Abstract
Burn injury is a significant source of morbidity and mortality in the pediatric population. Although 40,000 pediatric patients in the United States are admitted to the hospital with burn wounds annually, significant differences exist in the management and treatment of these patients, even among highly specialized burn centers. Some aspects of pediatric burn research, such as metabolic changes and nutritional support following burn injury have been studied extensively; however, in many aspects of burn care, pediatric research lags behind the study of adult populations. This review compares and contrasts a wide array of physiologic and immune responses between children and adults after burn injury. Such a review elucidates where robust research has been conducted, where adult research is applicable to pediatric patients, and where additional pediatric burn research needs to be conducted.
Keywords: pediatric burn, thermal injury, immunology, critical care
1. INTRODUCTION
Burn injury affects patients of all ages, but children (particularly young children) are affected disproportionately1. Some aspects of patient care differ significantly between adults and children based on differences in anatomy, physiology, and response to burn injury. Research of pediatric burn populations has increased significantly in the last several decades, but is still overshadowed by the amount of existing adult burn research. In some instances, it is appropriate to treat these patient populations similarly based on their underlying response; however, it is not uncommon for burn literature performed in adult populations to be applied to pediatric patients without considering potential physiologic differences.
It is the purpose of this review to gather many of the known differences between pediatric and adult burned patients across several aspects of care, emphasizing immunology and critical care physiology. The topic is vast and efforts have been made to succinctly establish the responses of children to burn injury before comparing and contrasting this with available information in the adult population, allowing for a broad overview from which to begin learning about a child’s response to burn injury.
2. PATIENT CHARACTERISTICS
2.1. Epidemiology of Burns
One million children suffer burn injuries annually2. Thankfully, both hospitalization and mortality rates are decreasing due to a combination of prevention and advancements in care, but burn injury still remains the fourth leading cause of death of children in the United States3. The makeup of the pediatric burned population differs significantly from adults. The most common mechanism of injury in children is scald injury, followed by contact injury4, whereas in adults, flame burn injuries are most common5. Flame burns are more likely to be deeper and inflict concordant inhalation injury, which results in fundamentally different management algorithms.
Among pediatric admissions, half of injuries occur in patients younger than four years of age1. While teenagers can be similar in body habitus and physiology to adults (interestingly, the most common mechanism for burns in adolescents is flame injury, as in adults), the same cannot be said for the majority of younger pediatric burn patients, necessitating completely different management in some respects. Regarding location of injury, two-thirds of pediatric burns take place at home3,6, compared to half of adult burns (due to a significant proportion of injury at the workplace) 5. Rates of intentional burn injury (abuse and self-harm) are also significantly higher in children (up to 20%) compared to adults (2%) 7,8.
Adults are more likely than children to have pre-existing medical conditions, which contributes to an overall higher mortality (4%) 9. Comparatively, pediatric burn mortality is 0.9–2% 3,10, and overall mortality in this population has decreased by 50% in the twenty-first century1. High-volume pediatric burn centers (>200 pediatric burn admissions per year) have the lowest mortality and early transfer to these institutions shortens hospital stay11,12. Interestingly, burn center volume does not correlate with improved outcomes in adult burn patients, indicating that pediatric patients have unique responses to burn injury that benefit from treatment by specialists with an understanding of their pathophysiology11.
Some epidemiologic commonalities exist between children and adult burn patients. Extremities are the most common region of the body involved regardless of age13. Males are more likely to suffer burn injury compared to females6. Burn care overall is becoming more centralized and more protocolized. The epidemiologic differences of these populations reflect the need to treat them as distinct groups. The following discussion of anatomy will continue to highlight these differences.
2.2. Biologic Response to Burn Injury
2.2.1. Anatomic Differences
Pediatric patients exhibit significant anatomic and physiologic differences compared to adults, which lessen as the child develops. These differences call for special considerations in pediatric groups, particularly for neonates and young children.
Anatomic Differences
Children have thinner skin with fewer dermal appendages which affects both their local tissue response to burns as well as consideration for skin grafting14. Because of this, they have an increased risk of deeper burns compared to adults4. Children have a higher body-surface-area-to-mass ratio, which predisposes them to hypothermia and results in higher relative fluid requirements per percent burn14.
Children are also proportionally different: the ‘rule of 9s’ is used to estimate burn wound size in adults, whereas in children, the Lund and Browder Chart is used (Figure 1). This dynamic chart allows for more accurate quantification of burns based on changes in development as children age. Young children have larger relative head sizes and smaller relative leg sizes. As in adults, however, the palm of the patient’s hand still represents approximately 1% total body surface area (TBSA) burn size4.
Figure 1.
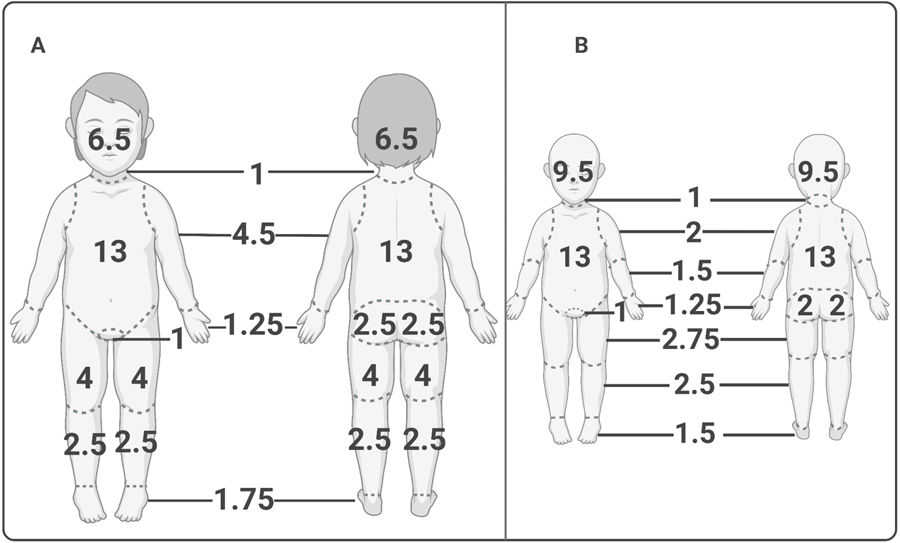
Lund and Browder burn estimation chart for child (A) and infant (B). Developmental differences in body proportion preclude the use of the traditional “rule of 9’s” used to estimate total body surface area (TBSA) percentages, as in adults. The Lund and Browder chart reflects the increased head to body ratio seen in young children (A) and especially infants (B), as well as their smaller relative limb sizes. Despite these differences in body part proportions, the hand still represents approximately 1% TBSA in both children and infants. Created with biorender.com.
Concerning the pediatric airway, the smaller relative diameter, shorter trachea, anteriorly displaced pharynx, and larger tonsils result in an airway which is predisposed to obstruction secondary to edema (Figure 2) 4,15. Children are also at greater risk for bronchospasm compared to adults after suffering inhalation injury16. Lung development in children occurs throughout the first eight years of life, so burn injury to the respiratory system in young children affects development4. The external anatomic differences are visually apparent, but the physiologic differences in response to burn injury between children and adults are just as profound.
Figure 2.
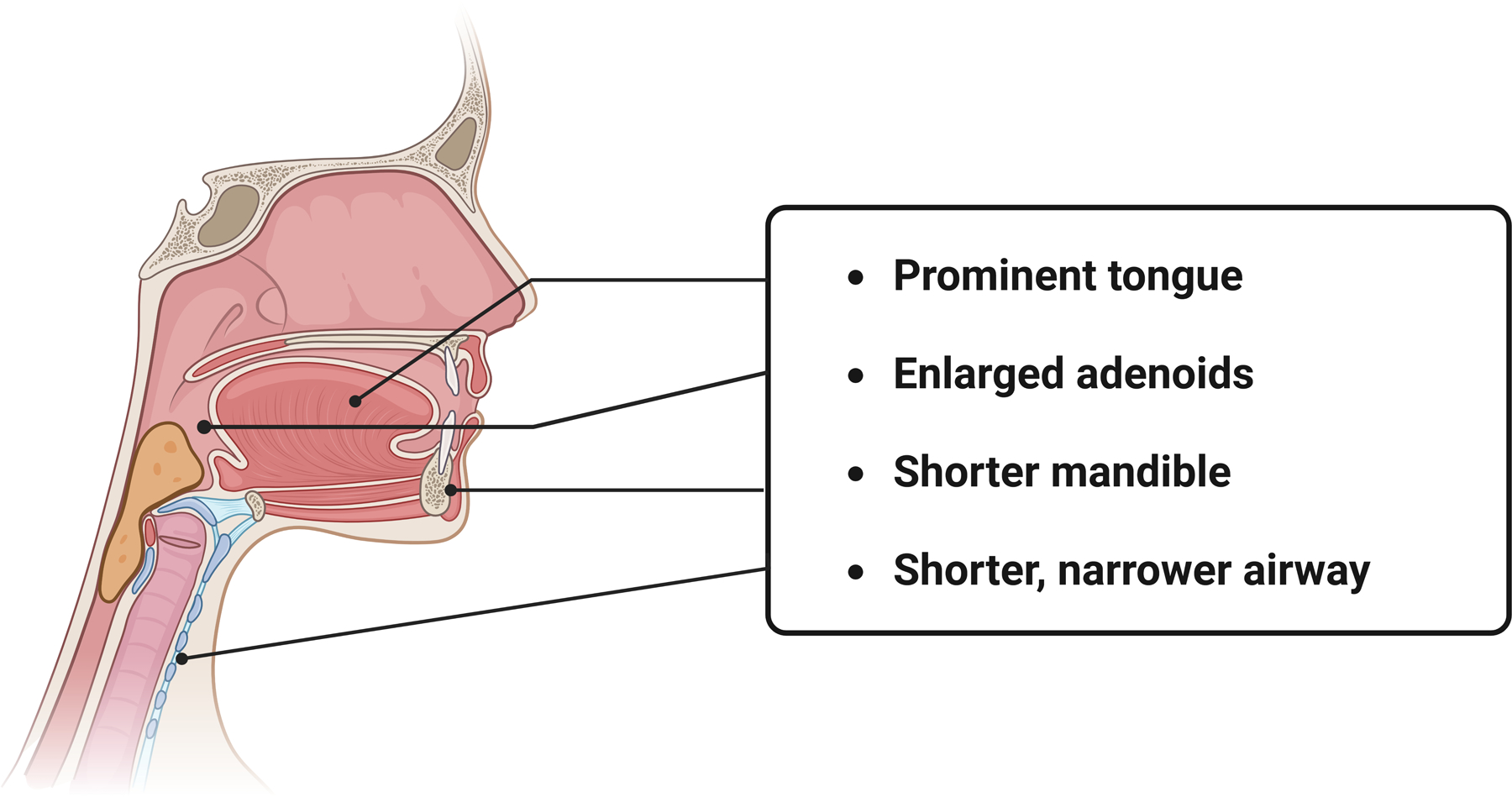
Anatomy of the pediatric airway. Special consideration should be taken in assessment and securing of the pediatric airway, as several anatomic distinctions predispose this population to more rapid airway compromise. The upper airway is characterized by a shorter mandible length, a more prominent tongue as well as proportionally larger tonsils and adenoids. In cases of inhalation injury, the resulting edema can lead to rapid airway obstruction and collapse. The naturally shorter, narrower airway seen in this age group additionally contributes to increased airway resistance. Created with biorender.com.
2.2.2. Physiologic Differences
Initial Response
The initial physiologic response after a burn injury is similar between children and adults. Burn injury results in coagulation at the site of the burn, along with vasoconstriction and capillary thrombosis adjacent to the burned tissue17. Histamine is released from mast cells, which increases membrane and vascular permeability, while serotonin and thromboxane A2 are released systemically, acting to increase pulmonary vascular resistance and enact mesenteric vasoconstriction, respectively2. The tissue adjacent to the area of necrosis is threatened, and may convert to a deeper burn wound, particularly in the setting of hypotension, hypoxia, and infection18.
The systemic effects of burn injury occur at approximately 30% TBSA involvement18. With respect to their cardiovascular system, infants have limited ability to modulate contractility and are unable to significantly increase their stroke volume4. They experience increased capillary permeability, reduced myocardial contractility, and selective vasoconstriction, while their respiratory system is subject to bronchoconstriction18. Relative blood volume is increased in children. Children are also more likely to experience hyponatremia, particularly if they are less than one year of age due to urinary sodium losses because of a relative inability to concentrate urine14,17. Young children have less glycogen stores and therefore require supplemental dextrose as part of fluid administration. Children react differently to treatment plans and interventions and require different management systems for optimal outcomes during recovery.
Differences in Metabolism
Wolf et al. provides an extensive review of the physiology of patients during the initial hypermetabolic response to burn injury2. These changes include increases in energy expenditure, stroke volume, temperature, and heart rate fueled by protein and fat breakdown. Macroendocrine hormones are released systemically in large burns and include catecholamines, cortisol, and glucagon. This results in proteolysis, lipolysis, gluconeogenesis, and glycogenolysis, leading to a loss of lean body mass and body fat19,20. Catecholamines increase gluconeogenesis, glycogenolysis, lipolysis, acute phase reactant production, thermogenesis, and heart rate21. Catecholamines also, along with the systemic release of IL-1β and IL-6, increase the temperature set point in pediatric and adult patients through action on the hypothalamus21. Cortisol production leads to an increase in glucose mobilization and utilization, which results in hyperglycemia. Muscle tissue can decrease by 50% through breakdown of protein. Peripheral lipolysis leads to increased deposition of fat in the liver. The negative nitrogen balance which exists in burn patients is a combination of protein storage breakdown and decreased synthesis. This loss of muscle mass which particularly affects children can hinder rehabilitation efforts following acute resuscitation22. A review by Chan et al. focuses on additional differences between children and adults after burn injury, including limited relative energy reserves and a higher minute volume in children compared to adult patients21. Adults, on the other hand, have a relative delay in collagen synthesis which can manifest as delayed wound healing or impaired wound contraction, and their skin has decreased tensile strength compared to children21.
Additional information regarding the hypermetabolic state and nutritional demands in pediatric and adult burn patients have previously been extensively reported and are beyond the scope of this review.
Alterations in Bone Formation
Reduced bone formation also occurs in patients and persists chronically, which leads to increased fracture risk following discharge23. These changes are multifactorial, and are influenced in part by glucocorticoids, inflammatory cytokines, hypoparathyroidism, and pervasive vitamin D deficiency, which is of special concern for pediatric burn patients suffering from suppressed osteoblast differentiation24–26.
2.2.3. Growth and Development
Growth and development in children after burn injury are inherently different than in fully developed adults. In a problem unique to children, severe burn injury leads to growth arrest which can persist up to three years27. Alterations in energy and protein metabolism have been studied to the point where therapeutic interventions have been widely introduced into practice. The testosterone-analogue oxandrolone is associated with an increase in lean body mass and bone mineral density in pediatric patients with large burns28. Oxandrolone combined with propranolol shortens the period of growth arrest by 3 months in burned children29. Long-term use of oxandrolone results in further increases in bone mineral content, bone density, and a greater height velocity which is more efficacious compared to short-term use30.
With regards to spinal development, contractures can develop following significant full thickness burns to the trunk which lead to spinal deformities in growing children who may require operative intervention31. Growth and developmental changes in pediatric burn patients have been extensively evaluated, and the existence of multiple therapeutic interventions which are starting to generate long-term data is indicative of the depth of understanding with which other aspects of pediatric burn research should be conducted.
2.2.4. Acute and Chronic Immune Response to Pediatric Burn Injury
Burn injury results in changes in the host immune milieu and generates a durable inflammatory response as a result of the incipient tissue damage generated by thermal, chemical, or electrical injury32. While there remains a relative paucity of information in the pediatric population, investigations into the differences in host response in younger patients does demonstrate several key differences in both acute and chronic immunologic changes as a response to burn injury32,33.
Local inflammatory response to burn injury
Burn injury results in compromise of the body’s first line of host defense, its protective skin barrier. The result of this local barrier disruption leads to increased susceptibility to infectious microbes and imbalances in thermoregulation and metabolic homeostasis34. Local capillary damage, as well as local and systemic vasodilation, leads to tissue edema (with its associated detrimental effects on wound healing) as well as significant insensible fluid losses34. Local tissue damage increases permeability to invasion by infectious microbes, increasing susceptibility to host infection, which can be further compounded by alterations in production of antimicrobial proteins and peptides (APPs)35.
Local tissue damage caused by burn injury generates a cascade of pro- and anti-inflammatory signaling, alterations in the local immune milieu, and longer-term transcriptomic changes in both the adaptive and innate immune systems which can persist for years after the initial insult36,37. The disruption of this first line of defense also results in an inherent reliance on cellular immune defense mechanisms to protect against invasive pathogens. We will review what is known regarding the local and systemic inflammatory response in pediatric burn patients, how these responses contribute to wound healing, as well as the acute, chronic, and pathologic immune changes that can be seen in this population.
Acute host immune response to burn injury
Following burn injury, protein degradation and catabolism are exacerbated by a systemic inflammatory response syndrome (SIRS). This pro-inflammatory environment is eventually attenuated by a compensatory anti-inflammatory response syndrome (CARS)38. The interplay between the pro- and anti-inflammatory responses in burn patients affects morbidity and mortality in the acute setting, and an imbalance in the two processes contributes to higher rates of sepsis, multisystem organ failure, and death (reviewed in: 38). Elevated levels of IL-10 have been associated with increased rates of sepsis and death39. Conversely, an increase in pro-inflammatory cytokine production (i.e., IL-6, IL-8, IL-1β, and IFN-γ) is associated with worsening renal and hepatic function in burned children, and has been associated with an increase in mortality in rodents40. Cytokine levels have also been analyzed as biomarkers for identification of burn patients at risk for poor outcome, with significant derangements of IL-8 associated with increased incidences of multisystem organ failure, sepsis, and mortality41.
Disruptions in homeostasis in these children continue as they heal their burn wounds and become well enough for hospital discharge. The effects of excessive inflammation or inflammatory suppression, measured by overall cytokine production, has been studied in pediatric patients with varying burn sizes up to two months after burn injury42. Jeschke et al. measured seventeen serum cytokines associated with the inflammatory response in burned children, and found that all were significantly changed, particularly G-CSF, IL-6, IL-8, MCP-1, and MIB-1β42. The significant perturbations in cytokine expression months after injury suggest a component of persistent immune dysregulation and dysfunction which could ultimately result in a state of relative immunosuppression, potentially increasing susceptibility to infections and sepsis even after the initial hospitalization.
Acute adaptations in innate immunity after burn injury
In adult burn patients, most innate immune effector cells, including neutrophils, monocytes, macrophages, mast cells, and dendritic cells (DCs) are significantly dysregulated after burn injury43. An overall reduction of DCs has been found in adult burn patients with persistent DC depletion in septic burn patients44. Mast cells localize to areas of burn injury and release histamine, heparin, chymase, cathepsin G, and hydroxypeptiase A which promote wound healing43. The ability of monocytes to perform phagocytosis is disrupted following burn injury45. Macrophage hyperactivity following burns is associated with a pro-inflammatory state which disrupts immunologic homeostasis and predisposes patients to sepsis46. Neutrophils normally migrate to injured tissue and perform integral tasks for wound healing; however, in patients with burn injuries, neutrophils become less able to perform chemotaxis, phagocytosis, and generate reactive oxygen species43. Natural Killer (NK) cell function is compromised with effects proportional to burn size32. NK cells are particularly helpful in defending against viral infections; however, this ability may be lessened following burn injury47. Impaired NK cell capability persists at least 40 days following injury, indicating that immunologic changes in this patient population can become chronically deranged48. The data presented in this section is overwhelmingly representative of adult patients, revealing a need for increased immunologic research into pediatric-specific innate immune responses.
Adaptive immune response: Th1 vs Th2 phenotypes following burn injury
T helper type 1 (Th1) cells are CD4+ T cells which are overall pro-inflammatory and produce TNF-α, IFN-γ, IL-2, as well as other cytokines which regulate the cellular immune response49. This pathway primes the body to defend itself against pathogens, which is useful as the barrier function provided by the skin is compromised in burn patients. T helper type 2 (Th2) cells produce IL-4 and IL-10 and facilitate the humoral immune response50. This pathway dampens the inflammatory response through changes in cytokine production, macrophage suppression, and limiting of lymphocyte differentiation51. This decreases the inflammatory environment promoted by Th1 cells and promotes the development of a more durable humoral response to infection. Burns may favor a Th1 response immediately following injury; however, the body rapidly adapts to a Th2-phenotypic response. This switch in phenotype increases susceptibility to sepsis52. It is unknown how long it takes for the anti-inflammatory response to normalize in burned pediatric patients. The re-establishment of homeostasis may be more gradual across this population than previously suspected. It is also possible that sub-segments of pediatric burn patients exist, with some patients experiencing more rapid recovery and others experiencing persistent immunosuppression. This could result in increased susceptibility to infection long after hospital discharge. More research is needed in this area to develop understanding of this issue.
Other immune cell responses
Cytotoxic T-cell activity is suppressed acutely following burn injury, with subsequent recovery and increased activity two weeks following insult53. This interplay between increased cytotoxic T-cell activity and Th2 cells dampening the inflammatory response is part of the complex balance between the pro- and anti-inflammatory states that exist following burn injury. Regulatory T cells become unregulated following burn trauma, which may lead to overall suppression of T cells and subsequent immunosuppression54. B cell immunoglobulin production can also be depressed following burn insult in the pediatric population55.
The inflammatory cascade and subsequent alterations in the immune system of these patients represents a potential avenue for therapy. Murine studies have looked at propranolol and insulin in an attempt to attenuate derangements in homeostasis and rebalance the pro- and anti-inflammatory systems with promising results56,57. Differences in these patient populations and the long-term prevalence of indolent infections have not yet been adequately studied.
Hypertrophic burn scars
Burn scars complicate the long-term outcomes of many burn patients58. They can lead to perceived disfigurement and interfere with joint mobility, resulting in chronic contractures. Inflammation plays a key role in initial wound healing which can result in scarring. Finnerty et al. reviewed the foundation of wound healing, which occurs through inflammation, proliferation, and remodeling (Figure 3) 58. During the inflammatory phase, fibrin clot establishes a scaffold while cytokines (i.e., PDGF, TGF-β, EGF, and IGF-1) recruit cells to the site of injury. During proliferation, fibroblasts first produce collagen and synthesize the eventual extracellular matrix, followed by differentiation into myofibroblasts which lead to wound contraction. In remodeling, the extracellular matrix is degraded and type III collagen is modified into type I collagen. Hypertrophic scars arise from excess dermal collagen accumulation, as well as aberrant alignment of collagen bundles59. Pathologic scars are more likely to form in the presence of inflammation, or if wound healing is delayed60. This is particularly important in a pediatric population that is still growing, especially given the complex interplay between scar appearance, pruritus, and effects on social normalization and school reintegration.
Figure 3.
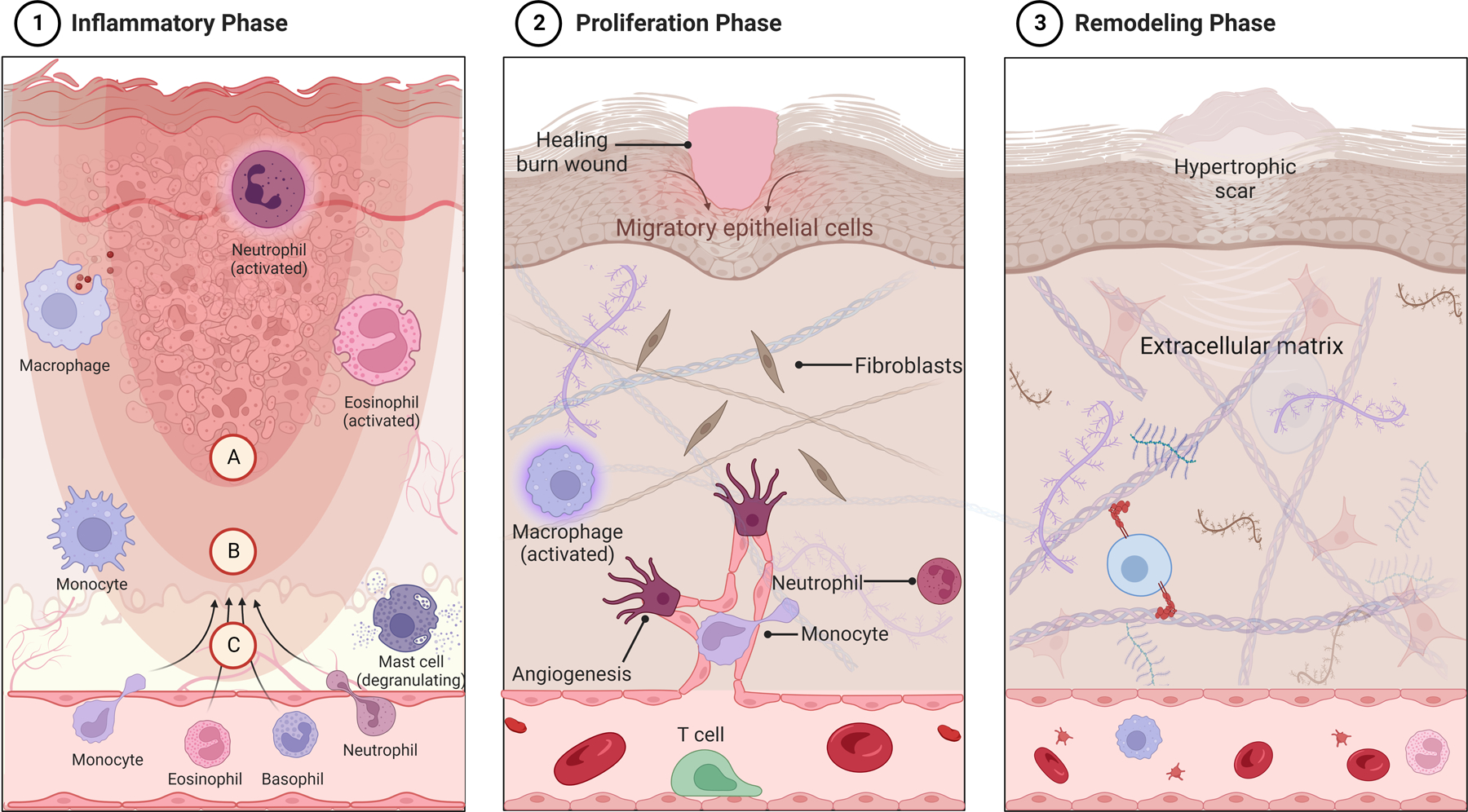
Immune response to burn injury and phases of healing. Burns, similar to other wounds, undergo three major phases of healing: the acute inflammatory phase (1), the proliferation phase (2), and the remodeling, or maturation phase (3). The inciting burn injury results in coagulation necrosis of the epidermis and underlying tissues, depending on burn depth, leading to degeneration of extracellular proteins and cell lysis (1). This is most prominent in the coagulation zone of injury (A), where irreversible tissue damage occurs. The zone of stasis (B), characterized by reduced tissue perfusion, remains potentially salvageable with assurance of adequate resuscitation and tissue perfusion, whereas the hyperemic zone (C) is characterized by increased tissue perfusion with increased vasodilation and capillary leak, contributing to tissue edema. Mast cells, neutrophils and monocytes are some of the first immune cells to migrate to the site of burn injury, followed by macrophages as initial neutrophil numbers decline (1). The initial cellular innate response triggers increased pro-inflammatory signaling, and their phagocytic activity helps with clearance of denatured proteins, dead tissue and toxins. The proliferative phase begins within hours of initial burn injury, with migration of keratinocytes from surrounding skin appendages. Growth and angiogenic factors released by responding immune cells trigger fibroblast activation and neovascularization within the dermis (2). In the final phase, or remodeling phase, increased collagen and elastin deposition strengthens the extracellular matrix, which is remodeled into scar tissue (3). Fibroblasts obtain a myofibroblast phenotype that contributes to scar contraction. In cases of prolonged wound healing (i.e., deep partial or full thickness burns allowed to heal by secondary intention), and in cases of excess dermal collagen accumulation, hypertrophic scars may develop (3). Created with biorender.com.
3. HOSPITALIZATION
3.1. Initial Treatment
3.1.1. Early Resuscitation
Resuscitation of acutely burned children sets the stage for their remaining hospitalization. Palmieri at al. reviewed principles of resuscitation in pediatric burn victims, which involves maintaining adequate organ perfusion while limiting excess fluid administration and pathologic edema4. General resuscitation of burned children differs from adults in airway management, fluid resuscitation, and choice of pharmacologic treatment4. Concerning airway management, endotracheal tube sizing is determined by the size child’s nares, small finger diameter, or the formula tube size = 4 + (age in years/4) 61. Surgical cricothyrotomy is contraindicated in children due to increased risk of stricture compared to adults. Children swallow air while crying and frequently require nasogastric tube placement4.
Fluid resuscitation is enacted when children have greater than approximately 15% TBSA burns, as opposed to the threshold of 20% TBSA burns seen in most adult burn protocols4. Two commonly accepted formulas for initial fluid resuscitation in children include the Parkland formula62:
And the Galveston formula2:
Modern resuscitation requirements are tailored to the individual burn patient through assessment of end organ perfusion, regardless of age. Overestimation of burn size occurs in up to 60% of pediatric patients in varying stages of development63. This increases the likelihood of over-resuscitation and fluid creep, increasing risk of pulmonary complications and abdominal compartment syndrome64. Factors which increase the amount of required resuscitation include inhalation injury, deeper burns, and delay in resuscitation17. Delay in care is also associated with increased length of stay, complications, renal failure, and mortality2,65.
Resuscitative fluids in children should be titrated to urine output as a marker of end-organ perfusion, which is similar to adults. The urine output goal is approximately 0.5–1 mL/kg/hr, which is an update from the prior practice of 1–2 mL/kg/hr2,17,66. Other methods for assessment of adequate resuscitation are not as commonly utilized. Lactated Ringer’s solution is an acceptable resuscitative fluid in all burned patients; however, infants may develop hypoglycemia during administration due to limited relative glycogen stores, necessitating a concomitant dextrose infusion for calories4. Colloids were shown in a randomized controlled trial in pediatric burns to decrease crystalloid infusion amount, fluid creep, and hospital length of stay67, however, other studies are equivocal, and the discussion of appropriate incorporation of colloids into resuscitation algorithms is ongoing within in the burn community68. Other methods of volume-reducing resuscitation have been largely abandoned with respect to acute burns in children: dextran due to coagulopathy, plasma due to disease transmission, and hypertonic saline due to possible increases in mortality and renal failure69,70. Multiple studies have compared different resuscitation formulas and variations with hypertonic saline or colloids with varying results, so it is difficult to develop a standardized method of resuscitation. Resuscitation is now understood as a dynamic and individualized process in which fluid administration is titrated based on evidence of end-organ perfusion in the pediatric burn patient.
3.1.2. Inhalation injury
Although occurring less frequently than in the adult burn population, inhalation injury remains prevalent in the pediatric burn population with an incidence of approximately 30%71. Inhalation injury is a major predictor of morbidity and mortality in pediatric patients. While the mainstays of treatment for inhalation injury are similar, key physiologic and anatomic distinctions in children require special consideration.
As in adults, early management of inhalation injury in children begins with a thorough airway assessment and confirmation of a secure airway. Ensuring a stable airway is of particular importance in infants and toddlers as these patients are particularly vulnerable to airway compromise secondary to the edema precipitated by inhalation injury. Their comparatively shorter mandibles, prominent tongues, and larger adenoids, as well as shorter, narrower airways, contribute to more rapid airway obstruction and increased resistance to airflow following inhalation injury (reviewed in: 72). Coupled with depressant medications administered for sedation and analgesia, these factors place children at a uniquely elevated risk of rapid airway collapse. A lower threshold for early intubation should be therefore be considered in pediatric patients presenting with significant facial burns or classical physical examination findings suggestive of inhalation injury, which include singed nares, soot in the airway, stridor, hoarseness, dysphonia, wheezing, or dyspnea73. It is important to avoid uncuffed endotracheal tubes in children to prevent air leaks, which is an update from prior practice74.
Additionally, given the developmental and physical limitations that may hinder a pediatric patient from escaping from a burn source, special care must be taken to assess for potential cyanide or carbon monoxide toxicity, which can be seen in up to 5% of all pediatric inhalation injuries and requires prompt attention75. Aggressive pulmonary hygiene, secretion management, bronchodilation, and prevention of cast formation with nebulized heparin and N-acetylcysteine, as in adults, remain important adjuncts in managing the resulting mucosal edema, sloughing, and increased secretion burden76.
Given increased rates of oxygen consumption and carbon dioxide production in infants and young children, along with increased susceptibility to developing pulmonary edema, tailoring of mechanical ventilation to allow for adequate gas exchange while minimizing ventilator-associated lung injury is a primary concern and often mandates higher respiratory rates compared to adults77. Beyond these basic principles, however, there remains no consensus on an optimal ventilation strategy for this age group even though a variety of strategies have been described, including conventional pressure and volume-controlled ventilatory modes, high-frequency oscillatory ventilation, and high tidal volume ventilation strategies (reviewed in: 72). Limited sample sizes and the retrospective nature of the existing literature underscores the need for more rigorous investigation in this domain.
3.1.3. Abdominal Compartment Syndrome
Patients undergoing resuscitation receive massive amounts of fluid in short time intervals. The subsequent leakage of fluid into the interstitium and resulting edema places the patient at risk of developing intra-abdominal hypertension that can progress to secondary abdominal compartment syndrome4,78. The volume of resuscitation which increases risk of abdominal compartment syndrome is 250mL/kg in the first day after burn injury, which is known as the Ivy Index79. A systematic review of burned adults and children observed the prevalence of intra-abdominal hypertension to be 65–75%, while the prevalence of abdominal compartment syndrome was 4–16%80. Increased abdominal pressures lead to physiologic changes, including compression of the cardiopulmonary system via the diaphragm and reduced venous return through external compression. Renal perfusion is decreased which lowers urine output; similarly, mesenteric flow is decreased81,82. Systemic inflammation during this time also can exacerbate injury82.
Intra-abdominal hypertension
Abdominal hypertension is defined as pressure within the abdominal cavity >12mmHg and is commonly measured with bladder pressures. Airway peak pressures can also be monitored. This diagnosis is associated with increased risk of kidney injury82. If intra-abdominal hypertension is diagnosed, steps such as escharotomy, paralysis, diuresis, and drain placement can be attempted in an attempt to halt progression to compartment syndrome81. Prior to laparotomy, percutaneous drain placement is recommended, particularly in children83.
Abdominal compartment syndrome
Abdominal compartment syndrome is defined as intra-abdominal pressures >20mmHg with evidence of end organ dysfunction. Children are more susceptible to the development of abdominal compartment syndrome in part due to their relatively smaller abdominal cavity84. Abdominal compartment syndrome is associated with increased mortality82. If non-surgical treatments fail, definitive treatment of abdominal compartment syndrome is laparotomy, in which a functional fascial release allows decompression. If decompressive laparotomy is performed, the goal should be closure as soon as appropriate; a study of adults and children showed 100% survival for patients closed within two days85. Mortality due to abdominal compartment syndrome in burn injury is 40–100% and is similar between children and adults86,87.
Associated complications include extremity compartment syndrome, pulmonary edema, and decreased perfusion of burn wounds through fluid creep64. Overall, abdominal compartment syndrome has not been studied enough in children to determine differences in parameters compared to adults. The available literature consists largely of case reports. It is possible that abdominal compartment syndrome occurs at a lower set point in children compared to adults, but this needs to be studied at a larger scale to determine how best to manage these patients84.
3.1.4. Escharotomy
Circumferential eschar secondary to burn injury is non-pliable and affects nerves and circulation in the extremities, as well as ventilation in the thorax88. Surgical release of eschar can lead to improvements in circulation and prevention of development of compartment syndrome. Escharotomies involve longitudinal incisions on lateral and medial aspects of extremities and digits, with a slightly more varied pattern across the thorax89. Crossing joint spaces in the extremities allows for adequate decompression. Children have different fat distribution compared to adults, so staying in the proper plane can be challenging4.
Following recovery from burn injuries, many children may require scar revision of their escharotomy sites. Escharotomies are, by necessity, full thickness injuries, but subsequent operative debridement in children may not require excision to subcutaneous fat, leading to an uneven appearance with differential healing. This process is also occurring in the setting of continued limb growth in pediatric patients89. As scar formation in children with burns has been strongly linked to social anxiety and negative self-image, it is important to be deliberate with the decision to perform an escharotomy, with care taken to minimize the number of incisions needed to effectively decompress the affected compartment89. Alternative methods include enzymatic debridement that is gaining popularity in the United States but is actively undergoing clinical trials and will not be covered in this review.
3.2. Critical Care Topics
3.2.1. Sepsis
Infection is the leading cause of morbidity and mortality in children and adults with large burns following the initial resuscitative period90. Adults are likely more susceptible to mortality from burn sepsis compared to children (50–84% compared to 55%) 91. Dysregulation in the immune response of the burn patient renders them susceptible to infection. The most common type of infection in pediatric patients is urinary tract infection, followed by pneumonia, burn wound infection, and central catheter infection92. Risk factors include burn depth, inhalation injury, indwelling devices, and TBSA burn size93,94. Identification of infection and sepsis is particularly challenging in burned patients in the setting of an active systemic inflammatory response. The American Burn Association held a Consensus Conference to define sepsis and infection, and differentiated between child and adult parameters given their state of development and differing physiology based on age95.
Physiologically, adult and pediatric patients respond to sepsis differently. Pediatric patient mortality is related to a low cardiac output leading to inadequate oxygen delivery96. Adults, on the other hand, develop decreased systemic vascular resistance with myocardial dysfunction, along with an inability to perform efficient oxygen extraction96. Most cases of sepsis in non-healed patients are related to burn wounds97. Early burn sepsis is typically caused by gram-positive bacteria, whereas late burn sepsis during hospitalization is caused by gram-negative bacterial and fungal infections98. If the burn wound is identified as the infectious source, then treatment involves excision97. Topical and enteral/parenteral antibiotics are also administered. Once a source is identified, it is important to de-escalate antibiotics therapy with a goal of decreasing rates of antibiotic resistance. As a child has many decades still to live, antibiotic stewardship is of particular importance. If vasopressor medications are required due to septic shock, then epinephrine is preferred in pediatric patients, which differs from norepinephrine recommended for adults96.
3.2.2. Multi-organ Dysfunction
Organ system dysfunction is a major source of morbidity during the hospitalization of the pediatric burn patient. Failure of multiple organ systems significantly increases risk of mortality99. These patients require careful assessment and multi-disciplinary management. The most common type of organ failure in pediatric burn patients is respiratory failure, followed by cardiac, hepatic, and renal failure99. Multisystem organ failure occurs in one in five pediatric burn patients in the ICU, and is correlated with increasing burn size, age, full-thickness burns, and inhalation injury. These patients suffer from an increased number of surgeries and major infections, longer lengths of stay, and higher rates of sepsis and mortality (41% vs 2%) 99. Sepsis is nearly always associated with multisystem organ failure100. However, compared to adults, children have a lower mortality rate after developing multisystem organ failure, in part due to pre-existing comorbidities in adult patients101. Studies in adults differ with respect to incidence of failure by organ system, but generally were similar in that respiratory failure was most common overall100,102.
Extracorporeal membrane oxygenation (ECMO) is a process that allows for externalized oxygenation of blood in the event of cardiopulmonary failure. Pooling various studies, the ECMO survival rate for pediatric burn patients is 52–77%103,104 whereas adult burn patient survival on ECMO was lower overall at 28–52%105,106.
3.2.3. Acute Kidney Injury
Pediatric patients are susceptible to acute kidney injury (AKI) at varying points in their hospitalization. Under-resuscitation can lead to pre-renal kidney failure. Drug-induced intrinsic kidney injury can also occur. Renal perfusion can be affected by blood loss, operations, or septic shock. Kidneys can also become injured secondary to rhabdomyolysis. Regardless of the etiology, acute kidney injury offers important principles in the care of the burned pediatric patient.
The incidence of AKI in pediatric burns admitted to the intensive care unit is 30–50% compared to 21–40% in adults, with risk factors including %TBSA burn, multiple surgical operations, sepsis, compartment syndrome, and increased ICU and hospital lengths of stay107–109. Mortality of burned children with AKI is increased six-fold compared to children without AKI, but is still half the mortality rate of adults with AKI107,110,111.
Incidence of kidney injury over time is bimodal, with early kidney largely associated with resuscitation and volume status, and late kidney injury occurring as a result of sepsis112. During sepsis, bacteria cause cytokine release which leads to endothelial damage and vasoplegia with resulting hypotension, which inflicts injury on renal tubules and vasculature112. Interestingly, late kidney injury in children is associated with increased mortality, whereas in adults, prognosis for early renal failure is worse than late renal failure107,113.
3.2.4. Blood Products
Blood products and burn patients are inexorably linked. Tangential excision and skin grafting causes significant blood loss despite meticulous attempts at hemostasis, and patients with major burns often receive greater than one blood volume worth of transfusions during their hospitalization114. Blood loss in pediatric burn operations is estimated at 3% per %TBSA excised and 2% per %TBSA grafted115 compared to 9% per %TBSA excised and grafted in adults116. Blood loss due to surgery is coupled with baseline anemia in the setting of critical illness, along with wounds, iron deficiency, hemodilution, and coagulopathy117.
Now that stewardship of blood products is at the forefront of research and hospital policy, it is pertinent to examine distinctions between pediatric and adult burns. In 1999, the TRICC Trial showed an effective restrictive transfusion strategy in the ICU, but excluded burn patients118. Voigt et al. performed a prospective randomized control trial in pediatric burns looking at a restrictive transfusion goal of 7mg/dL (prior standard 10mg/dL), and found that patients received 50% less blood in the OR and 25% outside of the OR with comparable outcomes119. The Transfusion Requirement in Burn Care Evaluation (TRIBE) trial also looked at a restrictive strategy in burn patients, with the restrictive group receiving one-third less blood transfusions with similar mortality, organ dysfunction, and infectious complications114. This restrictive strategy also decreased ventilator days and ICU days120. The use of whole blood in pediatric burns will soon become more widely studied as use of whole blood in trauma scenarios is regaining popularity.
4. CONCLUSION
Pediatric burn victims are a unique population with physiology that differs from adults. Advances in the study of hypermetabolism led to pharmacologic treatment in children that improved their quality of care. Research into the pediatric response to burn injury is critical to the understanding of the mechanisms involved in pediatric burns. This is followed by the development of tailored interventions specific to this population which allow for improved recovery. Impressive advancements have been made in reducing pediatric burn mortality. It is now time to take the next steps in the progression of research in pediatric burns.
Support:
This research was supported by the National Institutes of Health. ELB and VEP are supported by postgraduate training grant NIH NIGMS T32 GM008721 in burns, trauma, and perioperative injury. JCR and SDL are supported by NIH NIGMS R21 GM02452. PAE is supported by NIH NIGMS R35 GM140806. LLM is supported by RM1 GM139690.
REFERENCES
- 1.Armstrong M, Wheeler KK, Shi J, Thakkar RK, Fabia RB, Groner JI, Noffsinger D, Giles SA and Xiang H: Epidemiology and trend of US pediatric burn hospitalizations, 2003–2016. Burns 47(3):551–559, 2021. [DOI] [PubMed] [Google Scholar]
- 2.Wolf SE, Debroy M and Herndon DN: The cornerstones and directions of pediatric burn care. Pediatr Surg Int 12(5–6):312–20, 1997. [DOI] [PubMed] [Google Scholar]
- 3.Lee CJ, Mahendraraj K, Houng A, Marano M, Petrone S, Lee R and Chamberlain RS: Pediatric Burns: A Single Institution Retrospective Review of Incidence, Etiology, and Outcomes in 2273 Burn Patients (1995–2013). J Burn Care Res 37(6):e579–e585, 2016. [DOI] [PubMed] [Google Scholar]
- 4.Palmieri TL: Pediatric Burn Resuscitation. Crit Care Clin 32(4):547–59, 2016. [DOI] [PubMed] [Google Scholar]
- 5.Pham TN, Kramer CB, Wang J, Rivara FP, Heimbach DM, Gibran NS and Klein MB: Epidemiology and outcomes of older adults with burn injury: an analysis of the National Burn Repository. J Burn Care Res 30(1):30–6, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shields BJ, Comstock RD, Fernandez SA, Xiang H and Smith GA: Healthcare resource utilization and epidemiology of pediatric burn-associated hospitalizations, United States, 2000. J Burn Care Res 28(6):811–26, 2007. [DOI] [PubMed] [Google Scholar]
- 7.Strobel AM and Fey R: Emergency Care of Pediatric Burns. Emerg Med Clin North Am 36(2):441–458, 2018. [DOI] [PubMed] [Google Scholar]
- 8.Atwell K, Bartley C, Cairns B and Charles A: The epidemiologic characteristics and outcomes following intentional burn injury at a regional burn center. Burns 46(2):441–446, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Knowlin LT, Stanford LB, Cairns BA and Charles AG: The effect of preexisting respiratory co-morbidities on burn outcomes. Burns 43(2):366–373, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goverman J, Mathews K, Goldstein R, Holavanahalli R, Kowalske K, Esselman P, Gibran N, Suman O, Herndon D, Ryan CM, et al. : Pediatric Contractures in Burn Injury: A Burn Model System National Database Study. J Burn Care Res 38(1):e192–e199, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Palmieri TL, Taylor S, Lawless M, Curri T, Sen S and Greenhalgh DG: Burn center volume makes a difference for burned children. Pediatr Crit Care Med 16(4):319–24, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sheridan R, Weber J, Prelack K, Petras L, Lydon M and Tompkins R: Early burn center transfer shortens the length of hospitalization and reduces complications in children with serious burn injuries. J Burn Care Rehabil 20(5):347–50, 1999. [DOI] [PubMed] [Google Scholar]
- 13.Tiryaki C and Haksal MC: Comparison of clinical findings in adult and paediatric burn victims. Niger J Clin Pract 22(5):642–647, 2019. [DOI] [PubMed] [Google Scholar]
- 14.Mathias E and Srinivas Murthy M: Pediatric Thermal Burns and Treatment: A Review of Progress and Future Prospects. Medicines (Basel) 4(4), 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McNiece WL and Dierdorf SF: The pediatric airway. Semin Pediatr Surg 13(3):152–65, 2004. [DOI] [PubMed] [Google Scholar]
- 16.Fitzpatrick JC and Cioffi WG Jr.: Ventilatory support following burns and smoke-inhalation injury. Respir Care Clin N Am 3(1):21–49, 1997. [PubMed] [Google Scholar]
- 17.Sheridan RL: Burn Care for Children. Pediatr Rev 39(6):273–286, 2018. [DOI] [PubMed] [Google Scholar]
- 18.Hettiaratchy S and Dziewulski P: ABC of burns: pathophysiology and types of burns. Bmj 328(7453):1427–9, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Klein GL, Herndon DN, Goodman WG, Langman CB, Phillips WA, Dickson IR, Eastell R, Naylor KE, Maloney NA, Desai M, et al. : Histomorphometric and biochemical characterization of bone following acute severe burns in children. Bone 17(5):455–60, 1995. [DOI] [PubMed] [Google Scholar]
- 20.Senel E, Kizilgun M, Akbiyik F, Atayurt H, Tiryaki HT and Aycan Z: The evaluation of the adrenal and thyroid axes and glucose metabolism after burn injury in children. J Pediatr Endocrinol Metab 23(5):481–9, 2010. [DOI] [PubMed] [Google Scholar]
- 21.Chan MM and Chan GM: Nutritional therapy for burns in children and adults. Nutrition 25(3):261–9, 2009. [DOI] [PubMed] [Google Scholar]
- 22.Hart DW, Wolf SE, Mlcak R, Chinkes DL, Ramzy PI, Obeng MK, Ferrando AA, Wolfe RR and Herndon DN: Persistence of muscle catabolism after severe burn. Surgery 128(2):312–9, 2000. [DOI] [PubMed] [Google Scholar]
- 23.Klein GL, Herndon DN, Langman CB, Rutan TC, Young WE, Pembleton G, Nusynowitz M, Barnett JL, Broemeling LD, Sailer DE, et al. : Long-term reduction in bone mass after severe burn injury in children. J Pediatr 126(2):252–6, 1995. [DOI] [PubMed] [Google Scholar]
- 24.Schryver E, Klein GL, Herndon DN, Suman OE, Branski LK and Sousse LE: Bone metabolism in pediatric burned patients: A review. Burns 44(8):1863–1869, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Klein GL, Bi LX, Sherrard DJ, Beavan SR, Ireland D, Compston JE, Williams WG and Herndon DN: Evidence supporting a role of glucocorticoids in short-term bone loss in burned children. Osteoporos Int 15(6):468–74, 2004. [DOI] [PubMed] [Google Scholar]
- 26.Gottschlich MM, Mayes T, Khoury J and Warden GD: Hypovitaminosis D in acutely injured pediatric burn patients. J Am Diet Assoc 104(6):931–41, quiz 1031, 2004. [DOI] [PubMed] [Google Scholar]
- 27.Rutan RL and Herndon DN: Growth delay in postburn pediatric patients. Arch Surg 125(3):392–5, 1990. [DOI] [PubMed] [Google Scholar]
- 28.Murphy KD, Thomas S, Mlcak RP, Chinkes DL, Klein GL and Herndon DN: Effects of long-term oxandrolone administration in severely burned children. Surgery 136(2):219–24, 2004. [DOI] [PubMed] [Google Scholar]
- 29.Herndon DN, Voigt CD, Capek KD, Wurzer P, Guillory A, Kline A, Andersen CR, Klein GL, Tompkins RG, Suman OE, et al. : Reversal of Growth Arrest With the Combined Administration of Oxandrolone and Propranolol in Severely Burned Children. Ann Surg 264(3):421–8, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Reeves PT, Herndon DN, Tanksley JD, Jennings K, Klein GL, Mlcak RP, Clayton RP, Crites NN, Hays JP, Andersen C, et al. : FIVE-YEAR OUTCOMES AFTER LONG-TERM OXANDROLONE ADMINISTRATION IN SEVERELY BURNED CHILDREN: A RANDOMIZED CLINICAL TRIAL. Shock 45(4):367–74, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moulton DL, Yngve DA and Evans EB: Spinal Deformities in Pediatric Burn Patients. Spine Deform 4(2):149–155, 2016. [DOI] [PubMed] [Google Scholar]
- 32.Devine RA, Diltz Z, Hall MW and Thakkar RK: The systemic immune response to pediatric thermal injury. Int J Burns Trauma 8(1):6–16, 2018. [PMC free article] [PubMed] [Google Scholar]
- 33.Finnerty CC, Jeschke MG, Herndon DN, Gamelli R, Gibran N, Klein M, Silver G, Arnoldo B, Remick D and Tompkins RG: Temporal cytokine profiles in severely burned patients: a comparison of adults and children. Mol Med 14(9–10):553–60, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jeschke MG, van Baar ME, Choudhry MA, Chung KK, Gibran NS and Logsetty S: Burn injury. Nat Rev Dis Primers 6(1):11, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bhat S and Milner S: Antimicrobial peptides in burns and wounds. Curr Protein Pept Sci 8(5):506–20, 2007. [DOI] [PubMed] [Google Scholar]
- 36.Al-Tarrah K, Hewison M, Moiemen N and Lord JM: Vitamin D status and its influence on outcomes following major burn injury and critical illness. Burns Trauma 6:11, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xiao W, Mindrinos MN, Seok J, Cuschieri J, Cuenca AG, Gao H, Hayden DL, Hennessy L, Moore EE, Minei JP, et al. : A genomic storm in critically injured humans. J Exp Med 208(13):2581–90, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Korzeniowski T, Mertowska P, Mertowski S, Podgajna M, Grywalska E, Strużyna J and Torres K: The Role of the Immune System in Pediatric Burns: A Systematic Review. J Clin Med 11(8), 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Csontos C, Foldi V, Pálinkas L, Bogar L, Röth E, Weber G and Lantos J: Time course of pro- and anti-inflammatory cytokine levels in patients with burns--prognostic value of interleukin-10. Burns 36(4):483–94, 2010. [DOI] [PubMed] [Google Scholar]
- 40.Jeschke MG, Barrow RE, Suzuki F, Rai J, Benjamin D and Herndon DN: IGF-I/IGFBP-3 equilibrates ratios of pro- to anti-inflammatory cytokines, which are predictors for organ function in severely burned pediatric patients. Mol Med 8(5):238–46, 2002. [PMC free article] [PubMed] [Google Scholar]
- 41.Kraft R, Herndon DN, Finnerty CC, Cox RA, Song J and Jeschke MG: Predictive Value of IL-8 for Sepsis and Severe Infections After Burn Injury: A Clinical Study. Shock 43(3):222–7, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jeschke MG, Chinkes DL, Finnerty CC, Kulp G, Suman OE, Norbury WB, Branski LK, Gauglitz GG, Mlcak RP and Herndon DN: Pathophysiologic response to severe burn injury. Ann Surg 248(3):387–401, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sierawska O, Małkowska P, Taskin C, Hrynkiewicz R, Mertowska P, Grywalska E, Korzeniowski T, Torres K, Surowiecka A, Niedźwiedzka-Rystwej P, et al. : Innate Immune System Response to Burn Damage-Focus on Cytokine Alteration. Int J Mol Sci 23(2), 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.D’Arpa N, Accardo-Palumbo A, Amato G, D’Amelio L, Pileri D, Cataldo V, Mogavero R, Lombardo C, Napoli B and Conte F: Circulating dendritic cells following burn. Burns 35(4):513–8, 2009. [DOI] [PubMed] [Google Scholar]
- 45.Xiu F and Jeschke MG: Perturbed mononuclear phagocyte system in severely burned and septic patients. Shock 40(2):81–8, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schwacha MG: Macrophages and post-burn immune dysfunction. Burns 29(1):1–14, 2003. [DOI] [PubMed] [Google Scholar]
- 47.Klimpel GR, Herndon DN, Fons M, Albrecht T, Asuncion MT, Chin R and Stein MD: Defective NK cell activity following thermal injury. Clin Exp Immunol 66(2):384–92, 1986. [PMC free article] [PubMed] [Google Scholar]
- 48.Blazar BA, Rodrick ML, O’Mahony JB, Wood JJ, Bessey PQ, Wilmore DW and Mannick JA: Suppression of natural killer-cell function in humans following thermal and traumatic injury. J Clin Immunol 6(1):26–36, 1986. [DOI] [PubMed] [Google Scholar]
- 49.Ruterbusch M, Pruner KB, Shehata L and Pepper M: In Vivo CD4(+) T Cell Differentiation and Function: Revisiting the Th1/Th2 Paradigm. Annu Rev Immunol 38:705–725, 2020. [DOI] [PubMed] [Google Scholar]
- 50.Zang Y, Dolan SM, Ni Choileain N, Kriynovich SJ, Murphy TJ, Sayles P, Mannick JA and Lederer JA: Burn injury initiates a shift in superantigen-induced T cell responses and host survival. J Immunol 172(8):4883–92, 2004. [DOI] [PubMed] [Google Scholar]
- 51.Abbas AK, Murphy KM and Sher A: Functional diversity of helper T lymphocytes. Nature 383(6603):787–93, 1996. [DOI] [PubMed] [Google Scholar]
- 52.Rani M, Zhang Q and Schwacha MG: Burn wound γδ T-cells support a Th2 and Th17 immune response. J Burn Care Res 35(1):46–53, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hunt JP, Hunter CT, Brownstein MR, Giannopoulos A, Hultman CS, deSerres S, Bracey L, Frelinger J and Meyer AA: The effector component of the cytotoxic T-lymphocyte response has a biphasic pattern after burn injury. J Surg Res 80(2):243–51, 1998. [DOI] [PubMed] [Google Scholar]
- 54.Huang LF, Yao YM, Dong N, Yu Y, He LX and Sheng ZY: Association between regulatory T cell activity and sepsis and outcome of severely burned patients: a prospective, observational study. Crit Care 14(1):R3, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sobouti B, Fallah S, Ghavami Y and Moradi M: Serum immunoglobulin levels in pediatric burn patients. Burns 39(3):473–6, 2013. [DOI] [PubMed] [Google Scholar]
- 56.Muthu K, He LK, Szilagyi A, Stevenson J, Gamelli RL and Shankar R: Propranolol restores the tumor necrosis factor-alpha response of circulating inflammatory monocytes and granulocytes after burn injury and sepsis. J Burn Care Res 30(1):8–18, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jeschke MG, Einspanier R, Klein D and Jauch KW: Insulin attenuates the systemic inflammatory response to thermal trauma. Mol Med 8(8):443–50, 2002. [PMC free article] [PubMed] [Google Scholar]
- 58.Finnerty CC, Jeschke MG, Branski LK, Barret JP, Dziewulski P and Herndon DN: Hypertrophic scarring: the greatest unmet challenge after burn injury. Lancet 388(10052):1427–1436, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zuccaro J, Perez MM, Mohanta A, Fish JS and Doria AS: Quantification of Pediatric Burn Scar Stiffness Using Acoustic Radiation Force Impulse Ultrasound Elastography. Ultrasound Med Biol 45(8):1918–1923, 2019. [DOI] [PubMed] [Google Scholar]
- 60.Cho YS, Jeon JH, Hong A, Yang HT, Yim H, Kim DH, Hur J, Kim JH, Chun W, Lee BC, et al. : The effect of burn rehabilitation massage therapy on hypertrophic scar after burn: a randomized controlled trial. Burns 40(8):1513–20, 2014. [DOI] [PubMed] [Google Scholar]
- 61.Ahmed RA and Boyer TJ: Endotracheal Tube Treasure Island (FL): StatPearls Publishing; Copyright © 2022, StatPearls Publishing LLC., 2022. [PubMed] [Google Scholar]
- 62.Baxter CR and Shires T: Physiological response to crystalloid resuscitation of severe burns. Ann N Y Acad Sci 150(3):874–94, 1968. [DOI] [PubMed] [Google Scholar]
- 63.Goverman J, Bittner EA, Friedstat JS, Moore M, Nozari A, Ibrahim AE, Sarhane KA, Chang PH, Sheridan RL and Fagan SP: Discrepancy in Initial Pediatric Burn Estimates and Its Impact on Fluid Resuscitation. J Burn Care Res 36(5):574–9, 2015. [DOI] [PubMed] [Google Scholar]
- 64.Pruitt BA Jr.: Protection from excessive resuscitation: “pushing the pendulum back”. J Trauma 49(3):567–8, 2000. [DOI] [PubMed] [Google Scholar]
- 65.Barrow RE, Jeschke MG and Herndon DN: Early fluid resuscitation improves outcomes in severely burned children. Resuscitation 45(2):91–6, 2000. [DOI] [PubMed] [Google Scholar]
- 66.Warden GD: Burn shock resuscitation. World J Surg 16(1):16–23, 1992. [DOI] [PubMed] [Google Scholar]
- 67.Müller Dittrich MH, Brunow de Carvalho W and Lopes Lavado E: Evaluation of the “Early” Use of Albumin in Children with Extensive Burns: A Randomized Controlled Trial. Pediatr Crit Care Med 17(6):e280–6, 2016. [DOI] [PubMed] [Google Scholar]
- 68.Eljaiek R, Heylbroeck C and Dubois MJ: Albumin administration for fluid resuscitation in burn patients: A systematic review and meta-analysis. Burns 43(1):17–24, 2017. [DOI] [PubMed] [Google Scholar]
- 69.Huang PP, Stucky FS, Dimick AR, Treat RC, Bessey PQ and Rue LW: Hypertonic sodium resuscitation is associated with renal failure and death. Ann Surg 221(5):543–54; discussion 554–7, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sheridan R: Less Is More-Revisiting Burn Resuscitation Vol. 17. United States, 2016, pp. 578–9. [DOI] [PubMed] [Google Scholar]
- 71.Jeschke MG and Herndon DN: Burns in children: standard and new treatments. Lancet 383(9923):1168–78, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sen S: Pediatric inhalation injury. Burns Trauma 5:31, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Fidkowski CW, Fuzaylov G, Sheridan RL and Coté CJ: Inhalation burn injury in children. Paediatr Anaesth 19 Suppl 1:147–54, 2009. [DOI] [PubMed] [Google Scholar]
- 74.Sheridan RL: Uncuffed endotracheal tubes should not be used in seriously burned children. Pediatr Crit Care Med 7(3):258–9, 2006. [DOI] [PubMed] [Google Scholar]
- 75.Mintegi S, Clerigue N, Tipo V, Ponticiello E, Lonati D, Burillo-Putze G, Delvau N and Anseeuw K: Pediatric cyanide poisoning by fire smoke inhalation: a European expert consensus. Toxicology Surveillance System of the Intoxications Working Group of the Spanish Society of Paediatric Emergencies. Pediatr Emerg Care 29(11):1234–40, 2013. [DOI] [PubMed] [Google Scholar]
- 76.Foncerrada G, Culnan DM, Capek KD, González-Trejo S, Cambiaso-Daniel J, Woodson LC, Herndon DN, Finnerty CC and Lee JO: Inhalation Injury in the Burned Patient. Ann Plast Surg 80(3 Suppl 2):S98–s105, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Brambrink AM and Braun U: Airway management in infants and children. Best Pract Res Clin Anaesthesiol 19(4):675–97, 2005. [DOI] [PubMed] [Google Scholar]
- 78.Maxwell RA, Fabian TC, Croce MA and Davis KA: Secondary abdominal compartment syndrome: an underappreciated manifestation of severe hemorrhagic shock. J Trauma 47(6):995–9, 1999. [DOI] [PubMed] [Google Scholar]
- 79.Ivy ME, Atweh NA, Palmer J, Possenti PP, Pineau M and D’Aiuto M: Intra-abdominal hypertension and abdominal compartment syndrome in burn patients. J Trauma 49(3):387–91, 2000. [DOI] [PubMed] [Google Scholar]
- 80.Strang SG, Van Lieshout EM, Breederveld RS and Van Waes OJ: A systematic review on intra-abdominal pressure in severely burned patients. Burns 40(1):9–16, 2014. [DOI] [PubMed] [Google Scholar]
- 81.Jensen AR, Hughes WB and Grewal H: Secondary abdominal compartment syndrome in children with burns and trauma: a potentially lethal complication. J Burn Care Res 27(2):242–6, 2006. [DOI] [PubMed] [Google Scholar]
- 82.Sun K, Hancock BJ and Logsetty S: Ischemic bowel as a late sequela of abdominal compartment syndrome secondary to severe burn injury. Plast Surg (Oakv) 23(4):218–20, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kirkpatrick AW, Roberts DJ, De Waele J, Jaeschke R, Malbrain ML, De Keulenaer B, Duchesne J, Bjorck M, Leppaniemi A, Ejike JC, et al. : Intra-abdominal hypertension and the abdominal compartment syndrome: updated consensus definitions and clinical practice guidelines from the World Society of the Abdominal Compartment Syndrome. Intensive Care Med 39(7):1190–206, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Beck R, Halberthal M, Zonis Z, Shoshani G, Hayari L and Bar-Joseph G: Abdominal compartment syndrome in children. Pediatr Crit Care Med 2(1):51–6, 2001. [DOI] [PubMed] [Google Scholar]
- 85.Ramirez JI, Sen S, Palmieri TL and Greenhalgh DG: Timing of Laparotomy and Closure in Burn Patients with Abdominal Compartment Syndrome: Effects on Survival. J Am Coll Surg 226(6):1175–1180, 2018. [DOI] [PubMed] [Google Scholar]
- 86.Hershberger RC, Hunt JL, Arnoldo BD and Purdue GF: Abdominal compartment syndrome in the severely burned patient. J Burn Care Res 28(5):708–14, 2007. [DOI] [PubMed] [Google Scholar]
- 87.Struck MF, Reske AW, Schmidt T, Hilbert P, Steen M and Wrigge H: Respiratory functions of burn patients undergoing decompressive laparotomy due to secondary abdominal compartment syndrome. Burns 40(1):120–6, 2014. [DOI] [PubMed] [Google Scholar]
- 88.Butts CC, Holmes JH and Carter JE: Surgical Escharotomy and Decompressive Therapies in Burns. J Burn Care Res 41(2):263–269, 2020. [DOI] [PubMed] [Google Scholar]
- 89.Davenport LM, Cuttle L, McBride CA and Kimble R: The morbidity associated with paediatric burn wound escharotomies. ANZ J Surg 91(10):2139–2144, 2021. [DOI] [PubMed] [Google Scholar]
- 90.Partain KP, Fabia R and Thakkar RK: Pediatric burn care: new techniques and outcomes. Curr Opin Pediatr 32(3):405–410, 2020. [DOI] [PubMed] [Google Scholar]
- 91.Torres MJM, Peterson JM and Wolf SE: Detection of Infection and Sepsis in Burns. Surg Infect (Larchmt) 22(1):20–27, 2021. [DOI] [PubMed] [Google Scholar]
- 92.Sheridan RL: Sepsis in pediatric burn patients. Pediatr Crit Care Med 6(3 Suppl):S112–9, 2005. [DOI] [PubMed] [Google Scholar]
- 93.Demirdjian G: Adjusting a prognostic score for burned children with logistic regression. J Burn Care Rehabil 18(4):313–6, 1997. [DOI] [PubMed] [Google Scholar]
- 94.Rodgers GL, Mortensen J, Fisher MC, Lo A, Cresswell A and Long SS: Predictors of infectious complications after burn injuries in children. Pediatr Infect Dis J 19(10):990–5, 2000. [DOI] [PubMed] [Google Scholar]
- 95.Greenhalgh DG, Saffle JR, Holmes JHt, Gamelli RL, Palmieri TL, Horton JW, Tompkins RG, Traber DL, Mozingo DW, Deitch EA, et al. : American Burn Association consensus conference to define sepsis and infection in burns. J Burn Care Res 28(6):776–90, 2007. [DOI] [PubMed] [Google Scholar]
- 96.Greenhalgh DG: Sepsis in the burn patient: a different problem than sepsis in the general population. Burns Trauma 5:23, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Williams FN and Lee JO: Pediatric Burn Infection. Surg Infect (Larchmt) 22(1):54–57, 2021. [DOI] [PubMed] [Google Scholar]
- 98.Devrim İ, Kara A, Düzgöl M, Karkıner A, Bayram N, Temir G, Şencan A, Sorguç Y, Gülfidan G and Hoşgör M: Burn-associated bloodstream infections in pediatric burn patients: Time distribution of etiologic agents. Burns 43(1):144–148, 2017. [DOI] [PubMed] [Google Scholar]
- 99.Kraft R, Herndon DN, Finnerty CC, Shahrokhi S and Jeschke MG: Occurrence of multiorgan dysfunction in pediatric burn patients: incidence and clinical outcome. Ann Surg 259(2):381–7, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Saffle JR, Sullivan JJ, Tuohig GM and Larson CM: Multiple organ failure in patients with thermal injury. Crit Care Med 21(11):1673–83, 1993. [DOI] [PubMed] [Google Scholar]
- 101.Emr BM, Alcamo AM, Carcillo JA, Aneja RK and Mollen KP: Pediatric Sepsis Update: How Are Children Different? Surg Infect (Larchmt) 19(2):176–183, 2018. [DOI] [PubMed] [Google Scholar]
- 102.Nguyen LN and Nguyen TG: Characteristics and outcomes of multiple organ dysfunction syndrome among severe-burn patients. Burns 35(7):937–41, 2009. [DOI] [PubMed] [Google Scholar]
- 103.Thompson KB, Dawoud F, Castle S, Pietsch JB, Danko ME and Bridges BC: Extracorporeal Membrane Oxygenation Support for Pediatric Burn Patients: Is It Worth the Risk? Pediatr Crit Care Med 21(5):469–476, 2020. [DOI] [PubMed] [Google Scholar]
- 104.Pettignano R, Fortenberry JD, Heard ML, Labuz MD, Kesser KC, Tanner AJ, Wagoner SF and Heggen J: Primary use of the venovenous approach for extracorporeal membrane oxygenation in pediatric acute respiratory failure. Pediatr Crit Care Med 4(3):291–8, 2003. [DOI] [PubMed] [Google Scholar]
- 105.Chiu YJ, Huang YC, Chen TW, King YA and Ma H: A Systematic Review and Meta-Analysis of Extracorporeal Membrane Oxygenation in Patients with Burns. Plast Reconstr Surg 149(6):1181e–1190e, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Soussi S, Gallais P, Kachatryan L, Benyamina M, Ferry A, Cupaciu A, Chaussard M, Maurel V, Chaouat M, Mimoun M, et al. : Extracorporeal membrane oxygenation in burn patients with refractory acute respiratory distress syndrome leads to 28 % 90-day survival Vol. 42. United States, 2016, pp. 1826–1827. [DOI] [PubMed] [Google Scholar]
- 107.Steinvall I, Bak Z and Sjoberg F: Acute kidney injury is common, parallels organ dysfunction or failure, and carries appreciable mortality in patients with major burns: a prospective exploratory cohort study. Crit Care 12(5):R124, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Folkestad T, Brurberg KG, Nordhuus KM, Tveiten CK, Guttormsen AB, Os I and Beitland S: Acute kidney injury in burn patients admitted to the intensive care unit: a systematic review and meta-analysis. Crit Care 24(1):2, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Wu G, Xiao Y, Wang C, Hong X, Sun Y, Ma B, Wang G and Xia Z: Risk Factors for Acute Kidney Injury in Patients With Burn Injury: A Meta-Analysis and Systematic Review. J Burn Care Res 38(5):271–282, 2017. [DOI] [PubMed] [Google Scholar]
- 110.Lopes JA, Jorge S, Neves FC, Caneira M, da Costa AG, Ferreira AC and Prata MM: An assessment of the RIFLE criteria for acute renal failure in severely burned patients Vol. 22. England, 2007, p. 285. [DOI] [PubMed] [Google Scholar]
- 111.Brusselaers N, Monstrey S, Colpaert K, Decruyenaere J, Blot SI and Hoste EA: Outcome of acute kidney injury in severe burns: a systematic review and meta-analysis. Intensive Care Med 36(6):915–25, 2010. [DOI] [PubMed] [Google Scholar]
- 112.Clark A, Neyra JA, Madni T, Imran J, Phelan H, Arnoldo B and Wolf SE: Acute kidney injury after burn. Burns 43(5):898–908, 2017. [DOI] [PubMed] [Google Scholar]
- 113.Palmieri T, Lavrentieva A and Greenhalgh D: An assessment of acute kidney injury with modified RIFLE criteria in pediatric patients with severe burns. Intensive Care Med 35(12):2125–9, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Palmieri TL, Holmes JHt, Arnoldo B, Peck M, Potenza B, Cochran A, King BT, Dominic W, Cartotto R, Bhavsar D, et al. : Transfusion Requirement in Burn Care Evaluation (TRIBE): A Multicenter Randomized Prospective Trial of Blood Transfusion in Major Burn Injury. Ann Surg 266(4):595–602, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Housinger TA, Lang D and Warden GD: A prospective study of blood loss with excisional therapy in pediatric burn patients. J Trauma 34(2):262–3, 1993. [DOI] [PubMed] [Google Scholar]
- 116.Steadman PB and Pegg SP: A quantitative assessment of blood loss in burn wound excision and grafting. Burns 18(6):490–1, 1992. [DOI] [PubMed] [Google Scholar]
- 117.Palmieri TL: Burn injury and blood transfusion. Curr Opin Anaesthesiol 32(2):247–251, 2019. [DOI] [PubMed] [Google Scholar]
- 118.Hébert PC, Wells G, Blajchman MA, Marshall J, Martin C, Pagliarello G, Tweeddale M, Schweitzer I and Yetisir E: A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care. Transfusion Requirements in Critical Care Investigators, Canadian Critical Care Trials Group. N Engl J Med 340(6):409–17, 1999. [DOI] [PubMed] [Google Scholar]
- 119.Voigt CD, Hundeshagen G, Malagaris I, Watson K, Obiarinze RN, Hasanpour H, Woodson LC, Capek KD, Lee JO, Nunez Lopez O, et al. : Effects of a restrictive blood transfusion protocol on acute pediatric burn care: Transfusion threshold in pediatric burns. J Trauma Acute Care Surg 85(6):1048–1054, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Palmieri TL, Holmes JH, Arnoldo B, Peck M, Cochran A, King BT, Dominic W, Cartotto R, Bhavsar D, Tredget E, et al. : Restrictive Transfusion Strategy Is More Effective in Massive Burns: Results of the TRIBE Multicenter Prospective Randomized Trial. Mil Med 184(Suppl 1):11–15, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]


