Abstract
Background
Thromboinflammatory processes modulate the complex pathophysiology of cerebral ischemia-reperfusion (I/R) injury in ischemic stroke, but the exact underlying mechanisms remain poorly understood. Emerging evidence indicates that neutrophil extracellular traps (NETs) might play an important role in the thromboinflammatory cascade. In addition, the link between von Willebrand factor (VWF) and neutrophil recruitment in the ischemic brain might promote thromboinflammation, possibly by the formation of NETs.
Objectives
To study NET formation in a murine model of cerebral I/R injury in ischemic stroke.
Methods
The filament–induced transient middle cerebral artery occlusion model was used to induce 60 minutes of focal cerebral ischemia after which reperfusion was allowed. At different time points postischemia, NETs were identified in the ischemic mouse brain using quantitative immunofluorescence microscopy.
Results
NETs could be identified in the ipsilateral brain hemisphere. Interestingly, NETs could already be detected at 6 hours poststroke. Their presence increased at 12 hours, was highest at 24 hours, and decreased again 48 hours postischemia. Remarkably, NETs were predominantly localized within the brain vasculature postischemia, suggesting that NETs play a role in secondary microthrombosis. Strikingly, NET formation was significantly decreased in VWF–deficient mice compared to littermate wild-type mice 24 hours postischemia, indicating a possible role for VWF in promoting NETosis in the ischemic brain.
Conclusion
This study identified the spatiotemporal profile of NET formation in a mouse model of cerebral I/R injury in ischemic stroke. NETs, potentially in combination with VWF, might be attractive targets for the development of novel therapeutic strategies in ischemic stroke treatment.
Keywords: Extracellular traps, ischemic stroke, reperfusion injury, thromboinflammation, von willebrand factor
Essentials
-
•
Progression of brain injury in ischemic stroke patients remains a major health problem.
-
•
Neutrophil extracellular traps (NETs) are potential mediators of ischemia-reperfusion injury.
-
•
This study elucidated the spatiotemporal profile of NET formation in the ischemic brain.
-
•
von Willebrand factor is likely a driver of NETosis in ischemic stroke.
1. Introduction
Ischemic stroke is still a major cause of death and permanent disability worldwide [1]. It is caused by blood thrombi that obstruct cerebral blood flow, leading to irreversible damage in the associated brain tissue. Current treatment strategies focus on fast recanalization of the occluded artery, either through thrombolysis with recombinant tissue-plasminogen activator or via mechanical thrombectomy [2,3]. However, even when adequate blood supply to ischemic brain tissue is restored rapidly, progressive stroke brain injury can still occur through a process called ischemia-reperfusion (I/R) injury [[4], [5], [6]]. The exact underlying pathophysiological mechanisms of cerebral I/R injury are poorly understood, but several thromboinflammatory processes, including endothelial activation, secondary microthrombotic events, blood-brain-barrier (BBB) disruption, cerebral inflammation and dysregulation of the neurovascular unit, were shown to contribute to worse clinical outcomes in ischemic stroke patients, irrespective of early and successful reperfusion [7].
Neutrophil extracellular traps (NETs) are considered an important mediator of the thromboinflammatory cascade in cerebral I/R injury. NETs are released by activated neutrophils via a mechanism called NETosis, by creating a mesh of decondensed chromatin lined with granular components [8]. NETs are highly prothrombotic [9,10] and can also contribute to local tissue damage by exerting toxic effects on the surrounding cells [[11], [12], [13]]. Interestingly, elevated NET markers have been shown to correlate strongly with poor stroke patient outcomes regarding morbidity, disability, and mortality [[13], [14], [15], [16], [17], [18]]. Recently, the presence of NETs has been reported in postmortem brain tissues of ischemic stroke patients [13]. Similarly, increased levels of circulating DNA and nucleosomes were detected in mice subjected to ischemic stroke [19]. In addition, treatment of ischemic stroke mice with DNase (an endonuclease digesting DNA) or Cl-amidine (a peptidylarginine deiminase inhibitor suppressing NET formation) has been shown to be protective [12,[19], [20], [22], [23], [24]]. Yet, little is known about the spatiotemporal formation of NETs during brain ischemia and reperfusion in stroke. In the present study, we aimed to study the spatiotemporal profile of NET formation in a murine model of cerebral I/R injury in ischemic stroke via quantitative immunofluorescence microscopy. Since von Willebrand factor (VWF) was previously shown to promote neutrophil recruitment in the ischemic brain [[24], [25], [26]] and to induce NETosis in vitro [27], the impact of VWF deficiency on NET formation was also assessed.
2. Materials and Methods
2.1. Animals
Animal experiments were approved by the local Ethical Committee for Animal Experimentation (ECD, P050/2017 and P005/2021, KU Leuven, Leuven, Belgium) and were performed in accordance with the ARRIVE guidelines (http://www.nc3rs.org.uk) for the care and use of laboratory animals. Surgeries were randomized for each experimental condition. Experiments were performed on 8- to 12-week-old male and female wild-type (WT) and littermate VWF-knockout (KO) [28] mice with a C57Bl/6J background.
2.2. Cerebral I/R injury mouse model
Transient middle cerebral artery (MCA) occlusion was conducted as described earlier [29]. Briefly, anesthesia was induced with 5% isoflurane gas and maintained using 2% isoflurane gas mixed with medical oxygen. Body temperature during surgery was maintained at 37 °C (± 1 °C) using a temperature controller and heating pad. After a midline incision in the neck, the right common and right external carotid arteries were exposed and ligated. A standardized silicon rubber–coated 6.0 nylon monofilament (6021, Doccol Corp) was inserted into the right common carotid artery and advanced via the right internal carotid artery to occlude the origin of the right MCA and induce cerebral ischemia. After 60 minutes, the monofilament was withdrawn to allow reperfusion of the ischemic territory. Surgery time did not exceed 15 minutes per animal. All mice received following postoperative care: 1 mL saline was administered subcutaneously every 24 hours to prevent dehydration, and Vetergesic (buprenorphine hydrochloride, 0.1 mg/kg, subcutaneous) was administered as analgesic every 12 hours.
2.3. Neutrophil depletion
Neutrophils were depleted by an intraperitoneal injection of 10 mg/kg anti–mouse Ly6G (clone 1A8, Bio X Cell) 24 hours before induction of ischemia. Neutrophil depletion was confirmed via the identification of neutrophil count in whole blood (sampled from the orbital sinus) with the VetScan HM5 hematology analyzer (Zoetis).
2.4. Measurement of infarct volumes
After euthanasia of the animals through cervical dislocation, brains were quickly isolated and 3 coronal slices of 2-mm thickness were cut using a brain slice matrix and razor blades. To differentiate metabolic active tissue from dead brain tissue, brain slices were stained with 2% 2,3,5-triphenyl-tetrazolium chloride (Sigma-Aldrich) in phosphate–buffered saline at 37 °C for 10 minutes. Pictures were taken from the triphenyl-tetrazolium chloride–stained brain slices with a Nikon D70 digital camera, and cerebral lesions were quantified blinded for experimental condition in Image J software (National Institutes of Health). Infarct sizes were corrected for edema using the following formula: Vcorrected = Vuncorrected × [1 – (Vi – Vc)/Vc], where: Vi = ischemic hemisphere; Vc = contralateral hemisphere; Vi – Vc = volume difference between Vi and Vc; (Vi - Vc)/Vc = difference as a fraction of the contralateral hemisphere.
2.5. Immunofluorescent staining
Mouse brain slices were fixated in 4% paraformaldehyde for 24 hours at 4 °C. Next, slices were incubated in 30% sucrose in phosphate–buffered saline solution at 4 °C for 24 hours and then placed in optimal cutting temperature compound (Sakura) and frozen on dry ice. These frozen brain samples were used to cut cryosections of 9-μm thickness (Leica CM3050S Cryostat). Brain cryosections derived from the middle 2-mm coronal slice of the brain were stained for the presence of NETs. Prior to staining, brain sections were fixated for 15 minutes in ice-cold acetone (−20 °C); washed in Tris–buffered saline (TBS); and blocked for one hour at room temperature with blocking buffer containing 1% normal donkey serum, 1% normal goat serum, 3% bovine serum albumin, and 0.1% Triton X-100 in TBS. Brain sections were incubated overnight at 4 °C with the following primary antibodies: rat anti–mouse Ly6G (1/500, eBioscience), goat anti–mouse myeloperoxidase (1/500, R&D systems), and rabbit anti–mouse H3Cit (1/3000, Abcam). To stain the cerebral vasculature, an FITC–labeled tomato-lectin antibody (Lectin from Lycopersicon esculentum, 1/500, Merck Life Science B.V.) was used. After incubation with primary antibodies, brain sections were washed with TBS and incubated at room temperature with the following secondary antibodies: Alexa Fluor 555 goat anti–rat IgG (1/1000, ThermoFisher Scientific), Alexa Fluor 555 donkey anti–goat IgG (1/1000, Abcam), and Alexa Fluor 647 donkey anti–rabbit IgG (1/1000, Biolegend). Prolong Gold Antifade mounting solution with 4,6-diamidino-2-phenylindole (DAPI, Invitrogen) was used to stain DNA. As negative controls, following isotype control antibodies were used: rat IgG2B kappa isotype control (1/500, eBioscience) and rabbit IgG isotype control (1/3000, Biolegend).
2.6. Quantitative fluorescence microscopy
Images and tile scans were acquired using an Axio Observer Z1 inverted fluorescent microscope (Zeiss, Carl Zeiss AG) or a laser scanning confocal microscope (LSM710, Zeiss). Images were processed by the Zen software (blue edition, version 2.3, Zeiss). Individual NETs were defined by the colocalization of DNA (DAPI), citrullinated histones (H3Cit) and neutrophils (Ly6G), and thus included neutrophils that were undergoing NETosis and neutrophils that already had completely expelled their nuclear material. All NETs were counted in 3 different brain sections per mouse (front, middle, and back, within the middle 2-mm coronal slice of the brain).
2.7. Statistical analysis
All statistical analyses were conducted with GraphPad Prism version 8.0. Prior to statistical analysis, a Shapiro-Wilk test was used to test for normal data distribution. An unpaired Student’s t-test (parametric data) or Mann–Whitney test (nonparametric data) was used when applicable. In case of paired nonparametric data, a Wilcoxon test was performed. Spearman correlation analysis was used to investigate correlations. All data are represented as dot plots with a bar graph, including error bars indicating mean + SD.
3. Results
3.1. NETs are formed in stroke brain tissue with a peak at 24 hours postischemia
To gain more insight into the presence and spatiotemporal formation of NETs during the ischemia and reperfusion phase of the stroke brain, NET formation was studied at different time points. C57Bl/6J mice were subjected to a transient occlusion of the MCA, and NETs were visualized at the following 6 time points after induction of ischemia: 1 hour, 2 hours, 6 hours, 12 hours, 24 hours, and 48 hours. At the indicated time points, mouse brains were harvested, and brain sections of the middle 2-mm coronal slice of the mouse brains were prepared. These brain sections were stained for the presence of neutrophils (anti–Ly6G antibody), DNA (DAPI), and citrullinated histones (anti–H3Cit antibody), the colocalization of which is considered a hallmark of NET formation (Figure 1A).
Figure 1.
Neutrophil extracellular trap (NET) formation in affected mouse brain tissue during cerebral ischemia-reperfusion injury. (A) Transient focal cerebral ischemia was induced in C57Bl/6J mice by 1-hour occlusion of the right middle cerebral artery, followed by reperfusion of the ischemic territory. At different time points after induction of ischemia (1, 2, 6, 12, 24, and 48 hours), mouse brains were harvested, and infarct volumes were measured. To identify NETs, immunofluorescent staining for neutrophils (anti–Ly6G antibody, red), citrullinated histones (anti–H3Cit antibody, green), and DNA (DAPI, blue) was performed on 3 brain sections per mouse of the middle 2-mm part of the brain. Subsequently, NETs were visualized via fluorescence microscopy. (B) To quantify NETs, tile scans covering the complete brain section were obtained and carefully screened for NETs (indicated with white arrows), which were found within the ipsilateral hemisphere and mostly within the infarct core (white dotted line in the ipsilateral hemisphere) but not in the contralateral hemisphere. The red line indicates the division between the ipsilateral (i) and contralateral (c) hemisphere. The orange rectangles depict an example and magnification of a NET. Scale bars are 10 μm. (C) Temporal profile of NET formation. Data are presented as the sum of NETs in 3 different brain sections per mouse + SD and are represented for ipsi- and contralateral hemispheres. No NETs are present in the ischemic mouse brain at 1 hour and 2 hours postischemia. NETs were detected in the affected ipsilateral hemisphere at 6 hours, their number increased at 12 hours, peaked at 24 hours, and started to decrease again 48 hours postischemia. NETs were almost exclusively formed within the ipsilateral hemisphere at all time points. (D) NET formation is related to the infarct surface of the middle 2-mm part of the mouse brain (Spearman r = 0,6027, P < .0001). (E) Examples of high magnification pictures of NETs 24 hours poststroke. Scale bars are 10 μm.
NET formation could be clearly observed in affected mouse brain tissue at 6 hours, 12 hours, 24 hours, and 48 hours postischemia. To quantify these NETs, tile scans of 3 different brain sections per mouse were obtained (Figure 1B). Interestingly, NETs were absent at 1 hour and 2 hours postischemia in the affected mouse brain. NETs could be detected in the ipsilateral hemisphere at 6 hours, the number of NETs increased at 12 hours, was highest at 24 hours, and again lower at 48 hours after induction of ischemia (Figure 1C). NET formation was specific for the ipsilateral hemisphere and was as good as absent in the contralateral hemisphere at all investigated time points (Figure 1C). In the affected hemisphere, the vast majority of NETs was located within the infarct area (data not shown). Moreover, correlation analysis revealed that the intensity of NET formation in the infarct lesion at all time points correlated with the infarct size poststroke (Spearman r = 0.6027; p < .0001; Figure 1D).
High magnification pictures of NETs at 24 hours are shown in Figure 1E and are provided in the supplemental data set (Supplementary Figure 1 and Supplementary Movies 1, 2, and 3). NET formation was specific for MCA occlusion since NETosis was absent in sham–operated mice (Supplementary Figure 2). As an additional control, neutrophils were depleted 24 hours before stroke induction, after which NET formation was investigated. The number of NETs was clearly reduced or they were even absent 24 hours after induction of ischemia in the absence of neutrophils (Supplementary Figure 3). In addition, our microscopy data were verified using the neutrophil marker myeloperoxidase instead of Ly6G, revealing similar NET counts in all conditions (data not shown).
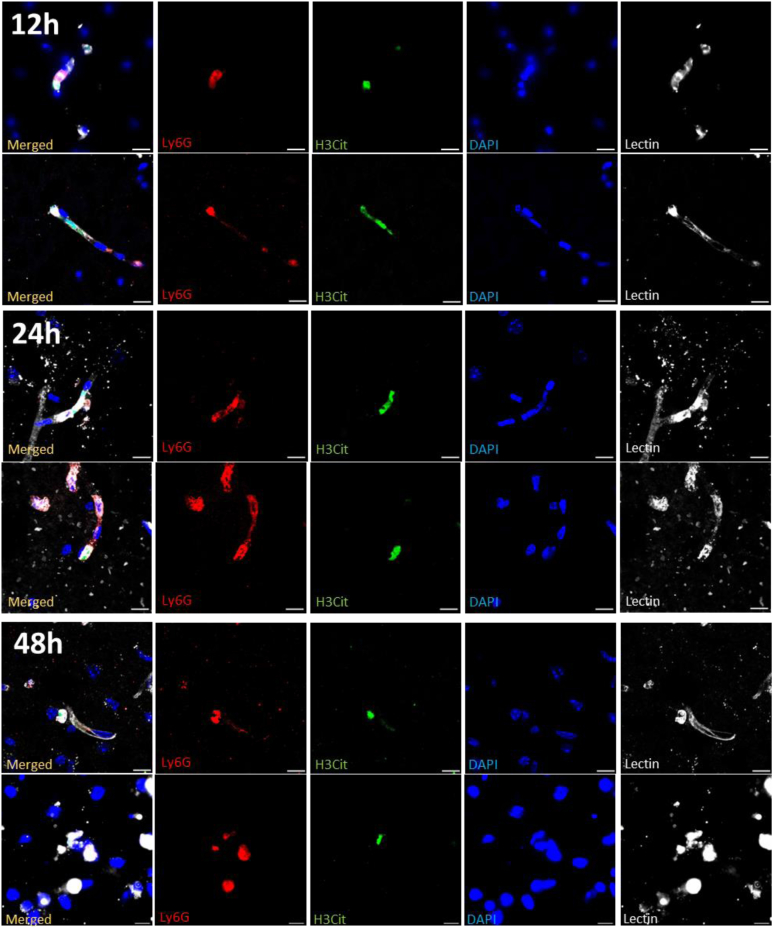
3.2. NETs are predominantly located within the cerebral vasculature during ischemic stroke
NETs hold the capacity to induce thrombotic events within circulation and can exert cytotoxic effects on surrounding cells or tissues [[9], [10], [11], [12], [13]]. To better understand the exact location of NET formation in the stroke brain, we assessed the formation of intra- and extravascular NETs. Apart from the immunofluorescent staining for NETs (Ly6G+, H3Cit+, and DNA+), cerebral vasculature was also stained (lectin) at different time points poststroke (6, 12, 24, and 48 hours). Representative images of NETs in the ipsilateral hemisphere are shown in Figure 2. Interestingly, the majority of NETs were found within the cerebral vessels. At 6 hours poststroke, all observed NETs were found intravascularly. At 12 hours, NETs could be detected within the brain parenchyma but the large majority (approximately 75%, data not shown) was still localized intravascularly. At 24 hours, the number of both intra- and extravascular NETs increased, with about one-third of NETs found in the brain parenchyma (data not shown). At 48 hours, most of the remaining NETs were found within the vasculature.
Figure 2.
Spatial characteristics of neutrophil extracellular trap (NET) formation during ischemic stroke. Transient focal cerebral ischemia was induced in C57Bl/6J mice by 60-minute occlusion of the right middle cerebral artery, followed by reperfusion. At different time points postischemia (12, 24, and 48 hours), mice brain sections were stained for neutrophils (Ly6G, red), citrullinated histones (H3Cit, green), DNA (DAPI, blue), and brain vasculature (lectin, white). NETs were predominantly located within the cerebral vasculature. Fluorescent images of intravascular NETs are shown at 12, 24, and 48 hours postischemia. Scale bars are 10 μm.
3.3. VWF deficiency is associated with significantly decreased NET formation in the ischemic brain
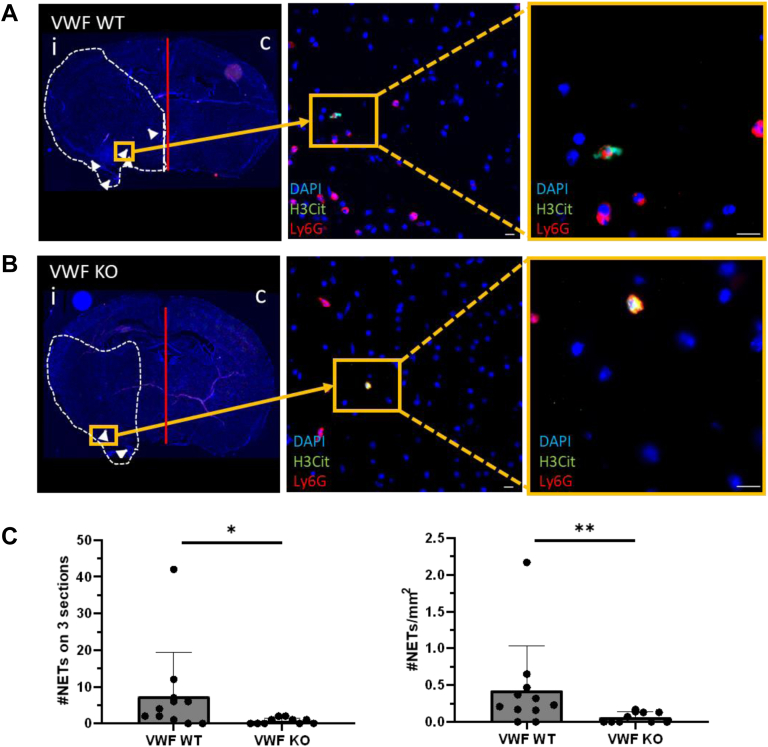
In recent years, a pathophysiological role for VWF in ischemic stroke has become apparent. Given the fact that we and other authors identified an inflammatory role for VWF in stroke by the recruitment of neutrophils via its A1 domain [[24], [25], [26]], our next aim was to investigate possible consequences of VWF deficiency on NET formation in ischemic stroke. Both WT and littermate VWF-KO mice were subjected to 60-minute occlusion of the right MCA, followed by 23 hours of reperfusion. Subsequently, brains were harvested, and brain sections were stained for the presence of NETs (Ly6G+, H3Cit+, and DNA+).
In agreement with previous studies, a significantly reduced cerebral infarct was observed in VWF-KO mice compared to WT mice 24 hours postischemia (2-fold reduction, P < .05, data not shown) [30,31]. NETs could be observed in the affected brain hemisphere of both WT and VWF-KO mice 24 hours after induction of ischemia (Figure 3A, B). However, quantification revealed a 10-fold reduction in number of NETs in VWF-KO mice compared to WT mice (P < .05, Figure 3C). To assess whether lower NET formation was not merely a consequence of the smaller infarct sizes observed in VWF-KO mice, the number of NETs was normalized for the infarct size of the middle 2-mm part of the mouse brain. After normalization for infarct size, the density of NETs was still 10 times lower in VWF-KO mice compared to WT mice 24 hours postischemia (P < .01, Figure 3D).
Figure 3.
The number of neutrophil extracellular traps (NETs) is reduced in the ischemic brain of von Willebrand factor (VWF)-knock-out (KO) mice. Transient focal cerebral ischemia was induced in wild-type (WT) and littermate VWF-KO mice by 60-minute occlusion of the right middle cerebral artery, followed by 23 hours of reperfusion. Brain sections were stained for neutrophils (Ly6G, red), citrullinated histones (H3Cit, green), and DNA (DAPI, blue), after which NETs were quantified. (A) Representative tile scan of a WT mouse with white arrows pointing toward NETs. The infarct core is depicted with the white dotted line. The orange rectangles depict fluorescent pictures of a NET, found within the infarct core. Scale bars are 10 μm. (B) Representative tile scan of a VWF-KO mouse with white arrows pointing toward NETs. The infarct core is depicted with the white dotted line. The orange rectangles depict fluorescent pictures of a NET, found within the infarct core. Scale bars are 10 μm. (C) Quantification of the number of NETs in the ipsilateral hemisphere revealed a significantly lower number of NETs in VWF-KO mice compared to WT mice 24 hours postischemia, both in absolute numbers (left) and in numbers corrected for infarct volume (right) (∗P < .05, ∗∗P < .01).
4. Discussion
In this study, NETs were visualized in the ischemic brain of mice subjected to transient occlusion of the MCA, and the tempo-spatial profile of NET formation was characterized. We could observe NET formation at 6 hours, and the numbers of NETs were highest at 24 hours postischemia. Moreover, NETs were primarily found within the brain vasculature poststroke and, to a lesser extent, in the brain parenchyma. Finally, NET formation was significantly reduced in the brains of VWF–deficient mice during ischemic stroke.
NETs are web-like structures of decondensed chromatin, released by activated neutrophils, and have been described as a potential mediator of ischemic stroke brain damage [12,13,23,32]. The formation of NETs has been described within the affected brain tissue in ischemic stroke in a few studies [11,12,20,[33], [34], [35], [36]]. However, the picture remains far from complete, as it is still not clear when and where exactly NETs are formed in the brain during ischemia and reperfusion. To gain more insights into the presence of NETs in cerebral I/R injury, we aimed to map the temporal profile of NETosis via quantitative immunofluorescence microscopy. Significant NETosis could be detected 6 hours postischemia. Given the fact that the in vitro process of NET formation takes at least 2 to 4 hours to complete [8], our results indicate early triggering of NETosis during or after ischemia. Using a rat model of permanent MCAO, Kim et al. [11] also reported the first signs of H3Cit induction at 6 hours, indicating that NETosis can indeed already start during ischemia and is thus not only a consequence of reperfusion. Typically, the transient MCA occlusion mouse model induces a peak volume of brain injury by 24 hours [37]. Considering that NETs could already be observed at 6 and 12 hours postischemia, before the cerebral lesion is fully established, it is possible that NETs have a pathophysiological role in cerebral I/R injury in ischemic stroke. In this context, targeting NETs in ischemic stroke has been shown to be protective [12,[19], [20], [21], [22], [23],32]. Indeed, treatment with DNase or Cl-amidine significantly improved stroke outcomes by decreasing infarct sizes and enhancing functional outcomes [12,[19], [20], [21], [22], [23]].
Rapid removal of the thrombus and consecutive vessel recanalization do not always go along with an improved clinical outcome in 20% to 60% of ischemic stroke patients [[4], [5], [6]]. Multiple processes, such as secondary microthrombosis, BBB dysfunction, and inflammation, are thought to be the causes of progressive brain damage in ischemic stroke, but the exact cellular and molecular mechanisms remain incompletely understood [7]. Interestingly, a novel finding of our study was that NETs were mainly observed within the mouse brain vasculature, further supporting the concept that NETs may contribute to or reinforce secondary microthrombosis, impairing efficient reperfusion of the microcirculation following recanalization. Indeed, using in vivo imaging techniques, it has already been reported that neutrophils, platelet aggregates, and red blood cells can plug microcapillaries in the salvageable penumbra after efficient reperfusion [[38], [39], [40]]. Furthermore, removal of circulating neutrophils using a depleting antibody restored cerebral blood flow by modulating these microcapillary stalls, along with decreased cellular damage and improved functional outcomes [40]. Of note, NETs were recently found within cerebral blood vessels trapped in neutrophil–rich thrombi in postmortem brain tissue of patients who succumbed from ischemic stroke and have previously been shown to mediate microvascular thrombosis in myocardial infarction and cancer–associated arterial microthrombosis, possibly by contributing to a procoagulant state [13,41,42]. Mechanistically, it is therefore tempting to speculate that intravascular NETs directly activate the coagulation cascade and act as a platform for blood cell adhesion, activation, and fibrin polymerization, leading to thrombus propagation [9,10,19,43]. Apart from microthrombus formation, the presence of intra- and extravascular NETs will likely impact stroke outcomes by harming the brain vasculature and aggravating neuronal injury. Extracellular histones together with proteolytic enzymes can exert a cytotoxic reaction, inducing neuronal and endothelial cell death, leading to BBB dysfunction [12,19,32,34,36,[44], [45], [46], [47]]. Additionally, during delayed phases of permanent ischemic stroke, NETs have been shown to impair vascular remodeling and revascularization, most likely by activating the STING pathway and increasing the production of IFN-β, thereby hampering stroke recovery [12]. Taken together, targeting NETs might be a valuable option in ischemic stroke management to minimize secondary injury cascades and thus improve patient outcomes. Future studies are however needed to further explore the potential contribution of NETs to stroke progression and outcomes, mainly using specific NET inhibitors.
VWF was demonstrated to have a detrimental role in ischemic stroke brain injury, both in clinical and preclinical studies [30,31,48,49]. The mechanisms by which VWF aggravates ischemic stroke outcomes are however not fully resolved, but recent insights indicate a thromboinflammatory role of primarily the VWF A1 domain, which is involved in the recruitment of platelets and leukocytes. We previously showed that blocking the VWF A1 domain significantly reduced the recruitment of neutrophils in the ischemic brain, which is associated with significantly smaller infarct sizes [24]. In this study, we assessed whether this also led to reduced NETosis. As expected, VWF deficiency is associated with a significantly reduced number of NETs present in the ischemic brain at 24 hours postischemia, which might be explained by the reduced recruitment of neutrophils in the absence of VWF [26]. In addition, it has been described that VWF directly promotes NETosis. Indeed, the Slc44a2 receptor on neutrophils was recently identified as a receptor for VWF A1 and platelet GPIIbIIIa [27,50]. Under flow, Slc44a2–mediated neutrophil adhesion to VWF, in combination with specific shear conditions, induced neutrophil activation and subsequent formation of NETs [27]. Moreover, the interaction of VWF with platelet GPIb induces the activation of platelet GPIIbIIIa, which, in turn, mediates neutrophil binding. Slc44a2–mediated platelet binding leads to the production of NETs in vitro under flow [50]. Whether such a direct effect of VWF on NETosis can also partly explain reduced NETosis in VWF–deficient animals is difficult to determine in our study. Similarly, whether reduced NETosis is a cause or consequence of the reduced infarct sizes observed in VWF–deficient mice is not easy to establish, but the fact that NETosis is still significantly lower in VWF–deficient mice even after correction for infarct size hints a possible direct involvement of VWF. Future studies addressing the VWF-Slc44a2 interaction can possibly better assess the direct role of VWF on NETosis and its possible effect on infarct progression in ischemic stroke. Another possible pathway through which VWF could promote NETosis is the recruitment of platelets and subsequent release of soluble factors. Platelet high mobility group box 1 has been shown to induce NET formation in an experimental stroke model [51]. In addition, NET formation was not induced by activated platelets of P-selectin–deficient mice and was inhibited after administration of anti–P-selectin antibodies in vitro [52]. Noteworthy, VWF–deficient mice have a defect in P-selectin translocation to the endothelial surface, affecting leukocyte recruitment after ischemic stroke [53].
In conclusion, this study sheds more light on the spatiotemporal profile of NETosis during the course of cerebral ischemia and reperfusion in a mouse model of ischemic stroke and indicates a role for VWF in promoting NET formation. Since NETs might be involved in the thromboinflammatory cascade, NETs, possibly together with VWF, might be attractive targets for the development of novel therapeutic strategies in stroke treatment.
Acknowledgments
Funding
This work was supported by the Fonds voor Wetenschappelijk Onderzoek - Vlaanderen (research grants G.0A86.13, G.0785.17, and 1509216N to S.F.D.M), by research grants from KU Leuven (OT/14/099 and ISP/14/02L2 to S.F.D.M), and by a research grant from the Queen Elisabeth Medical Foundation (to S.F.D.M).
Author contributions
M.D.W. designed the study, acquired, analyzed and interpreted the results and wrote the manuscript. S.F.D.M. conceived and designed the study, interpreted the results, and wrote the manuscript. L.D. performed experiments. C.T. and K.V. interpreted data, provided helpful discussions, and critically reviewed the manuscript.
Relationship Disclosure
There are no competing interests to disclose.
Footnotes
Funding information Fonds voor Wetenschappelijk Onderzoek – Vlaanderen, Grant/Award Number: G.0A86.13, G.0785.17, and 1509216N (to S.F.D.M); KU Leuven, Grant/Award Number: OT/14/099 and ISP/14/02L2 (to S.F.D.M); and Queen Elisabeth Medical Foundation, Grant/Award Number: (to S.F.D.M).
Handling Editor: Y. Senis
The online version contains supplementary material available at https://doi.org/10.1016/j.rpth.2022.100028
Supporting Information
References
- 1.Tsao C.W., Aday A.W., Almarzooq Z.I., Alonso A., Beaton A.Z., Bittencourt M.S., et al. Heart disease and stroke statistics—2022 update: a report from the American Heart Association. Circulation. 2022;146:153–639. doi: 10.1161/CIR.0000000000001052. [DOI] [PubMed] [Google Scholar]
- 2.Vanacker J.P., Lambrou J.D., Eskandari J.A., Mosimann J.P., Maghraoui J.A., Michel J.P. Eligibility and predictors for acute revascularization procedures in a stroke center. Stroke. 2016;47:1844–1849. doi: 10.1161/STROKEAHA.115.012577. [DOI] [PubMed] [Google Scholar]
- 3.Prabhakaran S., Ruff I., Bernstein R.A. Acute stroke intervention: a systematic review. JAMA. 2015;313:1451–1462. doi: 10.1001/jama.2015.3058. [DOI] [PubMed] [Google Scholar]
- 4.Mizuma A., You J.S., Yenari M.A. Targeting reperfusion injury in the age of mechanical thrombectomy. Stroke. 2018;49:1796–1802. doi: 10.1161/STROKEAHA.117.017286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Warach S., Latour L.L. Evidence of reperfusion injury, exacerbated by thrombolytic therapy, in human focal brain ischemia using a novel imaging marker of early blood-brain barrier disruption. Stroke. 2004;35:2659–2661. doi: 10.1161/01.STR.0000144051.32131.09. [DOI] [PubMed] [Google Scholar]
- 6.Schiphorst A.T., Charron S., Hassen W.B., Provost C., Naggara O., Benzakoun J., et al. Tissue no-reflow despite full recanalization following thrombectomy for anterior circulation stroke with proximal occlusion: a clinical study. J Cereb Blood Flow Metab. 2021;41:253–266. doi: 10.1177/0271678X20954929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lin L., Wang X., Yu Z. Ischemia-reperfusion injury in the brain: mechanisms and potential therapeutic strategies. Biochem Pharmacol. 2016;5:1–16. doi: 10.4172/2167-0501.1000213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fuchs T.A., Abed U., Goosmann C., Hurwitz R., Schulze I., Wahn V., et al. Novel cell death program leads to neutrophil extracellular traps. J Cell Biol. 2007;204:231–241. doi: 10.1083/jcb.200606027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Martinod K., Wagner D.D. Thrombosis: tangled up in NETs. Blood. 2014;123:2768–2776. doi: 10.1182/blood-2013-10-463646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tobias A.F., Alexander B., Daniel D., Daphne S., Marc M., Myers D.D., Jr., et al. Extracellular DNA traps promote thrombosis. Proc Natl Acad Sci. 2010;107 doi: 10.1073/pnas.1005743107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim S.W., Lee H., Lee H.K., Kim I.D., Lee J.K. Neutrophil extracellular trap induced by HMGB1 exacerbates damages in the ischemic brain. Acta Neuropathol Commun. 2019;7:94. doi: 10.1186/s40478-019-0747-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kang L., Yu H., Yang X., Zhu Y., Bai X., Wang R., et al. Neutrophil extracellular traps released by neutrophils impair revascularization and vascular remodeling after stroke. Nat Commun. 2020;11:2488. doi: 10.1038/s41467-020-16191-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Denorme F., Portier I., Rustad J.L., Cody M.J., de Araujo C.V., Hoki C., et al. Neutrophil extracellular traps regulate ischemic stroke brain injury. J Clin Invest. 2022;132 doi: 10.1172/JCI154225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Geiger S., Holdenrieder S., Stieber P., Hamann G.F., Bruening R., Ma J., et al. Nucleosomes in serum of patients with early cerebral stroke. Cerebrovasc Dis. 2006;21:32–37. doi: 10.1159/000089591. [DOI] [PubMed] [Google Scholar]
- 15.Lam N.Y.L., Rainer T.H., Wong L.K.S., Lam W., Lo Y.M.D. Plasma DNA as a prognostic marker for stroke patients with negative neuroimaging within the first 24 h of symptom onset. Resuscitation. 2006;68:71–78. doi: 10.1016/j.resuscitation.2005.05.021. [DOI] [PubMed] [Google Scholar]
- 16.Rainer T.H., Wong L.K.S., Lam W., Yuen E., Lam N.Y.L., Metreweli C., et al. Prognostic use of circulating plasma nucleic acid concentrations in patients with acute stroke. Clin Chem. 2003;49:562–569. doi: 10.1373/49.4.562. [DOI] [PubMed] [Google Scholar]
- 17.Tsai N.W., Lin T.K., Chen S. Der, Chang W.N., Wang H.C., Yang T.M., et al. The value of serial plasma nuclear and mitochondrial DNA levels in patients with acute ischemic stroke. Clin Chim Acta. 2011;412:476–479. doi: 10.1016/j.cca.2010.11.036. [DOI] [PubMed] [Google Scholar]
- 18.Valles J., Lago A., Santos M.T., Latorre A.M., Tembl J.I., Salom J.B., et al. Neutrophil extracellular traps are increased in patients with acute ischemic stroke: Prognostic significance. Thromb Haemost. 2017;117:1919–1929. doi: 10.1160/TH17-02-0130. [DOI] [PubMed] [Google Scholar]
- 19.De Meyer S.F., Suidan G.L., Fuchs T.A., Monestier M., Wagner D.D. Extracellular chromatin is an important mediator of ischemic stroke in mice. Arterioscler Thromb Vasc Biol. 2012;32:1884–1891. doi: 10.1161/ATVBAHA.112.250993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang S., Cao Y., Du J., Liu H., Chen X., Li M., et al. Neutrophil extracellular traps contribute to tissue plasminogen activator resistance in acute ischemic stroke. FASEB J. 2021;35 doi: 10.1096/fj.202100471RR. [DOI] [PubMed] [Google Scholar]
- 21.Deng J., Zhao F., Zhang Y., Zhou Y., Xu X., Zhang X., et al. Neutrophil extracellular traps increased by hyperglycemia exacerbate ischemic brain damage. Neurosci Lett. 2020;738 doi: 10.1016/j.neulet.2020.135383. [DOI] [PubMed] [Google Scholar]
- 22.Peña-Martínez C., Durán-Laforet V., García-Culebras A., Ostos F., Hernández-Jiménez M., Bravo-Ferrer I., et al. Pharmacological modulation of neutrophil extracellular traps reverses thrombotic stroke tpa (tissue-type plasminogen activator) resistance. Stroke. 2019;50:3228–3237. doi: 10.1161/STROKEAHA.119.026848. [DOI] [PubMed] [Google Scholar]
- 23.Peña-Martínez C., Durán-Laforet V., García-Culebras A., Cuartero M.I., Moro M.Á., Lizasoain I. Neutrophil extracellular trap targeting protects against ischemic damage after fibrin-rich thrombotic stroke despite non-reperfusion. Front Immunol. 2022;13 doi: 10.3389/fimmu.2022.790002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Denorme F., Martinod K., Vandenbulcke A., Denis C.V., Lenting P.J., Deckmyn H., et al. The von Willebrand Factor A1 domain mediates thromboinflammation, aggravating ischemic stroke outcome in mice. Haematologica. 2020;106:819. doi: 10.3324/haematol.2019.241042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Petri B., Broermann A., Li H., Khandoga A.G., Zarbock A., Krombach F., et al. von Willebrand factor promotes leukocyte extravasation. Blood. 2010;116:4712–4719. doi: 10.1182/blood-2010-03-276311. [DOI] [PubMed] [Google Scholar]
- 26.Khan M.M., Motto D.G., Lentz S.R., Chauhan A.K. ADAMTS13 reduces VWF-mediated acute inflammation following focal cerebral ischemia in mice. J Thromb Haemost. 2012;10:1665–1671. doi: 10.1111/j.1538-7836.2012.04822.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zirka G., Robert P., Tilburg J., Tishkova V., Maracle C.X., Legendre P., et al. Impaired adhesion of neutrophils expressing Slc44a2/HNA-3b to VWF protects against NETosis under venous shear rates. Blood. 2021;137:2256–2266. doi: 10.1182/blood.2020008345. [DOI] [PubMed] [Google Scholar]
- 28.Denis C., Methia N., Frenette P.S., Rayburn H., Ullman-Culleré M., Hynes R.O., et al. A mouse model of severe von Willebrand disease: defects in hemostasis and thrombosis. Proc Natl Acad Sci USA. 1998;95:9524–9529. doi: 10.1073/pnas.95.16.9524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.De Meyer S.F., Schwarz T., Schatzberg D., Wagner D.D. Platelet glycoprotein Ibα is an important mediator of ischemic stroke in mice. Exp Transl Stroke Med. 2011;3(1):9. doi: 10.1186/2040-7378-3-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kleinschnitz C., De Meyer S.F., Schwarz T., Austinat M., Vanhoorelbeke K., Nieswandt B., et al. Deficiency of von Willebrand factor protects mice from ischemic stroke. Blood. 2009;113:3600–3603. doi: 10.1182/blood-2008-09-180695. [DOI] [PubMed] [Google Scholar]
- 31.Zhao B., Chauhan A., Canault M., Patten I., Yang J., Dockal M., et al. vonWillebrand factor-cleaving protease ADAMTS13 reduces ischemic brain injury in experimental stroke. Blood. 2009;114:3329–3334. doi: 10.1182/blood-2009-03-213264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim S.W., Lee H., Lee H.K., Kim I.D., Lee J.K. Neutrophil extracellular trap induced by HMGB1 exacerbates damages in the ischemic brain. Acta Neuropathol Commun. 2019;7:94. doi: 10.1186/s40478-019-0747-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Otxoa-De-Amezaga A., Gallizioli M., Pedragosa J., Justicia C., Miró-Mur F., Salas-Perdomo A., et al. Location of neutrophils in different compartments of the damaged mouse brain after severe ischemia/reperfusion. Stroke. 2019;50:1548–1557. doi: 10.1161/STROKEAHA.118.023837. [DOI] [PubMed] [Google Scholar]
- 34.Cai W., Liu S., Hu M., Huang F., Zhu Q., Qiu W., et al. Functional dynamics of neutrophils after ischemic stroke. Transl Stroke Res. 2020;11:108–121. doi: 10.1007/s12975-019-00694-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Perez-de-Puig I., Miró-Mur F., Ferrer-Ferrer M., Gelpi E., Pedragosa J., Justicia C., et al. Neutrophil recruitment to the brain in mouse and human ischemic stroke. Pathol Mech Neurol Dis. 2015;129:239–257. doi: 10.1007/s00401-014-1381-0. [DOI] [PubMed] [Google Scholar]
- 36.Cai W., Wang J., Hu M., Chen X., Lu Z., Bellanti J.A., et al. All trans-retinoic acid protects against acute ischemic stroke by modulating neutrophil functions through STAT1 signaling. J Neuroinflammation. 2019;16:1–4. doi: 10.1186/s12974-019-1557-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu F., Schafer D.P., McCullough L.D. TTC, Fluoro-Jade B and NeuN staining confirm evolving phases of infarction induced by middle cerebral artery occlusion. J Neurosci Methods. 2009;179:1–8. doi: 10.1016/j.jneumeth.2008.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Erdener Ş.E., Tang J., Kılıç K., Postnov D., Giblin J.T., Kura S., et al. Dynamic capillary stalls in reperfused ischemic penumbra contribute to injury: A hyperacute role for neutrophils in persistent traffic jams. J. Cereb. Blood Flow Metab. 2021;41:236–252. doi: 10.1177/0271678X20914179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Erdener Ş.E., Tang J., Kılıç K., Postnov D., Giblin J.T., Kura S., et al. Neutrophil-mediated dynamic capillary stalls in ischemic penumbra: persistent traffic jams after reperfusion contribute to injury. bioRxiv. 2019:776427. [Google Scholar]
- 40.El Amki M., Glück C., Binder N., Middleham W., Wyss M.T., Weiss T., et al. Neutrophils Obstructing Brain Capillaries Are a Major Cause of No-Reflow in Ischemic Stroke. Cell Rep. 2020;33:108260. doi: 10.1016/j.celrep.2020.108260. [DOI] [PubMed] [Google Scholar]
- 41.Thålin C., Demers M., Blomgren B., Wong S.L., Von Arbin M., Von Heijne A., et al. NETosis promotes cancer-associated arterial microthrombosis presenting as ischemic stroke with troponin elevation. Thromb Res. 2016;139:56–64. doi: 10.1016/j.thromres.2016.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mangold A., Ondracek A.S., Hofbauer T.M., Scherz T., Artner T., Panagiotides N., et al. Culprit site extracellular DNA and microvascular obstruction in ST-elevation myocardial infarction. Cardiovasc Res. 2021;118:2006–2017. doi: 10.1093/cvr/cvab217. [DOI] [PubMed] [Google Scholar]
- 43.Steffen M., Lenka G., Marie-Luise Von B., Davit M., Susanne P., Christian G., et al. Reciprocal coupling of coagulation and innate immunity via neutrophil serine proteases. Nat Med. 2010;16:887. doi: 10.1038/nm.2184. [DOI] [PubMed] [Google Scholar]
- 44.Kim S.W., Davaanyam D., Seol S.I., Lee H.K., Lee H., Lee J.K. Adenosine triphosphate accumulated following cerebral ischemia induces neutrophil extracellular trap formation. Int J Mol Sci. 2020;21:1–19. doi: 10.3390/ijms21207668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Saffarzadeh M., Juenemann C., Queisser M.A., Lochnit G., Barreto G., Galuska S.P., et al. Neutrophil extracellular traps directly induce epithelial and endothelial cell death: a predominant role of histones. PLoS One. 2012;7 doi: 10.1371/journal.pone.0032366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Meegan J.E., Yang X., Beard R.S., Jannaway M., Chatterjee V., Taylor-Clark T.E., et al. Citrullinated histone 3 causes endothelial barrier dysfunction. Biochem Biophys Res Commun. 2018;503:1498–1502. doi: 10.1016/j.bbrc.2018.07.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li C., Xing Y., Zhang Y., Hua Y., Hu J., Bai Y. Neutrophil extracellular traps exacerbate ischemic brain damage. Mol Neurobiol. 2021:1–4. doi: 10.1007/s12035-021-02635-z. [DOI] [PubMed] [Google Scholar]
- 48.Taylor A., Vendramin C., Singh D., Brown M.M., Scully M. von Willebrand factor/ADAMTS13 ratio at presentation of acute ischemic brain injury is predictive of outcome. Blood Adv. 2020;4:398–407. doi: 10.1182/bloodadvances.2019000979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.De Meyer S.F., Schwarz T., Deckmyn H., Denis C.V., Nieswandt B., Stoll G., et al. Binding of von Willebrand factor to collagen and glycoprotein Ibα, but not to glycoprotein IIb/IIIa, contributes to ischemic stroke in mice-brief report. Arterioscler Thromb Vasc Biol. 2010;30:1949–1951. doi: 10.1161/ATVBAHA.110.208918. [DOI] [PubMed] [Google Scholar]
- 50.Constantinescu-Bercu A., Grassi L., Frontini M., Salles-Crawley, Woollard K.J., Crawley J.T.B. Activated αiibβ3 on platelets mediates flow-dependent netosis via slc44a2. Elife. 2020;9 doi: 10.7554/eLife.53353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Denorme F., Yost C.C., Campbell R.A. Neutrophil extracellular traps regulate ischemic stroke brain injury. J Clin Investig. 2022;132 doi: 10.1172/JCI154225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Etulain J., Martinod K., Wong S.L., Cifuni S.M., Schattner M., Wagner D.D. P-selectin promotes neutrophil extracellular trap formation in mice. Blood. 2015;126:242–246. doi: 10.1182/blood-2015-01-624023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Denis C.V., André P., Saffaripour S., Wagner D.D. Defect in regulated secretion of P-selectin affects leukocyte recruitment in von Willebrand factor-deficient mice. Proc Natl Acad Sci USA. 2001;98:4072–4077. doi: 10.1073/pnas.061307098. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.