Abstract
Background and Aim
To investigate the outcomes in eight Japanese patients with cancer treated with mycophenolate mofetil (MMF) and corticosteroids for immune checkpoint inhibitor treatment‐induced severe immune‐related hepatitis (ir‐hepatitis) and the efficacy and safety of MMF.
Methods
We retrospectively examined patient background, treatment course, as well as examination and imaging data using electronic medical records.
Results
The ratio of male to female patients was 7:1, and the median age was 60 years (27–72 years). There were five and two cases of kidney cancer and malignant melanoma, respectively, and one case of lung cancer. The median number of days until MMF administration in addition to systemic corticosteroid therapy after the onset of ir‐hepatitis was 14.5 (2–42). The patients were categorized as four “good responders” who showed an improvement in the liver function tests following MMF treatment and four “poor responders” who did not. Furthermore, the time from the onset of ir‐hepatitis to initial MMF administration was significantly shorter in good responders (median 3 days, range 2–15 days) than in poor responders (median 25.5 days, range 14–42 days) (P = 0.042). No significant intergroup difference was observed in other clinical factors. No serious adverse events caused by MMF were observed in any case.
Conclusions
According to these findings, early recognition of corticosteroid refractoriness and the use of MMF may be beneficial in patients with ir‐hepatitis.
Keywords: hepatitis, immune checkpoint inhibitor, immune‐related adverse event, mycophenolate mofetil
We investigated the efficacy and safety of mycophenolate mofetil (MMF) for eight Japanese patients with cancer treated with MMF and corticosteroids for immune checkpoint inhibitor treatment‐induced severe immune‐related hepatitis. The duration from the onset of immune‐related hepatitis (ir‐hepatitis) to initial MMF administration was significantly shorter in good responders than in poor responders (P = 0.042). Early recognition of steroid refractoriness and the usage of MMF may be beneficial in patients with ir‐hepatitis.
Introduction
Immune checkpoint inhibitors (ICIs) are promising drugs for treating diseases by strengthening the immune system of patients with cancer. They enhance antitumor effects by blocking endogenous factors such as cytotoxic T‐lymphocyte antigen‐4 (CTLA‐4) and programmed cell death‐1 (PD‐1). ICIs, including nivolumab, pembrolizuab, and ipilimumab, have been approved in many countries, as they prolong the overall survival of patients with various types of cancer. Their use may lead to unique adverse events known as immune‐related adverse events (irAEs), and early detection and proper management of irAEs is essential as they may lead to fatal outcomes. The “Cancer Immunotherapy Guidelines” created by the Japanese Society of Medical Oncology (JSMO) and guidelines on ICI‐induced irAEs issued by the American Society of Clinical Oncology (ASCO) describe treatments for immune‐related hepatitis (ir‐hepatitis). 1 , 2 Both guidelines state that ICI administration can be continued after the occurrence of grade 1 ir‐hepatitis with regular monitoring of liver function parameters. In case of deterioration to grade 2, ICI administration should be discontinued and liver function parameters should be monitored. Furthermore, corticosteroids should be administered if abnormal liver function test values persist for more than 5–7 days or worsen further. In case of deterioration of ir‐hepatitis to ≥grade 3, ICI administration should be discontinued and 1–2 mg/kg of methylprednisolone (mPSL) or an equivalent amount of corticosteroid should be given as an intravenous injection, and if liver function test values show no improvements for more than 3–5 days or deteriorate further, the combined use of 1 g of mycophenolate mofetil (MMF) twice daily or the use of other immunosuppressants should be considered. Meanwhile, the efficacy of MMF in treating liver dysfunctions occurring after ICI administration has not been established yet, and the use of MMF for this purpose is currently not covered by insurance in Japan. 3 Possible side effects of MMF include diarrhea and hyperuricemia. Side effects that require attention include malignant lymphoma and skin tumor; however, they have been reported to occur very rarely.
To date, there have been many case reports on the treatment of ICI‐induced ir‐hepatitis using MMF 4 , 5 , 6 , 7 , 8 , 9 , 10 , 11 , 12 , 13 , 14 , 15 , 16 ; however, we could find no reports that comprehensively describe when and how much MMF should be administered. In this study, we investigated the outcomes of Japanese patients with cancer treated using MMF combined with corticosteroids for ir‐hepatitis induced via various ICIs to clarify the efficacy and safety of MMF in ir‐hepatitis treatment.
Methods
Subjects
A total of 1227 patients underwent ICI treatment at the Osaka International Cancer Institute (hereinafter our institute) between January 2018 and December 2020. Of them, eight Japanese patients with cancer in whom MMF in combination with corticosteroids was used for treating ICI treatment‐induced ir‐hepatitis and whose treatment details could be reviewed using electronic medical records were included in this study.
Data collection and evaluation
Patient background, treatment course, and examination and imaging data were retrospectively collected from electronic medical records. Hepatitis was evaluated using the Common Terminology Criteria for Adverse Events ver 5.0. Diagnosis of ir‐hepatitis has been described in the previous reports from our instutition. 17 Briefly, it was done by the attending physician and hepatologists after the risk of other liver disorders had been eliminated based on various examinations, such as medical interviews; blood tests, including immunoglobulin G (Ig‐G), Ig‐M, anti‐nuclear antibody, anti‐mitochondrial antibody; and serological tests for hepatitis (A, B, C, and E), herpes simplex virus, and cytomegalovirus; or imaging modalities, such as ultrasonography, computed tomography (CT), or magnetic resonance imaging. MMF administration was determined according to guidelines at the discretion of the attending physician and hepatologist. The day of ir‐hepatitis onset was defined as day 0. Regarding liver function tests, total bilirubin (T‐Bil; normal range, ≤1.5 mg/dL), aspartate transaminase (AST; normal range, ≤30 IU/L), alanine transaminase (ALT; normal range, ≤30 IU/L), and alkaline phosphatase (ALP; normal range, ≤322 IU/L) were evaluated. In the present study, “good responders” were defined as those showing persistent improvement to grade 1 or less in liver function tests during MMF treatment, whereas “poor responders” were defined as those whose liver function tests did not improve to grade 1.
Ethical considerations
This study was conducted in compliance with the “Declaration of Helsinki” and the “Ethical Guidelines for Medical and Health Research Involving Human Subjects” and approved by the Ethical Review Board of our institute (approval number: 20061). This study gave due consideration to personal data protection, and data were handled following anonymization. Information regarding this study is available on our institute's website, and we adopted an opt‐out system that can respond to requests of withdrawal from research whenever necessary.
Statistical analysis
Mann–Whitney's U test, chi‐square test, and Fisher's exact test were used for intergroup comparisons as appropriate.
Results
Patient background of eight patients and treatment course for ir‐hepatitis
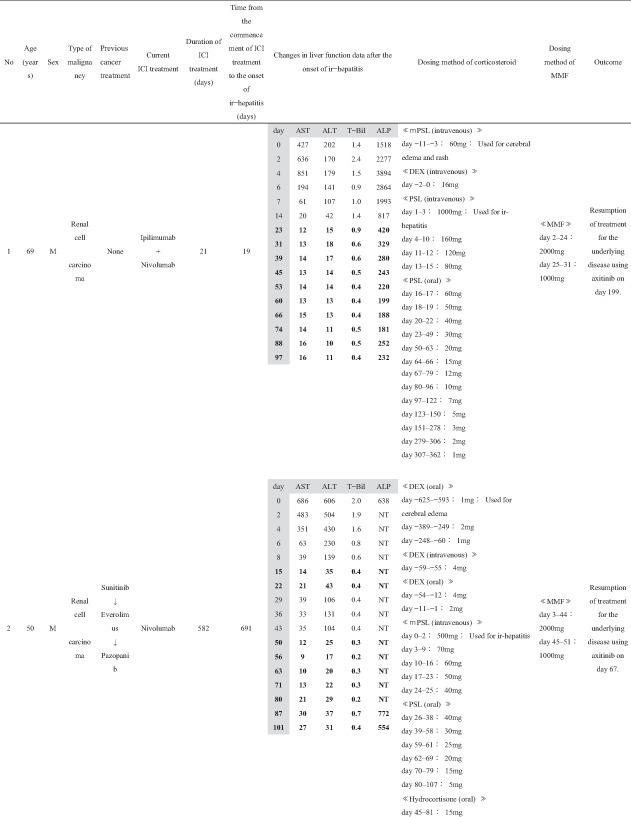
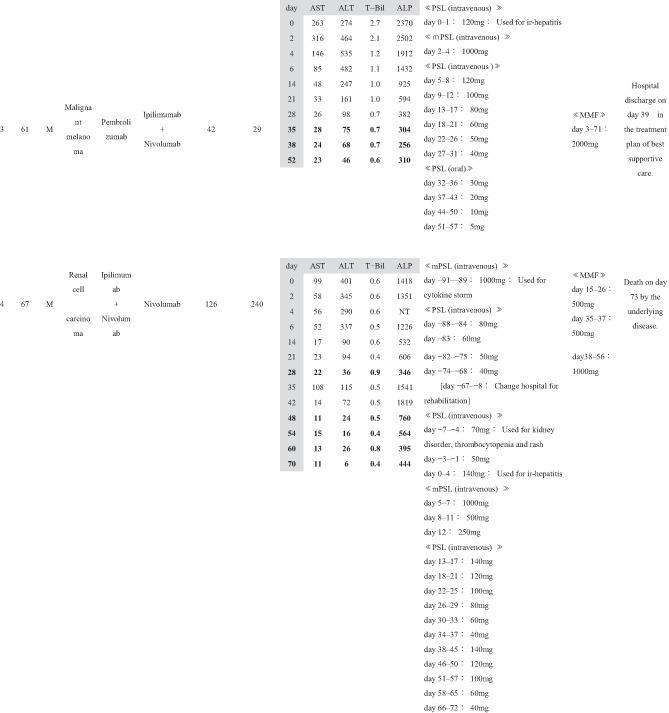
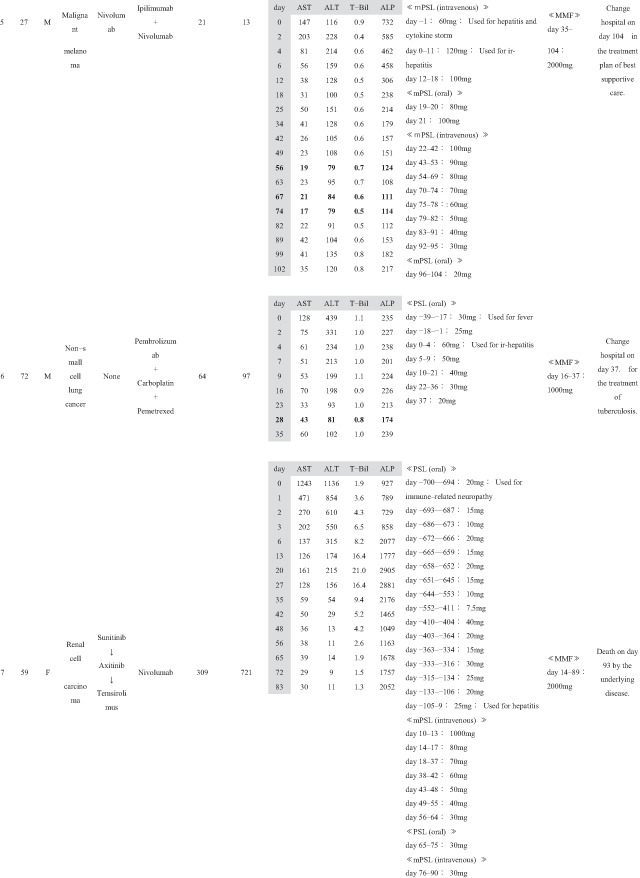
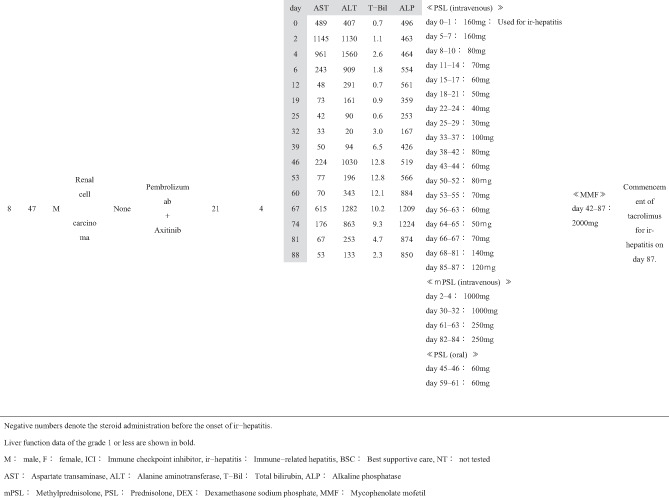
Patient background information is summarized in Table 1, and treatment course for ir‐hepatitis is summarized in Table 2. The ratio of male to female patients was 7:1. The patients had a median age of 60 years (range: 27–72 years). By cancer type, five patients, two patients, and one patient had renal cell carcinoma, malignant melanoma, and non‐small‐cell lung cancer, respectively. No patients had a previous history of chronic liver disease. Five patients had received previous cancer treatment before the current ICI treatment, three of whom had been treated with another ICI regimen (cases 3, 4, and 5). In the current ICI treatment, nivolumab, ipilimumab + nivolumab, and pembrolizumab were used in three, three, and two cases, respectively; in the two cases in which pembrolizumab was used, carboplatin and pemetrexed were used in combination in one case (case 6), whereas axitinib was administered concurrently in the other (case 8). The median duration of the ICI treatment was 53 days (range: 21–582 days). The median time from the commencement of ICI treatment to the onset of ir‐hepatitis was 63 days (range: 4–721 days). The ir‐hepatitis occurred in a median of 36 days (range: 4–426 days) after the final ICI administration. At the onset of ir‐hepatitis, median T‐Bil, AST, ALT, and ALP were 1.25 mg/dL (range: 0.6–2.7 mg/dL), 345 IU/L (range: 99–1243 IU/L), 404 IU/L (range: 116–1136 IU/L), and 829.5 IU/L (range: 235–2370 IU/L), respectively. Six and two patients presented with the CTCAE grade 3 and grade 4 liver injury.
Table 1.
Background of eight cancer patients with ir‐hepatitis who underwent MMF treatment
| Overall | Good responders | Poor responders | P‐value | |
|---|---|---|---|---|
| Number of cases | 8 | 4 | 4 | — |
| Age (years) | 60 (27–72) † | 64 (50–69) | 53 (27–72) | 0.387 |
| Sex (male/female) | 7/1 | 4/0 | 3/1 | 1.000 |
| Type of malignancy (renal cell carcinoma/malignant melanoma/non‐small‐cell lung cancer) | 5/2/1 | 3/1/0 | 2/1/1 | 0.549 |
| History of chronic liver disease (yes/no) | 0/8 | 0/4 | 0/4 | 1.000 |
| ICI that caused ir‐hepatitis (nivolumab/ipilimumab + nivolumab/pembrolizumab) | 3/3/2 | 2/2/0 | 1/1/2 | 0.264 |
| Duration of ICI treatment (days) | 53 (21–582) | 84 (21–582) | 42.5 (21–309) | 0.554 |
| Time from the commencement of ICI treatment to the onset of ir‐hepatitis (days) | 63 (4–721) | 134.5 (19–691) | 55 (4–721) | 0.772 |
| At baseline | ||||
| T‐Bil (mg/dL) | 0.5 (0.3–0.7) | 0.4 (0.3–0.6) | 0.5 (0.3–0.7) | 0.448 |
| AST (IU/L) | 21.5 (17–58) | 49 (20–58) | 20 (17–26) | 0.169 |
| ALT (IU/L) | 54.5 (12–330) | 89 (25–103) | 28 (12–330) | 0.655 |
| ALP (IU/L) | 230 (187–450) | 276 (201–450) | 230 (187–264) | 0.177 |
| At onset of ir‐hepatitis | ||||
| T‐Bil (mg/dL) | 1.25 (0.6–2.7) | 1.7 (0.6–2.7) | 1.0 (0.7–1.9) | 0.387 |
| AST (IU/L) | 345 (99–1243) | 345 (99–686) | 318 (128–1243) | 0.773 |
| ALT (IU/L) | 404 (116–1136) | 337.5 (202–606) | 423 (116–1136) | 0.564 |
| ALP (IU/L) | 829.5 (235–2370) | 1468 (638–2370) | 614 (235–927) | 0.083 |
| CTCAE grade of liver injury (3/4) | 6/2 | 3/1 | 3/1 | 1.000 |
| Duration from the onset of ir‐hepatitis to initial MMF administration (days) | 14.5 (2–42) | 3 (2–15) | 25.5 (14–42) | 0.042 |
| Starting dose of MMF (500 mg/1000 mg/2000 mg) | 1/1/6 | 1/0/3 | 0/1/3 | 0.368 |
| Duration of MMF administration (days) | 59 (29–90) | 45.5 (30–69) | 77 (29–90) | 0.248 |
Data expressed as median (range).
ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate transaminase; CTCAE, Common Terminology Criteria for Adverse Events; ICI, immune checkpoint inhibitor, ir‐hepatitis, immune‐related hepatitis; MMF, mycophenolate mofetil; T‐Bil, total bilirubin.
Table 2.
Treatment course of eight cancer patients with ir‐hepatitis who underwent MMF treatment
|
|
|
|
Concerning the medication for ir‐hepatitis, corticosteroid administration was started before the onset of ir‐hepatitis for treating irAEs other than ir‐hepatitis or other diseases in six cases (cases 1, 2, 4, 5, 6, and 7); the median number of days from the start of corticosteroid administration until the onset of ir‐hepatitis was 65 days (range: 1–700 days). In the remaining two cases (cases 3 and 8), corticosteroid administration was started at the onset of ir‐hepatitis. The median dosing period of corticosteroids after the onset of ir‐hepatitis was 88.5 days (range: 37–361 days). MMF was added after the patients were considered nonresponsive to corticosteroids. The median number of days until the start of MMF administration after the onset of ir‐hepatitis was 14.5 (range: 2–42 days). The daily MMF dose at introduction was 2000 mg/day in six cases, 1000 mg/day in one case (case 6), and 500 mg/day in one case (case 4), out of the total of eight cases. The duration of MMF administration was 47.5 days (range: 22–76 days).
Effectiveness and safety of MMF for ir‐hepatitis
Changes in the liver function tests in each of the eight cases before and after the MMF administration are shown in Table 2. In cases 1–4, MMF was effective and led to alleviation up to CTCAE grade 1 of all four laboratory tests, namely T‐Bil, AST, ALT, and ALP. In these four cases, the number of days required for ir‐hepatitis improvement to grade 1 following MMF administration was 23 in case 1, 50 in case 2, 35 in case 3, and 48 in case 4. The treatment of the primary disease through the next regimen was resumed in cases 1 and 2. The best supportive care (BSC) was then established in case 3. Case 4 died of exacerbation of the primary cancer disease. Therefore, these four cases (cases 1–4) who had ir‐hepatitis were regarded as “good responders” to MMF treatment.
However, the remaining four cases (cases 5–8) did not present with a good response to the MMF treatment; MMF did not result in sufficient decrease in the levels of T‐Bil, AST, ALT, and ALP to CTCAE grade 1 during follow‐up. These patients with ir‐hepatitis were regarded as “poor responders” to MMF treatment. As for the outcome of these patients, BSC was subsequently given in case 5. Case 6 was transferred to another hospital to treat the tuberculosis that occurred accidentally. Case 7 died of exacerbation of the primary cancer. In case 8, in which MMF was ineffective in treating ir‐hepatitis, tacrolimus was administered in addition to prednisolone (PSL) and MMF on day 87, and the treatment is still going on.
When the clinical factors were compared between the four “good responders” and four “poor responders” to the MMF treatment (Table 1), the duration from the onset of ir‐hepatitis to initial MMF administration was significantly shorter in good responders (median 3 days, range 2–15 days) than in poor responders (median 25.5 days, range 14–42 days) (P = 0.042). No significant difference was observed in the other clinical factors between good and poor responders.
As for adverse events of MMF, no serious adverse events were noted in any of the eight patients with ir‐hepatitis except for two patients who presented with infectious diseases (cases 4 and 6); however, the direct causal relationship with MMF remained unclear. In case 4, cytomegalovirus and aspergillus developed after the administration of corticosteroid and MMF, and treatment was performed in our facility. In case 6, tuberculosis developed after the administration of corticosteroid and MMF; therefore, tuberculosis treatment was given in another hospital.
Case presentation
Figure 1 shows the detailed clinical course of case 1 with right renal cell carcinoma along with multiple lung and brain metastase; the day of ir‐hepatitis onset was defined as day 0. The patient suddenly became unable to speak fluently and underwent a detailed head examination at another hospital on day 32. Since multiple brain metastases were found, gamma knife radiosurgery was performed, and the speech disorder improved. Subsequently, on day 21, the patient was transferred to our hospital for systemic treatment. The first course of ipilimumab + nivolumab combination therapy was performed. After 8 days, he was sent to our hospital on an emergency basis because he showed consciousness disorder, systemic rash, and fever. He was diagnosed with immune‐related dermatologic disorder, and 60 mg/day of mPSL was administered intravenously. The skin rash was ameliorated, whereas high fever continued, probably because of a thermoregulation disorder caused by irradiation‐related brain edema. mPSL was subsequently replaced by 16 mg/day of dexamethasone along with the administration of concentrated glycerin and fructose. On day 0, fever persisted, and abnormal blood test results were observed; AST, ALT, and ALP levels were 427, 202, and 1518 IU/L. Although lumboabdominal CT scan showed no abnormality in the liver and biliary tract, the patient was diagnosed with ir‐hepatitis based on the clinical symptoms and blood test results. On days 1–3, a steroid pulse treatment (mPSL, 1000 mg/day) was performed; furthermore, MMF administration was commenced on day 2. On day 2, fever subsided, and on day 3 the dose was changed from 1000 mg/day mPSL (i.v.) to 160‐mg/day PSL (i.v.). After day 9, ir‐hepatitis rapidly improved, and PSL was gradually reduced. MMF was administered at a reduced dose of 1000 mg/day on day 25 and then discontinued on day 31. Since then, PSL dose was gradually reduced and there was no recurrence of ir‐hepatitis. Treatment was resumed in the patient with the underlying disorder (axitinib was started on day 199). The patient subsequently continued to undergo the treatment for the primary disease, but died of respiratory failure caused by an exacerbation of the primary disease on day 1233.
Figure 1.
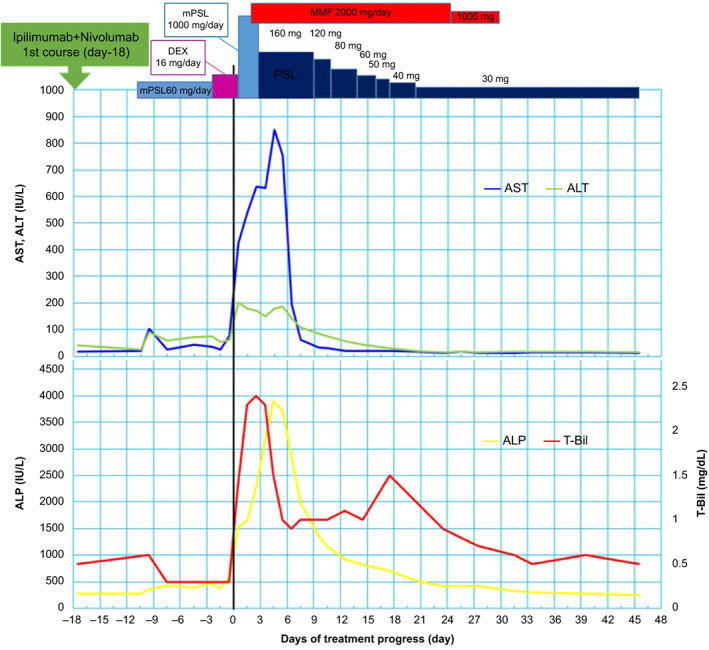
Clinical course after first course of ipilimumab + nivolumab combination therapy in case 1. The day of ir‐hepatitis onset was defined as day 0. The patient was diagnosed with immune‐related dermatologic disorder on day 11, and 60 mg/day of mPSL was administered intravenously. On day 0, fever persisted, and abnormal blood test results were observed: AST, ALT, and ALP levels were 427, 202, and 1518 IU/L. On days 1–3, a steroid pulse treatment (mPSL, 1000 mg/day) was given; furthermore, MMF administration was commenced on day 2. On day 2, fever subsided, and on day 3, the dose was changed from 1000 mg/day mPSL to 160 mg/day PSL. After day 9, ir‐hepatitis rapidly improved, and PSL was gradually reduced. MMF was administered at a reduced dose of 1000 mg/day on day 25 and then discontinued on day 31. Since then, PSL dose was gradually reduced and there was no recurrence of ir‐hepatitis.
Discussion
ICIs, including nivolumab, pembrolizuab, and ipilimumab, are currently used for treating malignant melanoma, non‐small‐cell lung cancer, and various other carcinomas, and a wide variety of irAEs have been reported. As the use of ICIs is expected to increase further, the incidence of ≥grade 3 hepatitis as a serious irAE is also likely to increase. Based on the results of clinical trials of ICIs involving patients with melanoma, the incidence of ir‐hepatitis, including all grades, was less than 5% in patients undergoing monotherapy with the anti‐PD‐1 antibody nivolumab but increased to about 20% in patients receiving nivolumab in combination with ipilimumab. 18 , 19 , 20
Based on its mechanism of pathogenesis, ir‐hepatitis is expected to cause a chronic liver disorder similar to autoimmune hepatitis (AIH), but it has been found to differ from AIH in terms of clinical course and pathological features. 21 Zen and Yeh, 22 Sue et al., 23 and Kleiner and Berman. 24 compared ir‐hepatitis with AIH in terms of pathological features and reported that while centrilobular hepatitis lesions with mild inflammation in the portal area were observed, the proliferation of CD4‐ and CD20‐positive cells in ICI‐related hepatitis was less than in AIH and infiltrating CD8‐positive cells were mainly found. These findings suggest that activated T lymphocytes damage hepatocytes and cause liver dysfunction in ir‐hepatitis and AIH; however, the types of lymphocytes responsible are different, including CD4‐ and CD20‐positive cells or CD8‐positive cells. Accordingly, MMF, which is a drug frequently used as an immunosuppressant and has been reported to be effective for treating AIH, 25 , 26 could also be effective in treating irAEs.
MMF is an antimetabolite that inhibits DNA synthesis by reversibly and noncompetitively inhibiting inosinate dehydrogenase in the purine biosynthesis pathway. 27 MMF inhibits the proliferation and activation of T and B lymphocytes, which depend mainly on the de novo purine synthesis pathway, and interferes with antibody production, cellular immunity, and cytokine production. Therefore, MMF can be expected to improve ir‐hepatitis by impairing lymphocyte function. 28 Various guidelines recommend corticosteroid administration when ir‐hepatitis of ≥grade 2 persists without improvement or is exacerbated. 1 , 2 In cases when the liver function test values do not improve after corticosteroid treatment, the combined use of MMF should be considered. In the present study, MMF was administered at doses of 500–2000 mg/day in addition to the corticosteroid in the eight patients with grade‐3 or higher ir‐hepatitis according to the dosage and administration criteria provided in the JSMO and ASCO guidelines. The median number of days until the start of MMF administration after the diagnosis of ir‐hepatitis was 14.5 days (range: 2–42 days). It is of particular interest to note that, among our patients, patients who received MMF treatment immediately after the onset of ir‐hepatitis showed a tendency to have a better response to the MMF treatment. Indeed, the median duration from the onset of ir‐hepatitis to initial MMF administration was 3 days (range, 2–15 days) in the “good responders,” compared with median 25.5 days (range, 14–42 days) in the “poor responders.” According to this, it would be better to start MMF treatment earlier in patients with ir‐hepatitis who do not respond to the preceding corticosteroid treatment. In three of our four “good responders” to MMF, corticosteroid was already administered before the onset of ir‐hepatitis. In such “steroid‐refractory” ir‐hepatitis patients, the treatment plan for MMF administration would be easier to decide. However, patients in whom ir‐hepatitis developed under the non‐administration of corticosteroid, both early recognition of refractoriness to the preceding corticosteroid treatment and the use of MMF may be useful.
When the MMF treatment together with corticosteroids does not show sufficient effectiveness for ir‐hepatitis, other drugs, such as antithymocyte globulin (ATG), 29 azathioprine, 30 and tacrolimus, 31 have been suggested to be used as third‐line treatment, although the priority of use of these drugs has not been established in any of the guidelines. In this study, oral tacrolimus administration was done in case 8, in whom the irAE was refractory to corticosteroids and MMF. In future, it will be necessary to investigate when to switch from MMF to next‐line treatment and evaluate the efficacy of next‐line treatment while treating ir‐hepatitis in patients nonresponsive or intolerant to MMF.
Adverse events of MMF, such as the development of secondary malignant tumor, progressive multifocal leukoencephalopathy, BK virus nephropathy, blood disorder, malignant tumor, gastrointestinal disorder, severe diarrhea, thrombosis, heart disorder, liver dysfunction, pulmonary edema, and convulsions, among others, have been reported. None of the above‐mentioned adverse events of MMF was observed.in our patients, suggesting the safety of the MMF treatment in ir‐hepatitis to some extent. However, serious infectious diseases, tuberculosis, and infection with cytomegalovirus and aspergillus developed during MMF treatment in two cases. Such infectious diseases may be induced through not only the direct immunosuppressive effect of MMF but also the cancer‐bearing condition and the systemic cancer treatment using ICIs and other drugs. During MMF treatment for ir‐hepatitis, it is imperative to exercise caution in terms of the development of infectious diseases that may be caused by the complex factors.
This study has some limitations. First, it included a small number of cases observed in a single institution. Second, this was a retrospective study conducted using electronic medical records. Thus, there could have been various biases due to the insufficient statistical power of the tests performed. A multicenter study is necessary to include more cases and perform more accurate evaluations.
Conclusion
In conclusion, we represented the case of eight patients having ir‐hepatitis who underwent MMF treatment in addition to corticosteroid in the present study. Better treatment response was found in patients in whom MMF was administered more promptly after the onset of ir‐hepatitis. Our results suggest that early recognition of corticosteroid refractoriness and the use of MMF in line with the guidelines 1 , 2 may be beneficial in patients with ir‐hepatitis. Further large‐scale studies should offer better understanding concerning the optimal use of MMF in ir‐hepatitis.
Ethics Statement
The present study was approved by the Ethical Review Board of the Osaka International Cancer Institute (Osaka, Japan) and conducted in accordance with the ethical guidelines on clinical research (approval no. 20061). Information regarding the present study is available at the institution's website.
Patient Consent Statement
Due consideration was given toward protecting personal information, and patient data was handled after ensuring anonymization. Informed consent was obtained from all patients included in the study. The patients could withdraw their consent for participation at any period throughout the study.
Declaration of conflict of interest: The authors declare that they have no conflict of interest to disclose.
Author contribution: Mari Takagi, Satoko Inoue, Akitoshi Tatsumi, Mayako Uchida, Keiko Fujita, Takako Inoue, Shuichi Ohe, Yasutomo Nakai, Yutaro Abe, Tasuku Nakabori, Tomoyuki Otsuka, Taiki Isei, Toru Kumagai, Kazuo Nishimura, and Kazuyoshi Ohkawa established comprehensive research goals and aims and developed and designed methodologies. Takako Inoue, Shuichi Ohe, Yasutomo Nakai, Yutaro Abe, Tasuku Nakabori, Tomoyuki Otsuka, Taiki Isei, Toru Kumagai, Kazuo Nishimura, and Kazuyoshi Ohkawa provided patient samples. Yukio Kadokawa, Mari Takagi, Satoko Inoue, Keiko Fujita, Takako Inoue, Shuichi Ohe, Yasutomo Nakai, Yutaro Abe, Tasuku Nakabori, Tomoyuki Otsuka, Taiki Isei, Toru Kumagai, Kazuo Nishimura, and Kazuyoshi Ohkawa performed the experiments, collected data, and conducted administrative activities to maintain the survey data. Yukio Kadokawa and Keiko Fujita conducted data analysis. Yukio Kadokawa drafted the manuscript with the help of Akitoshi Tatsumi, Mayako Uchida, Yutaro Abe, Tasuku Nakabori, Kazuyoshi Ohkawa. Takako Inoue, Shuichi Ohe, Yasutomo Nakai, Yutaro Abe, Tasuku Nakabori, Tomoyuki Otsuka, and Kazuo Nishimura assessed the authenticity of all the raw data to ensure their legitimacy. Mari Takagi, Satoko Inoue, Akitoshi Tatsumi, Mayako Uchida, Keiko Fujita, Takako Inoue, Shuichi Ohe, Yasutomo Nakai, Tomoyuki Otsuka, Yutaro Abe, Tasuku Nakabori, Taiki Isei, Toru Kumagai, Kazuo Nishimura, and Kazuyoshi Ohkawa conducted critical reviews, visualizations, and edits. Keiko Fujita, Yutaro Abe, Tasuku Nakabori, and Kazuyoshi Ohkawa were responsible for oversight and leadership in planning and carrying out research activities. All authors agreed to be accountable for all aspects of the work and ensure that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All authors read and approved the final manuscript.
Financial support: This study received no external funding.
Data availability statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
- 1. Japanese Society of Medical Oncology . Cancer Immunotherapy Guidelines, 2nd edn. Tokyo, Japan: Kanehara Shuppan, 2019. [Google Scholar]
- 2. Brahmer JR, Lacchetti C, Schneider BJ et al. Management of immune‐related adverse events in patients treated with immune checkpoint inhibitor therapy: American Society of Clinical Oncology Clinical Practice Guideline. J. Clin. Oncol. 2018; 36: 1714–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.CELLCEPT® package leaflet. Available from URL: https://pins.japic.or.jp/pdf/newPINS/00065980.pdf
- 4. De Martin E, Michot JM, Papouin B et al. Characterization of liver injury induced by cancer immunotherapy using immune checkpoint inhibitors. J. Hepatol. 2018; 68: 1181–90. [DOI] [PubMed] [Google Scholar]
- 5. Johncilla M, Misdraji J, Pratt DS et al. Ipilimumab‐associated hepatitis: clinicopathologic characterization in a series of 11 cases. Am. J. Surg. Pathol. 2015; 39: 1075–84. [DOI] [PubMed] [Google Scholar]
- 6. Forschner A, Schraml C, Pierchalla K et al. Pembrolizumab‐induced hepatitis: diagnosis and treatment. J. Dtsch. Dermatol. Ges. 2017; 15: 933–5. [DOI] [PubMed] [Google Scholar]
- 7. Abdel‐Wahab N, Shah M, Suarez‐Almazor ME. Adverse events associated with immune checkpoint blockade in patients with cancer: a systematic review of case reports. PLoS One. 2016; 11: e0160221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tanaka R, Fujisawa Y, Sae I et al. Severe hepatitis arising from ipilimumab administration, following melanoma treatment with nivolumab. Jpn. J. Clin. Oncol. 2017; 47: 175–8. [DOI] [PubMed] [Google Scholar]
- 9. Nakano K, Nishizawa M, Fukuda N et al. Mycophenolate mofetil as a successful treatment of corticosteroid‐resistant immune checkpoint inhibitor‐induced hepatitis. Oxf. Med. Case Reports. 2020; 2020: omaa027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Briggs WA, Choi MJ, Scheel PJ Jr. Successful mycophenolate mofetil treatment of a patient with severe steroid‐refractory hepatitis evoked by nivolumab plus ipilimumab treatment for relapsed bladder cancer. Clin. Case Rep. 2020; 9: 654–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tomyo F, Kobayashi K, Saito K, Tanaka A, Yamaguchi M, Nagase H. Pembrolizumab‐induced, possibly trimethoprim‐sulfamethoxazole‐related hepatitis requiring treatment with high dose prednisolone and mycophenolate mofetil. Ann. Jpn. Respir. Soc. 2020; 9: 99–103. [Google Scholar]
- 12. Kitagataya T, Suda G, Nagashima K et al. Prevalence, clinical course, and predictive factors of immune checkpoint inhibitor monotherapy‐associated hepatitis in Japan. J. Gastroenterol. Hepatol. 2020; 35: 1782–8. [DOI] [PubMed] [Google Scholar]
- 13. Rizell M, Åberg F, Perman M et al. Checkpoint inhibition causing complete remission of metastatic combined hepatocellular‐cholangiocarcinoma after hepatic resection. Case Rep. Oncol. 2020; 13: 478–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kopecký J, Kubecek O, Geryk T et al. Hepatic injury induced by a single dose of nivolumab ‐ a case report and literature review. Klin. Onkol. 2019; 32: 133–8. [DOI] [PubMed] [Google Scholar]
- 15. Nakashima K, Demura Y, Oi M et al. Infliximab was found to be effective for treating immunosuppressive drug‐resistant hepatitis due to durvalumab. Intern. Med. 2020; 59: 3055–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Motomura D, Baetz T, Grin A, Flemming JA. Severe refractory checkpoint inhibitor‐related hepatitis reversed with anti‐thymocyte globulin and N‐acetylcysteine. Hepatology. 2020; 72: 2235–8. [DOI] [PubMed] [Google Scholar]
- 17. Nakabori T, Abe Y, Higashi S et al. Feasibility of immunotherapy in cancer patients with persistent or past hepatitis B or C virus infection. JGH Open. 2022; 8: 309–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Larkin J, Chiarion‐Sileni V, Gonzalez R et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N. Engl. J. Med. 2015; 373: 23–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Postow MA, Chesney J, Pavlick AC et al. Nivolumab and ipilimumab versus ipilimumab in untreated melanoma. N. Engl. J. Med. 2015; 372: 2006–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Robert C, Long GV, Brady B et al. Nivolumab in putation. N. Engl. J. Med. 2015; 372: 320–30. [DOI] [PubMed] [Google Scholar]
- 21. Nishida N, Kudo M. Liver damage related to immune checkpoint inhibitors. Hepatol. Int. 2019; 13: 248–52. [DOI] [PubMed] [Google Scholar]
- 22. Zen Y, Yeh MM. Hepatotoxicity of immune checkpoint inhibitors: a histology study of seven cases in comparison with autoimmune hepatitis and idiosyncratic drug‐induced liver injury. Mod. Pathol. 2018; 31: 965–73. [DOI] [PubMed] [Google Scholar]
- 23. Sue M, Ueno M, Takabatake H et al. A case report of pembrolizumab‐induced the implication of CD8‐positive lymphocytes in the immune‐related adverse event. Kanzo. 2018; 59: 571–7. [Google Scholar]
- 24. Kleiner DE, Berman D. Pathologic changes in ipilimumab‐related hepatitis in patients with metastatic melanoma. Dig. Dis. Sci. 2012; 57: 2233–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Doycheva I, Watt KD, Gulamhusein AF. Autoimmune hepatitis: current and future therapeutic options. Liver Int. 2019; 39: 1002–13. [DOI] [PubMed] [Google Scholar]
- 26. De Lemos‐Bonotto M, Valle‐Tovo C, Costabeber AM, Mattos AA, Azeredo‐da‐Silva ALF. A systematic review and meta‐analysis of second‐line immunosuppressants for autoimmune hepatitis treatment. Eur. J. Gastroenterol. Hepatol. 2018; 30: 212–16. [DOI] [PubMed] [Google Scholar]
- 27. Mir R, Shaw HM, Nathan PD. Immunosuppressive agents and their role in managing immunotherapy toxicities in melanoma. Clin. Skin Cancer. 2017; 2: 18–23. [Google Scholar]
- 28. Heneghan MA, McFarlane IG. Current and novel immunosuppressive therapy for autoimmune hepatitis. Hepatology. 2002; 35: 7–13. [DOI] [PubMed] [Google Scholar]
- 29. Chmiel KD, Suan D, Liddle C et al. Resolution of severe ipilimumab‐induced hepatitis after antithymocyte globulin therapy. J. Clin. Oncol. 2011; 29: e237–40. [DOI] [PubMed] [Google Scholar]
- 30. Akimoto Y, Ishihara Y, Toyosawa J, Aoki H, Kato H. Azathioprine in steroid refractory immune checkpoint inhibitor‐induced cholangitis: a case report. Tando. 2020; 34: 223–31. [Google Scholar]
- 31. Ziogas DC, Gkoufa A, Cholongitas E et al. When corticosteroids are not enough in immune‐related hepatitis: current clinical challenges discussed on the basis of a case report. J. Immunother. Cancer. 2020; 8: e001322. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.


