Abstract
Squamous cell carcinoma of the oral cavity (OSCC) is the most common type of head and neck cancer; survival is poor and response to treatment varies. Metastasis or recurrence in the regional lymph nodes is associated with poor survival. Consequently, overt or occult spread to the lymph nodes is used to identify patients who will receive adjuvant radiation therapy. Perineural invasion and diameter of nerves exhibiting perineural invasion have also been suggested to be of prognostic significance. The explosion of interest in cancer neuroscience in the last two decades has led to novel biologic insights on interactions between nerves and tumor cells. However, our criteria for defining perineural invasion have lagged behind current knowledge. It is important to re-evaluate the concept of perineural invasion and identify other neural phenotypes in OSCC that could impact treatment selection and prognosis. In addition to perineural invasion, neural phenotypes that are of potential relevance to tumor progression include nerve-tumor distance, nerve diameter, and nerve density. This manuscript discusses the translational significance of recent mechanistic studies on progression of oral cancer.
Keywords: Squamous cell carcinoma, Perineural invasion, Nerve-tumor distance, Nerve density, Nerve diameter
Graphical Abstract
Squamous cell carcinoma of the oral cavity (OSCC) is the most common type of head and neck cancer. This paper discusses the clinical significance of recent studies on nerve-tumor distance, diameter of nerves within the tumor, and nerve density on progression of oral cancer and highlights gaps in knowledge.
Short Summary
Squamous cell carcinoma of the oral cavity (OSCC) is the most common type of head and neck cancer. Tumor recurrence and variability in response to treatment are persistent challenges to successful clinical outcomes. In an attempt to improve patient survival, histopathologic neural parameters such as perineural invasion and diameter of nerves exhibiting perineural invasion, have been suggested to be of prognostic significance. Moreover, mechanistic and translational studies highlight emerging neural parameters that could be of prognostic significance. This manuscript discusses the clinical significance of recent studies on nerve-tumor distance, diameter of nerves within the tumor, and nerve density on progression of oral cancer and highlights gaps in knowledge.
Introduction
Squamous cell carcinoma constitutes more than 90% of head and neck cancers, which include malignancies of the oral cavity, oropharynx, hypopharynx, larynx, and nasopharynx (1). There are more than 600,000 new cases each year (2, 3). Oral cavity squamous cell carcinoma (OSCC) is the most common type of head and neck cancer (3); OSCC occurs most frequently on the vermilion of the lip, particularly the lower lip. Excluding the vermilion, more than half of all OSCC occur on the tongue, with the posterolateral and ventral surfaces of the tongue being the predominant sites (4). Floor of mouth cancers are the next most frequent site of intraoral OSCC followed by cancers on the gingiva/ alveolar ridge (4).
OSCC usually occurs in individuals 60 years and older, and has a strong male predilection (4). Lip OSCC is linked to sun exposure whereas tobacco, alcohol, and intrinsic factors such as iron deficiency, are risk factors for other OSCC. Additionally, human papillomaviruses 16 (HPV16) has been linked to oral cancer; however, this association is stronger in squamous cell carcinoma of the oropharynx than the oral cavity. Although the incidence of HPV16-related oropharyngeal cancer has exceeded that of cervical cancer in the United States (5), OSCC is still the most common head and neck cancer, and is clinically and biologically distinct from HPV16-positive oropharyngeal cancer (6).
OSCC arise from pre-malignant lesions in the epithelial lining of the oral mucosa and vermillion region of the lip. In pre-malignant lesions, also known as epithelial dysplasia, transformed epithelial cells are retained within the surface epithelium, i.e. above the basement membrane (7). In OSCC, transformed epithelial cells destroy the basement membrane to invade the underlying fibrovascular tissue. From here, cancer cells can spread into adjacent sites, or enter vasculature and nerves to spread to regional and distant sites such as the lymph nodes and lung, respectively (8).
Clinically, OSCC presents as an ulcerated, exophytic, endophytic, white (leukoplakia), or red (erythroplakia) lesion (4). The patient may be asymptomatic with the lesion discovered on routine exam, or may complain of pain or paresthesia. Pain or paresthesia suggest neural involvement, an adverse phenotype that is associated with metastasis and poor survival. For example, neural invasion in lower lip cancers could lead to tumor spread to the mandible via the mental foramen. One fifth of patients with OSCC complain of pain as their initial symptom (9). In a prospective study of 339 patients, Scharpf et al (10) reported that ~54% of patients with head and neck cancer complained of pain, which is not surprising given the remarkable innervation of the head and neck. They reported that post-treatment pain was an independent predictor of recurrence in patients with head and neck cancer; higher post-treatment pain was associated with poorer survival than lower post-treatment pain. Paresthesia of the mental nerve region (“numb chin syndrome”) reported with OSCC of the gingiva or floor of mouth, has been attributed to invasion of OSCC into the mental or alveolar nerve (11). In this small group of eleven patients, the mean survival was ~2 years (11).
Pain may emanate from damaged nerves (neurotrophic pain) or in response to an external stimulus (nociceptive pain). Nociceptive pain, the nociceptor-mediated response to external injury, develops rapidly and disappears as the noxious stimulus is eliminated or nerve damage heals. In contrast, neurotrophic pain is persistent, prolonged, and due to damage within the nervous system such as that due to anti-cancer agents (12). Patients with OSCC may develop nociceptive or neurotrophic pain (13). While mild to moderate pain in patients with OSCC is treated with non-steroidal anti-inflammatory drugs, opioids are used to treat severe pain (14). The μ-opioid receptor, which is also present on tumor cells, is the target of prescription opioids (15). This receptor, which has two subtypes mediating pain and adverse effects of opioids, is present in sensory terminals of peripheral tissue, including oral mucosa (15). Based on heteromer formation with receptors of ligands that have a role in axon guidance and neurite outgrowth, the μ-opioid receptor may have a role in perineural invasion (15).
Neural involvement by OSCC is often associated with perineural spread or perineural invasion. Perineural spread is a clinical/radiographic phenotype whereby the cancer grows so extensively around the nerve that it is detectable radiographically (16). In contrast, perineural invasion denotes nerve involvement by cancer that is observed microscopically on tissue biopsies but not radiographically (16). Combining a standardized pain questionnaire and pathology reports, Salvo et al, reported that perineural invasion in OSCC is associated with mechanical allodynia and spontaneous pain in patients (17). In an independent study of 162 patients with oral cancer, there was a significant association of high levels of pain and perineural invasion, after adjusting for covariates (18).
In contrast to OSCC, neural phenotypes other than perineural invasion have not been investigated in HPV16-positive oropharyngeal cancer. Moreover, there are conflicting reports on association of perineural invasion with poor survival in oropharyngeal cancer (19–21); this is an area that requires further investigation. This review will focus on perineural invasion and other histopathologic neural phenotypes in OSCC, including nerve-tumor distance, nerve diameter, and nerve density, which are associated with tumor progression.
Innervation of the oral cavity
The oral mucosa is innervated by the peripheral nervous system, which is comprised of 12 pairs of cranial and 31 pairs of spinal nerves. The afferent (sensory) and efferent (motor) nerves of the peripheral nervous system transmit information towards or from the central nervous system (brain and spinal cord), respectively. The two main divisions of the peripheral nervous system, the viscero and somatic sub-divisions, have motor and sensory components (22). Thus, sensory nerves may be somatosensory or viscerosensory, and motor nerves may be somatic or visceral (autonomic) (22, 23). Somatic nerves innervate the skeletal muscle to control voluntary movement (24). The autonomic nervous system consists of sympathetic, parasympathetic, and enteric branches. The sympathetic branch controls ‘fight or flight’ responses whereas the parasympathetic branch controls involuntary physiologic functions of organs and tissues including salivary glands (22, 25).
Nerves innervating the oral cavity are also described as somatic or branchial depending on the embryologic derivation of the structures they innervate. For example, since the hypoglossal nerve innervates tongue muscles that are somite-derived, it is a somatic-efferent nerve. Since the muscles of mastication, facial expression and larynx/ pharynx are embryologically derived from the branchial arches, the nerves innervating these muscles are the branchial-efferent nerves (22). These include trigeminal (V) (26) and facial (VII) nerves, innervating muscles of mastication and facial expression, respectively, and glossopharyngeal (IX), vagus (X) and accessory (XI) nerves, which innervate laryngeal and pharyngeal muscles. All branchial-efferent nerves are mixed, i.e. both motor and sensory.
Cranial nerves, including some that innervate the oral region, arise in the brainstem and may be exclusively sensory, motor, or mixed, which contain afferent and efferent fibers (24). For example, the hypoglossal nerve (XII) is exclusively a motor cranial nerve whereas the trigeminal nerve (V) is both motor and sensory. Spinal nerves arise from the spinal cord and are mixed nerves containing sensory and motor components (23).
Neurons consist of the cell body or soma, single or multiple afferent processes termed dendrites, and a single efferent process termed axon. Pre-ganglionic neurons in the CNS are generally multipolar, characterized by a single axon and many dendrites (27). Post-ganglionic neurons connect to the periphery. Sensory post-ganglionic neurons are pseudounipolar; these have no dendrites and one single axon that connects the periphery to the pre-ganglionic neurons. Bipolar neurons, with a single axon and a single dendrite, are found in sensory organs such as the retina (27). Post-ganglionic sympathetic and parasympathetic neurons are multipolar. The pre-ganglionic neurons of the cranial parasympathetic system are in the brainstem whereas soma of the post-ganglionic neurons lie outside the central nervous system, in submandibular, pterygopalatine, and otic ganglia, and microganglia along facial and glossopharyngeal nerves. Soma of sympathetic post-ganglionic neurons innervating the oral cavity are primarily aggregated in superior cervical ganglia (28). The motor root of the trigeminal nerve originates in the brain and courses through the nerve; the trigeminal ganglion itself has only post-ganglionic sensory neurons. The soma for spinal sensory and motor neurons are in the dorsal root ganglia and grey matter respectively.
The nerve fiber is surrounded by Schwann cells that maintain, repair, and myelinate fibers in the peripheral nervous system (16). Nerve fibers and associated vasculature are surrounded by collagen, extracellular matrix, and fibroblasts that together constitute the endoneurium. Several nerve fibers with surrounding endoneurium are grouped into fascicles by perineurium, which consists of epithelial-like cells with basement membrane on both sides forming a protective barrier (29). The epineurium, consisting of connective tissue, collagen and elastic fibers, surrounds the nerve, wrapping several fascicles together.
Immunohistochemical markers used to characterize nerves include neuron-specific class III β-tubulin (TUJ1), or S100 (Schwann cells), or protein gene product 9.5 (PGP9.5, neuronal/neuroendocrine marker), (all nerves); calcitonin gene related peptide (CGRP) or Substance P or Nav1.8 or Receptor Potential Vanilloid type 1 channel (TRPV1), (sensory nerves); Tyrosine Hydroxylase (TH, sympathetic or adrenergic), Vasoactive Intestinal Polypeptide (VIP), or nNOS, or vesicular acetylcholine transporter (VAChT) or choline acetyl transferase (ChAT) (30) (parasympathetic or cholinergic); and growth associated protein 43 (GAP43, neurite growth). ChAT and VAChT are expressed by lower motor neurons connecting to the periphery, including branchial, visceral, and somatic motor neurons (31). In rat, light and heavy neurofilaments (NF-L and NF-H, respectively) are used to identify nascent and mature nerve fibers (30). For a more comprehensive review of nerve markers, please refer to Hernandez et al. (32).
Site-specific Innervation (Figure 1)
Figure 1:
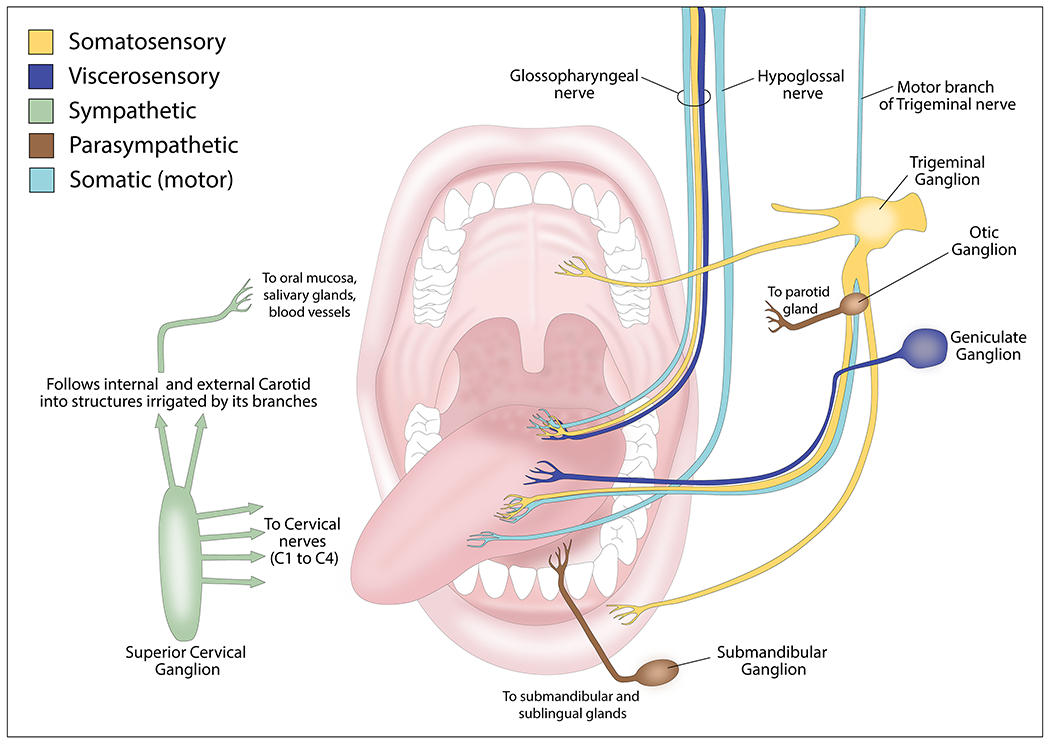
Innervation of the oral cavity.
The trigeminal (V), facial (VII), glossopharyngeal (IX), vagus (X), and hypoglossal (XII) cranial nerves and the spinal accessory nerve innervate the oral cavity (33). The maxillary and mandibular branches of the trigeminal nerve innervate the mucosal lining of the oral cavity. The lingual branch of the mandibular nerve provides general sensory innervation (pain and touch) for the anterior two-thirds of the tongue. The taste sensory innervation of this region is from the chorda tympani nerve, a branch of the facial nerve that arises from the geniculate ganglion (34). The posterior third of the tongue that differs in embryologic origin from the anterior two-thirds, has taste and general sensory innervation from the glossopharyngeal nerve. The base of the tongue is supplied by the superior laryngeal branch of the vagus nerve. The hypoglossal nerve innervates the tongue muscles, except the palatoglossus muscle that is innervated by the vagus nerve (33).
The maxillary and mandibular divisions of the trigeminal nerve innervate the gingiva (35). The maxillary nerve innervates the maxillary labial gingiva and teeth via the superior alveolar branch, whereas the nasopalatine and greater palatine branches innervate the palatal gingiva and hard palate. Sensory innervation of the soft palate is from the lesser palatine branch of the maxillary nerve. The buccal and inferior alveolar nerves, both from the mandibular nerve, innervate the mandibular labial gingiva and teeth, whereas the lingual nerve provides innervation of the lingual gingiva (35).
The labial branch of the infraorbital nerve from the maxillary division of the trigeminal nerve provides sensory supply to the upper lip. The mental branch of the inferior alveolar nerve from the mandibular division of the trigeminal nerve innervates the lower lip. The buccal branch of the mandibular nerve provides sensory innervation for the buccal mucosa (36).
The motor innervation of the muscles of the lips and cheek is from the buccal branch of the facial nerve. The mylohyoid branch of the inferior alveolar nerve innervates the mylohyoid and anterior belly of the digastric muscles. The glossopharyngeal and vagus nerves form the pharyngeal plexus that innervates the tonsils and soft palate muscles, except for the tensor veli palatine (37). The ganglionic branches of the maxillary nerve innervate the mucosa of the pharynx and palate. Thus, sensory, motor, and autonomic nerve fibers innervate the oral cavity and could interact with tumors arising in this location. The current literature on nerve-oral cancer interactions is described in the following section.
Neural influence in oral cavity cancer
Nerves are an essential component of the tumor microenvironment contributing to growth, progression, and spread of cancer. Nerves were historically considered passive bystanders in perineural invasion with cancer cells being the aggressor. However, in a landmark in vitro study in prostate cancer, Ayala et al, (38) showed dynamic interaction between cancer and nerves that led to cancer cells invading nerves, which project neurites towards cancer cells. In an in vitro model of perineural invasion, prostate cancer-related axonogenesis may be regulated by Semaphorin 4F (S4F) (39). PC-12-derived neuronal cells, treated with conditioned medium from a co-culture of dorsal root ganglia with DU145 human prostate cancer cells, showed higher neurite outgrowth when compared with PC-12 cells treated with conditioned medium from cancer cells alone or blank conditioned medium (39). Furthermore, N1E-115 cells stimulated with conditioned medium from prostate cancer cells overexpressing Semaphorin 4F developed more neurite sprouting, interestingly siRNA-mediated downregulation of Semaphorin 4F reversed this effect (39). More recently, in establishing the dynamic interaction between cancer and nerves in oral cancer, Scanlon et al (40) used in vitro and in vivo approaches to show that nerves release the neuropeptide galanin to induce galanin receptor 2 on cancer cells. Cancer cells reciprocate by releasing galanin and prostaglandin E2 (PGE2) to enhance neurite outgrowth and invasion. In an earlier study, the same group showed that galanin receptor 2 also enhances tumor growth and survival of oral cancer cells (41). In a subsequent study, Madeo et al (42) showed that exosomes released by head and neck cancer cells induce axon outgrowth from neuronal cells. Deletion of Rab27a/b, which blocks secretion, or biochemical inhibition of exosome release, suppresses this activity. Ephrin B1, an axon guidance molecule transported within exosomes, enhances axonal outgrowth from neuronal cells. Using TUJ1, TH, VIP and TRPV1 on tissue sections from 15 human head and neck SCC, Madeo et al (42) reported that these tumors are innervated by sensory (TUJ1 and TRPV1 positive) rather than sympathetic (TH) or parasympathetic nerves (VIP).
Recent studies exploring the interaction between nerves and cancer highlighted the role of the autonomic nervous system in tumor progression. In a ground breaking study, using chemical and surgical sympathectomy, Magnon et al, (43) showed that adrenergic nerves are required for tumor initiation, whereas the parasympathetic nervous system facilitates cancer progression. They used Hi-Myc transgenic mice, a prostate cancer model that has complete penetrance of intraepithelial neoplasia at post-natal week 2. Sympathectomy in neonate mice abrogated tumor initiation but these effects were attenuated if a sympathectomy was performed in 1 month old mice. In adult mice, sympathectomy had no effect in blocking tumor initiation. In mice with established prostate cancer, cholinergic agonists enhanced metastases to lymph nodes, which was abrogated with cholinergic antagonists. This carbachol-induced spread to lymph nodes was also reduced in cholinergic receptor knockout mice (Chrm1−/−). Together these biochemical and genetic studies established the role of the autonomic nervous system in stroma-dependent, initiation and progression of prostate cancer.
In the oral cavity, an in vivo study showed that sympathetic nerves also influence tumor progression and gene expression within the tumor. Sympathectomy reduced interstitial fluid pressure, lymphatic vessels and growth in rat tongue tumors (44), altering the gene profile of these tumors compared to control and sham groups (45). Genes related with cancer progression such as Akr1b8, Anxa1, Cdh3, Cxcl2, Hif1a, Itgb1, Mip2b, Mmp3, Pdpn, Postn, Timp1, and Pcna were found upregulated in the sham group (45). Cathecolamines such as norepinephrine are stress-derived molecules produced by the sympathetic nervous system. Norepinephrine acts through its α- and β-adrenergic receptors (ADRA, ADRB). Norepinephrine has been associated with OSCC proliferation and invasion through ADRB2/ERK/CREB signaling pathway (46). As expected, ERK and CREB inhibitors suppressed the norepinephrine-induced effects (46). Furthermore, an in vitro study reveals that norepinephrine-stimulated oral cancer cells reduced the cleavage of capsase-3, inhibited apoptosis, and consequently, induced resistance to cisplatin through the ADRB/Akt/ABCG2 (ATP-binding cassette subfamily G, isoform 2 protein) pathway (47). Since ADRB2 is present in oral cancer cells, β-blocker treatment has been explored (48). In an in vitro study, propranolol showed an anti-tumor effect, inhibiting cancer cell viability and the expression of pAkt, NF-κB, and VEGF in head and neck cancer cells (48). A subsequent elegant study from Amit et al (49), established the origin of sympathetic nerves that innervate oral cancer showing that these nerves are derived from trans-differentiation of sensory nerves. p53 is one of the most commonly mutated genes in OSCC (50). Using in vitro approaches, p53−/− mice, and injection of OSCC cell lines in mice, Amit et al (49), showed that cancer cells with mutant/inactive p53 induce neuritogenesis modulating the release of factors transported within exosomes. OSCC cells with mutant p53 do not secrete microRNA miR-34a; the absence of miR-34a coupled with the presence of miR-21 and miR-324 allows trans-differentiation of sensory to TH+ sympathetic neurons. This phenotypic switch is important for neural innervation of OSCC. However, Atherton et al. identified postganglionic sympathetic nerves in C57Bl/6 mouse tongue tumors using a retrograde tracer that labeled neurons in the superior cervical ganglion (51). Importantly, increased TH-positive nerve density is associated with aggressive OSCC (49).
Neural innervation of OSCC can be enhanced by a diet rich in palmitic acid. Pascual et al (52) showed that tumors from mice exposed to palmitic acid, or cancer cells briefly exposed to palmitic acid, acquire an aggressive metastatic phenotype. In response to dietary palmitic acid, tumor cells express CD36; these tumor cells then release galanin to stimulate Schwann cell colonization in the tumor bulk. This phenotype is retained even after several passages in mice, attributed to transcriptional and chromatin changes induced by palmitic acid. Included in these changes is a neural signature that leads to release of factors that induce a switch in intra-tumoral Schwann cells to a ‘proregenerative’ phenotype. The proregenerative Schwann cells release extracellular matrix factors that facilitate metastasis of OSCC (52).
Together these studies have provided major insights on the biology of neural involvement in oral cancer progression with significant implications for neural phenotypes observed in biopsies of human OSCC. For more information on other types of cancers and their interactions with the nervous system, please refer to recent review articles cited here (53–56).
Neural phenotypes in Oral Cancer
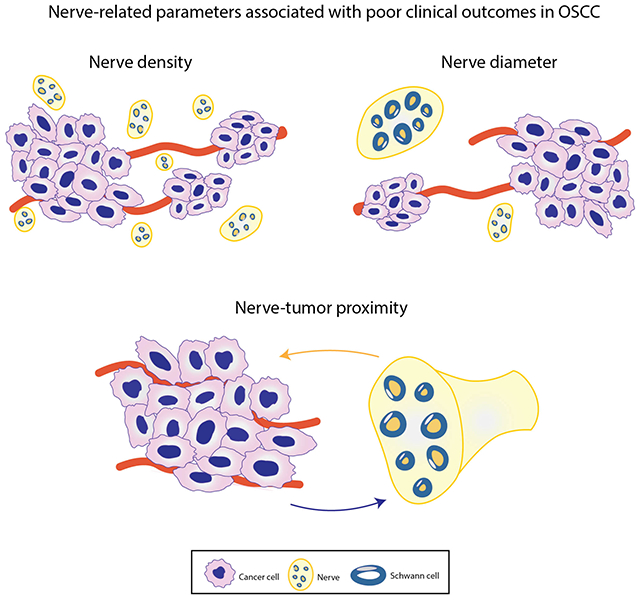
Given the emergence of the field of cancer neuroscience (57), with its elucidation of the mechanistic basis of cancer-nerve interactions, it is important to re-evaluate diagnostic criteria for perineural invasion and assess other neural phenotypes in the context of OSCC progression and patient survival. In addition to perineural invasion, neural phenotypes that have emerged as potentially important for prognosis in OSCC are nerve-tumor distance, nerve diameter, and nerve density (Figure 2). These phenotypes are supported by the biology of OSCC.
Figure 2:
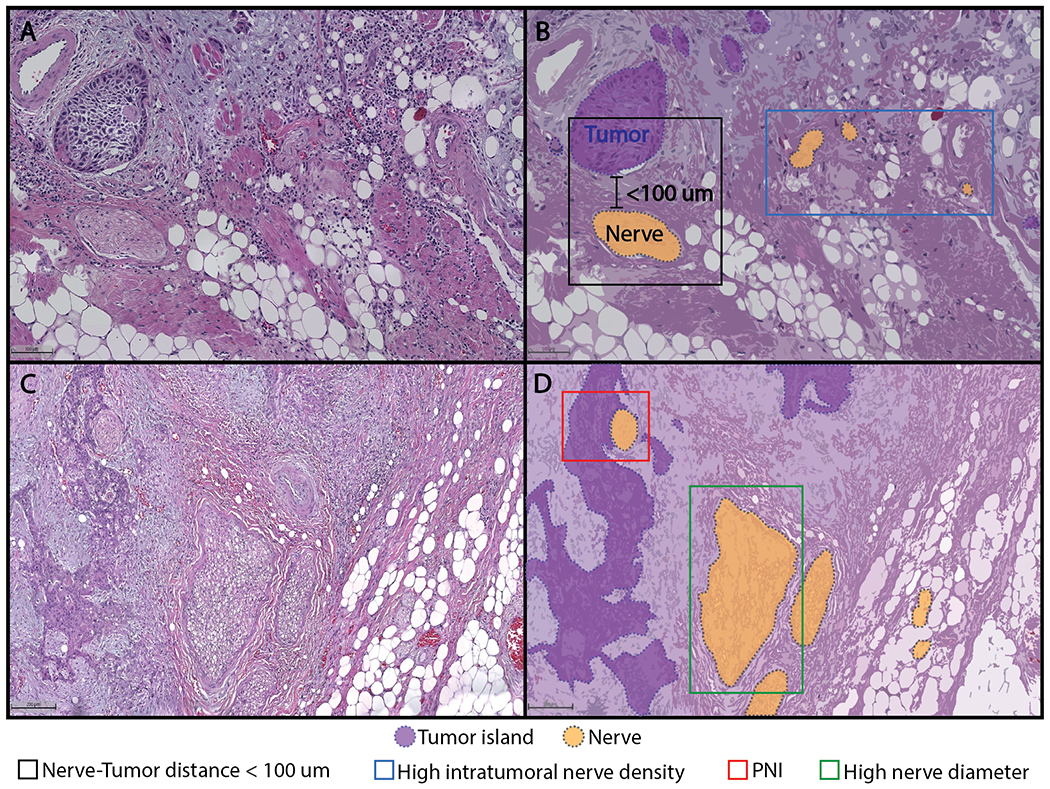
Neural phenotypes observed in human OSCC tissue sections that could be of relevance to prognosis and treatment selection in oral cavity cancer (panel A, bar = 100 μm; panel C, bar = 200 μm).
Perineural invasion is the best recognized histopathologic neural phenotype that highlights the interaction between nerves and cancer cells. Perineural invasion was originally defined as ‘cancer within, around or through the nerve’ (58). Subsequently, this definition was updated to cancer ‘surrounding at least 33% of the nerve’ or within the epineurium, endoneurium or perineurium (59), which is the current definition.
Perineural invasion has been repeatedly associated with poor outcomes in OSCC and multiple other cancers. Since OSCC with perineural invasion may be treated aggressively (60), it is important to accurately identify this phenotype in diagnostic biopsies. Using current criteria, detection of perineural invasion in SCC is enhanced by immunohistochemical staining with S100 or TUJ1, and cytokeratin to highlight nerves and tumor cells, respectively (61–64). In a study on biopsy tissue from 142 patients with OSCC, detection of perineural invasion was significantly increased from 26.1% on routine hematoxylin and eosin (H&E) stained sections to 43% when H&E was used in conjunction with immunohistochemistry (61, 62). Together these studies support the use of immunohistochemistry to enhance detection of perineural invasion in OSCC.
Previous studies reported perineural invasion in 5.2 to 90% of OSCC (16). This wide variability could be due to differences in interpretation between pathologists of what represents perineural invasion. As reported by Chi et al (65, 66), there is only fair to moderate agreement among pathologists in identifying perineural invasion in OSCC, possibly due to variations in interpretation of current criteria. The wide range in reported incidence of perineural invasion could also be due to reliance on pathology reports in these studies. These reports may not include perineural invasion in the microscopic description of the tumor; consequently, studies that use reports alone would underestimate this neural phenotype (16, 61, 62). Moreover, diagnostic criteria for perineural invasion have evolved thereby impacting conclusions in studies that relied solely on archival pathology reports. Standardization of reporting criteria in pathology reports or evaluation of tissue specimens rather than review of reports could provide more accurate information for this important phenotype.
Perineural invasion in OSCC has been reported as a surrogate marker for lymph node involvement. Metastases to the lymph nodes and locoregional recurrence in the lymph nodes after initial treatment, are associated with poor prognosis in head and neck SCC (62, 67). Consequently, patients with metastases to the lymph node at the time of diagnosis receive aggressive treatment (16, 68–71). It is important to accurately identify lymph node metastases due to the impact on the treatment plan. Perineural invasion could predict occult lymph node metastases at the time of diagnosis; for example, in tongue tumors, perineural invasion is associated with occult lymph node metastases (72). Perineural invasion in primary OSCC also increases the risk of recurrence in regional lymph nodes (60, 73). Perineural invasion is an independent risk factor for poor disease-specific survival (60–62). Studying biopsy tissue from 142 patients, Schmitd et al. (62), reported that OSCC with perineural invasion and negative lymph nodes (PNI+, N0), was associated with significantly poorer disease-specific survival, disease-free survival, and overall survival than in the absence of perineural invasion (PNI-, N0). Despite receiving adjuvant radiation, the PNI+, N0 group had a significantly higher hazard ratio than the PNI-, N0 group. An independent study also showed that perineural invasion is associated with worse disease-free interval, and locoregional control (60).
The impact of number of nerves exhibiting perineural invasion on survival has been disputed. Multifocal perineural invasion has been associated with poorer survival than unifocal involvement (74). However, two studies, including a large recent study showed no relationship between the number of nerves exhibiting perineural invasion and survival (60, 62). Even in patients with early stage OSCC (Stages I and II), no survival difference was observed between patients with less than 5 or more than 5 nerves exhibiting perineural invasion (62). This could be due to differences in treatment because early stage lesions with multifocal perineural invasion are more likely to receive adjuvant therapy in addition to surgery, than early lesions with unifocal nerve involvement (62).
In OSCC, perineural invasion in a nerve with a diameter of >1 mm has been linked to higher loco-regional recurrence (74, 75). However, Tarsitano et al (76) reported an association of perineural invasion with loco-regional recurrence regardless of nerve diameter. In a more recent study in OSCC, only 7 of more than 9000 nerves that were measured had a diameter larger than 1 mm (62). Moreover, no association was observed between nerve diameter and survival even when nerves exhibiting perineural invasion were grouped into tertiles of small (<29.2 μm), medium (29.2 to 47.5 μm) and large (>47.5 μm).
In summary, using current criteria, perineural invasion in OSCC is an independent risk factor for poor survival regardless of the diameter and number of nerves exhibiting this phenotype; immunohistochemical stains for cancer cells and nerves enhance detection of perineural invasion.
Nerve-Tumor Distance:
The pitfall of the current definition of perineural invasion requiring cancer to approximate at least 33% of the nerve, is that it does not consider recent findings of crosstalk between nerves and OSCC (1, 40). For example, studies in several cancers, including OSCC, show that cancer and nerves have dynamic interactions even prior to physical contact (38, 40). This suggests that the distance between nerves and cancer cells in OSCC tissue could be relevant to clinical outcome. To address this knowledge gap, recent studies in OSCC evaluated nerve-tumor distance with respect to survival (61, 62).
Nerve-tumor distance is defined as the shortest distance between any nerve and the nearest tumor island, regardless of whether the tumor surrounds the nerve. In a cohort of 142 patients with OSCC, the minimum nerve-tumor distance within a patient was linked to disease-specific survival, disease-free survival, and overall survival (62); the smaller the distance, the poorer the survival. Shorter nerve-tumor distance in patients whose tumors would be considered negative for perineural invasion under current criteria, was also associated with poor survival. Importantly, this study showed that shorter nerve-tumor distance is associated with poor disease-specific survival regardless of whether American Joint Commission on Cancer (AJCC) 7th or 8th edition criteria were used to adjust the Cox regression model. In contrast to the 7th edition, the 8th edition classification incorporates depth of invasion and extracapsular extension (77).
Assessing nerve-tumor distance in over 9000 nerves in OSCC from 142 patients, Schmitd et al (62), reported that the death rate decreases gradually with increasing nerve-tumor distance with the highest death rate at distance 0 μm (61, 62). Even in patients without lymph node involvement (N0), nerve-tumor distance < 27 μm, was associated with poor survival. Using a Cox generalized additive model to investigate the association between nerve-tumor distance and death, this study showed that the hazard of death decreases as nerve-tumor distance increases and stabilizes at ~500 μm. Not surprisingly, GAP43, a marker for nerve regeneration, was expressed more strongly in nerves close to tumor and in nerves exhibiting perineural invasion (61). These finding are consistent with mechanistic studies that show that nerves and OSCC cells in proximity to each other have very dynamic communication (40).
Together, these findings translating the biology of nerve-tumor interactions into clinical practice support inclusion of nerve-tumor distance as a neural phenotype of relevance to survival.
Nerve Diameter:
Nerve diameter is the smallest dimension of the nerve section. Large diameter of nerves in the tumor bulk is associated with poor survival. Schmitd et al (62) used regression tree methods to split subjects into two groups based on diameter that was most different with respect to survival. Patients with OSCC that did not exhibit perineural invasion but had nerves 32 μm or greater in the tumor bulk, had as poor survival as those whose tumors had perineural invasion. An inverse relationship between larger nerve diameter and disease-specific survival was observed even in N0 patients. The association between large nerves in the tumor bulk and poor survival was retained even after adjustments for comorbidities and AJCC 8th edition staging. Similar findings were also reported recently in pancreatic cancer; larger nerves were associated with poorer survival regardless of the presence of perineural invasion (78). It is possible that nerve diameter is a surrogate marker for nerve activity, i.e., the higher the activity, the stronger the attraction between cancer cells and nerves. In support of this possibility, GAP43 is expressed more strongly at the periphery of large nerves (61).
Nerve Density:
This is the area or number of nerves divided by the area analyzed in a tissue section (79). Changes in nerve density may be due to neurites growing into the tumor (axonogenesis), new neurons in the tumor due to differentiation of precursor cells (neurogenesis), or cancer cells growing towards nerves (neurotropism). Nerve fibers are quantified to determine nerve density due to axonogenesis and neurogenesis whereas nerve trunks or fascicles are quantified to assess nerve density due to neurotropism (79).
Factors secreted by nerves, including neurotransmitters, chemokines, neuropeptides, and neurotrophins, enhance neurite growth and cancer progression. In OSCC, the neuropeptide galanin enhances neurite outgrowth as well as growth, invasion, and metastasis of cancer cells (40, 52). This suggests that increased nerve density is associated with poor survival. In fact, using immunofluorescence to evaluate density of TH+ and VAChT+ nerves, Amit et al (49), reported that high density of TH+ nerves is associated with poor overall and recurrence-free survival.
Another study in OSCC determined that if the density of nerves exhibiting perineural invasion is >1, then patients have poor recurrence-free survival (80). This association could be due to perineural invasion itself rather than the number of nerves exhibiting perineural invasion. As mentioned earlier, other studies have shown no association between the number of nerves exhibiting perineural invasion and survival (60, 62). Since perineural invasion is usually detected in only one nerve in a section of OSCC (62), nerve density, regardless of perineural invasion, may be a more consistent phenotype of aggressive tumors. Additional studies and clear criteria for nerve density would help establish whether this phenotype is relevant for tumor progression.
Conclusions and Future Directions
Given the expanding literature elucidating the biology of interactions between nerves and cancer, it is important to translate these findings into clinical practice. Moreover, although perineural invasion and other neural phenotypes have been associated with progression of OSCC, all relevant neural phenotypes are not included in current assessment criteria for treatment selection and outcomes in OSCC. Given the neural phenotypes that have been associated with adverse outcomes, it is time to develop a comprehensive set of neural parameters that can be used to facilitate treatment selection and predict response to treatment of oral cancer. This could include perineural invasion, nerve-tumor distance, nerve diameter and potentially nerve density (Table I, Figure 2). Moreover, histopathologic criteria of neural phenotypes such as perineural invasion should be revised in the context of current knowledge of the biology of OSCC. With this motivation, replacement of perineural invasion by nerve-tumor distance could be valuable. Standardized approaches for quantification would enhance the value of nerve density as a neural parameter; this is an area that requires further investigation. It is likely that the significance of neural phenotypes in OSCC will vary by site due to differences in innervation in the oral cavity. Since tongue is one of the most common sites of OSCC, most studies focus on tongue or the majority of patients in a study have tongue OSCC. Additional studies would reveal if neural phenotypes at other intraoral sites that vary in innervation from the tongue, are clinically-relevant for survival. This would allow a more personalized approach to treatment selection for OSCC by site with the potential to improve outcomes. Importantly, this could provide cost-effective, rapid, and universally deployable criteria for assessing prognosis of OSCC.
Table I:
Summary of neural phenotypes that could be of relevance to clinical outcome.
| Phenotype | Definition | Criteria or Comments | Methods | References |
|---|---|---|---|---|
| Perineural invasion | Nerve invaded by or has at least 33% of its perimeter surrounded by tumor cells. | Detection enhanced by IHC. | H&E IHC: Pan-cytokeratin for epithelium, S100 or TUJ1 for nerve. |
(59) (61–63) |
| Nerve-tumor distance | Shortest distance between any nerve and nearest tumor island. | PNI negative according to current criteria but nerve-tumor distance less than 27 μm*; could increase up to ~100 μm. | H&E IHC: Pan-cytokeratin for epithelium, S100 or TUJ1 for nerve. Software to measure distance. |
(61, 62) |
| Nerve diameter | Smallest axis of cross-section of nerve. | Greater than 32 μm*; could increase up to ~50 μm. Nerves >2 mm from tumor bulk excluded. |
H&E Software to measure distance. |
(62) |
| Nerve density | Area or number of nerves divided by the area analyzed in a tissue section. | Requires further standardization | H&E IHC: Pan-cytokeratin for epithelium, S100 or TUJ1 for nerve. |
(49, 79) |
H&E: hematoxylin and eosin
IHC: immunohistochemistry
Acknowledgements
This work was supported by grants from NIH/NIDCR DE027551 and NIH/NCI CA250214 (NJD).
Biographies
Nisha D’Silva BDS, MSD, PhD is the Donald Kerr Endowed Collegiate Professor at the University of Michigan School of Dentistry, Professor of Pathology at the Medical School, and a Rogel Scholar at the Rogel Cancer Center. She is a dentist-scientist; her translational research in oral cancer focuses on mechanisms and biomarkers of tumor progression and treatment resistance. Her research is currently funded by the National Institute of Dental and Craniofacial Research/NIH and by the National Cancer Institute/NIH. She is a Diplomate of the American Board of Oral and Maxillofacial Pathology, which is her area of clinical practice.

Cindy Perez-Pacheco, DDS, MSc, PhD, is a postdoctoral research fellow in Nisha D’Silva’s laboratory at the University of Michigan School Of Dentistry. Dr. Perez-Pacheco received her dental degree at the Peruvian University Cayetano Heredia, Lima-Peru; her Masters and PhD degrees in Dentistry at State University of Ponta Grossa, Parana-Brazil, and at the Sao Paulo State University, Sao Paulo-Brazil, respectively. Currently, Dr. Perez-Pacheco’s research focuses on nerve-tumor interactions.

Ligia B. Schmitd obtained her dental and master’s degrees at the University of Sao Paulo, Brazil. She specializes in the field of Oral Medicine, and conducted her PhD at the Oral Health Sciences Program, University of Michigan School of Dentistry. Her PhD work focused on cancer neuroscience, the investigation of nerve-tumor interactions, more specifically in the context of oral cavity cancer. She is currently a postdoctoral fellow at the University of Michigan Medical School studying Schwann cell biology in peripheral nervous system injury and regeneration.

Footnotes
Conflict of interest: The authors have declared that no conflict of interest exists.
References
- 1.D’Silva NJ, and Gutkind JS (2019) Oral Cancer: Integration of Studies for Diagnostic and Therapeutic Precision. Adv Dent Res 30, 45–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Leemans CR, Snijders PJF, and Brakenhoff RH (2018) The molecular landscape of head and neck cancer. Nat Rev Cancer 18, 269–282 [DOI] [PubMed] [Google Scholar]
- 3.Ferlay J, Colombet M, Soerjomataram I, Parkin DM, Piñeros M, Znaor A, and Bray F (2021) Cancer statistics for the year 2020: An overview. Int J Cancer [DOI] [PubMed] [Google Scholar]
- 4.Neville BW, Damm DD, Allen C, and Chi AC (2015) Oral and maxillofacial pathology, Elsevier Health Sciences [Google Scholar]
- 5.Roman BR, and Aragones A (2021) Epidemiology and incidence of HPV-related cancers of the head and neck. J Surg Oncol 124, 920–922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Muller S, Boy SC, Day TA, Magliocca KR, Richardson MS, Sloan P, Tilakaratne WM, Zain RB, and Thompson LDR (2019) Data Set for the Reporting of Oral Cavity Carcinomas: Explanations and Recommendations of the Guidelines From the International Collaboration of Cancer Reporting. Arch Pathol Lab Med 143, 439–446 [DOI] [PubMed] [Google Scholar]
- 7.Scanlon CS, Van Tubergen EA, Inglehart RC, and D’Silva NJ (2013) Biomarkers of epithelial-mesenchymal transition in squamous cell carcinoma. J Dent Res 92, 114–121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Irani S (2016) Distant metastasis from oral cancer: A review and molecular biologic aspects. J Int Soc Prev Community Dent 6, 265–271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cuffari L, Tesseroli de Siqueira JT, Nemr K, and Rapaport A (2006) Pain complaint as the first symptom of oral cancer: a descriptive study. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 102, 56–61 [DOI] [PubMed] [Google Scholar]
- 10.Scharpf J, Karnell LH, Christensen AJ, and Funk GF (2009) The role of pain in head and neck cancer recurrence and survivorship. Arch Otolaryngol Head Neck Surg 135, 789–794 [DOI] [PubMed] [Google Scholar]
- 11.Sanchis JM, Bagan JV, Murillo J, Diaz JM, Poveda R, and Jimenez Y (2008) Mental neuropathy as a manifestation associated with malignant processes: its significance in relation to patient survival. J Oral Maxillofac Surg 66, 995–998 [DOI] [PubMed] [Google Scholar]
- 12.Battaglini E, Goldstein D, Grimison P, McCullough S, Mendoza-Jones P, and Park SB (2021) Chemotherapy-Induced Peripheral Neurotoxicity in Cancer Survivors: Predictors of Long-Term Patient Outcomes. J Natl Compr Canc Netw 19, 821–828 [DOI] [PubMed] [Google Scholar]
- 13.Vecht CJ, Hoff AM, Kansen PJ, de Boer MF, and Bosch DA (1992) Types and causes of pain in cancer of the head and neck. Cancer 70, 178–184 [DOI] [PubMed] [Google Scholar]
- 14.Ye Y, Jensen DD, Viet CT, Pan HL, Campana WM, Amit M, and Boada MD (2022) Advances in Head and Neck Cancer Pain. J Dent Res 101, 1025–1033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cata JP, Uhelski ML, Gorur A, Bhoir S, Ilsin N, and Dougherty PM (2022) The micro-Opioid Receptor in Cancer and Its Role in Perineural Invasion: A Short Review and New Evidence. Adv Biol (Weinh), e2200020. [DOI] [PubMed] [Google Scholar]
- 16.Schmitd LB, Scanlon CS, and D’Silva NJ (2018) Perineural Invasion in Head and Neck Cancer. J Dent Res 97, 742–750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Salvo E, Campana WM, Scheff NN, Nguyen TH, Jeong SH, Wall I, Wu AK, Zhang S, Kim H, Bhattacharya A, Janal MN, Liu C, Albertson DG, Schmidt BL, Dolan JC, Schmidt RE, Boada MD, and Ye Y (2020) Peripheral nerve injury and sensitization underlie pain associated with oral cancer perineural invasion. Pain 161, 2592–2602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Du KN, Shepherd AJ, Ma IV, Roldan CJ, Amit M, Feng LMS, Desai S, and Cata JP (2020) Lack of association between angiotensin-converting enzyme inhibitors and angiotensin receptor blockers and pain improvement in patients with oral cancer. Ecancermedicalscience 14, 1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kejner A, Gentile C, Porterfield Z, Carroll WR, and Buczek EP (2022) Positive Deep Initial Incision Margin Affects Outcomes in TORS for HPV+ Oropharynx Cancer. Laryngoscope [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Albergotti WG, Schwarzbach HL, Abberbock S, Ferris RL, Johnson JT, Duvvuri U, and Kim S (2017) Defining the Prevalence and Prognostic Value of Perineural Invasion and Angiolymphatic Invasion in Human Papillomavirus-Positive Oropharyngeal Carcinoma. JAMA Otolaryngol Head Neck Surg 143, 1236–1243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tassone P, Crawley M, Bovenzi C, Zhan T, Keane W, Cognetti D, Luginbuhl A, and Curry J (2017) Pathologic Markers in Surgically Treated HPV-Associated Oropharyngeal Cancer: Retrospective Study, Systematic Review, and Meta-analysis. Ann Otol Rhinol Laryngol 126, 365–374 [DOI] [PubMed] [Google Scholar]
- 22.Watson CK, Matthew; Paxinos George. (2010) The Brain: An Introduction to Functional Neuroanatomy, Elsevier [Google Scholar]
- 23.SEER Training Modules. Peripheral Nervous Sytem. U. S. National Institutes of Health, National Cancer Institute. 01, July 2022. [Google Scholar]
- 24.Akinrodoye MA, and Lui F (2022) Neuroanatomy, Somatic Nervous System. In StatPearls, StatPearls Publishing Copyright © 2022, StatPearls Publishing LLC., Treasure Island (FL) [PubMed] [Google Scholar]
- 25.Wehrwein EA, Orer HS, and Barman SM (2016) Overview of the Anatomy, Physiology, and Pharmacology of the Autonomic Nervous System. Compr Physiol 6, 1239–1278 [DOI] [PubMed] [Google Scholar]
- 26.Price S, and Daly DT (2022) Neuroanatomy, Trigeminal Nucleus. In StatPearls, StatPearls Publishing Copyright © 2022, StatPearls Publishing LLC., Treasure Island (FL) [PubMed] [Google Scholar]
- 27.Jennes L (2017) Cytology of the Central Nervous System. In Conn’s Translational Neuroscience pp. 1–10, Elsevier [Google Scholar]
- 28.Maningat AL, and Munakomi S (2022) Neuroanatomy, Superior Cervical Ganglion. In StatPearls, StatPearls Publishing Copyright © 2022, StatPearls Publishing LLC., Treasure Island (FL) [PubMed] [Google Scholar]
- 29.Lowe J, and Anderson P (2014) Stevens & Lowe’s Human Histology 4. baskı. London: Mosby [Google Scholar]
- 30.Carden MJ, Trojanowski JQ, Schlaepfer WW, and Lee VM (1987) Two-stage expression of neurofilament polypeptides during rat neurogenesis with early establishment of adult phosphorylation patterns. J Neurosci 7, 3489–3504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stifani N (2014) Motor neurons and the generation of spinal motor neuron diversity. Front Cell Neurosci 8, 293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hernandez S, Serrano AG, and Solis Soto LM (2022) The Role of Nerve Fibers in the Tumor Immune Microenvironment of Solid Tumors. Adv Biol (Weinh), e2200046 [DOI] [PubMed] [Google Scholar]
- 33.Kamrani P, and Sadiq NM (2022) Anatomy, Head and Neck, Oral Cavity (Mouth). In StatPearls, StatPearls Publishing Copyright © 2022, StatPearls Publishing LLC., Treasure Island (FL) [PubMed] [Google Scholar]
- 34.Sharabi AF, and Winters R (2022) Glossitis. In StatPearls, StatPearls Publishing Copyright © 2022, StatPearls Publishing LLC., Treasure Island (FL) [PubMed] [Google Scholar]
- 35.Koller A, and Sapra A (2022) Anatomy, Head and Neck, Oral Gingiva. In StatPearls, StatPearls Publishing Copyright © 2022, StatPearls Publishing LLC., Treasure Island (FL) [PubMed] [Google Scholar]
- 36.Piccinin MA, and Zito PM (2022) Anatomy, Head and Neck, Lips. In StatPearls, StatPearls Publishing Copyright © 2022, StatPearls Publishing LLC., Treasure Island (FL) [PubMed] [Google Scholar]
- 37.Helwany M, and Rathee M (2022) Anatomy, Head and Neck, Palate. In StatPearls, StatPearls Publishing Copyright © 2022, StatPearls Publishing LLC., Treasure Island (FL) [PubMed] [Google Scholar]
- 38.Ayala GE, Wheeler TM, Shine HD, Schmelz M, Frolov A, Chakraborty S, and Rowley D (2001) In vitro dorsal root ganglia and human prostate cell line interaction: redefining perineural invasion in prostate cancer. Prostate 49, 213–223 [DOI] [PubMed] [Google Scholar]
- 39.Ayala GE, Dai H, Powell M, Li R, Ding Y, Wheeler TM, Shine D, Kadmon D, Thompson T, Miles BJ, Ittmann MM, and Rowley D (2008) Cancer-related axonogenesis and neurogenesis in prostate cancer. Clin Cancer Res 14, 7593–7603 [DOI] [PubMed] [Google Scholar]
- 40.Scanlon CS, Banerjee R, Inglehart RC, Liu M, Russo N, Hariharan A, van Tubergen EA, Corson SL, Asangani IA, Mistretta CM, Chinnaiyan AM, and D’Silva NJ (2015) Galanin modulates the neural niche to favour perineural invasion in head and neck cancer. Nat Commun 6, 6885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Banerjee R, Henson BS, Russo N, Tsodikov A, and D’Silva NJ (2011) Rap1 mediates galanin receptor 2-induced proliferation and survival in squamous cell carcinoma. Cell Signal 23, 1110–1118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Madeo M, Colbert PL, Vermeer DW, Lucido CT, Cain JT, Vichaya EG, Grossberg AJ, Muirhead D, Rickel AP, Hong Z, Zhao J, Weimer JM, Spanos WC, Lee JH, Dantzer R, and Vermeer PD (2018) Cancer exosomes induce tumor innervation. Nat Commun 9, 4284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Magnon C, Hall SJ, Lin J, Xue X, Gerber L, Freedland SJ, and Frenette PS (2013) Autonomic nerve development contributes to prostate cancer progression. Science 341, 1236361. [DOI] [PubMed] [Google Scholar]
- 44.Raju B, Haug SR, Ibrahim SO, and Heyeraas KJ (2007) Sympathectomy decreases size and invasiveness of tongue cancer in rats. Neuroscience 149, 715–725 [DOI] [PubMed] [Google Scholar]
- 45.Raju B, Hultström M, Haug SR, Ibrahim SO, and Heyeraas KJ (2009) Sympathectomy suppresses tumor growth and alters gene-expression profiles in rat tongue cancer. Eur J Oral Sci 117, 351–361 [DOI] [PubMed] [Google Scholar]
- 46.Zhang B, Wu C, Chen W, Qiu L, Li S, Wang T, Xie H, Li Y, Li C, and Li L (2020) The stress hormone norepinephrine promotes tumor progression through β2-adrenoreceptors in oral cancer. Arch Oral Biol 113, 104712. [DOI] [PubMed] [Google Scholar]
- 47.Tjioe KC, Cardoso DM, Oliveira SHP, and Bernabé DG (2022) Stress hormone norepinephrine incites resistance of oral cancer cells to chemotherapy. Endocr Relat Cancer 29, 201–212 [DOI] [PubMed] [Google Scholar]
- 48.Shibuya CM, Tjioe KC, Oliveira SHP, and Bernabé DG (2022) Propranolol inhibits cell viability and expression of the pro-tumorigenic proteins Akt, NF-ĸB, and VEGF in oral squamous cell carcinoma. Arch Oral Biol 136, 105383. [DOI] [PubMed] [Google Scholar]
- 49.Amit M, Takahashi H, Dragomir MP, Lindemann A, Gleber-Netto FO, Pickering CR, Anfossi S, Osman AA, Cai Y, Wang R, Knutsen E, Shimizu M, Ivan C, Rao X, Wang J, Silverman DA, Tam S, Zhao M, Caulin C, Zinger A, Tasciotti E, Dougherty PM, El-Naggar A, Calin GA, and Myers JN (2020) Loss of p53 drives neuron reprogramming in head and neck cancer. Nature 578, 449–454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stransky N, Egloff AM, Tward AD, Kostic AD, Cibulskis K, Sivachenko A, Kryukov GV, Lawrence MS, Sougnez C, McKenna A, Shefler E, Ramos AH, Stojanov P, Carter SL, Voet D, Cortés ML, Auclair D, Berger MF, Saksena G, Guiducci C, Onofrio RC, Parkin M, Romkes M, Weissfeld JL, Seethala RR, Wang L, Rangel-Escareño C, Fernandez-Lopez JC, Hidalgo-Miranda A, Melendez-Zajgla J, Winckler W, Ardlie K, Gabriel SB, Meyerson M, Lander ES, Getz G, Golub TR, Garraway LA, and Grandis JR (2011) The mutational landscape of head and neck squamous cell carcinoma. Science 333, 1157–1160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Atherton M, Park S, Horan NL, Nicholson S, Dolan JC, Schmidt BL, and Scheff NN (2022) Sympathetic modulation of tumor necrosis factor alpha-induced nociception in the presence of oral squamous cell carcinoma. Pain [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pascual G, Domínguez D, Elosúa-Bayes M, Beckedorff F, Laudanna C, Bigas C, Douillet D, Greco C, Symeonidi A, Hernández I, Gil SR, Prats N, Bescós C, Shiekhattar R, Amit M, Heyn H, Shilatifard A, and Benitah SA (2021) Dietary palmitic acid promotes a prometastatic memory via Schwann cells. Nature 599, 485–490 [DOI] [PubMed] [Google Scholar]
- 53.Li J, Kang R, and Tang D (2021) Cellular and molecular mechanisms of perineural invasion of pancreatic ductal adenocarcinoma. Cancer Commun (Lond) 41, 642–660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zahalka AH, and Frenette PS (2020) Nerves in cancer. Nat Rev Cancer 20, 143–157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hunt PJ, Andújar FN, Silverman DA, and Amit M (2021) Mini-review: Trophic interactions between cancer cells and primary afferent neurons. Neurosci Lett 746, 135658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hutchings C, Phillips JA, and Djamgoz MBA (2020) Nerve input to tumours: Pathophysiological consequences of a dynamic relationship. Biochim Biophys Acta Rev Cancer 1874, 188411. [DOI] [PubMed] [Google Scholar]
- 57.Monje M, Borniger JC, D’Silva NJ, Deneen B, Dirks PB, Fattahi F, Frenette PS, Garzia L, Gutmann DH, Hanahan D, Hervey-Jumper SL, Hondermarck H, Hurov JB, Kepecs A, Knox SM, Lloyd AC, Magnon C, Saloman JL, Segal RA, Sloan EK, Sun X, Taylor MD, Tracey KJ, Trotman LC, Tuveson DA, Wang TC, White RA, and Winkler F (2020) Roadmap for the Emerging Field of Cancer Neuroscience. Cell 181, 219–222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Batsakis JG (1985) Nerves and neurotropic carcinomas. Ann Otol Rhinol Laryngol 94, 426–427 [PubMed] [Google Scholar]
- 59.Liebig C, Ayala G, Wilks JA, Berger DH, and Albo D (2009) Perineural invasion in cancer: a review of the literature. Cancer 115, 3379–3391 [DOI] [PubMed] [Google Scholar]
- 60.Chinn SB, Spector ME, Bellile EL, McHugh JB, Gernon TJ, Bradford CR, Wolf GT, Eisbruch A, and Chepeha DB (2013) Impact of perineural invasion in the pathologically N0 neck in oral cavity squamous cell carcinoma. Otolaryngol Head Neck Surg 149, 893–899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Schmitd LB, Beesley LJ, Russo N, Bellile EL, Inglehart RC, Liu M, Romanowicz G, Wolf GT, Taylor JMG, and D’Silva NJ (2018) Redefining Perineural Invasion: Integration of Biology With Clinical Outcome. Neoplasia 20, 657–667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Schmitd LB, Perez-Pacheco C, Bellile EL, Wu W, Casper K, Mierzwa M, Rozek LS, Wolf GT, Taylor JMG, and D’Silva NJ (2022) Spatial and Transcriptomic Analysis of Perineural Invasion in Oral Cancer. Clin Cancer Res [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kurtz KA, Hoffman HT, Zimmerman MB, and Robinson RA (2005) Perineural and vascular invasion in oral cavity squamous carcinoma: increased incidence on re-review of slides and by using immunohistochemical enhancement. Arch Pathol Lab Med 129, 354–359 [DOI] [PubMed] [Google Scholar]
- 64.Shen WR, Wang YP, Chang JY, Yu SY, Chen HM, and Chiang CP (2014) Perineural invasion and expression of nerve growth factor can predict the progression and prognosis of oral tongue squamous cell carcinoma. J Oral Pathol Med 43, 258–264 [DOI] [PubMed] [Google Scholar]
- 65.Chi AC, Katabi N, Chen HS, and Cheng YL (2016) Interobserver Variation Among Pathologists in Evaluating Perineural Invasion for Oral Squamous Cell Carcinoma. Head Neck Pathol 10, 451–464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yan F, Cheng YL, Katabi N, Nguyen SA, Chen HS, Morgan P, Zhang K, and Chi AC (2021) Interobserver Variation in Evaluating Perineural Invasion for Oral Squamous Cell Carcinoma: Phase 2 Survey Study. Head Neck Pathol 15, 935–944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Le Tourneau C, Velten M, Jung GM, Bronner G, Flesch H, and Borel C (2005) Prognostic indicators for survival in head and neck squamous cell carcinomas: analysis of a series of 621 cases. Head Neck 27, 801–808 [DOI] [PubMed] [Google Scholar]
- 68.Pfister DG, Spencer S, Adelstein D, Adkins D, Anzai Y, Brizel DM, Bruce JY, Busse PM, Caudell JJ, Cmelak AJ, Colevas AD, Eisele DW, Fenton M, Foote RL, Galloway T, Gillison ML, Haddad RI, Hicks WL, Hitchcock YJ, Jimeno A, Leizman D, Maghami E, Mell LK, Mittal BB, Pinto HA, Ridge JA, Rocco JW, Rodriguez CP, Shah JP, Weber RS, Weinstein G, Witek M, Worden F, Yom SS, Zhen W, Burns JL, and Darlow SD (2020) Head and Neck Cancers, Version 2.2020, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 18, 873–898 [DOI] [PubMed] [Google Scholar]
- 69.Tai SK, Li WY, Yang MH, Chang SY, Chu PY, Tsai TL, Wang YF, and Chang PM (2012) Treatment for T1–2 oral squamous cell carcinoma with or without perineural invasion: neck dissection and postoperative adjuvant therapy. Ann Surg Oncol 19, 1995–2002 [DOI] [PubMed] [Google Scholar]
- 70.Ross GL, Soutar DS, MacDonald DG, Shoaib T, Camilleri IG, and Robertson AG (2004) Improved staging of cervical metastases in clinically node-negative patients with head and neck squamous cell carcinoma. Ann Surg Oncol 11, 213–218 [DOI] [PubMed] [Google Scholar]
- 71.Sparano A, Weinstein G, Chalian A, Yodul M, and Weber R (2004) Multivariate predictors of occult neck metastasis in early oral tongue cancer. Otolaryngol Head Neck Surg 131, 472–476 [DOI] [PubMed] [Google Scholar]
- 72.Nair S, Singh B, Pawar PV, Datta S, Nair D, Kane S, and Chaturvedi P (2016) Squamous cell carcinoma of tongue and buccal mucosa: clinico-pathologically different entities. Eur Arch Otorhinolaryngol 273, 3921–3928 [DOI] [PubMed] [Google Scholar]
- 73.Tai SK, Li WY, Chu PY, Chang SY, Tsai TL, Wang YF, and Huang JL (2012) Risks and clinical implications of perineural invasion in T1–2 oral tongue squamous cell carcinoma. Head Neck 34, 994–1001 [DOI] [PubMed] [Google Scholar]
- 74.Aivazian K, Ebrahimi A, Low TH, Gao K, Clifford A, Shannon K, Clark JR, and Gupta R (2015) Perineural invasion in oral squamous cell carcinoma: quantitative subcategorisation of perineural invasion and prognostication. J Surg Oncol 111, 352–358 [DOI] [PubMed] [Google Scholar]
- 75.Brandwein-Gensler M, Teixeira MS, Lewis CM, Lee B, Rolnitzky L, Hille JJ, Genden E, Urken ML, and Wang BY (2005) Oral squamous cell carcinoma: histologic risk assessment, but not margin status, is strongly predictive of local disease-free and overall survival. Am J Surg Pathol 29, 167–178 [DOI] [PubMed] [Google Scholar]
- 76.Tarsitano A, Tardio ML, and Marchetti C (2015) Impact of perineural invasion as independent prognostic factor for local and regional failure in oral squamous cell carcinoma. Oral Surg Oral Med Oral Pathol Oral Radiol 119, 221–228 [DOI] [PubMed] [Google Scholar]
- 77.Amin Mahul B., E. SB, Greene Frederick L., Byrd David R., Brookland Robert K., Washington Mary Kay, Gershenwald Jeffrey E., Compton Carolyn C., Hess Kenneth R., Sullivan Daniel C., Jessup J. Milburn, Brierley James D., Gaspar Lauri E., Schilsky Richard L., Balch Charles M., Winchester David P., Asare Elliot A., Madera Martin, Gress Donna M., Meyer Laura R.. (2017) AJCC Cancer Staging Manual (8th Edition), Springer Cham [Google Scholar]
- 78.Ferdoushi A, Griffin N, Marsland M, Xu X, Faulkner S, Gao F, Liu H, King SJ, Denham JW, van Helden DF, Jobling P, Jiang CC, and Hondermarck H (2021) Tumor innervation and clinical outcome in pancreatic cancer. Sci Rep 11, 7390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Schmitd LB, Perez-Pacheco C, and D’Silva NJ (2021) Nerve density in cancer: Less is better. FASEB Bioadv 3, 773–786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Cracchiolo JR, Xu B, Migliacci JC, Katabi N, Pfister DG, Lee NY, Patel SG, Ghossein RA, and Wong RJ (2018) Patterns of recurrence in oral tongue cancer with perineural invasion. Head Neck 40, 1287–1295 [DOI] [PMC free article] [PubMed] [Google Scholar]


