Abstract
Profound environmental, hormonal, and neurobiological changes mark the transition to motherhood as a major biosocial life event. Despite the ubiquity of motherhood, the enduring impact of caregiving on cognition and the brain across the lifespan is not well characterized and represents a unique window of opportunity to investigate human neural and cognitive development. By integrating insights from the human and animal maternal brain literatures with theories of cognitive ageing, we outline a framework for understanding maternal neural and cognitive changes across the lifespan. We suggest that the increased cognitive load of motherhood provides an initial challenge during the peripartum period, requiring continuous adaptation; yet when these demands are sustained across the lifespan, they result in increased late-life cognitive reserve.
Keywords: Matrescence, Motherhood, Neurodevelopment, Cognition, Aging, Cognitive Reserve
Motherhood as a Neurocognitive Developmental Stage
The hormonal fluctuations of pregnancy, birth, and lactation initiate rapid and extreme physiological transformations that are unparalleled across the lifespan. These biological changes are accompanied by a dynamic restructuring of the physical, emotional, and social environment. In concert with these adaptations, the maternal brain undergoes significant structural and functional neuroplasticity[1, 2] as well as cognitive adaptations[3] across the peripartum period (see Glossary). The brain is transformed – in preparation for, and in response to – a developing child. These neural adaptations enable mothers to manage the new and demanding tasks of motherhood, and the development of strong bonds with their child (see [4–9] for recent reviews). Appropriate and sensitive maternal behaviors support child development and play a crucial role in establishing the wellbeing of the next generation. Reflecting the importance of the maternal influence on developing children, existing maternal brain studies tend to be infant-centric, rather than parent-centric, focusing on mothers’ neural and behavioral responses to infant cues, but overlooking the impact of motherhood on mothers themselves. Furthermore, the literature describing parental neurocognition tends to focus on birth-giving mothers (see Box 1).
Box 1: Birthing and Non-Birthing Parents.
Parenthood is an identity with both biologically- and socially determined dimensions, describing a person’s caregiving relationship to a child, with or without a biological relationship. Indeed, the term matrescence was coined by the anthropologist Dana Raphael as “the process of becoming a mother – a developmental passage where a woman transitions, through pre-conception, pregnancy and birth, surrogacy, or adoption to the postnatal period and beyond”[121]. From a social perspective, it may be more appropriate to define parents as mother, father, or parent based on how they identify with these terms, which may more closely align with gender identity (e.g., woman, man, non-binary), rather than sex (e.g., male, female, intersex), or gestational relationship (e.g., birth-giving, non-birth-giving).
In the field of parental neuroscience, the vast majority of research in this area has studied people whose gender and parental identity align with their sex. In other words, the maternal brain has been assumed to be female/woman, and the paternal brain has been assumed to be male/man. This reflects the discipline’s links with the preclinical literature, the ‘biological’ focus of many of the studies, as well as the societal norms when the studies were conducted. As such, here we use the terms ‘mother’ and ‘father’ accordingly. As we learn more about the distinctions between gender, sex, and parental experiences, and how these dimensions interact, these terms and norms may change. It may not be possible to fully disentangle the strictly biological from the strictly social dimensions of human parenthood.
Importantly, non-birthing parents, particularly those with young infants, are exposed to the environment of parenthood and the experience of caregiving. All parents are required to adapt to this environment and display novel caregiving behaviors. The isolated impact of caregiving has been shown in female virgin rodents exposed to pups[122, 123], suggesting that the caregiving environment alone can induce beneficial neural and cognitive changes in new parents. In the only study in human non-birthing mothers, foster mothers showed positive associations between oxytocin levels, and the quality of their maternal bond, and their brain response to their infant’s cues, similar to those of biological mothers[124]. However, no study to date has examined cognitive changes across the transition to parenthood for females who become parents without being pregnant and giving birth, or in people whose gender may not match their sex, representing a clear gap in the literature.
Profound biological and environmental changes mark the transition to motherhood as a major life event, reflecting an important developmental life stage for new mothers. Just as the hormonal and psychological changes of adolescence prepare a person for successful adulthood, matrescence is a time when the brain and body prepare for the transition to motherhood. These two life stages are characterized by shifting social roles, with adulthood marking the social transition from dependence to independence, and motherhood marking the transition to now supporting one’s own dependents.
In addition to hormonal and social similarities, changes to the maternal brain across pregnancy are morphologically similar to those occurring in adolescence, with one study showing comparable changes in gyrification, sulcal depth, and sulcal length across adolescence and matrescence[10]. The neuroplasticity of matrescence similarly constitutes a sensitive neurodevelopmental period, where the brain is primed to readily acquire experience-dependent skills and knowledge. While the developmental changes of childhood, adolescence, and ageing are the subject of intense study, the impact of motherhood as a life stage in humans remains poorly understood.
Characterizing ‘typical’ neural and cognitive development across the transition to motherhood, as well as deviations from normative processes, has implications for how we conceptualize maternal neurocognitive trajectories, both in early motherhood, and throughout the lifespan. Until recently, the prevailing rhetoric has been that the physiological and, by extension, neural changes of pregnancy, birth, and lactation fully resolve in the postpartum period. In other words, it is believed that as sex steroid hormones return to pre-pregnancy levels, women return to a pre-pregnancy state in body and mind. Critically, while the biological changes may resolve over time, the environmental and behavioral changes of motherhood are likely to continue throughout the lifespan. However, the longer-term and cumulative impacts of motherhood are not well characterized.
Here, we propose a framework for investigating cognition in motherhood, relevant for understanding both the initial changes across the peripartum period, and the enduring impacts of motherhood across the lifespan (Figure 1, Key Figure). We review maternal cognition across the peripartum period, using a neurodevelopmental framework to explain cognitive decrements in pregnancy[3], postpartum cognitive renormalization[11, 12], and potential cognitive improvements in middle and late-life. Specifically, we suggest that the increased environmental complexity of motherhood provides a cognitive challenge in early motherhood and results in increased cognitive reserve in late-life.
Figure 1, Key Figure: Reframing Matrescence as a Neurocognitive Developmental Stage in Humans.
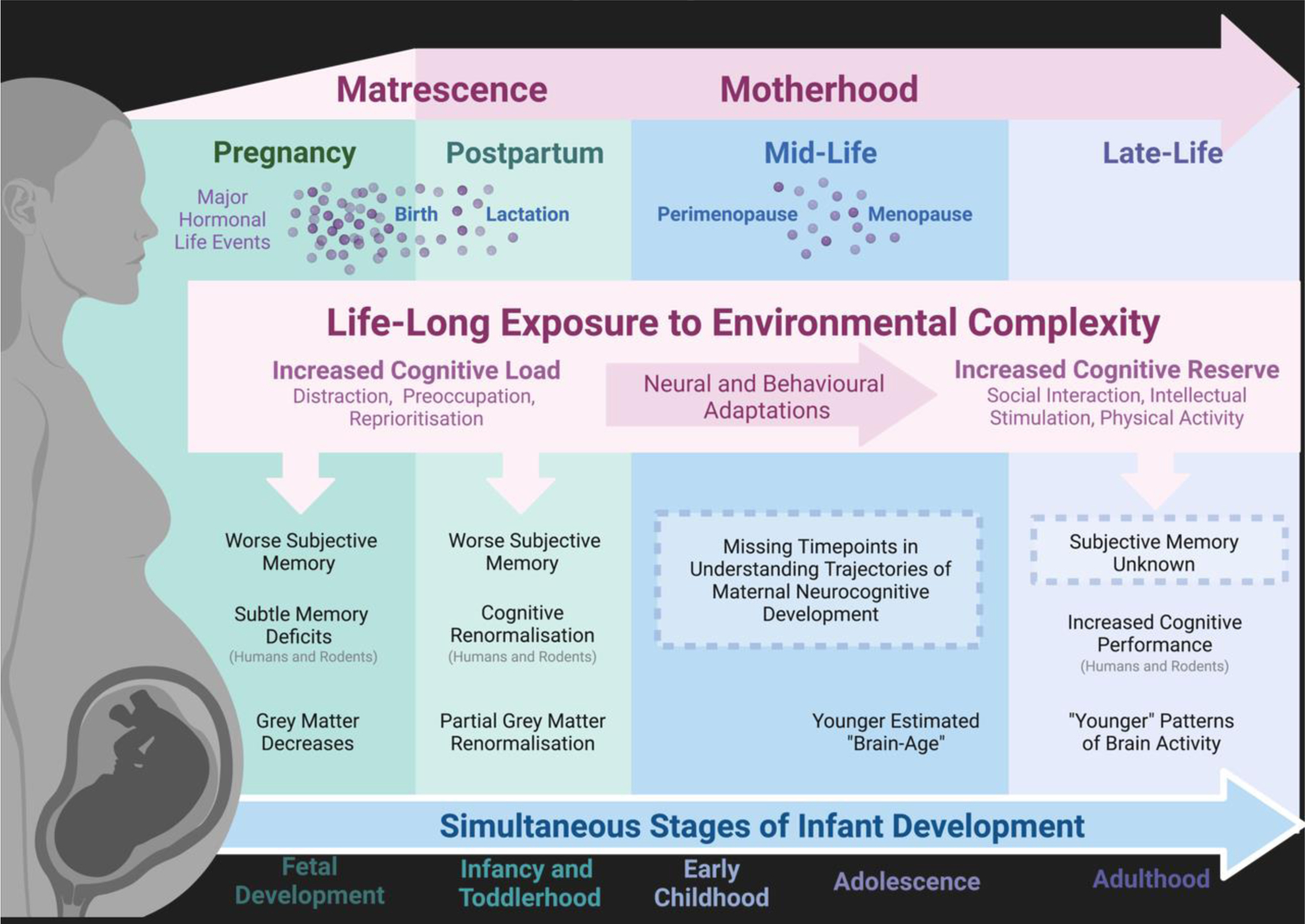
The transition to motherhood is associated with hormonal, neural, and cognitive changes both in the short-term (pregnancy and the postpartum period) and in the long-term (mid and late-life). Motherhood is also marked by dynamic and stage-specific exposure to increased environmental complexity. This increased environmental complexity may explain both cognitive challenges in the peripartum period, and cognitive improvements with increased parity in middle and late-life. Neural and cognitive changes across the maternal lifespan also likely interact with the major hormonal events of pregnancy, birth, lactation, perimenopause, and menopause, as well as the simultaneous stages of infant development. The influence of these interacting factors requires further investigation.
Early Motherhood and Cognition
Approximately 80% of new mothers report subjective experiences of cognitive decline across the transition to motherhood[3]. These self-reported declines span a range of cognitive domains, including impaired memory, concentration, and absentmindedness[3, 13]. Using neuropsychological assessments and cognitive tasks, many studies have also found objective cognitive decrements, in addition to self-reported memory decrements during pregnancy[13–19]. Three meta-analyses have synthesized the evidence for cognitive changes across pregnancy[3, 20, 21], revealing consistent memory decrements, strongest in the third trimester[3]. This is consistent with the rodent literature suggesting a cognitive decrement in the final week of pregnancy (equivalent to the third trimester of human pregnancy)[22–24]. In humans, these decrements are subtle and remain within the normative range of general cognitive functioning and memory[3, 25]. As such, while cognitive decrements may be salient to pregnant people themselves as a change from their own pre-pregnancy baseline, they are unlikely to significantly disrupt daily life[3, 25].
Unlike in pregnancy, where subjective cognitive decrements are also found with objective measures, postpartum mothers consistently report poorer memory[14, 18, 19, 26, 27] in the absence of cognitive differences[12]. Overall, the evidence for no cognitive decrement in motherhood[12, 14, 17–19, 26, 28–37] outweighs that showing significant differences[27, 38, 39]. This discrepancy suggests that mothers consistently report subjective memory impairments without measurable decrements in objective performance. Figure 2 shows a detailed breakdown of studies investigating cognition in the postpartum period. See Box 2 for a discussion of the potential impacts of sleep, mood, and nulliparous control groups on these effects.
Figure 2: Cognition in the Postpartum Period.
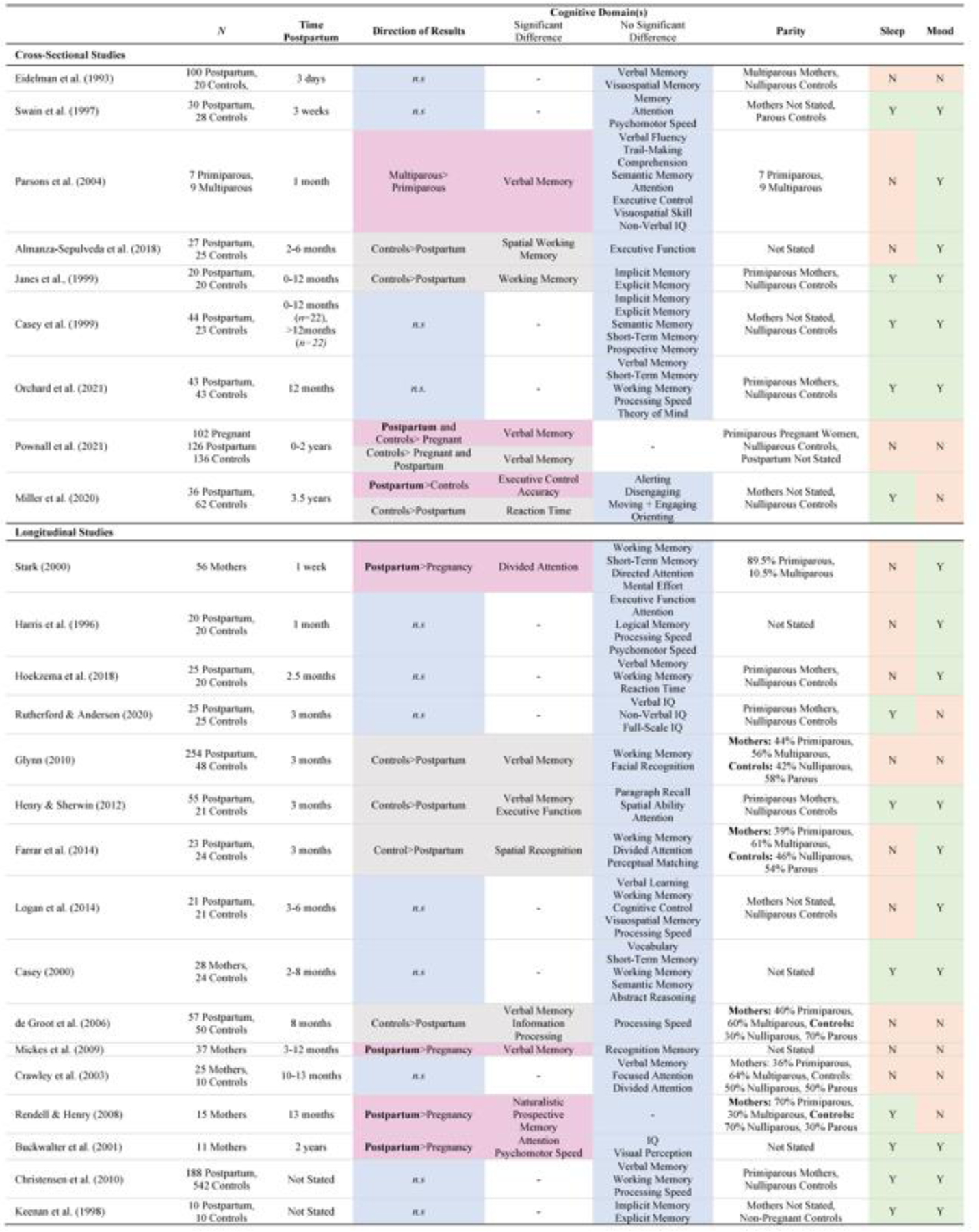
Results from the literature describing cognitive changes and null results in postpartum mothers [1, 12, 17–19, 26–39, 49, 50, 56, 63, 67, 120]. Direction of Results indicates which group or timepoint showed better performance, for example “Controls>Postpartum” indicates superior performance in the control group, “n.s” indicates non-significance. The significant differences column indicates the cognitive domain(s) which showed significant effects. Significant effects that suggest cognitive improvement in the postpartum period are colored pink, and those that suggest deficits are colored grey. No Significant Differences indicates the cognitive domains which did not show significant effects, shown in blue. Sleep and Mood indicate whether a study has collected (Y) measures of sleep or mood, or not (N).
Box 2: Important Methodological Considerations.
Mood and Sleep
There is a link between mood and memory, such that higher levels of depression are associated with poorer memory performance[125]. People with depression are also more likely to self-report memory problems than people without depression[126], even after adjusting for objective cognitive performance[127]. A similar link exists between cognition and sleep, where poor sleep is associated with poorer cognitive performance and self-reported memory[128, 129]. Given that mood disorders and sleep disturbance are common during pregnancy and the postpartum period, cognitive changes observed in the peripartum period may also be related to mood and sleep disturbance. Associations between subjective memory and disturbances in sleep and mood have been consistently demonstrated in the first year postpartum[12, 18, 19, 26, 29]. Some studies find that peripartum cognitive decrements disappear when including mood and sleep as covariates[130], or that cognitive differences are strongest in women with mood or sleep disturbance[131]. However, many studies of peripartum cognition do not report or account for sleep, depression, or anxiety outcomes, and further research is essential to further understand these associations.
Never-Pregnant Controls
Many studies of cognition in motherhood share one large problem: the control group consists of women who are not currently pregnant/postpartum but who are mothers and have experienced a previous pregnancy. Studies in rodents[25, 83] and humans[102, 103] suggest that reproductive experience confers cognitive benefits that are long-lasting, perhaps even permanent. Motherhood may also offer a cumulative effect on cognition, with multiparous women outperforming primiparous postpartum women[56]. Cumulative cognitive improvements with increasing parity have also been found in rats[25, 43, 123]. Given the long-term and cumulative impact of motherhood on cognition, a control group consisting of never-pregnant women are critical to achieving reliable comparisons and meaningful results.
Richly Phenotyped Datasets
Openly available datasets represent a valuable resource for population neuroscience, where large sample sizes provide requisite statistical power and robustness. The majority of maternal brain studies beyond the postpartum period characterize middle-aged parental effects using data sourced from one dataset: the UK Biobank (a large sample of adults from the United Kingdom)[102, 111–113]. These studies, and others[103, 119], are novel and provide valuable insight about the long-term impacts of parenthood. However, sparse information about caregiving and the limited racial and ethnic diversity of these samples limits interpretability. Specially collected data is needed to further characterize long-term brain changes in human parenthood, with rich phenotyping of parental factors (e.g., involvement in childcare, hormone levels, grandparenthood).
Trajectory of Cognitive Renormalization
A cognitive renormalization trajectory appears to occur in both rodent and human mothers, with initial cognitive decrements in late pregnancy and the early postpartum, followed by cognitive recovery, and even some cognitive improvements at the time of weaning. Compared to virgin females, maternal rats show impaired spatial memory in late pregnancy and the early postpartum period[22, 24], and improved memory performance[40–45], social learning[46], and reduced anxiety and stress[47, 48], after weaning. Collectively, these results suggest that rodent mothers experience some cognitive deficits in late pregnancy and the early postpartum, but that these decrements are resolved, and that cognition may even become enhanced following weaning and into the late postpartum period. The emerging human matrescence literature also indicates a similar cognitive trajectory. The few studies that do show cognitive decrements in the postpartum, examine mothers at 3–8 months postpartum[27, 38, 39, 49]. Conversely, studies reporting cognitive improvements test mothers a year or more after giving birth[34, 35, 50], consistent with common timelines for weaning in human mothers.
The timing of this cognitive trajectory is also consistent with neuroplasticity in the maternal brain. Importantly for understanding cognition, the hippocampus, a brain region crucial for memory ability, shows dramatic restructuring across the peripartum period in both humans and rodents. For example in rats, hippocampal neurogenesis is decreased during the early postpartum period[51], followed by increased hippocampal long term potentiation and improved hippocampal-dependent memory following weaning[45], aligning with the cognitive trajectory in maternal rodents[23, 24]. Similarly, in humans, the hippocampus shows reductions in grey matter across pregnancy[1], and subsequent increases in the postpartum period[1, 52]. Altered hippocampal structure and function are potentially related to changes in maternal cognition across pregnancy[3], however the associations between neural and cognitive adaptations have not been conclusively shown and care must be taken when extrapolating neural outcomes to cognitive changes, and vice versa, and when speculating about causal or mechanistic associations. Additionally, lower hippocampal volume at four-months postpartum is associated with positive mother-child interactions[53], suggesting broad implications in maternal caregiving behavior, beyond cognition. In addition to maternal brain changes, many biological, psychosocial, and environmental transitions occur in concert, which likely have simultaneous and interacting impacts on maternal cognition across the peripartum period.
Biological Factors
Hormonal fluctuations have been commonly cited as the causal factor for cognitive changes during the transition to motherhood[17, 54]. Levels of estrogens and progesterone, which increase during pregnancy and drop dramatically following birth, have been implicated in memory function outside of the context of pregnancy and motherhood. Like in other sensitive periods of hormonal fluctuation (e.g., adolescence[55]), neuroplasticity across pregnancy may come at an initial cost to memory, with benefits emerging longer-term, perhaps representing the reprioritization of crucial information (e.g., infant-related) or cognitive domains (e.g., social cognition)[20]. However, studies linking pregnancy hormones and cognition have not convincingly shown a causal association[25, 54, 56]. During late pregnancy and the first postpartum weeks, memory performance does not appear to correlate with levels of estradiol, progesterone, testosterone, cortisol or dehydroepiandrosterone (DHEA)[57]. Similarly, no association has been found between levels of oxytocin and cognitive performance in women tested during pregnancy and at three-months postpartum[54]. The few studies that have measured cognitive performance and hormone levels suggest that hormone exposure may not correlate with cognitive performance in pregnancy and the postpartum period[25, 56].
Psychosocial Factors
In addition to extreme biological changes, the transition to motherhood contains dramatic environmental and psychosocial changes, which may also contribute to cognitive decrements in pregnancy. The emotional adjustment required to prepare for a new baby’s arrival leads many expectant mothers to be preoccupied with this major life transition[58]. This preoccupation may account for the self-reported memory deficits in pregnancy, as attention is shifted away from information and tasks unrelated to motherhood[13]. Pregnancy can also be accompanied by additional stressors, including changing relationships, body image, adjustment to a new social role, and worry about labor and delivery[13, 59]. The shift in attention, motivation, and priorities towards one’s child may also account for objective cognitive decrements in pregnancy, with expectant mothers assigning laboratory testing a lower priority[60]. This is consistent with a dampened stress response to cognitive testing shown by pregnant women, where a certain amount of stress is required for optimal cognitive performance[20, 61]. A recent study elegantly showed pregnancy-related improvements in hippocampal-dependent spatial associative memory[11]. Specifically, when compared to nulliparous control women, pregnant women in their third trimester showed both a general enhancement in learning and retention, and a specific enhancement in memory of infant-related stimuli. This suggests both an increase in attention towards relevant stimuli as well as general improvements in hippocampal-dependent memory.
Environmental Factors
In addition to the challenges of low mood and poor sleep, new mothers are expected to undertake more responsibilities. Simultaneously tackling these challenges means that new mothers experience increased cognitive load whilst operating with fewer emotional and physical resources. This increased cognitive load represents a cognitive challenge. For example, leaving the house with an infant requires mothers to remember a multitude of items – a favorite toy, a change of clothes, a bottle – and to flexibly organize plans around naps and feeding timings. Furthermore, these responsibilities emerge in addition to a mother’s own personal needs and existing responsibilities. An increased cognitive load creates more opportunities to forget an item or task. Mothers may also develop a higher sensitivity to minor memory or concentration lapses, which would have otherwise been ignored or considered inconsequential before pregnancy[62]. Continuous re-evaluation and heightened awareness of memory ability may also be amplified by the societal expectation of cognitive decline for mothers in some cultures[63], contributing to a confirmation bias. Importantly, whilst in many cultures mothers are expected to undertake the majority of these responsibilities, this increased cognitive load likely impacts parents of all genders who share in the mental load of caregiving. However, our understanding of the impact of parenthood on fathers and non-birthing mothers is far less established and requires increased attention (See Box 3).
Box 3: Cognitive and Brain Changes in Early Fatherhood.
Compared to the already scarce literature describing neurocognitive changes across motherhood, even fewer studies investigate these adaptations in fathers. Only two studies have indirectly investigated cognition in early fatherhood. When comparing working memory in mothers and fathers longitudinally from pregnancy to six-weeks postpartum, no differences between mothers and fathers were found at any timepoint, nor any change across time[132]. This study used fathers as a control group for practice effects and did not compare the cognitive performance of fathers to childless men. The other study found that fathers performed worse on a measure of logical memory the day following their child’s birth, compared to non-pregnant control women, and interpreted this difference as related to fathers’ stress and anxiety[30].
A handful of studies have examined changes to fathers’ brain structure and function[103, 119, 133–135]. Similar to mothers, first time fathers show longitudinal reductions in grey matter volume after the birth of their child[135], and larger volume reductions in a subsample of these fathers were associated with stronger neural responses to pictures of their own child[136]. Additionally, longitudinal changes in paternal grey matter volume across the first four-months postpartum are associated with sensitive paternal caregiving behavior[134]. Fathers who spend more time in direct childcare also show patterns of brain activation resembling maternal brain changes[133], consistent with the preclinical literature[123, 137, 138], showing that neural changes can arise environmentally, flexibly activated by exposure to offspring[2]. Whilst these neural adaptations appear to support the behaviors of paternal caregiving, the associations between neural and cognitive adaptations are unclear in human fathers. See recent reviews for more details on the neurobiological changes in fathers[139], and for neuroendocrine changes supporting paternal care[140, 141].
The association between caregiving experience and enrichment is present in non-human fathers involved in the rearing of offspring. Paternal marmosets experience similar brain changes to animals living in enriched environments, and this effect is mediated by the amount of father-infant contact[84]. Furthermore, the degree of neural adaptation is proportionate to the amount of time spent in primary care: exposure to unfamiliar pups for ten minutes per day for four days is sufficient to induce neural changes and improve spatial memory for non-parental male rats[137]. Future research which includes non-birthing parents of all sexes will allow the investigation of the enriched environment hypothesis, uncovering whether and to what degree cognitive and neural effects occur in the absence of pregnancy, and the relative influence of endocrine and experiential factors.
New parents may adapt to the cognitive challenges of the postpartum period by developing mechanisms, tools, or strategies for maintaining their cognitive abilities. One such adaptive change may be an increase in executive functioning, critical for adaptive maternal behavior in humans[64–66], including the capacity for self-regulation and managing competing demands[65]. Increased reliance on executive functioning may strengthen cognitive abilities, which could generalize beyond the caregiving context. Recent evidence suggests that compared to nulliparous controls, mothers show superior executive function and attention three years following birth[67]. Therefore, executive function is a cognitive domain which is both important for understanding maternal care and may show improved ability over time. In addition to cognitive adaptations, mothers may also show neural compensation, where brain networks are activated in different ways, or additional neural mechanisms are recruited to support behavior[68]. This hypothesis is supported by studies of cognition and brain function in early motherhood[69, 70], showing differences in task-related brain activation in the absence of differences in task performance.
Sustained Environmental Complexity
Cognitive adaptation could be a mechanism for cognitive improvements in the postpartum period and beyond, as mothers continue to access advantageous adaptive and compensatory mechanisms. If so, the next question is when the benefits might begin to outweigh the challenges. The challenges of early motherhood resolve slowly over time – when infants start to sleep through the night, or when children start school, or perhaps even when they leave home. When considering the cumulative effects of multiparity, the challenges of early motherhood becomes more intense by the overlapping needs of infants born close together. Likewise, the experiences of early motherhood may be extended by children born further apart. The reality is that we do not know how long this stage of motherhood lasts, but the enduring environmental challenge of motherhood may represent a learning environment that is sustained for two or more decades of an individual’s life.
Whilst mothers may build resilience over time, and sleep and social support may increase as the child develops, the environmental complexity of motherhood never fully returns to pre-child levels. As children develop, their needs change, requiring continuous behavioral adaptations from mothers. For example, the tasks required to care for a fully-dependent newborn are very different from those required to care for a highly mobile toddler, or a school-aged child, and different still from those required to care for an adolescent or young adult. In this way, the environmental complexity of motherhood is influenced by the developmental stages of the child (Figure 1), resulting in continual adjustment to an ever-changing environment. Each subsequent child further increases this environmental complexity, requiring simultaneous care across different stages of child development. Additionally, as individual children display different temperaments and elicit distinctive behaviors in their mothers[71], the skills and strategies learned with one child may not generalize to the next, requiring a mother to re-learn and dynamically re-evaluate parenting styles.
Environmental Enrichment
The preclinical literature suggests that motherhood influences the rodent brain in a manner similar to the influence of an enriched environment. Environmental enrichment refers to housing conditions which involve enhanced sensory, cognitive, motor, or social stimulation[72]. Enriched environments stimulate synaptogenesis[73, 74], result in heavier brain weights[75], more complex dendritic branching[76], and may provide a protective effect against brain ageing[77]. Enriched animals also perform better on tasks of learning and memory[75], and show enhanced hippocampal neurogenesis[78, 79].
Foundational research suggests that maternal animals show cognitive and neural outcomes akin to enriched environments[80, 81]. Environmentally impoverished postpartum rats show greater cortical depth compared to rats in enriched environments[80], suggesting the maternal environment was enriching enough to negate the detrimental effects of an impoverished physical environment. This result was replicated, with maternal rats showing greater mean cortical depths than virgin females across all housing conditions[81]. Enhanced learning and memory, and increased neuroplasticity in maternal rodents relative to virgin females are widely interpreted as resulting from an enriching maternal environment[82, 83]. The association between caregiving experience and enrichment is also present in primate fathers involved in the rearing of offspring. Paternal marmosets experience similar brain changes to animals living in enriched environments, and this effect is mediated by the amount of father-infant contact[84].
Taken together, these studies demonstrate that reproductive experience confers neural and cognitive benefits similar to an environmental enrichment, suggesting that maternal experience may be stimulating and protective for brain health. We suggest that this may extend to human parenthood. Compared to laboratory rodents, humans live vastly more complex lives, and arguably already live in highly ‘enriched’ environments. In the same way that reproductive experience enriches the ‘housing conditions’ of rodents, human parenthood increases daily sensory, cognitive, social, and environmental complexity, though perhaps to a lesser extent. Two major elements of an enriched environment are complexity and novelty[72], which are both increased in the human caregiving environment, where new mothers are faced with novel child-related tasks and behaviors.
Cognitive Reserve
Environmental enrichment is primarily described in animal models; in humans, a related concept is cognitive reserve[85]. Cognitive reserve refers to the brain’s resilience to damage, illness, or cognitive decline by recruiting pre-existing cognitive processes[86]. The ability to flexibly recruit cognitive processes contributes to maintained function in the face of pathology and the ageing process[86]. For example, in healthy ageing, individuals with high cognitive reserve experience slower decline in memory, executive function, and language skills[87, 88]. Cognitive reserve is increased in people exposed to challenges and complex environments. Higher levels of education[89], occupational attainment and complexity[90, 91], being more socially active[92], engaging in cognitively demanding hobbies[93] and learning a foreign language[94] are all related to increased cognitive reserve in late-life. We suggest that motherhood comparably exerts environmental complexity across the lifespan. Consider the parallels with occupational complexity: if the type of job a person worked across their lifespan contributes to their cognitive reserve in late-life, then we would expect that motherhood, which provides novelty and complexity, requires a similar time commitment, and lasts for decades, would similarly increase cognitive reserve. In fact, the idea that parenthood adds to cognitive reserve has already been acknowledged in the cognitive reserve literature, albeit indirectly[95]. The Cognitive Reserve Index Questionnaire (CRIq) is a standardized measure of the cognitive reserve accumulated by an individual across their lifespan, based on their educational attainment, working activity, and leisure time. Individuals with more children, or more frequent caregiving responsibilities (for children or the elderly) are allocated a higher score on the CRIq, reflecting the impact of these activities on cognitive reserve[95]. In this way, the very same cognitive load that poses a challenge in early motherhood may be beneficial in the long-term, by exerting sustained environmental complexity across the lifespan.
Motherhood and Cognition Across the Lifespan
The positive association between reproductive experience and neurocognitive improvements is well documented and widely accepted in rodents. Maternal rodents show improved spatial learning and memory in mid-life, reduced hippocampal amyloid deposits, and attenuated memory decline in late-life[40, 41, 43–45, 82]. The understanding that reproductive experience is beneficial for long term cognition and brain health in rodents is uncontroversial, with hormonal and environmental changes that interact to produce a maternal brain that is healthier, more flexible, and more resistant to age-related decline[40, 43].
In contrast, the long-term association between cognitive performance and motherhood is less clear and understudied in humans. In older women, having fewer or no children is associated with better cognition in some studies[96, 97], but not in others[98, 99]. There also appears to be a distinction between studies examining biological and social determinants of motherhood and their relation to cognition. For example, some studies focus on biological factors such as age at first/last birth, breastfeeding duration, and other proxies for lifetime exposure to endogenous estrogens, whereas others focus on parity, encompassing the social exposure to children and the caregiving environment. Among parous women, younger age at first pregnancy is associated with worse cognition in late-life[99], and later age at first and last pregnancy is associated with improved cognition in late-life[100]. However, it is also important to recognize these biological associations in the context of broader social factors. For example, older age at first pregnancy is associated with higher socioeconomic status, income, educational attainment, and more participation in the labor force, factors which are all related to higher cognitive performance throughout the lifespan, and greater cognitive reserve in late-life[95].
Compared to these biologically-focused studies, a growing body of research examining parity suggests that motherhood is related to improved cognition in middle[98, 100–102] and late-life[103]. One study demonstrated this distinction in a sample of mid-life women (N=326, age=56.7±2.5), showing a positive association between parity and improved verbal memory performance[98]. In contrast, memory performance was not related to lifetime estrogen exposure, including menopausal status, use of hormonal replacement therapy, and reproductive period. As such, it appears that the biosocial influence of the caregiving environment impacts long-term maternal memory across the lifespan, rather than solely the biological exposure to estrogens[98]. Furthermore, it appears that the positive association between parity and cognition is non-linear: in a large sample of mid-life women (N=6,123, meanage= 55.0) and men (N=5,110, meanage=54.7), parity and cognition showed a U-shaped association, where both lower parity and grand multiparity (more than 5 children) were associated with worse cognitive performance, and optimal performance was observed in mothers and fathers with 2–3 children[100]. The association between grand multiparity and poorer cognition was largely accounted for by socioeconomic status, whereas the association between childlessness and poorer cognition was strengthened when controlling for socioeconomic status.
In another large sample of mid-life women (N=160,077, age=56.7±7.9) and men (N=143,119, age=57.5±8.1), higher parity was again associated with enhanced cognitive performance for both mothers and fathers[102]. Mothers and fathers with more children showed improved visual memory and processing speed. In both of these studies, cognitive improvements with parity were seen in parents of both sexes, with stronger effects detected in fathers[102]. Since males do not experience the same physiological changes that birth-giving parents do, these results indicate that the environmental changes of parenthood are important in the association between parity and cognition, at least in mid-life. In the only study to date in late-life mothers (N=235, age=73.86±3.50), we found that parity was positively associated with cognition, such that mothers with more children performed better on a task of verbal memory[103]. Consistent with this result, higher parity was also positively associated with the thickness of the parahippocampal gyrus, a brain region associated with memory performance.
In addition to cognition in healthy ageing, motherhood also shows a complex relationship with Alzheimer’s disease (AD), with many inconsistencies in the literature[101, 104]. Increased parity is associated with more severe AD pathology[105], and earlier AD onset[106], but not in all studies[107]. Interestingly, dementia risk also shows a U-shaped association with parity for women and men[107], with the lowest risk in those with two children. Apolipoprotein E (APOE) ε4 allele is a major genetic risk factor for AD, which interacts with parity to influence age of AD onset[108, 109]. Compared to women without an APOE-ε4 allele, women with one ε4 allele had on average one more child, and those with two ε4 alleles had 3.5 more children[110]. It is also possible that the association between parity and Alzheimer’s disease is influenced by fertility – not that having more children increases the risk of AD, but that those who are most at risk (APOE-ε4 carriers) are able to have more children. Taken together, genotype, parity, and lifetime estrogen exposure may interact to affect health later in life and should be taken into account in future studies.
The consistent U-shaped associations between parity and cognition, dementia risk, and brain structure may represent the myriad of social factors involved in parenthood, including the impact of high parity on financial pressures and increased stress[107]. Evidence from maternal rodents suggests that the beneficial effect of motherhood on hippocampal function and memory performance are abolished in maternal rats placed under high stress[45], suggesting that motherhood may only be enriching under optimal conditions. Furthermore, when interpreting findings from these large datasets it is important to note that the majority of participants in these samples have 2–3 children. Therefore, the confidence in predictions from the tails of these distributions is impacted by complex sociodemographic factors, and the reliability of statistical estimates themselves. Furthermore, we must also consider the potential factors causing people to not have children, including the influence of genetic or other biological factors influencing fertility. In other words, the U-shaped distribution in cognitive performance and dementia risk is difficult to interpret until more richly phenotyped and representative samples are collected and/or made available.
Parity and Brain Changes Across the Lifespan
Recent evidence suggests that motherhood confers lifelong changes to the structure of the human brain. Four studies of brain-age have explored grey matter differences in middle-aged mothers[102, 111, 112] and fathers[102], and white matter differences in middle-aged mothers[113]. Brain-age is a metric derived from MRI brain scans[114], using machine learning to measure deviations from normative ageing trajectories. The difference between chronological age and estimated age, based on brain structure, is considered a promising biomarker for accelerated ageing, Alzheimer’s disease, and cognitive impairment[114].
In middle age, mothers with more children show ‘younger-looking’ brain structure, suggesting a neuroprotective effect of motherhood[111], specifically in striatal and limbic regions[112]. These circuits are involved in reward processing and reinforcement learning, crucial for maternal behaviors in rodents[115, 116] and humans[1]. Mothers also show a negative association between hippocampal brain-age and parity[112]. This result is consistent with grey matter changes in the parahippocampal gyrus during pregnancy[1] and the postpartum period[52]. The only study of late-life parental brain structure[103] also found a positive association between parity and cortical thickness in the parahippocampal gyrus of elderly mothers. Rodent mothers show enhanced hippocampal neurogenesis in middle age in parous relative to nulliparous rats[117], and fewer amyloid deposits in the hippocampus of multiparous relative to primiparous and virgin animals[43, 44]. Due to its correlation with memory function and dementia risk[104], hippocampal volume is often used as a proxy for predicting brain health, suggesting parity may have a similarly beneficial impact on brain health in mid- and late-life mothers. Mothers to more children also show ‘younger’ white matter brain-age101 in the corpus collosum, consistent with increased myelination observed in healthy pregnant rats[118].
In the only study of late-life maternal brain function, patterns of brain activity were compared with three models of age-related decline, to test the hypothesis that motherhood confers functional neuroprotection on the late-life maternal brain[119]. For mothers with more children, patterns of functional connectivity related to parity were in the opposite direction to those usually associated with age-related cognitive decline, suggesting that motherhood may be beneficial for brain function in late-life[119]. These results are the first indication that the challenges and complexity of the parenting environment may contribute to a mother’s cognitive reserve across the lifespan[86]. Future research is needed to determine how the changes of motherhood endure throughout the lifespan and impact ageing trajectories.
Concluding Remarks
The rapid and extreme hormonal and environmental changes of pregnancy mark the transition to motherhood as a major biosocial life event, representing a sensitive neurocognitive developmental period: matrescence. If matrescence is framed as a sensitive neurodevelopmental period, many new questions emerge, including how this period influences the rest of a person’s life (see Outstanding Questions). The number and timing of pregnancies a person has is influenced to some degree by biology, and further influenced by sociodemographic factors, including socioeconomic status, cultural norms, and access to contraception. As such, parenthood is an ‘optional’ life stage which, due to choice, biology, and circumstance, does not occur for all people. We must be careful not to frame those without children as having ‘missed’ a developmental stage. However, it is also important to consider that the late-life parent is the most common form of late-life person, and that our understanding of normative ageing trajectories is based on samples where elderly parents comprise the vast majority. A lack of understanding of how motherhood impacts the ageing process therefore also has consequences for the lifelong health and wellbeing of those who are not parents.
Outstanding Questions.
What is the impact of parenthood across the lifespan? How does the experience of pregnancy and/or caregiving interact with subsequent life stages and the hormonal transitions of menopause? Does reproductive and/or caregiving experience alter ageing trajectories? What are the impacts of multiparity and pregnancy timings? How does maternal neurocognitive development interact with the simultaneous stages of child development? What are the impacts of grandparenthood, and caregiving responsibilities in late-life?
How can environmental and hormonal mechanisms be disentangled? Studies can be devised which include fathers and non-birthing parents, as well as people who have experienced pregnancy but do not parent a child (in the case of surrogacy, infant loss, or infants placed for adoption). This would allow the mechanistic understanding of the relative contributions of the hormones of pregnancy, and the caregiving environment.
What are appropriate control groups? Many studies of cognition in the peripartum period consist of women who are not currently pregnant but have experienced a previous pregnancy. Given the long-term and cumulative changes that motherhood bestows on cognition in rodents, and humans, a control group consisting of women who have never previously been pregnant is critical to achieving reliable comparisons and meaningful results.
What are the critical timepoints that need investigation? The current literature contains many missing timepoints that are crucial if we are to chart neural and cognitive trajectories across the lifespan. Specifically, the field lacks an understanding of the mid-life effects of motherhood (Figure 1). Almost all studies of maternal cognition focus on pregnant and postpartum mothers. Longitudinal studies targeting parents of toddlers, school-aged children, adolescents, and young adults who live at home are required to fill these knowledge gaps.
Motherhood involves increased cognitive load, with novel tasks and responsibilities, requiring continuous behavioral adaptation. Here, we outline a framework for understanding maternal neurocognition across the lifespan. The increased cognitive load across the peripartum period provides an initial challenge, requires continuous cognitive adaptation, and results in life-long environmental complexity and increased cognitive reserve in late-life. Combining evidence from animal and human studies, we propose that the increased lifetime complexity of motherhood may provide a form of enriched environment, positively contributing to cognitive reserve, and resilience to the ageing process.
Table 1:
Cognition in the Postpartum Period. Direction of Results indicates which group or timepoint showed better performance, for example “Controls>Postpartum” indicates superior performance in the control group, “n.s” indicates non-significance. The significant differences column indicates the cognitive domain(s) which showed significant effects. Significant effects that suggest cognitive improvement in the postpartum period are colored pink, and those that suggest deficits are colored grey. No Significant Differences indicates the cognitive domains which did not show significant effects, shown in blue. Sleep and Mood indicate whether a study has collected (Y) measures of sleep or mood, or not (N).
| N | Time Postpartum | Direction of Results | Significant Difference | No Significant Difference | Parity | Sleep | Mood | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cross-Sectional Studies | ||||||||||||
| Eidelman et al. (1993) | 100 Postpartum, 20 Controls, | 3 days | n.s | - | Verbal Memory Visuospatial Memory | Multiparous Mothers, Nulliparous Controls | N | N | ||||
| Swain et al. (1997) | 30 Postpartum, 28 Controls | 3 weeks | n.s | - | Memory Attention Psychomotor Speed | Mothers Not Stated, Parous Controls | Y | Y | ||||
| Parsons et al. (2004) | 7 Primiparous, 9 Multiparous | 1 month | Multiparous>Primiparous | Verbal Memory | Verbal Fluency Trail-Making Comprehension Semantic Memory Attention Executive Control Visuospatial Skill Non-Verbal IQ | 7 Primiparous, 9 Multiparous | N | Y | ||||
| Almanza-Sepulveda et al. (2018) | 27 Postpartum, 25 Controls | 2–6 months | Controls>Postpartum | Spatial Working Memory | Executive Function | Not Stated | N | Y | ||||
| Janes et al., (1999) | 20 Postpartum, 20 Controls | 0–12 months | Controls>Postpartum | Working Memory | Implicit Memory Explicit Memory | Primiparous Mothers, Nulliparous Controls | Y | Y | ||||
| Casey et al. (1999) | 44 Postpartum, 23 Controls | 0–12 months (n=22), >12months (n=22) | n.s | - | Implicit Memory Explicit Memory Semantic Memory Short-Term Memory Prospective Memory | Mothers Not Stated, Nulliparous Controls | Y | Y | ||||
| Orchard et al. (2021) | 43 Postpartum, 43 Controls | 12 months | n.s. | - | Verbal Memory Short-Term Memory Working Memory Processing Speed Theory of Mind | Primiparous Mothers, Nulliparous Controls | Y | Y | ||||
| Pownall et al. (2021) | 102 Pregnant 126 Postpartum 136 Controls | 0–2 years | - | Primiparous Pregnant Women, Nulliparous Controls, Postpartum Not Stated | N | N | ||||||
| Controls>Pregnant and Postpartum | Verbal Memory | |||||||||||
| Miller et al. (2020) | 36 Postpartum, 62 Controls | 3.5 years | Alerting Disengaging Moving + Engaging Orienting | Mothers Not Stated, Nulliparous Controls | Y | N | ||||||
| Controls>Postpartum | Reaction Time | |||||||||||
| Longitudinal Studies | ||||||||||||
| Stark (2000) | 56 Mothers | 1 week | Postpartum>Pregnancy | Divided Attention | Working Memory Short-Term Memory Directed Attention Mental Effort | 89.5% Primiparous, 10.5% Multiparous | N | Y | ||||
| Harris et al. (1996) | 20 Postpartum, 20 Controls | 1 month | n.s | - | Executive Function Attention Logical Memory Processing Speed Psychomotor Speed | Not Stated | N | Y | ||||
| Hoekzema et al. (2018) | 25 Postpartum, 20 Controls | 2.5 months | n.s | - | Verbal Memory Working Memory Reaction Time | Primiparous Mothers, Nulliparous Controls | N | Y | ||||
| Rutherford & Anderson (2020) | 25 Postpartum, 25 Controls | 3 months | n.s | - | Verbal IQ Non-Verbal IQ Full-Scale IQ | Primiparous Mothers, Nulliparous Controls | Y | N | ||||
| Glynn (2010) | 254 Postpartum, 48 Controls | 3 months | Controls>Postpartum | Verbal Memory | Working Memory Facial Recognition | Mothers: 44% Primiparous, 56% Multiparous, Controls: 42% Nulliparous, 58% Parous | N | N | ||||
| Henry & Sherwin (2012) | 55 Postpartum, 21 Controls | 3 months | Controls>Postpartum | Verbal Memory Executive Function | Paragraph Recall Spatial Ability Attention | Primiparous Mothers, Nulliparous Controls | Y | Y | ||||
| Farrar et al. (2014) | 23 Postpartum, 24 Controls | 3 months | Control>Postpartum | Spatial Recognition | Working Memory Divided Attention Perceptual Matching | Mothers: 39% Primiparous, 61% Multiparous, Controls: 46% Nulliparous, 54% Parous | N | Y | ||||
| Logan et al. (2014) | 21 Postpartum, 21 Controls | 3–6 months | n.s | - | Verbal Learning Working Memory Cognitive Control Visuospatial Memory Processing Speed | Mothers Not Stated, Nulliparous Controls | N | Y | ||||
| Casey (2000) | 28 Mothers, 24 Controls | 2–8 months | n.s | - | Vocabulary Short-Term Memory Working Memory Semantic Memory Abstract Reasoning | Not Stated | Y | Y | ||||
| de Groot et al. (2006) | 57 Postpartum, 50 Controls | 8 months | Controls>Postpartum | Verbal Memory Information Processing | Processing Speed | Mothers: 40% Primiparous, 60% Multiparous, Controls: 30% Nulliparous, 70% Parous | N | N | ||||
| Mickes et al. (2009) | 37 Mothers | 3–12 months | Postpartum>Pregnancy | Verbal Memory | Recognition Memory | Not Stated | N | N | ||||
| Crawley et al. (2003) | 25 Mothers, 10 Controls | 10–13 months | n.s | - | Verbal Memory Focused Attention Divided Attention | Mothers: 36% Primiparous, 64% Multiparous, Controls: 50% Nulliparous, 50% Parous | N | N | ||||
| Rendell & Henry (2008) | 15 Mothers | 13 months | Postpartum>Pregnancy | Naturalistic Prospective Memory | - | Mothers: 70% Primiparous, 30% Multiparous, Controls: 70% Nulliparous, 30% Parous | Y | N | ||||
| Buckwalter et al. (2001) | 11 Mothers | 2 years | Postpartum>Pregnancy | Attention Psychomotor Speed | IQ Visual Perception | Not Stated | Y | Y | ||||
| Christensen et al. (2010) | 188 Postpartum, 542 Controls | Not Stated | n.s | - | Verbal Memory Working Memory Processing Speed | Primiparous Mothers, Nulliparous Controls | Y | Y | ||||
| Keenan et al. (1998) | 10 Postpartum, 10 Controls | Not Stated | n.s | - | Implicit Memory Explicit Memory | Mothers Not Stated, Non-Pregnant Controls | Y | Y | ||||
Highlights.
The maternal brain undergoes significant structural and functional neuroplasticity as well as cognitive adaptations during the peripartum period, which are long-lasting and present throughout the lifespan.
The novel challenges of the postpartum period involve increased responsibilities, resulting in an increased cognitive load for new mothers. This cognitive load is increased across the lifespan, and dynamically adjusted as the needs of the child grow and change.
Long-term exposure to a more complex environment is beneficial for the brains of humans and animals, suggesting that increases in environmental complexity in motherhood may result in increased cognitive reserve in late-life.
Glossary
- Cognitive Reserve
the brain’s ability to cope with damage, illness, or cognitive decline by recruiting pre-existing cognitive processes.
- Enriched Environment
Animal housing conditions which consist of enhanced sensory, cognitive, motor, or social stimulation
- Executive Function
a set of higher-level cognitive processes, including working memory, inhibitory control, and set-shifting, which allow the control of behavior and emotion, and facilitate goal attainment
- Matrescence
The transition to motherhood.
- Multiparous
A person who has been pregnant or given birth more than once. This term can also be used to describe a multiple pregnancy (e.g., twins, triplets).
- Nulliparous
A person who has never been pregnant or given birth.
- Parity
The number of children a person has parented.
- Peripartum period
Pregnancy and the first 6–8 weeks postpartum.
- Postpartum period
The time following birth, also called the postnatal period. There is little consensus as to the length of time included in the postpartum period, with some defining postpartum as the 6–8 weeks following birth, and some up to the first six months, aligning with the resumption of menstruation and cessation of breastfeeding.
- Primiparous
A person who has been pregnant or given birth to one child.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hoekzema E, et al. , Pregnancy leads to long-lasting changes in human brain structure. Nat Neurosci, 2017. 20(2): p. 287–296. [DOI] [PubMed] [Google Scholar]
- 2.Feldman R, The adaptive human parental brain: implications for children’s social development. Trends Neurosci, 2015. 38(6): p. 387–99. [DOI] [PubMed] [Google Scholar]
- 3.Davies SJ, et al. , Cognitive impairment during pregnancy: a meta-analysis. Med J Aust, 2018. 208(1): p. 35–40. [DOI] [PubMed] [Google Scholar]
- 4.Martínez-García M, et al. , Characterizing the brain structural adaptations across the motherhood transition. Frontiers in Global Women’s Health, 2021: p. 76. DOI: 10.3389/fgwh.2021.742775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dahan O, The birthing brain: A lacuna in neuroscience. Brain and Cognition, 2021. 150: p. 105722. [DOI] [PubMed] [Google Scholar]
- 6.Luders E, Kurth F, and Poromaa IS, The Neuroanatomy of Pregnancy and Postpartum. NeuroImage, 2022: p. 119646. [DOI] [PubMed] [Google Scholar]
- 7.Cardenas EF, Kujawa A, and Humphreys KL, Neurobiological changes during the peripartum period: Implications for health and behavior. Soc Cogn Affect Neurosci, 2019. DOI: DOI: 10.1093/scan/nsz091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pawluski JL, et al. , Less Can Be More: Fine Tuning the Maternal Brain. Neuroscience & Biobehavioral Reviews, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barba-Müller E, et al. , Brain plasticity in pregnancy and the postpartum period: links to maternal caregiving and mental health. Archives of Women’s Mental Health, 2018. 22(2): p. 289–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carmona S, et al. , Pregnancy and adolescence entail similar neuroanatomical adaptations: a comparative analysis of cerebral morphometric changes. Human brain mapping, 2019. 40(7): p. 2143–2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Callaghan B, et al. , Evidence for cognitive plasticity during pregnancy via enhanced learning and memory. Memory, 2021: p. 1–18. [DOI] [PubMed] [Google Scholar]
- 12.Orchard ER, et al. , Evidence of Subjective, But Not Objective, Cognitive Deficit in New Mothers at 1-Year Postpartum. J Womens Health (Larchmt), 2022. 31(8): p. 1087–1096. [DOI] [PubMed] [Google Scholar]
- 13.Parsons CE and Redman S, Self-reported cognitive change during pregnancy. The Australian journal of advanced nursing: a quarterly publication of the Royal Australian Nursing Federation, 1991. 9(1): p. 20–29. [PubMed] [Google Scholar]
- 14.Crawley R, Self‐perception of cognitive changes during pregnancy and the early postpartum: salience and attentional effects. Applied Cognitive Psychology: The Official Journal of the Society for Applied Research in Memory and Cognition, 2002. 16(6): p. 617–633. [Google Scholar]
- 15.Brindle PM, et al. , Objective and subjective memory impairment in pregnancy. Psychological medicine, 1991. 21(3): p. 647–653. [DOI] [PubMed] [Google Scholar]
- 16.Sharp K, et al. , Memory loss during pregnancy. BJOG: An International Journal of Obstetrics & Gynaecology, 1993. 100(3): p. 209–215. [DOI] [PubMed] [Google Scholar]
- 17.Crawley R, Dennison K, and Carter C, Cognition in pregnancy and the first year post‐partum. Psychology and psychotherapy: Theory, research and practice, 2003. 76(1): p. 69–84. [DOI] [PubMed] [Google Scholar]
- 18.Casey P, et al. , Memory in pregnancy. II: Implicit, incidental, explicit, semantic, short-term, working and prospective memory in primigravid, multigravid and postpartum women. Journal of Psychosomatic Obstetrics & Gynecology, 1999. 20(3): p. 158–164. [DOI] [PubMed] [Google Scholar]
- 19.Janes C, et al. , Memory in pregnancy. I: Subjective experiences and objective assessment of implicit, explicit and working memory in primigravid and primiparous women. J Psychosom Obstet Gynaecol, 1999. 20(2): p. 80–7. [DOI] [PubMed] [Google Scholar]
- 20.Anderson MV and Rutherford M, Cognitive reorganization during pregnancy and the postpartum period: an evolutionary perspective. Evolutionary Psychology, 2012. 10(4): p. 147470491201000402. [PubMed] [Google Scholar]
- 21.Henry J and Rendell PG, A review of the impact of pregnancy on memory function. J Clin Exp Neuropsychol, 2007. 29(8): p. 793–803. [DOI] [PubMed] [Google Scholar]
- 22.Galea LA, et al. , Spatial working memory and hippocampal size across pregnancy in rats. Hormones and behavior, 2000. 37(1): p. 86–95. [DOI] [PubMed] [Google Scholar]
- 23.Pawluski JL, Lambert KG, and Kinsley CH, Neuroplasticity in the maternal hippocampus: Relation to cognition and effects of repeated stress. Hormones and Behavior, 2016. 77: p. 86–97. [DOI] [PubMed] [Google Scholar]
- 24.Darnaudéry M, et al. , Early motherhood in rats is associated with a modification of hippocampal function. Psychoneuroendocrinology, 2007. 32(7): p. 803–812. [DOI] [PubMed] [Google Scholar]
- 25.Macbeth AH and Luine VN, Changes in anxiety and cognition due to reproductive experience: a review of data from rodent and human mothers. Neurosci Biobehav Rev, 2010. 34(3): p. 452–67. [DOI] [PubMed] [Google Scholar]
- 26.Logan DM, et al. , How do memory and attention change with pregnancy and childbirth? A controlled longitudinal examination of neuropsychological functioning in pregnant and postpartum women. Journal of clinical and experimental neuropsychology, 2014. 36(5): p. 528–539. [DOI] [PubMed] [Google Scholar]
- 27.Glynn LM, Giving birth to a new brain: hormone exposures of pregnancy influence human memory. Psychoneuroendocrinology, 2010. 35(8): p. 1148–1155. [DOI] [PubMed] [Google Scholar]
- 28.Harris ND, et al. , Peripartal cognitive impairment: Secondary to depression? British Journal of Health Psychology, 1996. 1(2): p. 127–136. [Google Scholar]
- 29.Casey P, A longitudinal study of cognitive performance during pregnancy and new motherhood. Archives of Women’s Mental Health, 2000. 3(2): p. 65–76. [Google Scholar]
- 30.Eidelman A, Hoffmann NW, and Kaitz M, Cognitive deficits in women after childbirth. OBSTETRICS AND GYNECOLOGY-NEW YORK-, 1993. 81: p. 764–764. [PubMed] [Google Scholar]
- 31.Swain AM, et al. , A prospective study of sleep, mood, and cognitive function in postpartum and nonpostpartum women. Obstetrics & Gynecology, 1997. 90(3): p. 381–386. [DOI] [PubMed] [Google Scholar]
- 32.Christensen H, Leach LS, and Mackinnon A, Cognition in pregnancy and motherhood: prospective cohort study. Br J Psychiatry, 2010. 196(2): p. 126–32. [DOI] [PubMed] [Google Scholar]
- 33.Keenan P, et al. , Explicit memory in pregnant women. American journal of obstetrics and gynecology, 1998. 179(3): p. 731–737. [DOI] [PubMed] [Google Scholar]
- 34.Mickes L, et al. , The effects of pregnancy on memory: Recall is worse but recognition is not. Journal of clinical and experimental neuropsychology, 2009. 31(6): p. 754–761. [DOI] [PubMed] [Google Scholar]
- 35.Rendell PG and Henry JD, Prospective-memory functioning is affected during pregnancy and postpartum. Journal of Clinical and Experimental Neuropsychology, 2008. 30(8): p. 913–919. [DOI] [PubMed] [Google Scholar]
- 36.Rutherford M and Anderson MV, Changes In Intelligence Across Pregnancy And The Postpartum Period. 2020. [Google Scholar]
- 37.Stark MA, Is it difficult to concentrate during the 3rd trimester and postpartum? Journal of Obstetric, Gynecologic, & Neonatal Nursing, 2000. 29(4): p. 378–389. [DOI] [PubMed] [Google Scholar]
- 38.Henry J and Sherwin BB, Hormones and cognitive functioning during late pregnancy and postpartum: a longitudinal study. Behavioral neuroscience, 2012. 126(1): p. 73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.de Groot RH, et al. , Differences in cognitive performance during pregnancy and early motherhood. Psychological Medicine, 2006. 36(7): p. 1023–1032. [DOI] [PubMed] [Google Scholar]
- 40.Kinsley CH, et al. , Motherhood improves learning and memory. Nature, 1999. 402(6758): p. 137–138. [DOI] [PubMed] [Google Scholar]
- 41.Macbeth AH, Gautreaux C, and Luine V, Pregnant rats show enhanced spatial memory, decreased anxiety, and altered levels of monoaminergic neurotransmitters. Brain research, 2008. 1241: p. 136–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pawluski JL, et al. , First reproductive experience persistently affects spatial reference and working memory in the mother and these effects are not due to pregnancy or ‘mothering’alone. Behavioural brain research, 2006. 175(1): p. 157–165. [DOI] [PubMed] [Google Scholar]
- 43.Gatewood JD, et al. , Motherhood mitigates aging-related decrements in learning and memory and positively affects brain aging in the rat. Brain Res Bull, 2005. 66(2): p. 91–8. [DOI] [PubMed] [Google Scholar]
- 44.Love G, et al. , Maternal experience produces long-lasting behavioral modifications in the rat. Behavioral neuroscience, 2005. 119(4): p. 1084. [DOI] [PubMed] [Google Scholar]
- 45.Lemaire V, et al. , Motherhood‐induced memory improvement persists across lifespan in rats but is abolished by a gestational stress. European Journal of Neuroscience, 2006. 23(12): p. 3368–3374. [DOI] [PubMed] [Google Scholar]
- 46.Fleming AS, et al. , Olfactory-based social learning varies as a function of parity in female rats. Psychobiology, 1994. 22(1): p. 37–43. [Google Scholar]
- 47.Wartella J, et al. , Single or multiple reproductive experiences attenuate neurobehavioral stress and fear responses in the female rat. Physiology & Behavior, 2003. 79(3): p. 373–381. [DOI] [PubMed] [Google Scholar]
- 48.Byrnes EM and Bridges RS, Reproductive experience alters anxiety-like behavior in the female rat. Hormones and Behavior, 2006. 50(1): p. 70–76. [DOI] [PubMed] [Google Scholar]
- 49.Farrar D, et al. , Assessment of cognitive function across pregnancy using CANTAB: a longitudinal study. Brain and cognition, 2014. 84(1): p. 76–84. [DOI] [PubMed] [Google Scholar]
- 50.Buckwalter JG, et al. , Pregnancy and postpartum: changes in cognition and mood, in Progress in brain research. 2001, Elsevier. p. 303–319. [DOI] [PubMed] [Google Scholar]
- 51.Pawluski J and Galea L, Reproductive experience alters hippocampal neurogenesis during the postpartum period in the dam. Neuroscience, 2007. 149(1): p. 53–67. [DOI] [PubMed] [Google Scholar]
- 52.Kim P, et al. , The plasticity of human maternal brain: longitudinal changes in brain anatomy during the early postpartum period. Behav Neurosci, 2010. 124(5): p. 695–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Moses-Kolko EL, Banihashemi L, and Hipwell AE, Reduced postpartum hippocampal volume is associated with positive mother-infant caregiving behavior. Journal of Affective Disorders, 2021. 281: p. 297–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Silber M, et al. , Temporary peripartal impairment in memory and attention and its possible relation to oxytocin concentration. Life Sciences, 1990. 47(1): p. 57–65. [DOI] [PubMed] [Google Scholar]
- 55.Holder MK and Blaustein JD, Puberty and adolescence as a time of vulnerability to stressors that alter neurobehavioral processes. Frontiers in neuroendocrinology, 2014. 35(1): p. 89–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Parsons, et al. , Pregnancy history and cognition during and after pregnancy. International Journal of Neuroscience, 2004. 114(9): p. 1099–1110. [DOI] [PubMed] [Google Scholar]
- 57.Buckwalter JG, et al. , Pregnancy, the postpartum, and steroid hormones: effects on cognition and mood. Psychoneuroendocrinology, 1999. 24(1): p. 69–84. [DOI] [PubMed] [Google Scholar]
- 58.Winnicott DW, Primary maternal preoccupation. The maternal lineage: Identification, desire, and transgenerational issues, 1956: p. 59–66. [Google Scholar]
- 59.Christensen H, et al. , Pregnancy may confer a selective cognitive advantage. Journal of reproductive and infant psychology, 1999. 17(1): p. 7–25. [Google Scholar]
- 60.Cuttler C, et al. , Everyday life memory deficits in pregnant women. Can J Exp Psychol, 2011. 65(1): p. 27–37. [DOI] [PubMed] [Google Scholar]
- 61.Matthews KA and Rodin J, Pregnancy alters blood pressure responses to psychological and physical challenge. Psychophysiology, 1992. 29(2): p. 232–240. [DOI] [PubMed] [Google Scholar]
- 62.Anderson MV, Cognitive reorganization and protective mechanisms in pregnancy and the postpartum period. 2011. [Google Scholar]
- 63.Pownall M, Conner M, and Hutter RR, The effects of activating a “baby brain” stereotype on pregnant women’s cognitive functioning. Journal of Applied Social Psychology, 2021. [Google Scholar]
- 64.Rutherford HJ, et al. , Emotion regulation in parenthood. Developmental Review, 2015. 36: p. 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rutherford HJV, et al. , Executive Functioning Predicts Reflective Functioning in Mothers. Journal of Child and Family Studies, 2018. 27(3): p. 944–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rutherford HJ, et al. , Investigating the association between parental reflective functioning and distress tolerance in motherhood. Infant Behavior and Development, 2015. 40: p. 54–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Miller V, VanWormer LA, and Veile A, Assessment of attention in biological mothers using the attention network test-revised. Current Psychology, 2020: p. 1–10. [Google Scholar]
- 68.Stern Y, Brain Networks Associated with Cognitive Reserve in Healthy Young and Old Adults. Cerebral Cortex, 2005. 15(4): p. 394–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bak Y, et al. , Altered neural substrates within cognitive networks of postpartum women during working memory process and resting-state. Scientific reports, 2020. 10(1): p. 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zheng J-X, et al. , Disruption within brain default mode network in postpartum women without depression. Medicine, 2020. 99(18): p. e20045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ganiban JM, et al. , Understanding child-based effects on parenting: temperament as a moderator of genetic and environmental contributions to parenting. Developmental Psychology, 2011. 47(3): p. 676. [DOI] [PubMed] [Google Scholar]
- 72.Nithianantharajah J and Hannan AJ, Enriched environments, experience-dependent plasticity and disorders of the nervous system. Nature Reviews Neuroscience, 2006. 7(9): p. 697–709. [DOI] [PubMed] [Google Scholar]
- 73.Diamond MC, Enriching heredity: the impact of the environment on the anatomy of the brain. 1988: Free Press. [Google Scholar]
- 74.Diamond MC, Krech D, and Rosenzweig MR, The effects of an enriched environment on the histology of the rat cerebral cortex. Journal of Comparative Neurology, 1964. 123(1): p. 111–119. [DOI] [PubMed] [Google Scholar]
- 75.Cummins R, et al. , Environmentally-induced changes in the brains of elderly rats. Nature, 1973. 243(5409): p. 516–518. [DOI] [PubMed] [Google Scholar]
- 76.Volkmar FR and Greenough WT, Rearing complexity affects branching of dendrites in the visual cortex of the rat. Science, 1972. 176(4042): p. 1445–1447. [DOI] [PubMed] [Google Scholar]
- 77.Speisman RB, et al. , Environmental enrichment restores neurogenesis and rapid acquisition in aged rats. Neurobiol Aging, 2013. 34(1): p. 263–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Nilsson M, et al. , Enriched environment increases neurogenesis in the adult rat dentate gyrus and improves spatial memory. Journal of neurobiology, 1999. 39(4): p. 569–578. [DOI] [PubMed] [Google Scholar]
- 79.Kempermann G, Kuhn HG, and Gage FH, Experience-induced neurogenesis in the senescent dentate gyrus. Journal of Neuroscience, 1998. 18(9): p. 3206–3212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Diamond MC, Johnson RE, and Ingham C, Brain plasticity induced by environment and pregnancy. International Journal of Neuroscience, 1971. 2(4–5): p. 171–178. [DOI] [PubMed] [Google Scholar]
- 81.Hamilton WL, et al. , Effects of pregnancy and differential environments on rat cerebral cortical depth. Behavioral Biology, 1977. 19(3): p. 333–340. [DOI] [PubMed] [Google Scholar]
- 82.Kinsley CH, et al. , Motherhood induces and maintains behavioral and neural plasticity across the lifespan in the rat. Arch Sex Behav, 2008. 37(1): p. 43–56. [DOI] [PubMed] [Google Scholar]
- 83.Kinsley CH, Franssen RA, and Meyer EA, Reproductive experience may positively adjust the trajectory of senescence, in Behavioral Neurobiology of Aging. 2011, Springer. p. 317–345. [DOI] [PubMed] [Google Scholar]
- 84.Kozorovitskiy Y, et al. , Fatherhood affects dendritic spines and vasopressin V1a receptors in the primate prefrontal cortex. Nature neuroscience, 2006. 9(9): p. 1094–1095. [DOI] [PubMed] [Google Scholar]
- 85.Nithianantharajah J and Hannan AJ, The neurobiology of brain and cognitive reserve: mental and physical activity as modulators of brain disorders. Progress in neurobiology, 2009. 89(4): p. 369–382. [DOI] [PubMed] [Google Scholar]
- 86.Stern Y, What is cognitive reserve? Theory and research application of the reserve concept. Journal of the international neuropsychological society, 2002. 8(3): p. 448–460. [PubMed] [Google Scholar]
- 87.Manly JJ, et al. , Literacy and memory decline among ethnically diverse elders. Journal of clinical and experimental neuropsychology, 2003. 25(5): p. 680–690. [DOI] [PubMed] [Google Scholar]
- 88.Stern Y, Cognitive reserve. Neuropsychologia, 2009. 47(10): p. 2015–2028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Meng X and D’arcy C, Education and dementia in the context of the cognitive reserve hypothesis: a systematic review with meta-analyses and qualitative analyses. PloS one, 2012. 7(6): p. e38268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Boots EA, et al. , Occupational complexity and cognitive reserve in a middle-aged cohort at risk for Alzheimer’s disease. Archives of Clinical Neuropsychology, 2015. 30(7): p. 634–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Dekhtyar S, et al. , A life-course study of cognitive reserve in dementia—from childhood to old age. The American Journal of Geriatric Psychiatry, 2015. 23(9): p. 885–896. [DOI] [PubMed] [Google Scholar]
- 92.Evans IE, et al. , Social isolation, cognitive reserve, and cognition in healthy older people. PloS one, 2018. 13(8): p. e0201008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Marioni RE, et al. , Active cognitive lifestyle associates with cognitive recovery and a reduced risk of cognitive decline. Journal of Alzheimer’s Disease, 2012. 28(1): p. 223–230. [DOI] [PubMed] [Google Scholar]
- 94.Bialystok E, Bilingualism: Pathway to Cognitive Reserve. Trends in Cognitive Sciences, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Nucci M, Mapelli D, and Mondini S, Cognitive Reserve Index questionnaire (CRIq): a new instrument for measuring cognitive reserve. Aging clinical and experimental research, 2012. 24(3): p. 218–226. [DOI] [PubMed] [Google Scholar]
- 96.Heys M, et al. , Life long endogenous estrogen exposure and later adulthood cognitive function in a population of naturally postmenopausal women from Southern China: the Guangzhou Biobank Cohort Study. Psychoneuroendocrinology, 2011. 36(6): p. 864–873. [DOI] [PubMed] [Google Scholar]
- 97.Smith C, et al. , Lifelong estrogen exposure and cognitive performance in elderly women. Brain and cognition, 1999. 39(3): p. 203–218. [DOI] [PubMed] [Google Scholar]
- 98.Henderson V, et al. , Estrogen exposures and memory at midlife: a population-based study of women. Neurology, 2003. 60(8): p. 1369–1371. [DOI] [PubMed] [Google Scholar]
- 99.Ryan J, et al. , Life-time estrogen exposure and cognitive functioning in later life. Psychoneuroendocrinology, 2009. 34(2): p. 287–298. [DOI] [PubMed] [Google Scholar]
- 100.Read SL and Grundy EM, Fertility history and cognition in later life. Journals of Gerontology Series B: Psychological Sciences and Social Sciences, 2017. 72(6): p. 1021–1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Harville EW, et al. , Reproductive History and Cognitive Aging: The Bogalusa Heart Study. The American Journal of Geriatric Psychiatry, 2020. 28(2): p. 217–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ning K, et al. , Parity is associated with cognitive function and brain age in both females and males. Sci Rep, 2020. 10(1): p. 6100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Orchard ER, et al. , Relationship between parenthood and cortical thickness in late adulthood. PLOS ONE, 2020. 15(7): p. e0236031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Duarte-Guterman P, Leuner B, and Galea LAM, The long and short term effects of motherhood on the brain. Front Neuroendocrinol, 2019. 53: p. 100740. [DOI] [PubMed] [Google Scholar]
- 105.Colucci M, et al. , The number of pregnancies is a risk factor for Alzheimer’s disease. European journal of neurology, 2006. 13(12): p. 1374–1377. [DOI] [PubMed] [Google Scholar]
- 106.Tomasz S and Kloszewska I. Parity, number of pregnancies, and the age of onset of Alzheimer’s disease. in 7th World Congress of Biological Psychiatry, Jul, 2001, Berlin, Germany; Portions of this research were presented at the aforementioned conference. 2004. American Psychiatric Assn. [Google Scholar]
- 107.Gong J, et al. , Reproductive factors and the risk of incident dementia: A cohort study of UK Biobank participants. PLoS medicine, 2022. 19(4): p. e1003955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Corbo RM, et al. , Combined effect of apolipoprotein e genotype and past fertility on age at onset of Alzheimer’s disease in women. Dementia and geriatric cognitive disorders, 2007. 24(2): p. 82–85. [DOI] [PubMed] [Google Scholar]
- 109.Cui J, et al. , Amyloid precursor protein mutation disrupts reproductive experience-enhanced normal cognitive development in a mouse model of Alzheimer’s disease. Molecular neurobiology, 2014. 49(1): p. 103–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.van Exel E, et al. , Effect of APOE ε4 allele on survival and fertility in an adverse environment. PloS one, 2017. 12(7): p. e0179497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.de Lange A-MG, et al. , Population-based neuroimaging reveals traces of childbirth in the maternal brain. Proceedings of the National Academy of Sciences, 2019. 116(44): p. 22341–22346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.de Lange A-MG, et al. , The maternal brain: Region‐specific patterns of brain aging are traceable decades after childbirth. Human Brain Mapping, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Voldsbekk I, et al. , A history of previous childbirths is linked to women’s white matter brain age in midlife and older age. Hum Brain Mapp, 2021. 42(13): p. 4372–4386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Franke K and Gaser C, Ten Years of BrainAGE as a Neuroimaging Biomarker of Brain Aging: What Insights Have We Gained? Front Neurol, 2019. 10: p. 789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Brunton PJ and Russell JA, The expectant brain: adapting for motherhood. Nature Reviews Neuroscience, 2008. 9(1): p. 11–25. [DOI] [PubMed] [Google Scholar]
- 116.Numan M and Woodside B, Maternity: neural mechanisms, motivational processes, and physiological adaptations. Behavioral neuroscience, 2010. 124(6): p. 715. [DOI] [PubMed] [Google Scholar]
- 117.Eid RS, et al. , Early and late effects of maternal experience on hippocampal neurogenesis, microglia, and the circulating cytokine milieu. Neurobiology of aging, 2019. 78: p. 1–17. [DOI] [PubMed] [Google Scholar]
- 118.Gregg C, et al. , White matter plasticity and enhanced remyelination in the maternal CNS. Journal of Neuroscience, 2007. 27(8): p. 1812–1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Orchard ER, et al. , Neuroprotective Effects of Motherhood on Brain Function in Late Life: A Resting-State fMRI Study. Cerebral Cortex, 2021. 31(2): p. 1270–1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Almanza‐Sepulveda ML, et al. , Executive function in teen and adult women: Association with maternal status and early adversity. Developmental Psychobiology, 2018. 60(7): p. 849–861. [DOI] [PubMed] [Google Scholar]
- 121.Raphael D, The Tender Gift: Breastfeeding. 1973: Prentice-Hall. [Google Scholar]
- 122.Stolzenberg DS and Champagne FA, Hormonal and non-hormonal bases of maternal behavior: The role of experience and epigenetic mechanisms. Horm Behav, 2016. 77: p. 204–10. [DOI] [PubMed] [Google Scholar]
- 123.Kinsley CH, et al. , Motherhood improves learning and memory. Nature, 1999. 402(6758): p. 137–138. [DOI] [PubMed] [Google Scholar]
- 124.Bick J, et al. , Foster mother-infant bonding: associations between foster mothers’ oxytocin production, electrophysiological brain activity, feelings of commitment, and caregiving quality. Child Dev, 2013. 84(3): p. 826–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Burt DB, Zembar MJ, and Niederehe G, Depression and memory impairment: a meta-analysis of the association, its pattern, and specificity. Psychological bulletin, 1995. 117(2): p. 285. [DOI] [PubMed] [Google Scholar]
- 126.Kahn RL, et al. , Memory complaint and impairment in the aged: The effect of depression and altered brain function. Archives of general psychiatry, 1975. 32(12): p. 1569–1573. [DOI] [PubMed] [Google Scholar]
- 127.Schweizer S, et al. , Symptoms of depression in a large healthy population cohort are related to subjective memory complaints and memory performance in negative contexts. Psychological medicine, 2018. 48(1): p. 104–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Maquet P, The role of sleep in learning and memory. science, 2001. 294(5544): p. 1048–1052. [DOI] [PubMed] [Google Scholar]
- 129.Walker MP and Stickgold R, Sleep-dependent learning and memory consolidation. Neuron, 2004. 44(1): p. 121–133. [DOI] [PubMed] [Google Scholar]
- 130.Onyper SV, et al. , Executive functioning and general cognitive ability in pregnant women and matched controls. Journal of clinical and experimental neuropsychology, 2010. 32(9): p. 986–995. [DOI] [PubMed] [Google Scholar]
- 131.Ouellette SJ and Hampson E, Memory and affective changes during the antepartum: A narrative review and integrative hypothesis. Journal of clinical and experimental neuropsychology, 2019. 41(1): p. 87–107. [DOI] [PubMed] [Google Scholar]
- 132.Pieters S, et al. , Working memory from pregnancy to postpartum: Do women really change? Psychoneuroendocrinology, 2021. 126: p. 105169. [DOI] [PubMed] [Google Scholar]
- 133.Abraham E, et al. , Father’s brain is sensitive to childcare experiences. Proc Natl Acad Sci U S A, 2014. 111(27): p. 9792–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Kim P, et al. , Neural plasticity in fathers of human infants. Soc Neurosci, 2014. 9(5): p. 522–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Martínez-García M, et al. , First-time fathers show longitudinal gray matter cortical volume reductions: evidence from two international samples. Cerebral Cortex, 2022. [DOI] [PubMed] [Google Scholar]
- 136.Paternina-Die M, et al. , The Paternal Transition Entails Neuroanatomic Adaptations that are Associated with the Father’s Brain Response to his Infant Cues. Cerebral Cortex Communications, 2020. 1(1): p. tgaa082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Everette A, et al. , Paternal experience enhances behavioral and neurobiological responsivity associated with affiliative and nurturing responses. International Behavioral Neuroscience Society, Rio de Janeiro, 2007. [Google Scholar]
- 138.Lambert K, et al. , Pup exposure differentially enhances foraging ability in primiparous and nulliparous rats. Physiology & Behavior, 2005. 84(5): p. 799–806. [DOI] [PubMed] [Google Scholar]
- 139.Sobral M, et al. , Neurobiological Correlates of Fatherhood During the Postpartum Period: A Scoping Review. Frontiers in psychology, 2022: p. 84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Glasper E, et al. , More than just mothers: The neurobiological and neuroendocrine underpinnings of allomaternal caregiving. Frontiers in Neuroendocrinology, 2019. 53: p. 100741. [DOI] [PubMed] [Google Scholar]
- 141.Saltzman W and Ziegler TE, Functional significance of hormonal changes in mammalian fathers. J Neuroendocrinol, 2014. 26(10): p. 685–96. [DOI] [PMC free article] [PubMed] [Google Scholar]


