Abstract
Background & Aims
EASL guidelines recommend 8 weeks of treatment with sofosbuvir plus velpatasvir (SOF/VEL) for the treatment of acute or recently acquired HCV infection, but only 6- and 12-week data are available. Therefore, the aim of this study was to evaluate the safety and efficacy of a shortened 8-week SOF/VEL treatment for acute HCV monoinfection.
Methods
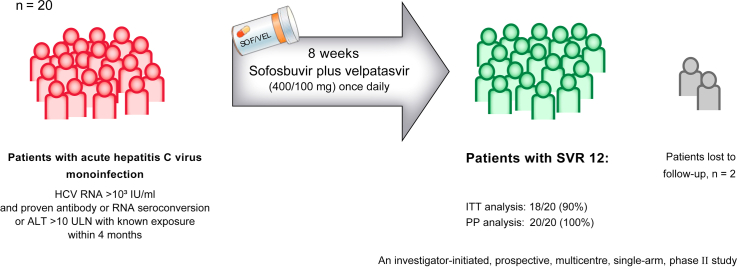
In this investigator-initiated, prospective, multicentre, single-arm study, we recruited 20 adult patients with acute HCV monoinfection from nine centers in Germany. Patients received SOF/VEL (400/100 mg) as a fixed-dose combination tablet once daily for 8 weeks. The primary efficacy endpoint was the proportion of patients with sustained virological response 12 weeks after the end of treatment (SVR12).
Results
The median HCV RNA viral load at baseline was 104,307 IU/ml; the distribution of HCV genotypes was as follows: GT1a/1b/2/3/4: n = 12/1/1/3/3. Thirteen (65%) of the 20 patients were taking medication for HIV pre-exposure prophylaxis. SVR12 was achieved in all patients who complied with the study protocol (n = 18/18 [100%], per protocol analysis), but the primary endpoint was not met in the intention-to-treat analysis (n = 18/20 [90%]) because two patients were lost to follow-up. One serious adverse event (unrelated to study drug) occurred during 12 weeks of post-treatment follow-up.
Conclusions
The 8-week treatment with SOF/VEL was well tolerated and highly effective in all adherent patients with acute HCV monoinfection. Early treatment of hepatitis C might effectively prevent the spread of HCV in high-risk groups.
Clinical Trial Number
Impact and implications
The HepNet acute HCV-V study (NCT03818308), an investigator-initiated, single-arm, multicenter pilot study, demonstrates the efficacy and safety of 8 weeks of daily treatment with the fixed-dose combination sofosbuvir/velpatasvir (400/100 mg) in patients with acute hepatitis C virus (HCV) infection. All patients who completed therapy and were followed-up achieved sustained virologic response. Thus, early treatment with SOF/VEL which might effectively prevent the spread of HCV in high-risk groups can be recommended for patients with acute HCV monoinfection.
Keywords: acute HCV infection, direct-acting antivirals, hepatitis C elimination, recently acquired infection
Abbreviations: ALT, alanine aminotransferase; HCV, hepatitis C virus; ITT, intention-to-treat; LLOQ, lower limit of quantification; PP, per protocol; SVR, sustained virologic response; ULN, upper limit of normal
Graphical abstract
Highlights
-
•
Sofosbuvir/velpatasvir for 8 weeks is effective in patients with acute hepatitis C, even those with high ALT levels.
-
•
Treatment of acute hepatitis C with sofosbuvir/velpatasvir was well tolerated.
-
•
Availability of a safe and effective treatment for acute HCV infection supports a simplified treatment cascade.
Introduction
Hepatitis C virus (HCV) infection is one of the leading global health problems. According to the WHO, there are an estimated 58 million chronically infected people worldwide and about 1.5 million new infections per year, resulting in an annual rate of more than 250,000 deaths (https://www.who.int/news-room/fact-sheets/detail/hepatitis-c).
Direct-acting antivirals are now available, and combination therapy of NS5A inhibitors with either a protease inhibitor or polymerase inhibitor results in sustained virologic response (SVR) in more than 95% of individuals with chronic hepatitis C.1 Therefore, it is theoretically possible to eradicate HCV infection globally, and the WHO has accordingly set elimination targets of a 90% reduction in HCV incidence by 2030 compared to 2015 baselines, resulting in a 65% reduction in HCV-related mortality.2 However, despite these successes in treating chronic hepatitis C, major challenges in the cascade of care must be overcome if we are to achieve HCV elimination. One of the main barriers is early diagnosis and treatment of high-risk groups, such as people who inject drugs or men who have sex with men, who contribute significantly to the ongoing transmission of HCV. As a result, more new acute HCV infections than cured chronic infections are observed in certain high-exposure risk populations, particularly because of high transmission and reinfection rates in these high-risk groups.3,4 Thus, if people with high-risk behaviors are not treated immediately and treatment programs are not scaled up, they will transmit HCV to many more individuals, and reinfections of already cured individuals will continue to occur.[5], [6], [7]
Since there is currently no prophylactic HCV vaccine, early treatment of high-risk groups is likely to be an important aspect of elimination strategies.8,9 Indeed, early treatment of acute hepatitis C has been shown to be safe and almost always to prevent chronic progression in numerous studies, including those conducted when interferon-alfa was still used for treatment.[10], [11], [12], [13], [14], [15], [16], [17] However, many studies on this topic have also been heterogeneous, with mixed populations of HCV-monoinfected or HCV/HIV-coinfected people and inconsistent definitions of acute HCV infection, with people often classified as having recently acquired and early chronic hepatitis C (e.g. infection for less than 12 months).14,15 Nevertheless, based on available data, the EASL guidelines recommend early treatment of HCV infection (acute or recently acquired) with pan-genotypic DAA therapy for 8 weeks with either glecaprevir/pibrentasivr (GLE/PIB) or sofosbuvir/velpatasvir (SOF/VEL).1 The efficacy of 8 weeks of GLE/PIB should be >95% based on data in individuals with chronic hepatitis C18 and a study reported high SVR rates of 96% even with 6 weeks of GLE/PIB in people with recent HCV infection without any safety concerns.15 For SOF/VEL, 6- and 12-week treatment regimens have been studied14 but not 8-week regimens as recommended by the EASL guidelines.1 In addition, treatment of acute HCV infection is not included in the marketing authorization label. Thus, the acute HCV-V study fills an important gap and provides data on the safety and efficacy of 8 weeks of SOF/VEL in people with acute hepatitis C without HIV coinfection.
Patients and methods
Study design and patients
The investigator-initiated HepNet acute HCV-V study was designed as a phase II, national, multicentre, prospective, single-arm pilot study and was coordinated by the HepNet Study-House, a project of the German Liver Foundation, funded by the German Center for Infection Research (DZIF). The trial was conducted at nine clinical sites in Germany. Each patient included in the study gave written informed consent. Detailed inclusion and exclusion criteria are listed in table S1. Briefly, eligible patients were at least 18 years of age with an acute HCV monoinfection, confirmed by HCV RNA levels >103 IU/ml at the time of the screening. Acute HCV infection was defined as documented seroconversion to HCV antibody within 4 months before screening or documented conversion to HCV RNA positivity 4 months before screening or a known or suspected exposure to HCV with raised alanine aminotransferase (ALT) concentration more than 10x the upper limit of normal (ULN) within 4 months preceding screening. Key exclusion criteria were presence of cirrhosis, clinical hepatic decompensation, solid organ transplantation, infection with HIV, uncontrolled drug abuse and clinically significant illness that might interfere with study treatment, assessment, or compliance with the protocol. Other causes of liver disease were excluded by standard clinical and laboratory criteria. Coinfection with HIV, significant illnesses other than HCV and any contraindication to the intake of SOF/VEL were exclusion criteria.
Procedures
After screening for inclusion and exclusion criteria, patients received SOF/VEL (dose 400/100 mg) once daily for 8 weeks in an open label one-arm trial design with a subsequent 12 weeks follow-up. Study visits occurred at screening, baseline, week 2, week 4, week 8 (end of treatment) of antiviral treatment and 12 weeks after the end of therapy. Blood and serum samples were obtained at each study visit and HCV RNA was quantified in the central laboratory of Hannover Medical School using the Hologic Aptima® HCV Quant Assay with a lower limit of quantification (LLOQ) of 10 IU/ml. Biochemical responses (alanine aminotransferase, aspartate aminotransferase, gamma-glutamyltransferase) and HCV genotype were assessed by the local laboratory of each center in serum samples using standard assays on automated platforms.
Outcomes
The primary efficacy endpoint was defined as the proportion of patients with HCV RNA below the LLOQ 12 weeks after stopping treatment. Secondary endpoints were virologic and biochemical kinetics at baseline, week 2, week 4, week 8, and week 12, and the proportion of patients achieving ALT normalization (ALT below the ULN) at 8 weeks of therapy and 12 weeks after the end of therapy. In addition, the safety and tolerability of SOF/VEL were evaluated based on an analysis of documented adverse events and serious adverse events. The study procedures and safety assessment are described in detail in the study protocol.
Ethics
The study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki as reflected in a priori approval by the institution´s human research committee (Hannover Medical School ethics committee, vote no. 8282_AMG_M_2019). The protocol was registered at ClinicalTrial.gov (NCT03818308).
Statistical analyses
This study was planned as a pilot trial with 20 patients and under the assumption that the proportion of patients who achieve a response will be close to 100% based on previous acute HCV-IV study data.12 The study is considered successful if the lower bound of the 95% Wilson CI is greater than 83%. The study had a power of 82% if the true response rate is greater than 98%. Baseline characteristics are reported as absolute and relative frequencies for categorical data and mean and SD for continuous data (except quantitative HCV RNA and liver enzymes). The primary analysis was conducted within the intention-to-treat (ITT) population (all patients who received at least one dose of study drug). The sensitivity analysis was conducted in the per protocol (PP) population (all patients that completed the study in accordance with the study protocol). Safety was assessed in all patients who received at least one dose of study drug. All analyses were performed with SAS version 9.4.
Patients with missing values for the primary endpoint SVR12 were counted as treatment failures for the ITT analysis. These patients were hence counted as patients who did not show a sustained viral response. All other missing virology values were imputed with locally assessed data. Patients with HCV RNA viral loads greater than or equal to the LLOQ were counted as HCV RNA positive and patients with HCV RNA viral loads below the LLOQ were counted as HCV RNA negative.
Results
Study cohort
Between March 2019 and June 2021, 31 patients were screened, and 20 patients entered the study. Eleven patients were ineligible at screening: five patients had HCV RNA viral load <10³ IU/ml at screening, five patients had no sufficient evidence of acute HCV infection, as defined in the inclusion criteria, and one patient had to be excluded due to COVID-19 restrictions at the respective study site (Fig. S1). Enrolled patients were predominantly men (95%), had a mean age of 37.4 years (SD 9.3) and most had HCV genotype 1a infection (60%). Two-thirds of the patients (n = 13 [65%]) were taking oral HIV pre-exposure prophylaxis with emtricitabine/tenofovir disoproxil and most patients had a primary acute HCV infection (95%) (Table 1). The exact route of transmission was not assessed in our study and information about any risk behavior could only be assumed due to concomitant medications like pre-exposure prophylaxis and drug substitutes.
Table 1.
Baseline characteristics.
| Patients (n = 20) | |
|---|---|
| Men | 19 (95%) |
| Age [years] | 37.4±9.3 |
| BMI [kg/m2] | 22.8 ±2.8 |
| HCV RNA [IU/ml] | 104,307 (7,842–1,726,734) |
| <50,000 | 8 (40%) |
| <10 | 2 (10%) |
| HCV genotype | |
| 1a | 12 (60%) |
| 1b | 1 (5%) |
| 2 | 1 (5%) |
| 3 | 3 (15%) |
| 4 | 3 (15%) |
| Patients with oral HIV pre-exposure prophylaxis | 13 (65%) |
| Patients with substitution treatment | 2 (10%) |
| Patients with confirmed HCV reinfection | 1 (5%) |
| Liver enzymes | |
| Alanine aminotransferase [U/L] | 249 (165–463) |
| Aspartate aminotransferase [U/L] | 133 (71–219) |
| Bilirubin [U/L] | 12.0 (8.0–17.1) |
| Gamma-glutamyltransferase [U/L] | 93 (52–160) |
Values as median (interquartile range) or mean ± SD.
Within the 4 months preceding screening, 10 patients had a documented seroconversion to HCV antibody (anti-HCV) positivity, four patients had a documented conversion to HCV RNA positivity and 10 patients had a known or suspected exposure to HCV with a documented peak ALT of more than 10x the ULN (median 719 U/L, IQR 590–988). In addition to anti-HCV seroconversion or new detection of HCV RNA, four patients also experienced peak ALT more than 10x the ULN with a documented infection event.
Median HCV RNA viral load at baseline was 104,307 IU/ml (IQR 7,842–1,726,734). Two patients had a viral load that was below the LLOQ at baseline but with HCV RNA >10³ IU/ml at screening visit (measured at local study sites according to inclusion criteria). We did not exclude these individuals because it has been reported that chronic HCV infection can develop despite intermittent HCV RNA negativity.19 Centrally measured HCV RNA levels were 1,426 IU/ml and 331 IU/ml for these two patients at screening. Four (20%) patients had jaundice with bilirubin concentration more than 1.5x the ULN at screening. Median ALT levels were 249 U/L (IQR 165–463).
Treatment efficacy
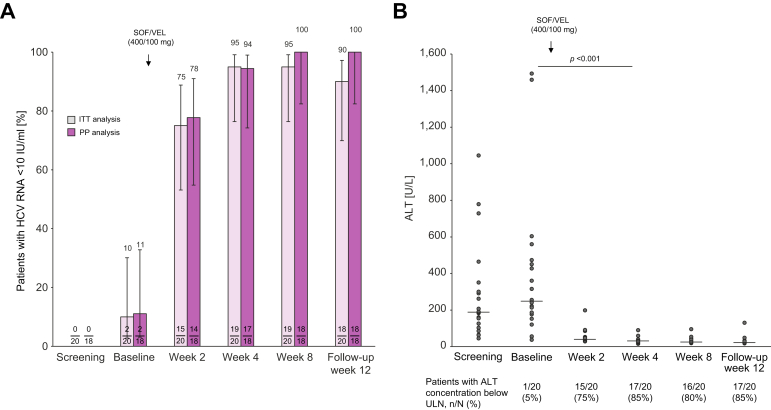
The mean time between first diagnosis of acute hepatitis C and start of antiviral therapy was 43.2 days (SD 25.6). In the ITT analysis (n = 20), HCV RNA levels were <10 IU/ml (LLOQ) in 19 patients (95%) after 4 weeks of antiviral treatment, in 19 patients (95%) at the end of therapy (treatment week 8), and in 18 patients (90%) after the 12-week follow-up period. The primary endpoint (ITT analysis, SVR12 100%, Wilson interval >83%) was not met, as the rate of SVR12 in the ITT analysis was 90% (95% Wilson interval CI [69.9%–97.2%]). During the study, two patients were lost to follow-up: one patient after week 4 of antiviral treatment and one patient during the 12-week follow-up period. Of note, both patients had HCV RNA levels below the LLOQ at their last study visit. In the PP analysis (n = 18), excluding the two patients lost-to-follow-up, HCV RNA levels were <10 IU/ml (LLOQ) in 17 patients (94%) after 4 weeks of treatment, and in all 18 patients at the end of treatment and 12 weeks after end of treatment. Thus, SVR12 was 100% (95% Wilson interval CI [82.41–100.00%]) in the PP analysis (Fig. 1).
Fig. 1.
Virological and biochemical response.
(A) Response to antiviral treatment by patients with serum HCV RNA levels <10 IU/ml LLOQ in ITT and PP analysis. Two patients were lost to follow-up. Percentage of patients ±95 Wilson confidence interval. (B) Decline of ALT by scatter plot with median line; Friedman test with post hoc analysis (n = 20). ALT, alanine aminotransferase; ITT, intention-to-treat; PP, per protocol; SOF/VEL, sofosbuvir/velpatasvir; ULN, upper limit of normal.
Biochemical response was rapid, with ALT concentrations declining into the normal range in 17 (85%) of 20 patients within the first 4 weeks of treatment, in 16 (80%) of 20 patients within the 8-week treatment period, and in 17 (85%) of 20 patients by follow-up week 12 (Fig. 1). One patient had abnormal ALT concentration during the whole study (ALT declined from baseline to week 2 by 62%, from 241 IU/ml to 91 IU/ml) but with persistently high ALT concentrations between 90 IU/ml and 131 IU/ml up to the end of follow-up. Bilirubin was above the ULN in four of 20 patients at baseline and normalized in all patients during the 8-week treatment period.
The individual HCV RNA and biochemical kinetics during the entire study period are displayed in Table S2.
Treatment safety
Treatment of acute hepatitis C with SOF/VEL was well tolerated. By 12 weeks post treatment, 28 adverse events were reported in total. Only six of these were reported as possible or probable drug-related adverse events, with very mild symptoms that occurred during medical treatment: 2/20 [10%] patients reported skin irritations (one twice), 1/20 [5%] patients reported sleeping disorders, 1/20 [5%] patients reported flatulence and 1/20 [5%] patients reported headache. There was only one serious adverse event that was classified as unrelated to the study drug; this patient presented with unusual behavior at the study site and was hospitalized due to toxicity to various agents (suspected intoxication with ketamine, benzodiazepines, and amphetamines based on external history). Overall, there were no significant changes in creatinine clearance, serum lipase concentration, or other safety-related biochemical or hematologic measures during or after therapy.
Discussion
There is still some controversy about whether patients with acute hepatitis C should be treated early because treatment may be unnecessary, leading to avoidable costs and side effects, as 20-50% of patients spontaneously eliminate HCV. However, studies have shown that an unrestricted access to early treatment leads to a substantial decline in new HCV infections in high-risk settings.8 In addition, it has been suggested that immediate treatment of acute HCV infection can actually be cost-effective.20 Therefore, we believe early treatment of acute HCV infection is an important tool to achieve WHO elimination goals. Current guidelines indeed recommend treatment of patients with acute or recently acquired HCV infection,1 although this is not included in the marketing authorization label of any EMA- and FDA-approved DAAs. Thus, safety and efficacy data in this setting remain important for possible indication expansion.
Our prospective study supports the EASL recommendation of 8 weeks SOF/VEL for patients with acute or recently acquired HCV infection. It is important to note that we only enrolled patients with acute HCV infection based on a very strict definition. This subsequently restricted our recruitment target to only 20 patients, which is a limitation of this study. Nevertheless, the definition of acute HCV infection in our and other studies cannot always reliably exclude chronic HCV infection. Fourteen patients had documented anti-HCV seroconversion or conversion to HCV RNA positivity, but in six patients with a 10-fold ALT elevation and known/suspected HCV exposure, we cannot be 100% certain of acute HCV infection because of the absence of HCV RNA testing in the past. Because of this limited sample size, the study narrowly failed to meet the primary endpoint (100% SVR, Wilson interval >83%), although no relapse occurred, because two patients were lost to follow-up. Nevertheless, it is important for the consideration of the extension of approval that we also have safety data on DAA therapy in patients with acute infection. From this study and the earlier acute HCV-IV study in 20 patients with acute HCV treated with sofosbuvir/ledipasvir,12 we can conclude that treatment with sofosbuvir plus a NS5A inhibitor is safe in patients with acute hepatitis C and high ALT, some even with elevated bilirubin. Importantly, early treatment with SOF/VEL was not only safe in patients with severe hepatitis activity, but also resulted in very rapid improvement of liver enzymes within a few weeks. Thus, early treatment likely shortened the duration of symptomatic disease, which could have major relevance for individual patients.
The high efficacy of 100% in the PP analysis in this study is not surprising and confirms the results of other studies12,13,[15], [16], [17] showing that a short duration of therapy is feasible in recently infected patients. Nevertheless, this confirmation is important because there was theoretical concern that 8 weeks of SOF/VEL was not sufficient, as 12 weeks is the standard treatment for patients with chronic hepatitis C and 6 weeks of therapy was associated with a higher relapse rate in a randomized trial of 188 patients with recently acquired hepatitis C that was published by Matthews et al. last year.14 However, it is important to note that unlike the authors of this paper,14 we did not include HIV-coinfected patients nor those with a duration of infection longer than 6 months, who still fall within the definition of recently acquired infection but do not meet the criteria for acute HCV infection. These patients may respond differently but must also be considered when making a clear recommendation for 8 weeks of SOF/VEL. One additional factor that may be associated with a suboptimal response after shorter treatment in this setting is very high baseline HCV RNA.21
Therefore, to avoid consideration of all these variables that might mitigate against shortened therapy in acute or recently acquired HCV infection, we propose that the word "chronic" simply be removed from the label of pan-genotypic DAA therapies so that all patients with HCV infection can be treated immediately, regardless of the stage of infection. This would greatly simplify treatment decisions, which is critical to achieving elimination goals. In addition, immediate treatment of acute HCV infection may reduce the risk of losing patients during follow-up, which is quite common in high-risk groups and in acute HCV infection.11 Even in our controlled trial with patients being treated by experienced centers and close monitoring, the lost-to-follow-up rate was still 10%. However, it is quite possible that all patients, including the lost-to-follow-up cases, have eliminated HCV, since they have received the therapy for a few weeks and even 4 weeks DAA therapy may be effective in a substantial number of patients.22
Finally, another important issue for HCV elimination that needs to be discussed in this context is the development of a prophylactic HCV vaccine,23 which we believe is also an important concept for reaching HCV elimination goals. The development of such a vaccine is challenging due to the lack of suitable animal models. Prospective trials in high-risk persons have been shown to be feasible but require huge efforts and resources and take quite some time.24 Therefore, a controlled human infection model such as those used for Influenza A or SARS-CoV-2 infections[25], [26], [27] would be a possibility for testing an early-stage HCV vaccine, and this is currently being discussed by renowned experts as a great opportunity to accelerate HCV vaccine development.28 It is essential for the discussion to have an effective and safe back-up treatment as early as possible in case the tested vaccine fails to prevent HCV infection. Therefore, the data from this study are also relevant to this discussion.
In conclusion, our data confirm that early treatment of acute HCV infection is safe and effective, and this supports current guideline recommendations for the treatment of acute or recently acquired HCV infection. These data are important as they support simplification of the treatment cascade, e.g. by removing “chronic” from the label of current pan-genotypic DAA regimens, and the consideration of controlled human infection models to accelerate HCV vaccine development.
Financial support
The study was funded by a grant of the DZIF (German Center for Infection Research, TTU Hepatitis) to the HepNet Study-House of the German Liver Foundation (DLS) and Gilead Sciences. The HepNet aHCV-V Study is an investigator-initiated trial. The HepNet Study-House coordinated the study. Gilead Sciences contributed with financial funding and provision of study medication. Gilead had no influence on the planning, conduct or analyis of the study.
Authors’ contributions
The study was designed and protocol was written by MC, BM, HW, MvK and MPM; the study was coordinated by MC, BM, JK, PD; patients were recruited and treated by MC, BM, PI, CDS, CC, HJS, JSzW, SS, KD, TM; data analysis and statistics were performed by MvK, MC, BM, JK. Drafting of the manuscript were done by MC, BM, PI, MvK, JK, PD and HW, critical revision of the manuscript was performed by all authors. MC, BM, MvK and JK has access to all data and can vouch for integrity of data analysis.
Data availability statement
All data is available from the corresponding authors upon special request.
Conflicts of interest
The authors declare no conflict of interest concerning the content of this manuscript.
Please refer to the accompanying ICMJE disclosure forms for further details.
Acknowledgements
The HepNet Study-House supported central study facilities, data acquisition and study management. Gilead Sciences provided medication and financial support. The legal sponsor of the study was Hannover Medical School. We thank all patients for taking part in this study. We thank all colleagues for their outstanding support during all phases of the study.
Footnotes
Author names in bold designate shared co-first authorship
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jhepr.2022.100650.
Supplementary data
The following are the supplementary data to this article:
References
- 1.European Association for the Study of the Liver EASL recommendations on treatment of hepatitis C: final update of the series☆. J Hepatol. 2020;73:1170–1218. doi: 10.1016/j.jhep.2020.08.018. [DOI] [PubMed] [Google Scholar]
- 2.Cooke G.S., Andrieux-Meyer I., Applegate T.L., Atun R., Burry J.R., Cheinquer H., et al. Accelerating the elimination of viral hepatitis: a lancet Gastroenterology & Hepatology commission. Lancet Gastroenterol Hepatol. 2019;4:135–184. doi: 10.1016/S2468-1253(18)30270-X. [DOI] [PubMed] [Google Scholar]
- 3.Rossi C., Butt Z.A., Wong S., Buxton J.A., Islam N., Yu A., et al. Hepatitis C virus reinfection after successful treatment with direct-acting antiviral therapy in a large population-based cohort. J Hepatol. 2018;69:1007–1014. doi: 10.1016/j.jhep.2018.07.025. [DOI] [PubMed] [Google Scholar]
- 4.Ingiliz P., Martin T.C., Rodger A., Stellbrink H.-J., Mauss S., Boesecke C., et al. HCV reinfection incidence and spontaneous clearance rates in HIV-positive men who have sex with men in Western Europe. J Hepatol. 2017;66:282–287. doi: 10.1016/j.jhep.2016.09.004. [DOI] [PubMed] [Google Scholar]
- 5.Martin N.K., Jansen K., An der Heiden M., Boesecke C., Boyd A., Schewe K., et al. Eliminating hepatitis C virus among human immunodeficiency virus-infected men who have sex with men in Berlin: a modeling analysis. J Infect Dis. 2019;220:1635–1644. doi: 10.1093/infdis/jiz367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mata-Marín J.A., de Pablos-Leal A.A., Mauss S., Arroyo-Anduiza C.I., Rodríguez-Evaristo M.S., Uribe-Noguéz L.A., et al. Risk factors for HCV transmission in HIV-positive men who have sex with men in México. PLoS One. 2022;17 doi: 10.1371/journal.pone.0269977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hosseini-Hooshyar S., Hajarizadeh B., Bajis S., Law M., Janjua N.Z., Fierer D.S., et al. Risk of hepatitis C reinfection following successful therapy among people living with HIV: a global systematic review, meta-analysis, and meta-regression. Lancet HIV. 2022;9:e414–e427. doi: 10.1016/S2352-3018(22)00077-7. [DOI] [PubMed] [Google Scholar]
- 8.Boerekamps A., van den Berk G.E., Lauw F.N., Leyten E.M., van Kasteren M.E., van Eeden A., et al. Declining hepatitis C virus (HCV) incidence in Dutch human immunodeficiency virus-positive men who have sex with men after unrestricted access to HCV therapy. Clin Infect Dis. 2018;66:1360–1365. doi: 10.1093/cid/cix1007. [DOI] [PubMed] [Google Scholar]
- 9.Day E., Hellard M., Treloar C., Bruneau J., Martin N.K., Øvrehus A., et al. Hepatitis C elimination among people who inject drugs: challenges and recommendations for action within a health systems framework. Liver Int. 2019;39:20–30. doi: 10.1111/liv.13949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jaeckel E., Cornberg M., Wedemeyer H., Santantonio T., Mayer J., Zankel M., et al. Treatment of acute hepatitis C with interferon alfa-2b. N Engl J Med. 2001;345:1452–1457. doi: 10.1056/NEJMoa011232. [DOI] [PubMed] [Google Scholar]
- 11.Deterding K., Grüner N., Buggisch P., Wiegand J., Galle P.R., Spengler U., et al. Delayed versus immediate treatment for patients with acute hepatitis C: a randomised controlled non-inferiority trial. Lancet Infect Dis. 2013;13:497–506. doi: 10.1016/S1473-3099(13)70059-8. [DOI] [PubMed] [Google Scholar]
- 12.Deterding K., Spinner C.D., Schott E., Welzel T.M., Gerken G., Klinker H., et al. Ledipasvir plus sofosbuvir fixed-dose combination for 6 weeks in patients with acute hepatitis C virus genotype 1 monoinfection (HepNet Acute HCV IV): an open-label, single-arm, phase 2 study. Lancet Infect Dis. 2017;17:215–222. doi: 10.1016/S1473-3099(16)30408-X. [DOI] [PubMed] [Google Scholar]
- 13.Chromy D., Mandorfer M., Bucsics T., Schwabl P., Scheiner B., Schmidbauer C., et al. High efficacy of interferon-free therapy for acute hepatitis C in HIV-positive patients. United Eur Gastroenterol J. 2019;7:507–516. doi: 10.1177/2050640619835394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Matthews G.V., Bhagani S., van der Valk M., Rockstroh J., Feld J.J., Rauch A., et al. Sofosbuvir/velpatasvir for 12 vs. 6 weeks for the treatment of recently acquired hepatitis C infection. J Hepatol. 2021;75:829–839. doi: 10.1016/j.jhep.2021.04.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Martinello M., Orkin C., Cooke G., Bhagani S., Gane E., Kulasegaram R., et al. Short-duration pan-genotypic therapy with glecaprevir/pibrentasvir for 6 Weeks among people with recent hepatitis C viral infection. Hepatology. 2020;72:7–18. doi: 10.1002/hep.31003. [DOI] [PubMed] [Google Scholar]
- 16.Boerekamps A., De Weggheleire A., van den Berk G.E., Lauw F.N., Claassen M.A.A., Posthouwer D., et al. Treatment of acute hepatitis C genotypes 1 and 4 with 8 weeks of grazoprevir plus elbasvir (DAHHS2): an open-label, multicentre, single-arm, phase 3b trial. Lancet Gastroenterol Hepatol. 2019;4:269–277. doi: 10.1016/S2468-1253(18)30414-X. [DOI] [PubMed] [Google Scholar]
- 17.Naggie S., Fierer D.S., Hughes M.D., Kim A.Y., Luetkemeyer A., Vu V., et al. Ledipasvir/sofosbuvir for 8 Weeks to treat acute hepatitis C virus infections in men with human immunodeficiency virus infections: sofosbuvir-containing regimens without interferon for treatment of acute HCV in HIV-1 infected individuals. Clin Infect Dis. 2019;69:514–522. doi: 10.1093/cid/ciy913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lampertico P., Carrión J.A., Curry M., Turnes J., Cornberg M., Negro F., et al. Real-world effectiveness and safety of glecaprevir/pibrentasvir for the treatment of patients with chronic HCV infection: a meta-analysis. J Hepatol. 2020;72:1112–1121. doi: 10.1016/j.jhep.2020.01.025. [DOI] [PubMed] [Google Scholar]
- 19.Bunchorntavakul C., Jones L.M., Kikuchi M., Valiga M.E., Kaplan D.E., Nunes F.A., et al. Distinct features in natural history and outcomes of acute hepatitis C. J Clin Gastroenterol. 2015;49:e31–e40. doi: 10.1097/MCG.0000000000000076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bethea E.D., Chen Q., Hur C., Chung R.T., Chhatwal J. Should we treat acute hepatitis C? A decision and cost-effectiveness analysis. Hepatology. 2018;67:837–846. doi: 10.1002/hep.29611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rockstroh J.K., Bhagani S., Hyland R.H., Yun C., Dvory-Sobol H., Zheng W., et al. Ledipasvir-sofosbuvir for 6 weeks to treat acute hepatitis C virus genotype 1 or 4 infection in patients with HIV coinfection: an open-label, single-arm trial. Lancet Gastroenterol Hepatol. 2017;2:347–353. doi: 10.1016/S2468-1253(17)30003-1. [DOI] [PubMed] [Google Scholar]
- 22.Madsen L.W., Christensen P.B., Hansen J.F., Røge B.T., Holm D.K., Dröse S., et al. Four weeks treatment with glecaprevir/pibrentasvir + ribavirin-A randomized controlled clinical trial. Viruses. 2022;14:614. doi: 10.3390/v14030614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cox A.L., El-Sayed M.H., Kao J.-H., Lazarus J.V., Lemoine M., Lok A.S., et al. Progress towards elimination goals for viral hepatitis. Nat Rev Gastroenterol Hepatol. 2020;17:533–542. doi: 10.1038/s41575-020-0332-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Page K., Melia M.T., Veenhuis R.T., Winter M., Rousseau K.E., Massaccesi G., et al. Randomized trial of a vaccine regimen to prevent chronic HCV infection. N Engl J Med. 2021;384:541–549. doi: 10.1056/NEJMoa2023345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shah S.K., Miller F.G., Darton T.C., Duenas D., Emerson C., Lynch H.F., et al. Ethics of controlled human infection to address COVID-19. Science. 2020;368:832–834. doi: 10.1126/science.abc1076. [DOI] [PubMed] [Google Scholar]
- 26.Killingley B., Mann A.J., Kalinova M., Boyers A., Goonawardane N., Zhou J., et al. Safety, tolerability and viral kinetics during SARS-CoV-2 human challenge in young adults. Nat Med. 2022;28:1031–1041. doi: 10.1038/s41591-022-01780-9. [DOI] [PubMed] [Google Scholar]
- 27.Han A., Czajkowski L., Rosas L.A., Cervantes-Medina A., Xiao Y., Gouzoulis M., et al. Safety and efficacy of CR6261 in an Influenza A H1N1 healthy human challenge model. Clin Infect Dis. 2021;73:e4260–e4268. doi: 10.1093/cid/ciaa1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liang T.J., Feld J.J., Cox A.L., Rice C.M. Controlled human infection model — fast track to HCV vaccine? New Engl J Med. 2021;385:1235–1240. doi: 10.1056/NEJMsb2109093. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data is available from the corresponding authors upon special request.