Abstract
Background:
Despite an aging population, little is known about racial disparities in aging-specific functional impairments and mortality among older adults hospitalized for acute myocardial infarction (AMI).
Methods:
We analyzed data from patients aged 75 years or older who were hospitalized for AMI at 94 U.S. hospitals from 2013 to 2016. Functional impairments and geriatric conditions were assessed in-person during the AMI hospitalization. The association between race and risk of mortality (primary outcome) was evaluated with logistic regression adjusted sequentially for age, clinical characteristics, and measures of functional impairment and other conditions associated with aging.
Results:
Among 2918 participants, 2668 (91.4%) self-identified as White and 250 (8.6%) as Black. Black participants were younger (80.8 yr. vs. 81.7 yr.; P=0.010) and more likely to be female (64.8% vs. 42.5%; P<0.001). Black participants were more likely to present with impairments in cognition (37.6% vs. 14.5%; P<0.001), mobility (66.0% vs. 54.6%; P<0.001) and vision (50.1% vs. 35.7%; P<0.001). Black participants were also more likely to report a disability in one or more activities of daily living (22.4% vs. 13.0%; P<0.001) and unintentional loss of more than 10 lbs. in the year prior to hospitalization (37.2% vs. 13.0%; P<0.001). The unadjusted odds of 6-month mortality among Black participants (OR 2.0, 95% CI 1.4-2.8) attenuated to non-significance after adjustment for age, clinical characteristics (OR 1.70, 95% CI 1.7, 1.2-2.5), and functional/geriatric conditions (OR 1.5, 95% CI 1.0-2.2).
Conclusions:
Black participants had a more geriatric phenotype despite a younger average age, with more functional impairments. Controlling for functional impairments and geriatric conditions attenuated disparities in 6-month mortality somewhat. These findings highlight the importance of systematically assessing functional impairment during hospitalization and also ensuring equitable access to community programs to support post-AMI recovery among Black older adults.
Keywords: Acute myocardial infarction, functional impairment, health care disparities, race, Black/African American
INTRODUCTION
Racial disparities among patients with acute myocardial infarction (AMI) have been extensively documented.1–4 Black patients with AMI present with more severe disease, receive percutaneous intervention (PCI) less frequently, and experience worse post-discharge outcomes. Despite the aging of the United States population, prior work examining racial disparities among patients with AMI has not focused on older adults (aged ≥75 years).5 Particularly notable is the absence of information about racial disparities in the prevalence of functional impairments, including those in cognition, vision, hearing, strength, and mobility among older patients with AMI. Information about these impairments can inform planning for post-discharge care, including need for home health care and visiting nursing services. Notably, functional impairments are powerful, independent predictors of mortality.6
The objective of this study was to examine racial disparities in a contemporary cohort of older patients hospitalized with AMI. We used data from SILVER-AMI, a multicenter observational study of older adults hospitalized with AMI that included a comprehensive, in-hospital functional assessment, as well as detailed information about post-discharge outcomes. We used these data to evaluate racial disparities in the baseline prevalence of functional impairments and 6-month mortality rates.
METHODS
Data Source
The design of SILVER-AMI has been described previously.7 The study enrolled 3041 participants aged 75 years or older who were hospitalized with acute myocardial infarction (AMI) at 94 U.S. hospitals from 2013 to 2016 (ClinicalTrials.gov identifier R01HL115295). AMI diagnosis was confirmed in accordance with the Third Universal Definition of Myocardial Infarction.8 All participants provided written informed consent. The University of California, San Diego Brief Assessment of Capacity to Consent was administered to participants with decisional capacity concerns, and proxy consent was obtained for participants with diminished capacity.9 Participants were ineligible if they had initial troponin elevation more than 24 hours after admission, were transferred from another hospital after more than 24 hours, were incarcerated, or were unable to provide informed consent, with no proxy available. Enrollment rates among eligible Black and White persons were 36.8% and 35.1%, respectively (P=0.42). All study protocols were approved by the institutional review boards at the coordinating site (Yale School of Medicine, New Haven, CT) and all participating sites.
Baseline Assessment
Participants underwent a structured interview and physical assessment during hospitalization. Timing of the assessment was left to the discretion on the site investigators and was scheduled so as not to conflict with diagnostic testing or procedures. The average time from admission to baseline assessment was 3.3 days for Black participants and 3.4 days for White participants (P=0.49). Participants reported demographic data, including age, sex, self-identified race (American Indian or Alaskan Native, Asian, Black, Native Hawaiian or Pacific Islander, White, or two or more races) and highest level of education during the baseline interview. Reporting race and ethnicity in this study was mandated by the US National Institutes of Health (NIH), consistent with the Inclusion of Women, Minorities, and Children policy. Participants were asked about their current marital status (married, divorced/separated, widowed, living as married/living with partner, never married) and finances at the end of the month (some money left over, just enough to make ends meet, not enough to make ends meet). The eight-item Patient Health Questionnaire depression scale (PHQ-8) and 12-Item Short-Form Health Survey (SF-12) were administered.10,11 We used an abridged 5-item version of the Medical Outcomes Study Social Support Survey (MOS-SSS) to measure 5 domains of social support: emotional support, informational support, tangible support, positive social interaction and affectionate support.12,13 The questionnaire assessed each domain of social support by asking about the presence of someone to provide each type of support on a scale from 1 (lowest) to 5.14
In-person, performance-based testing was used to ascertain cognitive impairment, muscle strength, and mobility. The Telephone Interview of Cognitive Status (TICS) was used to evaluate general cognitive function.15 Scores range from 0-41, with a score less than 27 indicating cognitive impairment. Upper-extremity strength was measured using a handheld dynamometer (B&L Engineering); impairment was defined as less than 18.5 kg for women and less than 28.5 kg for men on best of 3 dynamometer measurements.16,17 Mobility was measured with the Timed Get Up and Go (TUG) test, which required the participant to rise from a seated position, walk 3 meters, and then return to the chair and sit down.18 We applied cut points to classify participants into the following groups: preserved mobility (≤15 seconds to complete), mild impairment (>15 to ≤25 seconds to complete), moderate impairment (>25 seconds to complete) and severe impairment (unable to complete).19–22
Participants were asked select questions about vision from the National Eye Institute Visual Function Questionnaire.23 Responses of “poor,” “very poor,” or “completely blind” indicated visual impairment. They were also asked the extent to which their hearing interferes with normal day-to-day activities. Responses of “a lot” or “a moderate amount” were classified as hearing impairment. Participants were asked the degree to which they could independently perform activities of daily living (ADLs), including bathing, dressing, getting out of a chair, and ambulating 1 month prior to admission.24 Response options were “no help needed”, “help needed,” and “unable to do.” Participants were also asked how many falls they had experienced in the past year and whether they had unintentionally lost 10 or more pounds in the past year.
Site coordinators and a research nurse at the coordinating center abstracted data from participants’ medical records on presenting clinical characteristics (heart rate, blood pressure, hemoglobin, glomerular filtration rate, body mass index, Charlson comorbidity index, presence of chest pain prior to hospitalization, presence of ST segment length elevation, left ventricular ejection fraction, chronic kidney disease, and smoking history), and medical history (of arrhythmia, cerebrovascular disease, congestive heart failure, chronic obstructive pulmonary disease, coronary artery disease, diabetes mellitus, dyslipidemia, hypertension, prior myocardial infarction, prior percutaneous intervention, and peripheral artery disease).
Outcomes
The primary outcome was all-cause mortality at 6-months after hospital discharge. Mortality status was ascertained via medical records (in-hospital), death certificates (out of hospital), or obituaries (out of hospital). When these sources were not available (<5% cases), mortality status was determined through secondary report, such as from a family member. All deaths were double-adjudicated by physician investigators. Mortality status (dead or alive) at 6 months was determined for 99.9% of participants.
Statistical Analysis
Participants identifying as races other than Black or White, or missing information about race, were excluded from analyses. We focused on comparing Black and White participants because they were the two largest groups in our study. The categories excluded comprise small samples, limiting our ability to draw cross-group comparisons: 15 American Indian/Alaska Native, 40 Asian, 3 Native Hawaiian or Pacific Islander, 12 two or more races, and 48 of unknown race. We generated descriptive statistics for Black and White participants using percentages for categorical variables and means for continuous variables. To compare differences between Black and White participants, we used the chi-squared test for categorical variables and Student’s t-test for continuous variables. A 2-sided P value <0.05 was considered statistically significant. Descriptive analyses were performed using R.
We performed logistic regression to examine the association of race (Black versus White participants) with 6-month mortality after adjusting for validated risk factors specific to older AMI patients.25 We focused on 6-month mortality because it was the outcome for which a previously described risk model using data from the SILVER-AMI study had excellent discriminatory ability, providing precision for examining disparities.6,26 We built our models using covariates from the SILVER-AMI 6-month mortality model. Variables were evaluated for correlation to help avoid model overfitting. Missing data, <1% in all variables except ejection fraction and mobility impairment, was addressed via multiple imputation. Multivariable analyses were performed using Stata (College Station, TX).
We sequentially generated four models: model 1 included race only; model 2 included race and age; model 3 included race, age and clinical characteristics (length of stay, history of sleep apnea, history of peripheral arterial disease, hemoglobin, heart rate, glomerular filtration rate, ejection fraction and revascularization status); and model 4 included race, age, clinical characteristics, functional impairments and other conditions associated with aging (mobility impairment, hearing impairment, SF-12 score, and unintentional weight loss of 10 or more pounds in the past year).
RESULTS
Baseline Characteristics
Our analytic sample included 2918 participants, with 2668 identifying as White and 250 as Black. Black participants were younger (80.8 years vs. 81.7 years; P=0.010) and more likely to be women (64.8% vs. 42.5%; P<0.001) (Table 1). They also were more likely to have not completed high school (26.4% vs. 11.3%; P<0.001), not have enough money left over at the end of the month to make ends meet (10.8% vs. 5.6%; P<0.001) and to be unmarried (71.6% vs. 47.5%; P<0.001). Black participants were more likely to have a greater burden of comorbid disease, as indicated by their higher score on the Charlson comorbidity index (4.1 vs. 3.5; P<0.001), a prior history of cerebrovascular disease (23.2% vs. 14.8%; P<0.001), congestive heart failure (27.6% vs. 18.0%; P<0.001), diabetes mellitus (52.4% vs. 35.5%; P<0.001) and hypertension (95.6% vs. 84.3%; P<0.001). Black participants reported lower levels of overall social support (20.7 vs. 21.7 points; P=0.006).
Table 1: Participant characteristics: Black versus White older adults at hospitalization (N=2918).
For descriptive purposes, ‘depressive symptoms’ defined as 8-item Patient Health Questionnaire score ≥ 10. Chronic kidney disease defined as glomerular filtration rate < 60 mL/min/1.73m2. Social support score is calculated as the sum of the scores for 5 domains of social support (emotional, informational, tangible, social, and affectionate) scored on a scale from 1 (lowest) to 5. Data missing for < 5% of variables except for participant finances at the end of the month (8.0% among Black participants) and depressive symptoms (5.6% among Black participants).
| Black Participants (N=250) Mean (SD) or N (%) | White Participants (N=2668) Mean (SD) or N (%) | P-value | |
|---|---|---|---|
|
| |||
| Demographics | |||
|
| |||
| Calculated Age – Mean (SD) | 80.8 (5.13) | 81.7 (5.02) | 0.010 |
|
| |||
| Highest level of education completed | |||
| Less than high school | 66 (26.4%) | 302 (11.3%) | <0.001 |
| High school/GED | 103 (41.2%) | 1186 (44.5%) | |
| 2-year or 4-year college degree | 54 (21.6%) | 767 (28.7%) | |
| Graduate or post-graduate degree | 22 (8.8%) | 393 (14.7%) | |
|
| |||
| Sex | |||
| Male | 88 (35.2%) | 1534 (57.5%) | <0.001 |
| Female | 162 (64.8%) | 1134 (42.5%) | |
|
| |||
| Married or living as married | 71 (28.4%) | 1400 (52.5%) | <0.001 |
|
| |||
| Patient finances at the end of the month | |||
| Some money left over | 91 (36.4%) | 1738 (65.1%) | <0.001 |
| Just enough to make ends meet | 112 (44.8%) | 648 (24.3%) | |
| Not enough to make ends meet | 27 (10.8%) | 150 (5.6%) | |
| Depressive symptoms | 44 (17.6%) | 361 (13.5%) | 0.061 |
| Social support score – Mean (SD) | 20.7 (5.02) | 21.7 (4.41) | 0.006 |
|
| |||
| Clinical Presentation | |||
|
| |||
| Body mass index – Mean (SD) | 27.2 (5.97) | 27.6 (5.29) | 0.41 |
|
| |||
| Heart rate | 83.9 | 83.9 | 0.99 |
|
| |||
| Systolic blood pressure | 147.4 | 145.7 | 0.393 |
|
| |||
| Diastolic blood pressure | 78.5 | 78.0 | 0.666 |
|
| |||
| Charlson comorbidity index – Mean (SD) | 4.12 (2.61) | 3.52 (2.62) | <0.001 |
|
| |||
| Chest pain before hospitalization | 107 (42.8%) | 1052 (39.4%) | 0.260 |
|
| |||
| Chronic kidney disease | 149 (59.6%) | 1607 (60.2%) | 0.887 |
|
| |||
| Current or ever smoker | 141 (56.4%) | 1494 (56.0%) | 0.659 |
|
| |||
| Glomerular filtration rate – Mean (SD) | 52.5 (25.1) | 54.8 (19.3) | 0.158 |
|
| |||
| ST Elevation Myocardial Infarction | 59 (23.6%) | 707 (26.5%) | 0.357 |
|
| |||
| Medical History | |||
|
| |||
| Arrhythmia | 52 (20.8%) | 685 (25.7%) | 0.105 |
|
| |||
| Cerebrovascular disease | 58 (23.2%) | 395 (14.8%) | <0.001 |
|
| |||
| Congestive heart failure | 69 (27.6%) | 479 (18.0%) | <0.001 |
|
| |||
| Chronic obstructive pulmonary disease | 36 (14.4%) | 380 (14.2%) | 0.999 |
|
| |||
| Coronary artery disease | 129 (51.6%) | 1436 (53.8%) | 0.543 |
|
| |||
| Diabetes mellitus | 131 (52.4%) | 948 (35.5%) | <0.001 |
|
| |||
| Dyslipidemia | 168 (67.2%) | 1685 (63.2%) | 0.230 |
|
| |||
| Hypertension | 239 (95.6%) | 2249 (84.3%) | <0.001 |
|
| |||
| Myocardial infarction | 75 (30.0%) | 731 (27.4%) | 0.420 |
|
| |||
| Percutaneous intervention | 74 (29.6%) | 825 (30.9%) | 0.718 |
|
| |||
| Peripheral artery disease | 38 (15.2%) | 315 (11.8%) | 0.141 |
Functional Impairments
Most functional impairments were more common among Black participants than White participants (Figure 1). Black participants were more likely to have cognitive impairment (37.6% vs. 14.5%; P<0.001), mobility impairment (66.0% vs. 54.6%; P<0.001), and vision impairment (51.2% vs. 35.7%; P<0.001). Black participants were also more likely to report a disability in one or more activities of daily living (22.4% vs. 13.0%; P<0.001) and an unintentional loss of more than 10 lbs. in the past year (37.2% vs. 21.0%; P<0.001). However, Black participant were less likely to have hearing impairment (33.2% vs. 55.7%; P<0.001) and there was no statistically significant difference in fall history or muscle strength.
Figure 1: Functional impairments and geriatric conditions: Black versus White participants at hospitalization.
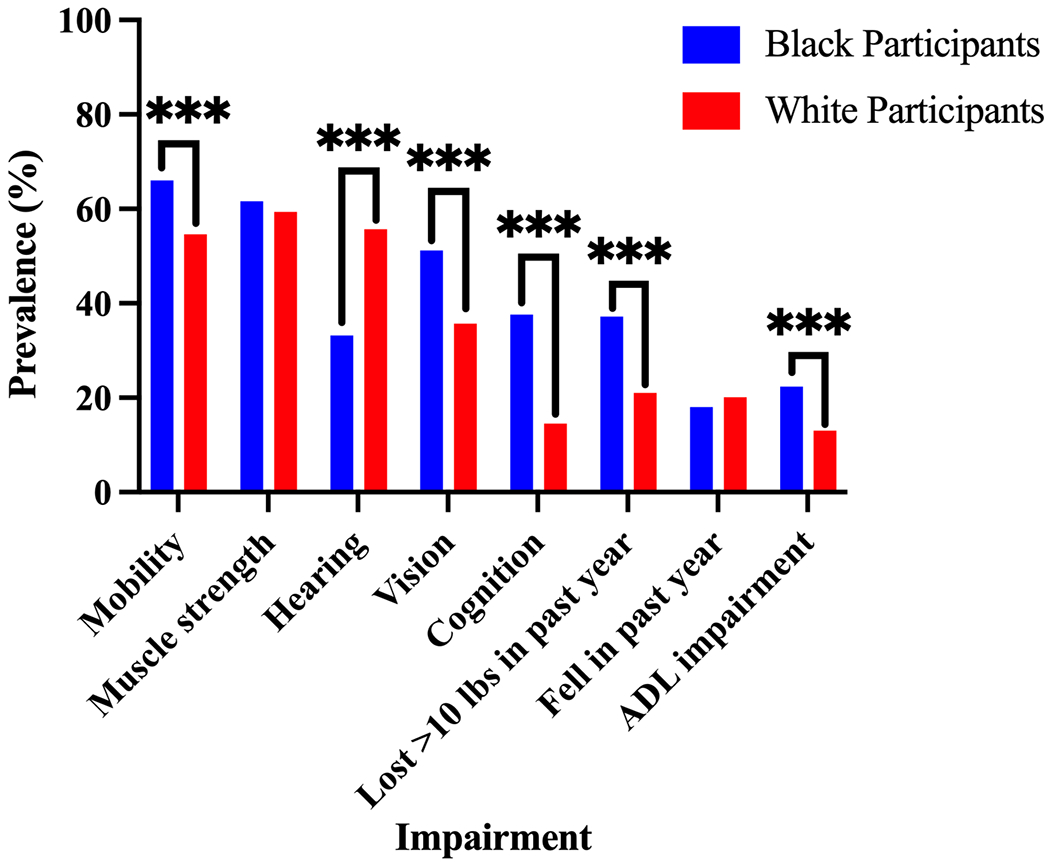
Mobility impairment defined as a Timed Get Up and Go (TUG) test time of > 15 seconds. Muscle strength impairment was defined as < 18.5 kg for women and < 28.5 kg for men on best of 3 handheld dynamometer measurements. Cognitive impairment defined as Telephone Interview of Cognitive Status (TICS) score of < 27. Hearing and vision impairment were self-reported. ADL, activity of daily living (bathing, dressing, getting out of a chair, and ambulating) 1 month prior to admission. Data missing for < 5% of participants for all variables except mobility impairment (19.6% of Black participants and 15.2% of White participants). P<0.001 (***).
6-Month Mortality
The rate of 6-month mortality was 16.8% among Black participants and 9.3% among White participants. In unadjusted analyses, Black participants were more likely to die at 6-months compared with White participants (OR 2.0, 95% CI 1.4-2.8) (Figure 2; Supplementary Table S1). This disparity increased when age was added as a covariate (OR 2.2, 95% CI 1.5-3.1). Adding clinical characteristics (length of stay, history of sleep apnea, history of peripheral arterial disease, hemoglobin, heart rate, glomerular filtration rate, ejection fraction and revascularization status) attenuated the mortality disparity (OR 1.7, 95% CI 1.2-2.5). Adding functional impairments and other geriatric conditions (mobility impairment, hearing impairment, SF-12 score, and unintentional weight loss of 10 or more pounds in the past year) further attenuated the mortality disparity, which then became borderline statistically non-significant (OR 1.5, 95% CI 1.0-2.2).
Figure 2. Sequentially adjusted odds ratios of 6-month mortality: Black versus White participants.
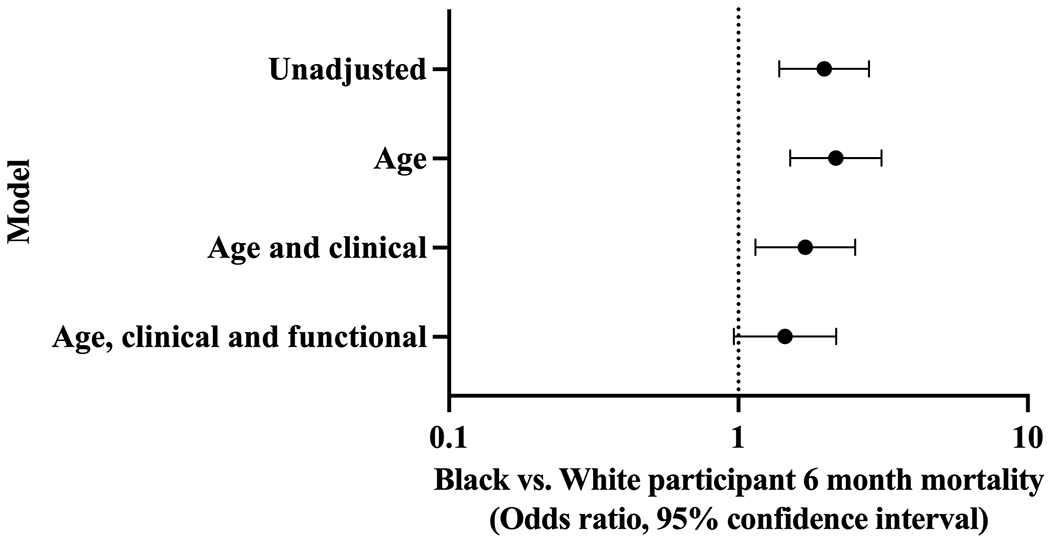
We generated four logistic regression models of race versus 6-month mortality, sequentially adding age, clinical characteristics (length of stay, history of sleep apnea, history of peripheral arterial disease, hemoglobin, heart rate, glomerular filtration rate, ejection fraction and revascularization status), and functional impairments and other geriatric conditions (mobility impairment, hearing impairment, SF-12 score, and unintentional weight loss of 10 or more pounds in the past year).
DISCUSSION
In this analysis of contemporary data on 2918 older adults hospitalized for AMI, we identified two important racial disparities. First, we found that Black participants had more functional impairments than White participants, despite their younger chronological age. Second, we observed a substantially greater risk of 6-month mortality in Black participants that attenuated after inclusion of functional impairments and other conditions associated with aging.
Our finding that older Black participants had a higher prevalence of functional impairments is consistent with those of prior studies performed in the broader Medicare eligible population. Using regional data from the Cardiovascular Health Study, Hirsch et al. found that Black Americans aged 65 and older had an approximately two-fold greater prevalence of frailty, defined as meeting three of five of the Fried Frailty Criteria, including decreased grip strength, exhaustion, unintentional weight loss, increased time to walk 15 feet, and low physical activity, than their White peers.27,28 A similar disparity was identified among participants enrolled in the National Health and Aging Trends Study, a nationally representative cohort of adults aged 65 and older living outside of nursing homes.29,30 In general, however, measures of physical and cognitive functional impairment are rarely included in studies of health disparities. Our study reinforces the importance of including these measures to understand racial disparities and builds upon prior work by focusing on older adults aged 75 and older at the time of hospitalization for AMI.
The weathering hypothesis provides one possible framework for interpreting our finding that older Black adults present with increased rates of functional impairments. According to the weathering hypothesis, Black Americans experience early health deterioration as a consequence of the stress endured living in a society that stigmatizes and disadvantages them.31 Accordingly, Black participants in SILVER-AMI were more likely to present with chronic stressors like financial hardship, lower educational attainment and fewer forms of social support. These signs of systematic inequality may indicate a role for weathering in contributing to observed disparities in functional impairments.
Previous studies using National Health and Nutrition Examination Survey (NHANES) data examined allostatic load scores, which indirectly measure the physiologic effects of weathering using measures such as blood pressure, glycated hemoglobin, glomerular filtration rate, albumin, triglycerides, C-reactive protein, homocysteine, total cholesterol, and waist to hip ratios, among Black and white adults aged less than 65.32,33 These studies found that Black adults have higher allostatic load scores independent of socioeconomic status and health behaviors. Researchers have proposed that allostatic load scores might serve as predictors of poor aging as higher scores have been shown to be associated with decline in functional domains including unintentional weight loss, grip, gait, and cognition in older adults.34,35 However, the converse relationship, in which individuals with functional impairments are more susceptible to chronic stressors, is also plausible. Future studies incorporating longitudinal data on allostatic load and functional impairment are needed to better understand this complex relationship.
Although the weathering hypothesis offers a compelling explanation for the disparities we observed in functional impairments, systemic racism likely influences aging in a myriad of complex and interrelated ways. For example, early life adversity is predictive of increased levels of inflammatory cytokines such as interleukin-6 at midlife in Black Americans, but not White Americans.36 Increased levels of inflammatory cytokines have in turn been associated with unintentional weight loss in older adults.37 In this manner consistent with the weathering hypothesis, Black Americans may undergo biological changes such as increased inflammation in response to systemic racism that increase their risk of developing functional impairments such as unintentional weight loss in old age. Alternatively, increased inflammation may serve as a sign of underlying conditions that, rather than occurring at higher rates due to stress, are underdiagnosed in Black older adults. For example, malignancy is a common etiology of unintentional weight loss in community-dwelling older adults, accounting for 19-36% of cases. Black Americans with cancer experience delays in care that lead to being diagnosed at a later stage.38 In this manner, poor access to timely and high quality medical care can lead to delays in identifying common medical etiologies of functional impairment among Black older adults.
Another important finding of our study is that functional impairments and geriatric conditions, along with age and clinical characteristics, were strongly influential in explaining observed racial disparities in 6-month mortality. In our sequential multivariable analysis, adding age as a covariate increased the mortality disparity. This finding may be explained by the lower average age of Black participants. We then observed a decrease in the mortality disparity after adjustment for clinical characteristics, which further diminished after adjustment for functional impairments.
The attenuation observed in the mortality disparity implies that clinical characteristics and functional impairments are important drivers of racial disparities in mortality among older participants hospitalized for AMI. Our finding that race attenuated to statistical non-significance does not necessarily imply that adjustment for clinical characteristics and functional impairments eliminated the racial disparity in mortality. The point estimate for the OR of 6-month mortality for Black older adults declined only modestly from 2.0 to 1.5 after full adjustment. Despite the large effect size, the lower bound of the confidence interval after full adjustment intersected with an OR of 1 likely due to our relatively small sample size. It may also be important to consider functional impairments as a mediator of the association between race and outcomes, not simply a confounder. While clinical characteristics are routinely considered as part of AMI management, functional impairments and other conditions associated with aging may receive less attention, particularly among Black Americans. Addressing functional impairments as part of standard clinical practice may help narrow racial disparities among older patients hospitalized for AMI.
The increased prevalence of functional impairments among older Black patients hospitalized for AMI may signal that they require (on average) more support in transitioning from the hospital to the home. For example, older patients with cognitive impairments might struggle to adhere to complex medication regimens, potentially leading to suboptimal treatment outcomes. Patients with mobility impairments may have difficulty adhering to recommendations for physical activity and exercise. Patients and their physicians can consider several strategies to mitigate the consequences of functional impairments following AMI. Patients with cognitive impairment may require periodic assistance from a home health aide in managing medications.39 Occupational therapy and home modifications may help patients with mobility or strength impairment improve physical function.40 The potential scope of these traditional strategies is limited by labor and resources available to a health system. As adoption of telemedicine grows following the COVID-19 pandemic, digital assistive technologies such as tablet-based medication self-management apps and bed-exit sensors may assist providers in remotely monitoring and assisting greater numbers of patients.41,42 Further research is needed to evaluate the cost-effectiveness of comparing novel assistive technologies to traditional home health services currently reimbursed by Medicare.
Despite their higher burden of functional impairments and geriatric conditions, evidence from prior studies suggests that older Black patients in the United States are less likely to receive services such as home health care.43 Recent evidence suggests that home health care reduces short-term mortality among older patients after hospitalization. A randomized clinical trial of comprehensive geriatric assessment followed by home nursing visits among older hospitalized patients found a significant reduction in 6-month mortality.44 In the US, a retrospective analysis found that Medicare beneficiaries discharged to the community who received transitional care management services, including home health care, experienced lower costs and 60-day mortality.45 Future research is needed to understand the relationship between access to transitional care services tailored to specific functional impairments and post-AMI mortality among older Black adults.
There are several important issues to consider in the interpretation of our findings. First, the overall percentage of Black participants in this study was small (8.6%). It is notable however that the proportion of Black participants in our study is representative of US demographics.46 Second, AMI participants with the highest level of clinical AMI severity, such as those presenting in cardiogenic shock, were less likely to be represented in our sample. However, rates of inclusion among Black and White persons screened for inclusion were similar. Third, we had limited information about other stressors, such as perceived discrimination and access to care, and other health behaviors in addition to smoking that may contribute to mortality disparities. Fourth, our multivariable model of 6-month mortality considered only baseline characteristics but not medications or procedures received in-hospital. Fifth, the traumatic nature of hospitalization (e.g., lack of sleep, increased time spent in bed) may confound the measurement of functional impairments. However, the timing of baseline assessment was consistent between Black and white participants. Furthermore, we did not collect data on availability or use of inpatient physical therapy, occupational therapy, or ambulatory assistance at sites. Variation in these practices may further confound the measurement of functional impairments during hospitalization. Sixth, we did not include geography in our analysis. Future research should include geographic measures because they are relevant to both the history of systemic racism in the United States and also contemporary social policy.
In conclusion, among older patients hospitalized for AMI, Black older adults presented with a more “geriatric” phenotype, despite being of younger age, with more functional impairments. They also had higher rates of 6-month mortality, which were attenuated substantially after adjustment for demographic and clinical and became non-significant after adjustment for functional variables. Recognizing preexisting disparities in access to services such as home health care, further attention is needed to develop and implement post-hospital care programs that address the specific needs of older Black patients.
Supplementary Material
Supplementary Table S1. Multivariable-Adjusted Odds Ratios for 6-Month Mortality.
Funding Sources:
This research was supported by grant R01 HL115295 from the National Heart, Lung, and Blood Institute. This work was conducted at the Yale Claude D. Pepper Older Americans Independence Center (grant P30 AG021342).
Conflict of Interest
PD reports a fellowship from the National Heart, Lung and Blood Institute (T35HL007649), an American Heart Association Quality of Care and Outcomes Research Scientific Session 2022 travel award, and Roivant Sciences stock options; SC reports grants from the National Heart, Lung, and Blood Institute and Yale Claude D. Pepper Older Americans Independence Center, and personal fees from CVS. AH, CO, and JD have nothing to disclose.
Sponsor’s Role
The sponsors were not involved in the design, methods, subject recruitment, data collections, analysis, or preparation of the paper.
Footnotes
Presentations: Moderated poster at the 2021 American College of Cardiology Scientific Session (Presentation Number: 1022-04).
REFERENCES
- 1.Pandey A, Keshvani N, Khera R, et al. Temporal Trends in Racial Differences in 30-Day Readmission and Mortality Rates After Acute Myocardial Infarction Among Medicare Beneficiaries. JAMA Cardiology. 2020;5(2):136–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen LM, Nallamothu BK, Spertus JA, Tang Y, Chan PS. Racial differences in long-term outcomes among older survivors of in-hospital cardiac arrest. Circulation. 2018;138(16):1643–1650. [DOI] [PubMed] [Google Scholar]
- 3.Polsky D, Jha AK, Lave J, et al. Short- and long-term mortality after an acute illness for elderly whites and blacks. Health Services Research. 2008;43(4):1388–1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barnato AE, Lucas FL, Staiger D, Wennberg DE, Chandra A. Hospital-Level Racial Disparities in Acute Myocardial Infarction Treatment and Outcomes. Medical Care. 2005;43(4):308–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Odden MC, Coxson PG, Moran A, Lightwood JM, Goldman L, Bibbins-Domingo K. The impact of the aging population on coronary heart disease in the United States. Am J Med. 2011;124(9):827–833.e825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Predicting 6-Month Mortality for Older Adults Hospitalized With Acute Myocardial Infarction. Annals of Internal Medicine. 2020;172(1):12–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dodson JA, Geda M, Krumholz HM, et al. Design and rationale of the comprehensive evaluation of risk factors in older patients with AMI (SILVER-AMI) study. BMC Health Serv Res. 2014;14:506–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thygesen K, Alpert JS, Jaffe AS, et al. Third universal definition of myocardial infarction. European Heart Journal. 2012;33(20):2551–2567. [DOI] [PubMed] [Google Scholar]
- 9.Jeste DV, Palmer BW, Appelbaum PS, et al. A New Brief Instrument for Assessing Decisional Capacity for Clinical Research. Archives of General Psychiatry. 2007;64(8):966–974. [DOI] [PubMed] [Google Scholar]
- 10.Kroenke K, Strine TW, Spitzer RL, Williams JB, Berry JT, Mokdad AH. The PHQ-8 as a measure of current depression in the general population. J Affect Disord. 2009;114(1-3):163–173. [DOI] [PubMed] [Google Scholar]
- 11.Ware J Jr., Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34(3):220–233. [DOI] [PubMed] [Google Scholar]
- 12.Sherbourne CD, Stewart AL. The MOS social support survey. Social Science & Medicine. 1991;32(6):705–714. [DOI] [PubMed] [Google Scholar]
- 13.McCarrier KP, Bushnell DM, Martin ML, Paczkowski R, Nelson DR, Buesching D. PRM16 VALIDATION AND PSYCHOMETRIC EVALUATION OF A 5-ITEM MEASURE OF PERCEIVED SOCIAL SUPPORT. Value in Health. 2011;14(3):A148. [Google Scholar]
- 14.Green YS, Hajduk AM, Song X, Krumholz HM, Sinha SK, Chaudhry SI. Usefulness of Social Support in Older Adults After Hospitalization for Acute Myocardial Infarction (from the SILVER-AMI Study). The American Journal of Cardiology. 2020;125(3):313–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brandt J, Spencer M, Folstein M. The Telephone Interview for Cognitive Status. Neuropsychiatry, Neuropsychology, & Behavioral Neurology. 1988;1(2):111–117. [Google Scholar]
- 16.Wang C-Y, Chen L-Y. Grip Strength in Older Adults: Test-Retest Reliability and Cutoff for Subjective Weakness of Using the Hands in Heavy Tasks. Archives of Physical Medicine and Rehabilitation. 2010;91(11):1747–1751. [DOI] [PubMed] [Google Scholar]
- 17.Rantanen T, Guralnik JM, Foley D, et al. Midlife Hand Grip Strength as a Predictor of Old Age Disability. JAMA. 1999;281(6):558–560. [DOI] [PubMed] [Google Scholar]
- 18.Viccaro LJ, Perera S, Studenski SA. Is timed up and go better than gait speed in predicting health, function, and falls in older adults? J Am Geriatr Soc. 2011;59(5):887–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barbeau SRJFH. Locomotor training and the effects of unloading on overground locomotion following stroke. In: Ruper G, ed. New Developments in Stroke Research. Hauppauge, NY: Nova Science Publishers; 2007:127–148. [Google Scholar]
- 20.Aubert CE, Folly A, Mancinetti M, Hayoz D, Donzé JD. Performance-based functional impairment and readmission and death: a prospective study. BMJ Open. 2017;7(6):e016207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Laver K, George S, Ratcliffe J, et al. Use of an interactive video gaming program compared with conventional physiotherapy for hospitalised older adults: a feasibility trial. Disability and Rehabilitation. 2012;34(21):1802–1808. [DOI] [PubMed] [Google Scholar]
- 22.Trombetti A, Hars M, Herrmann F, Rizzoli R, Ferrari S. Effect of a multifactorial fall-and-fracture risk assessment and management program on gait and balance performances and disability in hospitalized older adults: a controlled study. Osteoporosis International. 2013;24(3):867–876. [DOI] [PubMed] [Google Scholar]
- 23.Mangione CM, Lee PP, Pitts J, Gutierrez P, Berry S, Hays RD. Psychometric properties of the National Eye Institute Visual Function Questionnaire (NEI-VFQ). NEI-VFQ Field Test Investigators. Arch Ophthalmol. 1998;116(11):1496–1504. [DOI] [PubMed] [Google Scholar]
- 24.Katz S Assessing self-maintenance: activities of daily living, mobility, and instrumental activities of daily living. J Am Geriatr Soc. 1983;31(12):721–727. [DOI] [PubMed] [Google Scholar]
- 25.Dodson JA, Hajduk AM, Murphy TE, et al. Thirty-Day Readmission Risk Model for Older Adults Hospitalized With Acute Myocardial Infarction. Circulation: Cardiovascular Quality and Outcomes. 2019;12(5):e005320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hajduk AM, Murphy TE, Geda ME, et al. Association Between Mobility Measured During Hospitalization and Functional Outcomes in Older Adults With Acute Myocardial Infarction in the SILVER-AMI Study. JAMA Internal Medicine. 2019;179(12):1669–1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hirsch C, Anderson ML, Newman A, et al. The Association of Race With Frailty: The Cardiovascular Health Study. Annals of Epidemiology. 2006;16(7):545–553. [DOI] [PubMed] [Google Scholar]
- 28.Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56(3):M146–156. [DOI] [PubMed] [Google Scholar]
- 29.Usher T, Buta B, Thorpe RJ Jr., et al. Dissecting the Racial/Ethnic Disparity in Frailty in a Nationally Representative Cohort Study with Respect to Health, Income, and Measurement. The Journals of Gerontology: Series A. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bandeen-Roche K Frailty in Older Adults: A Nationally Representative Profile in the United States. The journals of gerontology Series A, Biological sciences and medical sciences.70(11):1427–1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Geronimus AT. The weathering hypothesis and the health of African-American women and infants: evidence and speculations. Ethn Dis. 1992;2(3):207–221. [PubMed] [Google Scholar]
- 32.Duru OK, Harawa NT, Kermah D, Norris KC. Allostatic load burden and racial disparities in mortality. J Natl Med Assoc. 2012;104(1–2):89–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Geronimus AT, Hicken M, Keene D, Bound J. “Weathering” and age patterns of allostatic load scores among blacks and whites in the United States. Am J Public Health. 2006;96(5):826–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Seeman TE, McEwen BS, Rowe JW, Singer BH. Allostatic load as a marker of cumulative biological risk: MacArthur studies of successful aging. Proceedings of the National Academy of Sciences. 2001;98(8):4770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gruenewald TL, Seeman TE, Karlamangla AS, Sarkisian CA. Allostatic load and frailty in older adults. J Am Geriatr Soc. 2009;57(9):1525–1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Slopen N, Lewis TT, Gruenewald TL, et al. Early life adversity and inflammation in African Americans and whites in the midlife in the United States survey. Psychosom Med. 2010;72(7):694–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gaddey HL, Holder K. Unintentional weight loss in older adults. Am Fam Physician. 2014;89(9):718–722. [PubMed] [Google Scholar]
- 38.O’Keefe EB, Meltzer JP, Bethea TN. Health Disparities and Cancer: Racial Disparities in Cancer Mortality in the United States, 2000–2010. Frontiers in Public Health. 2015;3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Campbell NL, Boustani MA, Skopelja EN, Gao S, Unverzagt FW, Murray MD. Medication Adherence in Older Adults With Cognitive Impairment: A Systematic Evidence-Based Review. The American Journal of Geriatric Pharmacotherapy. 2012;10(3):165–177. [DOI] [PubMed] [Google Scholar]
- 40.Szanton SL, Leff B, Wolff JL, Roberts L, Gitlin LN. Home-Based Care Program Reduces Disability And Promotes Aging In Place. Health Affairs. 2016;35(9):1558–1563. [DOI] [PubMed] [Google Scholar]
- 41.Khosravi P, Ghapanchi AH. Investigating the effectiveness of technologies applied to assist seniors: A systematic literature review. International Journal of Medical Informatics. 2016;85(1):17–26. [DOI] [PubMed] [Google Scholar]
- 42.Fotteler ML, Mühlbauer V, Brefka S, et al. The Effectiveness of Assistive Technologies for Older Adults and the Influence of Frailty: Systematic Literature Review of Randomized Controlled Trials. JMIR Aging. 2022;5(2):e31916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Smith JM, Jarrín OF, Lin H, Tsui J, Dharamdasani T, Thomas-Hawkins C. Racial Disparities in Post-Acute Home Health Care Referral and Utilization among Older Adults with Diabetes. International Journal of Environmental Research and Public Health. 2021;18(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Buurman BM, Parlevliet JL, Allore HG, et al. Comprehensive Geriatric Assessment and Transitional Care in Acutely Hospitalized Patients: The Transitional Care Bridge Randomized Clinical Trial. JAMA Internal Medicine. 2016;176(3):302–309. [DOI] [PubMed] [Google Scholar]
- 45.Bindman AB, Cox DF. Changes in Health Care Costs and Mortality Associated With Transitional Care Management Services After a Discharge Among Medicare Beneficiaries. JAMA Internal Medicine. 2018;178(9):1165–1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ortman JMV, Victoria A.; Hogan, Howard An Aging Nation: The Older Population in the United States. U.S. Census Bureau;2014. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table S1. Multivariable-Adjusted Odds Ratios for 6-Month Mortality.


