Abstract
Macrophages (MQs) pro-inflammatory phenotype is triggered by gliadin peptides. Akkermansia muciniphila (A. muciniphila) showed to enhance the anti-inflammatory phenotype of MQs. This study aimed to investigate the anti-inflammatory effects of A. muciniphila, on gliadin stimulated THP-1 derived macrophages. THP-1 cell line monocytes were differentiated into MQs by phorbol 12-myristate 13-acetate (PMA). MQs were treated with A. muciniphila before and after stimulation with gliadin (pre- and post-treat). CD11b, as a marker of macrophage differentiation, and CD206 and CD80, as M1 and M2 markers, were evaluated by flow cytometry technique. The mRNA expression of TGF-β, IL-6, and IL-10 and protein levels of IL-10 and TNF-α were measured by RT-PCR and ELISA techniques, respectively. Results show an increased percentage of M1 phenotype and release of proinflammatory cytokines (like TNF-α and IL-6) by macrophages upon incubation with gliadin. Pre- and post-treatment of gliadin-stimulated macrophages with A. muciniphila induced M2 phenotype associated with decreased proinflammatory (IL-6, TNF-α) and increased anti-inflammatory (IL-10, TGF-β) cytokines expression relative to the group that was treated with gliadin alone. This study suggests the potential beneficial effect of A. muciniphila on gliadin-stimulated MQs and the importance of future studies focusing on their exact mechanism of action on these cells.
Subject terms: Immunology, Molecular biology
Introduction
Macrophages (MQs) are mononuclear phagocytes with important roles in innate and adaptive immune responses. These cells are present in almost all body tissues and because of their ability to recognize pathogen-associated molecular patterns (PAMPs) and damage-associated molecular patterns (DAMPs), they can efficiently and rapidly detect pathogens and tissue destructions1,2. MQs can polarize to M1 or M2 functional phenotypes in response to their tissue microenvironment3. While the M1 phenotype of macrophages expresses numerous pro-inflammatory mediators, in particular tumor necrosis factor (TNF-α), interleukin (IL)-1, IL-6, reactive nitrogen and oxygen intermediates, the M2 phenotype of them expresses molecules with immunoregulatory functions including Arginase1, chitinase 3-like 3, resistin-like-α (also known as Fizz1), IL-10 and Mrc1 (also known as CD206)4. M1 and M2 phenotypes of macrophages can be converted into each other in response to microenvironmental stimuli5.
There are numerous MQs in the lamina propria of the small intestine, with a dual role of maintaining tissue homeostasis and participating in the development of intestinal inflammation. According to the reports, gliadin peptides, which trigger immune reactions in celiac disease (CeD), induce the activation of macrophages toward a pro-inflammatory M1 phenotype6–10. In vitro studies with murine MQ and human monocytic cell lines have shown that gliadin triggers the production of TNF-α, IL-8, RANTES, IL-1β and significantly increases nitric oxide (NO) upon activation of toll-like receptors 2 and 4 (TLR2/TLR4)9.
Celiac disease is an immune-mediated systemic disorder characterized by enteropathy of the small intestine and nutritional deficiencies affecting approximately 1% of the general population varying by continent11. The only therapeutic strategy for this disorder is strict adherence to a gluten free diet, which is difficult to maintain12. Therefore, researchers planned various in vitro and in vivo studies with the specific aim of finding a new therapeutic approach for this group of patients. Nowadays, gut dysbiosis (which is commonly observed in CeD patients) and its effect on immune system responses have become a subject of interest for finding an alternative therapeutic approach13.
Gut bacteria Akkermansia muciniphila is an anaerobic gram-negative bacterium that helps maintain homeostasis and barrier integrity in the gastrointestinal tract14,15. In addition, several studies have shown the anti-inflammatory effects of A. muciniphila16. These genera are known to break down mucus and produce short-chain fatty acids (SCFAs), which in turn support enterocyte health and inhibit intestinal inflammation17.
In the present study, gliadin stimulated THP-1 derived macrophage was treated with A. muciniphila. The idea of this treatment was to evaluate A. muciniphila enhanced phenotype switching of gliadin triggered M1 MQs to an anti-inflammatory phenotype. The results may give rise to more detailed studies on this gut bacterium's beneficial effects on CeD patients' treatment.
Results
Differentiation of THP-1 monocytes to macrophages
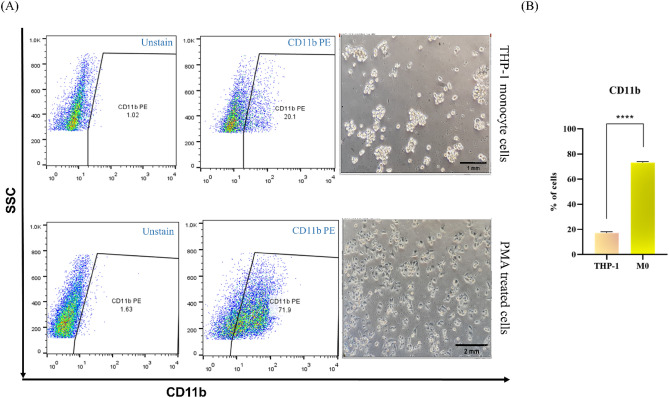
To investigate macrophage differentiation from monocytes, we measured the expression of CD11b as a differentiation marker in the THP-1 monocytes group and PMA-treated THP-1 cells. An increased expression of CD11b in the PMA-treated group in comparison to the THP-1 monocytes was observed, which suggested that THP-1 monocytes were well-differentiated to MQs by 50 ng/ml PMA for 48 h followed by 24 h rest (Fig. 1).
Figure 1.
PMA promotes the differentiation of human THP-1 monocytes into macrophages. (A) Representative images and flow cytometry analysis of THP-1 Monocytes cells and PMA-treated cells. (B) A significant increase in the number of CD11b + cells was detected in the PMA-treated group compared with the THP-1 monocytes cells (p < 0.0001). An unpaired t-test was used to make comparisons between groups (Triplicate assays). PMA, phorbol myristate acetate.
Gliadin induced up-regulation of IL-6 cytokine without any effect on TGF-β expression
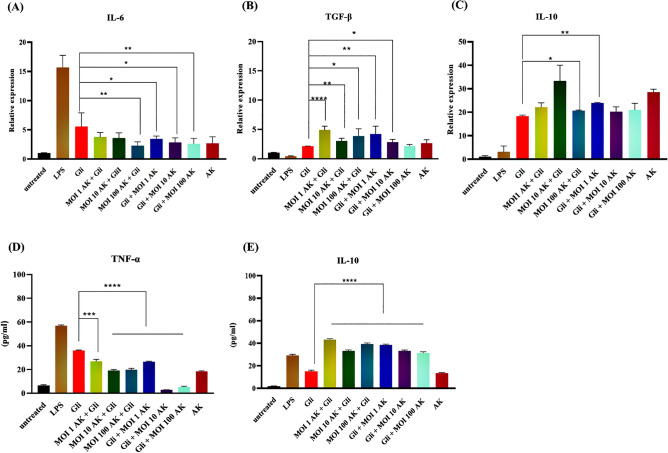
IL-6 and TGF-β were selected as markers for the immune activity of stimulated MQs. Results showed that the concentration of 200 and 400 μg/ml had the most efficacy in stimulating macrophages to produce IL-6 as an inflammatory cytokine (p < 0.0001) (Fig. 2A). In addition, there was no significant difference between various concentrations of gliadin in the expression of TGF-β (p = 0.8901) (Fig. 2B).
Figure 2.
Evaluation of the gliadin effect on THP-1 derived MQs. (A) Relative expression of IL-6 was significantly increased in all concentrations of gliadin. (B) Relative expression of TGF-β did not reach a statistically significant threshold. (****p < 0.0001, **p < 0.01). The Student’s t-test was used to make comparisons between groups (Triplicate assays).
A. muciniphila can reduce gliadin-induced inflammation in MQs
To characterize the preventive and protective effect of A. muciniphila, on gliadin treated MQs, A. muciniphila treated MQs (MOI = 1, 10, 100) were treated with gliadin (pre-treat) and gliadin stimulated MQ cells were treated with A. muciniphila (MOI = 1, 10, 100) for 24 h (post-treat), respectively. mRNA expressions of IL-6, TGF-β, and IL-10 were assessed by real-time RT-PCR and ELISA methods to evaluate the protein production of TNF-α and IL-10.
According to the real-time PCR results, pre-treatment of A. muciniphila for 24 h significantly reduced IL-6 mRNA expression (MOI = 100) (p < 0.01) and increased TGF-β (MOI = 1, 10, 100) (p < 0.0001, p < 0.01, p < 0.05) and IL-10 (MOI = 10) (p < 0.01) mRNA expressions relative to the MQ cells stimulated with gliadin. Post-treatment of A. muciniphila also significantly decreased IL-6 mRNA levels (MOI = 1,10,100) (p < 0.05, p < 0.05, p < 0.01 respectively) and increased TGF-β (MOI = 1, 10) (p < 0.01, p < 0.05) mRNA expressions. The changes towards untreated macrophages and LPS stimulated ones were significant in all groups (p˂0.05) (Fig. 3A–C).
Figure 3.
A. muciniphila modulates immune-regulatory cytokines. (A) The qRT-PCR analysis represented a significant reduction of IL-6 expression in the AK (MOI = 100) + Gli group compared to the Gli (gliadin) group. (B) Relative expression of TGF-β. (C) Relative expression of IL-10. (D) TNF-α and (E) IL-10 production by MQs with various treatments. Results were analyzed by One-way ANOVA (Triplicate assays) (*p < 0.05, **p < 0.01, ***p < 0.001 and ****p < 0.0001). AK, A. Muciniphila; Gli, gliadin; LPS, lipopolysaccharide; MOI, the multiplicity of infection.
As determined by ELISA, pre and post-treatment of gliadin stimulated macrophages with A. muciniphila (at all concentrations) could significantly reduce TNF-α and induce IL-10 expressions relative to gliadin-triggered macrophage cells (p < 0.0001) (Fig. 3D,E).
Increased frequency of M2 macrophages in response to A. muciniphila treatment
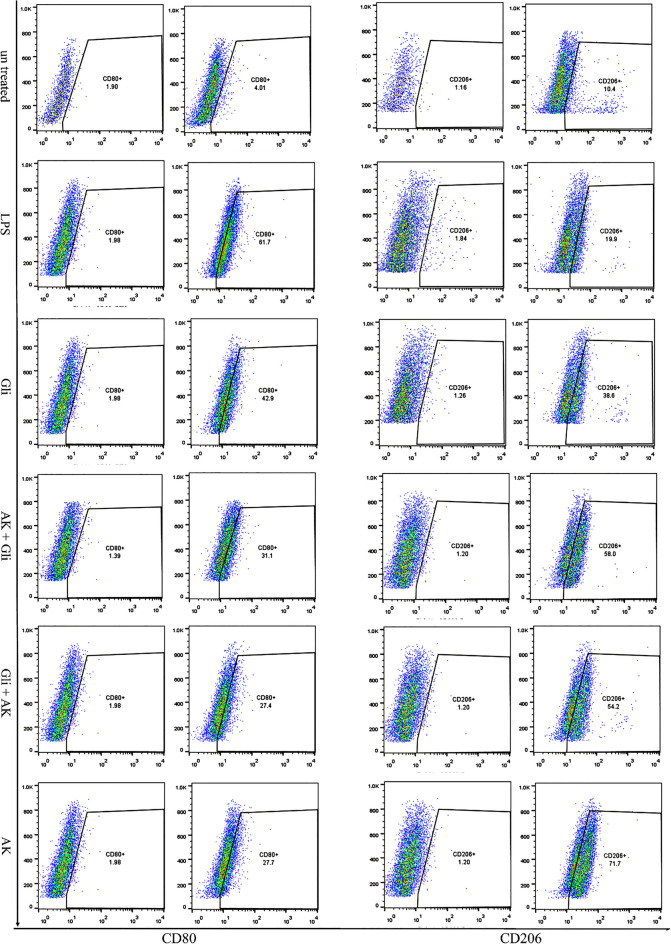
To characterize the effect of gliadin and A. muciniphila on the MQs cells phenotype, we assessed the CD80 levels, as an M1-specific marker, and CD206 levels, as an M2 specific marker, by flow cytometry.
Our results showed that incubation of macrophages with gliadin (200 µg/mL) induced a slight increase in the percentage of M1 phenotype compared to the group with no treatment (Fig. 4). Moreover, the results indicated that, pre-and post-treatment of gliadin-stimulated MQs with A. muciniphila induced upregulation in the percentage of CD206 expressing cells (M2 phenotype) relative to the group that incubated with gliadin alone (Fig. 4). Furthermore, MQs treatment with A. muciniphila increased the percentage of CD206 + cells compared to the untreated group (Fig. 4). The frequency of cells expressing CD80 (M1 phenotype) in the LPS group was increased relative to the untreated cells (Fig. 4).
Figure 4.
A. muciniphila increases the presence of M2 macrophages. Flow cytometry analysis of MQs treated with A. muciniphila and gliadin showed a significant decrease in the number of M1 MQ cells compared with the gliadin group. AK + Gli, A. muciniphila and gliadin; Gli + AK, gliadin and A. Muciniphila; AK, A. Muciniphila; Gli, gliadin; LPS, lipo poly sacharide. (Duplicate assays) (**p < 0.01, ****p < 0.0001).
A comparison of flow cytometry results in all groups is shown as a bar graph chart (Fig. 5).
Figure 5.

Bar graph chart representing the expression of CD80/CD206 markers in all groups. The student’s t-test was used to make comparisons between markers in each group. AK, A. Muciniphila; Gli, gliadin; LPS, lipo polysaccharide (*p < 0.05, **p < 0.01, ***p < 0.001 and ****p < 0.0001).
Discussion
Macrophages (MQs) are key players of innate immune response and provide adaptive immunity by acting as antigen-presenting cells (APCs)18. These cells can polarize to M1 (pro-inflammatory) or M2 (anti-inflammatory) functional phenotypes in response to their tissue microenvironment3. MQs can promote intestinal homeostasis and regulates immune responses and disease progression19. Therefore, targeting MQs polarization and inducing M2 phenotype may be a new strategy to treat intestinal inflammatory disorders like celiac disease. In this regard, potential targets that can affect M2 polarization becomes an attractive area of reaching mucosal healing which is the ultimate treatment goal of this kind of disorders18. In celiac disease, gliadin peptides induce activation of macrophages toward a pro-inflammatory M1 phenotype6–10. In vitro models of gluten toxicity are of particular importance for the development of novel treatment options for CeD. On this basis, in this study we evaluated the inflammatory and anti-inflammatory effects of A. muciniphila on THP-1 derived MQs stimulated with gliadin.
Our data showed that, gliadin stimulates pro-inflammatory cytokines (such as TNF-α and IL-6) expression in PMA-stimulated THP-1 MQs, similar to that induced by the potent inflammatory agent LPS and drives their differentiation toward the M1 phenotype. The effect of gliadin on MQ cells has been suggested by numerous in vitro studies conducted on human monocytic cell lines and murine macrophages which show that gliadin elicits an inflammatory response in these cells6,8,9. In line with our finding, the study of Serena et al. evaluated the response of primary human monocytes derived MQ to gliadin and indicated that gliadin triggers a potent inflammatory response on human primary MQs20.
The positive effects of A. muciniphila, on phenotype switching of gliadin triggered M1 MQs to an anti-inflammatory phenotype was also observed in this study. In our model, pre-treatment of gliadin-stimulated MQs with A. muciniphila (evaluating the preventive effect) caused increased M2 (CD206 +) phenotype MQ and reduced M1/M2 MQ ratio. This transition from M1 to M2 phenotype was associated with decreased proinflammatory (IL-6, TNF-α) and increased anti-inflammatory (IL-10, TGF-β) cytokines expression21.
The ability of A. muciniphila to induce an anti-inflammatory environment has been repeatedly reported in the context of different diseases22,23. In an in vivo study conducted by Mulhall et al. the effect of A. muciniphila and its pili-like protein was evaluated on the MQ’s polarization. In their study, increased alveolar bone loss was induced by Porphyromonas gingivalis gavage treatment of mice. The administration of gut commensal microbe A. muciniphila or its pili like protein Amuc_1100 to mice orally challenged with P. gingivalis, could protect alveolar bone loss and direct differentiation of MQs toward M2 phenotype and also increase IL-10 gene and protein expression within the gingival tissue24. Keshavarz Azizi Raftar et al. in their recent in vivo study evaluated the effects of live and pasteurized A. muciniphila and its extra cellular vesicles (EVs) on inflammatory markers involved in liver fibrosis. According to their results, Live A. muciniphila and its EVs had inhibitory effects on liver inflammation and hepatocytes damages. Moreover, four weeks of oral gavage with live and pasteurized A. muciniphila and its EVs could successfully maintain homeostasis in the colon tissue and enhances the epithelium and mucosal layer thickness and crypt depth accompanied by an increase in RNA level of tight junction proteins25. Ashrafian et al. also in their study showed that alive and pasteurized A. muciniphila had positive effect on tight junction and immune response-related genes in Caco-2 cell line26.
It is worth mentioning that A. muciniphila has mucin-degrading enzymes and utilizes mucin, a complex glycosylated protein, and degrades it as carbon, energy and nitrogen sources27. These beneficial by-products are involved in regulating the host immune system through different signals including TNF‐α, IL‐10, etc.14,28.
In the aspect of the protective effect of A. muciniphila, our results also showed that treating gliadin stimulated MQs with A. muciniphila leads to anti-inflammatory responses too. In general, no significant difference was observed between the protective and preventive effects of A. muciniphila.
This is also noteworthy that, different probiotics like some strains of Bifidobacterium have modulatory effects on immune responses through using different strategies, like reducing IL-6 levels29.
On the other hand, A. muciniphila alone also induced the increased levels of IL-6 and TNF-alpha in THP-1 cells, which may indicate its pivotal effect on the modulation of immune responses and point to the need for further investigation for finding its precise mechanisms. Actually, it can be said that, the best anti-inflammatory effect of A. muciniphila is achieved when it is used following inflammatory conditions.
Here, we gathered current suggestions regarding the anti-inflammatory and immune-modulatory mechanisms of A. muciniphila. As mentioned above, one observed mechanism is that purified Amuc_1100, as an outer-membrane protein of A. muciniphila, can activate TLR2 signaling which is associated with the production of specific cytokines and mainly leads to high levels of IL-10. Amuc_1100 is showed to be effective in reducing the inflammatory state of the immune responses, improving the barrier integrity and maintaining host immunological homeostasis16. Moreover, short-chain fatty acids (SCFAs) and branched-chain fatty acids (BCFAs) are the main metabolites of A. muciniphila, that may have a role in alleviating the inflammatory responses14,30. In general, SCFAs are reported to play a role in regulating the immune system and inflammatory responses and BCFAs are considered to have a beneficial role against inflammation and affect the expression of anti-inflammatory cytokines31–33. A. muciniphila-derived EVs have also been reported to have immune-modulatory and anti- inflammatory properties34. In this regard, the significant effects of EVs on the modulation of inflammatory and anti-inflammatory mediator’s gene expression, lipid metabolism and intestinal homeostasis has been reported in previous studies on different pathological conditions25,34. Additionally, it has been shown that, A. muciniphila adheres to the intestinal epithelium and increases the integrity of the epithelial cell layer. This observation suggests the ability of A. muciniphila to strengthen an impaired gut barrier and its linkage with intestinal health35.
The main limitation of this study was that we could not fully explain the exact beneficial mechanism of A. muciniphila on gliadin stimulated THP-1- derived MQ cells and further studies are highly recommended to expand our knowledge in this regard. Moreover, due to the significant difference between the in vitro and in vivo systems, the results should be used with caution and more extensive and detailed research is needed to confirm our results. Establishing a celiac disease animal model to prove these in vitro findings is also encouraged. Moreover, bone marrow-derived macrophages can be used to prove the equal anti-inflammatory effect of A. muciniphila ex vivo.
As celiac disease is accompanied by oral and intestinal dysbiosis, it is recommended that finding novel supplementary therapeutic targets with the aim of reducing dysbiosis harmful effects, can improve CeD patient’s quality of life. This study suggests that, A. muciniphila as a beneficial bacterium have the ability to change MQs response to gliadin and induce anti-inflammatory responses and may be considered as a supplemental therapy for CeD patients. The results might be expanded to next pre-clinical studies.
Methods
Cell culture
Human monocytic THP-1 cell line was purchased from Pasteur Institute of Iran. The cells were cultured in RPMI 1640 (Biosera, France) medium supplemented with 10% (v/v) fetal bovine serum (FBS) (Gibco, USA) and a 1% (v/v) mixture of penicillin and streptomycin (Gibco, USA). cells were maintained in a humidified incubator containing 5% CO2 at 37 °C. The culture medium was changed every 48 h.
Macrophage’s differentiation
For macrophage differentiation, 1× cells THP-1 was seeded in each well of 6-well tissue culture plates (SPL Life Sciences, Korea) and treated with 50 ng/ml phorbol 12-myristate 13-acetate (PMA; Santa Cruz Biotechnology Cat. No. sc-3576) for 36 h. To prove the differentiation of monocytes to macrophages, in addition to examining the morphology of cells, the CD11b expression assay was performed by flow cytometry technique36.
Bacterial culture
A. muciniphila MucT (ATCC BAA-835) was grown anaerobically (N2/CO2 80:20 v/v) in brain heart infusion broth supplemented with 3% commercial mucin (Sigma -Aldrich) in 10 ml Hungate anaerobic tubes. The concentration of bacteria was determined by measuring the optical density at 600 nm. After the OD600 reached ~ 1, bacterial suspension was centrifuged at 11,000 g for 20 min. Liquid cultures were washed and resuspended in anaerobic sterile PBS at the required concentration prior to each experiment34,37.
Gliadin and bacteria treatments
Gliadin (Sigma, USA) was dissolved in 1% Dimethyl sulfoxide (DMSO). In order to determine the appropriate concentration of gliadin with the most inflammatory effect on MQs, three different concentrations of gliadin (100, 200, 400 μg/ml) were used (although previous studies considered the concentration of 200 μg/ml as the best concentration for gliadin in this regard)6. Based on our findings, the concentration of 200 μg/ml of gliadin was used as the optimal concentration too.
A. muciniphila were added to MQ’s culture at a multiplicity of infection (MOI) of 1, 10 and 100 respectively25.
To investigate the effect of A. muciniphila on the MQ’s response to gliadin peptide, A. muciniphila was added to the MQ cells before stimulation with gliadin as pre-treat (preventive effect) and after stimulation with gliadin as post-treat (protective effect).
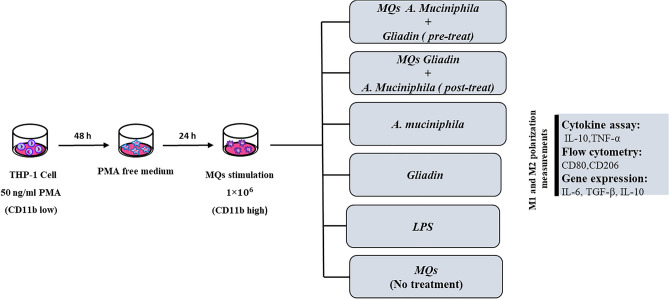
To evaluate the preventive effect of A. muciniphila on the inflammatory effect of gliadin, 1 × 106 of THP-1 cells were seeded in 6 well cell culture treated plate. After 48 h of PMA treatment, the culture media with PMA was changed with penicillin and streptomycin-free medium (to prevent bacterial death) followed by rest for 24 h prior to experiments. Cells were treated with A. muciniphila in three concentrations (MOI = 1, 10, 100) for 24 h and then the treatment with gliadin was done for the next 24 h (pre-treat). Furthermore, to evaluate the protective effect of A.muciniphila, after 24 h stimulation with gliadin, cells were treated with different concentrations (MOI = 1, 10, 100) of A. muciniphila (post-treat). A well was treated with 20 ng/ml LPS (Santa Cruz Biotechnology Cat No. sc-3535), as positive control, to compare its induction pattern with gliadin and bacteria. Two wells that were treated with gliadin alone and A. muciniphila alone and a well full of PMA-treated THP-1 cells without any treatment was considered as the negative control group (Fig. 6). Experiments were performed in triplicate (technical replicates).
Figure 6.
Schematic illustration of the study design.
RNA extraction and quantitative real -time PCR (qRT -PCR)
Total RNA was extracted from THP-1 monocytes derived macrophage cultures using the Total RNA Purification Mini kit for Blood/Cultured Cell/Tissue (Yekta Tajhiz Azma, Tehran, Iran) according to the provided protocol. Reverse transcription was performed using 2-step 2X RT-PCR Premix (Taq) kit (BioFact™, South Korea), according to the manufacturer's instructions. Quantitative RT-PCR was conducted on the thermocycler Rotor Gene Q MDx (QIAGEN) with SYBR Green Master Mix (BioFact™, South Korea). Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was measured as a housekeeping reference gene in each qPCR run and relative quantitation (RQ) for each gene expression was calculated by 2−ΔΔCt Method (ΔCt = Cttarget – Cthouse keeping). Specific human primers for IL-6, IL-10, and TGF-β were designed and analyzed by Gene Runner v. 3.05 software and used to evaluate gene expression (Table 1). All samples were assayed in triplicate.
Table 1.
Sequences for forward and reverse primers used to measure genes expression by real-time RT-PCR.
| Gene symbol | Primer sequence | Reference |
|---|---|---|
| IL-6 |
F: 5′-CTGGATTCAATGAGGAGACTTGC-3′ R: 5′-TCAAATCTGTTCTGGAGGTACTCTAGG-3′ |
Tian et al.38 |
| TGF-β |
F: 5′-CAATTCCTGGCGATACCTCAG-3′ R: 5′-GCACAACTCCGGTGACATCAA-3′ |
Jin et al.39 |
| IL-10 |
F: 5′-AAGAAGGCATGCACAGCTCA-3′ R: 5′-AAGTGGGTGCAGCTGTTCTC-3′ |
Aghamohamadi et al.40 |
| GAPDH |
F: 5′-TCTGACTTCAACAGCGACAC-3′ R: 5′-TACTCCTTGAGGCCATGT-3′ |
This study |
ELISA
Following stimulations, culture supernatants were harvested and stored at ˗70 °C until use. The level of IL-10 and TNF-α was determined using sandwich enzyme-linked immunosorbent assay (ELISA) kits (KPG. Kerman. Iran) according to the manufacturer’s instructions. Triplicate results were read by an ELISA reader at 450 nm (TECAN, Salzburg, Austria).
Flow cytometry
PE-conjugated anti-CD11b (BioLegend, San Diego, USA) was used as a difference marker between THP-1 cell line monocytes and differentiated MQs. MQs were stained with FITC-conjugated anti-CD80 (BioLegend, San Diego, USA) and PerCP-eFlour710 conjugated anti-CD206 (BD bioscience, USA) antibodies to evaluate the percentages of M1 and M2 MQs, respectively. The duplicate data were analyzed by FlowJo software v10 (FlowJo LCC, Ashland, OR, USA).
Statistics
Statistical analysis was performed using GraphPad Prism v8 (GraphPad Software, San Diego, CA, USA). Two groups were compared by Student's t-test, and One-way ANOVA was applied for comparisons between multiple experimental groups. p values were considered significant at less than 0.05. The criteria for significance were *p < 0.05, **p < 0.01, ***p < 0,0.001 and ****p < 0.0001.
Acknowledgements
The authors would like to acknowledge the Gastroenterology and Liver Diseases Research Center of Shahid Beheshti University of Medical Sciences for their support and contribution to this study.
Author contributions
M.R.N., K.B., and A.Y. designed the study. S.M.R., N.A. performed the experiments and statistical analysis. S.M.R., K.B., N.A., M.R.N., and A.S. drafted the manuscript. D.A. and A.S. conceived the study, and participated in its design. All authors read and approved the final manuscript.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Anna Sapone, Email: annasapone@yahoo.it.
Davar Amani, Email: amanid@sbmu.ac.ir.
Mohammad Rostami-Nejad, Email: m.rostamii@gmail.com.
References
- 1.Gordon S, et al. Localization and function of tissue macrophages. Ciba Found. Symp. 1986;118:54–67. doi: 10.1002/9780470720998.ch5. [DOI] [PubMed] [Google Scholar]
- 2.Matzinger P. Tolerance, danger, and the extended family. Annu. Rev. Immunol. 1994;12:991–1045. doi: 10.1146/annurev.iy.12.040194.005015. [DOI] [PubMed] [Google Scholar]
- 3.Liu H, et al. Macrophage functional phenotype can be consecutively and reversibly shifted to adapt to microenvironmental changes. Int. J. Clin. Exp. Med. 2015;8:3044–3053. [PMC free article] [PubMed] [Google Scholar]
- 4.Gordon S, Martinez FO. Alternative activation of macrophages: Mechanism and functions. Immunity. 2010;32:593–604. doi: 10.1016/j.immuni.2010.05.007. [DOI] [PubMed] [Google Scholar]
- 5.Wynn TA, Chawla A, Pollard JW. Macrophage biology in development, homeostasis and disease. Nature. 2013;496:445–455. doi: 10.1038/nature12034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jelínková L, Tucková L, Cinová J, Flegelová Z, Tlaskalová-Hogenová H. Gliadin stimulates human monocytes to production of IL-8 and TNF-alpha through a mechanism involving NF-kappaB. FEBS Lett. 2004;571:81–85. doi: 10.1016/j.febslet.2004.06.057. [DOI] [PubMed] [Google Scholar]
- 7.Tucková L, et al. Activation of macrophages by gliadin fragments: Isolation and characterization of active peptide. J. Leukoc Biol. 2002;71:625–631. doi: 10.1189/jlb.71.4.625. [DOI] [PubMed] [Google Scholar]
- 8.Thomas KE, Sapone A, Fasano A, Vogel SN. Gliadin stimulation of murine macrophage inflammatory gene expression and intestinal permeability are MyD88-dependent: Role of the innate immune response in Celiac disease. J. Immunol. 2006;176:2512–2521. doi: 10.4049/jimmunol.176.4.2512. [DOI] [PubMed] [Google Scholar]
- 9.Harris KM, Fasano A, Mann DL. Monocytes differentiated with IL-15 support Th17 and Th1 responses to wheat gliadin: Implications for celiac disease. Clin. Immunol. 2010;135:430–439. doi: 10.1016/j.clim.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim SO, Sheikh HI, Ha SD, Martins A, Reid G. G-CSF-mediated inhibition of JNK is a key mechanism for Lactobacillus rhamnosus-induced suppression of TNF production in macrophages. Cell. Microbiol. 2006;8:1958–1971. doi: 10.1111/j.1462-5822.2006.00763.x. [DOI] [PubMed] [Google Scholar]
- 11.Singh P, et al. Global prevalence of celiac disease: Systematic review and meta-analysis. Clin. Gastroenterol. Hepatol. 2018;16:823–836.e822. doi: 10.1016/j.cgh.2017.06.037. [DOI] [PubMed] [Google Scholar]
- 12.MacCulloch K, Rashid M. Factors affecting adherence to a gluten-free diet in children with celiac disease. Paediatr Child Health. 2014;19:305–309. doi: 10.1093/pch/19.6.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sellitto M, et al. Proof of concept of microbiome-metabolome analysis and delayed gluten exposure on celiac disease autoimmunity in genetically at-risk infants. PLoS ONE. 2012;7:e33387. doi: 10.1371/journal.pone.0033387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang T, Li Q, Cheng L, Buch H, Zhang F. Akkermansia muciniphila is a promising probiotic. Microb. Biotechnol. 2019;12:1109–1125. doi: 10.1111/1751-7915.13410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhai Q, Feng S, Arjan N, Chen W. A next generation probiotic, Akkermansia muciniphila. Crit. Rev. Food Sci. Nutr. 2019;59:3227–3236. doi: 10.1080/10408398.2018.1517725. [DOI] [PubMed] [Google Scholar]
- 16.Ottman N, et al. Pili-like proteins of Akkermansia muciniphila modulate host immune responses and gut barrier function. PLoS ONE. 2017;12:e0173004. doi: 10.1371/journal.pone.0173004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bodkhe R, et al. Comparison of small gut and whole gut microbiota of first-degree relatives with adult celiac disease patients and controls. Front. Microbiol. 2019;10:164. doi: 10.3389/fmicb.2019.00164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Martinez FO, Gordon S. The M1 and M2 paradigm of macrophage activation: Time for reassessment. F1000Prime Rep. 2014;6:13. doi: 10.12703/p6-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grainger JR, Konkel JE, Zangerle-Murray T, Shaw TN. Macrophages in gastrointestinal homeostasis and inflammation. Pflugers Arch. 2017;469:527–539. doi: 10.1007/s00424-017-1958-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Serena G, et al. Intestinal epithelium modulates macrophage response to gliadin in celiac disease. Front. Nutr. 2019;6:167. doi: 10.3389/fnut.2019.00167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Quero L, Hanser E, Manigold T, Tiaden AN, Kyburz D. TLR2 stimulation impairs anti-inflammatory activity of M2-like macrophages, generating a chimeric M1/M2 phenotype. Arthritis Res. Ther. 2017;19:245. doi: 10.1186/s13075-017-1447-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Earley H, et al. The abundance of Akkermansia muciniphila and its relationship with sulphated colonic mucins in health and ulcerative colitis. Sci. Rep. 2019;9:15683. doi: 10.1038/s41598-019-51878-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li J, Lin S, Vanhoutte PM, Woo CW, Xu A. Akkermansia Muciniphila protects against atherosclerosis by preventing metabolic endotoxemia-induced inflammation in Apoe-/- mice. Circulation. 2016;133:2434–2446. doi: 10.1161/circulationaha.115.019645. [DOI] [PubMed] [Google Scholar]
- 24.Mulhall H, et al. Akkermansia muciniphila and Its Pili-Like Protein Amuc_1100 modulate macrophage polarization in experimental periodontitis. Infect. Immun. 2020;89:e00500–e520. doi: 10.1128/iai.00500-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Raftar SKA, et al. The anti-inflammatory effects of Akkermansia muciniphila and its derivates in HFD/CCL4-induced murine model of liver injury. Sci. Rep. 2022;12:2453. doi: 10.1038/s41598-022-06414-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ashrafian F, et al. Comparative effects of alive and pasteurized Akkermansia muciniphila on normal diet-fed mice. Sci. Rep. 2021;11:17898. doi: 10.1038/s41598-021-95738-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Geerlings SY, et al. Genomic convergence between Akkermansia muciniphila in different mammalian hosts. BMC Microbiol. 2021;21:298. doi: 10.1186/s12866-021-02360-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Qu S, et al. Akkermansia muciniphila alleviates dextran sulfate sodium (DSS)-induced acute colitis by NLRP3 activation. Microbiol. Spectr. 2021;9:e0073021. doi: 10.1128/Spectrum.00730-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bozkurt HS, Bilen Ö. Oral booster probiotic bifidobacteria in SARS-COV-2 patients. Int. J. Immunopathol. Pharmacol. 2021;35:20587384211059677. doi: 10.1177/20587384211059677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li Z, et al. Study of growth, metabolism, and morphology of Akkermansia muciniphila with an in vitro advanced bionic intestinal reactor. BMC Microbiol. 2021;21:61. doi: 10.1186/s12866-021-02111-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ran-Ressler RR, Bae S, Lawrence P, Wang DH, Brenna JT. Branched-chain fatty acid content of foods and estimated intake in the USA. Br. J. Nutr. 2014;112:565–572. doi: 10.1017/s0007114514001081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nogal A, Valdes AM, Menni C. The role of short-chain fatty acids in the interplay between gut microbiota and diet in cardio-metabolic health. Gut Microbes. 2021;13:1–24. doi: 10.1080/19490976.2021.1897212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Budhram A, Parvathy S, Kremenchutzky M, Silverman M. Breaking down the gut microbiome composition in multiple sclerosis. Mult. Scler. J. 2016;23:628–636. doi: 10.1177/1352458516682105. [DOI] [PubMed] [Google Scholar]
- 34.Ashrafian F, et al. Akkermansia muciniphila-derived extracellular vesicles as a mucosal delivery vector for amelioration of obesity in mice. Front. Microbiol. 2019;10:2155. doi: 10.3389/fmicb.2019.02155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Reunanen J, et al. Akkermansia muciniphila adheres to enterocytes and strengthens the integrity of the epithelial cell layer. Appl. Environ. Microbiol. 2015;81:3655–3662. doi: 10.1128/aem.04050-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Starr T, Bauler TJ, Malik-Kale P, Steele-Mortimer O. The phorbol 12-myristate-13-acetate differentiation protocol is critical to the interaction of THP-1 macrophages with Salmonella Typhimurium. PLoS ONE. 2018;13:e0193601. doi: 10.1371/journal.pone.0193601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Derrien M, Vaughan EE, Plugge CM, de Vos WM. Akkermansia muciniphila gen. nov., sp. nov., a human intestinal mucin-degrading bacterium. Int. J. Syst. Evol. Microbiol. 2004;54:1469–1476. doi: 10.1099/ijs.0.02873-0. [DOI] [PubMed] [Google Scholar]
- 38.Tian B, et al. Analysis of the TGFβ-induced program in primary airway epithelial cells shows essential role of NF-κB/RelA signaling network in type II epithelial mesenchymal transition. BMC Genom. 2015;16:529. doi: 10.1186/s12864-015-1707-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jin Y, Lu X, Wang M, Zhao X, Xue L. X-linked inhibitor of apoptosis protein accelerates migration by inducing epithelial–mesenchymal transition through TGF-β signaling pathway in esophageal cancer cells. Cell Biosci. 2019;9:76. doi: 10.1186/s13578-019-0338-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Aghamohamadi E, et al. Gene expression analysis of intestinal IL-8, IL-17 A and IL-10 in patients with celiac and inflammatory bowel diseases. Mol. Biol. Rep. 2022;49:6085–6091. doi: 10.1007/s11033-022-07397-y. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.