Abstract
Corrosion inhibition performance of two synthesized Schiff base ligands; (E)-2-((2-methoxybenzylidene)amino)phenol L1 and (E)-2-((4-methoxybenzylidene)amino)phenol L2 were carried out by weight loss measurement in 0.1 M hydrochloric acid (HCl) solution. Density Functional Theory (DFT) and Molecular dynamics (MD) simulation were applied to theoretically explain the inhibitors’ intrinsic properties and adsorption mechanism in the corrosion study. The result of the inhibition performances carried out at varying concentrations and temperatures were compared. The corrosion inhibition efficiencies of L1 and L2 at an optimal concentration of 10 × 10–4 M were 75% and 76%. Langmuir isotherm model fits the data obtained from the experiment with a correlation coefficient (R2) value closer to unity. The adsorption mechanism of inhibitor on the surface of the Fe metal occurred via chemisorption inferred from the Gibbs free energy (ΔGads). Scanning electron microscopy showed a mild degradation on the surface of the mild steel immersed in the L1, and L2 inhibited acid solution, which could be due to surface coverage. The energy dispersive X-ray spectroscopy showed the metal surface’s elemental composition and the existence of the chlorine peak, which emanates from the HCl medium. DFT calculations revealed that the hybrid B3LYP functional performed better than the M06-2X meta-functional in estimating the energies of the synthesized Schiff bases for corrosion inhibition as seen in the lower ΔE values of 3.86 eV and 3.81 eV for L1 and L2. The MD simulation revealed that the orientation of inhibitors on the surface of the metal resulted in the coordination bond formation and that the interaction energy of L2 was −746.84 kJ/mol compared to −743.74 kJ/mol of L1. The DFT and MD results agreed with the observed trend of the experimental findings.
Subject terms: Computational models, Chemistry
Introduction
Iron-based materials and low-alloyed metals are useful in several industrial applications and, they play important roles due to their structural and mechanical strength1–3. Mild steel is a type of ferrous alloy that has been used extensively in various industrial applications such as in the fabrication of cooling systems, pipes, and space vehicles4,5. These metals and alloys become corroded due to the application of mineral acids6–8. Corrosion inhibition of mild steel using organic compounds, including natural products9,10, synthetic and some inorganic compounds7,11 has attracted much interest in recent years. These compounds serve as corrosion inhibitors because of the presence of heteroatoms such as nitrogen, oxygen, sulphur and π-electrons that promote adsorption on the metal surfaces thereby minimizing the deterioration of metals and their alloys in an acid medium 2. Schiff bases have been reported to be excellent corrosion inhibitors of corrosion due to the presence of the imine group that coordinates with the metal ions3. The inhibition efficiency of any given Schiff base ligand depends primarily on the type of P-orbitals, orbitals of the metal ions, and the acid medium12,13. The structural and electronic properties of compounds are important in the corrosion inhibition mechanism. Therefore, to comprehend the mechanism of inhibition between the surface of the metal and the inhibitor, Monte Carlo Simulation, molecular dynamics and quantum mechanics calculations are expedient14. Many accounts regarding Schiff bases as corrosion inhibitors have been reported, however, scanty work exists on the corrosion inhibition effect of ortho and para-directing groups in compounds as corrosion inhibitors. Therefore, this work reports the synthesis, and corrosion inhibition effects of ortho and para-substituted (E)-2-(methoxybenzylidene)amino)phenol Schiff base ligands on mild steel in 0.1 M HCl. In this study, the gravimetric (weight loss) method was adopted and scanning electron microscopy (SEM) and energy dispersive X-ray (EDX) spectroscopy were applied to analyse the metal surface. Density Functional Theory (DFT), Monte Carlo and molecular dynamics Simulation was used to understand the adsorptive capacity and mechanism inhibitor and metal ion interactions.
Experimental methods
Materials and methods
2-aminophenol, 2-methoxybenzaldehyde, and 4-methoxybenzaldehyde were purchased from Sigma-Aldrich and used without further purification. The 1H- and 13C-NMR spectra were acquired in CDCl3 at room temperature using a Bruker 400 MHz spectrometer, and their data were recorded in CDCl3, with a residual internal solvent signal of 7.26 and 77.00 ppm, respectively. On a Perkin Elmer FTIR spectrometer equipped with universal ATR, FT-IR spectra reported in wavenumbers (cm−1) were obtained. Mass spectra of the compounds were obtained from a Water synaptic GR electrospray positive spectrometer. The electronic absorption spectra were obtained using a UV-3600 Shimadzu UV–VIS-NIR spectrophotometer. Thermo Scientific FLASH 20 0 0 CHNS/O Analyzers were used for Elemental analyses.
General synthesis of the Schiff base ligands
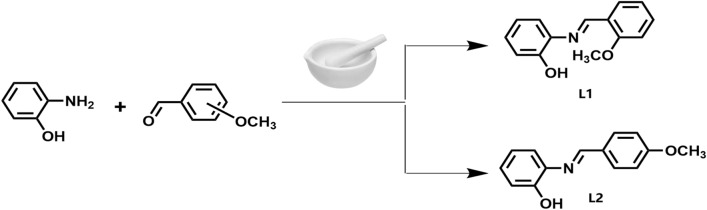
The Schiff base ligands (E)-2-((2-methoxybenzylidene)amino)phenol L1 and (E)-2-((4-methoxybenzylidene)amino)phenol L2 were obtained in excellent yields (97–98%) by solvent-free grinding using a mortar and pestle (Fig. 1) according to commonly described protocol in literature 15,16. The resultant products were dried in vacuo to completely remove the water and characterized spectroscopically using IR, NMR, and UV–Vis.
Figure 1.
Synthetic procedure for Schiff base ligands.
Synthesis of L1
2-Aminophenol (1 mmol, 0.109 g) was ground with 2-methoxybenzaldehyde (1 mmol, 0.136 g) for 10–12 min to obtain (E)-2-((2-methoxybenzylidene)amino)phenol L1 as a yellow solid. Yield (3.36 g, 97%), M.Pt.: 100–102 °C. 1H-NMR (CDCl3 400 MHz) δ (ppm) 3.95 (s, 3H), 6.92 (t, J = 7.66 Hz, 1H), 7.01 (dd, J = 8.24, 12.37 Hz, 2H), 7.08 (t, J = 7.56 Hz, 1H), 7.20 (t, J = 7.84 Hz, 1H), 7.35 (d, J = 7.92 Hz, 1H), 7.49 (t, J = 7.82 Hz, 1H), 8.18 (d, J = 7.72 Hz, 1H), 9.21 (s, 1H). 13C-NMR (CDCl3 400 MHz) δ (ppm) 153.1 (CH), 133.0 (C), 128.5 (C), 127.4 (C). 120.09 (CH), 120.0 (CH), 116.1 (CH), 114.8 (CH), 111.3 (CH), 55.6 (CH3). FT-IR (KBr, cm−1): 3329 ѵ (O–H), 2933 ѵ (sp3 C–H), 1587 ѵ (CH=N). MS-TOF m/z (%): 284.06 [M + H]+. Anal. Calcd. for C14H13NO2: C, 73.99; H 5.77; N 6.16%. Found; C, 73.80; H 5.59; N 6.06.
Synthesis of L2
2-Aminophenol (1 mmol, 0.109 g) was mixed with 2-methoxybenzaldehyde (1 mmol, 0.136 g) to obtain (E)-2-((4-methoxybenzylidene)amino)phenol L2 as a yellow solid. Yield (3.36 g, 98%), M.pt.: 83–85 °C. 1H-NMR (CDCl3 400 MHz) δ (ppm) 3.91 (s, 3H), 6.92 (t, J = 7.64 Hz, 1H), 7.02 (d, J = 8.68 Hz, 2H), 7.03 (d, J = 6.28 Hz, 1H), 7.22 (t, 1H), 7.29 (d, 1H), 7.49 (t, J = 7.82 Hz, 1H), 7.90 (d, J = 8.71 Hz, 2H), 8.65 (s, 1H). 13C-NMR (CDCl3 400 MHz) δ (ppm) 162.5 (CH), 156.6 (C), 152.1 (C), 135.9 (C), 130.6 (C), 128.9 (CH), 128.3 (CH), 120.1 (CH), 115.8 (CH), 114.8 (CH), 114.3 (CH), 55.5 (CH3). FT-IR (KBr, cm−1): 3338 ѵ (O–H), 2836 ѵ (sp3 C –H), 1589 ѵ (CH=N). MS-TOF m/z (%): 284.06 [M + H]+. Anal. Calcd. for C14H13NO2: C, 73.99; H 5.77; N 6.16%. Found; C, 73.85; H 5.55; N 6.10.
Metal and tests solution preparation
The carbon steel used in this corrosion study was procured commercially and cut to 4.0 cm × 3.0 cm × 0.7 cm in dimensions. The sample weight (%) composition is 0.17% C, 0.26% Si, 0.46% Mn, 0.017% S, 0.019% P, and the remaining Fe. The samples were abraded with emery papers of varying grades (grade 80–2000), degreased with ethanol and acetone to clean the surface of dust particles and stains, then rinsed with distilled water and further dried at room temperature and stored in moisture-free desiccators prior to the weight loss experiment. The test solution of 0.1 M HCl was prepared with distilled water by adequate dilution of analytical grade 37% HCl (from MERCK) with distilled water.
Weight loss measurement
The prepared mild steel samples were weighed accurately in triplicate with a sensitivity of ± 0.1 mg then immersed in the corrosive solution of (100 mL, 0.1 M HCl) blank and at different concentrations of the Schiff base inhibitors kept at 303 K, 333 K and 363 K respectively in a regulated thermostat water bath for 3 h. Afterwards, the samples were removed from the acidic solution, thoroughly washed with distilled water, dried in a vacuum oven, and then re-weighed. Analysis carried out was in triplicate after which the mean value of the weight loss measurements was reported. The average weight loss (ΔW), corrosion rate (CR), degree of surface coverage (θ) and inhibition efficiency () were calculated using Eqs. 1–4, respectively.
| 1 |
| 2 |
| 3 |
| 4 |
is the Weight loss (g) of the mild steel coupons, are the weights (g) of mild steel before immersion and after immersion; A is the area of the mild steel coupons (cm2), and t is the exposure time (h); CRblank is corrosion rates without the inhibitor and CRinh is corrosion rates in the presence of inhibitor.
Surface analysis
The prepared mild steel samples were immersed in a test solution of 0.1 M HCl (100 mL) with and without 1 mM concentration of L1 and L2 inhibitors in their respective beakers at 303 K and were subjected to an exposure period of 3 h in a thermostat water bath and afterwards removed from the solution and rinsed using distilled water and acetone respectively. It was allowed to dry and stored in a desiccator before the analysis of the surface morphology. The scanning electron microscope on (SEM, Quanta 450 FEI, voltage 10 kv, spot size 9 mm and magnification range 2228–2382 with 10 μm scale bar model: Σigma HD, Zeiss, Germany).
Quantum mechanics calculations
The DFT quantum mechanics calculations were performed to evaluate the molecule’s chemical properties, including atomic charge and intra-molecular geometry 17; stereo-electronic interactions accountable for conformational stability 18, and the selection of corrosion inhibitors 19 among others. In this study, the popular density functional theory (DFT)20 method embedded in Gaussian 16 program 21,22 was used to investigate the electronic properties of newly synthesized Schiff bases for their reactivity and mechanism as a corrosion inhibitor. The hybrid density functional B3LYP 23 and the highly parameterized exchange-correlated M06-2X 24 functionals in combination with the 6-31 + G(d,p) basis set 23 were chosen for the calculations and the results compared for their performances with respect to each variable. The Schiff bases L1 and L2 were modelled with GaussView 6 program and optimized to a minimum in the gas-phase. The frequency calculations were performed on optimized geometries both in gas-phase and hydrochloric, HCl (ε = 78.3, experimental solvent) media; and the results were compared for solvation effect. The fundamental conceptual DFT properties measured are the energies of the lowest unoccupied molecular orbital, ELUMO and the highest occupied molecular orbital, EHOMO. Other conceptual DFT parameters were determined from these basic properties in line with the work of Odewole et al. 3 as expressed in Eqs. (5) to (12).
| 5 |
| 6 |
| 7 |
| 8 |
| 9 |
| 10 |
| 11 |
| 12 |
where ΔE is the energy gap, A is the electron affinity, I is the ionization potential, η is the chemical hardness, δ is the softness, χ is the absolute electronegativity, CP is the chemical potential, and ω is the global electrophilicity index.
The mechanism of inhibition was measured in terms of how electrons were transferred vis-à-vis the inhibitor and metal surface. In this case, the fraction of electron transferred, (ΔN) onto the metal surface was determined from the electronegativity and chemical hardness of iron (Fe) and the inhibitor as expressed in Eq. (13).
| 13 |
χFe and χinh are the absolute electronegativities of iron and the inhibitor respectively while ηFe and ηinh are the chemical hardness of iron and inhibitor respectively. The theoretical values 7 for χFe and 0 for ηFe were used according to a previous study by Ebenso and co-workers 25.
The local reactivity at various sites of the ligands was treated from the Mulliken atomic charges. These charges form the basic variables for deriving Fukui functions, namely nucleophilicity and electrophilicity, of the molecules according to Eqs. (14) to (16) and were used to examine the potential adsorption sites on the Schiff base molecules.
| 14 |
| 15 |
| 16 |
where fk+ and fk− are the nucleophilic and electrophilic Fukui functions, respectively. qk(N + 1) and qk(N − 1) are the charges on the atoms in a molecule in its anionic and cationic states, respectively. qk(N) is the charge on the atoms in a molecule in its neutral state.
Molecular dynamics simulation
Molecular dynamics (MD) simulation is known and has been applied to investigate the interaction of metal (Fe) surfaces and inhibitors. The MD simulation was carried out using Material Studio (Accelrys Inc)26. For better stability and the nature of the packed surface of the chosen metal surface, Fe (1 1 0) was used in this study. Fe (1 1 0) surface also is one of the most active to stimulate the adsorption process. The relations between the inhibitor and the Fe (1 1 0) surface were performed in a simulation box size of 24.82 × 24.82 × 26.14 Å3 thru periodic boundary conditions. A vacuum slab of 6 Å height was set up on the Fe (1 1 0). 200 molecules of water, 9 of Cl− and 9 of H3O+ were included using the Adsorption locator module in Material Studio 27. Geometric optimization was performed to obtain minimal structures. 5 cycles (2000 steps) of simulated annealing were applied and the MD was performed via Berendsen thermostat temperature control at 298 K and simulation time of 50 ps with 1 fs time step28. The simulation was carried out under canonical ensemble (NVT) and Condensed Phase Optimized Molecular Potentials for Atomistic Simulation Studies (COMPASS) force field2,28,29. The interaction energy was obtained, and the Radial Distribution Function (RDF) was computed. The interaction energies were obtained based on Eq. 17.28
| 17 |
Etotal represents the total energy of the inhibitor molecule, surface, H2O, H3O and Cl−, Einhibitor designates the energy of absorbed inhibitor on the surface and the Esurface implies the energy of the metal surface involving H2O, H3O and Cl−.
| 18 |
Results and discussion
Spectroscopic studies
The condensation of 2-aminophenol with the methoxybenzaldehydes produced the imine (C=N) functionality observed by a stretching band at 1587 and 1589 cm−1 on the IR spectra for L1 and L2 respectively (Figures S1 and S2). Additional bands observed around 3371, 2836, 1616, and 1250 cm−1 were assigned to ν(O–H), ν(C–H), ν(C=C) and ν(C–O), respectively 3. Similarly, 1H- and 13C-NMR spectra of the ligands (Figs. S3–S6) afforded the imine proton and carbon peaks at 9.21 and 153.1 ppm for L1 as well as 8.65 and 162.5 ppm for L2 (Figures S5 and S6), which were further established the formation of the Schiff base 30. The electronic absorption spectra of the ligands obtained in dichloromethane are shown in Fig. 2. Due to intra-ligand charge transfer observed between 238 and 274 nm, absorption bands were assigned to π → π* while the band around 350 nm was assigned to n → π* transition 15.
Figure 2.
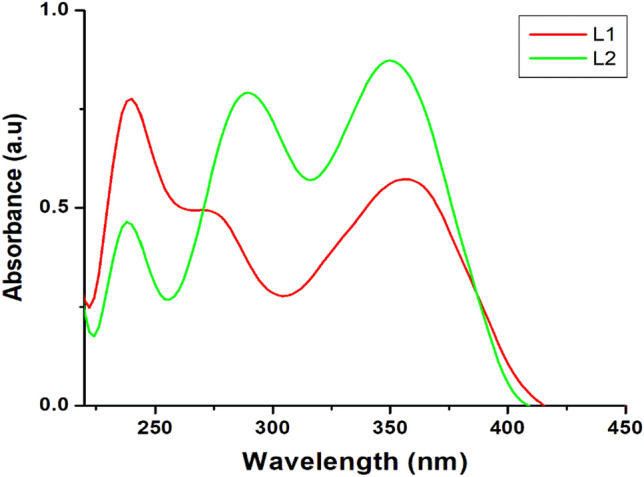
The electronic absorption spectra of the ligands obtained in dichloromethane.
Weight loss examination
Effect of inhibitors concentrations and temperature of test solution
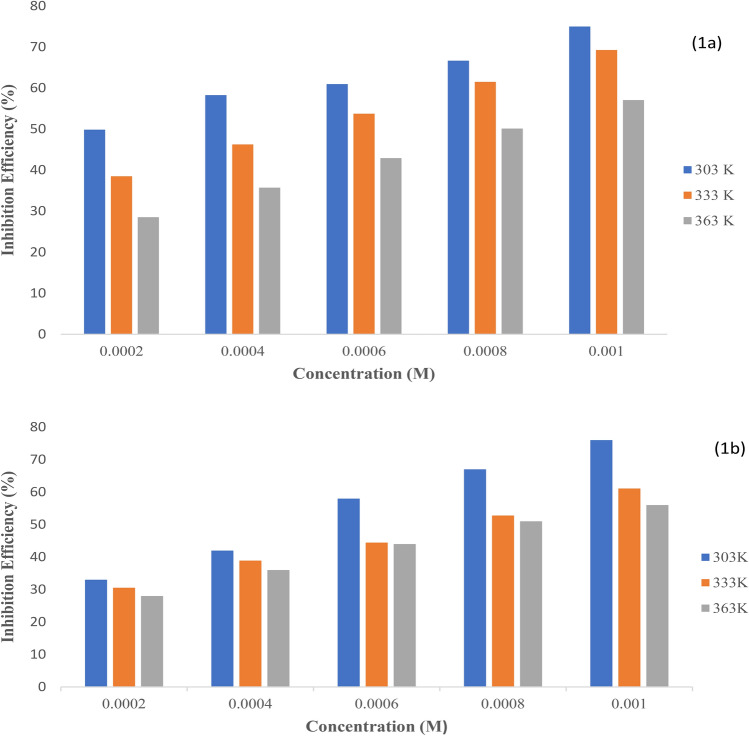
The corrosion rate values of mild steel without and with varying concentrations of L1 and L2 Schiff base inhibitors at different temperatures were computed based on Eq. 2. The obtained results from measurement by weight loss techniques presented in Table 1 revealed that the inhibitors retard the rate of corrosion rate since a decrease in corrosion rate values was observed following an increasing concentration of the inhibitors from 0.0002 M to 0.001 M and invariably resulted in an increase in inhibition efficiency. A concentration of 0.001 M gave inhibition efficiency of 75% and 76% for L1 and L2 respectively at 303 K. The increase in inhibition efficiency obtained with increasing inhibitor concentration is related to surface coverage increase in the inhibitor molecules on the surface of the mild steel. This trend is in agreement with previous work2,3.
Table 1.
Weight loss result of corrosion of mild steel in 0.1 mol L−1 HCl solution without and with varying concentrations of the inhibitors L1 and L2 at temperatures from 303 to 363 K.
| Inhibitors | 303 K | 333 K | 363 K | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cinh (ppm) | CR(mg/cm2h) | θ | η(%) | CR(g/cm2h) | θ | η (%) | CR(mg/cm2h) | θ | η (%) | |
| L1 | Blank | 0.0033 | – | – | 0.0036 | – | – | 0.0039 | – | – |
| 2 × 10–4 | 0.0017 | 0.50 | 50 | 0.0022 | 0.39 | 39 | 0.0028 | 0.29 | 29 | |
| 4 × 10–4 | 0.0014 | 0.58 | 58 | 0.0019 | 0.46 | 46 | 0.0025 | 0.36 | 36 | |
| 6 × 10–4 | 0.0014 | 0.58 | 58 | 0.0017 | 0.54 | 54 | 0.0022 | 0.43 | 43 | |
| 8 × 10–4 | 0.0011 | 0.67 | 67 | 0.0014 | 0.65 | 62 | 0.0019 | 0.50 | 50 | |
| 10 × 10–4 | 0.00083 | 0.75 | 75 | 0.0011 | 0.69 | 69 | 0.0017 | 0.56 | 56 | |
| Blank | 0.0033 | – | – | 0.0036 | – | – | 0.0039 | – | – | |
| L2 | 2 × 10–4 | 0.0022 | 0.33 | 33 | 0.0025 | 0.31 | 31 | 0.0028 | 0.28 | 28 |
| 4 × 10–4 | 0.0019 | 0.42 | 42 | 0.0022 | 0.39 | 39 | 0.0025 | 0.36 | 36 | |
| 6 × 10–4 | 0.0014 | 0.58 | 58 | 0.0020 | 0.47 | 47 | 0.0022 | 0.44 | 44 | |
| 8 × 10–4 | 0.0011 | 0.67 | 67 | 0.0017 | 0.53 | 53 | 0.0019 | 0.51 | 51 | |
| 10 × 10–4 | 0.0008 | 0.76 | 76 | 0.0014 | 0.69 | 69 | 0.0017 | 0.56 | 56 | |
Furthermore, it was observed that increasing solution temperature, led to a decline in inhibition efficiency for both L1 and L2 respectively (Fig. 3). This could be attributed to the desorption of molecules of the inhibitors from the surface of the mild steel which arises from kinetic energy increment of the inhibitor molecules which relapse the intermolecular force of interaction. An Increment in solution temperature could have also led to the decomposition of the molecule of the Schiff base inhibitors, hence, weakening surface coverage. The trend as observed in this study is in tandem with previous studies3,31,32. The Schiff base ligands showed comparable inhibitory efficiency at all temperatures (Table 1) with L2 slightly higher than L1 as a result of the electronic character of the methoxy substituent at the para and ortho positions respectively.
Figure 3.
Effect of varying concentration of (a) L1 and (b) L2, with varying temperature on inhibition efficiency.
Adsorption isotherm and thermodynamic parameters
The inhibitor’s efficiency is attributed to the degree of metal surface coverage by the inhibitor, which is a function of the concentration of the inhibitor. This explains the nature of adsorbed inhibitors unto mild steel surface revealed from several studies33,34. In this case, the adsorption nature was elucidated by fitting data obtained from the weight loss experiment, unto the adsorption isotherm models of Langmuir and Freundlich. For both inhibitors of L1 and L2, the Langmuir isotherm model (Eq. 19) gave a better correlation coefficient (R2) value closer to unity from the plot of Cinh/θ vs. Cinh (Fig. S7) when compared to the Freundlich model. Hence, the Langmuir model better described the nature of the adsorption process.
| 19 |
where Cinh is the concentration of the inhibitor (molL−1), Kads is the adsorptive equilibrium constant (L mol−1) and θ is the surface coverage. The equilibrium constant values (Kads) were computed from the intercept of the plots of Cinh/θ vs. Cinh (Figures S7). Table 2 revealed high Kads values for both L1 and L2 which signifies the strong adhesiveness of the inhibitor molecules on the surface of the mild steel. The values of the standard free energy (ΔGads) of the adsorption process were derived from the Kads values according to Eq. (20).
| 20 |
T is the absolute temperature (K), R = universal gas constant (8.314 J K−1 mol−1), 55.5 is the molar concentration of water in solution (mol/dm3). The computed values (Table 2) of the adsorption process obtained were all negative, thereby, stipulates the occurrence of a spontaneous adsorption process.
Table 2.
and Kads obtained from Langmuir Isotherm onto mild steel HCl (0.1 M) at varying concentrations, and temperatures of 303 K, 333 K and 363 K.
| Inhibitor | Temperature (K) | |||||
|---|---|---|---|---|---|---|
| Kads (103 L/mol) | (kJ/mol) | Slope | R2 | Intercept | ||
| L1 | 303 K | 5.0 | −31.57 | 1.1891 | 0.9799 | 0.0002 |
| 333 K | 2.5 | −32.78 | 1.1427 | 0.970 | 0.0004 | |
| 363 K | 2.0 | −35.06 | 1.2896 | 0.9609 | 0.0005 | |
| L2 | 303 K | 2.0 | −29.266 | 0.8306 | 0.9388 | 0.0005 |
| 333 K | 2.0 | −32.163 | 1.2254 | 0.9494 | 0.0005 | |
| 363 K | 2.0 | −35.060 | 1.3002 | 0.9793 | 0.0005 | |
In general, if values are about −20 kJ/mol or much lesser, it suggests that the process of adsorption occurs via the electrostatic interaction between the charged molecules and the charged metal surface. Hence, physisorption. Whereas, if values of is approximately −40 kJ/mol or higher, it stipulates the sharing of charge or electron transfer between or from organic inhibitors to the surface of the metal resulting in the formation of a coordinate bond which is chemisorption35. However, values between − 20 and − 40 kJ mol−1of stipulates the adsorption is a mixed type involving both physisorption and chemisorption36. Since the values of computed in this study is the range of − 20 and − 40 kJ mol−1, hence, it connotes the adsorption was governed by both chemisorption and physisorption. Chemisorption tends to predominate since ΔGads values for both L1 and L2 are much closer to − 40 kJ mol−1. This explains that there exists transfer of electrons from the heteroatoms of the L1 and L2 to the surface of the metal leading to the formation of coordinate bonds.
Thermodynamic parameters of the corrosion reaction
The Arrhenius Eq. (21) was used to further gain an understanding of the kinetics involved with respect to the varying temperature effect on the corrosion rate of the mild steel in the acidic medium both in the presence and/or absence of the inhibitors used.
| 21 |
where CR is the corrosion rate, Ea is the apparent activation energy, and A is the Arrhenius pre-exponential factor, T is the absolute temperature (K). Figures S8, revealed the Arrhenius plot of the corrosion rate of the mild steel in 0.1 mol L−1 HCl, in the absence and presence of the varying concentrations of the inhibitors at temperatures of 303 K, 333 K, and 363 K for 3 h. The computed Ea values obtained from the slope of the plots (Fig. S8) are presented in Table 3 which shows that the least Ea values occur in blank solution and then increase with the addition of inhibitors. The activation energy increase is assigned to an increased energy barrier of the dissolution of the mild steel correlated to the adsorbed inhibitors barring the active sites on the high-energy metal surface. Moreover, a slightly higher Ea value was observed in the presence of L2 at the highest concentration of 0.1 mM when compared to that of L1 (Table 3). Furthermore, values of enthalpy of activation, (ΔH∗corr) and entropy of activation, (ΔS∗corr) for the process of corrosion were computed from the slope and intercept in the straight-line plot of (Fig. S9) of the Eyring transition state Eq. (22)34.
| 22 |
where NA is the Avogadro number and h is the Planck constant. Other abbreviations have been defined in previous equations. The computed values of ΔH∗corr and ΔS∗corr are presented in Table 3. It was observed that the value of the activation enthalpy ΔH* is all positive, hence, revealing the endothermic nature of the mild steel dissolution reaction. Furthermore, it was observed that the least value of ΔH∗corr occurs in the blank and the highest value of ΔH∗corr occurs in the solution with the highest concentration of inhibitors. This could be best explained by that the minimum required energy needed by reactants to overcome energy barrier tend to increase with increasing inhibitors. The value of ΔH∗corr at the highest concentration of 10 mM for L2 > L1. In the case of the entropy of activation (ΔScorr∗), all the computed values were negative and quite large. This inclines that the activated complex is associative in nature in the determining step of the rate of reaction and the structure being more orderly, thereby resulting in entropy decline3,25. Also, Table 3 further shows that the computed value of ΔScorr in the blank is more negative as compared to the ΔScorr computed values of the varying concentration of the inhibited acid medium and this could be attributed to the presence of the adsorbed inhibitor species on the surface of the mild steel9.
Table 3.
Activation energy (Apparent) (Ea), A, ΔH∗, ΔS∗ of L1 and L2 at different concentrations derived from Arrhenius and Transition State Equations.
| Inhibitor | Concentration(M) | Activation parameter | |||
|---|---|---|---|---|---|
| Ea(kJ/mol) | A | ΔH≠ (J/mol) | ΔS≠ (J/mol K) | ||
| L1 | Blank | 2.37 | 0.126 | 23.44 | −197.53 |
| 2 × 10–4 | 7.77 | 0.238 | 32.42 | −197.51 | |
| 4 × 10–4 | 8.96 | 0.270 | 34.50 | −197.50 | |
| 6 × 10–4 | 8.12 | 0.225 | 33.73 | −−197.51 | |
| 8 × 10–4 | 8.44 | 0.221 | 34.68 | −197.507 | |
| 10 × 10–4 | 10.52 | 0.278 | 38.21 | −197.499 | |
| L2 | Blank | 2.54 | 0.129 | 23.68 | −197.539 |
| 2 × 10–4 | 3.67 | 0.132 | 26.50 | −197.539 | |
| 4 × 10–4 | 4.18 | 0.135 | 27.64 | −197.539 | |
| 6 × 10–4 | 6.99 | 0.195 | 32.12 | −197.537 | |
| 8 × 10–4 | 8.46 | 0.227 | 34.76 | −197.537 | |
| 10 × 10–4 | 11.63 | 0.342 | 39.71 | −197.535 | |
Surface analyses
The micrographs as shown from the scanning electron microscope (SEM) (Fig. 4) show the morphology and change in morphology on the surface of the mild steel prior to and after 3 h immersion in uninhibited and inhibited 0.1 M HCl solutions at 303 K. It was observed that the surface of the mild steel in the uninhibited acid solution was highly degraded with products of corrosion on its surface and this could be attributed to the increased dissolution rate from the direct attack on the surface. However, a mild degradation was observed on the surface of the mild steel immersed in the L1 and L2 inhibited acid solution, and this could be a result of mild steel surface coverage by both L1, and L2 inhibitors, therefore, protecting it from corrosion by the HCl. This agrees theoretical studies as well as the weight loss results.
Figure 4.
SEM micrographs of surface of mild steel (a) prior to immersion, (b) after 3 h immersion in 0.1 M HCl without inhibitors, (c) with 10 mM of L1 and(d) L2.
EDX analysis
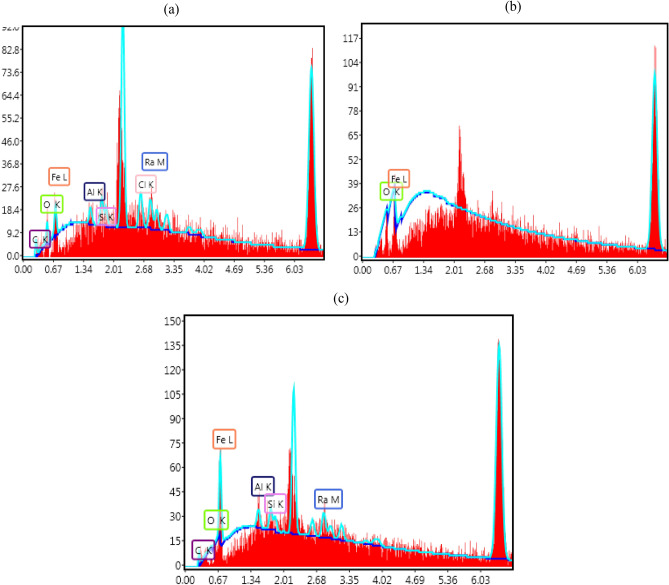
The EDX analysis was obtained to ascertain information with respect to the elemental constituent on the mild steel surface in 0.1 M HCl treated without and/or with inhibitors. The EDX spectrum of the mild steel coupons in 0.1 M HCl without and/or with 1 mM L1, and L2 inhibitors respectively is revealed in Fig. 5. It can be seen from Fig. 5a, the existence of the peak of chlorine indicates the presence of this element on the mild steel surface that emanates from the hydrochloric acid solution. Furthermore, the displayed spectrum in Fig. 5b-c revealed the absence of chlorine which could be attributed to the application of the inhibitors on the mild steel surface acting as a coverage to decrease the corrosive attack of the acidic solution.
Figure 5.
(a) Spectrum of mild steel in 0.1 M HCl (b) Spectrum of mild steel in 0.1 M HCl inhibited L1 Schiff base, (c) Spectrum of mild steel in 0.1 M HCl inhibited L2 Schiff base.
Mechanism of inhibition
The mechanism involved in the adsorption of the inhibitor molecules on the metal surface from the weight loss experiment as observed from the computed thermodynamic parameters conforms to mixed adsorption of both physisorption and chemisorption with the predominance of chemisorption over physisorption. This posits the contribution of the charged protonated molecules of the L1 and L2 inhibitors onto the metal surface. The mechanism in chemisorption occurs via by formation of coordination bonds between the active sites on the metal (Fe) surface and the inhibitor molecules, and this coordination is made possible between the centres that donate and withdraw electrons.
DFT analysis
Quantum mechanics method through conceptual DFT analysis has demonstrated suitability in the field of corrosion science for either acid or base medium used for metal corrosion inhibition study37. A number of studies27,38 have revealed the relationship between corrosion inhibition and the functional groups associated with the compounds under consideration. Analysis done with the B3LYP and M06-2X functional at 6-31 + G(d,p) basis set is presented.
Optimized geometries and energies analysis
Gaussian optimized structures of the Schiff bases are presented in Fig. 6.
Figure 6.
Optimized geometries of L1 and L2.
The work of Lgaz et al.38 supports the idea that inhibitor adsorption on the iron surface can proceed on a donor and acceptor reactions basis which links the unoccupied surface atom orbit and n electrons of the aromatic compounds. Table 4 shows the energies of the LUMO and HOMO orbitals plus other conceptual DFT parameters. The higher HOMO energies obtained for both ligands in HCl solvent compared to gas-phase medium speed up the molecule’s binding to the metallic surface and thus interferes with the electron transfer mechanism over the adsorbed layer. The energy gap, ΔE measures the minimum energy required for exciting an electron in the inhibitor molecule39. This study reveals that the hybrid B3LYP functional performs better than M06-2X functional in measuring the energies of Schiff bases for corrosion inhibition because, the former gave much lower ΔE values of 3.86 eV and 3.81 eV for L1 and L2, respectively as against 6.08 eV and 6.03 eV obtained with the later functional. Hence, the newly synthesized Schiff bases’ inhibitory efficiency is better measured and higher with hybrid B3LYP functional.
Table 4.
Electronic parameters and reactivity descriptors.
| ELUMO (eV) | EHOMO (eV) | ΔE (eV) | A (eV) | I (eV) | η (eV) | δ (eV−1) | χ (eV) | Cp (eV) | ω (eV) | ΔN | μ (Debye) | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| L1, B3LYP (gas) | −2.16 | −5.89 | 3.72 | 5.89 | 2.16 | 1.86 | 0.53 | 4.02 | −4.02 | 4.35 | 0.79 | 3.49 |
| L1, B3LYP (HCl) | −2.19 | −6.06 | 3.86 | 6.06 | 2.19 | 1.93 | 0.51 | 4.12 | −4.12 | 4.40 | 0.74 | 5.08 |
| L1, M06-2X (gas) | −1.23 | −7.17 | 5.94 | 7.17 | 1.23 | 2.97 | 0.33 | 4.20 | −4.20 | 2.97 | 0.47 | 3.53 |
| L1, M06-2X (HCl) | −1.28 | −7.36 | 6.08 | 7.36 | 1.28 | 3.04 | 0.32 | 4.32 | −4.32 | 3.07 | 0.43 | 5.00 |
| L2, B3LYP (gas) | −1.99 | −5.73 | 3.73 | 5.73 | 1.99 | 1.86 | 0.53 | 3.86 | −3.86 | 3.99 | 0.83 | 3.57 |
| L2, B3LYP (HCl) | −2.06 | −5.88 | 3.81 | 5.88 | 2.06 | 1.90 | 0.52 | 3.97 | −3.97 | 4.14 | 0.79 | 4.60 |
| L2, M06-2X (gas) | −1.05 | −7.02 | 5.96 | 7.02 | 1.05 | 2.98 | 0.33 | 4.03 | −4.03 | 2.73 | 0.49 | 3.37 |
| L2, M06-2X (HCl) | −1.14 | −7.18 | 6.03 | 7.18 | 1.14 | 3.01 | 0.33 | 4.16 | −4.16 | 2.87 | 0.46 | 4.16 |
Similarly, the chemical hardness is low in the hybrid B3LYP functional compared to M06-2X. This accounts for a relatively high softness and implies readily increased chemical reactivity as a corrosion inhibitor. The ΔN > 0 obtained for the ligand molecules reflects that they are very likely to donate electrons40. The viability and preference to use B3LYP functional over M06-2X for energies calculation are here confirmed since the former functional fraction of electron transferred as 0.74 and 0.79 (in HCl) for ligands L1 and L2 respectively as against 0.43 and 0.49 obtained using the later functional. The presence of aromatic rings and heteroatoms contributes to the corrosion inhibition efficacy of the molecules. By juxtaposing the two compounds using the energies analysis, L2 is more effective as a corrosion inhibitor than the L1 as demonstrated in the lower energy gap values and higher electron transferred fraction. In summary, the para-substituted ligand (L2) is relatively a stronger corrosion inhibitor of mild steel in HCl than its ortho-substituted (L1) derivative.
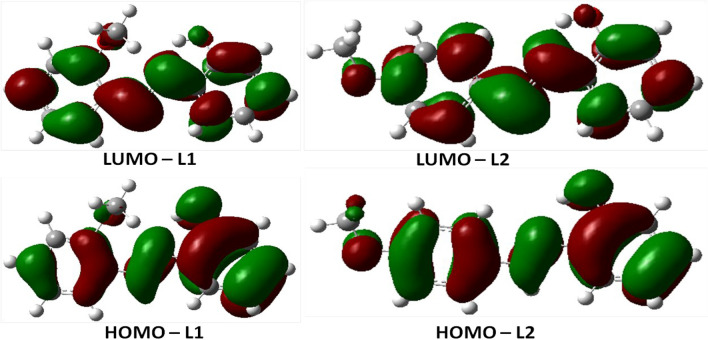
The frontier molecular orbital maps of the Schiff bases obtained with B3LYP functional and 6-31 + G(d,p) basis set in HCl solvent are presented in Fig. 7. Maps from this model were chosen over the M06-2X model because the former gave a lower band gap which demonstrated a better measure of the electronic properties of the Schiff bases. Ortho-methoxyl substitution in L1 partly affects the electron distribution in the phenyl group joined to C9 making the C9 atom electron-deficient and it is the preferred site for electrophilic attack. This is demonstrated in L1 where the electron cloud (HOMO-L1) spread from the C6 atom towards the phenyl group attached to it. In the same vein, the electron cloud in L2 spreads from the C6 atom towards the phenyl group attached to it, and the electron cloud in the phenyl group attached to C9 are evenly spread (HOMO-L2) and orbital maps facing each other. Thus, L2 has a larger surface area with well-spread electrons for transfer and to be adsorbed onto mild steel and this supports the previous suggestion that L2 relatively enhanced inhibition of mild steel corrosion than L1 as observed in the weight loss study. This is also buttressed in the fraction of electrons transferred since ΔN is higher in L2 than in L1.
Figure 7.
Frontier molecular orbitals of L1 and L2 calculated with B3LYP/6-31 + G(d,p).
Calculation of Fukui indices and Mulliken atomic charges
The Mulliken population analysis provides means of estimating partial atomic charges in evaluating the adsorption centers of inhibition and it is employed in determining the diffusion load across the whole inhibitor molecule system 27. The Mulliken atomic charges and the respective Fukui function indices obtained are presented in appendices B3LYP /6-31 + g(d,p) calculation for L1 shows that C6 (0.119) and C9 (−0.151) are the sites for nucleophilic and electrophilic attacks respectively in the gas-phase; while C15 (0.522) and C10 (−0.672) are the sites for nucleophilic and electrophilic attacks respectively in HCl solvent. On the other hand, using M06-2X/6-31 + G(d,p) for L1 suggests that C6 (0.158, 0.227) and C9 (−0.159, −0.180) are the respective sites for nucleophilic and electrophilic attacks in gas-phase and gas-phase HCl solvent.
Using B3LYP/6-31 + G(d,p) for L2 shows that C2 (0.104) and C10 (0.217) are the sites for nucleophilic attack both in gas-phase and in HCl solvent while C9 (−0.147, −0.205) is the preferred site for an electrophilic attack in both media. On the other hand, M06-2X/6-*31 + G(d,p) suggests that C2 (0.138) and C10 (0.385) are the sites for nucleophilic attack in gas-phase and HCl solvent respectively; while C11 (−0.205) and C9 (−0.445) are the sites for an electrophilic attack in gas-phase and HCl solvent. In summary, C6 and C9 are the respective preferred sites for a nucleophilic and electrophilic attack in L1; while C2 and C10 are the preferred sites for nucleophilic attack in gas-phase and HCl media while C9 is the preferred site for an electrophilic attack in L2.
Molecular dynamics (MD) simulation
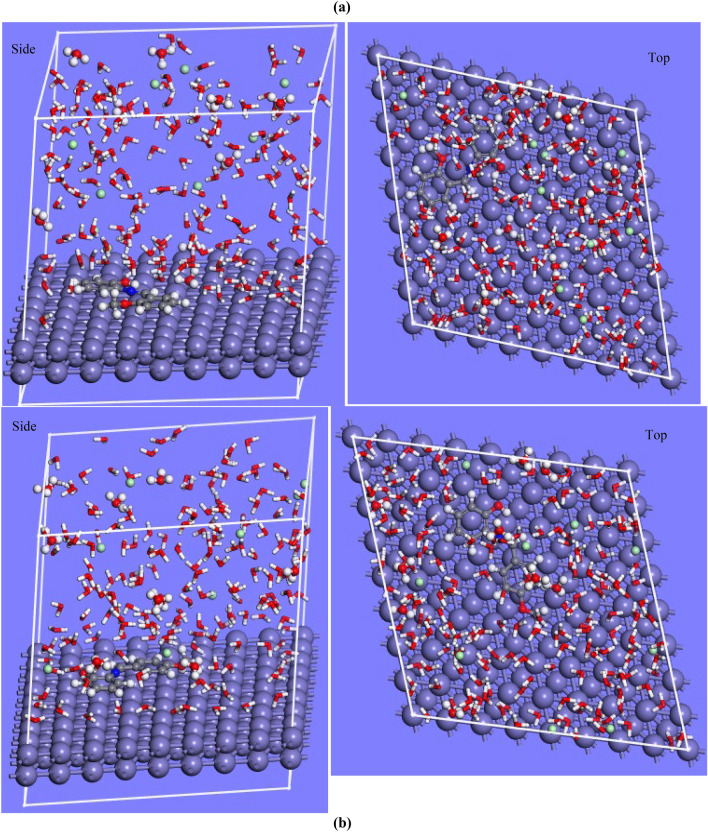
Molecular dynamics simulation has been reported to be a recognized method introduced to corrosion studies41. This approach gives insight into the inhibitors’ interactions, orientation, and binding energies involving the inhibitors and metal surface27,42. In this work, MD simulations were adopted to complement the experimental results obtained and to study the mechanism of interactions between the inhibitors and the Fe (1 1 0) surface as well as in the presence of 200 water, H3O+ and Cl−. The surface configurations of the optimized inhibitors in the aqueous medium are presented in Fig. 8. MD simulations showed that L1 and L2 inhibitors adsorb with a parallel configuration (Fig. 8) firmly on the Fe (1 1 0) surface. This configuration resulted in the bond formation between the donor active site of L1 and L2 and the vacant orbitals of the positively charged Fe (1 1 0) surface42. The MD simulation revealed that notwithstanding the presence of the corrosive elements such as H3O+, Cl− and the water molecules, a strong interaction exists between the surface of the metal and the heteroatoms (O, N, and the phenyl ring) of L1 and L2. The interaction energy of compounds unto the Fe (1 1 0) surface was calculated and L1 gave a value of −743.74 kJ/mol, while L2 had a value of −746.84 kJ/mol. The large positive value obtained mirrors the ability of the compounds to adsorb on the surface of the metal surface. The calculated interaction energy obtained is higher compared to that reported for other compounds27,43. The MD results indicated that the interaction energy for L1 and L2 agreed with the inhibition efficiency obtained from the weight loss experiment. Based on the simulation, it can be inferred that the high interaction energy shows slightly higher adsorption capabilities of L2 on the surface of the metal which also suggests better adsorption stability with higher inhibition efficiency44. The length of the bond between Fe and heteroatoms was measured to determine the mechanism of adsorption. For L1, Fe–O (2.89 Å), Fe–N (3.21 Å) and Fe–C (2.87 Å), while for L2, Fe–O (3.09 Å), Fe–N (3.33 Å) and Fe–C (2.73 Å). Bond lengths between 1 and 3 Å indicate chemisorption while ˃3.5 Å suggests a physisorption mechanism27. Analysis showed that all the bond lengths of studied molecules with Fe surfaces are less than 3.5 Å indicating a chemisorption mechanism.
Figure 8.
3D depiction of the Side and top views showing suitable conformation for adsorption of (a) L1 and (b) L2 on Fe (110) surface in aqueous solution.
Inhibition mechanism
The corrosion inhibition mechanism of inhibitors depends largely on the adsorption of molecules on the metal surface which can be traced to factors such as the chemical structure of molecules under study2,45. The corrosion inhibition mechanism of compounds has been studied experimentally and via a theoretical approach. In this section, the mechanism of adsorption is discussed putting into consideration other components in the solution and the type of adsorption that occurs between Fe and L1 and L2. L1 and L2 being Schiff base compounds could exist in the protonated form in 1.0 M Hydrochloric acid solution as seen in the following equilibrium:
| 23 |
where y = 1, 2.
The resulting protonated form as shown in Eq. (23), shows that the adsorption of inhibitors on the metal surface promotes chemisorption. This is due to the electrostatic attraction that exists between the hydrated negative charges of Cl− on the surface of Fe (1 1 0) and the positive charges from the ligands2,45. Hydrogen gas is released as the protonated form of the Schiff base competes with the aqueous H+, and the heteroatoms of the ligands return to their neutral form, which then transfers the unshared electron pair into the vacant orbitals of the Fe surface2. The series of transfers of electrons to the metal surface causes the accumulation of electrons in the metal. This results in a back the sequential transfer of electrons from the dd-orbitals of metal atoms to the unoccupied orbitals of the Schiff base leading to a phenomenon called chemisorption and retro-donation42,46,47. The isotherm study revealed that Langmuir Isotherm best fits the corrosion inhibition process and the values of further suggest that the mechanism of corrosion inhibition by L1 and L2 is by chemisorption. This results from the transfer of lone pair of electrons from the Schiff base formed and the pi electrons of the phenyl ring to the metal surfaces forming coordinate bonds. Figure 9 shows a pictorial explanation of the mechanism of corrosion inhibition.
Figure 9.
Schematic representation of the adsorption mechanism of inhibitors on the Fe (1 1 0) surface in 0.1 M HCl.
Conclusion
Schiff base ligands (E)-2-((2-methoxybenzylidene)amino)phenol and (E)-2-((4-methoxybenzylidene)amino)phenol were synthesized and characterized using NMR, IR and UV/Visible spectroscopy. The corrosion inhibition potential of these synthesized compounds was investigated by weight loss measurement. Computational approaches such as molecular dynamics and the DFT approach were applied to further explain the mechanism of inhibition. DFT calculations were also applied to elucidate their chemical reactivity and inherent properties. In this study, the inhibition efficiency increased with increasing inhibitor concentration, and decreased with an increased temperature. Langmuir Isotherm best fits the experimental data obtained for L1 and L2. DFT calculations showed that the presence of aromatic rings and heteroatoms contribute to the corrosion inhibition efficacy of the synthesized compounds. The interaction energies obtained for L1 and L2 from molecular dynamics studies agree with the DFT calculated parameters and the inhibition efficiencies obtained from the weight loss experiment. Chemisorption mechanism was inferred based on the value ΔGads and the close bond distance obtained between the heteroatoms of the ligands and the surface of the metal.
Supplementary Information
Acknowledgements
CUI, DCA and EOA are thankful to CHPC (www.chpc.ac.za) and UKZN for operational and infrastructural support.
Author contributions
C.U.I. designed and conceptualized, performed the molecular dynamics studies, and wrote part of the first draft. D.C.A., O.G.O., O.M.E., H.O.O., carried out the synthesis, the corrosion experiment studies and wrote part of the draft of the paper. E.O.A. performed the D.F.T. studies and wrote a draft of the paper. T.N. edited and revised the manuscript. All authors analyzed, read, edited, and approved the final manuscript.
Data availability
The data that support the findings of this study are available from Collins U. Ibeji, but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data are however available from the authors upon reasonable request and with permission of Collins U. Ibeji.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Collins U. Ibeji, Email: ugochukwu.ibeji@unn.edu.ng
Damilola C. Akintayo, Email: damilolaakintayo141@gmail.com
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-023-30396-3.
References
- 1.Aouniti A, Elmsellem H, Tighadouini S, Elazzouzi M, Radi S, Chetouani A, Hammouti B, Zarrouk A. Schiff's base derived from 2-acetyl thiophene as corrosion inhibitor of steel in acidic medium. J. Taibah Univ. Sci. 2016;10(5):774–785. doi: 10.1016/j.jtusci.2015.11.008. [DOI] [Google Scholar]
- 2.Okey NC, Obasi NL, Ejikeme PM, Ndinteh DT, Ramasami P, Sherif E-SM, Akpan ED, Ebenso EE. Evaluation of some amino benzoic acid and 4-aminoantipyrine derived Schiff bases as corrosion inhibitors for mild steel in acidic medium: Synthesis, experimental and computational studies. J. Mol. Liq. 2020;315:113773. doi: 10.1016/j.molliq.2020.113773. [DOI] [Google Scholar]
- 3.Odewole OA, Ibeji CU, Oluwasola HO, Oyeneyin OE, Akpomie KG, Ugwu CM, Ugwu CG, Bakare TE. Synthesis and anti-corrosive potential of Schiff bases derived 4-nitrocinnamaldehyde for mild steel in HCl medium: Experimental and DFT studies. J. Mol. Struct. 2021;1223:129214. doi: 10.1016/j.molstruc.2020.129214. [DOI] [Google Scholar]
- 4.Shivakumar S, Mohana K. Corrosion behavior and adsorption thermodynamics of some Schiff bases on mild steel corrosion in industrial water medium. International Journal of Corrosion. 2013;2013:1. doi: 10.1155/2013/543204. [DOI] [Google Scholar]
- 5.Lgaz H, Salghi R, Larouj M, Elfaydy M, Jodeh S, Rouifi Z, Lakhrissi B, Oudda H. Experimental, theoretical and Monte Carlo simulation of quinoline derivative as effective corrosion inhibitor for mild steel in 1 M HCl. J. Mater. Environ. Sci. 2016;7(12):4471–4488. [Google Scholar]
- 6.Abboud Y, Abourriche A, Saffaj T, Berrada M, Charrouf M, Bennamara A, Al Himidi N, Hannache H. 2, 3-Quinoxalinedione as a novel corrosion inhibitor for mild steel in 1 M HCl. Mater. Chem. Phys. 2007;105(1):1–5. doi: 10.1016/j.matchemphys.2007.03.037. [DOI] [Google Scholar]
- 7.Al-Amiery AA, Kadhum AAH, Mohamad AB, Junaedi S. A novel hydrazinecarbothioamide as a potential corrosion inhibitor for mild steel in HCl. Materials. 2013;6(4):1420–1431. doi: 10.3390/ma6041420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pandey A, Singh B, Verma C, Ebenso EE. Synthesis, characterization and corrosion inhibition potential of two novel Schiff bases on mild steel in acidic medium. RSC Adv. 2017;7(74):47148–47163. doi: 10.1039/C7RA08887F. [DOI] [Google Scholar]
- 9.Oguzie EE, Oguzie KL, Akalezi CO, Udeze IO, Ogbulie JN, Njoku VO. Natural products for materials protection: Corrosion and microbial growth inhibition using Capsicum frutescens biomass extracts. ACS Sustain. Chem. Eng. 2013;1(2):214–225. doi: 10.1021/sc300145k. [DOI] [Google Scholar]
- 10.Al-Haj-Ali AM, Jarrah NA, Mu'Azu ND, Rihan RO. Thermodynamics and kinetics of inhibition of aluminum in hydrochloric acid by date palm leaf extract. J. Appl. Sci. Environ. Manag. 2014;18(3):543–551. [Google Scholar]
- 11.Garcia-Ochoa E, Guzmán-Jiménez S, Hernández JG, Pandiyan T, Vásquez-Pérez JM, Cruz-Borbolla J. Benzimidazole ligands in the corrosion inhibition for carbon steel in acid medium: DFT study of its interaction on Fe30 surface. J. Mol. Struct. 2016;1119:314–324. doi: 10.1016/j.molstruc.2016.04.057. [DOI] [Google Scholar]
- 12.Patel A, Panchal V, Mudaliar G, Shah N. Impedance spectroscopic study of corrosion inhibition of Al-Pure by organic Schiff base in hydrochloric acid. J. Saudi Chem. Soc. 2013;17(1):53–59. doi: 10.1016/j.jscs.2011.06.003. [DOI] [Google Scholar]
- 13.Matar SA, Talib WH, Mustafa MS, Mubarak MS, AlDamen MA. Synthesis, characterization, and antimicrobial activity of Schiff bases derived from benzaldehydes and 3, 3′-diaminodipropylamine. Arab. J. Chem. 2015;8(6):850–857. doi: 10.1016/j.arabjc.2012.12.039. [DOI] [Google Scholar]
- 14.Kokalj A, Peljhan S. Density functional theory study of ATA, BTAH, and BTAOH as copper corrosion inhibitors: adsorption onto Cu (111) from gas phase. Langmuir. 2010;26(18):14582–14593. doi: 10.1021/la1019789. [DOI] [PubMed] [Google Scholar]
- 15.Yusuf TL, Oladipo SD, Zamisa S, Kumalo HM, Lawal IA, Lawal MM, Mabuba N. Design of new Schiff-base copper(II) complexes: Synthesis, crystal structures, DFT study, and binding potency toward cytochrome P450 3A4. ACS Omega. 2021;6(21):13704–13718. doi: 10.1021/acsomega.1c00906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Adeleke AA, Zamisa SJ, Omondi B. Crystal structure of dichlorido-bis ((E)-2-((pyridin-4-ylmethylene) amino) phenol) zinc (II), C24H20Cl2N4O2Zn. Zeitschrift für Kristallographie-New Crystal Structures. 2020;235(3):625–628. doi: 10.1515/ncrs-2019-0863. [DOI] [Google Scholar]
- 17.Wu HS, Sandler SI. Use of ab initio quantum mechanics calculations in group contribution methods. 1. Theory and the basis for group identifications. Ind. Eng. Chem. Res. 1991;30(5):881–889. doi: 10.1021/ie00053a010. [DOI] [Google Scholar]
- 18.Tormena CF. Conformational analysis of small molecules: NMR and quantum mechanics calculations. Prog. Nucl. Magn. Reson. Spectrosc. 2016;96:73–88. doi: 10.1016/j.pnmrs.2016.04.001. [DOI] [PubMed] [Google Scholar]
- 19.Costa J, Lluch J. The use of quantum mechanics calculations for the study of corrosion inhibitors. Corros. Sci. 1984;24(11–12):929–933. doi: 10.1016/0010-938X(84)90113-6. [DOI] [Google Scholar]
- 20.Argaman N, Makov G. Density functional theory: An introduction. Am. J. Phys. 2000;68(1):69–79. doi: 10.1119/1.19375. [DOI] [Google Scholar]
- 21.Robb, M. New Chemistry with Gaussian 16 & GaussView 6.
- 22.Frisch, M., Trucks, G., Schlegel, H., Scuseria, G., Robb, M., Cheeseman, J., Scalmani, G., Barone, V., Petersson, G., & Nakatsuji, H. Gaussian 16, revision B. 01; Wallingford, CT, 2016, Google Scholar There is no corresponding record for this reference.
- 23.Tirado-Rives J, Jorgensen WL. Performance of B3LYP density functional methods for a large set of organic molecules. J. Chem. Theory Comput. 2008;4(2):297–306. doi: 10.1021/ct700248k. [DOI] [PubMed] [Google Scholar]
- 24.Walker M, Harvey AJ, Sen A, Dessent CE. Performance of M06, M06–2X, and M06-HF density functionals for conformationally flexible anionic clusters: M06 functionals perform better than B3LYP for a model system with dispersion and ionic hydrogen-bonding interactions. J. Phys. Chem. A. 2013;117(47):12590–12600. doi: 10.1021/jp408166m. [DOI] [PubMed] [Google Scholar]
- 25.Ebenso EE, Isabirye DA, Eddy NO. Adsorption and quantum chemical studies on the inhibition potentials of some thiosemicarbazides for the corrosion of mild steel in acidic medium. Int. J. Mol. Sci. 2010;11(6):2473–2498. doi: 10.3390/ijms11062473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.A. Inc, Materials studio release notes, Release 17.1, Accelrys Software Inc., San Diego (2017).
- 27.Chafiq M, Chaouiki A, Lgaz H, Salghi R, Gaonkar SL, Bhat KS, Marzouki R, Ali IH, Khan MI, Shimizu H. Synthesis and corrosion inhibition evaluation of a new Schiff base hydrazone for mild steel corrosion in HCl medium: electrochemical, DFT, and molecular dynamics simulations studies. J. Adhes. Sci. Technol. 2020;34(12):1283–1314. doi: 10.1080/01694243.2019.1707561. [DOI] [Google Scholar]
- 28.Saha SK, Dutta A, Ghosh P, Sukul D, Banerjee P. Adsorption and corrosion inhibition effect of Schiff base molecules on the mild steel surface in 1 M HCl medium: A combined experimental and theoretical approach. Phys. Chem. Chem. Phys. 2015;17(8):5679–5690. doi: 10.1039/C4CP05614K. [DOI] [PubMed] [Google Scholar]
- 29.Hsissou R, Abbout S, Seghiri R, Rehioui M, Berisha A, Erramli H, Assouag M, Elharfi A. Evaluation of corrosion inhibition performance of phosphorus polymer for carbon steel in [1 M] HCl: Computational studies (DFT, MC and MD simulations) J. Market. Res. 2020;9(3):2691–2703. [Google Scholar]
- 30.Munzeiwa WA, Nyamori VO, Omondi B. N, O-Amino-phenolate Mg(II) and Zn(II) Schiff base complexes: Synthesis and application in ring-opening polymerization of ε-caprolactone and lactides. Inorg. Chim. Acta. 2019;487:264–274. doi: 10.1016/j.ica.2018.12.028. [DOI] [Google Scholar]
- 31.Shahabi S, Hamidi S, Ghasemi JB, Norouzi P, Shakeri A. Synthesis, experimental, quantum chemical and molecular dynamics study of carbon steel corrosion inhibition effect of two Schiff bases in HCl solution. J. Mol. Liq. 2019;285:626–639. doi: 10.1016/j.molliq.2019.04.137. [DOI] [Google Scholar]
- 32.Agbaffa EB, Akintemi EO, Uduak EA, Oyeneyin OE. Corrosion inhibition potential of the methanolic crude extract of Mimosa pudica leaves for mild steel in 1 M hydrochloric acid solution by weight loss method. Sci. Lett. 2021;15(1):23–42. doi: 10.24191/sl.v15i1.11791. [DOI] [Google Scholar]
- 33.Obot I, Ankah N, Sorour A, Gasem Z, Haruna K. 8-Hydroxyquinoline as an alternative green and sustainable acidizing oilfield corrosion inhibitor. Sustain. Mater. Technol. 2017;14:1–10. [Google Scholar]
- 34.Verma C, Olasunkanmi LO, Obot I, Ebenso EE, Quraishi M. 2, 4-Diamino-5-(phenylthio)-5 H-chromeno [2, 3-b] pyridine-3-carbonitriles as green and effective corrosion inhibitors: gravimetric, electrochemical, surface morphology and theoretical studies. RSC Adv. 2016;6(59):53933–53948. doi: 10.1039/C6RA04900A. [DOI] [Google Scholar]
- 35.Muthukrishnan P, Prakash P, Shankar K, Kathiresan A. Azo Schiff Base as Antiscaling Agent for Mild Steel in Hydrochloric Acid: Electrochemical, Non-electrochemical, and DFT Studies. J. Bio-and Tribo-Corros. 2019;5(1):1–13. doi: 10.1007/s40735-018-0208-2. [DOI] [Google Scholar]
- 36.Sığırcık G, Tüken T, Erbil M. Inhibition efficiency of aminobenzonitrile compounds on steel surface. Appl. Surf. Sci. 2015;324:232–239. doi: 10.1016/j.apsusc.2014.09.206. [DOI] [Google Scholar]
- 37.Lashgari M, Malek AM. Fundamental studies of aluminum corrosion in acidic and basic environments: Theoretical predictions and experimental observations. Electrochim. Acta. 2010;55(18):5253–5257. doi: 10.1016/j.electacta.2010.04.054. [DOI] [Google Scholar]
- 38.Lgaz H, Chung I-M, Salghi R, Ali IH, Chaouiki A, El Aoufir Y, Khan MI. On the understanding of the adsorption of Fenugreek gum on mild steel in an acidic medium: Insights from experimental and computational studies. Appl. Surf. Sci. 2019;463:647–658. doi: 10.1016/j.apsusc.2018.09.001. [DOI] [Google Scholar]
- 39.Wazzan NA. DFT calculations of thiosemicarbazide, arylisothiocynates, and 1-aryl-2, 5-dithiohydrazodicarbonamides as corrosion inhibitors of copper in an aqueous chloride solution. J. Ind. Eng. Chem. 2015;26:291–308. doi: 10.1016/j.jiec.2014.11.043. [DOI] [Google Scholar]
- 40.Obot I, Macdonald D, Gasem Z. Density functional theory (DFT) as a powerful tool for designing new organic corrosion inhibitors: Part 1: an overview. Corros. Sci. 2015;99:1–30. doi: 10.1016/j.corsci.2015.01.037. [DOI] [Google Scholar]
- 41.Lgaz H, Salghi R, Jodeh S, Hammouti B. Effect of clozapine on inhibition of mild steel corrosion in 10 M HCl medium. J. Mol. Liquids. 2017;225:271–280. doi: 10.1016/j.molliq.2016.11.039. [DOI] [Google Scholar]
- 42.Oukhrib R, Abdellaoui Y, Berisha A, Abou Oualid H, Halili J, Jusufi K, El Had MA, Bourzi H, El Issami S, Asmary FA. DFT, Monte Carlo and molecular dynamics simulations for the prediction of corrosion inhibition efficiency of novel pyrazolylnucleosides on Cu (111) surface in acidic media. Sci. Rep. 2021;11(1):1–18. doi: 10.1038/s41598-021-82927-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Singh A, Thakur S, Pani B, Chugh B, Lgaz H, Chung I-M, Chaubey P, Pandey A, Singh J. Solvent-free microwave assisted synthesis and corrosion inhibition study of a series of hydrazones derived from thiophene derivatives: Experimental, surface and theoretical study. J. Mol. Liq. 2019;283:788–803. doi: 10.1016/j.molliq.2019.03.126. [DOI] [Google Scholar]
- 44.Khaled K. Molecular modeling and electrochemical investigations of the corrosion inhibition of nickel using some thiosemicarbazone derivatives. J. Appl. Electrochem. 2011;41(4):423–433. doi: 10.1007/s10800-010-0252-1. [DOI] [Google Scholar]
- 45.Gupta NK, Verma C, Quraishi M, Mukherjee A. Schiff's bases derived from l-lysine and aromatic aldehydes as green corrosion inhibitors for mild steel: experimental and theoretical studies. J. Mol. Liq. 2016;215:47–57. doi: 10.1016/j.molliq.2015.12.027. [DOI] [Google Scholar]
- 46.Daoud D, Douadi T, Issaadi S, Chafaa S. Adsorption and corrosion inhibition of new synthesized thiophene Schiff base on mild steel X52 in HCl and H2SO4 solutions. Corros. Sci. 2014;79:50–58. doi: 10.1016/j.corsci.2013.10.025. [DOI] [Google Scholar]
- 47.Qiang Y, Guo L, Zhang S, Li W, Yu S, Tan J. Synergistic effect of tartaric acid with 2, 6-diaminopyridine on the corrosion inhibition of mild steel in 05 M HCl. Sci. Rep. 2016;6(1):1–14. doi: 10.1038/srep33305. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from Collins U. Ibeji, but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data are however available from the authors upon reasonable request and with permission of Collins U. Ibeji.