Abstract
Partially absorbable suture is useful for orthopedic repair as it possesses the capacity to promote a balance between strength, degradation rate and minimal inflammation. Still, the availability of partially absorbable suture is scarce. So far, no study has examined the mechanical strength and anti-microbial properties of partially absorbable monofilament suture made of low-density polyethylene (LDPE)/polylactide (PLA)/chitosan (CHS); hence, the reason for this study with a view to improve knot strength, antimicrobial property and degradation rate. In this study, monofilament suture was extruded using different weight fractions of LDPE, PLA and CHS. In vitro degradation studies were carried out using phosphate buffer solution (PBS). Mechanical and morphological changes were also examined. A standard Fourier transform infrared spectral of 3433, 2909–2840, 1738, 1452, 1174, 1062, 706 cm−1 were assigned to OH group, C–H stretch, C=O vibration of ester, CH3 bending, alkyl ester and CH2 stretch, respectively. Tensile strength of knotted neat LDPE (4.84 MPa) exhibited 48.7% improvement in LDPE/PLA/CHS (60/39.5/0.5). This suggests that a good knot can be achieved to 40% weight fraction of PLA. The monofilament suture also demonstrated better antimicrobial property as the monofilament, LDPE/PLA/CHS (60/39.5/0.5) and LDPE/PLA/CHS (50/49.5/0.5) covered 12.7 mm zone of inhibition which is greater than the standard 1 mm. The suture’s morphological phases show dark fibre-like rough surfaces with microstructural irregularities as PLA and CHS were added to the matrix, which is required for enhanced degradation. Thus, the partially absorbable suture produced in this study could serve as a suture for tendon repair.
Keywords: Extruded monofilament suture, Tendon, Staphylococcus, Biodegradation
Introduction
The quality of tissue repair depends on different variables, namely, the characteristics of the tissue, properties of the suture material, and the surgical technique. An ideal suture should possess excellent tensile strength, biologically inert, stretchable, accommodate wound edema and recoil to the original length with wound contraction, pliable, good antimicrobial property (Kim et al., 2007) and good knot security (Pillai et al. 2010). Suture security is the ability of knotted suture to hold tissue together, during the healing process without knot breaking (Hockenberger et al. 2004). Knot breakage is one of the causes of suture failure. The applications of suture material include, orthopedic surgeries to close wounds, repair facial muscles, tendons, ligaments and joint capsules, and to cerclage or perform tension bands in certain fractures (Kanimozhiet al. 2021). Orthopedic suture is an important device needed for the repair of athletic injury. Frequently used suture in repair of Achilles tendon, is a non-absorbable multifilament with high tensile strength, but excessive strength causes stress shielding which hinders tissue remodeling (Su et al. 2018). In this application, an absorbable suture material is more desirable compared to a non-absorbable suture material of equivalent strength and stiffness. However, absorbable suture has a low tensile strength (Muller et al. 2016). Therefore, the quest to find suture with the required tensile strength suitable for orthopedic repair has led to the development of partially absorbable suture. It should be noted that partially absorbable suture is commercially limited. Fiber-wire suture is an example of commercial partially absorbable suture made by braiding ultra-high-molecular-weight polyethylene (UHMWP) with polyester. However, ultra-high-molecular-weight polyethylene is relatively slippery. This inherent slipperiness often affects the knot integrity of a suture. It has also been observed that braided sutures are associated with high rate of surgical site infection. The most common complication in post-surgery is caused by surgical site infection, which increases hospital stay, financial cost, mortality and morbidity rate (Tucci et al. 2019; Anderson et al. 2014). Hospital acquired infection is related to the surgical site infection. In Nigeria, the estimate of hospital acquired infection is reported to have a prevalence rate between 2.5% and 6.3%. (Dayyab et al. 2018; Ige et al. 2011). Surgical site infection (SSI) remains a challenge in orthopedic surgery, in spite of preventive measures to curb it (Tucci et al. 2019). To reduce surgical site infection, triclosan coated suture was developed. Triclosan, a broad-spectrum antibacterial agent, is considered to interfere with bacterial lipid synthesis and consequently decrease bacterial colonization of the suture material (Ming et al. 2007; Ming et al. 2008). However, the potential toxicity and side-effects of triclosan is alarming and have raised increasing concerns regarding its biological safety. Different reports show that triclosan is physiologically harmful to the reproductive health, both in animal/human models (Wang et al. 2015; Hang et al. 2016; Aiello et al. 2011). Heavy metals, such as silver, have also been used to introduce antimicrobial activity in suture, but cell and tissue compatibility is yet to be achieved. In addition, their topography-based antibacterial property may not be suitable for combating contaminated wound (Serrano et al. 2015). Thus, there is a need for alternative suture material with good antimicrobial property. Chitosan, a natural polysaccharide is an attractive coating substance due to its unique properties, such as antimicrobial activity, wound healing ability, scar preventing propensity, biocompatibility (Gupta et al. 2011), low toxicity, and its ability to smoothing surface of various structures. Recently the incorporation of chitosan on the surface of sutures is one of the approaches adopted to improve the functional characteristics of various suture materials. To impart antimicrobial activity, chitosan was successfully applied onto various suture materials, namely, polypropylene sutures (Gupta et al. 2011), cotton sutures (Shanmugasundaram 2006). Literature on partially absorbable suture simply focuses on comparing mechanical strength of different sutures and their handling characteristics, such as pliability and co-branding of absorbable and non-absorbable suture. Nonetheless, information concerning synthesizing of partially absorbable suture is limited. Hence, there is a need to develop partially absorbable monofilament suture, made of low-density polyethylene (LDPE) and polylactic acid (PLA), with a definite property. PLA is considered an eco-friendly biomaterial with excellent properties, commonly investigated and widely used biodegradable polymer, due to non-toxicity of lactic acid which is naturally present in human body (Abdelaal et al. 2013). LDPE has excellent mechanical properties and is relatively cheap (Alnaimi 2004). Hence, to strike a balance between strength, rate of degradation and minimal inflammation suitable for orthopedic repair, there is a need to synthesize partially absorbable suture made of low-density polyethylene, polylactide and chitosan and to perform antimicrobial, knot and biodegradation assessments of the composite suture materials.
Materials and methods
Polylactide (PLA) (250,000 g/mol) resin was purchased from nature works in China; low-density polyethylene PFS 4020 was obtained from Petrochemical Plock located in Poland and partially soluble chitosan in PBS (66% degree of acetylation and crystallinity of 79%) was extracted from the exoskeleton of marine invertebrate at the University Lagos, Metallurgical and Materials Engineering Laboratory, Nigeria. U-Clear laboratory dry oven DHG-9030 (China), was used for drying of pellets. Water used in all the tests was Milli-Q water (Millipore, USA). Antimicrobial assay was done using Mannitol Salt Agar by Himedia Laboratories (India).
Preparation of LDPE/PLA partially absorbable suture
Low density polyethylene and polylactic acid was formulated in the weight ratio LDPE/PLA (100/0, 90/10, 80/20, 60/40, 50/50 w/w) and LDPE/PLA/CHS (60/39.5/0.5, 50/49.5/0.5 w/w) were weighed to 25 g. Thereafter the pellets were manually mixed and pre-dried in an oven at 103 °C for 4 h to remove moisture prior to extrusion. The pellets were immediately charged into the extruder hopper. The materials were extruded into a single filament using a desktop single screw extruder (wellzoom Desktop Extruder line ii Shenzen, China). The pre-heating temperature was set at 192 °C, while the extrusion temperature and speed were at 197 °C and 2 rev/min respectively. After the extrusion vernier caliper were used to measure the diameter (1.2 mm).
Mechanical test
The tensile test of the monofilament suture was performed using Instron computerized double column universal tensile machine model 3369 (USA), located at the Energy Center, Obafemi, Awolowo University, Ile-Ife, Nigeria. It has a capacity of 50 KN. The knot test was done using USP standard for tensile test of knot (50 mm gauge length and a crosshead speed of 100 mm/min) (USP 29 NF 24). The suture was knotted using a square knot before subjecting it to mechanical test.
Scanning electron microscopy (SEM)
The suture micrographs were produced by scanning electron microscopy model; Phenom Eindhoven. Netherlands. It works with an electron intensity beam of 15 kV, while the samples to be observed were usually mounted on a conductive carbon print left by the adhesive tape. This is usually prepared by placing the samples on the circular holder and coated for 5 min to enable it conduct electricity.
Fourier transform infrared spectroscopy (FTIR)
Functional groups in the partially absorbable composite suture were detected using Agilent Technologies Cary 630 spectrometer (India). Each sample of 10 mg was compressed to pellets after being dispersed in KBr. The spectral measurement was made in absorbance mode and processed at a resolution of 4 cm−1 between 500 and 4000 cm−1.
Antimicrobial test of partially absorbable composite suture
Agar diffusion method: antimicrobial activity of monofilament suture blended with PLA and CHS was determined against staphyloccus aureus ATCC 25,922 using standard agar plate method. S. aureus was grown in Nutrient Broth (NB). Inoculums were prepared by adjusting the turbidity of the medium to match 0.5 MC Farland Standard Dilutions. A 20 mL of nutrient Agar (NA) was sterilized in separated flasks and cooled to 45–50 °C. After injecting the microorganism cultures to sterile plate (1000 µL), the media were distributed and mixed homogenously. When the inoculated media solidified, monofilament sutures were placed onto the agar surfaces. Plates inoculated with S. aureus were incubated at 37 °C for 24 h. After the incubation period ended, antimicrobial activities were monitored and the studies were performed in triplicate.
Phosphate buffer solution (PBS)
There is no general recommendation for selecting simulated body environment for biodegradation; however, PBS is chosen for this study as it is one of the most commonly used media with respect to degradation experiments due to its widespread availability, simplicity and physiological pH (Suchy et al. 2021). The PBS was prepared using Girard et al. (2020) as modified by (Gbenebor 2017).
in vitro degradation studies of partially absorbable suture
The in vitro degradation properties of pure LDPE and its composite were evaluated by immersing a pre-weighed sample in 50 mL beaker containing PBS of pH 7.4 at 37 °C. The samples were left for 3 weeks and 8 weeks. Weight loss (WL) was computed using Eq. 1 (Pollini et al. 2009):
| 1 |
where Wo is initial weight and W is weight after immersion.
Statistical analysis
The One way ANOVA statistical analysis was performed using Minitab 16. The test was done in triplicate. All the values were expressed as mean ± standard deviation and the difference with values (p < 0.05) were considered statistically significant.
Results and discussion
Strength characteristics of knotted sutures
The tensile strength of the sutures is presented in Fig. 1. The tensile strength of knotted neat low-density polyethylene (LDPE) is 4.84 MPa. The knot strength of the suture increases steadily above that of neat LDPE to 7.3 MPa when being reinforced with LDPE/PLA/CHS (60/39.5/0.5), and then dropped beyond this weight percentage. The tensile strengths of knotted 60/39.5/0.5 blend show superior strengths of 40% compared to knotted neat LDPE suture (4.8 MPa). The greater tensile strength exhibited in this study could be attributed to the degree of crystallinity of partially soluble chitosan in the composite. The higher the crystallinity of a polymer, the greater the tensile strength of that material as opposed to amorphous structure, as the crystalline phase enhances the intermolecular bonding, which ultimately, restricts the degree of freedom for the molecular chains. This restriction of movement results in higher strength, thereby forming an oriented chain. However, the low knot strength of LDPE/CHS/PLA (50/49.5/0.5) composite is probably due to the non-homogeneity of chitosan particle distribution in the polymer composite matrix during melting, which may be because of the generation of voids in the suture as can be seen in the micrographs (Fig. 8). For LDPE/PLA suture composite, there exists a linear increment in the tensile strength (80/20 w/w), where the optimal tensile strength is observed. Thus, to partially replace LDPE in the suture matrix LDPE/PLA (80/20) and LDPE/PLA/CHS (60/29.5/0.5) is recommended, since the suture possesses the best knot performance at these weight fractions. In one-way ANOVA statistical analysis, the addition of PLA does not affect the knot strength of the composite, as it is statistically non-significant (p = 0.9). In addition, the error bar analysis using 95% confidence interval, showed that there is a minimum variability from the mean value of the knot strength of the suture composite at different weight fractions of PLA and CHS.
Fig. 1.
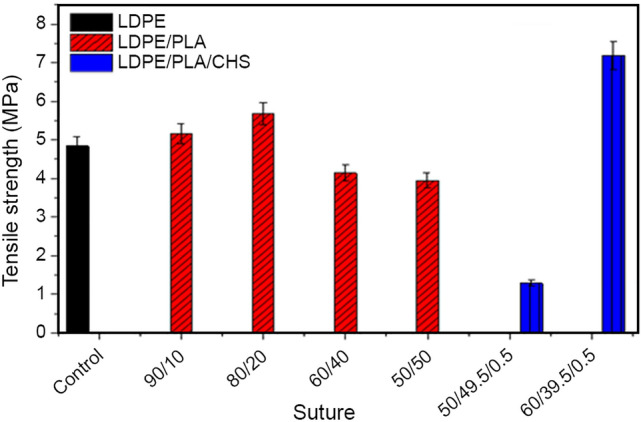
Tensile strength of knotted LDPE, LDPE/PLA and LDPE/PLA/CHS sutures
Fig. 8.
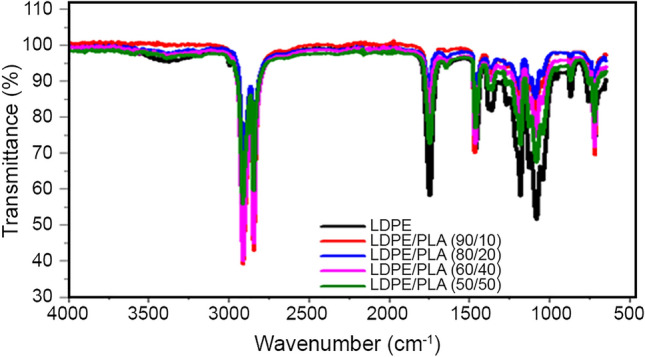
FTIR spectrum of low-density polyethylene (LDPE/PLA/CHS) suture
Biodegradation
The evaluation of the rate of biodegradation as measured by weight loss and loss of mechanical strength is shown in Figs. 2 and 3. The rate of degradation in neat LDPE suture (Fig. 2) showed a minimal loss in weight (1.8%) upon reinforcement with 90/10, 80/20 and 60/39.5/0.5 polymer blends, while the rate of degradations are improved by 6.7%, 5% and 8.6%, respectively. The highest level of degradation was observed in 60/39.5/0.5. This is because chitosan exhibits hydrophilic characteristics. This hydrophilicity is related to the degree of crystallinity of chitosan, which enables the suture composite to absorb water. The water molecule is absorbed in the amorphous domain of chitosan polymer, thereby diffusing into the composite and causing degradation. In other words, chitosan has poor water resistance, which has contributed to its hydrophilic nature (Xu et al. 2005; Abdurrahim 2019). The water absorption of CHS is due to a strong interaction of water molecules with its hydroxyl and amino groups (Cervera et al. 2004; Mali et al. 2005). Polymer undergoes either physical or chemical degradation (enzymatic and hydrolytic). Physical route involves bulk erosion, degradation, erosion, disintegration and dissolution. Physical degradation of polymer entails degradation (polymer chain scission cleaved into oligomers and monomers) and erosion (loss of material due to oligomers and monomers leaving the polymer). However, PLA also undergoes degradation on the surface of the polymer, and inside the polymer bulk, creating lactic acid (La) monomers and oligomers (Gopferich 1996). The diffusion of water into bulk polymer (PLA) degrades the polymer microstructure through the formation of internal cavities (Gopferichn 1996). This makes the cleaved monomers to diffuse out of the polymer with time (Silva et al. 2018). The combination of these factors promotes degradation of the suture in PBS, thereby leading to the loss in mass of the suture after immersion. The existence of debris in this study, after biodegradation implies that there has been no dissolution of degraded product in PBS solution. The rate of degradation by weight loss was also buttressed by reduction in mechanical strength of the monofilament suture. After immersion for 21 days there is a gain in tensile strength, as opposed to the loss of strength during in vitro degradation of suture. Since the suture undergoes bulk erosion, it absorbs fluid first, which is accompanied by increase in tensile strength, before the collapse of molecular structure leading to loss in strength. Nevertheless, 21 days of immersion was not sufficient to degrade the polymer. The tensile strength of neat LDPE (Fig. 3) does not show loss in mechanical strength and rather it gained strength for the period of 8 weeks immersion in PBS. Comparing the mechanical strength before and after immersion, it can be seen that the maximum loss in mechanical strength was exhibited by 60/39.5/0.5, which also revealed maximum weight loss. Thus, suggesting that the rate of degradation of LDPE suture can be improved by addition of PLA and chitosan. The one-way ANOVA statistical analysis showed that the rate of degradation evident from weight loss is dependent on the addition of PLA and CHS to LDPE composite. Thus, the rate of loss was statistically significant with increase in number of immersion days (p = 0.04). The statistical analysis was further confirmed by the error bar, which showed variance from the mean degradation, proving that the degradation was actually enhanced by reinforcing LDPE with PLA and CHS.
Fig. 2.

Weight-loss of neat low-density polyethylene (LDPE), LDPE/PLA and LDPE/PLA/CHS composite suture, after immersion for 8 weeks in PBS
Fig. 3.

Tensile strength of neat LDPE, LDPE/PLA and LDPE/PLA/CHS composite suture, before and after immersion in PBS
Agar diffusion method
Antimicrobial property of LDPE/PLA (100/0, 90/10, 80/20, 60/40, 50/50) and LPPE/PLA/CHS (60/39.5/0.5, 50/49.5/0.5) in terms of zone inhibition against S. aureus are shown in Fig. 4. Standard strain of S. aureus (ATCC 25,923), was used for this study. Polymer composite of 100/0, 90/10, 80/20, 60/40, 50/50 did not show antimicrobial activities against the tested strain of bacterial, after 24 h of incubation. Monofilament of 60/39.5/0.5 and 50/49.5/0.5 showed zone of inhibition of 12.7 mm (p = 0.001). The absence of inhibition zone in the rest threads showed that chitosan has inherent antimicrobial activity against staphyloccus aureus. According to standard Antibacterial test, SNV 195920-1992 (Phas et al. 2015), when the zone of inhibition is greater than 1 mm, a material is considered to have a strong antimicrobial property. S. aureus is known to be multi-resistant bacterial that is responsible for nosocomial infection (Wisplinghoff et al. 2004) and according to the National Noscomial Infections Survilance System (NNIS), the most frequently isolated pathogens from SSI are S. aureus (20%) and coagulase-negative staphylococci (Paocharoen et al. 2009). Thus, the obtained result showed that chitosan incoporated into suture could serve as a preventive measure, to protect surgical site from microbial biofilms formation. SSI occurs when pathogenic organisms grow in the surgical wounds, which slow down the wound healing and ultimately results in wound edge separation (Mingmalairak et al. 2009). Chitosan could support antibacterial activity while available in the media as a sol fraction for 12 h within the duration of this study. This is consistent with literature. Malinowaska-panczyk et al. (2015), stated that the antibacterial property of chitosan increases with contact time. Similarly, Li et al. (2012) and (2008) corroborated the antibacterial effect of chitosan solution to last for 12–24 h and 6 h, respectively.
Fig. 4.
Antimicrobial assay of monofilament using Agar diffusion method (A) neat, (J) 60/39.5/0.5, (L) 50/49.5/0.5
Functional group analysis of the suture
FTIR is a well-known method to investigate the intermolecular interaction and phase behavior between polymers. The FTIR of virgin LDPE (Fig. 5), showed a standard spectral of 3433, 2909, 2840, 1738, 1452, 1174, 1062, 706 cm−1 assigned to OH group, C–H stretch, C=O vibration of ester, CH3 bending, alkyl ester and CH2 stretch, respectively (Shivasharana et al. 2019; Pamela et al. 2019). Furthermore, the chitosan spectrum (Fig. 6) revealed peaks 3460 representing the OH stretching. The peak at 2931 is attributed to –CH and CH2 stretching of chitosan (Kandike et al. 2018); 1662 represents the N–H bending vibration of primary amine, peak 1558 representing secondary amine band II (Hamdan et al. 2020). CHS spectral used in this work is similar to the one reported by Abdou et al. (2008) and Rashmi et al. (2016). The spectral figures of LDPE/PLA and PE/PLA/CHS (Figs. 7 and 8) did not show additional functional group. However, the intensity at OH group shifted to a lower intensity when CHS and PLA were added to LDPE. Similar modification was observed by Khruengasi et al. (2021), when low density polyethylene film was biodegraded using fungal species from Thailand. The change in OH region was attributed to the presence of carboxylic acid produced from the hydrolysis reaction. The decrease in the peak intensity at 2840 cm−1 stretching vibration of C=O in the ester bond indicate the cleavage of ester bond. A shift in peak was also observed around 1461 and 1122 cm−1 corresponding to C–H bending and C–O stretching. Similar observation was also made by Sunilkumar et al. (2012). Wanchoo et al. (2003), affirmed that interactions among chemical groups in dissimilar polymers should, theoretically cause a shift in peak position. From the shift in peaks, it is obvious, that there are interactions among LDPE/PLA/CHS suture. Thus, the shift in peak is attributed to dissimilar chemical and geometrical linear structures of both LDPE and CHS (Sunilkumar et al. 2012) and chitosan. The FTIR data confirm changes in bond scission and chemical transformation. These changes indicate that the LDPE may be degraded by the presence of PLA and CHS. Furthermore, blending is used to enhance material property for specific application. However, in the case of two immiscible polymers, there is required a plasticizer. The spectral figures of LDPE and PLA blend (Fig. 7) showed characteristic spectral of homopolymers (LDPE and PLA) without significant changes, which indicate that there is little interaction between them. The peak shift in LDPE/PLA/CHS spectral (Fig. 8) shows that chitosan is acting as a plasticizing agent, which results in better miscibility of LDPE and PLA. This is also evident from the morphological analysis.
Fig. 5.

FTIR spectrum of low-density polyethylene (LDPE) suture
Fig. 6.

FTIR spectrum of chitosan
Fig. 7.

FTIR spectrum of low-density polyethylene (LDPE/PLA) suture
Morphological evaluation of partially absorbable composite suture after immersion in PBS solution
The micrograph of the suture after immersion in PBS (Fig. 9) reveals its structural change. Synthetic polymers undergo degradation by hydrolysis (Antoniac et al. 2021). PLA undergoes degradation by hydrolysis of ester bond backbone (Grizzi et al. 1995). The hydrolytic degradation is further catalyzed by the newly formed carboxylic groups at the terminal end of the cleaved PLA chains (Pitt et al. 1981). The hydrolysis of the partially absorbable suture, leads to the formation of flakes, cavities and cracks observed on the surface (Fig. 9). This suggests the gradual weakening of the bond holding the suture together as PLA and CHS are added to the LDPE matrix; cracks and cavities present in the fiber, increased, thereby enhancing the degradability of LDPE suture. Similar irregularities on the surface of the suture were also observed by Viscoet al. (2019) when they extruded a polymer blend of PLA/PCL. They suggested that surface irregularities could be attributed to ductile character of the polymer blend. In the same manner, Khruengsai et al. (2021) reported the presence different structural changes, such as cracks, pits, grooves, and roughening of surface, as LDPE was biodegraded by fungus species. Similar results have also been obtained in this result. The micrographs further agree with the FTIR result, confirming the degradation of LDPE/PLA/CHS suture. It is observed from the morphological features, that there is no clear phase separation within the polymer, proving that the composite is partially miscible. The improvement in the tensile strength of neat LDPE suture would suggest that the polymer blend is miscible.
Fig. 9.
Micrographs of suture after immersion in PBS for a*(neat LDPE), b*90/10, c*80/20, d*60/40, e*50/50, f*60/39.5/0.5, g*50/49.5/0.5)
Conclusions
Partially absorbable monofilament suture for orthopedic repair was extruded. The functional groups and biodegradation of low-density polyethylene suture using chitosan and PLA, which were evident by phase shift in the spectra was confirmed by FTIR. The knot strength of neat low-density polyethylene (LDPE) of 4.84 MPa improved to 7.3 MPa when reinforced with LDPE/PLA/CHS (60/39.5/0.5). The neat LDPE suture showed a weight loss of 1.8% but when reinforced as in 60/39.5/0.5 the weight loss increased to 8.6%, which shows an increase in the rate of degradation of LDPE. Antimicrobial assay of the monofilament of 60/39.5/0.5 and 50/49.5/0.5 sutures showed zone of inhibition of 12.7 mm. Morphological examination showed hydrolytic degradation of the suture with cracks and cavities, which is requred for improved degradaton. Thus, the developed sutures with good knot performance, good antimicrobial property and enhanced degradation have potential application in orthopedic repair.
Author contributions
All authors contributed to the study conception and design. Material preparation, data collection, analysis were performed by CCO, OPG. First draft of the manuscript was written by CCO. Second and third drafts were read and revised by MO, OIS and SOA. All authors have read and approved the final manuscript.
Funding
The authors declare that no fund, grants, or other support was received during the preparation of this manuscript.
Data availability
The data that supports the findings in this study, are available from the corresponding author, upon reasonable request.
Declarations
Conflict of interest
The authors declare that there is no competing interest during the preparation of this manuscript.
Ethical approval
This research, did not involve human or animal model, performed by any of the authors.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Abdelaal OA, Darwish SM. Review of rapid prototyping techniques for tissue engineering scaffolds fabrication: characterization and development of biosystems and biomat. Berlin: Springer; 2013. pp. 33–54. [Google Scholar]
- Abdou ES, Nagy KSA, Elsabee MZ. Extraction and characterization of chitin and chitosan from local sources. Bioresour Technol. 2008;199:1359–1367. doi: 10.1016/j.biortech.2007.01.051. [DOI] [PubMed] [Google Scholar]
- Abdurrahim KI. Water sorption, antimicrobial activity and thermal mechanical properties of chitosan/clay/glycerol nanocomposite film. Heliyon. 2019;5:e02342. doi: 10.1016/j.heliyon.2019.e02342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aiello AE, Clayton EMR, Todd M, Dowd JB. The impact of bisphenol A and triclosan on immune parameters in the U.S. population, NHANES 2003–2006. Environ Health Prospect. 2011;119(3):390–396. doi: 10.1289/ehp.1002883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alnaimi SM (2004) The optical and electrical properties of low-density polyethylene compact discs in CuSO4 solution. In: The 3rd Magneto Electronic International Symposium (MAGEL-3-La Rochelle-2004): La Rochelle, France, Jun 15–19
- Anderson DJ, Podgorny K, Berrı’os-Torres SI, Bratzler DW, Dellinger EP, Greene L, Nyquist A, Saiman L, Yokoe DS, Maragakis LL, Kaye KS. Strategies to prevent surgical site infections in acute care hospitals: 2014 update. Infect Control Hosp Epidemiol. 2014;35(6):605–627. doi: 10.1086/676022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antoniac I, Antoniac A, Gheorghita D. In vitro study on biodegradation of absorbable suture Materials used for surgical applications. Mater Plast. 2021;58(2):130–139. doi: 10.37358/matplast1964. [DOI] [Google Scholar]
- Cervera MF, Karjalainen M, Airaksinen S, Rantanen J, Krogars K, Heinamaki J, Colarte AI, Yliruusi J. Physical stability and moisture sorption of aqueous chitosan–amylose starch films plasticized with polyols. Eur J Pharm Biopharm. 2004;58:69–76. doi: 10.1016/j.ejpb.2004.03.015. [DOI] [PubMed] [Google Scholar]
- Dayyab FM, Iliyasu G, Aminu A, Habib ZG, Tiamiyu AB, Tambuwal SH, Borodo MM, Habib AG. A prospective study of hospital acquired infections among adults in tertiary hospital in North-Western Nigeria. Trans R Soc Trop Med Hyg. 2018;112(1):36–42. doi: 10.1093/trstmh/try020. [DOI] [PubMed] [Google Scholar]
- Gbenebor OP (2017) Potentials of polylactide-Chitin Composite as a Femoral Scaffold in Orthopedics. Ph.D thesis, university of lagos, Nigeria
- Girard E, Changnon G, Moreau-Gaudry A, Letoublon C, Favier D, Dejean S, Trilling B, Nottelet B. Evaluation of biodegradable PLA-PEG-PLA nterna biliary stent for liver transplantation: Invitro degradation and mechanical properties. J Biomed Mat Res Part B Appl Biomat. 2020 doi: 10.1002/jmb.b.34709.Hal-0296. [DOI] [PubMed] [Google Scholar]
- Gopferich A. Mechanism of polymer degradation and erosion of. Biomaterials. 1996;17:103–114. doi: 10.1016/0142-9612(96)85755-3. [DOI] [PubMed] [Google Scholar]
- Grizzi I, Garreau H, Li S, Vert M. Hydrolytic degradation of devices based on poly(DL-lactic acid). Size- dependence. Biomaterials. 1995;16:305–311. doi: 10.1016/0142-9612(95)93258-F. [DOI] [PubMed] [Google Scholar]
- Gupta B, Saxana S. Chitosan-polyethylene glycol coated cotton membranes for wound dressings. Indian J Fib Text Res. 2011;36:272–280. [Google Scholar]
- Hamdan IAA, Alhnon FJ, Hasan AA. Extraction characterization and bioactivity of chitosan from farms shrips of Barsa Province by chemical method. 1st Int Conf Pur Sci (ISCPS) Conf Ser J Phy. 2020 doi: 10.1088/1742-6596/1/012023. [DOI] [Google Scholar]
- Hang J, Ej W, Hwang UK, Kim IC, Yim JH, Lee JS. Triclosan (TCS) and Triclocarban (TCC) cause lifespan reduction and reproductive impairment through oxidative stress-mediated expression of the denfensome in the monogonont rotifer (Brachionus Koreanus) Comp Biochem Physio Toxi Pharmacol. 2016 doi: 10.1016/j.cbpc.2016.04.002. [DOI] [PubMed] [Google Scholar]
- Hockenberger AS, Karaca E (2004) Effect of suture structure on knot performance of polyamide suture. Ind J Fib Ten Res 271–277
- Ige OK, Adesanmi AA, Asuzu MC. Hospital acquired infections in a Nigeria tertiary health facility: an audit of surveillance reports. Nig Med J Nig Med Assoc. 2011;52(4):239. doi: 10.4103/0300-1652.93796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandile NG, Zaky HT, Mohammed MI, Nasr AS, Ali YG. Extraction and characterization of chitosan from shrimp shells. Open J Org Polym Mat. 2018;8:33–42. doi: 10.4236/ojopm.2018.83003. [DOI] [Google Scholar]
- Kanimozhi T, Pachiyappan KM, Kalaiselvi IE, Mahalakshmi VI, Gokarneshan N. GSC Adv Res and Rev. 2021;09(02):072–074. [Google Scholar]
- Khruengsai S, Sripahco T, Pripdeeveh T. Low-density polyethylene film biodegradation potential by gungal species from Thailand. J Fung. 2021;7:594. doi: 10.3390/jof7080594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JC, Lee YK, Lim BS, Rhee SH, Yang HC. Comparison of tensile and Knot security properties of surgical sutures. J Mater Sci Mater Med. 2007;18(12):2363–2369. doi: 10.1007/s10856-007-3114-6. [DOI] [PubMed] [Google Scholar]
- Li B, Wang X, Chen RX, Huangfu WG, Xie GL. Antibacterial activity of chitosan solution against Xanthomonas Pathogenic bacteria isolated from Euphorbia pulcherrima. Carbohydr Polym. 2008;72:287–292. doi: 10.1016/j.carbpol.2007.08.012. [DOI] [Google Scholar]
- Li B, Liu B, Shan C, Ibrahim M, Lou Y, Wang Y, Xie G, Li H, Sun G. Antibacterial activity of two chitosan solutions and their effect on rice bacterial leaf blight and leaf streak. Pest Manag Sci. 2012;69(2):312–320. doi: 10.1002/ps.3399. [DOI] [PubMed] [Google Scholar]
- Mali S, Sakanaka LS, Yamashita F, Grossmann MVE. Water sorption and mechanical properties of cassava starch films and their relation to plasticizing effect. Carbohydr Polym. 2005;60:283–289. doi: 10.1016/j.carbpol.2005.01.003. [DOI] [Google Scholar]
- Malinowska-Panczyk E, Staroszczyk H, Gottfried K, Kolodziejska I, Wojtasz-Pajak A. Antimicrobial properties of chitosan solutions, chitosan films and gelatin-chitosan film. Polimery-Warsaw. 2015;61:735–741. doi: 10.14314/polimery.2015.735. [DOI] [Google Scholar]
- Ming X, Nichols M, Rothenburger S. In vivo antibacterial efficacy of Monocryl plus antibacterial suture (Polgelcaprone 25 with triclosan) Surg Infect. 2007;8:209–214. doi: 10.1089/sur.2006.004. [DOI] [PubMed] [Google Scholar]
- Ming X, Rothenburger S, Nichols MM. In vivo and in vitro antibacterial efficacy of PDS plus (polydioxanone with triclosan) suture. Surg Infect. 2008;9:451–457. doi: 10.1089/sur.2007.061. [DOI] [PubMed] [Google Scholar]
- Mingmalairak C, Ungbhakorn P, Paocharoen V. Eficacy of antimicrobial coating suture coated polyglactin 910 with tricosan(vicryl plus) compareed with polyglactin 910(vicryl) in reduced surgical site infection of appendicities, double blind randomoze control trial, preliminary safety report. J Med Assoc Thai. 2009;92(6):770–775. [PubMed] [Google Scholar]
- Muller DA, Snedeker JG, Meyer DC. Two-month longitudinal study of mechanical properties of absorbable suture used in orthopedic surgery. J Orthop Surg Res. 2016;11(1):111. doi: 10.1186/s13018-016-0451-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pamela RPS, Thaís-Larissa-do AM, Orestes F, Fábio RP, Larissa SM. Protective low-density polyethylene residue from prepreg for the development of new nanocomposites with montmorillonite recycling and characterization. Recycl MDP. 2019;4(4):45. doi: 10.3390/recycling4040045. [DOI] [Google Scholar]
- Paocharoen V, Mingmalairak C, Pisarnthanarak A. Compaarison of surgical wounds infection after preoperative skin preparation with 4% chlorhexidine and povidone iodine:a prospectve randomized trial. Med Assoc Thai. 2009;92(7):898–902. [PubMed] [Google Scholar]
- Phas SP, Anbarasan S, Mukherjee A. Chandrasekaran N biobased nanocolloid coating on silk fibres for prevention of post-surgical wound infections. Int J Naomed. 2015;10(supply1):159–170. doi: 10.2147/IJN.S82211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillai CKS, Sharma CP. Review paper: absorbable polymeric surgical sutures: chemistry, production, properties, biodegradability, and performance. J Biomat Appl. 2010;25:291–366. doi: 10.1177/0885328210384890. [DOI] [PubMed] [Google Scholar]
- Pitt CG, Gratzi MM, Jimmel GL, Surles J, Schindler A. Aliphatic polyesters II. The degration of poly(Dl-Lactide, poly(epsilon-carprolactone) and their copolymers in vivo. Biomaterials. 1981;2:215–220. doi: 10.1016/0142-9612(81)90060-0. [DOI] [PubMed] [Google Scholar]
- Pollini M, Russo M, Licciulli A, Sannino A, Maffezzoli A. Characterizaton of antibacterial silver coated yarns. J Mat Sci Med. 2009;20(11):2361. doi: 10.1007/s10856-009-3796-z. [DOI] [PubMed] [Google Scholar]
- Rashmi SH, Biradar MB, Maladkar K, Kittur AA. Extraction of chitin from prawn shell and preparation of Chitosan. Res J Chem Environ Sci. 2016;4(4s):70–73. [Google Scholar]
- Serrano C, Garcia-Fernadez L, Fernadezze-Blazgue JP, Barbeck M, Ghanaati S, Unger R, Kirkpatrick J, Artz E, Funk L, Turon P, Ampo A. Nanostructured medical sutures with antibacterial properties. Biomat. 2015;52:291–300. doi: 10.1016/j.biomaterials.2015.02.039. [DOI] [PubMed] [Google Scholar]
- Shanmugasundaram OL. Chitosan coated cotton yarn and its effect on antimicrobial activity. Tech Manag. 2006;5:1–6. [Google Scholar]
- Shivasharana CT, Sheetal SK. Physical and mechanical characterization of low-density polyethylene and high-density polyethylene. J Adv Sci Res. 2019;3:30–34. [Google Scholar]
- Silva D, Kaduri M, Poley M, Adir O, Krinsky N, Shainsky-Roitman J. Schroeder. Biocompatibility, biodegradation and excretion of polylactic acid(PLA) in medical implants and theranostic systems. Chem Eng J. 2018;340:9–14. doi: 10.1016/j.cej.2018.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su W, Qi W, Li X, Zhao S, Jilang J, Zhao J. Effect of suture absorbability on rotator cuff healing in a rabbit rotator cuff repair model. Am J Spor Med. 2018;46:3243–2754. doi: 10.1177/0363546518787181. [DOI] [PubMed] [Google Scholar]
- Suchy T, Bartos M, Sedlacek R, Supova M, Zaloudkova M, Martynkova GS, Foltan R. Various simulated body fluids leading to significant differences in collagen tissue engineering scaffolds. Materials. 2021;14:4388. doi: 10.3390/ma14164388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunilkumar M, Francis T, Thachil ET, Sujith A. Low density polyethylene-Chitosan composite: a study on biodegradation. Chem Eng J. 2012;204–206:114–124. doi: 10.1016/j.cej.2012.07.058. [DOI] [Google Scholar]
- Tucci G, Romanini E, Zanoli G, Pavan L, Fantoni M, Venditti M. Prevention of Surgical site infections in orthopedic surgery: a synthesis of current recommendations. Eur Rev Med Pharma Sci. 2019;23(2):224–239. doi: 10.26355/eurrev_201904_17497. [DOI] [PubMed] [Google Scholar]
- Visco A, Scolaro C, Giamporcaro A, DeCaro S, Tranquillo E, Catauro M. Thread made with blended biopolymers: mechanical, physical and biological features. Polymers. 2019;11(5):901. doi: 10.3390/polym11050901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanchoo RK, Sharma PK. Viscometric study on the compatibility of some water solublepolymer-polymer mixture. Eur Polym J. 2003;39:1481–1490. doi: 10.1016/S0014-3057(02)00386-5. [DOI] [Google Scholar]
- Wang X, Chen X, Feng X, Chang F, Chen M, Xia Y, Cheng L. Triclosan causes spontaneous abortion accompanied by decline of estrogen sulfotransferase activity in humans and Mice. Sci Rep. 2015;5:18252. doi: 10.1038/srep18252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisplinghoff H, Bischoff T, Tallent SM, Seifert H, Wenzel RP, Edmond MB. Noscomia bloodstream infections in US hospitals:analysis of 24170 cases from a prospective nationwide survilance study. Clin Infect Dis. 2004;39(3):309–317. doi: 10.1086/421946. [DOI] [PubMed] [Google Scholar]
- Xu Y, Ren X, Hanna MA. Chitosan/clay nanocomposites film preparation and characterization. J Appl Polym Sci. 2005;99:1684–1691. doi: 10.1002/app.22664. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that supports the findings in this study, are available from the corresponding author, upon reasonable request.




