Abstract
Viruses play important roles in ecosystems by interfering with the central metabolic pathways of the host during infection via the expression of auxiliary metabolic genes (AMGs), altering the productivity of ecosystems and thus affecting geochemical cycling. In this study, the genetic diversity of phosphorus metabolism AMGs phoH, phoU and pstS was investigated by phylogenetic analysis, PCoA analysis, and alpha diversity analysis based on metagenomic data. It was found that the majority of the sequences were unique to Napahai plateau wetland. It was shown that the genetic diversity of phoH, phoU and pstS genes was independent of both habitats and host origins. In addition, the metabolic pathway of AMGs associated with the phosphorus cycling was identified based on metagenomic data. When phosphorus is deficient, virus utilizes AMGs to affect the metabolic pathway, contributing to higher phosphorus levels in the host and facilitating virus survival, replication, and propagation in the host cell.
Subject terms: Ecology, Biogeochemistry
Introduction
As the “kidneys of the earth”, wetlands have unique ecological functions and are the most active place for the energy flow and material circulation on earth. Napahai plateau wetland is located in the Three Parallel River Area of the Jinsha, Lancang and Nu rivers with unique geographic position, and possesses both terrestrial and aquatic characteristics. It belongs to a low-latitude (27° 47′ –27° 55′ ), high-altitude (average 3568 m), karst-landform seasonal swamp wetland, consisting of meadows, marshes, waters and forests. In addition, influenced by the southwest monsoon, Napahai plateau wetland forms a western monsoon climate in the low-temperate highland monsoon climate zone, with distinct dry and wet seasons. The unique geographic location, abundant water resources and rich nutrient lead to the rich biodiversity. However, due to geographical location, the species of Napahai plateau wetland are obviously different from other habitats, which determines the isolation, fragility and instability. Once affected by anthropogenic factors or external pressures, its ecosystem may undergo irreversible damage. The diversity of virus auxiliary metabolic genes (AMGs) can clarify the evolutionary history of organisms and the role they play in the ecosystem. However, the study on the genetic diversity of viral AMGs in plateau wetlands and how they regulate host metabolism has not been reported yet.
AMGs refers to a set of metabolism-related homologous genes carried by the viral genome1,2. AMGs include a number of genes that regulate host metabolism and life processes, and are currently divided into two main categories. The first class of AMGs is usually found in KEGG pathways (e.g. phosphate metabolism, carbon metabolism, nitrogen metabolism, sulfur metabolism, DNA biosynthesis, photosynthesis and other essential metabolic pathways3,4), i.e. AMGs that encode proteins with central metabolic functions. For example, genes involved in the electron transfer (psaA, petE, petF, ptoX, speD, hli) and light-harvesting processes (ho1, pcyA, pebS, cpeT), infected phage AMGs carrying photosynthetic system genes complements photosynthetic electron transport while transferring energy from the carbon fixation to the pentose phosphate pathway, leading to the increase of viral lysis5,6. Tsiola et al. conducted a metavirome study on coastal surface waters in the Ionian and South Aegean Seas, and revealed that viral AMGs participated in non-traditional energy production pathways (3HP, sulfur oxidation), Calvin cycle and TCA cycle7. Another class of AMGs does not usually appear in the KEGG pathway and encoded only proteins with general, unspecified metabolic roles or peripheral proteins involved in assembly and membrane transport functions. For example, AMGs associated with fatty acid metabolism in the Myxoviridae family can regulate host cytoplasmic or vesicle-like membrane fluidity. There are also AMGs involved in iron-sulfur cluster assembly, vitamin and cofactor synthesis, stress response, bacterial motility and chemotaxis, antioxidant and post-translational modifications8. In addition, AMGs are related to the community sensing, pathogenicity, host's defense, and spore formation involved in other ecosystems9. For example, abundant genes related to fatty acid metabolism and signaling were found in the viral genome, as well as those of fatty acid metabolism subsystem and components of the 3-hydroxypropionic acid (3HP) cycle. Phages that infect Prochlorococcus carried the phosphate acquisition gene pstS, which promoted phosphorus uptake by the host during infection10. Sabri et al. identified the gene que in the phage genome which could redirect host protein synthesis, enhanced the translation efficiency of the host, resulting in more efficient assembly of progeny phages11. The high abundance and genetic diversity of viral AMGs in the ocean suggested that virus played a significant role in microbial ecology by influencing the host's cellular functions via regulating host metabolism.
Phosphorus (P), an essential macronutrient for all life on Earth, has long been considered the second most limiting nutrient in some terrestrial ecosystems after nitrogen (N)12. In addition to being essential for DNA replication and nucleotide biosynthesis, it also critical for microbial growth. Unfortunately, there is growing evidence that P limitation is a general phenomenon occurring in various types of terrestrial ecosystems around the world, rather than in some specific terrestrial ecosystems13,14. The major viral AMGs currently associate with phosphorus metabolism include phoH, phoA, phoU, phoB, phoR, pstS, phoE, and ugpQ etc. Some phosphorus acquisition genes such as pstS and phoH, and alkaline phosphatase synthesis gene phoA existe in phages genome, which are beneficial to host cells to enhance phosphorus uptake and transport under low phosphorus environment. The phoH gene belongs to Pho regulon and is widely distributed in prokaryotes and viruses15. Li et al. identified phoH genes associated with phosphate metabolism in Northeastern wetland, which could be used as an effective biomarker gene16. To date, reports on phosphate metabolism-associated viral AMGs have focused on marine, lake, and rice paddy habitats. Few studies have been performed on phosphorus metabolism-associated viral AMGs in wetlands, especially plateau wetlands. In this paper, three genes related to phosphorus metabolism, phoH, phoU and pstS were obtained based on metagenomic data, which are expressed only in low or relatively phosphorus-deficient environments and thus regulate phosphorus homeostasis. Compared with the phosphorus content of other wetlands (Table S1 in the Supplementary Material), Napahai plateau wetland showed a low phosphorus level, so phoH, phoU and pstS were selected in this study.
In this paper, we investigated the genetic diversity of phoH, phoU, and pstS genes in Napahai plateau wetland based on metagenomic data by using phylogenetic analysis, PCoA analysis, and α-diversity analysis. We gained a brief understanding of the metabolic pathways AMGs involved in. It provides the foundation for subsequent studies on the genetic diversity of other metabolism-related AMGs in Napahai plateau wetland and the regulation of host metabolic pathways by viral AMGs, as well as virus-host co-evolution.
Results and discussion
Screening for viral AMGs
Viral protein annotation using VIBRANT and DRAM-v software combined with manual proofreading identified the viral AMGs in Napahai plateau wetland, including the viral AMGs phoH, phoU and pstS, which were associated with phosphorus metabolism.
Phylogenetic analysis of AMGs associated with phosphorus metabolism in Napahai plateau wetland
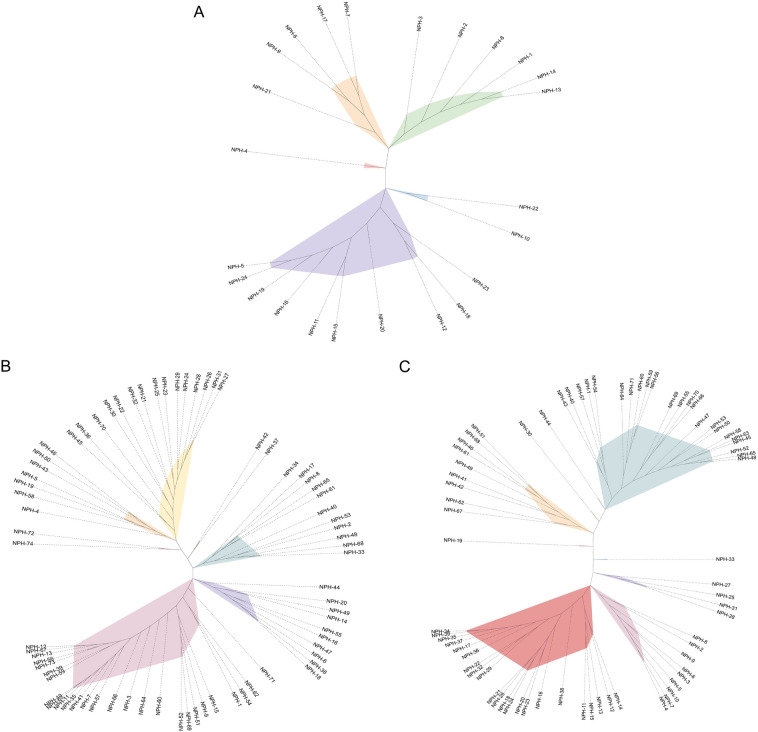
There were 24 amino acid sequences of phoH gene in Napahai plateau wetland (Fig. 1A). They were divided into 5 clusters, the largest of which had 10 sequences, while the smallest cluster had only 1 sequence. The remaining 3 clusters contained 6, 5 and 2 sequences, respectively. The phoH gene was genetically diverse in Napahai plateau wetland, which might be related to the different host origins. A total of 74 sequences of phoU gene could be found in seven clusters (Fig. 1B), with the largest cluster containing 27 sequences and the smallest cluster having two sequences. Similar to phoH, phoU was also genetically diverse, but richer than that of phoH. There were 71 pstS sequences forming 9 clusters, with the largest cluster of 23 sequences and the smallest cluster only 1 sequence (Fig. 1C). It could be seen that the genetic diversity of pstS was better than that of phoH and phoU, which might be related to the unique geographical location. Napahai plateau wetland is located in the Three Parallel Rivers of Yunnan protected areas, which forms a complex landscape, and then controls the evolution and characteristics of organisms, thus showing abundant biodiversity. Li et al. obtained 58 phoH gene sequences from Northeastern wetland sediments of China, which were 22%–99% consistent at the amino acid level, and found that the phoH gene could regulate phosphate uptake and metabolism under the low phosphate or phosphate limitation conditions16. However, the exact function remained unclear. The phoH gene clustered into five clusters in Napahai plateau wetland, indicating high genetic diversity. Additionally, water and soil samples were collected from eight separate sampling sites, and there were differences between samples environments, which might also have an impact on the genetic diversity of the three genes.
Figure 1.
Phylogenetic analysis of phosphorus metabolism AMGs in Napahai plateau wetland, different colors represent different branches. (A) Phylogenetic analysis of phoH genes. (B) Phylogenetic analysis of phoU genes. (C) Phylogenetic analysis of pstS genes.
Phylogenetic analysis and PCoA analysis of AMGs associated with phosphorus metabolism from different habitats and host origins
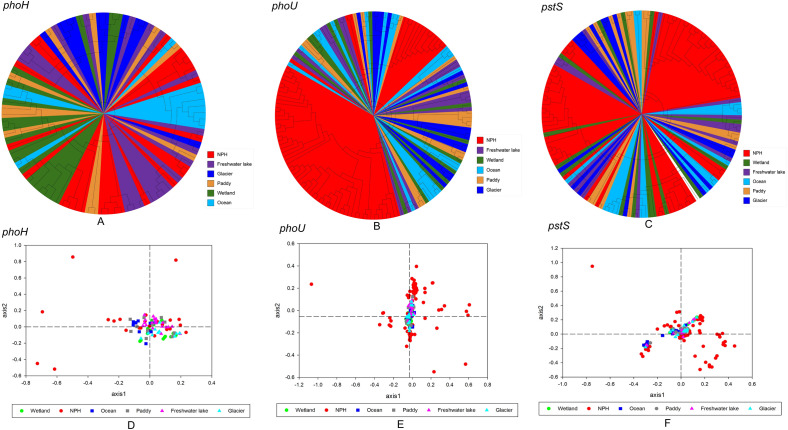
In order to understand the genetic diversity of viral AMGs (phoH, phoU, pstS) associated with phosphorus metabolism in Napahai plateau wetland, a phylogenetic tree of phosphorus metabolism AMGs from different habitats was constructed, and PCoA analysis was performed (Fig. 2). The results showed that most sequences of phoH, phoU and pstS genes in Napahai plateau wetland clustered individually, especially phoU and pstS genes, and only a few sequences were closely related to those of other habitats. In Fig. 2A, 14 sequences clustered individually and were relatively far from sequences of other habitats, whereas 7 sequences were close to those from freshwater lakes, and other 3 sequences were close to those from rice fields, oceans and other wetlands, respectively. Therefore, the genetic diversity of phoH in Napahai plateau wetland was independent of the habitat. Moreover, some of the phoH sequences were clustered with those of other habitats and distributed in the fourth quadrants (Fig. 2D). From Fig. 2B, apart from 3 sequences which clustered with those from the marine habitats and freshwater lakes, the rest were clustered separately. Whereas in Fig. 2E, apart from only a few sequences, most sequences of phoU were far away from those of different habitats, which was consistent with Fig. 2B. Thus, the genetic diversity of phoU gene in Napahai wetland was also independent of habitat, where the separately clustered sequences may be unique. From Fig. 2C, we can seen that apart from 8 sequences which more closely related to those from the freshwater lake, ocean, rice field, and other wetlands, all the rest were individually clustered. The result was consistent with that of Fig. 2F. Therefore, the genetic diversity of the pstS gene was also habitat-independent.
Figure 2.
Phylogenetic analysis and PCoA of phosphorus metabolism AMGs in different habitats, different colors represent different habitats. (A) Phylogenetic analysis of phoH genes in different habitats. (B) Phylogenetic analysis of phoU genes in different habitats. (C) Phylogenetic analysis of pstS genes in different habitats. (D) PCoA analysis of phoH genes in different habitats. (E) PCoA analysis of phoU genes in different habitats. (F) PCoA analysis of pstS genes in different habitats.
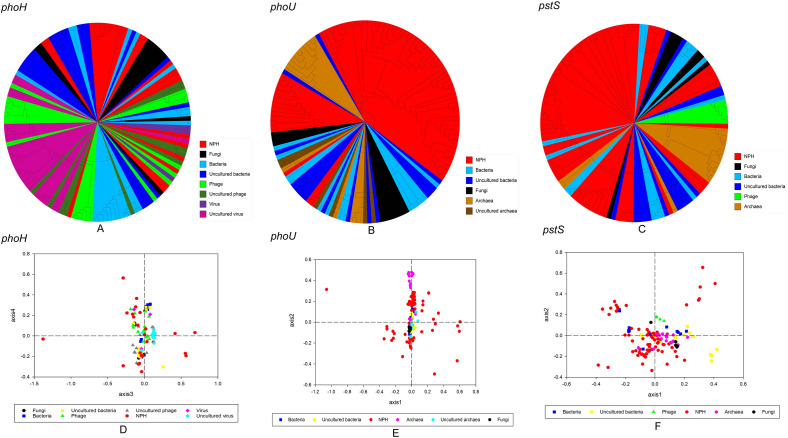
To study whether the genetic diversity was related to host origins, three AMGs associated with phosphorus metabolism were selected for phylogenetic and PCoA analyses with AMGs sequences from different host origins (Fig. 3). It showed that some sequences of all three genes were similar to those from different host origins, while the remaining were separately clustered. In Fig. 3A, apart from 14 sequences which clustered with those from fungi, bacteria, non-culturable phages, phages and viruses, all the rest were clustered separately. Whereas, most sequences were clustered with those from different host origins together, and only six sequences were far from other sequences of different host origins based on PCoA analysis (Fig. 3D). Only three sequences were clustered with those of archaea and uncultured archaea, and the rest were clustered together to form independent clusters (Fig. 3B). A small amount of sequences were gathered with bacteria, uncultured bacteria, archaea and uncultured archaea, and the rest were clustered individually (Fig. 3E). As can be seen in Fig. 3C, six sequences were clustered with those of archaea, fungi, bacteria, while the rest were clustered separately. Some sequences were gathered with bacteria, uncultured bacteria, archaea and uncultured archaea, and others were clustered separately (Fig. 3F). PCoA analysis was largely consistent with phylogenetic analysis. So the genetic diversity of phoH, phoU and pstS genes in Napahai plateau wetland was independent of the host origins.
Figure 3.
Phylogenetic analysis and PCoA of phosphorus metabolism AMGs from different host origins, different colors represent different host origins. (A) Phylogenetic analysis of phoH gene from different host origins. (B) Phylogenetic analysis of phoU gene from different host origins. (C) Phylogenetic analysis of pstS gene from different host origins. (D) PCoA analysis of phoH genes from different host origins. (E) PCoA analysis of phoU genes from different host origins. (F) PCoA analysis of pstS genes from different host origins.
Overall, the genetic diversity of phoH, phoU and pstS genes associated with phosphorus metabolism in Napahai plateau wetland was independent of both the habitats and host origins based on phylogenetic and PCoA analyses. It suggested that three genes showed relatively rich genetic diversity and were not genetically limited by differences in habitats or host origins. Han et al. showed that phoH sequences were widely distributed in soil, freshwater, and seawater environments in different locations around the world, indicating the genetic diversity independent of the environment17, which corroborated the conclusions in our study. Phylogenetic analysis of the 58 viral phoH gene sequences in Northeastern wetland of China revealed that some sequences were clustered with bacterial sequences and others clustered with phages sequences16. In Napahai plateau wetland, some phoH gene sequences were clustered with fungal, bacterial, phage, uncultured phage, and viruses. Hence, the genetic diversity of phoH gene was independent of the host origins in either Northeastern wetland or Napahai plateau wetland. Compared with Northeastern wetland, the phoH genes in Napahai plateau wetland showed more abundant genetic diversity, which may be related to geographical location and climate. Additionally, compared with sequences from different habitats and host sources, partial sequences from Napahai plateau wetland were clustered individually, thus they were unique, which might be related to the unique geography. Napahai plateau wetland is located in the Three Parallel Rivers with low latitude and high altitude, and shows specific characteristics which not found in other habitats, and then the species very different, thus providing the possibility for the emergence of unique genetic sequences. Of course, it would require further verification by subsequent study.
As far as the current studies are concerned, most reports on phosphorus AMGs focused on the function. Wang et al. mentioned that the phoH gene regulated phosphate uptake or metabolism under the low phosphorus or phosphate limitation conditions18. Kelly et al. isolated several phages from oligotrophic water bodies with low phosphorus condition, found that they contained the phosphate binding transporter gene pstS by sequencing, which enhanced the host cell with increasing the infection cycle of phages by increasing phosphate utilization19. Gardner et al. studied the PhoR-PhoB two-component regulatory system in E. coli, which regulated the expression of relevant genes according to environmental phosphate concentration and enabled cells to adapt the phosphate starvation20. The phoU existed in many bacteria and was identified as an auxiliary protein of the phosphate-specific transporter system, regulating phosphate metabolism in the host cell acting as phosphate regulators21. Few studies had been conducted on its genetic diversity, therefore, the information on the genetic diversity was relatively scarce.
α diversity analysis of phosphorus metabolism AMGs in different habitats and different host origins
Chao, Shannon and Simpson diversity indices are common mathematical measure of species alpha diversity in the community. Chao focuses on species richness. Shannon index and Simpson index measure species richness and evenness. Simpson reinforces evenness and Shannon reinforces richness22.
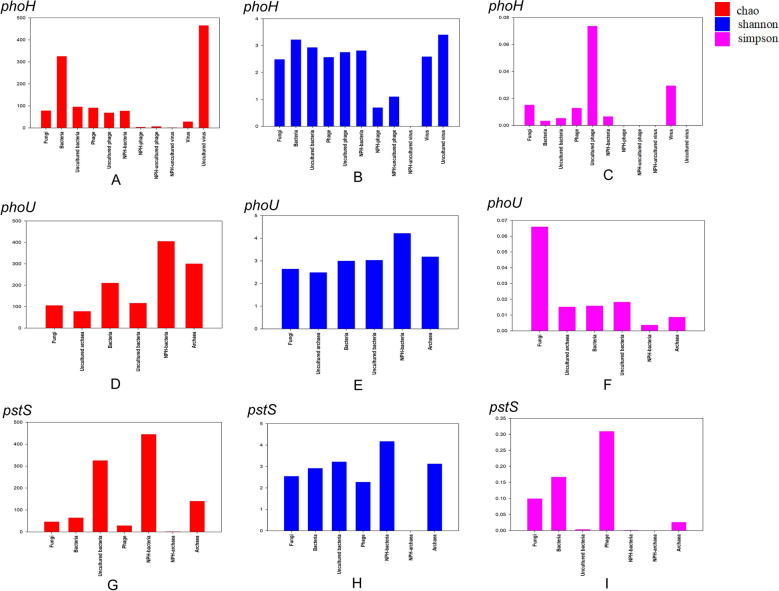
Sequences from different habitats, such as Napahai plateau wetland, Pacific Ocean, Lake Baikal, Northeast rice fields, glaciers, and wetlands, were selected for α-diversity analysis (Fig. 4). The genetic diversity indices, such as Chao, Shannon and Simpson, calculated based on the OUT dataset, were used to characterize the alpha diversity. Among them, larger Chao values, smaller Simpson values or larger Shannon values indicate higher genetic diversity. Only at the level of Chao values (Fig. 4A,D,G) and Shannon values (Fig. 4B,E,H), the values of phoH, phoU, and pstS in Napahai plateau wetland were greater than those from other habitats, indicating better heritable, which might be related to the unique geographical location and abundant water resources. The geographical location made it unique and less influenced by external factors, and abundant water resources created a rich biodiversity, thus providing a good genetic environment. From the Simpson values (Fig. 4C,F,I), the values of phoU and pstS genes were smaller than those of other habitats, indicating better inherited. For the phoH gene, the Simpson value was closer in magnitude and lower than those in Antarctic Lake and wetlands, indicating better heritable.
Figure 4.
Plots of genetic diversity indices analysis of phosphorus metabolism AMGs in different habitats, different colors represent different genetic diversity indices. (A, D, G) Represent respectively the Chao values of phoH, phoU, and pstS genes in different habitats. (B, E, H) Represent respectively the Shannon values of phoH, phoU, and pstS genes in different habitats. (C, F, I) Represent respectively the Simpson values of phoH, phoU, and pstS genes in different habitats.
Three AMGs associated with phosphorus metabolism in Napahai plateau wetland were selected for α-diversity analysis with AMGs sequences from different host origins (Fig. 5). In Fig. 5A, the Chao values of phoH gene from bacteria, phages, uncultured phages and uncultured viruses in Napahai plateau wetland were smaller than those of bacteria, phages, uncultured phages and uncultured viruses, indicating the poor genetic diversity. In addition, compared to the genetic diversity of sequences from other host sources, the genetic diversity of phoH gene from bacteria in Napahai plateau wetland was better. As can be seen in Fig. 5D, G, the Chao values of phoU and pstS genes from bacteria in Napahai plateau wetland were greater than those of other host origins, indicating better genetic diversity, while the Chao values of pstS genes from archaea in Napahai plateau wetland were smaller than those of other host origins, indicating poor genetic diversity.
Figure 5.
Plots of genetic diversity indices analysis of phosphorus metabolism AMGs from different host origins, different colors represent different genetic diversity indices. (A, D, G) Represent respectively the Chao values of phoH, phoU, and pstS genes from different host origins. (B, E, H) Represent respectively the Shannon values of phoH, phoU, and pstS genes from different host origins. (C, F, I) Represent respectively the Simpson values of phoH, phoU, and pstS genes from different host origins.
The Shannon value of phoH gene from bacteria in Napahai plateau wetland was smaller than that of bacteria and uncultured viruses, indicating poor diversity, but larger than other host sources, indicating better genetic diversity (Fig. 5B). The Shannon values of phoH gene from phages and uncultured phages in Napahai plateau wetland were lower than those of other host origins, indicating poor diversity. The Shannon value of phoH genes from uncultured viruses in Napahai plateau wetland was 0, probably due to sample size too small to calculate the Shannon value. In Fig. 5E, H, the Chao values of phoU and pstS genes from bacteria in Napahai plateaus wetland were greater than those from other host sources, indicating better diversity, while the Shannon value of pstS gene from archaea in the Napahai plateau wetland was 0, probably small sample size.
The Simpson values of phoH genes from phage, uncultured phage and uncultured virus in Napahai plateau wetland were smaller than those of other host origins (except uncultured virus), indicating better diversity. The smaller Simpson values of phoH genes related to fungi, phages, uncultured phages, and viruses indicated better diversity, while the larger Simpson values compared to bacteria, phages, and uncultured viruses indicated poor diversity (Fig. 5C). As can be seen in Figs. 5F,I, the Simpson values of phoU genes from bacteria and pstS genes from bacteria and archaea in Napahai plateau wetland were smaller than those of other host origins, indicating better genetic diversity.
Currently, most studies on phosphorus AMGs employed phylogenetic analysis16,23. In contrast, relatively few AMGs associated with phosphorus had been reported based on α-diversity analysis, so it was difficult to obtain specific values of α-diversity indices in other studies.
Biogeochemical cycling of AMGs associated with phosphorus metabolism in Napahai plateau wetland
Viruses are the gene carriers in susceptible hosts, and AMGs introduced by viruses into new hosts can enhance viral replication and/or influence key microbial metabolic pathways of the biogeochemical cycles24. It is well known that phosphorus is an essential nutrient and plays essential roles in cells25. Phosphorus deficiency leads to restricted cell division, down-regulation of photosynthesis, reduced protein and nitrogen content and chlorophyll synthesis26. To study the effect of AMGs associated with phosphorus metabolism, a phosphorus metabolic pathway containing phoH, phoU and pstS genes was constructed based on metagenomic data (Fig. 6). When phosphorus deficiency occurs in the host, it leads to the expression of phoH, phoU and pstS genes. phoH is a phosphate starvation inducible gene, while pstS acts as a phosphate transport gene and phoU belongs to a phosphate regulatory gene that produces dissolved inorganic phosphorus (DIPs), which then undergoes a series of reactions to produce ATP. The generated ATP becomes PolyP under the action of ppK which encoding polyphosphate kinase, or is used in Calvin cycle to provide energy for Ru5P to produce RuBP, or is used for DNA biosynthesis to provide energy. PolyP is regenerate into DIP with ppX which encoding exopolyphosphatase, and also involves in the biosynthesis process of DNA as Pi to provide phosphate for the nucleic acids synthesis. Thus, phosphorus metabolism of AMGs invoved plays a significant role in the life process of the virus and host. In addition, phoE and ugpQ genes also are identified in Napahai plateau wetland, but their roles in the phosphorus cycling are currently unknown and need further study.
Figure 6.

Biogeochemical cycling of AMGs associated with phosphorus metabolism in Napahai plateau wetland. Red line indicates the process of phosphorus metabolism.
Based on the phylogenetic and PCoA analyses, we found that the phoH, phoU, and pstS genes all showed unique sequences, which might be drive the microorganisms to produce the phosphorus metabolic pathway in Napahai plateau wetland. Of course, in order to prove this pathway, further validation might be done by metabolomics or metabolic flow method. Furthermore, the phosphorus metabolic pathway was poorly reported, so we could not compare with the phosphorus pathway from other environment to find commonalities and differences.
Materials and methods
Sample collection
According to the principle of typicality, water and soil samples were collected in Napahai plateau wetland, and eight sample points were chosen to sample soil. The depth of the soil layer at the sampling points was about 5–8 cm27, and 5 kg of soil samples were collected from each sample point. All the soil samples were mixed and divided into three portions, denoted as S1, S2 and S3. Water samples were collected along the lake, a total of eight sampling points. All water samples were mixed and divided equally into three parts as three replicates recorded as W1, W2 and W3. The latitude, longitude, altitude, air temperature, atmospheric pressure and pH value were recorded.
Nucleic acid extraction and high-throughput sequencing
The DNA of soil and water samples was extracted by QIAamp DNA kit (Qiagen), and the quality of the separated and purified DNA was examined and evaluated using 0.8% agarose gel electrophoresis and Nano Drop spectrophotometer. When a single band appeared on the gel without trailing, and the concentration reached 50 ng/μL, it could be used for subsequent analysis.
The fragments were randomly broken by ultrasonic fragmentation, and the sticky ends were repaired to flush ends by T4 Polymerase, Klenow DNA polymerase and T4 PNK, and then the ligated products were recovered with the addition of special Adapter ligation. The electrophoresis method was selected to recover the target fragment of about 300 bp in size. The DNA fragments with Adapter at both ends were amplified using PCR technique28–30. The qualified library was prepared and sequenced, and 125 bp Paired-End double-end sequencing was performed using HiSeq 2500 high-throughput sequencing platform with an insertion length of 300 bp by Guangzhou Magigene Biotechnology Co., Ltd.
Screening for viral AMGs
Firstly, CheckV31 was employed to distinguish and eliminate host contamination based on gene content for a single sequence. Then, based on VirSorter v2.032, viral contigs were further DRAM-v annotation33, and the required input files of DRAM-v were engendered. Next, the chosen viral contigs were worked through DRAM-v using default parameters, with gene definitions and auxiliary scores of < 4 considered putative AMGs. Conserved domains of viral AMGs were detected using the NCBI CD-search tool34. The Phyre2 web portal was used to search the protein structural homology35. Moreover, VIBRANT36 annotations were made on viral contigs based on KEGG using default parameters. KEGG annotations categorized into “metabolic pathways” were reported as potential AMGs36. No curations were executed on the VIBRANT output.
Phylogenetic analysis of AMGs associated with phosphorus metabolism in Napahai plateau wetland
The amino acid sequences of AMGs associated with phosphorus metabolism in Napahai plateau wetland were searched based on metagenomic data. The phylogenetic tree was constructed using the maximum likelihood method in MEGA X software37 with Bootstrap of 1000. To study the amino acid sequences of phoH, phoU, and pstS from Napahai plateau wetland with other habitats (including the ocean, lake, rice field, wetland, glacier, etc.) and other host origins (including bacteria, uncultured bacteria, fungi, phage, uncultured phages, viruses, uncultured viruses, archaea and uncultured archaea, etc.) as references, the amino acid sequences of phoH, phoU and pstS were sampled 1000 times at the amino acid level using the maximum likelihood method in MEGA X software to construct phylogenetic trees. Amino acid sequences information of phoH, phoU, and pstS from other habitats and host sources were detailed in Tables S2–S7 in the Supplementary Material.
PCoA analysis of AMGs associated with phosphorus metabolism in Napahai plateau wetland
Principal co-ordinates analysis (PCoA) is a non-constrained method of data dimensionality reduction analysis that can be used to study the similarity or difference of samples38,39. The nucleotide sequences corresponding to the amino acid sequences of different origins and host sources searched from NCBI were selected, and other specific nucleotide sequence information of different habitats and host sources can be referred to Tables S8–S13 in the Supplementary Material. Combined with the nucleotide sequences of phoH, phoU, and pstS from Napahai plateau wetland, the UniFrac distances of all phoH, phoU, and pstS nucleotide sequences were calculated using Mothur software34, and then the PCoA was plotted using the mapping software Sigma Plot 11.0.
α diversity analysis of AMGs associated with phosphorus metabolism in Napahai plateau wetland
The nucleotide sequences of phoH, phoU and pstS from different habitats and host sources were searched from NCBI, and specific information on nucleotide sequences could be found in Tables S8–S13 in the Supplementary Material. Combined with sequences from Napahai plateau wetland, the OTUs were typed using Mothur software, and genetic diversity indices, such as Chao, Shannon and Simpson indices, were used to characterize α-diversity.
Biogeochemical cycling of AMGs associated with phosphorus metabolism in Napahai plateau wetland
Based on the metagenomic data, the enzymes and their functions of AMGs associated with phosphorus metabolism were searched by KEGG to analyze the phosphorus cycling in Napahai plateau wetland.
Supplementary Information
Acknowledgements
We thank the National Natural Science Foundation of China (32160294, 31860147) for its support.
Author contributions
H.Y.: Design, Investigation, Data curation, Writing-original draft, L. X. and Y. L.: Data curation, editing, Y. W. and H. L.: Supervision, Writing-review & editing; Q. Z.: Supervision; X. J. and W. C.: Supervision, Writing-review & editing. All authors have contributed to the manuscript. All authors read and approved the manuscript.
Data availability
The datasets generated during the current study are available from the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Hang Yu and Lingling Xiong.
Contributor Information
Wei Chen, Email: wchen@kust.edu.cn.
Xiuling Ji, Email: jixiuling1023@126.com.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-023-28488-1.
References
- 1.Ruiz-Perez CA, Tsementzi D, Hatt JK, Sullivan MB, Konstantinidis KT. Prevalence of viral photosynthesis genes along a freshwater to saltwater transect in Southeast USA. Environ. Microbiol. Rep. 2019;11:672–689. doi: 10.1111/1758-2229.12780. [DOI] [PubMed] [Google Scholar]
- 2.Huang X, Jiao N, Zhang R. The genomic content and context of auxiliary metabolic genes in roseophages. Environ. Microbiol. 2021;23:3743–3757. doi: 10.1111/1462-2920.15412. [DOI] [PubMed] [Google Scholar]
- 3.Crummett LT, Puxty RJ, Weihe C, Marston MF, Martiny JBH. The genomic content and context of auxiliary metabolic genes in marine cyanomyoviruses. Virology. 2016;499:219–229. doi: 10.1016/j.virol.2016.09.016. [DOI] [PubMed] [Google Scholar]
- 4.Weynberg KD, et al. Coral-associated viral communities show high levels of diversity and host auxiliary functions. PeerJ. 2017;5:e4054. doi: 10.7717/peerj.4054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jiang T, et al. Isolation and complete genome sequence of a novel cyanophage, S-B05, infecting an estuarine Synechococcus strain: Insights into environmental adaptation. Adv. Virol. 2020;165:1397–1407. doi: 10.1007/s00705-020-04595-6. [DOI] [PubMed] [Google Scholar]
- 6.Puxty RJ, Millard AD, Evans DJ, Scanlan DJ. Shedding new light on viral photosynthesis. Photosynth. Res. 2015;126:71–97. doi: 10.1007/s11120-014-0057-x. [DOI] [PubMed] [Google Scholar]
- 7.Tsiola A, et al. Viral metagenomic content reflects seawater ecological quality in the coastal zone. Viruses. 2020;12:806. doi: 10.3390/v12080806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Anderson CL, Sullivan MB, Fernando SC. Dietary energy drives the dynamic response of bovine rumen viral communities. Microbiome. 2017;5:155. doi: 10.1186/s40168-017-0374-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Khot V, Strous M, Hawley AK. Computational approaches in viral ecology. Comput. Struct. Biotechnol. J. 2020;18:1605–1612. doi: 10.1016/j.csbj.2020.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhao F, et al. Biochemical and structural characterization of the cyanophage-encoded phosphate-binding protein: Implications for enhanced phosphate uptake of infected cyanobacteria. Environ. Microbiol. 2022;24:3037–3050. doi: 10.1111/1462-2920.16043. [DOI] [PubMed] [Google Scholar]
- 11.Sabri M, et al. Genome annotation and intraviral interactome for the Streptococcus pneumoniae virulent phage Dp-1. J. Bacteriol. 2011;193:551–562. doi: 10.1128/jb.01117-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mise K, Fujita K, Kunito T, Senoo K, Otsuka S. Phosphorus-mineralizing communities reflect nutrient-rich characteristics in Japanese arable andisols. Microbes Environ. 2018;33:282–289. doi: 10.1264/jsme2.ME18043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Augusto L, Achat DL, Jonard M, Vidal D, Ringeval B. Soil parent material-A major driver of plant nutrient limitations in terrestrial ecosystems. Glob. Change Biol. 2017;23:3808–3824. doi: 10.1111/gcb.13691. [DOI] [PubMed] [Google Scholar]
- 14.Hou E, et al. Global meta-analysis shows pervasive phosphorus limitation of aboveground plant production in natural terrestrial ecosystems. Nat. Commun. 2020;11:637. doi: 10.1038/s41467-020-14492-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gao S, et al. Patterns and ecological drivers of viral communities in acid mine drainage sediments across Southern China. Nat. Commun. 2022;13:2389. doi: 10.1038/s41467-022-30049-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li X, Sun Y, Liu J, Yao Q, Wang G. Survey of the bacteriophage phoH gene in wetland sediments in northeast China. Sci. Rep. 2019;9:911. doi: 10.1038/s41598-018-37508-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Han LL, et al. Distribution of soil viruses across China and their potential role in phosphorous metabolism. Environ. Microbiome. 2022;17:6. doi: 10.1186/s40793-022-00401-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang X, et al. Novel groups and unique distribution of phage phoH genes in paddy waters in northeast China. Sci. Rep. 2016;6:38428. doi: 10.1038/srep38428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kelly L, Ding H, Huang KH, Osburne MS, Chisholm SW. Genetic diversity in cultured and wild marine cyanomyoviruses reveals phosphorus stress as a strong selective agent. ISME J. 2013;7:1827–1841. doi: 10.1038/ismej.2013.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gardner SG, Johns KD, Tanner R, McCleary WR. The PhoU protein from Escherichia coli interacts with PhoR, PstB, and metals to form a phosphate-signaling complex at the membrane. J. Bacteriol. 2014;196:1741–1752. doi: 10.1128/jb.00029-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shang Y, et al. Staphylococcus aureus PhoU homologs regulate persister formation and virulence. Front. Microbiol. 2020;11:865. doi: 10.3389/fmicb.2020.00865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Avalos-Fernandez M, et al. The respiratory microbiota alpha-diversity in chronic lung diseases: First systematic review and meta-analysis. Respir. Res. 2022;23:214. doi: 10.1186/s12931-022-02132-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goldsmith DB, et al. Development of phoH as a novel signature gene for assessing marine phage diversity. Appl. Environ. Microbiol. 2011;77:7730–7739. doi: 10.1128/aem.05531-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mara P, et al. Viral elements and their potential influence on microbial processes along the permanently stratified Cariaco Basin redoxcline. ISME J. 2020;14:3079–3092. doi: 10.1038/s41396-020-00739-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu J, Zhou L, Ke Z, Li G, Tan Y. Phosphorus deficiency induced by aluminum in a marine nitrogen-fixing cyanobacterium Crocosphaera watsonii WH0003. Chemosphere. 2020;246:125641. doi: 10.1016/j.chemosphere.2019.125641. [DOI] [PubMed] [Google Scholar]
- 26.Markou G, Chatzipavlidis I, Georgakakis D. Carbohydrates production and bio-flocculation characteristics in cultures of Arthrospira (Spirulina) platensis: Improvements through phosphorus limitation process. BioEnergy Res. 2012;5:915–925. doi: 10.1007/s12155-012-9205-3. [DOI] [Google Scholar]
- 27.Li J. Sampling soils in a heterogeneous research plot. J. vis. Exp. JoVE. 2019 doi: 10.3791/58519. [DOI] [PubMed] [Google Scholar]
- 28.Qin J, et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature. 2010;464:59–65. doi: 10.1038/nature08821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hurwitz BL, Deng L, Poulos BT, Sullivan MB. Evaluation of methods to concentrate and purify ocean virus communities through comparative, replicated metagenomics. Environ. Microbiol. 2013;15:1428–1440. doi: 10.1111/j.1462-2920.2012.02836.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thurber RV, Haynes M, Breitbart M, Wegley L, Rohwer F. Laboratory procedures to generate viral metagenomes. Nat. Protoc. 2009;4:470–483. doi: 10.1038/nprot.2009.10. [DOI] [PubMed] [Google Scholar]
- 31.Nayfach S, et al. CheckV assesses the quality and completeness of metagenome-assembled viral genomes. Nat. Biotechnol. 2021;39:578–585. doi: 10.1038/s41587-020-00774-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Guo J, et al. VirSorter2: A multi-classifier, expert-guided approach to detect diverse DNA and RNA viruses. Microbiome. 2021;9:37. doi: 10.1186/s40168-020-00990-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shaffer M, et al. DRAM for distilling microbial metabolism to automate the curation of microbiome function. Nucleic Acids Res. 2020;48:8883–8900. doi: 10.1093/nar/gkaa621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lu S, et al. CDD/SPARCLE: The conserved domain database in 2020. Nucleic Acids Res. 2020;48:D265–d268. doi: 10.1093/nar/gkz991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kelley LA, Mezulis S, Yates CM, Wass MN, Sternberg MJ. The Phyre2 web portal for protein modeling, prediction and analysis. Nat. Protoc. 2015;10:845–858. doi: 10.1038/nprot.2015.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kieft K, Zhou Z, Anantharaman K. VIBRANT: Automated recovery, annotation and curation of microbial viruses, and evaluation of viral community function from genomic sequences. Microbiome. 2020;8:90. doi: 10.1186/s40168-020-00867-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kumar S, Stecher G, Li M, Knyaz C, Tamura K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018;35:1547–1549. doi: 10.1093/molbev/msy096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Checinska A, et al. Microbiomes of the dust particles collected from the international space station and spacecraft assembly facilities. Microbiome. 2015;3:50. doi: 10.1186/s40168-015-0116-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yang Z, Guo R, Xu X, Wang L, Dai M. Enhanced methane production via repeated batch bioaugmentation pattern of enriched microbial consortia. Biores. Technol. 2016;216:471–477. doi: 10.1016/j.biortech.2016.05.062. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during the current study are available from the corresponding author on reasonable request.