRS can be assessed by generating fluctuations in arterial BP with the help of various maneuvers such as the squat stand, sit stand, and oscillatory LBNP (OLBNP) application in different frequencies.10,11
It was previously believed that baroreflex control of arterial BP was ‘switched off’ during exercise, but later it was proven that baroreflex control of arterial BP is functional during exercise.12,13 Exercise training has become an integral part of healthy living, and exercise prescription including aerobic or resistance exercises or both are widely used for the prevention, treatment, and rehabilitation of various chronic diseases and musculoskeletal injuries.14,15 Exercise training has been shown to increase BRS in hypertensive and diabetic patients.16,17 Resistance exercises can be classified as static (isometric) or dynamic (isotonic).18 Isometric handgrip exercise (IHE) leads to a simultaneous increase in heart rate (HR), BP, and muscle sympathetic nerve activity through exercise pressure reflex. Furthermore, the intensity of exercise can also influence BRS differentially. Baroreflex resetting during exercise is mediated via modulation of the autonomic nervous system by the exercise pressure reflex and central command to fulfill the increased demand of oxygen and nutrients to the exercising muscle.19,20 There is limited literature on the effect of exercise on BRS at different frequencies, and the effect of exercise intensity on BRS at different frequencies has not been evaluated.
LBNP is widely used as an important tool to investigate the compensatory response of the baroreflex during hemodynamic changes.21 Spontaneous low-frequency oscillations (approximately 0.1 Hz) of arterial BP are seen in humans.22 These oscillations can be further augmented by LBNP. Furthermore, BP oscillations at higher frequencies such as 0.25 Hz can be generated by creating fluctuations in the central venous return with the help of an OLBNP device.23,24 This allows for estimation of BRS at both low and high frequencies.
The present study assessed the effects of IHE (20% and 30% of maximum voluntary contraction [MVC]) on baroreflex gain and evaluated the frequency dependence of BRS while different combinations of IHE (20% and 30% of MVC) and OLBNP (0.1 and 0.25 Hz frequencies) were applied. To the best of our knowledge, this is the first study to assess the effects of different IHE intensities on frequency dependence of BRS.
Materials and Methods
Participants
This study was approved by the Institute Ethics Committee of All India Institute of Medical Sciences, New Delhi (Reference No. IECPG-444/27.09.2018). A total of 19 healthy males were recruited for the study. The sample size was determined in line with previous studies on BRS and IHE.10,25 All participants were informed about the experimental protocol and provided written consent before data collection. In six participants, records could not be analyzed due to signal artifacts in BP or ECG. Final analyses were performed for 13 subjects. The anthropometric and hemodynamic characteristics of the subjects at rest are summarized in Table 1.
Table 1.
Anthropometric and hemodynamic characteristics of subjects at rest.
| Anthropometric characteristics (mean ± SD) | ||
|---|---|---|
| 1 | Age (years) | 27.38 ± 3.33 |
| 2 | Height (m) | 1.76 ± 3.24 |
| 3 | Weight (kg) | 71.61 ± 6.23 |
| 4 |
BMI (kg/m2) |
22.98 ± 2.38 |
| Hemodynamic parameters (mean ± SD) |
||
| 5 | SBP (mmHg) | 119.92 ± 5.09 |
| 6 | MBP (mmHg) | 85.81 ± 14.87 |
| 7 | DBP (mmHg) | 78.69 ± 4.64 |
| 7 | HR (beats per min) | 73.23 ± 4.55 |
Mean ± SD, SD, standard deviation; BMI, body mass index; SBP, systolic blood pressure; MBP, mean arterial blood pressure; DBP, diastolic blood pressure; HR, heart rate.
Inclusion criteria
Healthy male subjects between 18 and 35 years of age were included in the study.
Exclusion criteria
Subjects with a history of coronary artery disease, autonomic nervous system disorders, peripheral vascular disease, diabetes mellitus, and all causes of peripheral neuropathy (toxins, hepatic diseases, renal diseases, vitamin B12, and folic acid deficiency) and those on peripheral or central sympatholytic drugs were excluded from the study.
Protocol
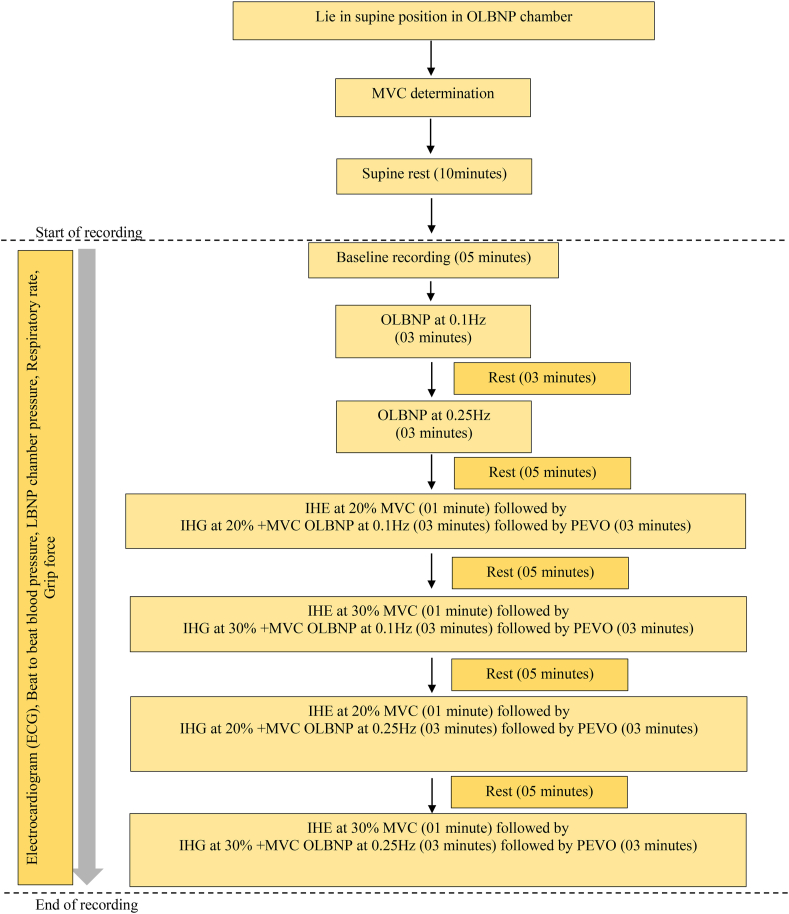
Each participant reported to the laboratory on one occasion at the same time of day and completed the recording within 2 h. After completion of the medical history and preliminary examination, the participants were asked to lie in a supine position inside the OLBNP chamber (VACUSPORT Regeneration System, Germany). Their legs were sealed inside the chamber at the level of the iliac crest. Beat-to-beat BP was recorded using the Human Non-Invasive BP System (Human NIBP; Finapres Medical Systems, The Netherlands). HR was recorded using an ECG in lead II configuration, using the PowerLab 15T System (AD Instruments, Bella Vista, Australia). A respiratory belt transducer (TN1132/ST) was connected to the PowerLab 4/30 System and placed on the chest at the level of the 5th intercostal space to record respiratory movements. The MVC of the dominant hand was determined using a hand grip force transducer (MLT/004 ST) connected to PowerLab 15T. After placing the participants in the OLBNP chamber, they were asked to press the handgrip dynamometer three times with maximum effort at intervals of 1 min, and the maximum value of three was taken as the MVC. After MVC determination, the participants rested for 10 min in the supine position before starting the investigation.
A digital pressure transducer (MLT1199 BP transducer) was placed inside the OLBNP chamber for the continuous monitoring of negative pressure generated inside. The Human NIBP System and digital pressure transducer were calibrated before each recording as per the manufacturer's instructions. After the 10-min rest period, the baseline recording of beat-to-beat BP, HR, respiratory rate, grip force, and OLBNP chamber pressure was started and continued for 5 min. Subsequently, recording of all parameters continued along with application of OLBNP at 0.1 and 0.25 Hz frequencies for the duration of 3 min each at an interval of 3 min. For application of OLBNP at 0.1 Hz frequency, the negative pressure was generated in such a manner that suction pressure was applied for 5 s followed by 5 s of release. Similarly, for application of OLBNP at 0.25 Hz frequency, the suction pressure was applied for 2 s followed by 2 s of release.
After a rest period of 5 min, the participants were asked to do IHE at 20% of their MVC with their right hand for 4 min and OLBNP at 0.1 Hz was added during the last 3 min of IHE, followed by post-exercise venous occlusion (PEVO) on the right arm with the help of an arm cuff of sphygmomanometer at 40 mmHg for 3 min to maintain the activation of muscle metaboreflex. After a rest period of 5 min, the participants again did IHE at 30% of MVC for 4 min and OLBNP at 0.1 Hz was added during the last 3 min of IHE, followed by PEVO on the right arm. A similar paradigm was repeated for IHE at 20% and 30% of MVC with OLBNP at 0.25 Hz followed by PEVO, as shown in Figure 1. Lab Chart Pro 8.0™ (AD Instruments) was used for data recording, and MATLAB (ver. R2018b; Mathworks, Natick, MA, USA) was used for data analyses.
Figure 1.
Study design.
BRS analysis
Beat-to-beat values of the R–R interval (RRI) and BP were extracted as .txt files for each experimental paradigm separately from recordings in Lab Chart Pro 8.0™ (AD instruments). The .txt files were imported into MATLAB (ver. R2018b; Mathworks) and the custom written baroreflex code was used for transfer function analysis. The signals were spline interpolated and resampled at 2 Hz. Transfer function analysis was done between SBP and RRI using a hamming window of length 100 and 50% overlap. The transfer function gain is calculated as the quotient of the cross spectral density of SBP and RRI and the power spectral density of SBP. BRS was quantified as the gain for 0.1 Hz (0.08–0.12 Hz band) and 0.25 Hz (0.22–0.27 Hz band) where coherence between SBP and RRI was higher than 0.5. Similarly, BRS gain for DBP and MBP was calculated. The coherence reflects the correlation and linear relationship between BP as the input variable and RRI as the output variable. Coherence value as a marker of RRI-BP coupling by baroreflex. BRS gain signifies the change in RRI (output) over the change in BP (input) and it denotes BRS.10
Statistical analyses
Statistical tests were done on GraphPad Prism version 8.0 (San Diego, CA, USA). Data were tested for normal distribution using Shapiro–Wilk test. The results are shown as the mean ± standard deviation and median ± quartile deviation. To understand the effect of IHE on BRS, a comparison was done for the BRS of SBP, MBP and DBP within same frequency band. To understand the frequency dependence of BRS, analysis was done to compare the BRS of SBP, MBP and DBP between 0.1 and 0.25 Hz frequency bands. The paired t-test was for the comparison of normally distributed data. For others, the Wilcoxon signed-rank test was used. P < 0.05 was considered statistically significant.
Results
BRS gain can be expressed as systolic BP (SBP), mean arterial BP (MBP), and diastolic BP (DBP). The SBP, MBP, and DBP all tend to rise significantly during isometric exercise, but during isotonic exercise, only SBP rises and both MBP and DBP either remain the same or reduce.
Effect of IHE on BRS
When the BRS gain of SBP and MBP was compared at 0.1 Hz or 0.25 Hz frequency band, there was no significant difference with IHE at 20% or 30% of MVC to OLBNP (Table 2). DBP BRS gain was significantly reduced in a higher frequency band when IHE at 20% or 30% of MVC was added to 0.25 Hz OLBNP as shown in Table 2.
Table 2.
BRS gain (ms/mmHg) of SBP, MBP, and DBP at 0.1 and 0.25 Hz OLBNP before and after IHE at 20% and 30% MVC.
| S. No. | Experimental conditions: Mean ± SD (Median ± QD) |
p-value | |||||
|---|---|---|---|---|---|---|---|
| A |
B |
C |
D |
E |
F |
||
| 0.1 Hz OLBNP | MVC 20% + 0.1 Hz OLBNP |
MVC 30% + 0.1 Hz OLBNP |
0.25 Hz OLBNP | MVC 20% + 0.25 Hz OLBNP |
MVC 30% + 0.25 Hz OLBNP |
||
| 1. SBP | 11.75 ± 7.28 (10.17 ± 4.69) | 10.80 ± 5.71 (11.57 ± 5.39) | 9.88 ± 5.98 (9.01 ± 2.47) | 11.43 ± 5.89 (11.41 ± 3.88) | 11.27 ± 7.66 (8.31 ± 7.99) | 11.71 ± 7.30 (10.80 ± 6.73) | 0.807ˆa, 0.071ˆb, 0.859ˆc, 0.968d |
| 2. DBP | 13.93 ± 7.71 (12.35 ± 5.38) | 15.15 ± 8.67 (14.08 ± 6.19) | 12.25 ± 5.37 (11.47 ± 3.82) | 25.21 ± 8.51 (26.71 ± 5.01) | 16.25 ± 6.27 (17.09 ± 4.94) | 15.44 ± 6.17 (15.20 ± 2.53) | 0.623a, 0.524b, 0.005c∗, 0.025d∗ |
| 3. MBP | 17.21 ± 14.34 (11.95 ± 6.83) | 16.40 ± 11.07 (14.75 ± 7.09) | 12.69 ± 8.99 (11.10 ± 3.32) | 33.86 ± 21.31 (28.97 ± 21.76) | 20.63 ± 13.08 (19.69 ± 8.67) | 19.14 ± 13.47 (16.15 ± 7.59) | 0.972ˆa, 0.117ˆb,0.203ˆc, 0.112d |
Mean ± SD (Median ± QD); SD = standard deviation, QD = quartile deviation. Paired t-test, ˆWilcoxon signed-rank test.∗p < 0.05; MVC, maximum voluntary contraction; SBP, systolic blood pressure; MBP, mean arterial blood pressure; DBP, diastolic blood pressure; OLBNP, oscillatory lower body negative pressure, acomparison between column A and B of the same row, bcomparison between column A and C of the same row, ccomparison between column D and E of the same row, dcomparison between column D and F of the same row.
Effect of IHE on frequency dependence of BRS
To assess the frequency dependence of the BRS, BRS gain of SBP and DBP was compared between 0.1 and 0.25 Hz frequency bands at baseline (i.e., without OLBNP and IHE). The data were normally distributed. The paired t-test was used to compare the gain, at baseline, between 0.1 and 0.25 Hz frequency bands. The BRS gain was significantly high in the 0.25 Hz frequency band for DBP as shown in Table 3. The BRS gain of SBP was also high in the 0.25 Hz frequency band, although it was not statistically significant. So it can be said that BRS is a frequency-dependent phenomenon.
Table 3.
BRS gain (ms/mmHg) for SBP, DBP, and MBP at baseline in 0.1 and 0.25 Hz frequency bands.
| S. No. | Mean ± SD (Median ± QD) |
p-value | |
|---|---|---|---|
| A. Baseline (0.1 Hz) | B. Baseline (0.25 Hz) | ||
| 1. SBP | 11.56 ± 5.93 (11.27 ± 3.68) | 17.83 ± 11.87 (14.26 ± 8.95) | 0.101 |
| 2. DBP | 17.31 ± 8.27 (15.92 ± 4.72) | 36.13 ± 18.05 (32.59 ± 16.23) | 0.008∗ |
| 3. MBP | 16.27 ± 8.12 (14.16 ± 3.94) | 33.86 ± 21.31 (28.97 ± 21.76) | 0.132 |
Mean ± SD (Median ± QD); SD = standard deviation, QD = quartile deviation. Paired t-test, ∗p < 0.05. SBP, systolic blood pressure; MBP, mean arterial blood pressure; DBP, diastolic blood pressure.
Effect of IHE at MVC 20% or MVC 30% on BRS at different frequencies
BRS gain of SBP and DBP was compared between 0.1 and 0.25 Hz frequency bands while applying OLBNP at 0.1 and 0.25 Hz along with IHE at 20% and 30% of MVC to see the effect of IHE on frequency dependence of BRS. The BRS gain for SBP and DBP was not significantly different in between both frequencies when IHE was applied at 20% and 30% of MVC as shown in Table 4.
Table 4.
BRS gain (ms/mmHg) for SBP, DBP, and MBP between 0.1 and 0.25 Hz OLBNP with IHE at 20% or 30% MVC.
| S. No. | Experimental conditions: Mean ± SD (Median ± QD) |
p-value | |||
|---|---|---|---|---|---|
| A. |
B. |
C. |
D. |
||
| MVC 20% + 0.1 Hz OLBNP | MVC 20% + 0.25 Hz OLBNP | MVC 30% + 0.1 Hz OLBNP | MVC 30% + 0.25 Hz OLBNP | ||
| 1. SBP BRS | 10.80 ± 5.71 (11.57 ± 5.39) | 11.27 ± 7.66 (8.31 ± 7.99) | 9.88 ± 5.98 (9.01 ± 2.47) | 11.71 ± 7.30 (10.80 ± 6.73) | 1.000ˆa, 0.480ˆb, 0.878ˆc, 0.155ˆd |
| 2. DBP BRS | 15.15 ± 8.67 (14.08 ± 6.19) | 16.25 ± 6.27 (17.09 ± 4.94) | 12.25 ± 5.37 (11.47 ± 3.82) | 15.44 ± 6.17 (15.20 ± 2.53) | 0.887a, 0.177b, 0.784c, 0.478d |
| 3. MBP BRS | 16.40 ± 11.07 (14.75 ± 7.09) | 20.63 ± 13.08 (19.69 ± 8.67) | 12.69 ± 8.99 (11.10 ± 3.32) | 19.14 ± 13.47 (16.15 ± 7.59) | 0.929ˆa, 0.084ˆb, 0.086ˆc, 0.594ˆd |
Mean ± SD (Median ± QD); SD = standard deviation, QD = quartile deviation. Paired t-test, ˆWilcoxon signed-rank test.∗p < 0.05; MVC, maximum voluntary contraction; SBP, systolic blood pressure; MBP, mean arterial blood pressure; DBP, diastolic blood pressure; OLBNP, oscillatory lower body negative pressure, acomparison between column A and B of the same row, bcomparison between column A and C of the same row, ccomparison between column B and D of the same row, dcomparison between column C and D of the same row.
Discussion
The current study examined the effect of IHE on BRS expressed as BRS gain and frequency dependence of BRS in the setting of induced oscillations in arterial BP at 0.1 and 0.25 Hz frequencies. The major findings of this study were as follows. (1) BRS gain for DBP significantly decreased at the parasympathetic frequency band (0.25 Hz) when IHE were added to OLBNP at the 0.25 Hz frequency band. (2) The BRS gain for DBP was not significantly different between the low and high frequency bands when IHE was applied at 20% and 30% of MVC along with OLBNP at 0.1 and 0.25 Hz.
It is worth highlighting that we obtained a significant reduction in BRS gain DBP during IHE because BP exhibits differential responses to isotonic and isometric exercises.27 In the isotonic exercise, the SBP rises but MBP and DBP remains the same or is reduced, but in isometric exercise SBP, MBP, and DBP rises.25 The different components of BP indicate various properties of circulation. The SBP correlates well with vascular structure and it has been seen that there is a rise in SBP with old age due to increased arterial stiffness. The MBP is a steady component of the BP and conveys more about static flow via the aorta, small artery resistance, and vascular elasticity averages over time. A low DBP is associated with poor coronary flow reserve and myocardial perfusion.29 The significant rise in DBP in isometric exercise might be due to a stronger chemoreflex response, which leads to sympathetic vasoconstriction and increases total peripheral resistance. On the other hand, DBP is either slightly raised, maintained or even decreased in isotonic exercises due to the accumulation of local metabolites from muscles resulting in peripheral arterial dilatation.26
In the current study, it was found that BRS is reduced during the IHE in the high-frequency band. This is in agreement with previous reports where BRS was reportedly reduced during dynamic exercise.23 Taken together, this study indicates that blunting of the baroreceptor occurs with addition of IHE in the parasympathetic frequency band (0.25 Hz) and the blunting increases with higher exercise intensity.27 These results are in agreement with a previous study, which found a reduction in BRS after resistance exercise and the recovery of BRS was delayed to a greater extent with high intensity exercise.28 Blunting of the baroreceptor might allow the rise of both BP and HR simultaneously during exercise.27
In another study on BRS, arterial stiffness and vascular tone were compared before and after 20 min of aerobic and resistance exercise. It was concluded that BRS was more significantly reduced after resistance exercise compared to aerobic exercise.27 The stiffness of central artery was described as the probable mechanism of diminished baroreceptor activation during exercise. The stiffer central arteries may result in reduced vessel wall deformation at the time of change in BP and finally lead to diminished baroreceptor activation.27
Normally, whenever there is a rise in BP, increased firing from baroreceptors leads to inhibition of rostral ventrolateral medulla (major excitatory output to sympathetic nerves controlling the vasculature) by caudal ventrolateral medulla, and a simultaneous increase in vagal tone by excitation of nucleus ambiguus and dorsal nucleus of vagus, ultimately leading to a decrease in HR and BP. But during exercise, the paraventricular nucleus of hypothalamus and vasopressinergic neuron of exercise pressure reflex directly inhibit baroreflex afferents causing decreased sympathetic inhibition, and less excitation of parasympathetic nervous system from nucleus of tractus solitarius, causing blunting of the baroreflex mechanism and resetting of the baroreflex function to a higher level, resulting in a simultaneous increase of BP and HR at the time of exercise.33 Thus, we can say that at the time of exercise, baroreceptor reflex gain is modulated and both the BP and HR rise simultaneously to meet the increase body demand compared to rest in which the change in BP is counteracted by change in HR by baroreceptor activation.31
In addition, we confirmed that the BRS is a frequency-dependent phenomenon because at baseline (i.e., no OLBNP or IHE) DBP, BRS gain was significantly more in the high frequency band (0.25 Hz frequency band) as shown in Table 3. The frequency-dependent phenomenon of BRS shown in the study is consistent with the findings of Munakata et al. (1994), who reported a higher BRS gain in the high-frequency band.27 Furthermore, the DBP BRS gain between low and high frequency bands was not significantly different when IHE at 20% and 30% of MVC was added to the OLBNP. This indicates that exercise might abolish the frequency-dependent nature of the BRS.34
It is worth highlighting that BRS gain for SBP and DBP was not significantly different when it was compared between low (0.1 Hz) and high frequency (0.25 Hz) for IHE at 20% and 30% of MVC as shown in Table 4. That means that the frequency dependence of BRS does not depend on the exercise intensity. Although previous studies have noted cardiovascular responses to different IHE intensities, the outcome variable compared before and after IHE was mainly HR and BP like Silva et al.35, who concluded that BP response does not change with different intensity of IHE.
Limitations of the study
The study was limited by the small sample size, due to the lack of the eligible volunteers during the relatively short duration of the study. Also, no adjustment in p-value for multiple comparisons was done, which might have reduced the number of statistically significant differences in the results. Still, the present results provide a direction for future well-designed studies with a larger sample size to confirm these findings.
Clinical justification – IHE along with OLBNP can be used as a technique for studying cardiovascular compensatory mechanisms during orthostatic stress in patients with orthostatic hypotension or autonomic disturbances, and whether adaptation to IHE training might influence tolerance during central hypovolemia produced due to application of OLBNP. LBNP can be used in patients with orthostatic hypotension or autonomic disturbances as a substitute for the lying to standing test or head-up tilt test to study the physiological factors contributing to the changes occurring during orthostatic stress. IHE reduced the DBS BRS gain at the high-frequency band, which indicated the blunting of BRS, with a rise in both BP and HR. Hence, isometric exercise might be useful in those with orthostatic hypotension or autonomic disturbances. Future well-designed large sample size studies are required for confirmation.
Conclusion
We conclude that the significant reduction in DBP BRS gain with the addition of IHE at the high-frequency band might indicate the blunting of BRS resulting in the rise of both BP and HR to fulfill the increased body demand of oxygen and nutrients during exercise. The frequency dependence of BRS is nullified by IHE and the phenomenon of frequency dependence of BRS is independent of IHE intensity.
Source of funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflict of interest
The authors have no conflict of interest to declare.
Ethical approval
No.: IECPG-444/27.09.2018.
Authors contributions
GKB – Conceived and designed the study, conducted the research, collected and organized the data, wrote the initial and final draft of the article. SB – Analyzed and interpreted the data, revised the final draft of the article. HBS – Analyzed and interpreted data, revised the final draft of the article. KKD – Provided research materials, revised the article and gave final approval of the submitted manuscript, provided logistic support. All authors have critically reviewed and approved the final draft and are responsible for the content and similarity index of the manuscript.
Footnotes
Peer review under responsibility of Taibah University.
References
- 1.Kazimierska A., Placek M.M., Uryga A., Wachel P., Burzyńska M., Kasprowicz M. Assessment of baroreflex sensitivity using time-frequency analysis during postural change and hypercapnia. Comput Math Methods Med. 2019 Feb 3;2019 doi: 10.1155/2019/4875231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.La Rovere M.T., Pinna G.D., Raczak G. Baroreflex sensitivity: measurement and clinical implications. Ann Noninvasive Electrocardiol. 2008 Apr;13(2):191–207. doi: 10.1111/j.1542-474X.2008.00219.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Swenne C.A. Baroreflex sensitivity: mechanisms and measurement. Neth Heart J. 2013 Feb;21(2):58–60. doi: 10.1007/s12471-012-0346-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schrezenmaier C., Singer W., Swift N.M., Sletten D., Tanabe J., Low P.A. Adrenergic and vagal baroreflex sensitivity in autonomic failure. Arch Neurol. 2007 Mar 1;64(3):381–386. doi: 10.1001/archneur.64.3.381. [DOI] [PubMed] [Google Scholar]
- 5.Oliveira R., Barker A., Debras F., O’doherty A., Williams C. Reliability of autonomic and vascular components of baroreflex sensitivity in adolescents. Clin Physiol Funct Imag. 2018 Feb 23:38. doi: 10.1111/cpf.12511. [DOI] [PubMed] [Google Scholar]
- 6.Dutoit A.P., Hart E.C., Charkoudian N., Wallin B.G., Curry T.B., Joyner M.J. Cardiac baroreflex sensitivity is not correlated to sympathetic baroreflex sensitivity within healthy, young humans. Hypertension. 2010 Dec;56(6):1118–1123. doi: 10.1161/HYPERTENSIONAHA.110.158329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Taboni A., Fagoni N., Fontolliet T., Vinetti G., Ferretti G. Dynamics of cardiovascular and baroreflex readjustments during a light-to-moderate exercise transient in humans. Eur J Appl Physiol. 2022;122(11):2343–2354. doi: 10.1007/s00421-022-05011-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carrara M., Bollen Pinto B., Baselli G., Bendjelid K., Ferrario M. Baroreflex sensitivity and blood pressure variability can help in understanding the different response to therapy during acute phase of septic shock. Shock. 2018 Jul;50(1):78–86. doi: 10.1097/SHK.0000000000001046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davies L.C., Francis D.P., Jurák P., Kára T., Piepoli M., Coats A.J.S. Reproducibility of methods for assessing baroreflex sensitivity in normal controls and in patients with chronic heart failure. Clin Sci. 1999 Sep 16;97(4):515–522. [PubMed] [Google Scholar]
- 10.Horsman H.M., Peebles K.C., Galletly D.C., Tzeng Y.C. Cardiac baroreflex gain is frequency dependent: insights from repeated sit-to-stand maneuvers and the modified Oxford method. Appl Physiol Nutr Metab. 2013 Jul;38(7):753–759. doi: 10.1139/apnm-2012-0444. [DOI] [PubMed] [Google Scholar]
- 11.Zhang R., Claassen J., Shibata S., Kilic S., Martin-Cook K., Diaz-Arrastia R., et al. Arterial-cardiac baroreflex function: insights from repeated squat-stand maneuvers. Am J Physiol Regul Integr Comp Physiol. 2009 Jul;297(1):R116–R123. doi: 10.1152/ajpregu.90977.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miki K., Yoshimoto M. Exercise-induced modulation of baroreflex control of sympathetic nerve activity. Front Neurosci. 2018 Jul 23;12:493. doi: 10.3389/fnins.2018.00493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Drew R.C. Baroreflex and neurovascular responses to skeletal muscle mechanoreflex activation in humans: an exercise in integrative physiology. Am J Physiol Regul Integr Comp Physiol. 2017 Dec 1;313(6):R654–R659. doi: 10.1152/ajpregu.00242.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pedersen B.K., Saltin B. Exercise as medicine - evidence for prescribing exercise as therapy in 26 different chronic diseases. Scand J Med Sci Sports. 2015 Dec;25(Suppl 3):1–72. doi: 10.1111/sms.12581. [DOI] [PubMed] [Google Scholar]
- 15.Sallis R.E. Exercise in the treatment of chronic disease: an underfilled prescription. Curr Sports Med Rep. 2017 Aug;16(4):225–226. doi: 10.1249/JSR.0000000000000378. [DOI] [PubMed] [Google Scholar]
- 16.Laterza M.C., de Matos L.D.N.J., Trombetta I.C., Braga A.M.W., Roveda F., Alves M.J.N.N., et al. Exercise training restores baroreflex sensitivity in never-treated hypertensive patients. Hypertension. 2007 Jun 1;49(6):1298–1306. doi: 10.1161/HYPERTENSIONAHA.106.085548. [DOI] [PubMed] [Google Scholar]
- 17.Loimaala A., Huikuri H.V., Kööbi T., Rinne M., Nenonen A., Vuori I. Exercise training improves baroreflex sensitivity in type 2 diabetes. Diabetes. 2003 Jul 1;52(7):1837–1842. doi: 10.2337/diabetes.52.7.1837. [DOI] [PubMed] [Google Scholar]
- 18.Kounoupis A., Papadopoulos S., Galanis N., Dipla K., Zafeiridis A. Are blood pressure and cardiovascular stress greater in isometric or in dynamic resistance exercise? Sports (Basel) 2020 Mar 28;8(4):41. doi: 10.3390/sports8040041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rowell L.B., O'Leary D.S., Kellogg D.L., Jr. Comprehensive Physiology [Internet] John Wiley & Sons, Ltd; 2011. Integration of cardiovascular control systems in dynamic exercise; pp. 770–838.https://onlinelibrary.wiley.com/doi/abs/10.1002/cphy.cp120117 [cited 2022 Jan 3]. Available from: [Google Scholar]
- 20.Katayama K., Kaur J., Young B.E., Barbosa T.C., Ogoh S., Fadel P.J. High-intensity muscle metaboreflex activation attenuates cardiopulmonary baroreflex-mediated inhibition of muscle sympathetic nerve activity. J Appl Physiol (1985) 2018 Sep 1;125(3):812–819. doi: 10.1152/japplphysiol.00161.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goswami N., Blaber A.P., Hinghofer-Szalkay H., Convertino V.A. Lower body negative pressure: physiological effects, applications, and implementation. Physiol Rev. 2019 01;99(1):807–851. doi: 10.1152/physrev.00006.2018. [DOI] [PubMed] [Google Scholar]
- 22.Julien C. The enigma of Mayer waves: facts and models. Cardiovasc Res. 2006 Apr 1;70(1):12–21. doi: 10.1016/j.cardiores.2005.11.008. [DOI] [PubMed] [Google Scholar]
- 23.Taylor J.A., Tan C.O., Hamner J.W. Assessing cerebral autoregulation via oscillatory lower body negative pressure and projection pursuit regression. JoVE. 2014 Dec 10;(94) doi: 10.3791/51082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rickards C.A., Sprick J.D., Colby H.B., Kay V.L., Tzeng Y.C. Coupling between arterial pressure, cerebral blood velocity, and cerebral tissue oxygenation with spontaneous and forced oscillations. Physiol Meas. 2015 Apr;36(4):785–801. doi: 10.1088/0967-3334/36/4/785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Iellamo F., Hughson R.L., Castrucci F., Legramante J.M., Raimondi G., Peruzzi G., et al. Evaluation of spontaneous baroreflex modulation of sinus node during isometric exercise in healthy humans. Am J Physiol. 1994 Sep;267(3 Pt 2):H994–H1001. doi: 10.1152/ajpheart.1994.267.3.H994. [DOI] [PubMed] [Google Scholar]
- 26.Tanaka S., Sugiura T., Yamashita S., Dohi Y., Kimura G., Ohte N. Differential response of central blood pressure to isometric and isotonic exercises. Sci Rep. 2014 Jun 25;4:5439. doi: 10.1038/srep05439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Munakata M., Imai Y., Takagi H., Nakao M., Yamamoto M., Abe K. Altered frequency-dependent characteristics of the cardiac baroreflex in essential hypertension. J Auton Nerv Syst. 1994 Sep;49(1):33–45. doi: 10.1016/0165-1838(94)90018-3. [DOI] [PubMed] [Google Scholar]
- 28.Weippert M., Behrens K., Rieger A., Stoll R., Kreuzfeld S. Heart rate variability and blood pressure during dynamic and static exercise at similar heart rate levels. PLoS One. 2013;8(12) doi: 10.1371/journal.pone.0083690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sesso H.D., Stampfer M.J., Rosner B., Hennekens C.H., Gaziano J.M., Manson J.E., et al. Systolic and diastolic blood pressure, pulse pressure, and mean arterial pressure as predictors of cardiovascular disease risk in men. Hypertension. 2000 Nov;36(5):801–807. doi: 10.1161/01.hyp.36.5.801. [DOI] [PubMed] [Google Scholar]
- 31.Heffernan K.S., Collier S.R., Kelly E.E., Jae S.Y., Fernhall B. Arterial stiffness and baroreflex sensitivity following bouts of aerobic and resistance exercise. Int J Sports Med. 2007 Mar;28(3):197–203. doi: 10.1055/s-2006-924290. [DOI] [PubMed] [Google Scholar]
- 33.Michelini L.C., O'Leary D.S., Raven P.B., Nóbrega A.C.L. Neural control of circulation and exercise: a translational approach disclosing interactions between central command, arterial baroreflex, and muscle metaboreflex. Am J Physiol Heart Circ Physiol. 2015 Aug 1;309(3):H381–H392. doi: 10.1152/ajpheart.00077.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.deBoer R.W., Karemaker J.M., Strackee J. Hemodynamic fluctuations and baroreflex sensitivity in humans: a beat-to-beat model. Am J Physiol. 1987 Sep;253(3 Pt 2):H680–H689. doi: 10.1152/ajpheart.1987.253.3.H680. [DOI] [PubMed] [Google Scholar]
- 35.Silva I., Sobrinho M., Ritti-Dias R., Sobral B., Pirauá A., Oliveira L., et al. Cardiovascular responses after isometric handgrip exercise at different intensities in healthy men. J Phys Educ. 2019 Jan 1;30 [Google Scholar]