Abstract
Background:
Heart failure with EF 41-49% is recognized as HF with “mildly reduced” EF (HFmrEF). However, existing knowledge of the HFmrEF phenotype is based on HF clinical trial and registry cohorts that may be limited by multiple forms of bias.
Methods and Results:
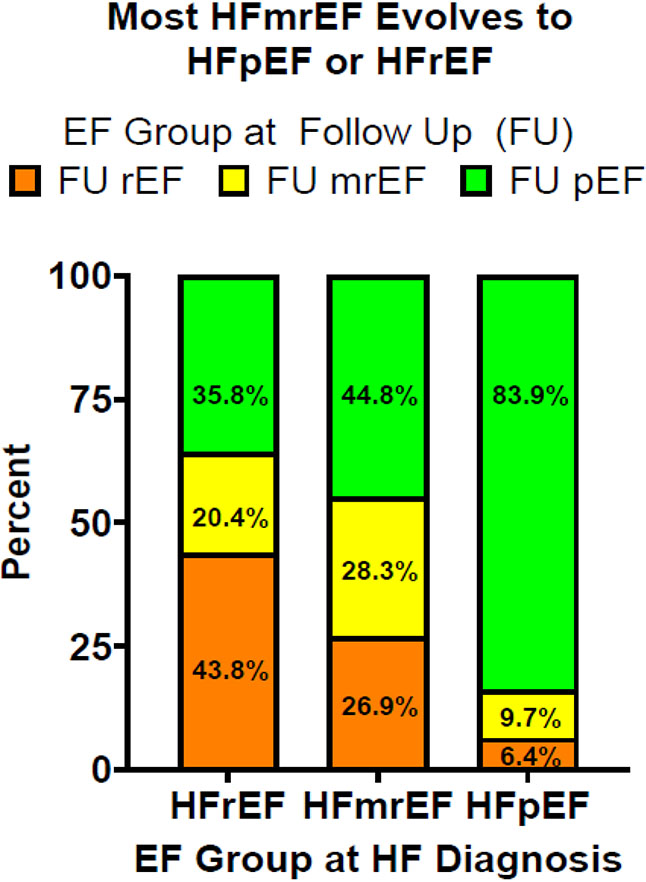
In a community-based, retrospective cohort study, adult residents of Olmsted County, Minnesota with validated (Framingham criteria) incident HF from 2007-2015 were categorized by echocardiographic EF at first HF diagnosis. Among 2035 adults with incident HF, 12.5% had HFmrEF, 29.9% had HFrEF, and 57.6% had HFpEF. Mean age and sex varied by EF group, with HFmrEF (75.6 years, 45.3% female), HFrEF (70.9 years, 36.5% female), and HFpEF (76.9 years, 59.7% female). Most comorbid conditions were more common in HFmrEF vs HFrEF, but similar in HFmrEF and HFpEF. After a mean (SD) follow-up of 4.6 (3.5) years, adjusting for age, sex and comorbidities, the risks of hospitalization and cardiovascular mortality did not differ by EF category. Of patients who began as HFmrEF, 26.9% declined to EF ≤ 40% and 44.8% improved to EF ≥50%.
Conclusions:
In this community cohort of incident HF, 12.5% have HFmrEF. Clinical characteristics in HFmrEF resemble HFpEF more than HFrEF. Adjusted hospitalization and mortality risks did not vary by EF group. Patients with incident HFmrEF usually transitioned to a different EF category on followup.
Keywords: HFmrEF, HFpEF, Epidemiology, Outcomes, Community
Graphical Abstract
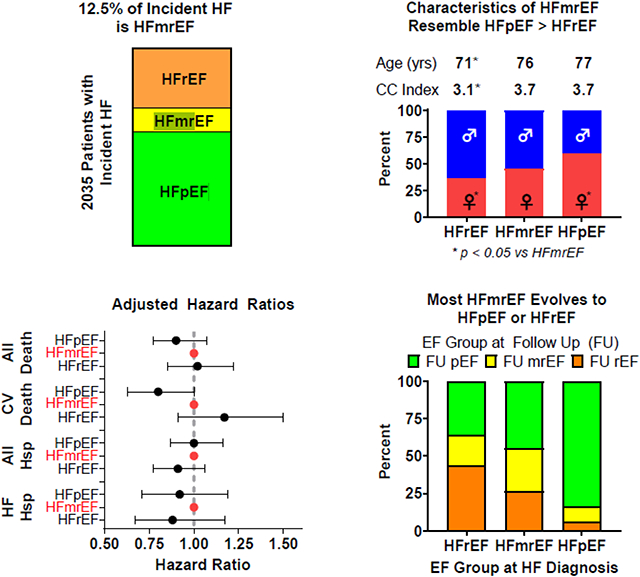
Take Home Graphic: 12.5% of incident HF cases are HFmrEF. Clinical characteristics of HFmrEF resemble HFpEF more than HFrEF. Adjusted outcomes are similar across EF groups in incident HF. Most cases of incident HFmrEF transition into the EF ranges used to define HFpEF or HFrEF. EF= ejection fraction, HF = heart failure, HFmrEF= heart failure with mildly reduced ejection fraction, HFpEF= heart failure with preserved ejection fraction, HFrEF= heart failure with reduced ejection fraction, CC Index = Charlson Comorbidity Index, HSP= hospitalization, CV = cardiovascular, FU = follow up.
Lay Summary
Heart failure (HF) refers to symptoms (shortness of breath, fatigue, and body swelling from fluid retention) due to heart dysfunction. Heart dysfunction can be described by the “squeezing function” of the heart, measured by the ejection fraction (EF). HF patients with low EF are treated differently than patients with high EF. In this study, there was no difference in outcomes (hospitalizations or deaths) between patients with the different values of EF. Many patients with reduced or mildly reduced EF transitioned to a different HF category over time while most HF patients with normal EF continued to have normal EF.
Introduction
The classification of patients with heart failure (HF) according to ejection fraction (EF) is evolving (1). Heart failure with an EF of 41-49% was previously termed HF with “borderline” or “mid-range” EF but is now proposed to represent HF with “mildly reduced” EF (HFmrEF) (2-4). This re-classification is critically important as post-hoc analyses of clinical trials have suggested that patients with HFmrEF may derive benefit from HFrEF guideline-directed medical therapies such as angiotensin receptor blockers (5), angiotensin receptor/neprilysin antagonists (6), mineralocorticoid receptor antagonists (7), and sodium-glucose co-transporter 2 inhibitors (8). This change would greatly expand the population treated with and benefitting from guideline-directed medical therapy for HFrEF (9).
As comprehensively reviewed (10), most existing knowledge of the HFmrEF phenotype is based on clinical trial and HF registry cohorts (11-16) that may be limited by several forms of bias (17). Many registries did not include all patients in a given institution, few were population-based, and many were restricted to inpatients or outpatients only. Most importantly, these registry and clinical trial studies of prevalent HF cases included patients with previous HFrEF and improvement in EF or previous HF with preserved EF (HFpEF) and deterioration in EF. A study pooling data from four community-based, longitudinal cohort studies conducted in the 1970’s to 2000’s with prospective HF ascertainment (categorization by EF in 75%) suggested that 10% of patients with incident HF had HFmrEF, with 39% having HFpEF and 51% had HFrEF (18).
To address these gaps in knowledge, the goal of the current study was to characterize a contemporary cohort of all patients with newly diagnosed HF in a single community and comparse the clinical characteristics and outcomes of patients with HFmrEF to those with incident HFrEF or HFpEF.
Methods:
Study Design.
This is a retrospective cohort study conducted in Olmsted County, Minnesota that used the resources of the Rochester Epidemiology Project (REP) (19). Population-based research is possible in this area as medical records from all sources of care for local residents are electronically linked, enabling longitudinal capture of patient care and outcomes (20). The age, sex, and ethnic characteristics of the local population are similar to those in the state of Minnesota and the upper Midwest United States (21). Patients were excluded from this analysis if they declined to provide Minnesota Research Authorization. The study was approved by the Mayo Clinic and Olmsted Medical Center Institutional Review Boards.
Study Population.
This analysis used previously published techniques to identify a cohort comprised of all adult (≥20 years) Olmsted County residents with incident (newly diagnosed) HF from 2007-2015 (22, 23). To develop the HF cohort, all patients with International Classification of Diseases, 9th and 10th revision codes for HF (ICD-9 code 428 and ICD-10 code I50) from the inpatient or outpatient setting were identified and their medical records were reviewed by trained nurse abstractors to validate the HF diagnosis using the Framingham criteria (24). Left ventricular EF was obtained from transthoracic echocardiograms performed within +/− 1 year of HF diagnosis; 90% of the echocardiograms were performed within 3 months of the HF incident date. When a patient had multiple echocardiograms within 1 year, EF from the one closest to the HF incident date was used. If multiple EF values were present on a single echocardiogram report, the average value was used. When an EF range was provided (e.g. 45-50%), the midpoint of the range was used. HFrEF was defined as an EF≤40%, HFmrEF 41-49%, and HFpEF ≥50% (2, 25). Follow-up EF measurement more than one year after the HF diagnosis was obtained from subsequent echocardiograms in a similar fashion.
Baseline clinical characteristics were captured from the electronic health record. The Charlson comorbidity index (26) was used to summarize the comorbidity burden; a comorbidity was defined as present with two codes separated by 30 days or more within the 5 years prior to HF to maximize the positive predictive value (27). Body mass index (BMI) was calculated using the earliest adult height and the last weight prior to HF diagnosis. Laboratory values closest to the date of HF diagnosis and within 1 year were used. Baseline medication use was as documented in the medical record at the most recent inpatient or outpatient visit prior to the HF diagnosis.
Outcomes.
Patients were followed until death or last clinical follow-up through 2020. The outcomes included mortality (all-cause and cardiovascular) and hospitalization (all-cause and HF-related). Surveillance for mortality in the REP occurs via several methods, including deaths noted in clinical care; death certificates from the State of Minnesota (28) and data from the National Death Index. Cardiovascular cause of death was defined by codes I00 to I99 on patient death certificates. Hospitalizations are captured from all hospitals in the community (Mayo Clinic hospitals and Olmsted Medical Center) via the REP. HF hospitalizations were defined as those with primary ICD-9 codes of 428.xx, 402.x1, 404.x3 or ICD-10 codes of I11.0x, I13.0x, I13.2x, I50.x. Followup EF values were collected over time as another outcome. Among those with at least one EF measurement during follow-up, EF range reassignment (EF≤40, EF 41-49%, and EF≥50%) was examined using the last EF obtained. As a sensitivity analysis, we also examined the number and percentage of patients who had a decline or improvement in EF of 10% or more over time by initial EF category (HFrEF, HFmrEF, HFpEF).
Statistical Analysis.
Differences in baseline characteristics by EF category (HFrEF, HFmrEF, HFpEF) were compared using ANOVA with specific group comparisons by ANOVA F-test or Mantel-Haenszel chi square where appropriate.
The associations of EF category with all-cause and cardiovascular mortality were analyzed using Kaplan Meier curves and Cox proportional hazard regression models. In models predicting cardiovascular mortality, we excluded patients where cause of death was unknown (n=39, 2.8%). For all time-to-event analyses, time 0 is the date of incident HF. The associations of EF category with all-cause and HF-specific hospitalizations were compared using mean cumulative frequency curves and analyzed using Andersen-Gill models, an extension of the Cox model allowing multiple hospitalizations per person. Models were adjusted for age, sex, and Charlson comorbidity index. Subjects were censored at non-cardiovascular death when modeling cardiovascular mortality and at death when modeling all-cause and HF-specific hospitalizations instead of modeling them as competing risks since the purpose of this study was to examine the relationship between EF category and outcomes and not prognostically predict the probability of each outcome. All analyses were performed using SAS version 9.4. A p-value of <0.05 was used as the level of significance in all analyses.
Results:
In total, 2211 patients were identified with incident HF in the study period. The median time from diagnosis of HF to LVEF assessment was −1 day (IQR −2 to 1 day). Of those, 176 (8%) had no EF measured within a year of HF diagnosis and were excluded. Among the remaining 2035 adults with incident HF, 254 (12.5%) had HFmrEF, 609 (29.9%) had HFrEF, and 1172 (57.6%) had HFpEF. The distribution of EF categories differed by sex, with the percent of patients with HFmrEF who were women being higher than in HFrEF but lower than in HFpEF (Table 1). Age varied by EF group with patients with incident HFmrEF being older than those with incident HFrEF but of similar age to those with HFpEF. This cohort was largely white. Patients with incident HFmrEF had a higher prevalence of hypertension, hyperlipidemia, diabetes, peripheral vascular disease, stroke, dementia, depression, atrial fibrillation, and higher BMI than those with HFrEF, but had a similar prevalence of these comorbidities as compared to HFpEF (Table 1). However, a history of coronary artery disease (CAD) was higher in those with HFmrEF than those with HFrEF or HFpEF. A history of smoking in patients diagnosed with HFmrEF was similar to HFrEF but higher than HFpEF.
TABLE 1.
Patient Baseline Characteristics
| HFrEF ≤40% |
HFmrEF 41- 49% |
HFpEF 50+% |
Total | P-value HFrEF vs HFmrEF |
P-value HFpEF vs HFmrEF |
|
|---|---|---|---|---|---|---|
| (N=609) | (N=254) | (N=1172) | (N=2035) | — | — | |
| Age in years, mean (SD) | 70.9 (15.9) | 75.6 (13.3) | 76.9 (13.0) | 74.9 (14.2) | <0.001 | 0.152 |
| Female Sex, n (%) | 222 (36.5%) | 115 (45.3%) | 700 (59.7%) | 1037 (51.0%) | 0.016 | <0.001 |
| Race, n (%) | — | — | — | — | 0.666 | 0.788 |
| Black/African American | 17 (2.8%) | 4 (1.6%) | 25 (2.1%) | 46 (2.3%) | ||
| White | 570 (93.6%) | 243 (95.7%) | 1102 (94.0%) | 1915 (94.1%) | ||
| Asian | 13 (2.1%) | 4 (1.6%) | 25 (2.1%) | 42 (2.1%) | ||
| Other | 9 (1.5%) | 3 (1.2%) | 20 (1.7%) | 32 (1.6%) | ||
| Marital Status, n (%) | — | — | — | — | 0.003 | 0.448 |
| Single | 91 (14.9%) | 22 (8.7%) | 77 (6.6%) | 190 (9.3%) | ||
| Married | 307 (50.4%) | 132 (52.0%) | 586 (50.0%) | 1025 (50.4%) | ||
| Divorced | 76 (12.5%) | 22 (8.7%) | 124 (10.6%) | 222 (10.9%) | ||
| Widowed | 130 (21.3%) | 78 (30.7%) | 379 (32.3%) | 587 (28.8%) | ||
| Unknown | 5 (0.8%) | 0 (0.0%) | 6 (0.5%) | 11 (0.5%) | ||
| History of smoking, n (%) | 369 (60.6%) | 163 (64.2%) | 633 (54.0%) | 1165 (57.3%) | 0.219 | 0.006 |
| CAD, n (%) | 211 (34.6) | 122 (48.0) | 453 (38.7) | 786 (38.6) | <0.001 | 0.006 |
| Hypertension, n (%) | 401 (65.8%) | 214 (84.3%) | 986 (84.1%) | 1601 (78.7%) | <.0001 | 0.961 |
| Hyperlipidemia, n (%) | 348 (57.1%) | 178 (70.1%) | 771 (65.8%) | 1297 (63.7%) | <0.001 | 0.189 |
| Diabetes, n (%) | 254 (41.7%) | 129 (50.8%) | 574 (49.0%) | 957 (47.0%) | 0.014 | 0.601 |
| COPD, n (%) | 83 (13.6%) | 45 (17.7%) | 249 (21.2%) | 377 (18.5%) | 0.124 | 0.208 |
| PVD, n (%) | 144 (23.6%) | 77 (30.3%) | 365 (31.1%) | 586 (28.8%) | 0.041 | 0.796 |
| Stroke, n (%) | 58 (9.5%) | 44 (17.3%) | 180 (15.4%) | 282 (13.9%) | 0.001 | 0.435 |
| Cancer, n (%) | 186 (30.5%) | 95 (37.4%) | 404 (34.5%) | 685 (33.7%) | 0.050 | 0.375 |
| Dementia, n (%) | 38 (6.2%) | 28 (11.0%) | 100 (8.5%) | 166 (8.2%) | 0.016 | 0.208 |
| Depression, n (%) | 99 (16.3%) | 60 (23.6%) | 285 (24.3%) | 444 (21.8%) | 0.011 | 0.815 |
| Charlson index | 3.1 (2.30) | 3.7 (2.57) | 3.7 (2.49) | 3.5 (2.46) | <0.001 | 0.817 |
| Atrial fibrillation, n (%) | 277 (45.5%) | 141 (55.5%) | 599 (51.1%) | 1017 (50.0%) | 0.007 | 0.203 |
| BMI, mean (SD) | 29.2 (6.73) | 30.6 (7.57) | 31.1 (8.09) | 30.5 (7.68) | 0.007 | 0.402 |
| Sodium, mmol/L, mean (SD) | 138.2 (4.6) | 138.2 (4.8) | 138.1 (4.7) | 138.1 (4.7) | 0.874 | 0.645 |
| eGFR (mL/min/1.73m^2), mean (SD) | 66.5 (27.5) | 64.1 (28.4) | 62.9 (29.0) | 64.1 (28.5) | 0.255 | 0.535 |
| Hemoglobin, g/dL, mean (SD) | 12.6 (2.2) | 11.9 (2.0) | 11.6 (2.1) | 12.0 (2.2) | <0.001 | 0.026 |
| Peak Troponin T, ng/mL, mean (SD) | 0.7 (2.3) | 0.6 (2.2) | 0.1 (0.6) | 0.4 (1.6) | 0.538 | <0.001 |
| NT-pro BNP, pg/mL, mean (SD) | 7671.4 (8866.9) | 6181.3 (6949.9) | 4151.8 (8185.6) | 5345.0 (8370.6) | 0.086 | 0.007 |
| BNP, pg/mL, mean (SD) | 1085.5 (788.6) | 1276.4 (1043.7) | 624.0 (544.2) | 820.2 (728.1) | 0.356 | <0.001 |
| Prior Beta blocker use, n (%) | 271 (44.5%) | 152 (59.8%) | 713 (60.8%) | 1136 (55.8%) | <0.001 | 0.769 |
| Prior ACE/ARB use, n (%) | 241 (26.8%) | 123 (13.7%) | 536 (59.6%) | 900 (44.2%) | 0.019 | 0.014 |
| Prior loop diuretic use, n (%) | 119 (19.5%) | 71 (28.0%) | 419 (35.8%) | 609 (29.9%) | 0.007 | 0.018 |
| Prior MRA use, n (%) | 16 (2.6%) | 6 (2.4%) | 44 (3.8%) | 66 (3.2%) | 0.821 | 0.272 |
| Prior thiazide diuretic use, n (%) | 127 (20.9%) | 69 (27.2%) | 311 (26.5%) | 507 (24.9%) | 0.044 | 0.8371 |
ACE= angiotensin converting enzyme inhibitor; ARB= angiotensin receptor blocker; MRA= mineralocorticoid receptor antagonists; BMI= body mass index; BNP= B-type natriuretic peptide; CAD= coronary artery disease; COPD= chronic obstructive pulmonary disease; eGFR= estimated glomerular filtration rate; HFmrEF= heart failure with mildly reduced ejection fraction; HFpEF= heart failure with preserved ejection fraction; HFrEF= heart failure with reduced ejection fraction; PVD= peripheral vascular disease; SD= standard deviation
The following variables had the indicated number of missing datapoints: marital status – 11 patients, sodium – 52 patients, eGFR – 25 patients, Hemoglobin- 44 patients, Peak TNT – 626 patients, NT-pro BNP – 950 patients, BNP – 1806 patients. All others had no missing data.
At the time of HF diagnosis, there was no difference in serum sodium or eGFR by EF group (Table 1). Patients with HFmrEF had similar levels of troponin T, NT-proBNP, and BNP but lower hemoglobin compared to patients with HFrEF. Patients with incident HFmrEF had higher baseline hemoglobin, troponin T, NT-proBNP, and BNP than patients with HFpEF.
At the time of HF diagnosis, patients with incident HFmrEF had higher rates of beta blocker, loop diuretic, and thiazide diuretic use than patients with HFrEF but lower rates of ACE/ARB use. Patients with HFmrEF had lower use of loop diuretics and ACE/ARB use, but similar use of thiazide diuretics and beta blockers compared to patients with HFpEF. The use of mineralocorticoid receptor antagonist was low and similar in all EF groups.
Hospitalization Risk.
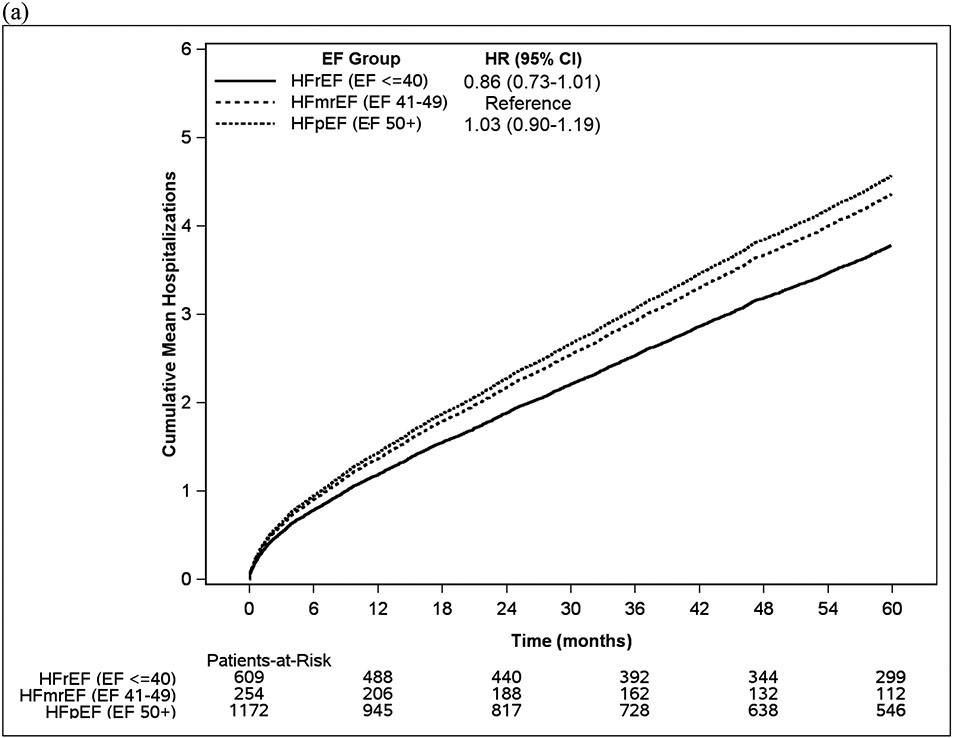
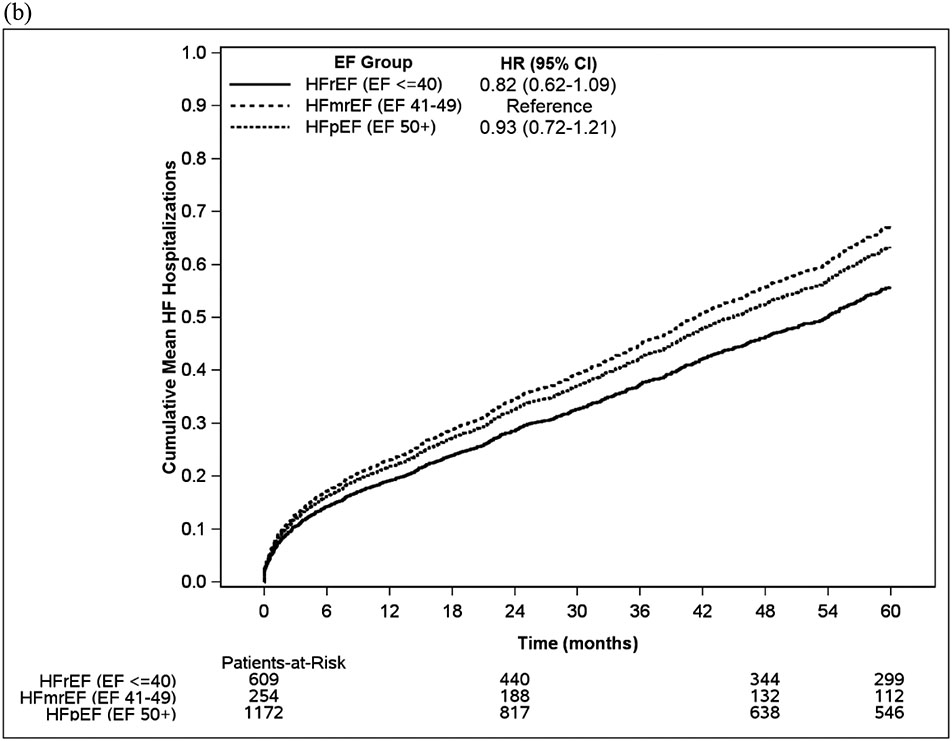
Over the course of a mean follow-up of 4.6 years (SD 3.5), there were a total of 7950 hospitalizations among 1716 patients, of which 1126 (14.2%) hospitalizations had a primary diagnosis of HF. In unadjusted analysis, patients with HFmrEF had a non-statistically significant trend toward a higher risk of both all-cause and HF hospitalizations (Figure 1, Figure 2, Table S1) compared to HFrEF but these trends became weaker after adjustment for age, sex and Charlson Comorbidity Index. Patients with HFmrEF had similar risks of all-cause hospitalization and HF hospitalizations as patients with HFpEF in both unadjusted and adjusted analyses. Patients with HFrEF and HFmrEF more often had percutaneous coronary intervention (25.3% vs. 28.7% vs. 18.5%, p<0.001) and coronary artery bypass grafting (20.7% vs. 28.7% vs. 17.4%, p<0.001), compared with patients with HFpEF, respectively, after HF diagnosis. Patients with HFrEF more often had cardiac resynchronization therapy (9.4% vs. 3.2% vs. 1.1%, p<0.001) than patients with HFmrEF and HFpEF, respectively, after HF diagnosis.
Figure 1. (a) Cumulative hospitalizations over time, (b) Cumulative heart failure hospitalizations over time.
EF= ejection fraction, HFmrEF= heart failure with mildly reduced ejection fraction, HFpEF= heart failure with preserved ejection fraction, HFrEF= heart failure with reduced ejection fraction
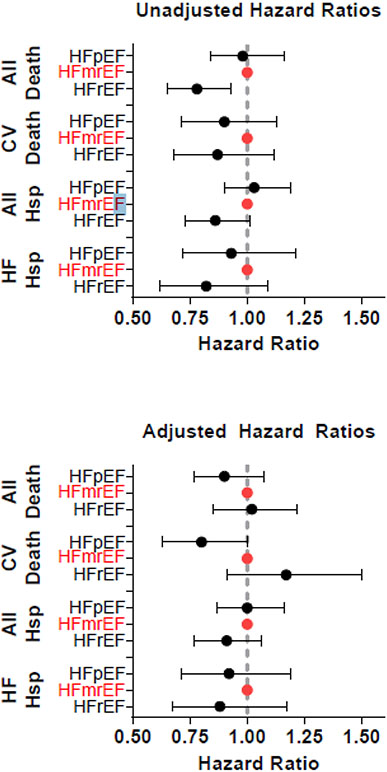
Figure 2: Hazard ratios for mortality and hospitalization in HFpEF, HFmrEF, and HFrEF populations that are a) unadjusted, and b) adjusted for age, sex, and Charlson Comorbidity Index.
HF= heart failure, HFmrEF= heart failure with mildly reduced ejection fraction, HFpEF= heart failure with preserved ejection fraction, HFrEF= heart failure with reduced ejection fraction
Mortality Risk.
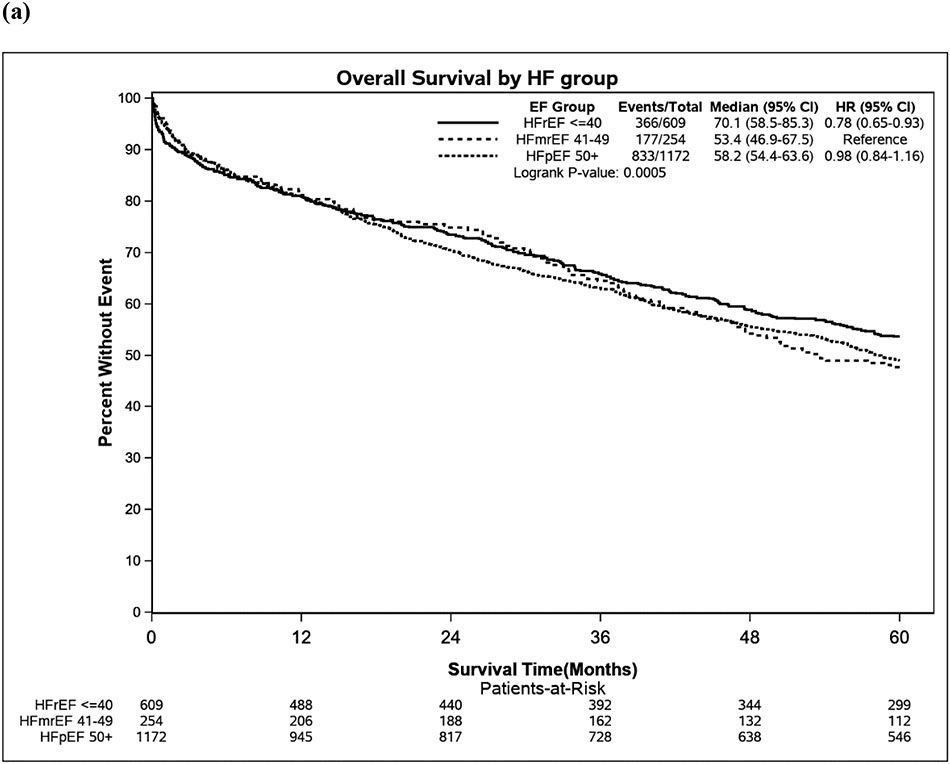
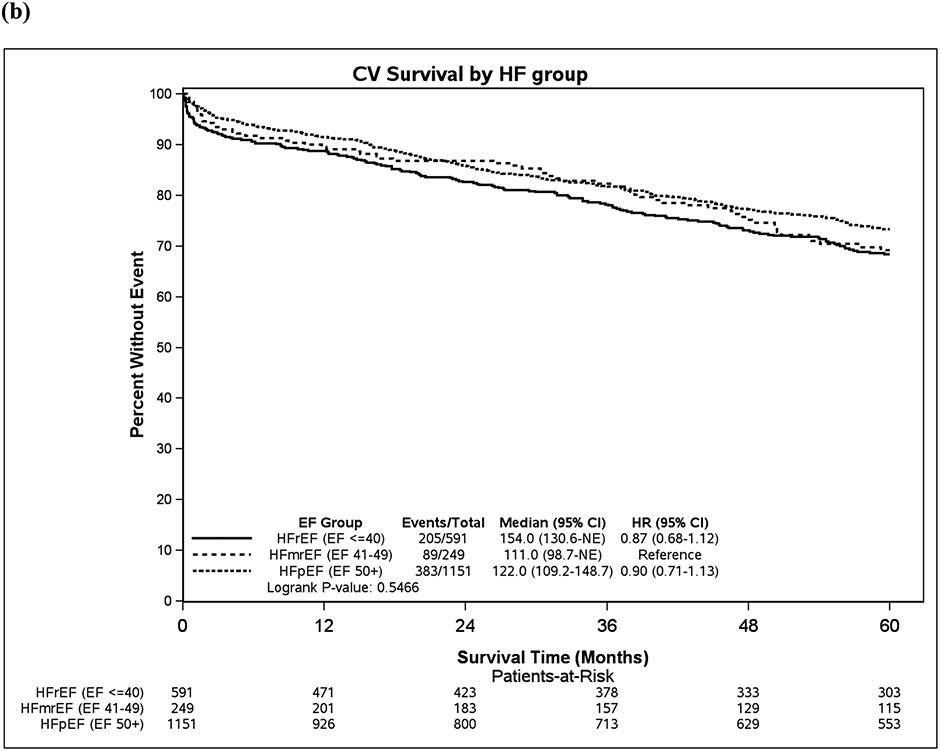
In total, 1376 patients died during follow-up, and 677 (49.2%) of those deaths were cardiovascular in nature, 660 (47.0%) were non-cardiovascular, and 39 (2.8%) did not have death certificates and cause of death was unknown. In unadjusted analysis, patients with HFmrEF had higher all-cause mortality than patients with HFrEF, though cardiovascular mortality was not significantly different. After adjustment for age, sex, and Charlson Comorbidity Index, there were no statistically significant differences in all-cause or cardiovascular mortality between HFmrEF and HFrEF (Figure 2, Figure 3, Table S1). In both unadjusted and adjusted analyses, there was no statistically significant difference in all-cause mortality between HFmrEF and HFpEF patients, though there was a trend toward lower cardiovascular mortality in HFpEF compared with HFmrEF patients (HR 0.80, 95% CI 0.63-1.00). The adjusted risk of the combined endpoint of cardiovascular death or HF hospitalization was lower in HFpEF compared with HFmrEF (HR 0.82, 95% CI 0.68-0.99, p=0.039, Table S1).
Figure 3. (a) Overall survival over time, (b) Cardiovascular survival over time.
CI= confidence interval, CV= cardiovascular, EF= ejection fraction, HF= heart failure, HFmrEF= heart failure with mildly reduced ejection fraction, HFpEF= heart failure with preserved ejection fraction, HFrEF= heart failure with reduced ejection fraction, NE= not estimated
In a sensitivity analysis defining HFmrEF as EF 41-54% and HFpEF as EF ≥55%, there were slight differences in outcomes by EF group observed. Patients with HFrEF had higher cardiovascular mortality than HFmrEF (HR 1.24 [1.00-1.53], p=0.046), and patients with HFpEF had lower cardiovascular mortality than HFmrEF (HR 0.82, 95% CI 0.68-1.00, p=0.046,Table S2).
Change in EF Over Time
Of the original 2035 patients, 1033 (50.8%) had at least one followup echocardiogram after diagnosis. Of these 1033 patients, 932 had a second EF measurement within 1 year after HF diagnosis. Of those, 245 patients had their EF increase by greater than 10%.
The median (IQR) number of followup echocardiograms was 2 (1, 3) with a maximum of 16 echocardiograms. Patients who had a followup echocardiogram were younger (mean age 71.5 years vs. 78.4 years) and a higher percentage were men (52.5% vs. 45.5%) compared to those without a followup echocardiogram.
When comparing first EF measurement to last EF measurement, of the 145 patients that had HFmrEF at incident HF diagnosis, 26.9% had EFs that declined to ≤ 40% and 44.8% had EFs that improved to ≥50% (Figure 4). Of the 372 patients with incident HFrEF, most experienced an improvement in EF with 20.4% of patients improving to 41-49% and 35.8% improving to ≥ 50%. In contrast, of patients with incident HFpEF, only 9.7% had a decline in EF to 41-49% and 6.4% had a decline in EF to ≤ 40%.
Figure 4: Evolution of Ejection Fraction Group Over Time.
Each column represents the heart failure ejection fraction category (≤40%, 41-49%, ≥50%) of the 1033 patients at diagnosis who have followup echocardiograms. Each color corresponds to the final EF category on echocardiogram for patients who were originally diagnosed within a given EF category.
EF= ejection fraction
Using a stricter definition of EF change (greater than 10 percentage points), the EF was stable in 76.2% of patients with HFpEF, 54.5% of patients with HFmrEF but only 38.4% of patients with HFrEF (Table S3). Using the definition in the current approach (change in currently accepted HFrEF, HFpEF or HFmrEF categorization), the EF categorization is stable in 83.9% of HFpEF, 28.3% of HFmrEF and 43.8% of HFrEF. Thus, rates of EF stability with the two definitions are similar for HFpEF (76.2% vs 83.9%) and HFrEF (38.4% vs 43.8%) but more patients with HFmrEF (54.5% vs 28.3%) are stable using the more stringent criteria.
Discussion:
In this contemporary, community-based cohort of all patients with newly diagnosed HF, 12.5% had HFmrEF, 29.9% had HFrEF, and 57.6% had HFpEF. In terms of clinical characteristics, incident HF patients with HFmrEF most resembled HFpEF patients with similar age and similar prevalence of most comorbidities; they were older and had a greater comorbidity burden than HFrEF. The sex distribution of HFmrEF patients was intermediate with a higher proportion of women than HFrEF but lower than in HFpEF. In contrast to the clinical characteristics, levels of cardiovascular biomarkers (NT-proBNP, BNP and troponin) in HFmrEF were similar to HFrEF and higher than in HFpEF. After adjustment for potential confounders, hospitalization and all-cause mortality risks did not vary by EF group. Lastly, in the 50% of patients where serial EF measurements were available, most patients with incident HFmrEF or HFrEF transitioned to another EF range, while most patients with incident HFpEF usually remained in the EF≥50 range over time.
Burden of HFmrEF
To the best of our knowledge, only Bhambhani et al have previously characterized HFmrEF in an incident and community-based HF cohort (18). Despite some differences between the elegant study of Bhambhani and ours (earlier era in the Bhambhani study, study design, frequency of missing EF [25% in Bhambhani study vs 8% here]), the two studies used the same HFmrEF definition, had a similar number of HF cases, validated HF according to a protocol, and found a roughly similar frequency of HFmrEF (9.7% in Bhambhani study vs 12.5% here). The study by Bhambhani et al included approximately 10% black persons vs 2.3 % here. Robust race/ethnicity/geographic data regarding HFmrEF burden are lacking. As comprehensively reviewed (10), many registry or clinical trial cohort studies have defined the frequency of HFmrEF in prevalent inpatient, outpatient, or mixed care setting HF cohorts with diverse study designs and slight differences in HFmrEF definition. The frequency of HFmrEF in these studies ranges from 10% to 24%, which is overall similar to the proportion of patients with newly diagnosed HF that have mildly reduced EF found herein (11-13, 15, 18, 29, 30).
Characteristics of HFmrEF:
Both incidence and prevalence studies have compared clinical characteristics and attempted to characterize HFmrEF as “most like HFrEF” or “most like HFpEF”. While results vary across the different study types, several consistent features emerge. Most of the registry/clinical trial prevalence studies (10) show that patients with HFmrEF have a sex distribution that is midway between HFrEF and HFpEF, that age more resembles HFpEF, and that CAD frequency more closely resembles HFrEF. These features were also seen here and in the Bhambhani incident cohort (18). Nearly all other clinical characteristics favor similarity between HFmrEF and HFpEF in both incident HF studies and the prevalent HF cohorts.
Less well studied are cardiac biomarkers at HF diagnosis by HF type. While not universally available, we found that at HF diagnosis, HFmrEF troponin and natriuretic peptide levels were more similar to those observed in HFrEF than HFpEF. Age, atrial fibrillation, and renal dysfunction would favor higher, and obesity would favor lower, natriuretic peptide levels but all these clinical characteristics in HFmrEF were similar to HFpEF. This is in contrast to the registry/clinical trial studies where natriuretic peptide levels (at study entry not HF diagnosis) in HFmrEF were more similar to HFpEF (10). The Bhambhani study did not characterize biomarkers at HF diagnosis.
Outcomes of HFmrEF
In the Bhambhani pooled incident HF study, unadjusted all-cause mortality in HFmrEF was similar to HFrEF and higher than HFpEF. Here, in somewhat older HFmrEF and HFpEF cohorts, unadjusted all-cause mortality was similar in HFmrEF and HFpEF and higher than in HFrEF. Adjusting for age, sex and comorbidities, there were no significant differences across EF groups but all-cause mortality in HFmrEF was closest to HFrEF and trended higher than HFpEF. There were no significant differences in unadjusted or adjusted cardiovascular mortality by HF type, though there were trends toward higher cardiovascular mortality in HFmrEF compared with HFpEF, and lower cardiovascular mortality in HFmrEF compared with HFrEF. Both of these trends became statistically significant when the definition of HFmrEF was expanded to EF 41-54%. Registry and clinical trial studies generally showed similar unadjusted all-cause and cardiovascular mortality in HFmrEF and HFpEF with higher rates in HFrEF in clinical trial but not registry studies (10).
In our study, while there were no statistically significant differences in all-cause and HF hospitalizations between EF groups, there was a trend toward higher risks of all-cause and HF hospitalizations in HFmrEF compared with HFrEF that were attenuated after adjustment for age, sex, and comorbidities. The Bhambhani study did not examine hospitalizations. In unadjusted analysis, the registry and clinical trial studies generally show HF hospitalization rates in HFmrEF are similar to HFpEF and lower than HFrEF (10). These differences likely reflect differences in study design and population characteristics (age, care setting, non-population based, etc).
Changes in EF versus HF type over time.
As documented in previous studies (30-32), categorizing HF by EF is complicated by the fact that EF changes over time and often into a range consistent with another HF type. Here, we saw 72% of patients with incident HFmrEF transition to EF≤40% (27%) or EF≥50% (45%) over time, while only 20% of patients with incident HFrEF and 10% of patients with incident HFpEF experience a change in EF to the EF 41-49% range over time. These EF transitions likely account for the differences in HFmrEF prevalence between incident and prevalent HF cohorts. Changes in EF over time will vary by the use of guideline-directed medical therapies for HF and comorbid conditions, CAD prevalence and therapy, age, sex distribution (as EF is higher in women), and the frequency of EF reassessment. Method of EF assessment, regression to the mean, and other technical aspects of quantifying EF by different modalities also contribute to differences in EF over time (10). These data also suggest that in prevalent HF cohort studies, many patients that were classified as having HFpEF likely previously had HFmrEF or HFrEF (whether recognized by previous EF measurement or not), underscoring an additional aspect of phenotypic diversity in HFpEF.
Optimal Incident HF Taxonomy and Clinical Implications:
Given the many factors contributing to variability in EF assessment, the transitory nature of the HFmrEF subtype observed here and the evidence that patients with “below normal” EF respond to HFrEF (5-7) therapies, one could argue that there should be just two EF sub-groups of incident HF (HFrEF and HFpEF). While the clinical profile of HFmrEF may resemble HFpEF and while adjusted outcomes did not vary by EF subgroup here, therapeutic response for some therapies does vary by EF (5-7) and that factor should drive taxonomy to simplify and promote optimal management. The definitions of these entities may need to be sex-specific (33). The likelihood of future EF transitions in HF must be recognized and distinguished from HF type in a the discrete manner as outlined in recent guidelines (3). Patients with incident HFmrEF or HFrEF who have improvements in EF to ≥50% should be characterized as HFwith improved EF and continue to be treated with guideline-directed medical therapies (34). In contrast, in a patient with HFpEF, deterioration in EF to below normal levels should trigger reclassification as HFrEF and initiation of appropriate HFrEF therapies. Lastly, when encountering a patient with established HF who has a normal EF or EF 41-50%, review of past EF assessments should be performed to determine if the patient has HF with improved EF and ensure continuation of HFrEF therapies in such patients (35).
Strengths and Limitations
The primary strength of this study is its status as a population-based study of newly diagnosed (incident) HF patients with longitudinal follow-up. This allows for characterizing the incidence, outcomes, and evolution of the HFmrEF population over time, without the many biases inherent in registry and clinical trial studies. Additionally, EF ascertainment was nearly universal (92%) whereas in other studies, EF was far more likely to be missing (up to 37%) (18, 36). Limitations of the study include the fact that patients were not required to have serial EF measurements at set intervals, which biases our longitudinal EF data towards patients who had more access to medical care or who may have had a change in clinical status. This may affect changes in EF over time. Furthermore, while most patients had their EF assessed in very close proximity to their HF diagnosis, it is possible that those whose EF assessments were delayed had already experienced changes in EF since diagnosis that were not captured. The generalizability of our findings in this study are limited by the fact it reflects a single community in the Upper Midwest United States, which is largely Caucasian.
Conclusion
In this community-based study of incident HF, HFmrEF represented 12.5% of HF cases. The clinical characteristics of incident HFmrEF were closest to HFpEF but female sex was less common, and biomarkers of myocardial stress and injury (BNP and troponin) were higher in HFmrEF than HFpEF. Adjusting for age, sex and comorbidity profile, there were no significant differences in cardiovascular or total hospitalization or mortality between patients who had incident HFmrEF, HFrEF, or HFpEF. Here, 20% of patients with incident HFrEF and 10% of patients with incident HFpEF transitioned to an EF of 41-49% (HFmrEF range) over time, whereas most patients (71.7%) with HFmrEF either dropped to an EF ≤40% or improved their EF ≥50% over time. These data expand our understanding of HFmrEF and the implications of the new HF taxonomy.
Supplementary Material
Highlights.
In this community cohort, 12.5% of incident HF was HFmrEF
Clinical characteristics of patients with HFmrEF resemble those with HFpEF
Outcomes of patients with HFmrEF are similar to patients with HFrEF and HFpEF
Over 70% of patients with incident HFmrEF will transition to a different EF category
Patients with newly diagnosed heart failure with mildly reduced EF have no difference in risk of death or hospitalization over time compared to patients with newly diagnosed heart failure with preserved or reduced EF.
Patients with heart failure with mildly reduced EF often have significant worsening or improvement in EF over time, which could be detected with serial imaging.
There are several medications that are recommended to improve outcomes in patients with heart failure with mildly reduced ejection fraction.
Funding Sources
This study was funded by the NIH (R01 HL144529, PI S.D.; R01 HL120859 PI Roger, and R21 AG045228, PI Roger). Dr. M.R’s time is supported by HL 160226-01. This study uses the resources of the Rochester Epidemiology Project (REP), which is supported by the National Institute on Aging (NIA; AG 058738).
Abbreviation and Acronyms
- HF
heart failure
- EF
left ventricular ejection fraction
- HFrEF
heart failure with reduced ejection fraction
- HFmrEF
heart failure with mildly reduced ejection fraction
- HFpEF
heart failure with preserved ejection fraction
Biography
VKumar photo

Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lam C, Yancy C. Universal Definition and Classification of Heart Failure: Is It universal? Does It Define Heart Failure? Journal of cardiac failure. 2021;27(5). [DOI] [PubMed] [Google Scholar]
- 2.McDonagh T, Metra M, Adamo M, Gardner R, Baumbach A, Böhm M, et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. European heart journal. 2021;42(36). [Google Scholar]
- 3.Bozkurt B, Coats A, Tsutsui H. Universal Definition and Classification of Heart Failure. Journal of cardiac failure. 2021. [DOI] [PubMed] [Google Scholar]
- 4.Heidenreich PA, Bozkurt B, Aguilar D, Allen LA, Byun JJ, Colvin MM, et al. 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J Am Coll Cardiol. 2022;79(17):e263–e421. [DOI] [PubMed] [Google Scholar]
- 5.Lund LH, Claggett B, Liu J, Lam CS, Jhund PS, Rosano GM, et al. Heart failure with mid-range ejection fraction in CHARM: characteristics, outcomes and effect of candesartan across the entire ejection fraction spectrum. Eur J Heart Fail. 2018;20(8):1230–9. [DOI] [PubMed] [Google Scholar]
- 6.Solomon SD, Vaduganathan M, B LC, Packer M, Zile M, Swedberg K, et al. Sacubitril/Valsartan Across the Spectrum of Ejection Fraction in Heart Failure. Circulation. 2020;141(5):352–61. [DOI] [PubMed] [Google Scholar]
- 7.Solomon SD, Claggett B, Lewis EF, Desai A, Anand I, Sweitzer NEC, et al. Influence of ejection fraction on outcomes and efficacy of spironolactone in patients with heart failure with preserved ejection fraction. Eur Heart J. 2016;37(5):455–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Anker SD, Butler J, Filippatos G, Ferreira JP, Bocchi E, Bohm M, et al. Empagliflozin in Heart Failure with a Preserved Ejection Fraction. N Engl J Med. 2021;385(16):1451–61. [DOI] [PubMed] [Google Scholar]
- 9.Vaduganathan M, Claggett B, Greene S, Aggarwal R, Bhatt A, McMurray J, et al. Potential Implications of Expanded US Food and Drug Administration Labeling for Sacubitril/Valsartan in the US. JAMA cardiology. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Savarese G, Stolfo D, Sinagra G, Lund L. Heart failure with mid-range or mildly reduced ejection fraction. Nature reviews Cardiology. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cheng RFC, Cox M, Neely ML, Heidenreich PA, Bhatt DL, Eapen ZJ, et al. Outcomes in patients with heart failure with preserved, borderline, and reduced ejection fraction in the Medicare population. Am Heart J. 2014;168(5):721–30. [DOI] [PubMed] [Google Scholar]
- 12.Koh A, Tay W, Teng T, Vedin O, Benson L, Dahlstrom U, et al. A comprehensive population-based characterization of heart failure with mid-range ejection fraction. European journal of heart failure. 2017; 19(12). [DOI] [PubMed] [Google Scholar]
- 13.Chioncel O, Lainscak M, Seferovic PM, Anker SD, Crespo-Leiro MG, Harjola VP, et al. Epidemiology and one-year outcomes in patients with chronic heart failure and preserved, mid-range and reduced ejection fraction: an analysis of the ESC Heart Failure Long-Term Registry. Eur J Heart Fail. 2017;19(12):1574–85. [DOI] [PubMed] [Google Scholar]
- 14.Shah K, Xu H, Matsouaka R, Bhatt D, Heidenreich P, Hernandez A, et al. Heart Failure With Preserved, Borderline, and Reduced Ejection Fraction: 5-Year Outcomes. Journal of the American College of Cardiology. 2017;70(20). [DOI] [PubMed] [Google Scholar]
- 15.Tsuji K, Sakata Y, Nochioka K, Miura M, Yamauchi T, Onose T, et al. Characterization of heart failure patients with mid-range left ventricular ejection fraction-a report from the CHART-2 Study. Eur J Heart Fail. 2017;19(10):1258–69. [DOI] [PubMed] [Google Scholar]
- 16.Kapoor JR, Kapoor R, Ju C, Heidenreich PA, Eapen ZJ, Hernandez AF, et al. Precipitating Clinical Factors, Heart Failure Characterization, and Outcomes in Patients Hospitalized With Heart Failure With Reduced, Borderline, and Preserved Ejection Fraction. JACC Heart Fail. 2016;4(6):464–72. [DOI] [PubMed] [Google Scholar]
- 17.Krumholz H. Registries and selection bias: the need for accountability. Circulation Cardiovascular quality and outcomes. 2009;2(6). [DOI] [PubMed] [Google Scholar]
- 18.Bhambhani V, Kizer J, Lima J, van der Harst P, Bahrami H, Nayor M, et al. Predictors and outcomes of heart failure with mid-range ejection fraction. European journal of heart failure. 2018;20(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rocca W, Yawn B, St Sauver J, Grossardt B, Melton L. History of the Rochester Epidemiology Project: half a century of medical records linkage in a US population. Mayo Clinic proceedings. 2012;87(12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.St Sauver J, Grossardt B, Yawn B, Melton L, Rocca W. Use of a medical records linkage system to enumerate a dynamic population over time: the Rochester epidemiology project. American journal of epidemiology. 2011;173(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.St Sauver J, Grossardt B, Leibson C, Yawn B, Melton L, Rocca W. Generalizability of epidemiological findings and public health decisions: an illustration from the Rochester Epidemiology Project. Mayo Clinic proceedings. 2012;87(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gerber Y, Weston SA, Redfield MM, Chamberlain AM, Manemann SM, Jiang R, et al. A contemporary appraisal of the heart failure epidemic in Olmsted County, Minnesota, 2000 to 2010. JAMA Intern Med. 2015;175(6):996–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cohen S, Roger V, Weston S, Jiang R, Movva N, Yusuf A, et al. Evaluation of claims-based computable phenotypes to identify heart failure patients with preserved ejection fraction. Pharmacology research & perspectives. 2020;8(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ho K, Anderson K, Kannel W, Grossman W, Levy D. Survival after the onset of congestive heart failure in Framingham Heart Study subjects. Circulation. 1993;88(1). [DOI] [PubMed] [Google Scholar]
- 25.Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail. 2016;18(8):891–975. [DOI] [PubMed] [Google Scholar]
- 26.Charlson M, Pompei P, Ales K, MacKenzie C. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. Journal of chronic diseases. 1987;40(5). [DOI] [PubMed] [Google Scholar]
- 27.St Sauver JL, Chamberlain AM, Bobo WV, Boyd CM, Finney Rutten LJ, Jacobson DJ, et al. Implementing the US Department of Health and Human Services definition of multimorbidity: a comparison between billing codes and medical record review in a population-based sample of persons 40-84 years old. BMJ Open. 2021;11(4):e042870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.St Sauver J, Grossardt B, Yawn B, Melton L, Pankratz J, Brue S, et al. Data resource profile: the Rochester Epidemiology Project (REP) medical records-linkage system. International journal of epidemiology. 2012;41(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lam C, Gamble G, Ling L, Sim D, Leong K, Yeo P, et al. Mortality associated with heart failure with preserved vs. reduced ejection fraction in a prospective international multi-ethnic cohort study. European heart journal. 2018;39(20). [DOI] [PubMed] [Google Scholar]
- 30.Savarese G, Vedin O, D'Amario D, Uijl A, Dahlström U, Rosano G, et al. Prevalence and Prognostic Implications of Longitudinal Ejection Fraction Change in Heart Failure. JACC Heart failure. 2019;7(4). [DOI] [PubMed] [Google Scholar]
- 31.Dunlay S, Roger V, Weston S, Jiang R, Redfield M. Longitudinal changes in ejection fraction in heart failure patients with preserved and reduced ejection fraction. Circulation Heart failure. 2012;5(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lupón J, Gavidia-Bovadilla G, Ferrer E, de Antonio M, Perera-Lluna A, López-Ayerbe J, et al. Dynamic Trajectories of Left Ventricular Ejection Fraction in Heart Failure. Journal of the American College of Cardiology. 2018;72(6). [DOI] [PubMed] [Google Scholar]
- 33.McMurray J, Jackson A, Lam C, Redfield M, Anand I, Ge J, et al. Effects of Sacubitril-Valsartan Versus Valsartan in Women Compared With Men With Heart Failure and Preserved Ejection Fraction: Insights From PARAGON-HF. Circulation. 2020;141(5). [DOI] [PubMed] [Google Scholar]
- 34.Gulati G, Udelson J. Heart Failure With Improved Ejection Fraction: Is it Possible to Escape One's Past? JACC Heart failure. 2018;6(9). [DOI] [PubMed] [Google Scholar]
- 35.Halliday B, Wassall R, Lota A, Khalique Z, Gregson J, Newsome S, et al. Withdrawal of pharmacological treatment for heart failure in patients with recovered dilated cardiomyopathy (TRED-HF): an open-label, pilot, randomised trial. Lancet (London, England). 2019;393(10166). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Senni M, Tribouilloy C, Rodeheffer R, Jacobsen S, Evans J, KR B, et al. Congestive heart failure in the community: a study of all incident cases in Olmsted County, Minnesota, in 1991. Circulation. 1998;98(21). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.