Highlights
-
•
Measures of Conduct Problems correlated with distinct brain connectivity at rest.
-
•
Shared brain connectivity mainly reflected dysconnectivity of the somatomotor network.
-
•
Functional characterization indicated altered motor, cognitive and reward processes.
Keywords: Functional connectivity, Somatomotor, Conduct disorder, Antisocial behaviors
Abstract
A recent meta-analysis of resting-state functional connectivity studies revealed that individuals exhibiting antisocial behaviors or conduct problems may show disrupted brain connectivity in networks underpinning socio-affective and attentional processes. However, studies included in the meta-analysis generally rely on small sample sizes and substantially differ in terms of psychometric scales and neuroimaging methodologies. Therefore, we aimed to identify reliable functional brain connectivity alterations associated with severity of conduct problems using a large sample of adolescents and two measures of conduct problems. In a sample of 1416 children and adolescents, mass-univariate analyses of connectivity measures between 333 cortical parcels were conducted to examine the relationship between resting-state functional cortical-cortical connectome and the severity of conduct problems using the Child Behavior Checklist (CBCL) and the Strengths and Difficulties Questionnaire (SDQ). At a liberal threshold, results showed that the functional brain connectivity significantly associated with conduct problems largely differ between the two scales. Indeed, only 21 pairs of brain regions overlapped between the CBCL and SDQ. Permutation feature importance of these 21 brain connectivity measures revealed that connectivity between precentral/postcentral gyri and lateral prefrontal cortex (both ventral and dorsal) were the most important features in explaining variance in conduct problems. The current study highlights that psychometric measures may yield distinct functional connectivity results. Moreover, severity of conduct problems in children and adolescents was mainly associated with deficient functional connectivity of somatomotor and ventral attention networks indicating potential alterations in motor, cognitive and reward processes.
1. Introduction
Conduct disorder (CD) is defined by serious and persistent patterns of behavior that violate the rights of others (i.e., aggressive, and rule-breaking behaviors) (APA, 2013). It has been suggested that approximately 5 % of children will display severe and persistent conduct problems (CP) and meet the criteria for CD (Bevilacqua et al., 2018, Fairchild et al., 2019). These children are known to display high levels of comorbid psychopathologies such as callous-unemotional traits (Frick and Thornton, 2014), but also attention-deficit/hyperactivity (ADHD) symptoms (Bird et al., 2006, Boylan et al., 2007, Costello et al., 2003, Nock et al., 2006, Wichstrøm et al., 2012). Past studies have shown that individuals with CP may demonstrate a variety of neurobiological impairments. Indeed, in a recent meta-analysis of functional neuroimaging studies, our research team observed that adolescents and adults exhibiting antisocial behaviors may be characterized by abnormal brain activity during fMRI tasks involving negative emotions processing, social cognition and cognitive control (Dugré et al., 2020). For example, scientific literature has extensively supported the role of the amygdala, medial and lateral prefrontal cortex, insula and cingulate cortex in our understanding of the neural correlates of CP during functional magnetic resonance imaging (fMRI) tasks (Alegria et al., 2016, Dugré et al., 2020, Noordermeer et al., 2016, Raschle et al., 2015, Wong et al., 2019). However, in comparison to other neuroimaging modalities, the functional brain connectivity underpinning CP remains largely understudied.
In the last decade, researchers have aimed to identify intrinsic functional networks which regroup reliable temporally correlated brain regions at rest. Indeed, these large-scale networks usually include the medial fronto-parietal (e.g. default-mode network), occipital (e.g. medial and lateral visual), pericentral (e.g. sensorimotor, somatomotor), dorsal fronto-parietal (e.g. dorsal attention), lateral fronto-parietal (e.g. cognitive control), midcingulo-insular (e.g. salience, ventral attention, cingulo-opercular) networks (Gordon et al., 2016, Schaefer et al., 2018, Uddin and Spreng, 2019, Yeo et al., 2011). Resting-state functional connectivity has recently gained considerable attention in the investigation of the neurobiological mechanisms involved in antisocial behaviors. In a recent meta-analysis of resting-state functional connectivity studies, we found that antisocial subjects exhibited prominent alterations in functional connectivity in nodes of the Default Mode Network (i.e., ventro- and dorso-medial PFC and posterior cingulate cortex), Dorsal Attention (i.e., Frontal eye field) and Ventral Attention regions (i.e., anterior midcingulate cortex/pre-supplementary motor area) as well as in the amygdala (Dugré and Potvin, 2021). Using data from the ABCD study (n = 9636), authors have recently showed that the severity of CP was significantly associated with average within-connectivity in the Dorsal Attention, whereas reduced connectivity within the Default Mode Network was rather associated with callous-unemotional traits (Umbach and Tottenham, 2020). Similarly, reduced PCC-vmPFC connectivity (within-Default Mode Netwrok) was found in inmates with compared to those without psychopathic traits (Motzkin et al., 2011). However, some recent meta-analytic evidence suggests that dysconnectivity of the Default Mode Network may not be specific to CP or callous-unemotional traits but may rather act as a transdiagnostic neurobiological markers (Doucet et al., 2020). These results justify the need to search for specific neurobiological markers of CP.
Growing evidence suggests that antisocial behaviors may be mostly associated with impairments of between- rather than within-network connectivity. In a large sample of adults (n = 1003), some researchers have found that anger-aggression was mainly correlated with connectivity between the PCC (Default Mode Network) and Visual, Somatomotor and Ventral Attention as well as between the Fronto-Parietal and Somatomotor (Weathersby et al., 2019). Antisocial behaviors appear to be also associated with deficient functional connectivity between Ventral Attention regions and Default Mode Network (Pujol et al., 2012) and Frontoparietal (Cohn et al., 2015) as well as between the Dorsal Attention and Default Mode Network and Ventral Attention (Shannon et al., 2011). An increasing number of studies also indicate that antisocial behaviors (e.g., aggression) may be mainly related with altered resting-state connectivity between the amygdala and brain regions involved in the Default Mode Network (Motzkin et al., 2011, Sukhodolsky et al., 2022), Frontoparietal and Ventral Attention networks (Sukhodolsky et al., 2022). Overall, these results indicate that most of the resting-state connectivity alterations are found between the Default Mode Network, Dorsal and Ventral Attention and Somatomotor. In comparison with other pediatric psychiatric disorders, several brain connectivity (e.g., Default Mode Network) associated with CP are also reported in ADHD (Sutcubasi et al., 2020), anxiety (Xu et al., 2019) and depressive (Kaiser et al., 2015) disorders. Once again, these highlight the importance of clarifying the deficits in resting-state connectivity that may be specifically associated with antisocial behaviors, compared to other psychopathologies.
Despite the relevance of the above-mentioned findings, there are several limitations that tamper scientific progress in the field. First, there are discrepancies in results across studies which may be explained by different methodologies such as restricting analyses to a priori defined seeds (e.g., amygdala) or a limited number of large-scale networks (e.g., Default Mode Network). Likewise, the diversity of psychometric scales used to assess antisocial behaviors and CP may contribute to discrepant results. Studies on resting-state functional connectivity usually include small sample sizes (median: 22 subjects, see Dugré and Potvin, 2021), which may increase the false positive rate. Recently, some have argued that in resting-state functional connectivity investigations, stability and reproducibility in brain-behavior relationships may require thousands of individuals (Marek et al., 2022), thus highlighting the need for larger sample size to investigate the neural correlates of CP.
Therefore, the purpose of the study was twofold. First, we aimed to address these issues by investigating the cortico-cortical and amygdala-cortical functional connectivity at rest associated with two distinct measures of CP, using a large sample of 1416 children and adolescents. We hypothesized that CP will be associated with disrupted functional connectivity within-Default Mode Network, and between Default Mode Network and Dorsal Attention, Ventral Attention, Somatomotor networks, as well as between the amygdala and these networks. Then, as exploratory analyses, we examined whether the significant brain connectivity associated with CP may also be related to other psychopathologies such as irritability, ADHD symptoms and callous-unemotional traits.
2. Methods
2.1. Participants and neuroimaging acquisition parameters
Data from 2200 participants were obtained from the Healthy Brain Network (HBN), an ongoing initiative in New York area (USA) that aims to investigate heterogeneity and impairment in developmental psychopathology (5–21 years old) (Alexander et al., 2017). The HBN adopted a community-referred recruitment model in which advertisements was provided to community members, educators, parents. Exclusion criteria were impairments that prevents full participation in the study (e.g., serious neurological disorders, hearing or visual impairments), neurodegenerative disorder, acute encephalopathy, acute intoxication, and serious psychiatric disorders (recent diagnosis of schizophrenia and/or manic episode). Supplemental information is provided elsewhere (Alexander et al., 2017).
From the 2200 participants included in the Data Release 7.0, 1583 participants contained available functional neuroimaging data. Written assent was obtained from participants younger than 18 years old, and written consent was obtained from their legal guardians. Written informed consent was obtained from participants aged 18 or older prior to enrolling in the study. The original HBN study was approved by the Chesapeake Institutional Review Board (now Advarra Inc., see https://www.advarra.com/). The current study was approved by the local ethics committee.
MRI acquisition took place at three different sites: mobile 1.5 T Siemens Avanto in Staten Island, 3 T Siemens Tim Trio at Rutgers University Brain Imaging Center (RUBIC), and 3 T Siemens Prisma at the CitiGroup Cornell Brain Imaging Center (CBIC) (acquisition protocols and parameters can be found in *** S1, in (Alexander et al., 2017) as well as https://fcon_1000.projects.nitrc.org/indi/cmi_healthy_brain_network/). Data at the CBIC were obtained using the same data acquisition protocol implemented at RUBIC. The acquisition of the two resting-state scans lasted 5 min each, during which participants viewed a fixation cross located at the center of the computer screen. Data for the Siemens Avanto were acquired in a single run lasting 10 min.
2.2. Main assessments
Conduct problems were assessed using the Child Behavior Checklist (CP-CBCL, Achenbach and Rescorla, 2001), which comprised 33 items from Aggressive (20 items) and Rule-Breaking (11 items) syndromes scales. Parents rated each item using a 3-point scale (0 = not true to 2 = very true)(α = 0.93). We also used the 5-item CP scale (2 items on aggressive and 3 on non-aggressive rule-breaking behaviors) of the Strength and Difficulties Questionnaire (CP-SDQ, Goodman, 2001), which showed acceptable internal consistency (α = 0.72). Pearson’s correlation between these two scales of CP revealed moderate-strong association (r = 0.788).
Exploratory analyses were conducted to investigate the association between brain connectivity and irritability, ADHD symptoms and callous-unemotional traits. Irritability was measured using the Parent-report form of the Affective Reactivity Index (Stringaris et al., 2012). This 6-item scale (0 = Not True to 2 = Certainly True) showed excellent internal consistency (α = 0.90). ADHD symptoms were measured using the total score of the parent-report form of the Strengths and Weakness of ADHD-symptoms and Normal-behavior (Swanson et al., 2012). This scale, which contains 18 items (+3 = Far Below Average to −3 = Far Above average), also showed excellent internal consistency (α = 0.95). Finally, callous-unemotional traits were measured using the parent-report form of the Inventory of Callous-Unemotional Traits (Essau, Sasagawa et Frick, 2006). In our study, we examined the relationship between brain connectivity and the three subscales of this 24-item questionnaire (i.e., Callousness, Uncaring, and Unemotional traits), separately, given that they are differently associated with CP and externalizing behaviors. Indeed, a recent meta-analysis showed that the unemotional subscale was weakly related with externalizing problems, compared to the two other subscales (Cardinale and Marsh, 2020). The Callousness (e.g., ‘Shows no remorse’, α = 0.74), Uncaring (e.g., reversed ‘Tries not to hurt others’ feelings’, α = 0.84) and Unemotional (e.g., ‘Does not show emotions’, α = 0.79) subscales demonstrated good internal consistency.
2.3. fMRI data preprocessing
Functional images were realigned, corrected for motion artifacts with the Artifact Detection Tool (Power et al., 2014)(ART, setting a threshold of 0.9 mm subject ART’s composite motion and a global signal threshold of Z = 5) with the implemented in CONN Toolbox (Whitfield-Gabrieli and Nieto-Castanon, 2012), bandpass filtered (0.01 Hz < f < 0.10 Hz) and co-registered to the corresponding anatomical image. The anatomical images were segmented (into grey matter, white matter, and cerebrospinal fluid) and normalized to the Montreal Neurological Institute (MNI) stereotaxic space. Functional images were then normalized based on structural data, spatially smoothed with a 6 mm full-width-at-half-maximum (FWHM) 3D isotropic Gaussian kernel and resampled to 2 mm3 voxels. For the preprocessing, the anatomical component-based noise correction method (aCompCor strategy, Behzadi et al., 2007), was employed to remove confounding effects from the BOLD time series, such as the physiological noise originating from the white matter and cerebrospinal fluid. This method was found to increase the validity and sensitivity of analyses (Chai et al., 2012). In the current study, preprocessed images were manually checked for each of the 1583 participants. We found pre-processing issues due to the poor quality of images in 108 participants, which resulted in the software unable to adequately detect & segment volumes into tissue classes (i.e., grey matter, white matter, and cerebrospinal fluid). In addition, 59 adolescents exhibited high movements (exceeding 3 mm) leaving a remaining sample of 1416 participants. Finally, given that the CBCL measures children and adolescent psychopathologies (<18 years old), 56 adults subjects were excluded, leaving a final sample size of 1360 adolescents.
2.4. Cortico-cortical and Amygdalo-cortical functional connectivity
To examine the cortico-cortical connectivity, we extracted functional connectivity between cortical parcels derived from the Gordon’s parcellation (i.e., 333 cortical parcels) covering the whole cortex (Gordon et al., 2016), as used in the ABCD study (Marek et al., 2019). These 333 cortical parcels are grouped into 13 intrinsic networks, namely Auditory, Cingulo-Opercular, Cingulo-Parietal, Default Mode Network, Ventral and Dorsal Attention, Frontoparietal, Somatomotor (Hand), Somatomotor (Mouth), Retrosplenial-Temporal, Salience, Visual, and Unassigned (None). We additionally included left and right amygdala from the FSL Harvard-Oxford Atlas, provided in the CONN Toolbox. Physiological noise, realignment parameters, and movement artifacts were regressed out as confounding effects from the BOLD time-series for each parcel.
2.5. Statistical analyses
2.5.1. Mass-Univariate analysis
In the first-level analysis, Pearson’s correlation coefficients between the residual BOLD time course from each parcel and the time course of all other 332 parcels, for each subject. The same was done for amygdala regions and all Gordon 333 parcels. Coefficients were converted to normally distributed z-scores using a Fisher Z-Transformation. Second-level analyses were conducted using mass-univariate linear regression to examine relationships with CP derived from the CBCL and the SDQ, removing the effect of age, sites, sex, percentage of valid scans and framewise displacement. To identify brain connectivity that were reliably associated with CP, we conducted mass-univariate linear regression analyses on 5,000 random subsamples using 90 % of the total sample at each iteration. Brain connectivity were then considered as statistically associated with CP if the average p-value across the 5,000 iterations met the uncorrected threshold of p < 0.005. This somewhat liberal threshold was used to keep brain connectivity that has acceptable association with CP. After having selected the most correlated brain connectivity across CBCL and the SDQ (p < 0.005), we kept only those overlapping between the two scales which may characterize the core features underpinning. These steps were conducted to adequately control for type II errors (i.e., false negative due to stringent thresholding) as well as decreasing type I errors by limiting spurious and scale-specific results. Indeed, selecting the most stable brain connectivity measures across the 5,000 subsampling iterations and those overlapping between scales measuring the same construct may reduce the risk for falsely accepting the null hypothesis on spurious brain connectivity.
2.5.2. Permutation feature importance
After having identified brain connectivity associated with CP across scales, we investigated whether brain connectivity results differed between scales regarding their importance in explaining variance of the CBCL and SDQ. We calculated feature importance by conducting a multivariate linear regression which included the resulting brain connectivity measures (independent variables) in association with CP severity (dependent variable), respectively. We permutated each brain connectivity measure 100 times on a test set (20 % of the data) and compared R2 scores between the baseline model on the train set (80 % of the data without permutations). Given that results may vary depending on the selected test set, we ran permutation importance on 1,000 randomly selected test set and averaged estimates. Compared to the base model, changes in R2 score would therefore indicate the relative importance of a particular feature. Finally, we examined differences in feature importance of each brain connectivity between the CBCL and SDQ with Fisher r-to-z transformation (p < 0.05, two-tailed). Subanalyses were performed using the same statiscal procedure to examine whether results may have been driven by developmental period (i.e., childhood, adolescence) and scanner strength (i.e., 3 Tesla, 1.5 Tesla).
2.6. Exploratory analyses
As exploratory analyses, we sought to examine whether the brain connectivity associated with severity of CP were also related to other psychopathologies, namely irritability, ADHD symptoms and callous-unemotional traits. To do so, we conducted partial correlations between brain connectivity and psychopathologies, adjusting for the effect of age, sites, sex, percentage of valid scans and framewise displacement.
2.7. Functional decoding
Functional decoding was conducted to examine the neurocognitive domains (i.e., task fMRI) underlying functional connectivity between two ROIs that are associated with CP, using the BrainMap environment. The BrainMap environment include a repository of neuroimaging studies which contain brain coordinates and metadata (e.g., sample size, behavioral categories) for more than 21,083 experiments. Brain coordinates (foci) and metadata (e.g., behavioral categories, sample size, contrasts) of papers included in the repository are coded by their research team, as well as authors of original papers via Scribe (http://brainmap.org/scribe/) and then verified by BrainMap staff. BrainMap ontology rely on 60 behavioral categories grouped into 5 domains: cognition, emotion, perception, action, interoception. The Behavioral Analysis plugin for Multi-image Analysis GUI (Lancaster et al., 2012) (ric.uthscsa.edu/mango) relies on the binomial “success” probabilities of activation foci within a ROI than expected for random spatial distribution for a behavioral subdomain. Z-score of 3.0 or more represent p < 0.05 Bonferroni corrected for multiple comparisons.
In the current study, 49 categories from 4 different neurocognitive domains were included (i.e., Action, Emotion, Cognition, Perception) given that none of the parcel was significantly associated with Interoception subcategories. Then, each parcel of a given brain connectivity (parcel A & parcel B) was characterized by a binary set of 49 behavioral categories (0 [Z < 3] and 1 [Z ≥ 3]). We created an adjacency matrix (49 categories-by-49 categories) representing the connected categories between parcel A and parcel B. Then, we summed adjacency matrices for all brain connectivity measure significantly associated with CP, separately for positive and negative correlations. Finally, behavioral categories at a node-level were ranked based on their number of edges (degree centrality) and influence across the network (betweenness centrality). We also examined what behavioral domains were the most frequently reported across brain connectivity. These analyses were conducted with python’s NetworkX package (Hagberg et al., 2008).
3. Results
3.1. Cortico-cortical and Amygdalo-cortical functional connectivity
Mass univariate analysis revealed significant functional brain connectivity associated with CP-CBCL (231 connections at a p < 0.005 uncorrected threshold) and CP-SDQ (269 connections at a p < 0.005 uncorrected threshold). From these results, only 21 brain connections were shared across both scales (i.e., 10 positively and 11 negatively associated with CP, see Fig. 1A-C and supplementary material). Overall, brain connectivity measures of CP were mainly driven by nodes of the Somatomotor (6 out of 21 connections) and Ventral Attention networks (4 out of 10 positive connections), but also with unassigned parcels from the Gordon Atlas (None: 4 connections). More precisely, severity of CP was positively associated with functional connectivity within-Somatomotor (2 connections), between Frontoparietal and unassigned parcels (i.e., bilateral posterior hippocampus – Frontal Eye Fields) but also between Ventral Attention and Default Mode Network, Dorsal Attention, Frontoparietal and Somatomotor. Furthermore, CP was negatively associated with functional connectivity between cingulo-opercular & visual (2 connections), Somatomotor & Salience network (2 connectivitiy between precentral & dACC), auditory & cingulo-opercular & Default Mode Network as well as within-Default Mode Network.
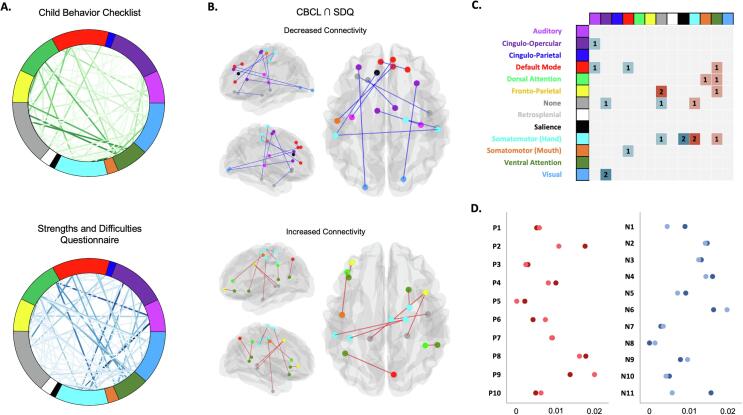
Fig. 1.
Associations between cortico-cortical connectivity and Conduct Problems across different scales. A. Weight (F-value) of each significant (p < 0.005) cortico-cortical connectivity across 13 networks of the Gordon (333 parcels, Gordon et al., 2015) after 5,000 random subsampling using 90 % of the sample in association with Conduct Problems scales derived from the Child Behavior Checklist (CP-CBCL) and the Strengths and Difficulties Questionnaire (CP-SDQ). B. Connectivity positively and negatively associated with Conduct Problems that intersected between the CBCL and SDQ (Red edges = positive associations; Blue edges = negative associations). C. Adjacency matrix showing significant within- and between-network connectivity results associated (Red = Positively; Blue = Negatively) with CP. D. Feature importance (R2 score with Standard Deviation) in association with severity of Conduct Problems for the Child Behavior Checklist (CP-CBCL) and the Strengths and Difficulties Questionnaire (CP-SDQ). Permutation importance was conducted by permutating each of the 21 cortico-cortical brain connectivity in a multivariate linear regression 100 times on a test set (20 % of the data) repeated 1,000 using Monte-Carlo cross-validation. Red dots = brain connectivity positively associated with CP; Blue dots = brain connectivity negatively associated with CP. Darker colors = CBCL & Lighter colors = SDQ. Please refer to Table 1 for more detailed information about brain connectivity. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
When examining feature importance of the 21 connections in a multivariate linear regression, we observed that between CBCL and SDQ, brain connectivity measures had roughly similar importance (see Table 1), except for P5 (CBCL > SDQ), N8 (SDQ > CBCL) and N11 (CBCL > SDQ). The top 5 most important features were (please refer to Table 1): 1) P8 connection: Premotor-Lateral OFC (R2 change 0.016-0.018); 2) N6 connection: Lateral OFC-SMA (R2 change 0.016-0.020); 3) P9 connection: Precentral-dlPFC (R2 change 0.014-0.020); 4) P2 connection: Lateral PFC-vlPFC (R2 change 0.011-0.018) and 5) N4 connection: dmPFC-Lateral PFC (R2 change 0.014-0.016). The least important feature was N8 connection: dACC-Postcentral (R2 change 0-0.001).
Table 1.
Multivariate Feature Importance (resting-state brain connectivity) associated with Conduct Problems across assessments tools.
| Names | Parcel 1 (Network) | Parcel 2 (Network) | Assessments |
Comparisons |
||
|---|---|---|---|---|---|---|
| r(CP-CBCL) | r(CP-SDQ) | Z | p | |||
| Positive Associations | ||||||
| P1 | pSMG (DA) | TPJ (VA) | 0.070 | 0.075 | 0.290 | 0.772 |
| P2 | lPFC (FP) | vlPFC (VA) | 0.132 | 0.104 | 1.630 | 0.103 |
| P3 | pHipp (None) | FEF (FP) | 0.052 | 0.046 | 0.350 | 0.728 |
| P4 | pHipp (None) | FEF (FP) | 0.100 | 0.089 | 0.640 | 0.523 |
| P5 | PoCG (SH) | PrCG (SH) | 0.043 | 0.000 | 2.480 | 0.013 |
| P6 | PoCG (SH) | PrCG (SH) | 0.063 | 0.085 | 1.270 | 0.203 |
| P7 | PrCG(SH) | pITG (None) | 0.095 | 0.094 | 0.060 | 0.954 |
| P8 | PMC (SH) | lOFC (VA) | 0.133 | 0.127 | 0.350 | 0.727 |
| P9 | PrCG(SM) | dlPFC (DA) | 0.117 | 0.141 | 1.400 | 0.162 |
| P10 | AG (VA) | PCUN (DMN) | 0.068 | 0.078 | 0.580 | 0.563 |
| Negative Associations | ||||||
| N1 | pINS (A) | dmPFC (DMN) | 0.095 | 0.066 | 1.680 | 0.093 |
| N2 | aINS (CO) | LG (V) | 0.121 | 0.119 | 0.120 | 0.907 |
| N3 | SMA (CO) | Heschl (A) | 0.115 | 0.112 | 0.170 | 0.861 |
| N4 | dmPFC (DMN) | lPFC (DMN) | 0.127 | 0.120 | 0.410 | 0.684 |
| N5 | lOFC (None) | ITG (None) | 0.096 | 0.084 | 0.700 | 0.487 |
| N6 | lOFC (None) | SMA (CO) | 0.128 | 0.141 | 0.760 | 0.448 |
| N7 | dACC (S) | PoCG (SH) | 0.052 | 0.057 | 0.290 | 0.772 |
| N8 | dACC (S) | PoCG (SH) | 0.000 | 0.034 | 1.960 | 0.050 |
| N9 | PrCg (SH) | pMTG (None) | 0.088 | 0.098 | 0.580 | 0.562 |
| N10 | PoCg(SM) | pgACC (DMN) | 0.070 | 0.063 | 0.410 | 0.685 |
| N11 | V2 (V) | lPFC (CO) | 0.125 | 0.075 | 2.900 | 0.004 |
Regarding amygdalo-cortical functional brain connectivity, analyses revealed that the CP-CBCL was negatively associated with functional connectivity between the right amygdala and the left (F = 10.38, p = 0.002) and right (F = 9.16, p = 0.004) ventral PCC (BA 23). Additionally, the CP-SDQ only showed negative association between the right amygdala and the pMTG (FP) (F = 12.57, p < 0.001 uncorrected). Thus, no significant connectivity intersected between the two scales, which suggest low reliability in amygdala connectivity across CP scales.
3.2. Testing for effects of developmental stage and scanning sites
Testing for differences in features importance across developmental periods yielded significant differences when examining the features’ relationships with CP-CBCL. Indeed, importance of N2 (aINS-Lingual, Z = 4.44) and N3 (SMA-Heschl, Z = 2.4) significantly increased in adolescence, whereas importance of P3 (pHipp-FEF, Z = 3.13), P10 (AG-PCUN, Z = 2.6), N4 (dmPFC-lPFC, Z = 2.45), N5 (lOFC-ITG, Z = 3.64), and N10 (PoCg-pgACC, Z = 3.1) decreased in adolescence. However, when examining feature importance between developmental stages using the CP-SDQ, no significant differences were observed (see supplementary material for complete results).
Moreover, when comparing features importance between scanner strengths (i.e., 3-tesla versus 1.5-tesla), importance of N4 (dmPFC-lPFC) and N6 (lOFC-SMA) were significantly stronger in the 3 T subsample for both CBCL and SDQ. Also, P4 (pHipp-FEF), N1 (pINS-dmPFC) and N9 (PrCG-pMTG) showed stronger importance in the 3 T subsample compared to the 1.5 T subsample when using the CP-CBCL, whereas N10 (PoCG-pgACC) showed stronger importance in the 1.5 T compared to the 3 T when using the CP-SDQ (see supplementary material).
3.3. Exploratory analyses
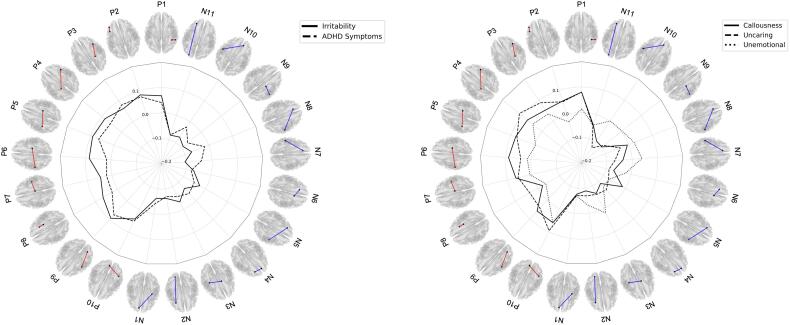
Partial correlations revealed that irritability, ADHD symptoms, callousness, uncaring but not unemotional traits were associated with brain connectivity (see Fig. 2 & Table 2). Indeed, irritability showed strong associations with N7 (dACC-PoCG, r = -0.107), P9 (PrCG-dlPFC, r = 0.094), and P5 (PoCG-PrCG, r = 0.092). Top brain connectivity measures associated with ADHD symptoms were N11 (V2-lPFC, r = -0.081), N6 (lOFC-SMA, r = -0.08), and P3 (pHipp-FEF, r = 0.08).
Fig. 2.
Relationship between functional brain connectivity associated with Conduct Problems and other psychopathologies. Please refer to Table 1 for complete list of brain connectivity measure. Red lines = Positive associations with conduct problems; Blue lines = Negative associations with conduct problems. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Table 2.
Relationship between resting-state brain connectivity associated with Conduct Problems and other psychopathologies.
| Names | Brain Connectivity |
Psychopathologies |
|||||
|---|---|---|---|---|---|---|---|
| Parcel 1 (Network) | Parcel 2 (Network) | Irritability r | ADHD Symptoms r | Callousness r | Uncaring r | Unemotional r | |
| Positive Associations | |||||||
| P1 | pSMG (DA) | TPJ (VA) | 0.068 | 0.041 | 0.08 | 0.08 | 0.014 |
| P2 | lPFC (FP) | vlPFC (VA) | 0.084 | 0.076 | 0.056 | 0.053 | −0.03 |
| P3 | pHipp (None) | FEF (FP) | 0.064 | 0.080 | 0.053 | 0.088 | 0.034 |
| P4 | pHipp (None) | FEF (FP) | 0.085 | 0.056 | 0.073 | 0.114 | 0.046 |
| P5 | PoCG (SH) | PrCG (SH) | 0.092 | 0.066 | 0.080 | 0.074 | −0.005 |
| P6 | PoCG (SH) | PrCG (SH) | 0.086 | 0.017 | 0.089 | 0.041 | 0.015 |
| P7 | PrCG(SH) | pITG (None) | 0.046 | 0.022 | 0.066 | 0.057 | 0.009 |
| P8 | PMC (SH) | lOFC (VA) | 0.066 | 0.025 | −0.022 | 0.036 | −0.037 |
| P9 | PrCG(SM) | dlPFC (DA) | 0.094 | 0.076 | 0.058 | 0.042 | −0.008 |
| P10 | AG (VA) | PCUN (DMN) | 0.042 | 0.052 | 0.061 | 0.096 | 0.061 |
| Negative Associations | |||||||
| N1 | pINS (A) | dmPFC (DMN) | −0.062 | −0.042 | −0.076 | −0.071 | −0.069 |
| N2 | aINS (CO) | LG (V) | −0.061 | −0.069 | −0.089 | −0.069 | −0.032 |
| N3 | SMA (CO) | Heschl (A) | −0.033 | −0.056 | −0.067 | −0.063 | 0.018 |
| N4 | dmPFC (DMN) | lPFC (DMN) | −0.067 | −0.04 | −0.09 | −0.056 | −0.073 |
| N5 | lOFC (None) | ITG (None) | −0.026 | −0.051 | −0.014 | −0.077 | −0.073 |
| N6 | lOFC (None) | SMA (CO) | −0.069 | −0.08 | −0.07 | −0.089 | −0.022 |
| N7 | dACC (S) | PoCG (SH) | −0.107 | −0.042 | −0.036 | −0.068 | 0.039 |
| N8 | dACC (S) | PoCG (SH) | −0.072 | −0.017 | −0.006 | −0.036 | 0.023 |
| N9 | PrCg (SH) | pMTG (None) | −0.091 | −0.068 | −0.098 | −0.088 | 0.003 |
| N10 | PoCg(SM) | pgACC (DMN) | −0.073 | −0.025 | −0.095 | −0.127 | 0.001 |
| N11 | V2 (V) | lPFC (CO) | −0.082 | −0.081 | −0.043 | −0.048 | −0.043 |
Regarding CU traits, severity of uncaring traits was mainly associated with N10 (PoCG-pgACC, r = -0.127), P4 (pHipp-FEF, r = 0.114), and P10 (AG-PCUN, r = 0.096), whereas callousness show stronger correlation with N9 (PrCG-pMTG, r = -0.098), N10 (PoCG, r = -0.095), and N4 (dmPFC-lPFC, r = -0.09).
3.4. Functional decoding
As shown in Fig. 3, the functional brain connectivity measures associated with CP were characterized by a variety of behavioral categories. First, positive brain connectivity measures were mainly related to interaction between Action and Cognition as well as within-Cognition domains. Indeed, the most frequent connection of behavioral domains was between Speech Execution (Action) and Working Memory (Cognition) with 4 out of 11 pairs of parcels. Also, the top 5 categories with the largest number of connections (node centrality) included: Unspecified (Action), Speech Execution (Action), Working Memory (Cognition), Attention (Cognition) and Semantics (Cognition). The top 5 categories that had the most influence (betweenness centrality) on the network were: Working Memory (Cognition), Unspecified (Action), Speech Execution (Action), Explicit Memory (Cognition) and Semantics (Cognition).
Fig. 3.
Circular layout displaying the relationship between the behavioral domains significantly associated with functional brain connectivity. Red graph = brain connectivity positively related to CP. Blue Graph = brain connectivity negatively related to CP. Thicker line represents larger number of connected behavioral categories across pairs of brain connectivity. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Second, negative brain connectivity measures rather showed a widespread relationship between the four behavioral domains. Indeed, the most frequent connections were 1) Action Execution (Unspecified) & Reward, 2) Attention & Reward, 3) Attention & Somesthesis (Unspecified), and 4) Reward & Somesthesis (Unspecified) with each 3 out of 10 pairs of parcels (Fig. 3). Moreover, the top 5 categories with the largest number of connections (node centrality) were: Orthography (Cognition), Shape (Visual), Unspecified (Visual), Speech Execution (Action) and Attention (Cognition). Finally, the top 5 categories that had the most influence on the network were: Orthography (Cognition), Shape (Visual), Unspecified (Visual), Speech Execution (Action) but also Reward (Emotions).
4. Discussion
Using a large sample of adolescents, we aimed to clarify the role of cortico-cortical and amygdalo-cortical functional brain connectivity associated with CP. More precisely, we investigated the reliability of the relationship between resting-state brain connectivity measures and severity CP using two different psychometric scales (CBCL and SDQ). We observed that both scales show distinctive association with brain connectivity measures. Indeed, using a liberal statistical threshold (p < 0.005 uncorrected), only 21 cortico-cortical resting-state connectivity measures associated with CP significantly overlapped between the two scales. These mainly included regions involved in the Somatomotor, Ventral Attention and Frontoparietal networks (positive associations) as well as Cingulo-Opercular, Salience and Default Mode Network regions (negative associations). Additional analyses revealed that these regions were characterized by interactions between Action & Cognition (i.e., Positive association with CP) as well as between Reward and Cognition, Perception and Action (i.e., Negative associations with CP). Finally, exploratory analyses revealed most brain connectivity were also associated with other psychopathologies such as irritability, ADHD symptoms, callousness, uncaring but not unemotional traits.
In our recent meta-analysis of resting-state connectivity studies, we showed that antisocial subjects exhibited hyperconnectivity with ventral attention network (ie., aMCC/pre-SMA) and amygdala, and hypoconnectivity regions of the Default Mode Network (i.e., mPFC and PCC/Precuneus) and Dorsal attention network (i.e., PMC, SPL), compared to healthy controls (Dugré et Potvin, 2021). In line with these results, we found that CP was positively associated with 4 brain connectivity including regions of the ventral attention network and negatively associated with 2 brain connectivity that involved parcels of the Default Mode Network. However, contrasting with results from the meta-analysis, we found that CP was rather prominently associated with disrupted connectivity from the Somatomotor network (7 connections), from brain regions unassigned to any of the Gordon Networks such as posterior hippocampus and inferior/middle temporal gyri (6 connections), Frontoparietal (3 connections) as well as cingulo-opercular networks (3 connections). Moreover, we found no reliable evidence of amygdala-cortical connectivity across scales. It is noteworthy to mention that studies included in our prior meta-analysis restricted their analyses on a priori seeds and did not investigate the whole connectome, and this may largely explain the discrepancies between results. Second, the functional connectivity alterations associated with CP may differ between a case-control design versus a study examining severity of CP, dimensionally. In our recent meta-analysis using case-control analysis, we found that antisocial population was characterized by disrupted socio-affective and attentional processes (Dugré and Potvin, 2021). Here, we rather found that CP was dimensionally related to somatomotor and ventral attention, salience and cingulo-opercular networks. While the results of the former meta-analysis may help understanding the shared features across subjects, the results of the latter may represent brain connectivity associated with severity of the pathology.
In the current study, we also investigated whether our results may have been driven by distinct developmental periods. Subanalyses revealed that importance of most brain connectivity associated with CP were relatively robust across developmental periods (i.e., childhood and adolescence). Indeed, the top 5 brain connectivity that were the most strongly associated with CP (i.e., P8) Primary Motor Cortex (Somatomotor) - Lateral OFC (Ventral Attention); N6) Lateral OFC (None) - SMA (Cingulo-opercular); P9) Precentral (Somatomotor) – dlPFC (Dorsal Attention); P2) Lateral PFC (Frontoparietal) - vlPFC (Ventral Attention); and N4) dmPFC (Default Mode Network) - lateral PFC (Default Mode Network)) show no significant change across developmental stages (except N4 using the CBCL). However, subtle differences between developmental periods were only observed using the CBCL. Although these intriguing results are difficult to interpret and further research is needed, it is possible that the CBCL-CP may capture the heterogeneity of CP (i.e., aggression and rule-breaking behaviors), and thus allows variations in item endorsement rates depending on the time period (i.e., childhood versus adolescence), while the SDQ don’t. For instance, contrasting to the longitudinal measurement invariance of the broad SDQ-CP items (Murray et al., 2022), adolescents (Mean = 3.18) may be more likely than children (Mean = 2.8) to endorse CBCL items underpinning rule-breaking behaviors (e.g., runaway, sets fire, steals, truancy, vandalism). It is thus possible that these variations in the endorsement of items may alter the relationship between some brain connectivity and CP. It is unequivocal that further research is needed to adequately address this.
Interestingly, we provided evidence that brain connectivity measures that were positively associated with CP were mainly characterized by interaction between Action Execution & Cognition behavioral domains. First, brain connectivity measures that were positively associated with CP included lateral PFC regions (i.e., ventro and dorsolateral) and the postcentral/precentral gyri. According to a recent meta-analysis, both the lateral PFC and precentral gyrus co-activate during n-back working memory tasks (Wang et al., 2019). Indeed, it has been shown that the lateral PFC (ventral and dorsal parts) plays a major role in the reception, maintenance and monitoring of sensory inputs and sending outputs to the motor system (Müller et al., 2002, Passingham and Sakai, 2004), whereas the precentral gyrus may rather be involved in action preparation and the processing of motor movements (Yang, 2015). As such, these results are in line with a recent meta-analysis of task-based fMRI studies showing that antisocial subjects exhibit aberrant co-activation of these particular brain regions (i.e., precentral and ventrolateral prefrontal cortex) during cognitive control tasks (Dugré et al., 2020). Interestingly, past results suggest that from 10 to 26 years old, changes in functional connectivity of the somatomotor and cingulo-opercular/salience networks may reflect development of cognitive control (Grayson and Fair, 2017, Marek et al., 2015). More precisely, deficient activity and connectivity of the somatomotor network might be a transdiagnostic neurobiological marker of general psychopathology in children and adolescent (Dugré et al., 2022, Schwarzlose et al., 2023) as well as in adults (Van Dam et al., 2017). While some have found that deficient activity in somatomotor regions may confer an increased risk for general externalizing behavior (Castellanos-Ryan et al., 2014), others showed that a latent component of resting-state connectivity, mostly characterized by positive connectivity within the somatomotor network and between the somatomotor and VentAttn/Salience, was strongly associated with impulsivity features (e.g., functional impulsivity, novelty seeking, motor impulsivity, persistence, impulsiveness) (Kebets et al., 2019). Given these results, it could be hypothesized that the connectivity of somatomotor network may be a neurobiological substrate of motor impulsiveness. Indeed, motor impulsiveness is prominently correlated with early criminality (Pechorro, Maroco, Ray, & Gonçalves, 2015), higher risk for CD and ASPD (Pechorro et al., 2015, Swann et al., 2009), a greater severity of crime (Pechorro et al., 2015), especially proactive and reactive aggression (Azevedoet al., 2018, Azevedo et al., 2020, Chen and Qian, 2013, Pechorro et al., 2015). Concurring with the idea that motor impulsiveness may be transdiagnostic (or co-occurring feature), we found that brain connectivity of somatomotor regions, both within- and between-network, were also associated with irritability, ADHD symptoms and callousness. Even though it may represent a transdiagnostic neurobiological marker, future studies should seek to investigate the specific role of somatomotor network in the development of antisocial behaviors.
Second, brain connectivity measures that were negatively associated with CP mainly included the pg- & dACC, the SMA and the aINS and lateral PFC, which were mainly represented by interactions between Reward and Action Execution, Attention & Somesthesis. These brain regions are involved in the ventral attention, salience and cingulo-opercular networks (Uddin and Spreng, 2019). Although they are systematically observed across meta-analyses on reward tasks (Diekhof et al., 2012, Liu and Schrier, 2011, Oldham et al., 2018, Sescousse et al., 2013, Silverman et al., 2015), the ACC and aINS are not specific to any particular neurocognitive domain (Shackman et al., 2011, Yarkoni et al., 2011), are known to be generally involved in detecting behaviorally relevant stimuli in the environment (Uddin and Spreng, 2019), and may play an interacting role between internally (i.e., DMN) and externally directed actions (i.e., frontoparietal network) (Uddin, 2015). In contrast with the functional decoding suggesting their implications in reward processing tasks, we recently found that antisocial subjects exhibited reduced brain activity in these regions (i.e., pg- & dACC extending to the aMCC/pre-SMA as well as the aINS) during acute threat response (Dugré et al., 2020). Moreover, recent studies support the association of functional brain dysconnectivity at a region-level (i.e., aINS-pgACC & pgACC-Supramarginal Gyrus, see Afzali et al., 2020) and a network-level (i.e., increased salience-ventral attention connectivity, see Lees et al., 2021) with broad externalizing problems. In our study, we observed that CP was negatively associated with functional connectivity of brain regions underpinning Cingulo-Opercular network (i.e., aINS, lPFC, SMA) and those corresponding to visual (i.e., Area 2 and lingual gyrus), Auditory (i.e., Heschl gyrus) and unlabeled (i.e., Lateral OFC). Furthermore, we found that CP was positively associated with functional connectivity between nodes of VentAttn and those from FP, DorsAttn and SomMot. Although we found no evidence of dysconnectivity between the core regions of the Salience, VentAttn and Cingulo-Opercular networks, namely the dACC, aMCC/pre-SMA, vlPFC and aINS, we nonetheless found that most pairs of brain connectivity which included one of these regions also correlated with severity of irritability and ADHD symptoms. These findings suggest a potential role of these regions in externalizing pathology. Indeed, brain connectivity which included regions of the Cingulo-Opercular network also correlated with ADHD symptoms (r ranging from −0.056 to −0.081) and irritability symptoms (r ranging from −0.033 to −0.082), whereas those involving regions of the VentAttn were rather associated with irritability (r ranging from 0.042 to 0.084) compared to ADHD symptoms (r ranging from 0.025 to 0.076). More importantly, we also found that reduced connectivity between the dACC (Salience) and the postcentral gyrus (Somatomotor) network were specifically associated with irritability but not the other psychopathologies. These results somewhat converge with recent findings showing that a latent component of resting-state connectivity, mainly characterized by altered connectivity within of the somatomotor network and between the somatomotor and salience/ventral attention networks, was strongly associated with features underpinning neuroticism (i.e., mood lability, dysfunctional impulsivity, anxiety) (Kebets et al., 2019). Of interest for the symptoms of irritability, activity of the dACC is frequently thought to be a core brain region during frustrative non-reward (Bertsch et al., 2020, Dugre et al., 0000, Leibenluft, 2017), whereas activity of the postcentral gyrus is observed when initiating aggressive and retaliatory behaviors (Dugre et al., 0000, Wong et al., 2019). It could be hypothesized that a weaker dACC-postcentral gyrus connectivity may increase the proneneness for CP (i.e., aggressive behaviors) by disinhibiting action initiation processes. It is unequivocal that future studies should seek to investigate this relationship more specifically.
5. Limitations
In our study, we aimed to address several limitations of current literature on resting-state functional connectivity such as the usually low sample size and the variability in the psychometric scales used across studies. Despite the strengths of our study, few limitations need to be acknowledged. Indeed, the sample contains a relatively wide age range spanning from childhood to late adolescence. This could have introduced biases in our results. However, we took additional measures to minimize the effects of age and conducted subanalyses that examined the effect of age on the reliability of our results. Indeed, developmental period only altered a few results, and these were only reported for CBCL, whereas the SDQ showed no significant differences. Secondly, neuroimaging data was collected in 3 different sites (two with identical scanning parameter) that may have altered results. We also tested whether differences in scanner strengths may have altered our results. We found that importance of some brain connectivity was significantly stronger when using 3 T scanner compared to 1.5 T. Third, the proximity of Gordon’s parcels and the amygdala (Harvard Oxford Atlas) may have caused autocorrelation issues which could explain lack of significant relationship between CP and functional connectivity between the amygdala and other structures such as the anterior medial temporal lobe. Fourth, the HBN adopted a community-referred recruitment model. Therefore, careful interpretations should be made when comparing study results with population-based cohorts. Although the sample size was relatively large in our study, further examination with other samples is needed to validate the generalizability of our results.
Conclusions.
In conclusion, we found that brain connectivity associated with CP largely depends on the measure used. In fact, only 21 connections were shared between the CBCL and SDQ even if both scales show relatively strong phenotypic correlation (r = 0.79). Nonetheless, these 21 connections mainly spanned the SomMot, VentAttn and FP (positive) and Cingulo-Opercular, Salience and DMN (negative) networks. Results of this study indicate that severity CP may principally be associated brain connectivity underpinning cognitive and motor control (positive) and emotional inhibition (negative association). Finally, the brain connectivity associated with CP were also related to other psychopathologies, suggesting that irritability, ADHD symptoms, callousness and uncaring may play a central role in neural features underpinning CP. This concurs with recent research showing that from 6 to 12 years old, the developmental co-occurrence of irritability, hyperactivity and CU traits plays an additive role in the risk for CP (Dugré et Potvin, Preprint). Future research should seek to investigate the replicability of our findings in different samples.
CRediT authorship contribution statement
Jules R. Dugré: Conceptualization, Methodology, Formal analysis, Writing – original draft. Stéphane Potvin: Conceptualization, Methodology, Supervision, Writing – review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
JRD is holder of a PhD scholarship from the Fonds de Recherche du Québec en Santé. SP is holder of the Eli Lilly Canada Chair on schizophrenia research. We thank the Healthy Brain Network to have made available this dataset.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.nicl.2023.103346.
Contributor Information
Jules R. Dugré, Email: jules.dugre@umontreal.ca.
Stéphane Potvin, Email: stephane.potvin@umontreal.ca.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Data availability
The authors do not have permission to share data.
References
- Achenbach, T.M., Rescorla, L.A., 2001. Manual for the ASEBA school-age forms & profiles: child behavior checklist for ages 6-18, teacher's report form, youth self-report: an integrated system of multi-informant assessment. University of Vermont, research center for children youth & families.
- Afzali M.H., Dagher A., Edalati H., Bourque J., Spinney S., Sharkey R.J., Conrod P. Adolescent resting-state brain networks and unique variability of conduct problems within the externalizing dimension. J. Pers. Disord. 2020;34:609–627. doi: 10.1521/pedi.2020.34.5.609. [DOI] [PubMed] [Google Scholar]
- Alegria A.A., Radua J., Rubia K. Meta-analysis of fMRI studies of disruptive behavior disorders. Am. J. Psychiatry. 2016;173:1119–1130. doi: 10.1176/appi.ajp.2016.15081089. [DOI] [PubMed] [Google Scholar]
- Alexander L.M., Escalera J., Ai L., Andreotti C., Febre K., Mangone A., Vega-Potler N., Langer N., Alexander A., Kovacs M., Litke S., O'Hagan B., Andersen J., Bronstein B., Bui A., Bushey M., Butler H., Castagna V., Camacho N., Chan E., Citera D., Clucas J., Cohen S., Dufek S., Eaves M., Fradera B., Gardner J., Grant-Villegas N., Green G., Gregory C., Hart E., Harris S., Horton M., Kahn D., Kabotyanski K., Karmel B., Kelly S.P., Kleinman K., Koo B., Kramer E., Lennon E., Lord C., Mantello G., Margolis A., Merikangas K.R., Milham J., Minniti G., Neuhaus R., Levine A., Osman Y., Parra L.C., Pugh K.R., Racanello A., Restrepo A., Saltzman T., Septimus B., Tobe R., Waltz R., Williams A., Yeo A., Castellanos F.X., Klein A., Paus T., Leventhal B.L., Craddock R.C., Koplewicz H.S., Milham M.P. An open resource for transdiagnostic research in pediatric mental health and learning disorders. Sci. Data. 2017;4 doi: 10.1038/sdata.2017.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association, 2013. Diagnostic and statistical manual of mental disorders (5th ed.). Arlington, VA: Author.
- Azevedo V.-C., Castelo-Branco C., Figueiredo-Braga Impulsive and premeditated aggression in male offenders with antisocial personality disorder. PLoS One. 2020;15(3):e0229876. doi: 10.1371/journal.pone.0229876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azevedo, Pais-Ribeiro, Coelho, Figueiredo-Braga Validation of the Portuguese Version of Impulsive-Premeditated Aggression Scale in an Inmate Population. Front Psychiatry. 2018;9:10. doi: 10.3389/fpsyt.2018.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behzadi Y., Restom K., Liau J., Liu T.T. A component based noise correction method (CompCor) for BOLD and perfusion based fMRI. Neuroimage. 2007;37:90–101. doi: 10.1016/j.neuroimage.2007.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertsch K., Florange J., Herpertz S.C. Understanding Brain Mechanisms of Reactive Aggression. Curr. Psychiatry Rep. 2020;22:81. doi: 10.1007/s11920-020-01208-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevilacqua, L., Hale, D., Barker, E.D., Viner, R.J.E.c., psychiatry, a., 2018. Conduct problems trajectories and psychosocial outcomes: a systematic review and meta-analysis. 27, 1239-1260. [DOI] [PubMed]
- Bird H.R., Davies M., Duarte C.S., Shen S., Loeber R., Canino G.J. A study of disruptive behavior disorders in Puerto Rican youth: II. Baseline prevalence, comorbidity, and correlates in two sites. J. Am. Acad. Child Adolesc. Psychiatry. 2006;45:1042–1053. doi: 10.1097/01.chi.0000227879.65651.cf. [DOI] [PubMed] [Google Scholar]
- Boylan K., Vaillancourt T., Boyle M., Szatmari P. Comorbidity of internalizing disorders in children with oppositional defiant disorder. Eur. Child Adolesc. Psychiatry. 2007;16:484–494. doi: 10.1007/s00787-007-0624-1. [DOI] [PubMed] [Google Scholar]
- Cardinale E.M., Marsh A.A. The Reliability and Validity of the Inventory of Callous Unemotional Traits: A Meta-Analytic Review. Assessment. 2020;27(1):57–71. doi: 10.1177/1073191117747392. [DOI] [PubMed] [Google Scholar]
- Castellanos-Ryan N., Struve M., Whelan R., Banaschewski T., Barker G.J., Bokde A.L., Bromberg U., Büchel C., Flor H., Fauth-Bühler M., Frouin V., Gallinat J., Gowland P., Heinz A., Lawrence C., Martinot J.L., Nees F., Paus T., Pausova Z., Rietschel M., Robbins T.W., Smolka M.N., Schumann G., Garavan H., Conrod P.J. Neural and cognitive correlates of the common and specific variance across externalizing problems in young adolescence. Am. J. Psychiatry. 2014;171:1310–1319. doi: 10.1176/appi.ajp.2014.13111499. [DOI] [PubMed] [Google Scholar]
- Chai X.J., Castañón A.N., Ongür D., Whitfield-Gabrieli S. Anticorrelations in resting state networks without global signal regression. Neuroimage. 2012;59:1420–1428. doi: 10.1016/j.neuroimage.2011.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, Yang, Qian Chinese Version of Impulsive/Premeditated Aggression Scale: Validation and its Psychometric Properties. J. Aggress. Maltreat. Trauma. 2013;22(2):175–191. doi: 10.1080/10926771.2013.741664. [DOI] [Google Scholar]
- Cohn, Pape, Schmaal, van den Brink, van Wingen, Vermeiren, Doreleijers, Veltman, Popma Differential relations between juvenile psychopathic traits and resting state network connectivity. Hum. Brain Mapp. 2015;36:2396–2405. doi: 10.1002/hbm.22779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costello E.J., Mustillo S., Erkanli A., Keeler G., Angold A. Prevalence and development of psychiatric disorders in childhood and adolescence. Arch. Gen. Psychiatry. 2003;60:837–844. doi: 10.1001/archpsyc.60.8.837. [DOI] [PubMed] [Google Scholar]
- Diekhof E.K., Kaps L., Falkai P., Gruber O. The role of the human ventral striatum and the medial orbitofrontal cortex in the representation of reward magnitude - an activation likelihood estimation meta-analysis of neuroimaging studies of passive reward expectancy and outcome processing. Neuropsychologia. 2012;50:1252–1266. doi: 10.1016/j.neuropsychologia.2012.02.007. [DOI] [PubMed] [Google Scholar]
- Doucet G.E., Janiri D., Howard R., O'Brien M., Andrews-Hanna J.R., Frangou S. Transdiagnostic and disease-specific abnormalities in the default-mode network hubs in psychiatric disorders: A meta-analysis of resting-state functional imaging studies. Eur Psychiatry. 2020;63:e57. doi: 10.1192/j.eurpsy.2020.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dugré J.R., Radua J., Carignan-Allard M., Dumais A., Rubia K., Potvin S. Neurofunctional abnormalities in antisocial spectrum: A meta-analysis of fMRI studies on Five distinct neurocognitive research domains. Neurosci. Biobehav. Rev. 2020 doi: 10.1016/j.neubiorev.2020.09.013. [DOI] [PubMed] [Google Scholar]
- Dugré J.R., Eickhoff S.B., Potvin S. Meta-analytical transdiagnostic neural correlates in common pediatric psychiatric disorders. Sci. Rep. 2022;12:4909. doi: 10.1038/s41598-022-08909-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dugré, J. R., & Potvin, S. Preprint. Neural bases of Frustration-Aggression Theory: A multi-domain meta-analysis of functional neuroimaging studies. medRxiv. [DOI] [PubMed]
- Dugré J.R., Potvin S. Impaired attentional and socio-affective networks in subjects with antisocial behaviors: a meta-analysis of resting-state functional connectivity studies. Psychol Med. 2021:1–11. doi: 10.1017/S0033291721001525. [DOI] [PubMed] [Google Scholar]
- Essau C.A., Sasagawa S., Frick P.J. Callous-Unemotional Traits in a Community Sample of Adolescents. Assessment. 2006;13(4):454–469. doi: 10.1177/1073191106287354. [DOI] [PubMed] [Google Scholar]
- Fairchild, G., Hawes, D.J., Frick, P.J., Copeland, W.E., Odgers, C.L., Franke, B., Freitag, C.M., De Brito, S.A.J.N.R.D.P., 2019. Conduct disorder. 5, 1-25. [DOI] [PubMed]
- Frick R., Thornton K. Annual research review: A developmental psychopathology approach to understanding callous-unemotional traits in children and adolescents with serious conduct problems. J. Child Psychol. Psychiatry. 2014;55:532–548. doi: 10.1111/jcpp.12152. [DOI] [PubMed] [Google Scholar]
- Goodman R. Psychometric properties of the strengths and difficulties questionnaire. J. Am. Acad. Child Adolesc. Psychiatry. 2001;40:1337–1345. doi: 10.1097/00004583-200111000-00015. [DOI] [PubMed] [Google Scholar]
- Gordon E.M., Laumann T.O., Adeyemo B., Huckins J.F., Kelley W.M., Petersen S.E. Generation and evaluation of a cortical area parcellation from resting-state correlations. Cereb. Cortex. 2016;26:288–303. doi: 10.1093/cercor/bhu239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grayson D.S., Fair D.A. Development of large-scale functional networks from birth to adulthood: A guide to the neuroimaging literature. Neuroimage. 2017;160:15–31. doi: 10.1016/j.neuroimage.2017.01.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagberg, A., Swart, P., S Chult, D., 2008. Exploring network structure, dynamics, and function using NetworkX. Los Alamos National Lab.(LANL), Los Alamos, NM (United States).
- Kaiser R.H., Andrews-Hanna J.R., Wager T.D., Pizzagalli D.A. Large-scale network dysfunction in major depressive disorder: a meta-analysis of resting-state functional connectivity. JAMA Psychiat. 2015;72:603–611. doi: 10.1001/jamapsychiatry.2015.0071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kebets V., Holmes A.J., Orban C., Tang S., Li J., Sun N., Kong R., Poldrack R.A., Yeo B.T.T. Somatosensory-Motor Dysconnectivity Spans Multiple Transdiagnostic Dimensions of Psychopathology. Biol. Psychiatry. 2019;86(10):779–791. doi: 10.1016/j.biopsych.2019.06.013. [DOI] [PubMed] [Google Scholar]
- Lancaster J.L., Laird A.R., Eickhoff S.B., Martinez M.J., Fox P.M., Fox P.T. Automated regional behavioral analysis for human brain images. Front. Neuroinf. 2012;6:23. doi: 10.3389/fninf.2012.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lees B., Squeglia L.M., McTeague L.M., Forbes M.K., Krueger R.F., Sunderland M., Baillie A.J., Koch F., Teesson M., Mewton L. Altered Neurocognitive Functional Connectivity and Activation Patterns Underlie Psychopathology in Preadolescence. Biol. Psychiatry Cogn. Neurosci. Neuroimaging. 2021;6:387–398. doi: 10.1016/j.bpsc.2020.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leibenluft E. Pediatric Irritability: A Systems Neuroscience Approach. Trends Cogn. Sci. 2017;21(4):277–289. doi: 10.1016/j.tics.2017.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H., Schrier F. Common and distinct networks underlying reward valence and processing stages: a meta-analysis of functional neuroimaging studies. Neurosci. Biobehav. Rev. 2011;35:1219–1236. doi: 10.1016/j.neubiorev.2010.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marek S., Hwang K., Foran W., Hallquist M.N., Luna B. The Contribution of Network Organization and Integration to the Development of Cognitive Control. PLoS Biol. 2015;13(12) doi: 10.1371/journal.pbio.1002328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marek S., Tervo-Clemmens B., Calabro F.J., Montez D.F., Kay B.P., Hatoum A.S., Donohue M.R., Foran W., Miller R.L., Hendrickson T.J., Malone S.M., Kandala S., Feczko E., Miranda-Dominguez O., Graham A.M., Earl E.A., Perrone A.J., Cordova M., Doyle O., Moore L.A., Conan G.M., Uriarte J., Snider K., Lynch B.J., Wilgenbusch J.C., Pengo T., Tam A., Chen J., Newbold D.J., Zheng A., Seider N.A., Van A.N., Metoki A., Chauvin R.J., Laumann T.O., Greene D.J., Petersen S.E., Garavan H., Thompson W.K., Nichols T.E., Yeo B.T.T., Barch D.M., Luna B., Fair D.A., Dosenbach N.U.F. Reproducible brain-wide association studies require thousands of individuals. Nature. 2022;603:654–660. doi: 10.1038/s41586-022-04492-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marek S., Tervo-Clemmens B., Nielsen A.N., Wheelock M.D., Miller R.L., Laumann T.O., Earl E., Foran W.W., Cordova M., Doyle O., Perrone A., Miranda-Dominguez O., Feczko E., Sturgeon D., Graham A., Hermosillo R., Snider K., Galassi A., Nagel B.J., Ewing S.W.F.…Dosenbach N.U.F. Identifying reproducible individual differences in childhood functional brain networks: An ABCD study. Dev. Cognit. Neurosci. 2019;40:100706. doi: 10.1016/j.dcn.2019.100706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motzkin J.C., Newman J.P., Kiehl K.A., Koenigs M. Reduced prefrontal connectivity in psychopathy. J. Neurosci. 2011;31:17348–17357. doi: 10.1523/JNEUROSCI.4215-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller N.G., Machado L., Knight R.T. Contributions of subregions of the prefrontal cortex to working memory: evidence from brain lesions in humans. J. Cogn. Neurosci. 2002;14:673–686. doi: 10.1162/08989290260138582. [DOI] [PubMed] [Google Scholar]
- Murray A.L., Speyer L.G., Hall H.A., Valdebenito S., Hughes C. A Longitudinal and Gender Invariance Analysis of the Strengths and Difficulties Questionnaire Across Ages 3, 5, 7, 11, 14, and 17 in a Large U.K.-Representative Sample. Assessment. 2022;29(6):1248–1261. doi: 10.1177/10731911211009312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nock M.K., Kazdin A.E., Hiripi E., Kessler R.C. Prevalence, subtypes, and correlates of DSM-IV conduct disorder in the National Comorbidity Survey Replication. Psychol. Med. 2006;36:699–710. doi: 10.1017/S0033291706007082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noordermeer S.D., Luman M., Oosterlaan J. A systematic review and meta-analysis of neuroimaging in oppositional defiant disorder (ODD) and conduct disorder (CD) taking attention-deficit hyperactivity disorder (ADHD) into account. Neuropsychol. Rev. 2016;26:44–72. doi: 10.1007/s11065-015-9315-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldham S., Murawski C., Fornito A., Youssef G., Yücel M., Lorenzetti V. The anticipation and outcome phases of reward and loss processing: A neuroimaging meta-analysis of the monetary incentive delay task. Hum. Brain Mapp. 2018;39:3398–3418. doi: 10.1002/hbm.24184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passingham D., Sakai K. The prefrontal cortex and working memory: physiology and brain imaging. Curr. Opin. Neurobiol. 2004;14:163–168. doi: 10.1016/j.conb.2004.03.003. [DOI] [PubMed] [Google Scholar]
- Pechorro P., Maroco J., Ray J.V., Gonçalves R.A. Psychometric properties of the Barratt Impulsiveness Scale version 11 among a Portuguese sample of incarcerated juvenile offenders. Psychol. Crime Law. 2015;21(9):854–870. doi: 10.1080/1068316X.2015.1054386. [DOI] [Google Scholar]
- Power, J.D., Mitra, A., Laumann, T.O., Snyder, A.Z., Schlaggar, B.L., Petersen, S.E.J.N., 2014. Methods to detect, characterize, and remove motion artifact in resting state fMRI. 84, 320-341. [DOI] [PMC free article] [PubMed]
- Pujol J., Batalla I., Contreras-Rodríguez O., Harrison B.J., Pera V., Hernández-Ribas R., Real E., Bosa L., Soriano-Mas C., Deus J. Breakdown in the brain network subserving moral judgment in criminal psychopathy. Soc. Cogn. Affect. Neurosci. 2012;7:917–923. doi: 10.1093/scan/nsr075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raschle N.M., Menks W.M., Fehlbaum L.V., Tshomba E., Stadler C. Structural and Functional Alterations in Right Dorsomedial Prefrontal and Left Insular Cortex Co-Localize in Adolescents with Aggressive Behaviour: An ALE Meta-Analysis. PLoS One. 2015;10:e0136553. doi: 10.1371/journal.pone.0136553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer A., Kong R., Gordon E.M., Laumann T.O., Zuo X.-N., Holmes A.J., Eickhoff S.B., Yeo B.T. Local-global parcellation of the human cerebral cortex from intrinsic functional connectivity MRI. Cereb. Cortex. 2018;28:3095–3114. doi: 10.1093/cercor/bhx179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarzlose R.F., Tillman R., Hoyniak C.P., Luby J.L., Barch D.M. Sensory Over-responsivity: A Feature of Childhood Psychiatric Illness Associated With Altered Functional Connectivity of Sensory Networks. Biol. Psychiatry. 2023;93:92–101. doi: 10.1016/j.biopsych.2022.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sescousse G., Caldú X., Segura B., Dreher J.C. Processing of primary and secondary rewards: a quantitative meta-analysis and review of human functional neuroimaging studies. Neurosci. Biobehav. Rev. 2013;37:681–696. doi: 10.1016/j.neubiorev.2013.02.002. [DOI] [PubMed] [Google Scholar]
- Shackman A.J., Salomons T.V., Slagter H.A., Fox A.S., Winter J.J., Davidson R.J. The integration of negative affect, pain and cognitive control in the cingulate cortex. Nat. Rev. Neurosci. 2011;12:154–167. doi: 10.1038/nrn2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shannon, B.J., Raichle, M.E., Snyder, A.Z., Fair, D.A., Mills, K.L., Zhang, D., Bache, K., Calhoun, V.D., Nigg, J.T., Nagel, B.J., 2011. Premotor functional connectivity predicts impulsivity in juvenile offenders. Proceedings of the National Academy of Sciences 108, 11241-11245. [DOI] [PMC free article] [PubMed]
- Silverman M.H., Jedd K., Luciana M. Neural networks involved in adolescent reward processing: An activation likelihood estimation meta-analysis of functional neuroimaging studies. Neuroimage. 2015;122:427–439. doi: 10.1016/j.neuroimage.2015.07.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stringaris A., Goodman R., Ferdinando S., Razdan V., Muhrer E., Leibenluft E., Brotman M.A. The Affective Reactivity Index: a concise irritability scale for clinical and research settings. J. Child Psychol. Psychiatry, Allied Disciplines. 2012;53(11):1109–1117. doi: 10.1111/j.1469-7610.2012.02561.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sukhodolsky D.G., Ibrahim K., Kalvin C.B., Jordan R.P., Eilbott J., Hampson M. Increased amygdala and decreased frontolimbic r esting- s tate functional connectivity in children with aggressive behavior. Soc. Cogn. Affect Neurosci. 2022;17:634–644. doi: 10.1093/scan/nsab128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutcubasi B., Metin B., Kurban M.K., Metin Z.E., Beser B., Sonuga-Barke E. Resting-state network dysconnectivity in ADHD: A system-neuroscience-based meta-analysis. World J. Biol. Psychiatry. 2020:1–74. doi: 10.1080/15622975.2020.1775889. [DOI] [PubMed] [Google Scholar]
- Swann A.C., Lijffijt M., Lane S.D., Steinberg J.L., Moeller F.G. Trait impulsivity and response inhibition in antisocial personality disorder. J. Psychiatr. Res. 2009;43(12):1057–1063. doi: 10.1016/j.jpsychires.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson J.M., Schuck S., Porter M.M., Carlson C., Hartman C.A., Sergeant J.A., Clevenger W., Wasdell M., McCleary R., Lakes K., Wigal T. Categorical and Dimensional Definitions and Evaluations of Symptoms of ADHD: History of the SNAP and the SWAN Rating Scales. Int. J. Educ. Psychol. Assess. 2012;10(1):51–70. [PMC free article] [PubMed] [Google Scholar]
- Uddin L.Q. Salience processing and insular cortical function and dysfunction. Nat. Rev. Neurosci. 2015;16:55–61. doi: 10.1038/nrn3857. [DOI] [PubMed] [Google Scholar]
- Uddin Y., Spreng Towards a universal taxonomy of macro-scale functional human brain networks. Brain Topogr. 2019;32:926–942. doi: 10.1007/s10548-019-00744-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umbach R.H., Tottenham N. Callous-unemotional traits and reduced default mode network connectivity within a community sample of children. Dev. Psychopathol. 2020:1–14. doi: 10.1017/S0954579420000401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dam N.T., O'Connor D., Marcelle E.T., Ho E.J., Cameron Craddock R., Tobe R.H., Gabbay V., Hudziak J.J., Xavier Castellanos F., Leventhal B.L., Milham M.P. Data-Driven Phenotypic Categorization for Neurobiological Analyses: Beyond DSM-5 Labels. Biol. Psychiatry. 2017;81:484–494. doi: 10.1016/j.biopsych.2016.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H., He W., Wu J., Zhang J., Jin Z., Li L. A coordinate-based meta-analysis of the n-back working memory paradigm using activation likelihood estimation. Brain Cogn. 2019;132:1–12. doi: 10.1016/j.bandc.2019.01.002. [DOI] [PubMed] [Google Scholar]
- Weathersby F.L., King J.B., Fox J.C., Loret A., Anderson J.S. Functional connectivity of emotional well-being: Overconnectivity between default and attentional networks is associated with attitudes of anger and aggression. Psychiatry Res. Neuroimaging. 2019;291:52–62. doi: 10.1016/j.pscychresns.2019.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitfield-Gabrieli S., Nieto-Castanon A. Conn: a functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connect. 2012;2:125–141. doi: 10.1089/brain.2012.0073. [DOI] [PubMed] [Google Scholar]
- Wichstrøm L., Berg-Nielsen T.S., Angold A., Egger H.L., Solheim E., Sveen T.H. Prevalence of psychiatric disorders in preschoolers. J. Child Psychol. Psychiatry. 2012;53:695–705. doi: 10.1111/j.1469-7610.2011.02514.x. [DOI] [PubMed] [Google Scholar]
- Wong T.Y., Sid A., Wensing T., Eickhoff S.B., Habel U., Gur R.C., Nickl-Jockschat T. Neural networks of aggression: ALE meta-analyses on trait and elicited aggression. Brain Struct. Funct. 2019;224:133–148. doi: 10.1007/s00429-018-1765-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J., Van Dam N.T., Feng C., Luo Y., Ai H., Gu R., Xu P. Anxious brain networks: A coordinate-based activation likelihood estimation meta-analysis of resting-state functional connectivity studies in anxiety. Neurosci. Biobehav. Rev. 2019;96:21–30. doi: 10.1016/j.neubiorev.2018.11.005. [DOI] [PubMed] [Google Scholar]
- Yang J. The influence of motor expertise on the brain activity of motor task performance: A meta-analysis of functional magnetic resonance imaging studies. Cogn. Affect Behav. Neurosci. 2015;15:381–394. doi: 10.3758/s13415-014-0329-0. [DOI] [PubMed] [Google Scholar]
- Yarkoni T., Poldrack R.A., Nichols T.E., Van Essen D.C., Wager T.D. Large-scale automated synthesis of human functional neuroimaging data. Nat. Methods. 2011;8:665–670. doi: 10.1038/nmeth.1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeo B.T., Krienen F.M., Sepulcre J., Sabuncu M.R., Lashkari D., Hollinshead M., Roffman J.L., Smoller J.W., Zöllei L., Polimeni J.R. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J. Neurophysiol. 2011 doi: 10.1152/jn.00338.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors do not have permission to share data.