Abstract
Black patients suffer worse outcomes after percutaneous coronary intervention (PCI) than White patients. Inequities in antiplatelet prescribing may contribute to this health disparity. We compared P2Y12 inhibitor prescribing by race following CYP2C19 genotyping to guide antiplatelet therapy selection after PCI. Patients from nine sites that performed clinical CYP2C19 genotyping after PCI were included. Alternative therapy (e.g., prasugrel or ticagrelor) was recommended for CYP2C19 no-function allele carriers, in whom clopidogrel is predicted to be less effective. The primary outcome was choice of P2Y12 inhibitor (clopidogrel versus alternative therapy) based on genotype. Of 3342 patients included, 2448 (73%) were White, and 659 (20%) were Black. More Black than White patients had a no-function allele (34.3% versus 29.7%, p=0.024). At hospital discharge following PCI, 44.2% of Black and 44.0% of White no-function allele carriers were prescribed alternative therapy. At the time of last follow-up within 12 months, numerically fewer Black (51.8%) than White (56.7%) no-function allele carriers were prescribed alternative therapy (p=0.190). However, the difference was not significant after accounting for other factors associated with P2Y12 inhibitor selection (odds ratio 0.79, 95% confidence interval 0.58–1.08). Alternative therapy use did not differ between Black (14.3%) and White (16.7%) patients without a no-function allele (p=0.232). Among real-world patients who received CYP2C19 testing after PCI, P2Y12 inhibitor prescribing rates did not differ between Black and White patients. Our data suggest an absence of racial disparity in genotype-guided antiplatelet prescribing among patients receiving CYP2C19 testing.
Keywords: Clopidogrel, CYP2C19 genotype, Percutaneous coronary intervention, Black race
Introduction
Ischemic heart disease remains the leading cause of morbidity and mortality in the United States.[1] Percutaneous coronary intervention (PCI) and use of dual antiplatelet therapy (DAPT), consisting of aspirin and a P2Y12 receptor antagonist (clopidogrel, prasugrel, or ticagrelor), significantly improve outcomes, particularly following an acute coronary syndrome (ACS).[2,3] Some studies have shown that Black patients are at increased risk for poor outcomes, including death, myocardial infarction, and stent thrombosis, following PCI compared to White patients.[4,5] Black patients are also under-represented in randomized clinical trials (RCTs) trials of DAPT following PCI.[5–7]
With growing evidence of genetic underpinnings of drug response and guidance available for applying genotype results to prescribing decisions, a number of institutions have implemented pharmacogenetic testing into clinical care, with CYP2C19 genotyping to guide post-PCI P2Y12 inhibitor therapy being one of the most common examples.[8–12] Clopidogrel, a prodrug, is metabolized to its active form by the cytochrome P450 2C19 (CYP2C19) enzyme. Clopidogrel-treated patients with a CYP2C19 no-function allele (most commonly *2 [rs4244285] and *3 [rs4986893]) are at increased risk for major adverse cardiovascular events following PCI compared to similarly treated patients without a no-function allele.[13–15] CYP2C19 genotype does not influence the effectiveness of either prasugrel or ticagrelor, which are recommended in patients known to carry a no-function allele in the absence of contraindications.[9,16,17]
The effectiveness of CYP2C19-guided DAPT, where prasugrel or ticagrelor is prescribed to those with one or two no-function alleles (deemed intermediate metabolizers [IMs] and poor metabolizers [PMs], respectively) and clopidogrel prescribed to those without a no-function allele (i.e., normal metabolizers [NMs], rapid metabolizers [RMs], or ultra-rapid metabolizers [UMs]) has been demonstrated in randomized controlled trials[18–20] observational studies,[21] and recent meta-analyses.[22–24] As part of the Implementing GeNomics In pracTicE (IGNITE) Network, we conducted real world effectiveness and cost effectiveness analyses of PCI patients who were genotyped for CYP2C19 as part of their clinical care, with alternative therapy (e.g., prasugrel or ticagrelor) recommended in IMs and PMs.[21,25–27] Among IM/PMs, treatment with ticagrelor or prasugrel, in accordance with recommendations, was associated with a reduced risk for major adverse cardiovascular events versus treatment with clopidogrel, whereas outcomes were similar among those with other phenotypes treated with either clopidogrel or alternative therapy.[21,27] Moreover, the approach of treating IM/PMs with prasugrel or ticagrelor while treating NM/RM/UMs with clopidogrel was found to be cost-effective.[26]
Despite robust data available on the association between CYP2C19 genotype and cardiovascular outcomes and evidence of improved outcomes with CYP2C19-guided P2Y12 inhibitor therapy,[9] no study has evaluated prescriber response to genotype results in underrepresented patient groups. This is critical to assess given evidence of greater use of clopidogrel versus prasugrel or ticagrelor among underrepresented minority populations,[28,29] and the potential for prescribing differences by race or ethnicity to widen health disparities. The IGNITE dataset, now expanded to include 3342 patients (approximately 20% of whom identify as Black or African American) across nine U.S. institutions,[25] provides an opportunity to assess whether choice of P2Y12 inhibitor based on genotype results reported in the electronic health record (EHR) varies by race in a real-world clinical setting.
Methods
Study population
The IGNITE cohort includes adult patients (age ≥18 years) who underwent PCI and received CYP2C19 testing, with results reported in the EHR, across nine institutions: University of Florida, Gainesville; University of Florida, Jacksonville; University of North Carolina, Chapel Hill; University of Maryland, Baltimore; University of Alabama, Birmingham; University of Pennsylvania; University of Pittsburgh; University of Illinois, Chicago; and Indiana University. All patients received a P2Y12 inhibitor (clopidogrel, prasugrel, or ticagrelor) following PCI. The processes for providing CYP2C19-guided therapy across our sites have been described.[11] Briefly, genotyping was performed at each institution in a Clinical Laboratory Improvement Amendments (CLIA)-licensed laboratory. All sites genotyped for the CYP2C19*2 and *3 no-function alleles and the *17 increased function allele, in accordance with Association of Molecular Pathology recommendations.[30] Additional no-function alleles (e.g., *4, *6, *8) were detected at some sites.[11] CYP2C19 phenotypes were assigned in accordance with Clinical Pharmacogenetics Implementation Consortium (CPIC) guidelines, with IMs and PMs defined as those with one or two no-function alleles, respectively.[9] Those with the *1/*1, *1/*17, or *17/*17 genotype were classified as NMs, RMs, or UMs, respectively. Alternative antiplatelet therapy, consisting of prasugrel or ticagrelor in the absence of contraindications, was recommended for IMs and PMs according to CPIC guidelines.[9] No recommendations were made for NMs, RMs, or UMs. The ultimate prescribing decision was left to the discretion of the provider.
Data abstraction and classification
Data were manually abstracted from the EHR, using a common data collection form, through review of hospitalizations and outpatient encounters starting from the time of index PCI, defined as the PCI performed in association with genotyping. Data were collected up to 12 months after PCI. Clinical outcomes of interest, including death, myocardial infarction, ischemic stroke, stent thrombosis, and hospitalization for unstable angina, occurring within 12 months of the index PCI were identified from provider-reported diagnoses at each encounter or clinical notes in the event of death. Race and ethnicity data were collected as required by the funding agency (i.e., National Institutes of Health). Additional data collected included age and clinical data at the time of the index PCI and antiplatelet therapy at the time of discharge from the index PCI and at the occurrence of an event of interest, or, in patients without an event, at the time of the last follow-up within 12 months of the index PCI. Data collection procedures were approved by the Institutional Review Board at each institution. Data curation, aggregation, and analysis occurred at the University of Florida and were approved by the University of Florida Institutional Review Board.
Race and ethnicity for each patient were based on documentation in the EHR. Race (White; Black or African American; Asian; Native Hawaiian or other Pacific Islander; American Indian or Alaska Native; multiracial; unknown race) and ethnicity (Hispanic or Latino; not Hispanic or Latino) categories were defined according to the Office of Management and Budget Revisions to the Standards for the Classification of Federal Data on Race and Ethnicity.[31,32] These data were used to create the following groups for comparison in the current study: Black, White, or Other Race. Those with race documented as Black or African American in the EHR were included in the group of Black patients. Those with race documented as White, Caucasian, or European were classified as White patients. Patients with another documented race, unknown race, or multiracial were classified accordingly and classified as Other Race.
Data analysis
The primary outcome was the choice of P2Y12 inhibitor therapy based on CYP2C19 metabolizer phenotype. Given that any disparity in P2Y12 inhibitor selection may contribute to differing outcomes after PCI, we also assessed occurrence of major atherothrombotic events within 12 months of the index PCI as an exploratory analysis. Major atherothrombotic events were defined as the composite of all-cause death or first occurrence of myocardial infarction, ischemic stroke, stent thrombosis, or hospitalization for unstable angina.
Patient characteristics at the time of index PCI and P2Y12 inhibitor therapy (clopidogrel versus alternative therapy) at both discharge from the index PCI hospitalization and at the time of clinical event or last follow-up within 12 months of the index PCI, were compared between Black and White patients using chi-square analysis for categorical variables and the two-independent sample t-test for continuous variables. Our primary focus was therapy at time of event or last follow-up given previous investigations of outcomes based on therapy at this time point.[21,25,27] Baseline characteristics and P2Y12 inhibitor therapy for patients with another documented race or unknown race were summarized using descriptive statistics for qualitative comparison, but were not formally compared to Black and White patients using inferential statistics. Genotype turnaround time, defined as the difference between the date genotype was ordered and date genotype resulted in the EHR, and time to change in P2Y12 inhibitor therapy, defined as the difference between date of discharge from the index PCI and date of switch in therapy, were compared between Black and White patients with the PM or IM phenotype via the Wilcoxon rank sum test. Changes in P2Y12 inhibitor therapy from discharge to time of event or last follow-up were assessed via the McNemar test for paired proportions. The odds ratio (OR) of being prescribed an alternative P2Y12 inhibitor versus clopidogrel at the time of event or last follow-up was estimated using logistic regression. A multivariable model of factors associated with use of alternative therapy versus clopidogrel at the time of event or last follow-up was constructed using a stepwise selection method. A p value <0.2 was required to enter the model, and p <0.05 was required to remain in the model. The final model only included Black race and covariates independently associated with P2Y12 inhibitor selection.
For the exploratory outcomes analysis, data were censored at the time of the event or last follow-up in which treatment with a P2Y12 inhibitor was documented. Major atherothrombotic event rates were compared between Black and White patients using time-to-event analysis. Comparisons were completed in the overall study population and within each of three genotype-antiplatelet therapy groups: PMs and IMs treated with alternative therapy, PMs and IMs treated with clopidogrel, and NM/RM/UMs irrespective of antiplatelet treatment. Cox regression analyses were performed to estimate the hazard ratios and 95% confidence intervals (CIs) for major atherothrombotic events, adjusting for demographics, comorbidities, and concurrent medications. The analysis of outcomes in the overall population was also adjusted for CYP2C19 phenotype and P2Y12 inhibitor medication at the time of the event or last follow-up. For each comparison, event rates per 100 patient-years were reported. P values < 0.05 were considered statistically significant. All analyses were performed using SAS version 9.4 (Cary, NC).
Results
Patient population
As shown in Table 1, of the 3342 patients included, 2448 (73%) were White, 659 (20%) were Black, and 235 (7%) were of another race, unknown race, or multiracial (grouped as Other Race). In addition, 118 (3.5%) patients were of Hispanic ethnicity, of whom 36 had their race documented in the EHR as unknown, and 30 had their race documented as multiracial. The majority of patients underwent PCI for an acute coronary syndrome (n=2290, 68.5%), and 2868 (85.8%) received a drug eluting stent. Compared to White patients, Black patients were younger, had a higher body mass index, and were more likely to be female, smoke, and have a history of diabetes, hypertension, stroke or transient ischemic attack, heart failure, and more severe renal impairment. History of atrial fibrillation and cancer were more common in White patients.
Table 1.
Patient characteristics at the time of PCI stratified by race
| Characteristic | Overall Population (n=3342) |
White (n=2448) |
Black (n=659) |
Other Race* (n=235) |
P-value for comparison of Black vs. White patients |
|---|---|---|---|---|---|
| Age (years) | 63 ± 12 | 64 ± 12 | 60 ± 12 | 62 ± 12 | <0.001 |
| Age ≥75 years | 557 (16.7) | 451 (18.4) | 69 (10.5) | 37 (15.7) | <0.001 |
| BMI (kg/m2) | 30 ± 6 | 30 ± 6 | 31 ± 7 | 29 ± 5 | <0.001 |
| Weight <60 kg | 203 (6.1) | 146 (6.0) | 40 (6.1) | 17 (7.2) | 0.993 |
| Male sex | 2267 (67.8) | 1737 (71.0) | 370 (56.1) | 160 (68.1) | <0.001 |
| Hispanic ethnicity | 118 (3.5) | 50 (2.0) | 2 (0.3) | 66 (28.4) | 0.002 |
| Indication for PCI | |||||
| ACS | 2290 (68.5) | 1660 (67.8) | 475 (72.1) | 155 (66.0) | 0.375 |
| ST-Elevation MI | 628 (18.8) | 456 (18.6) | 132 (20.0) | 40 (17.0) | |
| Non-ST-Elevation MI | 939 (28.1) | 665 (27.2) | 207 (31.4) | 67 (28.5) | |
| Unstable Angina | 723 (21.6) | 539 (22.0) | 136 (20.6) | 48 (20.4) | |
| Elective (non-ACS) | 1052 (31.5) | 788 (32.2) | 184 (27.9) | 80 (34.0) | |
| Drug eluting stent placement | 2868 (85.8) | 2112 (86.3) | 551 (83.6) | 205 (87.2) | 0.083 |
| Past Medical History | |||||
| Diabetes | 1354 (40.5) | 924 (37.7) | 319 (48.4) | 111 (47.2) | <0.001 |
| Hypertension | 2704 (80.9) | 1937 (79.1) | 583 (88.5) | 184 (78.3) | <0.001 |
| Dyslipidemia | 2317 (69.3) | 1730 (70.7) | 432 (65.6) | 155 (66.0) | 0.110 |
| MI | 866 (25.9) | 659 (26.9) | 173 (26.3) | 34 (14.5) | 0.731 |
| Prior stent | 770 (23.0) | 554 (22.6) | 156 (23.7) | 60 (25.5) | 0.572 |
| Stroke or TIA | 348 (10.4) | 243 (9.9) | 87 (13.2) | 18 (7.7) | 0.015 |
| PVD | 335 (10.0) | 243 (9.9) | 79 (12.0) | 13 (5.5) | 0.123 |
| Heart failure | 533 (15.9) | 371 (15.2) | 136 (20.6) | 26 (11.1) | <0.001 |
| Atrial fibrillation | 316 (9.5) | 253 (10.3) | 48 (7.3) | 15 (6.4) | 0.019 |
| Cancer | 184 (5.5) | 157 (6.4) | 25 (3.8) | 2 (0.9) | 0.011 |
| Current smoker | 969 (29.0) | 666 (27.2) | 258 (39.2) | 45 919.2) | <0.001 |
| Gastrointestinal or intracranial hemorrhage | 111 (3.3) | 78 (3.2) | 26 (4.0) | 7 (3.0) | 0.336 |
| eGFR distribution (ml/min/1.73m2) | |||||
| eGFR ≥ 60 | 2377 (71.3) | 1732 (70.8) | 480 (73.0) | 165 (70.2) | <0.001 |
| eGFR 30–59 | 741 (22.2) | 601 (24.6) | 93 (14.1) | 47 (20.0) | |
| eGFR<30 | 224 (6.7) | 115 (4.7) | 86 (13.0) | 23 (9.8) | |
| Co-medications at discharge | |||||
| Aspirin | 3269 (97.8) | 2405 (98.2) | 641 (97.3) | 223 (94.9) | 0.149 |
| OAC | 301 (9.0) | 223 (9.1) | 60 (9.1) | 18 (7.7) | 0.999 |
| Aspirin plus OAC | 276 (8.3) | 209 (8.5) | 53 (8.0) | 14 (6.0) | 0.744 |
| Statin | 3143 (94) | 2290 (93.5) | 631 (95.8) | 222 (94.5) | 0.034 |
| ACE inhibitor or ARB | 2245 (67) | 1621 (66.2) | 476 (72.2) | 148 (63.0) | 0.003 |
| β-blocker | 2852 (85) | 2079 (84.9) | 572 (86.8) | 201 (85.5) | 0.228 |
| Proton pump inhibitor | 1049 (31) | 808 (33.0) | 187 (28.4) | 54 (23.0) | 0.024 |
Data are presented as number (%) or mean ± SD.
Other race is comprised of Asian (n=38), Native Hawaiian or Pacific Islander (n=5); American Indian or Alaska Native (n=36); multiracial (n=32); and unknown race (n=124)
ACE, angiotensin converting enzyme; ACS, acute coronary syndrome; ARB, angiotensin receptor blocker; BMI, body mass index; CKD, Chronic kidney disease; eGRF, estimated glomerular filtration rate; IM, intermediate metabolizer; MI, myocardial infarction; OAC, oral anticoagulant (i.e., warfarin, dabigatran, rivaroxaban, apixaban, edoxaban); PCI, percutaneous coronary intervention; PM, poor metabolizer; PVD, peripheral vascular disease; RM, rapid metabolizers; TIA, transient ischemic attack; UM, ultra-rapid metabolizer
Genotype and phenotype frequencies by race
CYP2C19 genotypes and phenotypes by EHR-documented race are shown in Table S1 and Table 2. The median (interquartile range) genotype turnaround time was 1 (4 to 31) day in White IM/PMs and 1 (5 to 30) day in Black IM/PMs. There was a significant difference in phenotype distribution across groups, with a higher prevalence of IM/PMs among Black versus White patients (34.3% versus 29.7%, p=0.024). The prevalence of IM/PMs was also high among Asian, Native Hawaiian, or Pacific Islander patients (46.5%) and among American Indian or Alaska Native patients (47.2%). Among Hispanic patients, 20.3% were IMs or PMs compared to 31.3% among patients reported as non-Hispanic (p=0.011).
Table 2.
CYP2C19 metabolizer phenotype and P2Y12 inhibitor at time of event or last follow-up by race and phenotype
| Patient Group | Total No. | No. (%) IM/PMs | No. (%) NM/RM/UMs | IM/PM | NM/RM/UM | ||
|---|---|---|---|---|---|---|---|
| Clopidogrel | Alternative Therapy | Clopidogrel | Alternative Therapy | ||||
| All Patients | 3342 | 1032 (30.9) | 2310 (69.1) | 461 (44.7) | 571 (55.3)† | 1932 (83.6) | 378 (16.4) |
| White | 2448 | 728 (29.7) | 1720 (70.3) | 315 (43.3) | 413 (56.7)† | 1433 (83.3) | 287 (16.7) |
| Black | 659 | 226 (34.3) | 433 (65.7) | 109 (48.2) | 117 (51.8)† | 371 (85.7) | 62 (14.3) |
| Asian, Native Hawaiian or Pacific Islander* | 43 | 20 (46.5) | 23 (53.5) | 10 (50) | 10 (50) | 19 (82.6) | 4 (17.4) |
| American Indian or Alaska Native | 36 | 17 (47.2) | 19 (52.8) | 11 (64.7) | 6 (35.3) | 13 (68.4) | 6 (31.6) |
| Multiracial | 32 | 9 (28.1) | 23 (71.9) | 5 (55.6) | 4 (44.4) | 20 (87.0) | 3 (13.0) |
| Unknown race | 124 | 32 (25.8) | 92 (74.2) | 11 (34.4) | 21 (65.6) | 76 (82.6) | 16 (17.4) |
Data are presented as number (%)
Combined because of the small number of Native Hawaiian or Pacific Islander patients (n=5)
p,0.001 for comparison of alternative therapy in IM/PMs versus NM/RM/UMs.
IM, intermediate metabolizer; NM, normal metabolizer; PM, poor metabolizer; RM, rapid metabolizer; UM, ultra-rapid metabolizer
P2Y12 inhibitor prescribing by phenotype and race
At the time of hospital discharge after the index PCI, use of alternative therapy was significantly higher in IM/PMs versus NM/RM/UMs in the overall study population (44.0% vs 19.6%, p<0.0001) as well as among White patients (44.0% vs 20.0%, p<0.0001), Black patients (44.3% vs 15.9%, p<0.0001), patients of another race or unknown race (43.6% vs 24.8%, p=0.0034) and Hispanic patients (45.8% vs 13.8%, p = 0.0005). When comparing the use of alternative therapy at discharge between White and Black patients, there was no significant difference among either IM/PMs (44.0% and 44.2%, p=0.939; Figure 1A) or NM/RM/UMs (20.0% and 15.9%, p=0.055), respectively (Figure 1B).
Figure 1. P2Y12 inhibitor therapy at hospital discharge following PCI and at time of event or last follow-up stratified by race and CYP2C19 phenotype.
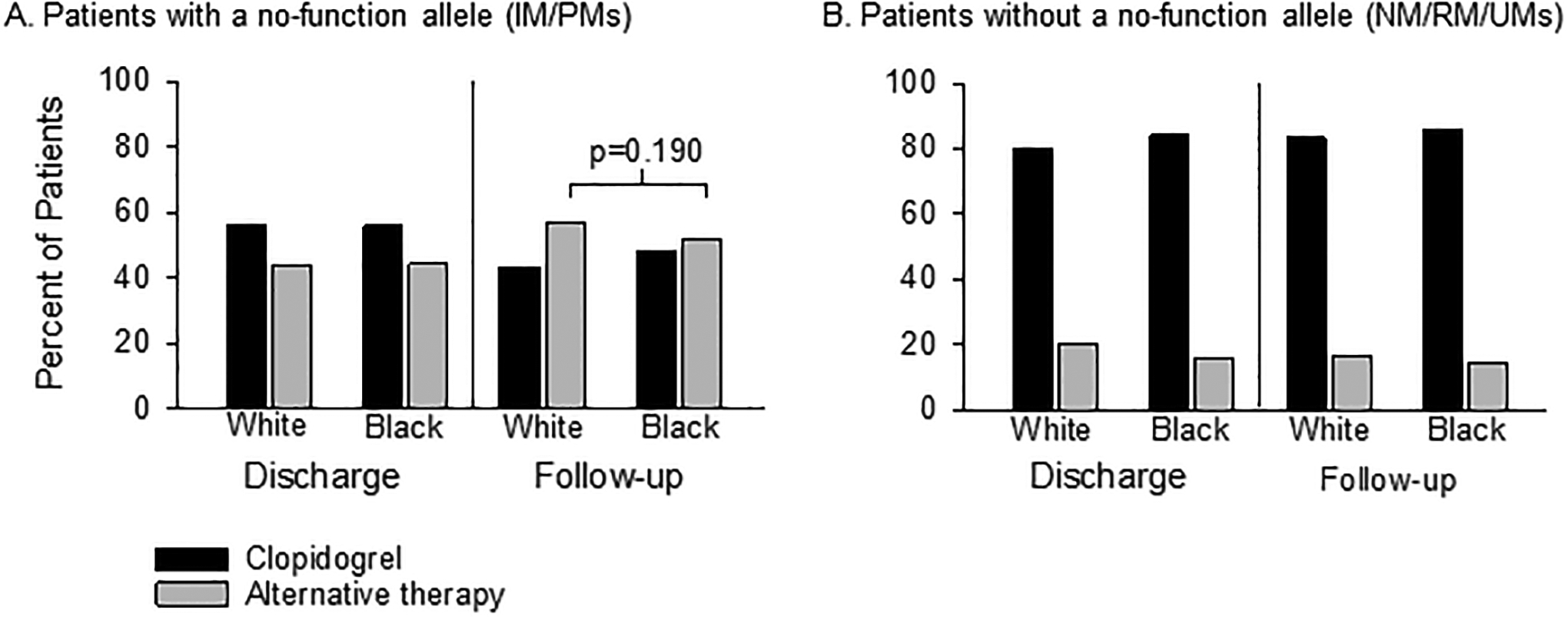
Data are shown for patients with a no-function allele (A) and without a no-function allele (B). Unadjusted p value shown. PCI, percutaneous coronary intervention; IM, intermediate metabolizer. NM, normal metabolizer; PM, poor metabolizers; RM, rapid metabolizer; UM, ultra-rapid metabolizer
Use of alternative therapy increased after discharge in IM/PMs, and was higher in IM/PMs compared to those with other phenotypes at the time of event or last follow-up in the overall study population (55.3% vs 16.4%, p<0.001) and among both White (56.7% vs 16.7%, p<0.001) and Black (51.8% vs 14.3%, p<0.001) patients (Table 2). The median (interquartile range) time to change in P2Y12 inhibitor therapy was 11 (4 to 31) days in White IM/PMs and 13 (5 to 30) days in Black IM/PMs (p=0.510). Alternate therapy use was also higher in IM/PMs versus NM/RM/UMs in patients of another race or unknown race (52.6% versus 18.5%, p<0.001) and in Hispanic patients (54.2% versus 10.6%, p<0.001). In comparing therapy in IM/PMs at the time of event or last follow-up by race, the percent of Black IM/PM patients receiving alternative therapy was numerically lower than the percent of White IM/PM patients (51.8% versus 56.7%, respectively, p=0.190; unadjusted odds ratio 0.82, 95% confidence interval 0.61 to 1.10, Figure 1A). Although not statistically significant, these differences appeared consistent in the subset of IMs carrying one no-function allele (50.0% versus 54.2%, p=0.296) and PMs carrying two no-function alleles (70% versus 82.1%, p=0.242) (Table S2). Alternative therapy use at event/last follow-up appeared lower among American Indian or Alaska Native and multiracial IM/PMs compared to White patients (Table 2), but formal statistical comparisons were not conducted because of the limited sample sizes. Most patients without a no-function allele remained on clopidogrel at the time of event or last follow-up (83.6%), and there was no difference in alternative P2Y12 inhibitor therapy use between Black and White patients without a no-function allele (14.3% vs 16.7%, p=0.232, Figure 1B).
Among the subset of White or Black patients, demographic and clinical characteristics differed between IM/PMs prescribed alternative therapy versus clopidogrel at the time of event or follow-up (Table S3). IM/PMs treated with alternative therapy were younger and less likely to be receiving oral anticoagulant therapy. In addition, alternative therapy-treated IM/PMs had a lower prevalence of diabetes, hypertension, chronic kidney disease, stroke or transient ischemic attack, peripheral vascular disease, heart failure, and atrial fibrillation. Factors independently associated with alternative P2Y12 inhibitor versus clopidogrel selection among IM/PMs were age, diabetes, prior stroke or transient ischemic attack, and oral anticoagulant prescription at discharge. After accounting for these factors on logistic regression analysis, there was no association between Black race and likelihood of prescribing alternative antiplatelet therapy at the time of event or follow-up among IM/PMs (adjusted odds ratio 0.79; 95% CI, 0.58–1.08; p=0.146; Figure 2).
Figure 2. Logistic regression analysis of factors associated with alternative therapy versus clopidogrel use at the time of event or last follow-up in CYP2C19 intermediate and poor metabolizers.
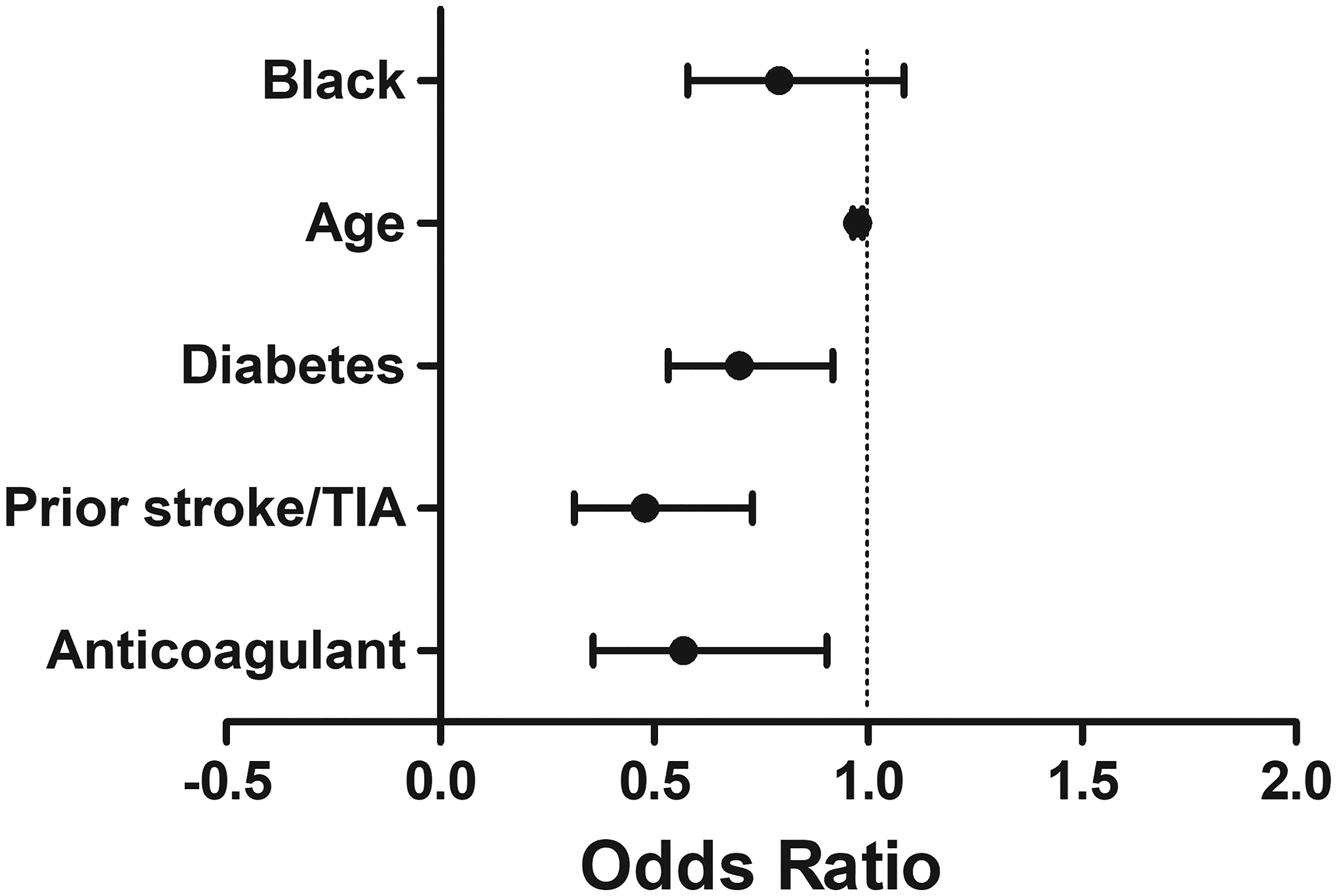
The model includes variables that remained independently associated (p<0.05) with choice of P2Y12 inhibitor therapy. The odds ratio and 95% confidence interval for each variable remaining in the model after stepwise selection are shown in Table S3.
Exploratory outcome analysis
Differences in baseline demographic and clinical characteristics between phenotype-antiplatelet therapy groups in the population overall and within the White and Black patient subgroups are shown in Table S4. Results of the outcomes analyses after adjusting for these differences are shown in Table 3. The median (interquartile range) follow-up period was 6.3 (1.0 to 11.0) months for White patients and 7.4 (1.2 to 11.1) months for Black patients. Over this time, there was no significant difference in the rate of major atherothrombotic events between Black (21.1 per 100 patient-years) and White (20.8 per 100 patient-years) patients overall (adjusted hazard ratio 0.95, 95% CI 0.73–1.23, p=0.706) or within any of the phenotype-antiplatelet therapy groups (Table 3).
Table 3.
Cumulative incidence rates of major atherothrombotic events at 12 months in White and Black patients
| Overall (n=3,342) |
White (n=2,448) |
Black (n=659) |
||||||
|---|---|---|---|---|---|---|---|---|
| Follow-up (months, median ± IQR) | 6.3 (1.0 to 11.0) | 6.3 (1.0 to 11.0) | 7.4 (1.2 to 11.1) | |||||
| Event No. (%) | Rate per 100 pt-yrs | Event No. (%) | Rate per 100 pt-yrs | Event No. (%) | Rate per 100 pt-yrs | Adjusted HR (Black vs. White) [95% CI] | P-value | |
| Major adverse cardiovascular events | ||||||||
| All patients | 353 (10.6) | 21.0 | 257 (10.5) | 20.8 | 75 (11.4) | 21.1 | 0.95 (0.73–1.23) | 0.706 |
| IM/PM-Clopidogrel | 71 (15.4) | 34.4 | 46 (14.6) | 34.1 | 22 (20.2) | 39.4 | 1.05 (0.61–1.83) | 0.853 |
| IM/PM-Alternative | 51 (8.9) | 17.1 | 34 (8.2) | 15.4 | 13 (11.1) | 22.0 | 1.60 (0.80–3.18) | 0.183 |
| NM/RM/UM-Clopidogrel | 231 (10.0) | 19.6 | 177 (10.3) | 20.1 | 40 (9.2) | 16.6 | 0.76 (0.54–1.07) | 0.121 |
Data are presented as the number (percent) of patients in each group who experienced the event over 12 months of follow-up after the index PCI
Event rates were calculated as the number of events per 100 patients-years (pt-yrs) of follow-up
CI, confidence interval; HR, hazard ratio; IM, intermediate metabolizer; NM, normal metabolizer; PM, poor metabolizer; RM, rapid metabolizer; UM, ultra-rapid metabolizer
Discussion
In our real-world cohort of patients across nine institutions who received CYP2C19 genotyping at the time of PCI to guide antiplatelet therapy, we observed no difference in P2Y12 inhibitor prescribing patterns at hospital discharge following PCI between Black and White patients, with greater prescribing of alternative therapy in IM/PMs versus NM/RM/UMs in both groups. Use of alternative therapy in IM/PMs, but not in those with other phenotypes, increased in the 12-month period following PCI in both Black and White patients, with no significant difference by race in time to a switch in P2Y12 inhibitor therapy after discharge. At the time of event or last follow-up, a numerically lower percentage of Black versus White patients with the IM or PM phenotype were prescribed alternative therapy, but there was no difference between groups after accounting for other factors associated with P2Y12 inhibitor selection.
Racial disparities in outcomes post-PCI have been well documented.[4,5] In a meta-analyses of 10 trials and over 22,000 patients who underwent PCI, the rates of major atherothrombotic events, including death and myocardial infarction, were significantly higher in Black compared to White patients.[5] Similar to the current study, Black patients in the meta-analysis had a higher prevalence of cardiovascular risk factors at baseline, including diabetes and hypertension, compared to White patients. Even after adjusting for these differences, worse outcomes remained among Black patients in the meta-analysis. Observational studies have also shown an increased risk for MACE in Black versus White patients following PCI.[33,34] However, this difference was attenuated after adjusting for clinical factors (e.g., comorbidities, disease severity) as well as income, education, and health insurance in one study,[33] suggesting that both clinical factors and socioeconomic status contribute to racial disparities in post-PCI outcomes.
Black patients comprised just 4.1% of all participants in the meta-analysis described above.[5] Black patients are similarly underrepresented in trials of DAPT after PCI, with fewer than 2% of patients included in the PLATO trial, which compared the efficacy of clopidogrel versus ticagrelor in reducing cardiovascular events after PCI.[6] The percent of Black participants was not reported for TRITON TIMI-38, which compared clopidogrel to prasugrel, but 92% of participants were reported as White.[7] Both trials demonstrated superiority of alternative therapy versus clopidogrel, and thus, guidelines state preference for prasugrel or ticagrelor over clopidogrel following an acute coronary syndrome and PCI.[35]
Importantly, a high percentage of both White and Black patients in our study had a CYP2C19 no-function allele (29.7% of White and 34.3% of Black patients), which is associated with reduced clopidogrel effectiveness after PCI and may have contributed to the inferiority of clopidogrel compared to alternative agents in the PLATO and TRITON TIMI-38 trial.[15,36] Because of the increased risk for atherothrombotic events with clopidogrel treatment in those with a no-function allele,[15] the U.S. Food and Drug Administration and Clinical Pharmacogenetics Implementation Consortium (CPIC) recommend alternative antiplatelet therapy after PCI in the absence of contraindications for both IMs and PMs.[8,9] Data from clinical trials and meta-analyses of genotype-guided therapy support this recommendation.[18–20,23,24] The POPular Genetics trial showed that, compared to universal use of alternative therapy, reserving alternative therapy for IM/PMs and treating those without a no-function allele with clopidogrel was noninferior in preventing ischemic events and superior in reducing risk for clinical significant bleeding.[18] The TAILOR PCI trial did not show a benefit with genotype-guided therapy at 12 months compared to universal clopidogrel use.[37] However, a prespecified sensitivity analysis showed a reduction in total events per patient, and a post-hoc analysis showed a reduction in events at 90 days with genotype-guided therapy. Meta-analyses of randomized controlled trial and observational data also demonstrated improved outcomes with alternative therapy versus clopidogrel in IM/PMs, but similar outcomes across P2Y12 inhibitors in those without a no-function allele.[22–24]
Similar to TRITON TIMI-38 and PLATO, Black patients were underrepresented in trials of CYP2C19-guided antiplatelet therapy; only 2% of TAILOR-PCI participants were Black, and <0.5% of POPular Genetics trial participants were reported as African.[18,37] Black patients comprised 20% of our study population; this serves as one of the largest cohorts of underrepresented minorities receiving CYP2C19 genotype testing to guide antiplatelet therapy after PCI to date. We observed that rates of major atherothrombotic events did not differ between Black and White patients overall or within any genotype-antiplatelet therapy subgroup. While data on outcomes from our exploratory analysis are promising, given our limited sample size, confirmation that genotype-guided therapy reduces the risk for adverse cardiovascular events in Black patients as has been observed in predominately White cohorts is needed, as is examination of the effect of genotype-guided therapy on bleeding events in Black patients. In particular, an appropriately powered study comparing outcomes in Black IM/PMs treated with clopidogrel versus alternative therapy is needed to better discern the benefit of genotype-guided therapy in this population.[38]
Previous studies have demonstrated greater use of medications addressed in pharmacogenetic guidelines, including clopidogrel, among Black patients than those of other race groups.[28,39,40] These data suggest that Black patients may be especially at risk for poor outcomes if they do not receive pharmacogenetic testing and subsequent adjustment of their drug therapy regimen if warranted based on test results. Therefore, ensuring equitable access to pharmacogenetic testing and individualized therapy based on pharmacogenetic test results, are necessary to prevent further worsening of health disparities. Our data from a diverse real-world population receiving CYP2C19 testing to guide antiplatelet therapy suggest equitable prescribing of alternative P2Y12 inhibitor therapy in Black patients in accordance with genotype guided prescribing recommendations. However, given the limited sample size and number of institutions involved, this requires further study.
In addition to our relatively small sample size of Black patients, which prevented appropriately powered assessment of outcomes in this group, our data are limited by the reliance on EHR documentation of race and ethnicity, which we recognize is an imperfect surrogate for patient self-report. Moreover, our sample size was limited to more rigorously evaluate genotype-guided prescribing in other underrepresented populations, including patients of Hispanic ethnicity. We also recognize that race is a social construct, and our intent in this regard was to examine whether inequities exists in prescribing practices when genetic information is available.[32] Further, data on health insurance, which may have influenced drug selection, were not collected. Prescription and outcome data were limited to what was available within the EHR at most institutions. It is possible that patients had additional encounters with outside providers and received additional medications that could not be captured with our study design. Lastly, our results were derived from nine academic institutions that were early adopters of genotype-guided antiplatelet therapy prescribing, and may not be generalizable to other settings. We also did not assess whether CYP2C19 genotyping after PCI was performed equitably across race and ethnic groups. Given that equitable access to genotype-guided therapy is critical to prevent worsening of health disparities, future research will be critical to identify, understand, and address disparities in the practice of precision medicine.
In summary, in a real-world population of patients who received CYP2C19 testing after PCI, P2Y12 inhibitor selection did not significantly differ between Black and White patients. Our data suggest that genotype-guided therapy has the potential to lessen previously reported disparities in antiplatelet prescribing. Whether genotype-guided therapy helps to reduce racial disparities in post-PCI outcomes requires further analysis.
Supplementary Material
Study Highlights.
What is the current knowledge on the topic?
Black patients are at increased risk for adverse outcomes after percutaneous coronary intervention (PCI) compared to White patients. There are reports of inequities in antiplatelet prescribing, which could contribute to this health disparity.
What question did this study address?
Does selection of antiplatelet therapy after PCI vary by race among real-world patients receiving CYP2C19 genotyping to guide antiplatelet prescribing decisions?
What does this study add to our knowledge?
Across nine medical centers that had implemented CYP2C19-guided antiplatelet therapy after PCI, antiplatelet drug therapy did not differ by race.
How might this change clinical pharmacology or translational science?
These data suggest that genotype-guided prescribing may be a means to eliminating previously reported disparities in antiplatelet prescribing.
Conflict of Interest Statement:
F.F. declares that he has received payment as an individual for consulting fee or honorarium from AstraZeneca, Bayer and Sanofi. Institutional payments for grants from PLx Pharma, and The Scott R. MacKenzie Foundation. R.P.K. receives research funding from Idorsia and consulting fees from Haemonetics. T.C.S. receives consulting fees from Indiana University Health. J.G. receives honoraria from Astra Zeneca and Inari Medical. D.J.A. has received consulting fees or honoraria from Abbott, Amgen, AstraZeneca, Bayer, Biosensors, Boehringer Ingelheim, Bristol-Myers Squibb, Chiesi, Daiichi-Sankyo, Eli Lilly, Haemonetics, Janssen, Merck, PhaseBio, PLx Pharma, Pfizer, and Sanofi. D.J.A. also declares that his institution has received research grants from Amgen, AstraZeneca, Bayer, Biosensors, CeloNova, CSL Behring, Daiichi-Sankyo, Eisai, Eli Lilly, Gilead, Janssen, Matsutani Chemical Industry Co., Merck, Novartis, Osprey Medical, Renal Guard Solutions, and the Scott R. MacKenzie Foundation. JAJ is a consultant for United Health Group. JG has served as an advisory and research funds to the institution from Boston Scientific, Inari Medical, Recor Medical, and Abbott Vascular. All other authors declared no competing interests for this work.
Sources of Funding:
This work was supported by grants from the National Institutes of Health (NIH, U01 HG007269 [J.A.J, L.H.C., J.D.D., C.W.M. and Y.G.], U01 HG007775 [A.L.B.], and U01 HG007762 and U01 HG010245 [T.C.S. and R.P.K], and by the NIH IGNITE Network (https://gmkb.org/ignite/). Additional support provided by NIH grants R01 HL149752 (L.H.C., C.R.L., A.L.B., Y.G., and C.W.M.), T32 HG008958 (C.D.T.), and K01 HL141690 (C.W.M.); NIH grants U01 GM074492 and U01 HL105198 (both part of the NIH Pharmacogenomics Research Network); and by substantial institutional support from the University of Florida and its Clinical Translational Science Institute (NIH grants UL1 TR000064 and UL1 TR001427) (L.H.C., J.A.J., J.D.D., C.W.M., Y.G.); NIH UL1 TR002489 (C.R.L., G.A.S.); Penn Center for Precision Medicine at the Perelman School of Medicine at the University of Pennsylvania (S.T., J.G.); NIH R01 HL092173, K24 HL133373, UL1 TR000165 and Hugh Kaul Precision Medicine Institute at the University of Alabama, Birmingham (N.A.L.); NIH U54 CA233444 (J.M.A.); NIH UL1 TR0000005, the University of Pittsburgh/UPMC Institute for Precision Medicine, American Society of Health System Pharmacists, American Heart Association grant 17MCPRP33400175, and by an Anonymous Donor (P.E.E., J.C.C., J.M.S.); NIH U54 MD010723 (J.C.L.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
SUPPORTING INFORMATION
Supplementary information accompanies this paper on the Clinical Pharmacology & Therapeutics website (www.cpt-journal.com).
References:
- 1.Mokdad AH, et al. The State of US Health, 1990–2016: Burden of diseases, injuries, and risk factors among US states. JAMA. 319, 1444–1472 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Keeley EC, Boura JA, Grines CL. Primary angioplasty versus intravenous thrombolytic therapy for acute myocardial infarction: a quantitative review of 23 randomised trials. Lancet. 361, 13–20 (2003). [DOI] [PubMed] [Google Scholar]
- 3.Angiolillo DJ, Galli M, Collet JP, Kastrati A, O’Donoghue ML. Antiplatelet therapy after percutaneous coronary intervention. EuroIntervention. 17, e1371–e1396 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Collins SD, et al. Does black ethnicity influence the development of stent thrombosis in the drug-eluting stent era? Circulation. 122, 1085–1090 (2010). [DOI] [PubMed] [Google Scholar]
- 5.Golomb M, et al. Prognostic impact of race in patients undergoing PCI: analysis from 10 randomized coronary stent trials. JACC Cardiovasc Interv. 13, 1586–1595 (2020). [DOI] [PubMed] [Google Scholar]
- 6.Wallentin L, et al. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 361, 1045–1057 (2009). [DOI] [PubMed] [Google Scholar]
- 7.Wiviott SD, et al. TRITON-TIMI Investigators. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 357, 2001–2015 (2007). [DOI] [PubMed] [Google Scholar]
- 8.Food and Drug Administration. Table of Pharmacogenetic Associations. ,https://www.fda.gov/medical-devices/precision-medicine/table-pharmacogenetic-associations>. Accessed 1 August 2020., Vol. 2020.
- 9.Lee CR, et al. Clinical Pharmacogenetics Implementation Consortium Guideline for CYP2C19 Genotype and Clopidogrel Therapy: 2022 Update. Clin Pharmacol Ther. doi: 10.1002/cpt.2526 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cavallari LH, et al. The IGNITE Pharmacogenetics Working Group: an opportunity for building evidence with pharmacogenetic implementation in a real-world setting. Clin Transl Sci. 10, 143–146 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Empey PE, et al. Multisite investigation of strategies for the implementation of CYP2C19 genotype-guided antiplatelet therapy. Clin Pharmacol Ther. 104, 664–674 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harada S, et al. Precision medicine at the University of Alabama at Birmingham: laying the foundational processes through implementation of genotype-guided antiplatelet therapy. Clin Pharmacol Ther. 102, 493–501 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Collet JP, et al. Cytochrome P450 2C19 polymorphism in young patients treated with clopidogrel after myocardial infarction: a cohort study. Lancet. 373, 309–317 (2009). [DOI] [PubMed] [Google Scholar]
- 14.Mega JL, et al. Cytochrome p-450 polymorphisms and response to clopidogrel. N Engl J Med. 360, 354–362 (2009). [DOI] [PubMed] [Google Scholar]
- 15.Mega JL, et al. Reduced-function CYP2C19 genotype and risk of adverse clinical outcomes among patients treated with clopidogrel predominantly for PCI: a meta-analysis. JAMA. 304, 1821–1830 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mega JL, et al. Cytochrome P450 genetic polymorphisms and the response to prasugrel: relationship to pharmacokinetic, pharmacodynamic, and clinical outcomes. Circulation. 119, 2553–2560 (2009). [DOI] [PubMed] [Google Scholar]
- 17.Wallentin L, et al. Effect of CYP2C19 and ABCB1 single nucleotide polymorphisms on outcomes of treatment with ticagrelor versus clopidogrel for acute coronary syndromes: a genetic substudy of the PLATO trial. Lancet. 376, 1320–1328 (2010). [DOI] [PubMed] [Google Scholar]
- 18.Claassens DMF, et al. A genotype-guided strategy for oral P2Y12 inhibitors in primary PCI. N Engl J Med. 381, 1621–1631 (2019). [DOI] [PubMed] [Google Scholar]
- 19.Notarangelo FM, et al. Pharmacogenomic approach to selecting antiplatelet therapy in acute coronary syndromes: PHARMCLO trial. J Am Coll Cardiol. 71, 1869–1877 (2018). [DOI] [PubMed] [Google Scholar]
- 20.Xie X, et al. Personalized antiplatelet therapy according to CYP2C19 genotype after percutaneous coronary intervention: a randomized control trial. Int J Cardiol. 168, 3736–3740 (2013). [DOI] [PubMed] [Google Scholar]
- 21.Cavallari LH, et al. Multisite investigation of outcomes with implementation of CYP2C19 genotype-guided antiplatelet therapy after percutaneous coronary intervention. JACC Cardiovasc Interv. 11, 181–191 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Galli M, et al. Guided versus standard antiplatelet therapy in patients undergoing percutaneous coronary intervention: a systematic review and meta-analysis. Lancet. 397, 1470–1483 (2021). [DOI] [PubMed] [Google Scholar]
- 23.Pereira NL, et al. Effect of CYP2C19 genotype on ischemic outcomes during oral P2Y12 inhibitor therapy: a meta-analysis. JACC Cardiovasc Interv. 14, 739–750 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Galli M, et al. Comparative effects of guided vs. potent P2Y12 inhibitor therapy in acute coronary syndrome: a network meta-analysis of 61 898 patients from 15 randomized trials. Eur Heart J. 43, 959–967 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee CR, et al. Impact of the CYP2C19*17 allele on outcomes in patients receiving genotype-guided antiplatelet therapy after percutaneous coronary intervention. Clin Pharmacol Ther. 109, 705–715 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Limdi NA, et al. Cost-effectiveness of CYP2C19-guided antiplatelet therapy in patients with acute coronary syndrome and percutaneous coronary intervention informed by real-world data. Pharmacogenomics J. 20, 724–735 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Beitelshees AL, et al. CYP2C19 genotype-guided antiplatelet therapy after percutaneous coronary intervention in diverse clinical settings. J Am Heart Assoc. 11, e024159 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hicks JK, et al. Opportunity for genotype-guided prescribing among adult patients in 11 US health systems. Clin Pharmacol Ther. 110, 179–188 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nathan AS, et al. Identifying racial, ethnic, and socioeconomic inequities in the use of novel P2Y12 inhibitors after percutaneous coronary intervention. J Invasive Cardiol. 34, E171–E178 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pratt VM, et al. Recommendations for clinical CYP2C19 genotyping allele selection: a report of the Association for Molecular Pathology. J Mol Diagn. 20, 269–276 (2018). [DOI] [PubMed] [Google Scholar]
- 31.Racial and Ethnic Categories and Definitions for NIH Diversity Programs and for Other Reporting Purposes. Notice Number: NOT-OD-15-089. Available through https://grants.nih.gov/grants/guide/notice-files/NOT-OD-15-089.html. Accessed February 19, 2022.
- 32.Flanagin A, Frey T, Christiansen SL, Committee, AMAMoS. Updated guidance on the reporting of race and ethnicity in medical and science journals. JAMA. 326, 621–627 (2021). [DOI] [PubMed] [Google Scholar]
- 33.Cai A, et al. Risk of major adverse cardiovascular events and major hemorrhage among White and Black patients undergoing percutaneous coronary intervention. J Am Heart Assoc. 8, e012874 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kobayashi T, et al. Comparative outcomes after percutaneous coronary intervention Among Black and White patients treated at US Veterans Affairs hospitals. JAMA Cardiol. 2, 967–975 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Levine GN, et al. 2016 ACC/AHA guideline focused update on duration of dual antiplatelet therapy in patients with coronary artery disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 68, 1082–1115 (2016). [DOI] [PubMed] [Google Scholar]
- 36.Mega JL, et al. Genetic variants in ABCB1 and CYP2C19 and cardiovascular outcomes after treatment with clopidogrel and prasugrel in the TRITON-TIMI 38 trial: a pharmacogenetic analysis. Lancet. 376, 1312–1319 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pereira NL, et al. Effect of genotype-guided oral P2Y12 inhibitor selection vs conventional clopidogrel therapy on ischemic outcomes after percutaneous coronary intervention: the TAILOR-PCI randomized clinical trial. JAMA. 324, 761–771 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nguyen AB, Cavallari LH, Rossi JS, Stouffer GA, Lee CR. Evaluation of race and ethnicity disparities in outcome studies of CYP2C19 genotype-guided antiplatelet therapy. Front Cardiovasc Med. 9, 991646 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dalton R, Brown JD, Duarte JD. Patients with geographic barriers to health care access are prescribed a higher proportion of drugs with pharmacogenetic testing guidelines. Clin Transl Sci. 14, 1841–1852 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nathan AS, et al. Racial, ethnic, and socioeconomic inequities in the prescription of direct oral anticoagulants in patients with venous thromboembolism in the United States. Circ Cardiovasc Qual Outcomes. 12, e005600 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


