Abstract
Cognition in preverbal human infants must be inferred from overt motor behaviors such as gaze shifts, head turns, or reaching for objects. However, infant mammals—including human infants—show protracted postnatal development of cortical motor outflow. Cortical control of eye, face, head, and limb movements is absent at birth and slowly emerges over the first postnatal year and beyond. Accordingly, the neonatal cortex in humans cannot generate the motor behaviors routinely used to support inferences about infants’ cognitive abilities, and thus claims of developmental continuity between infant and adult cognition are suspect. Recognition of the protracted development of motor cortex should temper rich interpretations of infant cognition and motivate more serious consideration of the role of subcortical mechanisms in early cognitive development.
Keywords: motor cortex, motor maps, visual system, eye movements, infant cognition, core knowledge
Motor Behavior As a Window on Cognition
It is said that our eyes reveal our thoughts, but the same can be said of any movement. Accordingly, researchers routinely use infant eye and head movements, facial expressions, reaching behaviors, and locomotion to infer what is happening in the infant mind—knowledge, emotions, morals, and goals (e.g., [1–4]). And when researchers couple inferences about infant cognition with the assumption that the cognitive processes are instantiated in the cerebral cortex, they must also conclude that the infant cortex is the source of motor outflow that crystallizes cognition in movement (Figure 1A).
Figure 1. The implications of protracted development of cortical motor outflow for neonatal cognition.
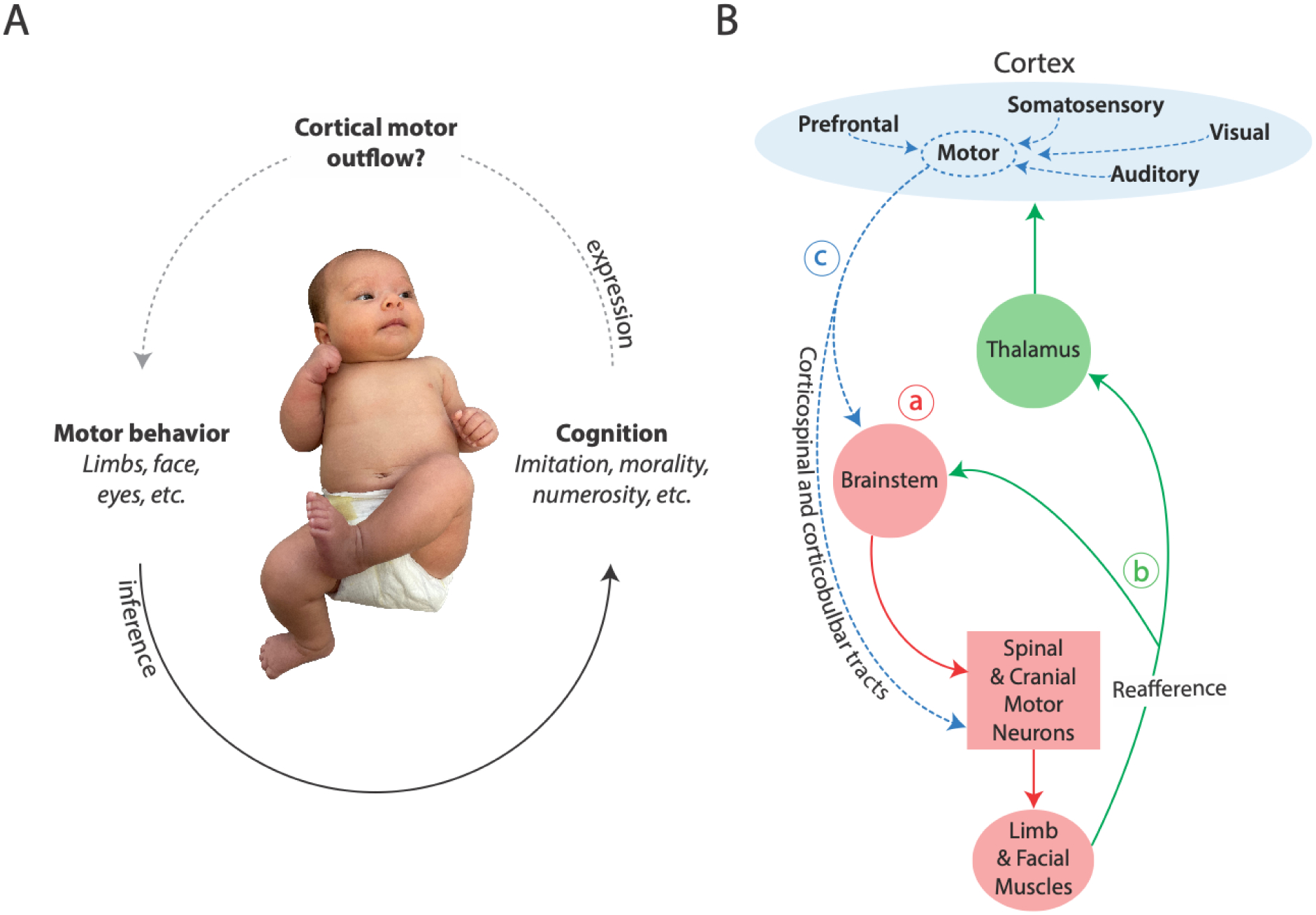
(A) The assumption of cortical motor outflow is central to inferences about cortically mediated cognition—especially for claims of developmental continuity between infant and adult cognition. Bottom solid arrow: Researchers use overt motor behavior to draw inferences about complex cognition in young, preverbal infants. Top dashed arrow: Cortically mediated cognition, in turn, can only be expressed as motor behavior if cortical motor outflow exists. Without cortical motor outflow, infant behavior must be mediated subcortically, and thus claims that infant cognition is developmentally continuous are suspect. Indeed, cortical motor outflow is absent in early postnatal development. Photo of infant courtesy of Jaya Rachwani. (B) Highly simplified diagram of motor outflow to the limb and facial muscles and associated sensory feedback (reafference) across early development. (a) Initially, newborn limb and facial movements are produced by brainstem motor systems (solid red lines). (b) Reafference is conveyed to the brainstem and somatosensory regions in thalamus and cortex (green lines). (c) Later in development, cortical motor outflow produces movement via the corticospinal and corticobulbar tracts and also modulates brainstem motor networks (dashed blue lines). In addition, other cortical regions develop the ability to influence cortical motor outflow. A similar developmental trajectory exists for cortical control of eye movements.
But what if this conclusion is wrong? Here, we present evidence that the cortical capacity for adult-like motor control is absent in newborns and only begins to emerge around 3 to 6 months of age. Of course, like adults, newborns can move all their body parts, including the eyes, face, head, torso, limbs, and fingers. But in adults, cortical control of movement is fully developed and functionally integrated, whereas in newborns it is not. In newborns, the brainstem produces movements throughout the body, and there is no evidence that the cortex “speaks” to the brainstem so as to influence motor behaviors. The initial absence of cortical motor outflow argues against theories about the developmental and neurobiological continuity of infant cognition, such that infants possess the same “core knowledge” present in adults. Specifically, if the cortex does not organize and execute newborn motor behaviors, and if cortical motor outflow only emerges gradually over the first six months and beyond, then either knowledge, emotions, morals, goals, and the like are produced subcortically (and thus are not developmentally continuous with adult cognition) or the behavioral indices of early infant cognition are unreliable. Something’s gotta give.
Note that absence of cortical motor outflow does not mean that the neonatal cortex lies dormant, awaiting the opportunity to “turn on” or “come online.” The quantity and patterning of early cortical activity are incompatible with such a notion [5–9] (Box 1). Thus, before the emergence of motor outflow, the cortex receives abundant sensory and non-sensory input that lays the foundation for the development of the cortical specializations and functionally integrated cortical-subcortical networks that will support later-developing cognition and behavior.
Box 1. The Surprising Sensory Origins of Primary Motor Cortex.
If motor cortex (M1) does not contribute significantly to motor control during much of early development, what is it doing? Long before M1 plays any role in motor control, neural recordings in rat pups show that M1 initially functions like a prototypical sensory structure [91,92]. For example, during REM (or active) sleep, the brainstem in postnatal day (P) 8 rats generates hundreds of thousands of brief and discrete limb and whisker twitches daily. Twitches trigger discrete pulses of proprioceptive feedback that, in turn, initiate a cascade of neural activity throughout the sensorimotor system, including primary somatosensory cortex (S1) and M1 [93]. Even in P20 rats, which is near the time of weaning, neurons in the forelimb region of M1 respond exclusively to sensory feedback from sleep- and wake-related movements [9] (Figure I). Such findings suggest that the early-developing and topographically organized sensory map in M1 lays the foundation for its later-developing motor map. The findings also counsel against assuming that the function of a developing cortical structure corresponds in an obvious way with its function in adults.
Figure I. Sensory origins of primary motor cortex.
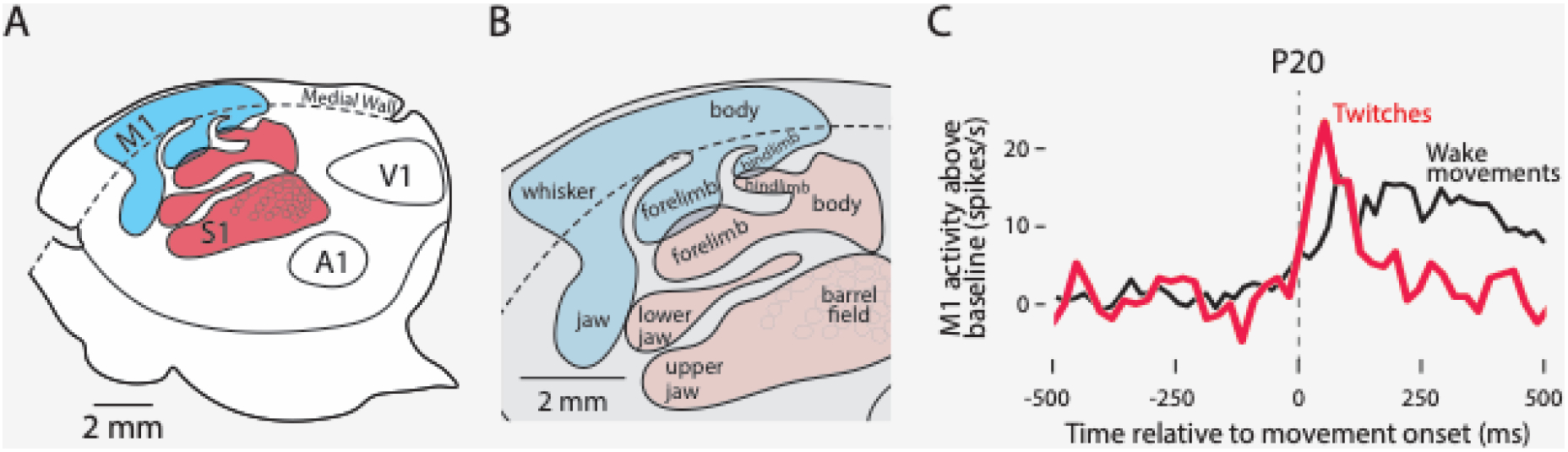
(A) Boundaries of primary cortical areas in rats—primary somatosensory cortex (S1, red) and primary motor cortex (M1, blue), and primary auditory (A1) and visual (V1) cortex. (B) Enlargements of red and blue regions in (A) show the somatotopic organization of S1 and M1. Adapted from Dooley et al., 2018. (C) Peri-event histogram showing sensory responsiveness of an individual neuron in the forelimb region of M1 at P20. The neuron’s firing rate is shown in relation to movement onset (vertical dashed line) for twitches (red) and wake movements (black). This neuron is representative of all M1 neurons recorded at this age. Neurons fire above baseline (0 on the y-axis) after—not before—movement onset during both sleep and wake, indicative of sensory responding. Adapted from Dooley et al., 2021.
Development of Cortical Control of Body Movements Is Protracted
Primary motor cortex (M1) gets its name from its unambiguous role in adult motor control: M1 neurons fire before self-generated movement, and electrical stimulation of M1 produces both simple and complex movements [10,11]. As shown in Figure 1B, M1 is a major source of the corticospinal tract that projects directly to spinal motor neurons controlling the limbs and trunk [12], and the corticobulbar tract that projects to cranial nerves controlling the face, jaw, and tongue [13] (but not the eyes; see next section). The corticospinal tract also projects to motor structures in the brainstem that, in turn, project to the spinal cord.
In human newborns and the young of other mammalian species, M1 and its descending projections do not exhibit the signature anatomical and functional characteristics with which they are associated in adults [14,15]. Development of these motor systems entails the initial establishment of anatomical connections between cortical axons and brainstem and spinal motor neurons, refinement of established connections, myelination, and formation of topographically precise motor maps [15]. Critically, anatomical evidence of cortical connectivity with downstream targets does not necessarily mean that these connections contribute to behavior. For example, within a few days after birth in rats, M1 has established direct corticospinal connections with spinal motor neurons; nonetheless, M1 does not assume motor functions until at least three weeks later [16,17]. In anesthetized rats at postnatal day (P) 25, intracortical microstimulation in M1 produces only the simplest forelimb movements (e.g., wrist extension) (Figure 2A); by P30, more complex forelimb movements are produced (e.g., grasping), and movement complexity continues to increase through P60 (i.e., adulthood).
Figure 2. Protracted development of motor maps in M1.
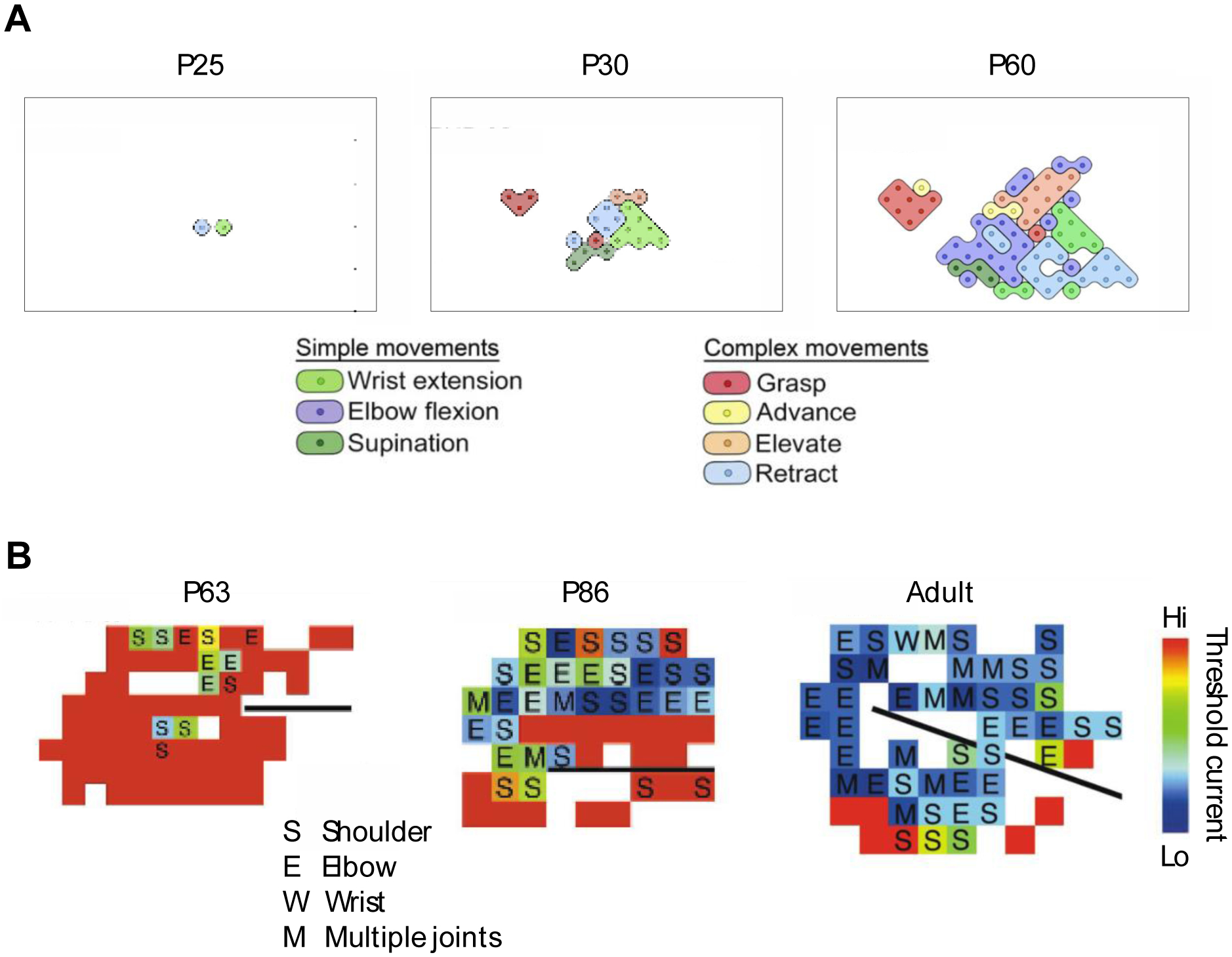
(A) Representative motor maps in rat pups at P25 and P30, and in adult rats at P60. Each map was produced using intracortical microstimulation in the forelimb region of M1 in anesthetized animals. The legends indicate the simple (i.e., single joint) and complex (i.e., multijoint) movements evoked at each stimulation site. Rectangles around the maps demarcate identical cortical surface areas. Adapted from Singleton et al., 2021. (B) Representative motor maps in kittens at P63 and P86, and in adult cats. Each map was produced using intracortical microstimulation in the forelimb region of M1 in anesthetized animals. The legend indicates the single-joint forelimb movements (shoulder, elbow, wrist) and multi-joint movements evoked at each stimulation site. Movements of the digits occurred with movements of other joints. Colors denote the threshold electric current for movement production as indicated by the color bar at right. The black lines show the location of the cruciate sulcus. Adapted from Chakrabarty and Martin, 2000. Both figures used with permission of the American Physiological Society; permission conveyed through Copyright Clearance, Inc.
If M1 is only beginning to contribute to behavior at P25 in rats, what does it do before then? Despite its name, M1 initially functions exclusively as a sensory structure (Box 1). Moreover, M1’s early sensory map is somatotopically aligned with its later-developing motor map, which means that the former lays the foundation for the latter. Thus, developmental changes in M1 powerfully illustrate that cortical activity—even task-relevant cortical activity—occurring contemporaneously with a movement does not imply that cortex plays a causal role in the production of that movement.
The inability of M1 in infant rats to produce movement contrasts with subcortical motor structures. For example, in week-old rats, neurons in the red nucleus—a midbrain motor nucleus—increase their activity before the onset of a forelimb movement and, moreover, stimulation of the red nucleus evokes movements [18,19]. Thus, through at least the first 3–4 postnatal weeks in rats, brainstem networks are sufficient to support complex postural and locomotor skills [20,21] (Box 2).
Box 2. Brainstem Networks Produce Complex Behavior.
Given the limited motor capabilities of the cerebral cortex in early development, the question naturally arises as to whether subcortical structures are sufficient to produce complex motor behavior. Here, we consider one midbrain structure—the superior colliculus—that integrates multisensory input and produces complex behavior in mammals and other vertebrates [94].
The superior colliculus, which forms the roof of the midbrain, is closely associated with many aspects of functioning in the visual system. It receives direct and prominent input from the retina and projects to oculomotor nuclei (to influence saccadic eye movements) and the pulvinar (a thalamic nucleus dedicated to visual processing). The superior colliculus also processes and integrates multimodal sensory input from the visual, auditory, and somatosensory systems [95] and is implicated in a subcortical network that supports face recognition in human infants [55] and adult monkeys [96].
Beyond eye movements, the superior colliculus can produce a wide variety of motor behaviors [97,98]. For example, activation of the superior colliculus in adult rats and mice elicits orienting responses and defensive reactions, including pursuit of moving objects. In adult monkeys, stimulation of the superior colliculus produces defensive behaviors, eye-head gaze shifts, and reaching movements. In adult humans, fMRI shows increased neural activity in the superior colliculus while reaching to visual targets [99].
Thus, based on evidence across multiple species, the superior colliculus can produce complex behaviors, even behaviors often assumed to require cortical involvement. But are these functions of the superior colliculus available to the young animal? Anatomical and functional evidence in newborn kittens suggests that they are [100]. For example, by 2 days of age, electrical stimulation of the superior colliculus evokes saccadic eye movements and movements of kittens’ limbs, neck, whiskers, and pinnae [101]; the system exhibits several features resembling those in adults (e.g., topographic organization), with less developed features achieving adult-like characteristics by 6 to 8 weeks, long before M1 has achieved adult-like function [24].
Relative to human newborns, rats are extremely immature at birth, but cats are born nearly as mature as humans [22]. Nonetheless, like rat pups, kittens show protracted M1 development [23]. Before P60 in kittens, electrical microstimulation in M1 produces movements at only 5% of electrode sites; adult-like levels (~67% of sites) are not achieved until P81–90—several weeks after weaning [24]. Along with these changes in stimulation efficacy, the threshold of activation decreases and the representation of the forelimb expands in its motor map (Figure 2B). Finally, as in rat pups, corticospinal connections are established in kittens long before M1 influences movement.
The findings in rats and cats tell a similar story about protracted M1 development. But are the findings relevant for human infants who, many assume, have more complex brains and developmental timelines? Assumptions about human exceptionalism should be met with skepticism [25]. Indeed, in a detailed analysis of 271 developmental events across 18 mammals—including rats, cats, and humans—a single model is sufficient to predict the timing of all events with great accuracy [22]. The ordering of the 271 events, which includes measures of brain growth, synaptogenesis, myelination, eye opening, and walk onset, was conserved across species, with the relative timing of events showing the most between-species variability. In other words, the order of developmental events is similar in rats, cats, and humans, but the timing varies from days in rats to weeks in cats to months in humans—thus, the first evidence of cortical motor outflow begins around P25 in rats, P81–90 in cats, and 3–6 months in humans.
In human fetuses, corticospinal axons first reach the cervical spinal cord by 24 weeks post-conception, and activation of M1 neurons using transcranial magnetic stimulation elicits movements in preterm and full-term newborns [26–28]. However, as in rats and cats, the onset in humans of anatomical or functional connectivity does not constitute evidence that M1 contributes to motor behavior [15,29].
In humans, compelling evidence of limited cortical motor outflow comes from infants who incurred brain damage from perinatal stroke, the leading cause of cerebral palsy [30–33]. In contrast to the immediate and often devastating paralysis that follows cortical stroke in adults, similar strokes around birth do not produce immediate paralysis or any other detectable motor disability. In fact, the disabling effects of cerebral palsy typically do not appear until at least 6 months after birth [34,35].
Microstimulation studies, such as those performed in rat pups and kittens (see Figure 2), cannot be performed in human infants to assess the development of cortical motor maps. Instead, researchers rely on neural imaging, such as functional near-infrared spectroscopy (fNIRS). For example, fNIRS shows diffuse, rather than topographically precise, M1 motor maps as human infants reach for an object or step on a motorized treadmill [36]. When reaching at 6 months, M1 activation patterns are diffusely organized (i.e., not topographically precise); activation at 12 months is less diffuse, but still more diffuse than in adults. During stepping, M1 activity at 6 and 12 months is as diffuse as that of 6-month-olds during reaching. As with rat pups and kittens, the absence in human infants of topographically precise motor maps provides converging evidence of protracted development of cortical motor outflow.
In summary, rats, cats, and humans show protracted M1 development. Initially, M1 is incapable of producing movement. And when motor outflow from M1 begins to emerge, activation thresholds are high and motor maps are not yet organized. In contrast, long before (and after) the emergence of cortical motor outflow, brainstem networks can organize and implement complex motor behaviors.
Development of Cortical Control of Eye Movements Is Also Protracted
When human adults respond to visual stimuli, a synergistic network of cortical and subcortical structures supports saccadic and pursuit eye movements [37–40]. Visual input is conveyed sequentially from primary visual cortex (V1) to the parietal eye field and then to the frontal eye field, a region in frontal cortex that abuts M1; the frontal eye field also receives input from dorsolateral prefrontal cortex. Accordingly, the frontal eye field integrates visuospatial information, short-term spatial memory, and decision processes, and it sends projections to the superior colliculus whose neurons project to the oculomotor nuclei. Other cortical regions involved in visual processing, including the parietal eye field and V1, also project to the superior colliculus.
In human newborns, the components required for the cortical control of eye movements are not yet established. In addition to the substantial postnatal development of V1 itself [41–43], horizontal connections from the output layers in V1 to secondary visual cortex do not appear to develop until after 4 months of age [43], suggesting that V1 cannot yet influence eye movements via the parietal eye field, the frontal eye field, or the dorsolateral prefrontal cortex. Further, the white matter tracts that connect parietal cortex with the frontal eye field and dorsolateral prefrontal cortex are not evident until three or more months of age [44,45]. In fact, one of these tracts—the superior longitudinal fasciculus—is among the slowest-developing tracts in the infant brain.
Developmental analyses of the event-related spike potential in parietal cortex provide converging electrophysiological evidence for the protracted development of V1’s ability to influence the downstream structures that control eye movements. In adults, this spike potential reliably precedes the onset of planned saccadic eye movements. But this spike potential is absent in 6-month-olds, and its amplitude is not yet adult-like even in 12-month-olds [46]. Moreover, the development of covert visual attention also indicates that control of eye movements by cortical structures downstream of V1 emerges between 3 and 6 months of age [47–49].
The process by which infants learn to reach for objects provides additional evidence that the ability to convey visual information to downstream structures is developmentally protracted. In human adults, reaches and grasps are mediated by separate cortical pathways, both of which begin in visual cortex and terminate in M1 [50]. Infants’ first successful reaches (defined as arm extensions that result in contact with the object) appear at approximately 3 months of age under the guidance of proprioceptive (not visual) feedback [51]. Visually guided reaching develops over the next two months [52], consistent with the emergence of horizontal connections from V1 to downstream structures [43]. The ability to actually grasp an object develops even later [53].
With minimal or absent cortical contributions to eye movements in the early postnatal period, subcortical structures must be responsible for organizing and implementing functional visuomotor behavior [41,47,54] (Box 2). However, we stress again that the absence of cortical participation in the control of movement does not imply that the cortex does nothing: Even at early ages, the cortex receives and processes modality- and task-specific input. Accordingly, as Johnson [55] put it, “[the] activation of visual cortical areas in the first months might have little influence over the visually guided behaviour of the infant” (p. 770). Why might such visual cortical activity occur when it cannot influence behavior? Likely reasons include the development and maintenance of local neural circuits and the interdigitation of functionally related cortical and subcortical networks. Indeed, Johnson [55] argued that newborn looking preferences in face-detection tasks are initially supported by a subcortical foundation upon which adult-like cortical mechanisms—including those in the specialized fusiform face area—are later built.
Implications of Protracted Cortical Motor Outflow for Claims About Cognitive Development
Delayed onset of cortical motor outflow sets neurobiological constraints on what can plausibly support cognition in early human infancy. Assessments of plausibility are on a continuum that varies with infant age. Given that cortical motor outflow is absent in newborns, claims about newborn cognition that assume cortical processing should be met with skepticism. But as cortical motor outflow gradually emerges over the first postnatal year, such claims become increasingly plausible. That is, extraordinary claims about cognitive capacities in newborns might be quite ordinary when applied to one-year-olds. Bottom line: Either the motor behaviors used to index infant cognition are unreliable or the behaviors are produced subcortically (Figure 1). Either way, given the limitations inherent in current methods, the available evidence does not support claims of developmental continuity between early infant and adult cognition.
The examples below illustrate how neurobiological plausibility can inform inferences about infant cognition based on motor behavior.
Newborn orientation discrimination
In the 1980s, some developmental psychologists were convinced that visual cortex is necessary for newborn visual discrimination. These researchers considered claims to the contrary (i.e., that visual processing in the brainstem is sufficient) as “doomed to founder on the rocks of a stubborn neonate who refuses to be relegated to the status of an involuntary, passive reactor” [56] (p. 13). To demonstrate the necessity of visual cortex, researchers chose a task—discriminating lines at different orientations—that requires engagement of orientation-selective neurons that, as assumed by some researchers at the time, exist only in visual cortex [57]. Newborns were visually habituated to bars oriented at 45°, meaning that infants looked repeatedly at the display before turning their eyes and head away due to “boredom.” Then, when tested with bars oriented at 135°, newborns dishabituated to the new line orientation (i.e., they showed a recovery in looking time to the display), suggesting that their eye and head movements depended on cortical discrimination.
But if cortical control of eye and head movements is unavailable to newborns, where does that leave the assumption that the cortex is necessary for orientation-specific discrimination? In fact, based on visually evoked potentials, orientation selectivity in visual cortex begins around 6 weeks of age, suggesting that vision relies on subcortical mechanisms at younger ages [58]. Moreover, it turns out that orientation-selective neurons are not exclusive to cortex: Such neurons exist at other locations in the visual system, including the superior colliculus and even the retina [59].
Neonatal imitation
For decades, researchers have argued passionately about whether human neonates can imitate facial expressions—stick out their tongue, for example, after seeing an adult do the same [60–62]. Some labs find behavioral evidence of neonatal imitation, but others do not [63]. Why the controversy? First, imitation is not trivial for a newborn: It requires the conversion of a visual stimulus into a sensory representation in body space followed by the production of a complementary motor response. None of this is easy for newborns given their poor visual acuity and limited experience linking visual, proprioceptive, and motor systems.
Controversy aside, some researchers propose cortical maps as contributors to neonatal imitation [64]. For cortical maps to support an imitative act like tongue protrusion—the behavior most reliably associated with neonatal imitation—neurons in primary visual cortex (V1) must activate neurons in motor cortex, resulting in the transmission of motor signals via the corticobulbar tract to the tongue muscles that produce protrusion. But, such long-range horizontal connections from V1 are not available to the newborn [43]. Moreover, some researchers go further and invoke cortical mirror neurons to explain newborns’ purported imitative abilities [65]. This last claim requires functional motor outflow from the neonatal motor cortex and functional outflow from mirror neurons to motor cortex. Evidence for neither exists.
In fact, cortical control of the tongue exhibits protracted development; moreover, the development of tongue protrusion (and retraction) is connected in complex ways with the development of suckling, breathing, swallowing, and eating solid food [66]. Tongue protrusion is initially produced spontaneously, showing its highest rates at birth and declining over the first three months [67].
Thus, consideration of cortical motor outflow further supports the notion that neonatal imitation is not the same sort of imitation produced by older children and adults [61,62]. Accordingly, to explain neonatal imitation, researchers should look to subcortical structures, including the superior colliculus [66] (Box 2). As with face processing in newborns [55], an appeal to subcortical mechanisms does not make a phenomenon less interesting. Rather, such an appeal simply points to more plausible neurobiological mechanisms.
Neonatal “crawling” in response to speech
Newborn arm and leg movements are grossly uncoordinated compared with the limb movements required for crawling, cruising, and walking in older infants [68]. The spinal cord, which contains the complex circuitry to enable precisely timed limb alternation in vertebrates [69], has received the bulk of attention from researchers focused on the neural basis of locomotion across human development [70,71]. However, some researchers asked whether supraspinal mechanisms can modulate newborn limb movements by assessing behavioral responses to visual [72] or auditory [73] stimuli. For example, using a mini-skateboard designed to enable newborns to move their arms and legs in a prone posture, researchers found that newborns born to French mothers moved more in response to French speech compared to English [73]. Because the researchers discounted the ability of brainstem mechanisms to discriminate French from English, they concluded that “typically developing newborns possess cortical networks ready to recognize their native language” (p. 4).
However, if cortical motor outflow is not available to support the observed movements, neonates must use subcortical mechanisms to discriminate French from English and convert that discrimination into movement. In fact, speech stimuli, including phonemes, are differentially processed within the brainstem [74]. Thus, it is plausible that neonates can discriminate native and non-native speech using subcortical mechanisms alone. Or, it is possible that the neonatal limb movements were overinterpreted: Indeed, the researchers failed to replicate the effect in newborns born to English-speaking mothers.
Numerosity in 3-month-olds
To investigate newborn perception of abstract number, researchers used a cross-modal matching task in which infants were familiarized to auditory stimuli composed of the same syllables repeated 4 or 12 times [75]. Then infants were tested with visual arrays composed of 4 or 12 objects. Infants familiarized to 4 syllables looked longer at the array of 4 objects, and infants familiarized to 12 syllables looked longer at the array of 12 objects, suggestive of “abstract numerical representations at the start of human life” (p. 10384).
In human children and adults, and in non-human animals, the intraparietal cortex is involved in the perception of numerosity [75]. Because 3-month-olds activate this area of cortex when detecting a change in the number of objects in a visual array [76], researchers suggested that “this parietal sensitivity arises from a predisposition of parietal cortex for spatial and numerical transformations, possibly present since birth” (p. 275). However, it is neurobiologically implausible that intraparietal cortex contributes to the behavioral expression of numerosity in 3-month-olds, let alone since birth. But if intraparietal cortex isn’t involved in the looking behavior in this task, how to explain its early activation when 3-month-olds detect a change in the number of objects in a visual array? One possibility is that this early activation reflects feedforward input from the subcortical structures responsible for the behavior, similar to what was proposed for the early development of face perception [55].
Social evaluations in 3-month-olds
Adults routinely evaluate other people’s proclivities to help or to harm. But do babies? For example, after watching a “helper” character (a geometric shape with eyes) “assist” another character up a hill, and a “hinderer” character “thwart” the other character’s efforts to go up the hill, 6- and 10-month-old infants reached more frequently for the helper character [77], leading the authors to conclude that this preference “may serve as the foundation for moral thought and action” (p. 557). These findings were subsequently extended to 3-month-olds using preferential looking, in which infants turn their eyes and head to look at a display [78].
Can we conclude that 3-month-olds have an “innate moral core” [3]? The answer to this question depends on whether 3-month-olds can make social evaluations—presumably dependent on cortical structures—and then use those evaluations to execute the required eye movements. Given that cortical mechanisms for controlling motor behavior are unavailable to 3-month-olds, two other possibilities must be considered: either that infants’ social evaluations are enabled initially by subcortical structures (and are thus not continuous with that of adults) or that concerns about whether the findings truly reflect social evaluation—as opposed to low-level perceptual discrimination—should be given more credence [79].
High-level infant cognition
Prefrontal cortex (PFC) is a computationally complex network implicated in high-level cognitive processes, such as working memory, decision-making, and motor planning [80]. Given the centrality of PFC for human cognition, researchers asked when and under what conditions PFC first develops its functional capabilities [81–83]. For example, using a task that taps into the ability to “hold a goal in mind” when an object is no longer visually accessible, researchers showed that the performance of human infants improves substantially from 7.5 to 12 months of age [84]. Indeed, performance at 12 months of age was similar to that of adult rhesus monkeys, and the performance of monkeys with lesions of dorsolateral prefrontal cortex was similar to that of human infants at 7.5 to 9 months of age. Additional support for protracted PFC development comes from consideration of that structure’s functional co-dependence with the cerebellum, which is also a late-developing structure [85–87].
In contrast, other researchers argue that PFC contributes to higher-order cognition at birth, enabling newborns to make active choices about where they direct their attention and how they experience the world [88]. Noting the newborn’s limited ability to reach, grasp, and point, the researchers claim that the cognitive contributions of the PFC are evidenced by infants’ “control over their gaze and visual attention from the first hours after birth” (p. 251). To the contrary, there is no evidence that the newborn PFC can influence visuomotor behavior.
Finally, to further support claims about a functional newborn PFC, researchers point to neuroimaging evidence of adult-like frontoparietal network in human infants before 1 month of age [89]. But again, evidence of early complex organization does not bear on the network’s ability to produce behavior. Indeed, it is more likely that subcortical mechanisms provide structured input to the newborn PFC long before it can actively contribute to the expression of behavior.
Concluding Remarks
Large gaps remain in understanding the development of cortical and subcortical motor control, particularly in human infants (see Outstanding Questions). For now, we suggest humility when making claims about when and how the cerebral cortex translates cognition into action. Likewise, we suggest caution in asserting that human newborns possess core knowledge, especially if that knowledge depends on a supporting neural architecture that is developmentally continuous with that of adults [1,90]. By carefully considering neurobiological plausibility across age, developmental psychologists will be better positioned to design experiments in which questions, tasks, and interpretations are suitably matched. Perhaps, then, the field can advance on surer footing and avoid needless controversies.
Outstanding Questions.
‘Can the developmental onset of cortical control of the limbs (M1) and eyes (frontal and parietal eye fields) be established with greater precision?’ To gain adequate spatial and temporal resolution to address this question, multimodal imaging should be used. For example, by combining fMRI with electroencephalography or magnetoencephalography (MEG), it should be possible to assess more precisely the temporal relations between brain activity and behavior.
‘Can high-density fNIRS with frequent sampling across the first postnatal year provide more detailed and precise assessments of the developmental emergence of motor maps in M1?’ Such information is needed to better understand the increasing contributions of M1 to behavior across age.
‘Which subcortical structures are involved in the earliest behaviors of human infants? And what are the temporal relations in the activity of subcortical and cortical structures?’ Answering these questions will require tools (e.g., MEG) that can record deep-brain and cortical activity with sufficient temporal precision.
At birth and for several months thereafter, the brainstem is largely responsible for motor behavior in human infants. But the brainstem does not simply hand off responsibility to the cortex and fade away. Instead, development entails the gradual interdigitation of cortical and subcortical networks into a functionally integrated whole. Current imaging methods are not yet able to capture this developmental process and thus cannot resolve the issue of developmental continuity in infant cognition and behavior.
Imaging studies in infants do reveal cortical activity suggestive of area-specific processing of relevant information. But evidence of cortical processing is not evidence of cortical involvement in motor behavior, even when the cortical processing fits with researchers’ adult-centric expectations. Indeed, in infant rats, individual neurons in M1 increase their activity only after the production of movement, indicative of sensory processing [9] (Box 1). Now consider the likely interpretation of this observation had the M1 recordings been performed using fMRI, a method with poor temporal resolution compared with single-cell recordings? Given that M1 is so closely tied to adult motor control, it is likely that researchers would have inferred erroneously that any fMRI-detected increase in M1 activity contemporaneous with movement reflects the role of M1 in the production of that movement. Similar problems confront researchers as they investigate cortical processing and cognition in human infants.
To conclude, researchers should be aware that behavioral indicators of cognitive sophistication may not reconcile with the neurobiological mechanisms available to support the behaviors. Ultimately, a complete and accurate account of the origins of human cognition requires greater understanding of the complementary relations among cortical and subcortical circuits and how those relations emerge across early development to support motor behavior and cognition.
HIGHLIGHTS.
Researchers routinely use motor behaviors (e.g., eye, face, and limb movements) to index cognition in the human neonate.
When developmental researchers use infant movements to index cognition, they often assume that cortex is involved in producing the behavior.
However, cortical control of movement is absent at birth, emerging gradually over the first several postnatal months and beyond; before cortical outflow emerges, brainstem networks produce complex motor behavior.
Thus, cortical control of the motor behaviors used to infer cognition in neonates is not neurobiologically plausible.
Researchers should be cautious when making claims about developmental continuity between newborn and adult cognition (i.e., “core knowledge”) and its supporting neural architecture.
Acknowledgments
We thank Dima Amso, Jimmy Dooley, Rick Gilmore, Mark Johnson, John Richards, and Linda Smith for helpful discussions and numerous suggestions for improving the manuscript. Preparation of this article was made possible by NICHD grant R37 HD081168 to MSB and by NICHD grant R01 HD33486 and DARPA grant N66001-19-2-4035 to KEA.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Spelke ES and Kinzler KD (2007) Core knowledge. Dev Sci 10, 89–96 [DOI] [PubMed] [Google Scholar]
- 2.Baillargeon R (2008) Innate ideas revisited: For a principle of persistence in infants’ physical reasoning. Perspect Psychol Sci 3, 2–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hamlin JK (2013) Moral judgment and action in preverbal infants and toddlers: Evidence for an innate moral core. Curr Dir Psychol Sci 22, 186–193 [Google Scholar]
- 4.Haith M (1998) Who put the cog in infant cognition? Is rich interpretation too costly. Infant Behav Dev 21, 167–179 [Google Scholar]
- 5.Luhmann HJ (2022) Neurophysiology of the developing cerebral cortex: What we have learned and what we need to know. Front Cell Neurosci 15, 814012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Glanz RM et al. (2021) Sensory coding of limb kinematics in motor cortex across a key developmental transition. J Neurosci 41, 6905–6918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sokoloff G et al. (2021) Twitches emerge postnatally during quiet sleep in human infants and are synchronized with sleep spindles. Curr Biol DOI: 10.1016/j.cub.2021.05.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ackman JB et al. (2012) Retinal waves coordinate patterned activity throughout the developing visual system. Nature 490, 219–225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dooley JC et al. (2021) Movements during sleep reveal the developmental emergence of a cerebellar-dependent internal model in motor thalamus. Curr Biol 31, 5501–5511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Graziano M (2006) The organization of behavioral repertoire in motor cortex. Annu Rev Neurosci 29, 105–134 [DOI] [PubMed] [Google Scholar]
- 11.Peters AJ et al. (2017) Learning in the rodent motor cortex. Annu Rev Neurosci 40, 77–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lemon R (2008) Descending pathways in motor control. Annu Rev Neurosci 31, 195–218 [DOI] [PubMed] [Google Scholar]
- 13.Morecraft RJ et al. (2004) The motor cortex and facial expression: New insights from neuroscience. Neurologist 10, 235–249 [DOI] [PubMed] [Google Scholar]
- 14.Sarnat H (2003) Functions of the corticospinal and corticobulbar tracts in the human newborn. J Pediatric Neurology 01, 003–008 [DOI] [PubMed] [Google Scholar]
- 15.Martin JH (2005) The corticospinal system: From development to motor control. Neurosci 11, 161–173 [DOI] [PubMed] [Google Scholar]
- 16.Young NA et al. (2012) Development of motor maps in rats and their modulation by experience. J Neurophysiol 108, 1309–1317 [DOI] [PubMed] [Google Scholar]
- 17.Singleton AC et al. (2021) Development and plasticity of complex movement representations. J Neurophysiol 125, 628–637 [DOI] [PubMed] [Google Scholar]
- 18.Rio-Bermudez CD et al. (2015) Sensorimotor processing in the newborn rat red nucleus during active sleep. J Neurosci 35, 8322–8332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mukherjee D et al. (2018) Corollary discharge in precerebellar nuclei of sleeping infant rats. eLife 7, e38213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shriner AM et al. (2009) The development of skilled walking in the rat. Behav Brain Res 205, 10–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Altman J and Sudarshan K (1975) Postnatal development of locomotion in the laboratory rat. Anim Behav 23, 896–920 [DOI] [PubMed] [Google Scholar]
- 22.Workman AD et al. (2013) Modeling transformations of neurodevelopmental sequences across mammalian species. J Neurosci 33, 7368–7383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Martin JH et al. (2007) Activity- and use-dependent plasticity of the developing corticospinal system. Neurosci Biobehav Rev 31, 1125–1135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chakrabarty S and Martin JH (2000) Postnatal development of the motor representation in primary motor cortex. J Neurophysiol 84, 2582–2594 [DOI] [PubMed] [Google Scholar]
- 25.Finlay BL and Workman AD (2013) Human exceptionalism. Trends Cogn Sci 17, 199–201 [DOI] [PubMed] [Google Scholar]
- 26.Eyre JA (2003) Development and plasticity of the corticospinal system in man. Neural Plast 10, 93–106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Eyre JA et al. (2000) Functional corticospinal projections are established prenatally in the human foetus permitting involvement in the development of spinal motor centres. Brain 123, 51–64 [DOI] [PubMed] [Google Scholar]
- 28.Koh TH and Eyre JA (1988) Maturation of corticospinal tracts assessed by electromagnetic stimulation of the motor cortex. Arch Dis Child 63, 1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Eyre JA et al. (2007) Is hemiplegic cerebral palsy equivalent to amblyopia of the corticospinal system? Ann Neurol 62, 493–503 [DOI] [PubMed] [Google Scholar]
- 30.Raju TNK et al. (2007) Ischemic perinatal stroke: summary of a workshop sponsored by the National Institute of Child Health and Human Development and the National Institute of Neurological Disorders and Stroke. Pediatrics 120, 609–616 [DOI] [PubMed] [Google Scholar]
- 31.Nelson KB and Lynch JK (2004) Stroke in newborn infants. Lancet Neurol 3, 150–158 [DOI] [PubMed] [Google Scholar]
- 32.Kirton A and deVeber G (2015) Paediatric stroke: pressing issues and promising directions. Lancet Neurol 14, 92–102 [DOI] [PubMed] [Google Scholar]
- 33.Kirton A and deVeber G (2013) Life after perinatal stroke. Stroke 44, 3265–3271 [DOI] [PubMed] [Google Scholar]
- 34.Golomb MR et al. (2001) Presumed pre- or perinatal arterial ischemic stroke: risk factors and outcomes. Ann Neurol 50, 163–168 [DOI] [PubMed] [Google Scholar]
- 35.Bouza H et al. (1994) Evolution of early hemiplegic signs in full-term infants with unilateral brain lesions in the neonatal period: A prospective study. Neuropediatrics 25, 201–207 [DOI] [PubMed] [Google Scholar]
- 36.Nishiyori R et al. (2016) Developmental changes in motor cortex activity as infants develop functional motor skills. Dev Psychobiol 58, 773–783 [DOI] [PubMed] [Google Scholar]
- 37.Pierrot-Deseilligny C et al. (2004) Eye movement control by the cerebral cortex. Curr Opin Neurol 17, 17–25 [DOI] [PubMed] [Google Scholar]
- 38.Sparks DL (2002) The brainstem control of saccadic eye movements. Nat Rev Neurosci 3, 952–964 [DOI] [PubMed] [Google Scholar]
- 39.Vernet M et al. (2014) Frontal eye field, where art thou? Anatomy, function, and non-invasive manipulation of frontal regions involved in eye movements and associated cognitive operations. Frontiers Integr Neurosci 8, 66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Goettker A and Gegenfurtner KR (2021) A change in perspective: The interaction of saccadic and pursuit eye movements in oculomotor control and perception. Vision Res 188, 283–296 [DOI] [PubMed] [Google Scholar]
- 41.Johnson MH (1990) Cortical maturation and the development of visual attention in early infancy. J Cogn Neurosci 2, 81–95 [DOI] [PubMed] [Google Scholar]
- 42.Siu CR and Murphy KM (2018) The development of human visual cortex and clinical implications. Eye Brain 10, 25–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Burkhalter A et al. (1993) Development of local circuits in human visual cortex. J Neurosci 13, 1916–1931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hermoye L et al. (2006) Pediatric diffusion tensor imaging: Normal database and observation of the white matter maturation in early childhood. Neuroimage 29, 493–504 [DOI] [PubMed] [Google Scholar]
- 45.Kulikova S et al. (2015) Multi-parametric evaluation of the white matter maturation. Brain Struct Funct 220, 3657–3672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Csibra G et al. (2000) Cortical development and saccade planning. NeuroReport 11, 1069–1073 [DOI] [PubMed] [Google Scholar]
- 47.Richards JE (2005) Localizing cortical sources of event‐related potentials in infants’ covert orienting. Developmental Sci 8, 255–278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Richards JE (2000) Localizing the development of covert attention in infants with scalp event-related potentials. Dev Psychol 36, 91–108 [PubMed] [Google Scholar]
- 49.Reynolds GD et al. (2013) The development of attention. In The Oxford Handbook of Cognitive Psychology (Reisberg D, ed), Oxford University Press [Google Scholar]
- 50.Karl JM and Whishaw IQ (2013) Different evolutionary origins for the reach and the grasp: An explanation for dual visuomotor channels in primate parietofrontal cortex. Front Neurol 4, 208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Clifton RK et al. (1993) Is visually guided reaching in early infancy a myth? Child Dev 64, 1099–110 [PubMed] [Google Scholar]
- 52.Corbetta D et al. (2014) Mapping the feel of the arm with the sight of the object: on the embodied origins of infant reaching. Front Psychol 5, 576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Thomas BL et al. (2015) Independent development of the reach and the grasp in spontaneous self-touching by human infants in the first 6 months. Front Psychol 5, 1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bronson G (1974) The postnatal growth of visual capacity. Child Dev 45, 873–890 [PubMed] [Google Scholar]
- 55.Johnson MH (2005) Subcortical face processing. Nat Rev Neurosci 6, 766–774 [DOI] [PubMed] [Google Scholar]
- 56.Bushnell IWR et al. (1989) Neonatal recognition of the mother’s face. Brit J Dev Psychol 7, 3–15 [Google Scholar]
- 57.Slater A et al. (1987) Orientation discrimination and cortical function in the human newborn. Perception 17, 597–602 [DOI] [PubMed] [Google Scholar]
- 58.Braddick OJ et al. (1986) Orientation-specific cortical responses develop in early infancy. Nature 320, 617–619 [DOI] [PubMed] [Google Scholar]
- 59.Antinucci P and Hindges R (2018) Orientation-selective retinal circuits in vertebrates. Front Neural Circuits 12, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Meltzoff AN and Moore M (1977) Imitation of facial and manual gestures by human neonates. Science 198, 75–78 [DOI] [PubMed] [Google Scholar]
- 61.Jones S (2016) Can newborn infants imitate? Wiley Interdiscip Rev Cogn Sci 8, e1410. [DOI] [PubMed] [Google Scholar]
- 62.Slaughter V (2021) Do newborns have the ability to imitate? Trends Cogn Sci 25, 377–387 [DOI] [PubMed] [Google Scholar]
- 63.Davis J et al. (2021) Does neonatal imitation exist? Insights from a meta-analysis of 336 effect sizes. Perspect Psychol Sci 16, 1373–1397 [DOI] [PubMed] [Google Scholar]
- 64.Meltzoff AN and Marshall PJ (2018) Human infant imitation as a social survival circuit. Curr Opin Behav Sci 24, 130–136 [Google Scholar]
- 65.Simpson EA et al. (2014) The mirror neuron system as revealed through neonatal imitation: presence from birth, predictive power and evidence of plasticity. Philos Trans R Soc Lond B Biol Sci 369, 20130289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Keven N and Akins KA (2017) Neonatal imitation in context: Sensorimotor development in the perinatal period. Behav Brain Sci 40, e381. [DOI] [PubMed] [Google Scholar]
- 67.Heimann M et al. (1989) Neonatal imitation of tongue protrusion and mouth opening: Methodological aspects and evidence of early individual differences. Scand J Psychol 30, 90–101 [DOI] [PubMed] [Google Scholar]
- 68.Adolph KE and Robinson SR (2015) Motor development. In Handbook of Child Psychology and Developmental Science: Vol. 2: Cognitive processes (7th edn) (Liben LS and Mueller U, eds), pp. 113–157, Wiley [Google Scholar]
- 69.Kiehn O (2011) Development and functional organization of spinal locomotor circuits. Curr Opin Neurobiol 21, 100–109 [DOI] [PubMed] [Google Scholar]
- 70.Dominici N et al. (2011) Locomotor primitives in newborn babies and their development. Science 334, 997–999 [DOI] [PubMed] [Google Scholar]
- 71.Sylos-Labini F et al. (2020) Distinct locomotor precursors in newborn babies. Proc National Acad Sci 597, 201920984–9612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Forma V et al. (2018) Effect of optic flows on newborn crawling. Dev Psychobiol 60, 497–510 [DOI] [PubMed] [Google Scholar]
- 73.Hym C et al. (2022) Newborns modulate their crawling in response to their native language but not another language. Dev Sci DOI: 10.1111/desc.13248. [DOI] [PubMed] [Google Scholar]
- 74.Hornickel J et al. (2009) Subcortical differentiation of stop consonants relates to reading and speech-in-noise perception. Proc National Acad Sci USA 106, 13022–13027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Izard V et al. (2009) Newborn infants perceive abstract numbers. Proc Natl Acad Sci USA 106, 10382–10385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Izard V et al. (2008) Distinct cerebral pathways for object identity and number in human infants. PLoS Biol 6, e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hamlin JK et al. (2007) Social evaluation by preverbal infants. Nature 450, 557–559 [DOI] [PubMed] [Google Scholar]
- 78.Hamlin JK et al. (2010) Three‐month‐olds show a negativity bias in their social evaluations. Dev Sci 13, 923–929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Scarf D et al. (2012) Social evaluation or simple association? Simple associations may explain moral reasoning in infants. PLoS ONE 7, e42698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chafee MV and Heilbronner SR (2022) Prefrontal cortex. Curr Biol 32, R346–R351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Werchan DM and Amso D (2017) A novel ecological account of prefrontal cortex functional development. Psychol Rev 124, 720–739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kolb B et al. (2012) Experience and the developing prefrontal cortex. Proc Natl Acad Sci USA 109, 17186–17193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Johnson MH et al. (2015) Brain adaptation and alternative developmental trajectories. Dev Psychopathol 27, 425–442 [DOI] [PubMed] [Google Scholar]
- 84.Diamond A and Goldman-Rakic PS (1989) Comparison of human infants and rhesus monkeys on Piaget’s AB task: evidence for dependence on dorsolateral prefrontal cortex. Exp Brain Res 74, 24–40 [DOI] [PubMed] [Google Scholar]
- 85.Sathyanesan A et al. (2019) Emerging connections between cerebellar development, behaviour and complex brain disorders. Nat Rev Neurosci 20, 298–313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Diamond A (2000) Close interrelation of motor development and cognitive development and of the cerebellum and prefrontal cortex. Child Dev 71, 44–56 [DOI] [PubMed] [Google Scholar]
- 87.Wang SSH et al. (2014) The cerebellum, sensitive periods, and autism. Neuron 83, 518–532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Raz G and Saxe R (2020) Learning in infancy is active, endogenously motivated, and depends on the prefrontal cortices. Annu Rev Dev Psychol 2, 1–22 [Google Scholar]
- 89.Eyre M et al. (2021) The developing human connectome project: Typical and disrupted perinatal functional connectivity. Brain 144, awab118- [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Dehaene-Lambertz G and Spelke ES (2015) The infancy of the human brain. Neuron 88, 93–109 [DOI] [PubMed] [Google Scholar]
- 91.Tiriac A et al. (2014) Self-generated movements with “unexpected” sensory consequences. Curr Biol 24, 2136–2141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Dooley JC and Blumberg MS (2018) Developmental “awakening” of primary motor cortex to the sensory consequences of movement. eLife 7, e41841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Blumberg MS et al. (2020) The developing brain revealed during sleep. Curr Opin Physiol 15, 14–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Isa T et al. (2021) The tectum/superior colliculus as the vertebrate solution for spatial sensory integration and action. Curr Biol 31, R741–R762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Stein BE et al. (2014) Development of multisensory integration from the perspective of the individual neuron. Nat Rev Neurosci 15, 520–535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Le QV et al. (2020) A prototypical template for rapid face detection is embedded in the monkey superior colliculus. Frontiers Syst Neurosci 14, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Dean P et al. (1989) Event or emergency? Two response systems in the mammalian superior colliculus. Trends Neurosci 12, 137–147 [DOI] [PubMed] [Google Scholar]
- 98.Cooper B and McPeek RM (2021) Role of the superior colliculus in guiding movements not made by the eyes. Annu Rev Vis Sc 7, 1–22 [DOI] [PubMed] [Google Scholar]
- 99.Linzenbold W and Himmelbach M (2012) Signals from the deep: Reach-related activity in the human superior colliculus. J Neurosci 32, 13881–13888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Stein BE (1984) Development of the superior colliculus. Annu Rev Neurosci 7, 95–125 [DOI] [PubMed] [Google Scholar]
- 101.Stein BE et al. (1980) Superior colliculus: Control of eye movements in neonatal kittens. Science 210, 78–80 [DOI] [PubMed] [Google Scholar]


