Abstract
Sepsis is a major health issue and a leading cause of death in hospitals globally. The treatment of sepsis is largely supportive and there are no therapeutics available that target the underlying pathophysiology of the disease. The development of therapeutics for the treatment of sepsis is hindered by the heterogeneous nature of the disease. The presence of multiple, distinct immune phenotypes ranging from hyperimmune to immunosuppressed can significantly impact the host response to infection. Recently, omics, biomarkers, cell surface protein expression and immune cell profiles have been utilized to classify immune status of sepsis patients. However, there has been limited studies of immune cell function during sepsis and even fewer correlating omics and biomarker alterations to functional consequences. In this review, we will discuss how the heterogeneity of sepsis and associated immune cell phenotypes result from changes in the omic make-up of cells and its correlation with leukocyte dysfunction. We will also discuss how emerging techniques such as in silico modeling and machine learning can help in phenotyping sepsis patients leading to precision medicine.
Keywords: Sepsis, Heterogeneous, Omics, Immunophenotyping, Immune Cells, Organ-on-chip
Introduction
Sepsis is a major health issue and a leading cause of death in hospitals. The global incidence of sepsis is over 49 million cases/year and 11 million deaths, accounting for 20% of all global deaths1,2. Sepsis is a clinical syndrome that is defined as life-threatening organ dysfunction due to dysregulated host response to infection (Sepsis-3)3. The origin of infection can be bacterial (Gram-negative or Gram-positive bacteria), fungal, parasitic or viral, or the result of secondary infections following non-infectious insults such as burn or trauma. Sepsis patients often die of organ failure and leukocyte-endothelial cell (EC) interactions leading to increased neutrophil influx and endothelial barrier disruption have a critical role in the early course of organ damage. While neutrophils are vital to host defense, neutrophil dysregulation has a critical role in the early course of organ damage through release of proteases, neutrophil extracellular traps (NETs), and reactive oxygen species (ROS), which damage ECs leading to multiple organ failure and increased mortality4,5.
Treatment of sepsis is largely supportive and there are no therapeutics available that target the underlying pathophysiology of sepsis6. Therapeutic development is hindered as a result of the heterogeneous nature of the disease7-9 and the presence of multiple distinct immune phenotypes ranging from hyperimmune to immunosuppressed that can impact function and response to infection8-12. Recently, omics, biomarkers, cell surface protein expression, and immune cell profiles have been utilized to classify the immune status of sepsis patients7,9-14. However, there has been limited functional immune studies and few linking and correlating functional consequences to omics and biomarker alterations. In this review, we will discuss how changes in omics of immune cells and the corresponding functional consequences lead to leukocyte dysfunction during sepsis. We will also discuss how emerging techniques such as in silico modeling and machine learning can help in phenotyping sepsis patients leading to precision medicine.
Sepsis is a Heterogeneous Disease
In sepsis, significant heterogeneity exists between patients that impacts immune function and response to infection8-11,15. In the last decade, interventions supported by data from animal or in vitro sepsis models have had little success in clinical trials, as these models have failed to fully replicate the underlying pathophysiology and account for the heterogeneity of the disease16. Individual factors such as age, sex, infection source, (epi)genetics, comorbidities, demographics and interventions are often not fully considered in animal models and in vitro studies but could significantly impact the clinical course of the disease3,7. In addition, the redundant biological signals in interconnecting pathways make it difficult to predict the clinical outcome and to establish a clear understanding of the underlying disease. Thus, a single standard treatment for the heterogeneous cohort of sepsis patients has proven to be problematic and underscores the importance of categorizing sepsis patients into distinct endotype classes by defining distinct host response subgroups is now well recognized7.
Neutrophil Dysfunction in Sepsis
Neutrophils are the most abundant leukocyte in the circulation and are critical effector cells in the innate immune system. They are characterized as a primary defense against invading pathogens and are one of the leading immune effector cells in sepsis. Multiple neutrophil subpopulations that differ in cell markers and function, have been described in sepsis patients. Four distinct subsets have been identified that include the conventional or high-density neutrophils, immature neutrophils, and two neutrophil populations that co-localize with peripheral blood mononuclear cells during density gradient centrifugation (identified as granulocyte myeloid-derived suppressor cells and low-density neutrophils (LDN))17-19. LDNs have distinct biological characteristics compared to conventional neutrophils, but the characteristics of these different neutrophil subpopulations in sepsis have not been well delineated. Whether these neutrophils exhibit plasticity to transform to other subtypes during the course of the disease is also not known.
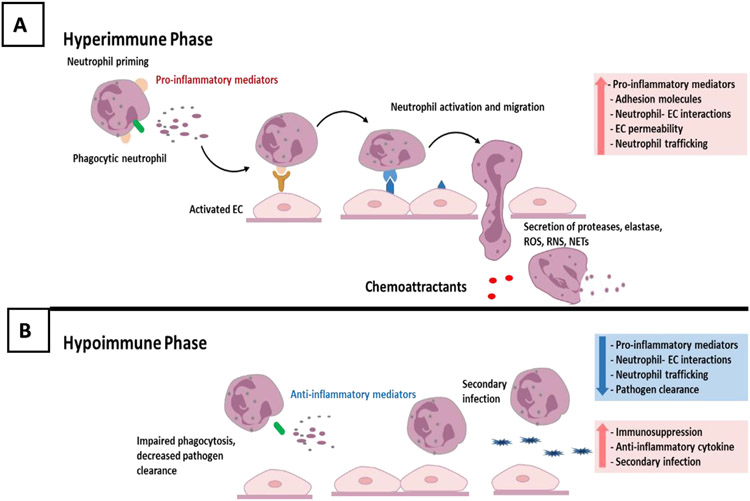
During the progression of sepsis, immune status often evolves as the pathophysiology develops. The initial hyperimmune phase (Figure 1A) is characterized by systemic inflammation, activation of immune cells, EC dysfunction and excessive neutrophil trafficking into vital organs often resulting in organ damage20,21. During this acute phase, pathogen-associated molecular patterns (PAMPs) are recognized by pattern recognition receptors (PRR) located on immune cells and EC, triggering activation of the endothelium and immune cells, and development of a pro-inflammatory phenotype. Activated EC have enhanced neutrophil–EC interactions, induction of apoptosis, and disruption of barrier integrity. Increased crosstalk between neutrophils and the endothelium results in neutrophil rolling, adhesion, and migration across EC through a multifactorial process controlled by concurrent chemoattractant-dependent signals, hemodynamic shear forces and adhesive events22, resulting in excessive neutrophil trafficking into critical organs. Activated neutrophils are critical regulators of endothelial function via secretion-dependent and adhesion-dependent events, which can damage EC, leading to organ dysfunction4. Neutrophils can damage EC through the formation of NETs, degranulation and release of proteases, and the secretion of ROS and reactive nitrogen species (RNS)23,24. The release of elastase, matrix metalloproteases, and myeloperoxidase (MPO) from neutrophil granules can cleave the protective glycocalyx, exposing the EC surface, resulting in enhanced leukocyte adhesion, EC activation, and increased barrier permeability4. The binding of NETs to vascular endothelium also increases permeability through disruption of adherens junctions and cytoskeleton reorganization4. Neutrophil-derived extracellular vesicles containing barrier disrupting cargo can also produce junctional disorganization, barrier disruption, and increased permeability4. Thus, while neutrophils are critical to host defense, neutrophil dysregulation in sepsis can play a critical role in the development of EC damage, leading to multiple organ dysfunction syndrome (MODS) and increased mortality.
Figure 1. The hyperimmune and hypoimmune phase during the progression of sepsis.
(A) The acute hyperimmune phase is characterized by systemic inflammation, immune cell and endothelial cell activation, and the release of proinflammatory mediators resulting in disrupted vascular endothelial barrier integrity, increased leukocyte trafficking, and organ damage.
(B) This hyperimmune phase often transitions into an immune compromised or hypoimmune phase characterized by decreased pathogen clearance, a shift in immune subpopulations, increased anti-inflammatory cytokines, and enhanced susceptibility to secondary infections.
The initial hyperimmune phase often transitions into an immunocompromised or hypoimmune phase (Figure 1B) characterized by decreased pathogen clearance, a shift in immune cell subpopulations, increased production of anti-inflammatory cytokines, a blunted response to inflammatory stimuli, and increased susceptibility to secondary infections21,25,26. Some sepsis patients develop a hybrid phenotype with both hyper- and hypoimmune characteristics of persistent inflammation and immunosuppression20,27. These different phases vary between individuals and even within the same individual, depending on the clinical course of the disease. This varying immune status could explain the observed heterogeneity of response to available immunomodulating treatments. As clinical care has improved, more sepsis patients are surviving the initial hyperimmune phase and transitioning to the hypoimmune state, often succumbing to secondary infections18,21,28.
Neutrophil Phenotyping in Sepsis
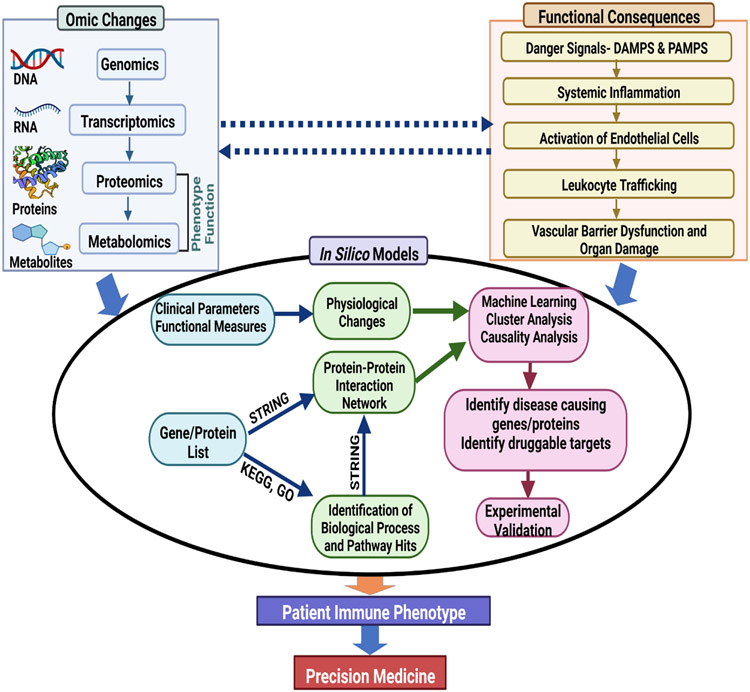
After the initial completion of the human genome in 2003, which sequenced ~92% of the human genome project, followed by sequencing of the remaining 8% of the genome, advances in systems biology (e.g., omics technologies) tools have permitted researchers to characterize and quantify a large number of biomolecules (e.g., DNA, RNA, proteins, metabolites)29,30. Omics (i.e. high-dimensional data) enables quantification of gene or protein expression in interconnected pathways during disease progression to help identify biomolecules of interest across time and different levels of biological systems (i.e., cells, tissues)29. A key outcome of omics is the creation of innovative hypotheses from data-driven experiments that could be tested in experimental models to explore the role of the quantified biomolecule(s) or signaling pathway(s) in question29. This emerging field has contributed to the generation of “big data” for application in machine learning, which we discuss later in this review, and can aid in understanding mechanisms of poorly characterized diseases such as sepsis and identify novel therapeutic targets that would have not been discovered via reductionist methods alone31. Given the dynamic nature of sepsis, omics can provide insight on temporal and spatial evolution of signals and how this evolution can alter immune function. Figure 2 illustrates the relationship between omics and functional consequences of leukocyte dysfunction in sepsis and how in silico models can be used to systematically integrate this information to identify key genes/proteins involved in the pathophysiology, identify druggable targets, and simulate therapeutic responses via machine learning. Furthermore, these models must be validated experimentally so they can be used to help identify a patient’s immune phenotype and advance the goal of precision medicine to select the right drug for the right patient at the right time.
Figure 2. Integration of Omics and Functional Changes into In Silico Modeling.
Understanding the relationship between omic changes and functional consequences can help contribute to the development of novel in silico models to detect potential sites of immune dysregulation and novel therapeutic targets. After experimental validation, in silico models could help identify a patient’s immune phenotype and advance precision medicine efforts.
Use of genomics and transcriptomics to classify sepsis endotypes
Genomics and transcriptomics have been used predominantly to classify sepsis patients into different endotypes based on genome wide profiling of whole blood but have not specifically focused on the omic expression patterns in leukocytes29. The classification of patients into respective endotypes based on omics is important for creating therapeutics for individual patients, since the identification of a critical pathway (or multiple interconnected pathways in the case of sepsis) to target in individual patients is the overall goal of precision medicine29. For example, recent studies have classified sepsis patients into different endotypes based on the finding that patients in low mortality groups had increased adaptive immune signaling while high mortality groups had mitigated immune function29, highlighting that patients fall into a hyperinflammatory or hypoinflammatory group across endotype studies. Nevertheless, the correlation between omics and functional consequences has not been clearly identified as omic changes do not always result in functional consequences.
Use of genomics and transcriptomics to classify leukocyte phenotypes in sepsis
Genomics and transcriptomics have been used to reveal mechanisms of innate immune cell function, specifically neutrophils, in sepsis12,32. For example, a retrospective study used publicly available gene expression profiles (i.e., GEO database) of neutrophils from whole blood to identify key genes and pathways associated with neutrophils during sepsis via bioinformatics32. Authors identified DEGs from neutrophils (day 3-4 and day 6-8 post septic shock) in patients with sepsis-induced immunosuppression. Upregulated DEGs such as Mmp8, IL-15, Nfkbia and downregulated DEGs including HLA genes (e.g., Hla-dma) and IFN-related genes (e.g., Ifi6, Ifit1) were identified as potential targets for immunotherapy management and demonstrated that neutrophils are the primary target cells for immune-stimulating therapies (e.g., anti-programmed cell death 1 (antiPD1), IL-7, IL-15)32. Both studies identified Akt1 as significantly dysregulated and thus warrant further functional investigation in neutrophils. On a transcriptional level, circulating human neutrophils have been found (via single-cell RNA sequencing (scRNA-seq)) to undergo different states as they transition from an immature (defined as Nh0) to an intermediate phenotype (Nh1) followed by one of two end points: a state of transcriptional inactivity (Nh2) or a state of expression of Interferon-induced genes (Nh3)33. RNA-seq was also used to identify another neutrophil phenotype (in a CLP-based mouse model) with strong immunosuppressive activity due to high PD-L1 expression under septic-like conditions12. Neutrophils were found to suppress innate immune responses by upregulating T regulatory cell production upon direct contact in vitro. Thus, this newly identified neutrophil phenotype may play a role in sepsis-induced immunosuppression or immunoparalysis and requires further functional investigation. These studies provide insight into neutrophil dysregulation during sepsis but require validation in prospective studies that genomic and transcriptomic responses have functional consequences. Moreover, proteomics can yield even more valuable insight on the phenotypic and mechanistic changes in biological systems and help to bridge the genotype-phenotype gap29.
Proteomic studies of leukocytes in sepsis
Proteomic studies of monocytes
Investigating the functionality of key innate immune cells during sepsis or septic-like conditions and correlating it to functional/phenotypic omics (e.g., proteomic) analysis, is critical for a comprehensive understanding of the underlying molecular expression within the cells and how they can significantly affect immune function, clinical parameters and treatment plans. The application of proteomics to study clinical samples from sepsis patients is relatively new and most have analyzed plasma samples34,35. However, a recent study profiled the proteome of isolated monocytes from septic shock patients and performed functional enrichment analysis to examine the underlying biological processes and the role of differentially expressed protein (DEP) pathways compared to healthy/control subjects34. Interestingly, proteins involved in glycolysis were increased with a reduction in proteins related to oxidative phosphorylation. An increase in lactase dehydrogenase was also observed as well as negative regulation of fatty acid beta-oxidation. These events indicate a significant metabolic shift from aerobic metabolism to anerobic metabolism in monocytes, potentially highlighting a key trait of septic shock.
Proteomic studies of neutrophils
Proteomic analysis was also used to identify DEPs associated with neutrophil functionality from the plasma of septic patients with cirrhosis and conducted functional activity of neutrophils (e.g., oxidative burst or phagocytic activity)36. Gene ontology (GO) Biological Processes (BP) such as neutrophil degranulation, neutrophil activation and neutrophil-mediated activity involved in immune response were upregulated in septic patients. Patients with sepsis had decreased oxidative burst activity which coincides with the bioinformatic results by indicating that although neutrophil numbers were increased in septic patients (i.e., neutrophil activation GO BP - the alteration of behavior of neutrophils after exposure to inflammatory stimuli leading to the development or progression of an immune response37), overall functionality (e.g., phagocytic and oxidative burst activity) was decreased. Ontologies (i.e., cellular compartment-based) associated with the decreased functionality were azurophil granule (azurophilic granules in neutrophils), azurophil granule lumen (the volume within an azurophil granule) and secretory granule lumen (the volume in a secretory granule) 37.
Proteomic studies of peripheral blood mononuclear cells
In another study, the transcriptome and proteome of leukocytes (peripheral blood mononuclear cells (PBMCs)) from sepsis patients were profiled38. Proteomic analysis indicated that the first module (i.e., cluster of genes/proteins related in biological function), in particular, contained upregulated genes and proteins related to neutrophil collagenase, neutrophil degranulation etc. These results complement previous functional studies which demonstrated increased nitric oxide and ROS production in leukocytes isolated from septic patients39. Further functional studies of LDNs in septic patients demonstrated elevated circulating cell numbers but decreased phagocytic and chemotaxis activity, increased lifespan and CXCR4 expression, and associated with neutrophil degranulation compared to conventional neutrophils18. Though these studies corroborate our current understanding of the role that innate immune cells play in sepsis progression on a molecular and functional level, additional studies examining the proteomes of innate immune cells of heterogeneous cohorts of sepsis patients during different disease stages are needed to investigate differential regulation of protein expression in these important pathways to gain insight into mechanisms involved in sepsis progression and to identify potential therapeutic targets. Thus, omics can advance sepsis research by 1) elucidating mechanisms of the pathophysiology and guiding therapies, 2) developing bench-to-bedside diagnostics and personalized therapeutics, and 3) reveal clusters or endotypes within diverse groups of sepsis patients29.
Emerging in vitro models to study neutrophil function and neutrophil-endothelial interaction
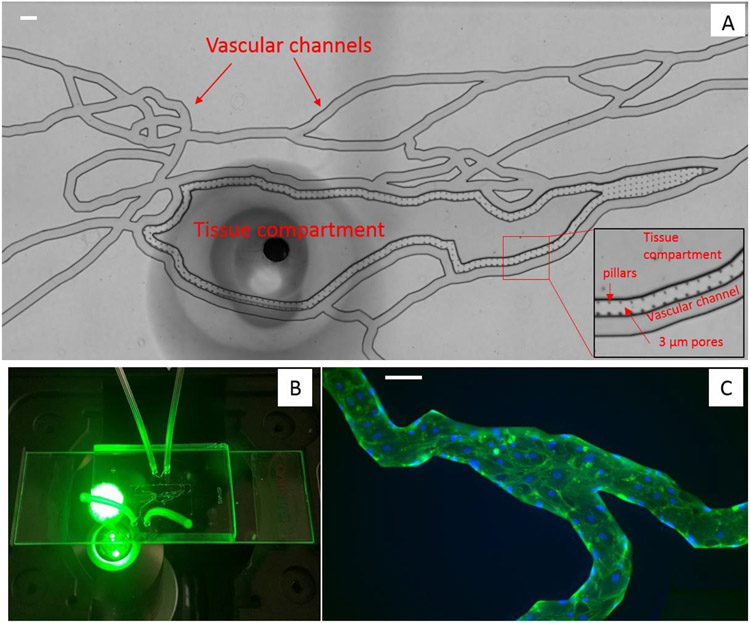
Traditional in vitro static models (e.g., transwell assays) do not provide a suitable, physiologically realistic environment to assess immune cell function during disease. Microphysiological systems (MPS), or organ-on-chip, are able to overcome shortcomings of static cell culture models by better representing the 3-D, in vivo microenvironment for a particular biological system and have the potential to screen therapeutics and increase translatability40. For example, our group has developed a unique, bioinspired MPS (Figure 3) that replicates the leukocyte trans-endothelial migration cascade including circulation, rolling, adhesion and migration of leukocytes under a biologically-relevant environment 22. This assay has been used to evaluate leukocyte-EC interactions during inflammation22. Employing this MPS model, we demonstrated that in response to proinflammatory cytokine activation, human neutrophil adherence to human ECs was significantly increased and greatest in vessels under low shear stress and at vessel bifurcations22. This enhanced adhesion was associated with cytokine-induced upregulation of adhesion molecule expression and a significant increase in neutrophil migration across human ECs, mimicking processes observed in vivo during inflammatory events.
Figure 3. Overview of a microphysiological system (MPS) also referred to as “Organ-on-Chip”.
(A) Bright-field image of the MPS network shows vascular channels are connected to the tissue compartment via 3 μm pores. (B) A MPS chip on the microscope stage, showing the outline of the vascular channels and tubing connected to inlet and outlet ports which allow introduction of various fluids and cells into MPS. (C) Fluorescence images show human lung microvascular endothelial cells grown to confluency in the vascular channel forming a complete 3D lumen. F-actin is labeled green using phalloidin and nuclei is labeled blue using Draq5. (Scale bar = 100 μm)
Other groups have also used MPS to assess neutrophil function and to study neutrophil chemotaxis/migration during sepsis. Ex vivo neutrophil migration was measured using MPS and found that neutrophil migration velocity from septic patients was significantly lower compared to control counterparts41. MPS have also been used to measure circulating NETs in blood from rodent models of burn injury and sepsis (cecal ligation puncture), reporting that the concentration of NETs increased early in inflammation, followed by a gradual decrease42. These functional studies of neutrophils are not only beneficial in furthering our understanding of how innate immune cells are dysregulated following sepsis or burn injury but also can provide novel methodologies for immunophenotyping and omics analysis of these cells. These MPS-based studies can provide a more comprehensive perspective on the relationship between omics expression of innate immune cells and the functional consequences of these distinct transcriptional states during sepsis.
In silico modeling and machine learning
Computational tools are required to analyze the large volume of data often generated in omics studies to map biological pathways in various cell types, identify druggable targets and predict how drugs alter inflammatory signaling29. In silico models can potentially be used to integrate omics findings with functional measures from novel tools such as MPS and clinical parameters to produce testable hypotheses, hasten the drug discovery process by identifying druggable targets, and help elucidate multi-faceted mechanisms of action that may not be immediately apparent from traditional analytical techniques such as statistical analysis29 (see Figure 2). In particular, network models are commonly constructed by overlapping omics data onto the interactome (e.g., functional interactions between genes, proteins etc. within a biological system) and do not necessitate a priori quantitative information of reactions between biological species which is often difficult to obtain43. For example, as shown in Figure 4, we have used in silico modeling to show that the number of proteins shared between the top 5 GO BPs increases after 24 hours of exposure of mouse lung, liver and kidney ECs to a clinically relevant mixture of cytokines thus indicating that over time, sepsis begins to affect protein expression of different EC phenotypes similarly44. The GO Consortium is the world’s most comprehensive, bioinformatics repository that maintains and updates a bioinformatics database which characterizes the roles of genes and gene products in organisms45. One of its popular features is its ability to take list of genes/proteins of interest provided by the user to identify significant BPs, molecular functions, or cellular components, which are the 3 ontologies of the software. This information help characterize the roles of biomolecules in various processes. Identifying significant BPs, molecular functions and cellular compartments in a list of biomolecules of interest can also be done in a programming language such as R. Specifically, Bioconductor is the organization or vendor that regulates and maintains bioinformatic and omic-related packages in R. One can also create in silico models in R using a Bioconductor package to show the interaction of significant ontologies with each other along with the corresponding proteins as we show in Figure 4. Different network analysis methods (e.g., weighted gene correlation network analysis (WGCNA), protein-protein interaction, etc.) have been used to elucidate immune cell (e.g., neutrophils) responses to septic plasma. In one such study, it was found that septic plasma impacted the neutrophil regulatory network to a greater degree than dendritic cell and peripheral blood mononuclear cell networks, with neutrophils having the highest number of DEGs, thus indicating a critical role in innate immune responses in sepsis46. However, to our knowledge, in silico network analysis has not been used to differentiate neutrophil subtypes in sepsis, such as high density vs low density neutrophils47, CD10-CD64+PD-L1+ neutrophils vs CD10−CD64+CD16low/-CD123+ immature neutrophils14, inhibitory neutrophils exhibiting high PDL1 expression12 etc. WGCNA is a bioinformatic approach that describes the correlation patterns among genes or proteins and can be used to identify clusters of related biological functions among highly correlated genes48. WCGCNA can be performed using a Bioconductor package48 in R. Protein-protein interactions (PPI) are normally generated from omics experiment or in silico predictions and are curated in various databases such as the Search Tool for the Retrieval of Interacting Genes/Proteins Database (STRING) software29, which is maintained by a community of academic, bioinformatic researchers (i.e., the vendors) at the University of Zurich, European Molecular Biology Laboratory and the Jensen lab. STRING can also be performed using Bioconductor in R. Databases such as HumanBase (maintained by the Flatiron Institute) contain tissue-specific PPIs49, which is valuable in studies where the disease of interest involves more than one tissue, such as sepsis. Additional pathway databases such as the Kyoto Encyclopedia of Genes and Genomes (KEGG) can be used for mapping genes/proteins of interest onto biological pathways43; KEGG is specifically maintained by the Bioinformatics Center and the Human Genome Center at Kyoto University and the University of Tokyo respectively. KEGG analysis can be performed in R using a variety of Bioconductor packages. Furthermore, findings from in silico models have not been systematically validated using functional and experimental data. Thus, studies such as these are needed to advance our understanding on the critical role that neutrophil subtypes play in sepsis progression.
Figure 4. Protein network map highlighting protein-protein interactions.
Gene concept network plot (cnetplot) indicates that the number of proteins shared between the top 5 Gene Ontology (GO) Biological Processes (BP) increases after mouse lung, liver and kidney endothelial cells are exposed to a mixture of clinically relevant cytokines for 24 hours. Each cnetplot indicates how the top 5 GO BPs in the lung, liver and kidney mouse endothelial cells interact with each other. The large dots represent the GO BPs, the small dots represent the proteins, and the color represents the organ52
Machine learning (ML), another type of in silico modeling, has the potential to improve the diagnosis, prognosis and monitoring of sepsis in patients50. Although there is no consensus within the scientific community on the definition of machine learning, it was classically defined by Arthur Samuel in 1959 (a pioneer in the field) as “a field of study that gives computers the ability to learn without being explicitly programmed”51. This definition holds true today as the essence of ML is to design computational models that can predict the behavior/outcome of a real-world event or phenomenon (e.g., therapeutic treatment for a patient). In general, this is accomplished by two different methods. One method is to provide an appropriately designed computer system with large amounts of clearly labeled data so that the computer system can “learn” how to correctly label new data that does not contain labels associated with each data point. This so-called supervised learning is the most common paradigm in the field. Conversely, unsupervised learning aims to identify undefined or unlabeled patterns in datasets by, for example, clustering similar observations together52. These techniques can be defined as dimensional reduction algorithms which include principal component analysis, k-means clustering and others 52. The number of applications of ML in sepsis research is expanding rapidly, from using algorithms to compose a proteomic signature that differentiates septic patients with or without acute respiratory distress syndrome53, to developing systems for sepsis prognosis and early accurate prediction of the disease54. Though ML has incorporated genomics and transcriptomics of immune cells in revealing gene signatures and biomarkers for sepsis prediction and prognosis55, studies using machine learning to integrate omics, functional analysis and clinical parameters to phenotype innate immune cells are urgently needed.
Conclusions
Understanding omics in combination with functional analysis of immune cells can characterize the progression of sepsis and advance the field of precision medicine. Proteomics and bioinformatic tools can help identify the underlying protein-protein interactions, biological processes and pathways of innate immune cells that contribute to a disease phenotype which would not be possible to achieve by using reductionist methods alone. In this regard, further studies of the role of post-translation modifications (e.g. glycosylation, phosphorylation, sumoylation etc.) on disease pathophysiology and how these different modifications could be targeted therapeutically are urgently needed. The addition of functional and validation studies of the immune cells in sepsis following (or prior to) omics analysis will determine if the changes at the molecular and subcellular level correlate with the phenotype of the cell as changes in omics do not always have functional consequences. Understanding how differential transcript and protein expression changes could reprogram the function of immune cells can outline the critical roles of these cells in disease progression. The orchestra of omics analysis combined with functional analysis may help investigators locate novel therapeutic targets for treating sepsis. Translating these advances in omics and functional studies to clinical practice has been hindered by a lack of 1) rapid testing from bench to bedside, 2) validation in heterogeneous cohorts, 3) tools to integrate omics, functional and clinical measures, 4) consensus on methodological techniques and clinical standards, and most importantly 5) studies combining and correlating omics and functional studies. Addressing these challenges, especially in profiling innate immune cells and elucidating the role of these cells in various pathways, will not only permit the translation of omics and functional studies to the clinic for identifying patient phenotypes but also advance precision medicine.
Support:
J.C.L. is an NIH NRSA F31 Predoctoral Fellow (1-F31AI164870-01). This work was supported by the Defense Threat Reduction Agency (HDTRA11910012) and the National Institutes of Health (GM134701).
Abbreviations
- BPs
Biological processes
- DEGs
Differentially expressed genes
- DEPS
Differentially expressed proteins
- EC/ECs
Endothelial cell(s)
- GO
Gene ontology
- KEGG
Kyoto Encyclopedia of genes and genomes
- LDNs
Low density neutrophils
- ML
Machine learning
- MODS
Multiple organ dysfunction syndrome
- MPO
Myeloperoxidase
- MPS
Microphysiological System
- NETs
Neutrophil extracellular traps
- PAMPs
Pathogen associated molecular patterns
- PPI
Protein-protein interaction
- PRRs
Pathogen recognition receptors
- RNS
Reactive nitrogen species
- ROS
Reactive oxygen species
- STRING
Search tool for the retrieval of interacting genes/proteins
- WGCNA
Weighted gene correlation network analysis
References
- 1.Rudd KE, Johnson SC, Agesa KM, et al. Global, regional, and national sepsis incidence and mortality, 1990–2017: analysis for the Global Burden of Disease Study. The Lancet. 2020;395(10219):200–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chiu C, Legrand M. Epidemiology of sepsis and septic shock. Current Opinion in Anesthesiology. 2021;34(2):71–76. [DOI] [PubMed] [Google Scholar]
- 3.Singer M, Deutschman CS, Seymour CW, et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA. 2016;315(8):801–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ma Y, Yang X, Chatterjee V, Meegan JE, Beard RS Jr, Yuan SY. Role of neutrophil extracellular traps and vesicles in regulating vascular endothelial permeability. Frontiers in immunology. 2019;10:1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Luan Y-Y, Dong N, Xie M, Xiao X-Z, Yao Y-M. The significance and regulatory mechanisms of innate immune cells in the development of sepsis. Journal of Interferon & Cytokine Research. 2014;34(1):2–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Drake AC. Of mice and men: what rodent models don't tell us. Cell Mol Immunol. 2013;10(4):284–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leligdowicz A, Matthay MA. Heterogeneity in sepsis: new biological evidence with clinical applications. Critical care. 2019;23(1):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Seymour CW, Kennedy JN, Wang S, et al. Derivation, validation, and potential treatment implications of novel clinical phenotypes for sepsis. Jama. 2019;321(20):2003–2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Scicluna BP, van Vught LA, Zwinderman AH, et al. Classification of patients with sepsis according to blood genomic endotype: a prospective cohort study. Lancet Respiratory Medicine. 2017;5(10):816–826. [DOI] [PubMed] [Google Scholar]
- 10.Davenport EE, Burnham KL, Radhakrishnan J, et al. Genomic landscape of the individual host response and outcomes in sepsis: a prospective cohort study. The Lancet Respiratory Medicine. 2016;4(4):259–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sweeney TE, Azad TD, Donato M, et al. Unsupervised analysis of transcriptomics in bacterial sepsis across multiple datasets reveals three robust clusters. Critical Care Medicine. 2018;46(6):915–925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Qi XX, Yu Y, Sun R, et al. Identification and characterization of neutrophil heterogeneity in sepsis. Critical Care. 2021;25(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Seree-Aphinan C, Vichitkunakorn P, Navakanitworakul R, Khwannimit B. Distinguishing sepsis from infection by neutrophil dysfunction: a promising role of CXCR2 surface level. Frontiers in Immunology. 2020;11:608696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Meghraoui-Kheddar A, Chousterman BG, Guillou N, et al. Two new neutrophil subsets define a discriminating sepsis signature. American Journal of Respiratory and Critical Care Medicine. 2022;205(1):46-+. [DOI] [PubMed] [Google Scholar]
- 15.Fenner BP, Darden DB, Kelly LS, et al. Immunological endotyping of chronic Critical Illness After Severe Sepsis. Frontiers in Medicine. 2021;7(1087). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Itenov TS, Murray DD, Jensen JUS. Sepsis: Personalized medicine utilizing ‘omic’technologies—A paradigm shift? Healthcare. 2018; 6(3):111. 10.3390/healthcare6030111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chatfield SM, Thieblemont N, Witko-Sarsat V. Expanding neutrophil horizons: new concepts in inflammation. Journal of Innate Immunity. 2018;10(5–6):422–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sun R, Huang J, Yang Y, et al. Dysfunction of low-density neutrophils in peripheral circulation in patients with sepsis. Scientific Reports. 2022;12(1):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wei Y, Kim J, Ernits H, Remick D. The septic neutrophil—friend or foe. Shock. 2021;55(2):147–155. [DOI] [PubMed] [Google Scholar]
- 20.Vachharajani V, McCall CE. Epigenetic and metabolic programming of innate immunity in sepsis. Innate Immunity. 2019;25(5):267–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Torres LK, Pickkers P, van der Poll T. Sepsis-induced immunosuppression. Annual Review of Physiology. 2022;84(1):157–181. [DOI] [PubMed] [Google Scholar]
- 22.Soroush F, Zhang T, King DJ, et al. A novel microfluidic assay reveals a key role for protein kinase C delta in regulating human neutrophil-endothelium interaction. J Leukoc Biol. 2016;100(5):1027–1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Burn GL, Foti A, Marsman G, Patel DF, Zychlinsky A. The neutrophil. Immunity. 2021;54(7):1377–1391. [DOI] [PubMed] [Google Scholar]
- 24.Janicova A, Relja B. Neutrophil phenotypes and functions in trauma and trauma-related sepsis. Shock. 2021;56(1):16–29. [DOI] [PubMed] [Google Scholar]
- 25.Zhang Z, Wang Y, Shan Y, Zhou R, Yin W. Oroxylin A alleviates immunoparalysis of CLP mice by degrading CHOP through interacting with FBXO15. Scientific Reports. 2020;10(1):19272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hotchkiss RS, Monneret G, Payen D. Sepsis-induced immunosuppression: from cellular dysfunctions to immunotherapy. Nat Rev Immunol. 2013;13(12):862–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Horiguchi H, Loftus TJ, Hawkins RB, et al. Innate immunity in the persistent inflammation, immunosuppression, and catabolism syndrome and its implications for therapy. Front Immunol. 2018;9:595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cavaillon JM, Singer M, Skirecki T. Sepsis therapies: learning from 30 years of failure of translational research to propose new leads. EMBO Mol Med. 2020;12(4):e10128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Langston JC, Rossi MT, Yang Q, et al. Omics of endothelial cell dysfunction in sepsis. Vascular biology (Bristol, England). 2022;4(1):R15–R34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nurk S, Koren S, Rhie A, et al. The complete sequence of a human genome. Science. 2022; 376:6588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Veenstra TD. Omics in systems biology: Current progress and future outlook. Proteomics. 2021;21(3–4). [DOI] [PubMed] [Google Scholar]
- 32.Liu ZJ, Chen Y, Pan TT, et al. Comprehensive analysis of common different gene expression signatures in the neutrophils of sepsis. Biomed Research International. 2021. Apr 17;2021:6655425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wigerblad G, Cao Q, Brooks S, et al. Single-cell analysis reveals the range of transcriptional states of circulating human neutrophils. Journal of Immunology. 2022; ji2200154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rodrigues PMD, Valente RH, Brunoro GVF, et al. Proteomics reveals disturbances in the immune response and energy metabolism of monocytes from patients with septic shock. Scientific Reports. 2021;11(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Miao H, Chen S, Ding RY. Evaluation of the molecular mechanisms of sepsis using proteomics. Frontiers in Immunology. 2021;12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sehgal R, Kaur N, Maiwall R, Ramakrishna G, Maras JS, Trehanpati N. Plasma Proteomic analysis identified proteins associated with faulty neutrophils functionality in decompensated cirrhosis patients with sepsis. Cells. 2022;11(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Carbon S, Ireland A, Mungall CJ, et al. AmiGO: online access to ontology and annotation data. Bioinformatics. 2009;25(2):288–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Leite GGF, Ferreira BL, Tashima AK, et al. Combined transcriptome and proteome leukocyte's profiling reveals up-regulated module of genes/proteins related to low density neutrophils and impaired transcription and translation processes in clinical sepsis. Frontiers in Immunology. 2021;12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Santos SS, Brunialti MKC, Rigato O, Machado FR, Silva E, Salomao R. Generation of nitric oxide and reactive oxygen species by neutrophils and monocytes from septic patients and association with outcomes. Shock. 2012;38(1):18–23. [DOI] [PubMed] [Google Scholar]
- 40.Yang Q, Wijerathne H, Langston JC, Kiani MF, Kilpatrick LE. Emerging approaches to understanding microvascular endothelial heterogeneity: A roadmap for developing anti-inflammatory therapeutics. International Journal of Molecular Sciences. 2021;22(15). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Muldur S, Marand AL, Ellett F, Irimia D. Measuring spontaneous neutrophil motility signatures from a drop of blood using microfluidics. Microfluidics in Cell Biology, Pt B: Microfluidics in Single Cells. 2018;147:93–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Otawara M, Roushan M, wang X, Ellett F, Yu YM, Irimia D. Microfluidic assay measures increased neutrophil extracellular traps circulating in blood after burn injuries. Scientific Reports. 2018;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bloomingdale P, Van Anh N, Niu J, Mager DE. Boolean network modeling in systems pharmacology. Journal of Pharmacokinetics and Pharmacodynamics. 2018;45(1):159–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rossi MT, Langston JC, Singh N, et al. Molecular framework of mouse endothelial cell dysfunction during inflammation: A proteomics approach. International Journal of Molecular Sciences. 2022;23(15). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Carbon S, Douglass E, Good BM, et al. The Gene Ontology resource: enriching a GOld mine. Nucleic Acids Research. 2021;49(D1):D325–D334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Godini R, Fallahi H, Ebrahimie E. Network analysis of inflammatory responses to sepsis by neutrophils and peripheral blood mononuclear cells. Plos One. 2018;13(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Blanco-Camarillo C, Aleman OR, Rosales C. Low-Density neutrophils in healthy individuals display a mature primed phenotype. Frontiers in Immunology. 2021;12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Langfelder P, Horvath S. WGCNA: an R package for weighted correlation network analysis. Bmc Bioinformatics. 2008;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Greene CS, Krishnan A, Wong AK, et al. Understanding multicellular function and disease with human tissue-specific networks. Nature Genetics. 2015;47(6):569–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Giacobbe DR, Signori A, Del Puente F, et al. Early detection of sepsis with machine learning techniques: a brief clinical perspective. Frontiers in Medicine. 2021;8:617486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Samuel AL. Some studies in machine learning using the game of checkers. Ibm Journal of Research and Development. 1959;3(3):211. [Google Scholar]
- 52.Sidey-Gibbons JAM, Sidey-Gibbons CJ. Machine learning in medicine: a practical introduction. Bmc Medical Research Methodology. 2019;19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yehya N, Fazelinia H, Taylor DM, et al. Differentiating children with sepsis with and without acute respiratory distress syndrome using proteomics. American Journal of Physiology-Lung Cellular and Molecular Physiology. 2022;322(3):L365–L372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Moor M, Rieck B, Horn M, Jutzeler CR, Borgwardt K. Early Prediction of sepsis in the ICU using machine learning: A systematic review. Frontiers in Medicine. 2021;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Banerjee S, Mohammed A, Wong HR, Palaniyar N, Kamaleswaran R. Machine learning identifies complicated sepsis course and subsequent mortality based on 20 genes in peripheral blood immune cells at 24 H post-ICU admission. Frontiers in Immunology. 2021;12. [DOI] [PMC free article] [PubMed] [Google Scholar]