Abstract
Cannabidiol (CBD) reportedly exerts protective effects against many psychiatric disorders and neurodegenerative diseases, but the mechanisms are poorly understood. In this study, we explored the molecular mechanism of CBD against cerebral ischemia. HT-22 cells or primary cortical neurons were subjected to oxygen-glucose deprivation insult followed by reoxygenation (OGD/R). In both HT-22 cells and primary cortical neurons, CBD pretreatment (0.1, 0.3, 1 μM) dose-dependently attenuated OGD/R-induced cell death and mitochondrial dysfunction, ameliorated OGD/R-induced endoplasmic reticulum (ER) stress, and increased the mitofusin-2 (MFN2) protein level in HT-22 cells and primary cortical neurons. Knockdown of MFN2 abolished the protective effects of CBD. CBD pretreatment also suppressed OGD/R-induced binding of Parkin to MFN2 and subsequent ubiquitination of MFN2. Overexpression of Parkin blocked the effects of CBD in reducing MFN2 ubiquitination and reduced cell viability, whereas overexpressing MFN2 abolished Parkin’s detrimental effects. In vivo experiments were conducted on male rats subjected to middle cerebral artery occlusion (MCAO) insult, and administration of CBD (2.5, 5 mg · kg−1, i.p.) dose-dependently reduced the infarct volume and ER stress in the brains. Moreover, the level of MFN2 within the ischemic penumbra of rats was increased by CBD treatment, while the binding of Parkin to MFN2 and the ubiquitination of MFN2 was decreased. Finally, short hairpin RNA against MFN2 reversed CBD’s protective effects. Together, these results demonstrate that CBD protects brain neurons against cerebral ischemia by reducing MFN2 degradation via disrupting Parkin’s binding to MFN2, indicating that MFN2 is a potential target for the treatment of cerebral ischemia.
Keywords: cannabidiol, MFN2, Parkin, cerebral ischemia, oxidative stress
Introduction
Ischemic stroke remains one of the leading causes of death and adult disability in humans worldwide [1]. Recombinant tissue plasminogen activators (rtPAs) and endovascular recanalization are beneficial to an extent in improving the outcomes of patients who can be sent to the hospital promptly [2]. However, owing to the short therapeutic windows of rtPAs, most patients cannot benefit from these therapies [3]. In addition, despite the successful application of rtPA or recanalization, the outcomes are often unsatisfactory, and most stroke patients still need additional pharmacological therapy for neuroprotection [2]. Therefore, the identification of novel therapeutic targets and neuroprotective drugs remains an unmet urgent need.
Mitofusin-2 (MFN2), a protein in the outer mitochondrial membrane, tethers the endoplasmic reticulum (ER) to mitochondria [4]. Loss of MFN2 leads to disrupted mitochondrial morphology, impaired calcium homeostasis in the mitochondria, and accelerated cell death [5]. MFN2 also affects the activation of ER stress and acts as a regulator of the unfolded protein response (UPR) [6, 7]. Specifically, MFN2 deficiency exacerbates ER stress and the subsequent activation of UPR [6], thus promoting neuronal death. In recent years, the role of MFN2 in the central nervous system has attracted wide interest. MFN2 mutation is viewed as a contributor to neurodegenerative Charcot-Marie-Tooth disease type 2A, the most common inheritable peripheral sensorimotor neuropathy [8]. In other neurological disorders, ablation of MFN2 in the cortex and hippocampus causes excessive oxidative stress and neuronal cell death [9]. By contrast, overexpression of MFN2 exerts protective effects against ischemia-reperfusion and reduces neuronal apoptosis caused by ischemia or hypoxia [10, 11]. Given the pivotal role of MFN2 in protecting against neurological disorders, whether MFN2 is a potential target for relieving neuronal damage during cerebral ischemia injury needs further investigation.
Cannabis sativa ingredients are a widely analyzed group of compounds. The two most extensively studied phytocannabinoids are Δ9-tetrahydrocannabinol (THC) and cannabidiol (CBD) [12, 13]. In recent years, increasing attention has been paid to those phytocannabinoids that do not show any psychoactive effects but have antioxidant and anti-inflammatory properties. THC is the main psychoactive constituent of cannabis, and CBD is a non-psychoactive cannabinoid derived from Cannabis sativa [12]. Besides the difference in psychoactive effects, cannabinoids may play different roles during the process of neuronal death. For example, studies have shown that THC exacerbates neuronal injury, whereas CBD attenuates it in rat hippocampal slices exposed to oxygen-glucose deprivation (OGD) [14]. Additionally, CBD has been reported to exert protective effects on psychiatric disorders and neurodegenerative diseases, such as cognitive impairment [15], aging proteinopathies [16], Alzheimer’s disease [17], and Parkinson’s disease [18]. As CBD has advantages over THC in both psychoactive effects and neuroprotection, we are interested in the effect and mechanism of CBD against cerebral ischemia. Increasing studies have shown that CBD exerts anti-apoptosis roles in cerebral ischemia [19, 20]. CBD treatment also attenuates ischemia-induced memory deficits [21], lipid peroxidation [20], astrogliosis, and microglial activation [22]. However, the mechanisms underlying its neuroprotection are largely unknown. Most studies have indicated that the CBD effect does not depend on cannabinoid receptors because CBD has a low affinity for both the cannabinoid CB1 and CB2 receptors [23]. More likely, CBD acts on multiple targets, including cannabinoid receptors, neurotransmitter transporters and receptors, and ion channels [24]. Importantly, it has been proposed that the mechanism of action of CBD is complicated and needs to be explored in the disease context [24]. Collectively, current findings confirm an important role of CBD against cerebral ischemia. However, the primary molecule(s) by which CBD mediates protective effects on cerebral ischemia remain poorly defined. Interestingly, our previous study showed that CBD treatment restored mitochondrial membrane potential (MMP) and enhanced the level of MFN2 in microglial cells subjected to lipopolysaccharide [25]. We were thus interested in whether MFN2 mediates the protective effects of CBD in models of cerebral ischemia. This study aimed to determine the essential role of MFN2 in the protective effects of CBD as well its potential mechanisms in experimental models of cerebral ischemia.
Materials and methods
Reagents
CBD (#C302806) was obtained from Aladdin (Shanghai, China). HT-22 neuronal cells (#SCC129), 2,3,5-triphenyltetrazolium chloride (TTC, #T8877), and dithiothreitol (DTT, #10197777001) were purchased from Merck (Darmstadt, Germany). Antibody against caspase 3 (#9662), parkin (#4211), ubiquitin (#3936), phosphorylated eukaryotic translation initiation factor 2α (p-eIF2α, ser51, #3597), eIF2α (#5324), glucose-regulated protein 78 (GRP78, #3177), Mouse Anti-rabbit IgG (Conformation Specific, HRP Conjugate, for co-IP, #5127) and IgG Isotype Control (#3900) were purchased from Cell Signaling Technology (Massachusetts, USA). Antibody against MFN2 (#12186-1-AP) was purchased from Proteintech (Rosemont, USA). Antibodies against phosphorylated inositol-requiring enzyme-1α (p-IRE1α, Ser724, #ab48187), and microtubule-associated protein 2 (MAP2, #ab11268) were obtained from Abcam (Cambridge, UK). Protein A/G Magnetic Beads (#B23202) and SYBR Green qPCR Master Mix (#B21202) were obtained from Bimake (Houston, Texas, USA). ShMFN2, MFN2 plasmid, and Parkin plasmid were obtained from OBiO Technology (Shanghai, China). Small interfering RNA (siRNA) targeted MFN2 was obtained from GenePharma (Shanghai, China). A Pierce™ Rapid Gold BCA Protein Assay Kit (#A53225), fetal bovine serum (FBS, #A3161001C), Opti-MEM™ Reduced Serum Medium (#31985070), high glucose Dulbecco’s modified Eagle’s medium (DMEM) (#11965092), glucose-free DMEM (#11966025), Lipofectamine 3000 Reagent (#L3000008), Lipofectamine 2000 Reagent (#11668027), Live Cell Imaging Solution (#A14291DJ), StemPro™ Accutase™ Cell Dissociation Reagent (#A1110501), Neurobasal (#21103049), B27 (#17504001), GlutaMAX™ Supplement (#35050038), Lipofectamine™ RNAiMAX Transfection Reagent (#13778150), tetramethylrhodamine ethyl ester perchlorate (TMRE, #T669), Mito-tracker (#M7512), CellROX™ Deep Red Reagent (#C10422), and TRIzol™ reagent (#15596026) were obtained from Thermo Fisher Scientific (Waltham, MA, USA). Cell Counting Kit-8 (CCK-8, #FD3788), antibodies against GAPDH (#FD0063), β-tubulin (#FD0064), goat anti-rabbit IgG (H + L)-HRP (#FDR007), goat anti-mouse IgG (H + L)-HRP (#FDM007), and RIPA buffer (high strength, #FD009; weak strength, #FD011) were purchased from FDBIO SCIENCE (Hangzhou, Zhejiang, China). Goat anti-mouse IgG Dylight 549 (#A23310) were obtained from Abbkine Scientific (Wuhan, Hubei, China). FastKing-RT SuperMix (#KR118) was obtained from TIANGEN (Beijing, China).
Cell culture
HT-22 cells were obtained from Merck (Darmstadt, Germany) and were cultured in DMEM supplemented with 10% FBS. The cells were maintained in a humidified atmosphere containing 5% CO2 at 37 °C. Before the experiments, the cells were tested for mycoplasma. Passages from 3 to 15 were used in the experiment.
Culturing of primary cortical neurons was conducted as previously reported [26]. Briefly, cortices of E16–18 fetal Sprague-Dawley (SD) rats were collected by micromanipulation, and the cortices were detached to single cell by digestion with Accutase for 3 min at 37 °C. The digestion was ended with DMEM supplemented with 5% FBS. After centrifugation, cells were resuspended to the desired density and seeded on plates. Then, 4 h after seeding, the medium was replaced with the NeurobasalTM medium supplemented with 2% B27 and 1% GlutaMAX™ Supplement.
Establishment of the oxygen-glucose deprivation/reperfusion (OGD/R) model
The OGD/R model was established as previously reported [26]. Briefly, after the culture medium had been washed with phosphate-buffered saline (PBS), it was replaced with glucose-free DMEM. Then, the cells were placed in a hypoxia chamber (Billups-Rothenberg, Inc., San Diego, CA, USA) containing 5% CO2 and 95% N2 at 37 °C. After OGD, the medium was changed to DMEM, and cells were returned to the normoxic environment and incubated for the desired time.
Cell viability detection
The variance of cell viability was detected by the CCK-8 assay [26]. Briefly, after 24 h of reoxygenation, CCK-8 was added to each well. After 4 h of incubation, absorbance at 450 nm was measured with a multifunctional microplate reader (Synergy HT; Biotech, Winooski, VT, USA). To avoid unwanted variance, the original OD value was normalized to the control group in each independent experiment.
Quantitative polymerase chain reaction (PCR)
Total RNA was isolated from the cell culture using the TRIzol™ reagent and then reverse transcribed using FastKing-RT SuperMix. Quantitative PCR (qPCR) was conducted by using the SYBR Green qPCR Master Mix on the Roche LightCycler 480 system (Roche, Basel, Switzerland). For Hot-Start DNA polymerase activation, the reaction was heated at 95 °C for 5 min. The PCR program (40 cycles) consisted of denaturation at 95 °C for 15 s, and annealing/extension at 60 °C for 30 s. The sequences of MFN2 and GAPDH primers were as follows: MFN2: forward primer “5’-AGCAAGTTGACATCACCCGA-3’,” reverse primer “5’-TGTCCAACCAGCCAGCTTTA-3’”; GAPDH: forward primer “5’-TGTGAACGGATTTGGCCGTA-3’,” reverse primer “5’-ACTGTGCCGTTGAATTTGCC-3’.” The gene expression level of MFN2 was calibrated with GAPDH and normalized to the control group.
Immunocytochemistry
Immunocytochemistry was conducted as described previously [26]. Briefly, after treatment, cells were fixed in 4% paraformaldehyde for 1 h at room temperature followed by permeabilization with 0.5% Triton X-100. Then, cells were incubated with 5% goat serum at room temperature for 1 h to block nonspecific binding sites and washed three times with PBS with 0.1% Tween 20. Cells were incubated with antibodies against MAP2 at 4 °C overnight. After three washes, cells were incubated with secondary antibodies coupled to a fluorescent probe at room temperature for 1 h. Finally, cells were imaged with a confocal microscope (ECLIPSE Ti, Nikon).
Overexpression of MFN2 and Parkin
In vitro overexpression of MFN2 and Parkin was conducted by transfecting with pEGFP-N1(Mfn2-3xFLAG-P2A-EGFP) and pEGFP-N1(Prkn-P2A-EGFP) plasmid. Briefly, the plasmid was incubated with the P3000 reagent in Opti-MEM for 5 min; and then incubated with Lipofectamine 3000 pre-diluted in Opti-MEM. After 20 min of incubation, this mixture was added to cells and incubated for 24 h. The overexpression was verified by immunoblotting.
Silencing of MFN2
In vitro silencing of MFN2 was achieved through transfection of siRNA specific to MFN2. siRNA transfection was performed as previously reported [27]. Briefly, for HT-22 cell transfection, the siRNAs and Lipofectamine 2000 pre-diluted in Opti-MEM were mixed and incubated for 20 min at room temperature. Then, this siRNA-Lipofectamine mixture was added to the cells and incubated for 6 h. Subsequently, the medium was changed to DMEM containing 1% FBS. The sequences of the siRNA for MFN2 were as follows: Sense: “5’-GCGCCAGCUUCCUUGAAGATT-3’” and antisense: “5’-UCUUCAAGGAAGCUGGCGCTT-3’”.
For neuron transfection, the siRNA and Lipofectamine™ RNAiMAX were diluted in Opti-MEM independently and then incubated to form a siRNA-Lipofectamine mixture for 5 min at room temperature. Then, this RNA-Lipofectamine mixture was added to the cells and incubated for 24 h. The siRNA sequences for MFN2 were as follows: sense: “5’-CUGCCAUGAACAAGAAAGUTT-3’” and antisense: “5’-ACUUUCUUGUUCAUGGCAGTT-3’”.
Mitochondrial membrane potential (MMP) detection
Changes in the MMP were visualized by TMRE staining [27]. After treatment, HT-22 cells were incubated with TMRE for 30 min and then washed with PBS, and the medium was changed to Live Cell Imaging Solution. Finally, the variance of TMRE fluorescence was imaged using a confocal microscope. Data were analyzed using ImageJ software.
Intracellular reactive oxygen species (ROS) detection
The level of intracellular ROS was detected using CellROX staining [26]. After treatment, cells were incubated with the CellROX Deep Red Reagent for 30 min. Then, cells were washed with PBS, and the medium was changed to Live Cell Imaging Solution. Finally, the change of intracellular ROS was visualized using a confocal microscope. Data were analyzed using ImageJ software.
Mito-tracker staining
To image the variance of mitochondrial morphology, Mito-tracker red dye was used to visualize mitochondria [28]. After OGD/R insult, HT-22 cells were incubated with Mito-tracker red dye at 37 °C for 30 min and then washed with PBS. Finally, the cells were imaged with a confocal microscope, and images were analyzed with ImageJ software.
Animals
It is well documented that sex affects the infarct volume, mortality, and sensory-motor impairment in experimental stroke animal models [29]. To avoid variations, SD male rats were used in the present studies. Rats (260–300 g) were obtained from the Laboratory Animal Center of Southern Medical University (Guangzhou, China). Six rats were housed per cage in a 12-h light/dark cycle at 23–25 °C. All animal care and experimental procedures were performed following the NIH Guide for the Care and Use of Laboratory Animals (NIH, revised 1996) and approved by the Laboratory Animal Ethics Committee of Southern Medical University. Animal studies were conducted in compliance with the ARRIVE guidelines. Similarly to how calculations were conducted in our previous studies [27, 30], power calculations were conducted to determine the lowest number of rats. Using the two-tailed test, a sample of 8 rats per group was needed to detect a difference in infarct sizes between vehicle and CBD by TTC staining with 95% confidence and 80% power. A total of 132 rats were used in this study; 27 rats were excluded from the analysis (25 rats were dead after surgery, and 2 rats showed no observable neurological deficit), and 105 rats were included in the final analysis (Table 1).
Table 1.
The total number of rat in experiment.
| Figure | Experiment | Surgery | Total number (n) | ICH caused (n) | NDS < 1 (n) | Mortality (n) | Rats included (n) |
|---|---|---|---|---|---|---|---|
| 7 | TTC/NDS | MCAO | 37 | 0 | 2 | 5 | 30 |
| Sham | 10 | 0 | 0 | 0 | 10 | ||
| WB/IP | MCAO | 13 | 0 | 0 | 5 | 8 | |
| Sham | 4 | 0 | 0 | 0 | 4 | ||
| 8, S4 | TTC | MCAO | 36 | 0 | 0 | 10 | 26 |
| WB (8f) | MCAO | 14 | 0 | 0 | 2 | 12 | |
| WB (8a, S4) | NA | 8 | 0 | 0 | 0 | 8 | |
| S3, S5, S6 | IHC | NA | 3 | 0 | 0 | 0 | 3 |
| S7 | WB | MCAO | 7 | 0 | 0 | 3 | 4 |
ICH intracerebral hemorrhage, NDS neurological deficit scores.
Drug preparation
For the in vitro study, CBD was dissolved in dimethyl sulfoxide, and the final concentration of dimethyl sulfoxide was less than 0.1%. The concentrations of CBD (0.1, 0.3, 1 μM) used in in vitro studies were based on a previous study showing that CBD produced neuroprotection in an in vitro cellular model of ischemic stroke [31]. For the in vivo study, CBD was dissolved in 5% HS15 containing 5% ethanol at the concentrations of 0.417 mg/mL and 0.833 mg/mL. Specifically, 2.5 mg/kg and 5 mg/kg CBD were administered intraperitoneally (ip) at the dose of 6 mL/kg. The doses were selected according to a previous study showing the protective effect of CBD in rats subjected to ischemic stroke [22].
Establishment of the middle cerebral artery occlusion/reperfusion (MCAO/R) model
The MCAO/R model was established as previously reported [26]. SD rats were anesthetized using isoflurane. An anal thermometer was used to maintain the body temperature at 37 ± 0.5 °C. Then, the internal carotid artery (ICA) was exposed and a 0.36-mm silicone-coated suture was inserted into ICA to block the MCA. After 2 h of MCAO, the suture was removed for reperfusion. Animals were excluded from experiments under the following conditions: neurological deficit score = 0, subarachnoid hemorrhage, or death.
Measurement of neurological deficit scores
Neurological functional recovery was evaluated by detecting neurological deficit scores 24 h after the MCAO insults. As previously reported [30], a five-point scoring system was used, where no behavioral deficit is “0”; failure to extend the contralateral forepaw freely is represented as “1”; circling to the contralateral side while walking as “2”; falling toward the contralateral side during walking as “3”; and no spontaneous walking as “4.” The evaluation was conducted by an experimenter who was blind to the experiment.
Detection of infarct volume
TTC staining is a classical method for detecting cerebral infarction [32]. The specific method is as follows: after measurement of neurological deficit scores, SD rats were anesthetized with isoflurane to death. Then, the brain was quickly collected, frozen at −30 °C for ~10 min, and cut into 2-mm-thick coronal slices using a brain matrix. Brain slices were incubated in 2% TTC solution at 37 °C for 30 min in the dark. Finally, the brain slices were fixed with 4% PFA for 30 min, placed on a glass plate in an orderly manner, and scanned with a scanner. The infarct volume was analyzed by ImageJ. To eliminate the influence of cerebral edema on the cerebral infarction calculation, we used the following correction formula: infarct volume (%) = (contralateral volume − non-infarct ipsilateral volume)/contralateral volume × 100%.
In vivo silencing of MFN2 expression
The sequence used for short hairpin RNA (shRNA) targeting MFN2 was “5’-CUGCCAUGAACAAGAAAGU-3’”. shRNA targeting MFN2 and the negative control (NC) were cloned into a vector (pAAV-U6-shRNA-CMV-EGFP-WPRE,) and then packed into adeno-associated virus (AAV) 2/9. This product was obtained from OBiO Technology (Shanghai, China). Then, 2–3 weeks before the MCAO procedure, AAV coding shMFN2 or NC was injected intracerebroventricularly (i.c.v.). After anesthetization with isoflurane and exposure of the skull, rats were fixed on the stereotaxic apparatus (Stoelting, Chicago, IL, USA). Then, AAV was slowly injected ipsilaterally into the lateral ventricle. The stereotaxic coordinate was −1 mm anteroposterior, 2 mm lateral, and −3.8 mm dorsoventral relative to bregma. AAV was injected into the lateral ventricle with a microsyringe pump (KD Scientific Inc., Holliston, MA, USA) at a constant speed. The microsyringe was held in place for 10 min in case of leakage. The knockdown efficacy was verified by immunoblotting.
Immunoblotting
Immunoblotting was performed as described previously [26]. Cells and penumbra cortex were lysed with the RIPA buffer containing protease and phosphatase inhibitor cocktails and homogenized using an ultrasonic breaker (VCX130; Sonics, Newtown, CT, USA). After crushing, samples were centrifugated at 20,000 × g for 20 min at 4 °C, and the supernatants were collected. The protein levels were measured with the BCA protein assay and normalized to the same level. Then, the samples were denatured by boiling at 95 °C for 10 min. The samples were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (8%–12%), and the proteins were electro-transferred to PVDF membranes. Subsequently, the nonspecific binding site was blocked using 5% skim milk for 1 h at room temperature. After blocking, the blots were incubated with the primary antibody at 4 °C overnight, followed by incubation with the secondary antibody conjugated to horseradish peroxidase. Finally, blots were imaged with a chemiluminescence imager (5200, Tanon, Shanghai, China).
Immunoprecipitation
Immunoprecipitation was performed as previously described [28]. Cells and brain tissues were lysed in the RIPA buffer containing protease and phosphatase inhibitor followed by centrifuge at 20,000 × g at 4 °C for 30 min. Then, the supernatants were cleared with protein A/G at 4 °C for 4 h to remove nonspecific binding and incubated with the primary antibody to form an immune complex at 4 °C overnight. After 12 h of incubation, to capture the immune complex, we added 25 μL of protein A/G magnetic beads to the samples and incubated them at 4 °C for 4 h. Subsequently, the protein A/G magnetic bead was washed with an immunoprecipitation buffer five times, and then the target antigens were eluted with the 1× SDS-PAGE loading buffer at 95 °C for 5 min.
Statistical analyses
Data are presented as the mean ± standard deviation. All statistical tests were two-tailed using GraphPad Prism 8.3 (GraphPad Prism Software, San Diego, CA, USA). Student’s t test was used for comparisons between two groups. For more than two groups, a one-way analysis of variance was performed, followed by Tukey’s post hoc test. P < 0.05 was considered statistically significant. Animals were assigned to each group using the random number program in SPSS software, and the experiments were done in a blinded manner. Experimenters did not know the animal group assignments or treatment conditions.
Results
CBD ameliorates HT-22 neuronal cell death and mitochondrial damage caused by OGD/R insult
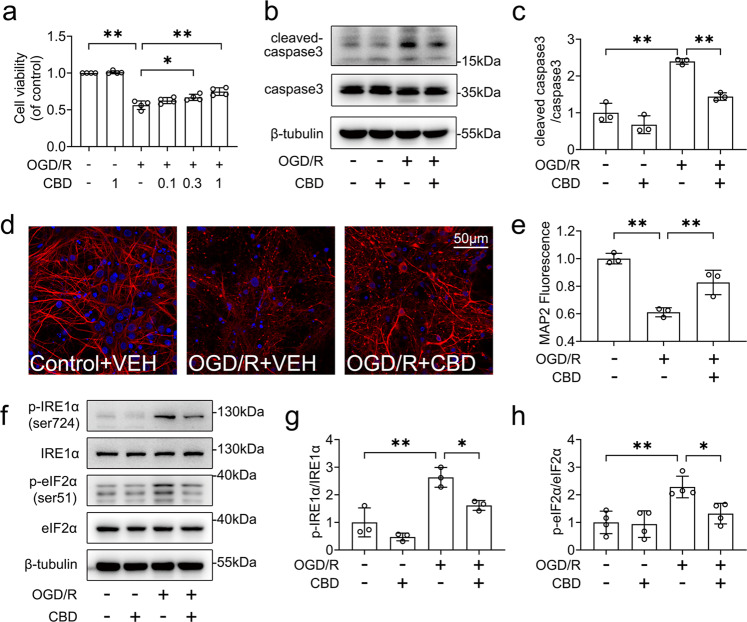
The chemical structure of CBD is shown in Fig. 1a. The OGD/R model was adopted to mimic neuronal injury following cerebral ischemia-reperfusion in vitro. HT-22 cells were exposed to OGD at various times (0–9 h) followed by 24 h of re-oxygenation. OGD/R insult caused HT-22 cell death in a time-dependent manner (Fig. 1b), with loss of ~50% of cell viability at 6 h of OGD insult followed by 24 h of reoxygenation (P < 0.01). Next, we investigated the protective effects of CBD against OGD/R-induced injury in HT-22 neuronal cells. OGD/R insult significantly reduced cell viability (P < 0.01), whereas treatment with CBD (0.1–1 μM) increased the viability of HT-22 cells in a concentration-dependent manner (Fig. 1c). These results were confirmed by immunoblotting, which showed that CBD treatment was effective in decreasing the apoptosis of cells subjected to OGD/R, as evidenced by reduced levels of cleaved caspase 3 (P < 0.05, Fig. S1a, b) and B-cell lymphoma 2-associated X protein (Bax) (P < 0.05, Fig. S1c, d). Calcein-AM/propidium iodide (PI) staining further showed that CBD treatment reduced the ratio of PI-positive cells (P < 0.01, Fig. S1e, f).
Fig. 1. CBD attenuated OGD/R-induced HT-22 cell death and mitochondrial dysfunction.
a The chemical structure of CBD. b HT-22 cells were subjected to OGD insult at various times (0, 3, 6, and 9 h) followed by 24 h of reoxygenation. The cell viability was detected by the CCK-8 assay (n = 4). c HT-22 cells pretreated with CBD (0.1, 0.3, 1 μM) were subjected to 6 h of OGD followed by 24 h of reoxygenation, and then cell viability was detected by the CCK-8 assay (n = 4). d, e HT-22 cells pretreated with CBD (0.1, 0.3, 1 μM) were subjected to 6 h of OGD followed by 1 h of reoxygenation. The variance of intracellular ROS was detected by CellROX staining (n = 3). f, g HT-22 cells pretreated with CBD (0.1, 0.3, 1 μM) were subjected to 6 h of OGD followed by 1 h of reoxygenation. The variance of MMP was detected by TMRE staining (n = 3). h, i HT-22 cells pretreated with CBD (1 μM) were subjected to 6 h of OGD followed by 1 h of reoxygenation. The variance of mitochondrial morphology was visualized with Mito-tracker staining. The mitochondrial area was calculated with ImageJ (n = 3). *P < 0.05, **P < 0.01, versus the indicated group. n represents the number of independent cultures.
Then, we detected the variance of intracellular ROS levels. OGD/R caused the accumulation of ROS, and CBD (0.1–1 μM) decreased the level of ROS in a dose-dependent manner (Fig. 1d, e). In addition, we investigated the role of CBD in the morphology and functions of mitochondria. Here, we found that CBD (0.1–1 μM) reversed the MMP loss after OGD/R in a dose-dependent manner (Fig. 1f, g). Simultaneously, Mito-tracker dyes were then used to visualize the morphological changes of the mitochondria. Compared to the control group, OGD/R induced fragmentation and loss of mitochondria, while treatment of CBD (1 μM) attenuated the fragmentation of mitochondrial and enhanced the mitochondrial area (P < 0.05, Fig. 1h, i). These results showed that CBD protected HT-22 cells against OGD/R insult.
CBD reduces ER stress in HT-22 neuronal cells subjected to OGD/R insult
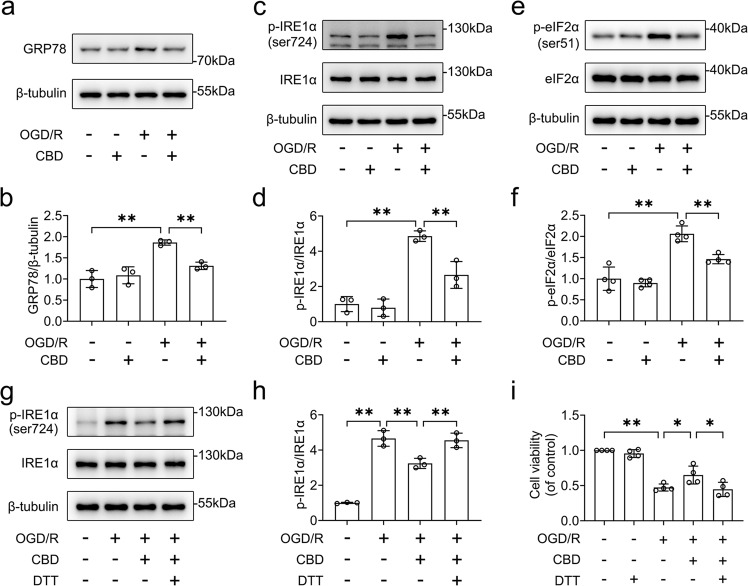
We further determined whether CBD attenuated ER stress caused by OGD/R insult. The protein levels of ER stress sensor proteins, including GRP78, p-IRE1α, and p-eIF2α, were detected by Western blotting. We found that CBD reduced the activation of ER stress in HT-22 cells, as evidenced by the decrease in levels of GRP78 (P < 0.01), p-IRE1α (P < 0.01), and p-eIF2α (P < 0.01, Fig. 2a–f). To further clarify the role of ER stress, DTT, an ER stress activator, was used to induce ER stress. The dose of DTT was based on the fact that 100 μM DTT was not cytotoxic to HT-22 cells. Our results showed that DTT abolished the role of CBD in the phosphorylation of IREα (P < 0.01, Fig. 2g, h). Similarly, DTT blocked the protective effects of CBD against OGD/R-induced cell death in HT-22 cells (P < 0.05, Fig. 2i). Taken together, these results indicate that treatment with CBD reduces ER stress induced by OGD/R insult.
Fig. 2. CBD ameliorated OGD/R-induced ER stress in HT-22 cells.
HT-22 cells pretreated with CBD (1 μM) were subjected to 6 h of OGD followed by 1 h of reoxygenation. a–f Then the variances of GRP78, p-IRE1α, IRE1α, p-eIF2α, and eIF2α were detected by immunoblotting. The changes in GRP78/β-tubulin (n = 3), p-IRE1α/IRE1α (n = 3), and p-eIF2α/eIF2α (n = 4) were determined by densitometry of the blots. g, h HT-22 cells pretreated with DTT (100 μM) were treated with CBD (1 μM) and then subjected to 6 h of OGD followed by 1 h of reoxygenation. The variance of p-IRE1α and IRE1α was detected by immunoblotting, and the change in p-IRE1α/IRE1α was determined by densitometry of the blots (n = 3). i HT-22 cells pretreated with DTT (100 μM) were treated with CBD (1 μM) and then subjected to 6 h of OGD followed by 24 h of reoxygenation. The change in cell viability was determined by the CCK-8 assay (n = 4). *P < 0.05, **P < 0.01, versus the indicated group. n represents the number of independent cultures.
MFN2 is essential for the protective effects of CBD against OGD/R-induced injury
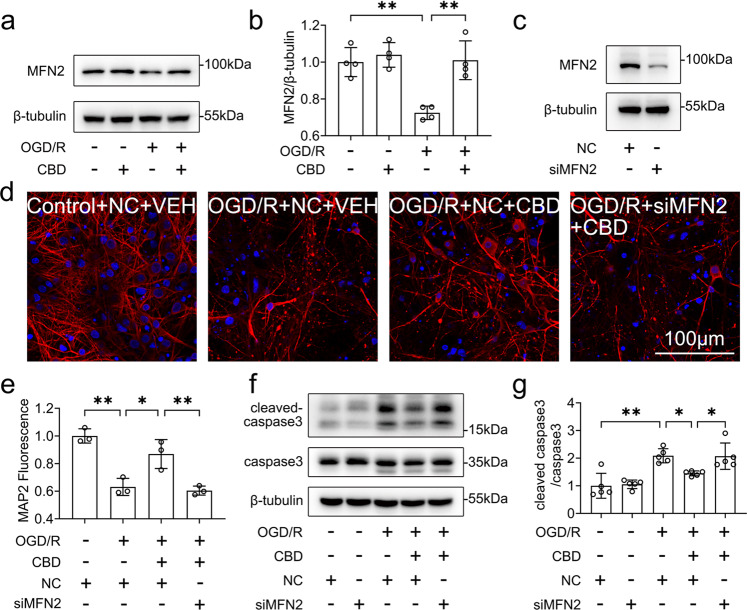
MFN2 is a pivotal regulator of mitochondria–ER contacts and calcium buffering [33]. We investigated whether MFN2 is involved in the protective effects of CBD. First, we found that OGD/R decreased the expression of MFN2 in HT-22 cells, while CBD (0.1–1 μM) attenuated the loss of MFN2 in a dose-dependent manner (Fig. 3a, b). Then, we knocked down the expression of MFN2 with a specific siRNA targeting MFN2 (Fig. S2a–c). Silencing of MFN2 abolished the role of CBD in the loss of the MMP (P < 0.01, Fig. 3c, d), and cell survival (P < 0.01, Fig. 3e). Moreover, knocking down MFN2 inhibited the effects of CBD against cell death (P < 0.01, Fig. S2d, e), the accumulation of intracellular ROS (P < 0.01, Fig. S2f, g), and neuronal apoptosis (P < 0.01, Fig. S2h, i). At the same time, rescue experiments were conducted by overexpressing MFN2 in siMFN2-transfected HT-22 neuronal cells (Fig. S2j). We found that overexpression of MFN2 reversed the reduction of cell viability (P < 0.05, Fig. 3f) and the increase in intracellular ROS (P < 0.01, Fig. 3g, h) caused by OGD/R and MFN2 knockdown. Finally, we found that knocking down MFN2 inhibited the effects of CBD against ER stress, as evidenced by the increase in the level of p-IRE1α (P < 0.01, Fig. 3i, j) and p-eIF2α (P < 0.05, Fig. 3k, l). These data indicate that MFN2 is critical to the protective effects of CBD against OGD/R-induced neuronal injury.
Fig. 3. MFN2 conferred protective effects of CBD against ER and mitochondrial dysfunction.
a, b HT-22 cells pretreated with CBD (0.1, 0.3, 1 μM) were subjected to 6 h of OGD followed by 1 h of reoxygenation. Then, the variance of MFN2 was detected by immunoblotting, and the change in MFN2/β-tubulin was determined by densitometry of the blots (n = 5). HT-22 cells pre-transfected with siMFN2 were treated with CBD (1 μM) and subjected to 6 h of OGD. c, d At 1 h after reoxygenation, the change of MMP was measured with TMRE staining (n = 3). e At 24 h after reoxygenation, cell viability was measured with the CCK-8 assay (n = 3). HT-22 cells pre-transfected with siMFN2 or MFN2 plasmid were treated with CBD (1 μM) and then subjected to 6 h of OGD. f At 24 h after reoxygenation, cell viability was measured with the CCK-8 assay (n = 3). g, h At 1 h after reoxygenation, the variance of intracellular ROS was detected with CellROX staining and quantified with ImageJ software (n = 3). HT-22 cells pre-transfected with siMFN2 were treated with CBD (1 μM) and subjected to 6 h of OGD. i–l At 1 h after reoxygenation, the variances of p-IRE1α, IRE1α, p-eIF2α, and eIF2α were detected by immunoblotting. Then, the changes of p-IRE1α/IRE1α (n = 3) and p-eIF2α/eIF2α (n = 4) were determined by densitometry of the blots. *P < 0.05, **P < 0.01, versus the indicated group. n represents the number of independent cultures.
CBD reduces cell death and ER stress in primary cortical neurons subjected to OGD/R insult
To further verify the protective effects of CBD against OGD/R insult, we evaluated the role of CBD in primary cultured cortical neurons. Consistent with the data obtained in HT-22 neuronal cells, treatment with CBD (0.1–1 μM) improved cell viability in a dose-dependent manner (Fig. 4a). Consistently, treatment with CBD (1 μM) decreased the level of cleaved caspase 3 (P < 0.01, Fig. 4b, c). Additionally, CBD treatment also restored the damage of dendrites caused by OGD/R (P < 0.01, Fig. 4d, e), as evidenced by the enhanced expression of MAP2 in neurons. We also found that CBD exerted similar inhibitory effects on OGD/R-induced ER stress in primary cortical neurons. Treatment with CBD decreased the protein levels of p-IRE1α (P < 0.05, Fig. 4f, g) and p-eIF2α (P < 0.05, Fig. 4f, h). Taken together, our data indicate that CBD protects against OGD/R-induced injury and ameliorates ER stress in primary neurons.
Fig. 4. CBD reduced OGD/R-induced cellular death and ER stress in primary cortical neurons.
a Primary cortical neurons pretreated with CBD (0.1, 0.3, 1 μM) were subjected to 1 h of OGD followed by 24 h of reoxygenation, and then cell viability was detected by the CCK-8 assay (n = 3). Primary cortical neurons pretreated with CBD (1 μM) were subjected to 1 h of OGD. b, c At 1 h after reoxygenation, the levels of caspase 3 and cleaved caspase 3 were detected by immunoblotting, and the change in cleaved caspase 3/caspase 3 was determined by densitometry of the blots (n = 3). d, e At 6 h after reoxygenation, the morphological change of dendrite was visualized with MAP2 staining (n = 3). f–h At 1 h after reoxygenation, the levels of p-IRE1α, IRE1α, p-eIF2α, and eIF2α were detected by immunoblotting. The changes of p-IRE1α/IRE1α (n = 3) and p-eIF2α/eIF2α (n = 4) were determined by densitometry of the blots. *P < 0.05, **P < 0.01, versus the indicated group. n represents the number of independent cultures.
We also verified the key findings in primary cortical neurons. As expected, CBD enhanced the protein level of MFN2 in neurons following OGD/R (P < 0.01, Fig. 5a, b). Silencing of MFN2 attenuated the protective effects of CBD against dendrite damage (P < 0.01, Fig. 5c–e). Additionally, the knockdown of MFN2 diminished the protective role of CBD and promoted neuronal apoptosis (P < 0.05, Fig. 5f, g). Collectively, these results indicate that MFN2 is critical to the protective effects of CBD against OGD/R-induced neuronal cell injury.
Fig. 5. MFN2 mediated the protective effects of CBD against OGD/R-induced injury in primary cultured neurons.
a, b Primary cortical neurons pretreated with CBD (1 μM) were subjected to 1 h of OGD followed by 6 h of reoxygenation. The levels of MFN2 were detected by immunoblotting, and the change in MFN2/β-tubulin was determined by densitometry of the blots (n = 3). c Primary cortical neurons were transfected with siMFN2, and then the knockdown efficacy was verified by immunoblotting. Primary cortical neurons pre-transfected with siMFN2 were treated with CBD (1 μM) and then subjected to 1 h of OGD. d, e At 6 h after reoxygenation, the dendrite morphological variance was detected with MAP2 staining (n = 3). f, g The levels of caspase 3 and cleaved caspase 3 were detected by immunoblotting, and the change in cleaved caspase 3/caspase 3 was determined by densitometry of the blots (n = 5). *P < 0.05, **P < 0.01, versus the indicated group.
CBD reduces the ubiquitination and degradation of MFN2 through disrupting the binding of Parkin to MFN2
Because CBD enhanced the level of MFN2 in neuronal cells, we then evaluated the potential mechanisms. OGD/R led to a decline in MMP in HT-22 cells (Fig. 1f). Parkin is commonly involved in the ubiquitination of proteins in the damaged depolarized mitochondria [34]. Thus, we determined whether CBD affects the binding of Parkin to MFN2. Our results showed that OGD/R strengthened the binding of Parkin to MFN2 (P < 0.01), while CBD weakened the interaction between them (P < 0.01, Fig. 6a–c). Moreover, we also found that OGD/R triggered the ubiquitination of MFN2 (P < 0.01), and the treatment of CBD abolished the ubiquitination of MFN2 (P < 0.05, Fig. 6d, e). The above results indicate that CBD decreases the loss of MFN2 by reducing the degradation of MFN2 through the ubiquitin-protease system (UPS). Generally, the intracellular protein level is controlled by its synthesis and degradation. We further detected the variance of MFN2 mRNA. Interestingly, OGD/R decreased the level of MFN2 mRNA (P < 0.01), while CBD did not affect the MFN2 mRNA level (P > 0.05, Fig. 6f). To further determine the involvement of UPS in MFN2 degradation, MG132, a proteasome inhibitor, was used. Both CBD (P < 0.01) and MG132 (P < 0.05) reduced the loss of MFN2 caused by OGD/R (Fig. 6g, h). Both CBD and MG132 conferred anti-apoptotic effects against OGD/R-induced injury (P < 0.05, Fig. 6g, i). To further confirm the involvement of Parkin, we transfected a plasmid encoding Parkin into HT-22 cells (Fig. S2k). Overexpression of Parkin abolished the effects of CBD on maintaining the expression of MFN2 (P < 0.05, Fig. 6j, k), the ubiquitination of MFN2 (P < 0.05, Fig. 6l, m), and cell survival (P < 0.05, Fig. 6n). Finally, we found that the overexpression of MFN2 abolished the effects of Parkin and increased cell viability (P < 0.05, Fig. 6o). These results indicate that CBD attenuates Parkin-mediated ubiquitination and degradation of MFN2 under the condition of OGD/R.
Fig. 6. CBD ameliorated Parkin-mediated ubiquitination of MFN2 induced by OGD/R.
HT-22 cells pretreated with CBD (1 μM) were subjected to 6 h of OGD followed by 1 h of reoxygenation. a, b The change in Parkin binding to MFN2 was detected by co-immunoprecipitation, and the variance of Parkin/MFN2 was determined by densitometry of the blots (n = 3). c The input level of Parkin and MFN2 was detected by immunoblotting. d, e The ubiquitination of MFN2 was detected by immunoprecipitation, and the variance of ubiquitin/MFN2 was determined by densitometry of the blots (n = 3). f The change in MFN2 mRNA level was detected with qPCR (n = 5). HT-22 cells pretreated with CBD (1 μM) or MG132 (20 μM) were subjected to 6 h of OGD followed by 1 h of reoxygenation. g, h, i The changes in MFN2 and cleaved caspase 3 were detected by immunoblotting, and the variance of MFN2/β-tubulin (n = 4) and cleaved caspase 3/caspase 3 (n = 3) were determined by densitometry of the blots. HT-22 cells pre-transfected with Parkin plasmid were treated with CBD (1 μM) and then subjected to 6 h of OGD followed by 1 h of reoxygenation. j, k The variance of MFN2 was detected by immunoblotting, and the variance of MFN2/β-tubulin was determined by densitometry of the blots (n = 4). l, m The ubiquitination of MFN2 was detected by immunoprecipitation, and the variance of Ubiquitin/MFN2 was determined by densitometry of the blots (n = 3). n HT-22 cells pre-transfected with Parkin plasmid were treated with CBD (1 μM) and then subjected to 6 h of OGD followed by 24 h of reoxygenation. Cell viability was measured with the CCK-8 assay (n = 3). o HT-22 cells pre-transfected with Parkin plasmid or MFN2 plasmid were treated with CBD (1 μM) and then subjected to 6 h of OGD followed by 24 h of reoxygenation. Cell viability was measured with the CCK-8 assay (n = 3). *P < 0.05, **P < 0.01, versus the indicated group. n represents the number of independent cultures.
MFN2 confers neuroprotection of CBD in rats following MCAO/R insult
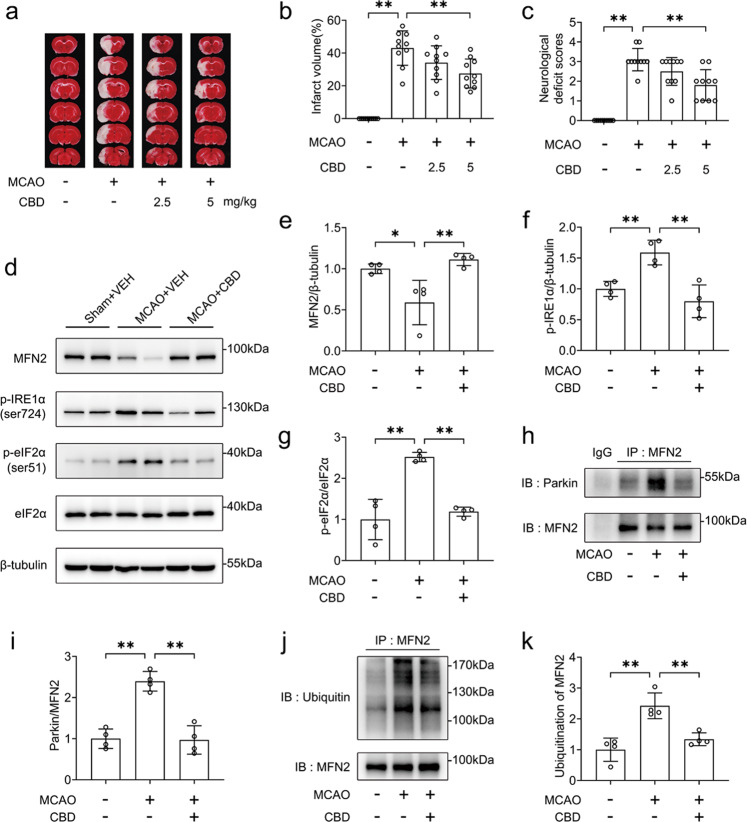
We next evaluated the effects of CBD on the expression of MFN2 in rats subjected to MCAO/R. Sex hormones may impact the recovery and outcomes of stroke treatment [35], so male animals are usually used for the study of cerebral ischemia [36]. To avoid the influence of sex differences on the neuroprotection of CBD, male rats were used in the current studies. CBD reduced infarct volume (Fig. 7a, b) and neurological deficit scores (Fig. 7c). Moreover, CBD reversed the loss of MFN2 in the penumbra of the cortex (P < 0.01, Fig. 7d, e). CBD also suppressed ER stress in vivo, as the levels of p-IRE1α and p-eIF2α were decreased after treatment with CBD (P < 0.01, Fig. 7d, f, g). Then, we found that treatment with CBD reduced the binding of Parkin to MFN2 (P < 0.01, Fig. 7h, i). Thus, the ubiquitination of MFN2 was decreased (P < 0.01, Fig. 7j, k). These results indicate that CBD can suppress ER stress in rats subjected to MCAO/R.
Fig. 7. CBD reduced ER and ubiquitination of MFN2 in the cortex penumbra of SD rats subjected to MCAO.
SD rats were subjected to 2 h of MCAO. At the time of reperfusion, rats were administered CBD (2.5 mg/kg or 5 mg/kg) or the vehicle. a, b At 24 h after reperfusion, the infarct size was detected by TTC staining (n = 10 each group). c Before TTC staining, the neurological deficit scores were recorded (n = 10, each group). SD rats were subjected to MCAO for 2 h. At the time of reperfusion, CBD (5 mg/kg) or the vehicle was administered. At 24 h after reperfusion, the cortex penumbra was collected. d–g The levels of MFN2, p-IRE1α, p-eIF2α, and eIF2α were detected by immunoblotting, and the variances of MFN2/β-tubulin, p-IRE1α/β-tubulin, and p-eIF2α/eIF2α were determined by densitometry of the blots (n = 4). h, i The change in Parkin binding to MFN2 was detected by co-immunoprecipitation, and the variance of Parkin/MFN2 was determined by densitometry of the blots (n = 4). j, k The ubiquitination of MFN2 was detected by immunoprecipitation, and the variance of ubiquitin/MFN2 was determined by densitometry of the blots (n = 4). *P < 0.05, **P < 0.01, versus the indicated group.
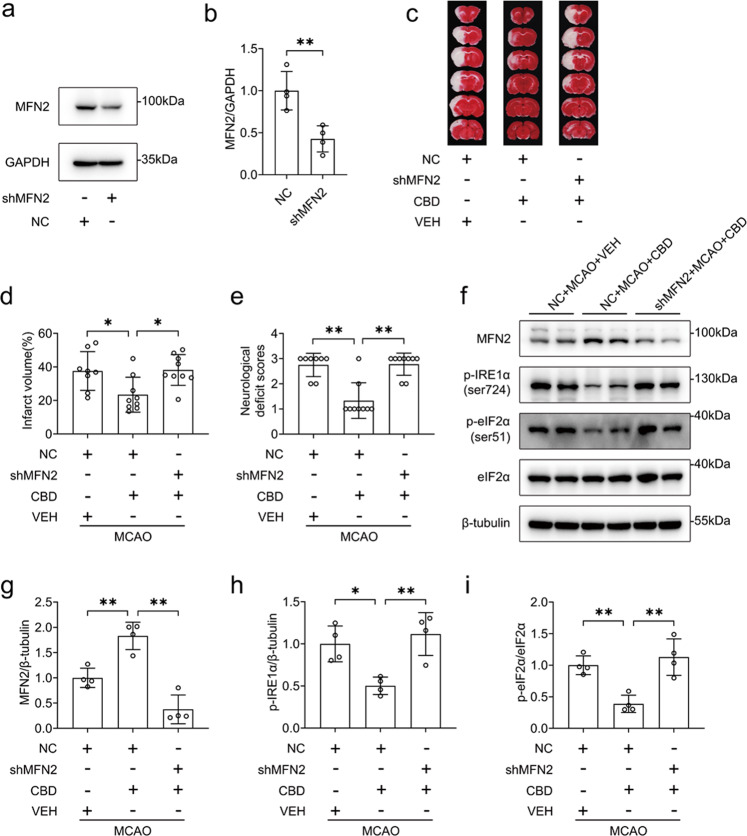
To verify the essential role of MFN2 in vivo, we performed the AAV delivery of shRNA against MFN2 or control intracerebroventricularly. The infected brain regions are shown in Fig. S3. The knockdown efficiency was confirmed by Western blotting. We found that the expression of MFN2 in the striatum (P < 0.01, Fig. 8a, b), cortex (P < 0.05, Fig. S4a, b), and hippocampus (P < 0.05, Fig. S4c, d) was efficiently knocked down by AAV-sh-MFN2. We also found that both neurons and astrocytes were infected by AAV-sh-MFN2 (Fig. S5). The knockdown efficiency of AAV-sh-MFN2 in neurons was further determined by immunofluorescence staining (Fig. S6). Similarly, infection of AAV-sh-MFN2 resulted in the reduction of MFN2 expression in the ischemia territory (Fig. S7). Silencing of MFN2 abolished the effect of CBD on attenuating infarct volume (P < 0.05, Fig. 8c, d) and neurological deficit scores (P < 0.01, Fig. 8e). Consistent with the data from the in vitro studies, silencing of MFN2 also blocked the effects of CBD on reducing ER stress, as evidenced by the decrease in the expression levels of p-IRE1α (P < 0.01, Fig. 8f–h), and p-eIF2α (P < 0.01, Fig. 8f, i). Taken together, our results indicate that the MFN2 is critical to the protective effects of CBD against brain injury caused by MCAO/R.
Fig. 8. MFN2 conferred protective effects of CBD against MCAO-induced SD rat injury.
a, b SD rats were transfected with AAV2/9 loading shMFN2 or NC intracerebroventricularly at least for 2 weeks. Then, knockdown efficacy was verified by immunoblotting, and the level of MFN2/GAPDH was determined by densitometry of the blots (n = 4). SD rats were subjected to MCAO for 2 h. At the time of reperfusion, CBD (5 mg/kg) or the vehicle was administered. c–e At 24 h after reperfusion, the infarct size was measured with TTC staining (n = 8, 9, 9 for each group), and the neurological deficit scores were recorded (n = 8, 9, 9 for each group). At 24 h after reperfusion, the cortex penumbra was collected. f–i The variance of MFN2, p-IRE1α, p-eIF2α, and eIF2α was detected by immunoblotting, and the levels of MFN2/β-tubulin, p-IRE1α/β-tubulin and p-eIF2α/eIF2α were determined by densitometry of the blots (n = 4). *P < 0.05, **P < 0.01, versus the indicated group.
Discussion
The results of this study indicate that MFN2 plays a vital role in mediating the protective effect of CBD against ischemia-induced neuron death. To demonstrate this effect, we applied an in vitro OGD/R model and in vivo MCAO/R model to clarify the essential involvement of MFN2. We proposed that CBD can reduce ER stress and mitochondrial dysfunction caused by OGD/R insult. We further demonstrated that CBD ameliorated the loss of MFN2, and the silencing of MFN2 abolished the protective effects of CBD against ER and mitochondrial dysfunction, while overexpressing MFN2 showed a rescue effect. Moreover, OGD/R induced the binding of Parkin to MFN2 and further induced the ubiquitination of MFN2. Treatment of CBD reduced the binding between Parkin and MFN2 and subsequent ubiquitination of MFN2. By contrast, the overexpression of Parkin blocked the effect of CBD in reducing MFN2 ubiquitination and improving cell viability. More importantly, the detrimental effect of overexpressing Parkin was rescued by the overexpression of MFN2. Finally, our in vivo results confirm the important role of MFN2 in the protective effects of CBD on ameliorating ER and mitochondrial dysfunction, and cerebral infarction in rats subjected to MCAO/R.
Cerebral ischemia causes dysfunctional mitochondria that further promote neuronal damage from cerebral ischemia and reperfusion [37]. This study verified that CBD effectively attenuated the loss of MMP and ameliorated mitochondrial morphology. These data are consistent with a previous study showing that CBD was capable of enhancing mitochondrial bioenergetics and regulating glucose metabolism to attenuate HT-22 cell death caused by OGD/R [20]. These findings emphasize the critical role of mitochondria in the normal functions of neurons. However, mitochondria dynamically interact with ER to determine cellular fate under the condition of neurological disorders [38]. In other words, both mitochondrial dysfunction and ER stress contribute to the pathology of cerebral ischemia. Hence, restoring the homeostasis of protein(s) linking the mitochondria and ER may be an ideal therapeutic strategy for rescuing cerebral ischemia. Our results showed that treatment with CBD reduced the expression of ER stress markers (GRP78, p-IRE1α, and p-eIF2α) in neurons following ischemia. It was encouraging to find that treatment with CBD reduced ER stress induced by hypoxia or ischemia. We then explored the specific molecule responsible for the regulation of ER stress by CBD. We found that CBD appreciably enhanced the level of MFN2 in both in vitro and in vivo cerebral ischemic models. Importantly, the knockdown of MFN2 blocked the protective effects of CBD against mitochondrial dysfunction and ER stress. These results indicate that CBD exerts neuroprotective effects in an MFN2-dependent way. Because MFN2 tethers ER to mitochondria [39], it is rational to speculate that restoring the expression of MFN2 during cerebral ischemia helps maintain ER and mitochondrial functions. Hence, ameliorating cerebral ischemic injury by intervening with MFN2 is a potential strategy. MFN2 is viewed as a promising target for the treatment of ischemia in other organs. For example, MFN2 attenuates injury caused by myocardial ischemia and renal ischemia [40, 41]. We would like to point out that the relationship between MFN2 and these markers under the condition of ischemic stroke deserves further study. For example, our results showed that MFN2 siRNA blocked the role of CBD on the phosphorylation of IRE1α at Ser724. However, whether the mutation of IRE1α (Ser724 site) affects CBD disrupting the interaction between Parkin and MFN2 needs to be studied further. Collectively, more studies on the interplay between CBD treatment and the number of ER–mitochondrial contact sites will help uncover the underlying mechanism of CBD regulating ER stress and mitochondrial dysfunction.
Additionally, it is necessary to understand how CBD affects the expression of MFN2. Our results indicate that MFN2 is an effector protein of CBD under the condition of stroke. However, we found that CBD did not affect the MFN2 mRNA level. Hence, we presume that the loss of MFN2 during ischemia is probably mediated by protein degradation. It has been proposed that Parkin, an E3 ubiquitin ligase, mediates the ubiquitination of MFN2 upon mitochondrial depolarization [42]. In our study, we found that hypoxia or ischemia-induced substantial binding of Parkin to MFN2. Moreover, CBD reversed the loss of MFN2 by attenuating the binding of Parkin to MFN2. Therefore, the effect of CBD is mediated in part by inhibiting the ubiquitination of MFN2. Besides the UPS, protein degradation may also occur through the autophagy–lysosome pathway [43, 44]. Interestingly, MFN2 and Parkin also regulate autophagy and mitophagy [45, 46]. Autophagy contributes to the progression of cerebral ischemia as well [47]. Whether CBD decreases the degradation of MFN2 through the autophagy–lysosome system deserves further consideration. In particular, PINK1/Parkin signaling is one of the most investigated pathways for mitophagy in mammalian cells. PINK1 can enhance the activity of Parkin and recruit Parkin to mitochondria [48]. Then, it triggers mitophagy [48]. Activation of PINK1/Parkin-mediated mitophagy should produce neuroprotective effects during cerebral ischemia [49]. We verified that CBD treatment restored the MMP and disrupted the binding of Parkin to MFN2. However, the effects of CBD on the expression of PINK1 and the phosphorylation of Parkin are largely unknown. Whether CBD decreases the degradation of MFN2 through the autophagy–lysosome system deserves further consideration. Nevertheless, our results support the role of MFN2 in the protective effect of CBD against cerebral ischemia. Importantly, they reveal that CBD can reduce ER and mitochondrial dysfunction via modulation of MFN2. However, further research is needed to determine whether CBD modulates MFN2 directly or indirectly. In addition, it has been shown that MFN2 attenuates excitotoxicity and mitochondrial dysfunction in neurons induced by glutamate [50], which plays an important role in neuronal death during cerebral ischemia [51]. Glutamate also triggers ER and mitochondrial accumulation of Parkin [52]. Thus, whether CBD can regulate the level of glutamate through MFN2 deserves further consideration.
In addition to MFN2, many protein complexes have been proposed to tether ER with mitochondria. Specifically, inositol 1,4,5-trisphosphate receptors, voltage-dependent anion channels, and glucose-regulated protein 75 are also engaged in the ER–mitochondria contacts [53]. It is commonly believed that dysfunction of ER–mitochondria contacts is a major lethal event during cerebral ischemia [54]. However, the effects of CBD on the expression, function, and interaction of mitochondrial proteins at the ER–mitochondrial interface have not been fully elucidated. In addition, MFN2 is expressed in both neurons and microglial cells. Our present study focused on the role of CBD in the degradation of MFN2 in neurons. Our previous study verified that CBD suppressed neuroinflammation by enhancing the expression of MFN2 in microglial cells treated with lipopolysaccharide [25]. Neuroinflammation is an important pathological event that occurs during the development of ischemic stroke [55], and impaired mitochondria can also trigger pro-inflammatory signals [56]. Whether the inhibitory effect of MFN2 on microglial activation contributes to the protective effect of CBD in the ischemic stroke model needs to be further studied. Collectively, this study uncovered a novel and essential role of MFN2 in mediating the neuroprotective effects of CBD. Importantly, MFN2 is a pivotal protein that acts as a promising therapeutic target for cerebral ischemia.
In conclusion, CBD exerts neuroprotective effects against cerebral ischemia, the mechanism of which needs to be clearly understood. For the first time, we showed that CBD reduced ischemia-induced neuronal apoptosis by enhancing the expression of MFN2. Furthermore, the effects of CBD on the expression of MFN2 are mediated by preventing MFN2 degradation via blocking the binding of Parkin to MFN2 and the subsequent ubiquitination. These findings indicate that CBD is a promising candidate for the treatment of ischemic stroke. In addition, the activation of MFN2 or the enhancement of MFN2 expression presents a promising therapeutic option for attenuating neuronal cell death after cerebral ischemia.
Supplementary information
Acknowledgements
This work was supported by National Natural Science Foundation of China (Nos. 82173802 and 82273902), Science and Technology Program of Guangzhou (No. 202002030494), Guangdong Basic and Applied Basic Research Foundation (No. 2022A1515012317).
Author contributions
BTX performed CCK-8 assay, immunoblotting, immunocytochemistry, co-IP, PCR, transfection, and partial animal experiments. MFL performed immunoblotting, PCR, and helped with animal experiments. KCC performed immunocytochemistry, PI staining and helped with animal experiments. XL performed immunoblotting and helped with cell culture. NBC performed animal experiments. BTX, MFL, XL, and KCC analyzed the data. HTW and JPX designed the experiments and supervised the project. BTX and HTW wrote the manuscript. All authors commented to the manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
These authors contributed equally: Bing-tian Xu, Meng-fan Li, Ke-chun Chen
Contributor Information
Jiang-ping Xu, Email: jpx@smu.edu.cn.
Hai-tao Wang, Email: wht821@smu.edu.cn.
Supplementary information
The online version contains supplementary material available at 10.1038/s41401-022-01004-3.
References
- 1.Hankey GJ. Stroke. Lancet. 2017;389:641–54. doi: 10.1016/S0140-6736(16)30962-X. [DOI] [PubMed] [Google Scholar]
- 2.Amar AP, Sagare AP, Zhao Z, Wang Y, Nelson AR, Griffin JH, et al. Can adjunctive therapies augment the efficacy of endovascular thrombolysis? A potential role for activated protein C. Neuropharmacology. 2018;134:293–301. doi: 10.1016/j.neuropharm.2017.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Campbell BCV, De Silva DA, Macleod MR, Coutts SB, Schwamm LH, Davis SM, et al. Ischaemic stroke. Nat Rev Dis Prim. 2019;5:70. doi: 10.1038/s41572-019-0118-8. [DOI] [PubMed] [Google Scholar]
- 4.Larrea D, Pera M, Gonnelli A, Quintana-Cabrera R, Akman HO, Guardia-Laguarta C, et al. MFN2 mutations in Charcot-Marie-Tooth disease alter mitochondria-associated ER membrane function but do not impair bioenergetics. Hum Mol Genet. 2019;28:1782–800. doi: 10.1093/hmg/ddz008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Martorell-Riera A, Segarra-Mondejar M, Munoz JP, Ginet V, Olloquequi J, Perez-Clausell J, et al. Mfn2 downregulation in excitotoxicity causes mitochondrial dysfunction and delayed neuronal death. EMBO J. 2014;33:2388–407. doi: 10.15252/embj.201488327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Munoz JP, Ivanova S, Sanchez-Wandelmer J, Martinez-Cristobal P, Noguera E, Sancho A, et al. Mfn2 modulates the UPR and mitochondrial function via repression of PERK. EMBO J. 2013;32:2348–61. doi: 10.1038/emboj.2013.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xin Y, Wu W, Qu J, Wang X, Lei S, Yuan L, et al. Inhibition of Mitofusin-2 promotes cardiac fibroblast activation via the PERK/ATF4 pathway and reactive oxygen species. Oxid Med Cell Longev. 2019;2019:3649808. doi: 10.1155/2019/3649808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rocha AG, Franco A, Krezel AM, Rumsey JM, Alberti JM, Knight WC, et al. MFN2 agonists reverse mitochondrial defects in preclinical models of Charcot-Marie-Tooth disease type 2A. Science. 2018;360:336–41. doi: 10.1126/science.aao1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jiang S, Nandy P, Wang W, Ma X, Hsia J, Wang C, et al. Mfn2 ablation causes an oxidative stress response and eventual neuronal death in the hippocampus and cortex. Mol Neurodegener. 2018;13:5. doi: 10.1186/s13024-018-0238-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Peng C, Rao W, Zhang L, Wang K, Hui H, Wang L, et al. Mitofusin 2 ameliorates hypoxia-induced apoptosis via mitochondrial function and signaling pathways. Int J Biochem Cell Biol. 2015;69:29–40. doi: 10.1016/j.biocel.2015.09.011. [DOI] [PubMed] [Google Scholar]
- 11.Peng C, Rao W, Zhang L, Gao F, Hui H, Wang K, et al. Mitofusin 2 exerts a protective role in ischemia reperfusion injury through increasing autophagy. Cell Physiol Biochem. 2018;46:2311–24. doi: 10.1159/000489621. [DOI] [PubMed] [Google Scholar]
- 12.Romer Thomsen K, Thylstrup B, Kenyon EA, Lees R, Baandrup L, Feldstein Ewing SW, et al. Cannabinoids for the treatment of cannabis use disorder: new avenues for reaching and helping youth? Neurosci Biobehav Rev. 2022;132:169–80. doi: 10.1016/j.neubiorev.2021.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liktor-Busa E, Keresztes A, LaVigne J, Streicher JM, Largent-Milnes TM. Largent-Milnes TM. Analgesic potential of terpenes derived from Cannabis sativa. Pharmacol Rev. 2021;73:98–126. doi: 10.1124/pharmrev.120.000046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Landucci E, Mazzantini C, Lana D, Davolio PL, Giovannini MG, Pellegrini-Giampietro DE. Neuroprotective effects of cannabidiol but not delta(9)-tetrahydrocannabinol in rat hippocampal slices exposed to oxygen-glucose deprivation: studies with cannabis extracts and selected cannabinoids. Int J Mol Sci. 2021;22:9773. doi: 10.3390/ijms22189773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Osborne AL, Solowij N, Weston-Green K. A systematic review of the effect of cannabidiol on cognitive function: relevance to schizophrenia. Neurosci Biobehav Rev. 2017;72:310–24. doi: 10.1016/j.neubiorev.2016.11.012. [DOI] [PubMed] [Google Scholar]
- 16.Dash R, Ali MC, Jahan I, Munni YA, Mitra S, Hannan MA, et al. Emerging potential of cannabidiol in reversing proteinopathies. Ageing Res Rev. 2021;65:101209. doi: 10.1016/j.arr.2020.101209. [DOI] [PubMed] [Google Scholar]
- 17.Khodadadi H, Salles EL, Jarrahi A, Costigliola V, Khan MB, Yu JC, et al. Cannabidiol ameliorates cognitive function via regulation of IL-33 and TREM2 upregulation in a murine model of Alzheimer’s disease. J Alzheimers Dis. 2021;80:973–7. doi: 10.3233/JAD-210026. [DOI] [PubMed] [Google Scholar]
- 18.de Almeida CMO, Brito MMC, Bosaipo NB, Pimentel AV, Tumas V, Zuardi AW, et al. Cannabidiol for rapid eye movement sleep behavior disorder. Mov Disord. 2021;36:1711–5. doi: 10.1002/mds.28577. [DOI] [PubMed] [Google Scholar]
- 19.Khaksar S, Bigdeli M, Samiee A, Shirazi-Zand Z. Antioxidant and anti-apoptotic effects of cannabidiol in model of ischemic stroke in rats. Brain Res Bull. 2022;180:118–30. doi: 10.1016/j.brainresbull.2022.01.001. [DOI] [PubMed] [Google Scholar]
- 20.Sun S, Hu F, Wu J, Zhang S. Cannabidiol attenuates OGD/R-induced damage by enhancing mitochondrial bioenergetics and modulating glucose metabolism via pentose-phosphate pathway in hippocampal neurons. Redox Biol. 2017;11:577–85. doi: 10.1016/j.redox.2016.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Meyer E, Bonato JM, Mori MA, Mattos BA, Guimaraes FS, Milani H, et al. Cannabidiol confers neuroprotection in rats in a model of transient global cerebral ischemia: impact of hippocampal synaptic neuroplasticity. Mol Neurobiol. 2021;58:5338–55. doi: 10.1007/s12035-021-02479-7. [DOI] [PubMed] [Google Scholar]
- 22.Ceprian M, Jimenez-Sanchez L, Vargas C, Barata L, Hind W, Martinez-Orgado J. Cannabidiol reduces brain damage and improves functional recovery in a neonatal rat model of arterial ischemic stroke. Neuropharmacology. 2017;116:151–9. doi: 10.1016/j.neuropharm.2016.12.017. [DOI] [PubMed] [Google Scholar]
- 23.Thomas A, Baillie GL, Phillips AM, Razdan RK, Ross RA, Pertwee RG. Cannabidiol displays unexpectedly high potency as an antagonist of CB1 and CB2 receptor agonists in vitro. Br J Pharm. 2007;150:613–23. doi: 10.1038/sj.bjp.0707133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morales P, Jagerovic N. Synthetic and natural derivatives of cannabidiol. Adv Exp Med Biol. 2021;1297:11–25. doi: 10.1007/978-3-030-61663-2_2. [DOI] [PubMed] [Google Scholar]
- 25.Li M, Xu B, Li X, Li Y, Qiu S, Chen K, et al. Mitofusin 2 confers the suppression of microglial activation by cannabidiol: insights from in vitro and in vivo models. Brain Behav Immun. 2022;104:155–70. doi: 10.1016/j.bbi.2022.06.003. [DOI] [PubMed] [Google Scholar]
- 26.Xu B, Xu J, Cai N, Li M, Liu L, Qin Y, et al. Roflumilast prevents ischemic stroke-induced neuronal damage by restricting GSK3beta-mediated oxidative stress and IRE1alpha/TRAF2/JNK pathway. Free Radic Biol Med. 2021;163:281–96. doi: 10.1016/j.freeradbiomed.2020.12.018. [DOI] [PubMed] [Google Scholar]
- 27.Zhong J, Dong W, Qin Y, Xie J, Xiao J, Xu J, et al. Roflupram exerts neuroprotection via activation of CREB/PGC-1alpha signalling in experimental models of Parkinson’s disease. Br J Pharmacol. 2020;177:2333–50. doi: 10.1111/bph.14983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhong J, Li M, Xu J, Dong W, Qin Y, Qiu S, et al. Roflupram attenuates alpha-synuclein-induced cytotoxicity and promotes the mitochondrial translocation of Parkin in SH-SY5Y cells overexpressing A53T mutant alpha-synuclein. Toxicol Appl Pharmacol. 2022;436:115859. doi: 10.1016/j.taap.2021.115859. [DOI] [PubMed] [Google Scholar]
- 29.El-Hakim Y, Mani KK, Eldouh A, Pandey S, Grimaldo MT, Dabney A, et al. Sex differences in stroke outcome correspond to rapid and severe changes in gut permeability in adult Sprague-Dawley rats. Biol Sex Differ. 2021;12:14. doi: 10.1186/s13293-020-00352-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xu B, Wang T, Xiao J, Dong W, Wen HZ, Wang X, et al. FCPR03, a novel phosphodiesterase 4 inhibitor, alleviates cerebral ischemia/reperfusion injury through activation of the AKT/GSK3beta/ beta-catenin signaling pathway. Biochem Pharmacol. 2019;163:234–49. doi: 10.1016/j.bcp.2019.02.023. [DOI] [PubMed] [Google Scholar]
- 31.Hind WH, England TJ, O’Sullivan SE. Cannabidiol protects an in vitro model of the blood-brain barrier from oxygen-glucose deprivation via PPARgamma and 5-HT1A receptors. Br J Pharmacol. 2016;173:815–25. doi: 10.1111/bph.13368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen J, Yu H, Zhong J, Feng H, Wang H, Cheng Y, et al. The phosphodiesterase-4 inhibitor, FCPR16, attenuates ischemia-reperfusion injury in rats subjected to middle cerebral artery occlusion and reperfusion. Brain Res Bull. 2018;137:98–106. doi: 10.1016/j.brainresbull.2017.11.010. [DOI] [PubMed] [Google Scholar]
- 33.Gbel J, Engelhardt E, Pelzer P, Sakthivelu V, Jahn HM, Jevtic M, et al. Mitochondria-endoplasmic reticulum contacts in reactive astrocytes promote vascular remodeling. Cell Metab. 2020;31:791–808. doi: 10.1016/j.cmet.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang XL, Feng ST, Wang ZZ, Yuan YH, Chen NH, Zhang Y. Parkin, an E3 ubiquitin ligase, plays an essential role in mitochondrial quality control in Parkinson’s disease. Cell Mol Neurobiol. 2021;41:1395–411. doi: 10.1007/s10571-020-00914-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Di Domenico F, Casalena G, Jia J, Sultana R, Barone E, Cai J, et al. Sex differences in brain proteomes of neuron-specific STAT3-null mice after cerebral ischemia/reperfusion. J Neurochem. 2012;121:680–92. doi: 10.1111/j.1471-4159.2012.07721.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang H, Du YS, Xu WS, Li CJ, Sun H, Hu KR, et al. Exogenous glutathione exerts a therapeutic effect in ischemic stroke rats by interacting with intrastriatal dopamine. Acta Pharmacol Sin. 2022;43:541–51. doi: 10.1038/s41401-021-00650-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.He Z, Ning N, Zhou Q, Khoshnam SE, Farzaneh M. Mitochondria as a therapeutic target for ischemic stroke. Free Radic Biol Med. 2020;146:45–58. doi: 10.1016/j.freeradbiomed.2019.11.005. [DOI] [PubMed] [Google Scholar]
- 38.Veeresh P, Kaur H, Sarmah D, Mounica L, Verma G, Kotian V, et al. Endoplasmic reticulum-mitochondria crosstalk: from junction to function across neurological disorders. Ann N Y Acad Sci. 2019;1457:41–60. doi: 10.1111/nyas.14212. [DOI] [PubMed] [Google Scholar]
- 39.de Brito OM, Scorrano L. Mitofusin 2 tethers endoplasmic reticulum to mitochondria. Nature. 2008;456:605–10. doi: 10.1038/nature07534. [DOI] [PubMed] [Google Scholar]
- 40.Gall JM, Wang Z, Bonegio RG, Havasi A, Liesa M, Vemula P, et al. Conditional knockout of proximal tubule mitofusin 2 accelerates recovery and improves survival after renal ischemia. J Am Soc Nephrol. 2015;26:1092–102. doi: 10.1681/ASN.2014010126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen QM. Nrf2 for protection against oxidant generation and mitochondrial damage in cardiac injury. Free Radic Biol Med. 2022;179:133–43. doi: 10.1016/j.freeradbiomed.2021.12.001. [DOI] [PubMed] [Google Scholar]
- 42.McLelland GL, Goiran T, Yi W, Dorval G, Chen CX, Lauinger ND, et al. Mfn2 ubiquitination by PINK1/parkin gates the p97-dependent release of ER from mitochondria to drive mitophagy. Elife. 2018;7:e32866. doi: 10.7554/eLife.32866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sun-Wang JL, Ivanova S, Zorzano A. The dialogue between the ubiquitin-proteasome system and autophagy: implications in ageing. Ageing Res Rev. 2020;64:101203. doi: 10.1016/j.arr.2020.101203. [DOI] [PubMed] [Google Scholar]
- 44.Wu M, Lu G, Lao YZ, Zhang H, Zheng D, Zheng ZQ, et al. Garciesculenxanthone B induces PINK1-Parkin-mediated mitophagy and prevents ischemia-reperfusion brain injury in mice. Acta Pharmacol Sin. 2021;42:199–208. doi: 10.1038/s41401-020-0480-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Puri R, Cheng XT, Lin MY, Huang N, Sheng ZH. Mul1 restrains Parkin-mediated mitophagy in mature neurons by maintaining ER-mitochondrial contacts. Nat Commun. 2019;10:3645. doi: 10.1038/s41467-019-11636-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gong Y, Tang N, Liu P, Sun Y, Lu S, Liu W. Newcastle disease virus degrades SIRT3 via PINK1-PRKN-dependent mitophagy to reprogram energy metabolism in infected cells. Autophagy. 2022;18:1503–21. doi: 10.1080/15548627.2021.1990515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wen H, Li L, Zhan L, Zuo Y, Li K, Qiu M, et al. Hypoxic postconditioning promotes mitophagy against transient global cerebral ischemia via PINK1/Parkin-induced mitochondrial ubiquitination in adult rats. Cell Death Dis. 2021;12:630. doi: 10.1038/s41419-021-03900-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li J, Lai M, Zhang X, Li Z, Yang D, Zhao M, et al. PINK1-parkin-mediated neuronal mitophagy deficiency in prion disease. Cell Death Dis. 2022;13:162. doi: 10.1038/s41419-022-04613-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mao Z, Tian L, Liu J, Wu Q, Wang N, Wang G, et al. Ligustilide ameliorates hippocampal neuronal injury after cerebral ischemia reperfusion through activating PINK1/Parkin-dependent mitophagy. Phytomedicine. 2022;101:154111. doi: 10.1016/j.phymed.2022.154111. [DOI] [PubMed] [Google Scholar]
- 50.Wang W, Zhang F, Li L, Tang F, Siedlak SL, Fujioka H, et al. MFN2 couples glutamate excitotoxicity and mitochondrial dysfunction in motor neurons. J Biol Chem. 2015;290:168–82. doi: 10.1074/jbc.M114.617167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhou L, Li F, Xu HB, Luo CX, Wu HY, Zhu MM, et al. Treatment of cerebral ischemia by disrupting ischemia-induced interaction of nNOS with PSD-95. Nat Med. 2010;16:1439–43. doi: 10.1038/nm.2245. [DOI] [PubMed] [Google Scholar]
- 52.Van Laar VS, Roy N, Liu A, Rajprohat S, Arnold B, Dukes AA, et al. Glutamate excitotoxicity in neurons triggers mitochondrial and endoplasmic reticulum accumulation of Parkin, and, in the presence of N-acetyl cysteine, mitophagy. Neurobiol Dis. 2015;74:180–93. doi: 10.1016/j.nbd.2014.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yuan M, Gong M, He J, Xie B, Zhang Z, Meng L, et al. IP3R1/GRP75/VDAC1 complex mediates endoplasmic reticulum stress-mitochondrial oxidative stress in diabetic atrial remodeling. Redox Biol. 2022;52:102289. doi: 10.1016/j.redox.2022.102289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ouyang YB, Giffard RG. ER-mitochondria crosstalk during cerebral ischemia: molecular chaperones and ER-mitochondrial calcium transfer. Int J Cell Biol. 2012;2012:493934. doi: 10.1155/2012/493934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Candelario-Jalil E, Dijkhuizen RM, Magnus T. Neuroinflammation, stroke, blood-brain barrier dysfunction, and imaging modalities. Stroke. 2022;53:1473–86. doi: 10.1161/STROKEAHA.122.036946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bader V, Winklhofer KF. Mitochondria at the interface between neurodegeneration and neuroinflammation. Semin Cell Dev Biol. 2020;99:163–71. doi: 10.1016/j.semcdb.2019.05.028. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.