Abstract
Although at least 25 million adults are transgender worldwide, few Phase 3 clinical trials have enrolled transgender and gender diverse (TGD) people. HIV is the only therapeutic area to include TGD people intentionally in Phase 3 randomized clinical trials during the development of certain newer HIV pharmacologic prevention interventions. Pharmacologic assessments for HIV prevention efficacy in TGD populations are important, as there may be specific considerations for product use and potential interactions with hormone therapies. Herein, we summarize ongoing and completed Phase 3 HIV trials that included TGD people as part of the study population, we examine investigators’ strategies for recruiting and engaging TGD priority populations in these Phase 3 trials, and we comment on the implications of these studies for prioritizing TGD populations in clinical pharmacology research within the Phase 3 clinical trial landscape.
Keywords: HIV, sex, gender, Phase 3, clinical trials, transgender
INTRODUCTION
“Transgender and gender diverse” (TGD) is an umbrella term to describe people whose gender identity or gender expression differs from the cultural expectation associated with the sex assigned at birth.1 This term includes nonbinary people (gender identity outside the binary of woman or man), and it is used irrespective of an individual’s desire to receive gender-affirming medical treatments for their gender expression goals. From 2006 to 2017, US HIV prevalence estimates (laboratory confirmed and self-reported) were 18.8% among transgender women and 2.0% among transgender men.2 Several factors contribute to the HIV burden among transgender people, including poverty, homelessness, substance abuse, survival sex work, and prevalence of polyamorous culture/multiple sexual partners.3–5 Although beyond the scope of this review, we refer readers to Scheim et al.1 and Baral et al.6 for comprehensive discussions on structural stigma, social determinants of health, and implications for TGD people in the US and globally.
Language in this review
We use “TGD” throughout this manuscript to recognize diverse gender identities, but we also use language consistent with specific Phase 3 randomized controlled trials (RCTs) as appropriate. We include the terms transgender women (or transfeminine adult) to describe people identifying as women or along the feminine spectrum and assigned male at birth. Transgender men (or transmasculine adult) describe people identifying as men or along the masculine spectrum and assigned female at birth. We used “estrogen treatment” and “testosterone treatment” to characterize specific hormone regimens that TGD people may take. These approaches are not intended to overlook or minimize the diversity of gender identities; rather, they reflect prior literature and clinical trials inclusive of these populations.
TGD people are underrepresented in all aspects of healthcare and the research enterprise
TGD people represent an increasingly visible population globally, with an estimated 25 million individuals self-identifying in this manner.7 Many TGD people are active participants in their health and remain resilient while navigating healthcare settings.8,9 However, TGD people face stigma and discrimination, leading to barriers to accessing healthcare and health disparities.1,10 Systemic discrimination within the healthcare system impedes access, adherence, and compliance with social and biomedical interventions to prevent HIV infection, including HIV pre-exposure prophylaxis (PrEP).11 As one example, from 2014–2015, 35.6% of US transgender women and 31.6% of transgender men reported having ever had an HIV test in their lifetime, which was numerically similar to the prevalence of HIV testing for cisgender, heterosexual adults (35.2%).12 In addition to confounders that prevent access to care, there are additional barriers to recruiting and enrolling TGD people in HIV-focused research, including research and medical mistrust, fear of mistreatment, and concerns about safety, exploitation, and confidentiality.13
Based on global prevalence estimates, transgender women have a 66-times higher odds, and transgender men have a 6.8-times higher odds of HIV acquisition than the general population.14 HIV prevalence data are unavailable for nonbinary people. Although HIV prevalence estimates are available for transgender women and transgender men, knowledge about HIV care for these populations are limited by several factors including non-standardized terminology for describing gender identity and history, limited data collection strategies that do not collect gender identity data accurately, and exclusion of TGD people from national public health surveillance systems.1,15,16 Investigators have made efforts to address these gaps in knowledge. One example is the American Cohort to Study HIV Acquisition among Transgender Women in High-Risk Area (LITE), the first multi-site US cohort study that will characterize HIV acquisition, risk factors for HIV, and access to HIV prevention and linkage to HIV care for a diverse cohort of more than 1000 transgender women exclusively.17
Importance of HIV pharmacology research in transgender medicine
Hormone therapy (testosterone treatment or estrogen treatment) is one part of the standard of medical care for TGD people.18 Although not all TGD people choose to access hormone therapies, based on the 2015 US Transgender Health Survey, which was a non-probability survey of approximately 30,000 TGD adults, nearly 80% of respondents wanted to take hormone therapy at some point in their lives.19 Because TGD people accessing hormone therapy may prioritize gender affirming hormone treatment over PrEP,9,11,20 integrated HIV care and clinical medical gender affirmation management (including hormone therapy) can serve as the “bedrock of gender-affirmative healthcare.”5 Researchers observed that active hormone therapy use was associated with 1.5-higher odds of linkage to HIV primary care (P=0.04), 2.04-higher odds of retention in HIV care (P<0.001), and 1.89-higher odds of viral suppression (P<0.001) for transgender women living with HIV relative to transgender women not using hormones.21
Despite the prominent role of hormone therapy in transgender medicine, pharmacologic knowledge gaps remain for gender affirming hormone therapies, which hamper investigators’ ability to predict the effects of hormone therapy on drug safety and efficacy for TGD people.22 Hormone therapy may markedly alter body composition (e.g., increased total fat during estrogen treatment), which may increase the volume of distribution of lipid-soluble medications.22 Hormone therapy may also alter drug-metabolizing enzyme or drug transporter activities (e.g., cytochrome P450 3A or P-glycoprotein efflux transporter),23 potentially increasing or decreasing systemic concentrations of antiretrovirals handled by these pathways. The effects of hormone therapy on kidney function for TGD people remain to be determined.24 Some investigators have observed higher estimated creatinine clearance for transgender women taking estrogen treatment compared with cisgender men (60%, P=0.04).25 Clinical trial simulations observed that estimated creatinine clearance significantly influenced tenofovir and emtricitabine clearance for transgender women on estrogen treatment.26 HIV prevention is the only therapeutic area to integrate drug-hormone interaction studies specific for TGD participants in post-marketing pharmacokinetic studies.
PRIORITIZING TGD POPULATIONS IN HIV CLINICAL TRIALS
Although the US FDA continues to prioritize diverse clinical trial representation based on published guidance to promote clinical trial diversity,27,28 this guidance falls short of acknowledging the inclusion of TGD people in clinical trials.29 TGD people are underrepresented in all clinical trials (Phases 1, 2, and 3), and most published transgender health-focused research is limited to cross-sectional or retrospective study designs using convenience sampling strategies.15 Over several decades, despite concerns regarding their participation in research, transgender women have advocated for increased representation in HIV research.30 Several Phase 1 or open-label studies have responded to concerns voiced by TGD community members and characterized antiretroviral drug pharmacology for transgender persons, predominantly among transgender women undergoing estrogen treatment.31,32
The National Institutes of Health (NIH) has issued requests for applications (RFAs) calling for research with transgender populations. Several NIH-funded HIV clinical trials networks have recruited transgender women in their study populations, including the Adolescent Medicine Trials Network for HIV/AIDS Interventions (ATN) and the HIV Prevention Trials Network (HPTN). For example, HPTN 091, an ongoing open-label study, is evaluating the feasibility, acceptability, and impact of integrated HIV prevention services, gender-affirming medical care (hormone therapy), and peer health navigation to increase pre-exposure prophylaxis (PrEP) uptake and persistence (i.e., duration of prescribed PrEP use).33 HPTN 091 focuses on transgender health and HIV prevention, and it is the first HIV network trial to exclusively enroll transgender women. These activities highlight efforts to expand research initiatives among transgender women across clinical phases of drug development.
Although investigators have focused primarily on transgender women due to the HIV epidemic, representation is still needed for all TGD people in Phase 3 HIV prevention efficacy trials. Further, TGD people should not be analyzed as an extension of cisgender populations, as behavioral, social, and biomedical needs differ between these groups.34–36 The Phase 3 RCTs summarized below highlight advancements and limitations in Phase 3 HIV pharmacologic prevention studies inclusive of TGD populations.
PHASE 3 CLINICAL TRIALS: DAILY ORAL PRE-EXPOSURE PROPHYLAXIS (PrEP)
Emtricitabine/tenofovir disoproxil fumarate (F/TDF)
The HIV Pre-Exposure Prophylaxis Initiative (iPrEx) was one of two Phase 3 RCTs that supported 2012 FDA approval of daily oral emtricitabine/tenofovir disoproxil fumarate (F/TDF) for HIV prevention. iPrEx enrolled transgender women and cisgender men at risk of HIV and reported a 44% relative HIV risk reduction among participants randomized to F/TDF (Table 1).37 During study screening procedures, investigators collected participant-reported gender identity information via a computer-assisted self-interview (participant reads each question and provides their responses directly into a computer). Questions included whether participants “identified as a man or a woman,” and separately, “How do you identify yourself?” with a “check all that apply” response list that included both “trans” and “woman.”37 Although the iPrEx RCT and the iPrEx open-label extension mentioned inclusion of transgender women in the primary publication, investigators did not stratify HIV incidence between transgender women and cisgender men. Baseline participant characteristics were not disaggregated between groups.37
Table 1.
Summary of Phase 3 clinical trials in HIV pharmacologic prevention interventions inclusive of transgender women, transgender men, or nonbinary people
| iPrEx (36, 37) | DISCOVER (44) | HPTN 083 (48) | PURPOSE-2 (52) | |
|---|---|---|---|---|
| Countries | Peru, Ecuador, Thailand, Brazil, South Africa, US | Austria, Denmark, France, Germany, Ireland, Italy, Netherlands, Spain, the UK, Canada, and US | Argentina, Brazil, Peru, South Africa, Thailand, Vietnam, US | Brazil, Peru, South Africa, US |
| Intervention(s) | Daily oral FDC F/TDF vs. placebo | Daily oral FDC F/TAF vs. F/TDF | Oral/injectable CAB (oral lead-in) vs. F/TDF | Oral/injectable LEN (oral lead-in) vs. F/TDF |
| % TGD people in study population (total enrolled population) | 14% (2599) – transgender women only | 1% (5387) – transgender women only | 12.5% (4566) – transgender women only | 20% (3000) – transgender women (transgender men and nonbinary people eligible) |
| Enriched for TGD people? | No | No | Yes | Yes |
Abbreviations: CAB, cabotegravir; FDC, fixed-dose combination; F/TAF, emtricitabine/tenofovir alafenamide fumarate; F/TDF, emtricitabine/tenofovir disoproxil fumarate; LEN, lenacapavir; TGD, transgender and gender diverse; UK, United Kingdom; US, United States
Following advocacy within the transgender community,36,37 Deutsch et al.37 re-analyzed gender identity data and identified 339 transgender people within the iPrEx RCT (14% total participants) and iPrEx open-label extension study populations: 296 participants identified as “transgender,” (48 reported taking hormone therapy), 29 identified as “woman,” (5 reported taking hormone therapy), and 14 “male-identified participants [who] reported exogenous female hormone use of some kind.”37 Hormone therapies reported by participants included synthetic or bioidentical estrogens, progestogens, and/or antiandrogens. Among 192 transgender women enrolled in the iPrEx open-label extension, 17% participants had tenofovir-diphosphate concentrations associated with “protective” concentrations (i.e., intraerythrocytic concentrations of tenofovir-diphosphate, the active tenofovir anabolite, in dried blood spots consistent with at least four F/TDF doses/week) compared with 35% cisgender men (33/200 person years vs. 464/1332 person years, respectively, P <0.0001).37 Investigators attributed this finding to low F/TDF uptake among transgender women participants.36,37
Motivated by findings from iPrEx and by the unknown effects between hormone therapy and PrEP,9,11,20 several investigators evaluated oral F/TDF concentrations among transgender women undergoing estrogen treatment25,32,38–43 and transgender men and nonbinary people on testosterone treatment.32,40,42 Most studies observed modest decreases in tenofovir and emtricitabine plasma area under the concentration-time curves among transgender women undergoing estrogen treatment (12%-27%),25,32,38–40 although clinical implications for PrEP efficacy remain to be determined. Investigators observed no significant difference in tenofovir or emtricitabine exposures during testosterone treatment.32,40,42 These data provide reassurance for transgender women and transgender men undergoing hormone therapy and taking daily oral F/TDF as PrEP. However, investigators have not performed extensive bi-directional drug-hormone interaction analyses within the Phase 3 F/TDF efficacy trials.
Emtricitabine/tenofovir alafenamide (F/TAF)
In 2019, the US FDA approved oral, fixed-dose F/TAF for daily HIV prevention. DISCOVER, a Phase 3 non-inferiority trial, compared fixed-dose regimens of once-daily F/TAF 200 mg/25 mg to F/TDF 200 mg/300 mg among adults at high risk of HIV, recruiting both transgender women and cisgender men who have sex with men (Table 1).44 TAF, a tenofovir prodrug, was developed as an alternative to the TDF prodrug and resulted in 90% lower plasma tenofovir concentrations compared with TDF, reducing tenofovir-related adverse effects.44 In DISCOVER, investigators collected information about “transgender status” during study screening via a computer-assisted self-interview.45 However, investigators neither enriched recruitment for transgender women nor stratified analyses of the primary study endpoint (HIV incidence) between transgender women and cisgender men. Thus, although nearly 5400 participants enrolled in DISCOVER, only 1% were transgender women (74 participants). Investigators did not comment whether they collected information about nonbinary identities within the study population.
In a subset of 27 transgender women randomized in DISCOVER who reported hormone use, investigators observed no differences in emtricitabine-triphosphate or tenofovir-diphosphate concentrations in peripheral blood mononuclear cells during daily oral F/TAF or F/TDF, as compared with concentrations from cisgender men.31 Although investigators did not directly observe the administration of medications in this exploratory analysis (including hormone therapies, F/TDF, or F/TAF), these data align with earlier pharmacokinetic data and suggest neither F/TDF nor F/TAF concentrations are significantly altered for transgender women undergoing estrogen treatment. Studies have not characterized the effect of testosterone treatment for transgender men or nonbinary people on F/TAF disposition.
An exploratory analysis examined the effects of estrogen treatment on daily oral F/TAF in a subset of 17 transgender women randomized in DISCOVER.39 Relative to cisgender men, investigators observed no differences in tenofovir-diphosphate and emtricitabine-triphosphate concentrations in peripheral blood mononuclear cells during daily oral F/TAF for transgender women undergoing estrogen treatment.39 Although investigators did not directly observe the administration of hormone therapies or F/TAF, these findings align with earlier F/TDF outcomes and suggest neither F/TDF nor F/TAF concentrations are significantly altered for transgender women undergoing estrogen treatment. No studies have characterized the effect of testosterone treatment on F/TAF disposition for transgender men or nonbinary people.
PHASE 3 CLINICAL TRIALS: LONG-ACTING PREP
Cabotegravir (HPTN 083)
In December 2021, the US FDA approved long-acting injectable cabotegravir (CAB-LA) as the first long-acting pharmacologic intervention to reduce the risk of sexually acquired HIV-1 transmission. Cabotegravir (CAB) is an integrase strand transfer inhibitor with favorable physicochemical characteristics for a long-acting antiretroviral agent, including low aqueous solubility and a high melting point, as well as favorable pharmacologic characteristics including high antiviral potency and a long half-life (5.6 to 11.5 weeks).46,47 The drug was formulated as an extended-release injectable nanosuspension for intramuscular delivery.46
HPTN 083, an ongoing Phase 2b/3 non-inferiority trial, compared cabotegravir-based regimens to daily oral F/TDF (Table 1). Overall, HPTN 083 demonstrated superiority of CAB-LA in preventing HIV via sexual transmission.48,49 During recruitment, investigators enriched enrollment for transgender women globally through education and outreach at participating study sites and by collaborating with local community advisory boards at each site.50 Among 4566 participants in the HPTN 083 intent-to-treat analysis, 570 (12.5%) were transgender women, exceeding the protocol-specified enrollment goal of 10%.48,50 Although not included as part of the primary, secondary, or tertiary study objectives, investigators disaggregated and reported incident HIV infections between transgender women and cisgender men who have sex with men, leading to product labeling that disaggregated efficacy data between transgender women and cisgender men.47
Among transgender women, 330 participants reported taking estrogen treatment during the study. In an exploratory drug-hormone interaction analysis of a subset of transgender women in HPTN 083, investigators observed no difference in plasma cabotegravir concentrations over 83 weeks post-randomization among 30 transgender women undergoing hormone therapy compared with 23 transgender women not reporting hormone use.51 These findings were a positive signal that transgender women can take estrogen treatment and cabotegravir without an impact on PrEP efficacy. CAB-LA pharmacology in the background of exogenous testosterone therapy has not been assessed.
Lenacapavir (PURPOSE-2)
Lenacapavir is an investigational, long-acting HIV capsid inhibitor administered as a twice-yearly injection for HIV prevention. A novel first-in-class agent, lenacapavir inhibits HIV capsid assembly and prevents viral transport and maturation.46 Results of Phase 3 trials of lenacapavir for HIV treatment are described elsewhere.46 For HIV prevention, PURPOSE-1 and 2 trials are ongoing Phase 3 RCTs that will compare a lenacapavir-based regimen (600 mg oral lenacapavir lead-in for 2 days plus subcutaneous 927 mg lenacapavir injections every 26 weeks) to either daily oral F/TAF or F/TDF (PURPOSE-1) or daily oral F/TDF only (PURPOSE-2) (Table 1). While PURPOSE-1 is recruiting cisgender women only, PURPOSE-2’s priority populations are transgender women and cisgender men (all ages ≥16 years and at risk of HIV). PURPOSE-2’s design includes a blinded phase over at least 52 weeks, an open-label extension, and a pharmacokinetic analysis during the lenacapavir “tail” phase.
The PURPOSE-2 enrollment goal is 3000 adults, with investigators establishing a 20% study-wide enrollment goal for transgender women based on country-specific HIV prevalence estimates specifically for transgender women.52 PURPOSE-2 enrollment is open to transgender men and nonbinary individuals. However, investigators could not set data-driven enrollment goals for these populations due to a lack of local-level HIV incidence data for these populations.52 The PURPOSE-2 study team utilized several evidence-based approaches to engage historically underrepresented individuals during protocol development.52 These efforts included engaging US and global stakeholders through a Global Community Advisory and Accountability Group and including members of the priority population within the study team. Although the investigators did not specify a plan to stratify the primary endpoint (HIV incidence) between transgender women and cisgender men,53 the study design indicates a positive movement toward inclusion of TGD people throughout study design and completion. Primary findings are anticipated in 2024.
Islatravir (IMPOWER 24)
Islatravir, an investigational, once-monthly oral nucleoside reverse transcriptase translocation inhibitor, was in Phase 3 development for both HIV treatment and prevention.46 Investigators planned to include transgender women in the Phase 3 non-inferiority trial between islatravir and F/TDF or F/TAF among 1500 adults at high risk for HIV (IMPOWER 24). However, as of July 2022, FDA paused islatravir-based clinical trials because of decreased lymphocyte and CD4+ T-cell counts. Further information about the inclusion of transgender women in IMPOWER 24, and islatravir’s future role in HIV prevention for TGD people, remains unclear.
CURRENT GAPS AND OPPORTUNITIES FOR INCREASING INVOLVEMENT OF TGD PEOPLE IN PHASE 3 TRIALS
Various studies and meta-analyses have highlighted the inclusion, in small numbers in some cases, of transgender women in HIV prevention research.4 Many Phase 3 trials have conflated transgender women with cisgender men who have sex with men.35,54 Beyond the inherent invalidation of referring to transgender women as “men who have sex with men,” this approach fails to convey differences in social determinants that may drive HIV exposure between cisgender men and transgender women who have sex with men.36
Transgender men and nonbinary people remain underrepresented in clinical research.4,11 US CDC population-based HIV/AIDs surveillance surveys have not included transgender men as a priority population, and knowledge about HIV prevalence and risk-related behaviors for transmasculine and nonbinary people is limited.55 Further, transmasculine persons were thought to have sex primarily with cisgender women at low risk for HIV.3 Nearly 600 transmasculine adults in a large cross-sectional clinic-based study reported having diverse sexual partnerships, and 32% reported having at least one cisgender male sex partner.56 Additional survey data suggest that up to 55% of transmasculine respondents met the eligibility criteria for HIV PrEP.3,57
Although nonbinary and gender-diverse people are historically underrepresented in HIV surveillance and prevention efforts, investigators have begun to include these populations in HIV research and identify HIV-associated risk factors specific to gender-diverse people.58 PURPOSE-2 expanded its priority populations to include transgender men and nonbinary people based on community advisory board feedback.52 Still, more work is needed to include these populations in Phase 3 HIV trials. Phase 3 trials should consider opportunities to enrich for all populations at risk of acquiring HIV, including transmasculine and nonbinary persons. Several opportunities exist for addressing and increasing the inclusion of TGD people across randomized clinical trials broadly, exclusive of cisgender persons, as described below.
Hormone Knowledge Gaps in Phase 3 RCTs
Although the aforementioned Phase 3 RCTs made efforts to recruit and enroll TGD in clinical trials, an ongoing gap lies with the need for robust data to evaluate the bi-directional relationship between hormone therapy and approved or investigational PrEP agents. In efficacy studies, investigators typically capture gender via self-report. Some trials captured data regarding specific gender affirming hormone therapeutic agents accessed by study participants. Measurement of hormone concentrations is uncommon in RCTs. Consequently, investigators have relied on small phase 1, open-label trials to understand the relationship between PrEP agents and hormone therapies. Several small exploratory pharmacokinetic analyses observed no clinically significant effect of F/TDF on hormone concentrations for TGD adults undergoing either estrogen or testosterone treatment.43,59,60 However, given diversity of hormone therapy regimens provided globally,22 it is unclear whether these small studies can be extrapolated to broader TGD communities. Thoughtful inclusion of sub-studies focused on drug-hormone interactions would enhance the richness of data collected from RCTs and mitigate potential concerns within the TGD community and among clinical providers regarding the impact of PrEP agents on hormone therapy efficacy.
Opportunities for recruitment and enrollment
HPTN 083 and PURPOSE-2 used enrichment to increase the number of transgender women enrolled in both trials (Table 1). Specifically, investigators recruited and enrolled transgender people at risk of HIV acquisition (prognostic enrichment).48,52,61 Given this population’s disproportionate HIV burden, investigators have prioritized the enrichment of transgender women in Phase 3 HIV prevention trials. To adequately enrich future trials on antiretrovirals, studies on HIV prevalence estimates inclusive of all TGD persons must be prioritized.
Although not described extensively in Phase 3 clinical trials above, investigators have recommended using a “2-step” method for collecting gender expression data for study populations (Figure 1).62 The 2-step method includes collecting information on a participant’s gender identity or expression and their sex assigned at birth as two variables.62 Investigators have implemented this approach in survey-based research.36 However, some TGD community members have pushed back on the 2-step method,63 as asking a TGD person to identify their sex assigned at birth draws focus to a potentially harmful and triggering aspect of their lived experience, while reinforcing biological essentialism on social constructs.
Figure 1.
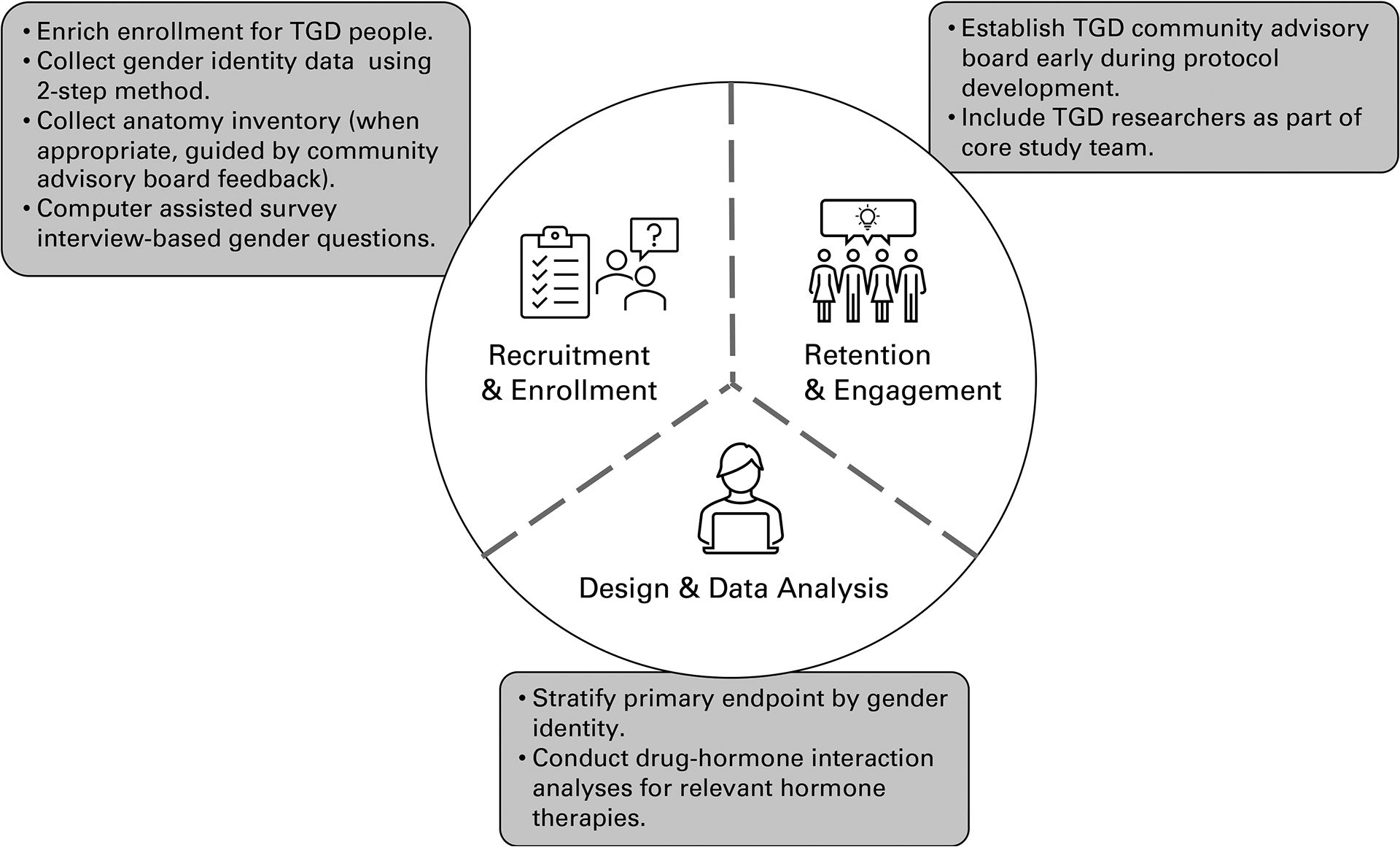
Strategies for including transgender and gender diverse (TGD) participants in randomized clinical trials throughout recruitment and enrollment, retention and engagement, and design and data analysis.
Investigators have recommended using surgical history (“anatomical inventory”) paired with open gender identification as part of baseline demographic data collection.1,52 Briefly, an anatomical inventory may include an electronic health record-based checklist of organs and surgeries intended to guide patient-centered health screenings and post-surgical care plans.64 Anatomical inventories provide information about a participant’s anatomy directly, rather than holding to the cultural assumptions corresponding with sex assigned at birth, while highlighting rates of genital affirmation surgeries among the population. This statistic would go unexamined if sex assigned at birth were collected only. Because studies on HIV transmission via neovagina or neophallus are lacking,2 this information could prove invaluable in determining the efficacy of antiretroviral therapies in a post-surgical population. Finally, incomplete gender identity data collection can underestimate the number of TGD people in clinical research.37 Consistent data collection, including an open question for participant-articulated gender and an anatomical inventory when appropriate, is important for these reasons.
Opportunities for participant retention and engagement
Reisner et al.15 suggested researchers focus on “patient-centeredness” when engaging with TGD participants in clinical studies, conducting research “with,” and not “on,” community members. Study-specific community advisory and accountability groups are evidence-based approaches to increase study retention for underrepresented clinical trial participants and to support patient-centeredness throughout the trial design (Figure 1).52 PURPOSE-2 and HPTN 083 worked with either local or global community advisory boards during protocol development and study recruitment.50,52 While PURPOSE-2 participant data are forthcoming, HPTN 083 exceeded its recruitment goal and illustrated the positive impact of enhanced recruitment efforts for transgender women. Additionally, TGD community members have advocated for their inclusion within study teams.30,34,65–67 Using Global Community Advisory and Accountability Group feedback, PURPOSE-2 included study investigators and staff who were members of the study’s priority population.52 Diverse study teams can support participant retention efforts while minimizing potential harm to the communities participating in the research.34 Finally, although not addressed directly in the RCTs covered here, experts have advocated for applying a trauma informed research approach, in which investigators acknowledge TGD participants’ experiences of trauma and minimize trauma-related stress throughout the HIV research process.68
CONCLUSION AND DIRECTIONS FOR RESEARCH
TGD people have been underrepresented in Phase 3 clinical trials. Although HIV pharmacologic prevention is one area in which investigators have increased TGD representation in randomized clinical trials, additional work remains. Recent randomized clinical trials for HIV pharmacologic prevention interventions have increased study team engagement with TGD communities and team members. These approaches have included (1) establishing or leveraging existing community and global advisory boards comprised of TGD community members, (2) enriching enrollment for transgender women, and (3) modifying protocol language and recruitment efforts to include transgender men and nonbinary people in the study priority population. These approaches should be considered widely for Phase 3 trials across any therapeutic area that relates to disparities prominent in the TGD population.
FUNDING INFORMATION
LRC is supported by the National Institute of General Medical Sciences of the National Institutes of Health under Award Number K23GM147350.
CONFLICT OF INTEREST
MAM receives funded research support from Gilead Sciences, Merck and ViiV Healthcare. All other authors declared no competing interests for this work.
Footnotes
DISCLAIMER
The opinions expressed in this manuscript are those of the authors and should not be interpreted as the position of the National Institutes of Health.
REFERENCES
- 1.Scheim AI, Baker KE, Restar AJ & Sell RL Health and health care among transgender adults in the United States. Annu. Rev. Public Health 43, 503–23 (2022). [DOI] [PubMed] [Google Scholar]
- 2.Poteat TC & Radix A HIV antiretroviral treatment and pre-exposure prophylaxis in transgender individuals. Drugs 80, 965–72 (2020). [DOI] [PubMed] [Google Scholar]
- 3.Golub SA, Fikslin RA, Starbuck L & Klein A High rates of PrEP eligibility but low rates of PrEP access among a national sample of transmasculine individuals. J. Acquir. Immune. Defic. Syndr 82, e1–e7 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Van Gerwen OT, Jani A, Long DM, Austin EL, Musgrove K & Muzny CA Prevalence of sexually transmitted infections and human immunodeficiency virus in transgender persons: a systematic review. Transgend. Health 5, 90–103 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reisner SL, Radix A & Deutsch MB Integrated and gender-affirming transgender clinical care and research. J. Acquir. Immune. Defic. Syndr. (1999) 72, S235 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baral SD, Poteat T, Stromdahl S, Wirtz AL, Guadamuz TE & Beyrer C Worldwide burden of HIV in transgender women: a systematic review and meta-analysis. Lancet Infect. Dis 13, 214–22 (2013). [DOI] [PubMed] [Google Scholar]
- 7.Winter S et al. Transgender people: health at the margins of society. Lancet 388, 390–400 (2016). [DOI] [PubMed] [Google Scholar]
- 8.Lacombe-Duncan A, Logie CH, Newman PA, Bauer GR & Kazemi M A qualitative study of resilience among transgender women living with HIV in response to stigma in healthcare. AIDS Care 32, 1008–13 (2020). [DOI] [PubMed] [Google Scholar]
- 9.Sevelius JM, Patouhas E, Keatley JG & Johnson MO Barriers and facilitators to engagement and retention in care among transgender women living with human immunodeficiency virus. Ann. Behav. Med 47, 5–16 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lacombe‐Duncan A et al. A qualitative exploration of barriers to HIV prevention, treatment and support: perspectives of transgender women and service providers. Health Soc. Care. Community 29, e33–e46 (2021). [DOI] [PubMed] [Google Scholar]
- 11.Dang M et al. Barriers and facilitators to HIV pre-exposure prophylaxis uptake, adherence, and persistence among transgender populations in the United States: a systematic review. AIDS Patient Care STDs 36, 236–48 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pitasi MA, Oraka E, Clark H, Town M & DiNenno EA HIV testing among transgender women and men—27 states and Guam, 2014–2015. MMWR Morb. Mortal. Wkly. Rep 66, 883 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Reisner SL, Chaudhry A, Cooney E, Garrison-Desany H, Juarez-Chavez E & Wirtz AL ‘It all dials back to safety’: A qualitative study of social and economic vulnerabilities among transgender women participating in HIV research in the USA. BMJ Open 10, e029852 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stutterheim SE, van Dijk M, Wang H & Jonas KJ The worldwide burden of HIV in transgender individuals: An updated systematic review and meta-analysis. PLoS One 16, e0260063 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reisner SL et al. Advancing methods for US transgender health research. Curr. Opin. Endocrinol. Diabetes Obes 23, 198–207 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baker KE, Streed CG Jr, & Durso LE Ensuring that LGBTQI+ people count-collecting data on sexual orientation, gender identity, and intersex status. N. Engl. J. Med 384, 1184–1186 (2021). [DOI] [PubMed] [Google Scholar]
- 17.Wirtz AL et al. American cohort to study HIV acquisition among transgender women in high-risk areas (The LITE Study): protocol for a multisite prospective cohort study in the eastern and southern united states. JMIR Res. Protoc 8, e14704 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Coleman E et al. Standards of care for the health of transgender and gender diverse people, version 8. Int. J. Transgend. Health 23, S1–S259 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.James SE & Herman J The report of the 2015 US transgender survey: executive summary. National Center for Transgender Equality; <https://transequality.org/sites/default/files/docs/usts/USTS-Full-Report-Dec17.pdf> (2017). Accessed September 1, 2022. [Google Scholar]
- 20.Braun HM et al. Transgender women living with HIV frequently take antiretroviral therapy and/or feminizing hormone therapy fifferently than prescribed due to drug-drug Interaction concerns. LGBT Health 4, 371–5 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sevelius JM, Xavier J, Chakravarty D, Keatley J, Shade S & Rebchook G Correlates of engagement in HIV care among transgender women of color in the United States of America. AIDS Behav. 25, 3–12 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cirrincione LR & Huang KJ Sex and gender differences in clinical pharmacology: implications for transgender medicine. Clin. Pharmacol. Ther 110, 897–908 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Le A, Huang KJ & Cirrincione LR Regulation of drug-metabolizing enzymes by sex-related hormones: clinical implications for transgender medicine. Trends Pharmacol. Sci 43, 582–592 (2022). [DOI] [PubMed] [Google Scholar]
- 24.Krupka E et al. The effect of gender-affirming hormone therapy on measures of kidney function: A systematic review and meta-analysis. Clin. J. Am. Soc. Nephrol 17, 1305–15 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shieh E et al. Transgender women on oral HIV pre-exposure prophylaxis have significantly lower tenofovir and emtricitabine concentrations when also taking oestrogen when compared to cisgender men. J. Int. AIDS Soc 22, e25405 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tanaudommongkon A et al. Population pharmacokinetics of tenofovir, emtricitabine and intracellular metabolites in transgender women. Br. J. Clin. Pharmacol 88, 3674–3682 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Johnson-Williams B, Jean D, Liu Q & Ramamoorthy A The importance of diversity in clinical trials. Clin. Pharmacol. Ther doi: 10.1002/cpt.2707 (2022). [DOI] [PubMed] [Google Scholar]
- 28.Food & Administration, D. Enhancing the diversity of clinical trial populations—eligibility criteria, enrollment practices, and trial designs guidance for industry. US Department of Health and Human Services; <https://www.fda.gov/media/127712/download> (2020). Accessed September 1, 2022. [Google Scholar]
- 29.Gross AS, Harry AC, Clifton CS & Della Pasqua O Clinical trial diversity: An opportunity for improved insight into the determinants of variability in drug response. Br. J. Clin. Pharmacol 88, 2700–17 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Scheim AI et al. Transgender HIV research: nothing about us without us. Lancet HIV 6, e566–e7 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yager JL & Anderson PL Pharmacology and drug interactions with HIV PrEP in transgender persons receiving gender affirming hormone therapy. Expert Opin. Drug Metab. Toxicol 16,463–474 (2020). [DOI] [PubMed] [Google Scholar]
- 32.Grant RM et al. Sex hormone therapy and tenofovir diphosphate concentration in dried blood spots: primary results of the interactions between antiretrovirals and transgender hormones study. Clin. Infect Dis 73, e2117–e23 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.HPTN 091: integrating HIV prevention, gender-affirmative medical care, and peer health navigation to prevent HIV acquisition and HIV transmission for transgender women in the Americas: a vanguard feasibility and acceptability study. <https://www.hptn.org/sites/default/files/inline-files/HPTN_091_Protocol_Final_Version_1.0_13Apr2020.pdf> (2022). Accessed September 1 2022.
- 34.Veale JF et al. Setting a research agenda in trans health: An expert assessment of priorities and issues by trans and nonbinary researchers. Int. J. Transgend 23, 392–408 doi: 10.1080/26895269.2022.2044425 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Poteat TC, van der Merwe LLA, Sevelius J & Keatley J Inclusion as illusion: erasing transgender women in research with MSM. J. Int. AIDS Soc 24, e25661 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Grant RM, Sevelius JM, Guanira JV, Aguilar JV, Chariyalertsak S & Deutsch MB Transgender women in clinical trials of pre-exposure prophylaxis. J. Acquir. Immune Defic. Syndr 72, S226–9 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Deutsch MB et al. HIV pre-exposure prophylaxis in transgender women: a subgroup analysis of the iPrEx trial. Lancet HIV 2, e512–e9 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cirrincione LR et al. Plasma and intracellular pharmacokinetics of tenofovir disoproxil fumarate and emtricitabine in transgender women receiving feminizing hormone therapy. J. Antimicrob. Chemother 75, 1242–9 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cespedes MS et al. Gender affirming hormones do not affect the exposure and efficacy of F/TDF or F/TAF for HIV preexposure prophylaxis: a subgroup analysis from the DISCOVER trial. Transgend. Health 10.1089/trgh.2022.0048 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yager J et al. Pharmacokinetics of emtricitabine/tenofovir disoproxil fumarate among transgender adolescents and young adults without HIV receiving gender affirming hormones. AIDS Res. Hum. Retroviruses 38, 840–846 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cattani VB et al. Impact of feminizing hormone therapy on tenofovir and emtricitabine plasma pharmacokinetics: a nested drug–drug interaction study in a cohort of Brazilian transgender women using HIV pre-exposure prophylaxis. J. Antimicrob. Chemother 77, 2729–2736 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Blumenthal et al. The Bi-directional effects of hormone therapy and PrEP in transgender individuals. https://www.croiconference.org/abstract/the-bidirectional-effects-of-hormone-therapy-and-prep-in-transgender-individuals/. (2022). Accessed 1 July 2022.
- 43.Hiransuthikul A et al. Drug-drug interactions between feminizing hormone therapy and pre-exposure prophylaxis among transgender women: the iFACT study. J. Int. AIDS Soc 22, e25338–e (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mayer KH et al. Emtricitabine and tenofovir alafenamide vs emtricitabine and tenofovir disoproxil fumarate for HIV pre-exposure prophylaxis (DISCOVER): primary results from a randomised, double-blind, multicentre, active-controlled, phase 3, non-inferiority trial. Lancet 396, 239–54 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Study to evaluate the safety and efficacy of emtricitabine and tenofovir alafenamide (F/TAF) fixed-dose combination once daily for pre-exposure prophylaxis in men and transgender women who have sex with men and are at risk of HIV-1 infection (DISCOVER). <https://clinicaltrials.gov/ct2/show/NCT02842086?term=discover+hiv&draw=2&rank=2>. Accessed September 1, 2022.
- 46.Bernice F & Kilcrease C Novel and investigational HIV therapies for treatment and prevention: focus on cabotegravir, islatravir, and lenacapavir. Curr. Infect. Dis. Rep 24, 89–96 (2022). [Google Scholar]
- 47.Apretude (cabotegravir). <https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/215499s000lbl.pdf> Accessed July 1, 2022.
- 48.Landovitz RJ et al. Cabotegravir for HIV prevention in cisgender men and transgender women. N. Engl. J. Med 385, 595–608 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Marzinke MA et al. Characterization of human immunodeficiency virus (HIV) infection in cisgender men and transgender women who have sex with men receiving injectable cabotegravir for HIV prevention: HPTN 083. J. Infect. Dis 224, 1581–92 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.HPTN 083: a phase 2b/3 double blind safety and efficacy study of injectable cabotegravir compared to daily oral tenofovir disoproxil fumarate/emtricitabine (TDF/FTC), for pre-exposure prophylaxis in HIV-uninfected cisgender men and transgender women who have sex with men. <https://www.hptn.org/sites/default/files/inline-files/HPTN%20083_FINAL%20Version%205.0_28Apr2022.pdf>. (2022). Accessed July 1, 2022.
- 51.Grinsztejn B, Hanscom B, Wang Z, et al. Transgender women (TGW) in HPTN 083: an evaluation of safety, efficacy, and gender affirming hormonal therapy (GAHT) interactions with long-acting cabotegravir (CAB-LA). <https://programme.aids2022.org/Abstract/Abstract/?abstractid=12707>. (2022). Accessed August 14 2022.
- 52.Cespedes M et al. Proactive strategies to optimize engagement of Black, Hispanic/Latinx, transgender, and nonbinary individuals in a trial of a novel agent for HIV pre-exposure prophylaxis (PrEP). PLoS One 17, e0267780 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Study to assess the effectiveness and safety of lenacapavir for human immunodeficiency virus (HIV) pre-exposure prophylaxis (PURPOSE 2). <https://clinicaltrials.gov/ct2/show/NCT04925752?term=purpose+lenacapavir&draw=2&rank=1>. Accessed September 1, 2022.
- 54.Del Río-González AM et al. Global scoping review of HIV prevention research with transgender people: Transcending from trans-subsumed to trans-centred research. J. Int. AIDS Soc 24, e25786 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.McFarland W, Wilson EC & Raymond HF HIV prevalence, sexual partners, sexual behavior and HIV acquisition risk among trans men, San Francisco, 2014. AIDS Behav. 21, 3346–52 (2017). [DOI] [PubMed] [Google Scholar]
- 56.Radix AE, Larson EL, Harris AB & Chiasson MA HIV prevalence among transmasculine individuals at a New York City Community Health Centre: a cross‐sectional study. J. Int. AIDS Soc 25, e25981 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Reisner SL et al. High risk and low uptake of pre-exposure prophylaxis to prevent HIV acquisition in a national online sample of transgender men who have sex with men in the United States. J. Int. AIDS Soc 22, e25391 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.De Brier N et al. Prevalence and associated risk factors of HIV infections in a representative transgender and non-binary population in Flanders and Brussels (Belgium): Protocol for a community-based, cross-sectional study using time-location sampling. PLoS One 17, e0266078 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yager J et al. Gender-affirming hormone pharmacokinetics among adolescent and young adult transgender persons receiving daily emtricitabine/tenofovir disoproxil fumarate. AIDS Res. Hum. Retroviruses doi: 10.1089/AID.2022.0044. (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cattani V, Jalil E, Torres T, et al. No impact of tenofovir/emtricitabine in estradiol exposure among transwomen on oral PrEP: results from the 12-week drug-drug interaction PrEParadas substudy. Abstract OALC0601. International AIDS Society 2021 Conference on HIV Science <https://theprogramme.ias2021.org/Abstract/Abstract/2207.> (2022). Accessed November 15, 2022. [Google Scholar]
- 61.Temple R Enrichment of clinical study populations. Clin. Pharmacol. Ther 88, 774–8 (2010). [DOI] [PubMed] [Google Scholar]
- 62.Siskind RL et al. Engaging transgender people in NIH-funded HIV/AIDS clinical trials research. J. Acquir. Immune Defic. Syndr 72, S243 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Alpert AB, Ruddick R & Manzano C Rethinking sex-assigned-at-birth questions. BMJ. 373, n1261 (2021). [DOI] [PubMed] [Google Scholar]
- 64.Grasso C, Goldhammer H, Thompson J & Keuroghlian AS Optimizing gender-affirming medical care through anatomical inventories, clinical decision support, and population health management in electronic health record systems. J Am Med Inform Assoc 28, 2531–5 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Klein A & Golub SA Ethical HIV research with transgender and non‐binary communities in the United States. J. Int. AIDS Soc 25, e25971 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Restar AJ & Operario D The missing trans women of science, medicine, and global health. Lancet 393, 506–8 (2019). [DOI] [PubMed] [Google Scholar]
- 67.Asquith A, Sava L, Harris AB, Radix AE, Pardee DJ & Reisner SL Patient-centered practices for engaging transgender and gender diverse patients in clinical research studies. BMC Med. Res. Methodol 21, 1–15 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Allison SM, Parker KL & Senn TE Incorporating a trauma-informed perspective in HIV-related research with transgender and gender diverse individuals. J. Int. AIDS Soc 25, e25976 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]


