Abstract
Nivolumab, the programmed cell death 1 inhibitor, is a kind of immune checkpoint inhibitor commonly used to treat advanced cancers. Unfortunately, such drugs often induce various immune-related adverse events involving different body systems, with psoriasis being one of the skin toxicities. We report the clinical features of an exacerbated psoriasis induced by using nivolumab after three days in a patient with stage IIIc gastric adenocarcinoma. At the same time, we searched 27 case reports published from 2015 to 2021 over the world and systematically summarized the clinical manifestation of a total of 44 cases with psoriasis caused or exacerbated by Nivolumab. Commonly traditional treatment could be useful, and small molecule drugs such as apremilast are effective among some patients. However, more studies are needed to evaluate the efficacy and safety of biologics or small molecule drugs in treating psoriasis induced by nivolumab.
Keywords: Nivolumab, Psoriasis, PD-1, Apremilast
Highlights
-
•
Nivolumab is programmed cell death 1 inhibitor of the immune checkpoint inhibitor.
-
•
Nivolumab often induces immune-related adverse events in treating cancers.
-
•
Nivolumab could induce or exacerbate skin diseases such as psoriasis.
-
•
Commonly traditional treatment and small molecule drugs could effectively treat psoriasis.
1. Introduction
Immunotherapy is a new milestone for people fighting cancer [1], aiming to downgrade the immune system of humans attacking themselves as well as the cancer cells [2]. Immune checkpoint inhibitors (ICIs) occupy an important part of it [3]. ICIs commonly include Cytotoxic T-Lymphocyte Antigen-4 (CTLA-4) inhibitors, programmed cell death 1 (PD-1), and programmed cell death ligand 1(PD-L1) inhibitors [4,5]. Nivolumab targets the PD-1 on T cells applying to melanoma, non-small cell lung cancer, squamous cell carcinoma of head and neck, renal cell carcinoma, classical Hodgkin lymphoma, urothelial carcinoma, gastric adenocarcinoma, and so on [[3], [4], [5], [6], [7], [8], [9]].
Although Nivolumab is efficient in curing various cancers, it causes a variety of immune-related adverse events (irAEs) including its toxicities to the skin [1,[10], [11], [12], [13]]. Treating with ICIs included with Nivolumab makes Patients with preexisting immune disease prone to have their previous disease exacerbated [14]. As it is known to all that psoriasis is related to the immune system of humans [[15], [16], [17]]. Eruption of psoriasis or psoriasiform rash has been reported in various cancers treated with nivolumab, such as melanoma, lung cancer, renal cancer, Hodgkin's lymphoma, and hepatoma. Herein we reported the first patient with gastric cancer treated with nivolumab leading to the exacerbation of psoriasis. We systematically summarize the psoriasis cases of new-onset and exacerbated induced by nivolumab that have been reported previously.
2. Case report
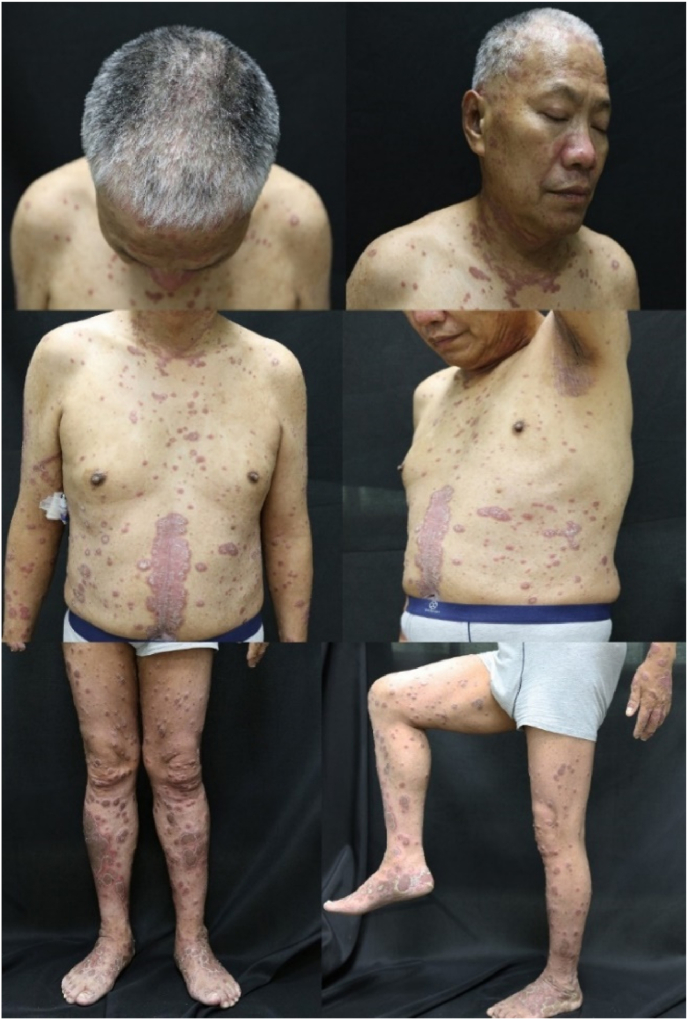
A 56-year-old man with a history of psoriasis of nearly 25 years, which had been controlled with topical therapy. The patient underwent a check of gastroscopy in April 2021, and the clinic-pathological and immunohistochemical results exhibited a poorly differentiated adenocarcinoma of the stomach. The patient has diagnosed with stage IIIc gastric adenocarcinoma (pT4N3M0, stage IIIc) in May 2021. After the first course of chemotherapy with mFOLFOX6, the patient experienced an acute severe liver injury. Nivolumab was used as an adjuvant immunotherapy combination with the chemotherapy on July 2021, after the treatment of the liver injury. Three days after using nivolumab, the patient developed generalized erythematous plaques with an overlying scale with a body surface area (BSA) of 30%. The scaly plaques involve areas of the scalp, face, axilla, trunk, limbs, palmoplantar, and nails (Fig. 1). To manage the exacerbation of psoriasis, the patient came to our hospital. Given his psoriatic history and the clinical manifestation, the exacerbation of psoriasis induced by applying Nivolumab was diagnosed. During the period, the patient has no pain in joints. The patient was treated with topical agents of steroids and tacalcitol in combination with oral compound Glycymhizin tablets.
Fig. 1.
Plaques with the scale of the patients involving the scalp, face, inverse areas, trunk, limbs, palmoplantar, and nails.
3. Literature review
Recently, there has been more new-onset or exacerbated psoriasis induced by monoclonal antibodies since the prevalence of chemical therapy in combination with immunotherapy in treating cancer. Herein, we summarized the features of 44 cases with different cancers that suffered psoriasis or psoriasiform reactions induced by nivolumab. At the same time, we try to review the studies of possible mechanisms and novel therapeutic strategies for this adverse event.
3.1. Method
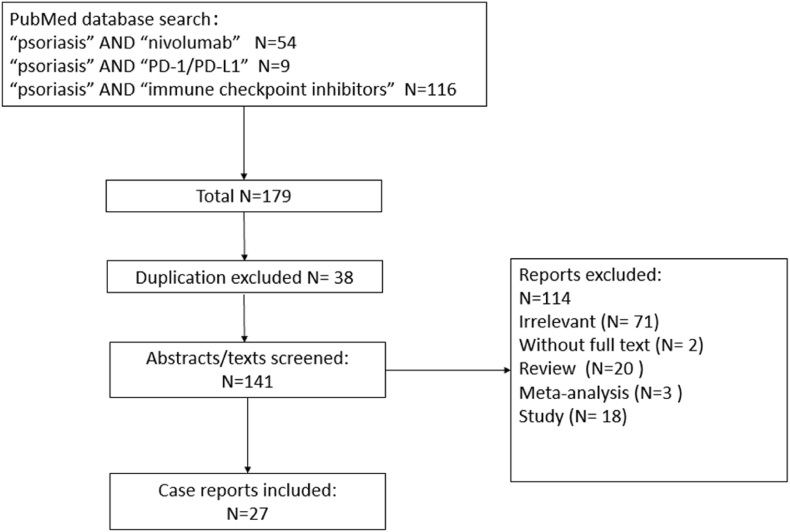
For this review, we selected “psoriasis” AND “Nivolumab”, “psoriasis” AND “PD-1/PD-L1”, “psoriasis” AND “immune checkpoint inhibitors” as the keywords to search mainly on the PubMed database 179 articles were identified. After eliminating duplicate cases (N = 38), 114 articles were excluded for the reasons of irrelevant (N = 71), no full text (N = 2), review (N = 20), meta-analysis (N = 3), study (N = 18).27 case reports remained from 2015 to 2021 Aug. 7th (Fig. 2). Plus with the case we reported, a total of 44 cases all experienced psoriasis as an adverse event induced by nivolumab. The information was recorded in Table 1.
Fig. 2.
Literature search flow diagram.
Table 1.
Psoriasis induced by immunotherapy of Nivolumab.
| number | sex | age/years old | cancer | latent period/day (s) | subtype of psoriasis | new-onset (N) or exacerbated (E) | family history | treatment | the outcome of psoriasis lesion | continue(C) or discontinue(D) using nivolumab | reported year |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | male | 80 | melanoma | 63 | plaque psoriasis | N | no | topical treatment + oral prednisolone | improved | C | 2015 [18] |
| 2 | male | 65 | melanoma | 21 | plaque psoriasis | E | NI | topical treatment + oral etretinate | improved | C | 2016 [19] |
| 3*** | male | 80 | lung cancer | 112 | plaque psoriasis and psoriatic arthritis | N | no | topical treatment + oral prednisone and MTX | improved | D | 2016 [20] |
| 4 | male | 87 | melanoma | 21 | plaque psoriasis | E | NI | topical treatment + oral prednisolone | improved | D | 2016 [21] |
| 5 |
male |
77 |
melanoma |
77 |
plaque and nail psoriasis |
NI |
NI |
topical treatment + oral retinoids |
improved |
D |
2017 [22] |
| 6 | male | 80 | melanoma | 28 | plaque psoriasis | NI | NI | topical treatment + oral prednisolone | improved | D | 2017 [22] |
| 7 | male | 64 | lung cancer | NE | guttate psoriasis and nail psoriasis | N | yes | topical treatment | improved | C | 2017 [23] |
| 8 | male | 72 | lung cancer | NE | plaque psoriasis and psoriatic arthritis | N | yes | topical treatment + oral prednisone and MTX | improved | C | 2017 [23] |
| 9 | male | 67 | lung cancer | 16 | plaque and pustular palmoplantar psoriasis | E | NI | topical treatment | improved | C | 2017 [23] |
| 10 | male | 59.2 | melanoma | 31 | plaque and palmoplantar psoriasis | E | NI | topical treatment | improved | C | 2017 [23] |
| 11 |
male |
81 |
lung cancer |
16 |
plaque and guttate psoriasis |
E |
NI |
topical treatment |
improved |
C |
2017 [23] |
| 12 | male | 58.9 | melanoma | 29 | plaque and guttate psoriasis | E | NI | topical treatment | improved | C | 2017 [23] |
| 13 | male | 67.5 | melanoma | 50 | plaque and guttate psoriasis | E | NI | topical treatment + oral acitretin | improved | C | 2017 [23] |
| 14 | male | 61.8 | lung cancer | 28 | plaque psoriasis | E | NI | topical treatment | improved | D | 2017 [23] |
| 15 | female | 59.3 | lung cancer | 36 | plaque and palmoplantar psoriasis | E | NI | topical treatment | improved | C | 2017 [23] |
| 16 | male | 81.5 | lung cancer | 98 | plaque and guttate psoriasis | E | NI | topical treatment + oral acitretin | improved | C | 2017 [23] |
| 17 | male | 65.9 | lung cancer | 19 | plaque psoriasis | E | NI | topical treatment + oral acitretin | improved | D | 2017 [23] |
| 18 |
male |
87 |
melanoma |
21 |
plaque and guttate psoriasis |
E |
NI |
oral general corticosteroid |
improved |
D |
2017 [23] |
| 19** | male | 65 | melanoma | 21 | plaque psoriasis | E | NI | topical treatment + oral acitretin | improved | C | 2017 [23] |
| 20 | male | 68 | lung cancer | 28 | palmoplantar psoriasis and psoriatic arthritis | N | no | topical treatment + oral prednisone and MTX | improved | D | 2017 [24] |
| 21 | male | 66 | lung cancer | 42 | plaque psoriasis | N | no | topical treatment + oral prednisolone | improved | C | 2017 [25] |
| 22 | male | 66 | lung cancer | NE | plaque psoriasis and psoriatic arthritis | N | NI | NI | improved | C | 2017 [25] |
| 23 | male | 47 | lung cancer | 154 | psoriatic arthritis and nail psoriasis | E | no | topical treatment + inject triamcinolone acetonide, methylprednisolone, and MTX | improved | C | 2017 [26] |
| 24*** |
male |
45 |
metastatic renal cell carcinoma |
14 |
plaque psoriasis |
N |
NI |
topical treatment |
NI |
D |
2017 [27] |
| 25** | male | 89 | melanoma | 14 | plaque psoriasis | N | NI | topical treatment | improved | C | 2017 [28] |
| 26 | male | 75 | lung cancer | 84 | plaque psoriasis | N | no | topical treatment | improved | C | 2018 [29] |
| 27 | male | 53 | lymphoma | 84 | plaque psoriasis | N | NI | topical treatment | improved | D | 2018 [30] |
| 28*** | female | 65 | melanoma | 154 | plaque psoriasis | E | NI | topical treatment | improved | D | 2018 [31] |
| 29 | male | 60 | lung cancer | 7 | plaque psoriasis | N | no | topical treatment + oral steroids | improved | NI | 2018 [32] |
| 30 | male | 58 | tongue carcinoma | 28 | inverse psoriasis | N | no | topical treatment + oral acitretin + phototherapy | improved | D | 2018 [33] |
| 31 | female | 74 | lung cancer | 180 | plaque psoriasis | E | NI | oral apremilast | improved | NI | 2019 [34] |
| 32* | male | 62 | hepatoma | NE | plaque psoriasis | NI | NI | oral etoposide | improved | D | 2019 [35] |
| 33 |
male |
64 |
lung cancer |
21 |
plaque and scalp psoriasis |
N |
no |
topical treatment |
clear |
D |
2019 [36] |
| 34 | female | 51 | lung cancer | 35 | plaque psoriasis | N | no | topical treatment | improved | C | 2020 [37] |
| 35*** | male | 54 | laryngeal carcinoma | 11 | plaque and nail psoriasis | E | NI | oral acitretin + phototherapy | improved | D | 2020 [38] |
| 36 | male | 59 | melanoma | NI | palmoplantar and nail psoriasis | N | no | topical treatment | improved | D | 2020 [39] |
| 37 | female | 71 | lung cancer | NI | plaque psoriasis | E | NI | topical treatment + oral acitretin + phototherapy | improved | D | 2020 [40] |
| 38 | male | 66 | metastatic renal cell carcinoma |
210 | palmoplantar psoriasis | N | no | oral retinoids and apremilast | improved | C | 2021 [41] |
| 39 | female | 61 | melanoma | 14 | plaque psoriasis | N | no | oral risankizumab-rzaa | clear | D | 2021 [42] |
| 40 |
male |
50 |
melanoma |
150 |
plaque psoriasis and psoriatic arthritis |
N |
no |
oral apremilast |
improved |
C |
2021 [43] |
| 41 | male | 70 | laryngeal carcinoma | 14 | plaque psoriasis | E | NI | oral apremilast | improved | C | 2021 [43] |
| 42** | male | 60 | lung cancer | 7 | plaque psoriasis | E | NI | oral apremilast | improved | C | 2021 [43] |
| 43** | male | 62 | melanoma | 90 | plaque psoriasis | E | NI | oral apremilast | improved | C | 2021 [44] |
| 44[this case] | male | 56 | gastric adenocarcinoma | 3 | plaque, scalp, palmoplantar, inverse, and nail psoriasis | E | no | topical treatment + oral compound Glycymhizin tablets | improved | C | 2021 |
NI: no information NE: not exactly.
No.32* This is an HIV(+) patient. No19,25,42,43**These four patients died of progression of cancer.
No.3,24,28,35***These four patients discontinued Nivolumab for a few weeks, then restarted it after treatment without exacerbation.
4. Results
Among the 44 cases, the number of male patients was more than that of female patients (86% vs 14%) (Fig. 3.1). The average age of the occurrence is 66.2 years old (45–89 years old). Given the data we researched, there were 8 types of cancers involving melanoma, lung cancer, laryngeal carcinoma, renal cell carcinoma, lymphoma, tongue carcinoma, hepatoma, gastric adenocarcinoma (Fig. 3.2). Patients with lung cancer occupied the majority of the number (46%), and the second type is melanoma, while, there is only 1 case for each type--lymphoma, tongue carcinoma, hepatoma, and gastric adenocarcinoma. Nearly half of the patients were new-onset (19/44) (Fig. 3.3). The latent period of the eruption ranges from 3 days to 210 days with average days of 53.5 days (cases without exact description were excepted), while that of patients with new-onset psoriasis is relatively longer than that of those who have exacerbated psoriasis (average days: 60.4 vs 48.6). About 84.1% (37/44) patients suffered plaque psoriasis, and 15.9% (7/44) patients suffered psoriatic arthritis. Only 1 case was reported with pustular palmoplantar psoriasis.
Fig. 3.
Part of the results: 3.1 the incidence is higher in males than females (38 vs 6), and the male-to-female ratio is about 6:1; 3.2 eight cancers have been reported--lung caner had the majority of the number (20/44), the second type is melanoma (16/44), renal cell carcinoma and laryngeal carcinoma both have 2 cases, while, there is only 1 case for lymphoma, tongue carcinoma, hepatoma, and gastric adenocarcinoma; 3.3 19 patients with new-onset psoriasis and 22 patients were exacerbated; 3.4 more than a half patients need not to stop Nivolumab.
For the treatment, 34.1% (15/44) patients were improved merely with topical steroids, vitamin D3 analogue, tacrolimus, and so on. Some patients needed oral steroids or acitretin (or both of them), and phototherapy could help the symptom. When it comes to patients with psoriatic arthritis, oral methotrexate and prednisolone or apremilast seem to be effective [[20], [23], [24], [25], [26], [43]]. Apremilast seems to provide a promising choice for those whose psoriasis is refractory as 5 patients got improved after being treated with apremilast [[34], [41], [43]]. Risankizumab-rzaa also helped a patient to get improved [42]. 18 of all the patients discontinued the immunotherapy of nivolumab for the severe eruption. Among all the patients, nearly a half (18/44) needed to discontinue the immunotherapy of Nivolumab (Fig. 3.4), and some of them (4/44) could restart Nivolumab later.
5. Discussion
We reported a patient with 25 years history of psoriasis which was controlled well with topical treatment before but was exacerbated by the adjuvant therapy of nivolumab because of gastric adenocarcinoma. To our knowledge, he was the first patient with a gastric tumor reported. We searched on the PubMed database and found another 43 cases with different cancers but all used nivolumab and suffered different subtypes of psoriasis. Nivolumab is an IgG4 monoclonal antibody targeting PD-1 of cytotoxic T-lymphocytes to destroy tumor cells [4,18,32]. Since it could activate the T-lymphocytes, nivolumab potentially may cause immune-related adverse events (irAEs). Based on the case reports and randomized clinical trials, nivolumab affected a lot of body systems, such as skin, gastrointestinal, endocrine, hepatic, pulmonary, renal, and so on [4,45]. While skin toxicity seems to be the first sign compared with other body systems, the most prevalent manifestations are erythema, maculopapular and pustulopapular rash [45]. New-onset psoriasis or exacerbation induced by immunotherapy of nivolumab is a rare result but has been reported previously [32], one review had summarized the data of psoriasis and psoriasiform reactions caused by immune checkpoint inhibitors [4], and the other one had reviewed 12 psoriasis reactions caused by PD-1/PD-L1 checkpoint inhibitors [31], another one had reviewed 5 exacerbated psoriasis cases caused by PD-1/PD-L1 checkpoint inhibitors [23]. This review was an update on nivolumab-caused psoriasis and mentioned the 44 cases in total.
We found the incidence is higher in males than females (38 vs 6), and the male-to-female ratio is about 6:1. Despite there being no gender difference in the incidence of psoriasis [16], the gender-associated differences of severity were observed that male patients who were prone to psoriasis of PASI ≥10 (moderate/severe) and PGA (severe/very severe) [46]. However, since this condition happens frequently among elder patients, maybe these features derive from tumors. Nevertheless, larger epidemiological research is needed to provide more information.
As many factors could trigger psoriasis, the pathogenesis remains unclear [16], with it is similar to this reaction. So some researchers tried to find out the molecular pathogenesis and its mechanisms [21,22,31,47]. Some believed that Nivolumab, the PD-1 inhibitor, could lead to the overactivity of T-lymphocytes to the stimulation, which could help to kill the tumor cells. However, because TNF-α, IL-17, and IL-22 secreted by Th1 and Th17 cells play a crucial role in this disease, it leads to these cells' overactivation resulting in the incidence of psoriasis [21,31]. Recently, others observed increased serum IL-6 levels in patients with psoriasis induced by nivolumab, and the level decreased among those who were not afflicted [22]. A team demonstrated in vivo that the mechanism of PD-1 inhibitors accelerating psoriasiform dermatitis is activating cytotoxic CD8 T cells, which results in the production of IL-6 [47].
As it is widely noticed that plaque psoriasis is the most common subtype of psoriasis [16], this reaction is no exception, while, patients with inverse and pustular palmoplantar psoriasis are rare. The patient reported by us had multiple rare manifestations for lesions involving the scalp, face, inverse areas, and palmoplantar. When it comes to the treatment strategy, the main features were summarized as follows.
Firstly, most patients could alleviate without stopping nivolumab, but as the management of skin toxicities recommended, stopping Nivolumab is needed if the rash covers over 30% of body surface area (BSA) with associated symptoms such as erythema, purpura, epidermal detachment [45].
Secondly, typical treatment of primary psoriasis is effective in this secondary situation, such as topical treatment with steroids or Vitamin D3 analogues in combination with oral prednisolone or acitretin are effective for most patients; phototherapy seems to be safe and effective for psoriasis inversa; methotrexate is needed for psoriatic arthritis [23]. While given the immunosuppressive effect may be adverse in treating tumor cells, cyclosporine seems to be inappropriate [23,43].
Thirdly, for some patients being insensitive to traditional treatment, the immunomodulating agent—apremilast seems to be a good choice [43,34,44]. Apremilast is an oral phosphodiesterase 4 inhibitor that could decrease serum levels of IL-17 A, IL-22, and TNF-α [[48], [49], [50], [51]]. Recently, A phase 3, double-blind placebo-controlled study evaluated the efficacy and safety of apremilast showing that 30 mg twice daily makes significant improvement among patients with mild to moderate psoriasis [49]. The most common treatment-emergent adverse events were diarrhea, headache, nausea, nasopharyngitis, and upper respiratory tract infection [49]. Another retrospective study evaluated the efficacy and safety of biological therapy or apremilast in treating psoriasis of patients with hematologic malignancy [48]. 76.2% (16/21) patients showed no recurrence of their hematologic malignancy, and only 2 patients showed an evolution [48]. While due to the limited data, biological therapy or apremilast should be applied cautiously to those patients with psoriasis induced by nivolumab.
6. Conclusion
Despite significant efficacy in treating various advanced cancers, nivolumab could trigger various skin toxicities such as psoriasis—one of the most depressing skin diseases. This secondary condition could be treated without discontinuing nivolumab for the majority of the patients. Generally, traditional treatment such as topical treatment with oral prednisolone or acitretin, and patients with psoriatic arthritis are recommended with methotrexate, while due to the bad condition of patients with cancers, more effective and safe treatments should be introduced. In addition, more studies with a larger population are needed to evaluate the efficacy and safety of using biologics or apremilast to treat psoriasis induced by Nivolumab.
Funding
None.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Handling Editor: Y Renaudineau
Contributor Information
Xiaojie Sun, Email: 769539972@qq.com.
Xiaole Mei, Email: pymeixiaole728@student.pumc.edu.cn.
Yi Liu, Email: liuyi@pumcderm.cams.cn.
References
- 1.de Miguel M., Calvo E. Clinical challenges of immune checkpoint inhibitors. Cancer Cell. 2020;38:326–333. doi: 10.1016/j.ccell.2020.07.004. [DOI] [PubMed] [Google Scholar]
- 2.Aikins M., Xu C., Moon J. Engineered nanoparticles for cancer vaccination and immunotherapy. Acc. Chem. Res. 2020;53:2094–2105. doi: 10.1021/acs.accounts.0c00456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Esfahani K., Roudaia L., Buhlaiga N., Del Rincon S.V., Papneja N., Miller W.H. A review of cancer immunotherapy: from the past, to the present, to the future. Curr. Oncol. 2020;27:87–97. doi: 10.3747/co.27.5223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cutroneo P., Ingrasciotta Y., Isgro V., et al. Psoriasis and psoriasiform reactions secondary to immune checkpoint inhibitors. Dermatol. Ther. 2021;34 doi: 10.1111/dth.14830. [DOI] [PubMed] [Google Scholar]
- 5.Carlino M.S., Larkin J., Long G.V. Immune checkpoint inhibitors in melanoma. Lancet. 2021;398:1002–1014. doi: 10.1016/s0140-6736(21)01206-x. [DOI] [PubMed] [Google Scholar]
- 6.Long G.V., Atkinson V., Lo S., et al. Combination nivolumab and ipilimumab or nivolumab alone in melanoma brain metastases: a multicentre randomised phase 2 study. Lancet Oncol. 2018;19:672–681. doi: 10.1016/s1470-2045(18)30139-6. [DOI] [PubMed] [Google Scholar]
- 7.Au L., Hatipoglu E., Robert de Massy M., et al. Cancer Cell; 2021. Determinants of Anti-PD-1 Response and Resistance in Clear Cell Renal Cell Carcinoma. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Doroshow D., Bhalla S., Beasley M., et al. PD-L1 as a biomarker of response to immune-checkpoint inhibitors, Nature reviews. Clin. Oncol. 2021;18:345–362. doi: 10.1038/s41571-021-00473-5. [DOI] [PubMed] [Google Scholar]
- 9.Braun D., Bakouny Z., Hirsch L., et al. Beyond conventional immune-checkpoint inhibition - novel immunotherapies for renal cell carcinoma, Nature reviews. Clin. Oncol. 2021;18:199–214. doi: 10.1038/s41571-020-00455-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Halle B.R., Betof Warner A., Zaman F.Y., et al. Immune checkpoint inhibitors in patients with pre-existing psoriasis: safety and efficacy. J Immunother Cancer. 2021;9 doi: 10.1136/jitc-2021-003066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhou X., Yao Z., Bai H., et al. Treatment-related adverse events of PD-1 and PD-L1 inhibitor-based combination therapies in clinical trials: a systematic review and meta-analysis. Lancet Oncol. 2021;22:1265–1274. doi: 10.1016/s1470-2045(21)00333-8. [DOI] [PubMed] [Google Scholar]
- 12.Tanaka R., Ichimura Y., Kubota N., et al. Differential involvement of programmed cell death ligands in skin immune responses. J. Invest. Dermatol. 2021 doi: 10.1016/j.jid.2021.06.026. [DOI] [PubMed] [Google Scholar]
- 13.Sangro B., Chan S., Meyer T., Reig M., El-Khoueiry A., Galle P. Diagnosis and management of toxicities of immune checkpoint inhibitors in hepatocellular carcinoma. J. Hepatol. 2020;72:320–341. doi: 10.1016/j.jhep.2019.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hoa S., Laaouad L., Roberts J., et al. Preexisting autoimmune disease and immune-related adverse events associated with anti-PD-1 cancer immunotherapy: a national case series from the Canadian Research Group of Rheumatology in Immuno-Oncology. Cancer Immunol. Immunother. 2021;70:2197–2207. doi: 10.1007/s00262-021-02851-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gran F., Kerstan A., Serfling E., Goebeler M., Muhammad K. Current developments in the immunology of psoriasis. Yale J. Biol. Med. 2020;93:97–110. [PMC free article] [PubMed] [Google Scholar]
- 16.Griffiths C., Armstrong A., Gudjonsson J., Barker J. Psoriasis, Lancet (London, England) 2021;397:1301–1315. doi: 10.1016/s0140-6736(20)32549-6. [DOI] [PubMed] [Google Scholar]
- 17.Greb J.E., Goldminz A.M., Elder J.T., et al. Psoriasis, Nat Rev Dis Primers. 2016;2 doi: 10.1038/nrdp.2016.82. [DOI] [PubMed] [Google Scholar]
- 18.Ohtsuka M., Miura T., Mori T., Ishikawa M., Yamamoto T. Occurrence of psoriasiform eruption during nivolumab therapy for primary oral mucosal melanoma. JAMA dermatology. 2015;151:797–799. doi: 10.1001/jamadermatol.2015.0249. [DOI] [PubMed] [Google Scholar]
- 19.Kato Y., Otsuka A., Miyachi Y., Kabashima K. Exacerbation of psoriasis vulgaris during nivolumab for oral mucosal melanoma. J. Eur. Acad. Dermatol. Venereol. : JEADV. 2016;30:e89–e91. doi: 10.1111/jdv.13336. [DOI] [PubMed] [Google Scholar]
- 20.Law-Ping-Man S., Martin A., Briens E., Tisseau L., Safa G. Psoriasis and psoriatic arthritis induced by nivolumab in a patient with advanced lung cancer. Rheumatology. 2016;55:2087–2089. doi: 10.1093/rheumatology/kew281. [DOI] [PubMed] [Google Scholar]
- 21.Matsumura N., Ohtsuka M., Kikuchi N., Yamamoto T. Exacerbation of psoriasis during nivolumab therapy for metastatic melanoma. Acta Derm. Venereol. 2016;96:259–260. doi: 10.2340/00015555-2212. [DOI] [PubMed] [Google Scholar]
- 22.Okiyama N., Tanaka R. Varied immuno-related adverse events induced by immune-check point inhibitors - nivolumab-associated psoriasiform dermatitis related with increased serum level of interleukin-6. Nihon Rinsho Men'eki Gakkai kaishi = Japanese journal of clinical immunology. 2017;40:95–101. doi: 10.2177/jsci.40.95. [DOI] [PubMed] [Google Scholar]
- 23.Voudouri D., Nikolaou V., Laschos K., et al. Anti-PD1/PDL1 induced psoriasis. Curr. Probl. Cancer. 2017;41:407–412. doi: 10.1016/j.currproblcancer.2017.10.003. [DOI] [PubMed] [Google Scholar]
- 24.Elosua-Gonzalez M., Pampin-Franco A., Mazzucchelli-Esteban R., et al. A case of de novo palmoplantar psoriasis with psoriatic arthritis and autoimmune hypothyroidism after receiving nivolumab therapy. Dermatol. Online J. 2017;23 [PubMed] [Google Scholar]
- 25.Sugiura Y., Fujimoto H., Yamamoto M., et al. [Psoriasis and psoriatic arthritis induced by nivolumab in a patient with advanced non-small-cell lung cancer], Gan to. Kagaku Ryoho. 2017;44:787–789. [PubMed] [Google Scholar]
- 26.Ruiz-Banobre J., Perez-Pampin E., Garcia-Gonzalez J., et al. Development of psoriatic arthritis during nivolumab therapy for metastatic non-small cell lung cancer, clinical outcome analysis and review of the literature. Lung Cancer. 2017;108:217–221. doi: 10.1016/j.lungcan.2017.04.007. [DOI] [PubMed] [Google Scholar]
- 27.Ruiz-Banobre J., Abdulkader I., Anido U., Leon L., Lopez-Lopez R., Garcia-Gonzalez J. Development of de novo psoriasis during nivolumab therapy for metastatic renal cell carcinoma: immunohistochemical analyses and clinical outcome. APMIS. 2017;125:259–263. doi: 10.1111/apm.12658. [DOI] [PubMed] [Google Scholar]
- 28.Murata S., Kaneko S., Harada Y., Aoi N., Morita E. Case of de novo psoriasis possibly triggered by nivolumab. J. Dermatol. 2017;44:99–100. doi: 10.1111/1346-8138.13450. [DOI] [PubMed] [Google Scholar]
- 29.Chujo S., Asahina A., Itoh Y., et al. New onset of psoriasis during nivolumab treatment for lung cancer. J. Dermatol. 2018;45:e55–e56. doi: 10.1111/1346-8138.14167. [DOI] [PubMed] [Google Scholar]
- 30.Kaloyannidis P., Al Shaibani E., Mashhour M., et al. De novo psoriasis vulgaris diagnosed after nivolumab treatment for refractory Hodgkin's lymphoma, completely resolved after autologous hematopoietic stem cell transplantation. Case Rep Hematol. 2018 doi: 10.1155/2018/6215958. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.De Bock M., Hulstaert E., Kruse V., Brochez L. Psoriasis vulgaris exacerbation during treatment with a PD-1 checkpoint inhibitor: case report and literature review. Case Rep. Dermatol. 2018;10:190–197. doi: 10.1159/000491572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Om A., Cardon B., Cohen G. Psoriasiform eruption on the face and extremities associated with nivolumab therapy. JAAD Case Rep. 2018;4:373–375. doi: 10.1016/j.jdcr.2017.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Troyanova-Slavkova S., Eickenscheidt L., Dumann K., Kowalzick L. [Initially undetected de novo psoriasis triggered by nivolumab for metastatic base of the tongue carcinoma] Hautarzt. 2018;69:674–680. doi: 10.1007/s00105-017-4109-y. [DOI] [PubMed] [Google Scholar]
- 34.Fattore D., Annunziata M.C., Panariello L., Marasca C., Fabbrocini G. Successful treatment of psoriasis induced by immune checkpoint inhibitors with apremilast. Eur. J. Cancer. 2019;110:107–109. doi: 10.1016/j.ejca.2019.01.010. [DOI] [PubMed] [Google Scholar]
- 35.Rios A., Cen P., Dinh B., Mays S.R., Patel A.B. Dramatic response of nivolumab-associated psoriasiform dermatitis to etoposide. Eur. J. Cancer. 2019;107:97–99. doi: 10.1016/j.ejca.2018.11.025. [DOI] [PubMed] [Google Scholar]
- 36.Tao Y., Sheng L., Ye-qiang L. A case of psoriasiform dermatitis induced by PD-1 inhibitor. J Clin Dermatol. 2019;48:668–670. doi: 10.16761/j.cnki.1000-4963.2019.11.006. [DOI] [Google Scholar]
- 37.Kilickap C.G.D.S., Guner G., Taban H., Dizdar O. Development of de novo psoriasis during nivolumab therapy in a patient with small cell lung cancer. J. Oncol. Pharm. Pract. 2020;26:256–258. doi: 10.1177/1078155219877234. [DOI] [PubMed] [Google Scholar]
- 38.George A., George R. Nivolumab (PD-1 inhibitor) induced exacerbation of psoriasis. Indian Dermatol Online J. 2020;11:261–262. doi: 10.4103/idoj.IDOJ_47_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Di Altobrando A., Bruni F., Alessandrini A., Starace M., Misciali C., Piraccini B.M. Severe de-novo palmoplantar and nail psoriasis complicating Nivolumab treatment for metastatic melanoma. Dermatol. Ther. 2020;33 doi: 10.1111/dth.13363. [DOI] [PubMed] [Google Scholar]
- 40.Politi A., Angelos D., Mauri D., Zarkavelis G., Pentheroudakis G. vol. 8. SAGE Open Med Case Rep; 2020. (A Case Report of Psoriasis Flare Following Immunotherapy: Report of an Important Entity and Literature Review). 2050313X19897707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mullangi S., Ponnam S., Lekkala M.R., Koya S. A case of de novo psoriasis secondary to nivolumab in a patient with metastatic renal cell carcinoma. Cureus. 2021;13 doi: 10.7759/cureus.15703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Glinos G.D., Fisher W.S., Morr C.S., Seminario-Vidal L. Nivolumab-induced psoriasis successfully treated with risankizumab-rzaa in a patient with stage III melanoma. JAAD Case Rep. 2021;11:74–77. doi: 10.1016/j.jdcr.2021.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mayor Ibarguren A., Enrique E.A., Diana P.L., Ana C., Pedro H.P. Apremilast for immune checkpoint inhibitor-induced psoriasis: a case series. JAAD Case Rep. 2021;11:84–89. doi: 10.1016/j.jdcr.2021.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Foti C., Tucci M., Stingeni L., et al. Successful treatment with apremilast of severe psoriasis exacerbation during nivolumab therapy for metastatic melanoma. Dermatol. Ther. 2021;34 doi: 10.1111/dth.14653. [DOI] [PubMed] [Google Scholar]
- 45.Haanen J., Carbonnel F., Robert C., et al. Management of toxicities from immunotherapy: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2017;28:iv119–iv142. doi: 10.1093/annonc/mdx225. [DOI] [PubMed] [Google Scholar]
- 46.Napolitano M., Mastroeni S., Fania L., et al. Sex- and gender-associated clinical and psychosocial characteristics of patients with psoriasis. Clin. Exp. Dermatol. 2020;45:705–711. doi: 10.1111/ced.14218. [DOI] [PubMed] [Google Scholar]
- 47.Tanaka R., Ichimura Y., Kubota N., et al. Activation of CD8 T cells accelerates anti-PD-1 antibody-induced psoriasis-like dermatitis through IL-6. Commun Biol. 2020;3:571. doi: 10.1038/s42003-020-01308-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cohen-Sors R., Fougerousse A.C., Reguiai Z., et al. Biological therapies or apremilast in the treatment of psoriasis in patients with a history of hematologic malignancy: results from a retrospective study in 21 patients. Clin. Cosmet. Invest. Dermatol. 2021;14:845–854. doi: 10.2147/CCID.S320098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gold L.S., Papp K., Pariser D., et al. Efficacy and safety of apremilast in patients with mild to moderate plaque psoriasis: results of a phase 3, multicenter, randomized, double-blind, placebo-controlled trial. J. Am. Acad. Dermatol. 2021 doi: 10.1016/j.jaad.2021.07.040. [DOI] [PubMed] [Google Scholar]
- 50.Papp K., Cather J., Rosoph L., et al. Efficacy of apremilast in the treatment of moderate to severe psoriasis: a randomised controlled trial. Lancet (London, England) 2012;380:738–746. doi: 10.1016/s0140-6736(12)60642-4. [DOI] [PubMed] [Google Scholar]
- 51.Edwards C., Blanco F., Crowley J., et al. Apremilast, an oral phosphodiesterase 4 inhibitor, in patients with psoriatic arthritis and current skin involvement: a phase III, randomised, controlled trial (PALACE 3) Ann. Rheum. Dis. 2016;75:1065–1073. doi: 10.1136/annrheumdis-2015-207963. [DOI] [PMC free article] [PubMed] [Google Scholar]