Abstract
A copper-mediated radiobromination of (hetero)aryl boronic pinacol esters is described. Cyclotron-produced [76/77Br]bromide was isolated using an anion exchange cartridge, wherein the pre-equilibration and elution solutions played a critical role in downstream deboro-bromination. The bromination tolerates a broad range of functional groups, labeling molecules with ranging electronic and steric effects. Bologically active radiopharmaceuticals were synthesized, including two radiobrominated inhibitors of poly ADP ribose polymerase, a clinically relevant chemotherapeutic target for ovarian, breast, and prostate cancers.
Graphical Abstract
INTRODUCTION
Nuclear medicine is a multidisciplinary clinical specialty utilizing radiopharmaceuticals for the detection, assessment, and treatment of disease. A critical research area in the field focuses on the incorporation of radioactive isotopes into small and large biological molecules for diagnostic and therapeutic purposes in neurology, cardiology, and oncology. Within nuclear medicine, the theranostic approach applies chemically matched diagnostic and therapeutic radiopharmaceuticals with interchangeable radioisotopes. This radiation oncology paradigm is becoming standard of care as highlighted by the recent FDA approvals of diagnostic/therapeutic radiopharmaceuticals Netspot1/Lutathera2 for neuroendocrine tumors and Pylarify3/Ga 68 PSMA-114/Pluvicto5 for prostate cancer. The predominant methods for chemically labeling radiopharmaceuticals are through direct small molecule incorporation of organic radionuclides6-8 or the incorporation of radiometals via chelators (DOTA, NOTA, etc.) coupled to targeting vectors.9-11 Of the former of these methods, radiohalogenation has proven particularly useful, exemplified through the synthesis of [18F]fluorodeoxyglucose ([18F]FDG) used in positron emission tomography (PET) diagnosis, staging, and monitoring treatment response of various oncologic diseases.
Incorporation of halogens into (hetero)aryl structural motifs has become prevalent in recent years for utility in radiopharmaceuticals used for both diagnostic imaging (18F, 76Br, 124I) and therapeutic treatment (77Br, 82Br, 123I, 125I, 131I, 211At).7,12,13 Radiobromine is unique within the halides as it has several isotopes (76Br, 77Br, 82Br) with suitable half-lives and radioactive decay pathways including positron emission (β+) used in PET imaging (76Br, t1/2 = 16.2 h), electron capture (EC) leading to Meitner Auger electron emission (77Br, t1/2 = 57.0 h), and beta (β−) emission (82Br, t1/2 = 35.3 h) for targeted radionuclide therapy (TRT).14 Meitner Auger electrons are low energy (eV – keV) electrons that are produced in a cascade fashion following EC decay. These electrons are of significant interest due to their high linear energy transfer (LET), dissipating their energy within a short distance in tissue (nm – μm) and delivering low off-target radiation dose.15 However, due to difficulty in the production/isolation or procurement of 76,77,82Br, methods for incorporating radiobromine into medicinal chemical products have not seen the same explosive growth as that of radiofluorine and radioiodine to date. Recently, we reported the improved cyclotron production and nuclear chemical isolation of 76,77Br resulting in the robust and reproducible production of clinical quantities of 76Br and pre-clinical quantities of 77Br.16
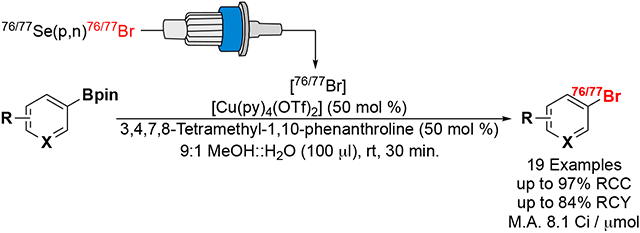
With the advances in transition metal mediated couplings of 18F, 12x/131I, and 211At to (hetero)aryl electrophiles (Scheme 1) and the improvement in production and isolation of 76/77Br, we sought to investigate the translation of these radiochemical methods to bromination. Pioneered by the Gouverneur group, copper-mediated radiofluorination of (hetero)aryl boronic esters17 / acids18 has become a versatile method due to its efficiency, extensive functional group tolerance, and low toxicity of boronic esters / acids.19 Furthermore, this work has been adapted for the incorporation of 123I20 and 211At13 by the Gouverneur and Mach groups respectively. While copper-mediated coupling of boronic pinacol esters16,21,22 / acids22,23 has facilitated incorporation of 77Br into a small selection of molecules, a systematic investigation of a general procedure for labeling (hetero)aryl boronic pinacol esters with radiobromine has yet to be reported. One of the challenges of these methods has been highlighted in radiofluorination wherein the cyclotron-produced [18F] fluoride (aq) must be concentrated through the use of a quaternary methyl ammonium (QMA) cartridge prior to deborylfluorination radiochemistry. It was observed that the presence of certain bases and phase transfer catalysts commonly used in liberating 18F from the QMA cartridge had detrimental effects to downstream labeling, especially when using transition metal mediated coupling methods.24 This manuscript investigates the effect 76/77Br QMA cartridge trap / release conditions has on downstream copper-mediated deboro-bromination, the radiochemistry’s tolerance to functional groups commonly found in medicinal compounds, the labeling of a wide substrate scope, and finally its application in the production of several theranostic radiopharmaceuticals.
Scheme 1. Copper-mediated (hetero)aryl deboro-radiohalogenation reactions and references.
RESULTS AND DISCUSSION
Recent advances in the production and isolation of 76,77Br have also brought challenges, including the dilution of 76,77Br in large volumes of water (~40 mL), requiring a QMA cartridge trap / release step prior to use in radiobromination reactions. Recent work investigating optimal QMA trapping and releasing conditions for aliphatic25,26 and aryl24 radiofluorination chemistry guided our studies with 76/77Br. Initially, NaHCO3 (10 mL, 0.5 M aq) followed by deionized water (10 mL) was used to pre-equilibrate the QMA cartridge leading to near quantitative 76/77Br trapping (99 ± 0.5%). Subsequent 76/77Br elution with K2CO3 (0.8 mL, 0.1 mM) led to good recovery (> 95%); however, after solvent evaporation, the resulting 76/77Br demonstrated low radiochemical conversion (RCC) (3 ± 2%, n = 2) under our general copper-mediated deboro-bromination reaction conditions (see Scheme 2).
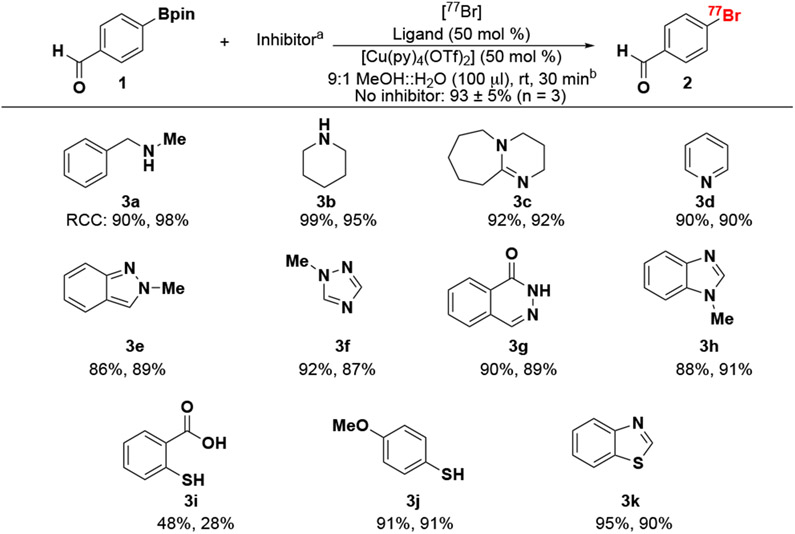
Scheme 2. Structural dependence of radiobromination on boronic pinacol esters via copper mediation.
aInhibitors were added 1:1 vs Bpin (1 μmol).
bReactions were performed on a 7.5 MBq [77Br]bromide with 1 μmol of 1, 0.5 μmol of [Cu(py)4(OTf)2] and 3,4,7,8-Tetramethyl-1,10-phenanthroline (Ligand) stirred in 100 μL of 9:1 MeOH::H2O for 30 minutes at room temperature (rt). All reactions were conducted in duplicate and RCC’s were calculated by activity trapped on C18 vs starting activity scaled by HPLC chromaogram rad peak integration to account for impurities.
To circumvent the use of Na2CO3 as releasing agent and transition to a volatile base, NH4OH (800 μL, 0.1 M) was used for elution (Table 1, entry 6); however, only 1% of the 76/77Br eluted from NaHCO3-equilibrated QMA cartridges. With the recent report by Scott et al.24 demonstrating greater 18F elution from sulfate equilibrated QMA cartridges, we investigated Na2SO4 as equilibration agent. Equilibrating with Na2SO4 (10 mL, 0.5 M) and eluting with NH4OH led to excellent 76/77Br trapping (96 ± 4%) and release (93 ± 7%) efficiencies (Table 1 entry 1). The 76/77Br eluted under these conditions led to improved but sporadic (5 – 80%) downstream deboro-bromination RCCs (reaction conditions in Scheme 3 forming compound 21).
Table 1.
QMA equilibration and elution solution investigation
| QMA Equilibration Agent |
76/77Br Trapping (%) | Entry | QMA Elution Agent |
pKaa |
76/77Br Eluted (%) |
n |
|---|---|---|---|---|---|---|
| Na2SO4 (0.5 M) | 96 ± 4 % n = 11 |
1 | NH4OH | 9.23 | 93 ± 7 | 9 |
| 2 | Me3N | 9.8 | 99 | 1 | ||
| 3 | MeNH2 | 10.66 | 98 | 1 | ||
| 4 | Me2NH | 10.73 | 100 | 1 | ||
| 5 | DBU | 13.5 | 99 | 1 | ||
| NaHCO3 (0.5 M) | 99 ± 0.5 % n = 9 |
6 | NH4OH | 9.23 | 1 | 1 |
| 7 | Me3N | 9.8 | 5 ± 1 | 2 | ||
| 8 | MeNH2 | 10.66 | 55 | 1 | ||
| 9 | Me2NH | 10.73 | 86 ± 18 | 8 | ||
| 10 | Me2NHb | 10.73 | 94 ± 6 | 6 | ||
| 11 | DBU | 13.5 | 100 | 1 |
pKa values in aqueous solution from CRC Handbook of Chemistry and Physics Online except DBU from Kaupmees et al. Croat. Chem. Acta 87(4): 385–395 (2014).
Eluted with 1.6 mL of QMA releasing agent instead of the standard 800 μL. 0.1 M 1:1 MeCN / H2O
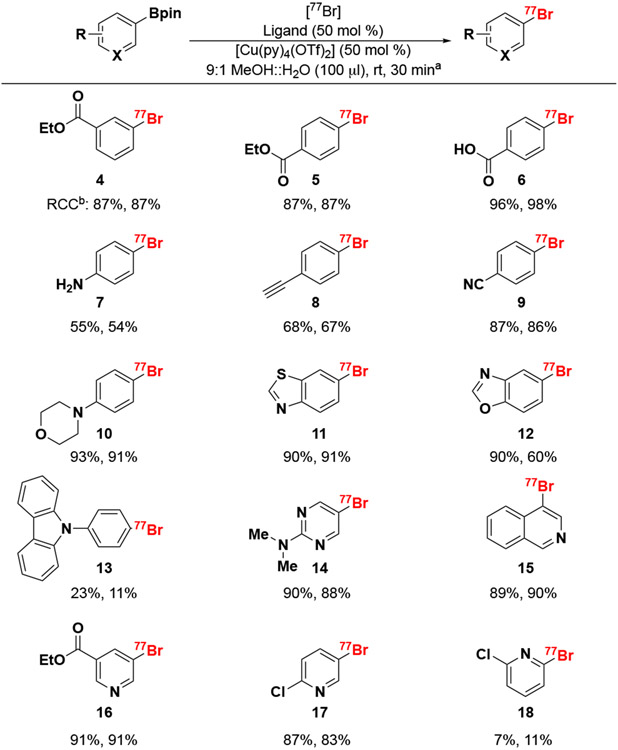
Scheme 3. Substrate scope of copper-mediated deboro-bromination.
aReactions were performed with 7.5 MBq [77Br]bromide, 1 μmol of boronic pinacol ester precursor, 0.5 μmol of [Cu(py)4(OTf)2], and 3,4,7,8-Tetramethyl-1,10-phenanthroline (Ligand) stirred in 100 μL of 9:1 MeOH::H2O for 30 minutes at room temperature (rt). bAll reactions were conducted in duplicate and RCC calculated as activity trapped on C18 cartridge versus starting activity scaled by HPLC radioactivity detector chromatogram product peak area versus all peak areas to account for impurities.
To determine if the releasing agent was causing the sporadic reaction yields, we screened a variety of volatile organic bases to elute 77Br from Na2SO4 equilibrated cartridges (entries 2 – 5). High levels (> 98%) of release were seen in all cases, however labeling was challenging (<5% RCC, n = 1 for elution conditions of Table 1, entry 4, reaction conditions in Scheme 2). We hypothesize this is due small amounts of sulfate (SO42−) being released off the QMA cartridge and complexing with the copper (Cu2+), thereby inhibiting catalytic turnover. This phenomenon has also been observed in copper-mediated radiofluorination of aryl boronic pinacol esters.24
We then screened the organic volatile base elution agents for releasing 76/77Br from NaHCO3 equilibrated QMA cartridges (entries 7 – 11). Higher pKa releasing agents resulted in more effective elution of 77Br, in agreement with radiofluoride trends observed by Scott et al.24 Eluting the QMA cartridge with NMe2H (0.8 mL) lead to good 76/77Br releasing efficiency (86 ± 18%) and increasing the amount of NMe2H (0.8 to 1.6 mL) led to improved, more consistent elution (94 ± 6%). Following NMe2H elution from NaHCO3 equilibrated QMA cartridges, 76/77Br reacted with high, consistent copper-mediated deboro-bromination RCC (93 ± 5%, n = 3, reaction conditions in Scheme 2) of our standard benzaldehyde-Bpin precursor. DBU (1,8-diazabicyclo[5.4.0]undec-7-ene) was also an effective elution agent from NaHCO3 equilibrated cartridges (100%, entry 11); however, the resulting 76/77Br was unreactive in copper- deboro-bromination reactions, likely due to the lack of volatility of the ~80 μmol of DBU elution agent. Thus, optimal QMA trap and release conditions are summarized by Table 1, entry 10, highlighted in red.
Upon determining optimal conditions for preparation of reactive [76/77Br]bromide, we sought to investigate the tolerance of the copper-mediated deboro-bromination reaction to various functional groups that are found in a range of radiopharmaceutical targets using the catalyst system (i.e. [Cu(py)4(OTf)2] / 3,4,7,8,-tetramethyl-1,10 phenanthroline) reported by Mach for radioiodination/astatination of aryl-boronic pinacol esters.12 Inspired by Gouveneur et al.’s de-risking approach for radiofluorination,27 we screened the functional groups in Scheme 2 by adding a 1:1 molar ratio of a functional-group-containing inhibitor molecule to our standard benzaldehyde 1 bromination reaction. It is hypothesized that if an inhibitor has an adverse effect on the standard radiolabeling, then radiopharmaceutical products containing this chemical functionality would similarly have low yield. Compared to radiofluorination, radiobromination is more tolerant to functional group variability.27 Secondary amines 3a and 3b did not inhibit the reaction with 76/77Br RCCs of 94 ± 4% and 97 ± 3%, respectively, comparable with the RCC of the non-inhibited reaction (93 ± 5%).
Interestingly, a 1:1 molar ratio of DBU 3c did not inhibit 76/77Br RCC (92.1 ± 0.2%) contradicting that when used as a QMA elution agent and present in an 80:1 molar ratio, DBU fully inhibits radiobromination (vide supra). 3e – 3h, common functional groups in a variety of oncologic radiotracers, such as small molecule chemotherapeutic inhibitors of poly ADP ribose polymerase (PARP), did not inhibit the reaction leading to 87 – 90% RCC. Deboro-bromination was significantly inhibited by addition of 2-mercaptobenzoic acid (3i, 38 ± 10% RCC). We postulate this is due to bidentate coordination to copper through the carboxylic acid and aryl thiol thereby inhibiting catalytic turnover. Non-bidentate chelating thiols (3j and 3k) did not significantly inhibit the radiobromination with (91.5 ± 0.3%) and (92.5 ± 2.5%), respectively.
To verify the inhibitor study, we labeled a range of substrates containing various structural motifs and electronic functional groups (Scheme 3). Initially, aryl-Bpin substrates were investigated (4 – 13), 77Br-aryl esters 4 and 5 labeled efficiently suggesting that labeling the para or meta positions does not impact deboro-bromination. 77Br-Benzoic acid 6 (97 ± 1%) was labeled in the para position with good RCC, however 77Br-aniline 7 (54 ± 1%), 77Br-ethynyl benzene 8 (67.5 ± 0.5%), and 77Br-benzo-nitrile 9 (85.5 ± 0.5%) showed lower RCC’s, which could be due to their coordination to the copper catalyst. Products 6 – 9 are important because they can be utilized in the subsequent aryl-radiobromination of amino acids and peptides via amide couplings (6, 7) and click chemistry (8 and 9). The thiazole-based extended 77Br-aryl system 11 labeled efficiently (90.5 ± 0.5% RCC) as predicted through the inhibitor study, but the similarly electron-rich 77Br-benzo-oxazole 12 had mixed results with 75 ± 15% RCC. Surprisingly, 77Br-phthalimide 13 only labeled in 17 ± 6%; we hypothesize this is due to the poor solubility of the precursor and subsequent product in the 9:1 MeOH/H2O reaction solvent.
Hetero(aryl) substrates containing nitrogen were subsequently examined. The electronically stabilized heterocyclic systems: 77Br-dimethylamino-pyrimidine 14 and 77Br-isoquinoline 15 showed good labeling with RCC’s of 89 ± 1% and 89.5 ± 0.5% respectively. The labeling position was significant when comparing 77Br-chloro-pyridine products, with bromination at the meta (17, 85 ± 2%) position showing higher RCC than at the ortho (18, 9 ± 2%) position. Interestingly, electron deficient 77Br-heterocycles (16 – 18) did not strictly follow radiofluorination trends where these radiofluorides often required higher quantities of catalyst loading to impart higher labeling efficiencies.17
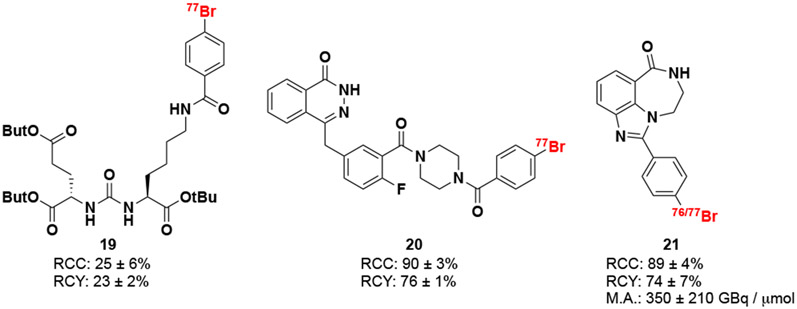
We applied our optimized method to the preparation of three radiotracers with biological application for the diagnosis and therapy of prostate (19), ovarian, and other cancers (20 and 21), shown in Scheme 4. Initially our focus centered on synthesizing and labeling a derivative of the 18F-DCFPyL (Pylarify) radioligand, used for the imaging of metastasis and recurrence in prostate cancer patients.28 We successfully labeled the Bpin precursor of the radiotracer’s tert-butyl ether-protected benzyl analogue with 77Br under our optimized conditions with 31 ± 4% RCC and non-decay corrected isolated radiochemical yield (n.d.c. RCY) of 23 ± 2% (n = 2). We also focused on derivatives of clinical chemotherapeutic inhibitors of the nuclear PARP proteins, which are involved in the cellular single-stranded DNA damage response mechanism.29 Pairing this biological mechanism with targeted Meitner-Auger electron emitting radionuclides is of significant interest because of its over-expression in a wide variety of cancers and the biological mechanism that brings, and in some cases traps,30 the radiopharmaceutical within nanometers of the DNA.31 Recent preclinical studies have investigated Meitner-Auger electron emitting radionuclide-labeled PARP inhibitors for the treatment of neuroblastoma,32 glioblastoma,33 ovarian,34 breast,35 and colon cancer.36 The olaparib derivative 20 was successfully labeled from its Bpin precursor with RCC of 90 ± 3% and isolated RCY of 76 ± 1% (n = 2). A rucaparib derivative 21 was labeled from its Bpin precursor leading to high RCC (89 ± 4%, n = 7). Subsequent isolation and formulation of 21 was conducted leading to good RCY (74 ± 7%, n = 7) and high molar activity (350 ± 210 GBq / μmol for [77Br]21, n = 6; ~700 GBq / μmol for [76Br]21, n =1). Syntheses starting with ~400 MBq 76/77Br produced 300 MBq of [77Br]21 and 280 MBq of [76Br]21 at end of synthesis (EOS). This production scale is suitable for pre-clinical therapeutic (with 77Br) and clinical diagnostic (with 76Br) studies. Current investigations are underway to translate this method to an automated synthesis module for routine production. Preliminary data investigating the dependence of reactant concentration, time, and temperature on [76/77Br]21 optimized reaction conditions is shown in the supporting information (SI Figure S5 - S7) which we hope to use to streamline the translation of this work into an automated synthesis module.
Scheme 4. Radiobrominated biological targeting vectors.
Reactions were performed on a 7.5 - 400 MBq scale with 1 μmol of boronic pinacol ester precursor, 0.5 μmol of [Cu(py)4(OTf)2] and 3,4,7,8-Tetramethyl-1,10-phenanthroline stirred in 100 μL of 9:1 MeOH::H2O for 30 minutes at room temperature. RCC’s were calculated by activity trapped on C18 vs starting activity scaled by HPLC chromaogram radioactivity peak integration to account for impurities. RCY was calculated based on activity isolated from preperatory HPLC versus starting activtiy of the reaction.
CONCLUSION
In conclusion, this work presents a reproducible, high yielding QMA-cartridge based method for preparing reactive [76/77Br]bromide for late-stage nucleophilic copper-mediated coupling to (hetero)aryl-boronic pinacol esters. This manuscript utilizes the approach to de-risking downstream labeling by conducting a competitive study of common functional groups seen in drug targets. The radiobromination method was observed to be amenable to a variety of aryl and heteroaryl compounds with varying steric and electronic substituents. Compared with previous deboro-radiobromination reports22,23, the method is effective with milder conditions, less precursor, and more radioactivity, as evidenced over a wide substrate scope. The utility of the developed methodology is demonstrated by synthesis of three radiobrominated medicinal targeted molecules with moderate to high yields and theranostic potential.
EXPERIMENTAL SECTION
Dry [76/77Br]bromide was dissolved in a mixture of [Cu(py)4OTf2] / 3,4,7,8-tetramethyl-1,10-phenanthroline (50 mol%, prepared fresh as 50 mM stock in MeOH) and an aryl Bpin precursor (1 μmol, prepared fresh as 12.5 mM stock in MeOH) in 9:1 MeOH/H2O (100 μL). The solution was stirred for 30 min at room temperature. Upon completion the solution was diluted in water (10 mL) and passed through a preconditioned C18 Sep-Pak to trap the radiolabeled compound. The cartridge was rinsed with water (10 mL) and the radio labeled compound was eluted from the C18 Sep-Pak with subsequent additions of pure ethanol (0.5 mL) and water (0.5 mL). The combined load/rinse solution (~20 mL), elution solution (~1 mL), and eluted C18 cartridge were assayed with a radioactive dose calibrator (CRC-15R setting #120 for 77Br, #690÷2 for 76Br, Capintec). A crude radiochemical conversion (RCC) was calculated by dividing the activity of the elution solution by the total summed activity. The elution solution was then injected onto a preparative high performance liquid chromatography (HPLC, Kinetix XB-C18, 5 μm, 100 Å, 10 x 250 mm) using the elution conditions detailed in the supporting information. Purified RCC was calculated by multiplying the crude RCC by the radiochemical purity of the product peak determined by integration of radioactivity peak areas of the HPLC chromatogram. Non-decay corrected radiochemical yield (RCY) was determined by the quantity of radiolabeled product isolated from the preparative HPLC divided by the starting activity in the reaction.
Supplementary Material
ACKNOWLEDGMENT
This research is supported by the U.S. Department of Energy Isotope Program, managed by the Office of Science for Isotope R&D and Production, grant number DE-SC0020960 and the U.S. Department of Defense Ovarian Cancer Research Program Pilot Award, grant number W81XWH2110351. J.C.M. is supported by the National Cancer Institute of the National Institutes of Health under Award Number T32CA009206. Aryl-boronic pinacol ester precursor for the radiobrominated rucaparib derivative was graciously provided by Professor Mehran Makvandi of the University of Pennsylvania.
Footnotes
Supporting Information
Supporting methods and reagents, analytical data, and figures. (PDF)
Data Availability Statement
The data underlying this study are available in the published article and its online supplementary material.
REFERENCES
- (1).Hennrich U; Benešová M [68Ga]Ga-DOTA-TOC: The First FDA-Approved 68Ga-Radiopharmaceutical for PET Imaging. Pharmaceuticals . 2020. 10.3390/ph13030038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Hennrich U; Kopka K Lutathera®: The First FDA- and EMA-Approved Radiopharmaceutical for Peptide Receptor Radionuclide Therapy. Pharmaceuticals 2019, 12 (3), 114. 10.3390/ph12030114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Keam SJ Piflufolastat F 18: Diagnostic First Approval. Mol. Diagn. Ther 2021, 25 (5), 647–656. 10.1007/s40291-021-00548-0. [DOI] [PubMed] [Google Scholar]
- (4).Hennrich U; Eder M [68Ga]Ga-PSMA-11: The First FDA-Approved 68Ga-Radiopharmaceutical for PET Imaging of Prostate Cancer. Pharmaceuticals 2021, 14 (8). 10.3390/ph14080713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Administration, U. S. F. and D.; Imaging, S. of N. M. and M. FDA Approves Pluvicto/Locametz for Metastatic Castration-Resistant Prostate Cancer. J. Nucl. Med 2022, 63 (5), 13N LP-- 13N. [PubMed] [Google Scholar]
- (6).Preshlock S; Tredwell M; Gouverneur V 18F-Labeling of Arenes and Heteroarenes for Applications in Positron Emission Tomography. Chem. Rev 2016, 116 (2), 719–766. 10.1021/acs.chemrev.5b00493. [DOI] [PubMed] [Google Scholar]
- (7).Dubost E; McErlain H; Babin V; Sutherland A; Cailly T Recent Advances in Synthetic Methods for Radioiodination. J. Org. Chem 2020, 85 (13), 8300–8310. 10.1021/acs.joc.0c00644. [DOI] [PubMed] [Google Scholar]
- (8).Goud NS; Bhattacharya A; Joshi RK; Nagaraj C; Bharath RD; Kumar P Carbon-11: Radiochemistry and Target-Based PET Molecular Imaging Applications in Oncology, Cardiology, and Neurology. J. Med. Chem 2021, 64 (3), 1223–1259. 10.1021/acs.jmedchem.0c01053. [DOI] [PubMed] [Google Scholar]
- (9).Cutler CS; Hennkens HM; Sisay N; Huclier-Markai S; Jurisson SS Radiometals for Combined Imaging and Therapy. Chem. Rev 2013, 113 (2), 858–883. 10.1021/cr3003104. [DOI] [PubMed] [Google Scholar]
- (10).Herrero Álvarez N; Bauer D; Hernéndez-Gil J; Lewis JS Recent Advances in Radiometals for Combined Imaging and Therapy in Cancer. ChemMedChem 2021, 16 (19), 2909–2941. 10.1002/cmdc.202100135. [DOI] [PubMed] [Google Scholar]
- (11).Brandt M; Cardinale J; Aulsebrook ML; Gasser G; Mindt TL An Overview of PET Radiochemistry, Part 2: Radiometals. J. Nucl. Med 2018, 59 (10), 1500 LP-- 1506. 10.2967/jnumed.117.190801. [DOI] [PubMed] [Google Scholar]
- (12).Jacobson O; Kiesewetter DO; Chen X Fluorine-18 Radiochemistry, Labeling Strategies and Synthetic Routes. Bioconjug. Chem 2015, 26 (1), 1–18. 10.1021/bc500475e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Reilly SW; Makvandi M; Xu K; Mach RH Rapid Cu-Catalyzed [211At]Astatination and [125I]Iodination of Boronic Esters at Room Temperature. Org. Lett 2018, 20 (7), 1752–1755. 10.1021/acs.orglett.8b00232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Eckerman KF; Endo A ICRP Publication 107. Nuclear Decay Data for Dosimetric Calculations. Ann. ICRP 2008, 38 (3), 7–96. 10.1016/j.icrp.2008.10.004. [DOI] [PubMed] [Google Scholar]
- (15).Ku A; Facca VJ; Cai Z; Reilly RM Auger Electrons for Cancer Therapy – a Review. EJNMMI Radiopharm. Chem 2019, 4 (1), 27. 10.1186/s41181-019-0075-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Ellison PA; Olson AP; Barnhart TE; Hoffman SLV; Reilly SW; Makvandi M; Bartels JL; Murali D; DeJesus T; Lapi SE; Bednarz B; Nickles RJ; Mach RH; Engle JW Improved Production of 76Br, 77Br and 80mBr via CoSe Cyclotron Targets and Vertical Dry Distillation. Nucl. Med. Biol 2020, 80–81, 32–36. https://doi.org/ 10.1016/j.nucmedbio.2019.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Tredwell M; Preshlock SM; Taylor NJ; Gruber S; Huiban M; Passchier J; Mercier J; Génicot C; Gouverneur V A General Copper-Mediated Nucleophilic 18F Fluorination of Arenes. Angew. Chemie Int. Ed 2014, 53 (30), 7751–7755. https://doi.org/ 10.1002/anie.201404436. [DOI] [PubMed] [Google Scholar]
- (18).Mossine AV; Brooks AF; Makaravage KJ; Miller JM; Ichiishi N; Sanford MS; Scott PJH Synthesis of [18F]Arenes via the Copper-Mediated [18F]Fluorination of Boronic Acids. Org. Lett 2015, 17 (23), 5780–5783. 10.1021/acs.orglett.5b02875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Wright JS; Kaur T; Preshlock S; Tanzey SS; Winton WP; Sharninghausen LS; Wiesner N; Brooks AF; Sanford MS; Scott PJH Copper-Mediated Late-Stage Radiofluorination: Five Years of Impact on Preclinical and Clinical PET Imaging. Clin. Transl. Imaging 2020, 8 (3), 167–206. 10.1007/s40336-020-00368-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Wilson TC; McSweeney G; Preshlock S; Verhoog S; Tredwell M; Cailly T; Gouverneur V Radiosynthesis of SPECT Tracers via a Copper Mediated 123I Iodination of (Hetero)Aryl Boron Reagents. Chem. Commun 2016, 52 (90), 13277–13280. 10.1039/C6CC07417K. [DOI] [PubMed] [Google Scholar]
- (21).Ordonez AA; Carroll LS; Abhishek S; Mota F; Ruiz-Bedoya CA; Klunk MH; Singh AK; Freundlich JS; Mease RC; Jain SK Radiosynthesis and PET Bioimaging of 76Br-Bedaquiline in a Murine Model of Tuberculosis. ACS Infect. Dis 2019, 5 (12), 1996–2002. 10.1021/acsinfecdis.9b00207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Zhou D; Chu W; Voller T; Katzenellenbogen JA Copper-Mediated Nucleophilic Radiobromination of Aryl Boron Precursors: Convenient Preparation of a Radiobrominated PARP-1 Inhibitor. Tetrahedron Lett. 2018, 59 (20), 1963–1967. 10.1016/j.tetlet.2018.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Kondo Y; Kimura H; Sasaki I; Watanabe S; Ohshima Y; Yagi Y; Hattori Y; Koda M; Kawashima H; Yasui H; Ishioka NS Copper-Mediated Radioiodination and Radiobromination via Aryl Boronic Precursor and Its Application to 125I/77Br-Labeled Prostate-Specific Membrane Antigen Imaging Probes. Bioorg. Med. Chem 2022, 69, 116915. https://doi.org/ 10.1016/j.bmc.2022.116915. [DOI] [PubMed] [Google Scholar]
- (24).Mossine AV; Brooks AF; Ichiishi N; Makaravage KJ; Sanford MS; Scott PJH Development of Customized [18F]Fluoride Elution Techniques for the Enhancement of Copper-Mediated Late-Stage Radiofluorination. Sci. Rep 2017, 7 (1), 233. 10.1038/s41598-017-00110-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Bratteby K; Shalgunov V; Battisti UM; Petersen IN; van den Broek SL; Ohlsson T; Gillings N; Erlandsson M; Herth MM Insights into Elution of Anion Exchange Cartridges: Opening the Path toward Aliphatic 18F-Radiolabeling of Base-Sensitive Tracers. ACS Pharmacol. Transl. Sci 2021, 4 (5), 1556–1566. 10.1021/acsptsci.1c00133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Inkster JAH; Akurathi V; Sromek AW; Chen Y; Neumeyer JL; Packard AB A Non-Anhydrous, Minimally Basic Protocol for the Simplification of Nucleophilic 18F-Fluorination Chemistry. Sci. Rep 2020, 10 (1), 6818. 10.1038/s41598-020-61845-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Taylor NJ; Emer E; Preshlock S; Schedler M; Tredwell M; Verhoog S; Mercier J; Genicot C; Gouverneur V Derisking the Cu-Mediated 18F-Fluorination of Heterocyclic Positron Emission Tomography Radioligands. J. Am. Chem. Soc 2017, 139 (24), 8267–8276. 10.1021/jacs.7b03131. [DOI] [PubMed] [Google Scholar]
- (28).Rowe SP; Macura KJ; Mena E; Blackford AL; Nadal R; Antonarakis ES; Eisenberger M; Carducci M; Fan H; Dannals RF; Chen Y; Mease RC; Szabo Z; Pomper MG; Cho SY PSMA-Based [18F]DCFPyL PET/CT Is Superior to Conventional Imaging for Lesion Detection in Patients with Metastatic Prostate Cancer. Mol. Imaging Biol 2016, 18 (3), 411–419. 10.1007/s11307-016-0957-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Scott CL; Swisher EM; Kaufmann SH Poly (ADP-Ribose) Polymerase Inhibitors: Recent Advances and Future Development. J. Clin. Oncol 2015, 33 (12), 1397–1406. 10.1200/JCO.2014.58.8848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Shen Y; Aoyagi-Scharber M; Wang B Trapping PARP. J. Pharmacol. Exp. Ther 2015. 10.1124/jpet.114.222448. [DOI] [PubMed] [Google Scholar]
- (31).Marie-France L; L. PJ; Swati R; M. PJ Structural Basis for DNA Damage–Dependent Poly(ADP-Ribosyl)Ation by Human PARP-1. Science (80-. ). 2012, 336 (6082), 728–732. 10.1126/science.1216338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Lee H; Riad A; Martorano P; Mansfield A; Samanta M; Batra V; Mach RH; Maris JM; Pryma DA; Makvandi M PARP-1-Targeted Auger Emitters Display High-LET Cytotoxic Properties in Vitro but Show Limited Therapeutic Utility in Solid Tumor Models of Human Neuroblastoma. J. Nucl. Med 2019. 10.2967/jnumed.119.233965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (33).Pirovano G; Jannetti SA; Carter LM; Sadique A; Kossatz S; Guru N; Demétrio De Souza França P; Maeda M; Zeglis BM; Lewis JS; Humm JL; Reiner T Targeted Brain Tumor Radiotherapy Using an Auger Emitter. Clin. Cancer Res 2020, 26 (12), 2871–2881. 10.1158/1078-0432.CCR-19-2440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).Riad A; Gitto SB; Lee H; Winters HD; Martorano PM; Hsieh C-J; Xu K; Omran DK; Powell DJ; Mach RH; Makvandi M PARP Theranostic Auger Emitters Are Cytotoxic in BRCA Mutant Ovarian Cancer and Viable Tumors from Ovarian Cancer Patients Enable Ex-Vivo Screening of Tumor Response. Molecules 2020, 25 (24). 10.3390/molecules25246029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (35).Sankaranarayanan RA; Peil J; Vogg ATJ; Bolm C; Terhorst S; Classen A; Bauwens M; Maurer J; Mottaghy F; Morgenroth A Auger Emitter Conjugated PARP Inhibitor for Therapy in Triple Negative Breast Cancers: A Comparative In-Vitro Study. Cancers (Basel). 2022, 14 (1). 10.3390/cancers14010230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (36).Wilson T; Pirovano G; Xiao G; Samuels Z; Roberts S; Viray T; Guru N; Zanzonico P; Gollub M; Pillarsetty NVK; Reiner T; Bargonetti J PARP-Targeted Auger Therapy in P53 Mutant Colon Cancer Xenograft Mouse Models. Mol. Pharm 2021, 18 (9), 3418–3428. 10.1021/acs.molpharmaceut.1c00323. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this study are available in the published article and its online supplementary material.