Abstract
Introduction
Juvenile systemic lupus erythematosus (j-SLE) is a rare chronic autoimmune disease affecting multiple organs. Ranging from minor features, such as headache or mild cognitive impairment, to serious and life-threatening presentations, j-neuropsychiatric SLE (j-NPSLE) is a therapeutic challenge. Thus, the diagnosis of NPSLE remains difficult, especially in pediatrics, with no specific biomarker of the disease yet validated.
Objectives
To identify central nervous system (CNS) disease biomarkers of j-NPSLE.
Methods
A 5-year retrospective tertiary reference monocentric j-SLE study. A combination of standardized diagnostic criteria and multidisciplinary pediatric clinical expertise was combined to attribute NP involvement in the context of j-SLE. Neopterin and interferon-alpha (IFN-α) protein levels in cerebrospinal fluid (CSF) were assessed, together with routine biological and radiological investigations.
Results
Among 51 patients with j-SLE included, 39% presented with j-NPSLE. J-NPSLE was diagnosed at onset of j-SLE in 65% of patients. No specific routine biological or radiological marker of j-NPSLE was identified. However, CSF neopterin levels were significantly higher in active j-NPSLE with CNS involvement than in j-SLE alone (p = 0.0008). Neopterin and IFN-α protein levels in CSF were significantly higher at diagnosis of j-NPSLE with CNS involvement than after resolution of NP features (respectively p = 0.0015 and p = 0.0010) upon immunosuppressive treatment in all patients tested (n = 10). Both biomarkers correlated strongly with each other (Rs = 0.832, p < 0.0001, n = 23 paired samples).
Conclusion
CSF IFN-α and neopterin constitute promising biomarkers useful in the diagnosis and monitoring of activity in j-NPSLE.
Supplementary Information
The online version contains supplementary material available at 10.1007/s10875-022-01407-1.
Keywords: Juvenile systemic lupus erythematosus, Neuropsychiatric, Central nervous system, Biomarker, Neopterin, Interferon-alpha
Introduction
Systemic lupus erythematosus (SLE) is a chronic auto-immune disease affecting multiple organs, including the central and peripheral nervous system. SLE is a rare disease with an estimated global prevalence of 47/100,000 [1]. Juvenile onset (i.e. onset before age 16 years) SLE (j-SLE) is even rarer, with an estimated prevalence of 3.76/100,000 [1]. Neuropsychiatric SLE (NPSLE), defined by the presence of SLE-related neuropsychiatric (NP) involvement, has a poorly known estimated prevalence of 22 to 95% in j-SLE, largely dependent on the level of stringency in definitions [2–4]. NP involvement can be difficult to diagnose and raises the question as to causality i.e. due (i) to direct neuroimmunological SLE involvement, (ii) to ‘indirect’ chronic-disease-induced stress, or (iii) to iatrogenic factors. Accurate diagnosis is important, since NPSLE can be associated with a significant social impact and care cost and may require specific, rapid, and aggressive treatment [5–7]. To assist clinicians, the American College of Rheumatology (ACR) has developed a standardized nomenclature system and case definition for 19 neuropsychiatric syndromes observed in SLE [8], also commonly applied to j-SLE. Several authors have proposed other criteria since then, but only in adult populations [9, 10]. To date, none of these nomenclatures or algorithms has been adopted as a gold standard.
Notably, diagnostic biomarkers of NPSLE with central nervous system (CNS) involvement are limited, particularly in children, and none have been validated as demonstrating robust clinical utility in the few studies undertaken of j-NPSLE [11–13]. In adults, many cerebrospinal or serum biomarkers have been assessed in heterogeneous populations (patients with variable peripheral and/or CNS impairment, partly due to the rarity of the different NPSLE syndromes), but none validated prospectively among the various cohorts. Of note, CSF α-Klotho, lipocalin-2, M-CSF and IgM, and serum IL-6, miR-23a, and miR-155 were reported to be the most promising diagnostic biomarkers of NPSLE [14]. Immunoinflammatory abnormalities have been recognized as having a potential role in the pathogenesis of psychiatric disease [15], and conversely, neuropsychiatric involvement is common in inflammatory diseases, mainly driven by cytokines [16]. Of note, the pathological effect of IFN-α on the brain is suggested by the occurrence of neuropsychiatric signs, such as psychosis, depressive or confusional syndrome in patients treated with this cytokine [17, 18]. Interestingly, elevated serum IFN-α has also been associated with SLE activity and severity [19, 20]. In addition, IFN-α is suggested to be involved in the pathophysiology of NPSLE [21]. Increased levels of cerebrospinal fluid (CSF) neopterin are indicative of intrinsic cellular immune activation and are suspected to be produced intrathecally by microglia and astrocytes in the context of various diseases of the CNS, reflecting CNS immunoinflammatory activation [22–24]. Furthermore, elevated neopterin and IFN-α has been reported in the CSF of patients affected with Aicardi-Goutières syndrome (AGS), a Mendelian type I interferonopathy sharing common pathological pathways with SLE [23, 25, 26]. For these reasons, we hypothesized that CSF IFN-α and neopterin might represent reliable biomarkers of j-NPSLE.
The aim of our study was to identify CNS biomarkers associated with j-NPSLE in order to provide accurate diagnostic tools for clinicians and help with monitoring of disease activity.
Methods
Patients
Patients identified through the French database of rare diseases who met the 2019 European League Against Rheumatism (EULAR) ACR classification criteria for SLE with onset < 16 years (j-SLE) followed at a tertiary reference center for autoimmune diseases in children, Robert-Debré hospital, Paris, France, from January 2017 to March 2022, were included. These patients were investigated according to the same routine-care procedure over the entire period: suspected cases of j-NPSLE were evaluated by a multidisciplinary pediatric team (rheumatologist, psychiatrist, psychologist, and neurologist) and longitudinally assessed during their follow-up. Standardized criteria (1999 ACR nomenclature, DSM V-Diagnostic and Statistical Manual of Mental Disorders) and multidisciplinary consensus were combined to attribute NP features to j-SLE. Isolated headaches were excluded. CSF neopterin and IFN-α were not considered for classification and only analyzed after group attribution. The study protocol followed ethics guidelines (Robert-Debré hospital ethical committee, authorization N˚ 2020–478). Data processing was approved by the AP-HP (Public Hospitals of Paris) data protection office (registration number N°20200227113056). All samples were collected with informed consent. The study was approved by the Comité de protection des personnes Ile de France II and the French advisory committee on data processing in medical research (ID-RCB: 2014-A01017-40).
Collected Data
Demographic information, past medical history, clinical features and laboratory findings, treatments, outcome, brain computed tomography scans, magnetic resonance imaging (MRI), and electroencephalogram (EEG) were recorded. All patients with j-NPSLE underwent brain MRI with non-contrast–enhanced MR angiography (TOF, time-of-flight) using either a 1.5 or 3 T MRI scanner. Global disease activity and cumulative organ damage were respectively quantified by the SLE Disease Activity Index (SLEDAI) and the Systemic Lupus International Collaborating Clinics (SLICC)/ACR damage index. Active NPSLE status was attributed with ACR and DSM V criteria and multidisciplinary expertise. Inactive NPSLE status was defined as the resolution of acute clinical signs which had led to the diagnosis of j-NPSLE.
Samples
Lumbar puncture analysis included cytology, protein, bacteriology, oligoclonal bands, CNS auto antibodies, neopterin, and IFN-α. CNS auto antibodies (including anti-N-methyl-D-aspartate receptor) were assessed by immunohistochemistry on rat brains slices. Neopterin was determined by liquid chromatography coupled with tandem mass spectrometry. IFN-α was quantified by different methods in serum and CSF: biological titration of antiviral activity [27, 28], and with a single-molecule array (Simoa) digital ELISA (enzyme-linked immunosorbent assay) using a monoclonal antibody pair isolated from patients with APECED with high specificity for all IFN-α subtypes (pan-IFN-α assay) as previously described [19]. Interferon stimulated gene (ISG) expression was assessed in whole blood and an ‘ISG score’ derived as previously described [29].
Statistical Analysis
Medians and interquartile ranges were used for quantitative variable description. Qualitative variables were described as number of patients (n) and percentages (%). Fisher’s exact test was used for categorical data. Given the small sample size, continuous data were compared using Mann–Whitney’s test. A paired data test was not performed in the principal analysis for neopterin and pan-IFN-α in j-NPSLE because of the consequences in terms of data loss. A p-value below 0.05 was considered statistically significant. Analyses were performed with GraphPad PRISM (version 9).
Results
Composition of the Study Group
Fifty-one j-SLE patients were included, 20 of whom were diagnosed with j-NPSLE with CNS involvement (39%) and shown in a flow chart (Figure S1). Median follow-up of all j-SLE patients was 34 months [20–59]. Patient demographic and clinical characteristics are summarized in Tables 1, S1, and S2. Of the 20 j-NPSLE patients, 13 demonstrated NP features at SLE onset (65%). The seven other patients received a diagnosis of j-NPSLE later in the course of their disease (median time: 15 months after SLE diagnosis, [2-36]). SLEDAI score at the time of CSF assessment did not differ significantly between both groups (j-NPSLE vs j-SLE-controls). On the contrary, SLICC/ACR damage score was significantly higher in the j-NPSLE group (Table 1: median = 1 [0;1] vs 0 [0;0], p = 0.0065; number of patients with a score ≥ 1 n = 11 [61%] vs 0; p = 0.0074) at last follow-up evaluation (median time follow-up from acute NP episode to last follow-up evaluation: 24 months [16;37] for j-NPSLE vs 14 months [12;29] for j-SLE-controls, p = 0.2627). We collected 38 CSF samples from 28 individuals, comprising 20 j-NPSLE patients with CNS involvement (19 and 11 samples during active and inactive disease respectively) and 8 controls (j-SLE where NPSLE was discarded). The following assessments were available: CSF IFN activity in 18 active and 9 inactive j-NPSLE and 8 controls, blood IFN activity in 19 active and 9 inactive j-NPSLE and 7 controls, CSF pan-IFN-α measured by Simoa in 9 active and 5 inactive j-NPSLE patients and 3 controls, serum pan-IFN-α measured by Simoa in 8 active and 5 inactive j-NPSLE and 3 controls, CSF neopterin in 18 active and 11 inactive j-NPSLE and 7 controls, 17 ISG scores in peripheral blood in 5 active and 7 inactive j-NPSLE and 5 controls, taken concomitant to CSF sampling (Figs. 1 and S1; Tables 1 and S3).
Table 1.
Comparison between group 1 (j-NPSLE +) and group 2 (j-NPSLE– but j-SLE +)
| Parameters | Group 1, j-NPSLE + (n = 20) | Group 2, j-NPSLE − (n = 8) | P value |
|---|---|---|---|
| Age at j-SLE diagnosis (years), median (Q1; Q3) | 14.0 (11.3; 15.1) | 13.9 (12.2;14.6) | 0.950 |
| Sex ratio (female/male), n/n | 19/1 | 6/2 | 0.188 |
| Comorbiditiesa | |||
| Neuropsychiatric personal history, n (%)b | 5 (25) | 3 (38) | 0.651 |
| Familial history of auto-immune disease in a first degree relativec, n (%) | 3 (15) | 1 (13) | 1 |
| Laboratory findings at j-SLE diagnosis | |||
| Anti-nuclear antibody, n (%) | 20 (100) | 8 (100) | 1 |
| Anti-SSA, n (%) | 11 (58) | 3 (38) | 0.420 |
| Anti-SSB, n (%) | 0 | 1 (13) | 0.296 |
| Anti-Sm, n (%) | 13 (72) | 5 (63) | 0.667 |
| Anti-RNP, n (%) | 13 (68) | 4 (50) | 0.415 |
| Anti-ribosome P, n (%) | 5 (31) | 2 (33) | 1 |
| Anti-scl70, n (%) | 1 (6) | 1 (14) | 0.507 |
| Laboratory findings at NP evaluation | |||
| Anti-DNA, n (%) | 17 (85) | 7 (100) | 0.545 |
| Anemia (hemoglobin < 12.0 g/dL) | 17 (85) | 7 (88) | 1 |
| Leukopenia (white blood cells < 4 × 109/l) | 6 (30) | 4 (50) | 0.400 |
| Lymphopenia (absolute lymphocyte count < 1.5 × 109/L) | 14 (70) | 5 (63) | 1 |
| Neutropenia (absolute neutrophil count < 1.5 × 109/L) | 2 (10) | 2 (25) | 0.555 |
| Thrombocytopenia (< 150 × 109/l) | 7 (35) | 1 (13) | 0.372 |
| Erythrocyte sedimentation rate (mm), median (Q1; Q3) | 58 (49;105) | 43 (11;77) | 0.345 |
| C-reactive protein (mg/L), median (Q1; Q3) | 0 (0;14) | 0 (0;0) | 0.564 |
| Lupus anticoagulant, n (%) | 1 (6) | 0 | 1 |
| Anticardiolipin antibody, n (%) | 1 (6) | 3 (43) | 0.059 |
| Anti-beta2 glycoprotein antibody, n (%) | 1 (6) | 0 | 1 |
| Low complement (C3, C4, or CH50), n (%) | 17 (89) | 5 (63) | 0.136 |
| Clinical features at NP evaluation | |||
| SLEDAI#, median (Q1; Q3) | 23 (18; 27) | 8 (4; 15) | 0.096 |
| Cutaneous-mucosal, n (%) | 16 (80) | 4 (50) | 0.172 |
| Alopecia, n (%) | 6 (30) | 2 (25) | 1 |
| Cutaneous ulcerations, n (%) | 3 (15) | 0 | 0.536 |
| Chilblains, n (%) | 8 (40) | 2 (25) | 0.669 |
| Raynaud’s phenomenon, n (%) | 0 | 0 | |
| Rheumatological, n (%) | 12 (60) | 4 (50) | 0.691 |
| Hematological, n (%) | 16 (80) | 6 (75) | 1 |
| Renal, n (%) | 13 (65) | 2 (25) | 0.096 |
| Pericarditis, n (%) | 5 (25) | 0 | 0.281 |
| HTAP, n (%) | 1 (5) | 0 | 1 |
| Myocarditis, n (%) | 1 (5) | 0 | 1 |
| Pulmonary, n (%) | 8 (40) | 2 (25) | 0.669 |
| Pleurisy, n (%) | 4 (20) | 0 | 0.295 |
| Parenchymal, n (%) | 7 (35) | 2 (25) | 1 |
| Glaucome, n (%) | 2 (10) | 0 | 1 |
| Cumulative lupus clinical features | |||
| Cutaneous-mucosal, n (%) | 19 (95) | 8 (100) | 1 |
| Alopecia, n (%) | 13 (65) | 4 (50) | 0.672 |
| Cutaneous ulcerations, n (%) | 6 (30) | 2 (25) | 1 |
| Chilblains, n (%) | 12 (60) | 3 (38) | 0.410 |
| Raynaud’s phenomenon, n (%) | 2 (10) | 4 (50) | 0.038 |
| Rheumatological, n (%) | 17 (85) | 8 (100) | 0.536 |
| Hematological, n (%) | 19 (95) | 7 (88) | 0.497 |
| Renal, n (%) | 16 (80) | 6 (75) | 1 |
| Pericarditis, n (%) | 6 (30) | 1 (13) | 0.633 |
| HTAP, n (%) | 1 (5) | 0 | 1 |
| Myocarditis, n (%) | 1 (5) | 0 | 1 |
| Pulmonary, n (%) | 10 (50) | 3 (38) | 0.686 |
| Pleurisy, n (%) | 6 (30) | 2 (25) | 1 |
| Parenchymal, n (%) | 7 (35) | 3 (38) | 1 |
| Glaucoma, n (%) | 3 (15) | 0 | 0.536 |
| Treatments (cumulative) | |||
| Corticosteroid infusions, n (%) | 20 (100) | 6 (75) | 0.074 |
| Oral corticosteroids, n (%) | 20 (100) | 8 (100) | 1 |
| Cyclophosphamide, n (%) | 13 (65) | 1 (13) | 0.033 |
| Aspirin, n (%); curative anticoagulant, n (%) | 9 (45); 1 (5) | 3 (38); 2 (25) | 1; 0.188 |
| Hydroxychloroquine, n (%) | 19 (95) | 8 (100) | 1 |
| Mycophenolate mofetil or mycophenolic acid, n (%) | 18 (90) | 6 (75) | 0.555 |
| Anti-CD20: rituximab, n (%); obinutuzumab, n (%) | 4 (20); 2 (10) | 2 (25); 0 | 1; 1 |
| Methotrexate, n (%); azathioprine, n (%) | 1 (5); 2 (10) | 2 (25); 1 (13) | 0.188; 1 |
| Janus kinase inhibitors (ruxolitinib), n (%) | 1 (5) | 0 | 1 |
| Immunoadsorption, n (%); plasma exchanges, n (%) | 4 (20); 1 (5) | 0; 0 | 0.295; 1 |
| Tacrolimus, n (%) | 2 (10) | 0 | 1 |
| Abatacept, n (%); infliximab, n (%) | 1 (5); 0 | 0; 1 (13) | 1; 0.286 |
| Belimumab, n (%); eculizumab, n (%) | 0; 1 (5) | 1 (13); 0 | 0.286; 1 |
| Immunomodulatory IV immunoglobulins, n (%) | 1 (5) | 1 (13) | 0.497 |
| Outcome | |||
| J-SLE evolution from j-SLE diagnosis to last follow-up evaluation (months), median (Q1; Q3) | 27 (19;47) | 35 (29;48) | 0.609 |
| J-SLE evolution from NP evaluation to last follow-up evaluation (months), median (Q1; Q3) | 24 (16;37) | 14 (12;29) | 0.263 |
| Death, n (%) | 0 | 0 | |
| SLICC/ACR damage index score at last follow up: median (Q1; Q3); ≥ 1; n (%) | 1 (0;1); 11 (61) | 0 (0;0); 0 | 0.007; 0.007 |
IV intravenous, j-NPSLE juvenile neuropsychiatric systemic lupus erythematosus, j-SLE juvenile systemic lupus erythematosus, NP neuropsychiatric, SLEDAI# Systemic Lupus Erythematosus Disease Activity Index without neuropsychiatric features, SLICC systemic lupus international collaborating clinics, ACR American College of Rheumatology
aComorbidities in group 1: severe hypertriglyceridemia (1), immune thrombocytopenic purpura (1). In group 2: sickle cell disease (1), precocious puberty (1)
bNeuro-psychiatric personal history in group 1: Aicardi-Goutières syndrome with panic attack (1), learning disabilities (1), epilepsy (1), hemorrhagic stroke related to idiopathic thrombocytopenic purpura (1), nystagmus (1). In group 2: borderline personality disorder (2), transient ataxia (1)
cFamilial history of auto-immune disease in a first degree relative in group 1: SLE (1), Crohn’s disease (1), thyroiditis and dermatomyositis (1). In group 2: idiopathic thrombocytopenic purpura (1)
Fig. 1.
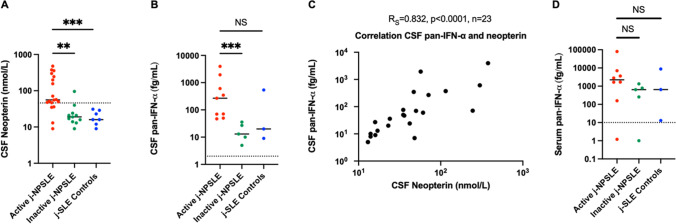
Comparison of CSF neopterin and IFN-α concentrations in non-NPSLE (j-SLE controls), active and inactive j-NPSLE patients. A Comparison of CSF neopterin concentration assessed by liquid chromatography coupled with mass spectrometry during active j-NPSLE in red (n = 18), inactive j-NPSLE in green (n = 11) and in non-NPSLE patients in blue (j-SLE controls, n = 7). B CSF pan-IFN-α concentration during active j-NPSLE in red (n = 9), inactive j-NPSLE in green (n = 5) and in non-NPSLE patients in blue (j-SLE controls, n = 3). C Correlation of pan-IFN-α measurement with neopterin assessment in the CSF in patients with j-NPSLE (n = 21) and j-SLE without NPSLE (n = 2). Spearman’s correlation was calculated Rs = 0.832, p < 0.0001, n = 23. D Serum pan-IFN-α concentration during active j-NPSLE in red (n = 8), inactive j-NPSLE in green (n = 5) and in non-NPSLE patients in blue (j-SLE controls, n = 3). Median levels are indicated by horizontal black bars, normal levels are indicated by horizontal dotted line. For A, B, D, tests were performed two by two with a Mann–Whitney’s test: the first compared active j-NPSLE and inactive j-NPSLE, the second test compared active j-NPSLE and j-SLE controls. *p < 0.05, ** < 0.01, ***p < 0.001, NS non-significant. CSF cerebrospinal fluid; IFN interferon; j-NPSLE juvenile neuropsychiatric systemic lupus erythematosus; j-SLE juvenile systemic lupus erythematosus; Simoa single-molecule array
Routine CSF Analysis, EEG and Cerebral MRI are Nonspecific in j-NPSLE
Electroencephalogram (EEG) nonspecific features were reported in 11/14 investigated j-NPSLE patients (79%): diffuse slow activity (n = 5), diffuse micro-voltage (n = 3), encephalopathy with disturbance in maintaining vigilance (n = 1), focal slow waves (n = 3), focal spikes (n = 2), and diffuse spikes (n = 1). CSF analysis was performed at first neuropsychiatric manifestation during the study period in all j-NPSLE patients (Table S3) except one due to profound thrombocytopenia (19/20). Moderate hyperproteinorachia (n = 3; 16%), associated with an increased white blood cell count (> 5 cells/mm3, n = 2; 11%), and isolated pleocytosis (n = 1; 5%) were the only abnormalities recorded. Oligoclonal bands were noted in 4 of 15 patients tested (27%). We observed positivity of CNS autoantibodies in none of the 12 patients tested. Brain MRI was performed in all j-NPSLE patients, and abnormal nonspecific morphological features were reported in 19 of 20 patients (95%): a mild enlargement of supratentorial anterior subarachnoid spaces associated with slight widening of sulci was seen in most patients (74%, n = 14), often after the use of corticosteroid treatment (64%, n = 9) and weight loss (64%, n = 9). White matter hyperintensities (WMH) on T2 or FLAIR were observed in 13 patients, without any correlation between their number or size and clinical features. Only one patient displayed ischemic brain lesions on MRI, after severe myocarditis and low brain blood-flow, without any feature of MRI inflammation.
Higher CSF IFN-Alpha and Neopterin Concentrations are Associated with Active j-NPSLE Associated with CNS Impairment and Decrease Upon Efficient Immunosuppressive Treatment
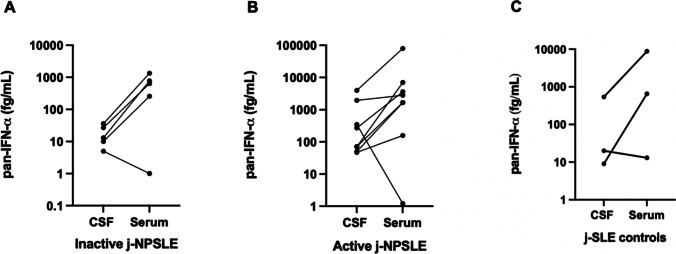
Only six j-NPSLE patients and three non-j-NPSLE patients had not received any immunosuppressive treatment the month before CSF assessment. Neopterin levels were significantly higher in active versus inactive j-NPSLE and non-NPSLE patients respectively (Fig. 1A, p = 0.0015, p = 0.0008, n = 36 samples of 26 patients assessed). ISG expression (IFN signature) was assessed in active j-NPSLE, inactive j-NPSLE, and j-SLE controls in 5, 7, and 5 patients, respectively, and median of IFN signature was not significantly different between active j-NPSLE patients and active j-SLE controls (median: 13 (n = 5) vs 19 (n = 5), p = 0.8968, data not shown). CSF IFN activity was assessed in active j-NPSLE, inactive j-NPSLE, and j-SLE controls in 18, 9, and 8 patients, respectively, as negative in 83% (n = 15/18) of active j-NPSLE (negative in all inactive j-NPSLE and j-SLE controls) and not statistically different (active vs inactive j-NPSLE p = 0.4554 and active j-NPSLE vs j-SLE controls p = 0.5292, data not shown). In contrast, levels of pan-IFN-α in the CSF measured using the more sensitive Simoa digital ELISA were significantly higher in active versus inactive j-NPSLE (Fig. 1B, p = 0.0010, n = 14 samples of 9 patients assessed). CSF neopterin and pan-IFN-α levels correlated strongly (Fig. 1C, R = 0.8323, p < 0.0001, n = 23 paired samples). On the contrary, levels of pan-IFN-α in the serum measured by Simoa were not significantly different in active versus inactive j-NPSLE, nor j-SLE controls (Fig. 1D, p = 0.0932 and p = 0.7758 respectively; n = 16 samples of 12 patients assessed). Interferon activity was higher in serum than in CSF for all j-NPSLE patients, except for one, who is currently being assessed for a monogenic cause of SLE by whole-genome sequencing. All serum IFN-α concentrations measured with the Simoa pan-IFN-α assay were higher than those in the CSF in j-NPSLE patients, except for one patient who had also a diagnosis of AGS by homozygous mutation c.529G > A, p.Ala177Thr in RNASEH2B (Fig. 2B). The analysis did not differ statistically, if groups were analyzed without the AGS patient associated to acute j-NPSLE episode (CSF neopterin j-NPSLE active vs inactive p = 0.0033; CSF neopterin controls vs j-NPSLE active p = 0.0011; CSF pan-IFN-α j-NPSLE active vs inactive p = 0.0040; CSF pan-IFN-α controls vs active j-NPSLE p = 0.1939). A decrease of CSF IFN-α concentrations, measured with the Simoa pan-IFN-α assay (n = 5), and CSF neopterin (n = 10) upon immunosuppressive treatment was observed in all patients tested, correlating with improvement of SLE and j-NPSLE features (Figs. 1, 2A, and S2).
Fig. 2.
Correlation of pan-IFN-α concentrations by Simoa in the CSF and in the serum during active and inactive j-NPSLE, and j-SLE controls. A pan-IFN-α concentration in the CSF and in the serum during active j-NPSLE (8 patients). B pan-IFN-α concentration in the CSF and in the serum during inactive j-NPSLE (5 patients). All serum concentrations were higher than those in the CSF, except for one patient with Aicardi-Goutières syndrome (P8). C pan-IFN-α concentration in the CSF and in the serum during j-SLE controls (3 patients). CSF cerebrospinal fluid; IFN interferon; j-NPSLE juvenile neuropsychiatric systemic lupus erythematosus; j-SLE juvenile systemic lupus erythematosus; Simoa single-molecule array
Discussion
In agreement with previous studies, we similarly reported fast-onset morbidity in our cohort: higher SLICC/ACR damage index score in the j-NPSLE group (61% of the j-NPSLE patients ≥ 1) after 24-month-median follow-up. Rapid diagnosis and subsequent appropriate treatments are therefore a major challenge. However, biological biomarkers are scarce in (j)-NPSLE. For instance, it has been suggested that anti-ribosomal P proteins or antiphospholipid antibodies may play a role in the pathogenesis of psychiatric complications of SLE, but this hypothesis remains controversial [12, 21]. No correlation between j-NPSLE and those antibodies was found in our study. Furthermore, other routine biological investigations in blood or CSF did not show any specificity for the diagnosis of j-NPSLE. Our study is the first to investigate novel diagnostic biomarkers in 20 j-NPSLE with CNS impairment in a global cohort of 51 j-SLE, thoroughly assessed by a collaborative team of pediatric multidisciplinary experts, and to compare them to 8 j-SLE controls.
We have first reported that CSF neopterin was able to distinguish active j-NPSLE from inactive j-NPSLE and j-SLE controls. Neopterin production is mainly mediated by IFN-gamma stimulation in response to Th1 cellular immune system activation, and CSF neopterin levels are already used to assess CNS inflammation [22, 23], and for therapeutic monitoring in the case of the neuroinflammatory disorder AGS [26]. CSF neopterin could therefore be considered as a promising diagnostic and activity biomarker for j-NPSLE.
We have also shown that CSF IFN-α levels assessed by Simoa, although mostly lower than concomitant blood concentration, are associated with active central j-NPSLE, decreasing with resolution of NP features upon immunosuppressive treatments. CSF IFN-α might therefore also constitute an interesting biomarker, as suggested in serum for SLE activity and relapse [20, 30]. While previous clinical studies have suggested a variable association of increased CSF IFN-α levels in adult NPSLE [31–33], we observed a positive correlation between CSF-IFN-α and central j-NPSLE activity. Our clinically homogeneous cohort (jNPSLE with CNS features) may explain the link with inflammatory CNS involvement reflected by both CSF biomarkers (neopterin and IFN-α), with a strong interdependency. Our patients displayed NP features, often associated with nonspecific neurological manifestations, whereas previous studies included heterogeneous populations of adult-NPSLE patients, some reported with isolated peripheral nervous system involvement, isolated seizure or headaches [33].
A statistically significant difference was observed for neopterin between NPSLE and non-NPSLE groups, but not for CSF pan-IFN-α levels — probably due to a lack of statistical power, since so few non-NPSLE patients were assessed using this test. For ethical reasons, CSF of patients without j-NPSLE suspicion was not collected. Moreover, as this is a retrospective study, lumbar puncture was often performed after starting immunosuppressive drugs, which may decrease these biomarkers. In addition, CSF was not always tested after disease resolution.
Among adult patients, other new NPSLE biomarkers have been identified in previous studies: CSF α-Klotho, lipocalin-2, macrophage colony-stimulating factor and IgM, and serum IL-6, miR-23a, and miR-155 [14]. However, they are not assessed in clinical practice, in contrast to neopterin which is measured in routine laboratories. As Simoa IFN-α assessment seems to be relevant for monitoring disease activity in the blood and CSF of (j)-(NP)SLE patients, we believe that it would have value as a routine test in (j)-SLE and (j)-NPSLE.
Regarding other biomarkers, non-specific brain MRI defects were noticed in almost all j-NPSLE patients. Cerebral atrophy and white matter FLAIR or T2 hyperintensities were the most common manifestations, as previously described in the literature [34, 35]. However, these abnormalities are not specific, and in some patients with punctiform WMH (< 3 mm), the differential diagnosis with Virchow-Robin spaces may be difficult. Therefore, normal or nonspecific brain imaging does not rule out NPSLE. Thus, other functional CNS assessments, such as brain fluorine-18 fluorodeoxyglucose-PET scan or MRI, should be further studied as more specific investigative tools for j-SLE [36].
Our study is limited by the retrospective design and its setting in a tertiary center, likely resulting in an over-representation of patients with severe SLE. Selection bias could also be linked to our nationally recognized expertise in the field of j-NPSLE, thereby influencing the prevalence of j-NPSLE that we observed.
Further research is necessary to better understand j-NPSLE-pathophysiology and offer personalized treatments, adapted to systematic clinical evaluation and accurate biomarker measurement in homogeneous cohorts. If CSF IFN-α is confirmed as being associated to NPSLE activity, this cytokine might be an interesting therapeutic target in the future, with the use of JAK-inhibitors or IFN-receptor antagonists in severe and refractory j-NPSLE, the latter best at crossing the blood–brain barrier [37, 38]. Moreover, if validated in prospective cohorts, CSF neopterin assessments and IFN-α monitoring by digital ELISA might be used as biomarkers to stratify severity and monitor treatment responses in j-NPSLE.
Conclusion
J-NPSLE is a diagnostic challenge for clinicians in the absence of current validated specific j-NPSLE markers. We have reported, to our knowledge for the first time, that patients with active j-NPSLE patients with CNS involvement display a specific biological profile with increased CSF pan-IFN-α and CSF neopterin levels. Further prospective investigations are warranted to assess the specific link between active j-NPSLE and these biomarkers, which could enable earlier targeted treatment and improve clinical outcome.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
DD thanks ImmunoQure for provision of the mAbs under an MTA for the Simoa pan-IFN-α assay.
Author Contribution
IM and PE designed the study. ML, SC, and VT collected clinical data. ML, VT, SB, SA, BBM, VB, OC, GD, CD, CD, AF, IH, VH, TK, UM, NO, CP, MP, FR, IS, PE, and IM participated in patient evaluation and provided resources. VB and DD quantified IFN-α. FR assessed IFN-activity. GIR assessed ISG scores/IFN signatures. AN and EM reviewed brain MRIs. ML, SC, PE, EL, and IM analyzed data. ML, SC, VT, PE, and IM wrote the paper. IM and PE supervised the study. All the authors have read final approval of the version published.
Funding
YJC acknowledges the European Research Council (GA309449 and 786142-E-T1IFNs) and a state subsidy managed by the National Research Agency (France) under the ‘Investments for the Future’ program bearing the reference ANR-10-IAHU-01. The project was supported by MSDAVENIR (Devo-Decode Project). YJC and DD acknowledge the Agence Nationale de la Recherche (grant CE17001002).
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics Approval
The study protocol followed ethics guidelines (N˚ 2020–478 from independent Robert Debré ethic committee). The data processing has been approved by AP-HP (Public Hospitals of Paris) data protection office and registered in general data processing register (N°20200227113056). Parents or patients, if they had reached the age of majority by the time of the study, have been informed in writing. All samples were collected with informed consent.
Consent to Participate
Written informed consent was obtained from all individual participants included in the study and their parents.
Consent for Publication
Not applicable.
Provenance and Peer Review
Patients and public were not involved in clinical research or co-production of research.
Competing Interests
The authors declare no competing interests.
Footnotes
Key Messages
What is already known about this subject?
• The diagnosis of juvenile neuropsychiatric systemic lupus erythematosus (j-NPSLE) remains difficult and a major challenge, without current validated biomarkers.
What does this study add?
• Cerebrospinal fluid (CSF) interferon alpha (IFN-α) and neopterin, assessed as novel biomarkers of central nervous system involvement, are elevated in active j-NPSLE.
• Both biomarkers decrease upon efficient treatment, correlating with clinical improvement.
How might this impact on clinical practice or future developments?
• CSF IFN-α and neopterin may constitute new diagnostic and activity biomarkers of j-NPSLE with central neuroinflammation.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Pierre Ellul and Isabelle Melki provided equal contribution
References
- 1.Arnaud L, Fagot J-P, Mathian A, Paita M, Fagot-Campagna A, Amoura Z. Prevalence and incidence of systemic lupus erythematosus in France: a 2010 nation-wide population-based study. Autoimmun Rev. 2014;13(11):1082–1089. doi: 10.1016/j.autrev.2014.08.034. [DOI] [PubMed] [Google Scholar]
- 2.Sibbitt WL, Brandt JR, Johnson CR, Maldonado ME, Patel SR, Ford CC, et al. The incidence and prevalence of neuropsychiatric syndromes in pediatric onset systemic lupus erythematosus. J Rheumatol. 2002;29(7):1536–1542. [PubMed] [Google Scholar]
- 3.Olfat MO, Al-Mayouf SM, Muzaffer MA. Pattern of neuropsychiatric manifestations and outcome in juvenile systemic lupus erythematosus. Clin Rheumatol. 2004;23(5):395–399. doi: 10.1007/s10067-004-0898-3. [DOI] [PubMed] [Google Scholar]
- 4.Giani T, Smith EM, Al-Abadi E, Armon K, Bailey K, Ciurtin C, et al. Neuropsychiatric involvement in juvenile-onset systemic lupus erythematosus: data from the UK juvenile-onset systemic lupus erythematosus cohort study. Lupus. 2021;2:9612033211045050. doi: 10.1177/09612033211045050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hanly JG, Urowitz MB, Su L, Bae SC, Gordon C, Wallace DJ, et al. Prospective analysis of neuropsychiatric events in an international disease inception cohort of patients with systemic lupus erythematosus. Ann Rheum Dis. 2010;69(3):529–535. doi: 10.1136/ard.2008.106351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lim LSH, Lefebvre A, Benseler S, Silverman ED. Longterm outcomes and damage accrual in patients with childhood systemic lupus erythematosus with psychosis and severe cognitive dysfunction. J Rheumatol. 2013;40(4):513–519. doi: 10.3899/jrheum.121096. [DOI] [PubMed] [Google Scholar]
- 7.Govoni M, Hanly JG. The management of neuropsychiatric lupus in the 21st century: still so many unmet needs? Rheumatology. 2020;59(Suppl5):v52–62. doi: 10.1093/rheumatology/keaa404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.The American College of Rheumatology nomenclature and case definitions for neuropsychiatric lupus syndromes. Arthritis Rheum. 1999;42(4):599–608. 10.1002/1529-0131(199904)42:4%3C599::AID-ANR2%3E3.0.CO;2-F [DOI] [PubMed]
- 9.Ainiala H, Hietaharju A, Loukkola J, Peltola J, Korpela M, Metsänoja R, et al. Validity of the new American College of Rheumatology criteria for neuropsychiatric lupus syndromes: a population-based evaluation. Arthritis Rheum. 2001;45(5):419–423. doi: 10.1002/1529-0131(200110)45:5<419::AID-ART360>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 10.Bortoluzzi A, Scirè CA, Govoni M. Attribution of neuropsychiatric manifestations to systemic lupus erythematosus. Front Med (Lausanne) 2018;14(5):68. doi: 10.3389/fmed.2018.00068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Soybilgic A. Neuropsychiatric systemic lupus erythematosus in children. Pediatr Ann. 2015;44(6):e153–e158. doi: 10.3928/00904481-20150611-11. [DOI] [PubMed] [Google Scholar]
- 12.Fernandes H, Brito I. Juvenile systemic lupus erythematosus: neuropsychiatric manifestations. Acta Reumatol Port. 2012;37(2):117–125. [PubMed] [Google Scholar]
- 13.Rubinstein TB, Putterman C, Goilav B. Biomarkers for CNS involvement in pediatric lupus. Biomark Med. 2015;9(6):545–558. doi: 10.2217/bmm.15.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lindblom J, Mohan C, Parodis I. Biomarkers in neuropsychiatric systemic lupus erythematosus: a systematic literature review of the last decade. Brain Sci. 2022;12(2):192. 10.3390/brainsci12020192 [DOI] [PMC free article] [PubMed]
- 15.Leboyer M, Berk M, Yolken RH, Tamouza R, Kupfer D, Groc L. Immuno-psychiatry: an agenda for clinical practice and innovative research. BMC Med. 2016;14(1):173. doi: 10.1186/s12916-016-0712-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gold SM, Köhler-Forsberg O, Moss-Morris R, Mehnert A, Miranda JJ, Bullinger M, et al. Comorbid depression in medical diseases. Nat Rev Dis Primers. 2020;6(1):69. doi: 10.1038/s41572-020-0200-2. [DOI] [PubMed] [Google Scholar]
- 17.Raison CL, Demetrashvili M, Capuron L, Miller AH. Neuropsychiatric adverse effects of interferon-alpha: recognition and management. CNS Drugs. 2005;19(2):105–123. doi: 10.2165/00023210-200519020-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fritz-French C, Tyor W. Interferon-α (IFNα) neurotoxicity. Cytokine Growth Factor Rev. 2012;23(1–2):7–14. doi: 10.1016/j.cytogfr.2012.01.001. [DOI] [PubMed] [Google Scholar]
- 19.Rodero MP, Decalf J, Bondet V, Hunt D, Rice GI, Werneke S, et al. Detection of interferon alpha protein reveals differential levels and cellular sources in disease. J Exp Med. 2017;214(5):1547–1555. doi: 10.1084/jem.20161451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mathian A, Mouries-Martin S, Dorgham K, Devilliers H, Barnabei L, Ben Salah E, et al. Monitoring disease activity in systemic lupus erythematosus with single-molecule array digital enzyme-linked immunosorbent assay quantification of serum interferon-α. Arthritis Rheumatol. 2019;71(5):756–765. doi: 10.1002/art.40792. [DOI] [PubMed] [Google Scholar]
- 21.Schwartz N, Stock AD, Putterman C. Neuropsychiatric lupus: new mechanistic insights and future treatment directions. Nat Rev Rheumatol. 2019;15(3):137–152. doi: 10.1038/s41584-018-0156-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Millner MM, Franthal W, Thalhammer GH, Berghold A, Aigner RM, Füger GF, et al. Neopterin concentrations in cerebrospinal fluid and serum as an aid in differentiating central nervous system and peripheral infections in children. Clin Chem. 1998;44(1):161–167. doi: 10.1093/clinchem/44.1.161. [DOI] [PubMed] [Google Scholar]
- 23.Dale RC, Brilot F, Fagan E, Earl J. Cerebrospinal fluid neopterin in paediatric neurology: a marker of active central nervous system inflammation. Dev Med Child Neurol. 2009;51(4):317–323. doi: 10.1111/j.1469-8749.2008.03225.x. [DOI] [PubMed] [Google Scholar]
- 24.Yan J, Kuzhiumparambil U, Bandodkar A, Bandodkar S, Dale RC, Fu S. Cerebrospinal fluid metabolites in tryptophan-kynurenine and nitric oxide pathways: biomarkers for acute neuroinflammation. Dev Med Child Neurol. 2021;63(5):552–559. doi: 10.1111/dmcn.14774. [DOI] [PubMed] [Google Scholar]
- 25.Blau N, Bonafé L, Krägeloh-Mann I, Thöny B, Kierat L, Häusler M, et al. Cerebrospinal fluid pterins and folates in Aicardi-Goutières syndrome: a new phenotype. Neurology. 2003;61(5):642–647. doi: 10.1212/01.WNL.0000082726.08631.E7. [DOI] [PubMed] [Google Scholar]
- 26.Han VX, Mohammad SS, Jones HF, Bandodkar S, Crow YJ, Dale RC, et al. Cerebrospinal fluid neopterin as a biomarker of treatment response to Janus kinase inhibition in Aicardi-Goutières syndrome. Dev Med Child Neurol. 2022;64(2):266–271. doi: 10.1111/dmcn.15025. [DOI] [PubMed] [Google Scholar]
- 27.Gresser I, Bandu MT, Brouty-boye D, Tovey M. Pronounced antiviral activity of human interferon on bovine and porcine cells. Nature. 1974;251(5475):543–545. doi: 10.1038/251543a0. [DOI] [PubMed] [Google Scholar]
- 28.Lebon P, Badoual J, Ponsot G, Goutières F, Hémeury-Cukier F, Aicardi J. Intrathecal synthesis of interferon-alpha in infants with progressive familial encephalopathy. J Neurol Sci. 1988;84(2–3):201–208. doi: 10.1016/0022-510X(88)90125-6. [DOI] [PubMed] [Google Scholar]
- 29.Rice GI, Melki I, Frémond M-L, Briggs TA, Rodero MP, Kitabayashi N, et al. Assessment of type I interferon signaling in pediatric inflammatory disease. J Clin Immunol. 2017;37(2):123–132. doi: 10.1007/s10875-016-0359-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mathian A, Mouries-Martin S, Dorgham K, Devilliers H, Yssel H, Garrido Castillo L, et al. Ultrasensitive serum interferon-α quantification during SLE remission identifies patients at risk for relapse. Ann Rheum Dis. 2019;78(12):1669–1676. doi: 10.1136/annrheumdis-2019-215571. [DOI] [PubMed] [Google Scholar]
- 31.Shiozawa S, Kuroki Y, Kim M, Hirohata S, Ogino T. Interferon-alpha in lupus psychosis. Arthritis Rheum. 1992;35(4):417–422. doi: 10.1002/art.1780350410. [DOI] [PubMed] [Google Scholar]
- 32.Fragoso-Loyo H, Atisha-Fregoso Y, Núñez-Alvarez CA, Llorente L, Sánchez-Guerrero J. Utility of interferon-α as a biomarker in central neuropsychiatric involvement in systemic lupus erythematosus. J Rheumatol. 2012;39(3):504–509. doi: 10.3899/jrheum.110983. [DOI] [PubMed] [Google Scholar]
- 33.Varley JA, Andersson M, Grant E, Berretta A, Zandi MS, Bondet V, et al. Absence of neuronal autoantibodies in neuropsychiatric systemic lupus erythematosus. Ann Neurol. 2020;88(6):1244–1250. doi: 10.1002/ana.25908. [DOI] [PubMed] [Google Scholar]
- 34.Al-Obaidi M, Saunders D, Brown S, Ramsden L, Martin N, Moraitis E, et al. Evaluation of magnetic resonance imaging abnormalities in juvenile onset neuropsychiatric systemic lupus erythematosus. Clin Rheumatol. 2016;35(10):2449–2456. doi: 10.1007/s10067-016-3376-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Inglese F, Kant IMJ, Monahan RC, Steup-Beekman GM, Huizinga TWJ, van Buchem MA, et al. Different phenotypes of neuropsychiatric systemic lupus erythematosus are related to a distinct pattern of structural changes on brain MRI. Eur Radiol. 2021;31(11):8208–8217. doi: 10.1007/s00330-021-07970-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Turpin S, Martineau P, Levasseur M-A, Meijer I, Décarie J-C, Barsalou J, et al. 18F-Flurodeoxyglucose positron emission tomography with computed tomography (FDG PET/CT) findings in children with encephalitis and comparison to conventional imaging. Eur J Nucl Med Mol Imaging. 2019;46(6):1309–1324. doi: 10.1007/s00259-019-04302-x. [DOI] [PubMed] [Google Scholar]
- 37.Neven B, Al Adba B, Hully M, Desguerre I, Pressiat C, Boddaert N, et al. JAK Inhibition in the Aicardi-Goutières Syndrome. N Engl J Med. 2020;383(22):2190–2191. doi: 10.1056/NEJMc2031081. [DOI] [PubMed] [Google Scholar]
- 38.Richardson PJ, Ottaviani S, Prelle A, Stebbing J, Casalini G, Corbellino M. CNS penetration of potential anti-COVID-19 drugs. J Neurol. 2020;267(7):1880–1882. doi: 10.1007/s00415-020-09866-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.