Abstract
4H-pyrans have been prepared through a mechanochemical multicomponent reaction (MCR) of different aldehydes, malononitrile, and various 1,3-dicarbonyl compounds, catalyzed by an amine-functionalized metal-organic framework (MOF) Cu2(NH2-BDC)2(DABCO) as a heterogeneous catalyst with good to excellent yields.
Keywords: Solvent-free, Ball milling, Mechanochemical, Metal-organic framework, 4H-pyrans, Heterogeneous catalyst
Graphical abstract
1. Introduction
Pyrans are non-aromatic heterocyclic six-membered rings with a molecular formula of RC5H6O, including five carbon atoms, one oxygen atom, and two double bonds. The two isomers of the pyrans differ in the double bond position. In 2H-pyran (1), saturated sp3 carbon is positioned at position 2, but in 4H-Pyran (2), it is at position 4 (Scheme 1). In 1962, 4H-pyrans were created for the first time through the thermal decomposition of 2-acetoxy-3,4-dihydro-2H-pyran, and their properties and applications were investigated [1,2].
Scheme 1.
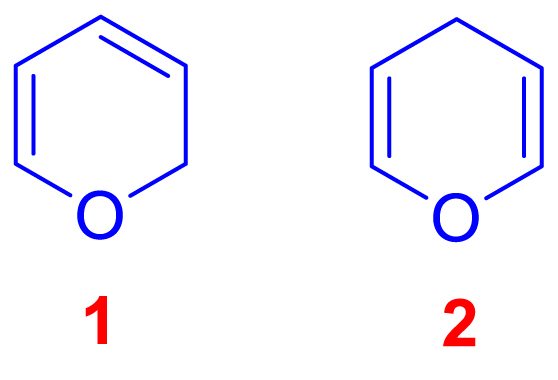
2H-Pyran and 4H-Pyran.
Heterocyclic compounds, including tetrahydrobenzo[b]pyrans and their derivatives, have been studied for their potential medicinal and pharmacological applications (see, for example, Scheme 2) [3]. They have attracted special attention, which can be attributed to their antioxidant [4], anticancer [5], antitumor [6] properties, etc. Tetrahydrobenzo[b]pyran-based compounds possess the potential for physical enhancers in treating neurological diseases such as Alzheimer's and Parkinson's diseases, Dawson's syndrome, amyotrophic lateral sclerosis, Huntington, Schizophrenia, AIDS-associated dementia, and myoclonus [7]. Also, these compounds are widely used in cosmetics and as pigments [8].
Scheme 2.
Pyran-based natural and synthetic drugs in clinical use [3].
Various methods have been reported to prepare tetrahydrobenzo[b]pyrans and their derivatives. Generally, the basis of their synthesis is the three-component condensation of variable 1,3-dicarbonyl derivatives, CH-active compounds, and aldehydes. Various heterogeneous and homogeneous catalysts have been reported so far for this condensation reaction, including magnetite l-proline [9], DABCO-CuCl complex [10], trisodium citrate [11], cesium carbonate [12], ninhydrin [13], ionic liquids based on choline hydroxide [Ch]+[OH]- [14] or DABCO [H2-DABCO][H2PO4]2} [15], starch solution [16], sulfonic acid functionalized silica [17], Fe(ClO4)3/SiO2 [18], ClO4−/Al-MCM-41 [19], poly(4-vinyl pyridine) (P4VP) [20], and silica-supported dodeca-tungstophosphoric acid DTP/SiO2 [21]. These methods may have disadvantages and problems, including using complex, toxic and destructive catalysts, tedious and challenging purification processes, low efficiency, and slow reaction rates.
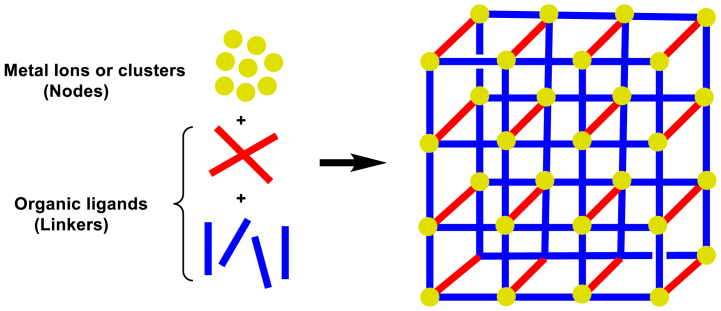
Crystalline compounds are known as metal-organic frameworks (MOFs), composed of inorganic moieties (metal ions/clusters) and organic linkers, creating a porous structure of different dimensions (Scheme 3) [[22], a), b)]. They have a low density, a large surface area, and a high proportion of catalytically active transition metals. Most often, metals in the lattice of MOFs act as Lewis acids. The solvent type, solvent concentration, opposite ion nature, metal-to-ligand ratio, metal quadratic geometry, pH, temperature, and the nature of guest molecules determine the MOF structure [[22], [23], [24], [25], [26], [27], [28]]. MOFs have many applications in drug delivery, sensors and luminescence, gas separation and storage, and catalytic reactions [[29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40]].
Scheme 3.
General scheme of MOF synthesis.
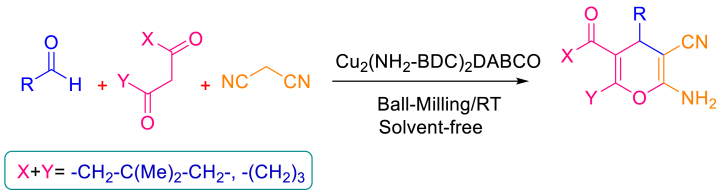
Mechanochemical reactions are considered nowadays as green methods of chemical synthesis. Among the others, ball-milling has been a widely accepted tool for solvent-free mechanochemical reactions in recent years. The ball milling method has numerous benefits over other procedures, including quick reaction times, solvent-free settings, high yields, and high atom efficacy [[41], a), b), c), d)]. Based on successful organic synthesis through ball milling in previous studies [[42], a), b), c), d)], Herein, we presented a three-component, solvent-free synthesis of 4H-pyrans derivatives using ball-milling with nanoporous Cu2(NH2-BDC)2(DABCO) as a heterogeneous catalyst with good to excellent yields (Scheme 4). These kinds of reactions can be considered as a base-catalyzed as well as an acid-catalyzed reactions. We have chosen Cu2(NH2-BDC)2(DABCO), to have a bifunctional catalyst, namely both a Lewis acid Cu2+, as well as Lewis base NH2 in a unique catalyst. The better activity of this catalyst has been shown in comparison to other catalysts.
Scheme 4.
The heterogeneous catalysis of Cu2(NH2-BDC)2(DABCO) in a three-component, solvent-free synthesis of 4H-pyran derivatives.
2. Experimental section
2.1. Materials and reagents
All chemicals, such as 2-aminoterephthalic acid (NH2-BDC), 1,4-diazabicyclo[2.2.2]octane (DABCO), metal salt Cu(OAc)2.H2O, aldehydes, and 1,3-dicarbonyl components were obtained through Sigma-Aldrich and Merck companies in reagent grade for direct use without additional purification. As eluents, EtOAc and n-hexane (1:1 or 1:2) were used for thin-layer chromatography (TLC).
2.2. Instrumental
Melting points have been measured using open capillaries (sealed at one end) using an Electrothermal 9100 instrument. FT-IR spectra were collected using a Shimadzu 8400S spectrometer. Spectra of 1HNMR were obtained using a Bruker 500 MHz spectrometer. DMSO‑d6 was used as solvent at ambient temperature. Using an X'pert MPD, XRD observations were conducted. Philips diffractometer with Cu radiation source (λ = 1.54050A) operating at 40 mA and40 KV. A MM400 Retsch ball milling device with two 10 mL jars and 7 mm stainless steel balls was utilized at a frequency of 28 Hz.
2.3. Synthesis of Cu2(NH2-BDC)2(DABCO)
With a molar ratio of 2:2:1, 0.6 mmol of Cu(OAc)2.H2O, 0.6 mmol of NH2-BDC, and 0.3 mmol of DABCO were forcefully grinded through solvent-free ball-milling (28 Hz) at ambient temperature for 2 h. This resulted in a green product washed three times with DMF (3 × 10 mL). Methanol was used for solvent exchange three times (10 mL each) at ambient temperature. In order to eliminate methanol molecules, the obtained powdered MOF was heated at 130 °C under a vacuum for 12 h [42].
2.4. Preparation of 2-amino-3-cyano-4H-pyran derivatives (5, 6, 8, or 9) zcatalyzed by Cu2(NH2-BDC)2(DABCO)
In order to synthesize 2-amino-3-cyano-4H-pyran derivatives (5, 6, 8, or 9), a mixture of malononitrile (1 mmol), Cu2(NH2-BDC)2(DABCO) (0.04 g), 1,3-dicarbonyl components (1 mmol), and aldehyde (1 mmol) was grinded through ball-mill forcefully at 27 Hz, at ambient temperature and solvent-free for the indicated intervals. After the reaction (monitored by TLC) was complete, the catalyst was separated by filtration, washed with (hot) ethanol, and dried for reuse. The product was obtained in pure form after evaporation of EtOH or recrystallization, if necessary.
3. Results and discussion
3.1. Synthesis and characterization of Cu2(NH2-BDC)2(DABCO)
Following the previously reported procedure, a combination of DABCO, copper (II) acetate, and 2-aminoterephthalic acid in a molar ratio of 1:2:2 was grinded using a ball mill at ambient temperature without the use of any solvent to produce the metal-organic framework Cu2 (NH2-BDC)2(DABCO) [42] (Scheme 5). The synthesis was finished in less than 2 h, yielding a green powder. Various methods were used to characterize the resultant MOF, including X-ray diffraction (XRD) and Fourier transform infrared spectroscopy (FT-IR).
Scheme 5.
Ball mill synthesis of Cu2(NH2-BDC)2(DABCO).
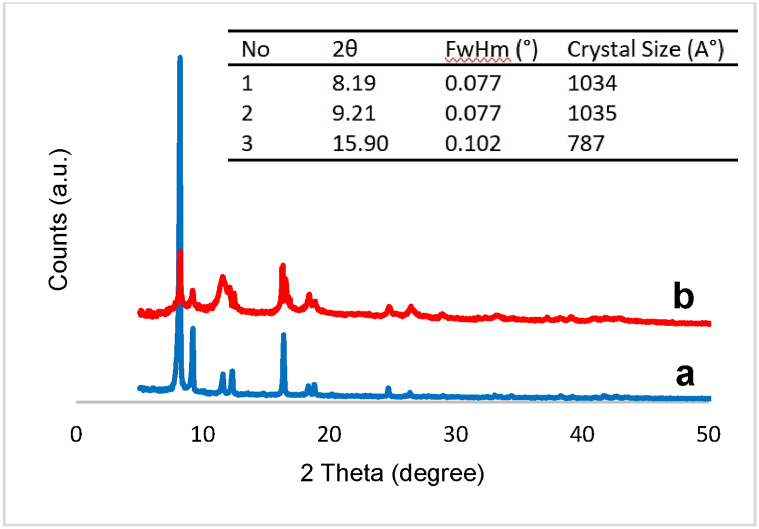
The as-synthesized XRD pattern of Cu2(NH2-BDC)2(DABCO) is shown in Fig. 1a,b. The significant peaks at 2θ values of 8, 9, 11, 12, and 16 align well with the previously reported data [42]. Comparing the patterns demonstrated the successful synthesis of a pure Cu2(NH2-BDC)2(DABCO).
Fig. 1.
XRD pattern of a) the prepared Cu2(NH2-BDC)2(DABCO) in this work, b) Simulated XRD pattern [42].
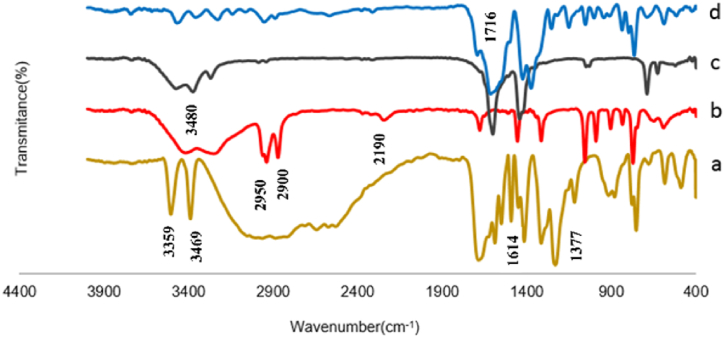
To describe the vibrational modes of the MOF, FT-IR spectroscopy was performed. Fig. 2 depicts the FT-IR spectrum of Cu2(NH2-BDC)2(DABCO), NH2-BDC, and DABCO. The asymmetric COO stretch mode (νas) is observed at 1614 cm−1, whereas CO symmetric stretching is observed at 1377 cm−1. The absorption bands at 3359 and 3469 cm−1 correspond to two NH stretching bands of –NH2 groups.
Fig. 2.
FT-IR spectra: a) 2-aminoterephthalic acid, b) DABCO, c) Cu(OAc)2, d) Cu2(NH2-BDC)2(DABCO).
The average particle size of the synthesized Cu2(NH2-BDC)2(DABCO) samples were determined to be less than 100 nm based on scanning electron microscopy (SEM) images. (Fig. 3a). Transmission electron microscopy was utilized to examine the morphology of Cu2(NH2-BDC)2(DABCO) (TEM). As depicted in Fig. 3b nano-scaled and crystalline material was generated. It is also important to note that the ball milling approach produced nanoparticles with the consistent distribution. As seen in the TEM photos, the presence of square units indicates the production of Cu2(NH2-BDC)2(DABCO) frameworks, consistent with the SEM images confirming the nano-cubic shape. Cu was detected in the particles of Cu2(NH2-BDC)2(DABCO) using energy dispersive X-ray spectroscopy (EDS) (Fig. 3c).
Fig. 3.
a) FESEM photographs, b) TEM photographs, c) EDS analysis and d) TGA spectra of Cu2(BDC)2(DABCO).
TGA was used to examine the thermal breakdown of Cu2(NH2-BDC)2(DABCO) at temperatures up to 500 °C at a heating rate of 10 °C min−1 in N2 flow (Fig. 3d). Cu2(NH2-BDC)2(DABCO) is stable up to 245 °C, and demonstrates distinct zones of weight loss commencing at approximately 110–150 °C (3.97% due to the loss of DMF and H2O. This step of the TGA study (starting at approximately 245 °C) demonstrates the decomposition of linkers (Fig. 3d). The storage capacity of molecules in the pores and channels of MOFs was evaluated using the Brunauer-Emmett-Teller (BET) technique. Based on the findings, the Cu2(NH2-BDC)2(DABCO) specific surface area was 143.35 ± 3.5 m2g-1, mesopore diameter at maximum pore volume was 19.568 nm, and total pore volume was 0.629 cm3 g−1in Cu2(NH2-BDC)2(DABCO). These results show that the catalyst includes many pores and channels which can be effective in catalytic functions. The average crystal size which has been represented in Fig. 7 based on XRD calculation, was 95.2 nm.
Fig. 7.
X-ray diffraction of powder of Cu2(NH2-BDC)2(DABCO): a) Fresh catalyst, b) Recovered catalyst after 6th runs.
3.2. Catalytic properties of Cu2(NH2-BDC)2(DABCO)
To evaluate the catalytic properties of Cu2(NH2-BDC)2(DABCO) in synthesizing 4H-pyran compounds, the three-component condensations of stoichiometric amounts of dimedone, 4-chlorobenzaldehyde, and malononitrile was studied. The findings data are reported in Table 1. The model reactions were investigated in a variety of solvents, including EtOH, THF, and CH3CN, using Cu2(NH2-BDC)2(DABCO) loading of 0.04 g at 75 °C temperature. The use of acetonitrile and tetrahydrofuran solvents resulted in increased reaction time and reduced efficiency; however, using ethanol as a solvent caused reduced reaction time and increased efficiency.
Table 1.
Optimizing the three-component reaction of 4-chlorobenzaldehyde (2a), malononitrile (3), and dimedone (4a) under various conditions.
 .
.
| Entry | Catalyst loading | Solvent | Temp. (°C) | Time (min) | Yieldb (%) |
|---|---|---|---|---|---|
| 1 | 0.04 | THF | 75 | 360 | 68 |
| 2 | 0.04 | MeCN | 75 | 300 | 85 |
| 3 | 0.04 | EtOH | 75 | 20 | 90 |
| 4 | 0.04 | EtOH | 50 | 30 | 85 |
| 5 | 0.04 | EtOH | r.t | 45 | 80 |
| 6 | – | Solvent-free | r.t | 80 | 88 |
| 7 | 0.02 | Solvent-free | r.t | 26 | 90 |
| 8 | 0.04 | Solvent-free | r.t | 5 | 96 |
| 9 | 0.04 | Solvent-free | r.t | 20 | 78 |
a Conditions of reaction: 4-chlorobenzaldehyde (2a, 1.0 mmol), malononitrile (3, 1.0 mmol), dimedone (4a, 1.0 mmol), grinding, ambient temperature.
Yield refers to isolated products.
It was then examined how temperature affected yield and reaction time. It was shown in Table 1 that low temperatures reduce the efficiency and decrease the reaction speed. The solvent and reaction temperature zoptimization indicated that a higher yield could be achieved in EtOH using a reflux condition over a shorter period. The solvent-free condition has shown that the reaction was carried out in the shortest possible time and with the highest efficiency (entry 6). The turnover frequency (TOF) of the reaction has been calculated based on the following equation:
The TON (Turnover number) indicates the maximum number of molecular reactions or reaction cycles that can occur at a catalyst's reactive centre before the activity of the catalyst begins to degrade. It has been calculated as:
Based on these equations, for the optimal conditions (Entry 8), the TOF was calculated as 15, and the TON as 5*10−2 s−1.
Generally, the ball milling method, except for the purification of the product, if necessary, no solvent is used, and hence it is considered a green synthetic method. Furthermore, the role of catalyst payload on reaction termination was studied. The best and highest yields are obtained with 0.04 g of catalyst (entry 8). The same reaction was performed with Cu2(BDC)2(DABCO) as a non-NH2 catalyst at a relatively higher time and with lower efficiency (entry 9).
To illustrate the extent of the usability of this catalyst, we expanded the zoptimized reaction conditions to various aldehydes and 1,3-cyclohexanedione compounds. Table 2 shows a summary of the findings. As shown in the table, the highest yield was obtained for producing products 5 and 6 under optimal conditions progressively. In addition, the catalyst was simply isolated by filtration and removed from the reaction mixture.
Table 2.
Preparation of 4H-pyrans derivatives by using Cu2(NH2-BDC)2(DABCO) as the catalysta
 .
.
| Entry | Aldehyde | Product | Time (min) | Yield (%)b | M. P. (°C) | M.P. (°C) [Lit.] |
|---|---|---|---|---|---|---|
| 1 | 4-Chloro benzaldehyde | 5a | 5 | 96 | 212–214 | 212-214 [45] |
| 2 | 2-Chloro benzaldehyde | 5b | 6 | 93 | 209–212 | 211-213 [46] |
| 3 | 4- Bromo benzaldehyde | 5c | 8 | 96 | 204–207 | 205-206 [43] |
| 4 | Benzaldehyde | 5d | 12 | 90 | 223–226 | 222-224 [44] |
| 5 | 2-Nitro benzaldehyde | 5e | 9 | 94 | 215–218 | 213-217 [15] |
| 6 | 3-Nitro benzaldehyde | 5f | 6 | 90 | 214–216 | 216-218 [47] |
| 7 | 4-Nitro benzaldehyde | 5g | 7 | 92 | 180–184 | 180-182 [48] |
| 8 | 4-Methyl benzaldehyde | 5h | 20 | 90 | 217–220 | 215-217 [48] |
| 9 | 3-Hydroxy benzaldehyde | 5i | 23 | 88 | 203–206 | 205-206 [49] |
| 10 | 4-Hydroxy benzaldehyde | 5j | 25 | 85 | 206–209 | 205-206 [50] |
| 11 | 2,4-Dichloro benzaldehyde | 5k | 6 | 94 | 189–192 | 192-194 [44] |
| 12 | 4-Cyano benzaldehyde | 5l | 8 | 95 | 220–224 | 221-224 [50] |
| 13 | 4-Chloro benzaldehyde | 6a | 20 | 94 | 242–244 | 241-244 [50] |
| 14 | 2-Chloro benzaldehyde | 6b | 25 | 90 | 210–212 | 210-212 [51] |
| 15 | 4- Bromo benzaldehyde | 6c | 22 | 90 | 235–237 | 236-238 [52] |
| 16 | Benzaldehyde | 6d | 40 | 86 | 237–240 | 241-242 [53] |
| 17 | 2-Nitro benzaldehyde | 6e | 25 | 85 | 198–200 | 197-199 [54] |
| 18 | 3-Nitro benzaldehyde | 6f | 30 | 92 | 234–237 | 234-236 [52] |
| 19 | 4-Nitro benzaldehyde | 6g | 20 | 88 | 223–225 | 222-224 [52] |
| 20 | 4-Methyl benzaldehyde | 6h | 40 | 90 | 224–228 | 225-226 [44] |
| 21 | 2,4-Dichloro benzaldehyde | 6i | 23 | 89 | 220–223 | 221-223 [13] |
| 22 | 4- Hydroxy benzaldehyde | 6j | 42 | 86 | 246–248 | 244-246 [50] |
Reaction conditions: aldehyde (2, 1.0 mmol), malononitrile (3, 1.0 mmol), dimedone or 1,3-cyclohexandione (4a-b, 1.0 mmol), Cu2(NH2-BDC)2(DABCO) (0.04 g), solvent-free and room temperature.
Isolated yield.
In the next step, ethyl acetoacetate was used as the changeable component for extending the scope of the protocol to the less active reagents. A summary of the data can be found in Table 3. It should be noted that in addition to ethyl acetoacetate, acetylacetone was also studied. Under optimum reaction conditions, the good and desirable yield of the intended products (5, 6, 8, and 9) was achieved within a brief reaction time.
Table 3.
Preparation of 4H-pyrans derivatives by using Cu2(NH2-BDC)2(DABCO) as the catalysta
 .
.
| Entry | Aldehyde | Product | Time (min) | Yield (%)b | M.P. (°C) | M.P. (°C) [Lit.] |
|---|---|---|---|---|---|---|
| 1 | 4-Chloro benzaldehyde | 8a | 22 | 92 | 160–163 | 162 [55] |
| 2 | 2-Chloro benzaldehyde | 8b | 25 | 90 | 188–192 | 190-191 [56] |
| 3 | 4-Bromo benzaldehyde | 8c | 25 | 92 | 179–182 | 179-180 [57] |
| 4 | Benzaldehyde | 8d | 60 | 80 | 185–188 | 189 [58] |
| 5 | 2-Nitro benzaldehyde | 8e | 42 | 90 | 178–180 | 177-178 [57] |
| 6 | 3-Nitro benzaldehyde | 8f | 40 | 87 | 186–190 | 187-188 [57] |
| 7 | 4-Nitro benzaldehyde | 8g | 40 | 89 | 175–178 | 174-176 [59] |
| 8 | 4-Methyl benzaldehyde | 8h | 18 | 90 | 173–176 | 175-176 [59] |
| 9 | 4-Chloro benzaldehyde | 9a | 30 | 65 | 149–154 | 153-155 [60] |
| 10 | 3-Nitro benzaldehyde | 9b | 42 | 63 | 160–164 | 166 [61] |
Reaction conditions: aldehyde (2, 1.0 mmol), malononitrile (3, 1.0 mmol), acetyl acetone or ethyl acetoacetate (7a-b, 1.0 mmol), Cu2(NH2-BDC)2(DABCO) (0.04 g), solvent-free and room Temperature.
Isolated yield.
Physical and spectroscopic data were compared to those of previously described compounds in the literature, allowing the clear labelling of all products. In FT-IR spectra of the products, the significant band at around 2190 cm−1 is related to the CN stretching band, and the broad band at 3200-3400 cm−1 approves the presence of the NH2 moiety (see Fig. 4d) [50]. As shown in Fig. 5 for the compound 6h, the characteristic H4 in 1H NMR spectra of these compounds was appeared as a singlet above 4.0 ppm [50].
Fig. 4.
FT-IR spectra of 2-amino-3-cyano-4-(4-methylphenyl)-5-oxo-5,6,7,8-tetrahydro-4H-1-benzopyran (6h).
Fig. 5.
1H NMR spectra of 2-amino-3-cyano-4-(4-methylphenyl)-5-oxo-5,6,7,8-tetrahydro-4H-1-benzopyran (6h).
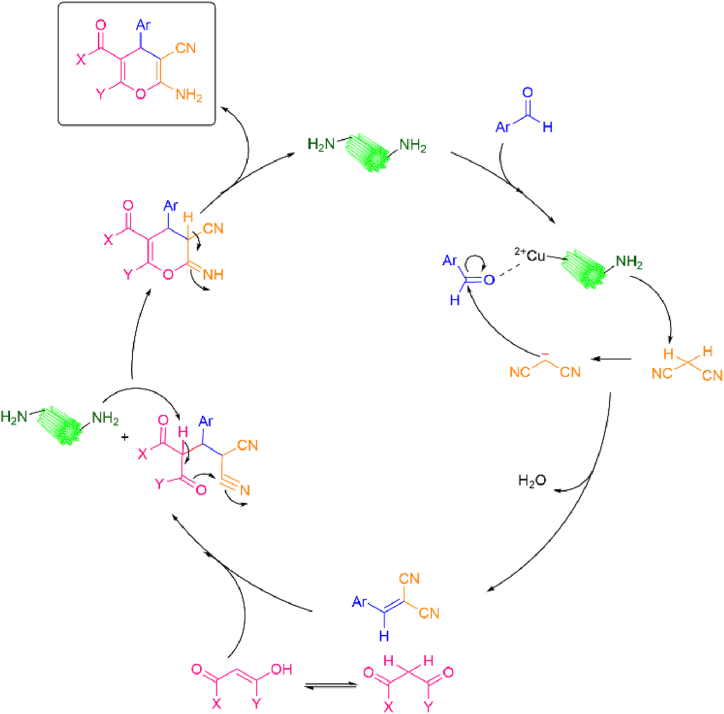
A plausible mechanism has been suggested in Scheme 6. The first step consists of forming a cyanocinnamonitrile Knoevenagel intermediate in the reaction of by Cu2+-activated aldehyde reacting with malononitrile. At this stage, the catalyst Cu2(NH2-BDC)2(DABCO) produces an anion by attacking the acid hydrogens of malononitrile. The produced anion is a nucleophile and attacks the carbonyl group of the aldehyde as an electrophile. Subsequently, by removing a water molecule, the Knoevenagel intermediate is formed. Then, 1,3-dicarbonyl components are added to the intermediate by Michael's addition of the enol form. Then the cyclization, and subsequent tautomerization of the imino-pyran intermediates is carried out on the amino-pyran.
Scheme 6.
The suggested mechanism of the 4H-pyrans derivatives synthesis using Cu2(NH2-BDC)2(DABCO).
3.3. Catalytic properties of Cu2(NH2-BDC)2(DABCO)
The recyclability and the reusability of Cu2(NH2-BDC)2(DABCO) were also studied for a minimum of six rounds in the model reaction zsynthesizing the product 5a. A simple filtration process separated the catalyst from the reaction after each run. A summary of the results can be found in Fig. 6. It has been demonstrated that Cu2(NH2-BDC)2(DABCO) is reusable without a substantial loss of activity in 4H-pyrans synthesis.
Fig. 6.
Reusability of Cu2(NH2-BDC)2(DABCO) catalyst for the MCR synthesis of 4H-pyran 5a.
XRD and FT-IR techniques were used to zanalyze the structure of the retrieved catalyst, which showed no degradation (Fig. 7, Fig. 8b). The recovered catalyst's FT-IR spectra and x-ray pattern show that its crystalline structure remains intact and stable.
Fig. 8.
FT-IR spectra of powder of Cu2(NH2-BDC)2(DABCO): a) Fresh catalyst, b) Recovered catalyst after 6th runs.
3.4. Comparison activity of Cu2(NH2-BDC)2(DABCO)
The new methodology for synthesizing various 4H-pyran derivatives was assessed by comparing several previous reports and accepted methods to demonstrate its efficacy and capabilities. Table 4 provides a comprehensive summary of the results of the present protocol, demonstrating its superiority over other approaches with respect to the yield of the product, reaction time, non-using of organic solvents as green chemistry, as well as simplified separation and reusability of the catalyst.
Table 4.
A comparison of the previously reported procedures for the synthesis of chemicals 5a, 6f, and 8a.
| Entry | Product | Catalyst | Catalyst loading | Solvent | Temp. (°C) | Time (min) | Yield (%) | Ref. |
|---|---|---|---|---|---|---|---|---|
| 1 | 5a | Cu2(NH2-BDC)2(DABCO) | 40 mg | Solvent-free | r.t | 5 | 96 | This work |
| 2 | 5a | Cu2(BDC)2(DABCO) | 40 mg | Solvent-free | r.t | 20 | 78 | This work |
| 3 | 5a | NH4Al(SO4)2.12H2O (Alum) | 20 mg | EtOH | 80 | 120 | 94 | [62] |
| 4 | 5a | PhB(OH)2 | 5 mol% | H2O/EtOH | Reflux | 30 | 84 | [63] |
| 5 | 5a | SiO2-Pr-SO3H | 30 mg | H2O/EtOH | Reflux | 15 | 97 | [17] |
| 6 | 5a | KF/Al2O3 | 250 mg | DMF | r.t | 60–180 | 48 | [60] |
| 7 | 5a | MPA-MDAZYb | 140 mg | EtOH | 80 | 70 | 90 | [64] |
| 8 | 5a | ChCl/Urea DESc | 1 mL | ChCl/Urea DESc | 80 | 60–240 | 92 | [65] |
| 9 | 5a | Amberlyst A21 | 30 mg | EtOH | r.t | 60 | 84 | [66] |
| 10 | 6f | Sodium Alginate | 10 mol% | EtOH | Reflux | 52 | 93 | [67] |
| 11 | 6f | MPA-MDAZYb | 140 mg | EtOH | 80 | 40 | 75 | [64] |
| 12 | 6f | Lipase from Porcine pancreas (PPL) | 30 mg | H2O/EtOH | 35 | 60 | 96 | [68] |
| 13 | 8a | ChCl/Urea DESc | 1 mL | ChCl/Urea DESc | 80 | 210 | 82 | [65] |
| 14 | 8a | Sodium Alginate | 10 mol% | EtOH | Reflux | 150 | 90 | [67] |
| 15 | 8a | nanocrystalline ZnO | 10 mol% | H2O/EtOH | r.t | 150 | 96 | [61] |
a Entries 1–8: Obtained outcomes for the synthesis of molecule5a, Entries 9–11: Obtained outcomes for the synthesis of molecule6f, Entries 12–14: Obtained findings for 8a compound synthesis.
12-Molybdophosphoric acid (MPA), modified dealuminated zeolite Y (MDAZY).
Deep eutectic solvent (DES).
4. Conclusion
In this paper, we developed a highly effective, environmentally friendly, and greenway for the multicomponent synthesis of 4H-pyran derivatives in the presence of Cu2(NH2-BDC)2(DABCO) as a heterogeneous and renewable catalyst. In addition to all of these, our method has many advantages, such as concise reaction time, the reaction at ambient temperature and non-solvent conditions, high yields of products, as well as easy separation of catalyst from the reaction mixture. It is noteworthy that the catalyst was synthesized in a solvent-free manner.
Author contribution statement
Zahra Akhlaghi, Mohammad R. Naimi-Jamal, Leila Panahi: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Mohammad G. Dekamin: Conceived and designed the experiments; Contributed reagents, materials, analysis tools or data.
Bahareh Farasati Far: Analyzed and interpreted the data; Wrote the paper.
Funding statement
Partial support by the Iran University of Science and Technology is acknowledged.
Data availability statement
Data will be made available on request.
Declaration of interest’s statement
The authors declare no conflict of interest.
References
- 1.Drygina O.V., Garnovskii A.D. 4H-pyrans. Chem. Heterocycl. Compd. 1983;19:807–821. [Google Scholar]
- 2.Drygina O.V., Garnovskii A.D., Kazantsev A.V. 2H-pyrans. Chem. Heterocycl. Compd. 1985;21:239–253. [Google Scholar]
- 3.Kumar D., Sharma P., Singh H., Nepali K., Gupta G.K., Jain S.K., Ntie-Kang F. The value of pyrans as anticancer scaffolds in medicinal chemistry. RSC Adv. 2017;7:36977–36999. [Google Scholar]
- 4.Narajji Ch, Karvekar M.D., Das A.K. Synthesis and antioxidant activity of 1,2-Bis(1-ethyl-2-substitutedphenylindolizin-3-yl)diselane. Asian J. Chem. 2008;20:6183–6188. [Google Scholar]
- 5.Skommer J., Wlodkowic D., Mättö M., Eray M., Pelkonen J. HA14-1, a small molecule Bcl-2 antagonist, induces apoptosis and modulates action of selected anticancer drugs in follicular lymphoma B cells. Leuk. Res. 2006;30:322–331. doi: 10.1016/j.leukres.2005.08.022. [DOI] [PubMed] [Google Scholar]
- 6.Mohr S.J., Chirigos M.A., Fuhrman F.S., Pryor J.W. Pyran copolymer as an effective adjuvant to chemotherapy against a murine leukemia and solid tumor. Cancer Res. 1975;35:3750–3754. [PubMed] [Google Scholar]
- 7.Jie H.W., Zhang L.Z., Malononitrile Á.Á. Highly efficient three-component , one-pot synthesis of dihydropyrano [3,2-c] chromene derivatives. Monatsh. Chem. 2010;141:1107–1112. [Google Scholar]
- 8.Kumar R., Yusuf M. Chromones and bischromones: an account of photoinduced reactions. Arkkitehti. 2006:239–264. [Google Scholar]
- 9.Aghahosseini H., Ramazani A. Magnetite L-proline as a reusable nano-biocatalyst for efficient synthesis of 4H-benzo[b]pyrans in water: a green protocols. Eurasian Chem. Commun. 2020;2(3):410–419. [Google Scholar]
- 10.Baghernejad B., Fiuzat M. A new strategy for the synthesis of 2-amino-4H-pyran derivatives in aqueous media using DABCO-CuCl complex as a novel and efficient catalyst. Eurasian Chem. Commun. 2020;2(11):1088–1092. [Google Scholar]
- 11.Zheng J., Li Y. One-pot synthesis of tetrahydrobenzo [ b ] pyran and dihydropyrano [c] chromene derivatives in aqueous media by using trisodium citrate as a green catalyst. Arch. Appl. Sci. Res. 2011;3:381–388. [Google Scholar]
- 12.Kamble V.T., Sadaf M., Samer B.S. Highly efficient synthesis of tetrahydrobenzo[b]pyrans promoted by cesium carbonate under visible light. Iran. Chem. Commun. 2017;5:167–172. [Google Scholar]
- 13.Baghernejad B. Application of ninhydrin as an efficient and novel catalyst for the preparation of 2-amino-4H-pyran derivatives. J. Appl. Organomet. Chem. 2021;1(1):17–21. [Google Scholar]
- 14.Hu H., Qiu F., Ying A., Yang J., Meng H. An environmentally benign protocol for aqueous synthesis of tetrahydrobenzo[b]pyrans catalysed by cost-effective ionic liquid. Int. J. Mol. Sci. 2014;15:6897–6909. doi: 10.3390/ijms15046897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shirini F., Langarudi M.S.N., Daneshvar N. Preparation of a new DABCO-based ionic liquid [H2-DABCO][H2PO4]2} and its application in the synthesis of tetrahydrobenzo[b]pyran and pyrano[2,3-d]pyrimidinone derivatives. J. Mol. Liq. 2017;234:268–278. [Google Scholar]
- 16.Hazeri N., Maghsoodlou T M., Mir F., Kangani M., Saravani H., Molashahi E. An efficient one-pot three-component synthesis of tetrahydrobenzo[b]pyran and 3,4-dihydropyrano[c]chromene derivatives using starch solution as catalyst. Chin. J. Catal. 2014;35:391–395. [Google Scholar]
- 17.Ziarani G., Abbasi A., Badiei A., Aslani Z. An efficient synthesis of tetrahydrobenzo[b]pyran derivatives using sulfonic acid functionalized silica as an efficient catalyst. E-Journal Chem. 2011;8:293. [Google Scholar]
- 18.Behbahani F.K., Naderi M. One-pot synthesis of 2-amino-4H-chromenes catalysed by Fe(ClO4)3/SiO2. Russ. J. Gen. Chem. 2016;86:2804–2806. [Google Scholar]
- 19.Abdollahi-Alibeik M., Sadeghi-Vasafi N., Moaddeli A., Rezaeipoor-Anari A. ClO4−/Al-MCM-41 nanoparticles as a solid acid catalyst for the synthesis of 2-amino-3-cyanopyridines. Res. Chem. Intermed. 2016;42:2867–2881. [Google Scholar]
- 20.Albadi J., Mansournezhad A. Poly (4-vinylpyridine) efficiently catalysed one-pot four-component synthesis of pyrano [2, 3-c] pyrazoles. Curr. Chem. Lett. 2014;3:221–227. [Google Scholar]
- 21.Gaikwad S., Unnamatla M.V.B. Simple, highly efficient synthesis 2-Amino-4-Phenyl-4,5,6,7-Tetrahydropyrano[3,2-c]Carbazole-3-Carbonitrile derivatives using silica supported dodeca-tungstophosphoric acid DTP/SiO2. J. Appl. Organomet. Chem. 2022;2(1):24–30. [Google Scholar]
- 22.a) Rowsell J.L.C., Yaghi O.M. Metal-organic frameworks: a new class of porous materials. Microporous Mesoporous Mater. 2004;73:3–14. [Google Scholar]; b) Zhou H.C., Susumu K. Metal–organic frameworks (MOFs) Chem. Soc. Rev. 2014;43:5415–5418. doi: 10.1039/c4cs90059f. [DOI] [PubMed] [Google Scholar]
- 23.Fernandez C.A., Nune S.K., Motkuri R.K., Thallapally P.K., Wang C., Liu J., Exarhos G.J., McGrail B.P. Synthesis, characterisation, and application of metal organic framework nanostructures. Langmuir. 2010;26:18591–18594. doi: 10.1021/la103590t. [DOI] [PubMed] [Google Scholar]
- 24.Schoedel A., Li M., Li D., O'Keeffe M., Yaghi O.M. Structures of metal-organic frameworks with rod secondary building units. Chem. Rev. 2016;116:12466–12535. doi: 10.1021/acs.chemrev.6b00346. [DOI] [PubMed] [Google Scholar]
- 25.Kurmoo M. Magnetic metal–organic frameworks. Chem. Soc. Rev. 2009;38:1353. doi: 10.1039/b804757j. [DOI] [PubMed] [Google Scholar]
- 26.Mondloch J.E., Karagiaridi O., Farha O.K., Hupp J.T. Activation of metal – organic framework materials. CrystEngComm. 2013;15:9258–9264. [Google Scholar]
- 27.Sarkisov L., Martin R.L., Haranczyk M., Smit B. On the flexibility of metal-organic frameworks. J. Am. Chem. Soc. 2014;136:2228–2231. doi: 10.1021/ja411673b. [DOI] [PubMed] [Google Scholar]
- 28.Zhou H.C., Long J.R., Yaghi O.M. Introduction to metal-organic frameworks. Chem. Rev. 2012;112:673–674. doi: 10.1021/cr300014x. [DOI] [PubMed] [Google Scholar]
- 29.Aijaz A., Xu Q. Catalysis with metal nanoparticles immobilised within the pores of metal − organic frameworks. J. Phys. Chem. Lett. 2014;5:1400–1411. doi: 10.1021/jz5004044. [DOI] [PubMed] [Google Scholar]
- 30.Manna B., Mukherjee S., Desai A.V., Sharma S., Krishna R., Ghosh S.K. A π-electron deficient diaminotriazine functionalised MOF for selective sorption of benzene over cyclohexane. Chem. Commun. 2015;51:15386–15389. doi: 10.1039/c5cc06128h. [DOI] [PubMed] [Google Scholar]
- 31.Corma A., García H., Llabrés i Xamena F.X. Engineering metal organic frameworks for heterogeneous catalysis. Chem. Rev. 2010;110:4606–4655. doi: 10.1021/cr9003924. [DOI] [PubMed] [Google Scholar]
- 32.Decoste J.B., Peterson G.W. Metal−Organic frameworks for air purification of toxic chemicals. Chem. Rev. 2014;114:5695–5727. doi: 10.1021/cr4006473. [DOI] [PubMed] [Google Scholar]
- 33.Safarifard V., Beheshti S., Morsali A. An interpenetrating amine-functionalised metal-organic framework as an efficient and reusable catalyst for the selective synthesis of tetrahydro-chromenes. CrystEngComm. 2015;17:1680–1685. [Google Scholar]
- 34.Giménez-Marqués M., Hidalgo T., Serre C., Horcajada P. Nanostructured metal-organic frameworks and their bio-related applications. Coord. Chem. Rev. 2015;307:342–360. [Google Scholar]
- 35.Li J., Sculley J., Zhou H. Metal organic frameworks for separations. Chem. Rev. 2012;112:869–932. doi: 10.1021/cr200190s. [DOI] [PubMed] [Google Scholar]
- 36.Burtch N.C., Jasuja H., Walton K.S. Water stability and adsorption in metal − organic frameworks. Chem. Rev. 2014;114:10575–10612. doi: 10.1021/cr5002589. [DOI] [PubMed] [Google Scholar]
- 37.Zhang M., Chen C., Wang Q., Fu W., Huang K., Zhou W. A metal–organic framework functionalised with piperazine exhibiting enhanced CH4 storage. J. Mater. Chem. A. 2017;5:349–354. [Google Scholar]
- 38.Lee K., Isley W.C., Dzubak A.L., Verma P., Stoneburner S.J., Lin L., Howe J.D., Bloch E.D., Reed D.A., Hudson M.R., Brown C.M., Long R., Neaton B., Smit B., Cramer C.J., Truhlar D.G., Gagliardi L. Design of a metal − organic framework with enhanced back bonding for separation of N2 and CH4. J. Am. Chem. Soc. 2013;136:698–704. doi: 10.1021/ja4102979. [DOI] [PubMed] [Google Scholar]
- 39.Chun J., Kang S., Park N., Park E.J., Jin X., Kim K., Seo H.O., Lee S.M., Kim H.J., Kwon W.H., Park Y., Kim J.M., Kim Y.D., Son S.U. Metal−Organic Framework@Microporous organic network: hydrophobic adsorbents with a crystalline inner porosity. J. Am. Chem. Soc. 2014;136:6786–6789. doi: 10.1021/ja500362w. [DOI] [PubMed] [Google Scholar]
- 40.Suh M.P., Park H.J., Prasad T.K., Lim D. Hydrogen storage in metal organic frameworks. Chem. Rev. 2011;112:782–835. doi: 10.1021/cr200274s. [DOI] [PubMed] [Google Scholar]
- 41.a) Claramunt R.M., Lopez C., Sanz D., Elguero J. Mechano heterocyclic chemistry: grinding and ball mills. Adv. Heterocycl. Chem. 2014;112:117–143. [Google Scholar]; b) Mahdian S., Naimi-Jamal M.R., Panahi L. Activity of M2(BDC)2(DABCO) (M= Co, Ni, Cu and Zn) metal-organic frameworks prepared via ball-milling solvent-free method in acylation of alcohols, amines and aldehydes. ChemistrySelect. 2018;3:11223–11229. [Google Scholar]; c) Cheng H., Hernández J.G., Bolm C. Mechanochemical cobalt-catalyzed C−H bond functionalizations by ball milling. Adv. Synth. Catal. 2018;360:1800–1804. [Google Scholar]; d) Liu Z., Xu H., Wang G.W. Palladium-catalyzed ortho-halogenations of acetanilides with N-halosuccinimides via direct sp2 C–H bond activation in ball mills. Beilstein J. Org. Chem. 2018;14:430–435. doi: 10.3762/bjoc.14.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.a) Alavi M.A., Morsali A., Joo S.W., Min B.K. Ultrasound and modulation assisted synthesis of {[Cu2(BDC-NH2)2(dabco)]DMF.3H2O} nanostructures; New precursor to prepare nanorods and nanotubes of copper(II) oxide. Ultrason. Sonochem. 2015;22:349–358. doi: 10.1016/j.ultsonch.2014.04.017. [DOI] [PubMed] [Google Scholar]; b) Panahi L., Naimi-Jamal M.R., Mokhtari J. Ultrasound-assisted Suzuki-Miyaura reaction catalysed by Pd@Cu2(NH2-BDC)2(DABCO. J. Organomet. Chem. 2018;868:36–46. [Google Scholar]; c) Naimi-Jamal M.R., Mashkouri S., Sharifi A. An efficient, multicomponent approach for solvent-free synthesis of 2-amino-4H-chromene scaffold. Mol. Divers. 2010;14:473–477. doi: 10.1007/s11030-010-9246-5. [DOI] [PubMed] [Google Scholar]; d) Amirnejad M., Naimi-Jamal M.R., Tourani H., Ghafuri H. A facile solvent-free one-pot three-component method for the synthesis of 2-amino-4H-pyrans and tetrahydro-4H-chromenes at ambient temperature. Monatsh. Chem. 2013;144:1219–1225. [Google Scholar]
- 43.Mahmoodi N.O., Khazaei Z. Preparation, characterisation and use of sulfonylbis(1,4-phenylene)bis(sulfamic acid) as an eco-benign, efficient, reusable and heterogeneous catalyst for the synthesis of mono- and bis-chromenes. J. Iran. Chem. Soc. 2017;14:1889–1898. [Google Scholar]
- 44.Kiyani H., Ghorbani F. Efficient tandem synthesis of a variety of pyran-annulated heterocycles, 3,4-disubstituted isoxazol-5(4H)-ones, and α,β-unsaturated nitriles catalysed by potassium hydrogen phthalate in water. Res. Chem. Intermed. 2015;41:7847–7882. [Google Scholar]
- 45.Mulik A.G., Chandam D.R., Patil D.R., Patil P.P., Mulik G.N., Salunkhe S.T., Deshmukh M.B. Protic ionic liquids: a lucid, rational tool for synthesis of phthalazinediones, quinoxalines and benzopyrans. Res. Chem. Intermed. 2015;41:10085–10096. [Google Scholar]
- 46.Tabrizian E., Amoozadeh A. A unique approach to magnetisation of metal oxides: nano-Fe3O4@TDI@TiO2 as a highly efficient, magnetically separable and recyclable heterogeneous nanocatalyst. Catal. Sci. Technol. 2016;6:6267–6276. [Google Scholar]
- 47.Ahad A., Farooqui M. Organocatalyzed domino reactions: diversity oriented synthesis of pyran-annulated scaffolds using in situ-developed benzylidenemalononitriles. Res. Chem. Intermed. 2017;43:2445–2455. [Google Scholar]
- 48.Zolfigol M.A., Yarie M., Baghery S. [4,4′-Bipyridine]-1,1′-diium tricyanomethanide as a nanostructured molten salt and its catalytic application in the synthesis of tetrahydrobenzo[ b ]pyrans, amido and aminoalkyl naphthol derivatives. J. Mol. Liq. 2016;222:923–932. [Google Scholar]
- 49.Guo R.-Y., An Z.-M., Mo L.-P., Wang R.-Z., Liu H.-X., Wang S.-X., Zhang Z.-H. Meglumine: a novel and efficient catalyst for one-pot, three-component combinatorial synthesis of functionalised 2-amino-4 H-pyrans. ACS Comb. Sci. 2013;15:557–563. doi: 10.1021/co400107j. [DOI] [PubMed] [Google Scholar]
- 50.Qareaghaj O.H., Mashkouri S., Naimi-Jamal M.R., Kaupp G. Ball milling for the quantitative and specific solvent-free Knoevenagel condensation + Michael addition cascade in the synthesis of various 2- amino-4-aryl-3-cyano-4H-chromenes without heating. RSC Adv. 2014;4:48191–48201. [Google Scholar]
- 51.Kalla R.M.N., Varyambath A., Kim M.R., Kim I. Amine-functionalized hyper-crosslinked polyphenanthrene as a metal-free catalyst for the synthesis of 2-amino-tetrahydro-4H-chromene and pyran derivatives. Appl. Catal. Gen. 2017;538:9–18. [Google Scholar]
- 52.Maheswari C.S., Ramesh R., Lalitha A. Synthesis, characterization, and catalytic behavior of bamboo rice husk ash. J. Chin. Chem. Soc. 2017;64:889–895. [Google Scholar]
- 53.Sun W.-B., Zhang P., Fan J., Chen S.-H., Zhang Z.-H. Lithium bromide as a mild, efficient, and recyclable catalyst for the one-pot synthesis of tetrahydro-4 H -chromene derivatives in aqueous media. Synth. Commun. 2010;40:587–594. [Google Scholar]
- 54.Nasr-Esfahani M., Abdizadeh T. Nanorod vanadatesulfuric acid as a novel, recyclable and heterogeneous catalyst for the one-pot synthesis of tetrahydrobenzopyrans. J. Nanosci. Nanotechnol. 2013;13:5004–5011. doi: 10.1166/jnn.2013.7592. [DOI] [PubMed] [Google Scholar]
- 55.Khalil K.D., Al-Matar H.M. Chitosan based heterogeneous catalyses: chitosan-grafted-poly(4- Vinylpyridne) as an efficient catalyst for michael additions and alkylpyridazinyl carbonitrile oxidation. Molecules. 2013;18:5288–5305. doi: 10.3390/molecules18055288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Li Z., Zhu A., Yang J. One-Pot three-component mild synthesis of 2-Aryl-3-(9-alkylcarbazol-3-yl)thiazolin-4-ones. J. Heterocycl. Chem. 2012;49:1458–1461. [Google Scholar]
- 57.Maleki B., Sheikh S. One-pot synthesis of 2-Amino-2-chromene and 2-Amino-3-cyano-4H-pyran derivatives promoted by potassium fluoride. Org. Prep. Proced. Int. 2015;47:368–378. [Google Scholar]
- 58.Gupta M., Gupta M., Rajnikant R., Gupta V.K. Salicyldimine-based Schiff's complex of copper(II) as an efficient catalyst for the synthesis of nitrogen and oxygen heterocycles. New J. Chem. 2015;39:3578–3587. [Google Scholar]
- 59.Maleki B., Baghayeri M., Jannat Abadi S.A., Tayebee R., Khojastehnezhad A. Ultrasound promoted facile one pot synthesis of highly substituted pyran derivatives catalysed catalryzed by silica-coated magnetic NiFe2O4 nanoparticle-supported H14[NaP5W30O110 ] under mil. RSC Adv. 2016;6:96644–96661. [Google Scholar]
- 60.Wang X.-S., Shi D.-Q., Tu S.-J., Yao C.-S. A convenient synthesis of 5-Oxo-5,6,7,8-tetrahydro-4H-benzo-[b]-pyran derivatives catalyzed by KF-alumina. Synth. Commun. 2003;33:119–126. [Google Scholar]
- 61.Bhattacharyya P., Pradhan K., Paul S., Das A.R. Nano crystalline ZnO catalysed one pot multicomponent reaction for an easy access of fully decorated 4H-pyran scaffolds and its rearrangement to 2-pyridone nucleus in aqueous media. Tetrahedron Lett. 2012;53:4687–4691. [Google Scholar]
- 62.Mohammadi A.A., Asghariganjeh M.R., Hadadzahmatkesh A. Synthesis of tetrahydrobenzo[b]pyran under catalysis of NH4Al(SO4)2·12H2O (Alum) Arab. J. Chem. 2017;10:S2213–S2216. [Google Scholar]
- 63.Ghashang M., Mansoor S.S., Aswin K., Sudhan S.P.N. Poly(4-vinylpyridinium)hydrogensulfate as an efficient and convenient catalyst for a three-component synthesis of 7-methyl-10-aryl-10H-5,8-dioxa-benzo[b]fluoren-9,11-diones. Res. Chem. Intermed. 2015;41:5239–5251. [Google Scholar]
- 64.Hojati S.F., Moosavifar M., Ghorbanipoor T. Improvement in nanocomposite host (nanocavity of dealuminated zeolite Y)-guest (12-molybdophosphoric acid) catalytic activity and its application to the one-pot three-component synthesis of tetrahydrobenzo[b]pyrans. Compt. Rendus Chem. 2017;20:520–525. [Google Scholar]
- 65.Azizi N., Dezfooli S., Khajeh M., Hashemi M.M. Efficient deep eutectic solvents catalysed synthesis of pyran and benzopyran derivatives. J. Mol. Liq. 2013;186:76–80. [Google Scholar]
- 66.Bihani M., Bora P.P., Bez G., Askari H. Amberlyst A21: a reusable solid catalyst for green synthesis of pyran annulated heterocycles at room temperature. Compt. Rendus Chem. 2013;16:419–426. [Google Scholar]
- 67.Dekamin M.G., Peyman S.Z., Karimi Z., Javanshir S., Naimi-Jamal M.R., Barikani M. Sodium alginate: an efficient biopolymeric catalyst for green synthesis of 2-amino-4H-pyran derivatives. Int. J. Biol. Macromol. 2016;87:172–179. doi: 10.1016/j.ijbiomac.2016.01.080. [DOI] [PubMed] [Google Scholar]
- 68.Xu J.C., Li W.M., Zheng H., Lai Y.F., Zhang P.F. One-pot synthesis of tetrahydrochromene derivatives catalysed by lipase. Tetrahedron. 2011;67:9582–9587. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.