Abstract
The Candida albicans INT1 gene is important for hyphal morphogenesis, adherence, and virulence (C. Gale, C. Bendel, M. McClellan, M. Hauser, J. M. Becker, J. Berman, and M. Hostetter, Science 279:1355–1358, 1998). The ability to switch between yeast and hyphal morphologies is an important virulence factor in this fungal pathogen. When INT1 is expressed in Saccharomyces cerevisiae, cells grow with a filamentous morphology that we exploited to gain insights into how C. albicans regulates hyphal growth. In S. cerevisiae, INT1-induced filamentous growth was affected by a small subset of actin mutations and a limited set of actin-interacting proteins including Sla2p, an S. cerevisiae protein with similarity in its C terminus to mouse talin. Interestingly, while SLA2 was required for INT1-induced filamentous growth, it was not required for polarized growth in response to several other conditions, suggesting that Sla2p is not required for polarized growth per se. The morphogenesis checkpoint, mediated by Swe1p, contributes to INT1-induced filamentous growth; however, epistasis analysis suggests that Sla2p and Swe1p contribute to INT1-induced filamentous growth through independent pathways. The C. albicans SLA2 homolog (CaSLA2) complements S. cerevisiae sla2Δ mutants for growth at 37°C and INT1-induced filamentous growth. Furthermore, in a C. albicans Casla2/Casla2 strain, hyphal growth did not occur in response to either nutrient deprivation or to potent stimuli, such as mammalian serum. Thus, through analysis of INT1-induced filamentous growth in S. cerevisiae, we have identified a C. albicans gene, SLA2, that is required for hyphal growth in C. albicans.
Candida albicans is the most prevalent fungal pathogen of humans. In addition to causing mucosal infections, such as thrush and vaginitis, in relatively healthy individuals, it causes life-threatening systemic infections in premature infants, surgical patients, chemotherapy patients, and other patients with weakened immune systems. Mortality from systemic infections approaches 30% despite appropriate therapy with the available antifungal agents (59). C. albicans grows in a number of morphologic forms, including ellipsoidal, yeast-form blastospores and filamentous forms that include elongated budding pseudohyphae and parallel-sided germ tubes that give rise to true hyphae (reviewed in reference 58). The ability of C. albicans to switch between these morphologies is correlated with its virulence (reviewed in references 16, 51, and 58). The transition from yeast to hyphal growth occurs in response to a broad range of environmental stimuli. Potent stimuli include one or more constituents of mammalian serum, the presence of specific compounds (such as N-acetylglucosamine), temperature of 37°C, and neutral pH (reviewed in reference 16). A slower filamentous growth response is induced by nutrient deprivation on solid media, such as Spider agar and milk-Tween agar (40).
Many genes that contribute to C. albicans morphogenesis have been identified. These include members of the mitogen-activated protein kinase (MAPK) cascade that is analogous to the MAPK cascade important for mating and pseudohyphal growth in Saccharomyces cerevisiae and members of the RAS-cyclic AMP (cAMP) signaling pathway, which also contributes to pseudohyphal growth in S. cerevisiae (13, 20). The MAPK and RAS-cAMP signaling pathways activate transcription factors, such as Cph1p and Efg1p, respectively (44). Other transcriptional activators and repressors, such as Tup1p, Rbf1p, Prr2p, and Czf1p, also contribute to C. albicans morphogenesis under some environmental conditions (reviewed in references 19 and 51). Loss of transcription factor function usually results in altered filamentation in response to a subset of the conditions that elicit filamentous growth (12, 20). The current working model is that there are several (≥3) independent pathways for triggering morphogenetic changes (12, 19). In addition, there is a significant amount of “cross-talk” between these pathways that may differ, depending upon the environmental stimuli present. Because the relationships between different elicitors, signaling pathways, and morphogenetic responses are complex and not well characterized, our goal is to improve our understanding of these complex relationships by determining the pathways and cellular processes regulated by the different morphogenetic signals.
Transcription factors, such as Efg1p and Tup1p, regulate cell morphogenesis, at least in part, by affecting the transcription of genes, such as those encoding cell wall components or cell wall maintenance functions (e.g., ALS1, CHS2, HWP1, and HYR1 [7, 12, 31, 47, 62, 66]). Ultimately, morphogenesis signals must be executed by the actin cytoskeleton and the secretory vesicles which deliver cell wall material to the cell surface in regions of cell growth. During polarized growth, the actin cytoskeleton delivers most vesicles to the distal pole of the bud, while during isotropic growth, actin and secretory vesicles are observed distributed around the entire periphery of the growing daughter cell.
C. albicans INT1 was originally cloned by virtue of its limited homology to vertebrate leukocyte integrins (23), adhesion proteins that bind the extracellular matrix and induce morphologic changes in response to extracellular signals (32). In C. albicans, INT1 is a virulence factor that contributes to the ability of the pathogen to adhere to epithelial cells (22). Like CPH1 and many other genes, INT1 is required for filamentous growth on milk-Tween and Spider medium, but is not required for filamentation on serum (22), which is considered to be a potent elicitor of the filamentation response. When expressed in S. cerevisiae, Int1p was detected on the cell surface, mediated adhesion to HeLa cell monolayers, and triggered the formation of highly polarized buds with a morphology similar to that of C. albicans germ tubes (22, 23; C. A. Gale, M. Gerami-Nejad, M. McClellan, M. S. Longtine, and J. Berman, submitted for publication). Unlike S. cerevisiae pseudohyphal growth, INT1-induced filamentous growth is independent of MAPK components, does not require specific genetic strain backgrounds, and occurs in either haploid or diploid cells (23). Based upon the integrin paradigm, these results are consistent with a model in which Int1p may be a C. albicans surface protein that responds to a subset of environmental stimuli and mediates a morphogenetic response to them.
In this study, we exploited the ability of C. albicans INT1 to induce filamentous growth in S. cerevisiae to ask about the actin cytoskeleton components required for INT1-induced filamentous growth. We asked if processes that are well characterized in S. cerevisiae are important for INT1-induced filamentous growth in S. cerevisiae and then asked if they are also important for hyphal growth in C. albicans. We identified components of the actin cytoskeleton that affect INT1-induced filamentous growth, including a small subset of actin mutations and a limited set of actin-interacting proteins. One of these is Sla2p, the S. cerevisiae protein most closely related to mouse talin. Interestingly, while SLA2 is required for INT1-induced filamentous growth, Sla2p is not required for S. cerevisiae to form highly polarized buds or mating projections, suggesting that Sla2p is not required for polarized growth per se. In addition, we determined that Sla2p does not trigger INT1-induced filamentous growth exclusively through the morphogenesis checkpoint mediated by Swe1p. In S. cerevisiae, CaSLA2 complemented S. cerevisiae sla2Δ mutants for growth at 37°C and INT1-induced filamentous growth. In C. albicans, disruption of both C. albicans SLA2 alleles resulted in strains that did not exhibit a hyphal growth response to mammalian serum or to nutrient deprivation. These results suggest that, in C. albicans, Sla2p is essential for hyphal growth in response to both potent and mild environmental stimuli.
MATERIALS AND METHODS
Plasmids, strains, culture conditions, and microscopy.
Plasmids and strains used in this study are listed in Table 1. pCG110 and pCG108, which express INT1 from the GAL10 promoter, were derived from pCG01 by using marker swap plasmids pUL9 and pUT11, respectively (17). pYES2-CaSLA2 contains a chromosomal copy of CaSLA2 obtained by PCR of fosmid 18B6 (obtained from the Candida albicans Mapping Project at the University of Minnesota, http://alces.med.umn.edu/candida/probeabout.html), using the following primers: forward, CGAGCTC(SacI)CCCCCCCTAGCCCAATG(Start)AG, and reverse, GCTGCTATTGTTTGTTC, which contains a sequence downstream of the EcoRI site 3′ of the stop codon. The PCR fragment was digested with SacI and EcoRI and cloned into SacI- and EcoRI-digested pYES2 (Invitrogen, Inc.) to drive expression of CaSLA2 from the GAL1 promoter of S. cerevisiae. YJB3857 was generated from YEF473 (yML97) (9) transformed with pWA9 (end4::HIS3) (68). YJB5565 and YJB5566 are sister progeny from a cross of YJB3857 and M-1623 (YEF473 cdc3-6) obtained by nine successive backcrosses of a cdc3-6 allele into the YEF473 strain background and was kindly provided by Mark Longtine, Oklahoma State University.
TABLE 1.
Plasmids and yeast strains used
| Strain or Plasmid | Relevant features or genotype | Source or reference |
|---|---|---|
| Plasmids | ||
| pBM272 | ampR URA3 CEN4 ARS1 pGAL1 pGAL10 | 33 |
| pCG01 | pBM272 pGAL10-INT1 | 23 |
| pCG110 | pCG01 ura3::LEU2 | This study |
| pCG108 | pCG108 ura3::TRP1 | This study |
| pYES2 | ampR URA3 2μm ori pGAL1 | Invitrogen, Inc. |
| pYES2-CaSLA2 | pYES2 pGAL1-CaSLA2 | This study |
| S. cerevisiae | ||
| Actin charge-to-alanine scanning mutants | ||
| YJB2603 | MATaACT1::HIS3 his3-Δ200 leu2-3,112 ura3-52 ade4 tub2-201 [pCG01] | 67 |
| YJB2604 | YJB2603 act1-101::HIS3 can1 [pCG01] | 67 |
| YJB2606 | YJB2603 act1-113::HIS3 can1 [pCG01] | 67 |
| YJB2607 | YJB2603 act1-119::HIS3 [pCG01] | 67 |
| YJB2608 | YJB2603 act1-120::HIS3 [pCG01] | 67 |
| YJB2609 | YJB2603 act1-122::HIS3 [pCG01] | 67 |
| YJB2610 | YJB2603 act1-124::HIS3 [pCG01] | 67 |
| YJB2611 | YJB2603 act1-129::HIS3 [pCG01] | 67 |
| YJB2612 | YJB2603 act1-133::HIS3 [pCG01] | 67 |
| YJB2614 | YJB2603 act1-104::HIS3 [pCG01] | 67 |
| YJB2615 | YJB2603 act1-115::HIS3 [pCG01] | 67 |
| YJB2617 | YJB2603 act1-117::HIS3 [pCG01] | 67 |
| YJB2618 | YJB2603 act1-123::HIS3 [pCG01] | 67 |
| Actin cytoskeleton mutants | ||
| YJB2714 | MATα ABP1 ura3-52 leu2-3 [pCG01] | 69 |
| YJB2715 | MATaabp1::LEU2 ura3-52 lys2-801 am leu2-3,112 [pCG01] | 69 |
| YJB3054 | MATaSAC6 his3 leu2 lys2 ura3 [pCG01] | A. Adams |
| YJB3053 | YJB3054 sac6::LEU2 [pCG01] | A. Adams |
| YJB3321 | MATa/α BNI1/BNI1 DFG9/DFG9 SRV2/SRV2 ura3-52/ura3-52 [pCG01] | 41 |
| YJB3331 | MATa/MATα bni1-100/bni1-100 ura3-52/ura3-52 leu2::hisG/leu2::hisG trp1::hisG/TRP1 [pCG01] | 53 |
| YJB3329 | MATa/MATα srv2-100/srv2-100 ura3-52/ura3-52 leu2::hisG/leu2::hisG trp1::hisG/TRP1 [pCG01] | 53 |
| YJB2457 | MATa/MATα dfg9-100/dfg9-100 ura3-52/ura3-52 leu2::hisG/leu2::hisG trp1::hisG/TRP1 [pCG01] | 53 |
| YJB3057 | MATα RVS167 his3Δ200 leu2-3,112 ura3-52 trp1-1(am) lys2-801(0c) [pCG01] | 39 |
| YJB3056 | MATα rvs167::TRP1 his3Δ200 leu2-3,112 ura3-52 trp1-1(am) lys2-801(0c) [pCG01] | 39 |
| YJB2623 | MATaVRP1 ura3-52 ade1 leu2-3,112 ile MEL1 [pCG01] | A. Hopper |
| YJB2622 | MATα vrp1::LEU2 leu2-3,112 ade1 ura3-52 ile MEL1 [pCG01] | 72 |
| YJB3156 | MATaSLA1 SLA2 leu2-3,112 ura3-52 [pCG110] | 28 |
| YJB3155 | MATasla1-Δ1::URA3 leu2-3,112 ura3-52 [pCG110] | 28 |
| YJB3157 | MATα ura3-52 leu2-3,112 sla2Δ::URA3 [pCG110] | 69 |
| YJB2489 | MATaura3-52 leu2-Δ2 his3-Δ200 trp1-Δ63 lys2-801 | Mark Longtine |
| YJB4786 | YJB2489 sla2::HIS3 | This study |
| cdc3 strains | ||
| YJB2757 | MATα ura3-52 leu2-Δ2 his3-Δ200 trp1-Δ63 lys2-801(SLA2) | 9 |
| YJB3857 | YJB2757 sla2::HIS3 | This work |
| YJB5565 | MATaura3-52 leu2-Δ2 his3-Δ200 trp1-Δ63 lys2-801 cdc3-6 | This work |
| YJB5566 | MATaura3-52 leu2-Δ2 his3-Δ200 trp1-Δ63 lys2-801 cdc3-6 sla2::HIS3 | This work |
| Sla2/End4 domain deletion strains | ||
| YJB4553 | MATa/MATα SLA2/SLA2 ura3-52/ura3-52 leu2-3,112/leu2-3,112 lys2-801/lys2-801 his3-Δ200/his3-Δ200/his3-Δ200 ade2-1/ADE2 [pCG110] | 70 |
| YJB5176 | YJB4553 sla2Δ::URA3/sla2Δ::URA3 [pCG110] | 70 |
| YJB4550 | YJB4553 sla2Δ768-968::URA3/sla2Δ768-968::URA3 [pCG110] | 70 |
| YJB4549 | YJB4553 sla2Δ501-968::URA3/sla2Δ501-968::URA3 [pCG110] | 70 |
| YJB4552 | YJB4553 sla2Δ360-968::URA3/sla2Δ360-968::URA3 [pCG110] | 70 |
| YJB5163 | MATasla2-Δ1::URA3 his3-Δ200 leu2-3,112 ura3-52 [pCG110, pRS313] | 70 |
| YJB5164 | YJB5163 [pCG110, pDD353 (SLA2)] | 70 |
| YJB5165 | YJB5163 [pCG110, pDD362 (sla2Δ33-750)] | 70 |
| YJB5166 | YJB5163 [pCG110, pDD364 (sla2Δ33-501)] | 70 |
| YJB5167 | YJB5163 [pCG110, pDD367 (sla2Δ33-359)] | 70 |
| YJB5168 | YJB5163 [pCG110, pDD369 (sla2Δ33-359, 576 stop)] | 70 |
| YJB5169 | YJB5163 [pCG110, pDD371 (sla2Δ360-575)] | 70 |
| YJB3149 | MATahis3 lys2 ura3 leu2 bar1 end4::HIS3 END4:TRP1 [pCG110] | 68 |
| YJB3146 | YJB3149 end4Δ114-284:TRP1 [pCG110] | 68 |
| YJB3154 | YJB3149 end4Δ286-301:TRP1 [pCG110] | 68 |
| YJB3147 | YJB3149 end4Δ318-373:TRP1 [pCG110] | 68 |
| YJB3150 | YJB3149 end4Δ376-501:TRP1 [pCG110] | 68 |
| YJB3151 | YJB3149 end4Δ495-573:TRP1 [pCG110] | 68 |
| YJB3152 | YJB3149 end4Δ376-573:TRP1 [pCG110] | 68 |
| YJB3153 | YJB3149 end4Δ376-440:TRP1 [pCG110] | 68 |
| YJB3148 | YJB3149 end4Δ767-968:TRP1 [pCG110] | 68 |
| Strains used in complementation studies | ||
| YJB4686 | MATalys2 his4 leu2 ura2 bar1 end4 sla2::LEU2 GAL | H. Reizman |
| YJB4896 | YJB2757 [pCG108, pYES-CaSLA2] | This study |
| YJB4897 | YJB3857 [pCG108, pBM272] | This study |
| YJB4897 | YJB3857 [pCG108, pYES2] | This study |
| YJB4898 | YJB3857 [pCG108, pYES-SLA2] | This study |
| C. albicans strains | ||
| YJB1873 (CAF2) | ura3::imm434/URA3 CaSLA2/CaSLA2 | 21 |
| YJB3018 (CAI4) | ura3::imm434/ura3::imm434 CaSLA2/CaSLA2 | 21 |
| YJB3400 | YJB3018 Casla2::hisG::URA3::hisG/CaSLA2 | This study |
| YCA37 | YJB3018 Casla2::hisG::URA3::hisG/CaSLA2 | This study |
| YJB3401 | YJB3400 Casla2::hisG/CaSLA2 | This study |
| YJB3611 | YCA37 Casla2::hisG/CaSLA2 | This study |
| YJB3402 | YJB3401 Casla2::hisG/Casla2::hisG::URA3::hisG | This study |
| YJB3612 | YJB3611 Casla2::hisG/Casla2::hisG::URA3::hisG | This study |
S. cerevisiae and C. albicans strains were grown in standard laboratory synthetic complete (SC) media with appropriate amino acid drop-outs (63). Media were supplemented after autoclaving with a carbon source as indicated in the text. C. albicans ura3 mutant strains were grown on medium supplemented with 2 μg of uridine/ml. Standard transformation protocols were used for both S. cerevisiae (24) and C. albicans (25). YJB5565 and YJB5566 were derived from progeny of YJB5126 crossed to YJB3857.
To assay INT1-induced filamentous growth, S. cerevisiae cells were grown overnight in 2% glucose at room temperature. Cells were diluted fivefold into SC medium containing 1% galactose and 1% raffinose to induce pGAL-INT1 expression, were grown overnight again, and were then examined to determine the percentage of cells exhibiting filaments and filament length by using a Nikon Eclipse E800 photomicroscope (Fryer Co., Huntley, Ill.) equipped with differential interference contrast optics. To assay C. albicans hyphal growth, cells were grown in yeast-peptone-dextrose medium (YPD) containing adenine, uridine, and 20% fetal calf serum or in RPMI medium (Life Technologies, Rockville, Md.) containing 20% fetal calf serum.
To examine the actin cytoskeleton, induced cultures were stained with rhodamine-phalloidin and examined using epifluorescence microscopy using a modification of the original protocol described by Adams and Pringle (2) as modified by David Amberg (http://genome-www.stanford.edu/group/botlab/protocols/rho_pha_calc.html).
Cells were photographed using a 40×, 0.75-na plan fluor objective. Digital images were collected using a CoolCam liquid-cooled, three-chip color charge-coupled device camera (Cool Camera Company, Decatur, Ga.) and captured to a Pentium II 300 MHz personal computer using Image Pro Plus version 4.0 software (Media Cybernetics, Silver Spring, Md.)
Complementation studies.
Strain YJB4686 was transformed with pYES2-CaSLA2 or pYES2, and transformants were selected on SC–plus glucose–lacking leucine medium at 25°C. Transformants were grown overnight, and 10-fold serial dilutions were spotted on SC–plus glucose–lacking leucine (to repress expression of CaSLA2) and SC plus galactose–lacking leucine (to induce expression of CaSLA2) media and grown at 25 and 37°C.
Generation of C. albicans sla2 disruptants.
The SLA2 disruption cassette was constructed from pJB1001, a plasmid containing the 2.9-kb SLA2 HindIII fragment inserted into the HindIII site of pUC18 digested with EcoRV, which cuts between codons 713 and 714 within the SLA2 open reading frame. URA-blaster plasmid pMB7 (21) was digested with PvuII to liberate a 4.1-kb fragment which was gel purified and ligated into EcoRV-digested pJB1001 to generate pJB1064.
For gene disruptions, CaSLA2-hisG::URA3::hisG-CaSLA2 was released from pJB1064 by digestion with PvuII and was used to transform C. albicans strain CAI4 (21) for uracil prototrophy. Insertion of the URA-blaster cassette within the CaSLA2 locus in strains YJB3400 and yCA37 was confirmed by PCR with primers P1 (5′-AGA TAA TGC TCT TGC TGA-3′), P2 (5′-TTC CCA TCG ATA ACA GCA-3′), and P3 (5′-CGA CTT CGA CAG AAC CAT-3′) and Southern analysis (data not shown). Independent heterozygotes were then plated onto SC medium containing 2% glucose, uridine, and 5-fluoroorotic acid (FOA) to select for loss of the URA3 marker (22). FOA-resistant CaSLA2/Casla2 (YJB3401 and YJB3611) strains were then retransformed with PvuII-digested pJB1064 to disrupt the second CaSLA2 allele. Homozygous Casla2/Casla2 strains were identified by restriction patterns on Southern blots, including the characteristic loss of the 2.7-kb HindIII band (data not shown), and identifications were verified by PCR. Two independent homozygous Casla2/Casla2 strains (YJB3402 and YJB3612) were generated to ensure that any phenotype was due to disruption of CaSLA2 rather than to spurious mutations that can occur during the transformation process.
RESULTS
Actin distribution is highly polarized in INT1-expressing cells.
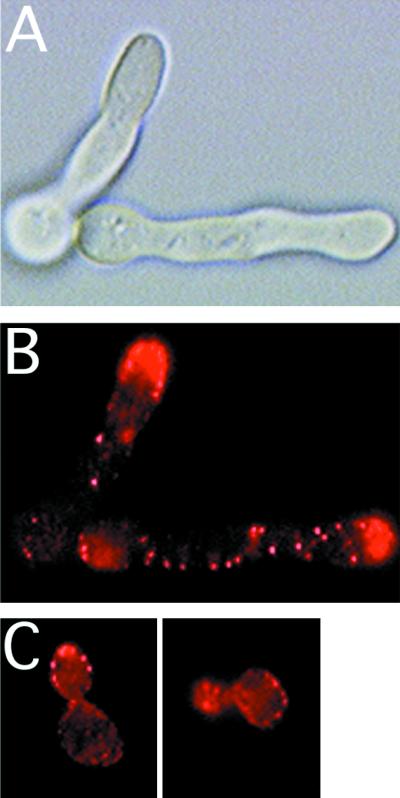
During hyphal growth in C. albicans, the actin cytoskeleton is also highly polarized to the growing hyphal tip (5). During both bud growth and shmoo formation, as well as during pseudohyphal growth, S. cerevisiae cells polarize their cytoskeleton towards zones of growth (18, 69). To monitor actin organization in S. cerevisiae cells expressing Int1p, we used rhodamine-phalloidin to visualize actin cables and cortical patches (1). In wild-type cells, actin cortical patches initially localize to the site of incipient bud growth and then to the tip of small buds. Once a critical bud size is reached, the cortical patches are distributed over the entire bud surface, resulting in isotropic growth of a round bud. Prior to cytokinesis, actin patches are concentrated at the mother-bud neck (reviewed in references 11 and 36). In INT1-induced filamentous cells, as in other polarized buds (14), the majority of the actin patches were usually at the growing tips of the cells (Fig. 1). We also observed a few actin patches distributed throughout the polarized bud. We did not observe any concentration of actin patches in the region where the mother and bud meet. Thus, expression of Int1p caused a reorganization of the actin cytoskeleton, such that it was often concentrated near the distal tip of the filamentous bud for a much longer period of time than in cells that do not express INT1. Actin patches in the INT1-expressing cells appeared similar in size to those in cells expressing only vector sequences.
FIG. 1.
Actin is highly polarized in S. cerevisiae cells expressing INT1. DIC images (A) and fluorescence images (B and C) of rhodamine-phalloidin-stained strain YJB2603 cells expressing INT1 (A and B) after 12 h of growth on galactose. (C) Rhodamine-phalloidin-stained YJB2603 cells were grown on glucose (to repress INT1 expression).
Two subdomains of actin are required for INT1-induced filamentous growth.
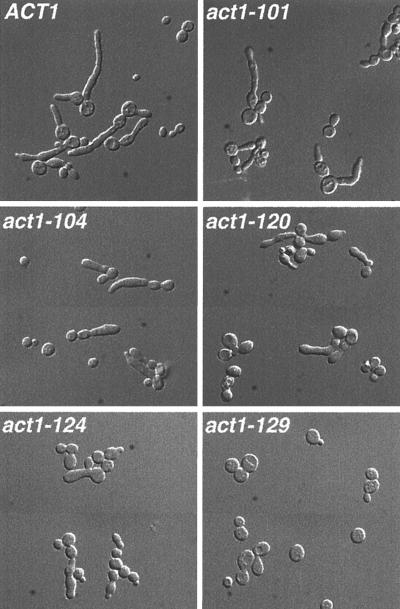
A large series of actin alleles have been generated to study the structure-function relationships of this important cytoskeleton component (11, 55, 67). We tested strains containing 13 different actin alleles for the ability to generate highly polarized buds in response to INT1 expression (Table 2). Only 2 of these 13 alleles, act1-124 and act1-129, caused a significant reduction in the extent of INT1-induced filamentous growth. In both of these strains, the proportion of cells producing polarized buds was significantly reduced with the most dramatic reduction seen with the act1-129 allele. Furthermore, when act1-124 or act1-29 cells that exhibited some polarized growth were observed, the polarized bud was much shorter than polarized buds induced by INT1 in otherwise wild-type cells (Fig. 2). The act1-124 allele maps to subdomain 2 of actin while the act1-129 allele maps to subdomain 3. The act1-124 and act1-129 alleles confer temperature sensitivity at 37°C. However, six other temperature-sensitive actin alleles did not have a significant effect on INT1-induced filamentous growth. Thus, it cannot be the ability to grow at high temperature, per se, that is required for the formation of polarized buds.
TABLE 2.
Some actin alleles affect INT1-induced filamentous growth
| Actin allelea | Amino acids changed to alanineb | Actin subdomain mutatedc | INT1-induced filamentous growthd | Filamentous colony growthe | Bud site selection patternf | Synthetic lethality withg |
|---|---|---|---|---|---|---|
| ACT1 | +++ | +++ | Unipolar | |||
| Temperature sensitivity | ||||||
| act1-101 | 363, 364 | 1 | +++ | sla2Δ | ||
| act1-113 | 210, 211 | 4 | +++ | +++ | Unipolar | sac6Δ |
| act1-119 | 116, 117, 118 | 1 | +++ | sac6Δ | ||
| act1-120 | 99, 100 | 1 | ++ | − | Random | sla2Δ |
| act1-122 | 80, 81 | 2 | +++ | |||
| act1-124 | 56, 57 | 2 | + | − | Random | sla2Δ, sac6Δ |
| act1-129 | 177, 179 | 3 | − | − | Random | sla2Δ, sac6Δ, abp1Δ |
| act1-133 | 24, 25 | 1 | +++ | sla1Δ, sla2Δ | ||
| Pseudo-wild type | ||||||
| act1-104 | 315, 316 | 3 | ++ | + | Unipolar | |
| act1-115 | 195, 196 | 4 | +++ | sla2Δ, sac6Δ | ||
| act1-117 | 183, 184 | 4 | +++ | ++ | Bipolar | |
| act1-123 | 68, 72 | 2 | +++ |
Strains used were YJB2603 to YJB2618, as listed in Table 1.
Phenotypes are as described by Wertman et al. (67).
Domains are as assigned by Botstein et al. (11).
Proportion of cells forming elongated filamentous cells. +++, like wild type; ++, 25 to 75% of wild-type levels; +, 5 to 25% of wild-type levels; −, less than 2% of wild-type levels.
Filamentous growth was described by Cali et al. (14). +++, like wild type; ++, 80 to 100% of colonies had filaments, but filaments were disorganized relative to the wild type; +, 1 to 10% of colonies had filaments; −, no filaments extending beyond the perimeter of the colony.
Bud site selection was determined by Cali et al. (14).
Data were reviewed by Botstein et al. (11).
FIG. 2.
INT1-induced filamentous growth is reduced by specific actin mutations. Shown are DIC micrographs of strains carrying the indicated ACT1 alleles (ACT1, YJB2603; act1-101, YJB2604; act1-104, YJB2614; act1-124, YJB2610; act1-129, YJB2611; act1-120, YJB2608) and expressing INT1 after growth on galactose for 16 h. Strains are listed in Tables 1 and 2.
The act1-124 and act1-129 alleles also cause a random budding pattern (14). However, the act1-120 allele, which also causes a randomized budding pattern, did not have a major effect on INT1-induced filamentous growth (Table 2). Furthermore, INT1-induced filamentous growth was observed in strains carrying act1 alleles that exhibit either unipolar or bipolar budding patterns (Table 2). Thus, INT1-induced filamentous growth occurs independently of any specific bud site selection pattern.
Interestingly, act1-120 is a temperature-sensitive actin allele that also is required for cell elongation and invasion of the agar during pseudohyphal growth (14). Yet, the act1-120 mutation did not have an obvious effect on INT1-induced filamentous growth (Fig. 2 and Table 2). The act1-120 allele is of particular interest because, unlike act1-124 and act-129, the temperature-sensitive phenotype of act1-120 is suppressed by specific alleles of SAC6, which encodes the S. cerevisiae fimbrin homolog. This suggests that the actin domain that interacts with Sac6p/fimbrin is not critical for INT1-induced filamentous growth.
INT1-induced filamentous growth requires a subset of actin-interacting proteins.
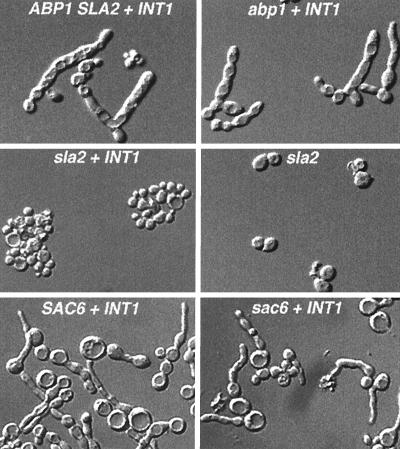
To determine which actin-interacting proteins are required for INT1-induced filamentous growth, we analyzed the ability of INT1 to generate highly polarized buds in strains with mutations in several actin-interacting proteins (Fig. 3; Table 3). Several of the mutants studied, including abp1::LEU2 and sac6::LEU2 mutants, had no discernable effect on INT1-induced filamentous growth. The observation that INT1-induced filamentous growth does not require SAC6 and that it occurs in the act1-120 strain is consistent with the idea that fimbrin and the interaction(s) between actin and fimbrin are not important for INT1-induced filamentous growth. Interestingly, the dfg9-100 allele of PEA2 (Table 3) which disrupts pseudohyphal growth (52) did not affect INT1-induced filamentous growth.
FIG. 3.
INT1-induced filamentous growth requires SLA2, but not ABP1 or SAC6. Shown are DIC micrographs of isogenic wild-type and mutant strains with the relevant genotype indicated. Strains are listed in Tables 1 and 3.
TABLE 3.
Some actin cortical patch mutations affect INT1-induced filamentous growth
| Strain name | Relevant genotypea | No. of INT1-induced filamentsb | Filament shapec |
|---|---|---|---|
| YJB2714 | Wild type | +++ | |
| YJB2715 | abp1Δ | +++ | wt |
| YJB3054 | Wild type | +++ | |
| YJB3053 | sac6Δ | +++ | |
| YJB3321 | Wild type | +++ | |
| YJB2520 | pea2/dfg9-100 | +++ | wt |
| YJB3329 | srv2-100 | + | sh |
| YJB3331 | bni1-100 | + | sh, shm |
| YJB2623 | Wild type | +++ | |
| YJB2622 | vrp1Δ | + | sh |
| YJB3057 | Wild type | +++ | |
| YJB3056 | rvs167Δ | ++ | sh |
| YJB3156 | wild type | ++++ | |
| YJB3155 | sla1-Δ1 | + | sh |
| YJB3156 | Wild type | +++ | |
| YJB3157 | sla2Δ | + | sh |
Complete genotypes of the strains are listed in Table 1.
Relative proportion of cells exhibiting INT1-induced filaments. In each case, the strain was compared with the appropriate isogenic or congenic wild-type strain listed in Table 1. +++, similar to wild type; ++, ∼25 to 75% of wild-type levels; +, less than 25% of wild-type levels of filament formation.
Appearance of filaments relative to how they appeared in the isogenic or congenic wild-type strains. wt, filaments were indistinguishable from wild-type filaments; sh, average filament length was shorter than in the relevant wild-type strain; shm, polarized cells appeared similar to mating projections (shmoos).
Importantly, there were several mutant strains in which INT1-induced filamentous growth was significantly reduced. Among this group of strains, the sla2/end4 strain had a dramatic effect on INT1-induced filamentous growth (Fig. 3). SLA2/END4 encodes a protein involved in membrane cytoskeleton assembly (38), the internalization phase of endocytosis (56, 68) and pseudohyphal growth (69). SLA2 was isolated, together with SLA1, in a screen for mutants synthetically lethal with a disruption allele of ABP1 (abp1::LEU2) (28). SLA2 was also isolated as END4, based on its role in endocytosis (60), and as MOP2, because it affects the accumulation and/or maintenance of plasma membrane H(+)-ATPase on the cell surface (57). SLA1 encodes a protein involved in the assembly of the cortical actin cytoskeleton (6, 28). Mutations in SLA1, as well as those in SRV2, VRP1, and BNI1, and to a lesser degree RVS167, all reduced the proportion of cells exhibiting INT1-induced filamentous growth and in many cases caused a significant reduction in the length of any INT1-induced filaments that were formed (Table 3). Thus, several components of the actin cortical patches contribute to efficient INT1-induced filamentous growth in S. cerevisiae.
Sla2p is specifically required for INT1 function in S. cerevisiae.
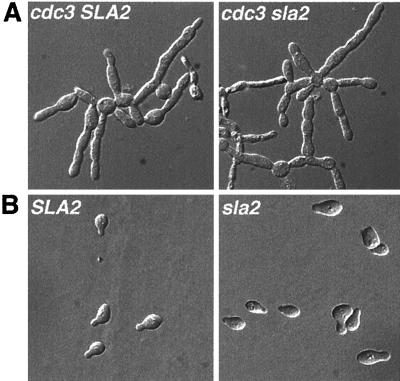
Because Sla2p is a component of actin cortical patches and because Sla2p is required for pseudohyphal growth (69), we asked if Sla2p is necessary for several types of polarized growth in S. cerevisiae. CDC3 encodes the Cdc3p septin protein found at the mother-bud neck. Loss of septin function causes the formation of cells with highly polarized buds (reviewed in reference 43) that are reminiscent of INT1-induced filamentous cells (Fig. 4A). We grew cdc3-6 and cdc3-6 sla2 strains and shifted them to 37°C for 12 h to determine if SLA2 is required for polarized growth. Interestingly, similar elongated buds were observed in both strains (Fig. 4A), indicating that SLA2 is not required for the generation of filamentous cells per se. Furthermore, this result demonstrates that mutation of SLA2 does not suppress a cdc3 mutation. In addition, we asked if Sla2p is required for the polarization of mating projections in response to mating pheromone. Consistent with a previous report (55), MATa sla2Δ strains form mating projections in response to α-factor treatment (Fig. 4B), indicating that they are not defective in the ability to polarize the actin cytoskeleton in response to some stimuli. Interestingly, like sla2 budding cells (55), the necks of the mating projections appeared to be wider than those in wild-type cells.
FIG. 4.
SLA2 is not required for all types of polarized growth. (A) DIC micrographs of strains carrying the cdc3-6 allele, which results in polarized growth in the presence (YJB5565; left) or absence (YJB5566; right) of SLA2. (B) DIC micrographs of MATa wild-type (YJB2489) and sla2Δ (YJB4786) strains exposed to 300 μg of α-factor/ml.
We also generated highly polarized cells by expressing high levels of SWE1, which encodes the kinase that phosphorylates Clb2/Cdc28 to execute the morphogenesis checkpoint (35), from the GAL1 promoter on plasmid pSWE1-19 (10). As seen with the cdc3-6 mutation, cells grown on galactose to induce SWE1 expression formed highly polarized buds, and the degrees of polarized growth were similar in SLA2 and sla2 strains (E. S. Bensen, data not shown). Thus, Sla2p is not required for all forms of polarized growth in S. cerevisiae and is not required for SWE1 to mediate a polarized growth response. Rather, Sla2p is required for certain types of polarized growth, such as filamentous growth, in response to INT1 expression and pseudohyphal growth in response to nutrient depletion (69), but Sla2p is not required for polarization of the cytoskeleton during early stages of bud growth or in response to pheromone stimulation during mating (Fig. 4B) (55).
SWE1 and SLA2 both contribute independently to INT1-induced filamentous growth.
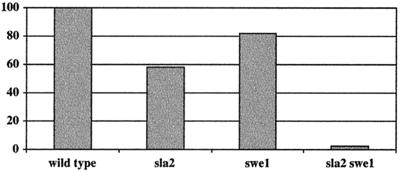
There are a number of mutations in S. cerevisiae that generate polarized buds by altering the progression of the mitotic cell cycle. In response to perturbations of the actin cytoskeleton, Swel kinase phosphorylates the Clb2/Cdc28p cyclin-dependent kinase, thereby preventing or delaying the normal transition from polarized growth to isotropic bud growth and delaying nuclear division (35, 37, 49). When stained with DAPI (4′,6′-diamidino-2-phenylindole), INT1-induced filamentous cells often have more than one nucleus (C. A. Gale, M. Gerami-Nejad, M. McClellan, M. S. Longtine, and J. Berman, submitted for publication), indicating that the nuclear cell cycle continues, albeit at a reduced rate in some genetic backgrounds. Since Sla2p is a component of actin cortical patches, we determined if INT1 expression activates the morphogenesis checkpoint by comparing INT1-induced filamentous growth in isogenic wild-type and swe1 null strains (Fig. 5). INT1-induced filamentous growth was reduced by 18% in a swe1Δ strain relative to growth in the wild-type strain, indicating that Swe1p, and presumably the morphogenesis checkpoint, contributes to, but is not absolutely required for, INT1-induced filamentous growth. In isogenic sla2 strains, INT1-induced filamentous growth was reduced by 42% relative to wild-type growth, suggesting that SLA2 makes an important contribution to, but is also not absolutely required for, this filamentous growth. We then asked if Sla2p is required to mediate INT1-induced filamentous growth through the SWE1-dependent pathway by comparing filamentous growth in isogenic sla2, swe1, or sla2 swe1 strains. If Sla2p activated INT1-induced filamentous growth only through a Swe1-dependent pathway, we would expect to find similar levels of INT1-induced filamentous growth in sla2 and sla2 swe1 mutant strains. In contrast to this expectation, we found that sla2 swe1 mutants displayed a much lower degree of INT1-induced filamentous growth (97.5% reduction) than did either the sla2 or swe1 mutant alone (Fig. 5). Thus, Swe1p and Sla2p do not mediate INT1-induced filamentous growth through a single, shared pathway. While these results do not rule out the possibility that Sla2p may trigger some INT1-induced filamentous growth by activating the morphogenesis checkpoint, it implies that Sla2p also acts in a Swe1p-independent manner to mediate filamentous growth in cells expressing INT1.
FIG. 5.
SLA2 and SWE1 contribute independently to INT1-induced filamentous growth. The percentage of INT1-induced filaments was determined by spreading cells onto plates containing 2% galactose and counting the cells producing filaments and the total number of cells on the plate 18 h after plating. Two isolates transformed with pGAL-INT1 were used for each experiment, and a minimum of 200 cells was counted for each strain. The wild-type strain produced 95% filamentous cells under these conditions.
The talin-like C terminus of Sla2p is not required for INT1-induced filamentous growth.
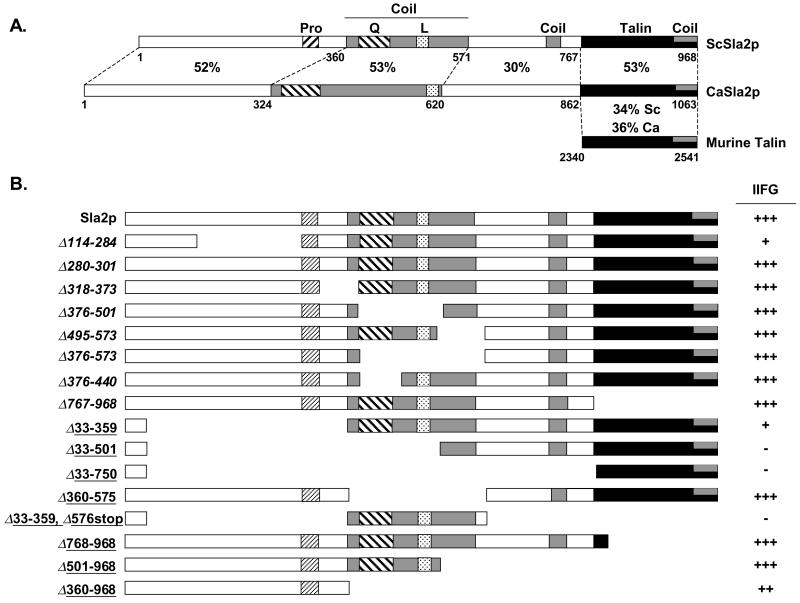
Sla2p is the S. cerevisiae protein most closely related to the actin-binding protein talin. Sla2p also shares significant similarity with the human Huntingtin interacting protein 1 (HIP1) and the Hip1-related protein (HIP1R). The C terminus of Sla2p is 45% similar to the C-terminal 197 residues of mouse talin (Fig. 6A) (46), a protein that mediates interactions between integrins at the cell surface and the actin cytoskeleton (30, 34, 54). The C-terminal I/LWEQ motif within the talin-like domain of Sla2p can bind to yeast or vertebrate F-actin in in vitro binding assays, cosediments with F-actin (46), and interacts with actin in two-hybrid assays (70). Because INT1 encodes a protein with limited similarity to vertebrate αX and αM integrins (23), the contribution of Sla2p to INT1-induced filamentous growth raised the intriguing possibility that the vertebrate paradigm of integrin-talin-actin interaction may hold for Int1p, Sla2p, and actin in budding yeasts. To test this possibility, we analyzed the domains of Sla2p that are required for INT1-induced filamentous growth using deletion alleles kindly provided by Wesp and coworkers (68) and by Yang and coworkers (70).
FIG. 6.
S. cerevisiae SLA2 and C. albicans SLA2 have a talin-like domain which is not required for Sla2p function in S. cerevisiae. (A) Comparison of ScSla2p, CaSla2p, and mouse talin domains. Amino acid positions of the domains illustrated are noted below the genes. Percent identity between domains of the proteins is indicated. Pro, proline-rich region; Q and L, glutamine- and leucine-rich regions within the long coil domain. Only the talin domain of murine talin has similarity to the Sla2 proteins. (B) Deletion analysis of ScSLA2 domains required for INT1-induced filamentous growth. Illustration of deletion alleles obtained from Reizman and colleagues (not underscored) and Drubin and colleagues (underscored). IIFG, INT1-induced filamentous growth determined using the scoring system described in Table 2.
Sla2p includes several distinct domains, including a 197-amino-acid (aa) coiled-coil domain required for homodimerization (70) and for an endocytic function that is redundant with the function(s) of Abp1p and Srv2p (68). A small region near the N terminus is required for endocytosis, growth at high temperature, and actin organization (Fig. 6A). Several conclusions can be reached from the analysis of INT1-induced filamentous growth in strains carrying deletion alleles of SLA2 (Fig. 6B). First, the same N-terminal domain that was required for other SLA2 functions was also an important contributor to INT1-induced filamentous growth (Fig. 6B, constructs Δ114-284 and Δ33-359). In addition, deletion of this N-terminal region together with deletion of the coiled-coil domain or the talin-like C terminus caused an almost complete loss of INT1-induced filamentous growth (Fig. 6B, Δ33-501 and Δ33-359+Δ576stop). Deletion of both the coil and the talin-like domains reduced INT1-induced filamentous growth to less than 50% of the wild-type level, but did not eliminate it completely (Fig. 6B, Δ360-968). Finally, the C-terminal talin-like domain alone was not necessary for INT1-induced filamentous growth (Fig. 6B, Δ767-968 and Δ768-968). The latter result is consistent with the previous work of others (68, 70) that indicated that the Sla2p talin-like domain is not required for any known Sla2p/End4p function, although the I/LWEQ boxes within the talin-like domain of Sla2p bind actin in vitro (46). While the Sla2p talin-like domain can interact with actin in vitro, it has been proposed that each Sla2p molecule may be involved in multiple protein-protein interactions with another molecule of Sla2p as well as with other cortical patch proteins (68, 71). Our results are consistent with the idea that multiple interactions with different actin patch components are partially redundant because deletion of any one of them is not sufficient to completely eliminate Sla2p function (68, 71). Thus, while the talin-like domain of Sla2p is not required for INT1-induced filamentous growth when other domains are intact, we cannot rule out the possibility that interaction(s) with the actin cortical patches are required for Sla2p to mediate INT1-induced filamentous growth. In this context, it is interesting that we detected two classes of cortical patch proteins: those that are required for optimal INT1-induced filamentous growth (e.g., Sla1p, Sla2p, Srv2p, and Rvs167p) and those that do not appear to contribute to INT1-induced filamentous growth (e.g., Abp1p and Sac6p).
C. albicans SLA2 can functionally complement an S. cerevisiae sla2Δ mutant.
C. albicans Sla2p (CaSla2p) is very similar to S. cerevisiae Sla2p (ScSla2p) across the entire length of the protein, including the essential N-terminal region, the coiled-coil domain (aa 376 to 573 in S. cerevisiae, aa 350 to 600 in C. albicans), and the talin-like domain (Fig. 6A). The C terminus of CaSla2p is even more similar to mouse talin (40.6% identity, 52.4% similarity) than ScSla2 is similar to mouse talin (37.6% identity, 47.9% similarity). ScSla2p and CaSla2p are also related to Sla2p's of other yeasts (50), talin proteins from Dictyostelium discoideum and Caenorhabditis elegans, human Hip1p, and other proteins that contain the highly conserved I/LWEQ boxes within the C termini (45).
To ask if CaSla2p executes the same functions as ScSla2p, we expressed CaSLA2 in an S. cerevisiae sla2 deletion strain and monitored growth at 37°C as well as the ability to form filamentous cells in response to INT1 expression. The sla2 strain expressing only vector sequences (YJB4686 plus pYES) was unable to grow at 37°C, while the same strain expressing pYES2-CaSLA2 (YJB4686 plus pYES-CaSLA2) was able to grow at 37°C (data not shown). In addition, the extents of INT1-induced filamentous growth (both the percentage of cells forming filaments and filament length) were similar in the Scsla2Δ strain expressing CaSLA2 (YJB4898) and an isogenic ScSLA2 strain (YJB4896) expressing CaSLA2 (Table 4). It should be noted that the CaSLA2 coding sequence includes one CUG codon (at aa 161), which encodes serine in C. albicans and leucine in all other organisms, and which does not appear to have an essential role in Sla2p function. Thus, ScSla2p and CaSla2p appear to have similar functions in S. cerevisiae, at least for growth at high temperature and in response to INT1 expression.
TABLE 4.
C. albicans SLA2 can complement S. cerevisiae sla2Δ for INT1-induced filamentous growth
| Strain name | Relevant genotype | % INT1-induced filamentous growtha |
|---|---|---|
| YJB4896 | SLA2 CaSLA2 | 100 |
| YJB4897 | sla2Δ | 47 |
| YJB4898 | sla2Δ CaSLA2 | 97 |
Data were normalized relative to INT1-induced filamentous growth in YJB4896.
CaSLA2 is required for filamentous growth in C. albicans.
C. albicans undergoes a more complex set of morphogenetic responses to the environment than does S. cerevisiae and we wanted to determine if CaSla2p is required for these responses. We sequentially generated disruption alleles of both copies of CaSla2p by insertion of the URA-blaster cassette (21) into the EcoRV site between codons 713 and 714 of CaSLA2, generating a protein lacking 351 C-terminal amino acids, including the 197-aa talin-like domain of the protein and all of the I/LWEQ boxes found between aa 865 and 1055. Several independent Casla2/CaSLA2 heterozygous strains were isolated and used to generate independent Casla2/Casla2 homozygous strains. Disruption of the gene was detected by PCR and confirmed with Southern blotting (data not shown). Two independent strains, YJB3612 and YJB3402, with disruptions only within both CaSLA2 alleles were chosen for continued study.
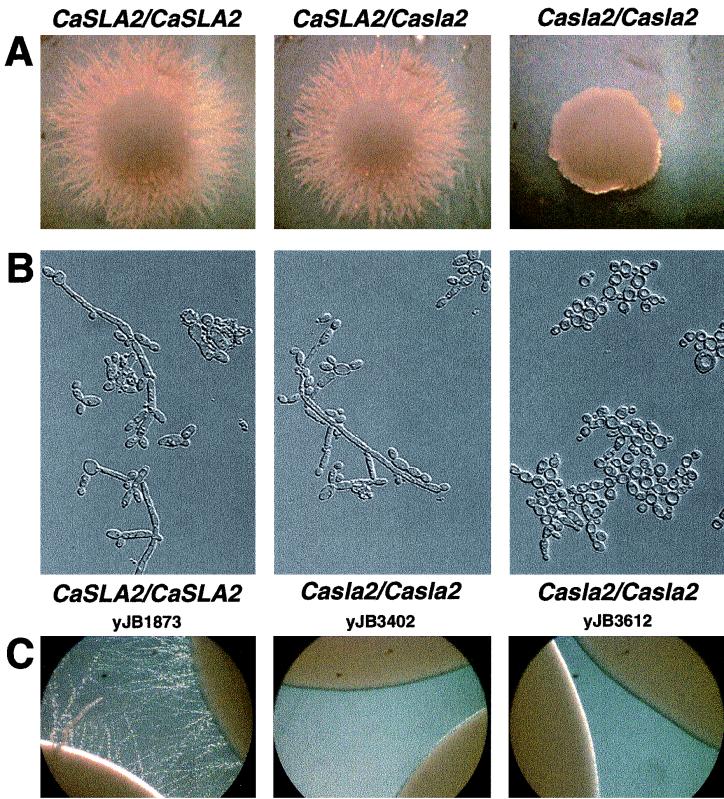
The growth and morphology of the heterozygous and homozygous strains were analyzed on different media that induce hyphal growth. On milk-Tween agar, wild-type strains produced lush filamentous growth emanating from the colony (Fig. 7A, left), as did both heterozygous CaSLA2/Casla2 strains (Fig. 7A, middle). In contrast, both of the homozygous Casla2/Casla2 disruption strains formed only smooth colonies (Fig. 7A, right). We also analyzed the effect of disruption of CaSLA2 on the morphology of individual cells grown in RPMI medium containing 20% fetal calf serum at 37°C. These conditions induced hyphal growth of wild-type cells (Fig. 7B, left panel), and hyphae were also evident in both heterozygous CaSLA2/Casla2 strains (Fig. 7B, middle panel). In contrast, the homozygous Casla2/Casla2 strains failed to form any true hyphae (cells with parallel side walls and perpendicular septa; Fig. 7B, right).
FIG. 7.
CaSLA2 is required for filamentous growth under several different hypha-inducing conditions. CaSLA2/CaSLA2 (YJB1873), CaSLA2/Casla2 (YJB3400), and Casla2/Casla2 (YJB3402) were grown on milk-Tween agar for 5 days (A), in RPMI medium–20% serum for 16 h at 37°C (B), or on YPD agar medium for 13 days (C). Similar results were observed with independently isolated yCA37 and YJB3612 heterozyogous and homozygous Casla2 strains. (C) Parental and two independent Casla2/Casla2 homozygote strains as indicated were photographed after 13 days of growth on YPAD at room temperature.
Immunoblot analysis of C. albicans Casla2/Casla2 strains revealed that disruption at the EcoRV site eliminated a band with an apparent molecular mass of ∼120 kDa and no new bands appeared in the gel (M. McClellan, unpublished data). There were no bands that appeared or displayed an obvious increase in intensity near the mobility expected (∼82 kDa) if the predicted truncation product was stable. Thus, the disruption of CaSla2p appears to have generated an unstable protein and the phenotypes of the Casla2/Casla2 strains are likely to be similar to the phenotypes of Casla2 null strains.
Many strains (including cph1/cph1 efg1/efg1 and ras1/ras1 strains) that are generally unable to form hyphae under strong induction conditions, such as serum at 37°C, still form filaments under microaerophilic or embedded agar conditions or incubation on YPD at room temperature (20, 65). This supports the currently favored model, which posits that different environmental stimuli trigger separate signal transduction pathways that converge on a common group of targets required for hyphal growth (19). We asked if Casla2/Casla2 strains would form filaments under these types of conditions as well. Even after 2 weeks on YPD, no filaments extended from colonies of either of the Casla2/Casla2 strains (Fig. 7C, middle and right). This result suggests that, in C. albicans, Sla2p is important for hyphal growth in response to both mild (e.g., milk-Tween agar and long incubation on YPD) and potent (serum at 37°C) environmental stimuli. In fact, in C. albicans, CaSla2p appears to be required for the formation of filaments under all hyphal induction conditions tested.
DISCUSSION
Contribution of the actin cytoskeleton to INT1-induced filamentous growth in S. cerevisiae.
We have used the heterologous expression of a C. albicans gene, INT1, in S. cerevisiae to study the contribution of the actin cytoskeleton to polarized growth. We found that actin patches appear normal in size and are often concentrated at the tips of the growing filaments. Furthermore, a small subset of actin mutations affects INT1-induced filamentous growth. Both actin alleles that disrupt INT1-induced filamentous growth confer temperature sensitivity at 37°C and can disrupt pseudohyphal growth when wild-type actin is also present (14). The act1-129 allele alters residues 177 and 179 in subdomain 3 of actin. These amino acids are predicted to stabilize actin-actin contacts, and mutations in them cause defects in actin-actin interactions in two-hybrid assays and can disrupt actin filament assembly in a dominant manner (3, 26, 44). These results also highlight differences in the genetic requirements for pseudohyphal growth and INT1-induced filamentous growth. For example, the act1-120 mutation, which perturbs interactions of actin with fimbrin (Sac6p) (3, 4, 27, 29) and is required for pseudohyphal growth (14), had very little effect on INT1-induced filamentous growth (Fig. 2). Consistent with this, sac6Δ strains that perturb pseudohyphal growth (14) did not perturb INT1-induced filamentous growth (Table 3). This result implies that interactions between actin and fimbrin are not important for INT1-induced filamentous growth.
In addition, we found that a subset of genes encoding cortical patch constituents is required for INT1-induced filamentous growth. These include SLA1, SLA2, and SRV2. In contrast, Abp1p and Sac6p, which colocalize to the cortical actin cytoskeleton together with Sla1p, Sla2p, and Srv2p (39), are not required for INT1-induced filamentous growth. Thus, INT1-induced filamentous growth is affected to different degrees by mutations in different proteins associated with the yeast cortical actin patches.
Because of its role in both pseudohyphal growth and INT1-induced filamentous growth, we asked if Sla2p is required for the execution of polarized bud growth under all circumstances. Interestingly, we found that SLA2 is not required for the formation of highly polarized buds in the absence of the Cdc3p septin or in the presence of excess Swe1p (35) and that Sla2p is not required for bud emergence or shmoo formation. Thus, in S. cerevisiae, Sla2p has a specific, rather than a general, role in executing polarized growth in response to specific signals.
The N-terminal domains of ScSla2p are essential for INT1 function.
Initially, we were intrigued by the relationship between Int1p, a protein with limited similarity to vertebrate integrins, and Sla2p, the S. cerevisiae protein most similar to vertebrate talins, because it raised the possibility that Sla2p might mediate interactions between Int1p and the actin cytoskeleton. The ScSla2p C-terminal talin-like domain binds actin in vitro and in two-hybrid assays (46, 70). However, by analyzing several sets of sla2 deletion strains for the ability to support Int1p-induced filamentation, we found that the talin-like C terminus of Sla2p, which is dispensable for all known Sla2p functions also, is not required for INT1-induced filamentous growth. Despite this finding, deletion of the talin-like domain enhanced the defects in INT1-induced filamentous growth seen when either the coiled-coil domain or the N-terminal essential domain were deleted (Fig. 6B). Thus, the talin-like domain may contribute to Sla2p function through actin interactions that are redundant with other interactions between different Sla2p domains and several different components of the cortical actin cytoskeleton (68, 70). Our data are consistent with the idea that the N-terminal domain of Sla2p is most important for function, that the central coil makes an important contribution, likely by facilitating the formation of homodimers (70), and that the talin-like domain makes only a minimal contribution to Sla2p function.
Perhaps Int1p, like Sla2p, interacts with Sla2p and/or other actin cytoskeleton components through more than one domain. For example, it is tempting to speculate that the coil domain of Sla2p and the predicted coiled-coil domains of Int1p (aa 347 to 363, 465 to 479, and 1512 to 1525) may interact. While we cannot rule out this possibility, two-hybrid experiments and several attempts at coprecipitation of Sla2p with Int1p failed to reveal strong evidence for direct, physical interactions between Sla2p and Int1p domains (E. Bensen and M. McClellan, unpublished results). Thus, we also must consider the alternative hypothesis that Int1p may stimulate filamentous growth in S. cerevisiae through indirect interactions with Sla2p. Given the large number of genetic and physical interactions between Sla2p and other cytoskeleton proteins, such as Pfy1p, Rvs167p, Sac6p, Abp1p, Ark1p, and actin (15, 27, 68–70), there are many proteins that might mediate an interaction between Int1p and Sla2p.
Role of SWE1 in INT1-induced filamentous growth.
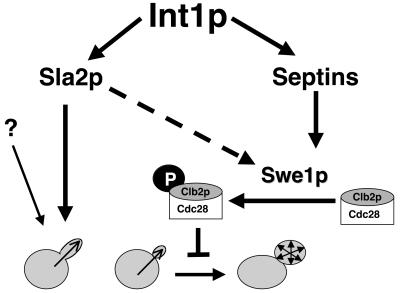
When the actin cytoskeleton is perturbed, for example, by cold shock or by treatment with Latrunculin A, the morphogenesis checkpoint is activated by the Swe1p kinase (49). Swe1p phosphorylates Clb2/Cdc28p, thereby preventing the switch to isotropic growth that normally occurs early in the cell cycle (64). Activation of the morphogenesis checkpoint causes cells to remain in the polarized growth state: mutations that trigger this checkpoint often result in highly polarized cells that resemble cells expressing INT1. Mutations that affect the septin ring also cause polarized growth in an Swe1-dependent manner (7, 48). Interestingly, the C terminus of Int1p has homology to S. cerevisiae Bud4p, which is localized to the septin rings at the mother-bud neck. In S. cerevisiae, Int1p colocalizes with septins (Gale et al., submitted), suggesting that INT1-induced filamentous growth might be dependent upon SWE1. While SWE1 contributes to INT1-induced filamentous growth, it is not absolutely required for it (Fig. 5 and 8). While our epistasis analysis indicates that Sla2p and Swe1p clearly make independent contributions to INT1-induced filamentous growth, we cannot rule out the possibility that Sla2p also mediates filamentous growth through a Swe1-dependent mechanism (Fig. 8, dashed arrow). Furthermore, there is a small amount of residual filamentous growth that occurs in sla2 swe1 cells expressing INT1, suggesting that there is a third, as-yet-uncharacterized mechanism that mediates filamentous growth in S. cerevisiae cells expressing INT1 (Fig. 8, question mark).
FIG. 8.
Int1p triggers filamentous growth through at least two pathways. Sla2p and Swe1p contribute independently to polarized growth in S. cerevisiae cells expressing INT1. Swe1p may also trigger the morphogenesis checkpoint via Swe1p.
CaSLA2 is required for hyphal growth under potent inducing conditions.
ScSla2p and CaSla2p have related structures, as revealed by the conservation of sequence motifs within the domains identified as important for Sla2p function in S. cerevisiae (50). Furthermore, expression of CaSLA2 in an S. cerevisiae sla2Δ strain restored growth at high temperature and restored INT1-induced filamentous growth, indicating functional similarity for the N-terminal domains of both proteins. Yet, the Casla2 disruption allele we used disrupted the gene ∼160 codons upstream of the Sla2p talin-like domain. Western analysis of proteins expressed in these mutants, using anti-ScSla2p antibodies (kindly provided by Drubin and coworkers), suggested that the C-terminally truncated protein, which lacks the talin domain, is unstable in these strains. Thus, the phenotypes observed in the Casla2/Casla2 strains most likely are due to loss of Sla2p function. This is different from what was seen in S. cerevisiae cells, where Sla2p that lacks the talin domain remains stable and retains most Sla2p functions.
In C. albicans, many different genes are required for hyphal growth in response to environmental stimuli, such as serum, or in response to nutrient deprivation. However, many of the genes that are required for filamentous growth on milk-Tween or Spider agar (e.g., CPH1 and INT1) are dispensable for filamentous growth at 37°C in serum (22, 40). Furthermore, genes such as EFG1 and RAS1, which are required for filamentous growth in response to serum, are not required for filamentous growth under other conditions (20, 65). Even triple-mutation strains lacking TUP1, EFG1, and CPH1 exhibit filamentous growth under some conditions (12, 61), suggesting that there are additional, as-yet-uncharacterized filamentous growth pathways in C. albicans (12). This is thought to be due to the activation of multiple independent filamentous growth signals under different sets of hypha-inducing conditions. In contrast to this, CaSLA2 appears to be required for hyphal growth in response to both nutrient deprivation or serum induction, suggesting that Sla2p may be absolutely required for hyphal growth in C. albicans.
ACKNOWLEDGMENTS
We thank David Drubin, Howard Reizman, Gerry Fink, David Botstein, Alison Adams, Anita Hopper, and Mark Longtine for providing strains and/or plasmids. We thank John Asleson and Mark McClellan for excellent technical assistance. We also thank Jaime Cope and Mark Longtine for helpful discussions.
This work was supported by Burroughs Wellcome Scholar Award no. 0677 to J. B., NIH grant T32-AI 07421 to C.M.A. and E.S.B., NIH Child Health Research Center grant P30 HD33692 to C.A.G., and European Union Programme BIOMED grant no. BMH4-96-0310 to C.K.
REFERENCES
- 1.Adams A E, Pringle J R. Relationship of actin and tubulin distribution to bud growth in wild-type and morphogenetic-mutant Saccharomyces cerevisiae. J Cell Biol. 1984;98:934–945. doi: 10.1083/jcb.98.3.934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adams A E, Pringle J R. Staining of actin with fluorochrome-conjugated phalloidin. Methods Enzymol. 1991;194:729–731. doi: 10.1016/0076-6879(91)94054-g. [DOI] [PubMed] [Google Scholar]
- 3.Amberg D C, Basart E, Botstein D. Defining protein interactions with yeast actin in vivo. Nat Struct Biol. 1995;2:28–35. doi: 10.1038/nsb0195-28. [DOI] [PubMed] [Google Scholar]
- 4.Amberg D C, Zahner J E, Mulholland J W, Pringle J R, Botstein D. Aip3p/Bud6p, a yeast actin-interacting protein that is involved in morphogenesis and the selection of bipolar budding sites. Mol Biol Cell. 1997;8:729–753. doi: 10.1091/mbc.8.4.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anderson J M, Soll D R. Differences in actin localization during bud and hypha formation in the yeast Candida albicans. J Gen Microbiol. 1986;132:2035–2047. doi: 10.1099/00221287-132-7-2035. [DOI] [PubMed] [Google Scholar]
- 6.Ayscough K R, Eby J J, Lila T, Dewar H, Kozminski K G, Drubin D G. Sla1p is a functionally modular component of the yeast cortical actin cytoskeleton required for correct localization of both Rho1p-GTPase and Sla2p, a protein with talin homology. Mol Biol Cell. 1999;10:1061–1075. doi: 10.1091/mbc.10.4.1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bailey D A, Feldmann P J, Bovey M, Gow N A, Brown A J. The Candida albicans HYR1 gene, which is activated in response to hyphal development, belongs to a gene family encoding yeast cell wall proteins. J Bacteriol. 1996;178:5353–5360. doi: 10.1128/jb.178.18.5353-5360.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barral Y, Parra M, Bidlingmaier S, Snyder M. Nim1-related kinases coordinate cell cycle progression with the organization of the peripheral cytoskeleton in yeast. Genes Dev. 1999;13:176–187. doi: 10.1101/gad.13.2.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bi E, Pringle J R. ZDS1 and ZDS2, genes whose products may regulate Cdc42p in Saccharomyces cerevisiae. Mol Cell Biol. 1996;16:5264–5275. doi: 10.1128/mcb.16.10.5264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Booher R N, Deshaies R J, Kirschner M W. Properties of Saccharomyces cerevisiae wee1 and its differential regulation of p34CDC28 in response to G1 and G2 cyclins. EMBO J. 1993;12:3417–3426. doi: 10.1002/j.1460-2075.1993.tb06016.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Botstein D, Amberg D, Mulholland J, Huffaker T, Adams A, Drubin D, Stearns T. The yeast cytoskeleton. In: Pringle J R, Broach J R, Jones E W, editors. The molecular and cellular biology of the yeast Saccharomyces. Plainview, N.Y: CSHL Press; 1997. pp. 1–90. [Google Scholar]
- 12.Braun B R, Johnson A D. TUP1, CPH1 and EFG1 make independent contributions to filamentation in Candida albicans. Genetics. 2000;155:57–67. doi: 10.1093/genetics/155.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brown A J, Gow N A. Regulatory networks controlling Candida albicans morphogenesis. Trends Microbiol. 1999;7:333–338. doi: 10.1016/s0966-842x(99)01556-5. [DOI] [PubMed] [Google Scholar]
- 14.Cali B M, Doyle T C, Botstein D, Fink G R. Multiple functions for actin during filamentous growth of Saccharomyces cerevisiae. Mol Biol Cell. 1998;9:1873–1889. doi: 10.1091/mbc.9.7.1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cope M J, Yang S, Shang C, Drubin D G. Novel protein kinases Ark1p and Prk1p associate with and regulate the cortical actin cytoskeleton in budding yeast. J Cell Biol. 1999;144:1203–1218. doi: 10.1083/jcb.144.6.1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Corner B E, Magee P T. Candida pathogenesis: unraveling the threads of infection. Curr Biol. 1997;7:R691–R694. doi: 10.1016/s0960-9822(06)00357-5. [DOI] [PubMed] [Google Scholar]
- 17.Cross F R. ‘Marker swap’ plasmids: convenient tools for budding yeast molecular genetics. Yeast. 1997;13:647–653. doi: 10.1002/(SICI)1097-0061(19970615)13:7<647::AID-YEA115>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 18.Drubin D G, Nelson W J. Origins of cell polarity. Cell. 1996;84:335–344. doi: 10.1016/s0092-8674(00)81278-7. [DOI] [PubMed] [Google Scholar]
- 19.Ernst J. Transcription factors in Candida albicans-environmental control of morphogenesis. Microbiology. 2000;146:1763–1774. doi: 10.1099/00221287-146-8-1763. [DOI] [PubMed] [Google Scholar]
- 20.Feng Q, Summers E, Guo B, Fink G. Ras signaling is required for serum-induced hyphal differentiation in Candida albicans. J Bacteriol. 1999;181:6339–6346. doi: 10.1128/jb.181.20.6339-6346.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fonzi W A, Irwin M Y. Isogenic strain construction and gene mapping in Candida albicans. Genetics. 1993;134:717–728. doi: 10.1093/genetics/134.3.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gale C, Bendel C, McClellan M, Hauser M, Becker J M, Berman J, Hostetter M. Linkage of adhesion, filamentous growth, and virulence in Candida albicans to a single gene, INT1. Science. 1998;279:1355–1358. doi: 10.1126/science.279.5355.1355. [DOI] [PubMed] [Google Scholar]
- 23.Gale C, Finkel D, Tao N, Meinke M, McCleland M, Olson J, Kendrick K, Hostetter M. Cloning and expression of a gene encoding an integrin-like protein in Candida albicans. Proc Natl Acad Sci USA. 1996;93:357–361. doi: 10.1073/pnas.93.1.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gietz R D, Schiestl R H. Transforming yeast with DNA. Methods Mol Cell Biol. 1995;5:255–269. [Google Scholar]
- 25.Gietz R D, Schiestl R H, Willems A R, Woods R A. Studies on the transformation of intact yeast cells by the LiAc/SS-DNA/PEG procedure. Yeast. 1995;11:355–360. doi: 10.1002/yea.320110408. [DOI] [PubMed] [Google Scholar]
- 26.Holmes K C, Popp D, Gebhard W, Kabsch W. Atomic model of the actin filament. Nature. 1990;347:44–49. doi: 10.1038/347044a0. [DOI] [PubMed] [Google Scholar]
- 27.Holtzman D A, Wertman K F, Drubin D G. Mapping actin surfaces required for functional interactions in vivo. J Cell Biol. 1994;126:423–432. doi: 10.1083/jcb.126.2.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Holtzman D A, Yang S, Drubin D G. Synthetic-lethal interactions identify two novel genes, SLA1 and SLA2, that control membrane cytoskeleton assembly in Saccharomyces cerevisiae. J Cell Biol. 1993;122:635–644. doi: 10.1083/jcb.122.3.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Honts J E, Sandrock T S, Brower S M, O'Dell J L, Adams A E. Actin mutations that show suppression with fimbrin mutations identify a likely fimbrin-binding site on actin. J Cell Biol. 1994;126:413–422. doi: 10.1083/jcb.126.2.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Horwitz A, Duggan K, Buck C, Beckerle M C, Burridge K. Interaction of plasma membrane fibronectin receptor with talin—a transmembrane linkage. Nature. 1986;320:531–533. doi: 10.1038/320531a0. [DOI] [PubMed] [Google Scholar]
- 31.Hoyer L L, Scherer S, Shatzman A R, Livi G P. Candida albicans ALS1: domains related to a Saccharomyces cerevisiae sexual agglutinin separated by a repeating motif. Mol Microbiol. 1995;15:39–54. doi: 10.1111/j.1365-2958.1995.tb02219.x. [DOI] [PubMed] [Google Scholar]
- 32.Hynes R O. Integrins: versatility, modulation, and signaling in cell adhesion. Cell. 1992;69:11–25. doi: 10.1016/0092-8674(92)90115-s. [DOI] [PubMed] [Google Scholar]
- 33.Johnston M, Davis R. Sequences that regulate the divergent GAL1-GAL10 promoter in Saccharomyces cerevisiae. Mol Cell Biol. 1984;4:1440–1448. doi: 10.1128/mcb.4.8.1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kaufmann S, Piekenbrock T, Goldmann W H, Barmann M, Isenberg G. Talin binds to actin and promotes filament nucleation. FEBS Lett. 1991;284:187–191. doi: 10.1016/0014-5793(91)80681-r. [DOI] [PubMed] [Google Scholar]
- 35.Lew D J, Reed S I. A cell cycle checkpoint monitors cell morphogenesis in budding yeast. J Cell Biol. 1995;129:739–749. doi: 10.1083/jcb.129.3.739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lew D J, Reed S I. Cell cycle control of morphogenesis in budding yeast. Curr Opin Genet Dev. 1995;5:17–23. doi: 10.1016/s0959-437x(95)90048-9. [DOI] [PubMed] [Google Scholar]
- 37.Lew D J, Reed S I. Morphogenesis in the yeast cell cycle: regulation by Cdc28 and cyclins. J Cell Biol. 1993;120:1305–1320. doi: 10.1083/jcb.120.6.1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li R, Zheng Y, Drubin D G. Regulation of cortical actin cytoskeleton assembly during polarized cell growth in budding yeast. J Cell Biol. 1995;128:599–615. doi: 10.1083/jcb.128.4.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lila T, Drubin D G. Evidence for physical and functional interactions among two Saccharomyces cerevisiae SH3 domain proteins, an adenylyl cyclase-associated protein and the actin cytoskeleton. Mol Biol Cell. 1997;8:367–385. doi: 10.1091/mbc.8.2.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu H, Köhler J, Fink G R. Suppression of hyphal formation in Candida albicans by mutation of a STE12 homolog. Science. 1994;266:1723–1726. doi: 10.1126/science.7992058. [DOI] [PubMed] [Google Scholar]
- 41.Liu H, Styles C A, Fink G R. Saccharomyces cerevisiae S288C has a mutation in FLO8, a gene required for filamentous growth. Genetics. 1996;144:967–978. doi: 10.1093/genetics/144.3.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lo H J, Kohler J R, DiDomenico B, Loebenberg D, Cacciapuoti A, Fink G R. Nonfilamentous C. albicans mutants are avirulent. Cell. 1997;90:939–949. doi: 10.1016/s0092-8674(00)80358-x. [DOI] [PubMed] [Google Scholar]
- 43.Longtine M S, DeMarini D J, Valencik M L, Al-Awar O S, Fares H, DeVirgilio C, Pringle J R. The septins: role in cytokinesis and other processes. Curr Opin Cell Biol. 1996;8:106–119. doi: 10.1016/s0955-0674(96)80054-8. [DOI] [PubMed] [Google Scholar]
- 44.Lorenz M, Popp D, Holmes K C. Refinement of the F-actin model against X-ray fiber diffraction data by the use of a directed mutation algorithm. J Mol Biol. 1993;234:826–836. doi: 10.1006/jmbi.1993.1628. [DOI] [PubMed] [Google Scholar]
- 45.McCann R O, Craig S W. Functional genomic analysis reveals the utility of the I/L WEQ module as a predictor of protein:actin interaction. Biochem Biophys Res Commun. 1999;266:135–140. doi: 10.1006/bbrc.1999.1776. [DOI] [PubMed] [Google Scholar]
- 46.McCann R O, Craig S W. The I/L WEQ module: a conserved sequence that signifies F-actin binding in functionally diverse proteins from yeast to mammals. Proc Natl Acad Sci USA. 1997;94:5679–5684. doi: 10.1073/pnas.94.11.5679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McCreath K J, Specht C A, Robbins P W. Molecular cloning and characterization of chitinase genes from Candida albicans. Proc Natl Acad Sci USA. 1995;92:2544–2548. doi: 10.1073/pnas.92.7.2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.McMillan J N, Longtine M S, Sia R A, Theesfeld C L, Bardes E S, Pringle J R, Lew D J. The morphogenesis checkpoint in Saccharomyces cerevisiae: cell cycle control of Swe1p degradation by Hsl1p and Hsl7p. Mol Cell Biol. 1999;19:6929–6939. doi: 10.1128/mcb.19.10.6929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.McMillan J N, Sia R A L, Lew D J. A morphogenesis checkpoint monitors the actin cytoskeleton in yeast. J Cell Biol. 1998;142:1487–1499. doi: 10.1083/jcb.142.6.1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Melms A S, Gausmann U, Swoboda R K, Dominguez A, Kurischko C. Sequence analysis of SLA2 of the dimorphic yeasts Candida albicans and Yarrowia lipolytica. Yeast. 1999;15:1519–1528. doi: 10.1002/(SICI)1097-0061(199910)15:14<1519::AID-YEA475>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 51.Mitchell A P. Dimorphism and virulence in Candida albicans. Curr Opin Microbiol. 1998;1:687–692. doi: 10.1016/s1369-5274(98)80116-1. [DOI] [PubMed] [Google Scholar]
- 52.Mosch H U, Fink G R. Dissection of filamentous growth by transposon mutagenesis in Saccharomyces cerevisiae. Genetics. 1997;145:671–684. doi: 10.1093/genetics/145.3.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mosch H U, Roberts R L, Fink G R. Ras2 signals via the Cdc42/Ste20/mitogen-activated protein kinase module to induce filamentous growth in Saccharomyces cerevisiae. Proc Natl Acad Sci USA. 1996;93:5352–5356. doi: 10.1073/pnas.93.11.5352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Muguruma M, Matsumura S, Fukazawa T. Direct interactions between talin and actin. Biochem Biophys Res Commun. 1990;171:1217–1223. doi: 10.1016/0006-291x(90)90815-5. [DOI] [PubMed] [Google Scholar]
- 55.Mulholland J, Wesp A, Riezman H, Botstein D. Yeast actin cytoskeleton mutants accumulate a new class of Golgi-derived secretary vesicle. Mol Biol Cell. 1997;8:1481–1499. doi: 10.1091/mbc.8.8.1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Munn A L, Riezman H. Endocytosis is required for the growth of vacuolar H(+)-ATPase-defective yeast: identification of six new END genes. J Cell Biol. 1994;127:373–386. doi: 10.1083/jcb.127.2.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Na S, Hincapie M, McCusker J H, Haber J E. MOP2 (SLA2) affects the abundance of the plasma membrane H(+)-ATPase of Saccharomyces cerevisiae. J Biol Chem. 1995;270:6815–6823. doi: 10.1074/jbc.270.12.6815. [DOI] [PubMed] [Google Scholar]
- 58.Odds F C. Candida and candidosis. 2nd ed. London, United Kingdom: Baillière Tindall; 1988. [Google Scholar]
- 59.Pfaller M A. Epidemiology of candidiasis. J Hosp Infect. 1995;30(Suppl.):329–338. doi: 10.1016/0195-6701(95)90036-5. [DOI] [PubMed] [Google Scholar]
- 60.Raths S, Rohrer J, Crausaz F, Riezman H. end3 and end4: two mutants defective in receptor-mediated and fluid-phase endocytosis in Saccharomyces cerevisiae. J Cell Biol. 1993;120:55–65. doi: 10.1083/jcb.120.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Riggle P J, Andrutis K A, Chen X, Tzipori S R, Kumamoto C A. Invasive lesions containing filamentous forms produced by a Candida albicans mutant that is defective in filamentous growth in culture. Infect Immun. 1999;67:3649–3652. doi: 10.1128/iai.67.7.3649-3652.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sharkey L L, McNemar M D, Saporito-Irwin S M, Sypherd P S, Fonzi W A. HWP1 functions in the morphological development of Candida albicans downstream of EFG1, TUP1, and RBF1. J Bacteriol. 1999;181:5273–5279. doi: 10.1128/jb.181.17.5273-5279.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sherman F, Fink G R, Hicks J B. Laboratory course manual for methods in yeast genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1986. [Google Scholar]
- 64.Sia R A, Herald H A, Lew D J. Cdc28 tyrosine phosphorylation and the morphogenesis checkpoint in budding yeast. Mol Biol Cell. 1996;7:1657–1666. doi: 10.1091/mbc.7.11.1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sonneborn A, Bockmuhl D P, Ernst J F. Chlamydospore formation in Candida albicans requires the Efg1p morphogenetic regulator. Infect Immun. 1999;67:5514–5517. doi: 10.1128/iai.67.10.5514-5517.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Staab J F, Ferrer C A, Sundstrom P. Developmental expression of a tandemly repeated, proline- and glutamine-rich amino acid motif on hyphal surfaces on Candida albicans. J Biol Chem. 1996;271:6298–6305. doi: 10.1074/jbc.271.11.6298. [DOI] [PubMed] [Google Scholar]
- 67.Wertman K F, Drubin D G, Botstein D. Systematic mutational analysis of the yeast ACT1 gene. Genetics. 1992;132:337–350. doi: 10.1093/genetics/132.2.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wesp A, Hicke L, Palecek J, Lombardi R, Aust T, Munn A L, Riezman H. End4p/Sla2p interacts with actin-associated proteins for endocytosis in Saccharomyces cerevisiae. Mol Biol Cell. 1997;8:2291–2306. doi: 10.1091/mbc.8.11.2291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yang S, Ayscough K R, Drubin D G. A role for the actin cytoskeleton of Saccharomyces cerevisiae in bipolar bud-site selection. J Cell Biol. 1997;136:111–123. doi: 10.1083/jcb.136.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yang S, Cope M J, Drubin D G. Sla2p is associated with the yeast cortical actin cytoskeleton via redundant localization signals. Mol Biol Cell. 1999;10:2265–2283. doi: 10.1091/mbc.10.7.2265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yang Y, Bauer C, Strasser G, Wollman R, Julien J P, Fuchs E. Integrators of the cytoskeleton that stabilize microtubules. Cell. 1999;98:229–238. doi: 10.1016/s0092-8674(00)81017-x. [DOI] [PubMed] [Google Scholar]
- 72.Zoladek T, Vaduva G, Hunter L A, Boguta M, Go B D, Martin N C, Hopper A K. Mutations altering the mitochondrial-cytoplasmic distribution of Mod5p implicate the actin cytoskeleton and mRNA 3′ ends and/or protein synthesis in mitochondrial delivery. Mol Cell Biol. 1995;15:6884–6894. doi: 10.1128/mcb.15.12.6884. [DOI] [PMC free article] [PubMed] [Google Scholar]