Abstract
Sepsis is a severe inflammatory disease syndrome caused by the dysregulated host response to infection. Neutrophils act as the first line of defense against pathogens by releasing effector molecules such as reactive oxygen species (ROS), myeloperoxidase (MPO), and neutrophil extracellular traps (NETs). However, uncontrolled activation of neutrophils and extensive release of effector molecules often cause a “friendly fire” to damage organ systems. Although neutrophils are considered a short-lived, terminally differentiated homogeneous population, recent studies have revealed its heterogeneity comprising different subsets or states implicated in sepsis pathophysiology. Besides the well-known N1 and N2 subsets of neutrophils, several new subsets including aged, antigen-presenting, reverse-migrated, ICAM-1+, low-density, OLFM4+, and Siglec-F+ neutrophils have been reported. These neutrophils potentially contribute to the pathogenesis of sepsis based on their pro-inflammatory and immunosuppressive functions. Damage-associated molecular patterns (DAMPs) are endogenous molecules to induce inflammation by stimulating pattern recognition receptors (PRRs) on immune cells. Different kinds of DAMPs have been shown to contribute to sepsis pathophysiology, including extracellular cold-inducible RNA-binding protein (eCIRP), high mobility group box 1 (HMGB1), extracellular histones, and heat shock proteins (HSP). In this review, we summarize the different subsets of neutrophils and their association with sepsis and discuss the novel roles of DAMPs on neutrophil heterogeneity.
Keywords: Sepsis, Neutrophil, DAMPs, eCIRP, HMGB1, NETs, Inflammation
Introduction
Sepsis is a life-threatening organ dysfunction caused by the maladaptive immune response to infection (1). The innate immune system is robustly activated, especially in the acute phase of sepsis (2, 3). Neutrophils comprise most of the leukocytes in our body and play a critical role in host defense against pathogens. With their arsenals such as neutrophil elastase (NE), myeloperoxidase (MPO), reactive oxygen species (ROS), and neutrophil extracellular traps (NETs), neutrophils combat and subsequently clear the pathogens by phagocytosis (4). On the other hand, aberrant effector functions of neutrophils can cause organ dysfunctions and death in sepsis (5). Although it is initially thought of as a terminally differentiated homogeneous cell population with a short life span, emerging evidence demonstrates neutrophils consist of several subtypes exhibiting extended half-lives (6, 7). Neutrophil subtypes contribute to sepsis differently based on their pro-inflammatory or immunosuppressive activities. As neutrophils are indispensable to combat infection and neutropenia instead worsens sepsis outcomes, identifying detrimental subsets of neutrophils as specific targets may pave the way for developing a novel therapy against dysregulated neutrophil activities in sepsis.
Cellular stress leading to cell death is a common feature of sepsis (8). Damage-associated molecular patterns (DAMPs) are endogenous molecules released from damaged cells to induce inflammation by stimulating pattern recognition receptors (PRRs). DAMPs can be actively secreted by exocytosis of exosomes and lysosomes or released passively by cell death such as necrosis, necroptosis, and NETosis (9). Different types of DAMPs have been shown to contribute to sepsis including, but not limited to, extracellular cold-inducible RNA-binding protein (eCIRP), high mobility group box 1 (HMGB1), extracellular histones, and heat shock proteins (HSPs) (9-12). These DAMPs have been shown to increase in sepsis patients and positively correlate with disease severity and mortality (9, 10). Moreover, inhibition of DAMPs in experimental sepsis has been shown to improve the outcomes (13). DAMPs influence neutrophils to alter their function by promoting their infiltration into tissue beds and inducing NET-formation in sepsis, potentially reflecting their phenotypic changes (10). Thus, it is plausible that DAMPs are closely associated with neutrophil heterogeneity during sepsis. Indeed, some DAMPs have been shown to directly induce certain subtypes of neutrophils in sepsis.
Here, we summarize different neutrophil subtypes including, N1/N2/Nreg, aged, antigen-presenting, reverse-migrated, intercellular adhesion molecule-1 (ICAM-1)+, low-density, olfactomedin 4 (OLFM4)+, and sialic-acid-binding immunoglobulin-like lectin (siglec)-F+ neutrophils in terms of their characteristics, mediators, and markers as well as their association with sepsis and DAMPs (Figure 1). Some of the neutrophil subsets exert pro-inflammatory activities, while others cause immunosuppression, both of which contribute to organ dysfunction and death in sepsis by producing different mediators and interacting with lymphocytes (Figure 2). Not all the subsets have been studied specifically in sepsis and/or DAMPs. However, we still discuss them based on their relevant mechanisms and possible implications when there is no direct evidence related to sepsis.
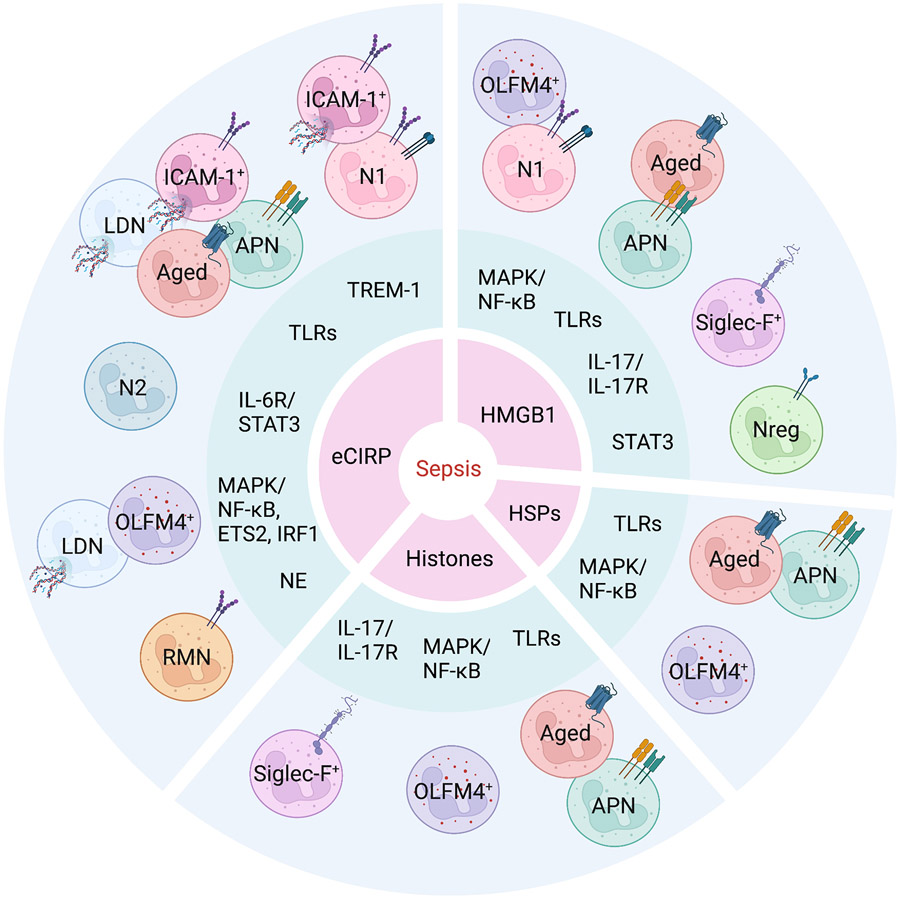
Figure 1. Stratification of neutrophil subtypes by DAMPs and mediators.
During sepsis, DAMPs (1st circle) activate their receptors and signaling pathways to induce the production of inflammatory mediators (2nd circle), which lead to neutrophil heterogeneity (3rd circle). APN, antigen presenting neutrophils; RMN, reverse-migrated neutrophils; LDN, low-density neutrophils; OLFM4, olfactomedin 4; Siglec-F, sialic-acid-binding immunoglobulin-like lectin-F; NE, neutrophil elastase; IRF1, interferon regulatory factor 1.
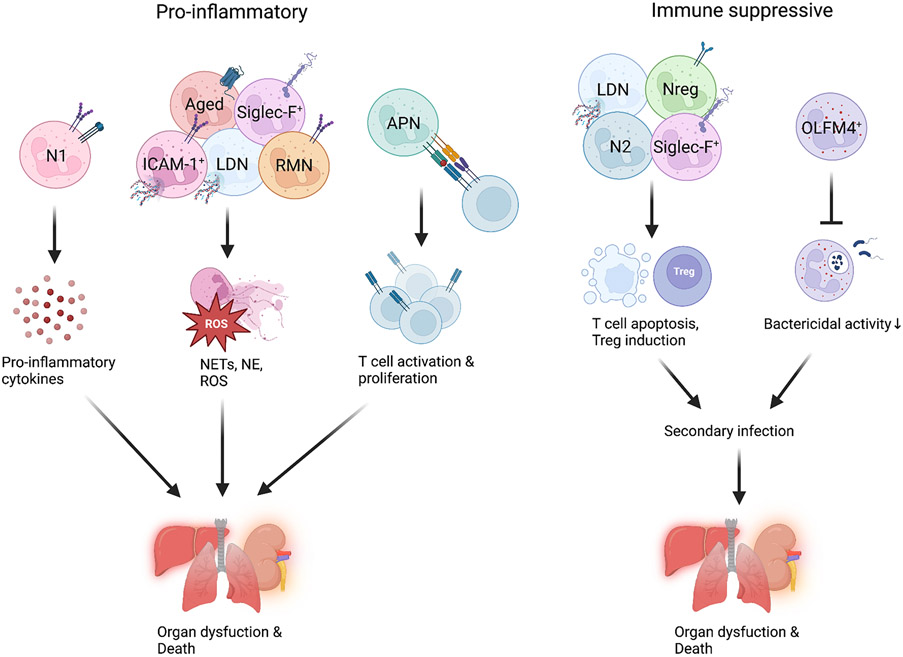
Figure 2. Impacts of neutrophil subtypes and their mechanisms of action.
Different subtypes of neutrophils (pro-inflammatory or immune suppressive) produce different mediators and directly interact with lymphocytes to promote organ injury and death in sepsis. APN, antigen presenting neutrophils; RMN, reverse-migrated neutrophils; LDN, low-density neutrophils; OLFM4, olfactomedin 4; Siglec-F, sialic-acid-binding immunoglobulin-like lectin-F; NE, neutrophil elastase; ROS, reactive oxygen species.
DAMPs
Cell stress causes the release of intracellular proteins and nucleic acids as well as lipids originating from the nucleus, cytoplasm, mitochondria, and granules. These molecules which have the potential to induce immune response are called DAMPs (14). DAMPs bind to PRRs, including, but not limited to, Toll-like receptor 4 (TLR4), TLR2, TLR7, TLR9, receptor for advanced glycation end products (RAGE), and triggering receptor expressed on myeloid cells-1 (TREM-1), to activate the downstream signaling pathways (14). Almost any immune and nonimmune cells can be affected by DAMPs, if not to the same extent. For example, DAMPs induce macrophage cytokine production, NET formation, and endothelial cell apoptosis (10). It has been shown that DAMPs contribute to various disorders such as sepsis, cancer, and autoimmune diseases. Therapeutic interventions to target DAMPs in the experimental modes of those disorders have been shown to improve the outcomes (13). However, further research is needed to apply these approaches widely in clinical settings at this time.
Neutrophil heterogeneity
Neutrophils are produced in the bone marrow from hematopoietic stem cells (15). Mature neutrophils are released into the blood and patrol the body. Neutrophils migrate to the inflamed tissues when they sense chemoattractants to exert their microbicidal and pro-inflammatory activities (2). Senescent neutrophils return to the bone marrow to be cleared by resident macrophages (15). During their maturation/aging and migration, neutrophils alter their phenotype, which is reflected by cellular markers such as chemokine receptors and adhesion molecules (15-17). These markers have been commonly used to identify distinct subsets of neutrophils (Table 1). Recently, an increasing number of studies have sought to identify new subsets of neutrophils using single-cell RNA sequencing, which can visualize distinct clusters within neutrophils unbiasedly (6, 18). This approach is expected to accelerate the understanding of neutrophil heterogeneity dramatically as even a single dataset can be analyzed in various ways to elucidate distinctive genes, pathways, and cell-cell interactions related to specific subtypes.
Table 1:
Markers of neutrophil subtypes.
| Subtypes | Markers | References |
|---|---|---|
| N1 | TNF-α, Fas, ICAM-1, CCL3 | 19 |
| N2 | Arginase, CCL2, CCL5 | 19 |
| Nreg | PD-L1, IL-10Rβ, TNF-α, TGF-β1, IRF5, TLR2 | 18 |
| Aged | CXCR4, CD62Llo, ICAM-1 | 16, 26 |
| APN | MHC-II, CD40, CD64, CD83, CD86 | 31-34 |
| RMN | ICAM-1, CXCR1lo | 17 |
| ICAM-1+ | iNOS, MPO | 24 |
| LDN | CD11bhi, CXCR4, ICAM-1, iNOS | 42 |
| OLFM4+ | CD64lo | 63 |
| Siglec-F+ | TNF-α, ICAM-1, PPIA, ProTα | 64 |
APN, antigen presenting neutrophils; RMN, reverse-migrated neutrophils; LDN, low-density neutrophils; OLFM4, olfactomedin 4; Siglec-F, sialic-acid-binding immunoglobulin-like lectin-F; CCL, CC chemokine ligand; CXCR, CXC chemokine receptors; IRF5, interferon regulatory factor 5; iNOS, inducible nitric oxide synthase; PPIA, peptidylprolyl isomerase A; ProTα, prothymosin alpha; iNOS, inducible nitric oxide synthase; MPO, myeloperoxidase.
N1, N2, and Nreg neutrophils
In analogy to macrophage M1/M2 polarization, neutrophils can be classified according to pro-inflammatory and anti-inflammatory properties, i.e., N1 and N2, respectively (19). N1 neutrophils produce pro-inflammatory cytokines such as interleukin (IL)-12, and tumor necrosis factor (TNF)-α, and express Fas, ICAM-1, and CC chemokine ligand (CCL) 3. N2 neutrophils can be identified by the expression of arginase, CCL2, and CCL5 (19). Although this paradigm has primarily been studied in oncology, neutrophils can be polarized into N1 and N2 phenotypes by different mediators closely related to sepsis. For example, N1 neutrophils can be induced by LPS, interferon (IFN)-γ, and IFN-β, whereas transforming growth factor (TGF)-β, IL-10, and L-lactate can induce N2 neutrophils (20). Besides N1/N2 paradigm, immunoregulation is also an important phenomenon of the immune system. Single-cell RNA sequencing of LPS-challenged neutrophils revealed a distinct cluster, regulatory neutrophils (Nreg), expressing programmed death-ligand 1 (PD-L1), which shows the representation of genes associated with cytokine production and signal transduction (18). The study has demonstrated PD-L1+ neutrophils can be differentiated via the p38α-MSK1/MK2 pathway. Nreg subset has been shown to exert immunosuppressive function, including inducing regulatory T cell (Treg) population, promoting T cell apoptosis, and inhibiting T cell activation (18). Furthermore, PD-L1−/− mice showed increased survival compared to WT mice in septic conditions induced by cecal ligation and puncture (CLP), indicating the detrimental role of Nreg in sepsis due to their immunosuppressive characteristics, given that prolonged immune-suppression renders the host susceptible to secondary infection (18). Although Tregs express the master transcription factor, FoxP3, in the context of Nregs, the expression of FoxP3 is unclear. However, neutrophils, regardless of their regulatory phenotype, Nregs, can express FoxP3, which is downregulated upon IL-8 stimulation, leading to the progression of oral squamous cell carcinoma (21). Elucidating FoxP3 expression in the Nreg population will provide more profound insight into the development and transcriptomics of these cells relevant to the other regulatory immune cells in sepsis induced by DAMPs.
Even though there have been few direct studies on DAMPs in terms of this N1/N2/Nreg paradigm, especially in sepsis, DAMPs have the potential to be involved in neutrophil polarization according to their capability of inducing the preceding mediators for each phenotype. Moreover, it has been implied by some studies that DAMPs may directly induce neutrophil polarization. HMGB1 upregulates TNF-α levels in neutrophils via the p38 MAPK/NF-κB pathway (22), suggesting HMGB1 may directly induce N1 polarization. HMGB1 has also been shown to upregulate PD-L1 on neutrophils via signal transducer and activator of transcription 3 (STAT3) (23), suggesting its potential implication in Nreg polarization. Similarly, eCIRP has been shown to induce neutrophils to express ICAM-1, which is a marker of N1 neutrophils (24). Conversely, since eCIRP promotes M2 polarization by inducing the expression of arginase via the IL-6 receptor (IL-6R)/STAT3 axis (25), it may generate N2 in sepsis. In fact, the DAMPs can act as double-edged swords because they can induce both pro- and anti-inflammatory cytokines production in sepsis, which directs the possibility of generating N1/N2/Nreg population.
Aged neutrophils
Neutrophils generally have a short lifespan and undergo apoptosis once they age or are activated to maintain homeostasis in a steady state. However, neutrophils acquire a longer lifespan and exhibit a distinct phenotype during certain inflammatory conditions such as sepsis. Aged neutrophils express elevated levels of CXCR4, routinely used to identify the population (16, 26). Downregulation of CD62L is also observed in aged neutrophils, at least in the steady state, but a study showed CD62L was not downregulated in aged neutrophils under LPS-treated condition, indicating CD62L downregulation may not serve as a marker to identify aged neutrophils during inflammation (16, 26). Induction of aged neutrophils depends on the TLRs-MyD88 pathway (16), suggesting the implication of DAMPs in this matter as different kinds of major DAMPs, e.g., eCIRP, HMGB1, histones, and HSPs are the potent agonists of TLRs (10, 27). Aged neutrophils are functionally active compared to the non-aged population, especially under inflammatory conditions. Aged neutrophils have shown to express elevated levels of β2 integrin Mac-1/CD11b in high-affinity conformation and migrate swiftly to the site of inflammation (26). Aged neutrophils also express other adhesion molecules, such as platelet endothelial cell adhesion molecule-1 (PECAM-1) and ICAM-1 (26), but the functional significance of these molecules has not yet been fully elucidated. Phagocytic capacity is enhanced in aged neutrophils in a CD11b- and SYK-dependent manner (26). It has also been indicated that aged neutrophils produce excess amounts of ROS and NETs (16).
Apoptosis impairment is thought to be one of the major mechanisms of inducing aged neutrophils by extending their life span. Bcl-xL, an anti-apoptotic protein, increases the life span of neutrophils within inflamed tissues (28). Some DAMPs have been shown to inhibit apoptosis, supporting their implication in neutrophil aging. For example, a study showed HSP27 inhibited human neutrophil apoptosis (29). HMGB1 has been shown to inhibit apoptosis in certain cell types (30), but it has not been confirmed in neutrophils. Besides directly assessing the role of DAMPs on the induction of aged neutrophils, their effects on apoptosis and other cell death would be of significant value in studying this subset. Considering apoptosis impairment leads to the increase in the lifespan of neutrophils otherwise being dead and cleared due to senescence, neutrophils can potentially be divided into three subgroups according to their aging and apoptosis status: group 1. Non-aged neutrophils, group 2. Aged neutrophils which undergo apoptosis over time, and group 3. Aged neutrophils which do not undergo apoptosis and live longer because of apoptosis impairment. It has been shown that aged neutrophils display different functions under different conditions even though being identified using the same markers (26). The difference between group 2 and group 3 could be the reason aged neutrophils exhibit different properties between the steady state, where group 2 is the main aged population, and inflammatory conditions, where group 3 takes over group 2 in aged population due to apoptosis impairment caused by pro-inflammatory mediators such as DAMPs (29, 30). Further studies evaluating the apoptotic state of aged neutrophils in different conditions would support this theory. In addition, the fate of aged neutrophils with impaired apoptosis would also be of interest. They may eventually undergo uncontrolled types of cell death accompanied by membrane rupture such as necrosis and necroptosis, which cause large amounts of DAMP release as opposed to apoptosis.
Antigen-presenting neutrophils
Neutrophils conventionally participate solely in innate immunity, unlike conventional antigen-presenting cells (APC). However, emerging evidence suggests neutrophils play a role in adaptive immunity by interacting with lymphocytes. It has been shown that neutrophils stimulated with certain cytokines such as IFN-γ or GM-CSF and co-cultured with T cells acquire MHC-II and costimulatory molecules, including CD80 and CD86 (31-33). Those neutrophils were functionally active and were able to present antigen and induce T cell proliferation, if not as efficient as professional APC, in an MHC-II-dependent manner (31-33). Circulating neutrophils of sepsis patients display an APC-like phenotype as represented by increased expression of CD40, CD64, and CD86 (34). The study has also shown neutrophils of some patients exhibited higher levels of CD83 and HLA-DR even though it was not significant across the cohort (34). TLR signaling has been shown to independently induce costimulatory molecules on neutrophils (33). Considering DAMPs’ capacity to activate TLRs, DAMPs may enhance the antigen-presenting function of neutrophils. DAMPs may support this matter as a recent study has shown that neutrophils require both an antigen and antigen-specific T cells to express MHC-II even when the TLR pathway is activated (33). The overall significance of antigen-presenting neutrophils in adaptive immunity, where professional APCs such as dendritic cells are thought to play more important roles, is barely understood. Antigen-presenting neutrophils may not only play dispensable roles in the adaptive immunity all the time, but they may play a major role under certain conditions. It would be of interest whether DAMPs influence the balance of APC and antigen-presenting neutrophils in terms of contribution to the adaptive immune system.
Reverse-migrated (RM) neutrophils
Traditionally, neutrophils are thought to be cleared by macrophages after they execute their antimicrobial functions in tissues. However, it is now known that neutrophil transendothelial migration is bidirectional, and some neutrophils return to the circulation from tissues. Reverse migration of neutrophils can be facilitated by the cleavage of junctional adhesion molecule-C (JAM-C) on endothelial cells by NE released from neutrophils (35, 36). A recent study has shown that NE is presented on Mac-1/CD11b expressed on the surface of neutrophils to cleave JAM-C (35). These phenomena have been demonstrated in the lung, an organ that attracts a large number of neutrophils during sepsis. The significance of this mechanism in lung injury can be supported by the clinical findings showing that the elevation of soluble JAM-C was observed in the blood of patients with acute respiratory distress syndrome (ARDS) and was correlated with the severity of multiple organ failure even though it was assessed in trauma patients (35). RM neutrophils can be identified as ICAM-1hiCXCR1lo population, whereas circulating neutrophils are ICAM-1loCXCR1high and tissue-resident neutrophils are ICAM-1loCXCR1lo (17). RM neutrophils have been found in the peripheral blood of patients with chronic inflammatory disease using these markers (17), but have not been confirmed in septic patients. Nevertheless, RM neutrophils have been shown to increase in the blood of septic mice induced by CLP, indicating their implication in sepsis (36). Reverse migration of neutrophils has been shown to be facilitated by different mediators related to sepsis, including a DAMP. eCIRP has been shown to contribute to RM neutrophil induction in sepsis as administration of eCIRP increased RM neutrophils in the blood, and CIRP−/− mice showed significantly decreased RM neutrophils in the blood compared to WT mice after CLP operation (36). The study further demonstrated that JAM-C on lung endothelial cells can be cleaved by NE released from neutrophils activated by eCIRP, resulting in reverse transendothelial migration of neutrophils (36). Besides by activating neutrophils, DAMPs may contribute to neutrophil reverse migration by acting on endothelial cells since endothelial cells also express PRRs such as TLR4 (10). Specifically, the effects of DAMPs on junctional molecules via the direct activation of endothelial cells would provide important insights. Taken together, DAMPs are expected to play a role in reverse transendothelial migration of neutrophils by affecting both active (i.e., neutrophils) and passive (i.e., endothelial cells) factors as to this matter.
ICAM-1+ neutrophils
ICAM-1, also known as CD54, is a cell surface glycoprotein mainly expressed on endothelial cells to serve as an adhesion molecule for facilitating leukocyte recruitment. ICAM-1 can also be found on neutrophils and is upregulated under inflammatory conditions (37). Even though ICAM-1 is an adhesion molecule, ICAM-1−/− neutrophils have been shown to migrate equivalently to WT neutrophils (37). Instead, ICAM-1+ neutrophils exhibit enhanced effector functions such as phagocytosis and production of ROS and NETs (24, 37). Soluble ICAM-1 has been shown to be elevated in the blood of septic patients and predicted mortality, but it has not been tested in neutrophils in the study (38). ICAM-1+ neutrophils are increased in the blood and lungs of endotoxemic mice as well as CLP-induced septic mice (24, 37, 39). Some studies have demonstrated the direct effects of DAMPs on ICAM-1+ neutrophils in sepsis. eCIRP induces ICAM-1+ neutrophils in TLR-4- and TREM-1-dependent manners (24, 39). ICAM-1+ neutrophils produce NETs via Rho GTPase activation upon eCIRP challenge (39). CIRP−/− mice displayed decreased ICAM-1+ neutrophils in the blood and lungs compared to WT mice after CLP, supporting the role of eCIRP on the induction of ICAM-1+ neutrophils during sepsis (24). It should be noted that ICAM-1 is also upregulated in other subsets of neutrophils, including N1, aged neutrophils, and RM neutrophils, as described earlier, indicating an overlap between these subtypes and ICAM-1+ neutrophils. The extent of this overlap remains elusive and requires further studies to determine it precisely. In addition, there can be a positive correlation between ICAM-1 expression and antigen-presenting function in neutrophils since ICAM-1+ neutrophils display enhanced phagocytosis (37), which is an early step for antigen processing. The antigen-presenting property of ICAM-1+ neutrophils would be of interest in future studies.
Low-density neutrophils
Gradient separation of cells, especially from blood, is a conventional way to isolate different cell types. Neutrophils are primarily found in the granulocyte layer, which contains cells with higher density than those in the buffy coat layer and are barely detectable in the peripheral blood mononuclear cell (PBMC) layer under the steady state. However, a fraction of neutrophils can be found in the PMBC layer during disease conditions, including sepsis, and are named LDN (40). The mechanisms by which neutrophils lose their density remain elusive, but it is speculated that it can be due to degranulation followed by the release of granule contents or water uptake regulated by aquaporin-9 (40). LDN have been shown to increase in septic patients compared to healthy volunteers (41). The study showed impaired function of these LDN as determined by decreased phagocytic and chemotactic activities (41). A recent study assessing the role of a DAMP on LDN induction in sepsis has revealed LDN can be divided into further subpopulations (42). eCIRP has been shown to induce Ly6G+CD11bhi subset within LDN population during sepsis (42). Ly6G+CD11bhi LDN exhibited pro-inflammatory function as observed by excessive amounts of ROS and NET production (42).
Another important cell population within the low-density fraction is myeloid-derived suppressor cells (MDSCs). MDSCs exert immunosuppressive activity and are associated with poor clinical outcomes for different diseases (43). MDSCs are divided into polymorphonuclear MDSCs (PMN-MDSCs) and monocytic MDSCs (M-MDSCs). However, there are no phenotypic cell surface markers to segregate PMN-MDSCs from classical neutrophils in mice, and it requires a functional assay to identify PMN-MDSCs by showing their ability to suppress other immune cells (43). Thus, existing studies about immunosuppressive LDN potentially overlap with PMN-MDSCs. Some studies have shown LDN as pro-inflammatory cells under certain conditions, while others have shown LDN as immunosuppressive population under different conditions (41, 42, 44). This discrepancy could be due to the difference in the balance of the pro-inflammatory and immunosuppressive phenotypes within LDN under different conditions. Considering the study showing a DAMP induces a pro-inflammatory subset of LDN as aforementioned (42), differences in the amounts and types of DAMPs present in different settings may explain the characteristic variation of LDN between the previous studies.
OLFM4+ neutrophils
OLFM4 is an olfactomedin domain-containing glycoprotein that plays a critical role in the innate immunity against infection. OLFM4 is expressed in epithelial cells and cancer cells as well as a subset of neutrophils (45). It has been shown that OLFM4 is a constituent of neutrophil-specific granules. OLFM4 interacts with its binding partners, such as nucleotide-binding oligomerization domain (NOD) 1/2 and cathepsin C to regulate cellular functions (45). OLFM4+ neutrophils are increased in the blood of septic patients and are independently associated with mortality (46). A study has demonstrated OLFM4 deficient mice were increased in survival in CLP-induced sepsis compared to WT mice, supporting the clinical finding (47). Another study has shown OLFM4−/− neutrophils had increased intracellular killing of Staphylococcus aureus and Escherichia coli, and OLFM4−/− mice had enhanced bacterial clearance upon intraperitoneal injection of S. aureus and E. coli, indicating OLFM4’s immunosuppressive function (48). The study has demonstrated that OLFM4 negatively regulates neutrophil bactericidal activity by restricting cathepsin C activity and its downstream serine proteases, which facilitate intracellular microbial killing and mediate inflammatory cell recruitment (48). OLFM4 expression can be upregulated by the MAPK/NF-κB and Wnt/β-catenin pathways (45), suggesting DAMPs known to activate these pathways, such as HMGB1, eCIRP, and histones may directly induce OLFM4+ neutrophils. Nonetheless, it would be valuable to specifically demonstrate each DAMP’s role in neutrophil OLFM4 expression. It has been shown that OLFM4 can be released as a component of NETs (49), however functional differences between OLFM4+ and OLFM4− NETs have barely been understood. Since extracellular OLFM4 has been shown to inhibit Wnt/β-catenin pathway (50), which negatively modulates Treg cell function (51), OLFM4+ neutrophils could induce Treg activation by producing OLFM4-containing NETs to cause immunosuppression. Given that DAMPs are also contained in NETs (9), OLFM4 and DAMPs present in NETs may concomitantly contribute to sepsis pathophysiology through different mechanisms.
Siglec-F+ neutrophils
Siglec-F is a mouse functional paralog of human Siglec-8 that induces eosinophil apoptosis (52). It has been known that neutrophils also express Siglec-F under certain conditions. Siglec-F+ neutrophils exhibited enhanced effector functions such as NET production (53). A study has shown that induction of Siglec-F+ neutrophils is dependent on IL-17 in mice infected with B. pertussis (53). Since IL-17 plays a significant role in sepsis pathophysiology (54), Siglec-F+ neutrophils could be found during sepsis and exhibit their effector functions to contribute to developing the disorder. TGF-β1 and GM-CSF have also been shown to induce Siglec-F+ neutrophils (55), thus evaluating the impacts of these mediators on sepsis would also support the implication of Siglec-F+ neutrophils in sepsis. Nevertheless, the confirmation of Siglec-F+ neutrophils in samples from septic patients and experimental models of septic animals is awaited. There have not been studies directly assessing the role of DAMPs in this subset. The association between some DAMPs and the mediators of Siglec-F+ neutrophils suggests the implication of DAMPs in this matter. For example, HMGB1 induces IL-17 production in the blood (56), and extracellular histones in NETs promote Th17 differentiation (57). It is still an open question whether DAMPs can directly induce Siglec-F+ neutrophils. Even though Siglec-F+ neutrophils exhibit enhanced effector functions to potentially cause organ injuries in sepsis, it should be noted that Siglecs are generally regarded as immunoregulatory receptors. Indeed, Siglec-F+ neutrophils have been shown to promote cancer by suppressing immune response (58). Thus, besides inducing organ injuries by the hyperactivity during the pro-inflammatory state, Siglec-F+ neutrophils may also contribute to the immunosuppressive state to cause secondary infection in sepsis. Studying DAMPs’ effects on both of these aspects of Siglec-F+ neutrophils may provide a better understanding of the pathophysiology of the different inflammatory states, which can coexist simultaneously in sepsis.
Therapeutic implications targeting DAMPs to ameliorate sepsis by preventing inflammatory neutrophil subsets
Therapeutics to target DAMPs in sepsis have been tested preclinically using inhibitors such as antibodies and antagonists (13). For example, treatments with anti-HMGB1 or anti-histone antibodies have shown to improve the outcomes of septic mice (59, 60). Small peptides that act as eCIRP’s inhibitors by interfering with the binding of eCIRP to TLR4 and TREM-1, named C23 and M3, respectively, mitigate sepsis (61, 62). As eCIRP has been shown to induce ICAM-1+ neutrophils via TLR4 and TREM-1 receptors (24, 39), C23 and M3 would theoretically inhibit this subtype. Both C23 and M3 have been shown to alleviate organ injuries and improve survival in septic mice (61, 62). These beneficial effects could be, at least in part, due to the inhibition of the detrimental subsets of neutrophils, considering the significance and close relation with eCIRP and ICAM-1+ neutrophils in sepsis. Different kinds of DAMPs are associated with NETosis, significantly contributing to sepsis pathophysiology by causing organ injuries, and some DAMPs such as HMGB1 and eCIRP have even been shown to directly induce NETosis (10). NETosis is also a key feature of multiple neutrophil subsets (16, 39, 42). Thus, inhibition of DAMPs by the preceding inhibitors may alleviate sepsis by preventing neutrophil subsets that release excessive amounts of NETs. Alternatively, several inhibitors of NETs have been identified, which could also be used to ameliorate sepsis by targeting NETs originating from different subtypes of neutrophils (10). It should be noted that the effects of DAMP inhibitors on neutrophil heterogeneity have barely been assessed directly in sepsis, and focused studies are awaited to confirm the efficacy of these inhibitors into this matter.
Conclusions and perspectives
Different subtypes of neutrophils play a critical role in sepsis and are associated with DAMPs. Even though there have been numerous articles about the role of DAMPs on macrophages in sepsis, more focused studies on each DAMP in neutrophil heterogeneity are awaited (Table 2). However, the recent advancement of transcriptomics by single-cell RNAseq has broadened the scope of the impact of different clusters/subtypes of neutrophils on sepsis pathophysiology (18). Focuses have also been given on the expression of non-canonical/unconventional markers in neutrophils, which usually are expressed in other cells. For example, elucidation of ICAM-1, which is a pan marker of endothelial cells, expression on neutrophils sheds lights on the implication of adhesion molecules in neutrophil biology (39). The intensity of NET formation among the various subtypes will enable us to know the degree of harmful effects of the particular subtype of neutrophils. Future studies should be carried out by depleting a specific neutrophil subtype using a conditional gene knockout strategy in neutrophil population to gain more deep insights on the pathway and the impact of that subset. Since the lifespan of neutrophils influences pathophysiology, in each subtype, the cellular half-life should be explored (26). In conclusion, our review demonstrated the current trend of neutrophil biology in terms of its heterogeneity, which reflects their behavior and functions in the experimental and clinical settings of sepsis. A comprehensive understanding of neutrophil heterogeneity directs novel pathophysiology and therapeutic interventions against this deadly disease syndrome.
Table 2:
Future directions for the study of neutrophil heterogeneity in sepsis.
| Subtypes | Future directions |
|---|---|
| N1, N2, Nreg | Detailed phenotypic identification of N1/N2/Nreg to confirm that DAMPs directly induce these subtypes. |
| Aged | Association with apoptosis impairment and DAMPs’ contribution. |
| APN | DAMPs’ influence on the balance of canonical APC and APN in the adaptive immune system. |
| RMN | Confirmation in sepsis patients using the existing markers (ICAM-1hi, CXCR1lo). |
| ICAM-1+ | Investigation of the overlap with other ICAM-1-expressing subtypes (i.e., N1, aged, RMN, APN). |
| LDN | Regulation of pro-inflammatory and immunosuppressive LDN phenotypes. |
| OLFM4+ | Role of DAMPs in OLFM4 expression of neutrophils. |
| Siglec-F+ | To determine if DAMPs can directly induce Siglec-F+ neutrophils. |
APN, antigen presenting neutrophils; RMN, reverse-migrated neutrophils; LDN, low-density neutrophils; OLFM4, olfactomedin 4; Siglec-F, sialic-acid-binding immunoglobulin-like lectin-F; APC, antigen-presenting cells.
Funding and acknowledgements
M.A. is supported by the National Institutes of Health (NIH) grants R01GM129633 and U01AI170018 and P.W. is supported by NIH grants R35GM118337, R01HL076179, R01AA028947, U01AI133655, and U01AI170018. We acknowledge the BioRender software service for preparing the figure.
Abbreviations
- DAMPs
damage-associated molecular patterns
- TLRs
Toll-like receptors
- PRRs
pattern recognition receptors
- eCIRP
extracellular cold-inducible RNA-binding protein
- HMGB1
high mobility group box 1
- HSP
heat shock protein
- TREM-1
triggering receptor expressed on myeloid cells-1
- ICAM-1
intercellular adhesion molecule-1
- APN
antigen presenting neutrophils
- RMN
reverse-migrated neutrophils
- LDN
low-density neutrophils
- OLFM4
olfactomedin 4
- Siglec-F
sialic-acid-binding immunoglobulin-like lectin-F
- NE
neutrophil elastase
- MPO
myeloperoxidase
- NETs
neutrophil extracellular traps
Footnotes
Competing financial and/or non-financial interests
The authors declared that they have no competing interests.
References
- 1.Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, Bellomo R, Bernard GR, Chiche JD, Coopersmith CM, et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). Jama 315(8):801–10, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aziz M, Jacob A, Yang WL, Matsuda A, Wang P. Current trends in inflammatory and immunomodulatory mediators in sepsis. J Leukoc Biol 93(3):329–42, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aziz M, Brenner M, Wang P. Therapeutic Potential of B-1a Cells in COVID-19. Shock 54(5):586–94, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Castanheira FVS, Kubes P. Neutrophils and NETs in modulating acute and chronic inflammation. Blood 133(20):2178–85, 2019. [DOI] [PubMed] [Google Scholar]
- 5.Lerman YV, Lim K, Hyun YM, Falkner KL, Yang H, Pietropaoli AP, Sonnenberg A, Sarangi PP, Kim M. Sepsis lethality via exacerbated tissue infiltration and TLR-induced cytokine production by neutrophils is integrin α3β1-dependent. Blood 124(24):3515–23, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xie X, Shi Q, Wu P, Zhang X, Kambara H, Su J, Yu H, Park SY, Guo R, Ren Q, et al. Single-cell transcriptome profiling reveals neutrophil heterogeneity in homeostasis and infection. Nat Immunol 21(9):1119–33, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Quail DF, Amulic B, Aziz M, Barnes BJ, Eruslanov E, Fridlender ZG, Goodridge HS, Granot Z, Hidalgo A, Huttenlocher A, et al. Neutrophil phenotypes and functions in cancer: A consensus statement. J Exp Med 219(6), 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bantel H, Schulze-Osthoff K. Cell death in sepsis: a matter of how, when, and where. Crit Care 13(4):173, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Murao A, Aziz M, Wang H, Brenner M, Wang P. Release mechanisms of major DAMPs. Apoptosis 26(3-4):152–62, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Denning NL, Aziz M, Gurien SD, Wang P. DAMPs and NETs in Sepsis. Front Immunol 10:2536, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Qiang X, Yang WL, Wu R, Zhou M, Jacob A, Dong W, Kuncewitch M, Ji Y, Yang H, Wang H, et al. Cold-inducible RNA-binding protein (CIRP) triggers inflammatory responses in hemorrhagic shock and sepsis. Nat Med 19(11):1489–95, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang H, Bloom O, Zhang M, Vishnubhakat JM, Ombrellino M, Che J, Frazier A, Yang H, Ivanova S, Borovikova L, et al. HMG-1 as a late mediator of endotoxin lethality in mice. Science 285(5425):248–51, 1999. [DOI] [PubMed] [Google Scholar]
- 13.Land WG. Use of DAMPs and SAMPs as Therapeutic Targets or Therapeutics: A Note of Caution. Mol Diagn Ther 24(3):251–62, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nofi CP, Wang P, Aziz M. Chromatin-Associated Molecular Patterns (CAMPs) in sepsis. Cell Death Dis 13(8):700, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cossío I, Lucas D, Hidalgo A. Neutrophils as regulators of the hematopoietic niche. Blood 133(20):2140–8, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang D, Chen G, Manwani D, Mortha A, Xu C, Faith JJ, Burk RD, Kunisaki Y, Jang JE, Scheiermann C, et al. Neutrophil ageing is regulated by the microbiome. Nature 525(7570):528–32, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Buckley CD, Ross EA, McGettrick HM, Osborne CE, Haworth O, Schmutz C, Stone PC, Salmon M, Matharu NM, Vohra RK, et al. Identification of a phenotypically and functionally distinct population of long-lived neutrophils in a model of reverse endothelial migration. J Leukoc Biol 79(2):303–11, 2006. [DOI] [PubMed] [Google Scholar]
- 18.Qi X, Yu Y, Sun R, Huang J, Liu L, Yang Y, Rui T, Sun B. Identification and characterization of neutrophil heterogeneity in sepsis. Crit Care 25(1):50, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Piccard H, Muschel RJ, Opdenakker G. On the dual roles and polarized phenotypes of neutrophils in tumor development and progression. Crit Rev Oncol Hematol 82(3):296–309, 2012. [DOI] [PubMed] [Google Scholar]
- 20.Ohms M, Möller S, Laskay T. An Attempt to Polarize Human Neutrophils Toward N1 and N2 Phenotypes. Front Immunol 11:532, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zeng C, Kuang H, Fan W, Chen X, Yu T, Tang Q, Zhou Z, Liang F. Downregulation of. Oncol Lett 18(5):4771–7, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Park JS, Arcaroli J, Yum HK, Yang H, Wang H, Yang KY, Choe KH, Strassheim D, Pitts TM, Tracey KJ, et al. Activation of gene expression in human neutrophils by high mobility group box 1 protein. Am J Physiol Cell Physiol 284(4):C870–9, 2003. [DOI] [PubMed] [Google Scholar]
- 23.Shi Y, Zhang J, Mao Z, Jiang H, Liu W, Shi H, Ji R, Xu W, Qian H, Zhang X. Extracellular Vesicles From Gastric Cancer Cells Induce PD-L1 Expression on Neutrophils to Suppress T-Cell Immunity. Front Oncol 10:629, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ode Y, Aziz M, Wang P. CIRP increases ICAM-1(+) phenotype of neutrophils exhibiting elevated iNOS and NETs in sepsis. J Leukoc Biol 103(4):693–707, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhou M, Aziz M, Denning NL, Yen HT, Ma G, Wang P. Extracellular CIRP induces macrophage endotoxin tolerance through IL-6R-mediated STAT3 activation. JCI Insight 5(5), 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Uhl B, Vadlau Y, Zuchtriegel G, Nekolla K, Sharaf K, Gaertner F, Massberg S, Krombach F, Reichel CA. Aged neutrophils contribute to the first line of defense in the acute inflammatory response. Blood 128(19):2327–37, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Calderwood SK, Gong J, Murshid A. Extracellular HSPs: The Complicated Roles of Extracellular HSPs in Immunity. Front Immunol 7:159, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Carrington EM, Louis C, Kratina T, Hancock M, Keenan CR, Iannarella N, Allan RS, Wardak AZ, Czabotar PE, Herold MJ, et al. BCL-XL antagonism selectively reduces neutrophil life span within inflamed tissues without causing neutropenia. Blood Adv 5(11):2550–62, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sheth K, De A, Nolan B, Friel J, Duffy A, Ricciardi R, Miller-Graziano C, Bankey P. Heat shock protein 27 inhibits apoptosis in human neutrophils. J Surg Res 99(1):129–33, 2001. [DOI] [PubMed] [Google Scholar]
- 30.Yuan S, Liu Z, Xu Z, Liu J, Zhang J. High mobility group box 1 (HMGB1): a pivotal regulator of hematopoietic malignancies. J Hematol Oncol 13(1):91, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lin A, Loré K. Granulocytes: New Members of the Antigen-Presenting Cell Family. Front Immunol 8:1781, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li Y, Wang W, Yang F, Xu Y, Feng C, Zhao Y. The regulatory roles of neutrophils in adaptive immunity. Cell Commun Signal 17(1):147, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vono M, Lin A, Norrby-Teglund A, Koup RA, Liang F, Loré K. Neutrophils acquire the capacity for antigen presentation to memory CD4. Blood 129(14):1991–2001, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Davey MS, Morgan MP, Liuzzi AR, Tyler CJ, Khan MWA, Szakmany T, Hall JE, Moser B, Eberl M. Microbe-specific unconventional T cells induce human neutrophil differentiation into antigen cross-presenting cells. J Immunol 193(7):3704–16, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Colom B, Bodkin JV, Beyrau M, Woodfin A, Ody C, Rourke C, Chavakis T, Brohi K, Imhof BA, Nourshargh S. Leukotriene B4-Neutrophil Elastase Axis Drives Neutrophil Reverse Transendothelial Cell Migration In Vivo. Immunity 42(6):1075–86, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jin H, Aziz M, Ode Y, Wang P. CIRP Induces Neutrophil Reverse Transendothelial Migration in Sepsis. Shock 51(5):548–56, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Woodfin A, Beyrau M, Voisin MB, Ma B, Whiteford JR, Hordijk PL, Hogg N, Nourshargh S. ICAM-1-expressing neutrophils exhibit enhanced effector functions in murine models of endotoxemia. Blood 127(7):898–907, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sessler CN, Windsor AC, Schwartz M, Watson L, Fisher BJ, Sugerman HJ, Fowler AA. Circulating ICAM-1 is increased in septic shock. Am J Respir Crit Care Med 151(5):1420–7, 1995. [DOI] [PubMed] [Google Scholar]
- 39.Murao A, Arif A, Brenner M, Denning NL, Jin H, Takizawa S, Nicastro B, Wang P, Aziz M. Extracellular CIRP and TREM-1 axis promotes ICAM-1-Rho-mediated NETosis in sepsis. FASEB J 34(7):9771–86, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hassani M, Hellebrekers P, Chen N, van Aalst C, Bongers S, Hietbrink F, Koenderman L, Vrisekoop N. On the origin of low-density neutrophils. J Leukoc Biol 107(5):809–18, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sun R, Huang J, Yang Y, Liu L, Shao Y, Li L, Sun B. Dysfunction of low-density neutrophils in peripheral circulation in patients with sepsis. Sci Rep 12(1):685, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Takizawa S, Murao A, Ochani M, Aziz M, Wang P. Frontline Science: Extracellular CIRP generates a proinflammatory Ly6G. J Leukoc Biol 109(6):1019–32, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Veglia F, Sanseviero E, Gabrilovich DI. Myeloid-derived suppressor cells in the era of increasing myeloid cell diversity. Nat Rev Immunol 21(8):485–98, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Blanco-Camarillo C, Alemán OR, Rosales C. Low-Density Neutrophils in Healthy Individuals Display a Mature Primed Phenotype. Front Immunol 12:672520, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu W, Rodgers GP. Olfactomedin 4 expression and functions in innate immunity, inflammation, and cancer. Cancer Metastasis Rev 35(2):201–12, 2016. [DOI] [PubMed] [Google Scholar]
- 46.Alder MN, Opoka AM, Lahni P, Hildeman DA, Wong HR. Olfactomedin-4 Is a Candidate Marker for a Pathogenic Neutrophil Subset in Septic Shock. Crit Care Med 45(4):e426–e32, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Alder MN, Mallela J, Opoka AM, Lahni P, Hildeman DA, Wong HR. Olfactomedin 4 marks a subset of neutrophils in mice. Innate Immun 25(1):22–33, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liu W, Yan M, Liu Y, McLeish KR, Coleman WG, Rodgers GP. Olfactomedin 4 inhibits cathepsin C-mediated protease activities, thereby modulating neutrophil killing of Staphylococcus aureus and Escherichia coli in mice. J Immunol 189(5):2460–7, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Welin A, Amirbeagi F, Christenson K, Björkman L, Björnsdottir H, Forsman H, Dahlgren C, Karlsson A, Bylund J. The human neutrophil subsets defined by the presence or absence of OLFM4 both transmigrate into tissue in vivo and give rise to distinct NETs in vitro. PLoS One 8(7):e69575, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liu W, Li H, Hong SH, Piszczek GP, Chen W, Rodgers GP. Olfactomedin 4 deletion induces colon adenocarcinoma in Apc. Oncogene 35(40):5237–47, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.van Loosdregt J, Fleskens V, Tiemessen MM, Mokry M, van Boxtel R, Meerding J, Pals CE, Kurek D, Baert MR, Delemarre EM, et al. Canonical Wnt signaling negatively modulates regulatory T cell function. Immunity 39(2):298–310, 2013. [DOI] [PubMed] [Google Scholar]
- 52.Nutku E, Aizawa H, Hudson SA, Bochner BS. Ligation of Siglec-8: a selective mechanism for induction of human eosinophil apoptosis. Blood 101(12):5014–20, 2003. [DOI] [PubMed] [Google Scholar]
- 53.Borkner L, Curham LM, Wilk MM, Moran B, Mills KHG. IL-17 mediates protective immunity against nasal infection with Bordetella pertussis by mobilizing neutrophils, especially Siglec-F. Mucosal Immunol 14(5):1183–202, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ge Y, Huang M, Yao YM. Biology of Interleukin-17 and Its Pathophysiological Significance in Sepsis. Front Immunol 11:1558, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ryu S, Shin JW, Kwon S, Lee J, Kim YC, Bae YS, Kim DK, Kim YS, Yang SH, Kim HY. Siglec-F-expressing neutrophils are essential for creating a profibrotic microenvironment in renal fibrosis. J Clin Invest 132(12), 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jhun J, Lee S, Kim H, Her YM, Byun JK, Kim EK, Lee SK, Cho ML, Choi JY. HMGB1/RAGE induces IL-17 expression to exaggerate inflammation in peripheral blood cells of hepatitis B patients. J Transl Med 13:310, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wilson AS, Randall KL, Pettitt JA, Ellyard JI, Blumenthal A, Enders A, Quah BJ, Bopp T, Parish CR, Brüstle A. Neutrophil extracellular traps and their histones promote Th17 cell differentiation directly via TLR2. Nat Commun 13(1):528, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Engblom C, Pfirschke C, Zilionis R, Da Silva Martins J, Bos SA, Courties G, Rickelt S, Severe N, Baryawno N, Faget J, et al. Osteoblasts remotely supply lung tumors with cancer-promoting SiglecF. Science 358(6367), 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Xu J, Zhang X, Pelayo R, Monestier M, Ammollo CT, Semeraro F, Taylor FB, Esmon NL, Lupu F, Esmon CT. Extracellular histones are major mediators of death in sepsis. Nat Med 15(11):1318–21, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yang H, Ochani M, Li J, Qiang X, Tanovic M, Harris HE, Susarla SM, Ulloa L, Wang H, DiRaimo R, et al. Reversing established sepsis with antagonists of endogenous high-mobility group box 1. Proc Natl Acad Sci U S A 101(1):296–301, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Denning NL, Aziz M, Murao A, Gurien SD, Ochani M, Prince JM, Wang P. Extracellular CIRP as an endogenous TREM-1 ligand to fuel inflammation in sepsis. JCI Insight 5(5), 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhang F, Brenner M, Yang WL, Wang P. A cold-inducible RNA-binding protein (CIRP)-derived peptide attenuates inflammation and organ injury in septic mice. Sci Rep 8(1):3052, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Stark JE, Opoka AM, Fei L, Zang H, Davies SM, Wong HR, Alder MN. Longitudinal characterization of olfactomedin-4 expressing neutrophils in pediatric patients undergoing bone marrow transplantation. PLoS One 15(5):e0233738, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Calcagno DM, Zhang C, Toomu A, Huang K, Ninh VK, Miyamoto S, Aguirre AD, Fu Z, Heller Brown J, King KR. SiglecF(HI) Marks Late-Stage Neutrophils of the Infarcted Heart: A Single-Cell Transcriptomic Analysis of Neutrophil Diversification. J Am Heart Assoc 10(4):e019019, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]